A Murine Model of Glioblastoma Initiating Cells and Human Brain Organoid Xenograft for Photodynamic Therapy Testing
Abstract
1. Introduction
2. Results
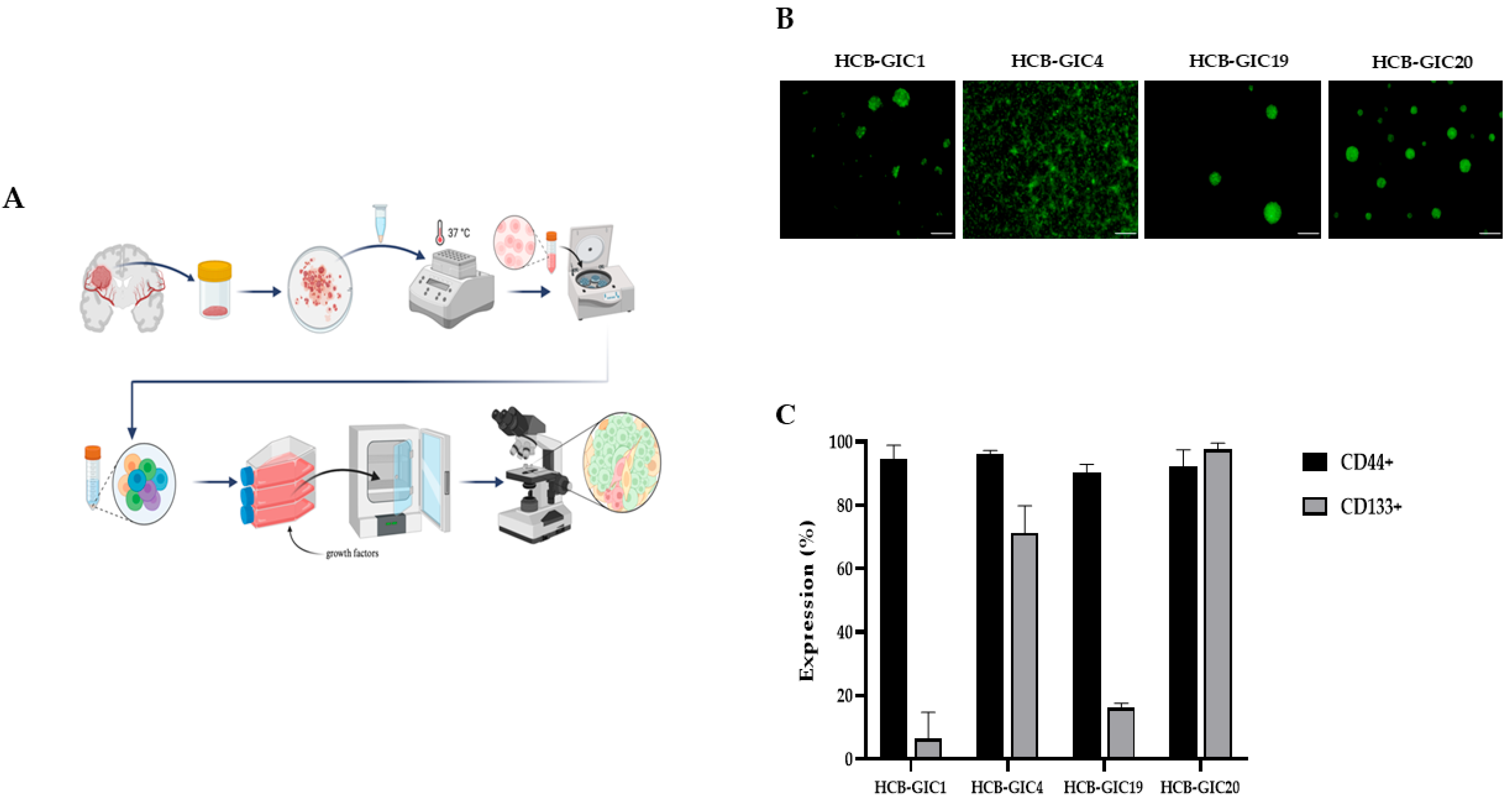
2.1. Patient-Derived HCB-GICs Malignant Phenotype
2.2. Patient-Derived HCB-GICs Genotype
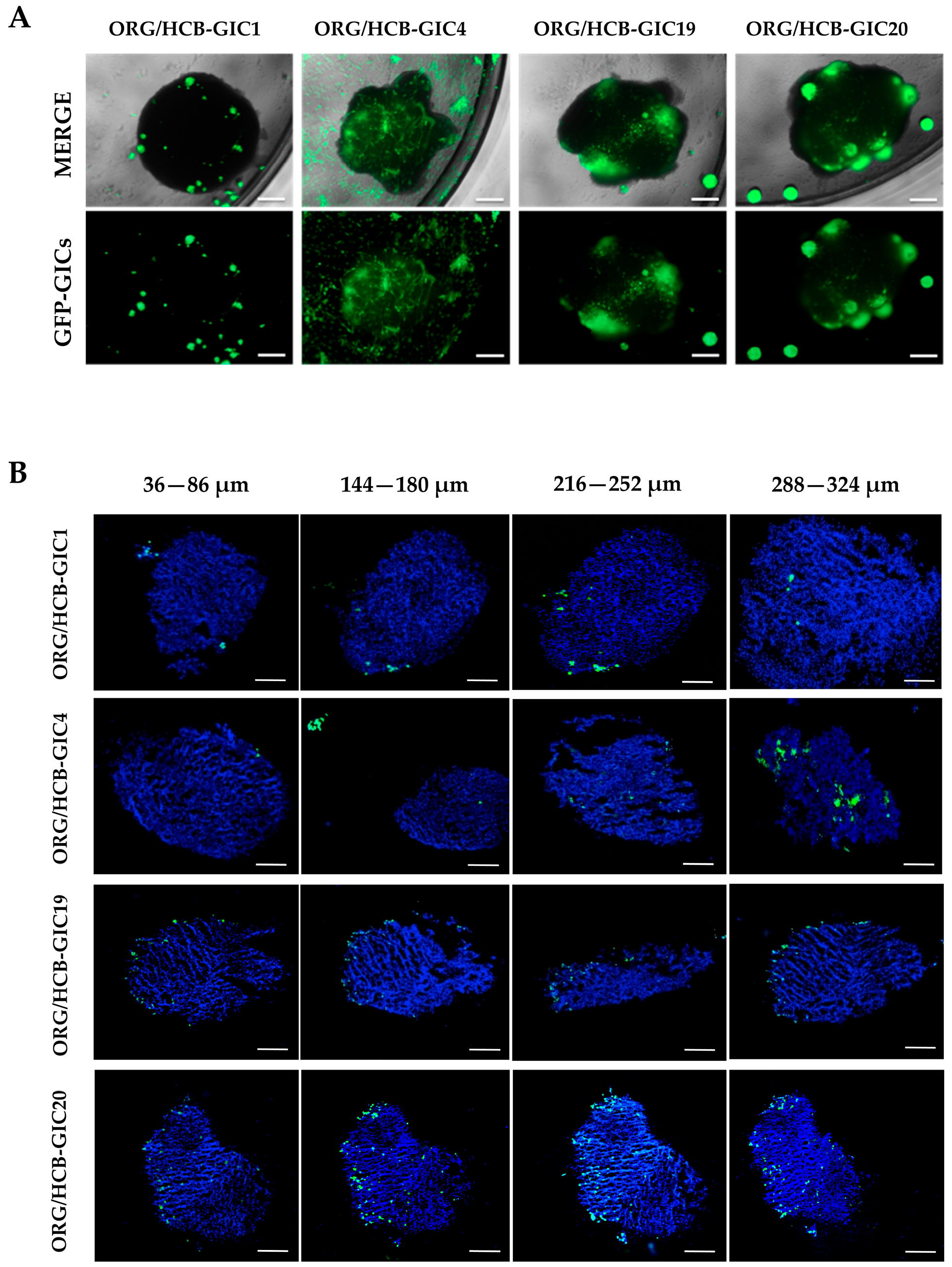
2.3. Organoid Cell Composition and HCB-GICs Differential Phenotype Determined Their Growth on Cerebral Organoid Co-Cultures
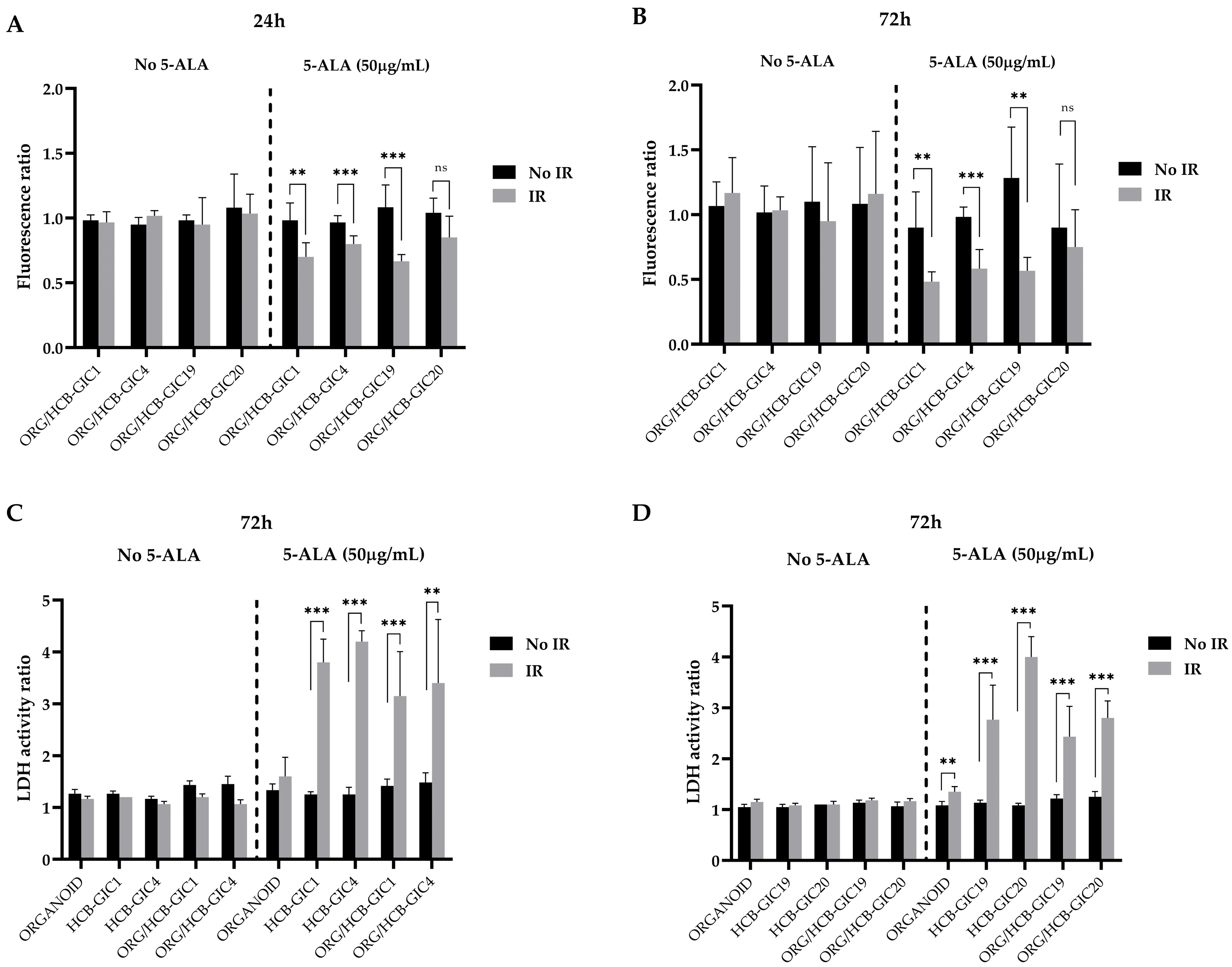
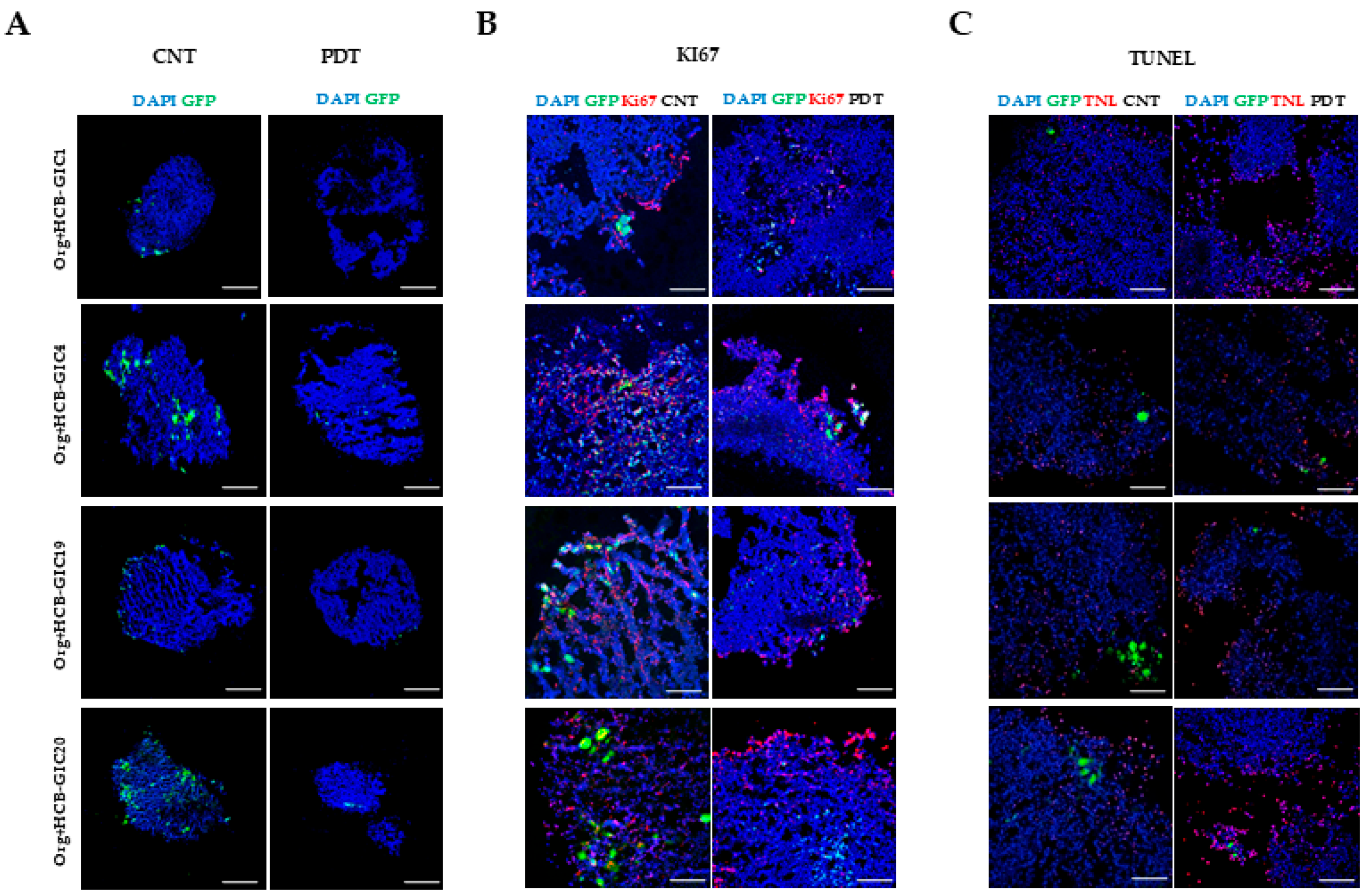
2.4. Photodynamic Therapy Effects In Vitro: HCB-GICs Infiltrating Brain Organoids
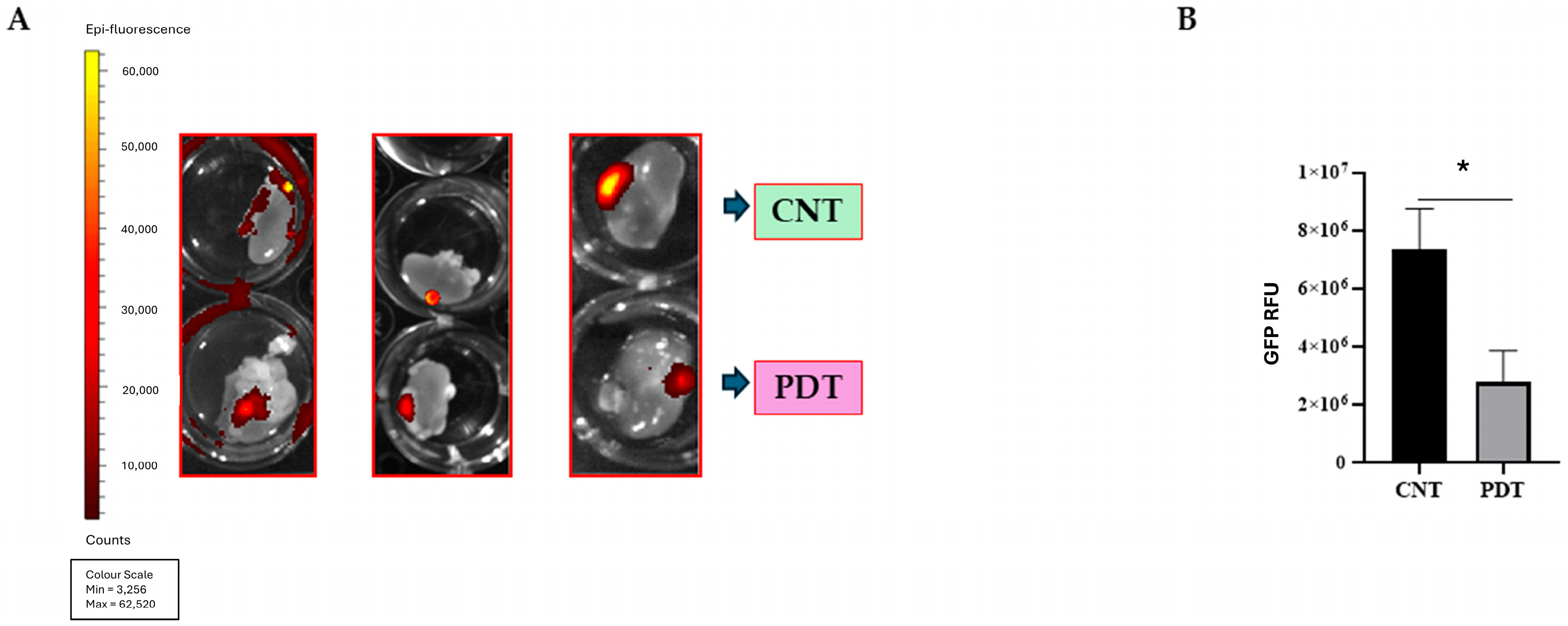
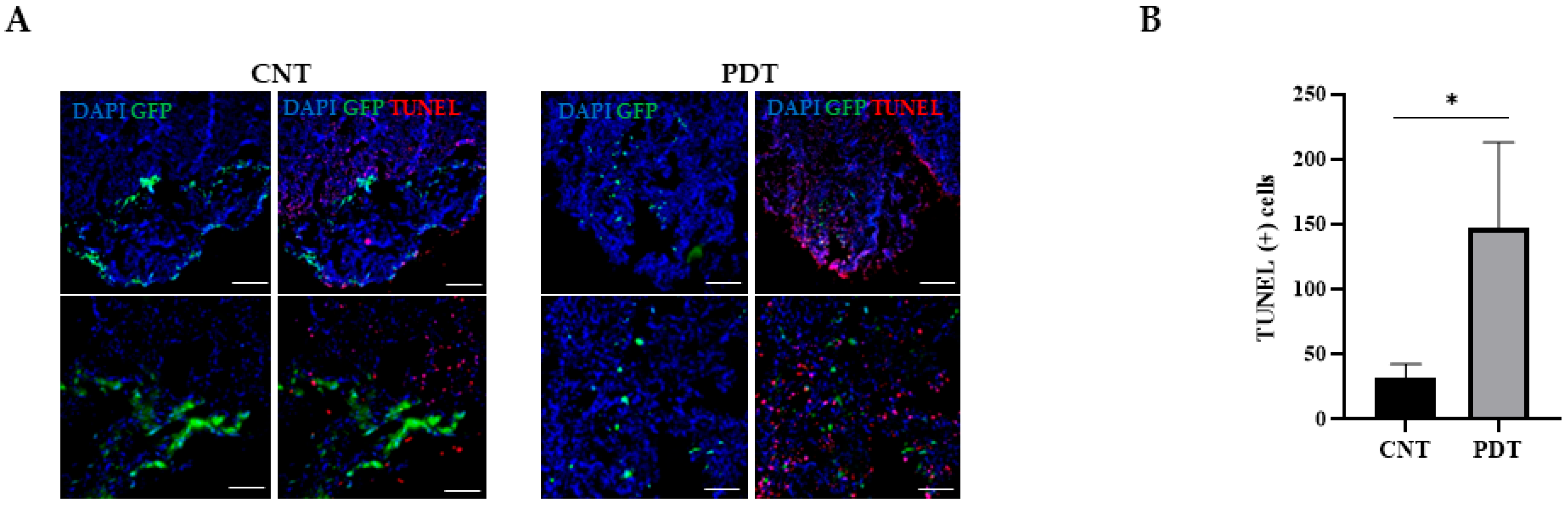
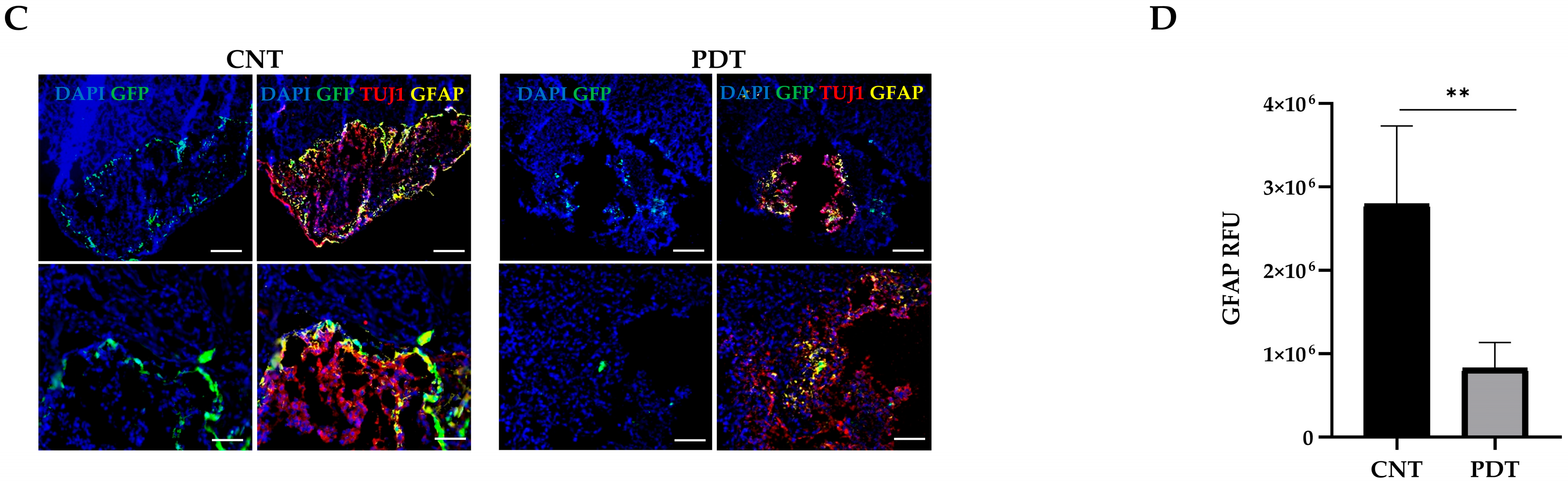
2.5. Photodynamic Therapy Effects In Vivo: HCB-GICs-Brain-Organoid Xenografts
3. Discussion
3.1. A New Concept in GB Preclinical Investigation Models
3.2. Profiling Patient-Specific HCB-GIC Cell Lines
3.3. Evaluating 5-ALA PDT Effect on HCB-GICs-Organoid In Vitro Model
3.4. Evaluating 5-ALA PDT Effect on HCB-GICs-Organoid In Vivo Model
3.5. Strengths and Limitations of the GICs-Organoid Xenograft Model
3.6. Immunological Implications of 5-ALA PDT Therapy
3.7. Limitations of the Study
4. Materials and Methods
4.1. Obtention of Glioblastoma Cell Lines and Organoids
4.2. Molecular Profiling of HCB-GICs
4.3. Cellular Components of Human Brain Organoids and GICs Co-Cultures
4.4. 5-ALA/PDT Testing In Vitro: HCB-GICs & Brain-Organoid Co-Cultures
4.5. Determination of PDT Efficacy In Vitro
4.6. Animal Subjects
4.7. 5-ALA/PDT Testing In Vivo: HCB-GICs Human-Brain-Organoid Xenograft
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GB | Glioblastoma |
| PDT | Photodynamic therapy |
| GIC | Glioblastoma initiating cells |
| CNS | Central nervous system |
| 5-ALA | 5-aminolevulinic acid |
| PDD | Photodynamic diagnosis |
| PS | Photosensitizer |
| PFS | Progression-free survival |
| CNA | Copy number alterations |
| cnLOH | Copy neutral loss of heterozygosity |
| RFU | Relative fluorescence units |
| MDM1/2 | Mouse double-minute 1 and 2 |
| GFAP | Glial fibrillary acid protein |
| TUJ1 | Tubulin betta class 3 |
| CD44 | Cluster of differentiation-44 |
| CD133 | Cluster of differentiation-133 |
| MGMT | O6-methylguanine-DNA methyltransferase |
| CDK4/6 | Cyclin-dependent kinase 4 and 6 |
| CDKN2A/B | Cyclin-dependent kinase inhibitor 2A/B |
| CCND2 | Cyclin D2 |
| Rb | Retinoblastoma protein |
| PIK3CA | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha |
| PIK3R | Phosphoinositide-3-kinase regulator |
| PTEN | Phosphatase and tensin homolog |
| EGFR | Epidermal growth factor receptor |
| PDGFR1 | Platelet derived growth factor receptor 1 |
| NF1 | Neurofibromin |
| SOX2 | Sex determining region Y-box 2 |
| LDH | Lactate Dehydrogenase |
| NSG | NOD scid gamma mouse |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP Nick End Labeling assay |
| WHO | World health organization |
| HCB | Hospital Clínic de Barcelona |
Appendix A. Patient-Derived HCB-GICs: Clinical Features
Appendix B. Primary Cell Lines Establishment
Appendix C
Appendix C.1. DNA Extraction and Analysis
Appendix C.2. Genetic Assessment
Appendix C.3. Double Immunofluorescence (IF) Analysis
Appendix C.4. TUNEL Analysis
Appendix C.5. Flow Cytometry
Appendix D
Appendix D.1. In Vivo Model of Renal Subcapsular HCB-GICs Brain-Organoid Engraftment
Appendix D.2. In Vivo 5-ALA/PDT Treatment
References
- Sanai, N.; Polley, M.Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef]
- Perez, A.; Huse, J.T. The Evolving Classification of Diffuse Gliomas: World Health Organization Updates for 2021. Curr. Neurol. Neurosci. Rep. 2021, 21, 67. [Google Scholar] [CrossRef]
- Diksin, M.; Smith, S.J.; Rahman, R. The Molecular and Phenotypic Basis of the Glioma Invasive Perivascular Niche. Int. J. Mol. Sci. 2017, 18, 2342. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Mosteiro, A.; Pedrosa, L.; Ferrés, A.; Diao, D.; Sierra, À.; González, J.J. The Vascular Microenvironment in Glioblastoma: A Comprehensive Review. Biomedicines 2022, 10, 1285. [Google Scholar] [CrossRef]
- Wang, J.; Cazzato, E.; Ladewig, E.; Frattini, V.; Rosenbloom, D.I.S.; Zairis, S.; Abate, F.; Liu, Z.; Elliott, O.; Shin, Y.-J.; et al. Clonal evolution of glioblastoma under therapy. Nat. Genet. 2016, 48, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, C.; Beck, J.A.; Horikawa, I.; Obiorah, I.E.; Von Muhlinen, N.; Vojtesek, B.; Lane, D.P.; Grunseich, C.; Chahine, J.J.; Ames, H.M.; et al. Radiation-induced astrocyte senescence is rescued by Δ133p53. Neuro-Oncology 2019, 21, 474–485. [Google Scholar] [CrossRef]
- Krammer, B.; Plaetzer, K. ALA and its clinical impact, from bench to bedside. Photochem. Photobiol. Sci. 2008, 7, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Pottier, R.H. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B Biol. 1992, 14, 275–292. [Google Scholar] [CrossRef]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2019, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, A.; Sofi, R.; Rahman, R.; Smith, S.J.; Teschemacher, A.G.; Kasparov, S. Using Light for Therapy of Glioblastoma Multiforme (GBM). Brain Sci. 2020, 10, 75. [Google Scholar] [CrossRef]
- Pedrosa, L.; Bedia, C.; Diao, D.; Mosteiro, A.; Ferrés, A.; Stanzani, E.; Martínez-Soler, F.; Tortosa, A.; Pineda, E.; Aldecoa, I.; et al. Preclinical Studies with Glioblastoma Brain Organoid Co-Cultures Show Efficient 5-ALA Photodynamic Therapy. Cells 2023, 12, 1125. [Google Scholar] [CrossRef]
- Van de Mark, D.; Kong, D.; Loncarek, J.; Stearns, T.; Chang, F. MDM1 is a microtubule-binding protein that negatively regulates centriole duplication. Mol. Biol. Cell 2015, 26, 3788–3802. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Varn, F.S.; Park, S.-H.; Yoon, B.W.; Park, H.R.; Lee, C.; Verhaak, R.G.W.; Paek, S.H. Perspective of mesenchymal transformation in glioblastoma. Acta Neuropathol. Commun. 2021, 9, 50. [Google Scholar] [CrossRef]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W.; et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006, 9, 391–403. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- da Silva, B.; Mathew, R.K.; Polson, E.S.; Williams, J.; Wurdak, H. Spontaneous Glioblastoma Spheroid Infiltration of Early-Stage Cerebral Organoids Models Brain Tumor Invasion. SLAS Discov. Adv. Sci. Drug Discov. 2018, 23, 862–868. [Google Scholar] [CrossRef]
- Sloan, A.R.; Silver, D.J.; Kint, S.; Gallo, M.; Lathia, J.D. Cancer stem cell hypothesis 2.0 in glioblastoma: Where are we now and where are we going? Neuro-Oncology 2024, 26, 785–795. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Jill, P.; Alexe, G.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Pavon, L.F.; Sibov, T.T.; de Oliveira, D.M.; Marti, L.C.; Cabral, F.R.; de Souza, J.G.; Boufleur, P.; Malheiros, S.M.; Neto, M.A.d.P.; da Cruz, E.F.; et al. Mesenchymal stem cell-like properties of CD133+ glioblastoma initiating cells. Oncotarget 2016, 7, 40546–40557. [Google Scholar] [CrossRef]
- Inoue, A.; Ohnishi, T.; Nishikawa, M.; Ohtsuka, Y.; Kusakabe, K.; Yano, H.; Tanaka, J.; Kunieda, T.A. Narrative Review on CD44’s Role in Glioblastoma Invasion, Proliferation, and Tumor Recurrence. Cancers 2023, 15, 4898. [Google Scholar] [CrossRef]
- Bian, S.; Repic, M.; Guo, Z.; Kavirayani, A.; Burkard, T.; Bagley, J.A.; Krauditsch, C.; Knoblich, J.A. Author Correction: Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods 2018, 15, 748, Correction in Nat. Methods 2018, 15, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Vermandel, M.; Quidet, M.; Vignion-Dewalle, A.-S.; Leroy, H.-A.; Leroux, B.; Mordon, S.; Reyns, N. Comparison of different treatment schemes in 5-ALA interstitial photodynamic therapy for high-grade glioma in a preclinical model: An MRI study. Photodiagn. Photodyn. Ther. 2019, 25, 166–176. [Google Scholar] [CrossRef]
- Curnow, A.; Haller, J.; Bown, S. Oxygen monitoring during 5-aminolaevulinic acid induced photodynamic therapy in normal rat colon. J. Photochem. Photobiol. B Biol. 2000, 58, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.O.; Piffaretti, D.; Marchi, F.; Burgio, F.; Faia-Torres, A.B.; Paganetti, P.; Pinton, S.; Pieles, U.; Reinert, M. Epithelial growth factor receptor expression influences 5-ALA induced glioblastoma fluorescence. J. Neuro-Oncol. 2017, 133, 497–507. [Google Scholar] [CrossRef]
- Ghantasala, S.; Bhat, A.; Agarwal, U.; Biswas, D.; Bhattarai, P.; Epari, S.; Moiyadi, A.; Srivastava, S. Deep proteome investigation of high-grade gliomas reveals heterogeneity driving differential metabolism of 5-aminolevulinic acid. Neuro-Oncol. Adv. 2023, 5, vdad065. [Google Scholar] [CrossRef]
- Pantazopoulou, V.; Jeannot, P.; Rosberg, R.; Berg, T.J.; Pietras, A. Hypoxia-Induced Reactivity of Tumor-Associated Astrocytes Affects Glioma Cell Properties. Cells 2021, 10, 613. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’cAllaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. Roles of neuropathology-associated reactive astrocytes: A systematic review. Acta Neuropathol. Commun. 2023, 11, 42. [Google Scholar] [CrossRef]
- Perelroizen, R.; Philosof, B.; Budick-Harmelin, N.; Chernobylsky, T.; Ron, A.; Katzir, R.; Shimon, D.; Tessler, A.; Adir, O.; Gaoni-Yogev, A.; et al. Astrocyte immunometabolic regulation of the tumour microenvironment drives glioblastoma pathogenicity. Brain 2022, 145, 3288–3307. [Google Scholar] [CrossRef]
- Frenster, J.D.; Placantonakis, D.G. Establishing Primary Human Glioblastoma Tumorsphere Cultures from Operative Specimens. Methods Mol. Biol. 2018, 1741, 63–69. [Google Scholar] [CrossRef]
- Han, J.-h.; Yoon, J.S.; Chang, D.-Y.; Cho, K.G.; Lim, J.; Kim, S.-S.; Suh-Kim, H. CXCR4-STAT3 Axis Plays a Role in Tumor Cell Infiltration in an Orthotopic Mouse Glioblastoma Model. Mol. Cells 2020, 43, 539–550. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosteiro, A.; Diao, D.; Bedia, C.; Pedrosa, L.; Caballero, G.A.; Aldecoa, I.; Mallo, M.; Solé, F.; Sevilla, A.; Ferrés, A.; et al. A Murine Model of Glioblastoma Initiating Cells and Human Brain Organoid Xenograft for Photodynamic Therapy Testing. Int. J. Mol. Sci. 2025, 26, 8889. https://doi.org/10.3390/ijms26188889
Mosteiro A, Diao D, Bedia C, Pedrosa L, Caballero GA, Aldecoa I, Mallo M, Solé F, Sevilla A, Ferrés A, et al. A Murine Model of Glioblastoma Initiating Cells and Human Brain Organoid Xenograft for Photodynamic Therapy Testing. International Journal of Molecular Sciences. 2025; 26(18):8889. https://doi.org/10.3390/ijms26188889
Chicago/Turabian StyleMosteiro, Alejandra, Diouldé Diao, Carmen Bedia, Leire Pedrosa, Gabriela Ailén Caballero, Iban Aldecoa, Mar Mallo, Francesc Solé, Ana Sevilla, Abel Ferrés, and et al. 2025. "A Murine Model of Glioblastoma Initiating Cells and Human Brain Organoid Xenograft for Photodynamic Therapy Testing" International Journal of Molecular Sciences 26, no. 18: 8889. https://doi.org/10.3390/ijms26188889
APA StyleMosteiro, A., Diao, D., Bedia, C., Pedrosa, L., Caballero, G. A., Aldecoa, I., Mallo, M., Solé, F., Sevilla, A., Ferrés, A., Cabrera, G., Muñoz-Tudurí, M., Centellas, M., Pineda, E., Jiménez, À. S., & González Sánchez, J. J. (2025). A Murine Model of Glioblastoma Initiating Cells and Human Brain Organoid Xenograft for Photodynamic Therapy Testing. International Journal of Molecular Sciences, 26(18), 8889. https://doi.org/10.3390/ijms26188889







