1. Introduction
Matrix metalloproteinases (MMPs) are a large family of zinc-dependent endopeptidases [
1]. As a family, MMPs can cleave virtually any component of the extracellular matrix to facilitate cell migration and affect cellular signaling, thereby regulating cell proliferation, differentiation, and cell death [
2]. In addition to cleaving the structural components of the extracellular matrix, MMPs are involved in the processing and signaling of growth factors and their receptors, cytokines, chemokines, adhesion molecules, and a variety of other enzymes such as caspases [
3,
4]. A subset of MMPs, often referred to as gelatinases, are composed of MMP-2 and MMP-9 and are capable of collagen degradation. MMP-2 and MMP-9 protein levels have been reported to be altered in the blood, skin, and/or cerebrospinal fluid (CSF) of ALS patients or in animal models of ALS [
5,
6,
7,
8,
9,
10]. Beuche and colleagues, using enzyme-linked immunosorbent assay (ELISA), identified increased levels of MMP-9 protein in the serum but not the CSF of ALS when compared to controls [
5]. A limited attempt was made using gelatin zymography to look at the active form of MMP-9, but only a few samples were examined, and no definitive conclusions were made. Lorenzl and colleagues measured the levels of MMP-2 and MMP-9 in the CSF, in addition to other biomarkers, and found no significant differences as compared to healthy controls [
6]. Demestre and colleagues reported elevated levels of both the inactive (proform) and active forms of MMP-9 in the serum of ALS as compared to healthy controls [
7]. Niebroj-Dobosz and colleagues reported significantly elevated levels of both MMP-9 and MMP-2 in the serum of ALS as compared to healthy controls. They also looked at the levels in CSF and found significantly elevated levels of MMP-2 but significantly decreased levels of MMP-9 [
8]. Fang and colleagues found significantly elevated levels of MMP-9 in the skin and CSF of ALS, but not in serum, as compared to healthy controls [
9]. They also examined MMP-2 and found no significant differences in serum, CSF, or skin as compared to healthy controls [
9]. Overall, levels of MMP-9 were typically elevated in ALS serum but were reduced or showed no change in CSF, whereas MMP-2 levels exhibited more variability across studies.
The current study seeks to further define MMP-2 and MMP-9 protein levels and enzyme activity in the serum and CSF of ALS and compare their levels to those in the serum of age-matched, healthy controls (HCs) and CSF of other neurological diseases (OND). We also measured other biomarkers associated with ALS disease progression, including neurofilament light (NfL), serum creatinine, and serum cystatin C [
11,
12,
13,
14,
15,
16,
17,
18]. Most importantly, we sought to determine whether the active forms of MMP-2 and MMP-9 are present in serum and/or CSF of ALS and whether they are elevated compared to HC or OND. Answering this question is more challenging than it first may seem. Enzyme-linked immunosorbent assay (ELISA) cannot differentiate between the active and proforms of these metalloenzymes, so other methods such as gelatin zymography and/or Western blotting have been employed [
5,
6,
7,
8,
9]. Those methods rely on the use of protein standards to confirm the presence or absence of these active forms via their band position on a gel that corresponds to their appropriate molecular weights. While these methods have allowed for the identification of the proforms of MMP-9 and MMP-2 with some degree of confidence, this has not been the case for their active forms. In many instances, protein standards for the active forms of MMP-2 and MMP-9 do not always align well with some of the observed bands presumed to be the active forms of MMP-2 and/or MMP-9 [
19,
20,
21,
22]. As such, these new observed bands have been attributed to being a partially cleaved isoform (i.e., formation of a 65 kD isoform of MMP-9 as a result of the cleavage of the C-terminal hemopexin portion), an active dimer/trimer form, or a charged variant. Other potential sources for these new bands have been attributed to being complexes composed of active MMP-9 and its corresponding tissue inhibitor of metalloproteinase-1 (TIMP1) or with neutrophil gelatinase-associated lipocalin (NGAL) [
6,
8,
20]. TIMPs are proteins that act as natural inhibitors of MMPs and serve as regulators of their activity. It has therefore been assumed that an observed increase in TIMP1 and/or TIMP2 in a patient’s blood implies an inflammatory state in which the body is responding to an increase in the levels of the active forms of MMP-2 and/or MMP-9 [
8]. NGAL is a small protein involved in the innate immune response, primarily functioning to bind and transport iron, and serves as an early biomarker for acute kidney injury and inflammation. An increase in the levels of NGAL has been thought by some to be a result of an increase in active MMP-9, resulting in the formation of an inactive NGAL/MMP-9 complex [
23]. As such, rather than using methods employing gel electrophoresis, we utilized a method that uses a capture antibody for both the pro and active forms of MMP-2 and/or MMP-9, followed by the addition of a labeled peptide substrate that generates a fluorescent signal that is quantified upon cleavage by active MMP-2 or MMP-9. In this way, one can quantify the amount of active form without needing to know what configuration (i.e., isoform, dimer/trimer, or charged variant) the protein is in. If active MMP-2 and/or MMP-9 were present and found elevated in ALS, another goal of our work was to further elucidate the state of their active site(s) to determine if they are accessible or are blocked from further binding/activity. Lastly, if active forms of MMP-2 and/or MMP-9 were present in ALS, a final goal was to determine how their levels compare to those of other inflammatory and/or neurodegenerative diseases. While this study focuses on understanding the relationship between MMP-2/-9 and ALS, other neurodegenerative diseases, such as Parkinson’s and Alzheimer’s disease, have also been reported to have elevated levels of various MMPs [
24]. As such, it was of interest to observe, in a limited manner, how any observed MMP changes in ALS compared to those in other neurodegenerative and non-neurological diseases, specifically diabetic nephropathy, which has an inflammatory component and may have bidirectional interactions between the kidneys and the nervous system.
3. Discussion
MMP-2 and MMP-9 exist in human biofluids and tissues as a mixture of both active and inactive forms (i.e., proforms). A cysteine located in the N-terminal pro-domain of the proform binds to the zinc atom in the active site of the enzyme, thus maintaining latency. Activation of the proform requires a disruption of the cysteine linkage with the zinc atom (via peptide fragment cleavage containing the cysteine or oxidation of the cysteine thiol), thus exposing the catalytic site. While it is rather straightforward to measure both the active forms and proforms via ELISA, differentiating the levels of the active form from that of the proform is somewhat challenging. Work by Aquilus and others have found that techniques such as Western blotting and gelatin zymography cannot, in many cases, adequately determine the levels of the various active forms of MMP-2 and MMP-9, as their locations on either Western or zymography gels do not always align with commercial standards [
27]. However, a different method was used for this study that utilizes a combination of selective antibody capture of both the pro and active forms of MMP-2 or MMP-9, followed by measuring the fluorescence of a peptide substrate cleaved by the bound active forms to thereby quantify their levels. In this way, one does not need to know the exact make-up of the active form (i.e., dimer or monomer with partial or total removal of C-terminal hemopexin-like domain) but only that it is functionally capable of proteolytically cleaving the FRET-labeled peptide.
For example, to measure active MMP-9 from a potential mixture containing various active forms of MMP-9 mixed with ProMMP-9 within serum or CSF, a Fluorokine E assay was used, which first exposes an immobilized anti-MMP-9 antibody that recognizes and captures all the active variants of MMP-9 and ProMMP-9 forms. Once all the forms have been captured, a FRET (fluorescence resonance energy transfer) peptide that is labeled with a fluorescence recorder is exposed to the bound enzymes. Only the active forms can cleave the FRET peptide, which in turn generates fluorescence upon peptide cleavage. The fluorescence measurement is then related to the concentration of the active forms of MMP-9 in the sample. By using a combination of standard ELISA for measuring total MMP-9 and the Fluorokine E assay for measuring only active MMP-9, one can obtain a clear picture of the levels of active and non-active forms. In this same manner, the level of active MMP-2 was measured using a slightly different assay (Quickzyme assay), which, like the Fluorokine E assay, uses an antibody to capture all of the active and non-active forms of MMP-2. However, unlike the Fluorokine E assay, the Quickzyme assay requires the active forms of MMP-2 to cleave and activate another proprietary Pro-Enzyme, which then cleaves the FRET peptide. This extra step enhances the specificity of the assay but also reduces the assay’s sensitivity.
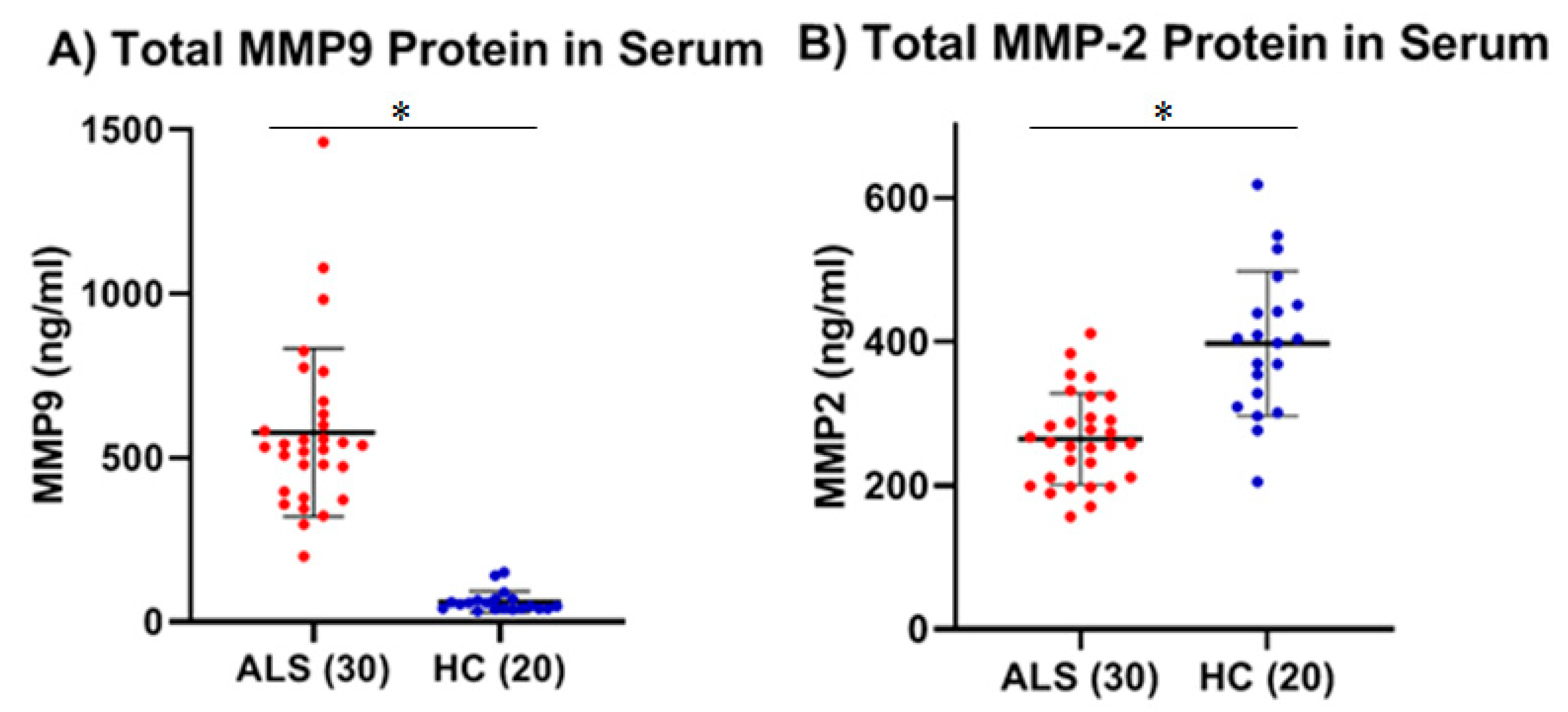
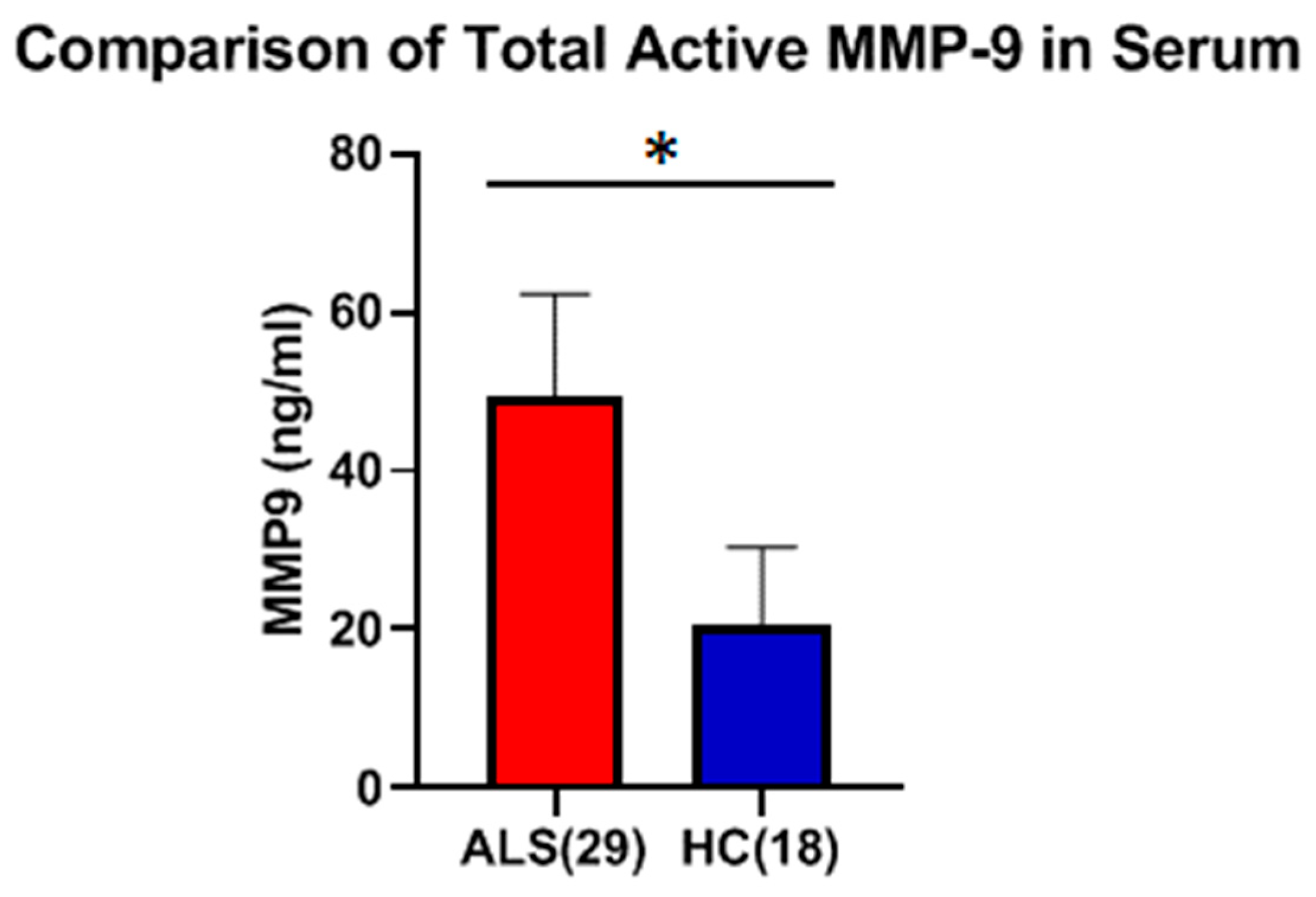
Using these approaches, the levels of total and active MMP-9 were measured in both the serum and CSF in ALS (Group 1,
Table 1) and compared to HC (Group 2,
Table 1). The results showed that there was a significant increase in both total (two studies, 7.5- and 9.5-fold; both
p < 0.0001) and active (2.4-fold;
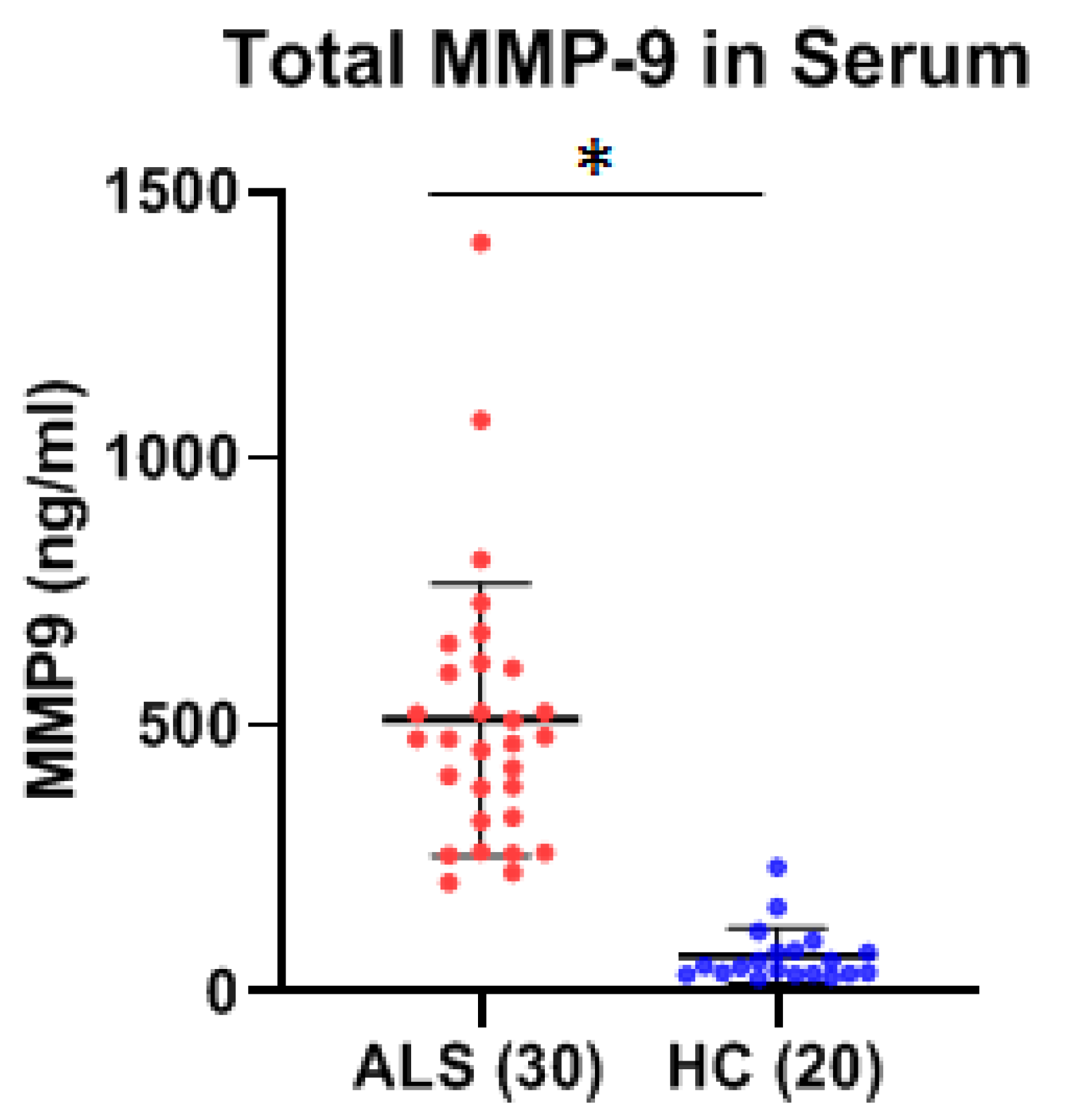
p < 0.0001) MMP-9 in the serum of ALS as compared to HC. Increased serum MMP-9 in ALS was confirmed in a separate cohort of serum samples (
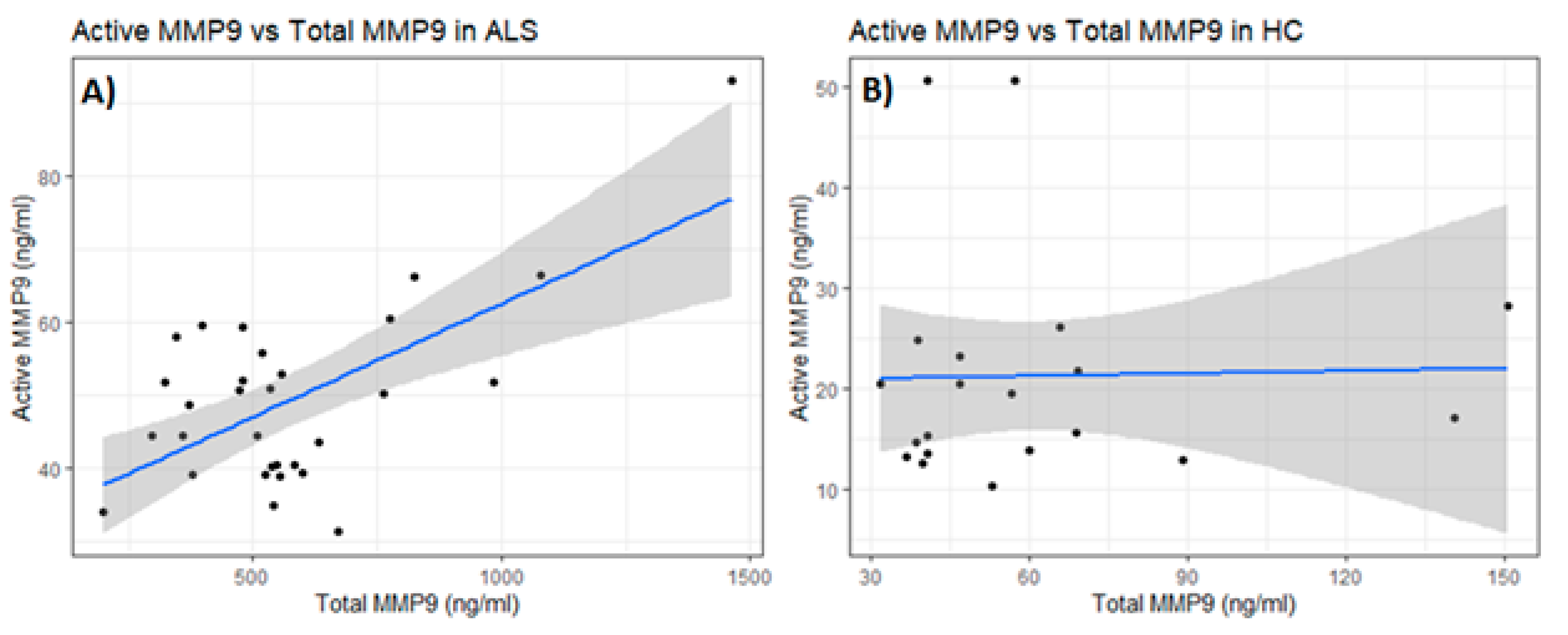
Figure 3). There was a positive correlation via Pearson’s correlation (Pearson’s
p = 0.0002, Spearman’s rank
p = 0.27) between the levels of total and active MMP-9 in ALS (Group 3,
Table 1) that was not observed in HC (Group 5,
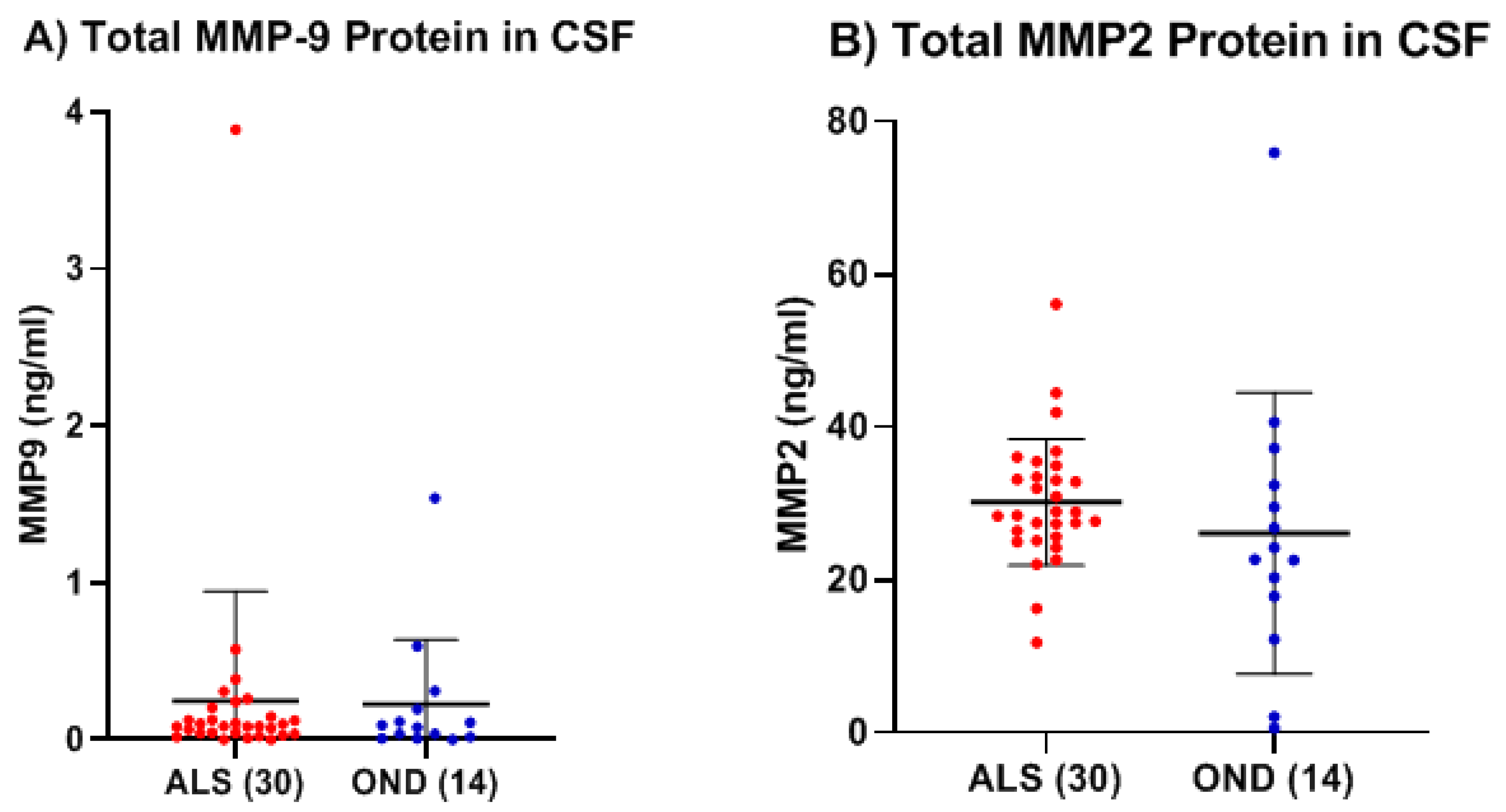
Table 1). In the CSF, low levels of both total and active MMP-9 were measured in both ALS (Group 4,
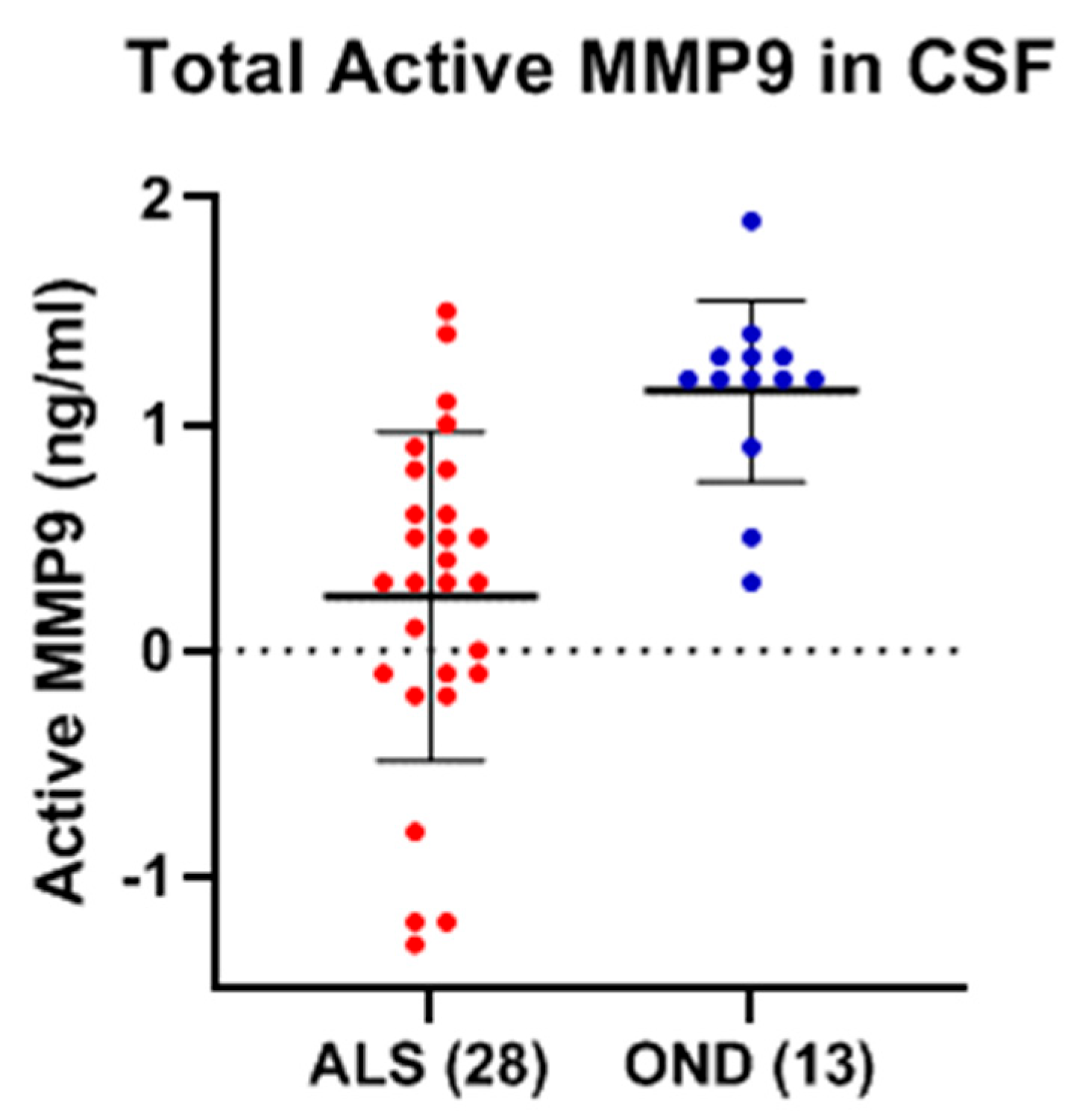
Table 1) and OND (Group 6,
Table 1), but there were no significant differences between the two groups, nor any correlation between their respective active and total MMP-9 levels, either in the ALS or OND groups. Comparing CSF from a specific neurological disease such as ALS to an OND group representing various other neurological diseases can increase biomarker variability due to diverse underlying pathologies. This added variability may obscure disease-specific signals, reducing the ability to detect meaningful differences. However, comparing MMP-9 in ALS with an OND group helps assess the specificity of a biomarker by determining whether it can distinguish the target disease, in this case, ALS, from other neurological conditions with overlapping symptoms or if the biomarker reflects a broader neurogenerative or inflammatory processes. From our results it is clear that the levels of total MMP-9 are not specific to ALS and that other neurological diseases are undergoing processes that are generating similar MMP-9 levels. How do these levels compare with the CSF of healthy adults? CSF from age-matched healthy controls are rare and were not available for this study but could be explored in future studies. However, in prior reports in the literature, where ELISA techniques had been optimized for the detection of MMP-9 in the CSF among recruited confirmed healthy adults (n = 27, median age = 33 years, range = 26–43 years of age), the levels of MMP-9 ranged from 0.156 to 0.189 ng/ml, which is lower than our observed mean MMP-9 levels for either the ALS (0.25 ng/ml) or OND (0.22 ng/ml) groups [
28].
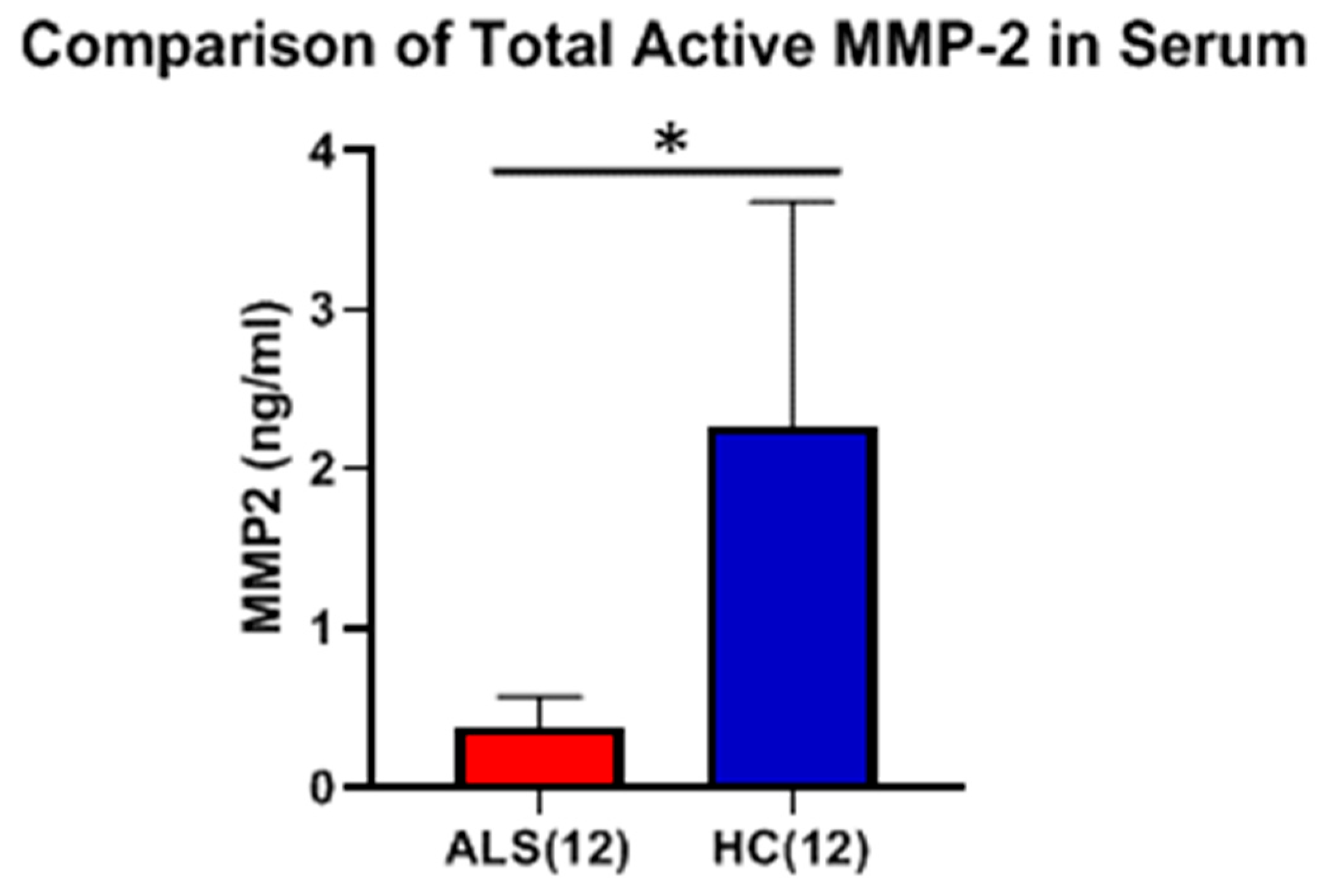
One observes a small, but significant, decrease in total MMP-2 (two studies, 26% and 33%; p < 0.001 and p = 0.0001, respectively) in the serum of ALS versus HC using standard ELISA. While active MMP-2 was detected in the serum of ALS using the Quickzyme assay, the levels were very low and not statistically different from HC. Unlike MMP-9, there was no observed correlation in serum between the levels of total and active MMP-2 in ALS nor in HC. In the CSF, the levels of total MMP-2 in ALS were not statistically different from those of OND. The levels of active MMP-2 in the CSF of both ALS and HC, while detectable in some cases, were too low to be measured with any degree of confidence. It is possible that the low levels of active MMP-2 measured in both serum and CSF may be due to the unique nature of the assay used, as it relies on an additional enzymatic step (compared to the assay used to measure active MMP-9), which reduces the overall sensitivity of the measurement.
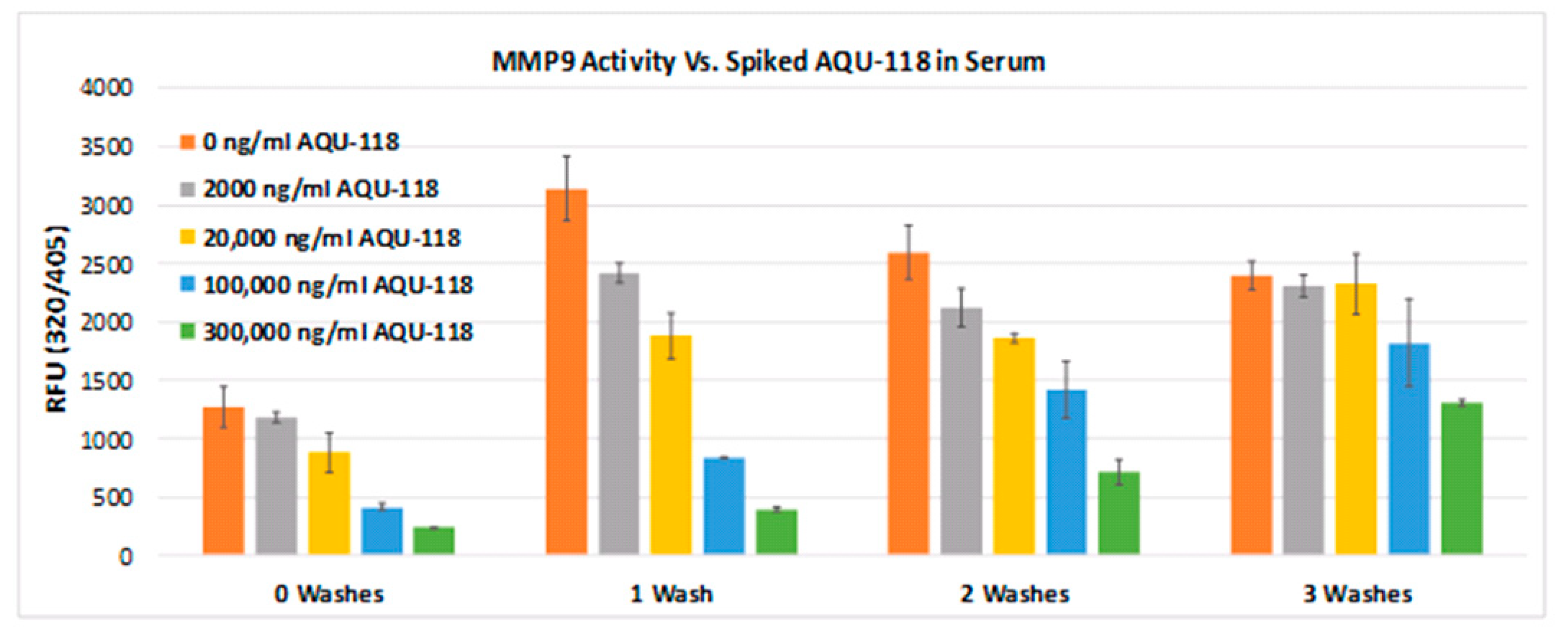
The finding that one can detect active MMP-9 in both the serum and CSF of ALS, with a significantly elevated level of active MMP-9 in the serum compared to HC, provides a potential pathway by which critical proteins may be broken down in the ALS disease state. However, as was mentioned in the introduction, there has been speculation that any active MMP-9 would most likely be regulated via the binding of an inhibitor protein such as TIMP1 and/or NGAL. Because the standard assay conditions for the Fluorokine E assay utilize several wash steps that result in the release of any potential non-covalent binders from the active site, it is possible that regulator proteins could be bound to the MMP-9 active site but are simply washed off. As such, we sought to determine whether there is any indication of the active site being blocked by exposing the active MMP-9 protein without any wash steps as well as to a competitive inhibitor in situ. By varying the number of washes and concentration of spiked inhibitor and comparing it to the resulting fluorescence from the cleaved peptide, one can discern information about the in situ state of the MMP-9 active site. Focusing on our data where there are no washes and no spiked inhibitor, one observes some level of fluorescence as a result of peptide cleavage. This clearly implies that there is some percentage of MMP-9 active sites that are not blocked in any significant manner and are capable of protease activity. After one wash and no spiked inhibitor, one observes a significant rise in fluorescence, which at first glance may indicate that potential binders are equilibrating out of the active site. However, such a conclusion must be tempered by the fact that concentration effects could also be impacting the level of fluorescence. In other words, the washes may be removing biological components that may be quenching the fluorescence generated by the peptide cleavage step. One also observes that after the first wash, some fluorescence is lost, indicating that possibly some of the antibody-bound active MMP-9 may be dislodging and washing away. Our AQU-118 data suggests that active MMP-9 is capable of being inhibited in situ in a concentration-dependent manner by the dual active MMP-2/9 inhibitor, AQU-118. This result clearly indicates that the active site is not strongly blocked by the binding of a high-affinity ligand and is both capable of proteolytic activity and amenable to inhibition, making a case for its regulation via dosing with an MMP inhibitor such as AQU-118.
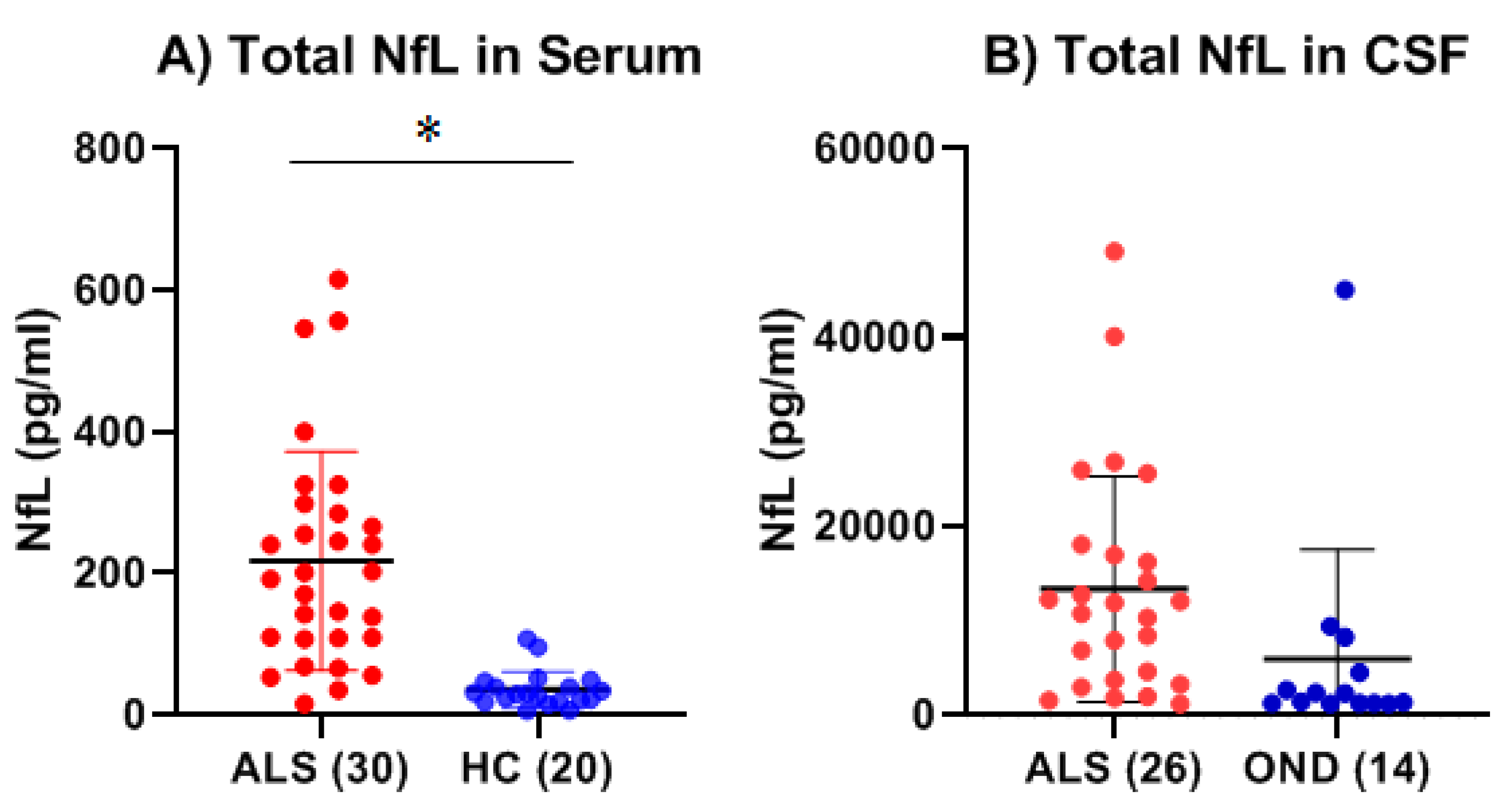
We detected significantly elevated serum NfL levels in ALS patients compared to healthy controls (HC) by as much as 6-fold. This is consistent with the existing literature identifying NfL as a sensitive and reliable biomarker for monitoring ALS progression [
12]. Its clinical significance was further underscored when it became the first biomarker used by the FDA to support the approval of Tofersen, a treatment for SOD1-ALS [
13]. However, no correlation was observed between the levels of total or active MMP-9 and the levels of NfL in either serum or CSF. One possible explanation is that these biomarkers originate from distinct inflammatory processes occurring in ALS. Although this may seem surprising, it aligns with findings from other studies on neuroinflammation. For example, in cases of optic neuritis associated with multiple sclerosis, elevated levels of both total MMP-9 and NfL were observed in the CSF of patients compared to healthy controls [
28]. However, while a strong correlation was found between MMP-9 and other markers such as CXCL10, no correlation was observed between MMP-9 and NfL. This led the authors to speculate that separate but parallel inflammatory processes were occurring [
28]. In the CSF, we did not observe a significant difference in NfL levels between ALS and OND groups, but these levels are significantly higher than those previously reported for NfL in the CSF of HC. For example, in one study, NfL in the CSF among recruited confirmed healthy adults (n = 27, median age = 33 years, range = 26–43 years of age), ranged from 1000 to 8532 pg/ml, which is lower than our observed mean NfL levels for the ALS (13,326 pg/ml) group [
28]. In another study where the recruited confirmed healthy adults (n = 75) were closer of age (mean age = 68.3 years ± 9.4 years) to the age in our study (mean 60.6 ± 5.5 years), the levels of NfL in the healthy control group ranged from 398 to 777 pg, which is even lower than the mean NfL observed in our study [
29].
Regarding the origins of the elevated levels of total and active MMP-9 in the serum, there has been speculation that a possible source of MMP-9 may be derived from the CNS through the breakdown of the blood–brain barrier [
30]. However, this is unlikely to be a driving factor since the quantity of total MMP-9 observed in the CSF of ALS is still only 0.04% of that found in serum, which seems rather low to account for such a large quantity in the serum. The markedly higher level of total MMP-9 in serum suggests that the reverse—MMP-9 migrating from serum to CSF—could be taking place. This is unlike the quantity of NfL, which was observed to be just over 60-fold higher in the CSF than in serum. Barring the possibility that the elevated levels of total and active MMP-9 in serum are solely derived from the CNS, it is more probable that the observed elevated levels are derived from peripheral tissues such as degenerating muscle. Others have speculated on muscle as a significant source of MMP-9, but evidence has been lacking [
5,
6]. It has been known that matrix metalloproteinases, such as MMP-2 and MMP-9, play a critical role in the homeostasis and maintenance of myofiber functional integrity and that inhibition of MMP-9 improves myofiber regeneration in rodent models of Duchenne muscular dystrophy [
31,
32,
33]. As such, we aimed to determine the level of muscle degeneration in ALS and see if this degeneration is related to the levels of total and/or active serum MMP-9.
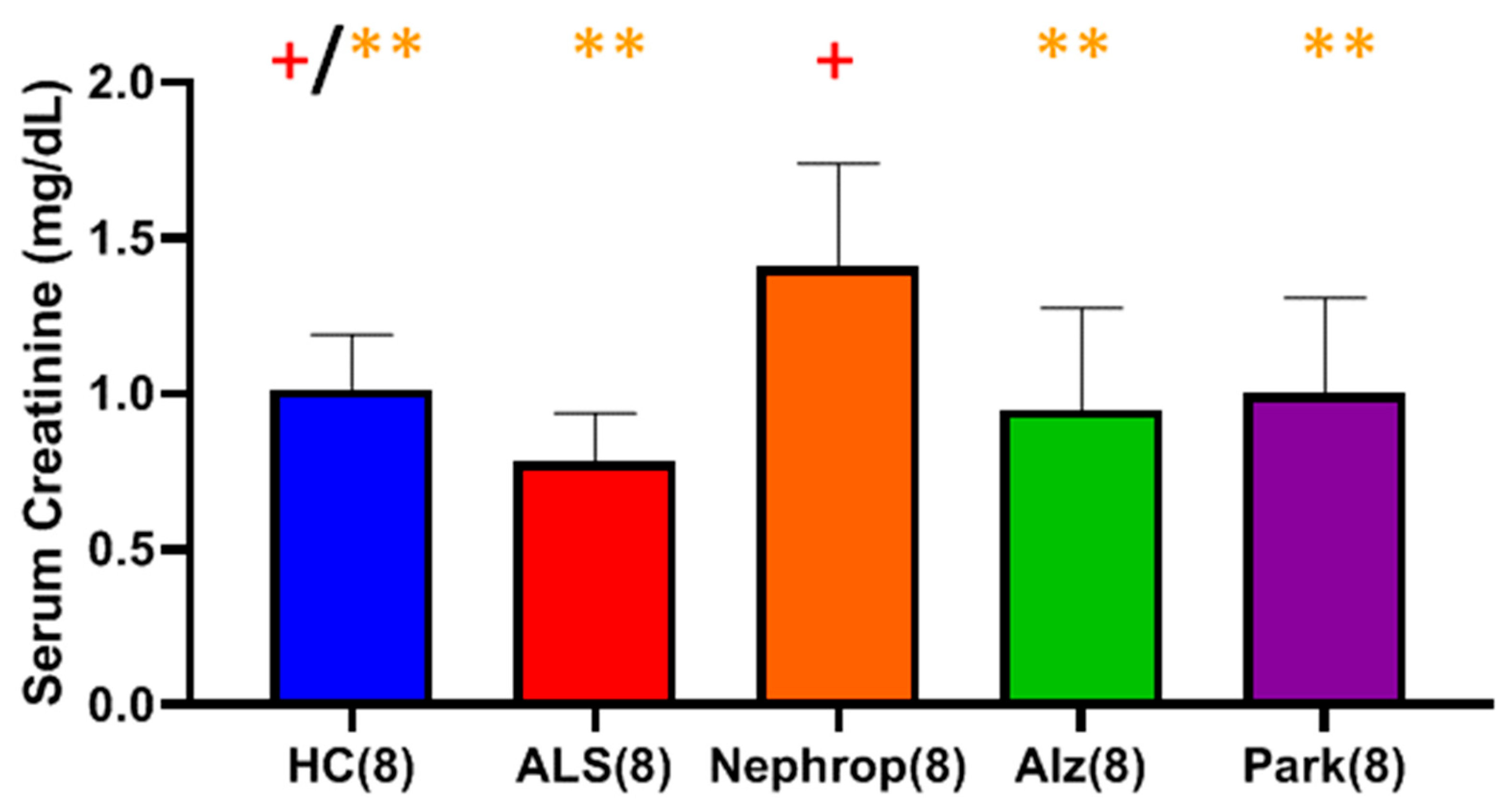
One way to determine muscle degeneration is to follow its mass via creatinine levels in the serum. Serum creatinine is a byproduct of the nonenzymatic breakdown of creatine phosphate, an energy source found in muscle tissue. It is produced at a relatively constant rate, with approximately 1–2% of muscle creatine converted to creatinine each day. Once formed, creatinine is transported through the bloodstream to the kidneys for excretion. Its levels in the blood are influenced primarily by an individual’s muscle mass and renal status [
14]. Several studies have reported significantly lower serum creatinine levels in ALS as compared to HC, with some studies noting reductions as early as one year before ALS diagnosis [
14,
15,
16]. These findings suggest that serum creatinine is a sensitive marker of ALS disease progression, with lower levels reflecting the loss of muscle mass. Our results confirmed that there is a statistically significant decrease of around 40% in the mean level of serum creatinine in ALS as compared to HC (
p < 0.0001). We observed no significant correlations between serum creatinine levels and NfL in ALS or in HC. We did observe a significant positive correlation between total serum MMP-9 levels and serum creatinine levels in ALS (Pearson’s
p = 0.028, Spearman’s rank
p = 0.005) that was not observed among HC (Pearson’s
p = 0.77, Spearman’s rank
p = 0.25). However, no correlation (positive or negative) was observed for active MMP-9 and serum creatinine. As such, a mechanism that can explain the positive correlation between total MMP-9 and serum creatinine within muscle remains unclear at present, though several possibilities exist. For instance, in the context of skeletal muscle injury, elevated serum MMP-9 levels may result from degranulating neutrophils that infiltrate the necrotic muscle early on, followed by macrophages that contribute additional MMP-9 as the inflammatory response progresses [
34]. This increase in MMP-9 reflects the breakdown of the local extracellular matrix. At the same time, muscle cell damage leads to a rise in serum creatinine, as it is continuously produced from muscle creatine and released when muscle fibers are lysed [
35]. In such cases, the positive correlation between MMP-9 and serum creatinine is likely a consequence of both muscle breakdown and inflammatory infiltration.
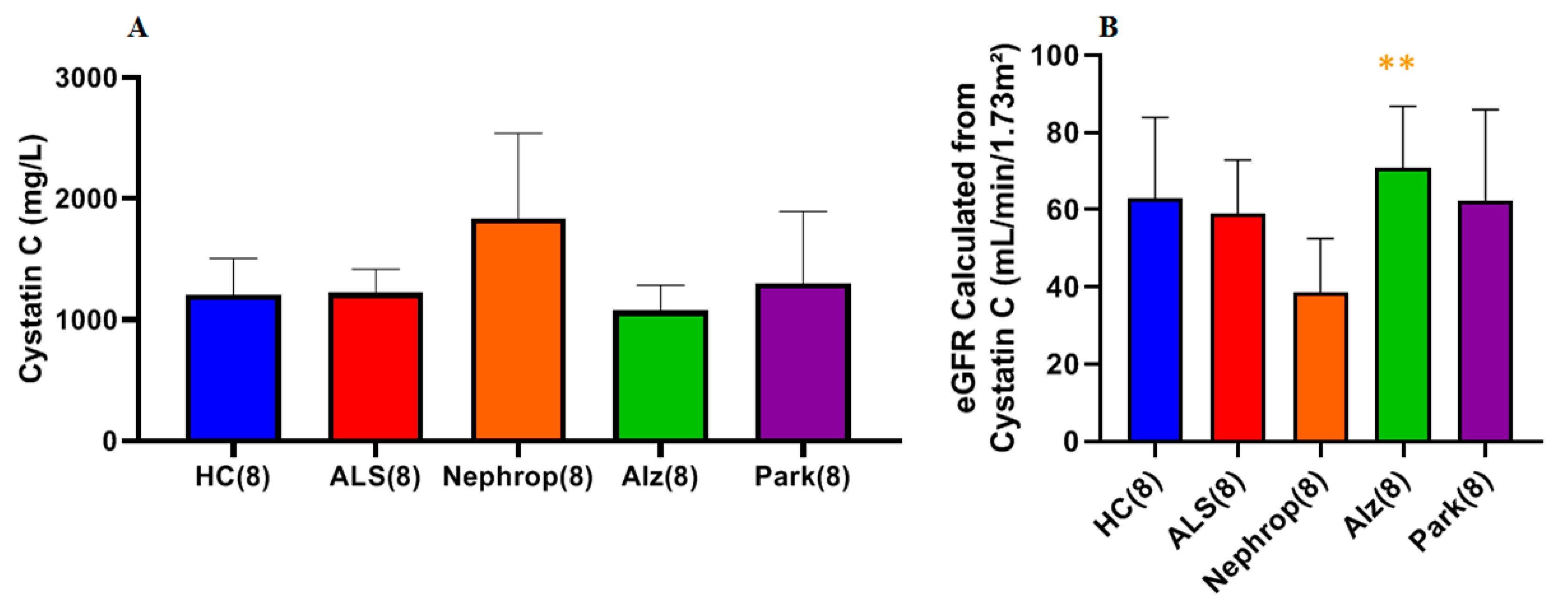
Another potential source for the elevated levels of total and active MMP-9 is the kidney via renal dysfunction. It is known that in ALS there is a slightly higher prevalence (~10%) of chronic kidney disease (CKD) [
36], whereby persistent inflammation and oxidative stress stimulate the upregulation of MMP-9 expression by immune cells such as macrophages, as well as renal tubular epithelial cells [
37]. This overexpression contributes to ongoing renal tissue remodeling and fibrosis, worsening kidney function. Simultaneously, as kidney function declines, the glomerular filtration rate (GFR) decreases, leading to the accumulation of creatinine in the blood [
37,
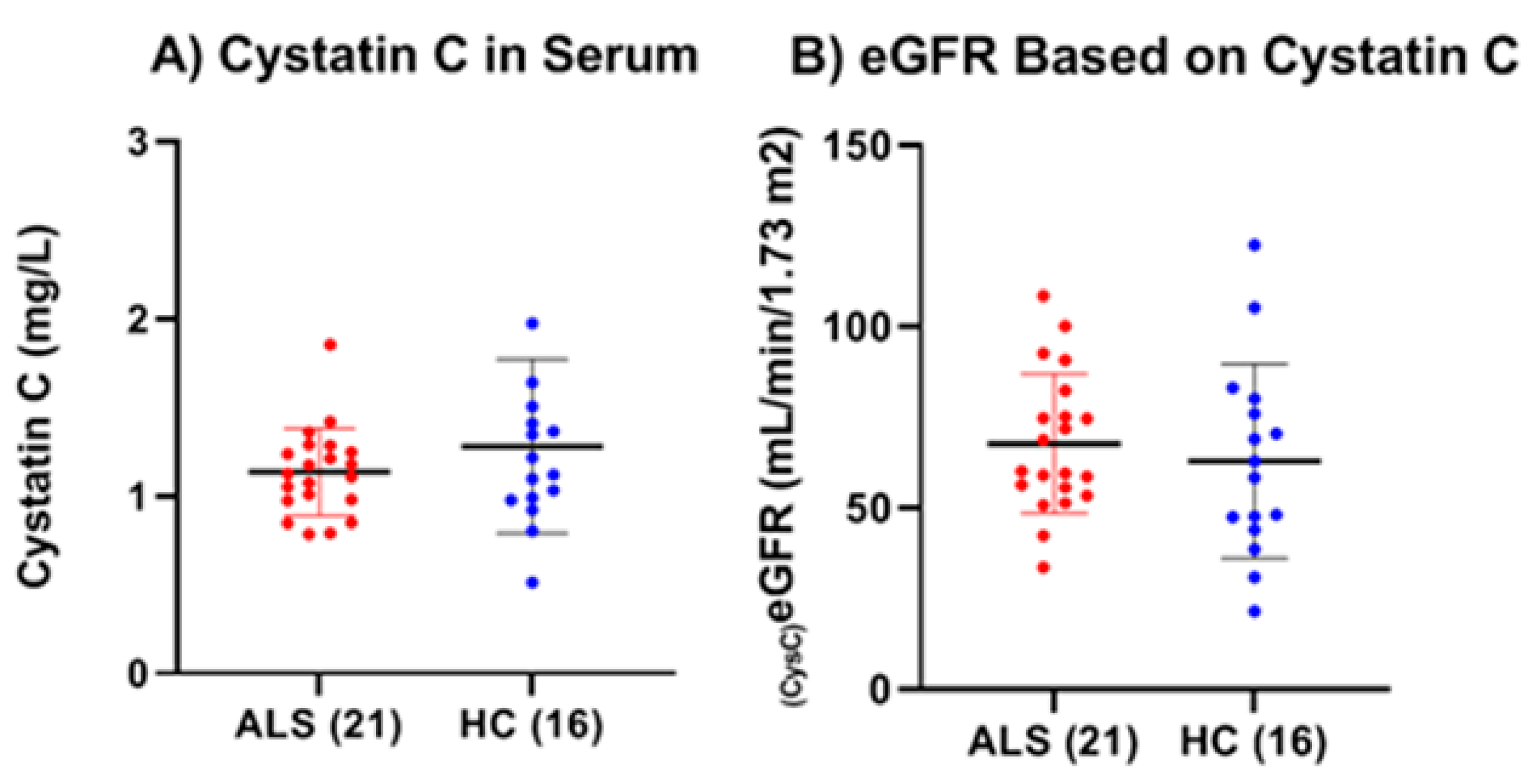
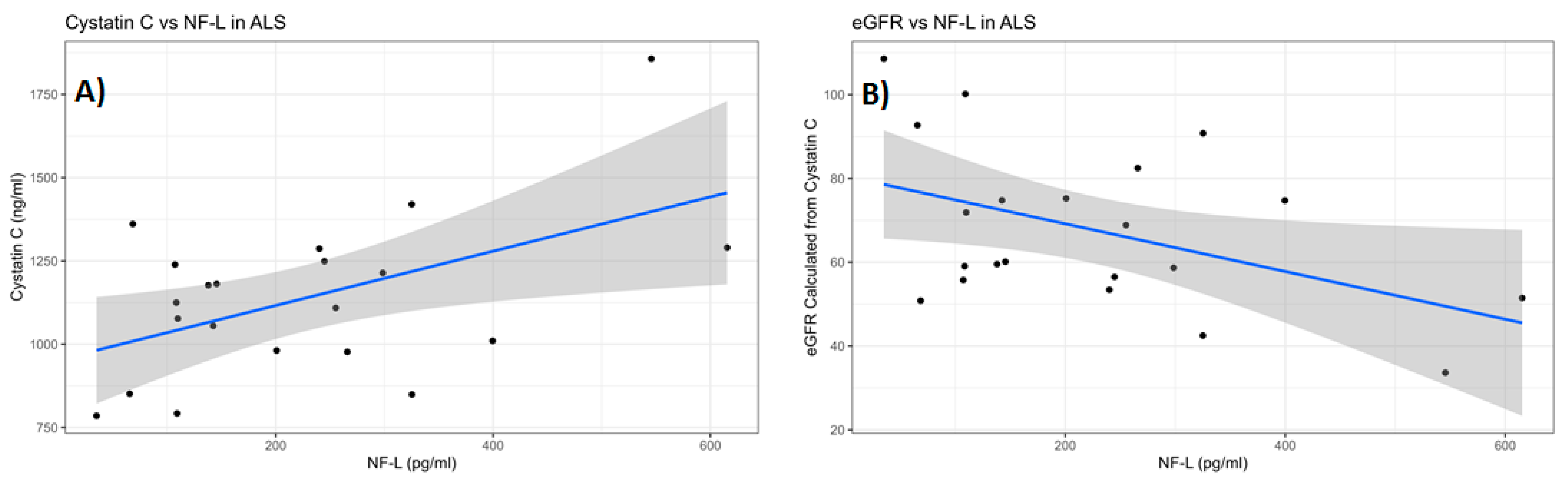
38]. For this reason, we examined serum cystatin C levels and calculated the cystatin C eGFR in ALS as a way to gauge, independently from serum creatinine, their overall kidney function compared to HC. The results showed that there were no statistical differences in either serum cystatin C level or the calculated eGFR between ALS and HC. Additionally, there were no significant correlations found between either total or active MMP-9 and either cystatin C or eGFR. Thus, with regard to cystatin C and eGFR, there seemed to be no indication of significant kidney dysfunction among the ALS group nor any notable impact of kidney function on the levels of total and active MMP-9. However, we did observe both a significant positive correlation between the levels of serum NfL and cystatin C and a significant negative correlation between serum NfL and eGFR in ALS, but not in HC. This indicates that the kidney may have some effect on the serum levels of NfL in ALS.
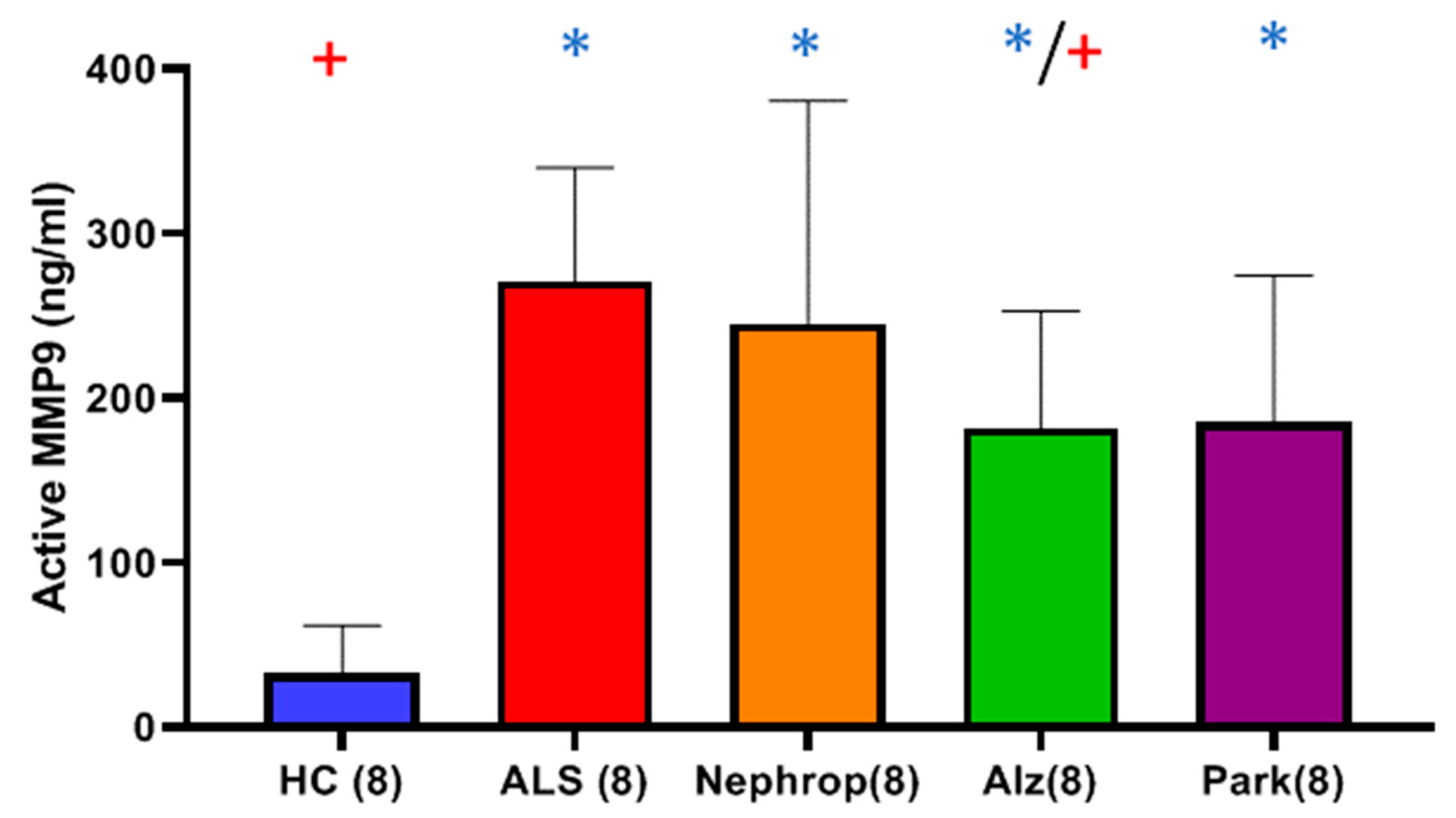
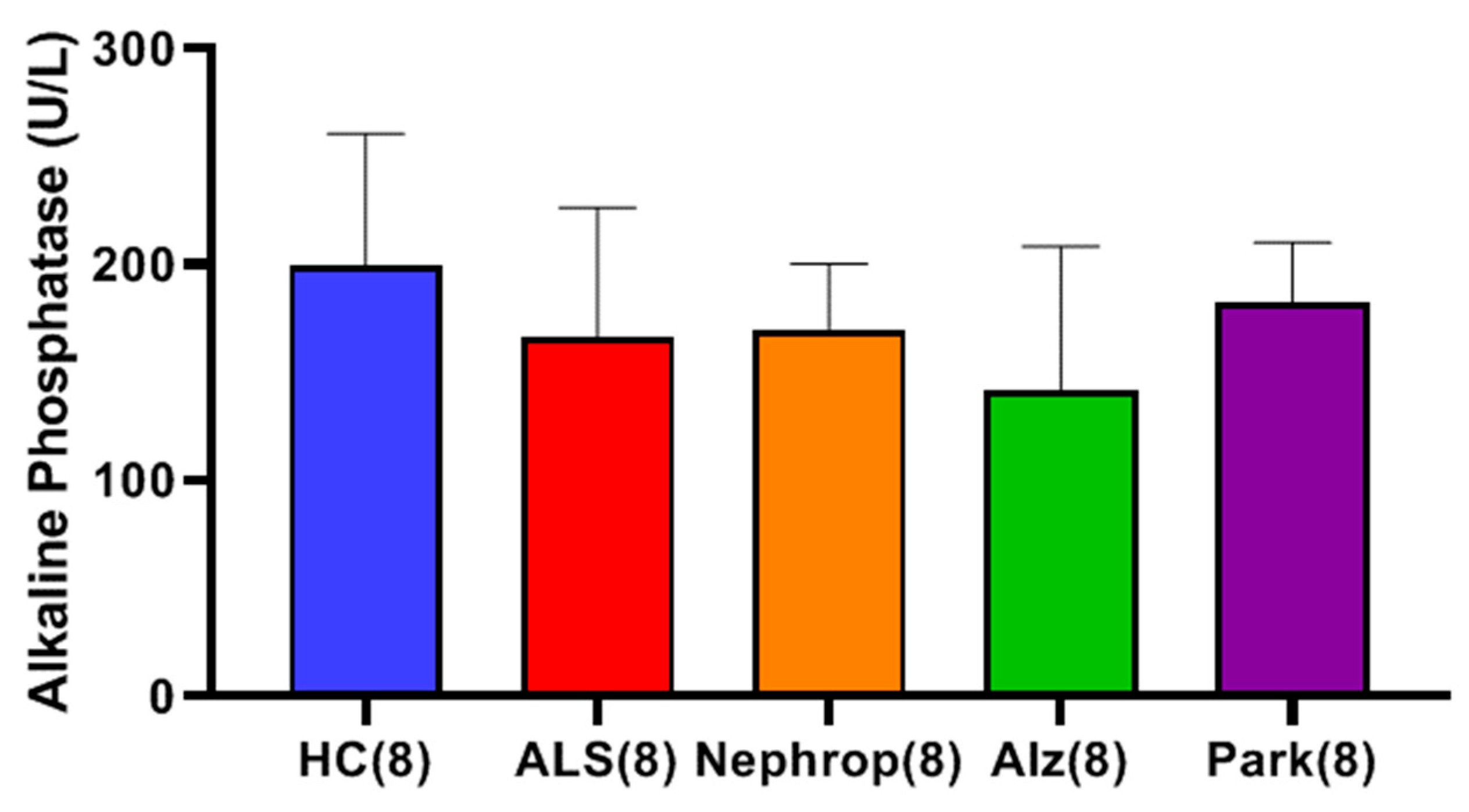
Finally, we were interested in learning how the elevated levels of active MMP-9 observed in the serum of ALS compared with other inflammatory diseases, such as diabetic nephropathy, Alzheimer’s disease, and Parkinson’s disease. Because our study was primarily focused on ALS and because we had a limited amount of ALS samples, we only chose to look at a small sampling (n = 8) of patients diagnosed with those other diseases and compare them with an equal number of ALS and HC, with a priority of balancing all the groups for gender and age. We found that all the disease groups exhibited significantly higher mean levels of active MMP-9 as compared to HC. The ALS group (n = 8) had the highest mean levels of active MMP-9 of all of the groups, which was significantly higher than the Alzheimer’s (p = 0.0459), and HC (p < 0.0001) groups. As before, we were interested in knowing where these elevated active MMP-9 levels were originating from. First, we looked at muscle as a potential source through the analysis of total serum creatinine levels among the various disease groups. As expected, the nephropathy group exhibited significantly higher mean levels of serum creatinine than all of the other groups. The ALS group was found to have the lowest mean level of serum creatinine of all of the groups, which was significantly lower than the HC (p = 0.0345) and nephropathy groups (p = 0.0064), indicating again that the ALS group do not exhibit signs of renal dysfunction., We next examined the levels of serum cystatin C and calculated the eGFR for all of the disease groups. The nephropathy group exhibited the highest mean levels of cystatin C and lowest eGFR of all groups. However, because of the limited sample size, the only group showing significance was the calculated eGFR for Alz (p = 0.00721) with the other groups trending toward significance. We next looked at the levels of ALP between the various disease groups as a way to gauge for potential liver disease as another possible source for the elevated levels of total and active MMP-9. The results showed that there were no significant differences between any of the groups indicating that there is no indication of any significant liver problems and/or bone disorder.
The earlier results, showing no statistical differences in either serum cystatin C levels or the calculated eGFR between ALS and HC, indicate that the elevated serum levels of total and active MMP-9 are most likely not derived from the kidneys. However, more work is needed to definitively prove that the elevated levels originate from degenerating muscle. For example, future work could include a longitudinal study examining MMP-9 levels in both serum and CSF among ALS patients to determine whether the levels in the CSF approach those observed in serum. One could also investigate creatine kinase levels in serum to see if there is any correlation with the levels of total and active MMP-9, as a way to more directly gauge muscle function. Finally, access to biopsied muscle samples from ALS patients could provide critical information to determine whether total and active MMP-9 levels can be detected and are elevated in muscle tissue. Schoser and colleagues, using immunohistochemistry on muscle biopsies from a small cohort (n = 5) of patients with ALS, spinal muscular atrophy, and chronic axonal neuropathies, observed strong MMP-9 immunoreactivity—and to a lesser extent, MMP-2 and MMP-7—in all samples [
39]. They also noted that the pattern of immunoreactivity differed markedly between the disease groups and normal muscle tissue. However, the method used did not allow for quantification or distinction between the pro- and active forms of the enzymes. Therefore, quantitative techniques would be necessary to definitively establish whether there is a link between total and active MMP-9 and degenerating muscle.
If the elevated levels of total and active MMP-9 are in fact derived from degenerating muscle, this may indicate that significant inflammatory processes are occurring peripherally to the CNS in ALS. Recently, there have been reports that phosphorylated TDP-43 (pTDP-43) aggregates have been detected in skeletal and cardiac muscle in ALS as well as in the GI tract and lymph nodes [
40,
41]. In some cases, these pTDP-43 aggregates are detected years before disease onset and formal ALS diagnosis [
42]. The formation of these pTDP-43 aggregates outside of the CNS, and specifically in muscle, could be the initiator of these inflammatory processes and explain the upregulation of both total and active MMP-9 outside of the CNS. Given that a significant portion of the MMP-9 is in its active state and capable of cleaving proteins such as myelin basic protein, matrix proteins and activating various inflammatory cytokines, it is most probable that it is a significant source of further inflammatory activation, providing a compelling rationale for its inhibition not only in the CNS, but systemically, with an inhibitor such as AQU-118 as a potential approach to reducing ALS induced muscle degeneration.
4. Materials and Methods
Serum and CSF samples (
Table 1). nVector provided the ALS serum samples and matched CSF samples (with only sex and age patient information available). The first group (Group 1,
Table 1), consisting of serum samples from thirty people with ALS, was balanced for gender (18 males, 12 females) and age (mean ± SD, 59.4 ± 6.1 years). A second validation group (Group 3,
Table 1), consisting of matching serum and CSF samples (Group 4,
Table 1) from thirty people with ALS, was also balanced for gender (19 males, 11 females) and age (mean 60.9 ± 7.9 years).
Two sets of healthy age-matched controls (HC) were used in the study. One group of twenty serum samples from HC (Group 2,
Table 1) was obtained from BioIVT and was balanced for gender (12 males, 8 females) and age (mean 56.5 ± 2.3 years). A second validation group of twenty serum samples from HC (Group 5,
Table 1) was obtained from BioIVT and was balanced for gender (12 males, 8 females) and age (mean 60.6 ± 5.5 years). Fourteen CSF samples from people with other neurological diseases (OND) (Group 6,
Table 1) was obtained from Precision for Medicine and were balanced for gender (8 males, 6 females) and age (mean 63.6 ± 9.8 years). The background information provided with samples included only the diagnosis or suspected diagnosis, the sex and age of the patients. This OND group included participants with optic neuritis, demyelinating disease of the CNS, idiopathic normal pressure hydrocephalus, acute cerebrovascular insufficiency, encephalopathy, hemiplegia, schizophrenia, and altered mental state.
Additionally, eight serum samples each (a total of 24 samples) were obtained from BioIVT from people diagnosed with diabetic nephropathy (Group, 9, 4 males, 4 females, 60.5 ± 6.8 years of age), Alzheimer’s disease (Group 10: 4 males, 4 females, 60.2 ± 5.5 years of age), and Parkinson’s disease (Group 11: 3 males, 5 females, 61.5 ± 2.9 years of age). The samples from diabetic nephropathy, Alzheimer’s disease and Parkinson’s disease were compared with an age-matched subset (Group 7: n = 8; 5 males, 3 females, 64.5 ± 8.4 years of age) taken from the second validation group of ALS serum samples and an age-matched subset (Group 8, n = 8; 4 males, 4 females, 61.1 ± 5.5 years of age) taken from the second validation group of HC serum samples.
The serum and CSF samples from ALS were obtained under a prior study protocol, which was ethically approved by an IRB (IRB#14BN059). The IRB approval included patient consent for the use of these samples for future research conducted by nVector. The HC samples obtained from BioIVT were collected under another study (Prospective Collection of Biological Specimens from Subjects Presenting at Specimen Donation Center for Research) by the commercial vendor, Seratrials/BioIVT, that was approved by an IRB (IRB#20161665) and included patient consent for the use of these samples for future research. The CSF samples from OND obtained from Precision for Medicine were collected under another study (PFM004, Remnant Biospecimen Program) by the commercial vendor, Precision for Medicine, that was approved by an IRB (IRB# Pro0051469) and included patient consent for the use of these samples for future research. All patient samples used in this study are legacy samples which included patient consent for their use in future research.
4.1. Chemical Materials
ProMMP-2 standard (Cat.444213-5 μg), active MMP-2 standard (Cat #PF023-5 μg), and ProMMP-9 standard (Cat #PF038-10 μg) were obtained from EMD Millipore (Burlington, VT, USA). 2× Zymogram Sample Buffer was obtained from G-BioSciences (St. Louis, MO, USA) (Cat # 786-483). Renaturing Buffer (Cat #LC2670) and Developing Buffer (Cat #LC2671) were Novex brand obtained from Thermo Fisher Scientific (Waltham, MA, USA). Simply Blue Stain was anInvitrogen brand obtained from Thermo Fisher Scientific (Waltham, MA, USA) (Cat #LC6060). Precision Plus Protein Dual Color Standards was obtained from Bio-Rad Laboratories (Waltham, MA, USA) (Cat. #161-0374). 10× TGS Running Buffer was obtained from Bio-Rad (Cat #161-0772). The electrophoresis tank was obtained from Invitrogen. The MMP inhibitor, AQU-118, was manufactured and purified by Aquilus Pharmaceuticals (Winchester, VA, USA) [
26].
4.2. Measurement of Total MMP-2 and MMP-9 via ELISA
Total MMP-2 protein levels were determined in both serum and CSF using a commercially obtained enzyme-linked immunosorbent assay (ELISA) kit obtained from R & D Systems, Minneapolis, MN, USA (Total MMP-2 Quantikine ELISA Kit, MMP200). The assay was performed following manufacturer instructions included with the kit. Total MMP-9 protein was determined in both serum and CSF using ELISA obtained from R & D Systems (Human MMP-9 Quantikine ELISA kit, DMP 900). The assay was performed following manufacturer instructions included with the kit.
4.3. Measurement of Active MMP-2 via Antibody Capture and Colorimetric Assay
Active MMP-2 was measured using a commercially obtained Human MMP-2 Activity Assay v2.0 Kit (Quickzyme, Biosciences, Leiden, The Netherlands). The assay uses a precoated plate containing an antibody that can recognize both the active and pro forms of MMP-2. After the sample is exposed to the antibody, all of the non-MMP-2 proteins are washed away, leaving both the active and pro forms of MMP-2 attached. A detection enzyme is added. This detection enzyme is converted into an active form by any active MMP-2 present. The active detection enzyme cleaves a chromogenic substrate, resulting in the generation of a yellow color that can be measured at 405 nm. The assay was performed following manufacturer instructions included with the kit.
4.4. Measurement of Active MMP-9 via Antibody Capture and Fluorometric Assay
Active MMP-9 was measured using a commercially obtained Fluorokine E, Human Active MMP-9 Assay Kit (R & D Systems, Minneapolis, MN, USA, Cat#F9M00). The assay uses a precoated plate containing an antibody that can recognize both the active and pro forms of MMP-9. After the sample is exposed to the antibody, all of the non-MMP-9 proteins are washed away, leaving both the active and pro forms of MMP-9 attached. A FRET (fluorescence resonance energy transfer) peptide that is labeled with a fluorescence recorder is added to the bound enzymes. Only the active form can cleave the FRET peptide, which in turn generates fluorescence upon the peptide cleavage. The fluorescence measurement is related to the concentration of MMP-9 in the sample. The excitation wavelength is set to 320 nm and the emission wavelength is set to 405 nm. The assay was performed following manufacturer instructions included with the kit.
4.5. Measurement of Neurofilament Light (NfL) via ELISA
NfL protein was determined in both serum and CSF using a commercial ELISA kit obtained from MSD, Rockville, USA (S-PLEX Human Neurofilament L Kit). The assay was performed following manufacturer instructions included with the kit.
4.6. Measurement of Serum Creatinine
Creatinine was determined in serum using a commercially available colorimetric kit from Arbor Assays, Ann Arbor, MI, USA (Creatinine DetectX Colorimetric Detection Kit, Catalog #KB02-H). The assay was performed following manufacturer instructions included with the kit, which proposes a 30 min room temperature incubation prior to reading on a plate reader at 490 nm.
4.7. Measurement of Cystatin C via ELISA
Cystatin C was determined in serum using a commercially available ELISA kit from Arbor Assays (Human Cystatin C ELISA Kit, Cat. # K012-H1). The assay was performed following manufacturer instructions included with the kit.
4.8. Measurement of Alkaline Phosphatase
Alkaline phosphatase was determined in serum using a commercially available colorimetric kit from Arbor Assays (Alkaline Phosphatase Colorimetric Activity Kit, Cat. #K082-H1). The assay was performed following manufacturer instructions included with the kit, which proposes a 30 min incubation at 37 °C prior to reading on a plate reader at 405 nm.
4.9. Measurement of Inhibition of Active MMP-9 in Human Serum When Exposed to the MMP Inhibitor, AQU-118
AQU-118 is a proprietary, semi-selective MMP inhibitor capable of inhibiting both MMP-2 (IC50 = 2.0 nM) and MMP-9 (IC50 = 9 nM) and was synthesized by Aquilus Pharmaceuticals [
26]. First, a 100 mg/ml solution of AQU-118 in triethylene glycol was prepared. The level of active MMP-9 was measured using the Fluorokine E, Human Active MMP-9 Assay Kit (R&D Systems, Minneapolis, MN, USA, Cat#F9M00) and a slightly modified procedure from that of the kit was used. Procedure: The samples from ALS were diluted to a final dilution of 1:100 into 1 × RD5-24 diluent containing a final concentration of inhibitor. Then, 50 microliters of Assay Diluent RD1N was added to each well of the plate. Then, 200 microliters of standard or sample was added to each well in duplicate. Each well was covered and shaken at 500 rpm for 2 h at room temperature. We washed 0/1/2/3 × with 300 microliters of 1× of Wash Buffer. We added 200 microliters of diluted substrate and incubated at 37 °C for 20 h. At the end of 20 h the Relative Fluorescence Unit (RFU) was measured using Ex/Em = 320/405 nm and 340/405 nm. The endpoint mode is present and the plate speed = 6.
4.10. Calculation of eGFR Levels
The eGFR was calculated using the method of Inker and colleagues [
25] using the equation below:
Abbreviations/Units
eGFR (estimated glomerular filtration rate) = mL/min/1.73 m2
Scys (standardized serum cystatin C) = mg/L
min = indicates the minimum of Scys/0.8 or 1
max = indicates the maximum of Scys/0.8 or 1
age = years
4.11. Statistical Analysis
With regard to the two data sets of thirty ALS (Groups 1, 3 and 4,
Table 1) and corresponding HC (Groups 2 and 5,
Table 1) and ONC (Group 6,
Table 1) groups, differences in means were compared by Welch’s
t-test. Effect sizes were assessed with Cohen’s d. Correlations were assessed with both Pearson’s correlation and Spearman’s rank correlation.
p values < 0.05 were considered significant. Prism version 8.02 (GraphPad Software, Boston, MS, USA) and R Studio 4.2.2 (developed by the R Foundation for Statistical Computing,
https://www.r-project.org/about.html) were used for data visualization and analysis. With regard to the data sets between the different disease groups (Groups 7–11), comparisons were assessed by pairwise Welch’s
t-test with Benjamini–Hockberg correction for multiple testing. All biomarker values are presented as mean ± standard deviation (SD).