Ecotoxicological Impacts of Perfluorooctane Sulfonate on the Freshwater Snail Lanistes carinatus: Oxidative Stress, Neurotoxicity, and Histopathological Alterations
Abstract
1. Introduction
2. Results
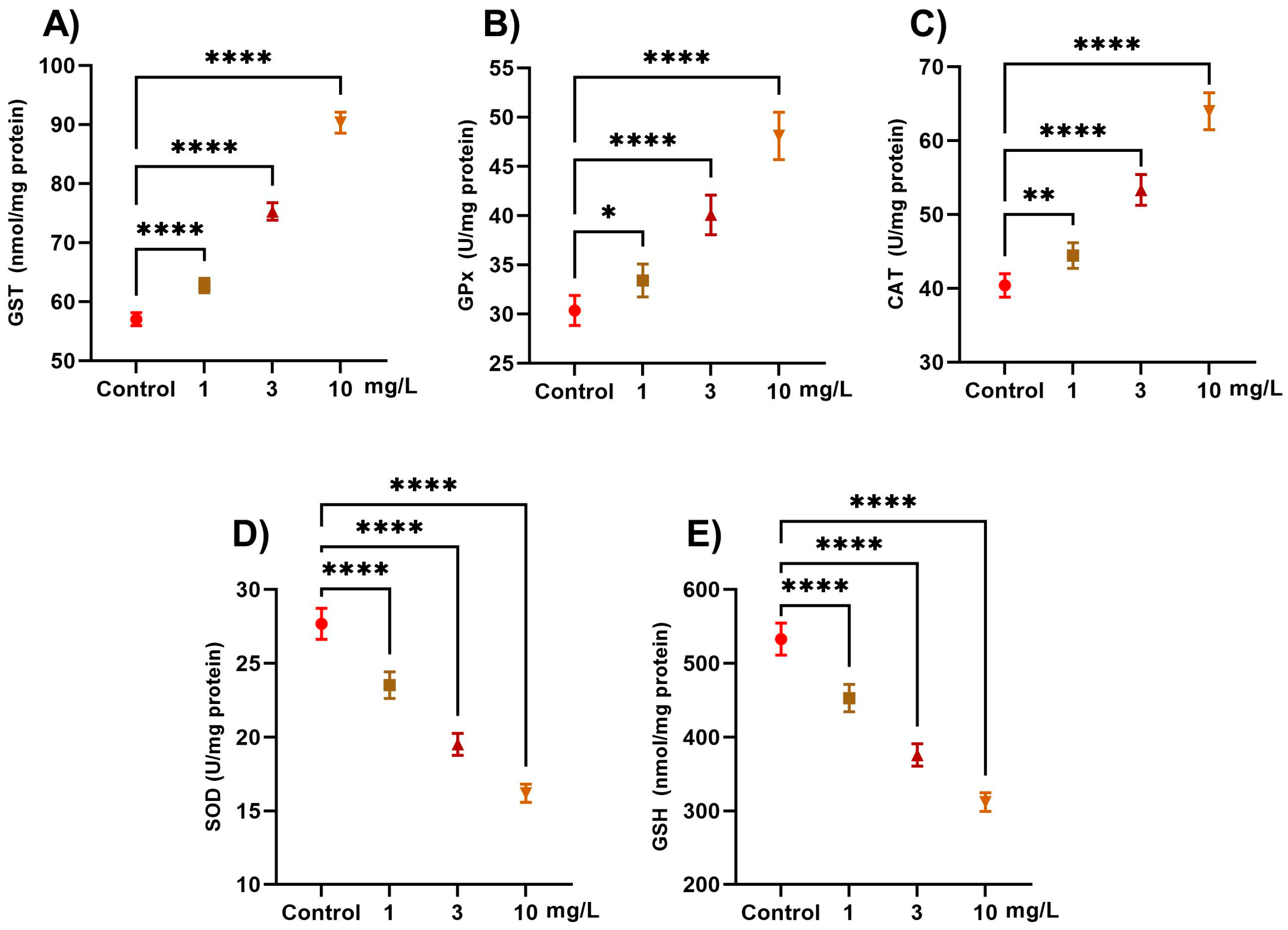
2.1. Antioxidant Defense Enzymes
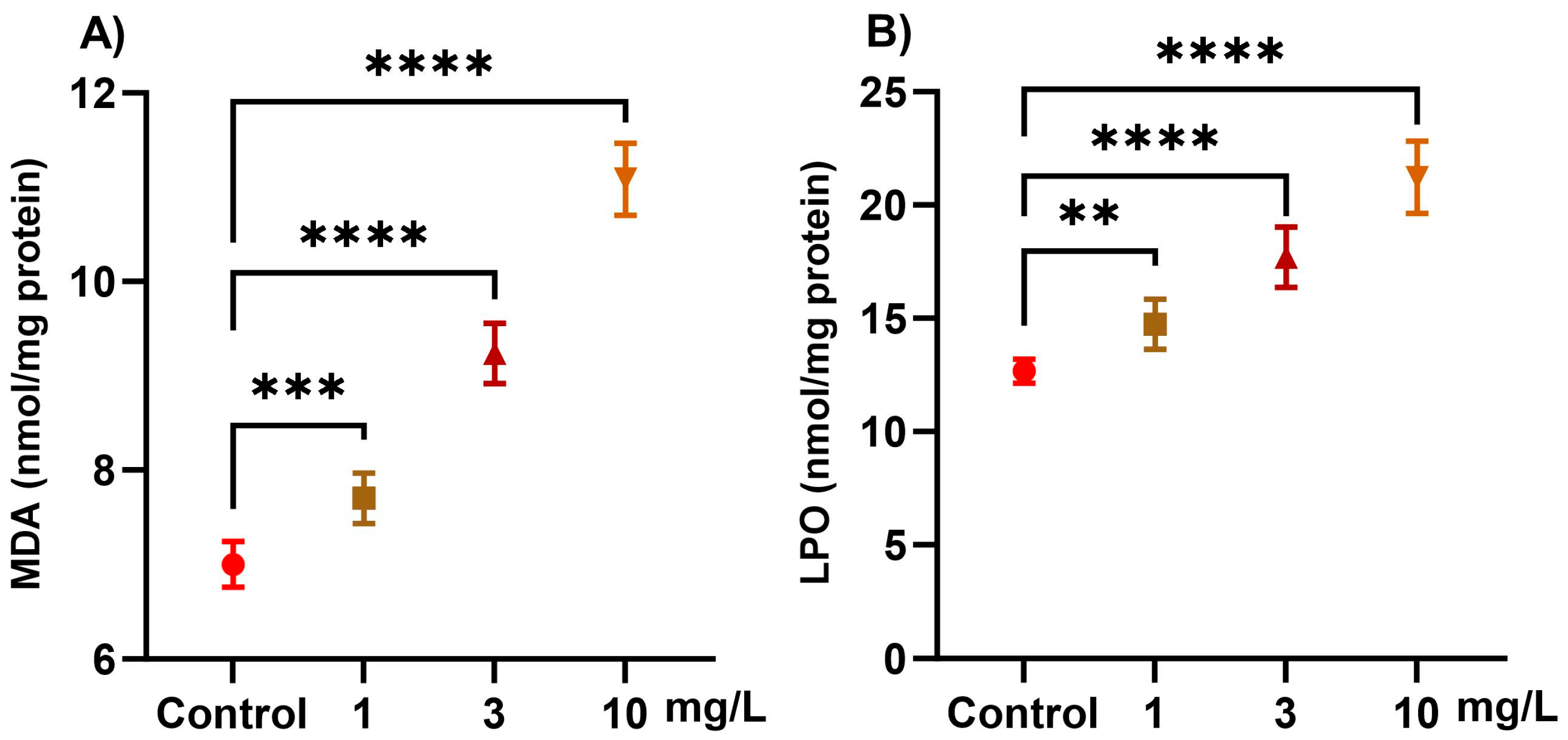
2.2. Oxidative Stress Markers
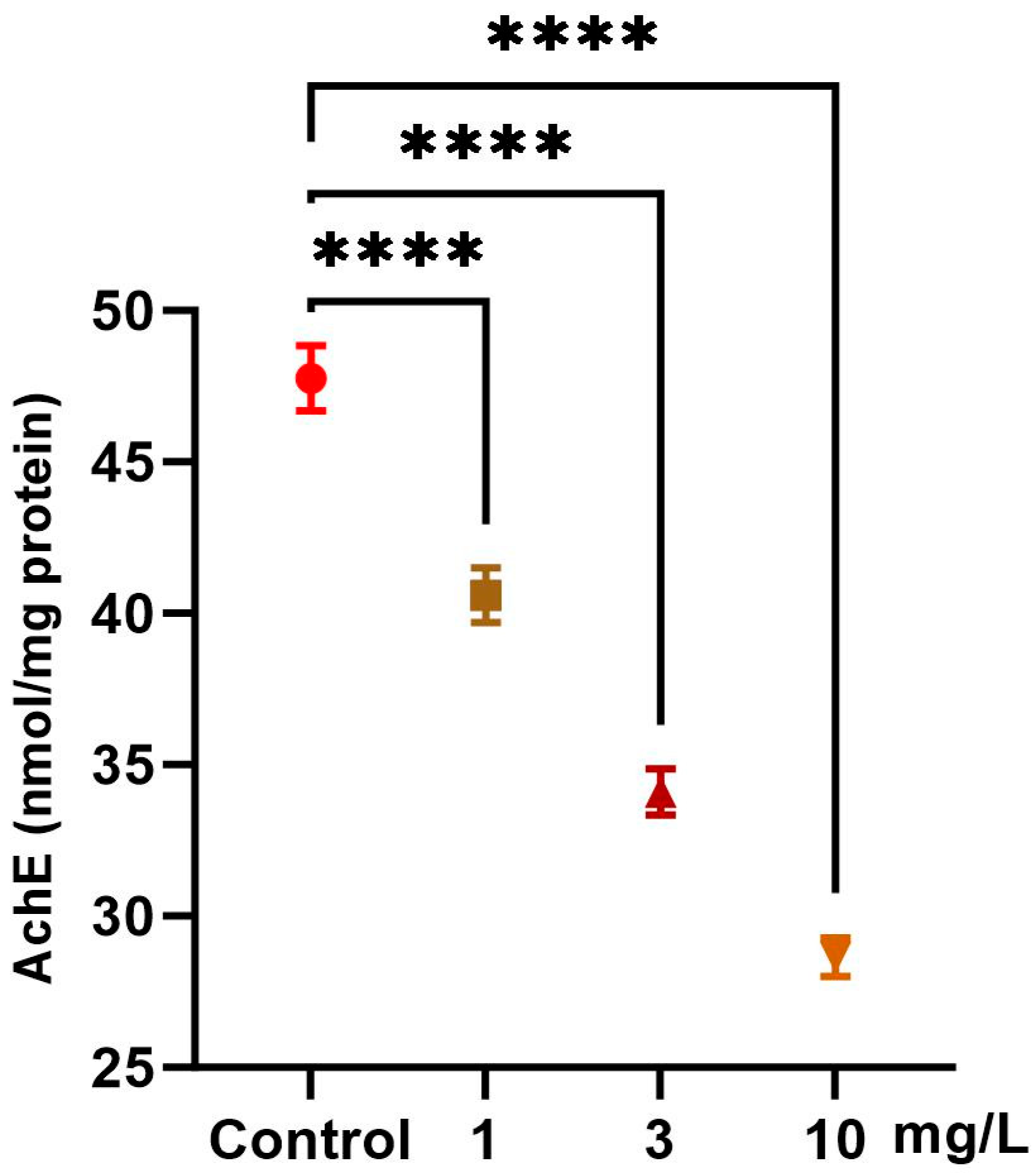
2.3. Neurotoxicity Markers
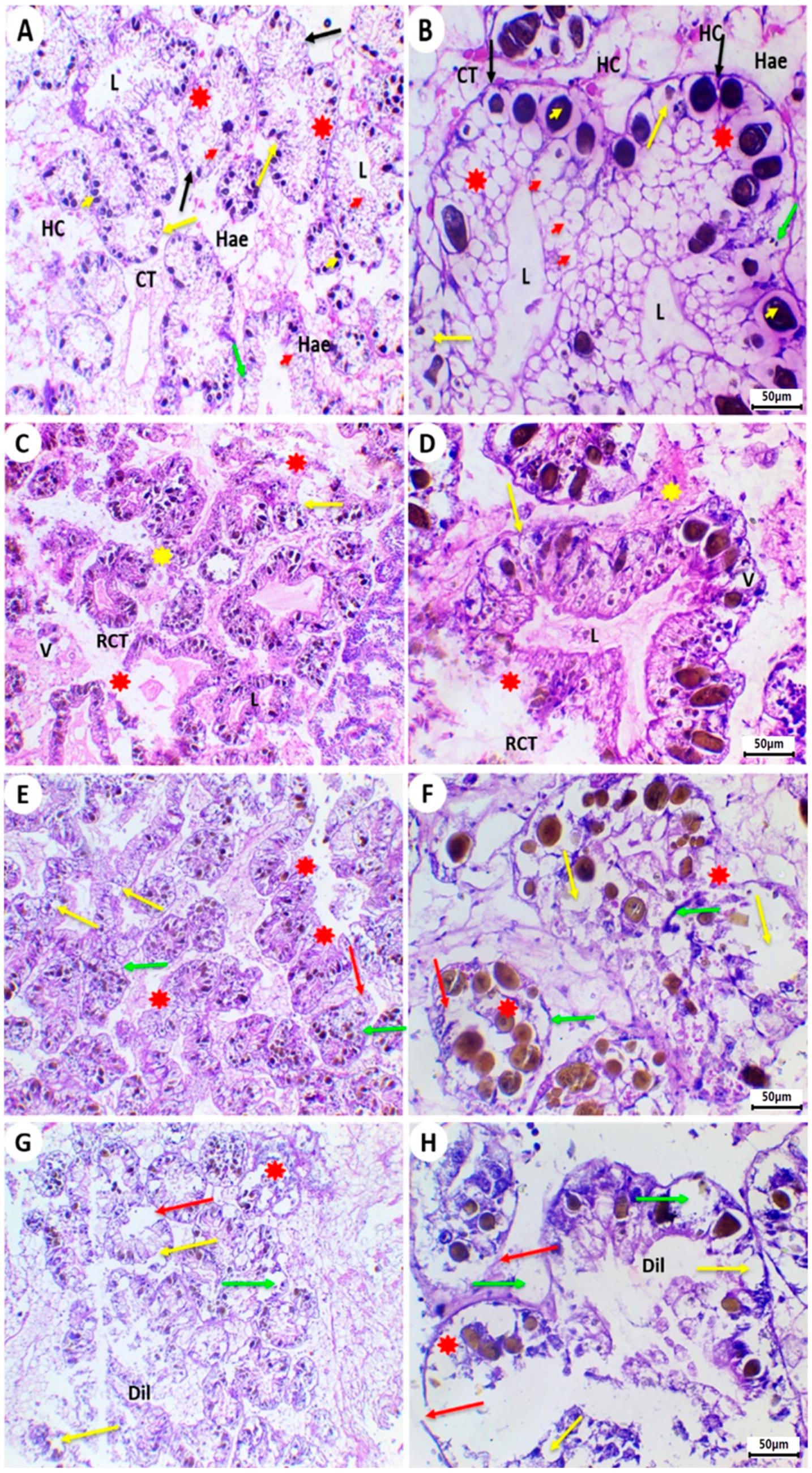
2.4. Histology of the Digestive Gland
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Experimental Animals
4.3. Antioxidant Enzymes
4.4. Oxidative Stress
4.5. Neurotoxicity Biomarker
4.6. Histopathology
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PFOS | Perfluorooctane sulfonate |
| PFAS | Per- and polyfluoroalkyl substances |
| PFOA | Perfluorooctanoic acid |
| C-F | Carbon–fluorine (bond) |
| LC50 | Lethal Concentration for 50% of test organisms |
| LPO | Lipid peroxidation |
| MDA | Malondialdehyde |
| GST | Glutathione S-transferase |
| GPx | Glutathione peroxidase |
| CAT | Catalase |
| GSH | Reduced glutathione |
| SOD | Superoxide dismutase |
| AchE (or AChE) | Acetylcholinesterase |
| ROS | Reactive oxygen species |
| POD | Peroxidase |
| GR | Glutathione reductase |
| ChE | Cholinesterase |
| DMSO | Dimethyl sulfoxide |
| H&E | Hematoxylin and eosin (stain) |
| SD | Standard deviation |
| ANOVA | Analysis of variance |
| LSD | Least significant difference (test) |
| L. carinatus | Lanistes carinatus (snail species, used in short form) |
| µg/L, mg/L, ng/L | Micrograms/milligrams/nanograms per liter |
References
- Schymanski, E.L.; Zhang, J.; Thiessen, P.A.; Chirsir, P.; Kondic, T.; Bolton, E.E. Per-and polyfluoroalkyl substances (PFAS) in PubChem: 7 million and growing. Environ. Sci. Technol. 2023, 57, 16918–16928. [Google Scholar] [CrossRef]
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef]
- Silva, A.V.; Ringblom, J.; Lindh, C.; Scott, K.; Jakobsson, K.; Öberg, M. A probabilistic approach to evaluate the risk of decreased total triiodothyronine hormone levels following chronic exposure to PFOS and PFHxS via contaminated drinking water. Environ. Health Perspect. 2020, 128, 076001, Erratum in Environ Health Perspect. 2020, 128, 89001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Z.; Li, J.; Zhang, X.; Wang, L.; Wang, J.; Lin, T. Perfluoroalkyl and polyfluoroalkyl substances (PFASs) in groundwater: Current understandings and challenges to overcome. Environ. Sci. Pollut. Res. 2022, 29, 49513–49533. [Google Scholar] [CrossRef]
- Oliaei, F.; Kriens, D.; Weber, R.; Watson, A. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ. Sci. Pollut. Res. 2013, 20, 1977–1992. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Aminot, Y.; Sayfritz, S.J.; Thomas, K.V.; Godinho, L.; Botteon, E.; Ferrari, F.; Boti, V.; Albanis, T.; Köck-Schulmeyer, M.; Diaz-Cruz, M.S. Environmental risks associated with contaminants of legacy and emerging concern at European aquaculture areas. Environ. Pollut. 2019, 252, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A never-ending story of per-and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518, Correction in Environ. Sci. Technol. 2018, 52, 3325. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per-and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.; Airaksinen, R.; Hallikainen, A.; Vuorinen, P.J.; Mannio, J.; Kiviranta, H. Perfluoroalkyl acids in various edible Baltic, freshwater, and farmed fish in Finland. Chemosphere 2015, 129, 186–191. [Google Scholar] [CrossRef]
- Kodavanti, P.R.S.; Loganathan, B.G. Organohalogen pollutants and human health. In The International Encyclopedia of Public Health; Quah, S.R., Cockerham, W.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 5, pp. 359–366. [Google Scholar]
- Rodil Rodríguez, M.d.R.; Villaverde de Sáa, M.E.; Cobas, J.; Quintana Álvarez, J.B.; Cela Torrijos, R.; Carro, N. Legacy and emerging pollutants in marine bivalves from the Galician coast (NW Spain). Environ. Int. 2019, 129, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Stahl, T.; Fliedner, A.; Rüdel, H.; Tarricone, K.; Brunn, H.; Koschorreck, J. Levels, accumulation patterns and retrospective trends of perfluoroalkyl acids (PFAAs) in terrestrial ecosystems over the last three decades. Environ. Pollut. 2019, 246, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Miao, X.; Fu, J.; Chen, Y.; Li, H.; Pan, W.; Fu, J.; Zhang, Q.; Zhang, A.; Jiang, G. Occurrence and trophic transfer of per-and polyfluoroalkyl substances in an Antarctic ecosystem. Environ. Pollut. 2020, 257, 113383. [Google Scholar] [CrossRef]
- Burkhard, L.P. Evaluation of published bioconcentration factor (BCF) and bioaccumulation factor (BAF) data for per-and polyfluoroalkyl substances across aquatic species. Environ. Toxicol. Chem. 2021, 40, 1530–1543, Correction in Environ. Toxicol. Chem. 2021, 40, 2935–2940. [Google Scholar] [CrossRef]
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per-and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003. [Google Scholar] [CrossRef]
- Tansel, B. Geographical characteristics that promote persistence and accumulation of PFAS in coastal waters and open seas: Current and emerging hot spots. Environ. Chall. 2024, 14, 100861. [Google Scholar] [CrossRef]
- Hamed, M.; Vats, A.; Lim, I.E.; Sapkota, B.; Abdelmoneim, A. Effects of developmental exposure to individual and combined PFAS on development and behavioral stress responses in larval zebrafish. Environ. Pollut. 2024, 349, 123912. [Google Scholar] [CrossRef]
- Soares, L.O.S.; de Araujo, G.F.; Gomes, T.B.; Júnior, S.F.S.; Cuprys, A.K.; Soares, R.M.; Saggioro, E.M. Antioxidant system alterations and oxidative stress caused by polyfluoroalkyl substances (PFAS) in exposed biota: A review. Sci. Total Environ. 2025, 977, 179395. [Google Scholar] [CrossRef]
- Zhang, J.; Xue, X.; Huang, C.; Chen, M.; Zhang, L.; Ren, M.; Hu, G.; Liu, S. Survey of perfluorooctanoic acid and perfluorooctane sulfonate in surface water from Tongsha Reservoir of Pearl River Delta, South China. Environ. Chem. 2017, 36, 2600–2608. [Google Scholar]
- Ololade, I.A.; Oladoja, N.A.; Ololade, O.O.; Oloye, F.F.; Adeola, A.O.; Alabi, A.B.; Ademila, O.; Adanigbo, P.; Owolabi, M.B. Geographical distribution of perfluorooctanesulfonate and perfluorooctanoate in selected rivers from Nigeria. J. Environ. Chem. Eng. 2018, 6, 4061–4069. [Google Scholar] [CrossRef]
- Picard, J.-C.; Munoz, G.; Duy, S.V.; Sauvé, S. Longitudinal and vertical variations of waterborne emerging contaminants in the St. Lawrence Estuary and Gulf during winter conditions. Sci. Total Environ. 2021, 777, 146073. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Stasinakis, A.S. Review on the occurrence, fate and removal of perfluorinated compounds during wastewater treatment. Sci. Total Environ. 2015, 524, 81–92. [Google Scholar] [CrossRef]
- Olsen, G.W. PFAS biomonitoring in higher exposed populations. In Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances; Springer: Berlin/Heidelberg, Germany, 2015; pp. 77–125. [Google Scholar]
- Poothong, S.; Papadopoulou, E.; Padilla-Sánchez, J.A.; Thomsen, C.; Haug, L.S. Multiple pathways of human exposure to poly-and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environ. Int. 2020, 134, 105244. [Google Scholar] [CrossRef]
- Mao, R.; Lu, Y.; Zhang, M.; Wang, C.; Sun, B.; Shi, Y.; Song, S.; Wang, P.; Yuan, J.; Zhao, J. Distribution of legacy and novel per-and polyfluoroalkyl substances in surface and groundwater affected by irrigation in an arid region. Sci. Total Environ. 2023, 858, 159693. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, J.; Yang, X.; Jiao, W.; Wang, T.; Zhu, L. Disclosing the bioaccumulation and biomagnification behaviors of emerging per/polyfluoroalkyl substances in aquatic food web based on field investigation and model simulation. J. Hazard. Mater. 2023, 445, 130566. [Google Scholar] [CrossRef]
- Wang, Q.; Ruan, Y.; Jin, L.; Tao, L.S.; Lai, H.; Li, G.; Yeung, L.W.; Leung, K.M.; Lam, P.K. Legacy and emerging per-and polyfluoroalkyl substances in a subtropical marine food web: Suspect screening, isomer profile, and identification of analytical interference. Environ. Sci. Technol. 2023, 57, 8355–8364. [Google Scholar] [CrossRef] [PubMed]
- Bartley, M.C.; Tremblay, T.; De Silva, A.O.; Kamula, C.M.; Ciastek, S.; Kuzyk, Z.Z.A. Sedimentary records of contaminant inputs in Frobisher Bay, Nunavut. Environ. Sci. Ecotechnol. 2024, 18, 100313. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Bao, H.; Zhang, L.; Wen, S.; Tan, W.; Zeeshan, M.; Sun, M.-K.; Chu, C.; Gui, Z.-H.; Lin, L.-Z.; et al. Health risk assessment of perfluorooctane sulfonate and perfluorooctanoic acid exposure in China based on epidemiological data. Hyg. Environ. Health Adv. 2023, 7, 100066. [Google Scholar] [CrossRef]
- Gou, X.; Tian, M.; Yan, L.; Xia, P.; Ji, H.; Tan, H.; Shi, W.; Yu, H.; Zhang, X. A novel molecular pathway of lipid accumulation in human hepatocytes caused by PFOA and PFOS. Environ. Int. 2024, 191, 108962. [Google Scholar] [CrossRef]
- Xie, L.-N.; Wang, X.-C.; Su, L.-Q.; Ji, S.-S.; Gu, W.; Barrett, H.; Dong, X.-J.; Zhu, H.-J.; Hou, S.-S.; Li, Z.-H. The association between per-/polyfluoroalkyl substances in serum and thyroid function parameters: A cross-sectional study on teenagers living near a Chinese fluorochemical industrial plant. Sci. Total Environ. 2024, 920, 170985. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Hao, G.; Zhou, D.; Fan, Y.; Wei, Z.; Li, D.; Shen, Y.; Fang, H.; Lin, F.; Zhao, M. Hepatotoxicity in Carp (Carassius auratus) Exposed to Perfluorooctane Sulfonate (PFOS): Integrative Histopathology and Transcriptomics Analysis. Animals 2025, 15, 610. [Google Scholar] [CrossRef]
- van Gerwen, M.; Colicino, E.; Guan, H.; Dolios, G.; Nadkarni, G.N.; Vermeulen, R.C.; Wolff, M.S.; Arora, M.; Genden, E.M.; Petrick, L.M. Per-and polyfluoroalkyl substances (PFAS) exposure and thyroid cancer risk. EBioMedicine 2023, 97, 104831. [Google Scholar] [CrossRef]
- Pickard, H.M.; Ruyle, B.J.; Thackray, C.P.; Chovancova, A.; Dassuncao, C.; Becanova, J.; Vojta, S.; Lohmann, R.; Sunderland, E.M. PFAS and precursor bioaccumulation in freshwater recreational fish: Implications for fish advisories. Environ. Sci. Technol. 2022, 56, 15573–15583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gu, X.; Mo, L.; Wan, N.; Wu, L.; Liu, S.; Zhang, M.; Li, M.; Liu, X.; Liu, Y. Per-and polyfluoroalkyl substances induce lipid metabolic impairment in fish: Integration on field investigation and laboratory study. Environ. Int. 2024, 187, 108687. [Google Scholar] [CrossRef]
- Hamed, M.; Said, R.E.; Shaalan, W.M.; Elbaghdady, H.A.M.; Sayed, A.E.-D.H. Immunological, neurological, and intestinal changes in red swamp crayfish (Procambarus clarkii) exposed to the combined toxicity of Pyrogallol and microplastics. Mar. Pollut. Bull. 2025, 213, 117641. [Google Scholar] [CrossRef]
- Said, R.E.; Hamed, M.; Shaalan, W.M.; Elbaghdady, H.A.M.; Sayed, A.E.-D.H. Exploring the Coexposure Effects of Pyrogallol and Microplastic on the Red Swamp Crayfish Procambarus clarkii. Aquac. Res. 2025, 2025, 6084150. [Google Scholar] [CrossRef]
- Ahrens, L.; Siebert, U.; Ebinghaus, R. Total body burden and tissue distribution of polyfluorinated compounds in harbor seals (Phoca vitulina) from the German Bight. Mar. Pollut. Bull. 2009, 58, 520–525. [Google Scholar] [CrossRef]
- Guo, C.; Hu, S.; Cheng, P.; Cheng, K.; Yang, Y.; Chen, G.; Wang, Q.; Wang, Y.; Liu, T. Speciation and biogeochemical behavior of perfluoroalkyl acids in soils and their environmental implications: A review. Eco-Environ. Health 2024, 3, 505–515. [Google Scholar] [CrossRef]
- Zhang, S.-Q.; Li, P.; Zhao, X.-L.; He, S.-W.; Xing, S.-Y.; Cao, Z.-H.; Zhang, H.-Q.; Li, Z.-H. Hepatotoxicity in carp (Cyprinus carpio) exposed to environmental levels of norfloxacin (NOR): Some latest evidences from transcriptomics analysis, biochemical parameters and histopathological changes. Chemosphere 2021, 283, 131210. [Google Scholar] [CrossRef] [PubMed]
- Belek, N.; Erkmen, B.; Dinçel, A.S.; Gunal, A.C. Does persistent organic pollutant PFOS (perfluorooctane sulfonate) negative impacts on the aquatic invertebrate organism, Astacus leptodactylus [Eschscholtz, 1823]. Ecotoxicology 2022, 31, 1217–1230. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Wang, W.; Yang, S.; Wang, C.; Shi, L.; Guo, H.; Chan, S. Gill transcriptomes reveal involvement of cytoskeleton remodeling and immune defense in ammonia stress response in the banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol. 2017, 71, 319–328. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Sun, T.; Ji, C.; Li, F.; Wu, H. Time is ripe for targeting per-and polyfluoroalkyl substances-induced hormesis: Global aquatic hotspots and implications for ecological risk assessment. Environ. Sci. Technol. 2024, 58, 9314–9327. [Google Scholar] [CrossRef]
- Bakr, Z.; Abdel-Wahab, M.; Thabet, A.A.; Hamed, M.; Abd El-Aal, M.; Saad, E.; Faheem, M.; Sayed, A.E.-D.H. Toxicity of silver, copper oxide, and polyethylene nanoparticles on the earthworm Allolobophora caliginosa using multiple biomarkers. Appl. Soil Ecol. 2023, 181, 104681. [Google Scholar] [CrossRef]
- Cossi, P.F.; Herbert, L.T.; Yusseppone, M.S.; Pérez, A.F.; Kristoff, G. Environmental concentrations of azinphos-methyl cause different toxic effects without affecting the main target (cholinesterases) in the freshwater gastropod Biomphalaria straminea. Ecotoxicol. Environ. Saf. 2018, 162, 287–295. [Google Scholar] [CrossRef]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, F.; Al Alam, J.; Fajloun, Z.; Millet, M. Snail as sentinel organism for monitoring the environmental pollution; a review. Ecol. Indic. 2020, 113, 106240. [Google Scholar] [CrossRef]
- El-Gawad, S. The mollusk gastropod Lanistes carinatus (Olivier, 1804) as abiomonitor for some trace metals in the Nile River. Int. J. Zool. Res. 2009, 5, 115–125. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.; Said, R.E.; Martyniuk, C.J.; Osman, A.G.; Sayed, A.E.-D.H. Oxidative stress, antioxidant defense responses, and histopathology: Biomarkers for monitoring exposure to pyrogallol in Clarias gariepinus. J. Environ. Manag. 2024, 351, 119845. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Soliman, H.A.M.; Eid, Z.; Al Naggar, Y.; Sayed, A.E.-D.H. Dietary Feeding Lycopene, Citric Acid, and Chlorella Alleviated the Neurotoxicity of Polyethylene Microplastics in African Catfish (Clarias gariepinus). Front. Environ. Sci. 2022, 10. [Google Scholar] [CrossRef]
- Liang, R.; Shao, X.; Shi, Y.; Jiang, L.; Han, G. Antioxidant defenses and metabolic responses of blue mussels (Mytilus edulis) exposed to various concentrations of erythromycin. Sci. Total Environ. 2020, 698, 134221. [Google Scholar] [CrossRef]
- Xu, D.; Li, C.; Wen, Y.; Liu, W. Antioxidant defense system responses and DNA damage of earthworms exposed to perfluorooctane sulfonate (PFOS). Environ. Pollut. 2013, 174, 121–127. [Google Scholar] [CrossRef]
- Amraoui, I.; Khalloufi, N.; Touaylia, S. Effects to perfluorooctane sulfonate (PFOS) on the mollusk Unio ravoisieri under laboratory exposure. Chem. Ecol. 2018, 34, 324–339. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, K.; Park, K.; Seong, C.; Do Yu, S.; Kim, P. Adverse effects of perfluoroalkyl acids on fish and other aquatic organisms: A review. Sci. Total Environ. 2020, 707, 135334. [Google Scholar] [CrossRef]
- Liu, C.; Yu, K.; Shi, X.; Wang, J.; Lam, P.K.; Wu, R.S.; Zhou, B. Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquat. Toxicol. 2007, 82, 135–143. [Google Scholar] [CrossRef]
- Yang, S.; Liu, S.; Ren, Z.; Jiao, X.; Qin, S. Induction of oxidative stress and related transcriptional effects of perfluorononanoic acid using an in vivo assessment. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 160, 60–65. [Google Scholar] [CrossRef]
- Ma, T.; Ye, C.; Wang, T.; Li, X.; Luo, Y. Toxicity of per-and polyfluoroalkyl substances to aquatic invertebrates, planktons, and microorganisms. Int. J. Environ. Res. Public Health 2022, 19, 16729. [Google Scholar] [CrossRef] [PubMed]
- De Vaufleury, A.G.; Pihan, F. Growing snails used as sentinels to evaluate terrestrial environment contamination by trace elements. Chemosphere 2000, 40, 275–284. [Google Scholar] [CrossRef]
- Ismail, R.F.; Hamed, M.; Sayed, A.E.-D.H. Lycopene supplementation: Effects on oxidative stress, sex hormones, gonads and thyroid tissue in tilapia Oreochromis niloticus during Harness® exposure. Front. Physiol. 2023, 14, 1237159. [Google Scholar] [CrossRef]
- Khalil, A.M. Toxicological effects and oxidative stress responses in freshwater snail, Lanistes carinatus, following exposure to chlorpyrifos. Ecotoxicol. Environ. Saf. 2015, 116, 137–142. [Google Scholar] [CrossRef] [PubMed]
- El-SiKaily, A.; Shabaka, S. Biomarkers in aquatic systems: Advancements, applications and future directions. Egypt. J. Aquat. Res. 2024, 50, 169–182. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiadomosci lekarskie. 2004, 57, 453–455. [Google Scholar]
- Mohamed, I.A.; Hamed, M.; Abdel-Tawab, H.S.; Mansour, S.; Soliman, H.A.; Lee, J.-S.; Sayed, A.E.-D.H. Multi-biomarkers approach to assess the toxicity of novel insecticide (Voliam flexi®) on Clarias gariepinus: From behavior to immunotoxicity. Fish Shellfish Immunol. 2022, 125, 54–64. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Revisiting the “forever chemicals”, PFOA and PFOS exposure in drinking water. NPJ Clean Water 2023, 6, 57. [Google Scholar] [CrossRef]
- Teng, J.; Tang, S.; Ou, S. Determination of perfluorooctanesulfonate and perfluorooctanoate in water samples by SPE-HPLC/electrospray ion trap mass spectrometry. Microchem. J. 2009, 93, 55–59. [Google Scholar] [CrossRef]
- Geng, Q.; Zou, L.; Guo, M.; Peng, J.; Li, F.; Bi, Y.; Jiang, S.; Qin, H.; Tan, Z. Insights into the combined toxicity and mechanisms of BDE-47 and PFOA in marine blue mussel: An integrated study at the physiochemical and molecular levels. Aquat. Toxicol. 2024, 273, 106999. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, K.; Li, J.; Chen, L.; Lin, K. Uptake and depuration kinetics of lead (Pb) and biomarker responses in the earthworm Eisenia fetida after simultaneous exposure to decabromodiphenyl ether (BDE209). Ecotoxicol. Environ. Saf. 2015, 113, 45–51. [Google Scholar] [CrossRef]
- Rijnders, J.; Bervoets, L.; Prinsen, E.; Eens, M.; Beemster, G.T.; AbdElgawad, H.; Groffen, T. Perfluoroalkylated acids (PFAAs) accumulate in field-exposed snails (Cepaea sp.) and affect their oxidative status. Sci. Total Environ. 2021, 790, 148059. [Google Scholar] [CrossRef]
- Ilhan, S.; Somuncu, S.; Atmaca, H. Effects of acute exposure to azoxystrobin on embryos and juveniles of the freshwater snail Lymnaea stagnalis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2025, 295, 110209. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Kuehl, D.W.; Kahl, M.D.; Jensen, K.M.; Linnum, A.; Leino, R.L.; Villeneuve, D.A. Reproductive and developmental toxicity and bioconcentration of perfluorooctanesulfonate in a partial life-cycle test with the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2005, 24, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, L.; Yue, J.-Q.; Lv, Z.-Q.; Xia, W.; Wan, Y.-J.; Li, Y.-Y.; Xu, S.-Q. Prenatal PFOS exposure induces oxidative stress and apoptosis in the lung of rat off-spring. Reprod. Toxicol. 2012, 33, 538–545. [Google Scholar] [CrossRef]
- Siwela, A.H.; Nyathi, C.; Naik, Y.S. A comparison of metal levels and antioxidant enzymes in freshwater snails, Lymnaea natalensis, exposed to sediment and water collected from Wright Dam and Lower Mguza Dam, Bulawayo, Zimbabwe. Ecotoxicol. Environ. Saf. 2010, 73, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Sayed, A.E.-D.H.; Said, R.E.M.; El-Aal, M.A.; Saad, E.; Kamel, W.A.; Hamed, M. Black sand nanoparticles and heat stress impacts the neurological and oxidative stress indices and splenic-renal histology of Clarias gariepinus. Sci. Rep. 2024, 14, 21993. [Google Scholar] [CrossRef]
- Liu, C.; Gin, K.Y.; Chang, V.W. Multi-biomarker responses in green mussels exposed to PFCs: Effects at molecular, cellular, and physiological levels. Environ. Sci. Pollut. Res. 2014, 21, 2785–2794. [Google Scholar] [CrossRef]
- Touaylia, S.; Khazri, A.; Ali, M.; Bejaoui, M. Effects of emerging persistent organic pollutant perfluorooctane sulfonate (PFOS) on the Crustacean Gammarus insensibilis. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 2133–2141. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, J.; Li, Q.; Jiang, R.; Yu, N.; Qin, J.; Chen, L. Effects of perfluorooctane sulfonate on the immune responses and expression of immune-related genes in Chinese mitten-handed crab Eriocheir sinensis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2015, 172, 13–18. [Google Scholar] [CrossRef]
- Lu, G.-h.; Liu, J.-C.; Sun, L.-S.; Yuan, L.-J. Toxicity of perfluorononanoic acid and perfluorooctane sulfonate to Daphnia magna. Water Sci. Eng. 2015, 8, 40–48. [Google Scholar] [CrossRef]
- Copeto, S.; Ganço, S.; Ferreira, I.J.; Sanchez, D.; Nunes, M.J.; Motta, C.; Silva, M.; Diniz, M. The Impact of Perfluorooctanoic Acid (PFOA) on the Mussel Mytilus galloprovincialis: A Multi-Biomarker Evaluation. In Proceedings of the Oceans, Halifax, NS, Canada, 23–26 September 2024; pp. 857–873. [Google Scholar]
- Yang, J.-H. Perfluorooctanoic acid induces peroxisomal fatty acid oxidation and cytokine expression in the liver of male Japanese medaka (Oryzias latipes). Chemosphere 2010, 81, 548–552. [Google Scholar] [CrossRef]
- Orbea, A.; Dariush Fahimi, H.; Cajaraville, M.P. Immunolocalization of four antioxidant enzymes in digestive glands of mollusks and crustaceans and fish liver. Histochem. Cell Biol. 2000, 114, 393–404. [Google Scholar] [CrossRef]
- Li, F.; Yu, Y.; Guo, M.; Lin, Y.; Jiang, Y.; Qu, M.; Sun, X.; Li, Z.; Zhai, Y.; Tan, Z. Integrated analysis of physiological, transcriptomics and metabolomics provides insights into detoxication disruption of PFOA exposure in Mytilus edulis. Ecotoxicol. Environ. Saf. 2021, 214, 112081. [Google Scholar] [CrossRef]
- Li, F.; Liu, Z.; Yao, L.; Jiang, Y.; Qu, M.; Yu, Y.; Gong, X.; Tan, Z.; Li, Z. Immunotoxicity of perfluorooctanoic acid to the marine bivalve species Ruditapes philippinarum. Environ. Toxicol. Chem. 2022, 41, 426–436. [Google Scholar] [CrossRef]
- Du, J.; Cai, J.; Wang, S.; You, H. Oxidative stress and apotosis to zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonate (PFOS) and ZnO nanoparticles. Int. J. Occup. Med. Environ. Health 2017, 30, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Miao, Z.; Gong, X.; Zhao, B.; Zhang, Y.; Ma, H.; Zhang, J.; Zhao, B. Changes on lipid peroxidation, enzymatic activities and gene expression in planarian (Dugesia japonica) following exposure to perfluorooctanoic acid. Ecotoxicol. Environ. Saf. 2017, 145, 564–568. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, S.; Mestre, N.C.; Abel, S.; Fonseca, T.G.; Carteny, C.C.; Cormier, B.; Keiter, S.H.; Bebianno, M.J. Ecotoxicological effects of chemical contaminants adsorbed to microplastics in the clam Scrobicularia plana. Front. Mar. Sci. 2018, 5, 143. [Google Scholar] [CrossRef]
- Jifa, W.; Yu, Z.; Xiuxian, S.; You, W. Response of integrated biomarkers of fish (Lateolabrax japonicus) exposed to benzo [a] pyrene and sodium dodecylbenzene sulfonate. Ecotoxicol. Environ. Saf. 2006, 65, 230–236. [Google Scholar] [CrossRef]
- Vidal-Liñán, L.; Bellas, J.; Fumega, J.; Beiras, R. Bioaccumulation of BDE-47 and effects on molecular biomarkers acetylcholinesterase, glutathione-S-transferase and glutathione peroxidase in Mytilus galloprovincialis mussels. Ecotoxicology 2015, 24, 292–300. [Google Scholar] [CrossRef]
- Miranda, A.F.; Trestrail, C.; Lekamge, S.; Nugegoda, D. Effects of perfluorooctanoic acid (PFOA) on the thyroid status, vitellogenin, and oxidant–antioxidant balance in the Murray River rainbowfish. Ecotoxicology 2020, 29, 163–174. [Google Scholar] [CrossRef]
- Jeong, T.-Y.; Yuk, M.-S.; Jeon, J.; Kim, S.D. Multigenerational effect of perfluorooctane sulfonate (PFOS) on the individual fitness and population growth of Daphnia magna. Sci. Total Environ. 2016, 569–570, 1553–1560. [Google Scholar] [CrossRef]
- Zhang, J.; Naveed, H.; Chen, K.; Chen, L. Toxicity of Per-and Polyfluoroalkyl Substances and Their Substitutes to Terrestrial and Aquatic Invertebrates—A Review. Toxics 2025, 13, 47. [Google Scholar] [CrossRef]
- Gülsever, G.; Parlak, H. Effects of perfluorooctane sulfonate compounds on the biochemical activities in mussels (Mytilus galloprovincialis). Ege J. Fish. Aquat. Sci. 2018, 35, 417–422. [Google Scholar] [CrossRef]
- Verma, R.S.; Mehta, A.; Srivastava, N. In vivo chlorpyrifos induced oxidative stress: Attenuation by antioxidant vitamins. Pestic. Biochem. Physiol. 2007, 88, 191–196. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.; Ju, B.; Wang, Y.; Wang, J.; Bai, D. Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamins C and E. Exp. Toxicol. Pathol. 2008, 59, 415–423. [Google Scholar] [CrossRef]
- Ahammad Sahib, I.K.; Sailatha, D.; Ramana Rao, K.V. Impact of malathion on acetylcholinesterase in the tissues of the fishTilapia mossambica (Peters)—A time course study. J. Biosci. 1980, 2, 37–41. [Google Scholar] [CrossRef]
- Moore, M.N.; Allen, J.I. A computational model of the digestive gland epithelial cell of marine mussels and its simulated responses to oil-derived aromatic hydrocarbons. Mar. Environ. Res. 2002, 54, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Bignell, J.; Dodge, M.; Feist, S.; Lyons, B.; Martin, P.; Taylor, N.; Stone, D.; Travalent, L.; Stentiford, G. Mussel histopathology: Effects of season, disease and species. Aquat. Biol. 2008, 2, 1–15. [Google Scholar] [CrossRef]
- Marigómez, I.; Garmendia, L.; Soto, M.; Orbea, A.; Izagirre, U.; Cajaraville, M.P. Marine ecosystem health status assessment through integrative biomarker indices: A comparative study after the Prestige oil spill “Mussel Watch”. Ecotoxicology 2013, 22, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Bignell, J.; Stentiford, G.; Taylor, N.; Lyons, B. Histopathology of mussels (Mytilus sp.) from the Tamar estuary, UK. Mar. Environ. Res. 2011, 72, 25–32. [Google Scholar] [CrossRef]
- Kuzukiran, O.; Yurdakok-Dikmen, B.; Erkmen, B.; Gunal, A.C.; Arslan, P.; Pacal, E.; Totan, F.E.; Filazi, A.; Yildirim, Z.; Erkoç, F. Sublethal responses of the indicator Unio species (mussel) to selected phthalate esters. Biologia 2022, 77, 851–864. [Google Scholar] [CrossRef]
- Lowe, D.; Clarke, K. Contaminant-induced changes in the structure of the digestive epithelium of Mytilus edulis. Aquat. Toxicol. 1989, 15, 345–358. [Google Scholar] [CrossRef]
- Carella, F.; Feist, S.; Bignell, J.; De Vico, G. Comparative pathology in bivalves: Aetiological agents and disease processes. J. Invertebr. Pathol. 2015, 131, 107–120. [Google Scholar] [CrossRef]
- Carella, F.; Villari, G.; Maio, N.; De Vico, G. Disease and disorders of freshwater unionid mussels: A brief overview of recent studies. Front. Physiol. 2016, 7, 489. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Environmental impacts, exposure pathways, and health effects of PFOA and PFOS. Ecotoxicol. Environ. Saf. 2023, 267, 115663. [Google Scholar] [CrossRef]
- Hong, M.-S.; Lee, J.-S.; Lee, M.-C.; Lee, J.-S. Ecotoxicological effects of per-and polyfluoroalkyl substances in aquatic organisms: A review. Mar. Pollut. Bull. 2025, 214, 117678. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H. Toxicity of perfluorooctane sulfonate and perfluorooctanoic acid to plants and aquatic invertebrates. Environ. Toxicol. Int. J. 2009, 24, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Valenzuela-Jiménez, M.; Durruty-Lagunes, C.; Cuzon, G.; Pacheco, E.; Arévalo, M.; Aguilera-Rivera, D.; Wasielesky, W.; Rodríguez-Fuentes, G.; Barreto, A.; Gaxiola, G. Effect of water salinity on the oxidative system of juveniles of the North Atlantic white shrimp Litopenaeus setiferus reared in biofloc technology. J. World Aquac. Soc. 2022, 53, 258–270. [Google Scholar] [CrossRef]
- Fox, C.J.; Blow, P.; Brown, J.H.; Watson, I. The effect of various processing methods on the physical and biochemical properties of shrimp head meals and their utilization by juvenile Penaeus monodon Fab. Aquaculture 1994, 122, 209–226. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Hamed, M.; Soliman, H.A.; Osman, A.G.; Sayed, A.E.-D.H. Antioxidants and molecular damage in Nile Tilapia (Oreochromis niloticus) after exposure to microplastics. Environ. Sci. Pollut. Res. 2020, 27, 14581–14588. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hamed, M.; Martyniuk, C.J.; Soliman, H.A.; Osman, A.G.; Said, R.E. Neurotoxic and cardiotoxic effects of pyrogallol on catfish (Clarias gariepinus). Environ. Toxicol. Pharmacol. 2024, 109, 104481. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.A.; Lightner, D.V. A Handbook of Normal Penaeid Shrimp Histology; World Aquaculture Society: Baton Rouge, LA, USA, 1988. [Google Scholar]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamed, M.; Abdel-Wahab, M.; Said, R.E.M.; Sayed, A.E.-D.H. Ecotoxicological Impacts of Perfluorooctane Sulfonate on the Freshwater Snail Lanistes carinatus: Oxidative Stress, Neurotoxicity, and Histopathological Alterations. Int. J. Mol. Sci. 2025, 26, 8898. https://doi.org/10.3390/ijms26188898
Hamed M, Abdel-Wahab M, Said REM, Sayed AE-DH. Ecotoxicological Impacts of Perfluorooctane Sulfonate on the Freshwater Snail Lanistes carinatus: Oxidative Stress, Neurotoxicity, and Histopathological Alterations. International Journal of Molecular Sciences. 2025; 26(18):8898. https://doi.org/10.3390/ijms26188898
Chicago/Turabian StyleHamed, Mohamed, Mohammed Abdel-Wahab, Rashad E. M. Said, and Alaa El-Din H. Sayed. 2025. "Ecotoxicological Impacts of Perfluorooctane Sulfonate on the Freshwater Snail Lanistes carinatus: Oxidative Stress, Neurotoxicity, and Histopathological Alterations" International Journal of Molecular Sciences 26, no. 18: 8898. https://doi.org/10.3390/ijms26188898
APA StyleHamed, M., Abdel-Wahab, M., Said, R. E. M., & Sayed, A. E.-D. H. (2025). Ecotoxicological Impacts of Perfluorooctane Sulfonate on the Freshwater Snail Lanistes carinatus: Oxidative Stress, Neurotoxicity, and Histopathological Alterations. International Journal of Molecular Sciences, 26(18), 8898. https://doi.org/10.3390/ijms26188898






