Effects of Cherry Consumption on Metabolic Health: A Pilot Clinical Study on Healthy Adults
Abstract
1. Introduction
2. Results and Discussion
2.1. Baseline Characteristics of the Participants
2.2. Metabolic Biomarkers
2.2.1. Glucose Regulation
2.2.2. Inflammation and Oxidative Stress Markers
2.2.3. Liver Function and Nutritional Status
2.2.4. Kidney Function
2.2.5. Iron Metabolism
2.2.6. Immune System
2.2.7. Body Weight and Waist Circumference
2.3. Participant Feedback
3. Materials and Methods
3.1. Study Design and Participant Population
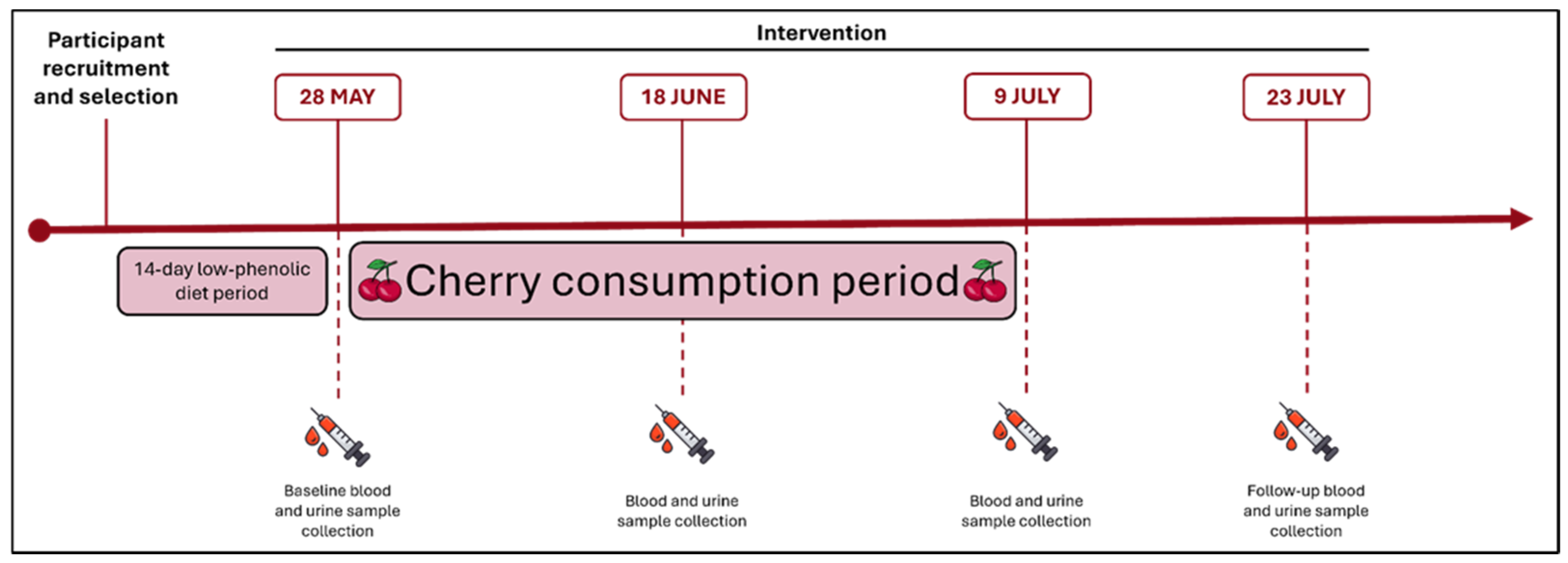
3.2. Intervention
3.3. Sample Collection and Biochemical Analysis
3.3.1. Glucose Regulation Markers
3.3.2. Lipid Metabolism Markers
3.3.3. Inflammation Markers
3.3.4. Liver Function and Nutritional Status Markers
3.3.5. Kidney Function Markers
3.3.6. Iron Metabolism Markers
3.3.7. Oxidative Stress Markers
3.3.8. Pancreatic Function Markers
3.3.9. Immunological Markers
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Chi-Square | df | p-Value | |
|---|---|---|---|
| Fasting glucose | 1.133 | 3 | 0.769 |
| HbA1c | 30.948 | 3 | <0.001 * |
| Estimated average glucose | 30.948 | 3 | <0.001 * |
| Insulin | 7.435 | 3 | 0.059 |
| Triglycerides | 1.633 | 3 | 0.652 |
| Total Cholesterol | 6.956 | 3 | 0.073 |
| HDL Cholesterol | 5.365 | 3 | 0.147 |
| LDL Cholesterol | 3.776 | 3 | 0.287 |
| CRP | 1.154 | 3 | 0.764 |
| IL-6 | 3.333 | 3 | 0.343 |
| AGP-1 | 20.088 | 3 | <0.001 * |
| AST | 1.679 | 3 | 0.642 |
| ALT | 2.069 | 3 | 0.558 |
| GGT | 11.296 | 3 | 0.010 * |
| Total Bilirubin | 0.658 | 3 | 0.883 |
| Conjugated bilirubin | 4.814 | 3 | 0.186 |
| Unconjugated bilirubin | 1.271 | 3 | 0.736 |
| LDH | 8.300 | 3 | 0.040 * |
| Total proteins | 4.108 | 3 | 0.250 |
| Albumin | 35.580 | 3 | <0.001 * |
| Urea | 6.781 | 3 | 0.079 |
| Creatinine | 17.979 | 3 | <0.001 * |
| Microalbumin | 7.612 | 3 | 0.055 |
| Uric acid | 2.823 | 3 | 0.420 |
| Iron | 2.868 | 3 | 0.412 |
| TIBC | 6.642 | 3 | 0.084 |
| Transferrin | 6.485 | 3 | 0.090 |
| Ferritin | 9.068 | 3 | 0.028 * |
| GPx | 51.783 | 3 | <0.001 * |
| GR | 18.926 | 3 | <0.001 * |
| GDH | 0.652 | 3 | 0.884 |
| Amylase | 5.095 | 3 | 0.165 |
| Lipase | 5.593 | 3 | 0.133 |
| IgA | 7.266 | 3 | 0.064 |
| IgG | 17.073 | 3 | <0.001 * |
| IgM | 4.577 | 3 | 0.205 |
| Total IgE | 30.252 | 3 | <0.001 * |
| Rheumatoid factor | 10.680 | 3 | 0.014 * |
| ASO | 8.453 | 3 | 0.038 * |
Appendix B
Appendix B.1
| Marker | t-Value/Z-Value (p-Value) | ||
|---|---|---|---|
| Day 21 | Day 42 | 2 Weeks After Intervention | |
| Triglycerides b | −0.613 (0.540) | −0.529 (0.597) | −0.091 (0.927) |
| Total Cholesterol a | −1.267 (0.216) | −1.642 (0.114) | 1.318 (0.201) |
| HDL Cholesterol a | −0.453 (0.654) | −0.333 (0.742) | 1.720 (0.099) |
| LDL Cholesterol b | −1.283 (0.200) | −1.529 (0.126) | −1.126 (0.260) |
Appendix B.2
| Marker | t-Value/Z-Value (p-Value) | ||
|---|---|---|---|
| Day 21 | Day 42 | 2 Weeks After Intervention | |
| Amylase b | −0.601 (0.548) | −0.872 (0.383) | −1.446 (0.148) |
| Lipase b | −0.529 (0.597) | −1.458 (0.145) | −1.332 (0.183) |
References
- Niewiadomska, J.; Gajek-Marecka, A.; Gajek, J.; Noszczyk-Nowak, A. Biological Potential of Polyphenols in the Context of Metabolic Syndrome: An Analysis of Studies on Animal Models. Biology 2022, 11, 559. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, M.; Zhang, W.; Liu, C.; Chen, S. Natural Polyphenols in Metabolic Syndrome: Protective Mechanisms and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 6110. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic Distribution of Metabolic Syndrome and Its Components in the General Adult Population: A Meta-Analysis of Global Data from 28 Million Individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef]
- Han, T.S.; Lean, M.E. A Clinical Perspective of Obesity, Metabolic Syndrome and Cardiovascular Disease. JRSM Cardiovasc. Dis. 2016, 5, 204800401663337. [Google Scholar] [CrossRef] [PubMed]
- Todowede, O.O.; Sartorius, B. Prevalence of Metabolic Syndrome, Discrete or Comorbid Diabetes and Hypertension in Sub-Saharan Africa among People Living with HIV versus HIV-Negative Populations: A Systematic Review and Meta-Analysis Protocol. BMJ Open 2017, 7, e016602. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Lahlou, R.A.; Silva, L.R. Phenolic Compounds from Cherries and Berries for Chronic Disease Management and Cardiovascular Risk Reduction. Nutrients 2024, 16, 1597. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, N.; Farrell, M.; O’Sullivan, L.; Langan, A.; Franchin, M.; Azevedo, L.; Granato, D. Effectiveness of Anthocyanin-Containing Foods and Nutraceuticals in Mitigating Oxidative Stress, Inflammation, and Cardiovascular Health-Related Biomarkers: A Systematic Review of Animal and Human Interventions. Food Funct. 2024, 15, 3274–3299. [Google Scholar] [CrossRef]
- Amini, A.M.; Spencer, J.P.E.; Yaqoob, P. Effects of Pelargonidin-3-O-Glucoside and Its Metabolites on Lipopolysaccharide-Stimulated Cytokine Production by THP-1 Monocytes and Macrophages. Cytokine 2018, 103, 29–33. [Google Scholar] [CrossRef]
- Vendrame, S.; Klimis-Zacas, D. Anti-Inflammatory Effect of Anthocyanins via Modulation of Nuclear Factor- B and Mitogen-Activated Protein Kinase Signaling Cascades. Nutr. Rev. 2015, 73, 348–358. [Google Scholar] [CrossRef]
- Ren, L.; Tan, N.; Ouyang, J.; Wang, R.; Tie, F.; Dong, Q.; Wang, H.; Hu, N. Hypoglycaemic Activity of the Anthocyanin Enriched Fraction of Lycium Ruthenicum Murr. Fruits and Its Ingredient Identification via UPLC–Triple-TOF-MS/MS. Food Chem. 2024, 461, 140837. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, P.; Li, Z.; Wu, W. Chemopreventive and Therapeutic Properties of Anthocyanins in Breast Cancer: A Comprehensive Review. Nutr. Res. 2022, 107, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Stoner, G.D. Anthocyanins and Their Role in Cancer Prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef]
- Tarozzi, A.; Morroni, F.; Hrelia, S.; Angeloni, C.; Marchesi, A.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective Effects of Anthocyanins and Their in Vivo Metabolites in SH-SY5Y Cells. Neurosci. Lett. 2007, 424, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Jo, M.G.; Badshah, H.; Kim, M.O. Anthocyanins Protect against LPS-Induced Oxidative Stress-Mediated Neuroinflammation and Neurodegeneration in the Adult Mouse Cortex. Neurochem. Int. 2016, 100, 1–10. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Huang, Y.; Evans, P.C.; Little, P.J.; Tian, X.; Weng, J.; Xu, S. Anthocyanins in Vascular Health and Disease: Mechanisms of Action and Therapeutic Potential. J. Cardiovasc. Pharmacol. 2024, 84, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Krga, I.; Vidovic, N.; Milenkovic, D.; Konic-Ristic, A.; Stojanovic, F.; Morand, C.; Glibetic, M. Effects of Anthocyanins and Their Gut Metabolites on Adenosine Diphosphate-Induced Platelet Activation and Their Aggregation with Monocytes and Neutrophils. Arch. Biochem. Biophys. 2018, 645, 34–41. [Google Scholar] [CrossRef]
- Nguyen, T.L.P.; Fenyvesi, F.; Remenyik, J.; Homoki, J.R.; Gogolák, P.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Vasvári, G.; Vecsernyés, M.; et al. Protective Effect of Pure Sour Cherry Anthocyanin Extract on Cytokine-Induced Inflammatory Caco-2 Monolayers. Nutrients 2018, 10, 861. [Google Scholar] [CrossRef]
- Zhou, Z.; Nair, M.G.; Claycombe, K.J. Synergistic Inhibition of Interleukin-6 Production in Adipose Stem Cells by Tart Cherry Anthocyanins and Atorvastatin. Phytomedicine 2012, 19, 878–881. [Google Scholar] [CrossRef]
- Ates, I. Type 2 Diabetes Mechanisms, Role of Cytokines and Their Variations in Disease Development. J. Pharm. Sci. 2018, 43, 3140. [Google Scholar]
- Eguchi, K.; Manabe, I. Macrophages and Islet Inflammation in Type 2 Diabetes. Diabetes Obes. Metab. 2013, 15, 152–158. [Google Scholar] [CrossRef]
- Ataie-Jafari, A.; Hosseini, S.; Karimi, F.; Pajouhi, M. Effects of Sour Cherry Juice on Blood Glucose and Some Cardiovascular Risk Factors Improvements in Diabetic Women. Nutr. Food Sci. 2008, 38, 355–360. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Maharat, M.; Chambari, M.; Moradi, F.; Rahimlou, M. Effects of Tart Cherry Juice Consumption on Cardio-Metabolic Risk Factors: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. Complement. Ther. Med. 2022, 71, 102883. [Google Scholar] [CrossRef]
- Guo, J.; Sun, D.; Xu, X.; Liu, P.; Sun, H. Relief Effects of Laoshan Cherry Extracts as a Dietary Supplement against the Symptoms of Acute Gouty Arthritis in Rats Induced by Urate Crystals. J. Food Sci. 2023, 88, 1188–1196. [Google Scholar] [CrossRef]
- Wang, C.; Sun, W.; Dalbeth, N.; Wang, Z.; Wang, X.; Ji, X.; Xue, X.; Han, L.; Cui, L.; Li, X.; et al. Efficacy and Safety of Tart Cherry Supplementary Citrate Mixture on Gout Patients: A Prospective, Randomized, Controlled Study. Arthritis Res. Ther. 2023, 25, 164. [Google Scholar] [CrossRef]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency Tart Cherry (Prunus cerasus L.) Consumption on Vascular Function in Men with Early Hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef]
- Johnson, S.A.; Navaei, N.; Pourafshar, S.; Jaime, S.J.; Akhavan, N.S.; Alvarez-Alvarado, S.; Proaño, G.V.; Litwin, N.S.; Clark, E.A.; Foley, E.M.; et al. Effects of Montmorency Tart Cherry Juice Consumption on Cardiometabolic Biomarkers in Adults with Metabolic Syndrome: A Randomized Controlled Pilot Trial. J. Med. Food 2020, 23, 1238–1247. [Google Scholar] [CrossRef]
- World Health Organization. A Healthy Lifestyle—WHO Recommendations. Available online: https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle---who-recommendations (accessed on 31 July 2024).
- Tchernof, A.; Després, J.-P. Pathophysiology of Human Visceral Obesity: An Update. Physiol. Rev. 2013, 93, 359–404. [Google Scholar] [CrossRef]
- Farmanfarma, K.; Ansari-Moghaddam, A.; Kaykhaei, M.; Mohammadi, M.; Adineh, H.; Aliabd, H.O. Incidence of and Factors Associated with Metabolic Syndrome, South-East Islamic Republic of Iran. East. Mediterr. Health J. 2021, 27, 1084–1091. [Google Scholar] [CrossRef]
- Nolan, P.B.; Carrick-Ranson, G.; Stinear, J.W.; Reading, S.A.; Dalleck, L.C. Prevalence of Metabolic Syndrome and Metabolic Syndrome Components in Young Adults: A Pooled Analysis. Prev. Med. Rep. 2017, 7, 211–215. [Google Scholar] [CrossRef]
- Halim, M.; Halim, A. The Effects of Inflammation, Aging and Oxidative Stress on the Pathogenesis of Diabetes Mellitus (Type 2 Diabetes). Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1165–1172. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Flores-Félix, J.D.; Alves, G.; Silva, L.R. Cherries and Blueberries-Based Beverages: Functional Foods with Antidiabetic and Immune Booster Properties. Molecules 2022, 27, 3294. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary Polyphenols and Type 2 Diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.; Clifton, P. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Hernández-Ruiz, R.G.; Olivares-Ochoa, X.C.; Salinas-Varela, Y.; Guajardo-Espinoza, D.; Roldán-Flores, L.G.; Rivera-Leon, E.A.; López-Quintero, A. Phenolic Compounds and Anthocyanins in Legumes and Their Impact on Inflammation, Oxidative Stress, and Metabolism: Comprehensive Review. Molecules 2025, 30, 174. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Nehring, S.M.; Goyal, A.; Patel, B.C. C Reactive Protein. Available online: https://www.ncbi.nlm.nih.gov/books/NBK441843/ (accessed on 31 January 2025).
- Dirienzo, W.; Stefanini, G.F.; Miribel, L.; Paulling, E.E.; Canonica, G.W.; Fudenberg, H.H. A1-Acid Glycoprotein (A1-AGP) on the Membrane of Human Lymphocytes: Possible Involvement in Cellular Activation. Immunol. Lett. 1987, 15, 167–170. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef]
- Lear, L.; Najjar, R.; Feresin, R.; Danh, J. Raspberry Polyphenol Extract Decreases NF-KB and IL-6 Expression in Lipopolysaccharide (LPS)-Induced RAW 264.7 Macrophages. Curr. Dev. Nutr. 2020, 4, 422. [Google Scholar] [CrossRef]
- Gholami, A.; Amirkalali, B.; Baradaran, H.R.; Hariri, M. The Beneficial Effect of Tart Cherry on Plasma Levels of Inflammatory Mediators (Not Recovery after Exercise): A Systematic Review and Meta-Analysis on Randomized Clinical Trials. Complement. Ther. Med. 2022, 68, 102842. [Google Scholar] [CrossRef]
- Gannon, B.M.; Glesby, M.J.; Finkelstein, J.L.; Raj, T.; Erickson, D.; Mehta, S. A Point-of-Care Assay for Alpha-1-Acid Glycoprotein as a Diagnostic Tool for Rapid, Mobile-Based Determination of Inflammation. Curr. Res. Biotechnol. 2019, 1, 41–48. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. The Role of Glutathione Peroxidase-1 in Health and Disease. Free Radic. Biol. Med. 2022, 188, 146–161. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The Role of Glutathione Reductase and Related Enzymes on Cellular Redox Homoeostasis Network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- Stachowska, E.; Wesołowska, T.; Olszewska, M.; Safranow, K.; Millo, B.; Domański, L.; Jakubowska, K.; Ciechanowski, K.; Chlubek, D. Elements of Mediterranean Diet Improve Oxidative Status in Blood of Kidney Graft Recipients. Br. J. Nutr. 2005, 93, 345–352. [Google Scholar] [CrossRef]
- Hohnholt, M.C.; Andersen, V.H.; Andersen, J.V.; Christensen, S.K.; Karaca, M.; Maechler, P.; Waagepetersen, H.S. Glutamate Dehydrogenase Is Essential to Sustain Neuronal Oxidative Energy Metabolism during Stimulation. J. Cereb. Blood Flow. Metab. 2018, 38, 1754–1768. [Google Scholar] [CrossRef]
- Tennant, B.C.; Center, S.A. Hepatic Function. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 2008; pp. 379–412. [Google Scholar]
- Hsieh, P.S.; Hsieh, Y.J. Impact of Liver Diseases on the Development of Type 2 Diabetes Mellitus. World J. Gastroenterol. 2011, 17, 5240–5245. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Harris, R.; Sattar, N.; Ebrahim, S.; Smith, G.D.; Lawlor, D.A. Gamma-Glutamyltransferase Is Associated With Incident Vascular Events Independently of Alcohol Intake. Arter. Thromb. Vasc. Biol. 2007, 27, 2729–2735, Correction in Arter. Thromb. Vasc. Biol. 2008, 28, e14. [Google Scholar] [CrossRef]
- Kasapoglu, B.; Turkay, C.; Yalcın, K.S.; Carlioglu, A.; Koktener, A. Role of γ-Glutamyl Transferase Levels in Prediction of High Cardiovascular Risk among Patients with Non-Alcoholic Fatty Liver Disease. Indian. J. Med. Res. 2016, 143, 30–36. [Google Scholar] [CrossRef]
- Fraser, A.; Harris, R.; Sattar, N.; Ebrahim, S.; Smith, G.D.; Lawlor, D.A. Alanine Aminotransferase, γ-Glutamyltransferase, and Incident Diabetes. Diabetes Care 2009, 32, 741–750. [Google Scholar] [CrossRef]
- Saha, P.; Talukdar, A.D.; Nath, R.; Sarker, S.D.; Nahar, L.; Sahu, J.; Choudhury, M.D. Role of Natural Phenolics in Hepatoprotection: A Mechanistic Review and Analysis of Regulatory Network of Associated Genes. Front. Pharmacol. 2019, 10, 509. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, A.; Kurokawa, M.; Kohjima, M.; Imoto, K.; Tashiro, S.; Suzuki, H.; Tanaka, M.; Okada, S.; Kato, M.; Ogawa, Y. Microcirculatory Disturbance in Acute Liver Injury. Exp. Ther. Med. 2021, 21, 596. [Google Scholar] [CrossRef]
- Farhana, A.; Lappin, S.L. Biochemistry, Lactate Dehydrogenase; StatPearls: Petersburg, FL, USA, 2025. [Google Scholar]
- Gupta, G.S. The Lactate and the Lactate Dehydrogenase in Inflammatory Diseases and Major Risk Factors in COVID-19 Patients. Inflammation 2022, 45, 2091–2123. [Google Scholar] [CrossRef]
- Mondal, M.; Kundu, S.K.; Islam, M.T.; Reiner, Ž.; Martorell, M.; Sharifi-Rad, J. Protective Effect of Bridelia Tomentosa Due to Its Phenolic Acids and Flavonoids against Oxidative Stress-Mediated Hepatic Toxicity Induced by Carbofuran. S. Afr. J. Bot. 2021, 141, 447–456. [Google Scholar] [CrossRef]
- Cho, H.M.; Kim, H.C.; Lee, J.M.; Oh, S.M.; Choi, D.P.; Suh, I. The Association between Serum Albumin Levels and Metabolic Syndrome in a Rural Population of Korea. J. Prev. Med. Public Health 2012, 45, 98–104. [Google Scholar] [CrossRef]
- Ishizaka, N.; Ishizaka, Y.; Nagai, R.; Toda, E.I.; Hashimoto, H.; Yamakado, M. Association between Serum Albumin, Carotid Atherosclerosis, and Metabolic Syndrome in Japanese Individuals. Atherosclerosis 2007, 193, 373–379. [Google Scholar] [CrossRef]
- Jia, F.; Sun, J.; Liu, X.; Liu, Y. Life Essentials 8 Score and Risk of Metabolic Syndrome: A Dose-Response Analysis in the US Population. PLoS ONE 2024, 19, e0312674. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fan, Y.; Su, J.; Yang, Z.; Liu, Z. The Association between Serum Albumin Levels and Metabolic Syndrome Based on the NHANES and Two Sample Mendelian Randomization Study. Sci. Rep. 2025, 15, 2861. [Google Scholar] [CrossRef]
- Wang, B.; Cui, S.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Tang, X.; Chen, W. Cyanidin Alleviated CCl4-Induced Acute Liver Injury by Regulating the Nrf2 and NF-ΚB Signaling Pathways. Antioxidants 2022, 11, 2383. [Google Scholar] [CrossRef]
- Thakur, S.; Kumar, V.; Das, R.; Sharma, V.; Mehta, D.K. Biomarkers of Hepatic Toxicity: An Overview. Curr. Ther. Res. Clin. Exp. 2024, 100, 100737. [Google Scholar] [CrossRef]
- Onopiuk, A.; Tokarzewicz, A.; Gorodkiewicz, E. Cystatin C: A kidney function biomarker. Adv. Clin. Chem. 2015, 68, 57–69. [Google Scholar]
- Lin, L.; Tan, W.; Pan, X.; Tian, E.; Wu, Z.; Yang, J. Metabolic Syndrome-Related Kidney Injury: A Review and Update. Front. Endocrinol. (Lausanne) 2022, 13, 904001. [Google Scholar] [CrossRef]
- Maringhini, S.; Zoccali, C. Chronic Kidney Disease Progression—A Challenge. Biomedicines 2024, 12, 2203. [Google Scholar] [CrossRef]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.K.; Vernekar, S.N. Markers of Renal Function Tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar] [PubMed]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507821/?utm_source=chatgpt.com (accessed on 19 February 2025).
- Ashkar, F.; Bhullar, K.S.; Wu, J. The Effect of Polyphenols on Kidney Disease: Targeting Mitochondria. Nutrients 2022, 14, 3115. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.M.; Zafar, S. Frequency of Microalbuminuria in Patients with Metabolic Syndrome. PJMHS 2014, 8, 616–618. [Google Scholar]
- Giordano, C.; Karasik, O.; King-Morris, K.; Asmar, A. Uric Acid as a Marker of Kidney Disease: Review of the Current Literature. Dis. Markers 2015, 2015, 382918. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Current Understanding of Iron Homeostasis. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1559S–1566S. [Google Scholar] [CrossRef]
- Simcox, J.A.; McClain, D.A. Iron and Diabetes Risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef]
- Ferńandez-Real, J.M.; Mcclain, D.; Review, M.M. Mechanisms Linking Glucose Homeostasis and Iron Metabolism Toward the Onset and Progression of Type 2 Diabetes. Diabetes Care 2015, 38, 2169–2176. [Google Scholar] [CrossRef]
- Sun, L.; Franco, O.H.; Hu, F.B.; Cai, L.; Yu, Z.; Li, H.; Ye, X.; Qi, Q.; Wang, J.; Pan, A.; et al. Ferritin Concentrations, Metabolic Syndrome, and Type 2 Diabetes in Middle-Aged and Elderly Chinese. J. Clin. Endocrinol. Metab. 2008, 93, 4690–4696. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, X.; Liu, Y.; Wang, H.; Luo, J.; Luo, Y.; An, P. Effects of Dietary Polyphenol Supplementation on Iron Status and Erythropoiesis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2021, 114, 780–793. [Google Scholar] [CrossRef]
- Das, S.K.; Wang, W.; Zhabyeyev, P.; Basu, R.; McLean, B.; Fan, D.; Parajuli, N.; DesAulniers, J.; Patel, V.B.; Hajjar, R.J.; et al. Iron-Overload Injury and Cardiomyopathy in Acquired and Genetic Models Is Attenuated by Resveratrol Therapy. Sci. Rep. 2015, 5, 18132. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef] [PubMed]
- Malesev, D.; Kuntic, V. Investigation of Metal-Flavonoid Chelates and the Determination of Flavonoids via Metal-Flavonoid Complexing Reactions. J. Serbian Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Correnti, M.; Gammella, E.; Cairo, G.; Recalcati, S. Iron Mining for Erythropoiesis. Int. J. Mol. Sci. 2022, 23, 5341. [Google Scholar] [CrossRef] [PubMed]
- Talukder, J. Role of Transferrin: An Iron-Binding Protein in Health and Diseases. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1011–1025. [Google Scholar]
- Kasvosve, I.; Delanghe, J. Total Iron Binding Capacity and Transferrin Concentration in the Assessment of Iron Status. Clin. Chem. Lab. Med. 2002, 40, 1014–1018. [Google Scholar] [CrossRef]
- Guo, R.; Gao, J.; Hui, L.; Li, Y.; Liu, J.; Fu, Y.; Shi, L.; Wang, Y.; Liu, B. An Improved Method for Quick Quantification of Unsaturated Transferrin. Biosensors 2022, 12, 708. [Google Scholar] [CrossRef]
- Sangeetha Vijayan, P.; Xavier, J.; Valappil, M.P. A Review of Immune Modulators and Immunotherapy in Infectious Diseases. Mol. Cell. Biochem. 2024, 479, 1937–1955. [Google Scholar] [CrossRef]
- Zmora, N.; Bashiardes, S.; Levy, M.; Elinav, E. The Role of the Immune System in Metabolic Health and Disease. Cell Metab. 2017, 25, 506–521. [Google Scholar] [CrossRef]
- Matarese, G.; Procaccini, C.; De Rosa, V. At the Crossroad of T Cells, Adipose Tissue, and Diabetes. Immunol. Rev. 2012, 249, 116–134. [Google Scholar] [CrossRef]
- Ahmed, J.; Choi, Y.; Ko, T.; Lim, J.; Hajjar, J. Use of Immunoglobulin Replacement Therapy in Clinical Practice: A Review. J. Immunother. Precis. Oncol. 2025, 8, 34–46. [Google Scholar] [CrossRef]
- Hand, T.W.; Reboldi, A. Production and Function of Immunoglobulin A. Annu. Rev. Immunol. 2025, 22, 59. [Google Scholar] [CrossRef]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.O.; Carbonero, F. Impact of Tart Cherries Polyphenols on the Human Gut Microbiota and Phenolic Metabolites in Vitro and in Vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Leusen, J.H.W.; Nimmerjahn, F. The Role of IgG in Immune Responses. In Molecular and Cellular Mechanisms of Antibody Activity; Springer: New York, NY, USA, 2013; pp. 85–112. [Google Scholar]
- Chaigne, B.; Mouthon, L. Mechanisms of Action of Intravenous Immunoglobulin. Transfus. Apher. Sci. 2017, 56, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Sujono, T.A.; Dian Kusumowati, I.T.; Munawaroh, R. Effects of Jamaican Cherry (Muntingia calabura L.) Fruits Extract on Immunoglobulin G Levels and Hematological Profiles in Mice. Pharmacogn. J. 2020, 13, 535–541. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Xiong, E.; Hong, R.; Lu, Q.; Ohno, H.; Wang, J.Y. Role of the IgM Fc Receptor in Immunity and Tolerance. Front. Immunol. 2019, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.A.; Rosenwasser, L.J. The Role of Immunoglobulin E and Immune Inflammation: Implications in Allergic Rhinitis. Curr. Allergy Asthma Rep. 2005, 5, 252–258. [Google Scholar] [CrossRef]
- Yano, S.; Umeda, D.; Yamashita, T.; Ninomiya, Y.; Sumida, M.; Fujimura, Y.; Yamada, K.; Tachibana, H. Dietary Flavones Suppresses IgE and Th2 Cytokines in OVA-Immunized BALB/c Mice. Eur. J. Nutr. 2007, 46, 257–263. [Google Scholar] [CrossRef]
- Gualtierotti, R.; Ciavarella, T.; Meroni, P.L. Rheumatoid Factors. In Autoantibodies; Elsevier: Amsterdam, The Netherlands, 2014; pp. 751–760. [Google Scholar]
- Blyth, C.C.; Robertson, P.W. Anti-Streptococcal Antibodies in the Diagnosis of Acute and Post-Streptococcal Disease: Streptokinase versus Streptolysin O and Deoxyribonuclease B. Pathology 2006, 38, 152–156. [Google Scholar] [CrossRef]
- Abid Ali, S.; Ahmad, R.; Ahmad, N.; Makhdoomi, M.; Parvaiz, Q. Augmentation of Immunocytes Functions by Prunus Cerasus Fruit and Its Biotherapeutic Potential in Mice Model. Biomed. Pharmacol. J. 2019, 12, 2071–2081. [Google Scholar] [CrossRef]
- Ziolkowska, S.; Binienda, A.; Jabłkowski, M.; Szemraj, J.; Czarny, P. The Interplay between Insulin Resistance, Inflammation, Oxidative Stress, Base Excision Repair and Metabolic Syndrome in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2021, 22, 11128. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.S.; Alvarez-Leite, J.I. Low-Grade Inflammation, Obesity, and Diabetes. Curr. Obes. Rep. 2014, 3, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H. Obese Visceral Fat Tissue Inflammation: From Protective to Detrimental? BMC Med. 2022, 20, 494. [Google Scholar] [CrossRef]
- Domínguez-Reyes, T.; Quiroz-Vargas, I.; Salgado-Bernabé, A.B.; Salgado-Goytia, L.; Muñoz-Valle, J.F.; Parra-Rojas, I. Las Medidas Antropométricas Como Indicadores Predictivos de Riesgo Metabólico En Una Población Mexicana. Nutr. Hosp. 2017, 34, 96–101. [Google Scholar] [CrossRef]
- Su, Y.; Sun, J.; Zhou, Y.; Sun, W. The Relationship of Waist Circumference with the Morbidity of Cardiovascular Diseases and All-Cause Mortality in Metabolically Healthy Individuals: A Population-Based Cohort Study. Rev. Cardiovasc. Med. 2024, 25, 212. [Google Scholar] [CrossRef]
- Miyatake, N.; Matsumoto, S.; Fujii, M.; Numata, T. Reducing Waist Circumference by at Least 3 Cm Is Recommended for Improving Metabolic Syndrome in Obese Japanese Men. Diabetes Res. Clin. Pr. 2008, 79, 191–195. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Morán-Ramos, S.; Siliceo-Bernardi, M.T.; Villalpando-Carrión, S.; Canizales-Quinteros, S.; Frigolet, M.E.; Gutiérrez-Aguilar, R. Gut Microbiota Composition after a Dietary and Physical Activity Intervention: A Pilot Study in Mexican Children with Obesity. Bol. Med. Hosp. Infant. Mex. 2022, 79, 318–325. [Google Scholar] [CrossRef]
- Godswill Awuchi, C.; Kate Echeta, C.; Godswill, C.; Kate, C. Current Developments in Sugar Alcohols: Chemistry, Nutrition, and Health Concerns of Sorbitol, Xylitol, Glycerol, Arabitol, Inositol, Maltitol, and Lactitol. Int. J. Adv. Acad. Res. 2019, 5, 31–66. [Google Scholar]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- González-Gómez, D.; Lozano, M.; Fernández-León, M.F.; Ayuso, M.C.; Bernalte, M.J.; Rodríguez, A.B. Detection and Quantification of Melatonin and Serotonin in Eight Sweet Cherry Cultivars (Prunus avium L.). Eur. Food Res. Technol. 2009, 229, 223–229. [Google Scholar] [CrossRef]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of Tart Cherry Juice (Prunus cerasus) on Melatonin Levels and Enhanced Sleep Quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Shaw, K.; Kaviani, M.; Gordon, J.; Zello, G.A.; Chilibeck, P.D. Glycemic Index of Lentil- and Cherry-Based Sport Nutrition Products for Endurance Athletes. Sci. Sports 2021, 36, 234.e1–234.e6. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.C.; Campos, G.; Alves, G.; Garcia-Viguera, C.; Moreno, D.A.; Silva, L.R. Physical and Phytochemical Composition of 23 Portuguese Sweet Cherries as Conditioned by Variety (or Genotype). Food Chem. 2021, 335, 127637. [Google Scholar] [CrossRef]
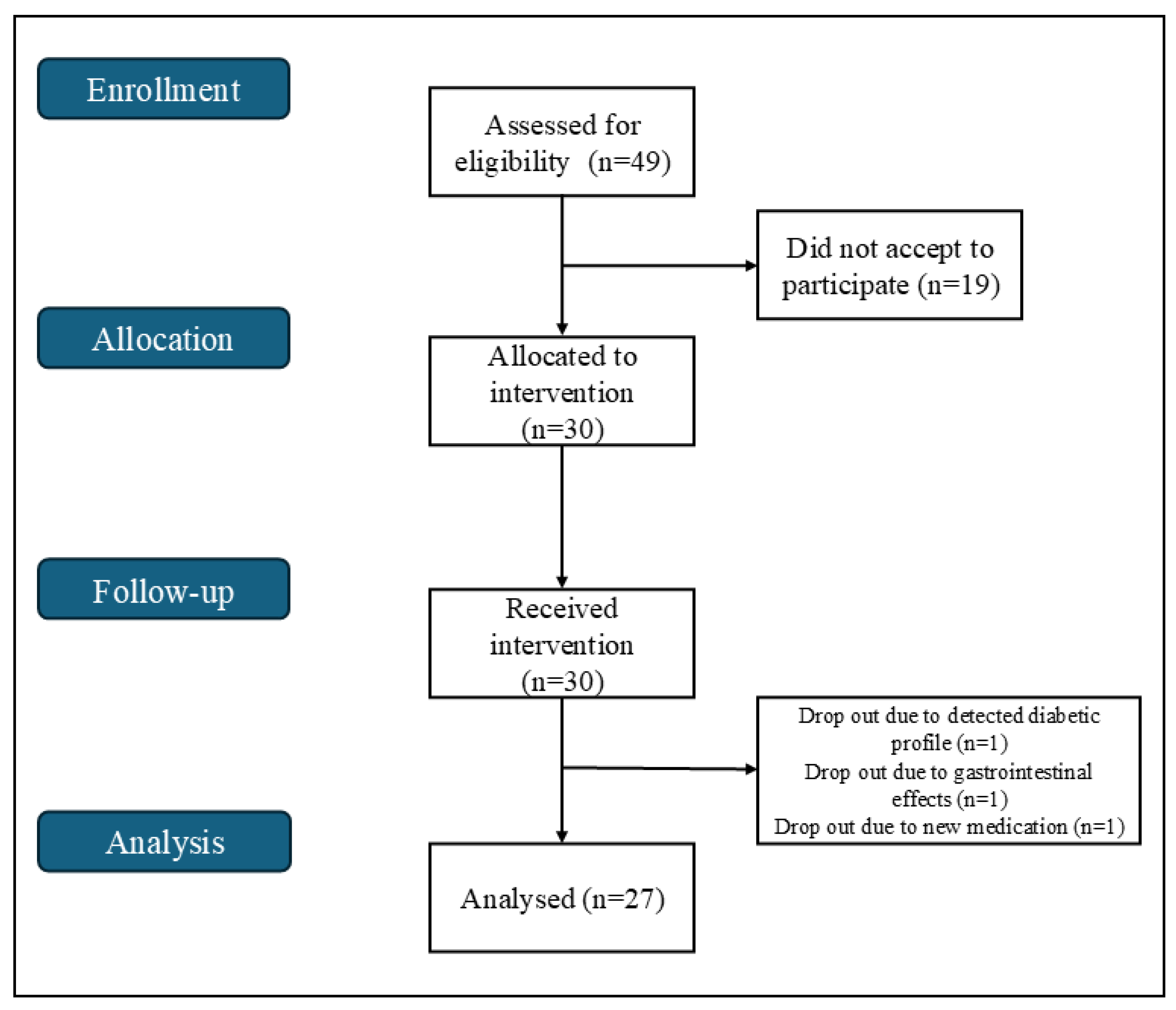
| Healthy Adults (n = 27) | ||
|---|---|---|
| Age, (range) | 47 ± 12 (18–64) | |
| BMI (kg/m2), (range) | 25.5 ± 3.9 (19.1–32.4) | |
| WC (cm), (range) | 86.0 ± 12.1 (64.0–110.0) | |
| Sex, n (%) | Male | 7 (25.9) |
| Female | 20 (74.1) | |
| Marital status, n (%) | Single | 7 (25.9) |
| Married | 16 (59.3) | |
| Divorced | 3 (11.1) | |
| Widowed | 1 (3.7) | |
| Education level, n (%) | Basic | 1 (3.7) |
| Secondary | 7 (25.9) | |
| Higher | 19 (70.4) | |
| Marker | t-Value/Z-Value (p-Value) | ||
|---|---|---|---|
| Day 21 | Day 42 | 2 Weeks After Intervention | |
| Fasting glucose b | −0.064 (0.949) | −0.761 (0.447) | −1.308 (0.191) |
| HbA1c a | −6.447 (<0.001) * | −3.864 (<0.001) * | −2.583 (0.017) * |
| Estimated average glucose a | −6.453 (0.001) * | −3.844 (0.001) * | −2.597 (0.016) * |
| Insulin b | −0.480 (0.631) | −0.525 (0.600) | −1.582 (0.114) |
| Marker | t-Value/Z-Value (p-Value) | ||
|---|---|---|---|
| Day 21 | Day 42 | 2 Weeks After Intervention | |
| CRP b | −0.349 (0.949) | −0.497 (0.447) | −1.049 (0.191) |
| IL-6 b | −0.447 (<0.001) * | −1.826 (<0.001) * | −1.604 (0.017) * |
| AGP-1 b | −8.59259 (0.001) * | −4.84 (0.001) * | −4.17391 (0.016) * |
| Marker | t-Value/Z-Value (p-Value) | ||
|---|---|---|---|
| Day 21 | Day 42 | 2 Weeks After Intervention | |
| GPx a | −1.833 (0.078) | −12.952 (<0.001) * | −8.918 (<0.001) * |
| GR a | −0.583 (0.563) | −3.910 (<0.001) * | −2.021 (0.056) |
| GDH b | −0.577 (0.564) | −0.444 (0.657) | −1.034 (0.301) |
| Marker | t-Value/Z-Value (p-Value) | ||
|---|---|---|---|
| Day 21 | Day 42 | 2 Weeks After Intervention | |
| AST b | −0.326 (0.744) | −1.347 (0.178) | −1.617 (0.106) |
| ALT b | −0.064 (0.949) | −0.383 (0.701) | −0.802 (0.423) |
| GGT b | −0.194 (0.846) | −1.852 (0.064) | −3.000 (0.003) * |
| Total bilirubin b | −0.096 (0.923) | −0.188 (0.850) | −0.174 (0.862) |
| Conjugated bilirubin b | −0.191 (0.849) | −0.624 (0.532) | −1.330 (0.183) |
| Unconjugated bilirubin b | −0.180 (0.857) | −0.081 (0.936) | −0.829 (0.407) |
| LDH b | −0.394 (0.694) | −2.194 (0.028) * | −1.582 (0.114) |
| Total proteins a | 0.591 (0.559) | −0.817 (0.422) | −1.286 (0.212) |
| Albumin b | −3.322 (<0.001) * | −3.396 (<0.001) * | −3.816 (<0.001) * |
| Marker | t-Value/Z-Value (p-Value) | ||
|---|---|---|---|
| Day 21 | Day 42 | 2 Weeks After Intervention | |
| Urea a | 0.178 (0.860) | 1.145 (0.264) | 3.296 (0.003) * |
| Creatinine b | −2.202 (0.028) * | −2.714 (0.007) * | −3.119 (0.002) * |
| Microalbumin b | −1.007 (0.314) | −1.867 (0.062) | −1.218 (0.223) |
| Uric acid a | 1.084 (0.288) | 1.797 (0.085) | 1.798 (0.086) |
| Marker | t-Value/Z-Value (p-Value) | ||
|---|---|---|---|
| Day 21 | Day 42 | 2 Weeks After Intervention | |
| Iron b | −0.961 (0.336) | −1.915 (0.055) | −0.152 (0.879) |
| TIBC b | −0.637 (0.524) | −1.534 (0.125) | −0.624 (0.533) |
| Transferrin b | −0.036 (0.971) | −1.709 (0.087) | −0.152 (0.879) |
| Ferritin b | −0.086 (0.931) | −2.788 (0.005) * | −1.829 (0.067) |
| Marker | t-Value/Z-Value (p-Value) | ||
|---|---|---|---|
| Day 21 | Day 42 | 2 Weeks After Intervention | |
| IgA b | −2.079 (0.038) * | −1.952 (0.051) | −1.382 (0.167) |
| IgG a | 3.317 (0.003) * | 2.247 (0.034) * | 4.164 (<0.001) * |
| IgM b | −0.025 (0.980) | −2.207 (0.027) * | −0.635 (0.526) |
| Total IgE b | −4.253 (<0.001) * | −4.114 (<0.001) * | −3.741 (<0.001) * |
| Rheumatoid factor b | −2.666 (0.008) * | −0.351 (0.726) | −1.403 (0.161) |
| ASO b | −0.344 (0.731) | −1.670 (0.095) | −1.872 (0.061) |
| Compound | Quantification (µg/g Dried Fruit) |
|---|---|
| Feruloyl di-hexose | 39.0 ± 2.18 |
| Feruloyl hexose | 93.5 ± 2.90 |
| Protocatechuic acid derivative | 57.3 ± 1.63 |
| Coumaroyl hexose derivative | 4.1 ± 0.20 |
| 3-O-caffeoylquinic acid | 274.5 ± 8.54 |
| Caffeoylquinic acid-glycoside 2 | 68.7 ± 1.18 |
| 3-Caffeoylquinic acid cis | 19.8 ± 0.43 |
| Procyanidin dimer B type 1 | 132.3 ± 4.05 |
| 3-Coumaroylquinic acid trans | 8.3 ± 0.38 |
| 3-Coumaroylquinic acid cis | 32.0 ± 0.91 |
| Caffeoyl hexose | 3.1 ± 0.06 |
| 5-Caffeoylquinic acid trans | 40.5 ± 1.88 |
| Taxifolin rutinoside | 7.7 ± 0.10 |
| Quercetin 7-O-glucoside-3-O-rutinoside | 3.7 ± 0.46 |
| Quercetin 3-O-rutinoside | 3.6 ± 0.32 |
| Quercetin 3-O-hexoside | 9.3 ± 0.13 |
| 3,5-Dicaffeoylquinic acid | 0.4 ± 0.18 |
| Quercetin derivative | 5.6 ± 0.72 |
| Total | 803.4 |
| Anthocyanins | |
| Cyanidin 3-Orutinoside | 3.6 ± 0.071 |
| Pelargonidin 3-O-rutinoside | 0.9 ± 0.13 |
| Peonidin 3-Orutinoside | 0.1 ± 0.01 |
| Total | 4.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, F.; Varges, A.; Lahlou, R.A.; Bárbara, E.; Santos, I.; Fonseca, C.; Silva, L.R. Effects of Cherry Consumption on Metabolic Health: A Pilot Clinical Study on Healthy Adults. Int. J. Mol. Sci. 2025, 26, 8891. https://doi.org/10.3390/ijms26188891
Carvalho F, Varges A, Lahlou RA, Bárbara E, Santos I, Fonseca C, Silva LR. Effects of Cherry Consumption on Metabolic Health: A Pilot Clinical Study on Healthy Adults. International Journal of Molecular Sciences. 2025; 26(18):8891. https://doi.org/10.3390/ijms26188891
Chicago/Turabian StyleCarvalho, Filomena, Alexandra Varges, Radhia Aitfella Lahlou, Eduardo Bárbara, Isa Santos, Cecília Fonseca, and Luís R. Silva. 2025. "Effects of Cherry Consumption on Metabolic Health: A Pilot Clinical Study on Healthy Adults" International Journal of Molecular Sciences 26, no. 18: 8891. https://doi.org/10.3390/ijms26188891
APA StyleCarvalho, F., Varges, A., Lahlou, R. A., Bárbara, E., Santos, I., Fonseca, C., & Silva, L. R. (2025). Effects of Cherry Consumption on Metabolic Health: A Pilot Clinical Study on Healthy Adults. International Journal of Molecular Sciences, 26(18), 8891. https://doi.org/10.3390/ijms26188891








