Switch from Ibalizumab to Lenacapavir in a Rescue Regimen for a Heavily Treatment-Experienced (HTE) Patient with Multidrug-Resistant (MDR) HIV-1 Infection
Abstract
1. Introduction
- 95% of all people living with HIV should know their HIV status.
- 95% of all people diagnosed with HIV should receive sustained antiretroviral therapy (ART).
- 95% of all people receiving ART should achieve viral suppression by 2025 [2].
- A low CD4 nadir at ART initiation.
- Co-infection with hepatitis C virus (HCV).
- A long treatment history.
- Ibalizumab (IBA)—a monoclonal antibody that inhibits the CD4-gp120 interaction [10].
- Fostemsavir—an entry inhibitor [11].
- Lenacapavir (LEN)—a capsid inhibitor [12].
- A control period (days 0–6), during which patients continued their failing ART regimen;
- A functional monotherapy period (days 7–13), during which participants received a 2000 mg intravenous loading dose of IBA;
- A maintenance period (day 14–week 25), in which patients started an optimized background regimen (OBR) and continued taking IBA at 800 mg intravenously every 14 days, beginning on day 21.
- A total of 62% of participants had HIV-1 RNA < 50 copies/mL.
- Excluding missing data, 82% achieved viral suppression.
- The mean CD4+ cell count increased by 122 cells/μL.
- The proportion of participants with CD4+ < 200 cells/μL decreased from 64% to 29%.
- A viral suppression rate of 90% in group 1.
- A viral suppression rate of 85% in groups 2 and 3,
- A viral suppression rate of 92% in group 4.
Case Report
2. Study Design
- HIV viral load evolution.
- CD4+ T-cell count.
- HIV-DNA levels.
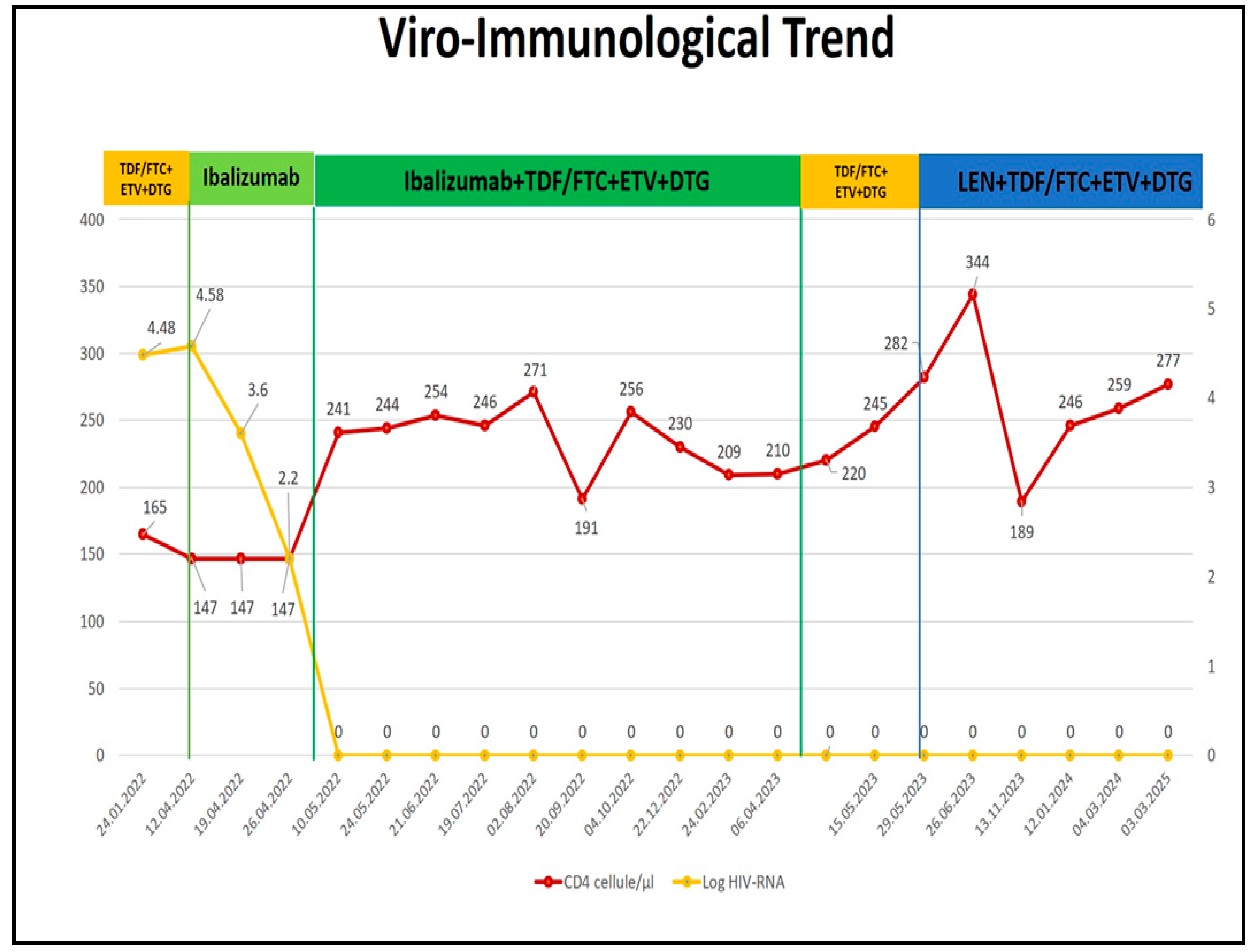
3. Result
- NRTIs: 0.75 (indicating potential low-level resistance).
- NNRTIs: 0 (indicating high-level resistance).
- PIs: 0.03 (indicating high-level resistance).
- INSTIs (RNA): no resistance mutations detected.
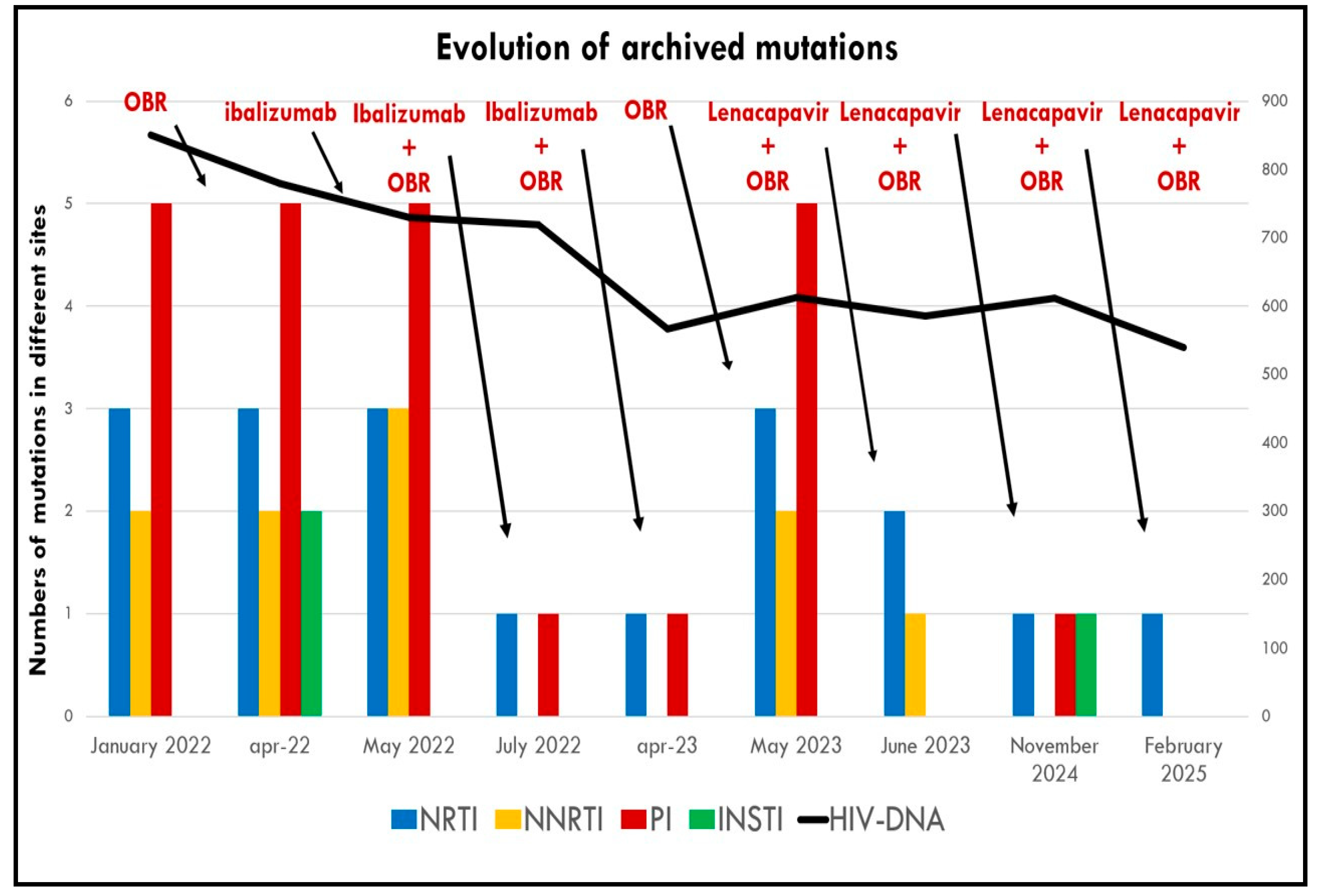
- After 1 month, INSTI resistance mutations were no longer detectable in proviral DNA.
- After 3 months, NNRTI mutations had also disappeared, along with a reduction in previously detected PI and NRTI mutations.
- The patient received 600 mg on Day 1 and Day 2.
- The patient received 300 mg on Day 8.
- After 1 month, archived PI mutations disappeared, with a reduction in NRTI and NNRTI mutations (Table 3).
- At 6 months, NNRTI mutations were no longer detectable, and only a single mutation remained in each of the PI, NRTI, and INSTI classes.
- After 12 months, the impact on the reservoir was comparable to that seen with IBA, with no detectable HIV-DNA mutations (see Figure 3).
4. Methods
- Clinical examination.
- Biochemical parameters.
- CD4+ T-cell count.
- HIV-RNA (plasma viral load).
- HIV-DNA quantification and amplification to detect resistance mutations in the viral reservoir.
4.1. Serological and Molecular Analysis
- Viral RNA was isolated from 200 μL of serum using the QIAamp RNA viral kit (Qiagen GmbH, Hilden, Germany).
- Genotyping was performed using a validated in-house methodology developed by the ANRS AC11 Resistance Study Group, including PCR and Sanger sequencing of the pol gene, covering:
- ○
- Protease (PR).
- ○
- Reverse transcriptase (RT).
- ○
- Integrase (IN).
- The IAS-USA mutation list.
- The Stanford HIV Drug Resistance Database (https://hivdb.stanford.edu, accessed on 21 August 2025).
- The Stanford CPR algorithm for primary resistance analysis (https://hivdb.stanford.edu/cpr/, accessed on 21 August 2025).
4.2. HIV-DNA Quantification and Resistance Testing
- Duplex PCR targeting both HIV-1 DNA and human telomerase reverse transcriptase (hTERT) for relative quantification and internal control.
- Robust performance in the presence of PCR inhibitors.
- Room temperature setup.
- Standard curve included with five levels of HIV-1 copy numbers and cell content.
4.3. Flow Cytometry
4.4. Quality of Life Assessment
5. Discussion
5.1. Switch from IBA to LEN to Optimize Durability and Adherence
5.2. HIV-DNA Reservoir Monitoring and Resistance Evolution
5.3. HIV-DNA Resistance Analysis and CD4+ Recovery: Markers of Deep Treatment Efficacy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambrosioni, J.; Levi, L.; Alagaratnam, J.; Van Bremen, K.; Mastrangelo, A.; Waalewijn, H.; Molina, J.; Guaraldi, G.; Winston, A.; Boesecke, C.; et al. Major revision version 12.0 of the European AIDS Clinical Society guidelines 2023. HIV Med. 2023, 24, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. Global AIDS Strategy 2021−2026: End Inequalities. End AIDS; Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2021; Available online: https://coilink.org/20.500.12592/pnvx21z (accessed on 21 August 2025).
- UNAIDS. Epidemiological Estimates. AIDSinfo. Available online: https://aidsinfo.unaids.org/ (accessed on 12 June 2025).
- ECDC. Progress towards reaching the Sustainable Development Goals related to HIV in the European Union and European Economic Area. In Monitoring the Implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in EUROPE and Central Asia—2023 Progress Report; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- Pelchen-Matthews, A.; Borges, Á.H.; Reekie, J.; Rasmussen, L.D.; Wiese, L.; Weber, J.; Pradier, C.; Degen, O.; Paredes, R.; Tau, L.; et al. Prevalence and Outcomes for Heavily Treatment-Experienced Individuals Living with Human Immunodeficiency Virus in a European Cohort. Am. J. Ther. 2021, 87, 806–817. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.K.; Fusco, J.S.; Henegar, C.E.; Vannappagari, V.; Clark, A.; Brunet, L.; Lackey, P.C.; Pierone, G.; Fusco, G.P. Heavily treatment-experienced people living with HIV in the OPERA® cohort: Population characteristics and clinical outcomes. BMC Infect. Dis. 2023, 23, 91. [Google Scholar] [CrossRef] [PubMed]
- Caputo, S.L.; Poliseno, M.; Tavelli, A.; Gagliardini, R.; Rusconi, S.; Lapadula, G.; Antinori, A.; Francisci, D.; Sarmati, L.; Gori, A.; et al. Heavily treatment-experienced persons living with HIV currently in care in Italy: Characteristics, risk factors, and therapeutic options—The ICONA Foundation cohort study. Int. J. Infect. Dis. 2024, 143, 106956. [Google Scholar] [CrossRef] [PubMed]
- Bajema, K.L.; Nance, R.M.; Delaney, J.A.; Eaton, E.; Davy-Mendez, T.; Karris, M.Y.; Moore, R.D.; Eron, J.J.; Rodriguez, B.; Mayer, K.H.; et al. Substantial decline in heavily treated therapy-experienced persons with HIV with limited antiretroviral treatment options. AIDS 2020, 34, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Cluck, D.B.; Chastain, D.B.; Murray, M.; Durham, S.H.; Chahine, E.B.; Derrick, C.; Dumond, J.B.; Hester, E.K.; Jeter, S.B.; Johnson, M.D.; et al. Consensus recommendations for the use of novel antiretrovirals in persons with HIV who are heavily treatment-experienced and/or have multidrug-resistant HIV-1: Endorsed by the American Academy of HIV Medicine, American College of Clinical Pharmacy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2024, 44, 360–382. [Google Scholar] [CrossRef] [PubMed]
- Beccari, M.V.; Mogle, B.T.; Sidman, E.F.; Mastro, K.A.; Asiago-Reddy, E.; Kufel, W.D. Ibalizumab, a Novel Monoclonal Antibody for the Management of Multidrug-Resistant HIV-1 Infection. Antimicrob. Agents Chemother. 2019, 63, e00110-19. [Google Scholar] [CrossRef] [PubMed]
- Seval, N.; Frank, C.; Kozal, M. Fostemsavir for the treatment of HIV. Expert. Rev. Anti-Infect. Ther. 2021, 19, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Dvory-Sobol, H.; Shaik, N.; Callebaut, C.; Rhee, M.S. Lenacapavir: A first-in-class HIV-1 capsid inhibitor. Curr. Opin. HIV AIDS 2022, 17, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Acosta, E.P.; Liang, L.; He, Y.; Yang, J.; Kerstner-Wood, C.; Zheng, Q.; Huang, J.; Wang, K. Current Status of the Pharmacokinetics and Pharmacodynamics of HIV-1 Entry Inhibitors and HIV Therapy. Curr. Drug Metab. 2017, 18, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Henrich, T.J.; Kuritzkes, D.R. HIV-1 entry inhibitors: Recent development and clinical use. Curr. Opin. Virol. 2013, 3, 51–57. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Trogarzo™ (Ibalizumab-Uiyk) Injection, for Intravenous Use: US Prescribing Information; US Food and Drug Administration: Silver Spring, MD, USA, 2018; p. 4230166. [Google Scholar]
- Norris, D.; Morales, J.; Gathe, J.; Godofsky, E.; Garcia, F.; Hardwicke, R.; Lewis, S. Phase 2 efficacy and safety of the novel entry inhibitor, TNX-355, in combination with optimized background regimen. In Proceedings of the 16th International AIDS Conference, Toronto, ON, Canada, 13–18 August 2006; p. TUPE0058. [Google Scholar]
- Khanlou, H.; Gathe, J.J.; Schrader, S.; Towner, W.; Weinheimer, S.; Lewis, S. Safety, efficacy, and pharmacokinetics of ibalizumab in treatment-experienced HIV-1 infected patients: A phase 2b study. In Proceedings of the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 17–20 September 2011; p. H2-794b. [Google Scholar]
- Emu, B.; Fessel, J.; Schrader, S.; Kumar, P.; Richmond, G.; Win, S.; Weinheimer, S.; Marsolais, C.; Lewis, S. Phase 3 Study of Ibalizumab for Multidrug-Resistant HIV-1. N. Engl. J. Med. 2018, 379, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Prokesch, R.C.; Schroeder, C.P.; Hardin, T.C.; Van Anglen, L.J. 2494. Real-world use of ibalizumab in physician office infusion centers (POICs). Open Forum Infect. Dis. 2019, 6, S865. [Google Scholar] [CrossRef]
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 2020, 584, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Bester, S.M.; Wei, G.; Zhao, H.; Adu-Ampratwum, D.; Iqbal, N.; Courouble, V.V.; Francis, A.C.; Annamalai, A.S.; Singh, P.K.; Shkriabai, N.; et al. Structural and mechanistic bases for a potent HIV-1 capsid inhibitor. Science 2020, 370, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Ogbuagu, O.; Molina, J.-M.; Chetchotisakd, P.; Ramgopal, M.N.; Sanchez, W.; Brunetta, J.; Castelli, F.; E Crofoot, G.; Hung, C.-C.; Ronot-Bregigeon, S.; et al. Efficacy and Safety of Long-Acting Subcutaneous Lenacapavir in Heavily Treatment-Experienced People with Multidrug-Resistant HIV-1: Week 104 Results of a Phase 2/3 Trial. Clin. Infect. Dis. 2024, 80, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Berhe, M.; Crofoot, G.; Benson, P.; Ramgopal, M.; Sims, J.; McDonald, C.; Ruane, P.; E Sanchez, W.; Scribner, A.; et al. Lenacapavir administered every 26 weeks or daily in combination with oral daily antiretroviral therapy for initial treatment of HIV: A randomised, open-label, active-controlled, phase 2 trial. Lancet HIV 2022, 10, e15–e23. [Google Scholar] [CrossRef] [PubMed]
- Nouchi, A.; Nguyen, T.; A Valantin, M.; Simon, A.; Sayon, S.; Agher, R.; Calvez, V.; Katlama, C.; Marcelin, A.G.; Soulie, C. Dynamics of drug resistance-associated mutations in HIV-1 DNA reverse transcriptase sequence during effective ART. J. Antimicrob. Chemother. 2018, 73, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Borjesson, R.P.; Zazzi, M.; Saladini, F.; Santoro, M.M.; Armenia, D.; Spagnuolo, V.; Castagna, A. PRESTIGIO RING: “A 28-year-old highly treatment- experienced man with vertical HIV infection on ibalizumab therapy: ART simplification perspectives”. New Microbiol. 2024, 47, 298–302. [Google Scholar]

| Patient Peculiarities | |
|---|---|
| Sex | Male |
| Risk factor | Reported occasional unprotected sexual intercourse with same sex partner |
| Year of diagnosis | 1998 |
| Age | 70 |
| CDC | A3 |
| HIV subtype | B |
| Nadir CD4+ | 225 |
| Number of therapeutic lines | 15 |
| Comorbidities | Dislipidemia, Hypertension |
| Resistance Mutations on HIV-RNA | 98 | 99 | 00 | 03 | 04 | 05 | 07 | 08 | 09 | 10 | 11 | 12 | 13 | 14 | 18 | 21 | 22 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REGIMEN | AZT 3TC SQV/r | AZT 3TC NFV | DDI IDV/r | TDF D4T NVP | TDF 3TC NVP | ABC 3TC FSP/r | ABC 3TC SQV/r | TDF 3TC LPV/r | RAL FTC DRV/r | ABC 3TC RAL FSP/r | TDF FTC RAL TPV/r | MVC DRV/r | RAL DRV/r | TDF ETV SQV/r ENF | TDF FTC DOR DRV/c | TDF ETV SQV/r | TDF FTC ETV DTG |
| NRTI | L210F | M41L, M184V, L210F, T215Y | M41L, M184V, T215Y | M41L, M184V, T215Y | |||||||||||||
| NNRTI | No Mutations | Y188L | Y188L | Y188L | |||||||||||||
| PI | Minor Mutations (L10V, L63P) | V32I, L33FL, M46I, I47V, I50V, F53FL, I54L | M46I, I47V | M46I, I47V | |||||||||||||
| INSTI | Not available | Not available | Not available | Q148H | |||||||||||||
| VIRAL TROPISM | R5 | X4/DM |
| Resistance Mutations | 01/22 on HIV-RNA | 04/22 on HIV-DNA | 05/22 on HIV-DNA | 07/22 on HIV-DNA | 04/23 on HIV-DNA | 05/23 on HIV-DNA | 06/23 on HIV-DNA | 11/24 on HIV-DNA | 02/25 on HIV-DNA |
|---|---|---|---|---|---|---|---|---|---|
| REGIMEN | TDF/FTC +ETV +DGT | IBA | TDF/FTC +ETV +DGT +IBA | TDF/FTC +ETV +DGT +IBA | TDF/FTC +ETV +DGT | TDF/FTC +ETV +DGT +LEN | TDF/FTC +ETV +DGT +LEN | TDF/FTC +ETV +DGT +LEN | TDF/FTC +ETV +DGT +LEN |
| NRTI | M41L, M184V, T215Y | M41L, M184V, T215Y | M41L, M184V, T215Y | M184MV | M184MV | M41L, M184V, T215Y | M184MV, T215TI | K70KR | K70KR |
| NNRTI | Y188L, V106I | Y188L, V106I | Y188L, V106I, G190GE | None | None | Y188L, V106I | Y188YH | None | None |
| PI | M46I, I47V, V32I, I50V, I54L | M46I, I47V, V32I, I50V, I54L | M46I, I47V, V32I, I50V, I54L | L90LM | L90LM | M46I, I47V, V32I, I50V, I54L | None | L90LM | None |
| INSTI | None | G140GS, Q148QH | None | None | None | None | None | G140GS | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, S.; Salmoni, L.; Palladino, R.; Russo, A.; Cuomo, N.; Raddi, A.; Starace, M.; Minichini, C.; Pisaturo, M.; Coppola, N. Switch from Ibalizumab to Lenacapavir in a Rescue Regimen for a Heavily Treatment-Experienced (HTE) Patient with Multidrug-Resistant (MDR) HIV-1 Infection. Int. J. Mol. Sci. 2025, 26, 8881. https://doi.org/10.3390/ijms26188881
Martini S, Salmoni L, Palladino R, Russo A, Cuomo N, Raddi A, Starace M, Minichini C, Pisaturo M, Coppola N. Switch from Ibalizumab to Lenacapavir in a Rescue Regimen for a Heavily Treatment-Experienced (HTE) Patient with Multidrug-Resistant (MDR) HIV-1 Infection. International Journal of Molecular Sciences. 2025; 26(18):8881. https://doi.org/10.3390/ijms26188881
Chicago/Turabian StyleMartini, Salvatore, Lorenzo Salmoni, Roberta Palladino, Antonio Russo, Nunzia Cuomo, Adriana Raddi, Mario Starace, Carmine Minichini, Mariantonietta Pisaturo, and Nicola Coppola. 2025. "Switch from Ibalizumab to Lenacapavir in a Rescue Regimen for a Heavily Treatment-Experienced (HTE) Patient with Multidrug-Resistant (MDR) HIV-1 Infection" International Journal of Molecular Sciences 26, no. 18: 8881. https://doi.org/10.3390/ijms26188881
APA StyleMartini, S., Salmoni, L., Palladino, R., Russo, A., Cuomo, N., Raddi, A., Starace, M., Minichini, C., Pisaturo, M., & Coppola, N. (2025). Switch from Ibalizumab to Lenacapavir in a Rescue Regimen for a Heavily Treatment-Experienced (HTE) Patient with Multidrug-Resistant (MDR) HIV-1 Infection. International Journal of Molecular Sciences, 26(18), 8881. https://doi.org/10.3390/ijms26188881








