Circulating Levels of SMPDL3B Define Metabolic Endophenotypes and Subclinical Kidney Alterations in Myalgic Encephalomyelitis
Abstract
1. Introduction
2. Results
2.1. Clinical and Demographic Characteristics of Participants
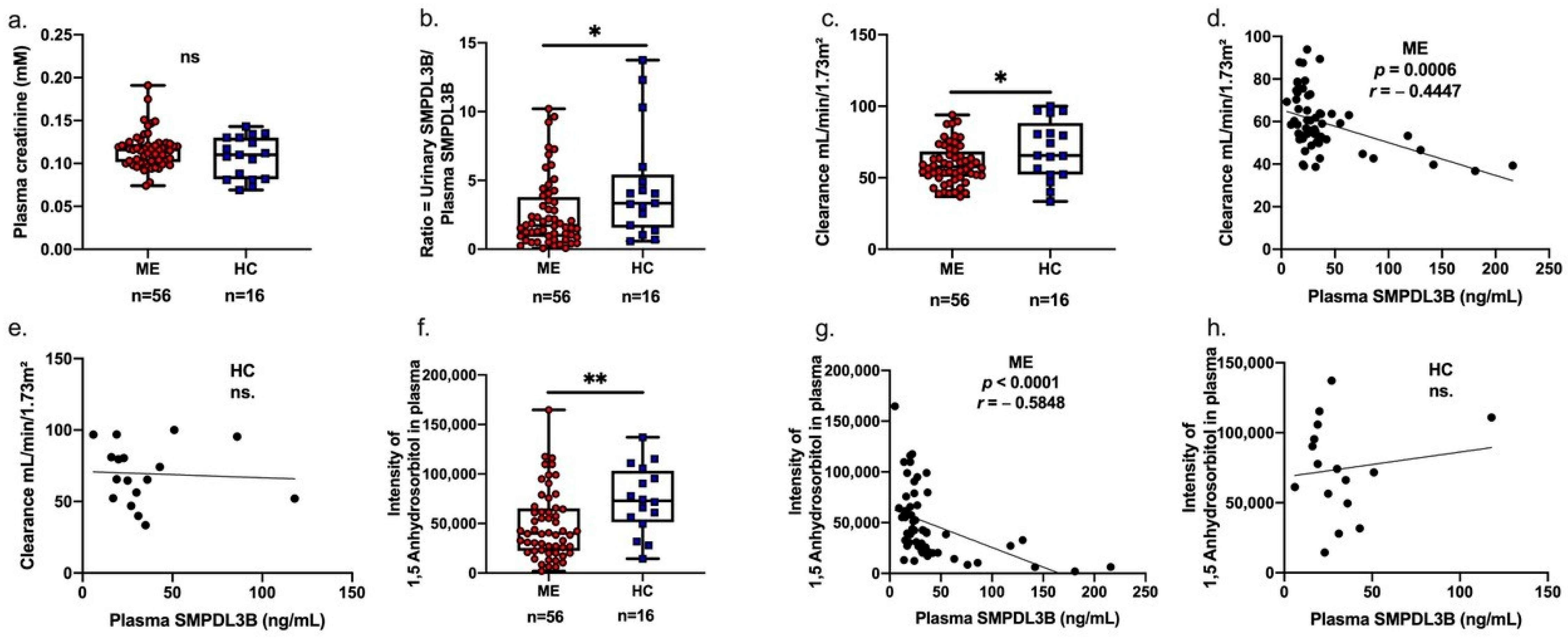
2.2. Associations Between Soluble SMPDL3B, 1,5-Anhydrosorbitol, and Renal Function in Patients with ME
2.3. Urinary-to-Plasma SMPDL3B Ratio as an Independent Predictor of Renal Function
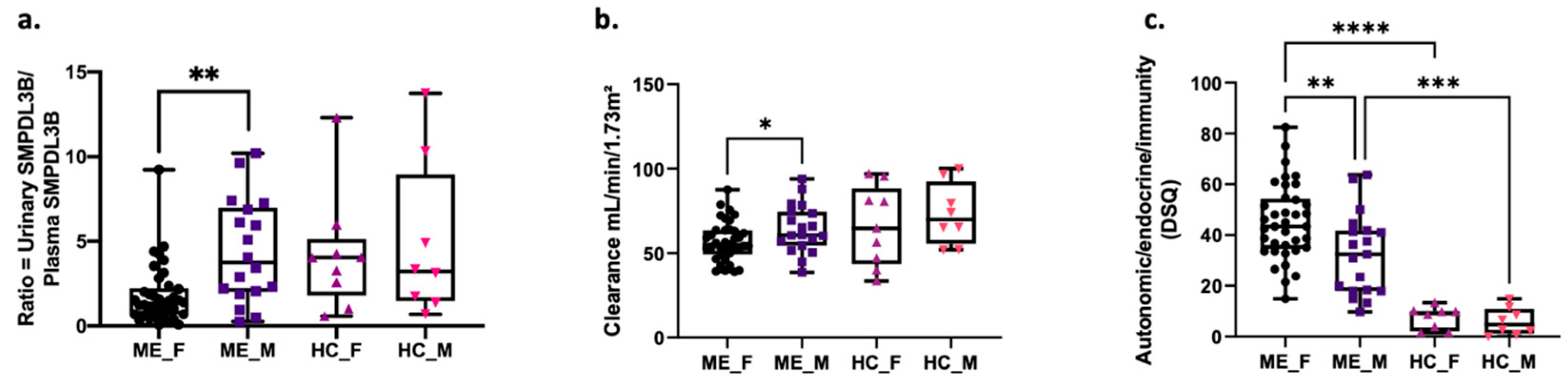
2.4. Sex-Specific Differences in SMPDL3B Levels, Renal Function and ME Symptoms
2.5. Sex-Specific Correlations Between Soluble SMPDL3B and Renal Metabolites
2.6. Plasma Metabolite Alterations Associated with Renal Dysfunction in ME
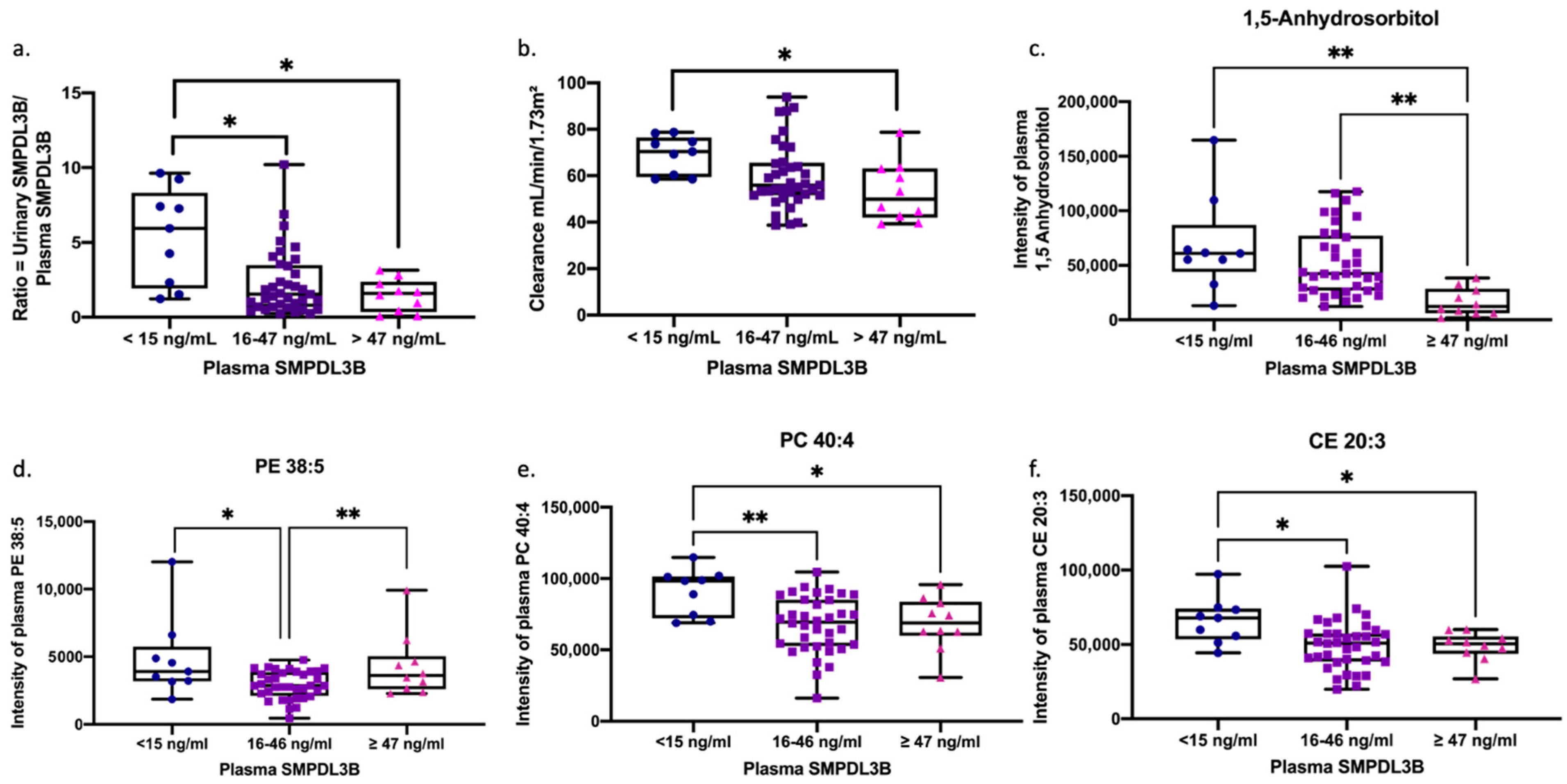
2.7. Renal and Lipidomic Signatures Across SMPDL3B-Defined Endophenotypes in ME
3. Discussion
4. Materials and Methods
4.1. Sex as a Biological Variable
4.2. Study Populations
4.3. Clinical and Demographic Data Collection
4.4. Assessment of Health Status, Symptoms, and Disease Severity
4.5. Sample Collection and Processing
4.6. Measurement of Plasma and Urinary SMPDL3B Levels
4.7. Plasma and Urinary Metabolite Profiling by NMR Spectroscopy
4.8. Plasma Metabolite Profiling by Mass Spectrometry (MS)
4.9. Estimation of Renal Clearance
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| [1H] | proton nuclear magnetic resonance |
| µL | microliter |
| μM | micromolar |
| ANOVA | analysis of variance |
| B | unstandardized regression coefficient |
| BMI | body mass index |
| BSA | body surface area |
| C24:1 | N-nervonoyl-D-erythro-sphingosine |
| CE20:3 | Cholesteryl homo-γ-linolenate |
| Cer(d18:1/24:1) | N-nervonoyl-D-erythro-sphingosine |
| CFS | Chronic Fatigue Syndrome |
| CHU Sainte-Justine | Centre Hospitalier Universitaire Sainte-Justine |
| CKD | chronic kidney disease |
| COVID-19 | coronavirus disease 2019 |
| DSQ | DePaul symptom questionnaire |
| EDTA | ethylenediaminetetraacetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| FC | fold change |
| FDR | false discovery rate |
| FM | Fibromyalgia |
| g | gravitational force |
| HC | healthy controls |
| HMDB ID | Human Metabolome Database Identification |
| HSD | honestly significant difference |
| kg/m | kilogram per meter |
| kg/m2 | kilograms per square meter |
| LC-MS/MS | liquid chromatography-tandem mass spectrometry |
| LPC(P-18:1) | lysophosphatidylcholine lipid with a 18:1 fatty acid chain with a phosphorus group |
| ME | Myalgic Encephalomyelitis |
| MFI-20 | multidimensional fatigue inventory |
| Mg | milligram |
| MHz | megahertz |
| mL/min/1.73 m2 | milliliters per minute per 1.73 square meters |
| mM | millimolar |
| MS | mass spectrometry |
| MS | multiple sclerosis |
| N/A | not applicable |
| ng/mL | nanogram per milliliter |
| NMR | nuclear magnetic resonance |
| n.s. | non-significant |
| NSAID | non-steroidal anti-inflammatory drugs |
| PBQC | pooled biological quality controls |
| PC 40:4 | phosphatidylcholine 40 carbons and double bonds in the two fatty acid chains attached to the glycerol backbone |
| PC(P-34:2) | phosphatidylcholine (plasmalogen-34 carbons in the two fatty acid chains attached to the glycerol backbone: 2 double bonds) |
| PE 38:5 | phosphatidylethanolamine with a total of 38 carbons and 5 double bonds |
| PEM | post-exertional malaise |
| PI-PLC | phosphatidylinositol-specific phospholipase C |
| p-value | probability value |
| QTOF-MS | quadrupole time-of-flight mass spectrometer |
| r | correlations |
| R2 | coefficient of determination |
| RAW264.4 | monocyte/macrophage cell line |
| SE | standard error of the regression coefficient |
| SEM | standard error of the mean |
| SF-36 | 36-Item Short Form Health Survey |
| SM 40:2 | oxygenated form of the sphingolipid, with 40 carbons and 2 double bonds |
| SM 42:2 | sphingomyelin with a total of 42 carbons and 2 double bonds in its fatty acid chains |
| SMPDL3B | Sphingomyelin Phosphodiesterase Acid-Like 3B |
| TCA | tricarboxylic acid |
| T-TEST | Student’s t-test |
| VIF | variance inflation factor |
| ZIC-pHILIC | Zwitterionic hydrophilic interaction |
References
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A., Jr.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front. Pediatr. 2019, 6, 412. [Google Scholar] [CrossRef]
- Canada, O.M.F. What Is ME/CFS? 2022. Available online: https://www.omfcanada.ngo/what-is-me-cfs/ (accessed on 9 May 2022).
- Hoel, F.; Hoel, A.; Pettersen, I.K.N.; Rekeland, I.G.; Risa, K.; Alme, K.; Sørland, K.; Fosså, A.; Lien, K.; Herder, I.; et al. A map of metabolic phenotypes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. JCI Insight 2021, 6, e149217. [Google Scholar] [CrossRef]
- Picariello, F.; Moss-Morris, R.; Macdougall, I.C.; Chilcot, A.J. The role of psychological factors in fatigue among end-stage kidney disease patients: A critical review. Clin. Kidney J. 2017, 10, 79–88. [Google Scholar] [CrossRef]
- Rostami-Afshari, B.; Elremaly, W.; Franco, A.; Elbakry, M.; Akoume, M.-Y.; Boufaied, I.; Moezzi, A.; Leveau, C.; Rompré, P.; Godbout, C.; et al. SMPDL3B a novel biomarker and therapeutic target in myalgic encephalomyelitis. J. Transl. Med. 2025, 23, 748, Correction in J. Transl. Med. 2025, 23, 911. https://doi.org/10.1186/s12967-025-06900-w. [Google Scholar] [CrossRef]
- Mitrofanova, A.; Mallela, S.K.; Ducasa, G.M.; Yoo, T.H.; Rosenfeld-Gur, E.; Zelnik, I.D.; Molina, J.; Santos, J.V.; Ge, M.; Sloan, A.; et al. SMPDL3b modulates insulin receptor signaling in diabetic kidney disease. Nat. Commun. 2019, 10, 2692. [Google Scholar] [CrossRef]
- Watanabe, S.; Hirono, K.; Aizawa, T.; Tsugawa, K.; Joh, K.; Imaizumi, T.; Tanaka, H. Podocyte sphingomyelin phosphodiesterase acid-like 3b decreases among children with idiopathic nephrotic syndrome. Clin. Exp. Nephrol. 2021, 25, 44–51. [Google Scholar] [CrossRef]
- Heinz, L.X.; Baumann, C.L.; Köberlin, M.S.; Snijder, B.; Gawish, R.; Shui, G.; Sharif, O.; Aspalter, I.M.; Müller, A.C.; Kandasamy, R.K.; et al. The Lipid-Modifying Enzyme SMPDL3B Negatively Regulates Innate Immunity. Cell Rep. 2015, 11, 1919–1928. [Google Scholar] [CrossRef]
- Yoo, T.H.; Pedigo, C.E.; Guzman, J.; Correa-Medina, M.; Wei, C.; Villarreal, R.; Mitrofanova, A.; Leclercq, F.; Faul, C.; Li, J.; et al. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J. Am. Soc. Nephrol. 2015, 26, 133–147. [Google Scholar] [CrossRef]
- Lim, E.J.; Ahn, Y.C.; Jang, E.S.; Lee, S.W.; Lee, S.H.; Son, C.G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef]
- LaForge, J.M.; Urso, K.; Day, J.M.; Bourgeois, C.W.; Ross, M.M.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. Non-steroidal Anti-inflammatory Drugs: Clinical Implications, Renal Impairment Risks, and AKI. Adv. Ther. 2023, 40, 2082–2096. [Google Scholar] [CrossRef]
- Beloborodova, N.; Pautova, A.; Sergeev, A.; Fedotcheva, N. Serum Levels of Mitochondrial and Microbial Metabolites Reflect Mitochondrial Dysfunction in Different Stages of Sepsis. Metabolites 2019, 9, 196. [Google Scholar] [CrossRef]
- Azzam, P.; Francis, M.; Youssef, T.; Mroueh, M.; Daher, A.A.; Eid, A.A.; Fornoni, A.; Marples, B.; Zeidan, Y.H. Crosstalk Between SMPDL3b and NADPH Oxidases Mediates Radiation-Induced Damage of Renal Podocytes. Front. Med. 2021, 8, 732528. [Google Scholar] [CrossRef] [PubMed]
- Copur, S.; Onal, E.M.; Afsar, B.; Ortiz, A.; van Raalte, D.H.; Cherney, D.Z.; Rossing, P.; Kanbay, M. Diabetes mellitus in chronic kidney disease: Biomarkers beyond HbA1c to estimate glycemic control and diabetes-dependent morbidity and mortality. J. Diabetes Complicat. 2020, 34, 107707. [Google Scholar] [CrossRef]
- Hering-Smith, K.S.; Hamm, L.L. Acidosis and citrate: Provocative interactions. Ann. Transl. Med. 2018, 6, 374. [Google Scholar] [CrossRef] [PubMed]
- McGregor, N.R.; Armstrong, C.W.; Lewis, D.P.; Butt, H.L.; Gooley, P.R. Widespread pain and altered renal function in ME/CFS patients. Fatigue Biomed. Health Behav. 2016, 4, 132–145. [Google Scholar] [CrossRef]
- König, R.S.; Albrich, W.C.; Kahlert, C.R.; Bahr, L.S.; Löber, U.; Vernazza, P.; Scheibenbogen, C.; Forslund, S.K. The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front. Immunol. 2022, 12, 628741, Erratum in Front Immunol. 2022, 12, 628741. https://doi.org/10.3389/fimmu.2022.878196. [Google Scholar] [CrossRef]
- Booth, N.E.; Myhill, S.; McLaren-Howard, J. Mitochondrial dysfunction and the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Int. J. Clin. Exp. Med. 2012, 5, 208–220. [Google Scholar]
- Lancaster, M.S.; Graham, B.H. Succinyl-CoA Synthetase Dysfunction as a Mechanism of Mitochondrial Encephalomyopathy: More than Just an Oxidative Energy Deficit. Int. J. Mol. Sci. 2023, 24, 10725. [Google Scholar] [CrossRef]
- Brial, F.; Chilloux, J.; Nielsen, T.; Vieira-Silva, S.; Falony, G.; Andrikopoulos, P.; Olanipekun, M.; Hoyles, L.; Djouadi, F.; Neves, A.L.; et al. Human and preclinical studies of the host-gut microbiome co-metabolite hippurate as a marker and mediator of metabolic health. Gut 2021, 70, 2105–2114. [Google Scholar] [CrossRef]
- Varesi, A.; Deumer, U.S.; Ananth, S.; Ricevuti, G. The Emerging Role of Gut Microbiota in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): Current Evidence and Potential Therapeutic Applications. J. Clin. Med. 2021, 10, 5077. [Google Scholar] [CrossRef]
- Robert, T.; Tang, E.; Kervadec, J.; Zaworski, J.; Daudon, M.; Letavernier, E. Kidney Injury and Hair-Straightening Products Containing Glyoxylic Acid. N. Engl. J. Med. 2024, 390, 1147–1149. [Google Scholar] [CrossRef]
- Knol, M.G.E.; Wulfmeyer, V.C.; Müller, R.U.; Rinschen, M.M. Amino acid metabolism in kidney health and disease. Nat. Rev. Nephrol. 2024, 20, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Kavyani, B.; Ahn, S.B.; Missailidis, D.; Annesley, S.J.; Fisher, P.R.; Schloeffel, R.; Guillemin, G.J.; Lovejoy, D.B.; Heng, B. Dysregulation of the Kynurenine Pathway, Cytokine Expression Pattern, and Proteomics Profile Link to Symptomology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Mol. Neurobiol. 2024, 61, 3771–3787. [Google Scholar] [CrossRef]
- Taylor, L.; Curthoys, N.P. Glutamine metabolism: Role in acid-base balance. Biochem. Mol. Biol. Educ. 2004, 32, 291–304. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Hu, C.A. Therapeutic Potential of Amino Acids in Inflammatory Bowel Disease. Nutrients 2017, 9, 920. [Google Scholar] [CrossRef]
- Tasseva, G.; Bai, H.D.; Davidescu, M.; Haromy, A.; Michelakis, E.; Vance, J.E. Phosphatidylethanolamine deficiency in Mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J. Biol. Chem. 2013, 288, 4158–4173. [Google Scholar] [CrossRef]
- St Germain, M.; Iraji, R.; Bakovic, M. Phosphatidylethanolamine homeostasis under conditions of impaired CDP-ethanolamine pathway or phosphatidylserine decarboxylation. Front. Nutr. 2023, 9, 1094273. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Oxidative and Nitrosative Stress and Immune-Inflammatory Pathways in Patients with Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Curr. Neuropharmacol. 2014, 12, 168–185. [Google Scholar] [CrossRef]
- Armstrong, C.W.; McGregor, N.R.; Lewis, D.P.; Butt, H.L.; Gooley, P.R. Metabolic profiling reveals anomalous energy metabolism and oxidative stress pathways in chronic fatigue syndrome patients. Metabolomics 2015, 11, 1626–1639. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Gowda, D.; Masum, M.A.; Gowda, S.G.B.; Shekhar, C.; Rubel, Z.U.; Kira, S.; Ichii, O.; Kon, Y.; Chiba, H.; Hui, S.-P. Lipidomic study of kidney in a mouse model with urine flow obstruction. Sci. Rep. 2024, 14, 18042. [Google Scholar] [CrossRef]
- Ware, J.E., Jr. SF-36 health survey update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Smets, E.M.; Garssen, B.; Bonke, B.; De Haes, J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J. Psychosom. Res. 1995, 39, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Jason, L.A.; Sunnquist, M.; Brown, A.; Furst, J.; Cid, M.; Farietta, J.; Kot, B.; Bloomer, C.; Nicholson, L.; Williams, Y.; et al. Factor Analysis of the DePaul Symptom Questionnaire: Identifying Core Domains. J. Neurol. Neurobiol. 2015, 1, 114. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.W.; McGregor, N.R.; Sheedy, J.R.; Buttfield, I.; Butt, H.L.; Gooley, P.R. NMR metabolic profiling of serum identifies amino acid disturbances in chronic fatigue syndrome. Clin. Chim. Acta 2012, 413, 1525–1531. [Google Scholar] [CrossRef]
- Al-Osali, M.E.; Al-Qassabi, S.S.; Al-Harthi, S.M. Assessment of glomerular filtration rates by cockcroft-gault and modification of diet in renal disease equations in a cohort of omani patients. Sultan Qaboos Univ. Med. J. 2014, 14, e72–e79. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
| Characteristics | ME (n = 56) | HC (n = 16) | p-Value |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 37 (66.1%) | 8 (50.0%) | |
| Male | 19 (33.9%) | 8 (50.0%) | |
| Age, years (mean ± SEM) | 51 ± 1.4 | 49 ± 3.0 | 0.6 |
| BMI, kg/m2 (mean ± SEM) | 25.0 ± 0.7 | 25.0 ± 1.1 | 0.6 |
| Illness duration, years (Mean ± SEM) | 10.87 ± 1.9 | N/A | N/A |
| Variable | Unstandardized Coefficient (B) | Standard Error (SE) | Standardized Coefficient (β) * | t-Value | p-Value | 95% Confidence Interval for B |
|---|---|---|---|---|---|---|
| Model: Clearance | ||||||
| Overall Model Fit: R2 = 0.644 | Adjusted R2 = 0.600 | F-statistic (6, 48) = 14.47 | p < 0.0001 | |||
| Predictors: | ||||||
| Intercept | 110 | 10.05 | N/A | 10.94 | <0.0001 **** | 89.75 to 130.2 |
| Creatinine Concentrations (mM) | −328.6 | 63.62 | N/A | −5.166 | <0.0001 **** | −456.5 to −200.7 |
| Ratio SMPDL3B (urinary/plasma) | 1.087 | 0.513 | N/A | 2.118 | 0.0394 * | 0.055 to 2.118 |
| 1,5-Anhydrosorbitol | 0.0001 | 3.96 × 10−5 | N/A | 2.86 | 0.0063 ** | 3.364 × 10−5 to 0.0001 |
| Age | −0.586 | 0.125 | N/A | −4.693 | <0.0001 **** | −0.837 to −0.335 |
| Illness duration | 0.161 | 0.097 | N/A | 1.665 | 0.1025 | −0.033 to 0.355 |
| Sex | 4.456 | 2.717 | N/A | 1.64 | 0.1075 | −1.006 to 9.919 |
| Variable (Soluble SMPDL3B vs.) | Plasma SMPDL3B All ME Patients (n = 56) | Plasma SMPDL3B Female ME Patients (n = 37) | Plasma SMPDL3B Male ME Patients (n = 19) | Urinary SMPDL3B All ME Patients (n = 56) | Urinary SMPDL3B Female ME Patients (n = 37) | Urinary SMPDL3B Male ME Patients (n = 19) |
|---|---|---|---|---|---|---|
| Citrate | r = −0.03 p = 0.84 | r = −0.24 p = 0.15 | r = 0.50 p = 0.03 * | r = −0.248 p = 0.06 | r = 0.384 p = 0.022 * | r = −0.577 p = 0.006 ** |
| Hippurate | r = 0.29 p = 0.03 * | r = 0.33 p = 0.04 * | r = 0.24 p = 0.32 | r = 0.308 p = 0.021 * | r = 0.3751 p = 0.026 * | r = 0.391 p = 0.07 |
| Threonine | r = 0.27 p = 0.05 * | r = 0.35 p = 0.03 * | r = 0.22 p = 0.37 | r = 0.305 p = 0.022 * | r = 0.211 p = 0.22 | r = 0.395 p = 0.07 |
| Metabolite Name (HMDB ID) | ME (n = 56) | HC (n = 16) | FC | Status (ME Relative to HC) | t-Test | Adjusted p-Value |
|---|---|---|---|---|---|---|
| Succinic acid_HMDB0000254 | 80,673 ± 5369 | 151,759 ± 20377 | 0.53 | Decreased | 7.68 × 10−6 | 0.002 |
| Benzoic acid_HMDB0001870 | 3,098,959 ± 170,374 | 4,658,580 ± 548,375 | 0.67 | Decreased | 0.001 | 0.006 |
| Phenyllactic acid_HMDB0000779 | 9450 ± 630 | 16,711 ± 3124 | 0.57 | Decreased | 0.001 | 0.005 |
| 1,5-Anhydrosorbitol_HMDB0002712 | 48,838 ± 4615 | 74,131 ± 8514 | 0.66 | Decreased | 0.012 | 0.035 |
| L-Tryptophan_HMDB0000929 | 521,597 ± 38,773 | 703,767 ± 76,957 | 0.74 | Decreased | 0.032 | 0.046 |
| L-Glutamine_HMDB0000641 | 653,315 ± 52,695 | 893,783 ± 99,977 | 0.73 | Decreased | 0.035 | 0.048 |
| L-Kynurenine_HMDB0000684 | 2664 ± 212 | 3705 ± 586 | 0.72 | Decreased | 0.042 | 0.047 |
| Citrate | 0.005 ± 0.0001 | 0.004 ± 0.0002 | 1.25 | Increased | 0.0015 | 0.045 |
| ID of Metabolites | Plasma SMPDL3B Levels <15 ng/mL | Plasma SMPDL3B Levels 16–46 ng/mL | Plasma SMPDL3B Levels ≥47 ng/mL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p-Value | FDR | r | p-Value | FDR | r | p-Value | FDR | |
| Cer(d18:1/24:1) | 0.796 | 0.026 | 0.043 | 0.003 | 0.985 | 2.463 | −0.709 | 0.027 | 0.029 |
| LPC(P-18:1) | 0.726 | 0.035 | 0.045 | 0.089 | 0.608 | 1.013 | −0.770 | 0.013 | 0.025 |
| PC(P-34:2) | 0.726 | 0.035 | 0.043 | 0.079 | 0.647 | 0.719 | −0.781 | 0.011 | 0.024 |
| SM 40:2 | 0.752 | 0.026 | 0.045 | −0.029 | 0.867 | 1.495 | −0.721 | 0.023 | 0.026 |
| SM 42:2 | 0.761 | 0.023 | 0.049 | 0.218 | 0.201 | 2.010 | −0.879 | 0.002 | 0.017 |
| SM 44:3 | 0.936 | 0.001 | 0.022 | 0.235 | 0.167 | 1.392 | −0.709 | 0.027 | 0.027 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rostami-Afshari, B.; Elremaly, W.; McGregor, N.R.; Huang, K.J.K.; Armstrong, C.W.; Franco, A.; Godbout, C.; Elbakry, M.; Abdelli, R.; Moreau, A. Circulating Levels of SMPDL3B Define Metabolic Endophenotypes and Subclinical Kidney Alterations in Myalgic Encephalomyelitis. Int. J. Mol. Sci. 2025, 26, 8882. https://doi.org/10.3390/ijms26188882
Rostami-Afshari B, Elremaly W, McGregor NR, Huang KJK, Armstrong CW, Franco A, Godbout C, Elbakry M, Abdelli R, Moreau A. Circulating Levels of SMPDL3B Define Metabolic Endophenotypes and Subclinical Kidney Alterations in Myalgic Encephalomyelitis. International Journal of Molecular Sciences. 2025; 26(18):8882. https://doi.org/10.3390/ijms26188882
Chicago/Turabian StyleRostami-Afshari, Bita, Wesam Elremaly, Neil R. McGregor, Katherine Jin Kai Huang, Christopher W. Armstrong, Anita Franco, Christian Godbout, Mohamed Elbakry, Rim Abdelli, and Alain Moreau. 2025. "Circulating Levels of SMPDL3B Define Metabolic Endophenotypes and Subclinical Kidney Alterations in Myalgic Encephalomyelitis" International Journal of Molecular Sciences 26, no. 18: 8882. https://doi.org/10.3390/ijms26188882
APA StyleRostami-Afshari, B., Elremaly, W., McGregor, N. R., Huang, K. J. K., Armstrong, C. W., Franco, A., Godbout, C., Elbakry, M., Abdelli, R., & Moreau, A. (2025). Circulating Levels of SMPDL3B Define Metabolic Endophenotypes and Subclinical Kidney Alterations in Myalgic Encephalomyelitis. International Journal of Molecular Sciences, 26(18), 8882. https://doi.org/10.3390/ijms26188882






