Understanding Endometriosis: A Broad Review of Its Causes, Management, and Impact
Abstract
1. Introduction
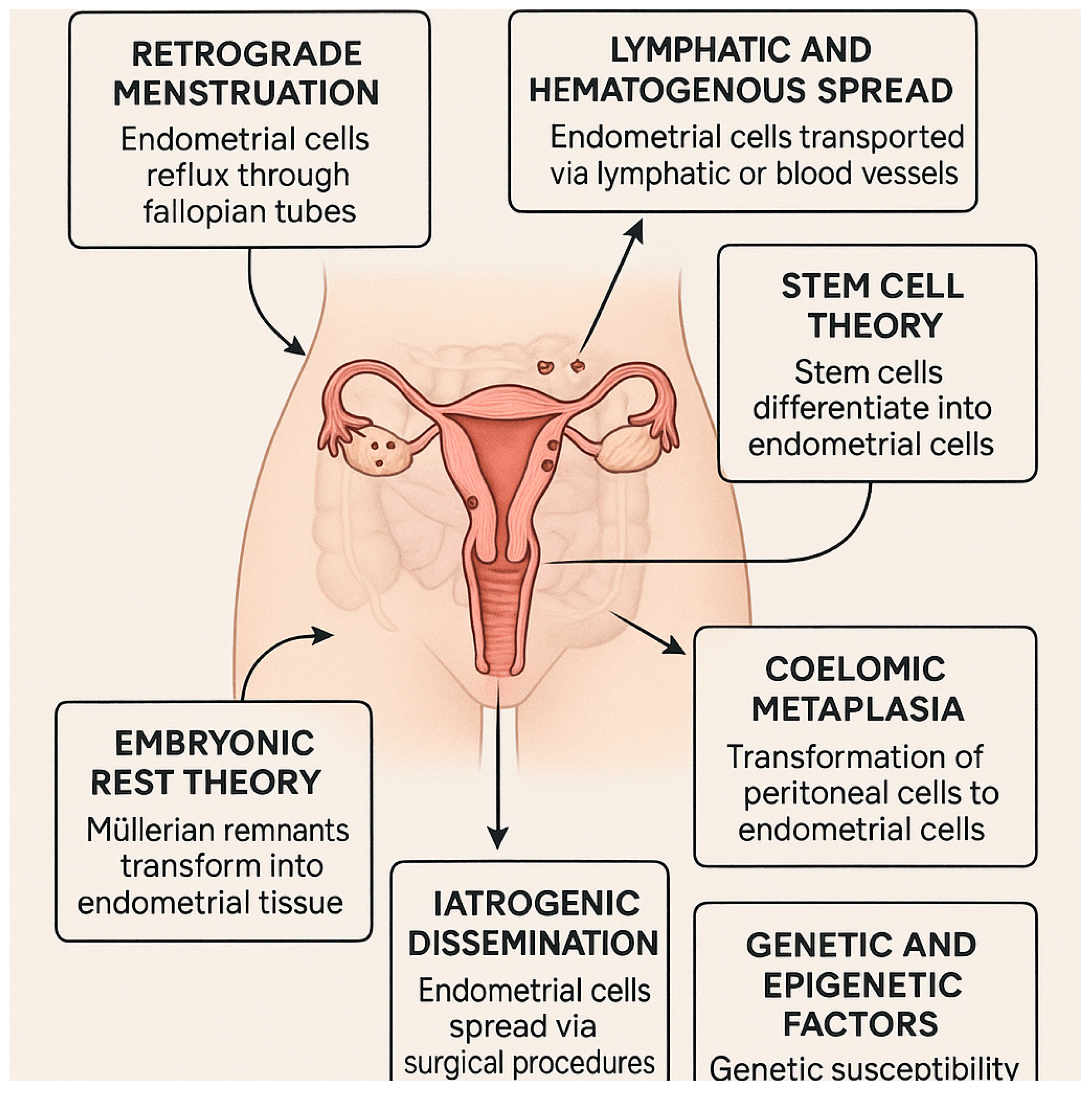
1.1. Theories of Endometriosis Development
- Retrograde menstruation
- Lymphatic and Hematogenous Spread
- Stem cell theory
- Coelomic metaplasia
- Embryonic rest theory
- Iatrogenic dissemination
- Genetic and epigenetic factors
1.2. Newly Proposed Mechanisms of Pathogenesis
- NETs—Neutrophils play a crucial role in the pathogenesis of endometriosis. They mainly kill pathogens through phagocytosis and degradation, but they can also kill pathogenic microorganisms by releasing a network structure called NETs. NETs are different from phagocytosis and degradation and are a new way of immune response. They play a crucial role in the occurrence and development of various diseases, including endometriosis. When the integrity of endometrial epithelial cells is destroyed, it is more conducive to the invasion and infection of pathogenic microorganisms. Neutrophils can reach the uterine cavity through the endometrium and make an early response to the infection caused by the pathogens. The number of neutrophils increases in the late pregnancy of healthy dairy cows, exhibiting a strong phagocytic ability. However, when endometritis and other uterine diseases occur, the numbers and phagocytic ability of neutrophils are reduced. The formation and regulation of NETs depend on the production of ROS mediated by NADPH oxidase [33].
- Chemokines—Chemokines play a crucial role in inflammation and immunity by stimulating the migration of immune cells, especially macrophages and granulocytes, through concentration gradients. The main sources of chemokines are activated monocytes, macrophages, and granulocytes; however, they can also be produced by many other cells of the immune system. About 50 chemokines are currently known, but the number continues to grow. The chemokines are subdivided into four main classes: the CC chemokines, the CXC chemokines, the C chemokines, and the CX3C chemokines. This classification is based on the location of the first two cysteine (C) residues and disulphide bonds in their protein sequence [36].
- Pattern-recognition receptors—PRRs are a type of receptor in the innate immune system that are able to detect pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) in both intracellular and external environments. In endometriosis, PRRs play a key role in the pathological processes associated with the disease by recognizing PAMPs and triggering a receptor ligand reaction, followed by the stimulation of host immune response. This helps to remove pathogenic microorganisms from the body. There are five groups of PRRs, namely toll-like receptors (TLRs), c-type lectin receptors (CLRs), nod-like receptors (NLRs), retinoic acid-inducible gene I-like receptors (RLRs), and absent in melanoma 2 (AIM2)-like receptors (ALRs). These receptors can be divided into two major classes: membrane-bound receptors and unbound intracellular receptors. PRRs, especially TLRs, may serve as potential therapeutic targets for alleviating pain in endometriosis patients [39].
2. Psychological Aspects of Pain Management in Endometriosis
3. Diagnosis and Biomarkers
3.1. The Classifications of Endometriosis
3.2. Protein Markers of Endometriosis
3.3. Other Markers
3.4. Diagnostic Tests
3.5. Examples of Commercially Available Tests
4. Treatment of Endometriosis
4.1. Classical Treatment Methods
4.1.1. Medical (Pharmacological) Management
Hormonal Therapy
GnRH Agonists and Antagonists
Aromatase Inhibitors
NSAIDs and Pain Management
4.2. Surgical Management
Postoperative Management
4.3. Sclerotherapy and Alternative Approaches
4.4. Natural Substances Used in the Treatment
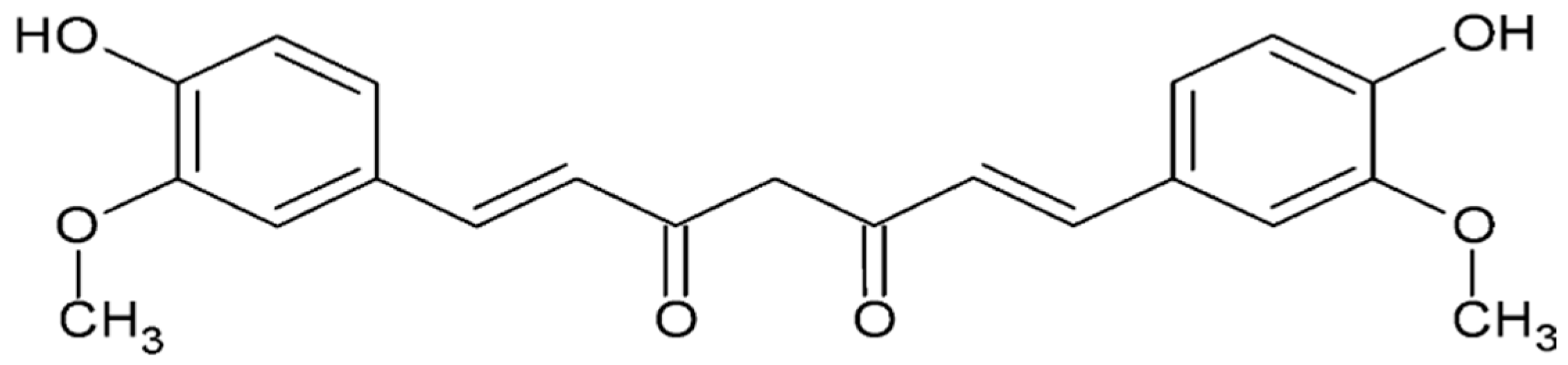
4.4.1. Curcumin
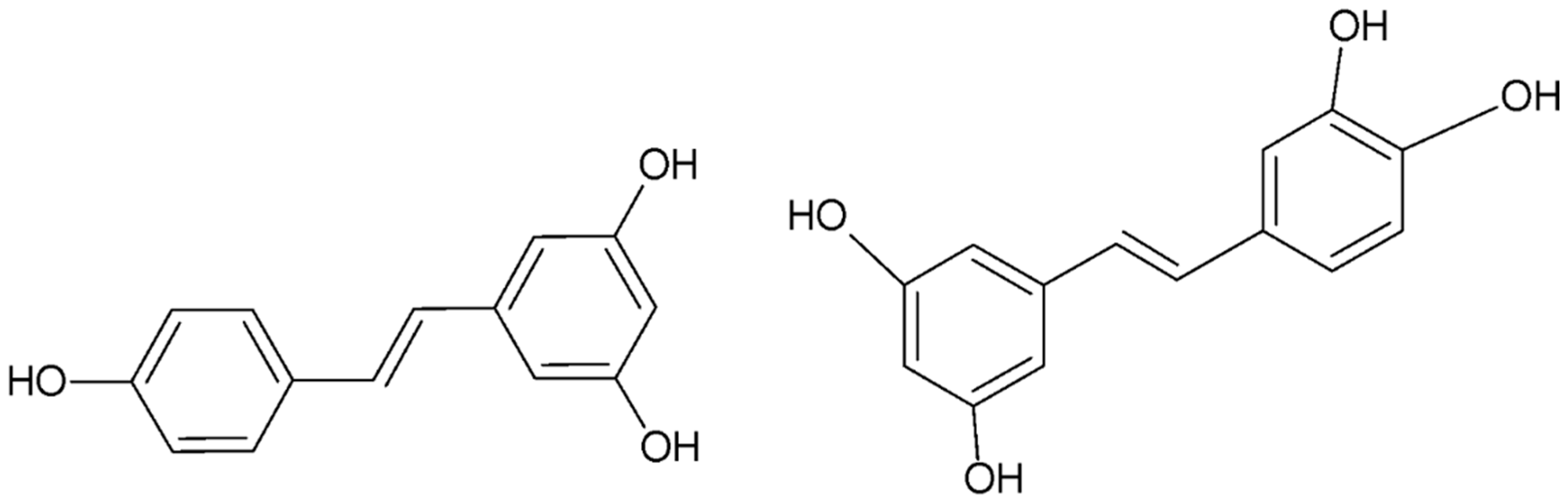
4.4.2. Polyphenols
- Resveratrol
- Epigallocatechin gallate
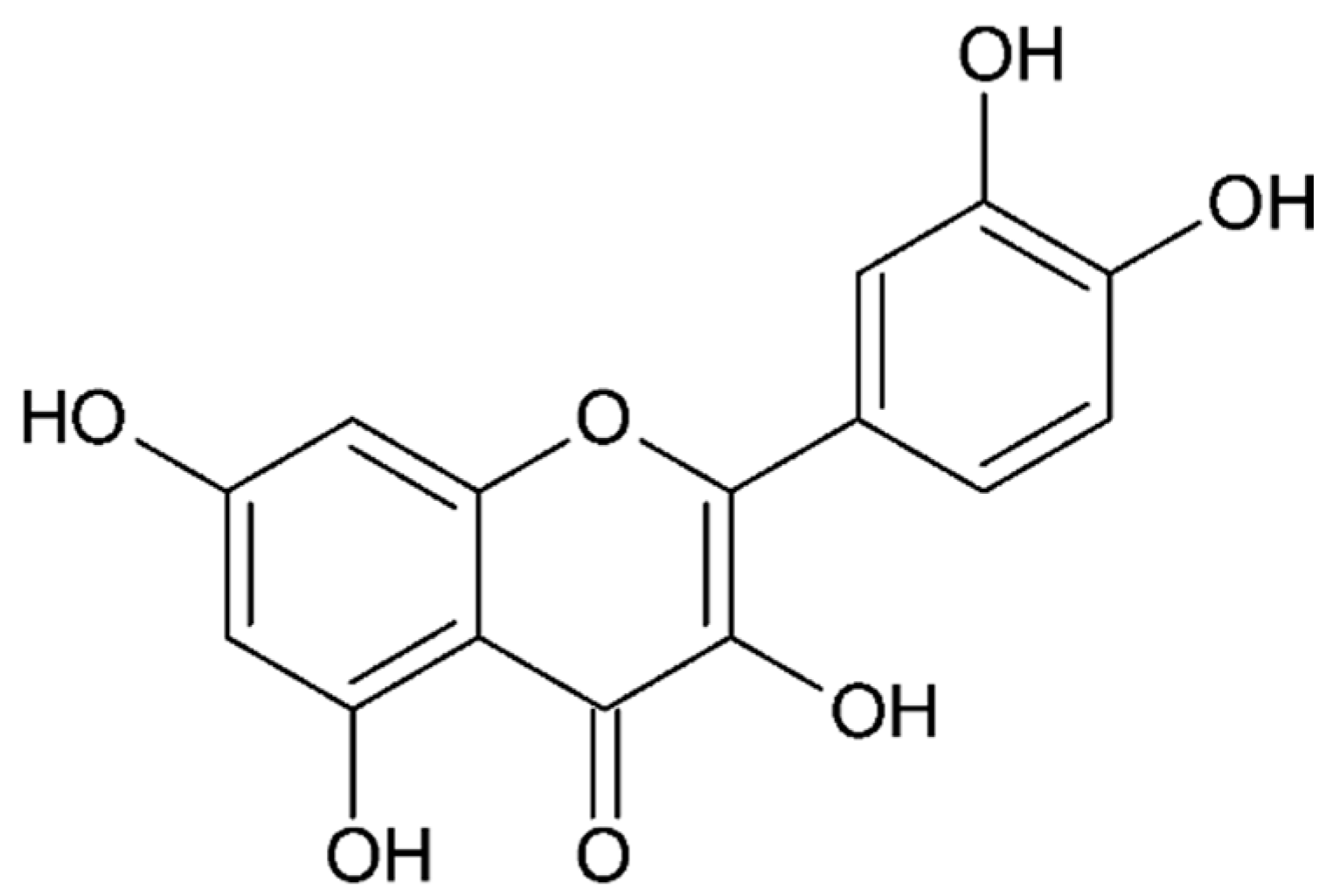
- Quercetin
4.4.3. Flavonoids and Derivatives
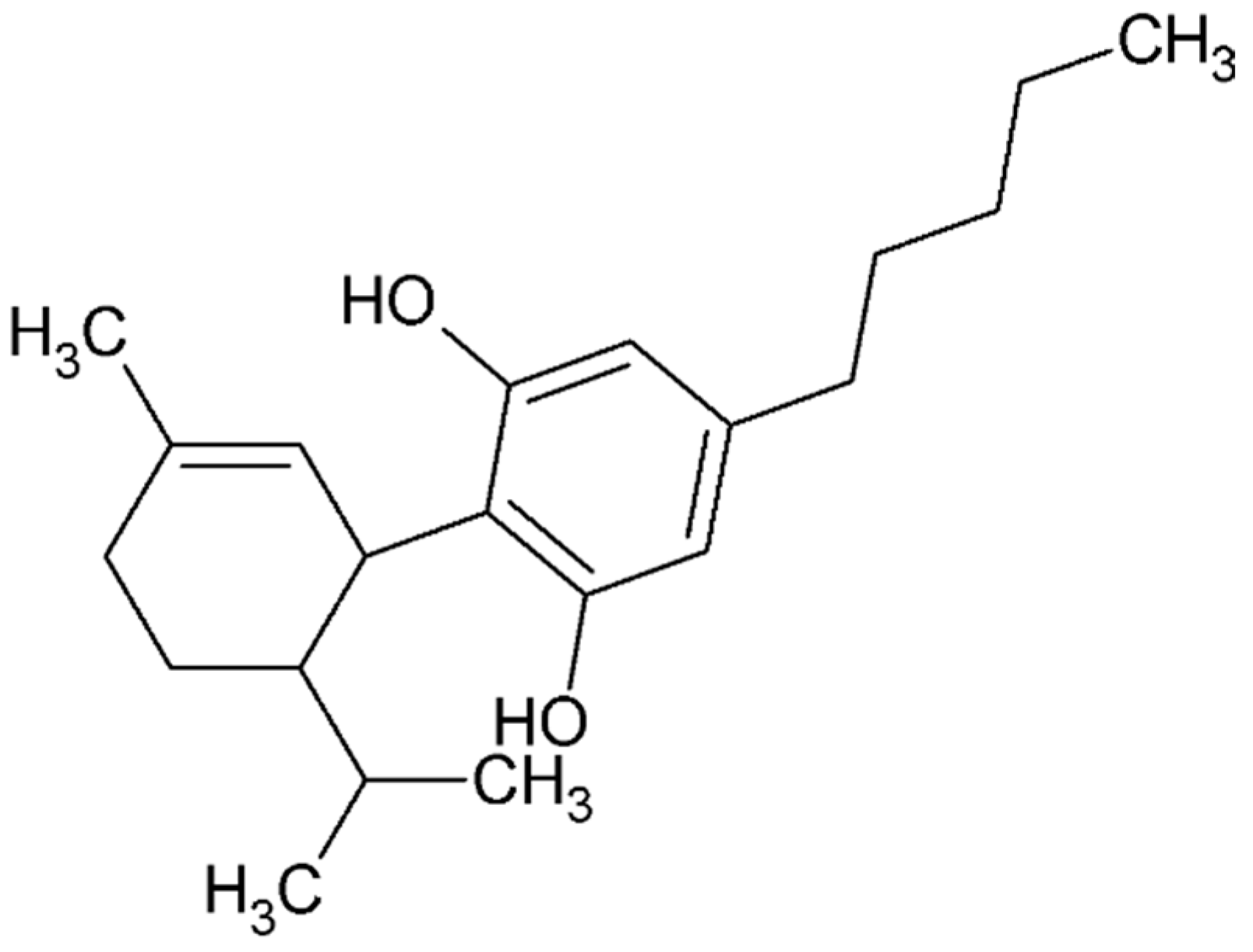
4.4.4. Ginsenoside
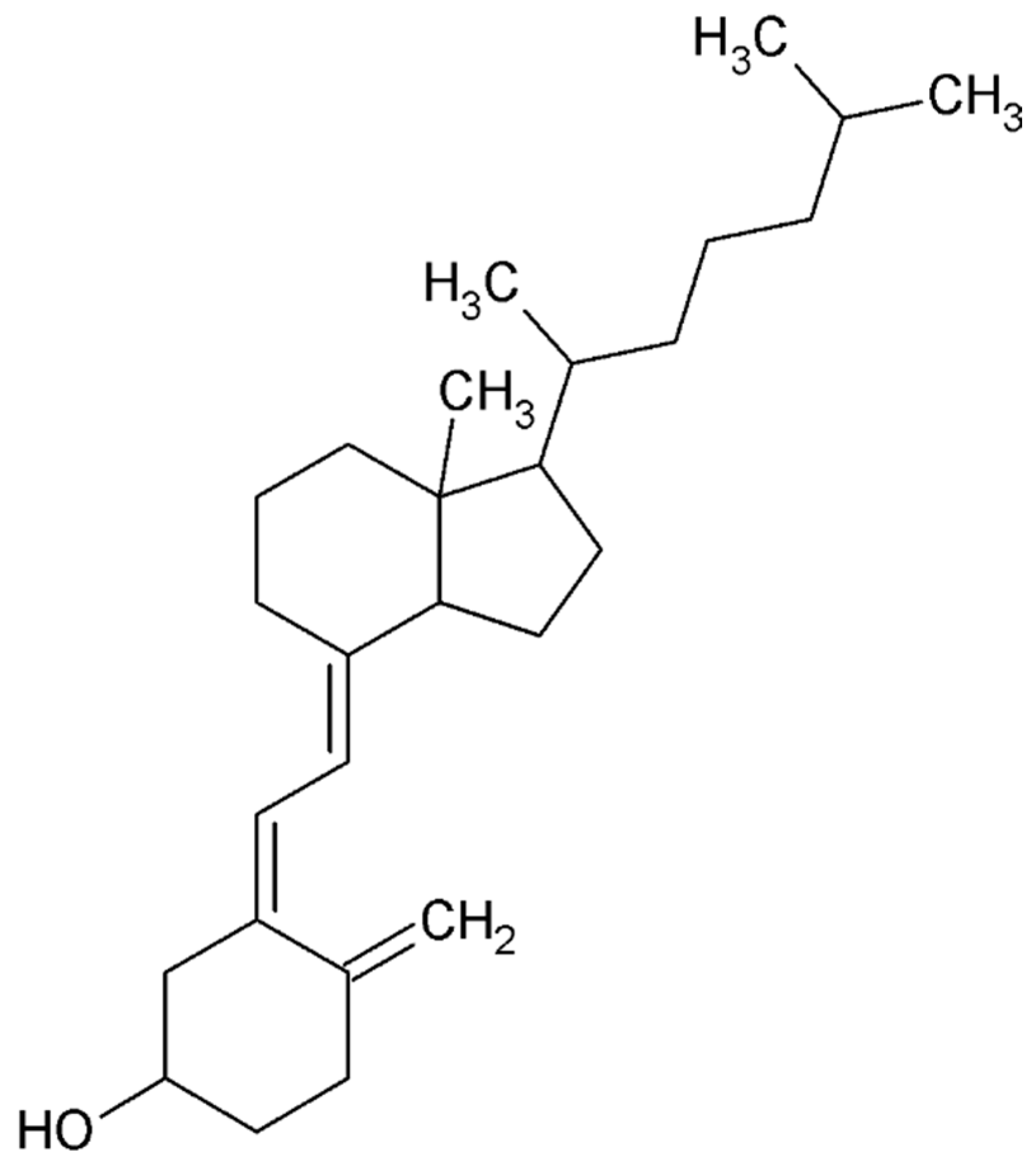
4.4.5. Cannabidiol
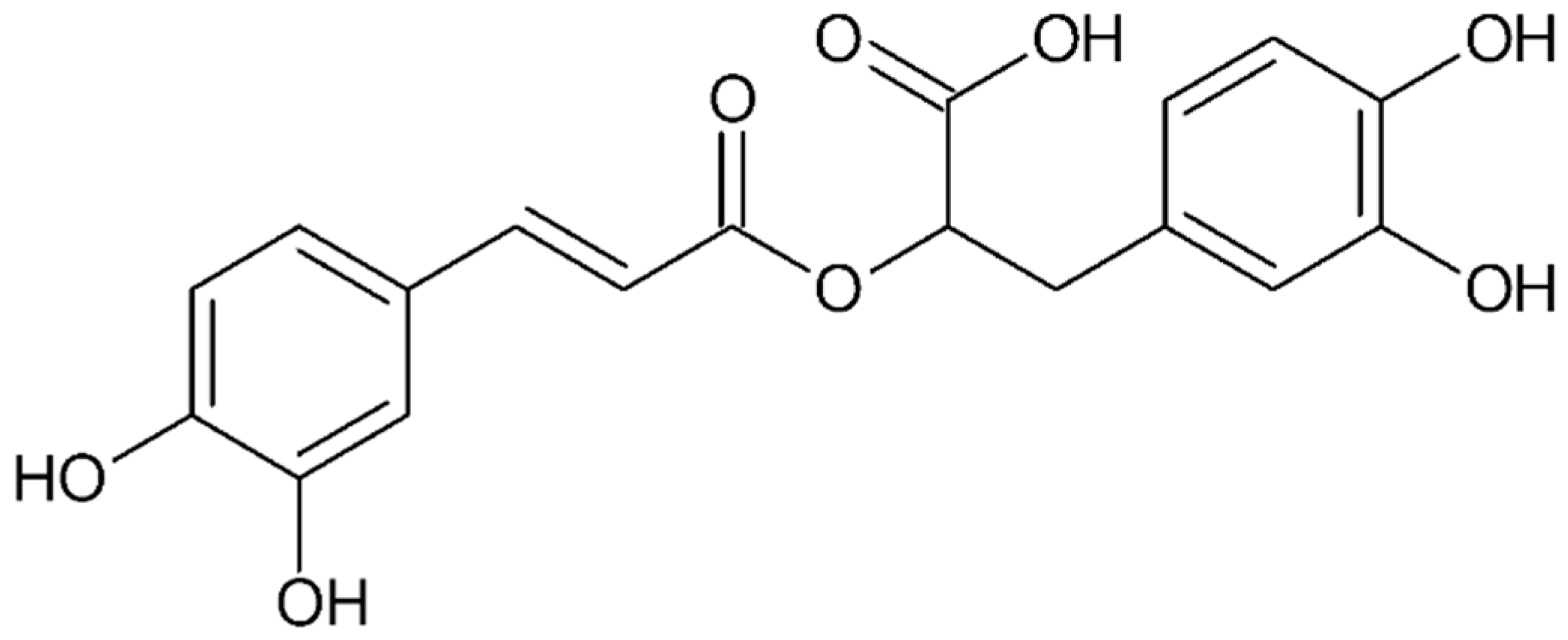
4.4.6. Rosmarinic Acid
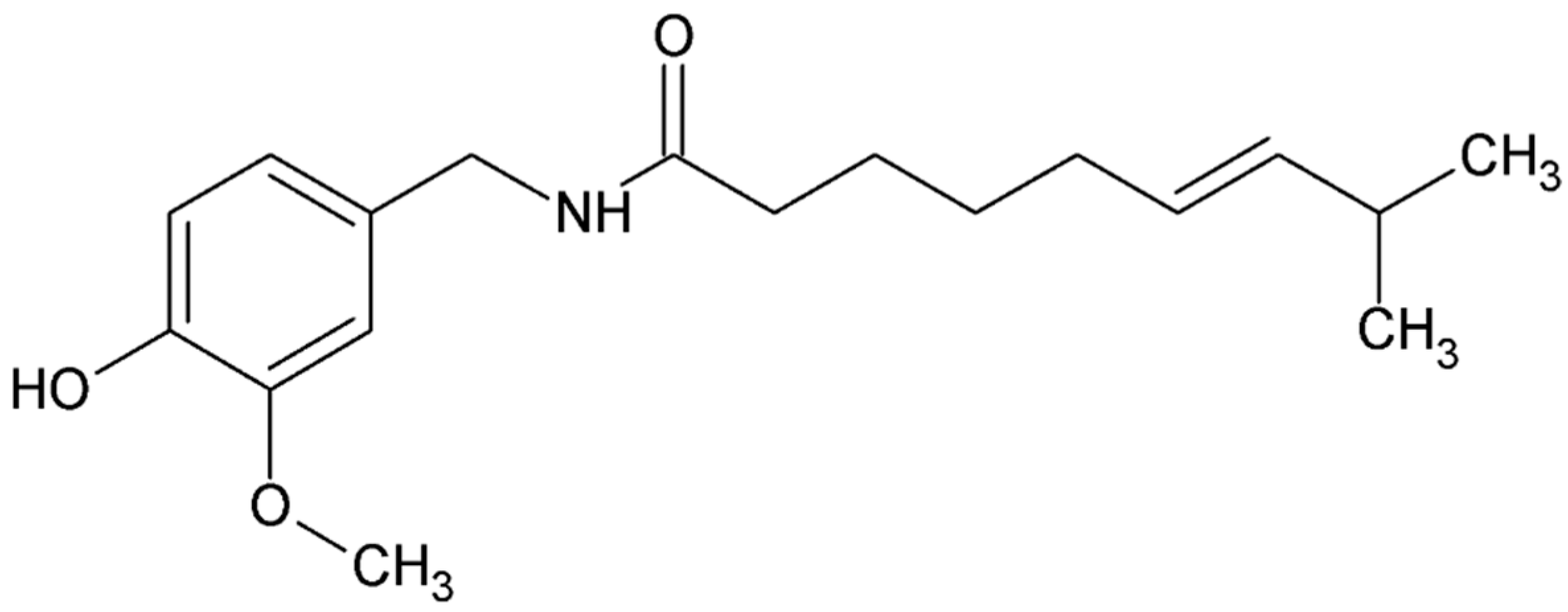
4.4.7. Capsaicin
4.4.8. Vitamins
4.5. Small Compounds Used in the Treatment
4.5.1. Selective Progesterone Receptor Modulators (SPRMs)
4.5.2. Estrogen Receptors
4.5.3. Steroid Sulfatase Inhibitors
4.5.4. Aromatase Inhibitors
4.5.5. GnRH Antagonists
4.5.6. Anti-Angiogenic Agents
4.5.7. Immune Modulators
4.6. Stem Cell Therapy
4.7. Gene Therapy
4.8. Clinical Trials
4.8.1. Relugolix Combination Therapy
4.8.2. Linzagolix
4.8.3. Gefapixant
4.8.4. Eliapixant
4.8.5. Anakinra
4.8.6. Quinagolide
4.8.7. Norethindrone Acetate (NETA) vs. Dienogest
4.8.8. 99mTc-Maraciclatide
5. Impact on Quality of Life
6. Conclusions
7. Future Perspective
8. Search Methodology
Funding
Conflicts of Interest
References
- Vlahos, N.F.; Economopoulos, K.P.; Fotiou, S. Endometriosis, in vitro fertilisation and the risk of gynaecological malignancies, including ovarian and breast cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2010, 24, 39–50. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sumimoto, K.; Kitanaka, T.; Yamada, Y.; Sado, T.; Sakata, M.; Yoshida, S.; Kawaguchi, R.; Kanayama, S.; Shigetomi, H.; et al. Ovarian endometrioma—Risks factors of ovarian cancer development. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 187–193. [Google Scholar] [CrossRef]
- Melin, A.; Sparén, P.; Persson, I.; Bergqvist, A. Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Hum. Reprod. 2006, 21, 1237–1242. [Google Scholar] [CrossRef]
- Vercellini, P.; Scarfone, G.; Bolis, G.; Stellato, G.; Carinelli, S.; Crosignani, P.G. Site of origin of epithelial ovarian cancer: The endometriosis connection. BJOG 2000, 107, 1155–1157. [Google Scholar] [CrossRef]
- Ogawa, S.; Kaku, T.; Amada, S.; Kobayashi, H.; Hirakawa, T.; Ariyoshi, K.; Kamura, T.; Nakano, H. Ovarian endometriosis associated with ovarian carcinoma: A clinicopathological and immunohistochemical study. Gynecol. Oncol. 2000, 77, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Munkarah, A.; Arabi, H.; Bandyopadhyay, S.; Semaan, A.; Hayek, K.; Garg, G.; Morris, R.; Ali-Fehmi, R. Prognostic analysis of ovarian cancer associated with endometriosis. Am. J. Obstet. Gynecol. 2011, 204, 63.e1–63.e7. [Google Scholar] [CrossRef]
- Giudice, L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Jabbour, H.N.; Kelly, R.W.; Fraser, H.M.; Critchley, H.O. Endocrine regulation of menstruation. Endocr. Rev. 2006, 27, 17–46. [Google Scholar] [CrossRef]
- Critchley, H.O.D.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R.W. Physiology of the Endometrium and Regulation of Menstruation. Physiol. Rev. 2020, 100, 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110.43. [Google Scholar]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noë, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef]
- Suda, K.; Nakaoka, H.; Yoshihara, K.; Ishiguro, T.; Tamura, R.; Mori, Y.; Yamawaki, K.; Adachi, S.; Takahashi, T.; Kase, H.; et al. Clonal Expansion and Diversification of Cancer-Associated Mutations in Endometriosis and Normal Endometrium. Cell Rep. 2018, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, T.H.; Leonova, A.; Lac, V.; Senz, J.; Tessier-Cloutier, B.; Nazeran, T.M.; Köbel, M.; Grube, M.; Kraemer, B.; Yong, P.J.; et al. Molecular analysis suggests oligoclonality and metastasis of endometriosis lesions across anatomically defined subtypes. Fertil. Steril. 2022, 118, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Jubanyik, K.J.; Comite, F. Extrapelvic endometriosis. Obstet. Gynecol. Clin. North. Am. 1997, 24, 411–440. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Yoshimura, Y. Stem cell theory for the pathogenesis of endometriosis. Front. Biosci. (Elite Ed.) 2012, 4, 2754–2763. [Google Scholar] [CrossRef]
- Gargett, C.E. Uterine stem cells: What is the evidence? Hum. Reprod. Update 2007, 13, 87–101. [Google Scholar] [CrossRef]
- Maruyama, T.; Masuda, H.; Ono, M.; Kajitani, T.; Yoshimura, Y. Human uterine stem/progenitor cells: Their possible role in uterine physiology and pathology. Reproduction 2010, 140, 11–22. [Google Scholar] [CrossRef]
- Sasson, I.E.; Taylor, H.S. Stem cells and the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 2008, 1127, 106–115. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 2007, 25, 2082–2086. [Google Scholar] [CrossRef]
- Hufnagel, D.; Li, F.; Cosar, E.; Krikun, G.; Taylor, H.S. The Role of Stem Cells in the Etiology and Pathophysiology of Endometriosis. Semin. Reprod. Med. 2015, 33, 333–340. [Google Scholar] [CrossRef]
- Duke, C.M.; Taylor, H.S. Stem cells and the reproductive system: Historical perspective and future directions. Maturitas 2013, 76, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Katabuchi, H.; Tohya, T.; Fukumatsu, Y.; Matsuura, K.; Okamura, H. Scanning electron microscopic and immunohistochemical studies of pelvic endometriosis. Hum. Reprod. 1993, 8, 2218–2226. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Ohtake, H.; Katabuchi, H.; Okamura, H. Coelomic metaplasia theory of endometriosis: Evidence from in vivo studies and an in vitro experimental model. Gynecol. Obstet. Investig. 1999, 47 (Suppl. 1), 18–20; discussion 20–22. [Google Scholar] [CrossRef]
- Lamceva, J.; Uljanovs, R.; Strumfa, I. The Main Theories on the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2023, 24, 4254. [Google Scholar] [CrossRef]
- Signorile, P.G.; Viceconte, R.; Baldi, A. New Insights in Pathogenesis of Endometriosis. Front. Med. 2022, 9, 879015. [Google Scholar] [CrossRef] [PubMed]
- Stefanko, D.P.; Eskander, R.; Aisagbonhi, O. Disseminated Endometriosis and Low-Grade Endometrioid Stromal Sarcoma in a Patient with a History of Uterine Morcellation for Adenomyosis. Case Rep. Obstet. Gynecol. 2020, 2020, 7201930. [Google Scholar] [CrossRef]
- Sepilian, V.; Della Badia, C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet. Gynecol. 2003, 102 Pt 2, 1125–1127. [Google Scholar]
- Cubuk, A.; Ozkaptan, O.; Neymeyer, J. Iatrogenic endometriosis following apical pelvic organ prolapse surgery: A case report. J. Med. Case Rep. 2020, 14, 3. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Mortlock, S.; Ghiasi, M.; Møller, P.L.; Stefansdottir, L.; Galarneau, G.; Turman, C.; Danning, R.; Law, M.H.; Sapkota, Y.; et al. The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat. Genet. 2023, 55, 423–436. [Google Scholar] [CrossRef]
- Bedrick, B.S.; Courtright, L.; Zhang, J.; Snow, M.; Amendola, I.L.S.; Nylander, E.; Cayton-Vaught, K.; Segars, J.; Singh, B. A Systematic Review of Epigenetics of Endometriosis. F S Rev. 2024, 5, 100070. [Google Scholar] [CrossRef]
- Pagliardini, L.; Gentilini, D.; Sanchez, A.M.; Candiani, M.; Viganò, P.; Di Blasio, A.M. Replication and meta-analysis of previous genome-wide association studies confirm vezatin as the locus with the strongest evidence for association with endometriosis. Hum. Reprod. 2015, 30, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, L.; Wang, J.; Zhao, W. The emerging role of neutrophil extracellular traps in endometritis. Front. Immunol. 2023, 14, 1153851. [Google Scholar] [CrossRef]
- Wilson, T.R.; Kasper, S.; Burns, K.A. An emerging role for neutrophils in the pathogenesis of endometriosis. npj Women’s Health 2025, 3, 9. [Google Scholar] [CrossRef]
- Xu, Z.; Ji, R.; Wang, M.; Li, T.; Hu, H. Neutrophil Extracellular Traps and Endometriosis: Insights from a Case-Control Study. Int. J. Women’s Health 2025, 17, 2121–2131. [Google Scholar] [CrossRef]
- Smycz-Kubańska, M.; Kondera-Anasz, Z.; Sikora, J.; Wendlocha, D.; Królewska-Daszczyńska, P.; Englisz, A.; Janusz, A.; Janusz, J.; Mielczarek-Palacz, A. The Role of Selected Chemokines in the Peritoneal Fluid of Women with Endometriosis—Participation in the Pathogenesis of the Disease. Processes 2021, 9, 2229. [Google Scholar] [CrossRef]
- Nishida, M.; Nasu, K.; Narahara, H. Role of chemokines in the pathogenesis of endometriosis. Front. Biosci. (Schol Ed.) 2011, 3, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Wang, M.J.; Wang, Y.X.; Shen, B. CXC chemokines influence immune surveillance in immunological disorders: Polycystic ovary syndrome and endometriosis. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166704. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chen, J.H.; Zhang, J.H.; Fang, Y.; Liu, X.J.; Zhang, J.; Zhu, H.Q.; Zhan, L. Pattern-recognition receptors in endometriosis: A narrative review. Front. Immunol. 2023, 14, 1161606. [Google Scholar] [CrossRef]
- Edwards, M.J. Functional neurological disorder: Lighting the way to a new paradigm for medicine. Brain 2021, 144, 3279–3282. [Google Scholar] [CrossRef]
- Laganà, A.S.; Condemi, I.; Retto, G.; Muscatello, M.R.; Bruno, A.; Zoccali, R.A.; Triolo, O.; Cedro, C. Analysis of psychopathological comorbidity behind the common symptoms and signs of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K. Patients presenting with somatic complaints: Epidemiology, psychiatric comorbidity and management. Int. J. Methods Psychiatr. Res. 2003, 12, 34–43. [Google Scholar] [CrossRef]
- Delanerolle, G.; Ramakrishnan, R.; Hapangama, D.; Zeng, Y.; Shetty, A.; Elneil, S.; Chong, S.; Hirsch, M.; Oyewole, M.; Phiri, P.; et al. A systematic review and meta-analysis of the Endometriosis and Mental-Health Sequelae; The ELEMI Project. Women’s Health 2021, 17, 17455065211019717. [Google Scholar] [CrossRef]
- Laganà, A.S.; La Rosa, V.L.; Rapisarda, A.M.C.; Valenti, G.; Sapia, F.; Chiofalo, B.; Rossetti, D.; Ban Frangež, H.; Vrtačnik Bokal, E.; Vitale, S.G. Anxiety and depression in patients with endometriosis: Impact and management challenges. Int. J. Women’s Health 2017, 9, 323–330. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Foster, W.G.; Groessl, E.J. Rethinking endometriosis care: Applying the chronic care model via a multidisciplinary program for the care of women with endometriosis. Int. J. Women’s Health 2019, 11, 405–410. [Google Scholar] [CrossRef]
- Mundo-López, A.; Ocón-Hernández, O.; Lozano-Lozano, M.; San-Sebastián, A.; Fernández-Lao, C.; Galiano-Castillo, N.; Cantarero-Villanueva, I.; Arroyo-Morales, M.; Artacho-Cordón, F. Impact of symptom burden on work performance status in Spanish women diagnosed with endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 261, 92–97. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- McPeak, A.E.; Allaire, C.; Williams, C.; Albert, A.; Lisonkova, S.; Yong, P.J. Pain Catastrophizing and Pain Health-Related Quality-of-Life in Endometriosis. Clin. J. Pain 2018, 34, 349–356. [Google Scholar] [CrossRef]
- Maulenkul, T.; Kuandyk, A.; Makhadiyeva, D.; Dautova, A.; Terzic, M.; Oshibayeva, A.; Moldaliyev, I.; Ayazbekov, A.; Maimakov, T.; Saruarov, Y.; et al. Understanding the impact of endometriosis on women’s life: An integrative review of systematic reviews. BMC Women’s Health 2024, 24, 524. [Google Scholar] [CrossRef]
- Della Corte, L.; Di Filippo, C.; Gabrielli, O.; Reppuccia, S.; La Rosa, V.L.; Ragusa, R.; Fichera, M.; Commodari, E.; Bifulco, G.; Giampaolino, P. The Burden of Endometriosis on Women’s Lifespan: A Narrative Overview on Quality of Life and Psychosocial Wellbeing. Int. J. Environ. Res. Public Health 2020, 17, 4683. [Google Scholar] [CrossRef] [PubMed]
- Pontoppidan, K.; Olovsson, M.; Grundström, H. Clinical factors associated with quality of life among women with endometriosis: A cross-sectional study. BMC Women’s Health 2023, 23, 551. [Google Scholar] [CrossRef]
- Gete, D.G.; Doust, J.; Mortlock, S.; Montgomery, G.; Mishra, G.D. Impact of endometriosis on women’s health-related quality of life: A national prospective cohort study. Maturitas 2023, 174, 1–7. [Google Scholar] [CrossRef]
- Warzecha, D.; Szymusik, I.; Wielgos, M.; Pietrzak, B. The Impact of Endometriosis on the Quality of Life and the Incidence of Depression-A Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 3641. [Google Scholar] [CrossRef]
- Leuenberger, J.; Kohl Schwartz, A.S.; Geraedts, K.; Haeberlin, F.; Eberhard, M.; von Orellie, S.; Imesch, P.; Leeners, B. Living with endometriosis: Comorbid pain disorders, characteristics of pain and relevance for daily life. Eur. J. Pain 2022, 26, 1021–1038. [Google Scholar] [CrossRef]
- Linton, S.J.; Shaw, W.S. Impact of psychological factors in the experience of pain. Phys. Ther. 2011, 91, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Gerdle, B.; Åkerblom, S.; Brodda Jansen, G.; Enthoven, P.; Ernberg, M.; Dong, H.J.; Stålnacke, B.M.; Äng, B.O.; Boersma, K. Who benefits from multimodal rehabilitation—An exploration of pain, psychological distress, and life impacts in over 35,000 chronic pain patients identified in the Swedish Quality Registry for Pain Rehabilitation. J. Pain Res. 2019, 12, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Fernandez, S.; Olive, L.; Payne, L.A.; Mikocka-Walus, A. Psychological and mind-body interventions for endometriosis: A systematic review. J. Psychosom. Res. 2019, 124, 109756. [Google Scholar] [CrossRef] [PubMed]
- Buggio, L.; Barbara, G.; Facchin, F.; Frattaruolo, M.P.; Aimi, G.; Berlanda, N. Self-management and psychological-sexological interventions in patients with endometriosis: Strategies, outcomes, and integration into clinical care. Int. J. Women’s Health 2017, 9, 281–293. [Google Scholar] [CrossRef]
- van Aken, M.A.W.; Oosterman, J.M.; van Rijn, C.M.; Ferdek, M.A.; Ruigt, G.S.F.; Peeters, B.W.M.M.; Braat, D.D.M.; Nap, A.W. Pain cognition versus pain intensity in patients with endometriosis: Toward personalized treatment. Fertil. Steril. 2017, 108, 679–686. [Google Scholar] [CrossRef]
- Dancet, E.A.; Apers, S.; Kremer, J.A.; Nelen, W.L.; Sermeus, W.; D’Hooghe, T.M. The patient-centeredness of endometriosis care and targets for improvement: A systematic review. Gynecol. Obstet. Investig. 2014, 78, 69–80. [Google Scholar] [CrossRef]
- Grundström, H.; Kilander, H.; Wikman, P.; Olovsson, M. Demographic and clinical characteristics determining patient-centeredness in endometriosis care. Arch. Gynecol. Obstet. 2023, 307, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Boersen, Z.; de Kok, L.; van der Zanden, M.; Braat, D.; Oosterman, J.; Nap, A. Patients’ perspective on cognitive behavioural therapy after surgical treatment of endometriosis: A qualitative study. Reprod. Biomed. Online 2021, 42, 819–825. [Google Scholar] [CrossRef]
- Aerts, L.; Grangier, L.; Streuli, I.; Dällenbach, P.; Marci, R.; Wenger, J.M.; Pluchino, N. Psychosocial impact of endometriosis: From co-morbidity to intervention. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E.; ESHRE Special Interest Group for Endometriosis and Endometrium Guideline Development Group. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Coyne, K.S.; Zaiser, E.; Castelli-Haley, J.; Fuldeore, M.J. The burden of endometriosis symptoms on health-related quality of life in women in the United States: A cross-sectional study. J. Psychosom. Obstet. Gynaecol. 2017, 38, 238–248. [Google Scholar] [CrossRef]
- Vannuccini, S.; Clemenza, S.; Rossi, M.; Petraglia, F. Hormonal treatments for endometriosis: The endocrine background. Rev. Endocr. Metab. Disord. 2022, 23, 333–355. [Google Scholar] [CrossRef]
- Exacoustos, C.; Malzoni, M.; Di Giovanni, A.; Lazzeri, L.; Tosti, C.; Petraglia, F.; Zupi, E. Ultrasound mapping system for the surgical management of deep infiltrating endometriosis. Fertil. Steril. 2014, 102, 143–150.e2. [Google Scholar] [CrossRef]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Hudelist, G.; Valentin, L.; Saridogan, E.; Condous, G.; Malzoni, M.; Roman, H.; Jurkovic, D.; Keckstein, J. What to choose and why to use—A critical review on the clinical relevance of rASRM, EFI and Enzian classifications of endometriosis. Facts Views Vis. Obgyn 2021, 13, 331–338. [Google Scholar] [CrossRef]
- Lee, S.Y.; Koo, Y.J.; Lee, D.H. Classification of endometriosis. Yeungnam Univ. J. Med. 2021, 38, 10–18. [Google Scholar] [CrossRef]
- Adamson, G.D.; Pasta, D.J. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil. Steril. 2010, 94, 1609–1615. [Google Scholar] [CrossRef]
- Abrao, M.S.; Andres, M.P.; Miller, C.E.; Gingold, J.A.; Rius, M.; Neto, J.S.; Carmona, F. AAGL 2021 Endometriosis Classification: An Anatomy-based Surgical Complexity Score. J. Minim. Invasive Gynecol. 2021, 28, 1941–1950.e1. [Google Scholar] [CrossRef]
- Barbieri, R.L.; Niloff, J.M.; Bast, R.C.; Scaetzl, E.; Kistner, R.W.; Knapp, R.C. Elevated serum concentrations of CA-125 in patients with advanced endometriosis. Fertil. Steril. 1986, 45, 630–634. [Google Scholar] [CrossRef]
- O’Brien, T.J.; Beard, J.B.; Underwood, L.J.; Shigemasa, K. The CA 125 gene: A newly discovered extension of the glycosylated N-terminal domain doubles the size of this extracellular superstructure. Tumour Biol. 2002, 23, 154–169. [Google Scholar]
- Yin, B.W.; Lloyd, K.O. Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16. J. Biol. Chem. 2001, 276, 27371–27375. [Google Scholar] [CrossRef] [PubMed]
- Anfosso, F.; Bardin, N.; Vivier, E.; Sabatier, F.; Sampol, J.; Dignat-George, F. Outside-in signaling pathway linked to CD146 engagement in human endothelial cells. J. Biol. Chem. 2001, 276, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.P.; Rothbächer, U.; Sers, C. The progression associated antigen MUC18: A unique member of the immunoglobulin supergene family. Melanoma Res. 1993, 3, 337–340. [Google Scholar] [CrossRef]
- Leñero, C.; Kaplan, L.D.; Best, T.M.; Kouroupis, D. CD146+ Endometrial-Derived Mesenchymal Stem/Stromal Cell Subpopulation Possesses Exosomal Secretomes with Strong Immunomodulatory miRNA Attributes. Cells 2022, 11, 4002. [Google Scholar] [CrossRef]
- Hilage, P.; Birajdar, A.; Marsale, T.; Patil, D.; Patil, A.M.; Telang, G.; Somasundaram, I.; Sharma, R.K.; Joshi, M.G. Characterization and angiogenic potential of CD146. Stem Cell Res. Ther. 2024, 15, 330. [Google Scholar] [CrossRef]
- Chiu, P.C.; Koistinen, R.; Koistinen, H.; Seppala, M.; Lee, K.F.; Yeung, W.S. Zona-binding inhibitory factor-1 from human follicular fluid is an isoform of glycodelin. Biol. Reprod. 2003, 69, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Loukovaara, S.; Immonen, I.R.; Loukovaara, M.J.; Koistinen, R.; Kaaja, R.J. Glycodelin: A novel serum anti-inflammatory marker in type 1 diabetic retinopathy during pregnancy. Acta Ophthalmol. Scand. 2007, 85, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Oehninger, S.; Coddington, C.C.; Hodgen, G.D.; Seppala, M. Factors affecting fertilization: Endometrial placental protein 14 reduces the capacity of human spermatozoa to bind to the human zona pellucida. Fertil. Steril. 1995, 63, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Rachmilewitz, J.; Riely, G.J.; Tykocinski, M.L. Placental protein 14 functions as a direct T-cell inhibitor. Cell Immunol. 1999, 191, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.C.; Chung, M.K.; Koistinen, R.; Koistinen, H.; Seppala, M.; Ho, P.C.; Ng, E.H.; Lee, K.F.; Yeung, W.S. Cumulus oophorus-associated glycodelin-C displaces sperm-bound glycodelin-A and -F and stimulates spermatozoa-zona pellucida binding. J. Biol. Chem. 2007, 282, 5378–5388. [Google Scholar] [CrossRef]
- Chiu, P.C.; Chung, M.K.; Tsang, H.Y.; Koistinen, R.; Koistinen, H.; Seppala, M.; Lee, K.F.; Yeung, W.S. Glycodelin-S in human seminal plasma reduces cholesterol efflux and inhibits capacitation of spermatozoa. J. Biol. Chem. 2005, 280, 25580–25589. [Google Scholar] [CrossRef]
- Kocbek, V.; Vouk, K.; Mueller, M.D.; Rižner, T.L.; Bersinger, N.A. Elevated glycodelin-A concentrations in serum and peritoneal fluid of women with ovarian endometriosis. Gynecol. Endocrinol. 2013, 29, 455–459. [Google Scholar] [CrossRef]
- Vodolazkaia, A.; El-Aalamat, Y.; Popovic, D.; Mihalyi, A.; Bossuyt, X.; Kyama, C.M.; Fassbender, A.; Bokor, A.; Schols, D.; Huskens, D.; et al. Evaluation of a panel of 28 biomarkers for the non-invasive diagnosis of endometriosis. Hum. Reprod. 2012, 27, 2698–2711. [Google Scholar] [CrossRef]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef]
- Drapkin, R.; von Horsten, H.H.; Lin, Y.; Mok, S.C.; Crum, C.P.; Welch, W.R.; Hecht, J.L. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005, 65, 2162–2169. [Google Scholar] [CrossRef]
- Huhtinen, K.; Suvitie, P.; Hiissa, J.; Junnila, J.; Huvila, J.; Kujari, H.; Setälä, M.; Härkki, P.; Jalkanen, J.; Fraser, J.; et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br. J. Cancer 2009, 100, 1315–1319. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Falcone, T.; Goldberg, J.M.; Sharma, R.K.; Nelson, D.R.; Agarwal, A. Peritoneal fluid leptin is associated with chronic pelvic pain but not infertility in endometriosis patients. Hum. Reprod. 2006, 21, 788–791. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Guo, F.; Lei, T.; Li, S.; Monaghan-Nichols, P.; Jiang, Z.; Xin, H.B.; Fu, M. TRIM65 E3 ligase targets VCAM-1 degradation to limit LPS-induced lung inflammation. J. Mol. Cell Biol. 2020, 12, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Taooka, Y.; Chen, J.; Yednock, T.; Sheppard, D. The integrin alpha9beta1 mediates adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J. Cell Biol. 1999, 145, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Marchese, M.E.; Berdnikovs, S.; Cook-Mills, J.M. Distinct sites within the vascular cell adhesion molecule-1 (VCAM-1) cytoplasmic domain regulate VCAM-1 activation of calcium fluxes versus Rac1 during leukocyte transendothelial migration. Biochemistry 2012, 51, 8235–8246. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Qin, X.; Xu, H.; Li, L.; Wang, Z.; Li, F.; Xie, X.; Zhou, H.; Shen, Y.; Long, J. Structural insights into Paf1 complex assembly and histone binding. Nucleic Acids Res. 2013, 41, 10619–10629. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Yi, D.; Lai, C.; Wang, H.; Zou, W.; Cao, B. Knockdown of vascular cell adhesion molecule 1 impedes transforming growth factor beta 1-mediated proliferation, migration, and invasion of endometriotic cyst stromal cells. Reprod. Biol. Endocrinol. 2019, 17, 69. [Google Scholar] [CrossRef]
- Chung, M.S.; Han, S.J. Endometriosis-Associated Angiogenesis and Anti-angiogenic Therapy for Endometriosis. Front. Glob. Women’s Health 2022, 3, 856316. [Google Scholar] [CrossRef]
- Arici, A.; Oral, E.; Attar, E.; Tazuke, S.I.; Olive, D.L. Monocyte chemotactic protein-1 concentration in peritoneal fluid of women with endometriosis and its modulation of expression in mesothelial cells. Fertil. Steril. 1997, 67, 1065–1072. [Google Scholar] [CrossRef]
- Jolicoeur, C.; Boutouil, M.; Drouin, R.; Paradis, I.; Lemay, A.; Akoum, A. Increased expression of monocyte chemotactic protein-1 in the endometrium of women with endometriosis. Am. J. Pathol. 1998, 152, 125–133. [Google Scholar] [PubMed]
- Anastasiu, C.V.; Moga, M.A.; Elena Neculau, A.; Bălan, A.; Scârneciu, I.; Dragomir, R.M.; Dull, A.M.; Chicea, L.M. Biomarkers for the Noninvasive Diagnosis of Endometriosis: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 1750. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Kubota, T.; Aso, T. Usefulness of CA19-9 versus CA125 for the diagnosis of endometriosis. Fertil. Steril. 2002, 78, 733–739. [Google Scholar] [CrossRef]
- Shen, A.; Xu, S.; Ma, Y.; Guo, H.; Li, C.; Yang, C.; Zou, S. Diagnostic value of serum CA125, CA19-9 and CA15-3 in endometriosis: A meta-analysis. J. Int. Med. Res. 2015, 43, 599–609. [Google Scholar] [CrossRef]
- Chantalat, E.; Valera, M.C.; Vaysse, C.; Noirrit, E.; Rusidze, M.; Weyl, A.; Vergriete, K.; Buscail, E.; Lluel, P.; Fontaine, C.; et al. Estrogen Receptors and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2815. [Google Scholar] [CrossRef]
- Bi, X.; Liu, S.; Liu, D.; Li, C. Elucidating the role of Brain-Derived Neurotrophic Factor (BDNF) and its receptor Tyrosine Receptor Kinase B (TrkB) in the development and symptoms of endometriosis. Int. J. Neurosci. 2025, 135, 63–69. [Google Scholar] [CrossRef]
- Herranz-Blanco, B.; Daoud, E.; Viganò, P.; García-Velasco, J.A.; Colli, E. Development and Validation of an Endometriosis Diagnostic Method Based on Serum Biomarkers and Clinical Variables. Biomolecules 2023, 13, 1052. [Google Scholar] [CrossRef]
- Żeberkiewicz, M.; Hyc, A.; Iwan, A.; Zwierzchowska, A.; Ścieżyńska, A.; Kalaszczyńska, I.; Barcz, E.; Malejczyk, J. Expression of Fucosyltransferase 4. J. Clin. Med. 2022, 11, 5606. [Google Scholar]
- Panir, K.; Schjenken, J.E.; Robertson, S.A.; Hull, M.L. Non-coding RNAs in endometriosis: A narrative review. Hum. Reprod. Update 2018, 24, 497–515. [Google Scholar] [CrossRef]
- Bendifallah, S.; Dabi, Y.; Suisse, S.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Jornea, L.; Bouteiller, D.; Touboul, C.; et al. Endometriosis Associated-miRNome Analysis of Blood Samples: A Prospective Study. Diagnostics 2022, 12, 1150. [Google Scholar] [CrossRef] [PubMed]
- Noar, M.; Mathias, J.; Kolatkar, A. Gastrointestinal Myoelectrical Activity (GIMA) Biomarker for Noninvasive Diagnosis of Endometriosis. J. Clin. Med. 2024, 13, 2866. [Google Scholar] [CrossRef] [PubMed]
- Bendifallah, S.; Suisse, S.; Puchar, A.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Jornea, L.; Bouteiller, D.; Touboul, C.; et al. Salivary MicroRNA Signature for Diagnosis of Endometriosis. J. Clin. Med. 2022, 11, 612. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Casper, R.F. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil. Steril. 2017, 107, 533–536. [Google Scholar] [CrossRef]
- Vercellini, P.; Bracco, B.; Mosconi, P.; Roberto, A.; Alberico, D.; Dhouha, D.; Somigliana, E. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: A before and after study. Fertil. Steril. 2016, 105, 734–743.e3. [Google Scholar] [CrossRef] [PubMed]
- Gurbuz, T.B.; Aslan, K.; Kasapoglu, I.; Muzii, L.; Uncu, G. Norethindrone acetate versus dienogest for pain relief in endometriosis related pain: A randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 310, 113940. [Google Scholar] [CrossRef] [PubMed]
- Surrey, E.S. GnRH agonists in the treatment of symptomatic endometriosis: A review. F S Rep. 2023, 4 (Suppl. 2), 40–45. [Google Scholar] [CrossRef]
- Shore, N.D.; Saad, F.; Cookson, M.S.; George, D.J.; Saltzstein, D.R.; Tutrone, R.; Akaza, H.; Bossi, A.; van Veenhuyzen, D.F.; Selby, B.; et al. Oral Relugolix for Androgen-Deprivation Therapy in Advanced Prostate Cancer. N. Engl. J. Med. 2020, 382, 2187–2196. [Google Scholar] [CrossRef]
- Wang, P.H.; Yang, S.T.; Chang, W.H.; Liu, C.H.; Lee, F.K.; Lee, W.L. Endometriosis: Part I. Basic concept. Taiwan. J. Obstet. Gynecol. 2022, 61, 927–934. [Google Scholar] [CrossRef]
- Saridogan, E.; Becker, C.M.; Feki, A.; Grimbizis, G.F.; Hummelshoj, L.; Keckstein, J.; Nisolle, M.; Tanos, V.; Ulrich, U.A.; Vermeulen, N.; et al. Working group of ESGE, ESHRE and WES, Recommendations for the surgical treatment of endometriosis-part 1: Ovarian endometrioma. Gynecol. Surg. 2017, 14, 27. [Google Scholar]
- Imperiale, L.; Nisolle, M.; Noël, J.C.; Fastrez, M. Three Types of Endometriosis: Pathogenesis, Diagnosis and Treatment. State of the Art. J. Clin. Med. 2023, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Viganò, P.; Somigliana, E.; Panina-Bordignon, P.; Vercellini, P.; Candiani, M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: From pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum. Reprod. Update 2014, 20, 217–230. [Google Scholar] [CrossRef]
- Falik, R.C.; Li, A.; Farrimond, F.; Meshkat Razavi, G.; Nezhat, C.; Nezhat, F. Endometrios: Classification and surgical management. OBG Manag. 2017, 29, 38–43. [Google Scholar]
- Martire, F.G.; Costantini, E.; D’Abate, C.; Schettini, G.; Sorrenti, G.; Centini, G.; Zupi, E.; Lazzeri, L. Endometriosis and Adenomyosis: From Pathogenesis to Follow-Up. Curr. Issues Mol. Biol. 2025, 47, 298. [Google Scholar] [CrossRef]
- Castellarnau Visus, M.; Ponce Sebastia, J.; Carreras Collado, R.; Cayuela Font, E.; Garcia Tejedor, A. Preliminary results: Ethanol sclerotherapy after ultrasound-guided fine needle aspiration without anesthesia in the management of simple ovarian cysts. J. Minim. Invasive Gynecol. 2015, 22, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Shiau, C.S.; Lo, L.M.; Hsieh, T.T.; Chang, M.Y. Effectiveness of ultrasound-guided aspiration and sclerotherapy with 95% ethanol for treatment of recurrent ovarian endometriomas. Fertil. Steril. 2009, 91, 2709–2713. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Clower, L.; Fleshman, T.; Geldenhuys, W.J.; Santanam, N. Targeting Oxidative Stress Involved in Endometriosis and Its Pain. Biomolecules 2022, 12, 1055. [Google Scholar] [CrossRef]
- Salehi, B.; Butnariu, M.; Corneanu, M.; Sarac, I.; Vlaisavljevic, S.; Kitic, D.; Rahavian, A.; Abedi, A.; Karkan, M.F.; Bhatt, I.D.; et al. Chronic pelvic pain syndrome: Highlighting medicinal plants toward biomolecules discovery for upcoming drugs formulation. Phytother. Res. 2020, 34, 769–787. [Google Scholar] [CrossRef]
- Chowdhury, I.; Banerjee, S.; Driss, A.; Xu, W.; Mehrabi, S.; Nezhat, C.; Sidell, N.; Taylor, R.N.; Thompson, W.E. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-κB signaling pathway. J. Cell Physiol. 2019, 234, 6298–6312. [Google Scholar] [CrossRef]
- Cao, W.G.; Morin, M.; Metz, C.; Maheux, R.; Akoum, A. Stimulation of macrophage migration inhibitory factor expression in endometrial stromal cells by interleukin 1, beta involving the nuclear transcription factor NFkappaB. Biol. Reprod. 2005, 73, 565–570. [Google Scholar] [CrossRef]
- Cao, H.; Wei, Y.X.; Zhou, Q.; Zhang, Y.; Guo, X.P.; Zhang, J. Inhibitory effect of curcumin in human endometriosis endometrial cells via downregulation of vascular endothelial growth factor. Mol. Med. Rep. 2017, 16, 5611–5617. [Google Scholar] [CrossRef]
- Gudarzi, R.; Shabani, F.; Mohammad-Alizadeh-Charandabi, S.; Naghshineh, E.; Shaseb, E.; Mirghafourvand, M. Effect of curcumin on painful symptoms of endometriosis: A triple-blind randomized controlled trial. Phytother. Res. 2024, 38, 147–155. [Google Scholar] [CrossRef]
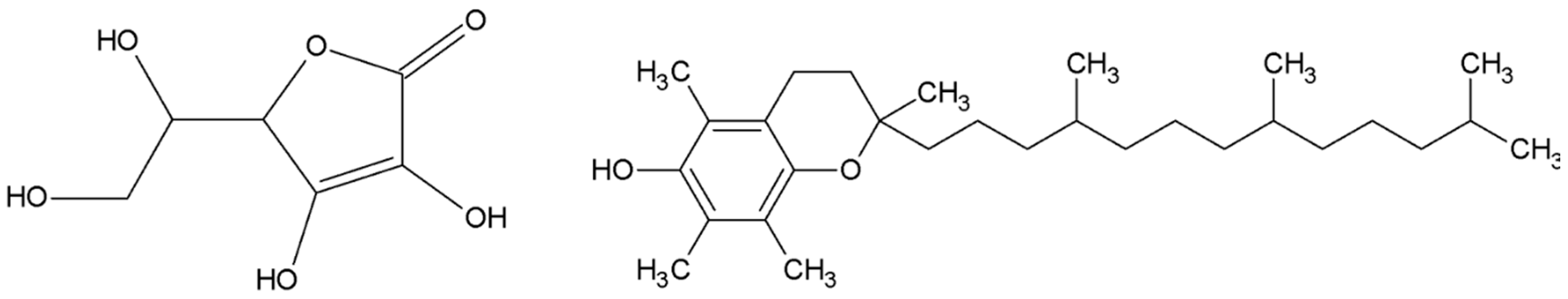
- Markowska, A.; Antoszczak, M.; Markowska, J.; Huczyński, A. The Role of Selected Dietary Factors in the Development and Course of Endometriosis. Nutrients 2023, 15, 2773. [Google Scholar] [CrossRef]
- Hipólito-Reis, M.; Neto, A.C.; Neves, D. Impact of curcumin, quercetin, or resveratrol on the pathophysiology of endometriosis: A systematic review. Phytother. Res. 2022, 36, 2416–2433. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Osteen, K.G.; Taylor, H.S.; Sokalska, A.; Haines, K.; Duleba, A.J. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol. Reprod. 2011, 84, 106–112. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Bahramsoltani, R.; Rahimi, R. Phytochemicals as Adjunctive with Conventional Anticancer Therapies. Curr. Pharm. Des. 2016, 22, 4201–4218. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.G.; Olivares, C.N.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I. Natural therapies assessment for the treatment of endometriosis. Hum. Reprod. 2013, 28, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Xu, H.; Man, G.C.; Zhang, T.; Chu, K.O.; Chu, C.Y.; Cheng, J.T.; Li, G.; He, Y.X.; Qin, L.; et al. Prodrug of green tea epigallocatechin-3-gallate (Pro-EGCG) as a potent anti-angiogenesis agent for endometriosis in mice. Angiogenesis 2013, 16, 59–69. [Google Scholar] [CrossRef]
- Delenko, J.; Xue, X.; Chatterjee, P.K.; Hyman, N.; Shih, A.J.; Adelson, R.P.; Safaric Tepes, P.; Gregersen, P.K.; Metz, C.N. Quercetin enhances decidualization through AKT-ERK-p53 signaling and supports a role for senescence in endometriosis. Reprod. Biol. Endocrinol. 2024, 22, 100. [Google Scholar] [CrossRef]
- Cao, Y.; Zhuang, M.F.; Yang, Y.; Xie, S.W.; Cui, J.G.; Cao, L.; Zhang, T.T.; Zhu, Y. Preliminary study of quercetin affecting the hypothalamic-pituitary-gonadal axis on rat endometriosis model. Evid. Based Complement. Altern. Med. 2014, 2014, 781684. [Google Scholar] [CrossRef]
- Jamali, N.; Zal, F.; Mostafavi-Pour, Z.; Samare-Najaf, M.; Poordast, T.; Dehghanian, A. Ameliorative Effects of Quercetin and Metformin and Their Combination Against Experimental Endometriosis in Rats. Reprod. Sci. 2021, 28, 683–692. [Google Scholar] [CrossRef]
- Zaurito, A.; Mehmeti, I.; Limongelli, F.; Zupo, R.; Annunziato, A.; Fontana, S.; Tardugno, R. Natural compounds for endometriosis and related chronic pelvic pain: A review. Fitoterapia 2024, 179, 106277. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Taştan, H.; Küpeli Akkol, E. The regression of endometriosis with glycosylated flavonoids isolated from Melilotus officinalis (L.) Pall. in an endometriosis rat model. Taiwan. J. Obstet. Gynecol. 2020, 59, 211–219. [Google Scholar] [CrossRef]
- Ilhan, M.; Ali, Z.; Khan, I.A.; Taştan, H.; Küpeli Akkol, E. Bioactivity-guided isolation of flavonoids from Urtica dioica L. and their effect on endometriosis rat model. J. Ethnopharmacol. 2019, 243, 112100. [Google Scholar] [CrossRef]
- Kohama, T.; Herai, K.; Inoue, M. Effect of French maritime pine bark extract on endometriosis as compared with leuprorelin acetate. J. Reprod. Med. 2007, 52, 703–708. [Google Scholar]
- Kim, J.H.; Woo, J.H.; Kim, H.M.; Oh, M.S.; Jang, D.S.; Choi, J.H. Anti-Endometriotic Effects of Pueraria Flower Extract in Human Endometriotic Cells and Mice. Nutrients 2017, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Park, M.Y.; Song, G.; Lim, W. 5,7-Dimethoxyflavone induces apoptotic cell death in human endometriosis cell lines by activating the endoplasmic reticulum stress pathway. Phytother. Res. 2020, 34, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Song, G.; Lim, W. Myricetin inhibits endometriosis growth through cyclin E1 down-regulation in vitro and in vivo. J. Nutr. Biochem. 2020, 78, 108328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Li, M.Z.; Shen, H.H.; Abudukeyoumu, A.; Xie, F.; Ye, J.F.; Xu, F.Y.; Sun, J.S.; Li, M.Q. Ginsenosides in endometrium-related diseases: Emerging roles and mechanisms. Biomed. Pharmacother. 2023, 166, 115340. [Google Scholar] [CrossRef]
- Uchendu, E.; Shukla, M.; Reed, B.; Brown, D.; Saxena, P.; MooYoung, M. Improvement of Ginseng by In Vitro Culture: Challenges and Opportunities. In Comprehensive Biotechnology, 2nd ed.; Volume 4: Agricultural and Related Biotechnologies; Elsevier: Amsterdam, The Netherlands, 2011; pp. 317–329. [Google Scholar]
- Lee, J.H.; Park, J.H.; Won, B.H.; Im, W.; Cho, S. Administration of red ginseng regulates microRNA expression in a mouse model of endometriosis. Clin. Exp. Reprod. Med. 2021, 48, 337–346. [Google Scholar] [CrossRef]
- Norooznezhad, A.H.; Norooznezhad, F. Cannabinoids: Possible agents for treatment of psoriasis via suppression of angiogenesis and inflammation. Med. Hypotheses 2017, 99, 15–18. [Google Scholar] [CrossRef]
- Brunetti, P.; Lo Faro, A.F.; Pirani, F.; Berretta, P.; Pacifici, R.; Pichini, S.; Busardò, F.P. Pharmacology and legal status of cannabidiol. Ann. Ist. Super. Sanita 2020, 56, 285–291. [Google Scholar]
- Kogan, N.M.; Blázquez, C.; Alvarez, L.; Gallily, R.; Schlesinger, M.; Guzmán, M.; Mechoulam, R. A cannabinoid quinone inhibits angiogenesis by targeting vascular endothelial cells. Mol. Pharmacol. 2006, 70, 51–59. [Google Scholar] [CrossRef]
- Genovese, T.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Caudullo, S.; Raffone, E.; Macrí, F.; Interdonato, L.; Gugliandolo, E.; Interlandi, C.; et al. Molecular and Biochemical Mechanism of Cannabidiol in the Management of the Inflammatory and Oxidative Processes Associated with Endometriosis. Int. J. Mol. Sci. 2022, 23, 5427. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cantelmo, A.R.; Cattaneo, M.G.; Cammarota, R.; Bartolini, D.; Cinquina, V.; Valenti, M.; Vicentini, L.M.; Noonan, D.M.; et al. Cannabidiol inhibits angiogenesis by multiple mechanisms. Br. J. Pharmacol. 2012, 167, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Anvari Aliabad, R.; Hassanpour, K.; Norooznezhad, A.H. Cannabidiol as a possible treatment for endometriosis through suppression of inflammation and angiogenesis. Immun. Inflamm. Dis. 2024, 12, e1370. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. 2017, 40, 248–255. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Terao, J. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma, and is degraded and metabolized to conjugated forms of caffeic acid, ferulic acid and m-coumaric acid. Life Sci. 2004, 75, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Natsume, M.; Yasuda, A.; Muto, Y.; Hiyoshi, K.; Takano, H.; Yoshikawa, T.; Terao, J. Absorption, metabolism, degradation and urinary excretion of rosmarinic acid after intake of Perilla frutescens extract in humans. Eur. J. Nutr. 2005, 44, 1–9. [Google Scholar] [CrossRef]
- Ferella, L.; Bastón, J.I.; Bilotas, M.A.; Singla, J.J.; González, A.M.; Olivares, C.N.; Meresman, G.F. Active compounds present inRosmarinus officinalis leaves andScutellaria baicalensis root evaluated as new therapeutic agents for endometriosis. Reprod. Biomed. Online 2018, 37, 769–782. [Google Scholar] [CrossRef]
- Holzer, P. Capsaicin: Cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991, 43, 143–201. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bolognese, J.; Calder, N.; Baxendale, J.; Kehler, A.; Cummings, C.; Connell, J.; Herman, G. Effect of morphine and pregabalin compared with diphenhydramine hydrochloride and placebo on hyperalgesia and allodynia induced by intradermal capsaicin in healthy male subjects. J. Pain 2008, 9, 1088–1095. [Google Scholar] [CrossRef]
- Wallace, M.; Duan, R.; Liu, W.; Locke, C.; Nothaft, W. A Randomized, Double-Blind, Placebo-Controlled, Crossover Study of the T-Type Calcium Channel Blocker ABT-639 in an Intradermal Capsaicin Experimental Pain Model in Healthy Adults. Pain Med. 2016, 17, 551–560. [Google Scholar] [CrossRef][Green Version]
- Lembeck, F. Columbus, Capsicum and capsaicin: Past, present and future. Acta Physiol. Hung. 1987, 69, 265–273. [Google Scholar] [PubMed][Green Version]
- Watson, C.P. Topical capsaicin as an adjuvant analgesic. J. Pain Symptom Manag. 1994, 9, 425–433. [Google Scholar] [CrossRef]
- Arora, V.; Campbell, J.N.; Chung, M.K. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol. Ther. 2021, 220, 107743. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules 2016, 21, 966. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Wu, Y.; Starzinski-Powitz, A.; Guo, S.W. Capsaicin inhibits proliferation of endometriotic cells in vitro. Gynecol. Obstet. Investig. 2008, 66, 59–62. [Google Scholar] [CrossRef]
- Qiu, Y.; Yuan, S.; Wang, H. Vitamin D status in endometriosis: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2020, 302, 141–152. [Google Scholar] [CrossRef]
- Delbandi, A.A.; Torab, M.; Abdollahi, E.; Khodaverdi, S.; Rokhgireh, S.; Moradi, Z.; Heidari, S.; Mohammadi, T. Vitamin D deficiency as a risk factor for endometriosis in Iranian women. J. Reprod. Immunol. 2021, 143, 103266. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinskaya, M.; Denisova, A.; Tkachenko, N.; Ivashenko, T.; Bespalova, O.; Tolibova, G.; Tral, T. Vitamin D significance in pathogenesis of endometriosis. Gynecol. Endocrinol. 2021, 37 (Suppl. 1), 40–43. [Google Scholar] [CrossRef]
- Harris, H.R.; Chavarro, J.E.; Malspeis, S.; Willett, W.C.; Missmer, S.A. Dairy-food, calcium, magnesium, and vitamin D intake and endometriosis: A prospective cohort study. Am. J. Epidemiol. 2013, 177, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Koga, K.; Izumi, G.; Sue, F.; Makabe, T.; Taguchi, A.; Nagai, M.; Urata, Y.; Takamura, M.; Harada, M.; et al. Effects of 1,25-Dihydroxy Vitamin D3 on Endometriosis. J. Clin. Endocrinol. Metab. 2016, 101, 2371–2379. [Google Scholar] [CrossRef]
- Baek, J.C.; Jo, J.Y.; Lee, S.M.; Cho, I.A.; Shin, J.K.; Lee, S.A.; Lee, J.H.; Cho, M.C.; Choi, W.J. Differences in 25-hydroxy vitamin D and vitamin D-binding protein concentrations according to the severity of endometriosis. Clin. Exp. Reprod. Med. 2019, 46, 125–131. [Google Scholar] [CrossRef]
- Somigliana, E.; Panina-Bordignon, P.; Murone, S.; Di Lucia, P.; Vercellini, P.; Vigano, P. Vitamin D reserve is higher in women with endometriosis. Hum. Reprod. 2007, 22, 2273–2278. [Google Scholar] [CrossRef] [PubMed]
- Mier-Cabrera, J.; Aburto-Soto, T.; Burrola-Méndez, S.; Jiménez-Zamudio, L.; Tolentino, M.C.; Casanueva, E.; Hernández-Guerrero, C. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod. Biol. Endocrinol. 2009, 7, 54. [Google Scholar] [CrossRef]
- Hoorsan, H.; Simbar, M.; Tehrani, F.R.; Fathi, F.; Mosaffa, N.; Riazi, H.; Akradi, L.; Nasseri, S.; Bazrafkan, S. The effectiveness of antioxidant therapy (vitamin C) in an experimentally induced mouse model of ovarian endometriosis. Women’s Health (Lond) 2022, 18, 17455057221096218. [Google Scholar] [CrossRef]
- Erten, O.U.; Ensari, T.A.; Dilbaz, B.; Cakiroglu, H.; Altinbas, S.K.; Çaydere, M.; Goktolga, U. Vitamin C is effective for the prevention and regression of endometriotic implants in an experimentally induced rat model of endometriosis. Taiwan. J. Obstet. Gynecol. 2016, 55, 251–257. [Google Scholar] [CrossRef]
- Ansariniya, H.; Hadinedoushan, H.; Javaheri, A.; Zare, F. Vitamin C and E supplementation effects on secretory and molecular aspects of vascular endothelial growth factor derived from peritoneal fluids of patients with endometriosis. J. Obstet. Gynaecol. 2019, 39, 1137–1142. [Google Scholar] [CrossRef]
- Amini, L.; Chekini, R.; Nateghi, M.R.; Haghani, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res. Manag. 2021, 2021, 5529741. [Google Scholar] [CrossRef]
- Mier-Cabrera, J.; Genera-García, M.; De la Jara-Díaz, J.; Perichart-Perera, O.; Vadillo-Ortega, F.; Hernández-Guerrero, C. Effect of vitamins C and E supplementation on peripheral oxidative stress markers and pregnancy rate in women with endometriosis. Int. J. Gynaecol. Obstet. 2008, 100, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Santanam, N.; Kavtaradze, N.; Murphy, A.; Dominguez, C.; Parthasarathy, S. Antioxidant supplementation reduces endometriosis-related pelvic pain in humans. Transl. Res. 2013, 161, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.C.; Lin, T.C.; Wu, M.H.; Tsai, S.J. Progesterone resistance in endometriosis: A pathophysiological perspective and potential treatment alternatives. Reprod. Med. Biol. 2024, 23, e12588. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Chodankar, R.R. 90 YEARS OF PROGESTERONE: Selective progesterone receptor modulators in gynaecological therapies. J. Mol. Endocrinol. 2020, 65, T15–T33. [Google Scholar] [CrossRef]
- Burns, K.A.; Rodriguez, K.F.; Hewitt, S.C.; Janardhan, K.S.; Young, S.L.; Korach, K.S. Role of estrogen receptor signaling required for endometriosis-like lesion establishment in a mouse model. Endocrinology 2012, 153, 3960–3971. [Google Scholar] [CrossRef]
- Kulak, J.; Fischer, C.; Komm, B.; Taylor, H.S. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology 2011, 152, 3226–3232. [Google Scholar] [CrossRef]
- Khine, Y.M.; Taniguchi, F.; Nagira, K.; Nakamura, K.; Ohbayashi, T.; Osaki, M.; Harada, T. New insights into the efficacy of SR-16234, a selective estrogen receptor modulator, on the growth of murine endometriosis-like lesions. Am. J. Reprod. Immunol. 2018, 80, e13023. [Google Scholar] [CrossRef]
- Harada, T.; Ohta, I.; Endo, Y.; Sunada, H.; Noma, H.; Taniguchi, F. SR-16234, a Novel Selective Estrogen Receptor Modulator for Pain Symptoms with Endometriosis: An Open-label Clinical Trial. Yonago Acta Med. 2017, 60, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Clemenza, S.; Vannuccini, S.; Ruotolo, A.; Capezzuoli, T.; Petraglia, F. Advances in targeting estrogen synthesis and receptors in patients with endometriosis. Expert. Opin. Investig. Drugs 2022, 31, 1227–1238. [Google Scholar] [CrossRef]
- Bulun, S.E.; Zeitoun, K.M.; Takayama, K.; Sasano, H. Estrogen biosynthesis in endometriosis: Molecular basis and clinical relevance. J. Mol. Endocrinol. 2000, 25, 35–42. [Google Scholar] [CrossRef]
- Attar, E.; Tokunaga, H.; Imir, G.; Yilmaz, M.B.; Redwine, D.; Putman, M.; Gurates, B.; Attar, R.; Yaegashi, N.; Hales, D.B.; et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J. Clin. Endocrinol. Metab. 2009, 94, 623–631. [Google Scholar] [CrossRef]
- Béliard, A.; Noël, A.; Foidart, J.M. Reduction of apoptosis and proliferation in endometriosis. Fertil. Steril. 2004, 82, 80–85. [Google Scholar] [CrossRef]
- Słopień, R.; Męczekalski, B. Aromatase inhibitors in the treatment of endometriosis. Prz. Menopauzalny 2016, 15, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Stanway, S.J.; Purohit, A.; Woo, L.W.; Sufi, S.; Vigushin, D.; Ward, R.; Wilson, R.H.; Stanczyk, F.Z.; Dobbs, N.; Kulinskaya, E.; et al. Phase I study of STX 64 (667 Coumate) in breast cancer patients: The first study of a steroid sulfatase inhibitor. Clin. Cancer Res. 2006, 12, 1585–1592. [Google Scholar] [CrossRef]
- Rižner, T.L.; Romano, A. Targeting the formation of estrogens for treatment of hormone dependent diseases-current status. Front. Pharmacol. 2023, 14, 1155558. [Google Scholar] [CrossRef]
- Pavone, M.E.; Bulun, S.E. Aromatase inhibitors for the treatment of endometriosis. Fertil. Steril. 2012, 98, 1370–1379. [Google Scholar] [CrossRef]
- Ailawadi, R.K.; Jobanputra, S.; Kataria, M.; Gurates, B.; Bulun, S.E. Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate: A pilot study. Fertil. Steril. 2004, 81, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, S.; Gillott, D.J.; Venturini, P.L.; Remorgida, V. Use of aromatase inhibitors to treat endometriosis-related pain symptoms: A systematic review. Reprod. Biol. Endocrinol. 2011, 9, 89. [Google Scholar] [CrossRef]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef]
- Sbracia, M.; Scarpellini, F. Use of depot GNRH antagonist (Degarelix) in the treatment of endometriomas (ovarian endometriosis) before IVF: A controlled trial. Fertil. Steril. 2015, 104, e158. [Google Scholar] [CrossRef]
- Franssen, A.M.; Kauer, F.M.; Chadha, D.R.; Zijlstra, J.A.; Rolland, R. Endometriosis: Treatment with gonadotropin-releasing hormone agonist Buserelin. Fertil. Steril. 1989, 51, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.L.; Reis, F.M.; Taylor, R.N. Angiogenesis and endometriosis. Obstet. Gynecol. Int. 2013, 2013, 859619. [Google Scholar] [CrossRef] [PubMed]
- Bouquet de Joliniere, J.; Fruscalzo, A.; Khomsi, F.; Stochino Loi, E.; Cherbanyk, F.; Ayoubi, J.M.; Feki, A. Antiangiogenic Therapy as a New Strategy in the Treatment of Endometriosis? The First Case Report. Front. Surg. 2021, 8, 791686. [Google Scholar] [CrossRef]
- Antônio, L.G.L.; Rosa-E-Silva, J.C.; Machado, D.J.; Westin, A.T.; Garcia, S.B.; Candido-Dos-Reis, F.J.; Poli-Neto, O.B.; Nogueira, A.A. Thalidomide Reduces Cell Proliferation in Endometriosis Experimentally Induced in Rats. Rev. Bras. Ginecol. Obstet. 2019, 41, 668–672. [Google Scholar] [CrossRef]
- He, Y.; Hung, S.W.; Liang, B.; Zhang, R.; Gao, Y.; Chu, C.Y.; Zhang, T.; Xu, H.; Chung, J.P.W.; Wang, C.C. Receptor Tyrosine Kinase Inhibitor Sunitinib as Novel Immunotherapy to Inhibit Myeloid-Derived Suppressor Cells for Treatment of Endometriosis. Front. Immunol. 2021, 12, 641206. [Google Scholar] [CrossRef]
- Selak, V.; Farquhar, C.; Prentice, A.; Singla, A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2000, 2, CD000068. [Google Scholar]
- Chen, P.; Mamillapalli, R.; Habata, S.; Taylor, H.S. Endometriosis stromal cells induce bone marrow mesenchymal stem cell differentiation and PD-1 expression through paracrine signaling. Mol. Cell Biochem. 2021, 476, 1717–1727. [Google Scholar] [CrossRef]
- Sobstyl, M.; Mertowska, P.; Mertowski, S.; Zaborek-Łyczba, M.; Dudziński, D.; Polak, G.; Grywalska, E. The PD-1/PD-L1 Gateway: Peripheral Immune Regulation in the Pathogenesis of Endometriosis. Int. J. Mol. Sci. 2024, 25, 6775. [Google Scholar] [CrossRef]
- Li, J.; Yan, S.; Li, Q.; Huang, Y.; Ji, M.; Jiao, X.; Yuan, M.; Wang, G. Macrophage-associated immune checkpoint CD47 blocking ameliorates endometriosis. Mol. Hum. Reprod. 2022, 28, gaac010. [Google Scholar] [CrossRef]
- D’Hooghe, T.M.; Bambra, C.S.; Raeymaekers, B.M.; De Jonge, I.; Hill, J.A.; Koninckx, P.R. The effects of immunosuppression on development and progression of endometriosis in baboons (Papio anubis). Fertil. Steril. 1995, 64, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Chatzianagnosti, S.; Dermitzakis, I.; Theotokis, P.; Kousta, E.; Mastorakos, G.; Manthou, M.E. Application of Mesenchymal Stem Cells in Female Infertility Treatment: Protocols and Preliminary Results. Life 2024, 14, 1161. [Google Scholar] [CrossRef]
- Laschke, M.W.; Giebels, C.; Nickels, R.M.; Scheuer, C.; Menger, M.D. Endothelial progenitor cells contribute to the vascularization of endometriotic lesions. Am. J. Pathol. 2011, 178, 442–450. [Google Scholar] [CrossRef]
- Wang, X.; Mamillapalli, R.; Mutlu, L.; Du, H.; Taylor, H.S. Chemoattraction of bone marrow-derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res. 2015, 15, 14–22. [Google Scholar] [CrossRef]
- Artemova, D.; Vishnyakova, P.; Gantsova, E.; Elchaninov, A.; Fatkhudinov, T.; Sukhikh, G. The prospects of cell therapy for endometriosis. J. Assist. Reprod. Genet. 2023, 40, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Yuxue, J.; Ran, S.; Minghui, F.; Minjia, S. Applications of nanomaterials in endometriosis treatment. Front. Bioeng. Biotechnol. 2023, 11, 1184155. [Google Scholar] [CrossRef]
- Othman, E.E.; Salama, S.; Ismail, N.; Al-Hendy, A. Toward gene therapy of endometriosis: Adenovirus-mediated delivery of dominant negative estrogen receptor genes inhibits cell proliferation, reduces cytokine production, and induces apoptosis of endometriotic cells. Fertil. Steril. 2007, 88, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Egorova, A.; Shubina, A.; Sokolov, D.; Selkov, S.; Baranov, V.; Kiselev, A. CXCR4-targeted modular peptide carriers for efficient anti-VEGF siRNA delivery. Int. J. Pharm. 2016, 515, 431–440. [Google Scholar] [CrossRef]
- Mazzone, M.; Dettori, D.; de Oliveira, R.L.; Loges, S.; Schmidt, T.; Jonckx, B.; Tian, Y.M.; Lanahan, A.A.; Pollard, P.; de Almodovar, C.R.; et al. Heterozygous deficiency of PHD2 restores tumor oxygenation and inhibits metastasis via endothelial normalization. Cell 2009, 136, 839–851. [Google Scholar] [CrossRef]
- Tapmeier, T.T.; Rahmioglu, N.; Lin, J.; De Leo, B.; Obendorf, M.; Raveendran, M.; Fischer, O.M.; Bafligil, C.; Guo, M.; Harris, R.A.; et al. Neuropeptide S receptor 1 is a nonhormonal treatment target in endometriosis. Sci. Transl. Med. 2021, 13, 839–851. [Google Scholar] [CrossRef]
- Arbelaez, F.; Joeng, H.K.; Hussain, A.; Sunga, S.; Guan, Y.; Chawla, A.; Carmona, F.; Lines, C.; Mendizabal, G. Randomized, controlled, proof-of-concept trial of gefapixant for endometriosis-related pain. Fertil. Steril. 2025, 123, 280–288. [Google Scholar] [CrossRef]
- Parke, S.; Gude, K.; Roth, K.; Messina, F. Efficacy and safety of eliapixant in endometriosis-associated pelvic pain: The randomized, placebo-controlled phase 2b SCHUMANN study. BMC Women’s Health 2024, 24, 353. [Google Scholar] [CrossRef]
- Sullender, R.T.; Agarwal, R.K.; Jacobs, M.B.; Wessels, J.M.; Foster, W.G.; Agarwal, S.K. Pilot Study of IL-1 Antagonist Anakinra for Treatment of Endometriosis. Int. J. Women’s Health 2024, 16, 1583–1593. [Google Scholar] [CrossRef]
- Pellicer, A.; Taylor, H.S.; Alberich-Bayarri, A.; Liu, Y.; Gamborg, M.; Barletta, K.E.; Pinton, P.; Heiser, P.W.; Bagger, Y.Z. Quinagolide vaginal ring for reduction of endometriotic lesions: Results from the QLARITY trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2025, 310, 113946. [Google Scholar] [CrossRef]
- As-Sanie, S.; Abrao, M.S.; Reznichenko, G.; Wilk, K.; Zhong, Y.; Perry, J.; Hunsche, E.; Soulban, G.; Becker, C.M. Impact of relugolix combination therapy on functioning and quality of life in women with endometriosis-associated pain. Fertil. Steril. 2024, 122, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Johnson, N.P.; As-Sanie, S.; Arjona Ferreira, J.C.; Abrao, M.S.; Wilk, K.; Imm, S.J.; Mathur, V.; Perry, J.S.; Wagman, R.B.; et al. Two-year efficacy and safety of relugolix combination therapy in women with endometriosis-associated pain: SPIRIT open-label extension study. Hum. Reprod. 2024, 39, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.D. Once Daily Oral Relugolix Combination Therapy Versus Placebo in Patients With Endometriosis-Associated Pain: Two Replicate Phase 3, Randomised, Double-Blind, Studies (SPIRIT 1 and 2). J. Obstet. Gynecol. Neonatal Nurs. 2024, 53, 590–593. [Google Scholar] [CrossRef]
- Donnez, J.; Becker, C.; Taylor, H.; Carmona Herrera, F.; Donnez, O.; Horne, A.; Paszkowski, M.; Petraglia, F.; Renner, S.P.; Patel, A.; et al. Linzagolix therapy versus a placebo in patients with endometriosis-associated pain: A prospective, randomized, double-blind, Phase 3 study (EDELWEISS 3). Hum. Reprod. 2024, 39, 1208–1221. [Google Scholar] [CrossRef]

| Biomarker | Uniprot Number | Description | Reference(s) |
|---|---|---|---|
| CA 125 | P04633 | A tumor-associated antigen overexpressed in ovarian cancer and endometriosis | [62,63,64] |
| Glycodelin | P04633 | A glycoprotein secreted by the testis that regulates sperm motility and capacitation | [69,70,71,72,73,74] |
| HE4 | P04633 | A secreted glycoprotein overexpressed in serous and endometrioid ovarian cancer | [75,76] |
| IL-6 | P04633 | A cytokine involved in inflammation and immune response | [77,78] |
| VCAM-1 | P04633 | A transmembrane glycoprotein involved in immune cell adhesion and recruitment | [79,80,81,82,83] |
| VEGF | P04633 | A growth factor involved in angiogenesis and endometriosis tissue growth | [84] |
| MCP-1 | P04633 | A chemokine involved in immune cell recruitment and inflammation | [85,86] |
| Urocortin | P04633 | A neuropeptide involved in stress response and inflammation | [87] |
| CA 19-9 | P04633 | A tumor-associated antigen is overexpressed in some types of cancer and endometriosis | [88,89] |
| Estrogen level | N/A | The level of estrogen in the body, which is increased in response to endometriosis | [90] |
| Pharmacological Treatments | |||
|---|---|---|---|
| Treatment | Route of Administration | Advantages | Disadvantages |
| NSAIDs (e.g., ibuprofen, naproxen) | Oral | Easily accessible; reduces inflammation and dysmenorrhea | Symptom-only relief; no effect on lesion growth; GI side effects |
| Combined Oral Contraceptives (COCs) | Oral, Vaginal ring, Transdermal patch | First-line therapy; regulates cycles; reduces pain and bleeding | Limited in severe disease; risk of VTE; may not prevent progression |
| Progestins (Dienogest, NETA, MPA) | Oral, Injectable, Intrauterine | Inhibits endometrial growth; suitable for long-term use; affordable | Breakthrough bleeding; weight gain; ↓ BMD; some forms carry VTE and liver risks |
| GnRH Agonists (leuprorelin, nafarelin) | Injection, Nasal spray | Highly effective pain relief; suppresses estrogen | Hypoestrogenism (hot flashes, osteopenia); requires add-back therapy; not suitable for conception |
| GnRH Antagonists (elagolix, relugolix) | Oral | Rapid onset; avoids flare effect of agonists | Similar side effects as agonists; limited long-term safety data |
| Danazol/Gestrinone | Oral | Induces pseudomenopause; halts lesion growth | Androgenic effects (weight gain, acne, voice change); liver toxicity |
| Aromatase Inhibitors (letrozole, anastrozole) | Oral | Suppresses local estrogen; used in refractory cases | Vaginal dryness, osteoporosis risk; usually combined with other agents |
| LNG-IUS (Levonorgestrel IUD) | Intrauterine | Local hormone delivery; reduces pelvic pain; low systemic exposure | Limited effect on extrauterine lesions; irregular bleeding; insertion discomfort |
| Resveratrol (experimental) | Oral (natural compound) | Potential anti-inflammatory and apoptotic effects | Limited clinical evidence; currently investigational |
| Surgical Procedure | Route/Approach | Advantages | Disadvantages |
|---|---|---|---|
| Laparoscopic Excision/Ablation | Minimally invasive surgery | Gold standard for diagnosis and treatment; removes lesions; symptom relief | Requires expertise; recurrence possible; operative risks |
| Ovarian Cystectomy (Endometrioma) | Laparoscopic surgery | Lower recurrence vs. drainage; preserves fertility | ↓ AMH; technical difficulty; risk of ovarian damage |
| Laser Vaporization | Laparoscopic (precision laser) | Minimal thermal damage; useful for superficial lesions | May not treat deep lesions; specialized equipment needed |
| Deep Infiltrating Endometriosis Resection | Laparoscopy or laparotomy | Improves pain and function in DIE; multidisciplinary approach recommended | Risk of complications (bowel, bladder); complex and time-consuming |
| Hysterectomy ± Oophorectomy | Laparoscopic, vaginal, or abdominal surgery | Option for severe pain in women done with childbearing | Permanent infertility; possible symptom persistence if extrauterine disease remains |
| Sclerotherapy (Endometrioma) | Ultrasound-guided aspiration + alcohol | Minimally invasive; preserves ovarian tissue | High recurrence; not standard of care; best for selected cases only |
| Postoperative Hormonal Suppression | Oral or IUD (COCs, progestins, GnRH analogs) | Prevents recurrence after surgery; improves long-term outcomes | Requires long-term compliance; not suitable for conception |
| Name | DRUGBANK Number | Short Description | Reference(s) |
|---|---|---|---|
| Mifepristone | DB00834 | Synthetic estrane steroid, originally designed as an anti-glucocorticoid drug | [171] |
| UPA | DB08867 | Steroidal selective progesterone receptor modulator (SPRM) was initially studied as an antifertility drug | [171] |
| Vilaprisan | DB11971 | Potent, orally active selective progesterone receptor modulator (SPRM) developed by Bayer AG | [171] |
| Bazedoxifene | DB06401 | Selective estrogen receptor modulator (SERM) that inhibits estrogen-induced stimulation of the uterine endometrium | [174] |
| SR-16234 | DB05966 | Treatment with SR significantly decreased both the total number and size of lesions per mouse without promoting endometrial growth | [175] |
| Irosustat | N/A | Steroid sulfatase inhibitor | [181,182] |
| Estrone-3-O-sulfamate | N/A | Steroid sulfatase inhibitor | [182] |
| E2MATE | N/A | Steroid sulfatase inhibitor | [182] |
| Letrozole | N/A | Aromatase inhibitor | [184] |
| Anastrozole | N/A | Aromatase inhibitor | [185] |
| Elagolix | N/A | GnRH antagonist | [186] |
| Degarelix | N/A | GnRH antagonist | [187] |
| Buserelin | N/A | GnRH antagonist | [188] |
| Bevacizumab | N/A | Anti-angiogenic agent | [189] |
| Thalidomide | N/A | Anti-angiogenic agent | [190] |
| Sunitinib | N/A | Anti-angiogenic agent | [191] |
| Danazol | N/A | Immune modulator | [192] |
| PD-1/PDL-1 | N/A | Immune modulator | [193] |
| CD47 inhibitors | N/A | Immune modulator | [194] |
| Azathioprine | N/A | Immune modulator | [195] |
| Substance | Study | Final Outcomes |
|---|---|---|
| Gefapixant | [224] | Inconclusive, results directionally favoring gefapixant but not demonstrating superiority to placebo |
| Eliapixant | [225] | Terminated early, no significant differences in pain reduction compared to placebo |
| Anakinra | [226] | Trends toward improvement in quality of life and dysmenorrhea, justifying further investigation |
| Quinagolide | [227] | No significant difference in reducing total lesion size compared to placebo |
| Norethindrone Acetate (NETA) | [115] | Effectively reduced pain, achieved a greater reduction in endometrioma size and had a lower dropout rate |
| 99mTc-maraciclatide | N/A | N/A |
| Relugolix Combination Therapy | [228,229,230] | Sustained improvements in endometriosis-associated pain, dyspareunia, and function over 2 years |
| Linzagolix | [231] | Significantly reduced dysmenorrhea and non-menstrual pelvic pain at 3 months with 200 mg linzagolix with add-back therapy, significantly decreased dysmenorrhea only with 75 mg linzagolix |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czubak, P.; Herda, K.; Niewiadomska, I.; Putowski, L.; Łańcut, M.; Masłyk, M. Understanding Endometriosis: A Broad Review of Its Causes, Management, and Impact. Int. J. Mol. Sci. 2025, 26, 8878. https://doi.org/10.3390/ijms26188878
Czubak P, Herda K, Niewiadomska I, Putowski L, Łańcut M, Masłyk M. Understanding Endometriosis: A Broad Review of Its Causes, Management, and Impact. International Journal of Molecular Sciences. 2025; 26(18):8878. https://doi.org/10.3390/ijms26188878
Chicago/Turabian StyleCzubak, Paweł, Karolina Herda, Iwona Niewiadomska, Lechosław Putowski, Mirosław Łańcut, and Maciej Masłyk. 2025. "Understanding Endometriosis: A Broad Review of Its Causes, Management, and Impact" International Journal of Molecular Sciences 26, no. 18: 8878. https://doi.org/10.3390/ijms26188878
APA StyleCzubak, P., Herda, K., Niewiadomska, I., Putowski, L., Łańcut, M., & Masłyk, M. (2025). Understanding Endometriosis: A Broad Review of Its Causes, Management, and Impact. International Journal of Molecular Sciences, 26(18), 8878. https://doi.org/10.3390/ijms26188878







