Antifibrotic Effects of an α7 Nicotinic Acetylcholine Receptor Agonist in Keloid Fibroblasts and a Rat Scar Model
Abstract
1. Introduction
2. Results
2.1. In Vitro Study
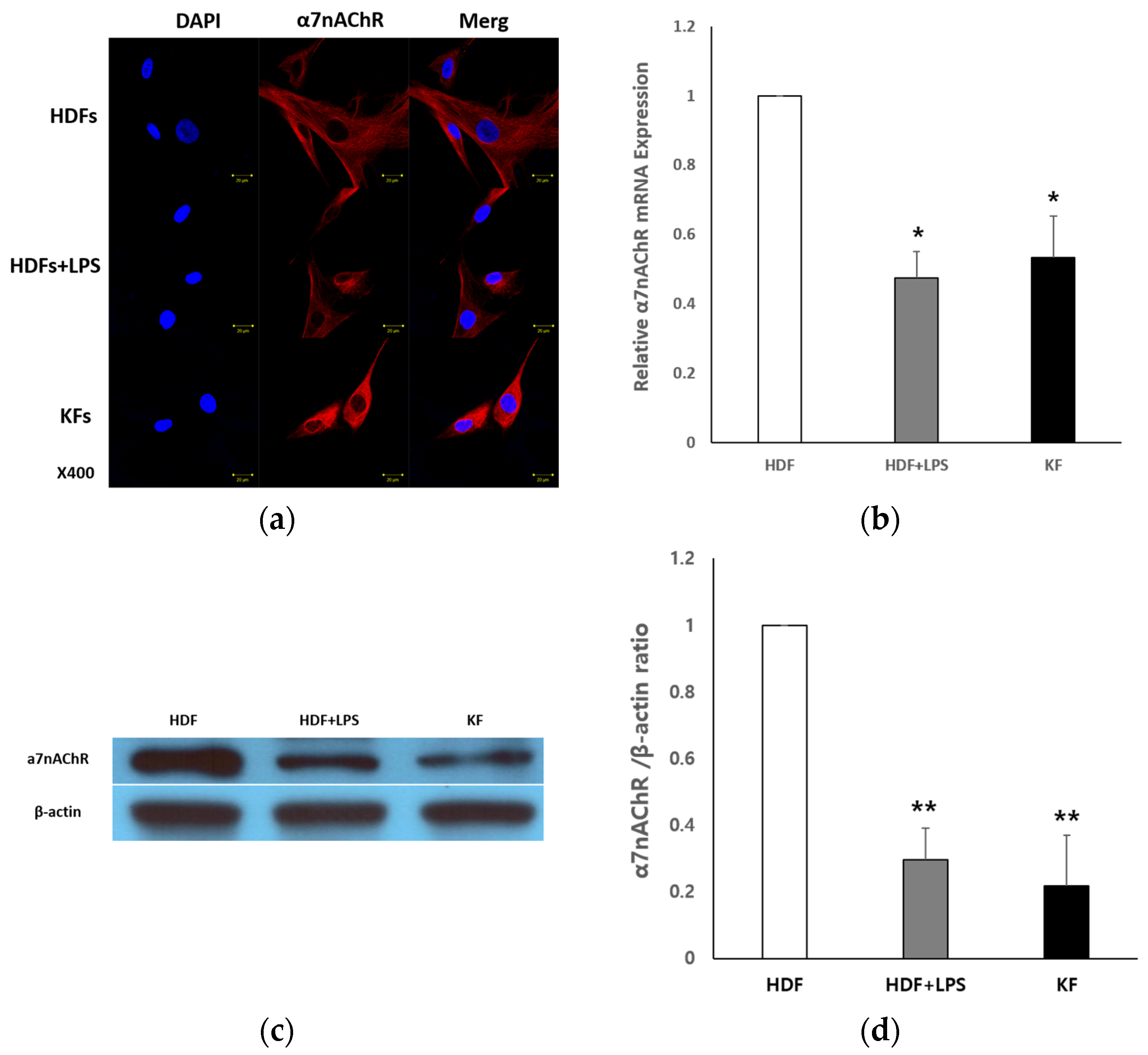
2.1.1. Expression of α7nAChR in HDFs and KFs
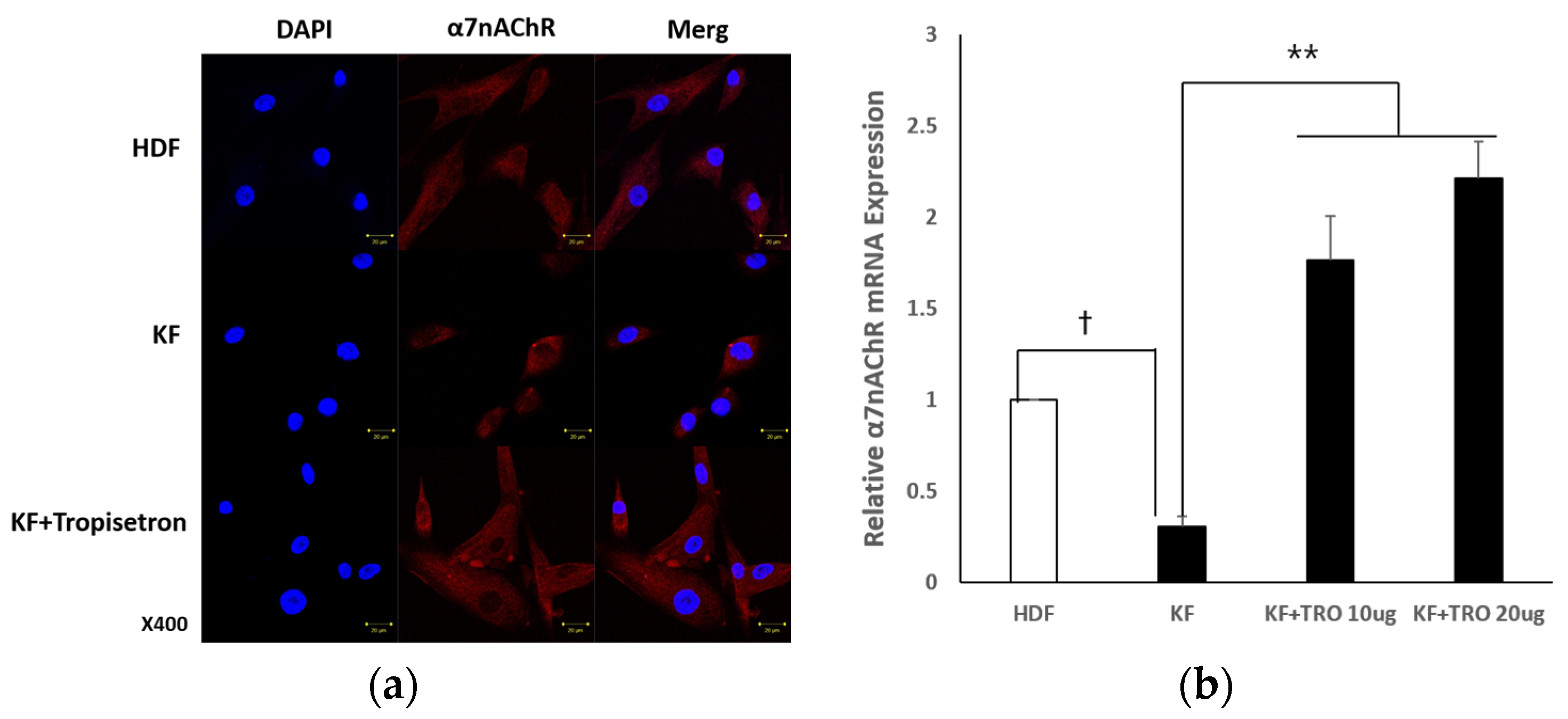
2.1.2. Tropisetron Enhances α7nAChR Expression in KFs
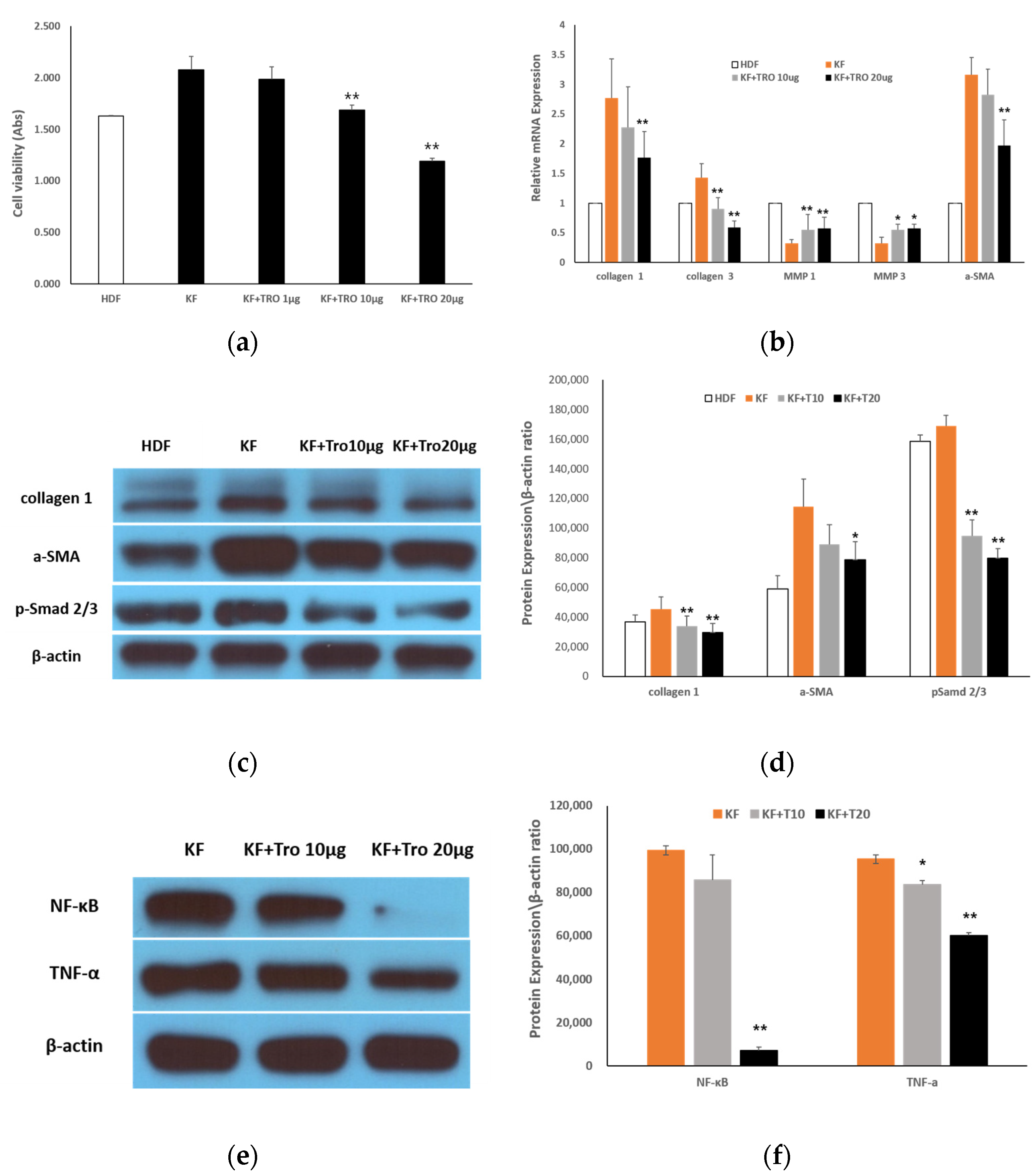
2.1.3. Effects of Tropisetron on KF Viability and Fibrotic Gene/Protein Expression
2.2. In Vivo Rat Incisional Scar Model
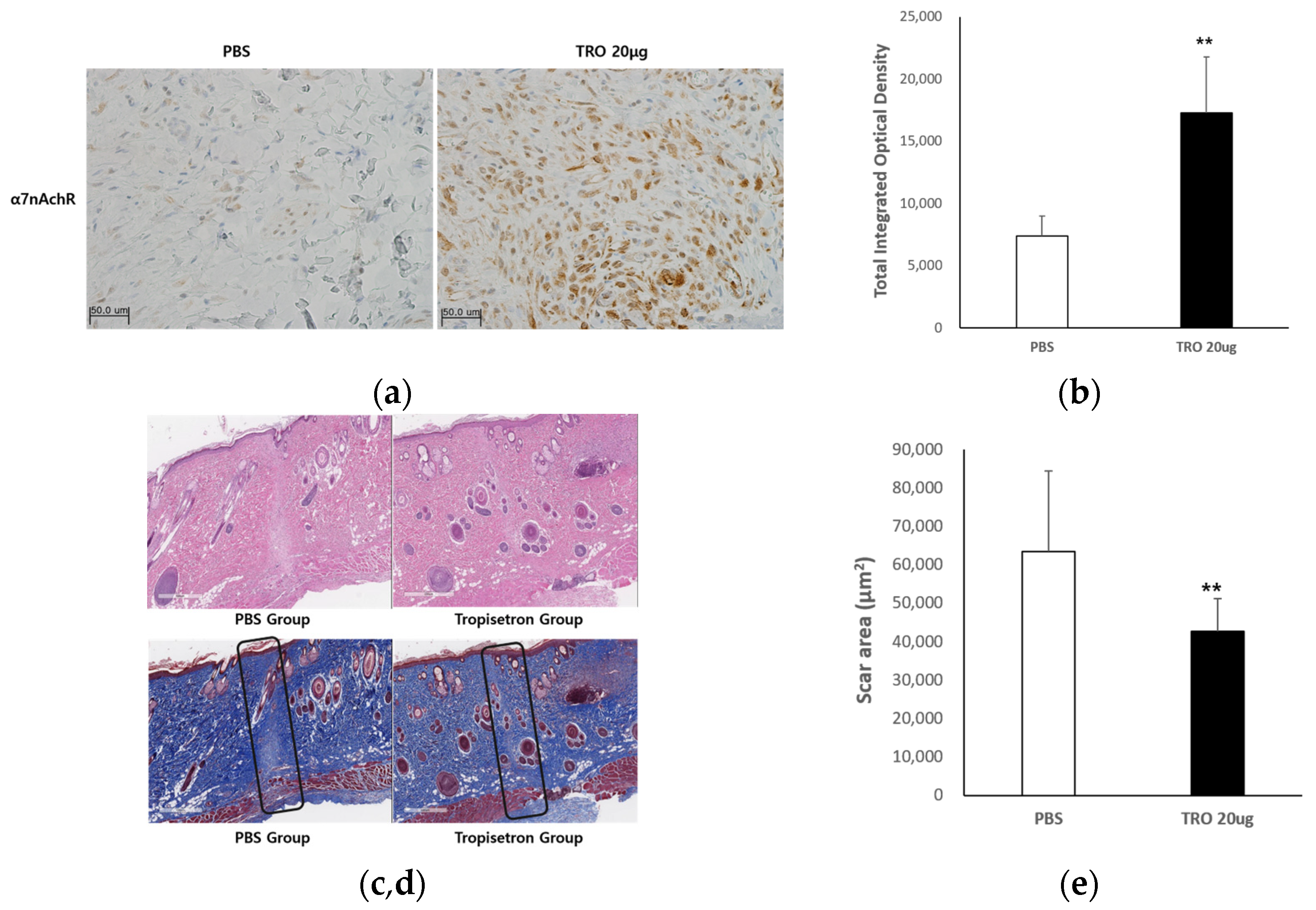
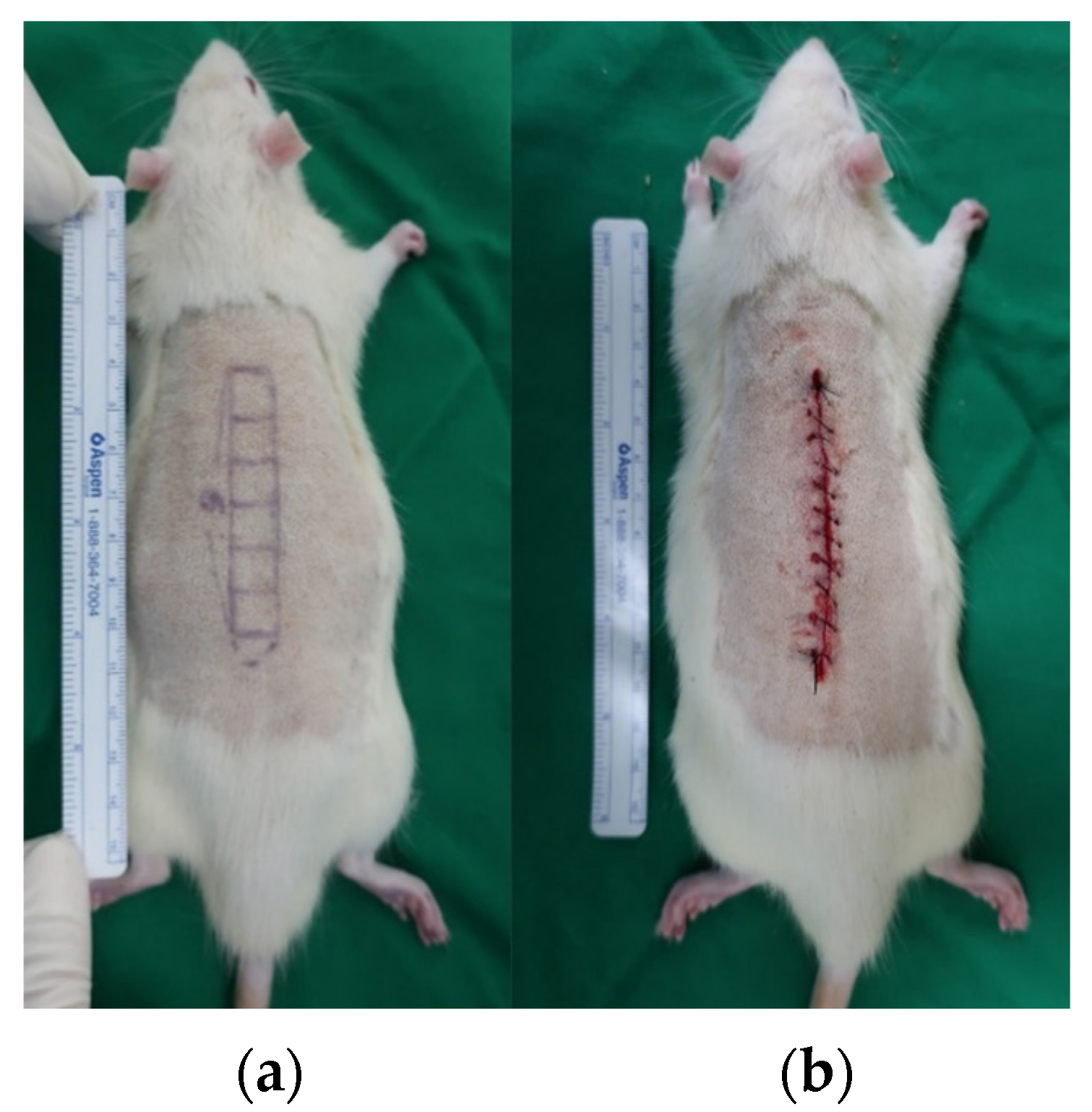
2.2.1. Evaluation of the Antifibrotic Effect of Tropisetron
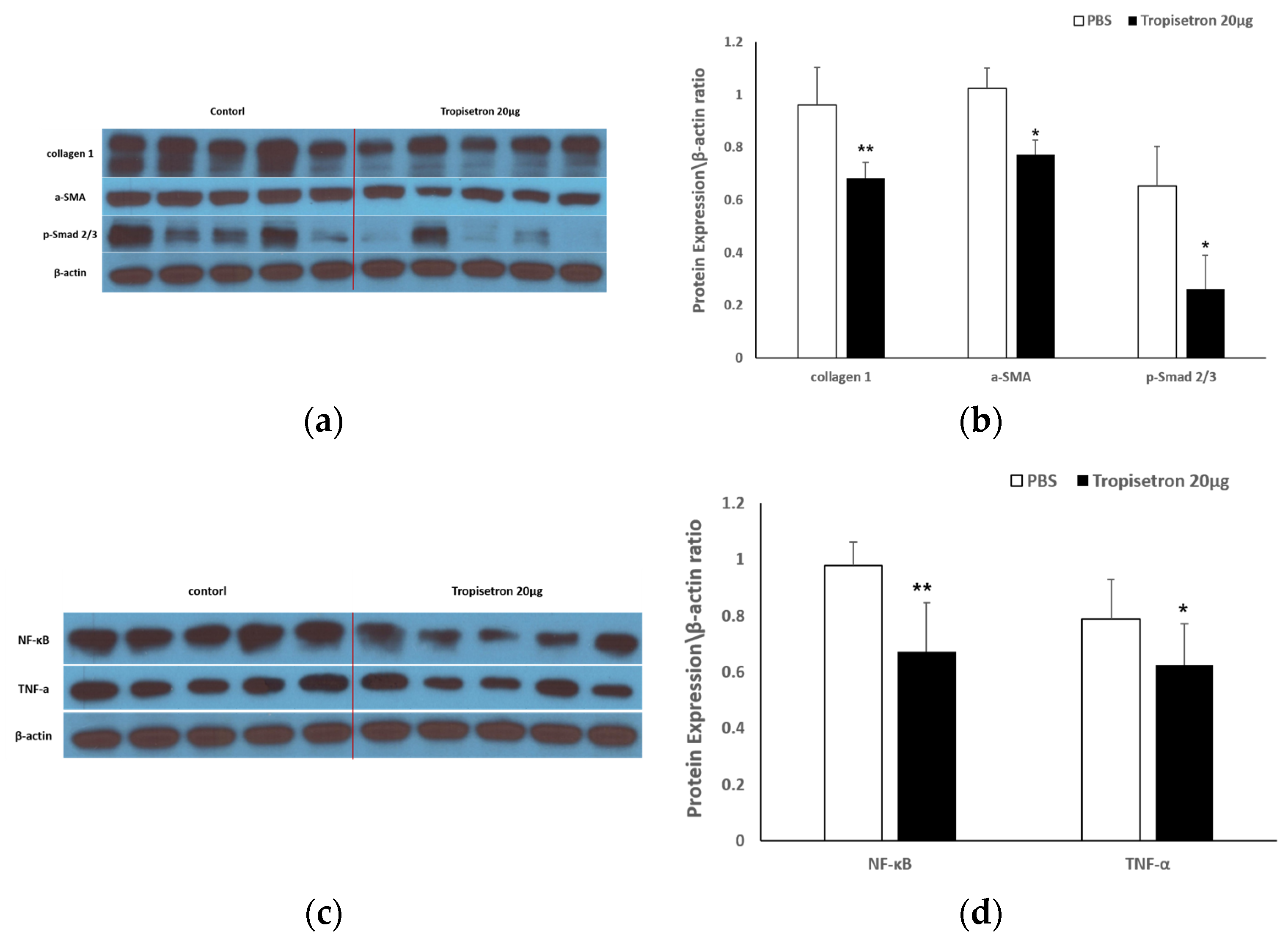
2.2.2. Tropisetron Reduces Fibrotic and Inflammatory Signaling in Incisional Wound Tissue
3. Discussion
4. Materials and Methods
4.1. In Vitro Studies
4.1.1. Cell Culture
4.1.2. α7nAChR Activation Assays
4.1.3. Immunofluorescence Assay
4.1.4. Cell Viability Assay
4.1.5. qRT-PCR
4.1.6. Western Blot Analysis
4.2. In Vivo Rat Incisional Scar Model
4.2.1. Histological Analysis
4.2.2. Immunohistochemistry for α7nAChR
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α7nAChR | Alpha-7 nicotinic acetylcholine receptor |
| ACTA2 | Alpha-smooth muscle actin gene |
| COL1A1 | Collagen type I alpha 1 chain gene |
| COL3A1 | Collagen type III alpha 1 chain gene |
| ECM | Extracellular matrix |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HDF | Human dermal fibroblast |
| KF | Keloid fibroblast |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| SMA | Smooth muscle actin |
| TGF-β | Transforming growth factor-beta |
| TNF-α | Tumor necrosis factor-alpha |
References
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Liu, Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int. 2006, 69, 213–217. [Google Scholar] [CrossRef]
- Ogawa, R. The Most Current Algorithms for the Treatment and Prevention of Hypertrophic Scars and Keloids: A 2020 Update of the Algorithms Published 10 Years Ago. Plast. Reconstr. Surg. 2022, 149, 79e–94e. [Google Scholar] [CrossRef]
- Al-Attar, A.; Mess, S.; Thomassen, J.M.; Kauffman, C.L.; Davison, S.P. Keloid pathogenesis and treatment. Plast. Reconstr. Surg. 2006, 117, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. The keloid disorder: Heterogeneity, histopathology, mechanisms and models. Front. Cell Dev. Biol. 2020, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.P.; Marttala, J.; Macarak, E.; Rosenbloom, J.; Uitto, J. Keloid pathogenesis: Potential role of cellular fibronectin with the EDA domain. J. Investig. Dermatol. 2015, 135, 1921–1924. [Google Scholar] [CrossRef]
- Tracey, K.J. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- de Jonge, W.J.; van der Zanden, E.P.; The, F.O.; Bijlsma, M.F.; van Westerloo, D.J.; Bennink, R.J.; Berthoud, H.R.; Uematsu, S.; Akira, S.; van den Wijngaard, R.M.; et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2–STAT3 signaling pathway. Nat. Immunol. 2005, 6, 844–851. [Google Scholar] [CrossRef]
- Stegemann, A.; Flis, D.; Ziolkowski, W.; Distler, J.H.W.; Steinbrink, K.; Böhm, M. The α7 nicotinic acetylcholine receptor: A promising target for the treatment of fibrotic skin disorders. J. Investig. Dermatol. 2020, 140, 2371–2379. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; Wang, H.; Abumrad, N.; Eaton, J.W.; Tracey, K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef]
- Stegemann, A.; Sindrilaru, A.; Eckes, B.; del Rey, A.; Heinick, A.; Schulte, J.S.; Müller, F.U.; Grando, S.A.; Fiebich, B.L.; Scharffetter-Kochanek, K.; et al. Tropisetron Suppresses Collagen Synthesis in Skin Fibroblasts via α7 Nicotinic Acetylcholine Receptor and Attenuates Fibrosis in a Scleroderma Mouse Model. Arthritis Rheum. 2013, 65, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Costantini, T.W.; Krzyzaniak, M.; Cheadle, G.A.; Putnam, J.G.; Hageny, A.-M.; Lopez, N.; Eliceiri, B.P.; Bansal, V.; Coimbra, R. Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: A cholinergic agonist prevents gut barrier failure after severe burn injury. Am. J. Pathol. 2012, 181, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Shytle, R.D.; Mori, T.; Townsend, K.; Vendrame, M.; Sun, N.; Zeng, J.; Ehrhart, J.; Silver, A.A.; Sanberg, P.R.; Tan, J. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J. Neurochem. 2004, 89, 337–343. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, K.; Hu, J.; Wang, X.; Feng, X.; Li, Y.; Ni, X.; Wen, S.; Wang, J. Activation of Nicotinic Acetylcholine α7 Receptor Attenuates Progression of Monocrotaline-Induced Pulmonary Hypertension in Rats by Downregulating the NLRP3 Inflammasome. Front. Pharmacol. 2019, 10, 128. [Google Scholar] [CrossRef]
- Sun, P.; Li, L.; Zhao, C.; Pan, M.; Qian, Z.; Su, X. Deficiency of α7 Nicotinic Acetylcholine Receptor Attenuates Bleomycin-Induced Lung Fibrosis in Mice. Mol. Med. 2017, 23, 34–49. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, W.; Zhang, R.; Yuan, Q.; Wu, R.; Liu, X.; Wuri, J.; Li, R.; Yan, T. Activation of Cholinergic Anti-Inflammatory Pathway Ameliorates Cerebral and Cardiac Dysfunction After Intracerebral Hemorrhage Through Autophagy. Front. Immunol 2022, 13, 870174. [Google Scholar] [CrossRef]
- Akcay, S.; Kumral, Z.N.O.; Cilingir-Kaya, O.T.; Eyüboglu, I.P.; Akkiprik, M.; Yegen, B.C.; Koc, M. Activation of the Alpha 7 Nicotinic Acetylcholine Receptor by GTS-21 Mitigates Contrast Nephropathy in a Rat Model. Kidney Blood Press. Res. 2024, 49, 646–656. [Google Scholar] [CrossRef]
- Rosas-Ballina, M.; Olofsson, P.S.; Ochani, M.; Valdés-Ferrer, S.I.; Levine, Y.A.; Reardon, C.; Tusche, M.W.; Pavlov, V.A.; Andersson, U.; Chavan, S.; et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011, 334, 98–101. [Google Scholar] [CrossRef]
- Papke, R.L.; Schiff, H.C.; Jack, B.A.; Horenstein, N.A. Molecular Dissection of Tropisetron, an Alpha7 Nicotinic Acetylcholine Receptor-Selective Partial Agonist. Neurosci. Lett. 2005, 378, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, A.; Böhm, M. The α7 Nicotinic Acetylcholine Receptor Agonist Tropisetron Counteracts Ultraviolet A-Mediated Oxidative Stress in Human Dermal Fibroblasts. Exp. Dermatol. 2020, 29, 836–844. [Google Scholar] [CrossRef]
- Callahan, P.M.; Bertrand, D.; Bertrand, S.; Plagenhoef, M.R.; Terry, A.V., Jr. Tropisetron Sensitizes α7-Containing Nicotinic Receptors to Low Levels of Acetylcholine in Vitro and Improves Memory-Related Task Performance in Young and Aged Animals. Neuropharmacology 2017, 112, 222–231. [Google Scholar] [CrossRef]
- Lee, H.J.; Jang, Y.J. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int. J. Mol. Sci. 2018, 19, 711. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.C.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Li, S.; Zhao, T.; Xin, H.; Ye, L.-H.; Zhang, X.; Tanaka, H.; Nakamura, A.; Kohama, K. Nicotinic acetylcholine receptor alpha7 subunit mediates migration of vascular smooth muscle cells toward nicotine. J. Pharmacol. Sci. 2004, 94, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Inaba, Y.; Watanabe, H.; Matsukawa, T.; Matsumoto, M.; Inoue, H. Nicotinic Alpha-7 Acetylcholine Receptor Deficiency Exacerbates Hepatic Inflammation and Fibrosis in a Mouse Model of Non-Alcoholic Steatohepatitis. J. Diabetes Investig. 2019, 10, 659–666. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Yu, T.-S.; Wang, T.; Liu, W.-W.; Zhao, R.; Zhang, S.-T.; Ma, W.-X.; Zheng, J.-L.; Guan, D.-W. Nicotinic acetylcholine receptor α7 subunit is time-dependently expressed in distinct cell types during skin wound healing in mice. Histochem. Cell Biol. 2011, 135, 375–387. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Zhang, S.-T.; Yu, L.-S.; Ye, G.-H.; Lin, K.-Z.; Wu, S.-Z.; Dong, M.-W.; Han, J.-G.; Feng, X.-P.; Li, X.-B. The time-dependent expression of α7 nicotinic acetylcholine receptor during skeletal muscle wound healing in rats. Int. J. Legal Med. 2014, 128, 779–786. [Google Scholar] [CrossRef]
- Messadi, D.V.; Doung, H.S.; Zhang, Q.; Kelly, A.P.; Tuan, T.L.; Reichenberger, E.; Le, A.D. Activation of NF-κB signal pathways in keloid fibroblasts. Arch. Dermatol. Res. 2004, 296, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, F.; Zhou, K.; Fang, L.; Wu, J.; Xia, Q.; Cen, Y.; Chen, J.; Qing, Y. Increased sensitivity to TNF-α promotes keloid fibroblast hyperproliferation by activating the NF-κB, JNK and p38 MAPK pathways. Exp. Ther. Med. 2021, 21, 502. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, H.; Cao, Q.; Guo, Z.; Ren, A.; Dai, Q. Stimulation of α7 nicotinic acetylcholine receptor attenuates nicotine induced upregulation of MMP, MCP 1, and RANTES through modulating ERK1/2/AP 1 signaling in RAW264.7 and MOVAS cells. Mediators Inflamm. 2017, 2017, 2401027. [Google Scholar] [CrossRef]
- Mei, Z.; Tian, X.; Chen, J.; Wang, Y.; Yao, Y.; Li, X.; Yang, C.; Zhang, S.; Xie, C. α7-nAChR agonist GTS-21 reduces radiation-induced lung injury. Oncol. Rep. 2018, 40, 2287–2297. [Google Scholar] [CrossRef] [PubMed]
- Shlepova, O.V.; Parfenova, A.A.; Andreeva, N.V.; Shulepko, M.A.; Dolgikh, D.A.; Tsetlin, V.I.; Kirpichnikov, M.P. Peptide Mimicking Loop II of the Human Epithelial Protein SLURP-2 Enhances the Viability and Migration of Skin Keratinocytes. Acta Naturae 2024, 16, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, L.; Shlepova, O.V.; Dolgikh, D.A.; Kirpichnikov, M.P.; Tsetlin, V.I. Human Epithelial Protein SLURP-2 as a Prototype of Drugs for Wound Healing. Russ. J. Bioorg. Chem. 2024, 50, 696–705. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, H.; Kim, Y.H.; Heo, K.J.; Hong, J.W.; Lee, W.J. Antifibrotic Effects of an α7 Nicotinic Acetylcholine Receptor Agonist in Keloid Fibroblasts and a Rat Scar Model. Int. J. Mol. Sci. 2025, 26, 8868. https://doi.org/10.3390/ijms26188868
Roh H, Kim YH, Heo KJ, Hong JW, Lee WJ. Antifibrotic Effects of an α7 Nicotinic Acetylcholine Receptor Agonist in Keloid Fibroblasts and a Rat Scar Model. International Journal of Molecular Sciences. 2025; 26(18):8868. https://doi.org/10.3390/ijms26188868
Chicago/Turabian StyleRoh, Hyun, Yo Han Kim, Kyung Jun Heo, Jong Won Hong, and Won Jai Lee. 2025. "Antifibrotic Effects of an α7 Nicotinic Acetylcholine Receptor Agonist in Keloid Fibroblasts and a Rat Scar Model" International Journal of Molecular Sciences 26, no. 18: 8868. https://doi.org/10.3390/ijms26188868
APA StyleRoh, H., Kim, Y. H., Heo, K. J., Hong, J. W., & Lee, W. J. (2025). Antifibrotic Effects of an α7 Nicotinic Acetylcholine Receptor Agonist in Keloid Fibroblasts and a Rat Scar Model. International Journal of Molecular Sciences, 26(18), 8868. https://doi.org/10.3390/ijms26188868





