Cannabis sativa Root Extract Exerts Anti-Nociceptive and Anti-Inflammatory Effects via Endocannabinoid Pathway Modulation In Vivo and In Vitro
Abstract
1. Introduction
2. Results
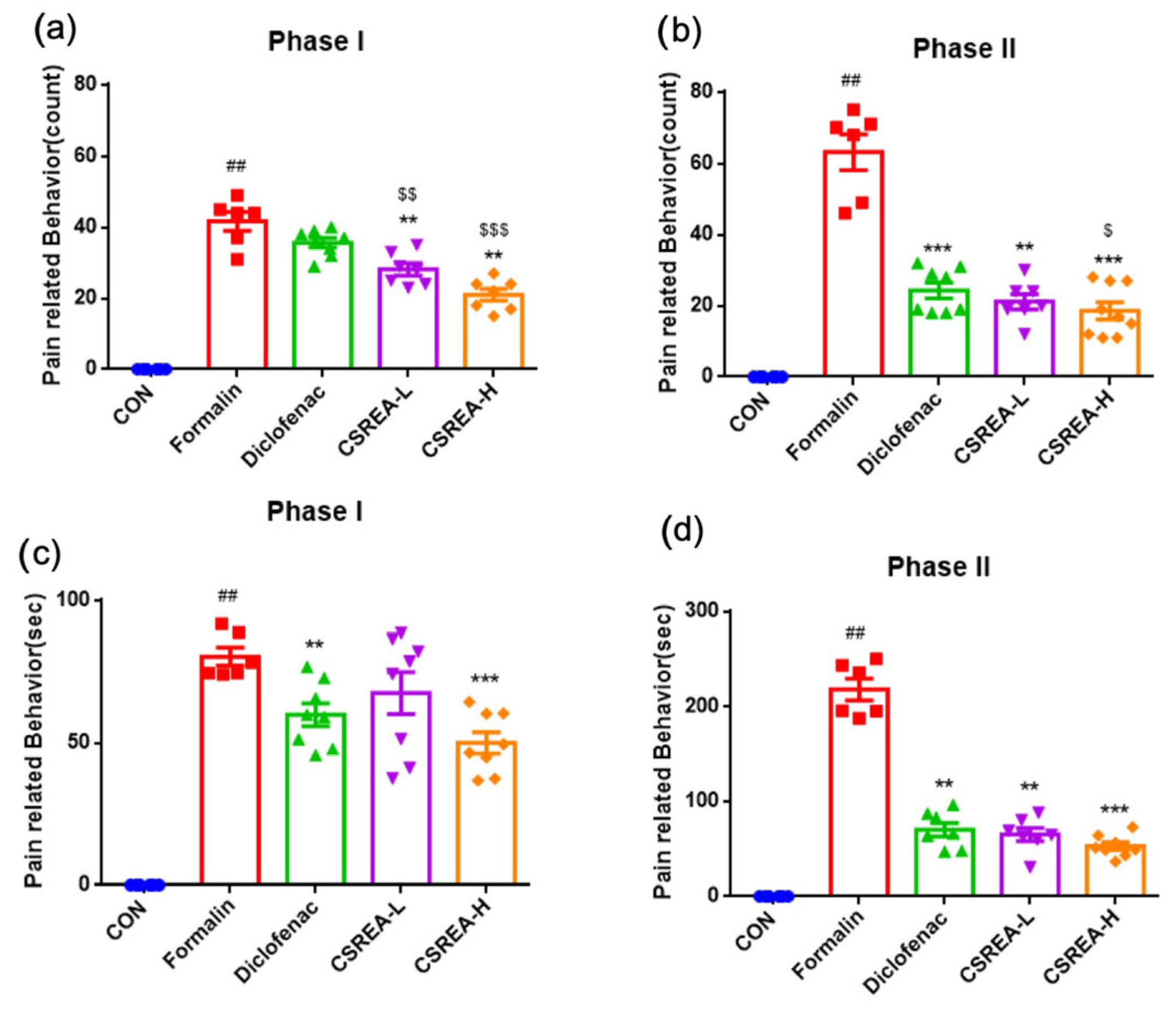
2.1. Effect of CSREA on the Formalin Behavioral Test in Mouse Model
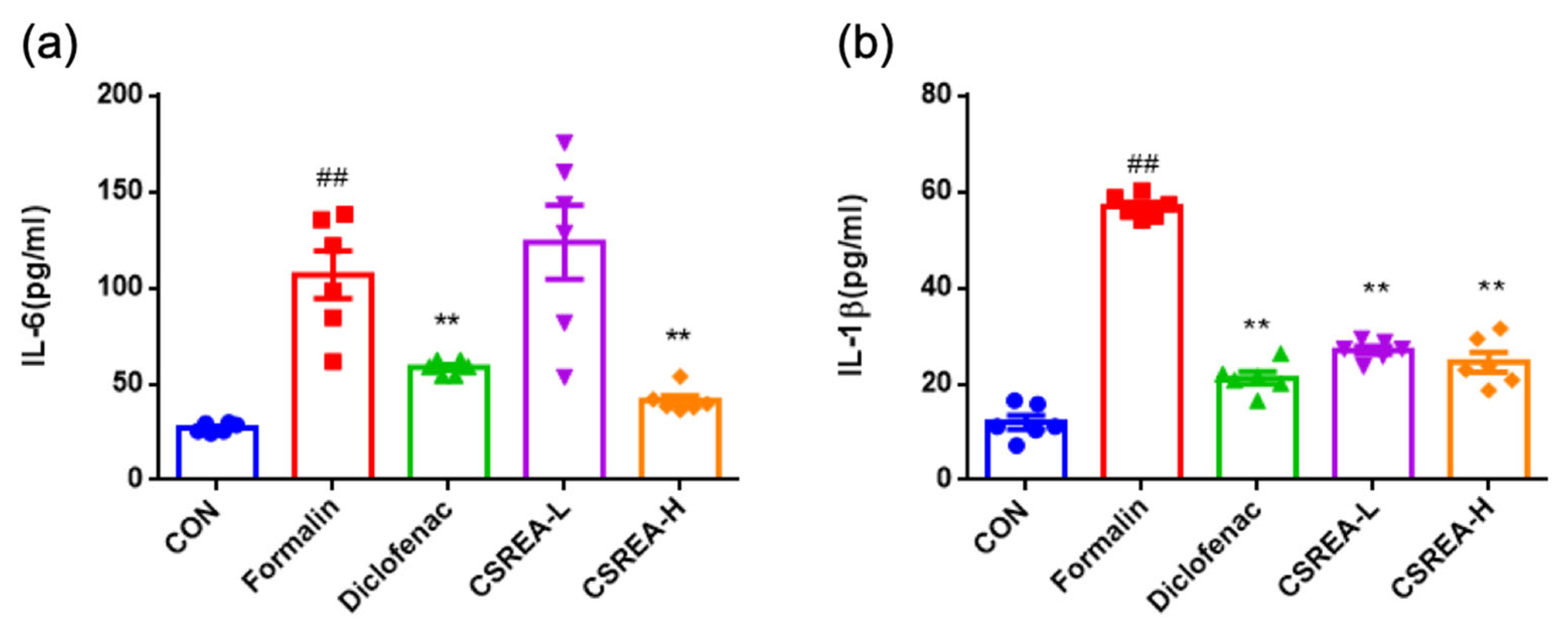
2.2. Anti-Inflammatory Effects of CSREA in Formalin-Induced Mouse Serum
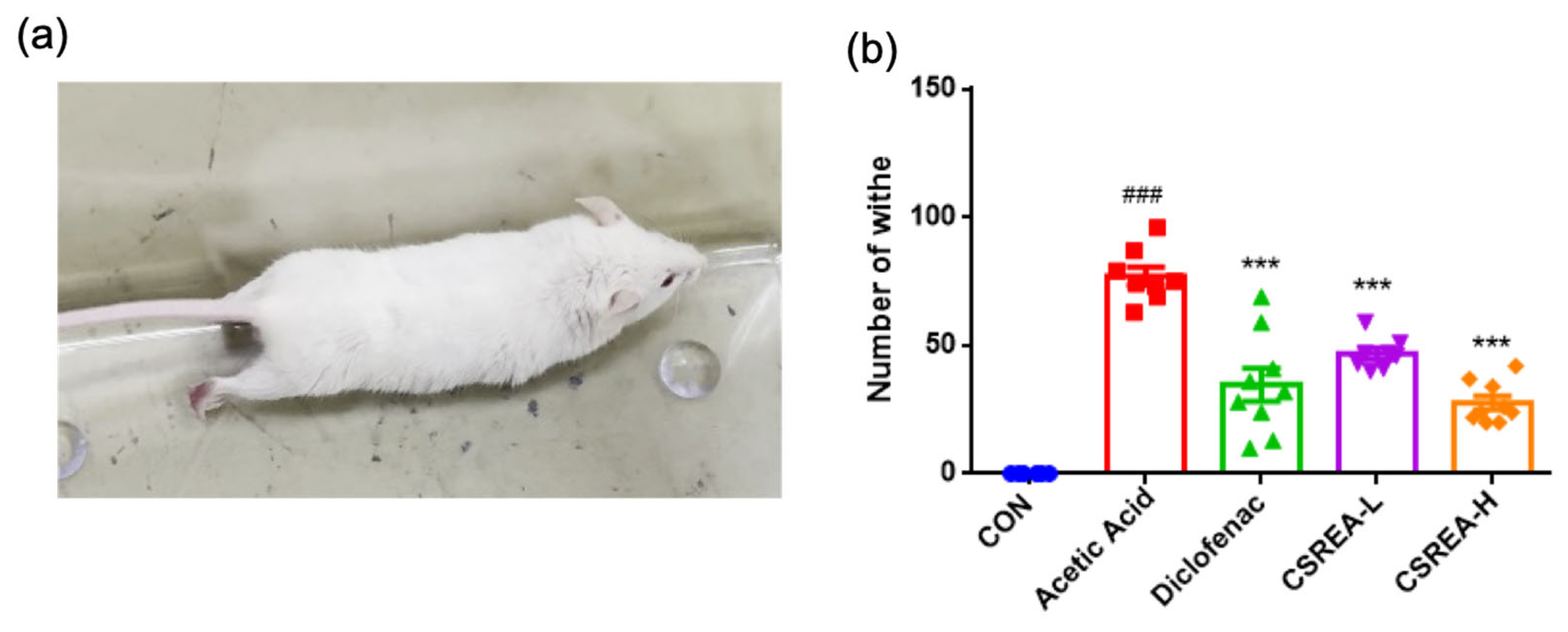
2.3. Effect of CSREA on the Acetic Acid-Induced Writhing Test in a Mouse Model
2.4. Effect of CSREA on Survival in Mice Following Acetic Acid-Induced Model
2.5. Effects of CSREA on Carrageenan-Induced Inflammation in Rat Model
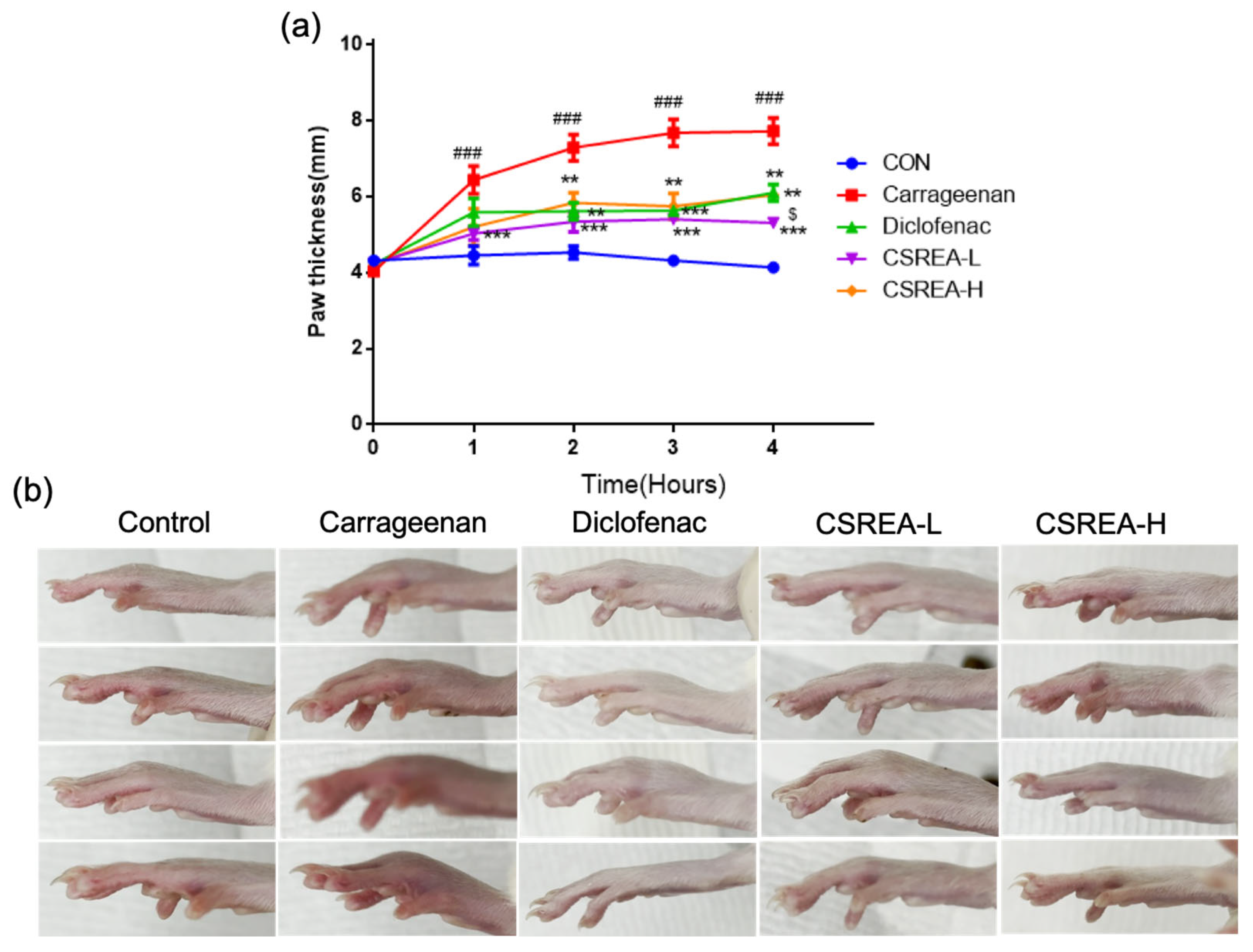
2.6. Effects of CSREA on Carrageenan-Induced Hind Paw Edema in a Rat Model
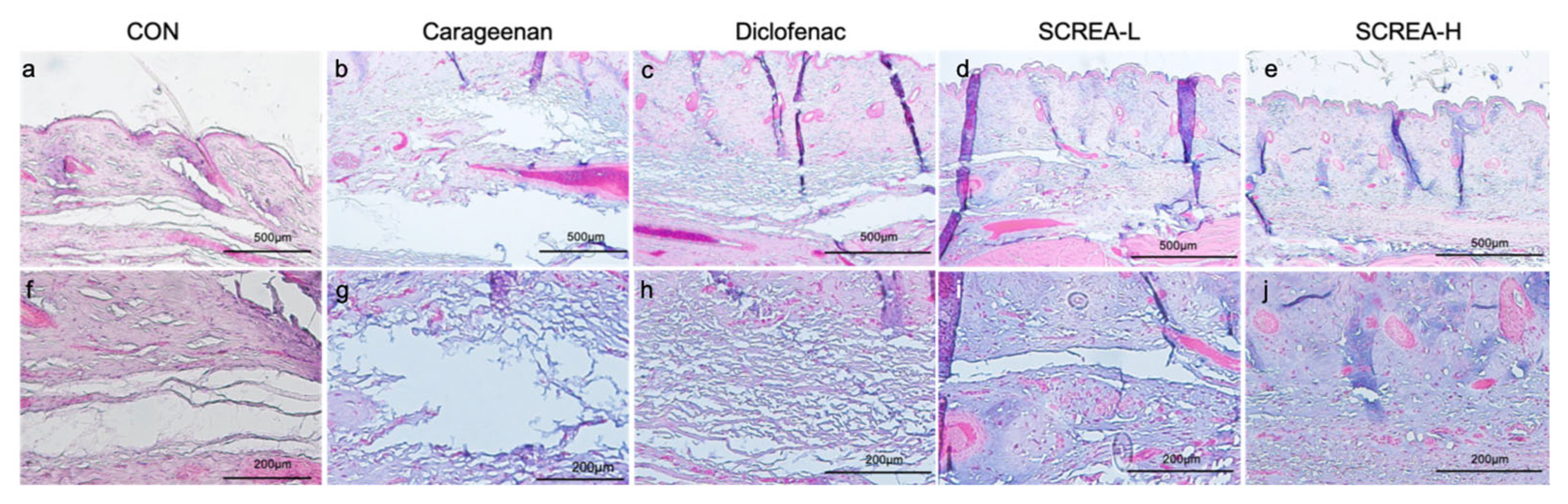
2.7. Anti-Inflammatory Effects of CSREA in a Carrageenan-Induced Inflammation Model in Rats
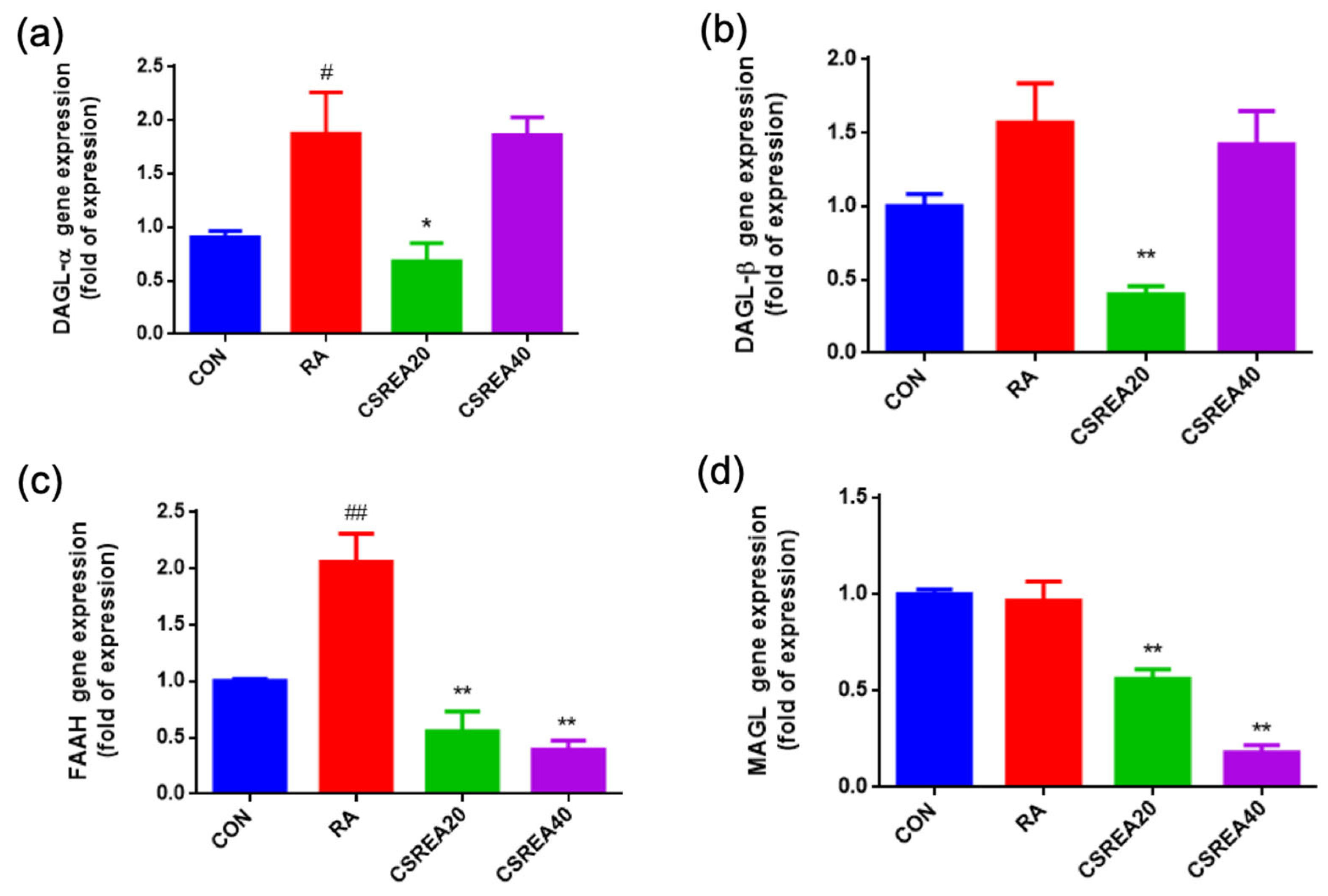
2.8. CSREA Regulates the Expression of Endocannabinoid-Related Genes in RA-Differentiated SH-SY5Y Cells
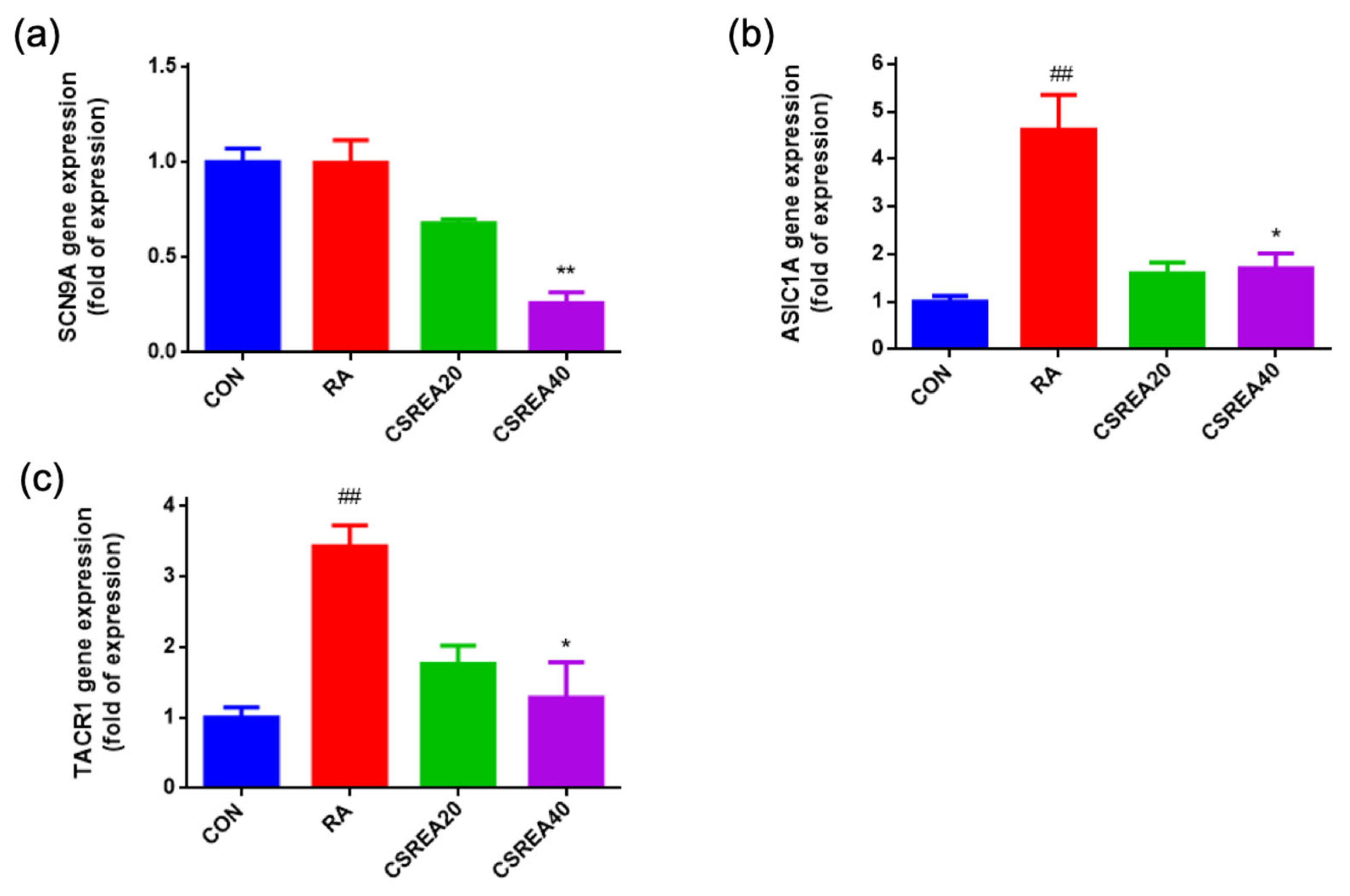
2.9. CSREA Decreased Expression Level of Pain-Related Genes in SH-SY5Y Cells
2.10. CSREA Regulates Endocannabinoid Receptors in SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Animal
4.2. Extraction of CSREA from Cannabis sativa L. Root
4.3. Formalin Test
4.4. Writhing Test
4.5. Evaluation of Carrageenan-Induced Hind Paw Edema
4.6. Biochemical Analysis of Serum and Tissue Collection
4.7. Cell Culture and Neuronal Differentiation
4.8. Gene Expression Analysis
4.9. Histological Evaluation of Edema in Rat Paw Tissue
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSREA | Cannabis sativa root ethyl acetate fraction |
| CR | Cannabis root |
| CBD | Cannabidiol |
| THC | Tetrahydrocannabinol |
| CBC | Cannabichromene |
| CB1R | Cannabinoid receptor1 |
| CB2R | Cannabinoid receptor2 |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Interleukin-6 |
| 2-AG | 2-arachidonoylglycerol |
| MAGL | Monoacylglycerol lipase |
| FAAH | Fatty acid amide hydrolase |
| DAGL-α | Diacylglycerol lipase alpha |
| DAGL-β | Diacylglycerol lipase beta |
| ASIC1A | Acid-sensing ion channel 1a |
| SCN9A | Sodium voltage-gated channel alpha subunit 9 |
| TACR1 | Tachykinin receptor1 |
| PPIA | Peptidylprolyl isomerase A |
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986, 3 (Suppl. S1), 226.
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef] [PubMed]
- El-Tallawy, S.N.; Nalamasu, R.; Salem, G.I.; LeQuang, J.A.K.; Pergolizzi, J.V.; Christo, P.J. Management of musculoskeletal pain: An update with emphasis on chronic musculoskeletal pain. Pain Ther. 2021, 10, 181–209. [Google Scholar] [CrossRef]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.M.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11, S105. [Google Scholar] [CrossRef]
- Jahromi, B.; Pirvulescu, I.; Candido, K.D.; Knezevic, N.N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics 2021, 13, 251. [Google Scholar] [CrossRef]
- On the Preparations of the Indian Hemp, or Gunjah (Cannabis Indica), Their Effects on the Animal System in Health, and Their Utility in the Treatment of Tetanus and Other Convulsive Diseases. Br. Foreign Med. Rev. 1840, 10, 225–228.
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. PKS activities and biosynthesis of cannabinoids and flavonoids in Cannabis sativa L. plants. Plant Cell Physiol. 2008, 49, 1767–1782. [Google Scholar] [CrossRef]
- Agarwal, N.; Pacher, P.; Tegeder, I.; Amaya, F.; Constantin, C.E.; Brenner, G.J.; Rubino, T.; Michalski, C.W.; Marsicano, G.; Monory, K.; et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat. Neurosci. 2007, 10, 870–879. [Google Scholar] [CrossRef]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R43. [Google Scholar] [CrossRef]
- Steffens, S.; Pacher, P. Targeting cannabinoid receptor CB(2) in cardiovascular disorders: Promises and controversies. Br. J. Pharmacol. 2012, 167, 313–323. [Google Scholar] [CrossRef]
- Jouanjus, E.; Lapeyre-Mestre, M.; Micallef, J.; French Association of the Regional, A.; Dependence Monitoring Centres Working Group on Cannabis, C. Cannabis use: Signal of increasing risk of serious cardiovascular disorders. J. Am. Heart Assoc. 2014, 3, e000638. [Google Scholar] [CrossRef]
- Ahmed, W.; Katz, S. Therapeutic Use of Cannabis in Inflammatory Bowel Disease. Gastroenterol Hepatol 2016, 12, 668–679. [Google Scholar]
- Nguyen, L.C.; Yang, D.; Nicolaescu, V.; Best, T.J.; Gula, H.; Saxena, D.; Gabbard, J.D.; Chen, S.N.; Ohtsuki, T.; Friesen, J.B.; et al. Cannabidiol inhibits SARS-CoV-2 replication through induction of the host ER stress and innate immune responses. Sci. Adv. 2022, 8, eabi6110. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Urits, I.; Orhurhu, V.; Peck, J.; Orhurhu, M.S.; Giacomazzi, S.; Smoots, D.; Piermarini, C.; Manchikanti, L.; Kaye, A.D. The role of the cannabinoid system in pain control: Basic and clinical implications. Curr. Pain Headache Rep. 2020, 24, 35. [Google Scholar] [CrossRef]
- Elmes, S.J.R.; Winyard, L.A.; Medhurst, S.J.; Clayton, N.M.; Wilson, A.W.; Kendall, D.A.; Chapman, V. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain 2005, 118, 327–335. [Google Scholar] [CrossRef]
- Fine, P.G.; Rosenfeld, M.J. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef]
- Xin, Q.; Xu, F.; Taylor, D.H.; Zhao, J.F.; Wu, J. The impact of cannabinoid type 2 receptors (CB2Rs) in neuroprotection against neurological disorders. Acta Pharmacol. Sin. 2020, 41, 1507–1518. [Google Scholar] [CrossRef]
- Nackley, A.G.; Makriyannis, A.; Hohmann, A.G. Selective activation of cannabinoid CB(2) receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience 2003, 119, 747–757. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2009, 153, 319–334. [Google Scholar] [CrossRef]
- Xiong, W.; Cui, T.; Cheng, K.; Yang, F.; Chen, S.R.; Willenbring, D.; Guan, Y.; Pan, H.L.; Ren, K.; Xu, Y.; et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J. Exp. Med. 2012, 209, 1121–1134. [Google Scholar] [CrossRef]
- Kasatkina, L.A.; Rittchen, S.; Sturm, E.M. Neuroprotective and immunomodulatory action of the endocannabinoid system under neuroinflammation. Int. J. Mol. Sci. 2021, 22, 5431. [Google Scholar] [CrossRef] [PubMed]
- Gagne, V.; Merindol, N.; Boucher, R.; Boucher, N.; Desgagne-Penix, I. Rooted in therapeutics: Comprehensive analyses of Cannabis sativa root extracts reveals potent antioxidant, anti-inflammatory, and bactericidal properties. Front. Pharmacol. 2024, 15, 1465136. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, H.; Xu, J.; Zhou, H.; Seeram, N.P.; Ma, H.; Gu, Q. Chemical constituents of industrial hemp roots and their anti-inflammatory activities. J. Cannabis Res. 2023, 5, 1. [Google Scholar] [CrossRef]
- Menezes, P.M.N.; Araujo, T.C.L.; Pereira, E.C.V.; Neto, J.A.; Silva, D.S.; Brito, M.C.; Lima, K.S.B.; Monte, A.; Matos, M.H.T.; Lima, R.S.; et al. Investigation of antinociceptive, antipyretic, antiasthmatic, and spasmolytic activities of Brazilian Cannabis sativa L. roots in rodents. J. Ethnopharmacol. 2021, 278, 114259. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef]
- Dubuisson, D.; Dennis, S.G. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 1977, 4, 161–174. [Google Scholar] [CrossRef]
- Gawade, S.P. Acetic acid induced painful endogenous infliction in writhing test on mice. J. Pharmacol. Pharmacother. 2012, 3, 348. [Google Scholar] [CrossRef]
- Gupta, A.K.; Parasar, D.; Sagar, A.; Choudhary, V.; Chopra, B.S.; Garg, R.; Ashish; Khatri, N. Analgesic and Anti-Inflammatory Properties of Gelsolin in Acetic Acid Induced Writhing, Tail Immersion and Carrageenan Induced Paw Edema in Mice. PLoS ONE 2015, 10, e0135558. [Google Scholar] [CrossRef]
- Lima, K.S.B.; Silva, M.; Araujo, T.C.L.; Silva, C.; Santos, B.L.; Ribeiro, L.A.A.; Menezes, P.M.N.; Silva, M.G.; Lavor, E.M.; Silva, F.S.; et al. Cannabis roots: Pharmacological and toxicological studies in mice. J. Ethnopharmacol. 2021, 271, 113868. [Google Scholar] [CrossRef]
- Henson, J.D.; Vitetta, L.; Hall, S. Tetrahydrocannabinol and cannabidiol medicines for chronic pain and mental health conditions. Inflammopharmacology 2022, 30, 1167–1178. [Google Scholar] [CrossRef]
- Safi, K.; Sobieraj, J.; Błaszkiewicz, M.; Żyła, J.; Salata, B.; Dzierżanowski, T. Tetrahydrocannabinol and Cannabidiol for Pain Treatment—An Update on the Evidence. Biomedicines 2024, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Gardner, E.L. Addictive potential of cannabinoids: The underlying neurobiology. Chem. Phys. Lipids 2002, 121, 267–290. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, D.J.; Doorenbos, N.J.; Harris, L.S.; Masoud, A.N.; Quimby, M.W.; Schiff, P.L., Jr. Chemical constituents of Cannabis sativa L. root. J. Pharm. Sci. 1971, 60, 1891–1892. [Google Scholar] [CrossRef]
- Guan, H.; Yan, T.; Wu, D.; Tripathi, A.S. Epifriedelinol ameliorates the neuropathic pain and recovers the function in spinal cord injury by downregulation of neuronal apoptosis and NMDA receptor. Tohoku J. Exp. Med. 2022, 258, 143–148. [Google Scholar] [CrossRef]
- Erami, E.; Azhdari-Zarmehri, H.; Imoto, K.; Furue, H. Characterization of nociceptive behaviors induced by formalin in the glabrous and hairy skin of rats. Basic Clin. Neurosci. 2017, 8, 37. [Google Scholar] [CrossRef]
- Lau, B.K.; Vaughan, C.W. Descending modulation of pain: The GABA disinhibition hypothesis of analgesia. Curr. Opin. Neurobiol. 2014, 29, 159–164. [Google Scholar] [CrossRef]
- Baggelaar, M.P.; Maccarrone, M.; van der Stelt, M. 2-Arachidonoylglycerol: A signaling lipid with manifold actions in the brain. Prog. Lipid Res. 2018, 71, 1–17. [Google Scholar] [CrossRef]
- Bari, M.; Battista, N.; Fezza, F.; Gasperi, V.; Maccarrone, M. New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev. Med. Chem. 2006, 6, 257–268. [Google Scholar] [CrossRef]
- Harding, E.K.; Souza, I.A.; Gandini, M.A.; Gadotti, V.M.; Ali, M.Y.; Huang, S.; Antunes, F.T.; Trang, T.; Zamponi, G.W. Differential regulation of Cav3. 2 and Cav2. 2 calcium channels by CB1 receptors and cannabidiol. Br. J. Pharmacol. 2023, 180, 1616–1633. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Verri, W.A.; Chiu, I.M. Nociceptor Sensory Neuron–Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Beissner, F.; Brandau, A.; Henke, C.; Felden, L.; Baumgartner, U.; Treede, R.D.; Oertel, B.G.; Lotsch, J. Quick discrimination of A(delta) and C fiber mediated pain based on three verbal descriptors. PLoS ONE 2010, 5, e12944. [Google Scholar] [CrossRef]
- Xu, Q.; Yaksh, T.L. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr. Opin. Anaesthesiol. 2011, 24, 400–407. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. The endocannabinoid system and pain. CNS Neurol. Disord. Drug Targets 2009, 8, 403–421. [Google Scholar] [CrossRef]
- Martin, W.J.; Loo, C.M.; Basbaum, A.I. Spinal cannabinoids are anti-allodynic in rats with persistent inflammation. Pain 1999, 82, 199–205. [Google Scholar] [CrossRef]
- Kelly, S.; Chapman, V. Selective cannabinoid CB1 receptor activation inhibits spinal nociceptive transmission in vivo. J. Neurophysiol. 2001, 86, 3061–3064. [Google Scholar] [CrossRef]
- Elmes, S.J.; Jhaveri, M.D.; Smart, D.; Kendall, D.A.; Chapman, V. Cannabinoid CB2 receptor activation inhibits mechanically evoked responses of wide dynamic range dorsal horn neurons in naive rats and in rat models of inflammatory and neuropathic pain. Eur. J. Neurosci. 2004, 20, 2311–2320. [Google Scholar] [CrossRef]
- Elhendawy, M.A.; Wanas, A.S.; Radwan, M.M.; Azzaz, N.A.; Toson, E.S.; ElSohly, M.A. Chemical and Biological Studies of Cannabis sativa Roots. Med. Cannabis Cannabinoids 2019, 1, 104–111. [Google Scholar] [CrossRef]
- Chandler, R.F.; Hooper, S.N. Friedelin and associated triterpenoids. Phytochemistry 1979, 18, 711–724. [Google Scholar] [CrossRef]
- Cherry, A.L.; Wheeler, M.J.; Mathisova, K.; Di Miceli, M. In silico analyses of the involvement of GPR55, CB1R and TRPV1: Response to THC, contribution to temporal lobe epilepsy, structural modeling and updated evolution. Front. Neuroinform. 2024, 18, 1294939. [Google Scholar] [CrossRef] [PubMed]
- Ricardi, C.; Barachini, S.; Consoli, G.; Marazziti, D.; Polini, B.; Chiellini, G. Beta-caryophyllene, a cannabinoid receptor type 2 selective agonist, in emotional and cognitive disorders. Int. J. Mol. Sci. 2024, 25, 3203. [Google Scholar] [CrossRef] [PubMed]
- Topuz, R.D.; Gunduz, O.; Karadag, C.H.; Ulugol, A. Non-opioid Analgesics and the Endocannabinoid System. Balk. Med. J. 2020, 37, 309–315. [Google Scholar] [CrossRef]
- Anthony, A.T.; Rahmat, S.; Sangle, P.; Sandhu, O.; Khan, S. Cannabinoid Receptors and Their Relationship with Chronic Pain: A Narrative Review. Cureus 2020, 12, e10436. [Google Scholar] [CrossRef]
- Manzanares, J.; Julian, M.D.; Carrascosa, A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr. Neuropharmacol. 2006, 4, 239–257. [Google Scholar] [CrossRef]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, T.; Meng, F.; Jiang, M.; Wu, S.; Xu, H. The endocannabinoid system in the brain undergoes long-lasting changes following neuropathic pain. iScience 2024, 27, 111409. [Google Scholar] [CrossRef]
- Lau, B.K.; Vaughan, C.W. Targeting the endogenous cannabinoid system to treat neuropathic pain. Front. Pharmacol. 2014, 5, 28. [Google Scholar] [CrossRef]
- Chen, C. Inhibiting degradation of 2-arachidonoylglycerol as a therapeutic strategy for neurodegenerative diseases. Pharmacol. Ther. 2023, 244, 108394. [Google Scholar] [CrossRef]
- Long, J.Z.; Nomura, D.K.; Vann, R.E.; Walentiny, D.M.; Booker, L.; Jin, X.; Burston, J.J.; Sim-Selley, L.J.; Lichtman, A.H.; Wiley, J.L.; et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 20270–20275. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Francavilla, M.; Zanaboni, A.M.; Tassorelli, C. Dual Inhibition of FAAH and MAGL Counteracts Migraine-like Pain and Behavior in an Animal Model of Migraine. Cells 2021, 10, 2543. [Google Scholar] [CrossRef]
- Kinsey, S.G.; Long, J.Z.; O’Neal, S.T.; Abdullah, R.A.; Poklis, J.L.; Boger, D.L.; Cravatt, B.F.; Lichtman, A.H. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J. Pharmacol. Exp. Ther. 2009, 330, 902–910. [Google Scholar] [CrossRef]
- Della Pietra, A.; Krivoshein, G.; Ivanov, K.; Giniatullina, R.; Jyrkkanen, H.K.; Leinonen, V.; Lehtonen, M.; van den Maagdenberg, A.; Savinainen, J.; Giniatullin, R. Potent dual MAGL/FAAH inhibitor AKU-005 engages endocannabinoids to diminish meningeal nociception implicated in migraine pain. J. Headache Pain 2023, 24, 38. [Google Scholar] [CrossRef] [PubMed]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.W.; Kim, Y.; Kang, J.H.; Kang, S.S.; Ahn, Y.K.; Park, C.S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Chiurchiu, V.; Diaz-Alonso, J.; Bari, M.; Guzman, M.; Maccarrone, M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 2013, 52, 633–650. [Google Scholar] [CrossRef]
- Voscopoulos, C.; Lema, M. When does acute pain become chronic? Br. J. Anaesth. 2010, 105 (Suppl. S1), i69–i85. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, P.; Jha, K.K.; Mishra, G.; Srivastava, S.; Khosa, R.L. Antiinflammatory, Analgesic and Antipyretic Activities of Aerial Parts of Costus speciosus Koen. Indian J. Pharm. Sci. 2013, 75, 83–88. [Google Scholar] [CrossRef]
- Radi, M.H.; El-Shiekh, R.A.; El-Halawany, A.M.; Abdel-Sattar, E. Friedelin and 3β-friedelinol: Pharmacological activities. Rev. Bras. De Farmacogn. 2023, 33, 886–900. [Google Scholar] [CrossRef]
- Kobtrakul, K.; Rani, D.; Binalee, A.; Udomlarp, P.; Srichai, T.; De-Eknamkul, W.; Vimolmangkang, S. Elicitation enhances the production of friedelin and epifriedelanol in hairy root cultures of Cannabis sativa L. Front. Plant Sci. 2023, 14, 1242584. [Google Scholar]
- Pina, L.T.; Serafini, M.R.; Oliveira, M.A.; Sampaio, L.A.; Guimarães, J.O.; Guimarães, A.G. Carvone and its pharmacological activities: A systematic review. Phytochemistry 2022, 196, 113080. [Google Scholar] [CrossRef]
- Porto, C.; Stüker, C.Z.; Mallmann, A.S.; Simionatto, E.; Flach, A.; Canto-Dorow, T.D.; da Silva, U.F.; Dalcol, I.I.; Morel, A.F. (R)-(-)-carvone and (1R, 4R)-trans-(+)-dihydrocarvone from poiretia latifolia vogel. J. Braz. Chem. Soc. 2010, 21, 782–786. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The Story of Beta-sitosterol—A Review. Eur. J. Med. Plants 2014, 4, 590–609. [Google Scholar] [CrossRef]
- Nazir, S.; Ahmad, I.; Mobashar, A.; Sharif, A.; Shabbir, A.; Chaudhary, W.A. Mechanistic evaluation of antiarthritic and anti-inflammatory effect of campesterol ester derivatives in complete Freund’s adjuvant-induced arthritic rats. Front. Pharmacol. 2024, 14, 1346054. [Google Scholar] [CrossRef] [PubMed]
- de Morais Oliveira-Tintino, C.D.; da Silva, F.E.F.; Santiago, G.M.P.; Pinto, F.D.C.L.; Pessoa, O.D.L.; da Fonseca, A.M.; Paulo, C.L.R.; Dos Santos, H.S.; Marinho, M.M.; Dos Santos, J.L.; et al. Molecular docking and antibacterial activity of campesterol derivatives against Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa multiresistant strains. Chem. Biodivers. 2025, 22, e202401073. [Google Scholar] [CrossRef]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.-H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health benefits and pharmacological properties of stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef]



| Group | Number of Survived Subjects/Total Subjects | Survival Rate |
|---|---|---|
| Control | 9/9 | 100% |
| Acetic acid | 0/9 | 0% |
| Diclofenac | 4/9 | 44.4% |
| CSREA-L | 7/9 | 77.7% |
| CSREA-H | 9/9 | 100% |
| Gene | Acceesion No. | Primer Forward Sequence(5′-3′) | Primer Reverse Sequence(5′-3′) |
| PPIA | NM_021130.5 | GCCGAGGAAAACCGTGTACT | TGTCTGCAAACAGCTCAAAGGA |
| FAAH | NM_001441.3 | TCAGCTTTCCTCAGCAACAT | CCGCAGACACAACTCTTCTT |
| DAGLα | NM_006133.3 | CTTCCTCTTTCTCCTGCATACC | TGGCTTGACCCTCCTCTAA |
| DAGLβ | NM_139179.4 | TCAGCATGAGAGGAACGATTT | GGCTCTTGGTTGTTCCTGATA |
| MAGL | NM_00138315.1 | CTCATTTCGCCTCTGGTTCT | GAAGACGGAGTTGGTGACTT |
| SCN9A | NM_002977.4 | GTCTCCCTGGTTGATGGACG | TGATTGGTCGTGCCCTCTGG |
| TACR1 | NM_001058.4 | ATGACAGGTTCCGTCTGGGC | TACACACTGCCCTGGGTCTG |
| ASIC1α | NM_001412756.1 | AGCGGCTGTCTCTGAAGC | AGCTCCCCAGCATGATACAG |
| CNR1 | NM_001370547.1 | CGTCGTTCAAGGAGAATGAGG | TGCCGATGAAGTGGTAGGAAG |
| CNR2 | NM_001841.3 | CGTGGCTGTGCTCTATCTGA | AGCCAGCTCAGCAGGTAGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.-Y.; Jin, H.-L.; Yu, G.-R.; Lim, D.-W.; Park, W.-H. Cannabis sativa Root Extract Exerts Anti-Nociceptive and Anti-Inflammatory Effects via Endocannabinoid Pathway Modulation In Vivo and In Vitro. Int. J. Mol. Sci. 2025, 26, 8863. https://doi.org/10.3390/ijms26188863
Jang S-Y, Jin H-L, Yu G-R, Lim D-W, Park W-H. Cannabis sativa Root Extract Exerts Anti-Nociceptive and Anti-Inflammatory Effects via Endocannabinoid Pathway Modulation In Vivo and In Vitro. International Journal of Molecular Sciences. 2025; 26(18):8863. https://doi.org/10.3390/ijms26188863
Chicago/Turabian StyleJang, Seo-Yul, Hye-Lin Jin, Ga-Ram Yu, Dong-Woo Lim, and Won-Hwan Park. 2025. "Cannabis sativa Root Extract Exerts Anti-Nociceptive and Anti-Inflammatory Effects via Endocannabinoid Pathway Modulation In Vivo and In Vitro" International Journal of Molecular Sciences 26, no. 18: 8863. https://doi.org/10.3390/ijms26188863
APA StyleJang, S.-Y., Jin, H.-L., Yu, G.-R., Lim, D.-W., & Park, W.-H. (2025). Cannabis sativa Root Extract Exerts Anti-Nociceptive and Anti-Inflammatory Effects via Endocannabinoid Pathway Modulation In Vivo and In Vitro. International Journal of Molecular Sciences, 26(18), 8863. https://doi.org/10.3390/ijms26188863






