Assessment of the Behavioral and Neurochemical Characteristics in a Mice Model of the Premotor Stage of Parkinson’s Disease Induced by Chronic Administration of a Low Dose of MPTP
Abstract
1. Introduction
2. Results
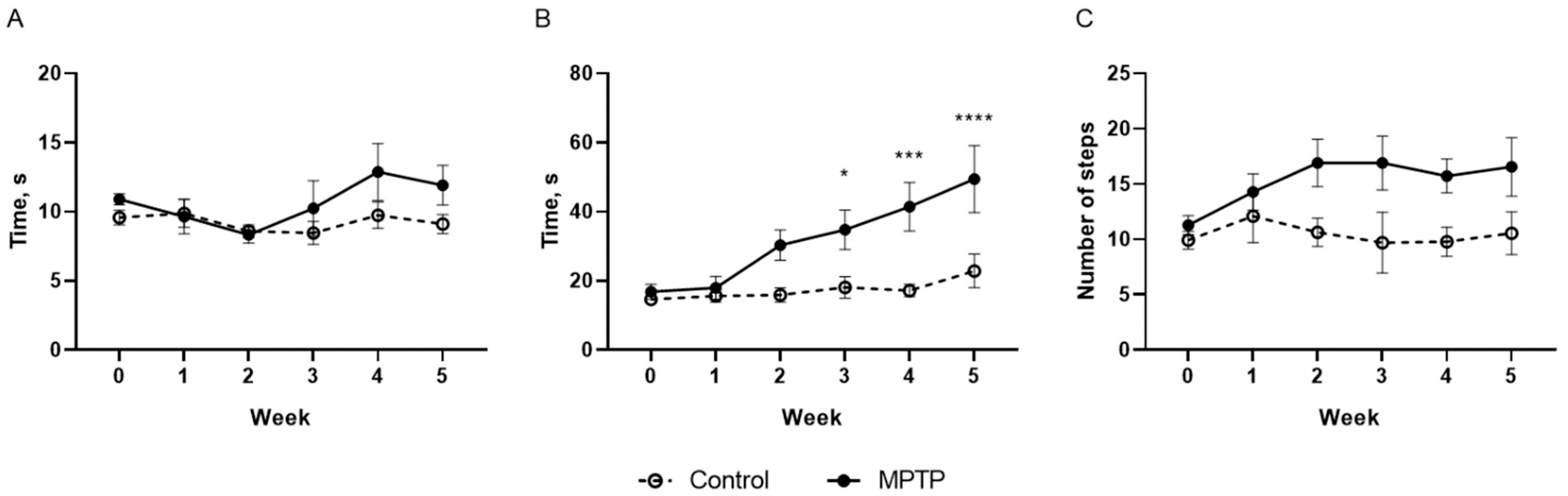
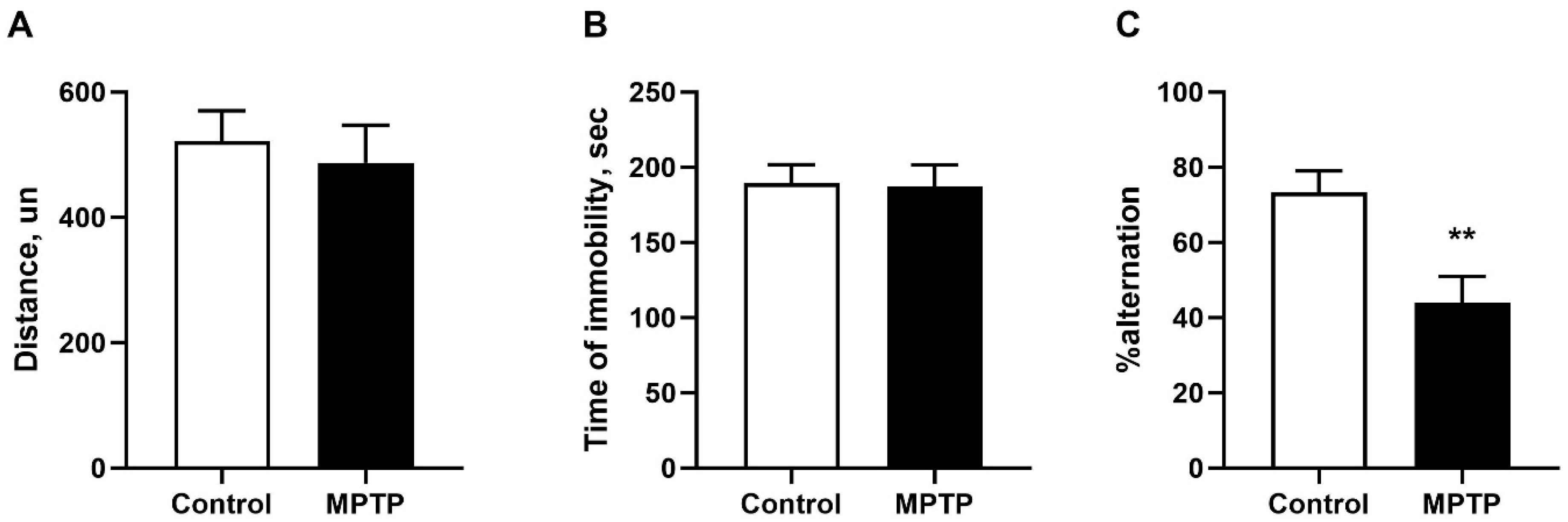
2.1. Evaluation of Motor and Non-Motor Deficits
2.2. The Content of Dopamine and Its Metabolites in Mice’s Striatum and Prefrontal Cortex
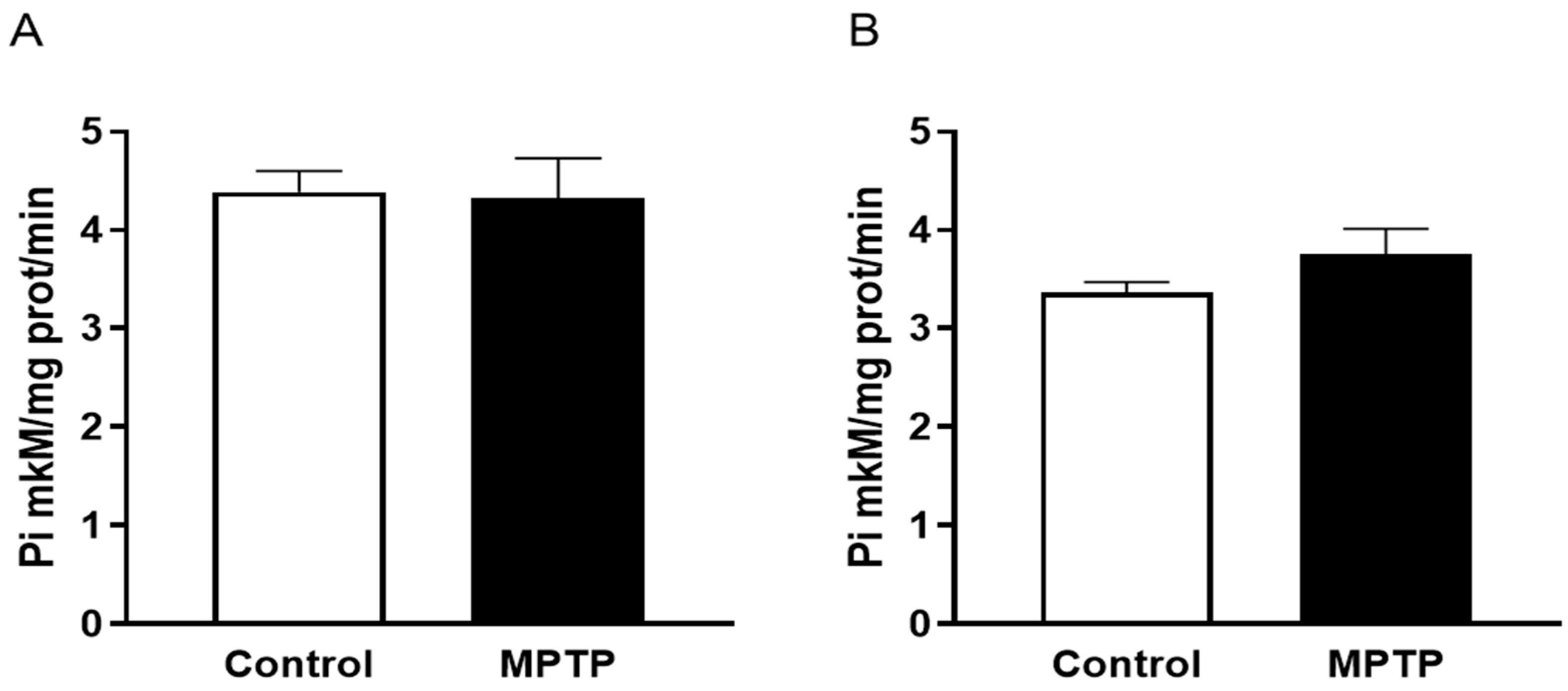
2.3. Na,K-ATPase Activity in the Midbrain and Cerebellum
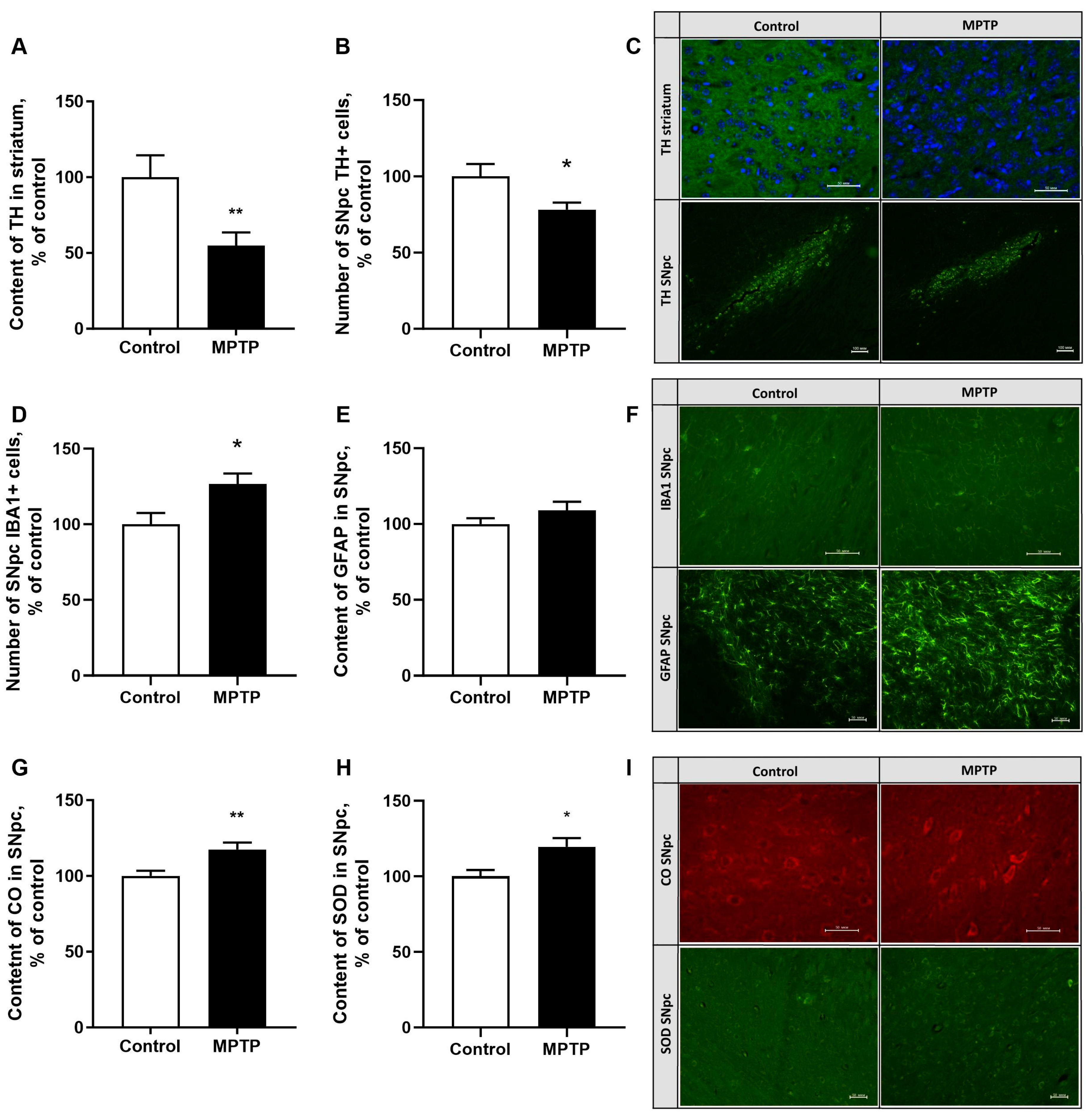
2.4. The Evaluation of Tyrosinohydroxylase Content in the Striatum and SNpc
2.5. The Assessment of the Neuroinflammatory Reaction Development in the SNpc
2.6. The Evaluation of Superoxide Dismutase and Cytochrome Oxidase (Isoform 1) Content in SNpc
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Behavioral Testing
4.4. The Content of Monoamines
4.5. The Activity of Na,K-ATPase
4.6. Immunohistochemistry
4.7. Statistical Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Leite Silva, A.B.R.; Gonçalves de Oliveira, R.W.; Diógenes, G.P.; de Castro Aguiar, M.F.; Sallem, C.C.; Lima, M.P.P.; de Albuquerque Filho, L.B.; Peixoto de Medeiros, S.D.; Penido de Mendonça, L.L.; de Santiago Filho, P.C.; et al. Premotor, nonmotor and motor symptoms of Parkinson’s Disease: A new clinical state of the art. Ageing Res. Rev. 2023, 84, 101834. [Google Scholar] [CrossRef]
- Golden, J.P.; Demaro, J.A., 3rd; Knoten, A.; Hoshi, M.; Pehek, E.; Johnson, E.M., Jr.; Gereau, R.W., 4th; Jain, S. Dopamine-dependent compensation maintains motor behavior in mice with developmental ablation of dopaminergic neurons. J. Neurosci. 2013, 33, 17095–17107. [Google Scholar] [CrossRef]
- Ledonne, A.; Massaro Cenere, M.; Paldino, E.; D’Angelo, V.; D’Addario, S.L.; Casadei, N.; Nobili, A.; Berretta, N.; Fusco, F.R.; Ventura, R.; et al. Morpho-Functional Changes of Nigral Dopamine Neurons in an α-Synuclein Model of Parkinson’s Disease. Mov. Disord. 2023, 38, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.J.; Tan, E.-K.; Chao, Y.-X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 24–64. [Google Scholar] [CrossRef]
- Meredith, G.E.; Rademacher, D.J. MPTP Mouse Models of Parkinson’s Disease: An Update. J. Park. Dis. 2011, 1, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, M.; Mat Taib, C.N. MPTP-induced mouse model of Parkinson’s disease: A promising direction of therapeutic strategies. Bosn. J. Basic Med. Sci. 2021, 21, 422–433. [Google Scholar] [CrossRef]
- Zeng, X.S.; Geng, W.S.; Jia, J.J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 1759091418777438. [Google Scholar] [CrossRef]
- Rai, S.N.; Singh, P. Advancement in the modelling and therapeutics of Parkinson’s disease. J. Chem. Neuroanat. 2020, 104, 101752. [Google Scholar] [CrossRef]
- Radad, K.; Al-Shraim, M.; Al-Emam, A.; Wang, F.; Kranner, B.; Rausch, W.-D.; Moldzio, R. Rotenone: From modelling to implication in Parkinson’s disease. Folia Neuropathol. 2019, 57, 317–326. [Google Scholar] [CrossRef]
- Magnard, R.; Vachez, Y.; Carcenac, C.; Krack, P.; David, O.; Savasta, M.; Boulet, S.; Carnicella, S. What can rodent models tell us about apathy and associated neuropsychiatric symptoms in Parkinson’s disease? Transl. Psychiatry 2016, 6, e753. [Google Scholar] [CrossRef]
- Drui, G.; Carnicella, S.; Carcenac, C.; Favier, M.; Bertrand, A.; Boulet, S.; Savasta, M. Loss of dopaminergic nigrostriatal neurons accounts for the motivational and affective deficits in Parkinson’s disease. Mol. Psychiatry 2014, 19, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Heinzen, E.L.; Arzimanoglou, A.; Brashear, A.; Clapcote, S.J.; Gurrieri, F.; Goldstein, D.B.; Jóhannesson, S.H.; Mikati, M.A.; Neville, B.; Nicole, S.; et al. ATP1A3 Working Group. Distinct neurological disorders with ATP1A3 mutations. Lancet Neurol. 2014, 13, 503–514. [Google Scholar] [CrossRef]
- Shrivastava, A.N.; Redeker, V.; Fritz, N.; Pieri, L.; Almeida, L.G.; Spolidoro, M.; Liebmann, T.; Bousset, L.; Renner, M.; Léna, C.; et al. α-synuclein assemblies sequester neuronal α3-Na+/K+-ATPase and impair Na+ gradient. EMBO J. 2015, 34, 2408–2423. [Google Scholar] [CrossRef]
- Khan, F.H.; Sen, T.; Chakrabarti, S. Dopamine oxidation products inhibit Na+, K+-ATPase activity in crude synaptosomalmitochondrial fraction from rat brain. Free Radic. Res. 2003, 37, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Hong, T.; Yang, Z.; Zhang, Y.; Wang, L.; Dong, M.; Zhao, J.; Mu, J.; Meng, Y. Effect of Quercetin in the 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Evid. Based Complement. Alternat. Med. 2012, 2012, 928643. [Google Scholar] [CrossRef]
- Lin, Q.S.; Chen, P.; Wang, W.X.; Lin, C.C.; Zhou, Y.; Yu, L.H.; Lin, Y.X.; Xu, Y.F.; Kang, D.Z. RIP1/RIP3/MLKL mediates dopaminergic neuron necroptosis in a mouse model of Parkinson disease. Lab. Investig. 2020, 100, 503–511. [Google Scholar] [CrossRef]
- Narmashiri, A.; Abbaszadeh, M.; Ghazizadeh, A. The effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on the cognitive and motor functions in rodents: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 140, 1047–1092. [Google Scholar] [CrossRef]
- Santoro, M.; Fadda, P.; Klephan, K.J.; Hull, C.; Teismann, P.; Platt, B.; Riedel, G. Neurochemical, histological, and behavioral profiling of the acute, sub-acute, and chronic MPTP mouse model of Parkinson’s disease. J. Neurochem. 2023, 164, 121–142. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, Z.; Li, Y.; Zhao, G.; Huang, J.; Zhang, Y.; Xue, J.; Tang, X. Whether the Subacute MPTP-Treated Mouse is as Suitable as a Classic Model of Parkinsonism. Neuromol. Med. 2023, 25, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.S.; Heng, Y.; Mou, Z.; Huang, J.Y.; Yuan, Y.H.; Chen, N.H. Reassessment of subacute MPTP-treated mice as animal model of Parkinson’s disease. Acta Pharmacol. Sin. 2017, 38, 1317–1328. [Google Scholar] [CrossRef]
- Berezhnoy, D.S.; Troshev, D.V.; Kulikova, O.I.; Abaimov, D.A.; Muzychuk, O.A.; Stvolinsky, S.L.; Fedorova, T.N. Comparison of Neurobehavioral Changes in Mice Treated with Mitochondrial Toxins—Rotenone and MPTP. Hum. Physiol. 2021, 47, 821–830. [Google Scholar] [CrossRef]
- Bezard, E.; Dovero, S.; Bioulac, B.; Gross, C.E. Kinetics of nigral degeneration in a chronic model of MPTP-treated mice. Neurosci. Lett. 1997, 234, 47–50. [Google Scholar] [CrossRef]
- Muñoz-Manchado, A.B.; Villadiego, J.; Romo-Madero, S.; Suárez-Luna, N.; Bermejo-Navas, A.; Rodríguez-Gómez, J.A.; Garrido-Gil, P.; Labandeira-García, J.L.; Echevarría, M.; López-Barneo, J.; et al. Chronic and progressive Parkinson’s disease MPTP model in adult and aged mice. J. Neurochem. 2016, 136, 373–387. [Google Scholar] [CrossRef]
- Burks, S.; Raymick, J.; Robinson, B.; Hanig, J.; Sarkar, S. Neuroprotective effects of acetyl-l-carnitine (ALC) in a chronic MPTP-induced Parkinson’s disease mouse model: Endothelial and microglial effects. Neurosci. Lett. 2019, 703, 86–95. [Google Scholar] [CrossRef]
- Jackson-Lewis, V.; Przedborski, S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protoc. 2007, 2, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Crampton, J.M.; Wilson, J.A. Urinary excretion of MPTP and its primary metabolites in mice. Life Sci. 1988, 43, 1459–1464. [Google Scholar] [CrossRef]
- Dionísio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef] [PubMed]
- Tatton, N.A.; Kish, S.J. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience 1997, 77, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, X.; Wang, X.; Luo, D.; Jia, J.; Wang, X. Electro- acupuncture stimulation improves spontaneous locomotor hyper-activity in MPTP intoxicated mice. PLoS ONE 2013, 8, 64403. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Chen, M.C.; Waugh, C.E.; Joormann, J.; Gotlib, I.H. Distinctive and common neural underpinnings of major depression, social anxiety, and their comorbidity. Soc. Cogn. Affect. Neurosci. 2015, 10, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.H.; Zhang, Q.J.; Liu, J.; Zhang, L.; Ali, U.; Hou, C.; Fan, L.L.; Sun, Y.N.; Wu, Z.H.; Hui, Y.P. Unilateral lesion of the nigrostriatal pathway decreases the response of fast-spiking interneurons in the medial prefrontal cortex to 5-HT1A receptor agonist and expression of the receptor in parvalbumin-positive neurons in the rat. Neurochem. Int. 2011, 59, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Matheus, F.C.; Rial, D.; Real, J.I.; Lemos, C.; Takahashi, R.N.; Bertoglio, L.J.; Cunha, R.A.; Prediger, R.D. Temporal Dissociation of Striatum and Prefrontal Cortex Uncouples Anhedonia and Defense Behaviors Relevant to Depression in 6-OHDA-Lesioned Rats. Mol. Neurobiol. 2016, 53, 3891–3899. [Google Scholar] [CrossRef]
- Han, N.R.; Kim, Y.K.; Ahn, S.; Hwang, T.Y.; Lee, H.; Park, H.J. A Comprehensive Phenotype of Non-motor Impairments and Distribution of Alpha-Synuclein Deposition in Parkinsonism-Induced Mice by a Combination Injection of MPTP and Probenecid. Front. Aging Neurosci. 2021, 13, 599045. [Google Scholar] [CrossRef]
- Kamińska, K.; Lenda, T.; Konieczny, J.; Czarnecka, A.; Lorenc-Koci, E. Depressive-like neurochemical and behavioral markers of Parkinson’s disease after 6-OHDA administered unilaterally to the rat medial forebrain bundle. Pharmacol. Rep. 2017, 69, 985–994. [Google Scholar] [CrossRef]
- Reijnders, J.S.A.M.; Ehrt, U.; Weber, W.E.J.; Aarsland, D.; Leentjens, A.F.G. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov. Disord. 2008, 23, 183–189. [Google Scholar] [CrossRef]
- Scatton, B.; Javoy-Agid, F.; Rouquier, L.; Dubois, B.; Agid, Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983, 275, 321–328. [Google Scholar] [CrossRef]
- Dremencov, E.; Newman, M.E.; Kinor, N.; Blatman-Jan, G.; Schindler, C.J.; Overstreet, D.H. Hyperfunctionality of serotonin-2C receptor-mediated inhibition of accumbal dopamine release in an animal model of depression is reversed by antidepressant treatment. Neuropharmacology 2005, 48, 34–42. [Google Scholar] [CrossRef]
- Mayeux, R.; Williams, J.B.; Stern, Y.; Cote, L. Depression and Parkinson’s disease. Adv. Neurol. 1984, 40, 241–250. [Google Scholar]
- Buchanan, A.M.; Mena, S.; Choukari, I.; Vasa, A.; Crawford, J.N.; Fadel, J.; Maxwell, N.; Reagan, L.; Cruikshank, A.; Best, J.; et al. Serotonin as a biomarker of toxin-induced Parkinsonism. Mol. Med. 2024, 30, 33. [Google Scholar] [CrossRef]
- Bezu, M.; Malikovic, J.; Kristofova, M.; Engidawork, E.; Höger, H.; Lubec, G.; Korz, V. Spatial Working Memory in Male Rats: Pre-Experience and Task Dependent Roles of Dopamine D1- and D2-Like Receptors. Front. Behav. Neurosci. 2017, 11, 196. [Google Scholar] [CrossRef]
- Morgan, R.G.; Gibbs, J.T.; Melief, E.J.; Postupna, N.O.; Sherfield, E.E.; Wilson, A.; Keene, C.D.; Montine, T.J.; Palmiter, R.D.; Darvas, M. Relative contributions of severe dopaminergic neuron ablation and dopamine depletion to cognitive impairment. Exp. Neurol. 2015, 271, 205–214. [Google Scholar] [CrossRef]
- Schneider, J.S.; Marshall, C.A.; Keibel, L.; Snyder, N.W.; Hill, M.P.; Brotchie, J.M.; Johnston, T.H.; Waterhouse, B.D.; Kortagere, S. A novel dopamine D3R agonist SK609 with norepinephrine transporter inhibition promotes improvement in cognitive task performance in rodent and non-human primate models of Parkinson’s disease. Exp. Neurol. 2021, 335, 113514. [Google Scholar] [CrossRef]
- Fallon, S.J.; Bor, D.; Hampshire, A.; Barker, R.A.; Owen, A.M. Spatial structure normalises working memory performance in Parkinson’s disease. Cortex 2017, 96, 73–82. [Google Scholar] [CrossRef]
- Sawamoto, N.; Piccini, P.; Hotton, G.; Pavese, N.; Thielemans, K.; Brooks, D.J. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain 2008, 131, 1294–12302. [Google Scholar] [CrossRef]
- Yoon, T.; Okada, J.; Jung, M.W.; Kim, J.J. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learn. Mem. 2008, 15, 97–105. [Google Scholar] [CrossRef]
- Loued-Khenissi, L.; Preuschoff, K. Apathy and noradrenaline: Silent partners to mild cognitive impairment in Parkinson’s disease? Curr. Opin. Neurol. 2015, 28, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Zarow, C.; Lyness, S.A.; Mortimer, J.A.; Chui, H.C. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch. Neurol. 2003, 60, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Sullivan, P.; Holmes, C. Catechols in postmortem brain of patients with Parkinson disease. Eur. J. Neurol. 2011, 18, 703–710. [Google Scholar] [CrossRef]
- Nayyar, T.; Bubser, M.; Ferguson, M.C. Cortical serotonin and norepinephrine denervation in parkinsonism: Preferential loss of the beaded serotonin innervation. Eur. J. Neurosci. 2009, 30, 207–216. [Google Scholar] [CrossRef] [PubMed]
- De Nuccio, F.; Cianciulli, A.; Porro, C.; Kashyrina, M.; Ruggiero, M.; Calvello, R.; Miraglia, A.; Nicolardi, G.; Lofrumento, D.D.; Panaro, M.A. Inflammatory Response Modulation by Vitamin C in an MPTP Mouse Model of Parkinson’s Disease. Biology 2021, 10, 1155. [Google Scholar] [CrossRef]
- Morales, I.; Puertas-Avendaño, R.; Sanchez, A.; Perez-Barreto, A.; Rodriguez-Sabate, C.; Rodriguez, M. Astrocytes and retrograde degeneration of nigrostriatal dopaminergic neurons in Parkinson’s disease: Removing axonal debris. Transl. Neurodegener. 2021, 10, 43. [Google Scholar] [CrossRef]
- Pajares, M.; Rojo, I.A.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.H.; Wang, X.; Zhu, X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013, 62, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Kadenbach, B.; Reimann, A. Cytochrome c oxidase: Tissue-specific expression of isoforms and regulation of activity. New Compr. Biochem. 1992, 10, 241–263. [Google Scholar]
- Cai, R.; Zhang, Y.; Simmering, J.E.; Schultz, J.L.; Li, Y.; Fernandez-Carasa, I.; Consiglio, A.; Raya, A.; Polgreen, P.M.; Narayanan, N.S.; et al. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J. Clin. Investig. 2019, 129, 4539–4549. [Google Scholar] [CrossRef]
- Haga, H.; Matsuo, K.; Yabuki, Y.; Zhang, C.; Han, F.; Fukunaga, K. Enhancement of ATP production ameliorates motor and cognitive impairments in a mouse model of MPTP-induced Parkinson’s disease. Neurochem. Int. 2019, 129, 104492. [Google Scholar] [CrossRef]
- Lin, K.H.; Li, C.Y.; Hsu, Y.M.; Tsai, C.H.; Tsai, F.J.; Tang, C.H.; Yang, J.S.; Wang, Z.H.; Yin, M.C. Oridonin, A natural diterpenoid, protected NGF-differentiated PC12 cells against MPP+- and kainic acid-induced injury. Food Chem. Toxicol. 2019, 133, 110765. [Google Scholar] [CrossRef]
- National Research Council (US). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- de l’Europe, C. European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes; European Treaty Series-No. 123; Strasbourg, 18.III. 1986; Conseil de l’Europe Section des Publications: Strasbourg, France, 1986. [Google Scholar]
- Chiu, K.; Lau, W.M.; Lau, H.T.; So, K.F.; Chang, R.C. Micro-dissection of rat brain for RNA or protein extraction from specific brain region. J. Vis. Exp. 2007, 7, 269. [Google Scholar] [CrossRef]
- Troshev, D.; Voronkov, D.; Pavlova, A.; Abaimov, D.; Latanov, A.; Fedorova, T.; Berezhnoy, D. Time course of neurobehavioral disruptions and regional brain metabolism changes in the rotenone mice model of Parkinson’s disease. Biomedicines 2022, 10, 466. [Google Scholar] [CrossRef]
- Rathbun, W.B.; Betlach, M.V. Estimation of enzymically produced orthophosphate in the presence of cysteine and adenosine triphosphate. Anal. Biochem. 1969, 28, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Keith, B.J.F. The Mouse Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]

| Number of Animals | Day of MPTP Injection | Test |
|---|---|---|
| control N = 12, MPTP N = 11 | 0, 7, 14, 21, 28, 35 | Beam walking test |
| control N = 12, MPTP N = 11 | 0, 7, 14, 21, 28, 35 | Inclined grid walking test |
| control N = 12, MPTP N = 11 | 35 | Open field |
| control N = 7, MPTP N = 6 | 35 | Tail-suspension test |
| control N = 7, MPTP N = 6 | 35 | Y-maze |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timoshina, Y.A.; Pavlova, A.K.; Voronkov, D.N.; Abaimov, D.A.; Latanov, A.V.; Fedorova, T.N. Assessment of the Behavioral and Neurochemical Characteristics in a Mice Model of the Premotor Stage of Parkinson’s Disease Induced by Chronic Administration of a Low Dose of MPTP. Int. J. Mol. Sci. 2025, 26, 8856. https://doi.org/10.3390/ijms26188856
Timoshina YA, Pavlova AK, Voronkov DN, Abaimov DA, Latanov AV, Fedorova TN. Assessment of the Behavioral and Neurochemical Characteristics in a Mice Model of the Premotor Stage of Parkinson’s Disease Induced by Chronic Administration of a Low Dose of MPTP. International Journal of Molecular Sciences. 2025; 26(18):8856. https://doi.org/10.3390/ijms26188856
Chicago/Turabian StyleTimoshina, Yulia A., Anastasia K. Pavlova, Dmitry N. Voronkov, Denis A. Abaimov, Alexander V. Latanov, and Tatiana N. Fedorova. 2025. "Assessment of the Behavioral and Neurochemical Characteristics in a Mice Model of the Premotor Stage of Parkinson’s Disease Induced by Chronic Administration of a Low Dose of MPTP" International Journal of Molecular Sciences 26, no. 18: 8856. https://doi.org/10.3390/ijms26188856
APA StyleTimoshina, Y. A., Pavlova, A. K., Voronkov, D. N., Abaimov, D. A., Latanov, A. V., & Fedorova, T. N. (2025). Assessment of the Behavioral and Neurochemical Characteristics in a Mice Model of the Premotor Stage of Parkinson’s Disease Induced by Chronic Administration of a Low Dose of MPTP. International Journal of Molecular Sciences, 26(18), 8856. https://doi.org/10.3390/ijms26188856






