In Vivo Immune Cell Responses and Long-Term Effects of Cold Atmospheric Plasma in the Upper Respiratory Tract
Abstract
1. Introduction
2. Results
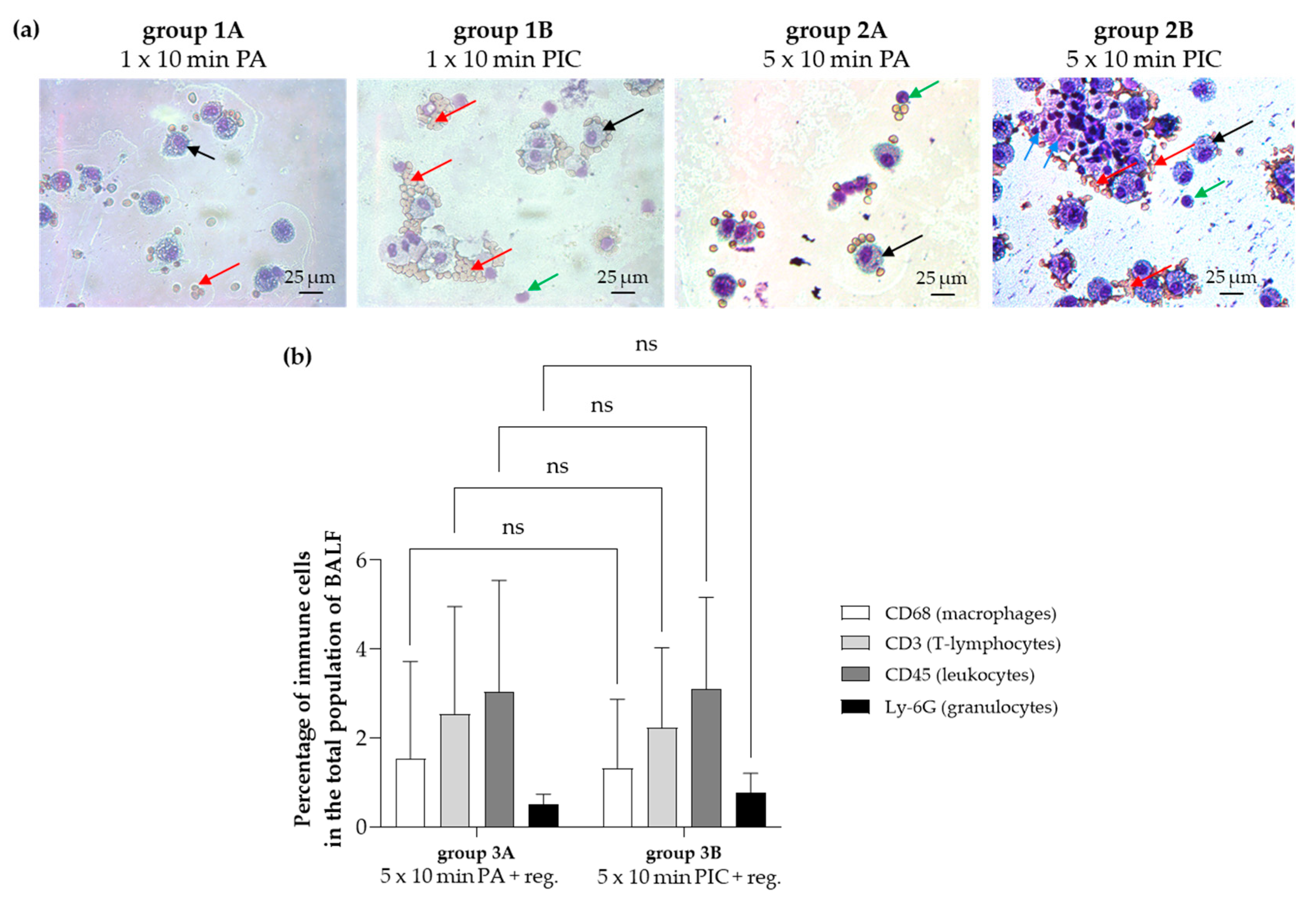
2.1. Evaluation of Bronchoalveolar Lavage Fluid (BALF) After PIC Treatment
2.1.1. Cell Cluster Formation and Erythrocyte Adhesion After PIC Treatment
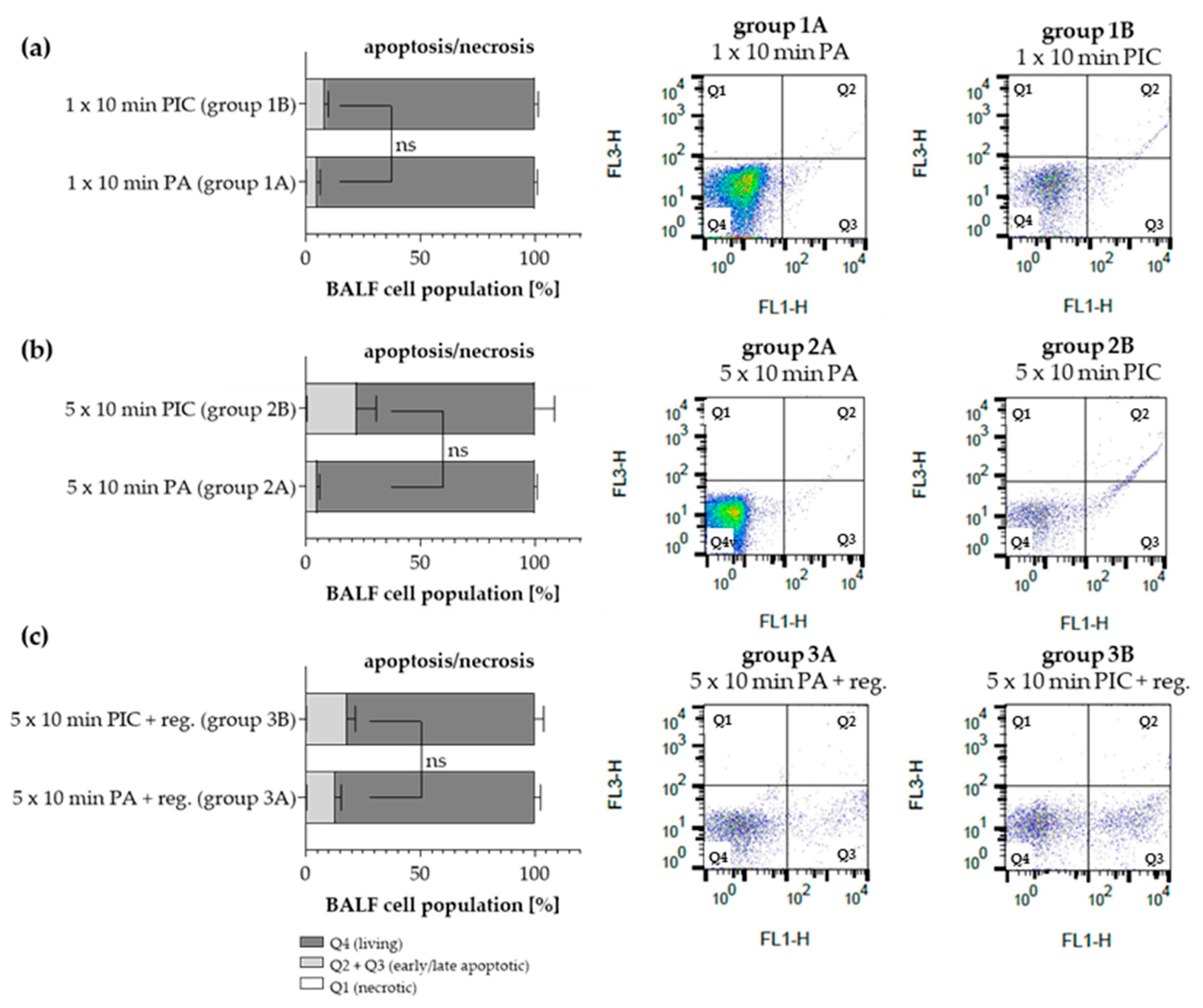
2.1.2. Apoptosis Detection in BALF Cells After PIC Treatment
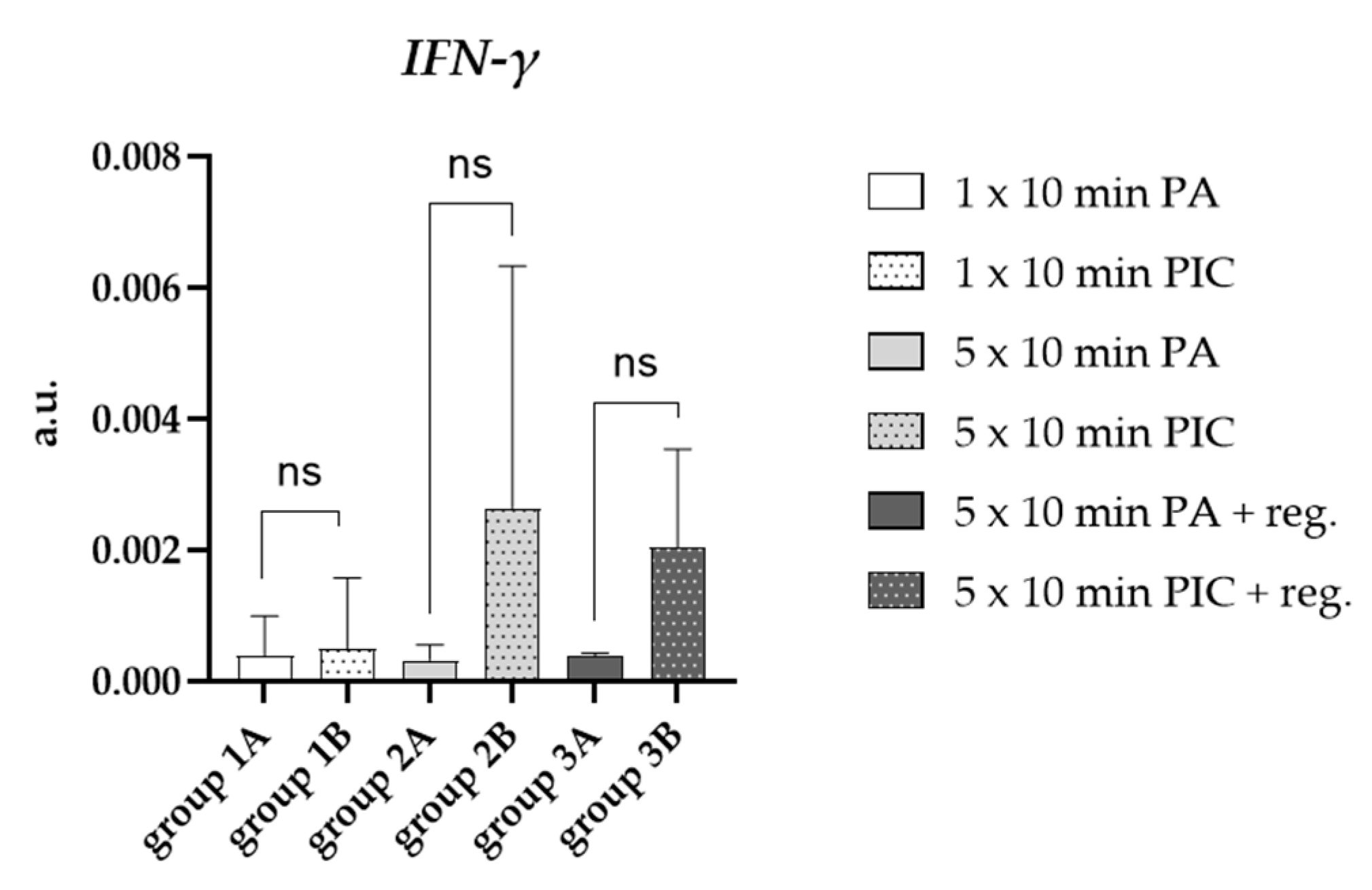
2.1.3. Cytokine Induction in BALF Cells After PIC Treatment
2.2. Evaluation of Lung Histology After PIC Treatment
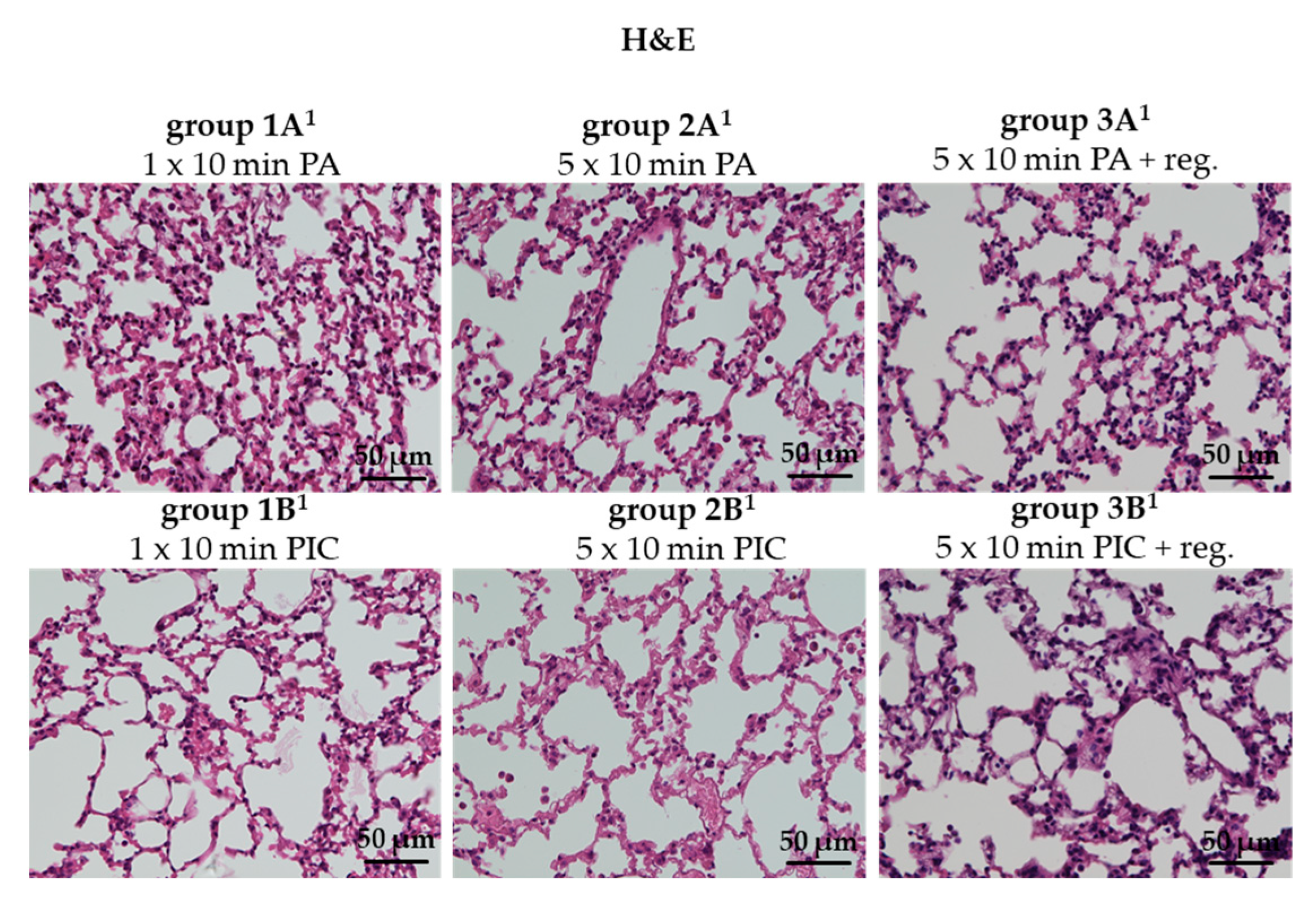
2.2.1. Lung Morphology Was Not Changed After PIC Treatment
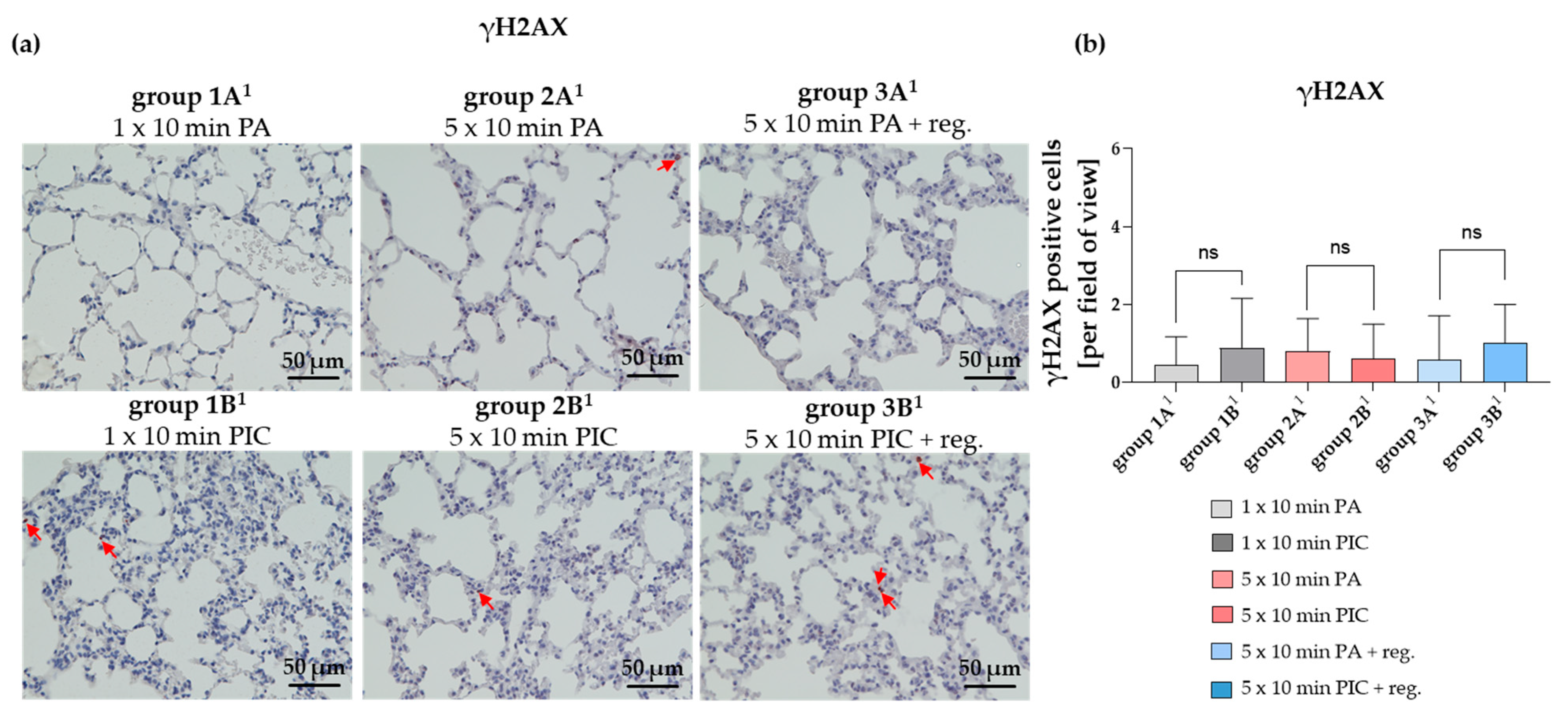
2.2.2. DNA Damage in Lung Tissue Was Not Significantly Altered After PIC Treatment
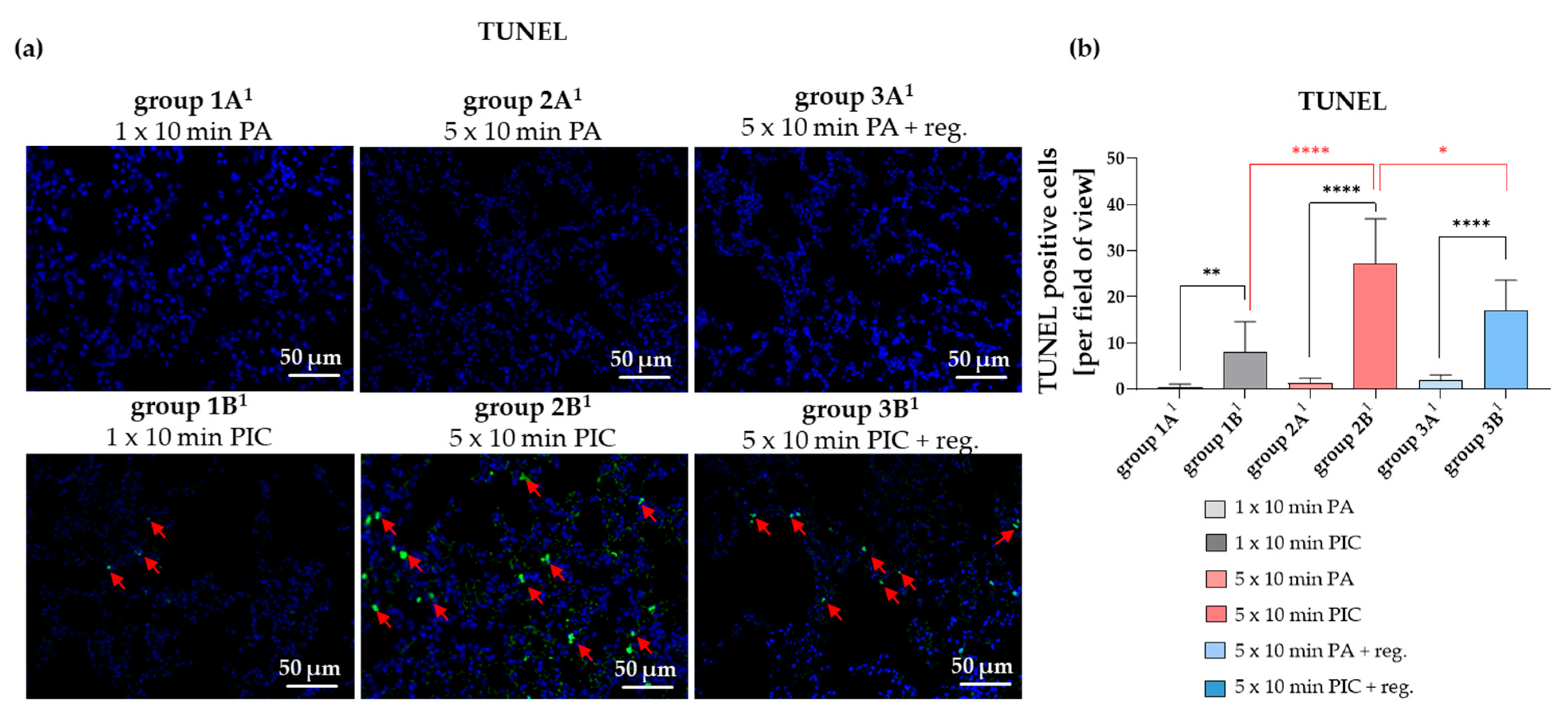
2.2.3. Cell Apoptosis in the Lungs Was Induced After PIC Treatment
2.2.4. Immune Cell Alterations Were Observed After PIC Treatment
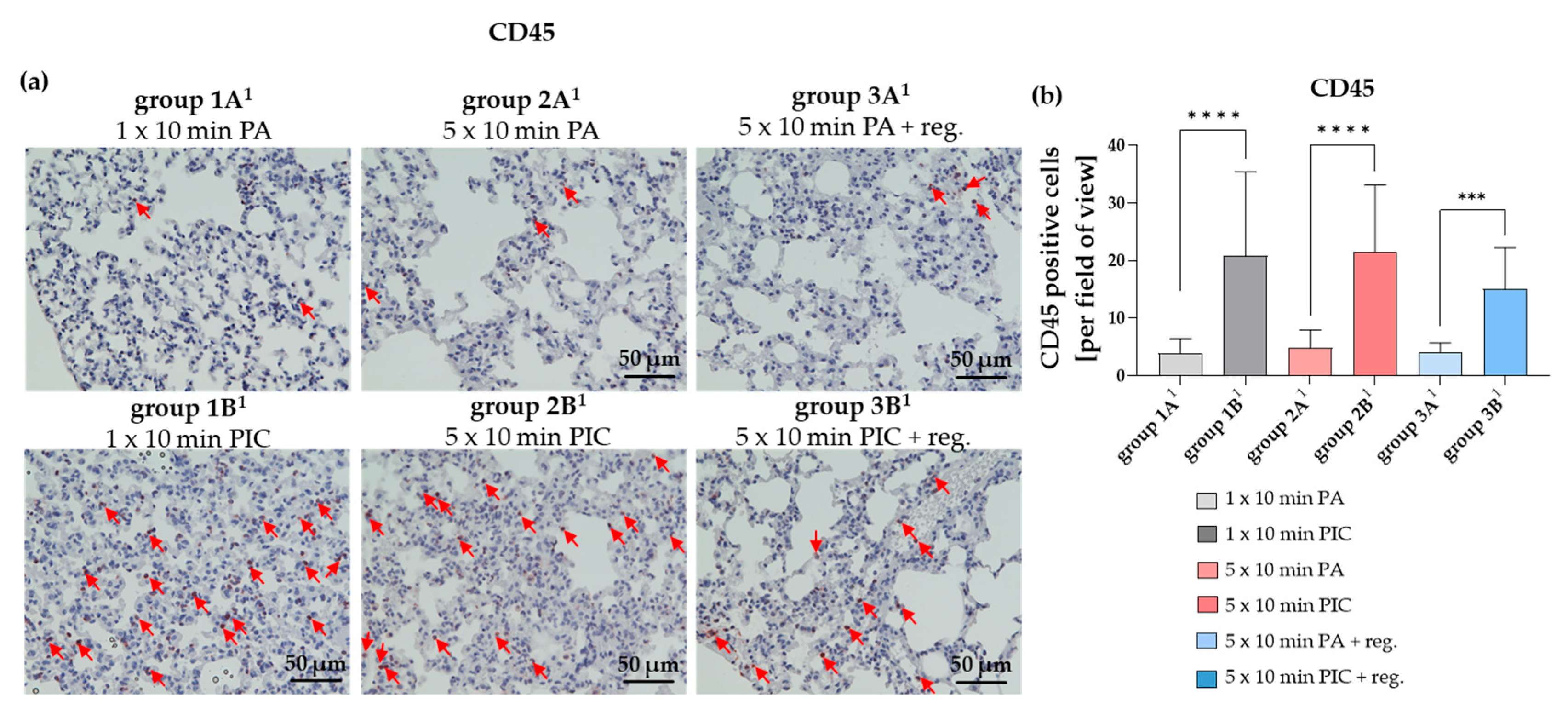
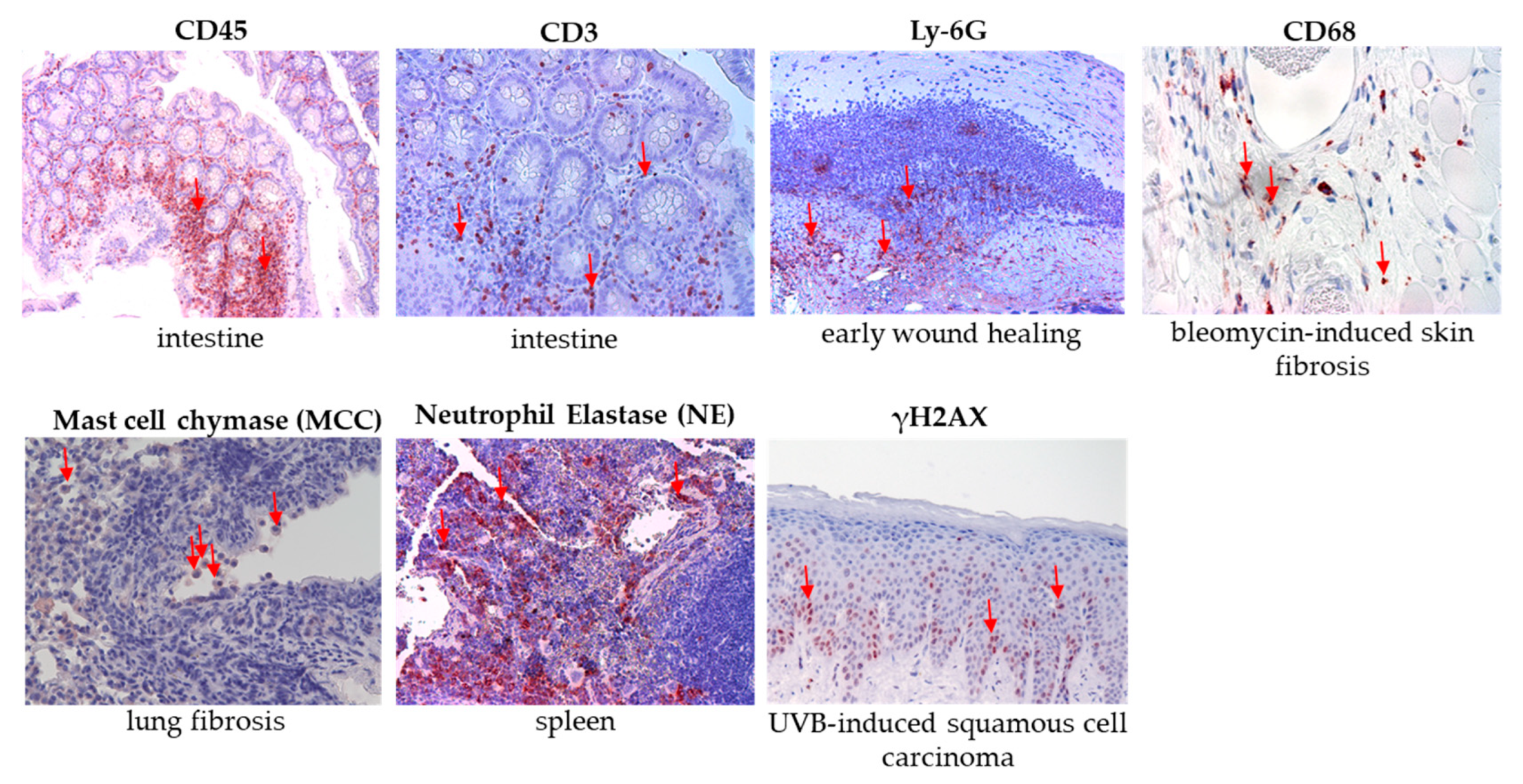
The Common Leukocyte Antigen CD45 Was Induced After PIC Treatment
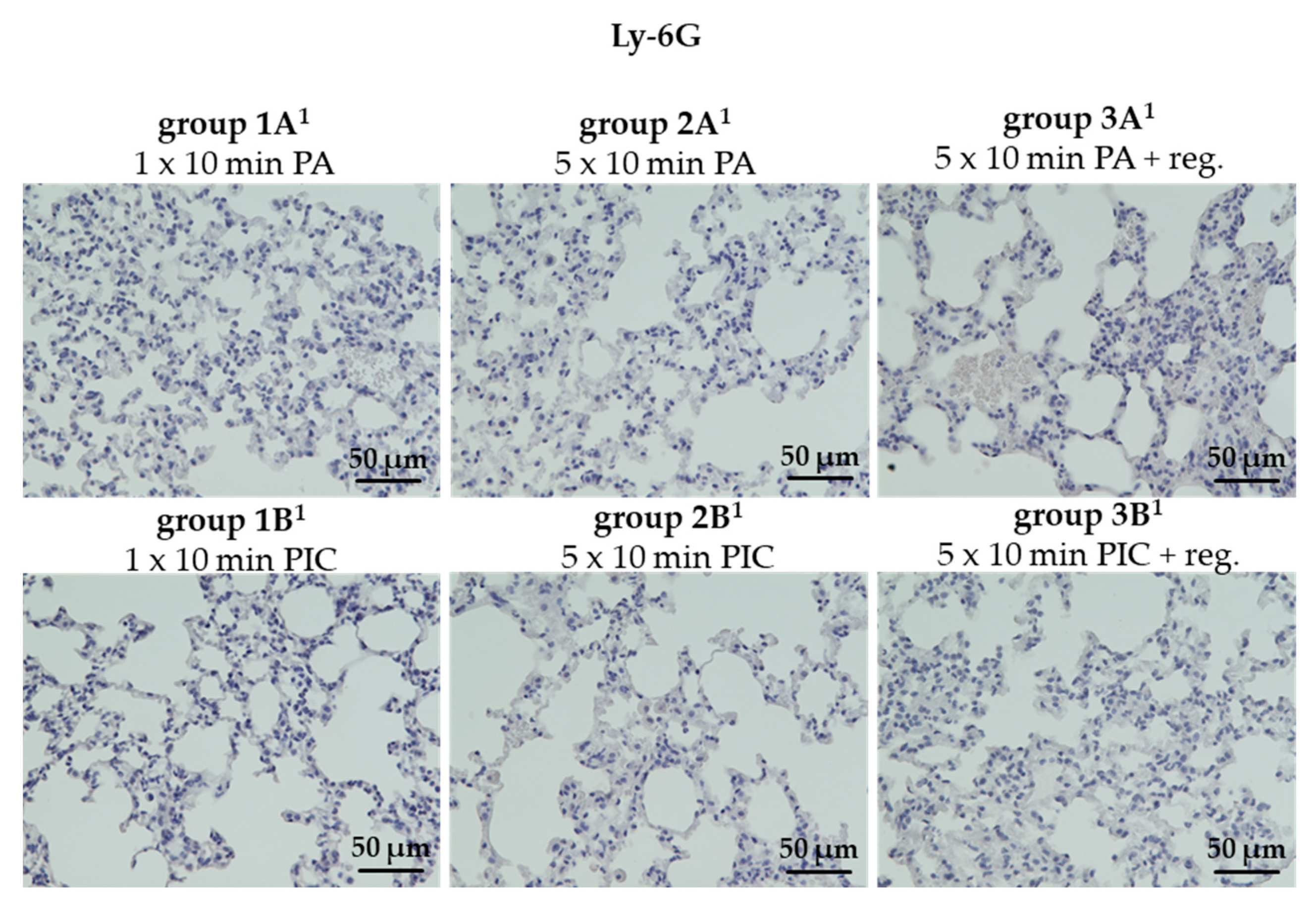
The Granulocyte Marker Ly-6G Was Not Detectable in Lung Tissues
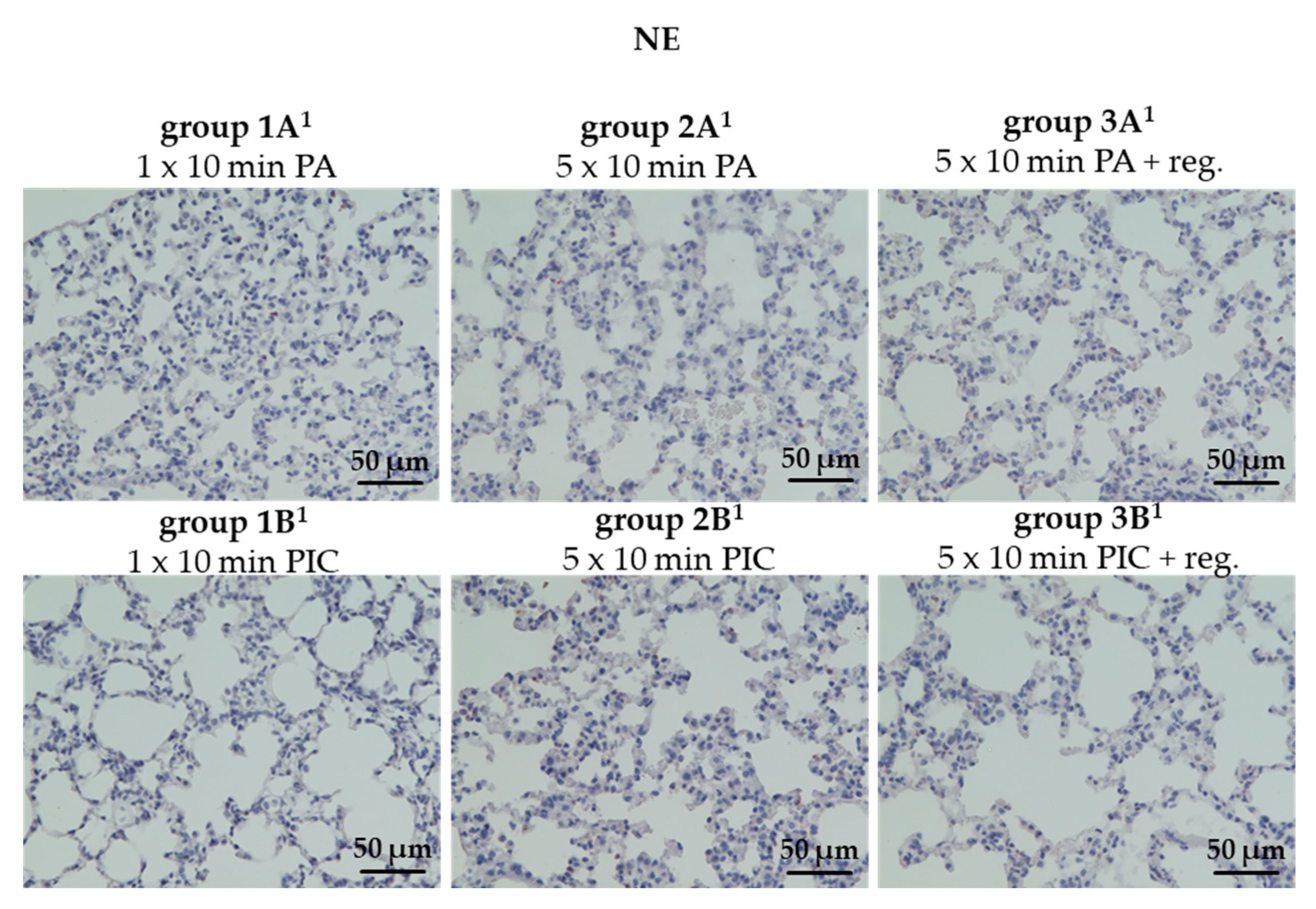
Neutrophil Elastase (NE) Was Not Detectable After PIC Treatment
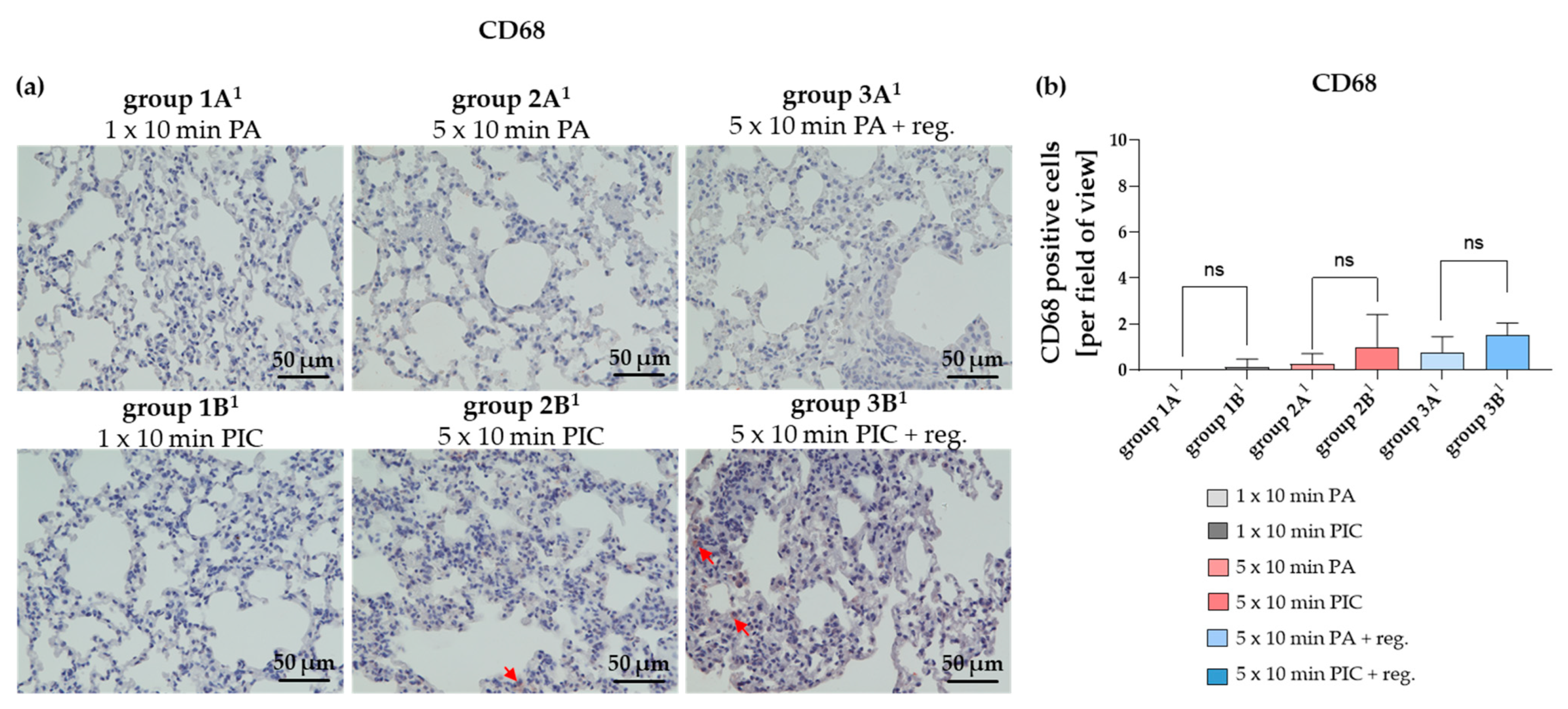
The Macrophage Marker CD68 Was Not Significantly Altered After PIC Treatment
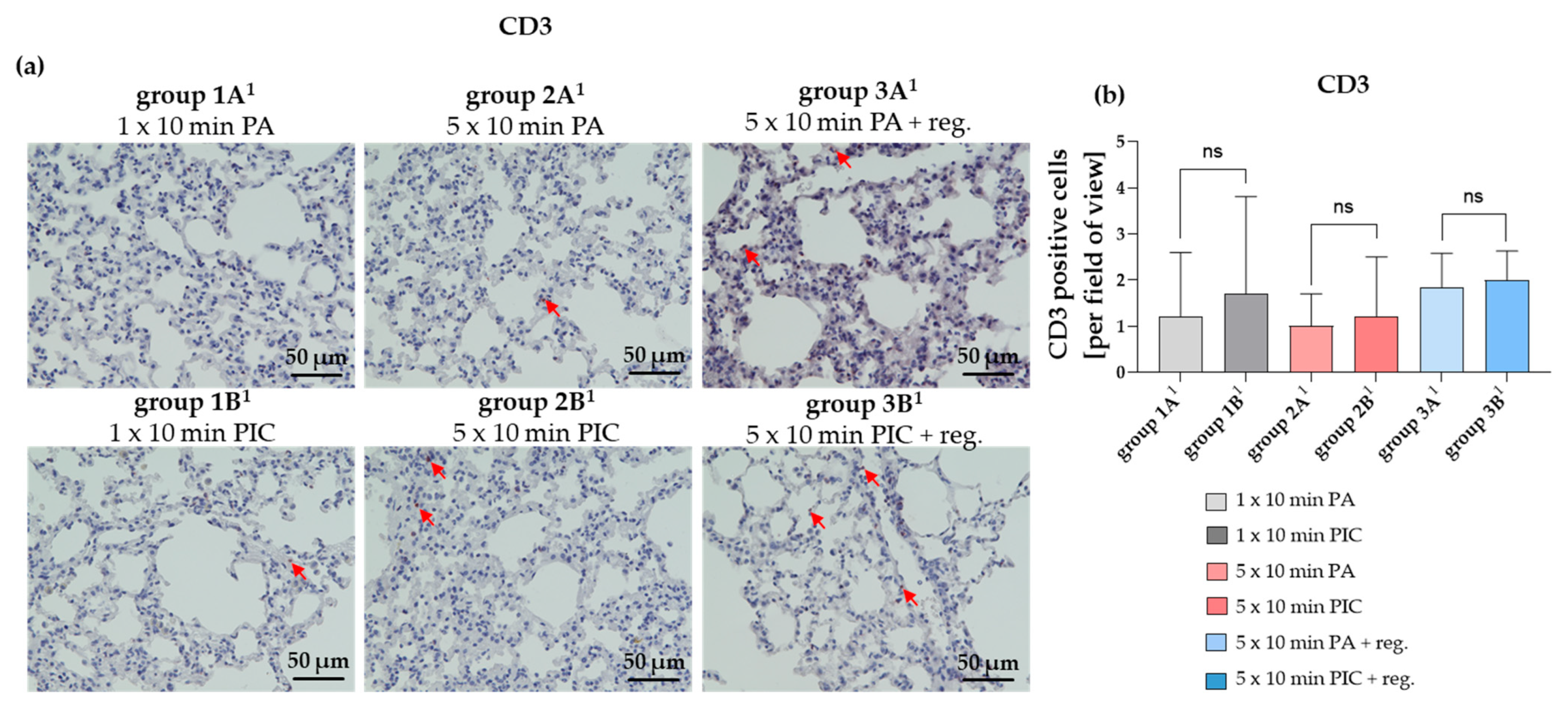
The T Lymphocyte Marker CD3 Is Not Significantly Changed After PIC Treatments
Mast Cell Chymase (MCC) Was Induced After PIC Treatment
2.2.5. Cytokine Expression Was Changed After PIC Treatment
Interleukin-6 (IL-6) Gene Expression Was Induced After PIC Treatment
Tumor Necrosis Factor-Alpha (TNF-α) Gene Expression Was Induced After PIC Treatment
3. Discussion
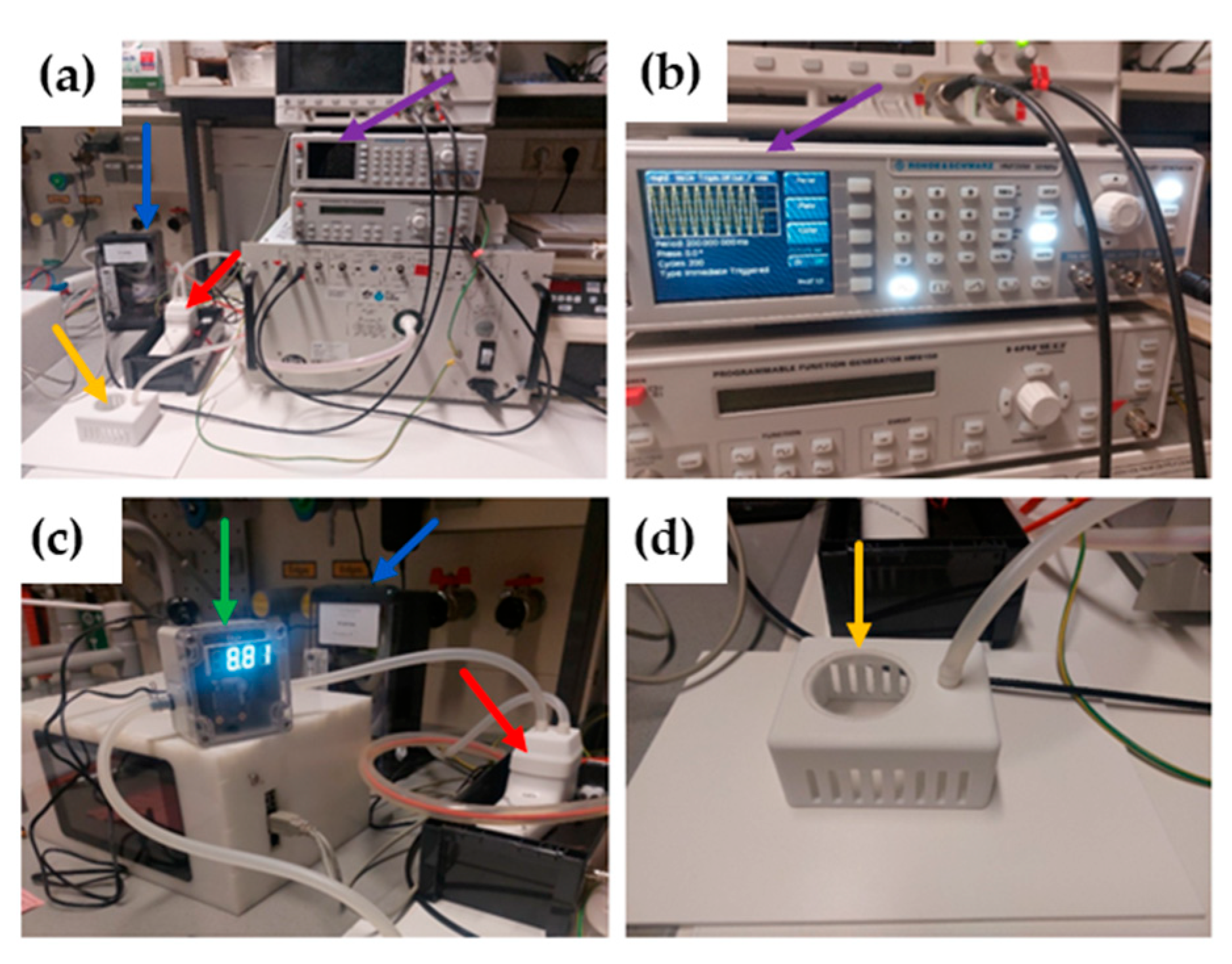
4. Materials and Methods
4.1. Animals
4.2. Plasma Device
4.3. Treatment of Mice with Cold Atmospheric Plasma or Pressurized Air
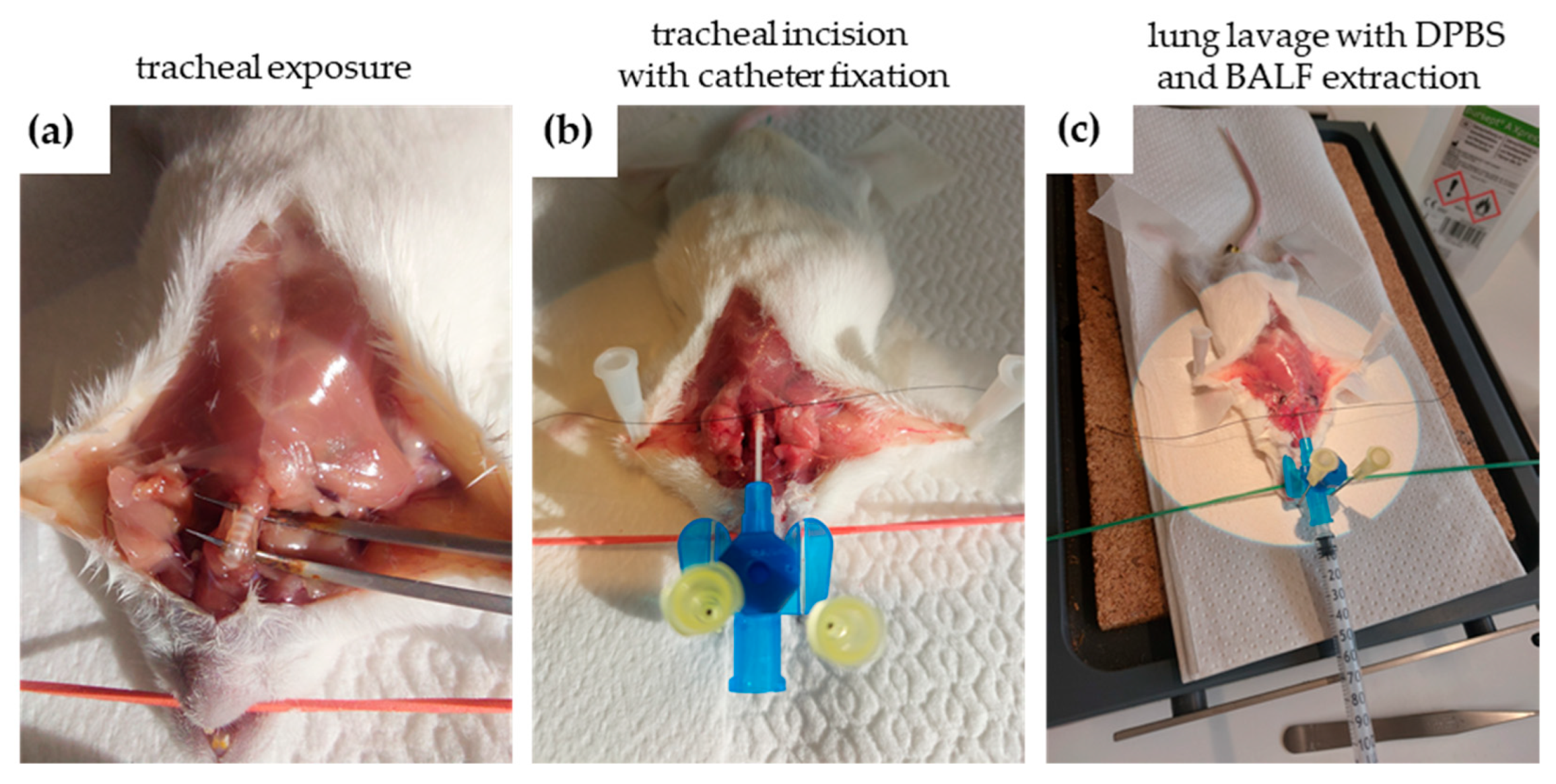
4.4. Bronchoalveolar Lavage Fluid (BALF) Evaluation
4.4.1. Bronchoalveolar Lavage Fluid (BALF) Extraction
4.4.2. Cytospin Preparation and Imaging
4.4.3. Leukocyte Profile Evaluated by Flow Cytometry
4.4.4. Measurement of Cell Apoptosis and Necrosis by Flow Cytometry
4.5. Histological Evaluation
4.5.1. Determination of Morphological Changes (H&E)
4.5.2. Determination of DNA Damage (γH2AX)
4.5.3. Determination of Apoptosis (TUNEL)
4.5.4. Determination of Lung Immune Cell Marker Alterations (CD45, Ly-6G, CD3, CD68, MCC)
4.5.5. Determination of Lung Fibrosis (NE)
4.6. Isolation of Ribonucleic Acid (RNA) and Reverse Transcription
4.7. Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
4.8. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAP | Cold atmospheric plasma |
| PA | Pressurized air |
| PIC | Plasma intensive care |
| ROS | Reactive oxygen species |
| RONS | Reactive oxygen and nitrogen species |
| TUNEL | Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling |
| BALF | Bronchoalveolar lavage fluid |
| URT | Upper respiratory tract |
| H&E | Hematoxylin and eosin |
| MCC | Mast cell chymase |
| NE | Neutrophil elastase |
| IFN-γ | Interferon-gamma |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-6 | Interleucin-6 |
| IL-8 | Interleucin-8 |
| VAP | Ventilator-associated pneumonia |
| SMD | Surface-microdischarge |
| 3D | Three-dimensional |
| PMNs | Polymorphonuclear neutrophils |
| CRS | Cytokine release syndrome |
| MAS | Macrophage activation syndrome |
| Slm | Standard liters per minute |
| MCP-1 | Monocyte chemoattractant protein-1 |
| AEC | 3-Amino-9-Ethylcarbazole |
| DPBS | Dulbecco’s Phosphate-Buffered Saline |
| COPD | Chronic obstructive pulmonary disease |
| DNA | Deoxyribonucleic acid |
| fMLP | Formyl-Methionyl-Leucyl-Phenylalanine |
| AC | Alternating Current |
| ppm | parts per million |
References
- Bassi, G.L.; Ferrer, M.; Marti, J.D.; Comaru, T.; Torres, A. Ventilator-associated pneumonia. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: New York, NY, USA, 2014; pp. 469–481. [Google Scholar]
- Charles, M.P.; Kali, A.; Easow, J.M.; Joseph, N.M.; Ravishankar, M.; Srinivasan, S.; Kumar, S.; Umadevi, S. Ventilator-associated pneumonia. Australas. Med. J. 2014, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; Fagon, J.-Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Kalanuria, A.A.; Zai, W.; Mirski, M. Ventilator-associated pneumonia in the ICU. Crit. Care 2014, 18, 208. [Google Scholar] [CrossRef]
- Cabrera-Tejada, G.G.; Chico-Sánchez, P.; Gras-Valentí, P.; Jaime-Sánchez, F.A.; Galiana-Ivars, M.; Balboa-Esteve, S.; Gómez-Sotero, I.L.; Sánchez-Payá, J.; Ronda-Pérez, E. Estimation of additional costs in patients with ventilator-associated pneumonia. Antibiotics 2023, 13, 2. [Google Scholar] [CrossRef]
- Safdar, N.; Dezfulian, C.; Collard, H.R.; Saint, S. Clinical and economic consequences of ventilator-associated pneumonia: A systematic review. Crit. Care Med. 2005, 33, 2184–2193. [Google Scholar] [CrossRef]
- Heinlin, J.; Isbary, G.; Stolz, W.; Zeman, F.; Landthaler, M.; Morfill, G.; Shimizu, T.; Zimmermann, J.L.; Karrer, S. A randomized two-sided placebo-controlled study on the efficacy and safety of atmospheric non-thermal argon plasma for pruritus. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 324–331. [Google Scholar] [CrossRef]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef]
- Maisch, T.; Bosserhoff, A.K.; Unger, P.; Heider, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.E.; Landthaler, M.; Karrer, S. Investigation of toxicity and mutagenicity of cold atmospheric argon plasma. Environ. Mol. Mutagen. 2017, 58, 172–177. [Google Scholar] [CrossRef]
- Brun, P.; Bernabe, G.; Marchiori, C.; Scarpa, M.; Zuin, M.; Cavazzana, R.; Zaniol, B.; Martines, E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: Membrane permeability, biofilm penetration and antimicrobial sensitization. J. Appl. Microbiol. 2018, 125, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Shimizu, T.; Li, Y.F.; Heinlin, J.; Karrer, S.; Morfill, G.; Zimmermann, J.L. Decolonisation of MRSA, S-aureus and E-coli by Cold-Atmospheric Plasma Using a Porcine Skin Model In Vitro. PLoS ONE 2012, 7, e34610. [Google Scholar] [CrossRef]
- De Backer, J.; Razzokov, J.; Hammerschmid, D.; Mensch, C.; Hafideddine, Z.; Kumar, N.; Van Raemdonck, G.; Yusupov, M.; Van Doorslaer, S.; Johannessen, C. The effect of reactive oxygen and nitrogen species on the structure of cytoglobin: A potential tumor suppressor. Redox Biol. 2018, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karrer, S.; Unger, P.; Gruber, M.; Gebhardt, L.; Schober, R.; Berneburg, M.; Bosserhoff, A.K.; Arndt, S. In vitro safety study on the use of cold atmospheric plasma in the upper respiratory tract. Cells 2024, 13, 1411. [Google Scholar] [CrossRef] [PubMed]
- Reichold, L.Z.; Gruber, M.; Unger, P.; Maisch, T.; Lindner, R.; Gebhardt, L.; Schober, R.; Karrer, S.; Arndt, S. Cellular Response of Immune Cells in the Upper Respiratory Tract After Treatment with Cold Atmospheric Plasma In Vitro. Int. J. Mol. Sci. 2024, 26, 255. [Google Scholar] [CrossRef]
- Young, H.A.; Hardy, K.J. Role of interferon-γ in immune cell regulation. J. Leukoc. Biol. 1995, 58, 373–381. [Google Scholar] [CrossRef]
- Mah, L.; El-Osta, A.; Karagiannis, T. γH2AX: A sensitive molecular marker of DNA damage and repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef]
- Donovan, J.A.; Koretzky, G.A. CD45 and the immune response. J. Am. Soc. Nephrol. 1993, 4, 976–985. [Google Scholar] [CrossRef]
- Lee, P.Y.; Wang, J.-X.; Parisini, E.; Dascher, C.C.; Nigrovic, P.A. Ly6 family proteins in neutrophil biology. J. Leukoc. Biol. 2013, 94, 585–594. [Google Scholar] [CrossRef]
- Doring, G. The role of neutrophil elastase in chronic inflammation. Am. J. Respir. Crit. Care Med. 1994, 150, S114. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Killingsworth, M.C.; Myasoedova, V.A.; Orekhov, A.N.; Bobryshev, Y.V. CD68/macrosialin: Not just a histochemical marker. Lab. Investig. 2017, 97, 4–13. [Google Scholar] [CrossRef]
- Lund, K.; Refsnes, M.; Sandstrøm, T.; Søstrand, P.; Schwarze, P.; Boe, J.; Kongerud, J. Increased CD3 positive cells in bronchoalveolar lavage fluid after hydrogen fluoride inhalation. Scand. J. Work Environ. Health 1999, 25, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cell chymase: Morphofunctional characteristics. Histochem. Cell Biol. 2019, 152, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Hirata, K.; Sugama, Y.; Ikura, Y.; Ohsawa, M.; Inoue, Y.; Yamamoto, S.; Kitaichi, M.; Ueda, M. Enhanced mast cell chymase expression in human idiopathic interstitial pneumonia. Int. J. Mol. Med. 2007, 19, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Choy, E.; Rose-John, S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J. Scleroderma Relat. Disord. 2017, 2, S1–S5. [Google Scholar] [CrossRef]
- Silva, L.B.; Dos Santos Neto, A.P.; Maia, S.M.; Dos Santos Guimarães, C.; Quidute, I.L.; Carvalho, A.D.A.; Júnior, S.A.; Leão, J.C. The role of TNF-α as a proinflammatory cytokine in pathological processes. Open Dent. J. 2019, 13, 332–338. [Google Scholar] [CrossRef]
- Kupke, L.S.; Arndt, S.; Lenzer, S.; Metz, S.; Unger, P.; Zimmermann, J.L.; Bosserhoff, A.-K.; Gruber, M.; Karrer, S. Cold atmospheric plasma promotes the immunoreactivity of granulocytes in vitro. Biomolecules 2021, 11, 902. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; Von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Poitout-Belissent, F.; Grant, S.N.; Tepper, J.S. Aspiration and inspiration: Using bronchoalveolar lavage for toxicity assessment. Toxicol. Pathol. 2021, 49, 386–396. [Google Scholar] [CrossRef]
- Arndt, S.; Wacker, E.; Li, Y.F.; Shimizu, T.; Thomas, H.M.; Morfill, G.E.; Karrer, S.; Zimmermann, J.L.; Bosserhoff, A.K. Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. J. Exp. Dermatol. 2013, 22, 284–289. [Google Scholar] [CrossRef]
- Maxeiner, J.H.; Karwot, R.; Hausding, M.; Sauer, K.A.; Scholtes, P.; Finotto, S. A method to enable the investigation of murine bronchial immune cells, their cytokines and mediators. Nat. Protoc. 2007, 2, 105–112. [Google Scholar] [CrossRef]
- Van Rijt, L.S.; Kuipers, H.; Vos, N.; Hijdra, D.; Hoogsteden, H.C.; Lambrecht, B.N. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J. Immunol. Methods 2004, 288, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Wacker, E.; Dorn, C.; Koch, A.; Saugspier, M.; Thasler, W.E.; Hartmann, A.; Bosserhoff, A.K.; Hellerbrand, C. Enhanced expression of BMP6 inhibits hepatic fibrosis in non-alcoholic fatty liver disease. Gut 2015, 64, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.; Unger, P.; Berneburg, M.; Bosserhoff, A.-K.; Karrer, S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. J. Dermatol. Sci. 2018, 89, 181–190. [Google Scholar] [CrossRef] [PubMed]


| Group (n = 6) | Treatment | Evaluation |
|---|---|---|
| 1A | 1 × 10 min PA | BALF |
| 1B | 1 × 10 min PIC | BALF |
| 2A | 5 × 10 min PA | BALF |
| 2B | 5 × 10 min PIC | BALF |
| 3A | 5 × 10 min PA; 7 days reg. | BALF |
| 3B | 5 × 10 min PIC; 7 days reg. | BALF |
| 1A1 | 1 × 10 min PA | histology |
| 1B1 | 1 × 10 min PIC | histology |
| 2A1 | 5 × 10 min PA | histology |
| 2B1 | 5 × 10 min PIC | histology |
| 3A1 | 5 × 10 min PA; 7 days reg. | histology |
| 3B1 | 5 × 10 min PIC; 7 days reg. | histology |

| Primer Name | Forward Primer 5′ → 3′ | Reverse Primer 5′ → 3 | Condition 1 |
|---|---|---|---|
| β-actin | AGTGTGACGTTGACATCCGT | GTAACAGTCCGCCTAGAAGC | ann. 60 °C |
| melt. 81 °C | |||
| IL-6 | GTCCTTCCTACCCCAATTTCCA | TAACGCACTAGGTTTGCCGA | ann. 60 °C |
| melt. 77 °C | |||
| TNF-α | AGCCCACGTCGTAGCAAACC | CGGGGCAGCCTTGTCCCTTG | ann. 60 °C |
| melt. 84 °C | |||
| IFN-γ | AGCAAGGCGAAAAAGGATGC | CTCATTGAATGCTTGGCGCT | ann. 60 °C |
| melt. 77 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arndt, S.; Unger, P.; Gebhardt, L.; Schober, R.; Berneburg, M.; Karrer, S. In Vivo Immune Cell Responses and Long-Term Effects of Cold Atmospheric Plasma in the Upper Respiratory Tract. Int. J. Mol. Sci. 2025, 26, 8852. https://doi.org/10.3390/ijms26188852
Arndt S, Unger P, Gebhardt L, Schober R, Berneburg M, Karrer S. In Vivo Immune Cell Responses and Long-Term Effects of Cold Atmospheric Plasma in the Upper Respiratory Tract. International Journal of Molecular Sciences. 2025; 26(18):8852. https://doi.org/10.3390/ijms26188852
Chicago/Turabian StyleArndt, Stephanie, Petra Unger, Lisa Gebhardt, Robert Schober, Mark Berneburg, and Sigrid Karrer. 2025. "In Vivo Immune Cell Responses and Long-Term Effects of Cold Atmospheric Plasma in the Upper Respiratory Tract" International Journal of Molecular Sciences 26, no. 18: 8852. https://doi.org/10.3390/ijms26188852
APA StyleArndt, S., Unger, P., Gebhardt, L., Schober, R., Berneburg, M., & Karrer, S. (2025). In Vivo Immune Cell Responses and Long-Term Effects of Cold Atmospheric Plasma in the Upper Respiratory Tract. International Journal of Molecular Sciences, 26(18), 8852. https://doi.org/10.3390/ijms26188852






