Plant-Derived Nutraceuticals in Mental Health and Brain Function: Mechanisms of Action and Therapeutic Potential
Abstract
1. Introduction
2. Bioactive Components of Nutraceuticals and Functional Foods
| Bioactive Component | Nutraceutical Effects | References |
|---|---|---|
| Minerals | - Minerals fulfill a variety of biological and physiological functions and have considerable potential in the maintenance of homeostasis and metabolism. | [53] |
| Vitamins | - Vitamins play a significant role in the prevention of various diseases and health conditions, including heart disease, vision loss, cancer, osteoporosis, immune system suppression, osteoarthritis, type 2 diabetes mellitus, and cardiovascular diseases. - Vitamins enhance quality of life and play a role in the oxidation process, impeding the actions of reactive oxygen species and free radicals, implicated in many acute and chronic diseases. | [56,57] |
| Essential fatty acids | - Essential fatty acids support a multitude of critical functions in humans, including cardioprotective, immune-boosting, antimicrobial, metabolic, cardiovascular-promoting, reduced risk of atherosclerosis, anti-inflammatory, and health-promoting effects. - Adequate intake of essential fatty acids enhances brain health and cognitive function, alleviates depression, and mitigates pain. | [58,60] |
| Dietary fiber | - Fiber binds with excess fat and glucose, facilitating their release via stool, and also mitigates the risk for cardiovascular diseases, obesity, and type 2 diabetes mellitus. - Fiber plays a crucial role in the body’s detoxification processes and prevents diverticulosis. | [61,62] |
| Prebiotics | - Prebiotics function as substrates for beneficial bacteria, increasing their growth, enhancing colon-pH reduction, and increasing bacterial short-chain fatty acid production. Short-chain fatty acids possess antioxidant, anti-proliferative, and anti-inflammatory properties. | [63,65] |
| Phytochemicals | - Many phytochemicals present antioxidant, cardioprotective, immune-boosting, antimicrobial, metabolic, heart-promoting, anti-inflammatory, and health-promoting properties. | [67,68] |
| Probiotics | - Probiotics are associated with the prevention and/or treatment of a wide range of diseases and conditions, including diarrhea, Crohn’s disease, lactose intolerance, Helicobacter pylori infection, ulcerative colitis, metabolic diseases (e.g., dyslipidemia, obesity, and diabetes mellitus), impaired immunity, respiratory tract infections, allergies (e.g., eczema and asthma), neurological and mental health conditions (e.g., depression and anxiety), bacterial vaginosis, high blood pressure, and inflammatory bowel disease. | [71,73,74,75,76,77,78,79,80,81] |
3. Nutraceuticals in the Context of Mental Health Conditions
3.1. Polyunsaturated Omega Fatty Acids
3.2. Hyperforin
3.3. Curcumin
3.4. Silexan
3.5. Ginkgo biloba Extracts
3.6. Ginseng Extracts
4. The Role of Nutraceuticals in Neurodegeneration
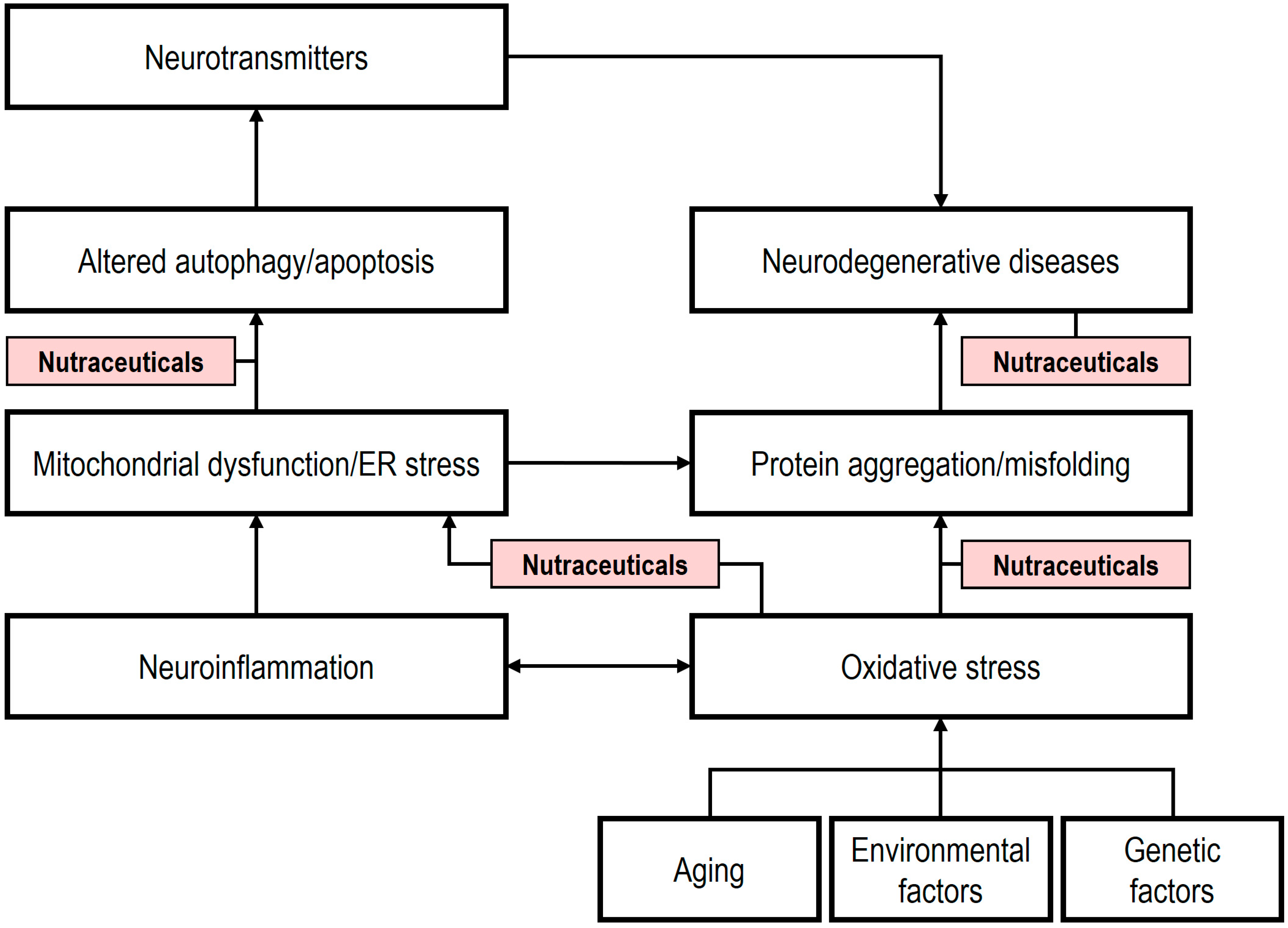
5. Novel Nutraceuticals: Mechanisms and Therapeutic Potential
5.1. Astaxanthin
5.2. Cannabidiol
5.3. Monk Fruit
5.4. Nigella Seeds
6. Gut Microbiome Interactions with Nutraceuticals and Phytochemicals
6.1. The Gut Microbiome and Nutraceuticals
6.1.1. Curcumin
6.1.2. Ginseng
6.1.3. Ginkgo biloba Extracts
6.1.4. Hypericum perforatum
6.1.5. Lavandula spp. Essential Oils
6.2. The Gut Microbiome and Phytochemicals
6.2.1. Polyunsaturated Omega Fatty Acids
6.2.2. Anthocyanins
6.2.3. Quercetins
6.2.4. Catechins
6.2.5. Chlorogenic Acid
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| AX | Astaxanthin |
| BBB | Blood–brain barrier |
| BD | Bipolar disorder |
| BDNF | Brain-derived neurotrophic factor |
| CBD | Cannabidiol |
| CGA | Chlorogenic acid |
| CNS | Central nervous system |
| COX-2 | Cyclooxygenase-2 |
| CREB | cAMP response element binding protein |
| DHA | Docosahexaenoic acid |
| EFAs | Essential fatty acids |
| EPA | Eicosapentaenoic acid |
| ERK | Extracellular signal-regulated kinase |
| FMT | Fecal microbiota transplantation |
| Gb | Ginkgo biloba |
| GBA | Gut–brain axis |
| GM | Gut microbiome |
| GPR55 | G-protein-coupled receptor 55 |
| 5-HT1A | Serotonin 1A receptor |
| iNOS | Inducible nitric oxide synthase |
| KRG | Korean red ginseng |
| LPS | Lipopolysaccharides |
| 5-LOX | 5-lipoxygenase |
| MAPK | Mitogen-activated protein kinase |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MDD | Major depressive disorder |
| NMDA | N-methyl-D-aspartate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2/HO-1 | Nuclear factor erythroid 2-related factor 2/heme oxygenase-1 |
| omega-3 PUFAs | Omega-3 polyunsaturated fatty acids |
| omega-6 PUFAs | Omega-6 polyunsaturated fatty acids |
| PD | Parkinson’s disease |
| PKA | Protein kinase A |
| PKB | Protein kinase B |
| PPAR | Peroxisome proliferator-activated receptor |
| PPD | Protopanaxadiol |
| PPT | Protopanaxatriol |
| RCTs | Randomized clinical trials |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| TDP-43 | Trans-active response DNA-binding protein-43 |
| THC | Δ9-tetrahydrocannabinol |
| TNF-α | Tumor necrosis factor-α |
| TQ | Thymoquinone |
| TRPC6 | Transient receptor potential C6 |
References
- Borrego-Ruiz, A.; Borrego, J.J. Nutritional Psychiatry: A Novel Approach to the Treatment of Mental Health Disorders. Actas Españolas Psiquiatr. 2025, 53, 443–445. [Google Scholar] [CrossRef]
- Santomauro, D.F.; Herrera, A.M.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A. Una revisión crítica sobre la aplicación de estimulación cognitiva en el contexto gerontológico [A critical review on the application of cognitive stimulation in the gerontological context]. Escr. Psicol.—Psychol. Writ. 2024, 17, 31–43. [Google Scholar] [CrossRef]
- DeFelice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar] [CrossRef]
- Stauffer, J.E. Nutraceuticals. Cereals Food World 1999, 44, 115–116. [Google Scholar]
- Awuchi, C.G.; Okpala, C.O.R. Natural nutraceuticals, especially functional foods, their major bioactive components, formulation, and health benefits for disease prevention—An overview. J. Food Bioact. 2022, 19, 97–123. [Google Scholar] [CrossRef]
- Nystrand, B.T.; Olsen, S.O. Relationships between functional food consumption and individual traits and values: A segmentation approach. J. Funct. Foods 2021, 86, 104736. [Google Scholar] [CrossRef]
- Hofmeyr, G.J.; Manyame, S.; Medley, N.; Williams, M. Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy. Cochrane Database Syst. Rev. 2019, 9, CD011192. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Mellem, J.J.; Sabiu, S. Medicinal foods and plants with antiaging properties: A review of in vitro and in vivo studies. Food Front. 2024, 5, 24–25. [Google Scholar] [CrossRef]
- Ramalingum, N.; Mahomoodally, M.F. The therapeutic potential of medicinal foods. Adv. Pharmacol. Sci. 2014, 2014, 354264. [Google Scholar] [CrossRef]
- Islam, S.U.; Ahmed, M.B.; Ahsan, H.; Lee, Y.S. Recent Molecular Mechanisms and Beneficial Effects of Phytochemicals and Plant-Based Whole Foods in Reducing LDL-C and Preventing Cardiovascular Disease. Antioxidants 2021, 10, 784. [Google Scholar] [CrossRef]
- Nayak, S.K.; Jena, S.; Sahu, P.; Tiwari, P.; Chaudhury, H.C. Nutraceuticals as supplements in the management of arthritis: A review. Int. J. Pharmaceut. Res. 2021, 13, 451–464. [Google Scholar]
- Pandita, D.; Pandita, A. Omics Technology for the Promotion of Nutraceuticals and Functional Foods. Front. Physiol. 2022, 13, 817247. [Google Scholar] [CrossRef]
- Hamid, S.B.; Hamid, A.F.A. Roles of nutraceuticals and functional food in prevention of cardiovascular disease: Sustaining health. In Nutraceutical and Functional Foods in Disease Prevention; Keservani, R.K., Sharma, A.K., Kesharwani, R.K., Eds.; IGI Global: Hershey, PA, USA, 2019; pp. 126–165. [Google Scholar]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Singh, S.; Kola, P.; Kaur, D.; Singla, G.; Mishra, V.; Panesar, P.S.; Mallikarjunan, K.; Krishania, M. Therapeutic Potential of Nutraceuticals and Dietary Supplements in the Prevention of Viral Diseases: A Review. Front. Nutr. 2021, 8, 679312. [Google Scholar] [CrossRef]
- Xu, D.; Fu, L.; Pan, D.; Lu, Y.; Yang, C.; Wang, Y.; Wang, S.; Sun, G. Role of Whole Grain Consumption in Glycaemic Control of Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 14, 109. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R.; Al Nishan, A.; Habiba, S.U.; Moon, I.S. Brain modulation by the gut microbiota: From disease to therapy. J. Adv. Res. 2023, 53, 153–173. [Google Scholar] [CrossRef]
- Ahmed, G.K.; Ramadan, H.K.A.; Elbeh, K.; Haridy, N.A. Bridging the gap: Associations between gut microbiota and psychiatric disorders. Middle East Curr. Psychiatr. 2024, 31, 2. [Google Scholar] [CrossRef]
- Liu, L.; Qi, W.; Zhang, N.; Zhang, J.; Liu, S.; Wang, H.; Jiang, L.; Sun, Y. Nutraceuticals for Gut-Brain Axis Health: A Novel Approach to Combat Malnutrition and Future Personalised Nutraceutical Interventions. Nutrients 2025, 17, 1551. [Google Scholar] [CrossRef] [PubMed]
- Banga, S.; Kumar, V.; Suri, S.; Kaushal, M.; Prasad, R.; Kaur, S. Nutraceutical Potential of Diet Drinks: A Critical Review on Components, Health Effects, and Consumer Safety. J. Am. Coll. Nutr. 2020, 39, 272–286. [Google Scholar] [CrossRef] [PubMed]
- Bulman, A.; D’Cunha, N.M.; Marx, W.; McKune, A.J.; Jani, R.; Naumovski, N. Nutraceuticals as Potential Targets for the Development of a Functional Beverage for Improving Sleep Quality. Beverages 2021, 7, 33. [Google Scholar] [CrossRef]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. An international regulatory review of food health-related claims in functional food products labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; BiBi, J.; Ali Kamboh, A.; Phil, L.; Chao, S. Potential nutraceutical and food additive properties and risks of coffee: A comprehensive overview. Crit. Rev. Food Sci. Nutr. 2019, 59, 3293–3319. [Google Scholar] [CrossRef]
- Islam, J.; Shirakawa, H.; Kabir, Y. Emerging roles of nutraceuticals from selected fermented foods in lifestyle-related disease prevention. In Herbal Medicine in India. Indigenous Knowledge, Practice, Innovation and Its Value; Sen, S., Chakraborty, R., Eds.; Springer: Singapore, 2019; pp. 479–488. [Google Scholar]
- Vasilean, I.; Aprodu, I.; Garnai, M.; Munteanu, V.; Patrașcu, L. Preliminary Investigations into the Use of Amylases and Lactic Acid Bacteria to Obtain Fermented Vegetable Products. Foods 2021, 10, 1530. [Google Scholar] [CrossRef]
- Yun, Y.-R.; Park, B.-Y.; Kim, S.-H.; Jung, J.-H. Antioxidant, Anti-Obesity, and Anti-Aging Activities of Jeju Citrus Blended Vinegar. Foods 2021, 10, 1441. [Google Scholar] [CrossRef]
- Arcusa, R.; Carrillo, J.Á.; Xandri-Martínez, R.; Cerdá, B.; Villaño, D.; Marhuenda, J.; Zafrilla, M.P. Effects of a Fruit and Vegetable-Based Nutraceutical on Biomarkers of Inflammation and Oxidative Status in the Plasma of a Healthy Population: A Placebo-Controlled, Double-Blind, and Randomized Clinical Trial. Molecules 2021, 26, 3604. [Google Scholar] [CrossRef]
- Keservani, R.K.; Sharma, A.K.; Kesharwani, R.K. Medicinal effect of nutraceutical fruits for the cognition and brain health. Scientifica 2016, 2016, 3109254. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Chang, L.S.; Fazry, S.; Aida, W.M.W.; Babji, A.S. Functional food & ingredients from seaweed, edible bird’s nest and tropical fruits: A translational research. LWT Food Sci. Technol. 2021, 151, 112164. [Google Scholar]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The current use and evolving landscape of nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef]
- Nemzer, B.; Kalita, D. Bioaccessibility, Bioavailability, Antioxidant Activities and Health Beneficial Properties of Some Selected Spices. In Herbs and Spices—New Advances; Ivanišová, E., Ed.; IntechOpen: London, UK, 2023. [Google Scholar]
- Ngwatshipane Mashabela, M.; Tshepiso Ndhlovu, P.; Otang Mbeng, W. Herbs and Spices’ Antimicrobial Properties and Possible Use in the Food Sector. In Herbs and Spices—New Advances; Ivanišová, E., Ed.; IntechOpen: London, UK, 2023. [Google Scholar]
- Berenbaum, M.R.; Calla, B. Honey as a Functional Food for Apis mellifera. Annu. Rev. Entomol. 2021, 66, 185–208. [Google Scholar] [CrossRef]
- Olas, B. Honey and Its Phenolic Compounds as an Effective Natural Medicine for Cardiovascular Diseases in Humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar]
- Barman, A.; Marak, C.M.; Barman, R.M.; Sangma, C.S. Nutraceutical Properties of Legume Seeds and Their Impact on Human Health. In Legume Seed Nutraceutical Research; Jimenez-Lopez, J.C., Clemente, A., Eds.; IntechOpen: London, UK, 2018. [Google Scholar]
- Carrera, C.S.; Salvagiotti, F.; Ciampitti, I.A. Benchmarking Nutraceutical Soybean Composition Relative to Protein and Oil. Front. Nutr. 2021, 8, 663434. [Google Scholar] [CrossRef]
- Kumar, S.; Dvya; Thakur, S.; Verma, M.; Hajam, Y.A.; Kumar, R.; Dhull, S.B.; Rose, P.K.; Muzaffar, N.; Gautam, D. Nutraceutical and Pharmacological Benefits of Some Leguminous Plants of North-Western Himalaya. Legume Sci. 2024, 6, e236. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Are Phytosterols Responsible for the Low-Density Lipoprotein-Lowering Effects of Tree Nuts? A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2015, 65, 2765–2767. [Google Scholar] [CrossRef][Green Version]
- Flore, G.; Deledda, A.; Lombardo, M.; Armani, A.; Velluzzi, F. Effects of Functional and Nutraceutical Foods in the Context of the Mediterranean Diet in Patients Diagnosed with Breast Cancer. Antioxidants 2023, 12, 1845. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Singh, A.; O’Keefe, J.H. Nuts: Natural Pleiotropic Nutraceuticals. Nutrients 2021, 13, 3269. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Naniwa-Kuroki, Y.; Sakuma, M.; Taketani, Y.; Takeda, E. Flaxseed oil intake reduces serum small dense low-density lipoprotein concentrations in Japanese men: A randomized, double blind, crossover study. Nutr. J. 2015, 14, 39. [Google Scholar] [CrossRef]
- Khosravi-Boroujeni, H.; Nikbakht, E.; Natanelov, E.; Khalesi, S. Can sesame consumption improve blood pressure? A systematic review and meta-analysis of controlled trials. J. Sci. Food Agric. 2017, 97, 3087–3094. [Google Scholar] [CrossRef]
- Singh, S.; Singh, L.; Singh, H.; Sangma, C.S. Nutraceutical Potential of Seed and Grain Proteins in Health. In Promotion Grain and Seed Proteins Functionality; Jiménez-López, J.C., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Arya, M.S.; Reshma, U.R.; Syama, S.T.; Karishma, S. Nutraceuticals in vegetables: New breeding approaches for nutrition, food and health: A review. J. Pharmacognosy Phytochem. 2019, 8, 677–682. [Google Scholar]
- Dhruv, J.J.; Patel, N.J.; Parmar, S. Nutraceutical importance of vegetables and their use for human health: A review. Ind. J. Agric. Biochem. 2019, 32, 132–142. [Google Scholar] [CrossRef]
- Saiwal, N.; Dahiya, M.; Dureja, H. Nutraceutical insight into vegetables and their potential for nutrition mediated healthcare. Curr. Nutr. Food Sci. 2019, 14, 441–453. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Priebe, M.G.; McMonagle, J.R. Effects of Ready-to-Eat-Cereals on Key Nutritional and Health Outcomes: A Systematic Review. PLoS ONE 2016, 11, e0164931. [Google Scholar] [CrossRef]
- Wei, X.; Yang, W.; Wang, J.; Zhang, Y.; Wang, Y.; Long, Y.; Tan, B.; Wan, X. Health Effects of Whole Grains: A Bibliometric Analysis. Foods 2022, 11, 4094. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The Importance of Minerals in Human Nutrition: Bioavailability, Food Fortification, Processing Effects, and Nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Gómez-Galera, S.; Rojas, E.; Sudhakar, D.; Zhu, C.; Pelacho, A.M.; Capell, T.; Christou, P. Critical evaluation of strategies for the mineral fortification of staple food crops. Transgenic Res. 2010, 19, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, D.; Chiranjib, K.; Kumar, S. A potential medicinal importance of zinc in human health and chronic. Int. J. Pharm. Bio Med. Sci. 2010, 1, 5–11. [Google Scholar]
- Öztürk, B. Nanoemulsions for food fortification with lipophilic vitamins: Production challenges, stability, and bioavailability. Eur. J. Lipid Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]
- Xiao, S.; Li, J. Study on functional components of functional food based on food vitamins. J. Phys. Conf. Ser. 2020, 1549, 032002. [Google Scholar] [CrossRef]
- Herrera Vielma, F.; Valenzuela, R.; Videla, L.A.; Zúñiga-Hernández, J. N-3 Polyunsaturated Fatty Acids and Their Lipid Mediators as A Potential Immune–Nutritional Intervention: A Molecular and Clinical View in Hepatic Disease and Other Non-Communicable Illnesses. Nutrients 2021, 13, 3384. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Joshi, A.; Hegde, M.; Zanwar, A. Flaxseed oil and palm olein blend to improve omega-6: Omega-3 ratio. J. Food Sci. Technol. 2022, 59, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Ambuja, S.R.; Rajakumar, S.N. Dietary Fiber Incorporated Dairy Foods: A Healthy Trend. Int. J. Eng. Res. Appl. 2018, 8, 34–40. [Google Scholar]
- He, M.; Van Dam, R.M.; Rimm, E.; Hu, F.B.; Qi, L. Whole grain, cereal fiber, bran, and germ intake and risks of all-cause and CVD specific mortality among women with type 2 diabetes. Circulation 2010, 121, 2162–2168. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Metabolic benefits of dietary prebiotics in human subjects: A systematic review of randomized controlled studies. Br. J. Nutr. 2014, 111, 1147–1161. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.R.; Naik, S.R.; Vakil, V.B. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Kamarul Zaman, M.; Chin, K.F.; Rai, V.; Majid, H.A. Fiber and prebiotic supplementation in enteral nutrition: A systematic review and meta-analysis. World J. Gastroenterol. 2015, 21, 5372–5381. [Google Scholar] [CrossRef]
- Awuchi, C.G. Medicinal Plants, Bioactive Compounds, and Dietary Therapies for Treating Type 1 and Type 2 Diabetes Mellitus. In Natural Drugs from Plants; El-Shemy, H.A., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Howes, M.R.; Perry, N.S.L.; Vásquez-Londoño, C.; Perry, E.K. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. Br. J. Pharmacol. 2020, 177, 1294–1315. [Google Scholar] [CrossRef]
- Yeh, K.C.; Hung, C.F.; Lee, H.L.; Hsieh, T.Y.; Wang, S.J. Soybean Meal Extract Preserves Memory Ability by Increasing Presynaptic Function and Modulating Gut Microbiota in rats. Mol. Neurobiol. 2022, 59, 1649–1664. [Google Scholar] [CrossRef] [PubMed]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- de Simone, C. The Unregulated Probiotic Market. Clin. Gastroenterol. Hepatol. 2018, 17, 809–817. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; González-Domenech, C.M.; Borrego, J.J. The Role of Fermented Vegetables as a Sustainable and Health-Promoting Nutritional Resource. Appl. Sci. 2024, 14, 10853. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Tabrizi, A.; Homayouni, A. The Effects of Probiotics and Prebiotics on Mental Disorders: A Review on Depression, Anxiety, Alzheimer, and Autism Spectrum Disorders. Curr. Pharm. Biotechnol. 2020, 21, 555–565. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Psychobiotics: A new perspective on the treatment of stress, anxiety, and depression. Ansiedad Estrés 2024, 30, 79–93. [Google Scholar] [CrossRef]
- Collinson, S.; Deans, A.; Padua-Zamora, A.; Gregorio, G.V.; Li, C.; Dans, L.F.; Allen, S.J. Probiotics for treating acute infectious diarrhea. Cochrane Database Syst. Rev. 2020, 12, CD003048. [Google Scholar] [PubMed]
- Cuello-Garcia, C.A.; Brożek, J.L.; Fiocchi, A.; Pawankar, R.; Yepes-Nuñez, J.J.; Terracciano, L.; Gandhi, S.; Agarwal, A.; Zhang, Y.; Schünemann, H.J. Probiotics for the prevention of allergy: A systematic review and meta-analysis of randomized controlled trials. J. Allergy Clin. Immunol. 2015, 136, 952–961. [Google Scholar] [CrossRef]
- Dolan, K.E.; Finley, H.J.; Burns, C.M.; Gasta, M.G.; Gossard, C.M.; Parker, E.C.; Pizano, J.M.; Williamson, C.B.; Lipski, E.A. Probiotics and Disease: A Comprehensive Summary—Part 1, Mental and Neurological Health. Integr. Med. 2016, 15, 46–58. [Google Scholar]
- Parker, E.C.; Gossard, C.M.; Dolan, K.E.; Finley, H.J.; Burns, C.M.; Gasta, M.G.; Pizano, J.M.; Williamson, C.B.; Lipski, E.A. Probiotics and Disease: A Comprehensive Summary—Part 2, Commercially Produced Cultured and Fermented Foods Commonly Available in the United States. Integr. Med. 2016, 15, 22–30. [Google Scholar]
- Saez-Lara, M.J.; Gomez-Llorente, C.; Plaza-Diaz, J.; Gil, A. The role of probiotic lactic acid bacteria and bifidobacteria in the prevention and treatment of inflammatory bowel disease and other related diseases: A systematic review of randomized human clinical trials. BioMed Res. Int. 2015, 2015, 505878. [Google Scholar] [CrossRef]
- Williamson, C.B.; Burns, C.M.; Gossard, C.M.; Pizano, J.M.; Dolan, K.E.; Finley, H.J.; Gasta, M.G.; Parker, E.C.; Lipski, E.A. Probiotics and Disease: A Comprehensive Summary—Part 3, Cardiometabolic Diseases and Fatigue Syndromes. Integr. Med. 2017, 16, 30–41. [Google Scholar]
- Lin, J.; Zhang, Y.; He, C.; Dai, J. Probiotics supplementation in children with asthma: A systematic review and meta-analysis. J. Paediatr. Child Health 2018, 54, 953–961. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; Romero, M.; Jiménez, R.; Sánchez, M.; Pérez-Vizcaíno, F.; Duarte, J. Antihypertensive Effects of Probiotics. Curr. Hypertens. Rep. 2017, 19, 26. [Google Scholar] [CrossRef]
- Bozzatello, P.; Novelli, R.; Montemagni, C.; Rocca, P.; Bellino, S. Nutraceuticals in Psychiatric Disorders: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 4824. [Google Scholar] [CrossRef]
- Gutte, R.G.; Deshmukh, V. Sectional study of nutritional psychology to identify the significance of the connection between mental health and nutraceutical functional ingredients. Funct. Food Ingred. Ment. Health 2023, 1, 1–13. [Google Scholar]
- Kumar, S.; Patel, R.; Seshadri, S. The Role of Nutraceuticals in Preventing and Managing Mental Disorders. In Functional Food Textbook Volume 7: Functional Foods and Mental Health; Martirosyan, D.M., Martirosyan, D., Eds.; Food Science Publisher: New York, NY, USA, 2017; pp. 50–71. [Google Scholar]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23, 8493. [Google Scholar] [CrossRef] [PubMed]
- Bjork, J.M.; Grant, S.J.; Chen, G.; Hommer, D.W. Dietary tyrosine/phenylalanine depletion effects on behavioral and brain signatures of human motivational processing. Neuropsychopharmacology 2014, 39, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Galizia, I.; Oldani, L.; Macritchie, K.; Amari, E.; Dougall, D.; Jones, T.N.; Lam, R.W.; Massei, G.J.; Yatham, L.N.; Young, A.H. S-adenosyl methionine (SAMe) for depression in adults. Cochrane Database Syst. Rev. 2016, 10, CD011286. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.; Tevatia, M.S.; Prakash, J.; Yadav, A.S.; Bajaj, S. Are vitamin D, B12, and folate deficiency associated with depressive disorder? A case-control study. Ind. Psychiatry J. 2023, 32, 100–105. [Google Scholar] [CrossRef]
- Firth, J.; Teasdale, S.B.; Allott, K.; Siskind, D.; Marx, W.; Cotter, J.; Veronese, N.; Schuch, F.; Smith, L.; Solmi, M.; et al. The efficacy and safety of nutrient supplements in the treatment of mental disorders: A meta-review of meta-analyses of randomized controlled trials. World Psychiatry 2019, 18, 308–324. [Google Scholar] [CrossRef]
- Mielech, A.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. Vitamins in Alzheimer’s Disease—Review of the Latest Reports. Nutrients 2020, 12, 3458. [Google Scholar] [CrossRef]
- Szewczyk, B.; Kubera, M.; Nowak, G. The role of zinc in neurodegenerative inflammatory pathways in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 693–701. [Google Scholar] [CrossRef]
- Al-Fartusie, F.S.; Al-Bairmani, H.K.; Al-Garawi, Z.S.; Yousif, A.H. Evaluation of Some Trace Elements and Vitamins in Major Depressive Disorder Patients: A Case-Control Study. Biol. Trace Elem. Res. 2019, 189, 412–419. [Google Scholar] [CrossRef]
- Anbari-Nogyni, Z.; Bidaki, R.; Madadizadeh, F.; Sangsefidi, Z.S.; Fallahzadeh, H.; Karimi-Nazari, E.; Nadjarzadeh, A. Relationship of zinc status with depression and anxiety among elderly population. Clin. Nutr. ESPEN 2020, 37, 233–239. [Google Scholar] [CrossRef]
- Botturi, A.; Ciappolino, V.; Delvecchio, G.; Boscutti, A.; Viscardi, B.; Brambilla, P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020, 12, 1661. [Google Scholar] [CrossRef]
- Sun, C.; Wang, R.; Li, Z.; Zhang, D. Dietary magnesium intake and risk of depression. J. Affect. Disord. 2019, 246, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Tarleton, E.K.; Kennedy, A.G.; Rose, G.L.; Crocker, A.; Littenberg, B. The Association between Serum Magnesium Levels and Depression in an Adult Primary Care Population. Nutrients 2019, 11, 1475. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Li, H.; Quan, Z.; Qing, H. Appropriate Macronutrients or Mineral Elements Are Beneficial to Improve Depression and Reduce the Risk of Depression. Int. J. Mol. Sci. 2023, 24, 7098. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Miura, A.; Nagahata, T.; Shibata, Y.; Okada, E.; Ojima, T. Low Zinc, Copper, and Manganese Intake is Associated with Depression and Anxiety Symptoms in the Japanese Working Population: Findings from the Eating Habit and Well-Being Study. Nutrients 2019, 11, 847. [Google Scholar] [CrossRef]
- Barks, A.; Hall, A.M.; Tran, P.V.; Georgieff, M.K. Iron as a model nutrient for understanding the nutritional origins of neuropsychiatric disease. Pediatr. Res. 2019, 85, 176–182. [Google Scholar] [CrossRef]
- Portugal-Nunes, C.; Castanho, T.C.; Amorim, L.; Moreira, P.S.; Mariz, J.; Marques, F.; Sousa, N.; Santos, N.C.; Palha, J.A. Iron Status is Associated with Mood, Cognition, and Functional Ability in Older Adults: A Cross-Sectional Study. Nutrients 2020, 12, 3594. [Google Scholar] [CrossRef]
- Björkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Tondo, L.; Vázquez, G.H. Pharmacological treatment of adult bipolar disorder. Mol. Psychiatry 2019, 24, 198–217. [Google Scholar] [CrossRef]
- Egeland, M.; Guinaudie, C.; Du Preez, A.; Musaelyan, K.; Zunszain, P.A.; Fernandes, C.; Pariante, C.M.; Thuret, S. Depletion of adult neurogenesis using the chemotherapy drug temozolomide in mice induces behavioural and biological changes relevant to depression. Transl. Psychiatry 2017, 7, e1101. [Google Scholar] [CrossRef]
- Mokhber, N.; Namjoo, M.; Tara, F.; Boskabadi, H.; Rayman, M.P.; Ghayour-Mobarhan, M.; Sahebkar, A.; Majdi, M.R.; Tavallaie, S.; Azimi-Nezhad, M.; et al. Effect of supplementation with selenium on postpartum depression: A randomized double-blind placebo-controlled trial. J. Matern. Fetal Neonatal Med. 2011, 24, 104–108. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. An updated overview on the relationship between human gut microbiome dysbiosis and psychiatric and psychological disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110861. [Google Scholar] [CrossRef]
- Farooq, R.K.; Alamoudi, W.; Alhibshi, A.; Rehman, S.; Sharma, A.R.; Abdulla, F.A. Varied Composition and Underlying Mechanisms of Gut Microbiome in Neuroinflammation. Microorganisms 2022, 10, 705. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress and the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Influence of human gut microbiome on the healthy and the neurodegenerative aging. Exp. Gerontol. 2024, 194, 112497. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An Inflammation-Centric View of Neurological Disease: Beyond the Neuron. Front. Cell. Neurosci. 2018, 12, 72, Correction in Front. Cell. Neurosci. 2020 13, 578. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Nobile, M.; Ciappolino, V.; Delvecchio, G.; Tesei, A.; Turolo, S.; Crippa, A.; Mazzocchi, A.; Altamura, C.A.; Brambilla, P. The Role of Omega-3 Fatty Acids in Developmental Psychopathology: A Systematic Review on Early Psychosis, Autism, and ADHD. Int. J. Mol. Sci. 2017, 18, 2608. [Google Scholar] [CrossRef] [PubMed]
- Bozzatello, P.; De Rosa, M.L.; Rocca, P.; Bellino, S. Effects of Omega 3 Fatty Acids on Main Dimensions of Psychopathology. Int. J. Mol. Sci. 2020, 21, 6042. [Google Scholar] [CrossRef]
- Saunders, E.F.; Ramsden, C.E.; Sherazy, M.S.; Gelenberg, A.J.; Davis, J.M.; Rapoport, S.I. Omega-3 and Omega-6 Polyunsaturated Fatty Acids in Bipolar Disorder: A Review of Biomarker and Treatment Studies. J. Clin. Psychiatry 2016, 77, e1301–e1308. [Google Scholar] [CrossRef]
- Bozzatello, P.; Brignolo, E.; De Grandi, E.; Bellino, S. Supplementation with Omega-3 Fatty Acids in Psychiatric Disorders: A Review of Literature Data. J. Clin. Med. 2016, 5, 67. [Google Scholar] [CrossRef]
- Mischoulon, D.; Freeman, M.P. Omega-3 fatty acids in psychiatry. Psychiatr. Clin. N. Am. 2013, 36, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, M.M.; Nolan, Y.M.; Green, H.F.; Robertson, R.C.; Stanton, C.; Kelly, P.; Cryan, J.F.; Dinan, T.G. The Omega-3 Polyunsaturated Fatty Acid Docosahexaenoic Acid (DHA) Reverses Corticosterone-Induced Changes in Cortical Neurons. Int. J. Neuropsychopharmacol. 2016, 19, pyv130. [Google Scholar] [CrossRef]
- Lange, K.W. Omega-3 fatty acids and mental health. Glob. Health J. 2020, 4, 18–30. [Google Scholar] [CrossRef]
- Müller, C.P.; Reichel, M.; Mühle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain membrane lipids in major depression and anxiety disorders. Biochim. Biophys. Acta 2015, 1851, 1052–1065. [Google Scholar] [CrossRef]
- Kim, O.Y.; Song, J. Important roles of linoleic acid and α-linolenic acid in regulating cognitive impairment and neuropsychiatric issues in metabolic-related dementia. Life Sci. 2024, 337, 122356. [Google Scholar] [CrossRef]
- Kurowska, A.; Ziemichód, W.; Herbet, M.; Piątkowska-Chmiel, I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients 2023, 15, 1436. [Google Scholar] [CrossRef]
- Sharif, S.N.; Darsareh, F. Impact of evening primrose oil consumption on psychological symptoms of postmenopausal women: A randomized double-blinded placebo-controlled clinical trial. Menopause 2020, 27, 194–198. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of omega-3 polyunsaturated fatty acids on brain functions: A systematic review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef]
- Regenthal, R.; Rong, P.; Krügel, U. Nutraceuticals in mental diseases—Bridging the gap between traditional use and modern pharmacology. Curr. Opin. Pharmacol. 2021, 61, 62–68. [Google Scholar]
- Friedland, K.; Harteneck, C. Hyperforin: To Be or Not to Be an Activator of TRPC(6). Rev. Physiol. Biochem. Pharmacol. 2015, 169, 1–24. [Google Scholar]
- Leuner, K.; Kazanski, V.; Müller, M.; Essin, K.; Henke, B.; Gollasch, M.; Harteneck, C.; Müller, W.E. Hyperforin—A key constituent of St. John’s wort specifically activates TRPC6 channels. FASEB J. 2007, 21, 4101–4111. [Google Scholar]
- Leuner, K.; Li, W.; Amaral, M.D.; Rudolph, S.; Calfa, G.; Schuwald, A.M.; Harteneck, C.; Inoue, T.; Pozzo-Miller, L. Hyperforin modulates dendritic spine morphology in hippocampal pyramidal neurons by activating Ca2+-permeable TRPC6 channels. Hippocampus 2013, 23, 40–52. [Google Scholar] [PubMed]
- Concerto, C.; Boo, H.; Hu, C.; Sandilya, P.; Krish, A.; Chusid, E.; Coira, D.; Aguglia, E.; Battaglia, F. Hypericum perforatum extract modulates cortical plasticity in humans. Psychopharmacology 2018, 235, 145–153. [Google Scholar] [PubMed]
- Marrelli, M.; Statti, G.; Conforti, F. Hypericum spp.: An Update on the Biological Activities and Metabolic Profiles. Mini Rev. Med. Chem. 2020, 20, 66–87. [Google Scholar] [PubMed]
- Saitgareeva, A.R.; Bulygin, K.V.; Gareev, I.F.; Beylerli, O.A.; Akhmadeeva, L.R. The role of microglia in the development of neurodegeneration. Neurol. Sci. 2020, 41, 3609–3615. [Google Scholar] [CrossRef]
- Bonaterra, G.A.; Mierau, O.; Hofmann, J.; Schwarzbach, H.; Aziz-Kalbhenn, H.; Kolb, C.; Kinscherf, R. In Vitro Effects of St. John’s Wort Extract Against Inflammatory and Oxidative Stress and in the Phagocytic and Migratory Activity of Mouse SIM-A9 Microglia. Front. Pharmacol. 2020, 11, 603575. [Google Scholar] [CrossRef]
- Traeger, A.; Voelker, S.; Shkodra-Pula, B.; Kretzer, C.; Schubert, S.; Gottschaldt, M.; Schubert, U.S.; Werz, O. Improved Bioactivity of the Natural Product 5-Lipoxygenase Inhibitor Hyperforin by Encapsulation into Polymeric Nanoparticles. Mol. Pharm. 2020, 17, 810–816. [Google Scholar] [CrossRef]
- Ozkan, E.E.; Ozsoy, N.; Ozden, T.Y.; Ozhan, G.; Mat, A. Evaluation of Chemical Composition and In-Vitro Biological Activities of Three Endemic Hypericum Species from Anatolia (H. thymbrifolium, H. spectabile and H. pseudolaeve). Iran. J. Pharm. Res. 2018, 17, 1036–1046. [Google Scholar] [PubMed]
- Orhan, I.E.; Senol Deniz, F.S.; Traedal-Henden, S.; Cerón-Carrasco, J.P.; den Haan, H.; Peña-García, J.; Pérez-Sánchez, H.; Emerce, E.; Skalicka-Wozniak, K. Profiling Auspicious Butyrylcholinesterase Inhibitory Activity of Two Herbal Molecules: Hyperforin and Hyuganin C. Chem. Biodivers. 2019, 16, e1900017. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Ak, G.; Sinan, K.I.; Elbasan, F.; Ferrarese, I.; Sut, S.; Yıldıztugay, E.; Peron, G.; Schievano, E.; Nancy Picot-Allain, M.C.; et al. Hypericum triquetrifolium and H. neurocalycinum as Sources of Antioxidants and Multi-Target Bioactive Compounds: A Comprehensive Characterization Combining In Vitro Bioassays and Integrated NMR and LC-MS Characterization by Using a Multivariate Approach. Front. Pharmacol. 2021, 12, 660735. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Cheng, P.; Yu, K.; Han, Y.; Song, M.; Li, Y. Hyperforin attenuates aluminum-induced Aβ production and Tau phosphorylation via regulating Akt/GSK-3β signaling pathway in PC12 cells. Biomed. Pharmacother. 2017, 96, 1–6. [Google Scholar] [CrossRef]
- Jiang, X.; Kumar, M.; Zhu, Y. Protective Effect of Hyperforin on β Amyloid Protein Induced Apoptosis in PC12 Cells and Colchicine Induced Alzheimer’s Disease: An Anti-oxidant and Anti-inflammatory Therapy. J. Oleo Sci. 2018, 67, 1443–1453. [Google Scholar] [CrossRef]
- Radulović, N.S.; Genčić, M.S.; Stojanović, N.M.; Randjelović, P.J.; Baldovini, N.; Kurteva, V. Prenylated β-diketones, two new additions to the family of biologically active Hypericum perforatum L. (Hypericaceae) secondary metabolites. Food Chem. Toxicol. 2018, 118, 505–513. [Google Scholar] [CrossRef]
- Chongtham, A.; Agrawal, N. Curcumin modulates cell death and is protective in Huntington’s disease model. Sci. Rep. 2016, 6, 18736. [Google Scholar] [CrossRef]
- Matias, J.N.; Achete, G.; Campanari, G.S.D.S.; Guiguer, É.L.; Araújo, A.C.; Buglio, D.S.; Barbalho, S.M. A systematic review of the antidepressant effects of curcumin: Beyond monoamines theory. Aust. N. Z. J. Psychiatry 2021, 55, 451–462. [Google Scholar]
- Ramaholimihaso, T.; Bouazzaoui, F.; Kaladjian, A. Curcumin in Depression: Potential Mechanisms of Action and Current Evidence—A Narrative Review. Front. Psychiatry 2020, 11, 572533. [Google Scholar] [CrossRef]
- Pan, X.; Chen, L.; Xu, W.; Bao, S.; Wang, J.; Cui, X.; Gao, S.; Liu, K.; Avasthi, S.; Zhang, M.; et al. Activation of monoaminergic system contributes to the antidepressant- and anxiolytic-like effects of J147. Behav. Brain Res. 2021, 411, 113374. [Google Scholar] [CrossRef] [PubMed]
- Kandezi, N.; Mohammadi, M.; Ghaffari, M.; Gholami, M.; Motaghinejad, M.; Safari, S. Novel Insight to Neuroprotective Potential of Curcumin: A Mechanistic Review of Possible Involvement of Mitochondrial Biogenesis and PI3/Akt/GSK3 or PI3/Akt/CREB/BDNF Signaling Pathways. Int. J. Mol. Cell. Med. 2020, 9, 1–32. [Google Scholar]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Voulgaropoulou, S.D.; van Amelsvoort, T.A.M.J.; Prickaerts, J.; Vingerhoets, C. The effect of curcumin on cognition in Alzheimer’s disease and healthy aging: A systematic review of pre-clinical and clinical studies. Brain Res. 2019, 1725, 146476. [Google Scholar] [CrossRef]
- Emmanuel, I.A.; Olotu, F.A.; Agoni, C.; Soliman, M.E.S. Deciphering the ‘Elixir of Life’: Dynamic Perspectives into the Allosteric Modulation of Mitochondrial ATP Synthase by J147, a Novel Drug in the Treatment of Alzheimer’s Disease. Chem. Biodivers. 2019, 16, e1900085. [Google Scholar] [CrossRef]
- Liu, Y.M.; Fan, H.R.; Ding, J.; Huang, C.; Deng, S.; Zhu, T.; Xu, T.L.; Ge, W.H.; Li, W.G.; Li, F. Curcumol allosterically modulates GABA(A) receptors in a manner distinct from benzodiazepines. Sci. Rep. 2017, 7, 46654. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.G.; Huang, C.; Zhu, M.X.; Xu, T.L.; Wu, D.Z.; Li, Y. Subunit-specific inhibition of glycine receptors by curcumol. J. Pharmacol. Exp. Ther. 2012, 343, 371–379. [Google Scholar] [CrossRef]
- Barati, S.; Yadegari, A.; Shahmohammadi, M.; Azami, F.; Tahmasebi, F.; Rouhani, M.R.; Kazemi, S.; Asl, E.R. Curcumin as a promising therapeutic agent for diabetic neuropathy: From molecular mechanisms to functional recovery. Diabetol. Metab. Syndr. 2025, 17, 314. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef]
- El-Rakabawy, O.M.; Elkholy, A.A.; Mahfouz, A.A.; Abdelsalam, M.M.; El Wakeel, L.M. Curcumin supplementation improves the clinical outcomes of patients with diabetes and atherosclerotic cardiovascular risk. Sci. Rep. 2025, 15, 28358. [Google Scholar] [CrossRef]
- Kroon, M.A.G.M.; van Laarhoven, H.W.M.; Swart, E.L.; van Tellingen, O.; Kemper, E.M. A pharmacokinetic study and critical reappraisal of curcumin formulations enhancing bioavailability. iScience 2025, 28, 112575. [Google Scholar] [CrossRef]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.; Sillani, G.; Schuwald, A.; Friedland, K. Pharmacological basis of the anxiolytic and antidepressant properties of Silexan®, an essential oil from the flowers of lavender. Neurochem. Int. 2021, 143, 104899. [Google Scholar] [CrossRef]
- Friedland, K.; Silani, G.; Schuwald, A.; Stockburger, C.; Koch, E.; Nöldner, M.; Müller, W.E. Neurotrophic Properties of Silexan, an Essential Oil from the Flowers of Lavender—Preclinical Evidence for Antidepressant-Like Properties. Pharmacopsychiatry 2021, 54, 37–46. [Google Scholar] [CrossRef]
- Dong, G.; Bai, X.; Aimila, A.; Aisa, H.A.; Maiwulanjiang, M. Study on Lavender Essential Oil Chemical Compositions by GC-MS and Improved pGC. Molecules 2020, 25, 3166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Y.; Fu, Y.; Lin, L.; Lin, Y.; Zhang, Y.; Ji, L.; Li, C. Anti-Alzheimer’s Disease Molecular Mechanism of Acori Tatarinowii Rhizoma Based on Network Pharmacology. Med. Sci. Monit. Basic Res. 2020, 26, e924203. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Panza, F. Plant-based nutraceutical interventions against cognitive impairment and dementia: Meta-analytic evidence of efficacy of a standardized Gingko biloba extract. J. Alzheimers Dis. 2015, 43, 605–611. [Google Scholar] [CrossRef]
- Howes, M.J.R.; Houghton, P.J. Traditional Medicine for Memory Enhancement. In Herbal Drugs: Ethnomedicine to Modern Medicine; Ramawat, K.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 239–291. [Google Scholar]
- Pagotto, G.L.d.O.; Santos, L.M.O.d.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.d.; Goulart, R.d.A.; Catharin, V.M.C.S.; et al. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants 2024, 13, 651. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Direito, R.; Laurindo, L.F.; Marton, L.T.; Guiguer, E.L.; Goulart, R.A.; Tofano, R.J.; Carvalho, A.C.A.; Flato, U.A.P.; Capelluppi Tofano, V.A.; et al. Ginkgo biloba in the Aging Process: A Narrative Review. Antioxidants 2022, 11, 525. [Google Scholar] [CrossRef]
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Koch, E. Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: Considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts. Phytomedicine 2005, 12, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Koltermann, A.; Hartkorn, A.; Koch, E.; Fürst, R.; Vollmar, A.M.; Zahler, S. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell. Mol. Life Sci. 2007, 64, 1715–1722. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Calabrese, V.; Tsatsakis, A.; Giordano, J.J. Hormesis and Ginkgo biloba (GB): Numerous biological effects of GB are mediated via hormesis. Ageing Res. Rev. 2020, 64, 101019. [Google Scholar] [CrossRef]
- Scuto, M.; Ontario, M.L.; Salinaro, A.T.; Caligiuri, I.; Rampulla, F.; Zimbone, V.; Modafferi, S.; Rizzolio, F.; Canzonieri, V.; Calabrese, E.J.; et al. Redox modulation by plant polyphenols targeting vitagenes for chemoprevention and therapy: Relevance to novel anti-cancer interventions and mini-brain organoid technology. Free Radic. Biol. Med. 2022, 179, 59–75. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Tao, Y.; Wang, M.; Huang, F.; Wu, X. Natural compounds as potential therapeutic candidates for multiple sclerosis: Emerging preclinical evidence. Phytomedicine 2024, 123, 155248. [Google Scholar] [CrossRef]
- Wang, C.; Wang, B.J.P.R. Ginkgo biloba extract attenuates oxidative stress and apoptosis in mouse cochlear neural stem cells. Phytother. Res. 2016, 30, 774–780. [Google Scholar] [CrossRef]
- Wang, A.; Yang, Q.; Li, Q.; Wang, X.; Hao, S.; Wang, J.; Ren, M. Ginkgo biloba L. Extract Reduces H2O2-Induced Bone Marrow Mesenchymal Stem Cells Cytotoxicity by Regulating Mitogen-Activated Protein Kinase (MAPK) Signaling Pathways and Oxidative Stress. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 3159. [Google Scholar] [CrossRef]
- Zuo, W.; Yan, F.; Zhang, B.; Li, J.; Mei, D. Advances in the Studies of Ginkgo biloba Leaves Extract on Aging-Related Diseases. Aging Dis. 2017, 8, 812–826. [Google Scholar] [CrossRef]
- Fehske, C.J.; Leuner, K.; Müller, W.E. Ginkgo biloba extract (EGb761) influences monoaminergic neurotransmission via inhibition of NE uptake, but not MAO activity after chronic treatment. Pharmacol. Res. 2009, 60, 68–73. [Google Scholar] [CrossRef]
- Naoi, M.; Wu, Y.; Maruyama, W.; Shamoto-Nagai, M. Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases. Int. J. Mol. Sci. 2025, 26, 2916. [Google Scholar] [CrossRef]
- Yoshitake, T.; Yoshitake, S.; Kehr, J. The Ginkgo biloba extract EGb 761(R) and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br. J. Pharmacol. 2010, 159, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: A review for use in cardiovascular diseases. J. Ginseng Res. 2018, 42, 264–269. [Google Scholar] [CrossRef]
- Ogawa-Ochiai, K.; Kawasaki, K. Panax ginseng for Frailty-Related Disorders: A Review. Front. Nutr. 2019, 5, 140. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362372. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Colalto, C.; Delfino, D.V.; Iriti, M.; Izzo, A.A. Herbal Dietary Supplements for Erectile Dysfunction: A Systematic Review and Meta-Analysis. Drugs 2018, 78, 643–673. [Google Scholar] [CrossRef]
- Arring, N.M.; Millstine, D.; Marks, L.A.; Nail, L.M. Ginseng as a Treatment for Fatigue: A Systematic Review. J. Altern. Complement. Med. 2018, 24, 624–633. [Google Scholar] [CrossRef]
- Lee, H.W.; Lim, H.J.; Jun, J.H.; Choi, J.; Lee, M.S. Ginseng for Treating Hypertension: A Systematic Review and Meta-Analysis of Double Blind, Randomized, Placebo-Controlled Trials. Curr. Vasc. Pharmacol. 2017, 15, 549–556. [Google Scholar] [CrossRef]
- Gui, Q.F.; Xu, Z.R.; Xu, K.Y.; Yang, Y.M. The Efficacy of Ginseng-Related Therapies in Type 2 Diabetes Mellitus: An Updated Systematic Review and Meta-Analysis. Medicine 2016, 95, e2584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, G.; Gong, J.; Lu, F.; Diao, Q.; Sun, J.; Zhang, K.; Tian, J.; Liu, J. Ginseng for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Top. Med. Chem. 2016, 16, 529–536. [Google Scholar] [CrossRef]
- Lee, N.H.; Jung, H.C.; Lee, S. Red Ginseng as an Ergogenic Aid: A Systematic Review of Clinical Trials. J. Exerc. Nutr. Biochem. 2016, 20, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Choi, H.-K.; Huang, L. State of Panax ginseng Research: A Global Analysis. Molecules 2017, 22, 1518. [Google Scholar] [CrossRef]
- Smith, I.; Williamson, E.M.; Putnam, S.; Farrimond, J.; Whalley, B.J. Effects and mechanisms of ginseng and ginsenosides on cognition. Nutr. Rev. 2014, 72, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Kezhu, W.; Pan, X.; Cong, L.; Liming, D.; Beiyue, Z.; Jingwei, L.; Yanyan, Y.; Xinmin, L. Effects of Ginsenoside Rg1 on Learning and Memory in a Reward-directed Instrumental Conditioning Task in Chronic Restraint Stressed Rats. Phytother. Res. 2017, 31, 81–89. [Google Scholar] [CrossRef]
- Lu, C.; Shi, Z.; Dong, L.; Lv, J.; Xu, P.; Li, Y.; Qu, L.; Liu, X. Exploring the Effect of Ginsenoside Rh1 in a Sleep Deprivation-Induced Mouse Memory Impairment Model. Phytother. Res. 2017, 31, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.H.; Zhang, Y.; Ding, G.N.; Hong, F.X.; Dong, P.; Tian, M. Ginsenoside Rb1 Attenuates Isoflurane/surgery-induced Cognitive Dysfunction via Inhibiting Neuroinflammation and Oxidative Stress. Biomed. Environ. Sci. 2017, 30, 363–372. [Google Scholar]
- Lu, C.; Lv, J.; Dong, L.M.; Jiang, N.; Wang, Y.; Fan, B.; Wang, F.; Liu, X. Neuroprotective Effect of Ginsenoside Rh1 on Scopolamine-Induced Cognitive Dysfunctions. Neuropsychiatry 2018, 8, 749–760. [Google Scholar]
- Hou, J.; Xue, J.; Wang, Z.; Li, W. Ginsenoside Rg3 and Rh2 protect trimethyltin-induced neurotoxicity via prevention on neuronal apoptosis and neuroinflammation. Phytother. Res. 2018, 32, 2531–2540. [Google Scholar] [CrossRef]
- Makkar, R.; Behl, T.; Bungau, S.; Zengin, G.; Mehta, V.; Kumar, A.; Uddin, M.S.; Ashraf, G.M.; Abdel-Daim, M.M.; Arora, S.; et al. Nutraceuticals in Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 4424. [Google Scholar] [CrossRef]
- Chauhan, N.B.; Mehla, J. Ameliorative Effects of Nutraceuticals in Neurological Disorders. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Watson, R.R., Preedy, V.R., Eds.; Elsevier: London, UK, 2015; pp. 245–260. [Google Scholar]
- Preethi Pallavi, M.C.; Sampath Kumar, H.M. Nutraceuticals in Prophylaxis and Therapy of Neurodegenerative Diseases. In Discovery and Development of Neuroprotective Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: London, UK, 2018; pp. 359–376. [Google Scholar]
- Chiu, H.F.; Venkatakrishnan, K.; Wang, C.K. The role of nutraceuticals as a complementary therapy against various neurodegenerative diseases: A mini-review. J. Tradit. Complement. Med. 2020, 10, 434–439. [Google Scholar] [CrossRef]
- Shukla, A.K.; Nilgirwar, P.S.; Bali, S.D. Current pharmacological treatments for neurodegenerative diseases. In The Neurodegeneration Revolution; Emerging Therapies and Sustainable Solutions; Koduru, T.S., Osmani, R.A.M., Singh, E., Dutta, S., Eds.; Academic Press: New York, NY, USA, 2024. [Google Scholar]
- Davì, F.; Iaconis, A.; Cordaro, M.; Di Paola, R.; Fusco, R. Nutraceutical Strategies for Targeting Mitochondrial Dysfunction in Neurodegenerative Diseases. Foods 2025, 14, 2193. [Google Scholar] [CrossRef]
- Da Silva, T.M.; Munhoz, R.P.; Alvarez, C.; Naliwaiko, K.; Kiss, Á.; Andreatini, R.; Ferraz, A.C. Depression in Parkinson’s Disease: A Double-Blind, Randomized, Placebo-Controlled Pilot Study of Omega-3 Fatty-Acid Supplementation. J. Affect. Disord. 2008, 111, 351–359. [Google Scholar] [CrossRef]
- de Waal, H.; Stam, C.J.; Lansbergen, M.M.; Wieggers, R.L.; Kamphuis, P.J.; Scheltens, P.; Maestú, F.; van Straaten, E.C. The effect of souvenaid on functional brain network organisation in patients with mild Alzheimer’s disease: A randomised controlled study. PLoS ONE 2014, 9, e86558. [Google Scholar] [CrossRef]
- Eriksdotter, M.; Vedin, I.; Falahati, F.; Freund-Levi, Y.; Hjorth, E.; Faxen-Irving, G.; Wahlund, L.O.; Schultzberg, M.; Basun, H.; Cederholm, T.; et al. Plasma Fatty Acid Profiles in Relation to Cognition and Gender in Alzheimer’s Disease Patients During Oral Omega-3 Fatty Acid Supplementation: The OmegAD Study. J. Alzheimers Dis. 2015, 48, 805–812. [Google Scholar] [CrossRef]
- Jernerén, F.; Cederholm, T.; Refsum, H.; Smith, A.D.; Turner, C.; Palmblad, J.; Eriksdotter, M.; Hjorth, E.; Faxen-Irving, G.; Wahlund, L.O.; et al. Homocysteine Status Modifies the Treatment Effect of Omega-3 Fatty Acids on Cognition in a Randomized Clinical Trial in Mild to Moderate Alzheimer’s Disease: The OmegAD Study. J. Alzheimers Dis. 2019, 69, 189–197. [Google Scholar] [CrossRef]
- Olde Rikkert, M.G.; Verhey, F.R.; Blesa, R.; von Arnim, C.A.; Bongers, A.; Harrison, J.; Sijben, J.; Scarpini, E.; Vandewoude, M.F.; Vellas, B.; et al. Tolerability and Safety of Souvenaid in Patients with Mild Alzheimer’s Disease: Results of Multi-Center, 24-Week, Open-Label Extension Study. J. Alzheimers Dis. 2015, 44, 471–480. [Google Scholar] [CrossRef]
- Phillips, M.A.; Childs, C.E.; Calder, P.C.; Rogers, P.J. No Effect of Omega-3 Fatty Acid Supplementation on Cognition and Mood in Individuals with Cognitive Impairment and Probable Alzheimer’s Disease: A Randomised Controlled Trial. Int. J. Mol. Sci. 2015, 16, 24600–24613. [Google Scholar] [CrossRef] [PubMed]
- Pomponi, M.; Loria, G.; Salvati, S.; Di Biase, A.; Conte, G.; Villella, C.; Righino, E.; Ciciarelli, C.; Bria, P.; La Torre, G.; et al. DHA Effects in Parkinson Disease Depression. Basal Ganglia 2014, 4, 61–66. [Google Scholar] [CrossRef]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R., Jr.; Weiner, M.; et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: A randomized trial. JAMA 2010, 304, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Shinto, L.; Quinn, J.; Montine, T.; Dodge, H.H.; Woodward, W.; Baldauf-Wagner, S.; Waichunas, D.; Bumgarner, L.; Bourdette, D.; Silbert, L.; et al. A Randomized Placebo-Controlled Pilot Trial of Omega-3 Fatty Acids and Alpha Lipoic Acid in Alzheimer’s disease. J. Alzheimers Dis. 2014, 38, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Daneshvar Kakhaki, R.; Bahmani, F.; Abolhassani, J.; Aarabi, M.H.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017, 108, 183–189. [Google Scholar] [CrossRef]
- Wang, X.; Hjorth, E.; Vedin, I.; Eriksdotter, M.; Freund-Levi, Y.; Wahlund, L.O.; Cederholm, T.; Palmblad, J.; Schultzberg, M. Effects of n-3 FA supplementation on the release of proresolving lipid mediators by blood mononuclear cells: The OmegAD study. J. Lipid Res. 2015, 56, 674–681. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.; Cheung, S.K. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer’s disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2014, 29, 642–651. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef]
- Chowdhury, D.; Roy, A.K.; Reddy, V.R.; Gupta, Y.K.; Nigam, P.; Hoerr, R. Multicenter, Open-Label, Prospective Study Shows Safety and Therapeutic Benefits of a Defined Ginkgo biloba Extract for Adults with Major Neurocognitive Disorder. Dement. Geriatr. Cogn. Disord. 2024, 53, 299–309. [Google Scholar] [CrossRef]
- García-Alberca, J.M.; Gris, E.; Mendoza, S. Combined treatment with Ginkgo biloba extract EGb 761 plus acetylcholinesterase inhibitors improved cognitive function and neuropsychiatric symptoms in patients with mild cognitive impairment. Alzheimers Dement. 2022, 8, e12338. [Google Scholar] [CrossRef] [PubMed]
- Herrschaft, H.; Nacu, A.; Likhachev, S.; Sholomov, I.; Hoerr, R.; Schlaefke, S. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J. Psychiatr. Res. 2012, 46, 716–723. [Google Scholar] [CrossRef]
- Ihl, R.; Bachinskaya, N.; Korczyn, A.D.; Vakhapova, V.; Tribanek, M.; Hoerr, R.; Napryeyenko, O.; GOTADAY Study Group. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2011, 26, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, G.; Yancheva, S.; Raychev, I.; Hoerr, R. PLAGIN Study Group Ginkgo biloba Extract in Dementia: A 22-Week Randomised, Placebo-Controlled, Double-Blind Trial. Bulg. Neurol. 2013, 14, 139–143. [Google Scholar]
- Rapp, M.; Burkart, M.; Kohlmann, T.; Bohlken, J. Similar treatment outcomes with Ginkgo biloba extract EGb 761 and donepezil in Alzheimer’s dementia in very old age: A retrospective observational study. Int. J. Clin. Pharmacol. Ther. 2018, 56, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Coley, N.; Ousset, P.J.; Berrut, G.; Dartigues, J.F.; Dubois, B.; Grandjean, H.; Pasquier, F.; Piette, F.; Robert, P. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. Lancet Neurol. 2012, 11, 851–859. [Google Scholar] [CrossRef]
- Yancheva, S.; Ihl, R.; Nikolova, G.; Panayotov, P.; Schlaefke, S.; Hoerr, R.; GINDON Study Group. Ginkgo biloba extract EGb 761(R), donepezil or both combined in the treatment of Alzheimer’s disease with neuropsychiatric features: A randomised, double-blind, exploratory trial. Aging Ment. Health 2009, 13, 183–190. [Google Scholar]
- Cheng, W.; Chen, W.; Wang, P.; Chu, J. Asiatic acid protects differentiated PC12 cells from Aβ25-35-induced apoptosis and tau hyperphosphorylation via regulating PI3K/Akt/GSK-3β signaling. Life Sci. 2018, 208, 96–101. [Google Scholar] [CrossRef]
- Guo, J.; Xue, J.; Ding, Z.; Li, X.; Wang, X.; Xue, H. Activated Phosphoinositide 3-Kinase/Akt/Mammalian Target of Rapamycin Signal and Suppressed Autophagy Participate in Protection Offered by Licochalcone A Against Amyloid-β Peptide Fragment 25-35-Induced Injury in SH-SY5Y Cells. World Neurosurg. 2022, 157, e390–e400. [Google Scholar] [CrossRef]
- Hu, S.; Han, R.; Mak, S.; Han, Y. Protection against 1-methyl-4-phenylpyridinium ion (MPP+)-induced apoptosis by water extract of ginseng (Panax ginseng C.A. Meyer) in SH-SY5Y cells. J. Ethnopharmacol. 2011, 135, 34–42. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Shim, J.S.; Song, M.Y.; Yim, S.V.; Lee, S.E.; Park, K.S. Proteomic analysis reveals that the protective effects of ginsenoside Rb1 are associated with the actin cytoskeleton in β-amyloid-treated neuronal cells. J. Ginseng Res. 2016, 40, 278–284. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.Y.; Zhao, J.; Dong, Z.; Feng, D.Y.; Wu, R.; Shi, M.; Zhao, G. Ginsenoside Rd Protects SH-SY5Y Cells against 1-Methyl-4-phenylpyridinium Induced Injury. Int. J. Mol. Sci. 2015, 16, 14395–14408. [Google Scholar] [CrossRef]
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. Br. J. Pharmacol. 2020, 177, 1227–1240. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications -A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Choi, C.I. Astaxanthin as a Peroxisome Proliferator-Activated Receptor (PPAR) Modulator: Its Therapeutic Implications. Mar. Drugs 2019, 17, 242. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential Anti-Atherosclerotic Properties of Astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Brown, D.R.; Gough, L.A.; Deb, S.K.; Sparks, S.A.; McNaughton, L.R. Astaxanthin in Exercise Metabolism, Performance and Recovery: A Review. Front. Nutr. 2018, 4, 76. [Google Scholar] [CrossRef]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef]
- Crippa, J.A.; Guimaraes, F.S.; Campos, A.C.; Zuardi, A.W. Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front. Immunol. 2018, 9, 2009. [Google Scholar] [CrossRef]
- Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Pina, S.D.; Tambaro, S.; Memo, M.; Mastinu, A. Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019, 224, 120–127. [Google Scholar] [CrossRef]
- Rong, C.; Lee, Y.; Carmona, N.E.; Cha, D.S.; Ragguett, R.M.; Rosenblat, J.D.; Mansur, R.B.; Ho, R.C.; McIntyre, R.S. Cannabidiol in medical marijuana: Research vistas and potential opportunities. Pharmacol. Res. 2017, 121, 213–218. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S.; Cannabidiol in Dravet Syndrome Study Group. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef]
- Silote, G.P.; Sartim, A.; Sales, A.; Eskelund, A.; Guimarães, F.S.; Wegener, G.; Joca, S. Emerging evidence for the antidepressant effect of cannabidiol and the underlying molecular mechanisms. J. Chem. Neuroanat. 2019, 98, 104–116. [Google Scholar] [CrossRef]
- Lee, J.L.C.; Bertoglio, L.J.; Guimaraes, F.S.; Stevenson, C.W. Cannabidiol regulation of emotion and emotional memory processing: Relevance for treating anxiety-related and substance abuse disorders. Br. J. Pharmacol. 2017, 174, 3242–3256. [Google Scholar] [CrossRef]
- Renard, J.; Norris, C.; Rushlow, W.; Laviolette, S.R. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: Implications for novel schizophrenia treatments. Neurosci. Biobehav. Rev. 2017, 75, 157–165. [Google Scholar] [CrossRef]
- Lowin, T.; Schneider, M.; Pongratz, G. Joints for joints: Cannabinoids in the treatment of rheumatoid arthritis. Curr. Opin. Rheumatol. 2019, 31, 271–278. [Google Scholar] [CrossRef]
- Ward, S.J.; McAllister, S.D.; Kawamura, R.; Murase, R.; Neelakantan, H.; Walker, E.A. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br. J. Pharmacol. 2014, 171, 636–645. [Google Scholar] [CrossRef]
- Jesus, C.H.A.; Redivo, D.D.B.; Gasparin, A.T.; Sotomaior, B.B.; de Carvalho, M.C.; Genaro, K.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Zanoveli, J.M.; et al. Cannabidiol attenuates mechanical allodynia in streptozotocin-induced diabetic rats via serotonergic system activation through 5-HT1A receptors. Brain Res. 2019, 1715, 156–164. [Google Scholar] [CrossRef]
- Belardo, C.; Iannotta, M.; Boccella, S.; Rubino, R.C.; Ricciardi, F.; Infantino, R.; Pieretti, G.; Stella, L.; Paino, S.; Marabese, I.; et al. Oral Cannabidiol Prevents Allodynia and Neurological Dysfunctions in a Mouse Model of Mild Traumatic Brain Injury. Front. Pharmacol. 2019, 10, 352. [Google Scholar] [CrossRef]
- De Gregorio, D.; McLaughlin, R.J.; Posa, L.; Ochoa-Sanchez, R.; Enns, J.; Lopez-Canul, M.; Aboud, M.; Maione, S.; Comai, S.; Gobbi, G. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain 2019, 160, 136–150. [Google Scholar] [CrossRef]
- Booz, G.W. Cannabidiol as an Emergent Therapeutic Strategy for Lessening the Impact of Inflammation on Oxidative Stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. [Google Scholar] [CrossRef]
- Khoury, J.M.; Neves, M.C.L.D.; Roque, M.A.V.; Queiroz, D.A.B.; Corrêa de Freitas, A.A.; de Fátima, Â.; Moreira, F.A.; Garcia, F.D. Is there a role for cannabidiol in psychiatry? World J. Biol. Psychiatry 2019, 20, 101–116. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. A Holistic Review of Cannabis and Its Potential Risks and Benefits in Mental Health. Psychiatry Int. 2025, 6, 92. [Google Scholar] [CrossRef]
- White, C.M. A Review of Human Studies Assessing Cannabidiol’s (CBD) Therapeutic Actions and Potential. J. Clin. Pharmacol. 2019, 59, 923–934. [Google Scholar] [CrossRef]
- Naftali, T.; Mechulam, R.; Marii, A.; Gabay, G.; Stein, A.; Bronshtain, M.; Laish, I.; Benjaminov, F.; Konikoff, F.M. Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn’s Disease, A Randomized Controlled Trial. Dig. Dis. Sci. 2017, 62, 1615–1620. [Google Scholar] [CrossRef]
- Irving, P.M.; Iqbal, T.; Nwokolo, C.; Subramanian, S.; Bloom, S.; Prasad, N.; Hart, A.; Murray, C.; Lindsay, J.O.; Taylor, A.; et al. A Randomized, Double-blind, Placebo-controlled, Parallel-group, Pilot Study of Cannabidiol-rich Botanical Extract in the Symptomatic Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 714–724. [Google Scholar] [CrossRef]
- Pandey, A.K.; Chauhan, O.P. Monk fruit (Siraitia grosvenorii)—Health aspects and food applications. Pantnagar J. Res. 2019, 17, 191–198. [Google Scholar]
- Tao, L.; Cao, F.; Xu, G.; Xie, H.; Zhang, M.; Zhang, C. Mogroside IIIE Attenuates LPS-Induced Acute Lung Injury in Mice Partly Through Regulation of the TLR4/MAPK/NF-κB Axis via AMPK Activation. Phytother. Res. 2017, 31, 1097–1106. [Google Scholar] [CrossRef]
- Engels, G.; Brinkmann, J. Nigella—Nigella sativa, Family Ranunculaceae. Herbal Profile 2017, 114, 8–16. [Google Scholar]
- Zou, C.; Zhang, Q.; Zhang, S. Mogroside IIIE attenuates gestational diabetes mellitus through activating of AMPK signaling pathway in mice. J. Pharmacol. Sci. 2018, 138, 161–166. [Google Scholar] [CrossRef]
- Tey, S.L.; Salleh, N.B.; Henry, C. Effects of aspartame-, monk fruit-, stevia- and sucrose- sweetened beverages on post-prandial glucose, insulin and energy intake. Int. J. Obes. 2017, 41, 450–457. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Havakhah, S.; Boskabady, M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J. Ethnopharmacol. 2016, 90, 372–386. [Google Scholar] [CrossRef]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, Molecular Pharmacology, and Safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef]
- Askari, G.; Rouhani, M.H.; Ghaedi, E.; Ghavami, A.; Nouri, M.; Mohammadi, H. Effect of Nigella sativa (black seed) supplementation on glycemic control: A systematic review and meta-analysis of clinical trials. Phytother. Res. 2019, 33, 1341–1352. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Ayati, M.H.; Abdollahi, M. The effects of Nigella sativa L. on obesity: A systematic review and meta-analysis. J. Ethnopharmacol. 2018, 219, 173–181. [Google Scholar] [CrossRef]
- Sahebkar, A.; Beccuti, G.; Simental-Mendía, L.E.; Nobili, V.; Bo, S. Nigella sativa (black seed) effects on plasma lipid concentrations in humans: A systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol. Res. 2016, 106, 37–50. [Google Scholar] [CrossRef]
- Sahebkar, A.; Soranna, D.; Liu, X.; Thomopoulos, C.; Simental-Mendia, L.E.; Derosa, G.; Maffioli, P.; Parati, G. A systematic review and meta-analysis of randomized controlled trials investigating the effects of supplementation with Nigella sativa (black seed) on blood pressure. J. Hypertens. 2016, 34, 2127–2135. [Google Scholar] [CrossRef]
- Ardakani Movaghati, M.R.; Yousefi, M.; Saghebi, S.A.; Sadeghi Vazin, M.; Iraji, A.; Mosavat, S.H. Efficacy of black seed (Nigella sativa L.) on kidney stone dissolution: A randomized, double-blind, placebo-controlled, clinical trial. Phytother. Res. 2019, 33, 1404–1412. [Google Scholar] [CrossRef]
- Nikkhah-Bodaghi, M.; Darabi, Z.; Agah, S.; Hekmatdoost, A. The effects of Nigella sativa on quality of life, disease activity index, and some of inflammatory and oxidative stress factors in patients with ulcerative colitis. Phytother. Res. 2019, 33, 1027–1032. [Google Scholar] [CrossRef]
- Alberts, A.; Moldoveanu, E.T.; Niculescu, A.G.; Grumezescu, A.M. Nigella sativa: A Comprehensive Review of Its Therapeutic Potential, Pharmacological Properties, and Clinical Applications. Int. J. Mol. Sci. 2024, 25, 13410. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.C.; Huus, K.E.; Finlay, B.B. Microbes and the mind: Emerging hallmarks of the gut microbiota-brain axis. Cell. Microbiol. 2016, 18, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef]
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics 2018, 15, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases. Neurochem. Int. 2018, 120, 149–163. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. N. Am. 2017, 46, 77–89. [Google Scholar] [CrossRef]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Appleton, J. The Gut-Brain Axis: Influence of Microbiota on Mood and Mental Health. Integr. Med. 2018, 17, 28–32. [Google Scholar]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Sampson, T.R.; Mazmanian, S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015, 17, 565–576. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wen, Q.; Jiang, J.; Li, H.-L.; Tan, Y.-F.; Li, Y.-H.; Zeng, N.-K. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J. Ethnopharmacol. 2016, 179, 253–264. [Google Scholar] [CrossRef]
- Dhama, K.; Tiwari, R.; Chakrabort, S.; Saminathan, M.; Kumar, A.; Karthik, K.; Wani, M.Y.; Amarpal; Singh, S.V.; Rahal, A. Evidence Based Antibacterial Potentials of Medicinal Plants and Herbs Countering Bacterial Pathogens Especially in the Era of Emerging Drug Resistance: An Integrated Update. Int. J. Pharmacol. 2013, 10, 1–43. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Shah, M.H.; Khan, M.A. Phytochemicals and Nutraceuticals. In Wild Edible Vegetables of Lesser Himalayas; Ethnobotanical and Nutraceutical Aspects; Springer: Cham, Switzerland, 2015; Volume 1, pp. 31–66. [Google Scholar]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneka, K.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Pharmacogenomic and Pharmacomicrobiomic Aspects of Drugs of Abuse. Genes 2025, 16, 403. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R. Rethinking pharmacogenomics in an ecosystem: Drug-microbiome interactions, pharmacomicrobiomics, and personalized medicine for the human supraorganism. Curr. Pharmacogenom. Pers. Med. 2012, 10, 258–261. [Google Scholar] [CrossRef]
- Kan, J.; Wu, F.; Wang, F.; Zheng, J.; Cheng, J.; Li, Y.; Yang, Y.; Du, J. Phytonutrients: Sources, bioavailability, interaction with gut microbiota, and their impacts on human health. Front. Nutr. 2022, 9, 960309. [Google Scholar] [CrossRef]
- Rathaur, P.; Johar, K.S.R. Metabolism and Pharmacokinetics of Phytochemicals in the Human Body. Curr. Drug Metab. 2019, 20, 1085–1102. [Google Scholar] [CrossRef]
- Dey, P. Gut microbiota in phytopharmacology: A comprehensive overview of concepts, reciprocal interactions, biotransformations and mode of actions. Pharmacol. Res. 2019, 147, 104367. [Google Scholar] [CrossRef] [PubMed]
- Koppel, N.; Maini Rekdal, V.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, eaag2770. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Young, J.D. Phytochemicals as prebiotics and biological stress inducers. Trends Biochem. Sci. 2020, 45, 462–471. [Google Scholar] [CrossRef]
- Pferschy-Wenzig, E.M.; Pausan, M.R.; Ardjomand-Woelkart, K.; Röck, S.; Ammar, R.M.; Kelber, O.; Moissl-Eichinger, C.; Bauer, R. Medicinal Plants and Their Impact on the Gut Microbiome in Mental Health: A Systematic Review. Nutrients 2022, 14, 2111. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Chen, H.; Hu, J.; Fan, S.; Nie, S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, F.; Abas, F.; Lajis, N.H.; Othman, I.; Naidu, R. Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer. Biomolecules 2019, 9, 270. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation Between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Pandey, A.; Chaturvedi, M.; Mishra, S.; Kumar, P.; Somvanshi, P.; Chaturvedi, R. Reductive metabolites of curcumin and their therapeutic effects. Heliyon 2020, 6, e05469. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Ulamek-Koziol, M. Mutual Two-Way Interactions of Curcumin and Gut Microbiota. Int. J. Mol. Sci. 2020, 21, 1055. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ji, H.F. Bidirectional interactions between dietary curcumin and gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 2896–2902. [Google Scholar] [CrossRef]
- Burapan, S.; Kim, M.; Han, J. Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota. J. Agric. Food Chem. 2017, 65, 3305–3310. [Google Scholar] [CrossRef]
- Peterson, C.T.; Vaughn, A.R.; Sharma, V.; Chopra, D.; Mills, P.J.; Peterson, S.N.; Sivamani, R.K. Effects of Turmeric and Curcumin Dietary Supplementation on Human Gut Microbiota: A Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Evid. Based Integr. Med. 2018, 23, 2515690X18790725. [Google Scholar] [CrossRef]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef]
- Zam, W. Gut Microbiota as a Prospective Therapeutic Target for Curcumin: A Review of Mutual Influence. J. Nutr. Metab. 2018, 2018, 1367984. [Google Scholar] [CrossRef] [PubMed]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Rupasinghe, T.W.; Tull, D.L.; Boughton, B.; Oliver, C.; McSweeny, C.; Gras, S.L.; Augustin, M.A. Degradation of curcuminoids by in vitro pure culture fermentation. J. Agric. Food Chem. 2014, 62, 11005–11015. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Zheng, J.; Hu, H.; Lee, J.; Zeng, S. Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 985, 38–47. [Google Scholar] [CrossRef]
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. Intestinal Microbiota and Metabolic Diseases: Pharmacological Implications. Trends Pharmacol. Sci. 2016, 37, 169–171. [Google Scholar] [CrossRef]
- Liu, J.; Luo, W.; Chen, Q.; Chen, X.; Zhou, G.; Sun, H. Curcumin sensitizes response to cytarabine in acute myeloid leukemia by regulating intestinal microbiota. Cancer Chemother. Pharmacol. 2022, 89, 243–253. [Google Scholar] [CrossRef]
- Li, R.; Yao, Y.; Gao, P.; Bu, S. The Therapeutic Efficacy of Curcumin vs. Metformin in Modulating the Gut Microbiota in NAFLD Rats: A Comparative Study. Front. Microbiol. 2020, 11, 555293. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.G.; Hu, J. Effect of Curcumin on the Diversity of Gut Microbiota in Ovariectomized Rats. Nutrients 2017, 9, 1146. [Google Scholar] [CrossRef]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzinska, B. Curcumin and Its Potential Impact on Microbiota. Nutrients 2021, 13, 2004. [Google Scholar] [CrossRef]
- Di Meo, F.; Donato, S.; Di Pardo, A.; Maglione, V.; Filosa, S.; Crispi, S. New Therapeutic Drugs from Bioactive Natural Molecules: The Role of Gut Microbiota Metabolism in Neurodegenerative Diseases. Curr. Drug Metab. 2018, 19, 478–489. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, M.; Seth, P.; Sharma, S.K. Tetrahydrocurcumin confers protection against amyloid beta-induced toxicity. Neuroreport 2011, 22, 23–27. [Google Scholar] [CrossRef]
- Sun, Z.Z.; Li, X.Y.; Wang, S.; Shen, L.; Ji, H.F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Maes, M.; Maker, G.L.; Hood, S.D.; Drummond, P.D. Curcumin for the treatment of major depression: A randomised, double-blind, placebo controlled study. J. Affect. Disord. 2014, 167, 368–375. [Google Scholar] [CrossRef]
- Sanmukhani, J.; Satodia, V.; Trivedi, J.; Patel, T.; Tiwari, D.; Panchal, B.; Goel, A.; Tripathi, C.B. Efficacy and safety of curcumin in major depressive disorder: A randomized controlled trial. Phytother. Res. 2014, 28, 579–585, Correction in Phytother. Res. 2024, 38, 2594. [Google Scholar] [CrossRef]
- Yu, S.; Chun, E.; Ji, Y.; Lee, Y.J.; Jin, M. Effects of red ginseng on gut, microbiota, and brain in a mouse model of post-infectious irritable bowel syndrome. J. Ginseng Res. 2021, 45, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, S.H.; Kim, M.S.; Ahn, K.S.; Kim, M. Effect of Lactobacillus dominance modified by Korean Red Ginseng on the improvement of Alzheimer’s disease in mice. J. Ginseng Res. 2022, 46, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Lee, D.Y.; Kim, J.Y.; Heo, K.; Shim, J.J.; Lee, J.L.; Kim, D.H. Effect of fermented red ginseng on gut microbiota dysbiosis- or immobilization stress-induced anxiety, depression, and colitis in mice. J. Ginseng Res. 2023, 47, 255–264, Correction in J. Ginseng Res. 2023, 47, 683. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, M.; Li, X.; Zhao, D.; Wang, M. Microbiota, co-metabolites, and network pharmacology reveal the alteration of the ginsenoside fraction on inflammatory bowel disease. J. Ginseng Res. 2023, 47, 54–64. [Google Scholar] [CrossRef]
- Iqbal, H.; Kim, Y.; Jin, M.; Rhee, D.K. Ginseng as a therapeutic target to alleviate gut and brain diseases via microbiome regulation. J. Ginseng Res. 2025, 49, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Z.; Liu, J.; Qi, H.; Li, J.; Chen, J.; Huang, Q.; Liu, Q.; Mi, J.; Li, X. Gut Microbiota: Therapeutic Targets of Ginseng Against Multiple Disorders and Ginsenoside Transformation. Front. Cell. Infect. Microbiol. 2022, 12, 853981. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Zuo, T.T.; Hu, Y.; Li, Z.; Wang, H.D.; Xu, X.Y.; Yang, W.Z.; Guo, D.A. Advances and challenges in ginseng research from 2011 to 2020: The phytochemistry, quality control, metabolism, and biosynthesis. Nat. Prod. Rep. 2022, 39, 875–909. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Fu, R.; Duan, Z.; Wang, P.; Zhu, C.; Fan, D. Ginsenoside Rk3 alleviates gut microbiota dysbiosis and colonic inflammation in antibiotic-treated mice. Food Res. Int. 2021, 146, 110465. [Google Scholar] [CrossRef]
- Bai, X.; Fu, R.; Duan, Z.; Liu, Y.; Zhu, C.; Fan, D. Ginsenoside Rh4 alleviates antibiotic-induced intestinal inflammation by regulating the TLR4-MyD88-MAPK pathway and gut microbiota composition. Food Funct. 2021, 12, 2874–2885. [Google Scholar] [CrossRef]
- Li, J.M.; Yu, R.; Zhang, L.P.; Wen, S.Y.; Wang, S.J.; Zhang, X.Y.; Xu, Q.; Kong, L.D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef]
- Lee, S.; Jung, S.; You, H.; Lee, Y.; Park, Y.; Lee, H.; Hyun, S. Effect of Fermented Red Ginseng Concentrate Intake on Stool Characteristic, Biochemical Parameters, and Gut Microbiota in Elderly Korean Women. Nutrients 2022, 14, 1693. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Wei, R.; Xie, X.; Wang, C.; Fan, S.; Zhang, X.; Su, J.; Liu, J.; Jia, W.; et al. Metabolome and gut microbiota variation with long-term intake of Panax ginseng extracts on rats. Food Funct. 2018, 9, 3547–3556. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, D.H. Lactobacillus mucosae and Bifidobacterium longum synergistically alleviate immobilization stress-induced anxiety/depression in mice by suppressing gut dysbiosis. J. Microbiol. Biotechnol. 2019, 29, 1369–1374. [Google Scholar] [CrossRef]
- Zhao, L.; Sui, M.; Zhang, T.; Zhang, K. The interaction between ginseng and gut microbiota. Front. Nutr. 2023, 10, 1301468. [Google Scholar] [CrossRef]
- Lee, S.; Rhee, D.K. Effects of ginseng on stress-related depression, anxiety, and the hypothalamic-pituitary-adrenal axis. J. Ginseng Res. 2017, 41, 589–594. [Google Scholar] [CrossRef]
- Seong, E.; Bose, S.; Han, S.Y.; Song, E.J.; Lee, M.; Nam, Y.D.; Kim, H. Positive influence of gut microbiota on the effects of Korean red ginseng in metabolic syndrome: A randomized, double-blind, placebo-controlled clinical trial. EPMA J. 2021, 12, 177–197. [Google Scholar] [CrossRef]
- Kim, Y.K.; Yum, K.S. Effects of red ginseng extract on gut microbial distribution. J. Ginseng Res. 2022, 46, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Xie, M.Z. A review of a potential and promising probiotic candidate-Akkermansia muciniphila. J. Appl. Microbiol. 2021, 130, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Xu, J.; Zhu, H.; Wu, J.; Xu, J.D.; Yan, R.; Li, X.Y.; Liu, H.H.; Duan, S.M.; Wang, Z.; et al. Gut microbiota-involved mechanisms in enhancing systemic exposure of ginsenosides by coexisting polysaccharides in ginseng decoction. Sci. Rep. 2016, 6, 22474. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shen, J.; Li, H.; Zheng, X.; Kang, D.; Xu, Y.; Chen, C.; Guo, H.; Xie, L.; Wang, G.; et al. Ginsenoside Rb1 exerts neuroprotective effects through regulation of Lactobacillus helveticus abundance and GABAA receptor expression. J. Ginseng Res. 2020, 44, 86–95. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Xiao, H.; Ye, J.; Cao, L.; Li, W.; Sun, G. Advancements in research on the effects of panax notoginseng saponin constituents in ameliorating learning and memory disorders. Heliyon 2024, 10, e28581. [Google Scholar] [CrossRef]
- Bell, L.; Whyte, A.; Duysburgh, C.; Marzorati, M.; Van den Abbeele, P.; Le Cozannet, R.; Fança-Berthon, P.; Fromentin, E.; Williams, C. A randomized, placebo-controlled trial investigating the acute and chronic benefits of American Ginseng (Cereboost®) on mood and cognition in healthy young adults, including in vitro investigation of gut microbiota changes as a possible mechanism of action. Eur. J. Nutr. 2022, 61, 413–428. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.F.; Huang, L.; et al. Altered gut microbiota in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2017, 60, 1241–1257. [Google Scholar] [CrossRef]
- Kohler, C.A.; Maes, M.; Slyepchenko, A.; Berk, M.; Solmi, M.; Lanctôt, K.L.; Carvalho, A.F. The Gut-Brain Axis, Including the Microbiome, Leaky Gut and Bacterial Translocation: Mechanisms and Pathophysiological Role in Alzheimer’s Disease. Curr. Pharm. Des. 2016, 22, 6152–6166. [Google Scholar] [CrossRef]
- Jimenez-García, A.M.; Villarino, M.; Arias, N. A systematic review and meta-analysis of basal microbiota and cognitive function in Alzheimer’s disease: A potential target for treatment or a contributor to disease progression? Alzheimers Dement. 2024, 16, e70057. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.; Zeng, Y.; Guo, Y.; Wu, C.; Zhao, H.; Zheng, H.; Jiao, J. Improving Alzheimer’s disease by altering gut microbiota in tree shrews with ginsenoside Rg1. FEMS Microbiol. Lett. 2020, 367, fnaa011. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, X.; Xu, Q.; Wei, G.; Zhang, L.; Fan, Y.; Wen, L.; Liu, Y.; Zhang, T.; Zhang, L.; et al. Qisheng Wan formula ameliorates cognitive impairment of Alzheimer’s disease rat via inflammation inhibition and intestinal microbiota regulation. J. Ethnopharmacol. 2022, 282, 114598. [Google Scholar] [CrossRef]
- Tysnes, O.B.; Storstein, A. Epidemiology of Parkinson’s disease. J. Neural Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.; Koskinen, K.; Paulin, L.; Pekkonen, E.; Haapaniemi, E.; Kaakkola, S.; Eerola-Rautio, J.; Pohja, M.; et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.; Kim, H.Y.; Bae, C.H.; Lee, Y.; Kim, S. Korean red ginseng regulates intestinal tight junction and inflammation in the colon of a Parkinson’s disease mouse model. J. Med. Food 2020, 23, 1231–1237. [Google Scholar] [CrossRef]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of gut microbiota in patients with Parkinson’s disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef]
- Zhou, R.; He, D.; Xie, J.; Zhou, Q.; Zeng, H.; Li, H.; Huang, L. The Synergistic Effects of Polysaccharides and Ginsenosides from American Ginseng (Panax quinquefolius L.) Ameliorating Cyclophosphamide-Induced Intestinal Immune Disorders and Gut Barrier Dysfunctions Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2021, 12, 665901. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.C.; Chiang, H.L.; Liou, J.M.; Chang, C.M.; Lu, T.P.; Chuang, E.Y.; Tai, Y.C.; Cheng, C.; Lin, H.Y.; et al. Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J. Neuroinflamm. 2019, 16, 129. [Google Scholar] [CrossRef]
- Aa, L.; Fei, F.; Tan, Z.; Aa, J.; Wang, G.; Liu, C. The pharmacokinetics study of ginkgolide A, B and the effect of food on bioavailability after oral administration of ginkgolide extracts in beagle dogs. Biomed. Chromatogr. 2018, 32, E4212. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, L.; Xu, F.; Sun, Y.; Du, F.; Ma, X.; Zhong, C.; Li, X.; Wang, F.; Zhang, N.; et al. Systemic and cerebral exposure to and pharmacokinetics of flavonols and terpene lactones after dosing standardized Ginkgo biloba leaf extracts to rats via different routes of administration. Br. J. Pharmacol. 2013, 170, 440–457. [Google Scholar] [CrossRef]
- Yan-Yan, Z.; Li-Li, G.; Guo-Ming, S.; Rong, R.; Jing-Zhen, T. Determination of Ginkgolides A, B, C, J and Bilobalide in Plasma by LC-ESI (-)/MS/MS (QQQ) and its Application to the Pharmacokinetic Study of Ginkgo biloba Extract in Rats. Drug Res. 2016, 66, 520–526. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef]
- Lin, W.; Wang, W.; Yang, H.; Wang, D.; Ling, W. Influence of Intestinal Microbiota on the Catabolism of Flavonoids in Mice. J. Food Sci. 2016, 81, H3026–H3034. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Noor-E-Tabassum; Das, R.; Lami, M.S.; Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Idroes, R.; Mohamed, A.A.; Hossain, M.J.; Dhama, K.; et al. Ginkgo biloba: A Treasure of Functional Phytochemicals with Multimedicinal Applications. Evid. Based Complement. Alternat. Med. 2022, 2022, 8288818. [Google Scholar]
- Zhang, L.; Zhang, S.; Jiang, M.; Ni, X.; Du, M.; Jiang, H.; Bi, M.; Wang, Y.; Liu, C.; Liu, S. Limosilactobacillus reuteri Alleviates Anxiety-like Behavior and Intestinal Symptoms in Two Stressed Mouse Models. Nutrients 2024, 16, 3209. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hei, M.; Kong, L.; Liu, Y.; Yang, Y.; Mu, H.; Zhang, X.; Zhao, S.; Duan, J. One water-soluble polysaccharide from Ginkgo biloba leaves with antidepressant activities via modulation of the gut microbiome. Food Funct. 2019, 10, 8161–8171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Xu, X.; Wang, H.; Wang, D.; Yan, W.; Zhu, J.; Hao, H.; Wang, G.; Cao, L.; et al. Ginkgo biloba extract ameliorates atherosclerosis via rebalancing gut flora and microbial metabolism. Phytother. Res. 2022, 36, 2463–2480. [Google Scholar] [CrossRef] [PubMed]
- Obrenovich, M.; Singh, S.K.; Li, Y.; Perry, G.; Siddiqui, B.; Haq, W.; Reddy, V.P. Natural Product Co-Metabolism and the Microbiota-Gut-Brain Axis in Age-Related Diseases. Life 2022, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xing, Y.; Gao, Q.; Wang, D.; Chen, H.; Wang, H.; Zhang, Y. Ginkgo biloba Extract Drives Gut Flora and Microbial Metabolism Variation in a Mouse Model of Alzheimer’s Disease. Pharmaceutics 2023, 15, 2746. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Venkatanarayanan, N.; Ho, C.Y.X. Clinical use of Hypericum perforatum (St John’s wort) in depression: A meta-analysis. J. Affect. Disord. 2017, 210, 211–221. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Tang, Z.; Shi, X.; Song, Z.; Cao, F.; Wei, P.; Li, M.; Li, X.; Jiang, D.; et al. Improvements in estrogen deficiency-induced hypercholesterolemia by Hypericum perforatum L. extract are associated with gut microbiota and related metabolites in ovariectomized (OVX) rats. Biomed. Pharmacother. 2020, 135, 111131. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, B.; Liu, X.; Shi, K.; Chen, Q. Hyperforin-induced gut microbiota metabolite carbocysteine protects against depressive-like behaviors in mice by modulating the colonic mucus barrier. J. Affect. Disord. 2025, 380, 620–630. [Google Scholar] [CrossRef]
- Tan, J.; Li, X.; Zhu, Y.; Sullivan, M.A.; Deng, B.; Zhai, X.; Lu, Y. Antidepressant Shugan Jieyu Capsule Alters Gut Microbiota and Intestinal Microbiome Function in Rats With Chronic Unpredictable Mild Stress -Induced Depression. Front. Pharmacol. 2022, 13, 828595. [Google Scholar] [CrossRef]
- López, V.; Nielsen, B.; Solas, M.; Ramírez, M.J.; Jäger, A.K. Exploring Pharmacological Mechanisms of Lavender (Lavandula angustifolia) Essential Oil on Central Nervous System Targets. Front. Pharmacol. 2017, 8, 280. [Google Scholar] [CrossRef]
- Diass, K.; Merzouki, M.; Elfazazi, K.; Azzouzi, H.; Challioui, A.; Azzaoui, K.; Hammouti, B.; Touzani, R.; Depeint, F.; Ayerdi Gotor, A.; et al. Essential Oil of Lavandula officinalis: Chemical Composition and Antibacterial Activities. Plants 2023, 12, 1571. [Google Scholar] [CrossRef]
- Islek, Z.; Fikrettin Sahin, F. In vitro Antileishmanial Activity of Lavandula angustifolia Essential Oil on Leishmania infantum Parasites. Experimed 2023, 13, 115–120. [Google Scholar] [CrossRef]
- Yao, N.; He, J.K.; Pan, M.; Hou, Z.F.; Xu, J.J.; Yang, Y.; Tao, J.P.; Huang, S.Y. In Vitro Evaluation of Lavandula angustifolia Essential Oil on Anti-Toxoplasma Activity. Front. Cell. Infect. Microbiol. 2021, 11, 755715. [Google Scholar] [CrossRef]
- Baker, J.; Brown, K.; Rajendiran, E.; Yip, A.; DeCoffe, D.; Dai, C.; Molcan, E.; Chittick, S.A.; Ghosh, S.; Mahmoud, S.; et al. Medicinal lavender modulates the enteric microbiota to protect against Citrobacter rodentium-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G825–G836. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.V.; Lieshchova, M.A.; Brygadyrenko, V.V. Impacts on gut microbiota of rats with high-fat diet supplemented by herbs of Melissa officinalis, Lavandula angustifolia and Salvia officinalis. Regul. Mech. Biosyst. 2023, 14, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiao, D.; Li, C.; Wu, T.; Li, L.; Li, T.; Pan, X.; Liu, Q.; Chi, M.; Li, R.; et al. Lavender essential oil alleviates depressive-like behavior in alcohol-withdrawn rats: Insights from gut metabolites and hippocampal transcriptome analysis. Biomed. Pharmacother. 2024, 176, 116835. [Google Scholar] [CrossRef]
- Jayapala, H.P.S.; Lim, S.Y. N-3 Polyunsaturated Fatty Acids and Gut Microbiota. Comb. Chem. High Throughput Screen. 2023, 26, 892–905. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Zhu, Y.; Huang, Y.; Zhang, Q.; Lin, S.; Fang, C.; Li, L.; Lv, Y.; Mei, W.; et al. Effect of Plant-Derived n-3 Polyunsaturated Fatty Acids on Blood Lipids and Gut Microbiota: A Double-Blind Randomized Controlled Trial. Front. Nutr. 2022, 9, 830960. [Google Scholar] [CrossRef] [PubMed]
- Anavi-Cohen, S.; Tsybina-Shimshilashvili, N.; Zandani, G.; Hovav, R.; Sela, N.; Nyska, A.; Madar, Z. Effects of High Oleic Acid Peanuts on Mice’s Liver and Adipose Tissue Metabolic Parameters and Gut Microbiota Composition. Front. Nutr. 2023, 10, 1205377. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]
- Cao, W.; Wang, C.; Chin, Y.; Chen, X.; Gao, Y.; Yuan, S.; Xue, C.; Wang, Y.; Tang, Q. DHA-phospholipids (DHA-PL) and EPA-phospholipids (EPA-PL) prevent intestinal dysfunction induced by chronic stress. Food Funct. 2019, 10, 277–288. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Kasper, L.H. The Second Brain: Is the Gut Microbiota a Link Between Obesity and Central Nervous System Disorders? Curr. Obes. Rep. 2016, 5, 51–64. [Google Scholar] [CrossRef]
- Warner, D.R.; Warner, J.B.; Hardesty, J.E.; Song, Y.L.; King, T.N.; Kang, J.X.; Chen, C.Y.; Xie, S.; Yuan, F.; Prodhan, M.A.I.; et al. Decreased ω-6:ω-3 PUFA ratio attenuates ethanol-induced alterations in intestinal homeostasis, microbiota, and liver injury. J. Lipid Res. 2019, 60, 2034–2049. [Google Scholar] [CrossRef]
- Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J. Anim. Sci. 2020, 98, skaa086. [Google Scholar] [CrossRef]
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Paul Ross, R.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37. [Google Scholar] [CrossRef]
- An, J.U.; Song, Y.S.; Kim, K.R.; Ko, Y.J.; Yoon, D.Y.; Oh, D.K. Biotransformation of polyunsaturated fatty acids to bioactive hepoxilins and trioxilins by microbial enzymes. Nat. Commun. 2018, 9, 128. [Google Scholar] [CrossRef]
- Blanchard, H.; Pédrono, F.; Boulier-Monthéan, N.; Catheline, D.; Rioux, V.; Legrand, P. Comparative effects of well-balanced diets enriched in α-linolenic or linoleic acids on LC-PUFA metabolism in rat tissues. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 383–389. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243, Correction in Gut 2023, 72, e7. [Google Scholar] [CrossRef] [PubMed]
- Cornick, S.; Tawiah, A.; Chadee, K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015, 3, e982426. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Ahl, D.; Liu, H.; Schreiber, O.; Roos, S.; Phillipson, M.; Holm, L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol. 2016, 217, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef]
- Roussel, C.; Guebara, S.A.B.; Plante, P.L.; Desjardins, Y.; Di Marzo, V.; Silvestri, C. Short-term supplementation with ω-3 polyunsaturated fatty acids modulates primarily mucolytic species from the gut luminal mucin niche in a human fermentation system. Gut Microbes 2022, 14, 2120344. [Google Scholar] [CrossRef]
- Ghosh, S.; DeCoffe, D.; Brown, K.; Rajendiran, E.; Estaki, M.; Dai, C.; Yip, A.; Gibson, D.L. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 2013, 8, e55468. [Google Scholar] [CrossRef]
- Wan, J.; Hu, S.; Jacoby, J.J.; Liu, J.; Zhang, Y.; Yu, L.L. The impact of dietary sn-2 palmitic triacylglycerols in combination with docosahexaenoic acid or arachidonic acid on lipid metabolism and host faecal microbiota composition in Sprague Dawley rats. Food Funct. 2017, 8, 1793–1802. [Google Scholar] [CrossRef]
- Ayee, M.A.A.; Bunker, B.C.; De Groot, J.L. Membrane modulatory effects of omega-3 fatty acids: Analysis of molecular level interactions. Curr. Top. Membr. 2020, 86, 57–81. [Google Scholar] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Faria, A.; Fernandes, I.; Norberto, S.; Mateus, N.; Calhau, C. Interplay between anthocyanins and gut microbiota. J. Agric. Food Chem. 2014, 62, 6898–6902. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, Y.; Hou, D.X.; Wu, S. The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B.; Yildiz, F. Phenolic compounds: Natural alternative in inflammation treatment. A review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Tena, N.; Martin, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Cheng, J.R.; Liu, X.M.; Chen, Z.Y.; Zhang, Y.S.; Zhang, Y.H. Mulberry anthocyanin biotransformation by intestinal probiotics. Food Chem. 2016, 213, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.O.; Carbonero, F. Impact of tart cherries polyphenols on the human gut microbiota and phenolic metabolites in vitro and in vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Ruiz del Castillo, M.L.; Costabile, A.; Klee, A.; Bigetti Guergoletto, K.; Gibson, G.R. In vitro fermentation of anthocyanins encapsulated with cyclodextrins: Release, metabolism and influence on gut microbiota growth. J. Funct. Foods. 2015, 16, 50–57. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, G.; Jing, N.; Liu, X.; Li, Q.; Liang, W.; Liu, Z. Bilberry anthocyanin extracts enhance anti-PD-L1 efficiency by modulating gut microbiota. Food Funct. 2020, 11, 3180–3190. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Liu, J.; Hao, W.; He, Z.; Kwek, E.; Zhu, H.; Ma, N.; Ma, K.Y.; Chen, Z.Y. Blueberry and cranberry anthocyanin extracts reduce bodyweight and modulate gut microbiota in C57BL/6J mice fed with a high-fat diet. Eur. J. Nutr. 2021, 60, 2735–2746. [Google Scholar] [CrossRef]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Liu, T.; Wang, X.; Huang, H.; Liu, W. Callistephin enhances the protective effects of isoflurane on microglial injury through downregulation of inflammation and apoptosis. Mol. Med. Rep. 2019, 20, 802–812. [Google Scholar] [CrossRef]
- Badshah, H.; Kim, T.H.; Kim, M.O. Protective effects of anthocyanins against amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochem. Int. 2015, 80, 51–59. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Ali, T.; Kim, M.J.; Rehman, S.U.; Ahmad, A.; Kim, M.O. Anthocyanin-loaded PEG-gold nanoparticles enhanced the neuroprotection of anthocyanins in an Aβ1–42 mouse model of Alzheimer’s disease. Mol. Neurobiol. 2017, 54, 6490–6506. [Google Scholar] [CrossRef] [PubMed]
- Frenț, O.D.; Stefan, L.; Morgovan, C.M.; Duteanu, N.; Dejeu, I.L.; Marian, E.; Vicaș, L.; Manole, F. A Systematic Review: Quercetin-Secondary Metabolite of the Flavonol Class, with Multiple Health Benefits and Low Bioavailability. Int. J. Mol. Sci. 2024, 25, 12091. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Babaei, F.; Mirzababaei, M.; Nassiri-Asl, M. Quercetin in Food: Possible Mechanisms of Its Effect on Memory. J. Food Sci. 2018, 83, 2280–2287. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Wu, J.C.; Huang, Q.; Shahidi, F.; Wang, Y.J.; Ho, C.T.; Pan, M.H. Metabolic and colonic microbiota transformation may enhance the bioactivities of dietary polyphenols. J. Funct. Foods 2014, 7, 3–25. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Z.; Zhang, N.; Liu, L.; Li, S.; Wei, H. In vitro catabolism of quercetin by human fecal bacteria and the antioxidant capacity of its catabolites. Food Nutr. Res. 2014, 58, 23406. [Google Scholar] [CrossRef]
- Santangelo, R.; Silvestrini, A.; Mancuso, C. Ginsenosides, catechins, quercetin and gut microbiota: Current evidence of challenging interactions. Food Chem. Toxicol. 2019, 123, 42–49. [Google Scholar] [CrossRef]
- Lan, H.; Hong, W.; Qian, D.; Peng, F.; Li, H.; Liang, C.; Du, M.; Gu, J.; Mai, J.; Bai, B.; et al. Quercetin modulates the gut microbiota as well as the metabolome in a rat model of osteoarthritis. Bioengineered 2021, 12, 6240–6250. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Bian, X.; Yao, Z.; Wang, Y.; Gao, W.; Guo, C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 2020, 11, 8003–8013. [Google Scholar] [CrossRef]
- Li, Q.; Van Herreweghen, F.; De Mey, M.; Goeminne, G.; Van de Wiele, T. The Donor-Dependent and Colon-Region-Dependent Metabolism of (+)-Catechin by Colonic Microbiota in the Simulator of the Human Intestinal Microbial Ecosystem. Molecules 2021, 27, 73. [Google Scholar] [CrossRef] [PubMed]
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Antioxidant Role of Catechin in Health and Disease. In Polyphenols Human Health and Disease; Watson, R.R., Preedy, V.R., Zipadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 267–271. [Google Scholar]
- Shabbir, U.; Rubab, M.; Daliri, E.B.; Chelliah, R.; Javed, A.; Oh, D.H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef]
- Liu, A.B.; Tao, S.; Lee, M.J.; Hu, Q.; Meng, X.; Lin, Y.; Yang, C.S. Effects of gut microbiota and time of treatment on tissue levels of green tea polyphenols in mice. Biofactors 2018, 44, 348–360. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Milenkovic, D.; Van de Wiele, T.; Rodriguez-Mateos, A.; de Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 1600557. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Li, X.M.; Liang, J.P.; Xiang, L.P.; Wang, K.R.; Shi, Y.L.; Yang, R.; Shi, M.; Ye, J.H.; Lu, J.L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef]
- Li, Q.; Van de Wiele, T. Gut microbiota as a driver of the interindividual variability of cardiometabolic effects from tea polyphenols. Crit. Rev. Food Sci. Nutr. 2021, 13, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Pastoriza, S.; Mesias, M.; Cabrera, C.; Rufian-Henares, J.A. Healthy properties of green and white teas: An update. Food Funct. 2017, 8, 2650–2662. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Sang, S. Biotransformation of tea polyphenols by gut microbiota. J. Funct. Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; Ni, L.; Vincken, J.P. Green and Black Tea Phenolics: Bioavailability, Transformation by Colonic Microbiota, and Modulation of Colonic Microbiota. J. Agric. Food Chem. 2018, 66, 8469–8477. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Kutschera, M.; Engst, W.; Blaut, M.; Braune, A. Isolation of catechin-converting human intestinal bacteria. J. Appl. Microbiol. 2011, 111, 165–175. [Google Scholar] [CrossRef]
- Hursel, R.; Westerterp-Plantenga, M.S. Catechin- and caffeine-rich teas for control of body weight in humans. Am. J. Clin. Nutr. 2013, 98, 1682S–1693S. [Google Scholar] [CrossRef]
- Jin, J.S.; Touyama, M.; Hisada, T.; Benno, Y. Effects of green tea consumption on human fecal microbiota with special reference to Bifidobacterium species. Microbiol. Immunol. 2012, 56, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Zhang, X.; Miao, Y.; Cao, J.; Wu, Z.; Weng, P. The modulatory effect of (-)-epigallocatechin 3-O-(3-O-methyl) gallate (EGCG3”Me) on intestinal microbiota of high fat diet-induced obesity mice model. Food Res. Int. 2017, 92, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tang, L.; Zhou, H.; Zhou, J.; Glenn, T.C.; Shen, C.L.; Wang, J.S. Long-term treatment with green tea polyphenols modifies the gut microbiome of female sprague-dawley rats. J. Nutr. Biochem. 2018, 56, 55–64. [Google Scholar] [CrossRef]
- Liao, Z.L.; Zeng, B.H.; Wang, W.; Li, G.H.; Wu, F.; Wang, L.; Zhong, Q.P.; Wei, H.; Fang, X. Impact of the Consumption of Tea Polyphenols on Early Atherosclerotic Lesion Formation and Intestinal Bifidobacteria in High-Fat-Fed ApoE−/− Mice. Front. Nutr. 2016, 3, 42. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Han, L.; Liu, L.; Gao, L.; Xue, B.; Wang, Y.; Ou, S.; Miller, M.; Peng, X. Catechin supplemented in a FOS diet induces weight loss by altering cecal microbiota and gene expression of colonic epithelial cells. Food Funct. 2018, 9, 2962–2969. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Nwafor, E.O.; Lu, P.; Zhang, Y.; Liu, R.; Peng, H.; Xing, B.; Liu, Y.; Li, Z.; Zhang, K.; Zhang, Y.; et al. Chlorogenic acid: Potential source of natural drugs for the therapeutics of fibrosis and cancer. Transl. Oncol. 2022, 15, 101294. [Google Scholar] [CrossRef]
- Cowan, T.E.; Palmnäs, M.S.; Yang, J.; Bomhof, M.R.; Ardell, K.L.; Reimer, R.A.; Vogel, H.J.; Shearer, J. Chronic coffee consumption in the diet-induced obese rat: Impact on gut microbiota and serum metabolomics. J. Nutr. Biochem. 2014, 25, 489–495. [Google Scholar] [CrossRef]
- Farah, A.; dePaula Lima, J. Consumption of Chlorogenic Acids through Coffee and Health Implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef]
- Song, J.; Zhou, N.; Ma, W.; Gu, X.; Chen, B.; Zeng, Y.; Yang, L.; Zhou, M. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019, 10, 2947–2957. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Yu, B.; Chen, D.; Mao, X.; Zheng, P.; Luo, J.; He, J. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 2018, 96, 1108–1118. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Zheng, P.; Luo, Y.; Huang, Z.; Luo, J.; Mao, X.; Yu, J.; He, J. Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation. Appl. Microbiol. Biotechnol. 2019, 103, 8157–8168. [Google Scholar] [CrossRef]
- Chen, J.L.; Zheng, P.; Zhang, C.; Yu, B.; He, J.; Yu, J.; Luo, J.Q.; Mao, X.B.; Huang, Z.Q.; Chen, D.W. Benzoic acid beneficially affects growth performance of weaned pigs which was associated with changes in gut bacterial populations, morphology indices and growth factor gene expression. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1137–1146. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.; García-Villalba, R.; Quartieri, A.; Raimondi, S.; Amaretti, A.; Leonardi, A.; Rossi, M. In vitro transformation of chlorogenic acid by human gut microbiota. Mol. Nutr. Food Res. 2014, 58, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Paz de Pena, M.; Concepcion, C.; Alan, C. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors 2013, 39, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic Acid-Induced Gut Microbiota Improves Metabolic Endotoxemia. Front. Endocrinol. 2021, 12, 762691. [Google Scholar] [CrossRef]
- Wang, Z.; Lam, K.L.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef]
- Shi, A.; Li, T.; Zheng, Y.; Song, Y.; Wang, H.; Wang, N.; Dong, L.; Shi, H. Chlorogenic Acid Improves NAFLD by Regulating Gut Microbiota and GLP-1. Front. Pharmacol. 2021, 12, 693048. [Google Scholar] [CrossRef]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2020, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Mon, M.A.; Ortega, M.A.; García-Montero, C.; Fraile-Martinez, O.; Monserrat, J.; Lahera, G.; Mora, F.; Rodriguez-Quiroga, A.; Fernandez-Rojo, S.; Quintero, J.; et al. Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects. Pharmaceuticals 2021, 14, 821. [Google Scholar] [CrossRef]
- Calfio, C.; Andrea Gonzalez, A.; Singh, S.K.; Rojo, L.E.; Maccionia, R.B. The Emerging Role of Nutraceuticals and Phytochemicals in the Prevention and Treatment of Alzheimer’s Disease. J. Alzheimers Dis. 2020, 77, 33–51. [Google Scholar] [CrossRef]
- Vaishnavi, P.; Surana, S.S. Nutraceuticals for Mental Health: A Review on Current and Future Potential. Int. J. Pharm. Sci. 2025, 3, 764–774. [Google Scholar]
- Romanenko, M.; Kholin, V.; Koliada, A.; Vaiserman, A. Nutrition, Gut Microbiota, and Alzheimer’s Disease. Front. Psychiatry 2021, 12, 712673. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. The Bidirectional Interplay Between Substances of Abuse and Gut Microbiome Homeostasis. Life 2025, 15, 834. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, L.; Liu, Y.; Zhan, S.; Wu, Z.; Zhang, X. Plant-derived bioactive components regulate gut microbiota to prevent depression and depressive-related neurodegenerative diseases: Focus on neurotransmitters. Trends Food Sci. Technol. 2022, 129, 581–590. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. The role of the gut microbiome in Alzheimer’s disease pathophysiology. Curr. Opin. Neurol. 2025, 38, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.K.; Gubert, C.; Hannan, A.J. The microbiota-inflammasome-brain axis as a pathogenic mediator of neurodegenerative disorders. Neurosci. Biobehav. Rev. 2025, 176, 106276. [Google Scholar] [CrossRef] [PubMed]
- Toni, M.; Massimino, M.L.; De Mario, A.; Angiulli, E.; Spisni, E. Metal Dyshomeostasis and Their Pathological Role in Prion and Prion-Like Diseases: The Basis for a Nutritional Approach. Front. Neurosci. 2017, 11, 3. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Human gut microbiome, diet, and mental disorders. Int. Microbiol. 2025, 28, 1–15. [Google Scholar] [CrossRef]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef]
- Rashid, S.M.; Razak, R.; Tantray, A.K.; James, A.W.; Showkat, N.; Shehjar, F.; Jan, F.; Ahmad, S.B.; Khan, A.; Shah, Z.A. Unraveling Alzheimer’s: Exploring the Gut Microbiota–Brain Axis as a New Frontier in Understanding. J. Dement. Alzheimers Dis. 2024, 1, 22–47. [Google Scholar] [CrossRef]
- Jiang, C.; Li, G.; Huang, P.; Liu, Z.; Zhao, B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 58, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Salinas, N.; Povolotsky, T.L.; Landau, M.; Kolodkin-Gal, I. Emerging Roles of Functional Bacterial Amyloids in Gene Regulation, Toxicity, and Immunomodulation. Microbiol. Mol. Biol. Rev. 2020, 85, e00062-20. [Google Scholar] [CrossRef] [PubMed]
- Mertsalmi, T.H.; Aho, V.T.E.; Pereira, P.A.B.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. More than constipation—Bowel symptoms in Parkinson’s disease and their connection to gut microbiota. Eur. J. Neurol. 2017, 24, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P.; Wüllner, U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017, 9, 39, Correction in Genome Med. 2017, 9, 61. [Google Scholar] [CrossRef]
- Fang, C.; Lv, L.; Mao, S.; Dong, H.; Liu, B. Cognition Deficits in Parkinson’s Disease: Mechanisms and Treatment. Parkinsons Dis. 2020, 2020, 2076942. [Google Scholar] [CrossRef]
- Lei, Q.; Wu, T.; Wu, J.; Hu, X.; Guan, Y.; Wang, Y.; Yan, J.; Shi, G. Roles of α synuclein in gastrointestinal microbiome dysbiosis related Parkinson’s disease progression. Mol. Med. Rep. 2021, 24, 734. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Ho, C.T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Chen, X.; McClements, D.J.; Wang, J.; Zou, L.; Deng, S.; Liu, W.; Yan, C.; Zhu, Y.; Cheng, C.; Liu, C. Coencapsulation of (-)-Epigallocatechin-3-gallate and Quercetin in Particle-Stabilized W/O/W Emulsion Gels: Controlled Release and Bioaccessibility. J. Agric. Food Chem. 2018, 66, 3691–3699. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, A.; Amal, T.C.; Sarvalingam, A.; Vasanth, K. A review on the influence of nutraceuticals and functional foods on health. Food Chem. Adv. 2024, 5, 100749. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Kruk, M.E.; Gage, A.D.; Arsenault, C.; Jordan, K.; Leslie, H.H.; Roder-DeWan, S.; Adeyi, O.; Barker, P.; Daelmans, B.; Doubova, S.V.; et al. High-Quality Health Systems in the Sustainable Development Goals Era: Time for a Revolution. Lancet Glob. Health 2017, 6, e1196–e1252, Correction in Lancet Glob. Health 2018, 6, e1162; Correction in Lancet Glob. Health 2021, 9, e1067. [Google Scholar] [CrossRef]
- Vignesh, A.; Amal, T.C.; Selvakumar, S.; Vasanth, K. Unraveling the role of medicinal plants and gut microbiota in colon cancer: Towards microbiota-based strategies for prevention and treatment. Health Sci. Rev. 2023, 9, 100115. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Colombo, F.; Di Lorenzo, C.; Biella, S.; Vecchio, S.; Frigerio, G.; Restani, P. Adverse effects to food supplements containing botanical ingredients. J. Funct. Foods 2020, 72, 103990. [Google Scholar] [CrossRef]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef]
- Ashrafpour, S.; Ashrafpour, M. The double-edged sword of nutraceuticals: Comprehensive review of protective agents and their hidden risks. Front. Nutr. 2025, 12, 1524627. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A. Vegetarian and ketogenic diets: Their relationship with gut microbiome and mental health, and their clinical applications. Food Nutr. Chem. 2025, 3, 278. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Influencia de la dieta vegetariana en el microbioma intestinal humano [Influence of the vegetarian diet on the human intestinal microbiome]. Nutr. Clín. Diet Hosp. 2024, 44, 149–157. [Google Scholar] [CrossRef]
- Vallboehmer, F.; Schoofs, H.; Rink, L.; Jakobs, J. Zinc supplementation among zinc-deficient vegetarians and vegans restores antiviral interferon-α response by upregulating interferon regulatory factor 3. Clin. Nutr. 2025, 51, 161–173. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A. Una revisión crítica sobre la influencia de la dieta vegetariana en la salud mental [A critical review on the influence of the vegetarian diet on mental health]. Rev. Esp. Nutr. Comun. 2024, 30. Available online: https://hdl.handle.net/20.500.14468/25971 (accessed on 15 August 2025).
- Ong, J.; Wu, Q.; Sasaki, K.; Isoda, H.; Szele, F.G. Nutraceuticals: Using Food to Enhance Brain Health by Modulating Postnatal Neurogenesis in Animal Models and Patient Populations. Stem Cells Transl. Med. 2025, 14, szaf006. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Bustillos-López, A. Intervención social dirigida al envejecimiento saludable: Revisión de estudios recientes [Social intervention aimed at healthy aging: Review of recent studies]. Anál. Modif. Conducta 2024, 50, 21–38. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Una revisión actual sobre enfoques terapéuticos microbianos destinados a mejorar las funciones cognitivas en adultos mayores [A current review on microbial therapeutic approaches aimed at improving cognitive functions in older adults]. Gerokomos 2024, 35, 235–243. [Google Scholar]
- Abbad-Gomez, D.; Carballo-Casla, A.; Beridze, G.; Lopez-Garcia, E.; Rodríguez-Artalejo, F.; Sala, M.; Comas, M.; Vetrano, D.L.; Calderón-Larrañaga, A. Dietary patterns and accelerated multimorbidity in older adults. Nat. Aging 2025, 5, 1481–1490. [Google Scholar] [CrossRef]
- Cimen, Y.A.; Elibol, B.; Korkmaz, N.D.; Yuzgulec, M.; Kinsiz, B.; Kutlu, S.; Ustunova, S. The Impact of Ketogenic Diet Consumption on the Sporadic Alzheimer’s Model Through MT1/MT2 Regulation. J. Neurosci. Res. 2025, 103, e70070. [Google Scholar] [CrossRef]
- Shu, Y.; Hong, W.; Liu, J.; Zhu, X. Exploring the Association of Dietary Index for Gut Microbiota with Parkinson’s Disease and Depression: Insights from NHANES. J. Affect. Disord. 2025, 386, 119461. [Google Scholar] [CrossRef] [PubMed]
- Tufail, T.; Ain, H.B.U.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Functional, Nutraceutical and Health Endorsing Perspectives of Saffron. Food Sci. Nutr. 2025, 13, e70721. [Google Scholar] [CrossRef] [PubMed]
- Walczak, K.; Kołodziejczyk, W.; Pszczołowska, M.; Kozłowska, M.; Beszłej, J.A.; Leszek, J. Klotho Protein in Alzheimer’s Disease: Diet Leading to Immortality? J. Alzheimers Dis. 2025, 106, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Epigenetic Mechanisms in Aging: Extrinsic Factors and Gut Microbiome. Genes 2024, 15, 1599. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.N.; Hodges, R.; Hanes, D.; Stack, E.; Cheishvili, D.; Szyf, M.; Henkel, J.; Twedt, M.W.; Giannopoulou, D.; Herdell, J.; et al. Potential reversal of epigenetic age using a diet and lifestyle intervention: A pilot randomized clinical trial. Aging 2021, 13, 9419–9432, Correction in Aging 2022, 14, 5959; Correction in Aging 2024, 16, 4943–4395. [Google Scholar] [CrossRef]
- Waziry, R.; Ryan, C.P.; Corcoran, D.L.; Huffman, K.M.; Kobor, M.S.; Kothari, M.; Graf, G.H.; Kraus, V.B.; Kraus, W.E.; Lin, D.T.S.; et al. Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults from the CALERIE trial. Nat. Aging 2023, 3, 248–257, Correction in Nat. Aging 2023, 3, 753. [Google Scholar] [CrossRef] [PubMed]

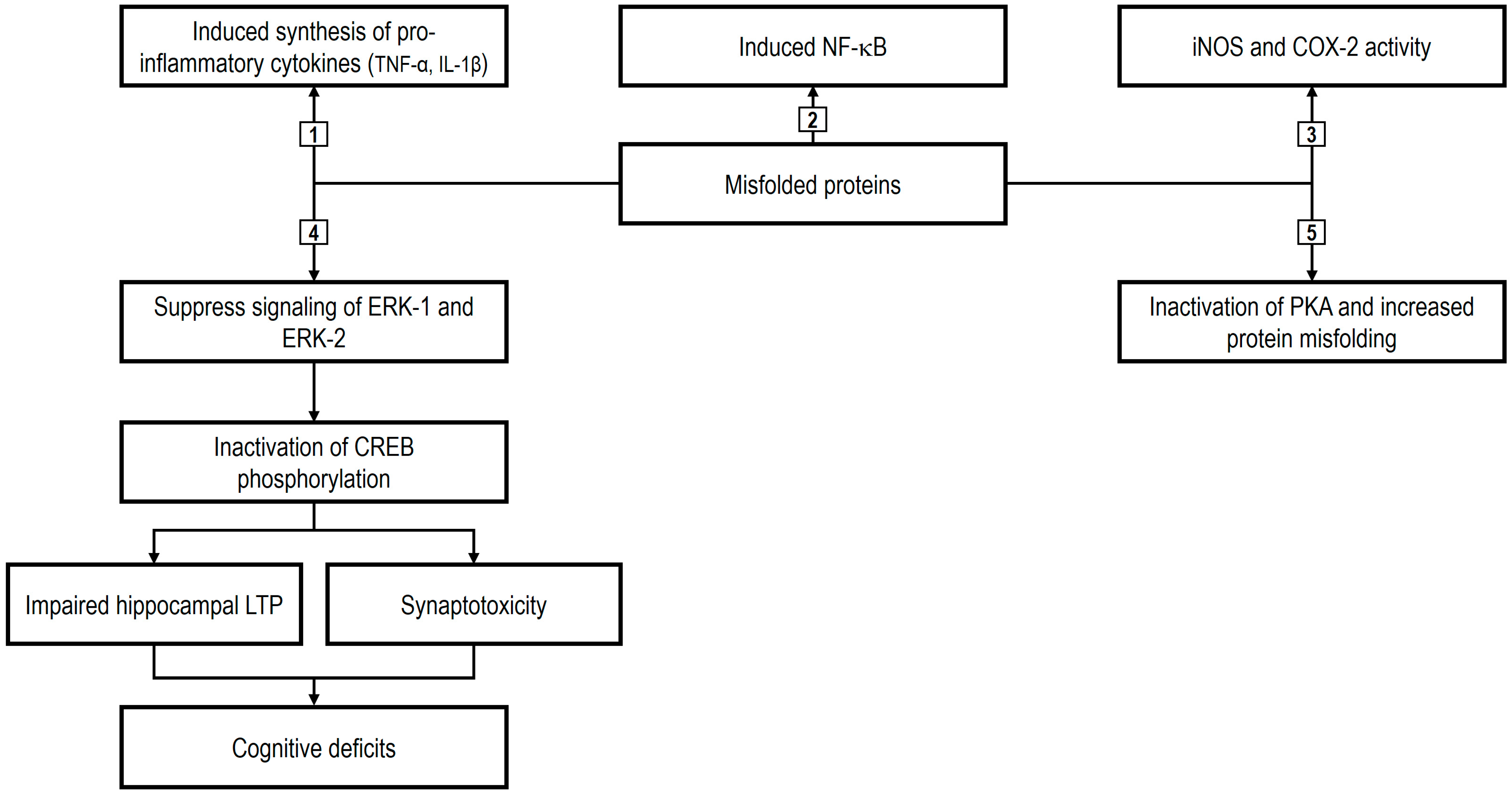
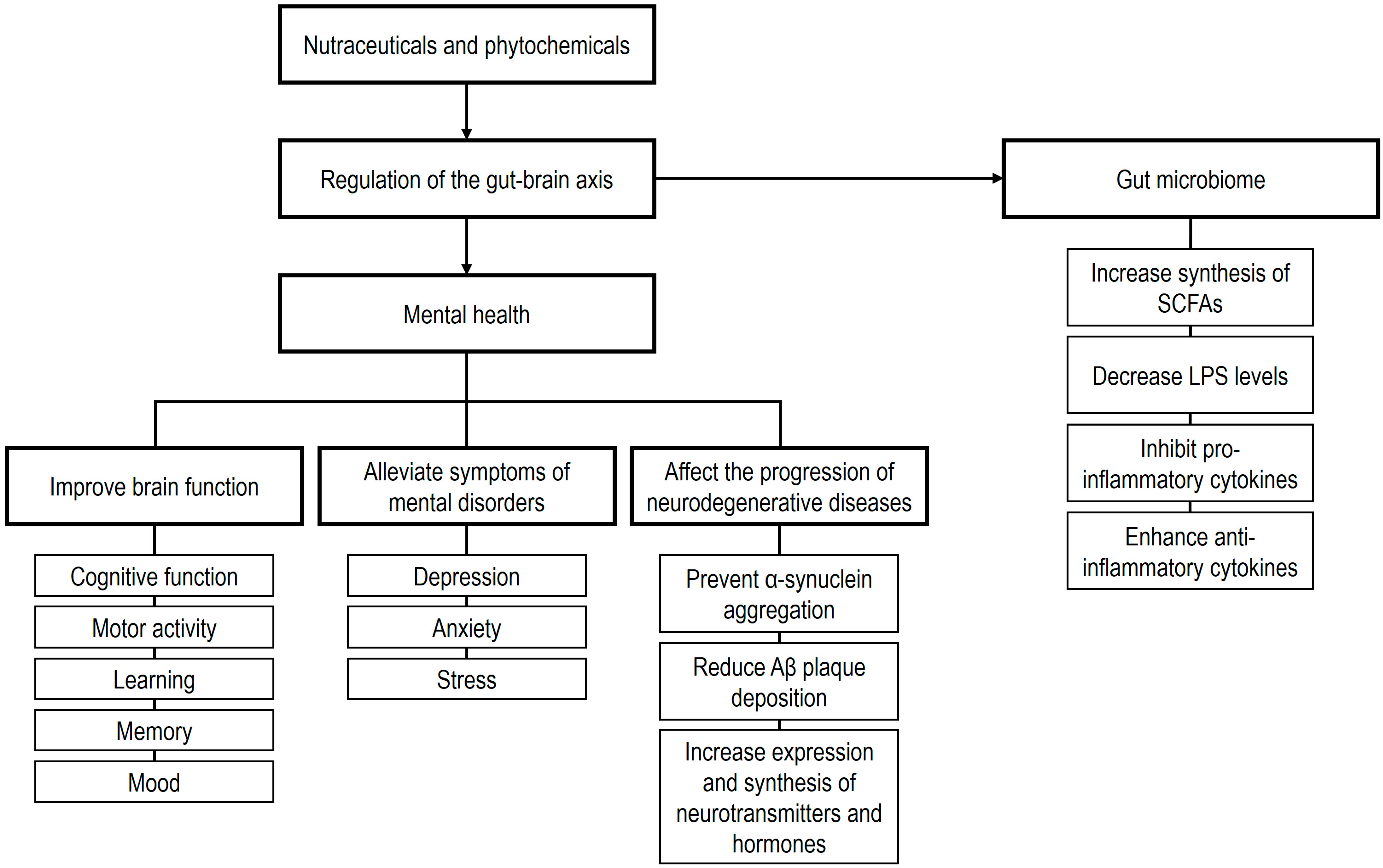
| Functional Foods | Bioactive Components | Nutraceutical/Functional Effects | References |
|---|---|---|---|
| Nutraceutical beverages | - Catechins (epigallocatechin-3-gallate and epicatechin gallate). - Alkaloids (caffeine). | - Improve digestion, enhance overall health, strengthen immunity, improve heart health, and increase energy levels. - Tea is a rich source of bioactive compounds and nutrients with significant antioxidant, anticancer, anti-inflammatory, immune-modulating, antihistamine, antimicrobial, antidepressant, cardioprotective, blood pressure-lowering, nervous system protective, and health-promoting properties. - The ingestion of coffee has a potential impact on the prevalence of various chronic conditions, including but not limited to mental health issues, liver disease, and elevated suicide risk. | [21,22,23,24] |
| Fermented foods | - Polyphenols. - Dietary fiber. - Probiotics and prebiotics. - Conjugated linoleic acid. - Vitamins (cobalamin). - Fatty acids. | - Immune-modulatory, antioxidant, chemoprotective, anti-inflammatory, cardioprotective, immune-boosting, anticancer, and antidiabetic properties. - The microorganisms used in the fermentation process can enhance the bioavailability of nutrients and augment the bioactive compounds present in food products. - Citrus-blended vinegars can be used as functional foods due to their anti-obesity, anti-aging, and antioxidant properties. | [25,26,27] |
| Fruits | - Polyphenols and alkaloids. - Vitamins and minerals. - Dietary fiber. - Tannins (ellagitannins, prodelphinidins). - Catechins (gallocatechins). - Anthocyanidins (pelargonidin, cyanidin, delphinidin). | - Antioxidant, cardioprotective, anti-inflammatory, antimicrobial, antidepressive, anticancer, immunomodulatory, nervous system-enhancing, antidiabetic, and anti-obesity properties. - Fruits contain various components, such as antioxidants and polyphenols, which reduce the effect of hormones that are associated with brain disorders. | [28,29,30] |
| Herbs and spices | - Curcumin. - Gingerol saponin and shogaols. - Dietary fiber. - Alkaloids (piperine). - Capsaicin. - Allicin. | - Antioxidant, antidiabetic, nutritional, cardioprotective, anti-hypercholesterolemic, antimicrobial, anti-inflammatory, anticancer, antihistamine, and blood pressure-lowering properties. | [31,32,33] |
| Honey and derivatives | - Organic acids. - Royalisin (defensin-1). - Hydrogen peroxide, methylglyoxal, eugenol, and p-coumaric acid. - Flavonoids (naringin, luteolin, chrysin, kaempferol, quercetin, galangin, apigenin, isorhamnetin, pinocembrin, pinobanksin). - Phenolic compounds (syringic acid, gallic acid, ferulic acid, caffeic acid). | - Enhance resistance to infections, alleviate cold symptoms, potentially extend lifespan, promote wound healing, and prevent certain diseases. - Possess antioxidant, anticancer, antidiabetic, anti-inflammatory, immune-boosting, blood pressure-lowering, antihistamine, antimicrobial, cognitive, cardioprotective, and nervous-system-improving properties. - Honey is topically used as an antibiotic in herbal and traditional medicine for the treatment of burns and skin injuries. Honey might play a beneficial role in the management of adverse effects associated with chemotherapy and radiation therapy used in cancer treatment regimes and for the treatment of osteoarthritis. | [34,35,36,37] |
| Legumes | - Dietary fiber and minerals. - Protein and peptides. - Isoflavones (genistein, daidzein, glycitein). - Phytosterols. - Saponins. - Resveratrol. | - Beneficial role in the prevention and treatment of a wide range of health conditions, including cancer, obesity, cardiovascular diseases, overweight, diabetes mellitus, digestive diseases, and nervous system disorders. - Regular consumption of soybeans can ameliorate menopause symptoms and can reduce the risk of certain types of cancer, such as breast and prostate cancers. | [38,39,40] |
| Nuts | - α-linolenic acid and polyunsaturated fatty acids. - Minerals and amino acids (L-arginine). - Dietary fiber. - Antioxidants (polyphenols, tocopherols). - Vitamins (folate). | - Synergistic and beneficial effect on vascular and metabolic pathways, improving cardiovascular prognosis and reducing the risk of cardiovascular and cancer diseases. - The ingestion of nuts also promotes moderate improvements in inflammation, endothelial function, blood pressure, and glycemic control, ameliorating and preventing the effects of diabetes, brain disorders, and obesity/overweight. | [41,42,43] |
| Seeds | - Dietary fiber and essential oils. - Omega-3 fats, polyunsaturated and monounsaturated fats. - Antioxidants (p-coumaric acid). - Phenolic compounds (ferulic acid, catechins, sesamin, caffeic acid, lignans, pinoresinol, sesamolin, lariciresinol). - Saponins (triterpenoid glycosides). - Phytosterols (stigmasterol, sitosterol, secoisolariciresinol diglucoside, campesterol). - Vitamins and minerals. - Triterpenes. - Cyanogenic glycosides (neolinustatin). | - Reduce blood pressure, control blood sugar, decrease LDL cholesterol, reduce the risk of cancer and cardiovascular diseases, and enhance antimicrobial activity. - Polyphenols contained in seeds possess antioxidant, anti-inflammatory, anticancer, immune-promoting, and cardioprotective properties. | [44,45,46] |
| Vegetables | - Omega-3 fatty acids. - Dietary fiber and oligoelements (zinc, selenium). - Vitamins. - Carotenoids (β-carotene, lycopene). - Xanthophylls (astaxanthin, zeaxanthin, canthaxanthin, cryptoxanthin). - Phenolic compounds (isoflavones, flavonoids). - Catechins (epigallocatechin-3-gallate, epicatechin, epigallocatechin, and epicatechin gallate). - Glucosinolates. - Phytosterols. | - Prevent numerous diseases in humans, including diabetes, cardiovascular diseases, and microbial infections. - The regular inclusion of vegetables in diets results in a decrease in the incidence of cardiovascular diseases, stroke, and cancer. | [28,47,48,49] |
| Whole grains | - Dietary fiber (β-glucans) and minerals. - Vitamins (folate, vitamin E, tocols). - Prebiotics (inulin). - Phenolic compounds (polyphenols, lignans). - Carotenoids (lutein, zeaxanthin, β-cryptoxanthin, β-carotene). - Amino acids (betaine). - Phytosterols. | - Reduce the risk of diseases such as gastrointestinal disorders, cognitive decline, obesity, cardiovascular diseases, type 2 diabetes mellitus, coronary heart disease, cancer, stroke, and diverticulosis. | [50,51,52] |
| Nutraceuticals | References | Intervention | Main Results |
|---|---|---|---|
| Omega-3 fatty acids | |||
| Da Silva et al. [195] | N = 31 PD and MDD patients (mean age 64.4 years). 480 mg/day DHA + 720 mg/day EPA. 12 weeks. | Treatment had no statistically significant effect on the rate of change on the Hoehn–Yahr Scale score, but there was a significant decrease in MADRS and CGI scores. | |
| De Waal et al. [196] | N = 179 mild AD patients (≥50 years). 1700 mg/day DHA + 6 mg/day EPA. 24 weeks. | The administration contributed to the maintenance of the organization of brain networks in mild AD patients. | |
| Eriksdotter et al. [197] | N = 174 AD patients (74 ± 9 years). 1720 mg/day DHA + 600 mg/day EPA 24 weeks. | The daily supplementation stabilized the cognitive performance of AD subjects, assessed by ADAS-cog and MMSE scores. | |
| Jernerén et al. [198] | N = 171 AD patients. 1720 mg/day DHA + 600 mg/day EPA. 24 weeks. | The effect of omega-3 supplementation on MMSE and CDR appeared to be influenced by homocysteine plasma levels. | |
| Olde Rikkert et al. [199] | N = 201 patients with mild AD. 1200 mg/day DHA + 300 mg/day EPA (Souvenaid). 24 weeks. | The intake of Souvenaid was well tolerated with a favorable safety profile. The adherence to Souvenaid was very high, reflecting its good tolerability and ease of use. | |
| Philips et al. [200] | N = 42 AD patients (mean age 71.1 years). 625 mg/day DHA + 600 mg/day EPA. 16 weeks. | The daily supplementation was associated with none or only negligible benefits on mood and cognition, assessed by MMSE, HVLT-R, BASDEC, and BADLS. | |
| Pomponi et al. [201] | N = 24 PD patients. 800 mg/day DHA + 290 mg/day EPA. 24 weeks. | No statistically significant effect on the rate of change on either the UPDRS or Hoehn–Yahr Scale score. In DHA-treated patients, the HDRS score was reduced by at least 50%. | |
| Quinn et al. [202] | N = 295 AD patients (mean age 76 years). 2000 mg/day DHA from seaweed. 18 months. | Supplementation with DHA compared with placebo did not slow the rate of cognitive and functional decline in patients with mild to moderate AD assessed by MMSE, ADAS-cog, CDR, ADS-ADL, and NPI. | |
| Shinton et al. [203] | N = 39 AD patients (>55 years). 675 mg/day DHA + 975 mg/day EPA. 675 mg/day DHA + 975 mg/day EPA + 600 mg/day LA. 12 months. | Active groups were not different from the placebo group in ADAS-cog. The omega-3 + LA group showed less decline assessed by MMSE. | |
| Taghizadeh et al. [204] | N = 60 PD patients. 1000 mg omega-3 fatty acids + 400 IU vitamin E. 12 weeks. | Treatment had favorable effects on the UPDRS score. | |
| Wang et al. [205] | N = 15 AD patients (mean age 72.5 years). 1720 mg/day DHA + 600 mg/day EPA. 24 weeks. | A decrease was observed in RvD1 and LXA4 production from peripheral blood mononuclear cells of AD patients who did not receive omega-3, but not in cells of AD subjects under omega-3 intake. | |
| Curcumin | |||
| Baum et al. [206] | N = 27 AD patients (>50 years). Dose CUR 1–3 g. 24 weeks. | No differences in cognitive decline were observed. | |
| Cox et al. [207] | N = 60 healthy elderly patients (60–85 years). Dietary Longvida with 400 mg/kg of CUR. 4 weeks. | Single-dose improved performance on working memory and sustained attention. | |
| Rainey-Smith et al. [208] | N = 96 healthy elderly people (40–90 years). Dose: CUR 1500 mg. 12 months. | Cognitive decline after 6 months in the placebo group, but not in the curcumin group. | |
| Ringman et al. [209] | N = 30 mild-to-moderate AD patients (>49 years). Dose: CUR 4 g. 24–48 weeks. | Non-significant change on MMSE scores after CUR administration. No alterations of AD biomarkers. | |
| Small et al. [210] | N = 40 non-demented subjects (51–84 years). Dose: CUR 90 mg. 18 months. | CUR improved memory and attention performance and prevented neuropathological deposition in the amygdala and hypothalamus. | |
| Ginkgo biloba | |||
| Chowdhury et al. [211] | N = 150 AD and vascular neurocognitive disorder patients (>50 years). EGb 761: extract of Ginkgo biloba leaves: 240 mg/day. 18 weeks. | Significant improvements compared to baseline were found in constructional praxis, memory, speed and executive functioning, and behavioral symptoms. In total, 22.0% of patients presented adverse drug reactions, with headache and diarrhea being the most frequent events. | |
| García-Alberca et al. [212] | N = 133 MCI patients (64–84 years). EGb 761: 240 mg/day + acetylcholinesterase inhibitors: donepezil (5–10 mg daily), galantamine (16–24 mg daily), or rivastigmine patch (9.5–13.3 mg daily). 12 months. | Combined therapy with EGb 761 plus AChEI may provide added cognitive and functional benefits in patients with MCI. | |
| Herrschaft et al. [213] | N = 200 AD and vascular dementia patients (>60 years). EGb 761: 240 mg/day. 24 weeks. | Patients treated with EGb 761 improved the SKT total score. The NPI composite score improved in the EGb 761-treated group. | |
| Ihl et al. [214] | N = 202 AD and vascular dementia patients (>60 years). EGb 761: 240 mg/day. 24 weeks. | Patients treated with EGb 761 improved SKT and NPI total scores. | |
| Nikolova et al. [215] | N = 165 AD and vascular dementia patients (>60 years). EGb 761: 240 mg/day. 22 weeks. | EGb 761 improved impaired mitochondrial function, hippocampal neurogenesis, and neuroplasticity. It also inhibited the aggregation and toxicity of Aβ protein. | |
| Rapp et al. [216] | N = 189 AD patients (>80 years). EGb 761: 240 mg/day; donepezil (5–10 mg/day). 12 months. | Results suggest similar effects on cognitive symptoms from the use of EGb 761 in the treatment of dementia in AD, together with favorable safety compared to donepezil. | |
| Vellas et al. [217] | N = 1406 elderly participants who spontaneously reported memory complaints (>70 years). EGb 761: 240 mg/day. 5 years. | Long-term use of standardized Gb extract did not reduce the risk of progression to AD compared to placebo. | |
| Yancheva et al. [218] | N = 96 probable AD patients (>50 years). EGb 761: 240 mg/day, donepezil (5–10 mg/day), and both treatments. 22 weeks. | Changes from baseline to week 22 and response rates were similar for all three treatment groups with respect to all outcome measures (SKT, NPI, GBSS, HDRS, CDT, and Verbal Fluency Test). | |
| Ginseng | |||
| Cheng et al. [219] | Effects of asiatic acid treatment on PC12 cells with Aβ25-35-induced injury. | Asiatic acid significantly increased the viability of differentiated PC12 cells but attenuated the mitochondria-mediated apoptosis dose-dependently when challenged with Aβ25-35. Asiatic acid protected differentiated PC12 cells from Aβ25-35-induced apoptosis and tau protein hyperphosphorylation, which might be partially mediated by the activation of the PI3K/Akt/GSK-3β signaling pathway. | |
| Guo et al. [220] | Effects of licochalcone A (LicA) treatment on SH-SY5Y cells with Aβ25-35-induced injury. | LicA improved cell viability and decreased lactate dehydrogenase leakage remarkably in Aβ25-35-induced injury in SH-SY5Y cells. After treatment with LicA, ROS, glutathione, and superoxide dismutase levels in cells all were significantly decreased, which indicated that LicA has an antioxidative effect on Aβ25-35-induced oxidative injury. LicA could also significantly reduce Aβ25-35-induced autophagy in SH-SY5Y cells. In the cells injured by Aβ25-35, LicA prevented the transformation from light chain protein 3-I to light chain protein 3-II and reduced the levels of proteins GRP78, GRP94, CHOP, and Bax, but increased the levels of antiapoptotic protein and phosphorylation of PI3K, Akt, and mTOR. | |
| Hu et al. [221] | Effects of WEG and ginsenoside Rb1 on SH-SY5Y cells treated with MPP. | WEG exhibited significant protective effects against MPP(+)-induced cytotoxicity in SH-SY5Y cells, possibly through the suppression of ROS generation and the inhibition of the mitochondria-dependent apoptotic pathway. | |
| Hwang et al. [222] | In vitro study utilizing SH-SY5Y cells treated with the ginsenoside Rb1. | Forty proteins were significantly changed in response to Rb1 treatment in β-amyloid-treated neuronal cells. Analysis of the interactions of altered proteins revealed that actin cytoskeleton proteins might be linked to the regulatory mechanisms of Rb1. The CAP1, CAPZB, TOMM40, and DSTN proteins showed potential as molecular target proteins for the functional contribution of Rb1 in AD. | |
| Liu et al. [223] | In vitro study utilizing SH-SY5Y cells treated with the ginsenoside Rd. | Rd could exert a beneficial effect in an experimental PD model in vitro, in which SH-SY5Y cells were injured by 1-methyl-4-phenylpyridinium (MPP+), an active metabolic product of the classical Parkinsonian toxin MPTP. Rd at 1 and 10 μM could significantly attenuate MPP+-induced cell death. This protective effect may be ascribed to its ability to reduce intracellular ROS levels, enhance antioxidant enzymatic activities, preserve the activity of respiratory complex I, stabilize the mitochondrial membrane potential, and increase intracellular ATP levels. Furthermore, the PI3K/Akt survival-signaling pathway was also involved in the protective effect of Rd. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrego-Ruiz, A.; Borrego, J.J. Plant-Derived Nutraceuticals in Mental Health and Brain Function: Mechanisms of Action and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 8849. https://doi.org/10.3390/ijms26188849
Borrego-Ruiz A, Borrego JJ. Plant-Derived Nutraceuticals in Mental Health and Brain Function: Mechanisms of Action and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(18):8849. https://doi.org/10.3390/ijms26188849
Chicago/Turabian StyleBorrego-Ruiz, Alejandro, and Juan J. Borrego. 2025. "Plant-Derived Nutraceuticals in Mental Health and Brain Function: Mechanisms of Action and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 18: 8849. https://doi.org/10.3390/ijms26188849
APA StyleBorrego-Ruiz, A., & Borrego, J. J. (2025). Plant-Derived Nutraceuticals in Mental Health and Brain Function: Mechanisms of Action and Therapeutic Potential. International Journal of Molecular Sciences, 26(18), 8849. https://doi.org/10.3390/ijms26188849






