The Novel Flax Cultivar Silesia Shows High Morphogenetic Capacity in Tissue Cultures
Abstract
1. Introduction
2. Results and Discussion
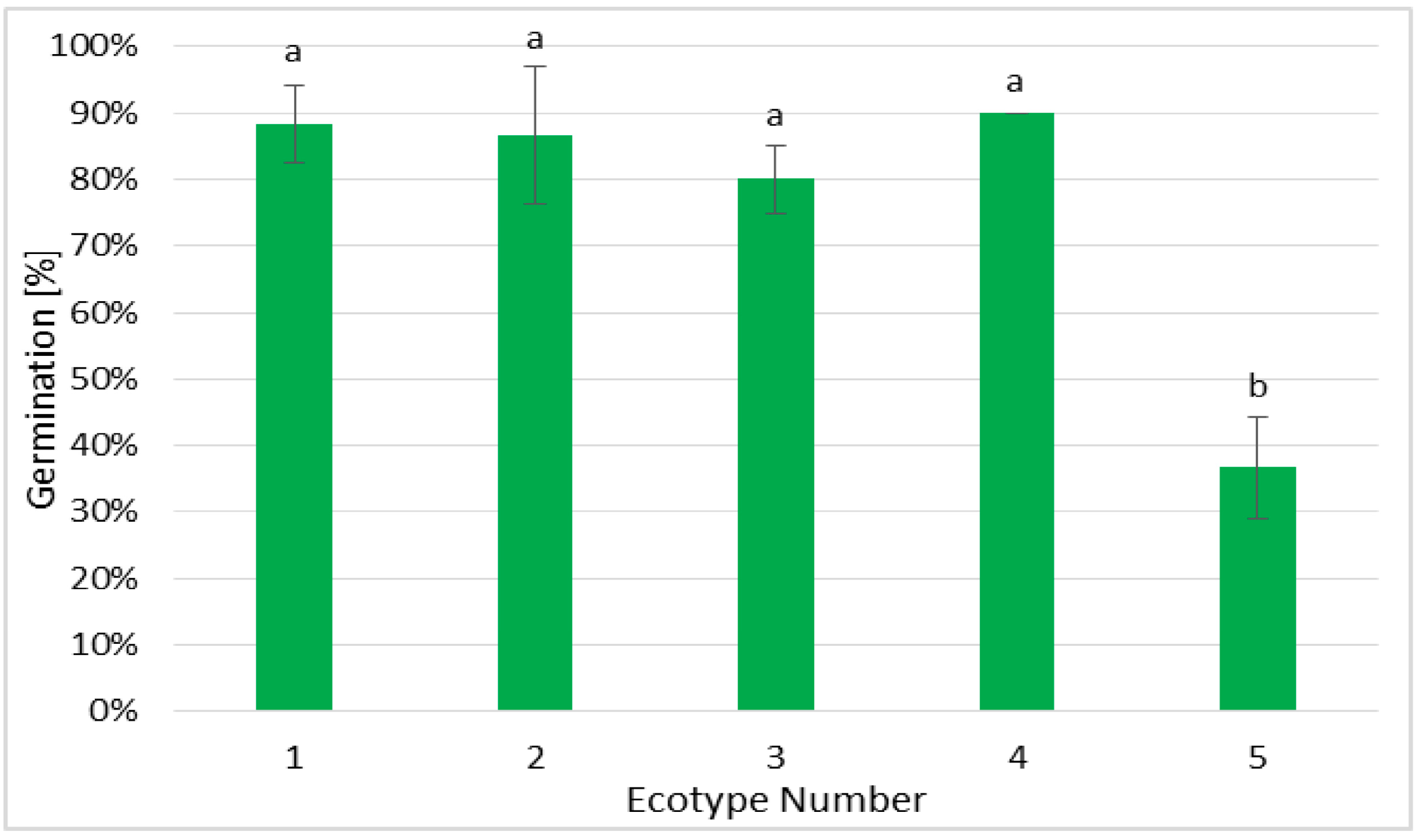
2.1. Establishment of Germination Efficiency
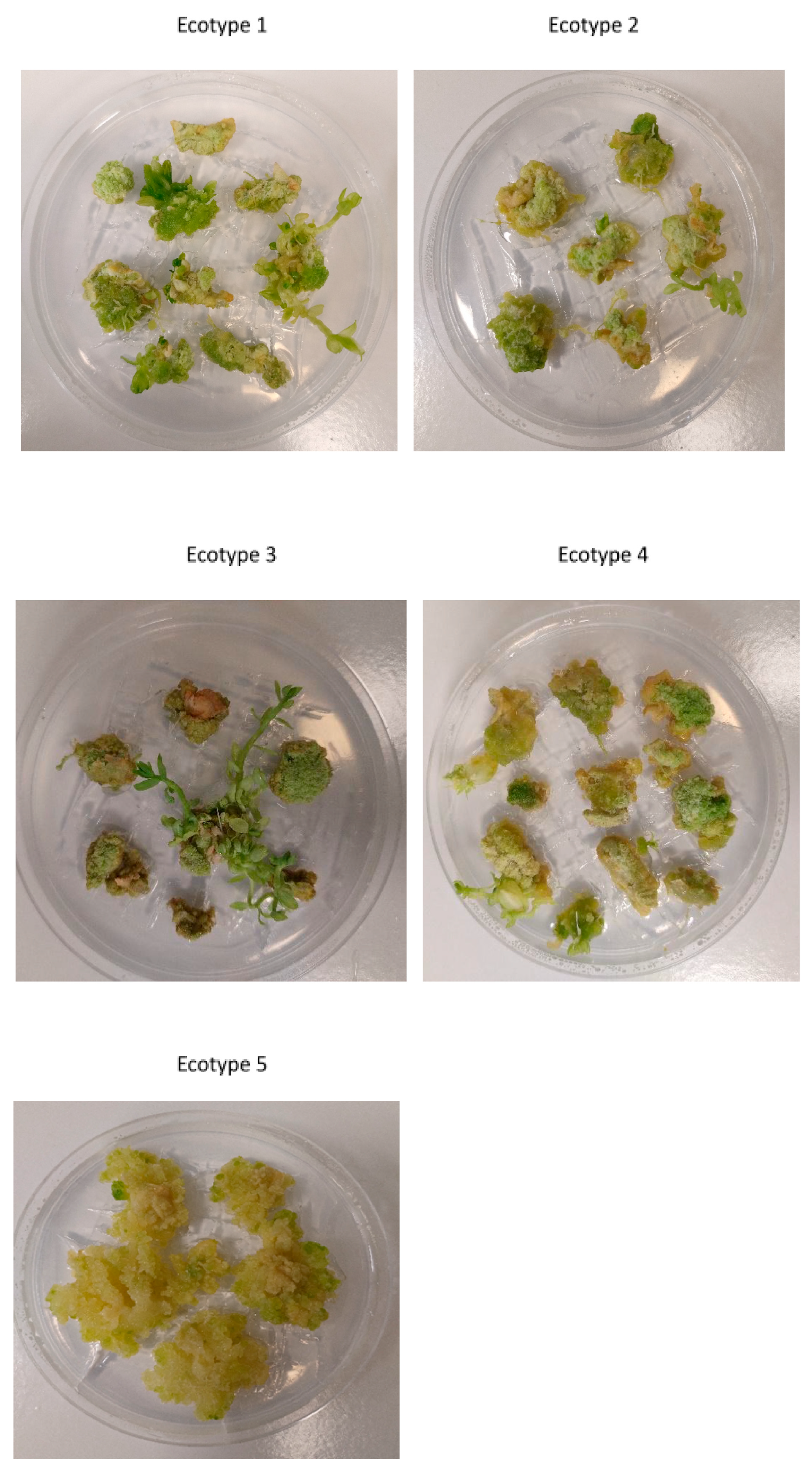
2.2. Frequency of Callus Induction
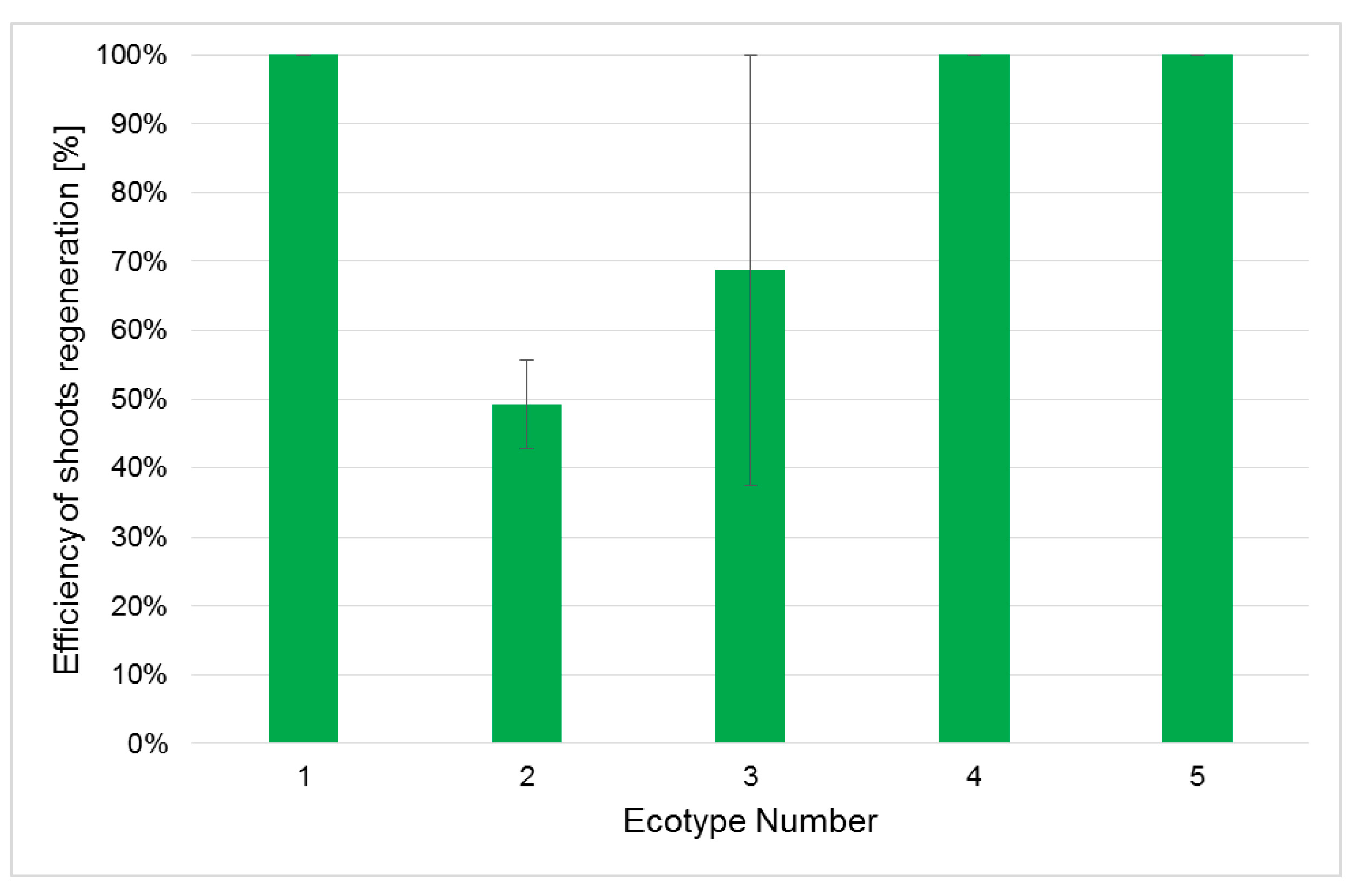
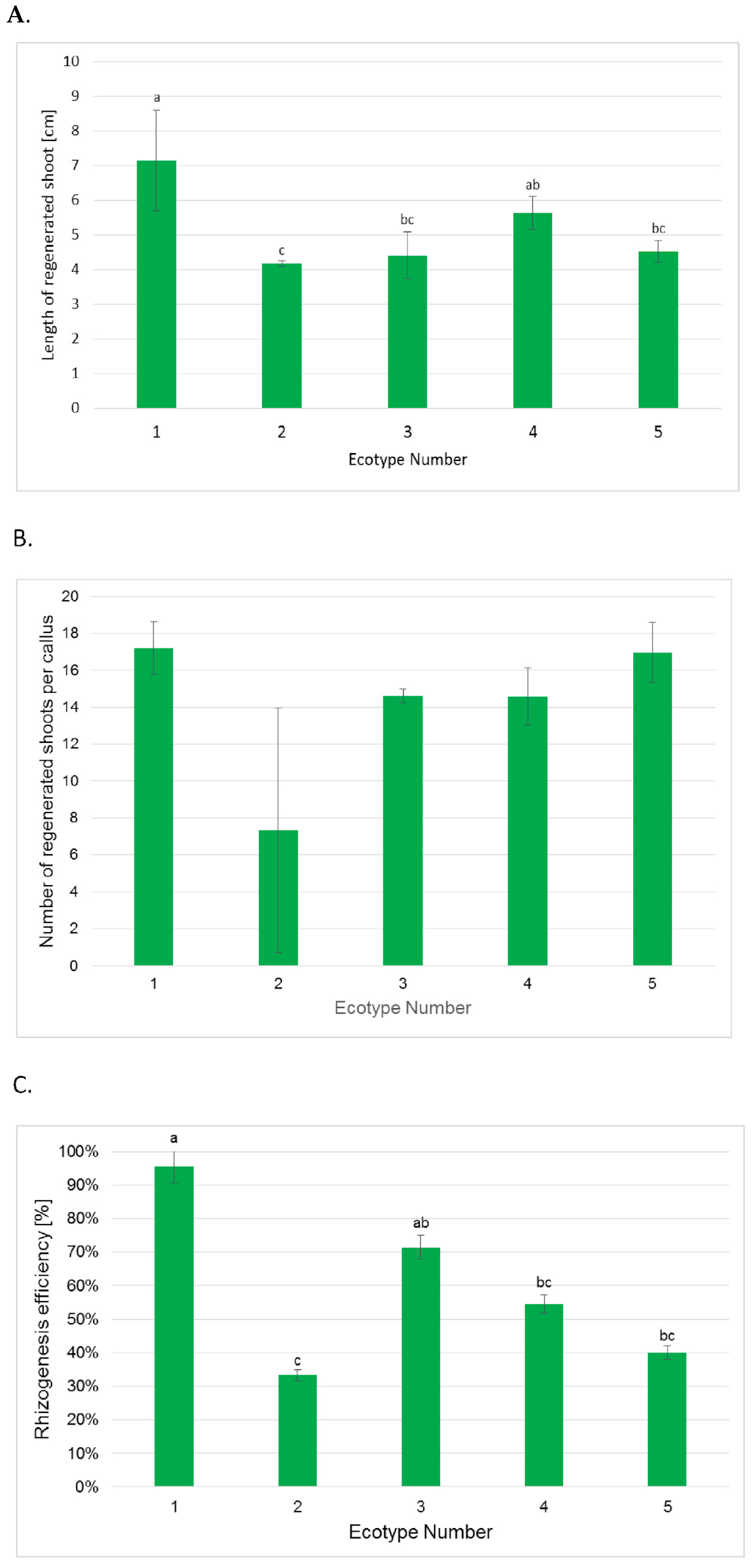
2.3. Efficiency of Shoot Regeneration and Rooting
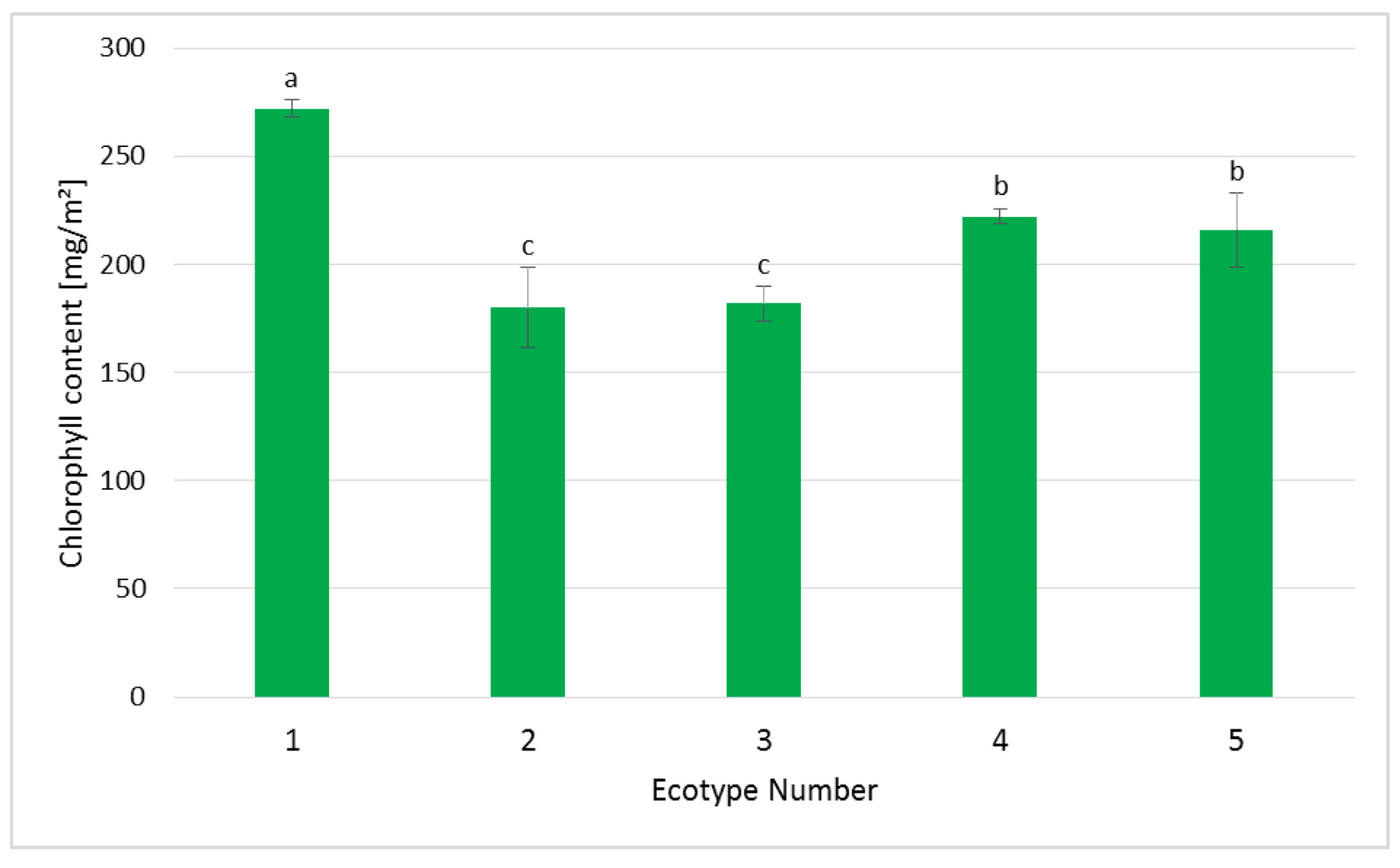
2.4. Chlorophyll Measurements in Regenerated Plants
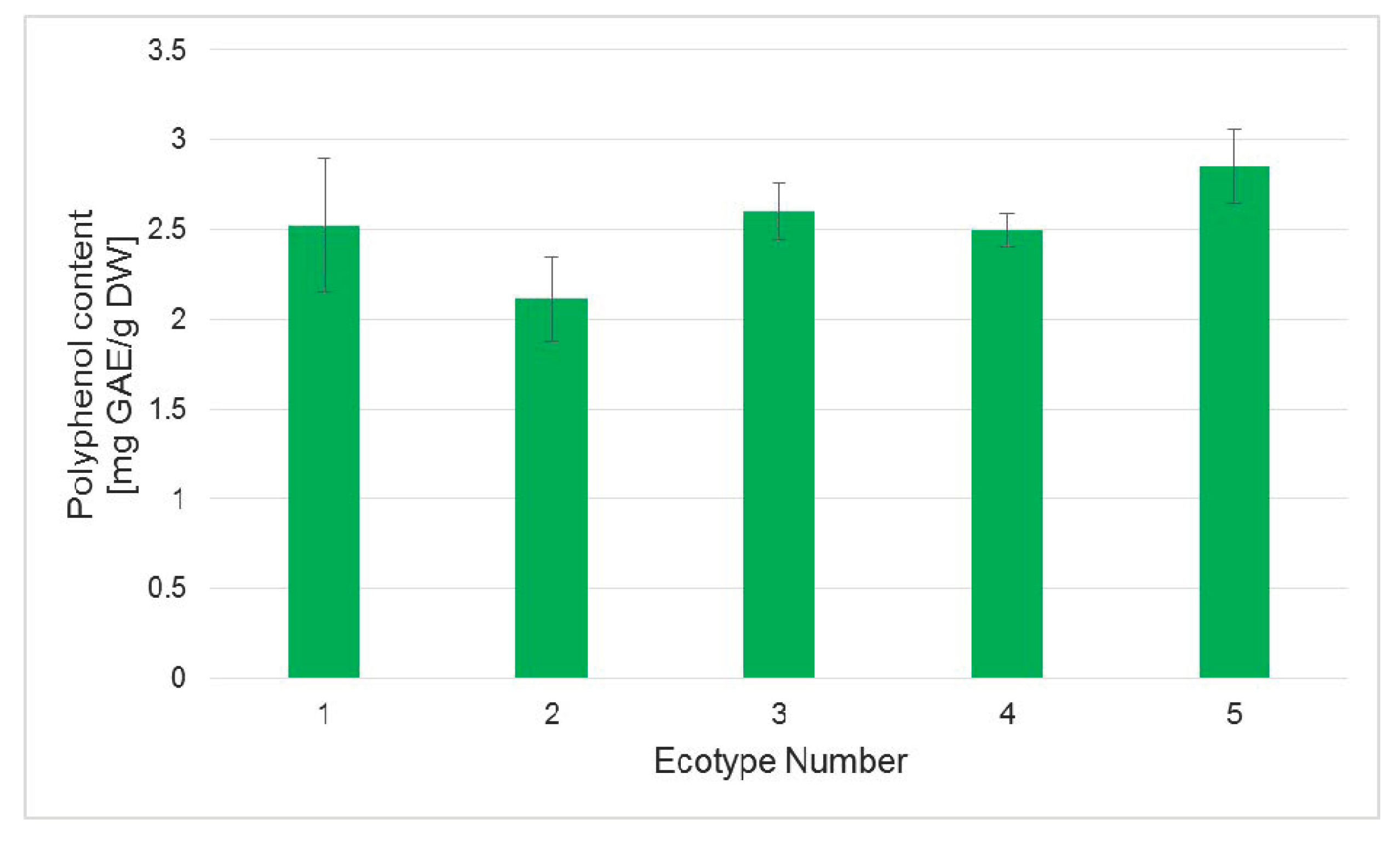
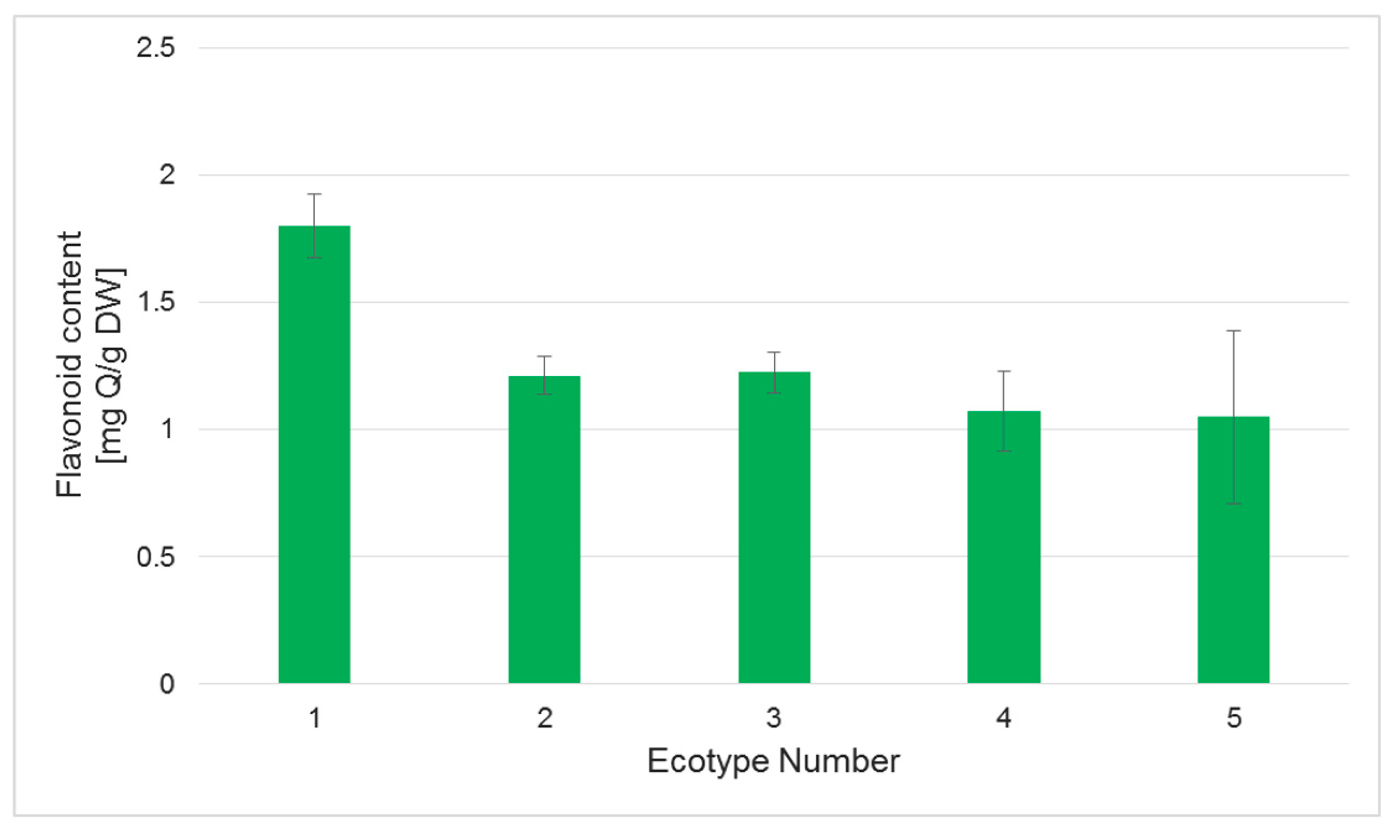
2.5. Polyphenol and Flavonoid Content in Regenerated Plants
3. Materials and Methods
3.1. Plant Material
3.2. Establishment of Flax Tissue Cultures
3.3. Establishment of Germination Efficiency
3.4. Induction of Callus
3.5. Regenerations of Shoots
3.6. In Vitro Rooting of Obtained Regenerants
- v—level of rooting assessed according to 5-step scale;
- n—number of shoots at each assessed level;
- V—the highest level of the used scale;
- N—total number of shoots taken for rooting.
3.7. Determination of Fatty Acid Profile in Silesia Seeds
3.8. Chlorophyll Level in Regenerated Plants
3.9. Total Polyphenol and Flavonoid Content in Regenerated Plants
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data availability Statement
Acknowledgments
Conflicts of Interest
References
- Shinde, N.V.; Sahane, N.S.; Bhosale, S.K.; Patole, V.P.; Satalkar, A.T. Golden Flaxseed (Linum Usitatissimum): Unveiling Its Botanical Marvels, Therapeutic Potentials, and Nanoscale Applications. Chin. J. Appl. Physiol. 2025, 41, e20250017. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Czemplik, M.; Kulma, A.; Żuk, M.; Kaczmar, J.; Dymińska, L.; Hanuza, J.; Ptak, M.; Szopa-Skórkowski, J. New biocomposites based on bioplastic flax fibers and biodegradable polymers. Biotechnol. Prog. 2012, 28, 1336–1346. [Google Scholar] [CrossRef]
- Wróbel-Kwiatkowska, M.; Kostyn, K.; Dymińska, L.; Hanuza, J.; Kurzawa, A.; Żuk, M.; Rymowicz, W. Spectroscopic and biochemical characteristics of flax transgenic callus cultures producing PHB. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 141, 489–497. [Google Scholar] [CrossRef]
- Khlifa, H.D.; Sweelam, H.T.M.; El-Desoky, A.H.; Raslan, M.A. Phytochemical characterization of callus cultures from the endangered plant Crocus scepusiensis (Rehm. & Woł.) Borbás ex Kulcz. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 158, 5. [Google Scholar]
- Pilarska, K.M.; Panić, M.; Redovniković, I.R.; Wróbel-Kwiatkowska, M. Characterization of carnivorous plants Sarracenia purpurea L. transformed with Agrobacterium rhizogenes. Appl. Sci. 2022, 12, 10289. [Google Scholar] [CrossRef]
- Lee, K.; Wang, K. Strategies for genotype-flexible plant transformation. Curr. Opin. Biotechnol. 2023, 79, 102848. [Google Scholar] [CrossRef]
- Smýkalová, I.; Doležal, K.; Plačková, L.; Plíhalová, L.; Zatloukal, M.; Griga, M. The effect of supplementing in vitro flax (Linum usitatissimum L.) culture media with a synthetic cytokinin derivative (BAP9 THP) and a cytokinin metabolism modulator (INCYDE). Plant Growth Regul. 2025, 105, 1125–1138. [Google Scholar] [CrossRef]
- Xu, L.; Huang, H. Genetic and epigenetic controls of plant regeneration. Curr. Top. Dev. Biol. 2014, 108, 1–33. [Google Scholar] [PubMed]
- Shang, B.; Xu, C.; Zhang, X.; Cao, H.; Xin, W.; Hu, Y. Very-long-chain fatty acids restrict regeneration capacity by confining pericycle competence for callus formation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 5101–5106. [Google Scholar] [CrossRef]
- Fehér, A. Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2015, 1849, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Meï, C.; Michaud, M.; Cussac, M.; Albrieux, C.; Gros, V.; Maréchal, E.; Ratet, P.; Rébeillé, F. Levels of polyunsaturated fatty acids correlate with growth rate in plant cell cultures. Sci. Rep. 2015, 5, 15207. [Google Scholar] [CrossRef]
- Czemplik, M.; Boba, A.; Kostyn, K.; Kulma, A.; Mituła, A.; Sztajnert, M.; Skórkowska-Telichowska, K. Flax Engineering for Biomedical Application. In Engineering Flax for Biomedical Applications; InTech: Rijeka, Croatia, 2011; Volume 17. [Google Scholar]
- Augustyniak, B.; Wojtasik, W.; Sawuła, A.; Burgberger, M.; Kulma, A. Spermidine treatment limits the development of the fungus in flax shoots by suppressing polyamine metabolism and balanced defence reactions, thus increasing flax resistance to fusariosis. Front. Plant Sci. 2025, 16, 1561203. [Google Scholar] [CrossRef]
- Jhala, A.J.; Hall, L.M. Flax (Linum usitatissimum L.): Current uses and future applications. Aust. J. Basic Appl. Sci. 2010, 4, 4304–4312. [Google Scholar]
- Zuk, M.; Szperlik, J.; Szopa, J. Linseed Silesia, diverse crops for diverse diets. New solutions to increase dietary lipids in crop species. Foods 2021, 10, 2675. [Google Scholar] [CrossRef]
- Ruiz-Lopez, N.; Usher, S.; Sayanova, O.V.; Napier, J.A.; Haslam, R.P. Modifying the lipid content and composition of plant seeds: Engineering the production of LC-PUFA. Appl. Microbiol. Biotechnol. 2015, 99, 143–154. [Google Scholar] [CrossRef]
- Zienkiewicz, A.; Saldat, M.; Zienkiewicz, K. Here, there and everywhere—The importance of neutral lipids in plant growth and development. Postep. Biochem. 2022, 68, 46–56. [Google Scholar] [CrossRef]
- Shimada, T.L.; Hayashi, M.; Hara-Nishimura, I. Membrane dynamics and multiple functions of oil bodies in seeds and leaves. Plant Physiol. 2018, 176, 199–207. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, L.; Chen, C.; Zhou, T.; Wu, Q.; Wen, F.; Zhang, S.; Yan, J. Comparative changes in sugars and lipids show evidence of a critical node for regeneration in safflower seeds during aging. Front. Plant Sci. 2022, 13, 1020478. [Google Scholar] [CrossRef] [PubMed]
- Balouchi, H.; Soltani Khankahdani, V.; Moradi, A.; Gholamhoseini, M.; Piri, R.; Heydari, S.Z.; Dedicova, B. Seed fatty acid changes germination response to temperature and water potentials in six sesame (Sesamum indicum L.) cultivars: Estimating the cardinal temperatures. Agriculture 2023, 13, 1936. [Google Scholar] [CrossRef]
- Binte Mostafiz, S.; Wagiran, A. Efficient callus induction and regeneration in selected indica rice. Agronomy 2018, 8, 77. [Google Scholar] [CrossRef]
- Ghobeishavi, H.; Uliaie, E.D.; Alavikia, S.S.; Valizadeh, M. The effect of plant growth regulators on embryogenic callus induction and regeneration from coleoptile in rice. Int. J. Biosci. 2014, 5, 144–150. [Google Scholar]
- Li, J.; Na, X.; Qi, C.; Shi, R.; Li, K.; Jin, J.; Yang, Z.; Bi, Y. Cytoplasmic G6PDs modulate callus formation in Arabidopsis root explants through regulation of very-long-chain fatty acids accumulation. Plant Physiol. Biochem. 2025, 220, 109526. [Google Scholar] [CrossRef]
- Smýkalová, I.; Vrbová, M.; Tejklová, E.; Větrovcová, M.; Griga, M. Large scale screening of heavy metal tolerance in flax/linseed (Linum usitatissimum L.) varieties tested in vitro. Ind. Crops Prod. 2010, 32, 527–533. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, C.; Pei, W.; Fan, K.; Shen, W. Chlorophyll a fluorescence as a tool to monitor physiological status in the leaves of Artemisia ordosica under root cutting conditions. Front. Plant Sci. 2024, 14, 1308209. [Google Scholar] [CrossRef]
- Rapacz, M.; Szewczyk-Taranek, B.; Bani, I.; Marcinkowski, P. The fitness of pelargonium cuttings affects the relationship between the photochemical activity of the photosynthetic apparatus and rooting ability. Sci. Rep. 2024, 14, 19716. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef]
- Casanova-Sáez, R.; Mateo-Bonmatí, E.; Ljung, K. Auxin metabolism in plants. Cold Spring Harb. Perspect. Biol. 2021, 13, a039867. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hasiewicz-Derkacz, K.; Kulma, A.; Czuj, T.; Prescha, A.; Żuk, M.; Grajzer, M.; Szopa, J. Natural phenolics greatly increase flax (Linum usitatissimum) oil stability. BMC Biotechnol. 2015, 15, 62. [Google Scholar] [CrossRef]
- Grajzer, M.; Wiatrak, B.; Gębarowski, T.; Matkowski, A.; Grajeta, H.; Rój, E.; Kulma, A.; Prescha, A. Chemistry, oxidative stability and bioactivity of oil extracted from Rosa rugosa (Thunb.) seeds by supercritical carbon dioxide. Food Chem. 2021, 335, 127649. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. The chlorophyll fluorescence ratio F735/F700 as an accurate measure of the chlorophyll content in plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Liszka, J.; Dymińska, L.; Łaba, W.; Wróbel-Kwiatkowska, M. The influence of the non-pathogenic Fusarium oxysporum Fo47 strain on flax resistance to pathogens. Int. J. Mol. Sci. 2025, 26, 4396. [Google Scholar] [CrossRef] [PubMed]
| Content of Individual Fatty Acids in Selected Ecotypes of the Silesia Flax | |||||
|---|---|---|---|---|---|
| Ecotype Number | Palmitic Acid (C16:0) | Stearic Acid (C18:0) | Oleic Acid (C18:1) | Linoleic Acid (C18:2) | α-Linolenic Acid (C18:3) |
| 1 | 4.99 ± 0.09 a | 5.93 ± 0.32 a | 15.82 ± 0.05 b | 42.29 ± 0.87 c | 30.96 ± 0.52 b |
| 2 | 4.78 ± 0.39 a | 3.39 ± 0.11 b | 11.91 ± 1.18 c | 78.50 ± 1.58 a | 1.42 ± 0.13 e |
| 3 | 4.47 ± 0.14 a | 6.47 ± 0.31 a | 25.44 ± 0.43 a | 16.96 ± 0.44 d | 46.66 ± 0.15 a |
| 4 | 5.21 ± 1.04 a | 6.76 ± 0.52 a | 14.44 ± 2.12 bc | 63.43 ± 0.58 b | 10.17 ± 0.03 d |
| 5 | 4.68 ± 0.46 a | 7.28 ± 1.633 a | 17.51 ± 1.03 b | 44.23 ± 1.25 c | 26.29 ± 1.11 c |
| Ecotype of Silesia | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Ratio of linoleic to α-linolenic acid | 1.36 | 55.28 | 0.36 | 6.23 | 1.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipiński, M.; Pilarska-Dudziak, K.; Czuj, T.; Prescha, A.; Łaba, W.; Skórkowska-Telichowska, K.; Wróbel-Kwiatkowska, M. The Novel Flax Cultivar Silesia Shows High Morphogenetic Capacity in Tissue Cultures. Int. J. Mol. Sci. 2025, 26, 8847. https://doi.org/10.3390/ijms26188847
Lipiński M, Pilarska-Dudziak K, Czuj T, Prescha A, Łaba W, Skórkowska-Telichowska K, Wróbel-Kwiatkowska M. The Novel Flax Cultivar Silesia Shows High Morphogenetic Capacity in Tissue Cultures. International Journal of Molecular Sciences. 2025; 26(18):8847. https://doi.org/10.3390/ijms26188847
Chicago/Turabian StyleLipiński, Mateusz, Kinga Pilarska-Dudziak, Tadeusz Czuj, Anna Prescha, Wojciech Łaba, Katarzyna Skórkowska-Telichowska, and Magdalena Wróbel-Kwiatkowska. 2025. "The Novel Flax Cultivar Silesia Shows High Morphogenetic Capacity in Tissue Cultures" International Journal of Molecular Sciences 26, no. 18: 8847. https://doi.org/10.3390/ijms26188847
APA StyleLipiński, M., Pilarska-Dudziak, K., Czuj, T., Prescha, A., Łaba, W., Skórkowska-Telichowska, K., & Wróbel-Kwiatkowska, M. (2025). The Novel Flax Cultivar Silesia Shows High Morphogenetic Capacity in Tissue Cultures. International Journal of Molecular Sciences, 26(18), 8847. https://doi.org/10.3390/ijms26188847






