Cytoprotective Potential of Annurca Apple Polyphenols on Mercury-Induced Oxidative Stress in Human Erythrocytes
Abstract
1. Introduction
2. Results
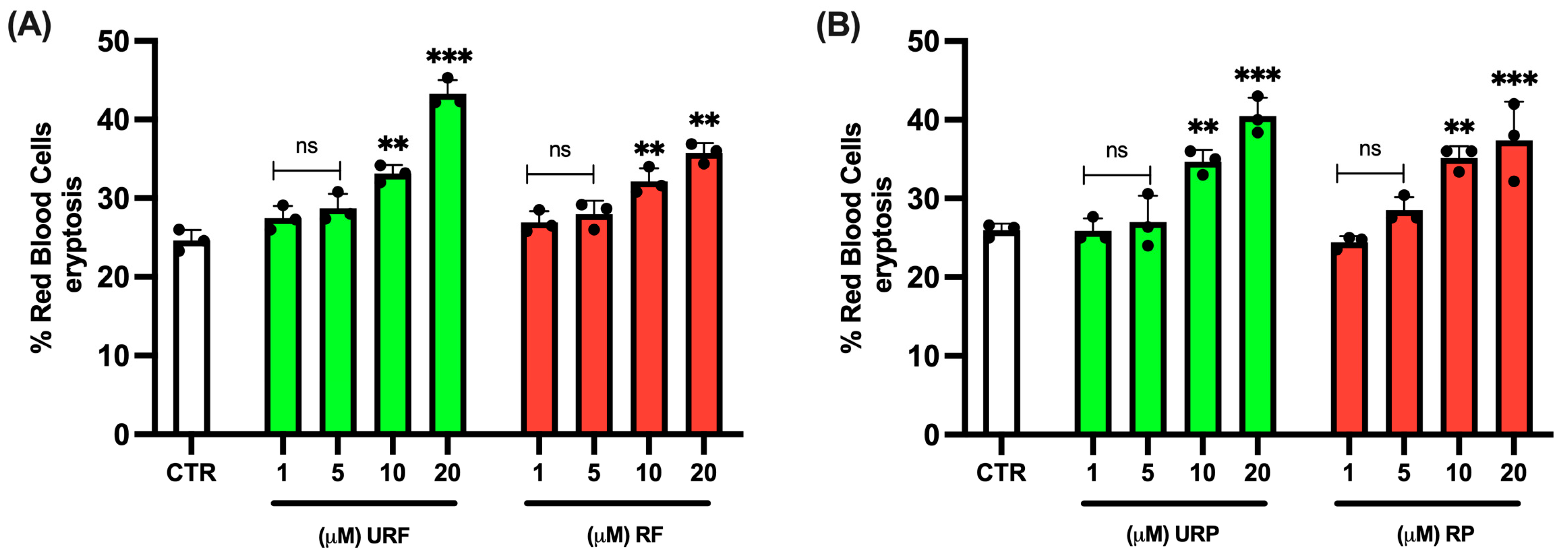
2.1. RBC Viability
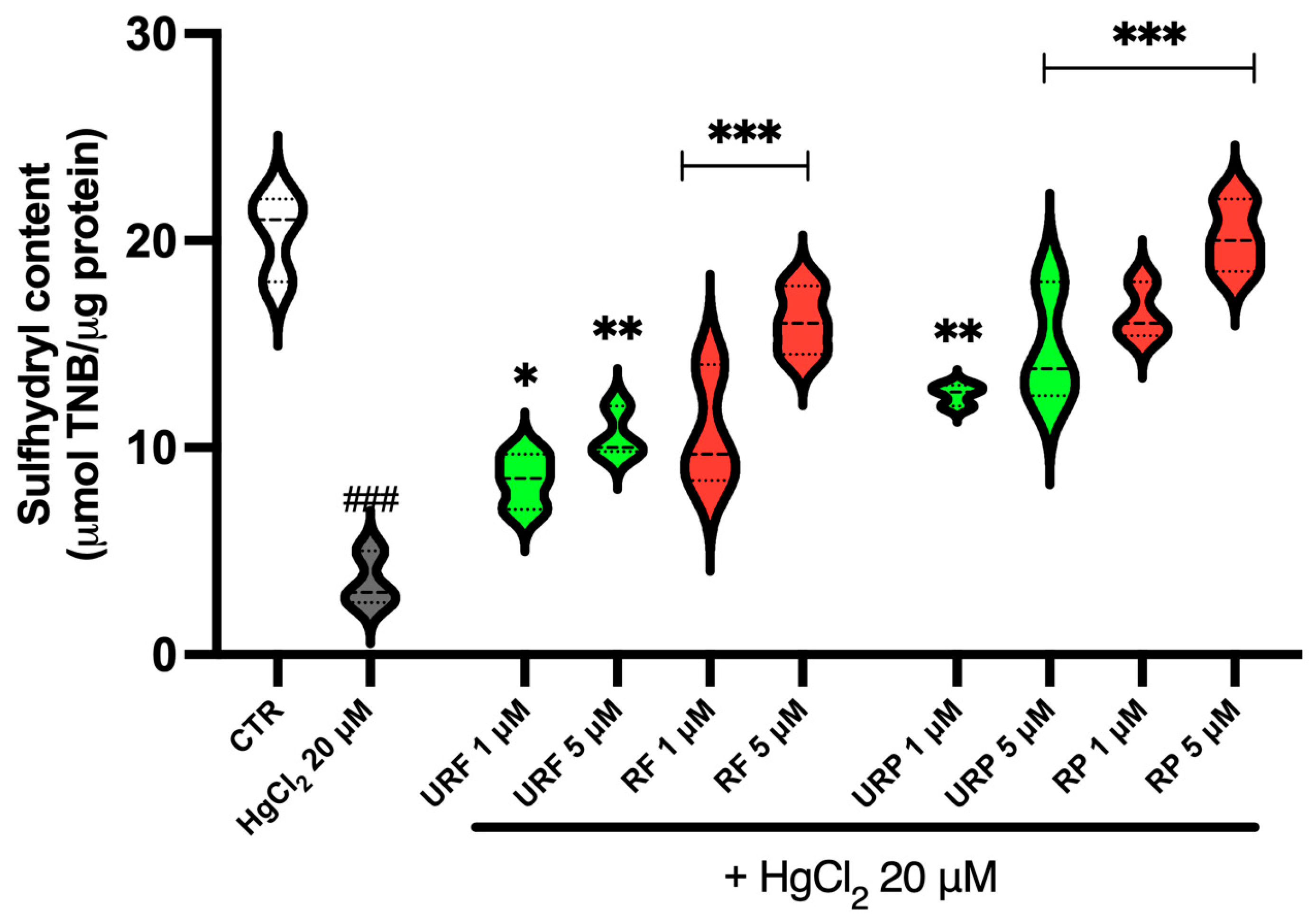
2.2. Total Sulfhydryl Group Content
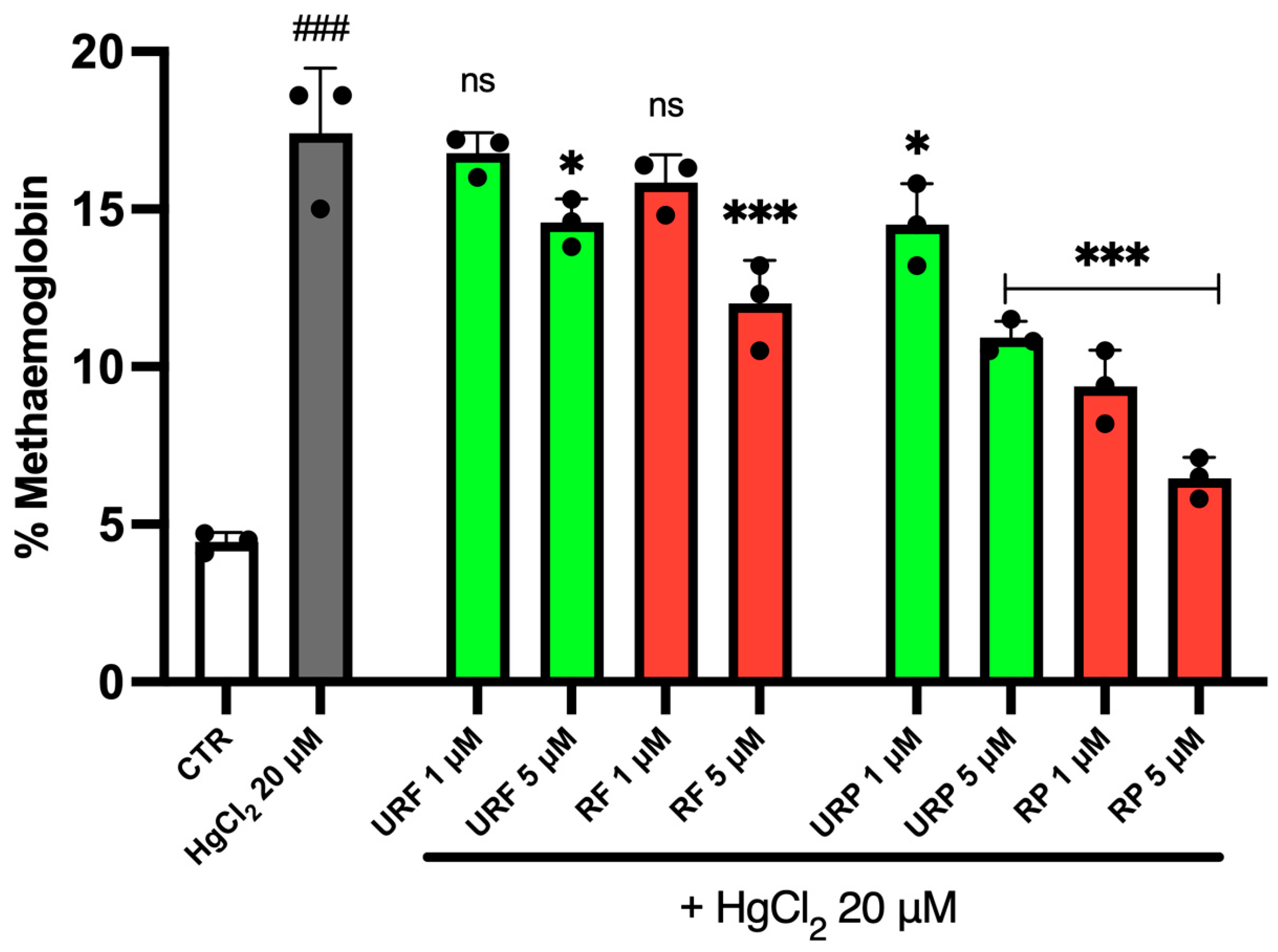
2.3. MetHb Levels
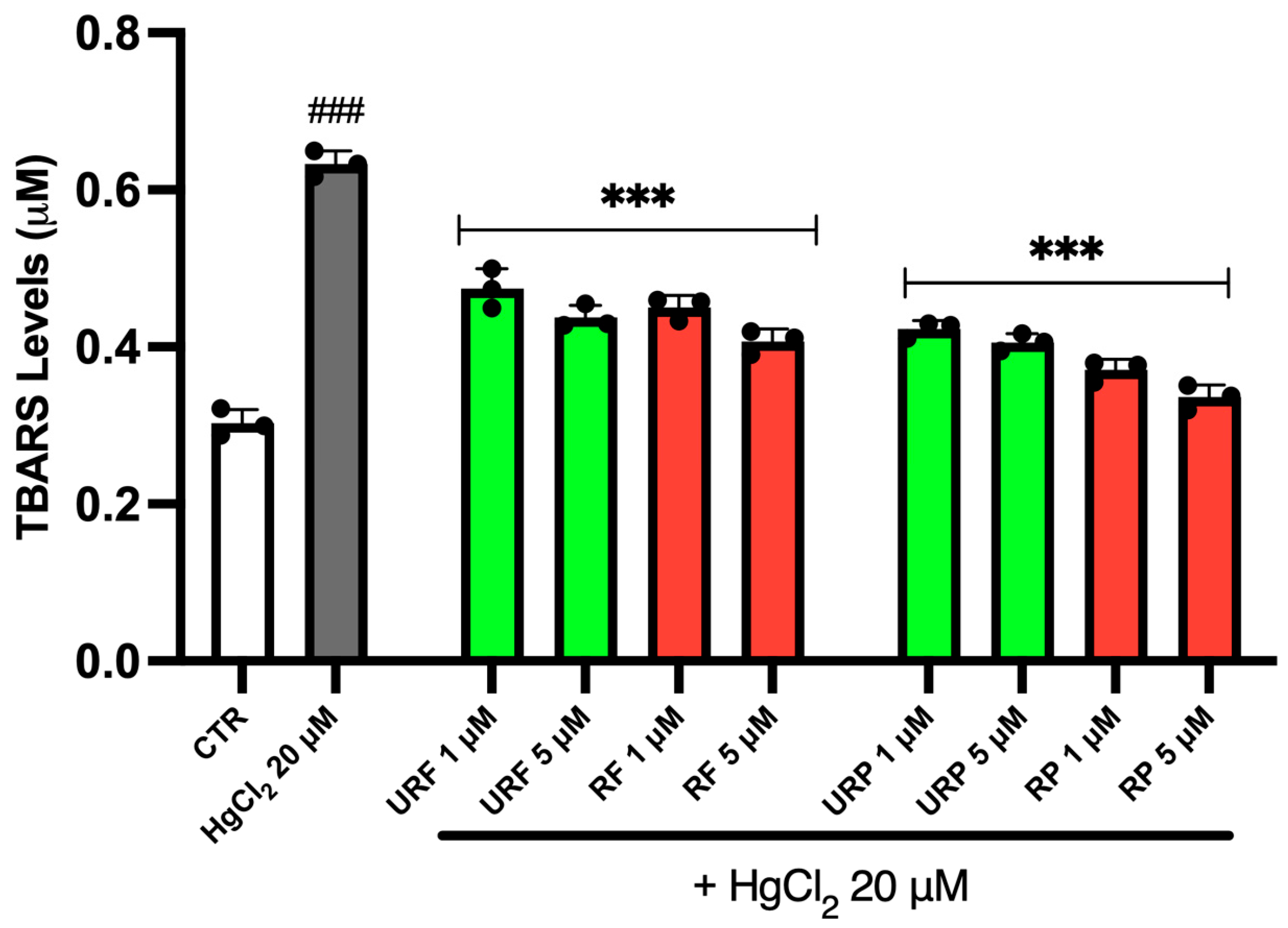
2.4. TBARS Levels
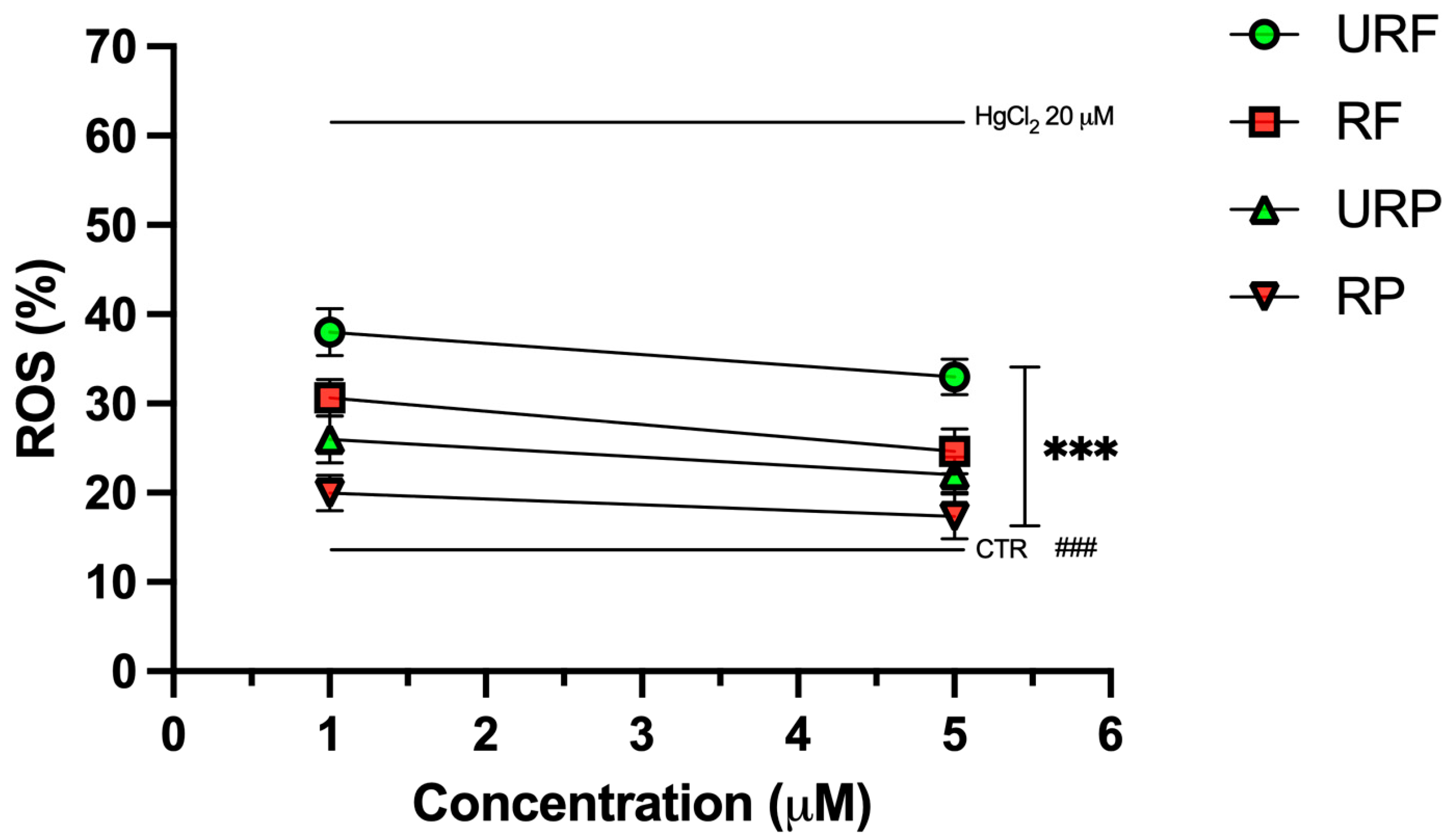
2.5. ROS Levels
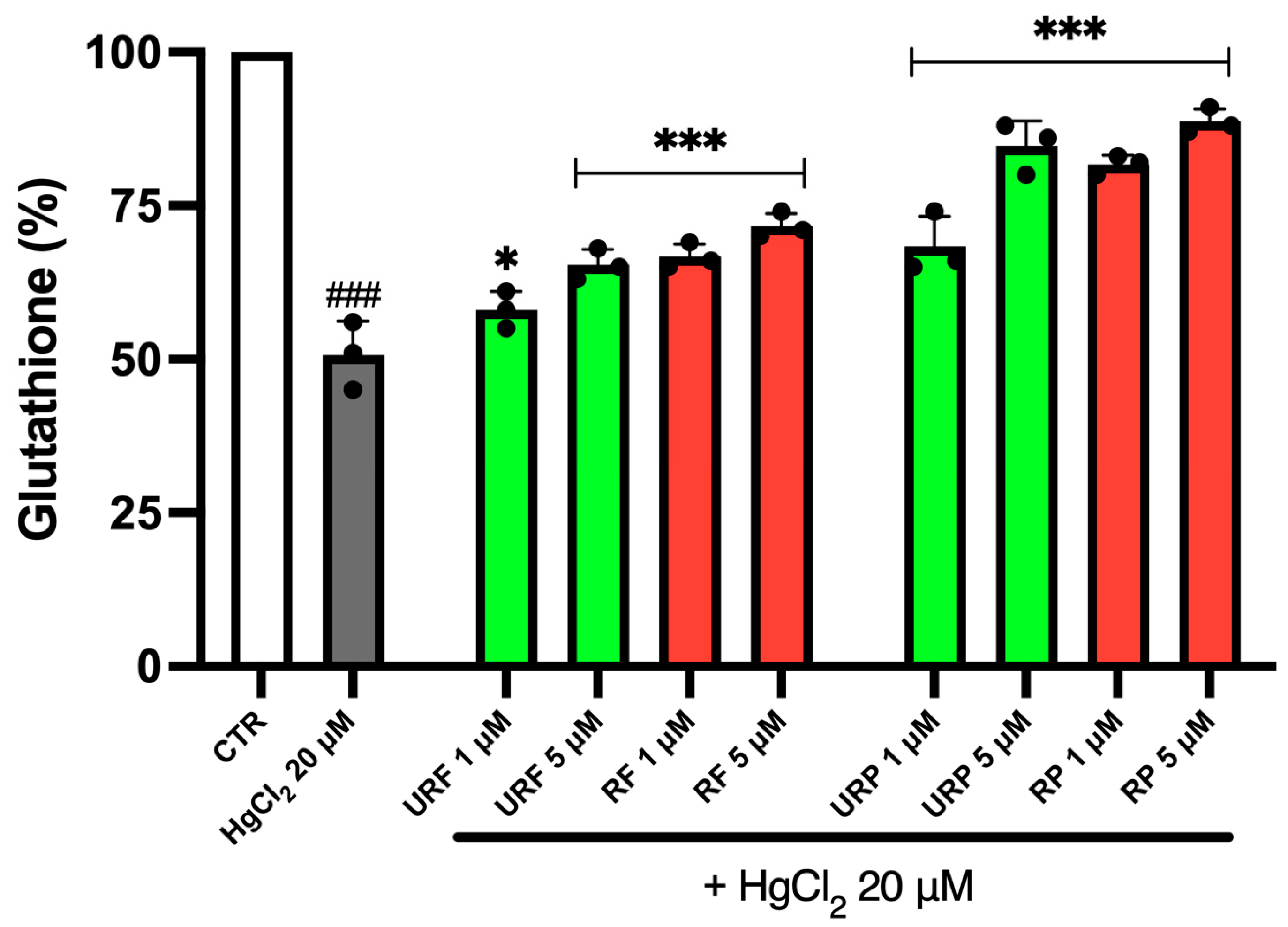
2.6. GSH Content
2.7. MV Generation
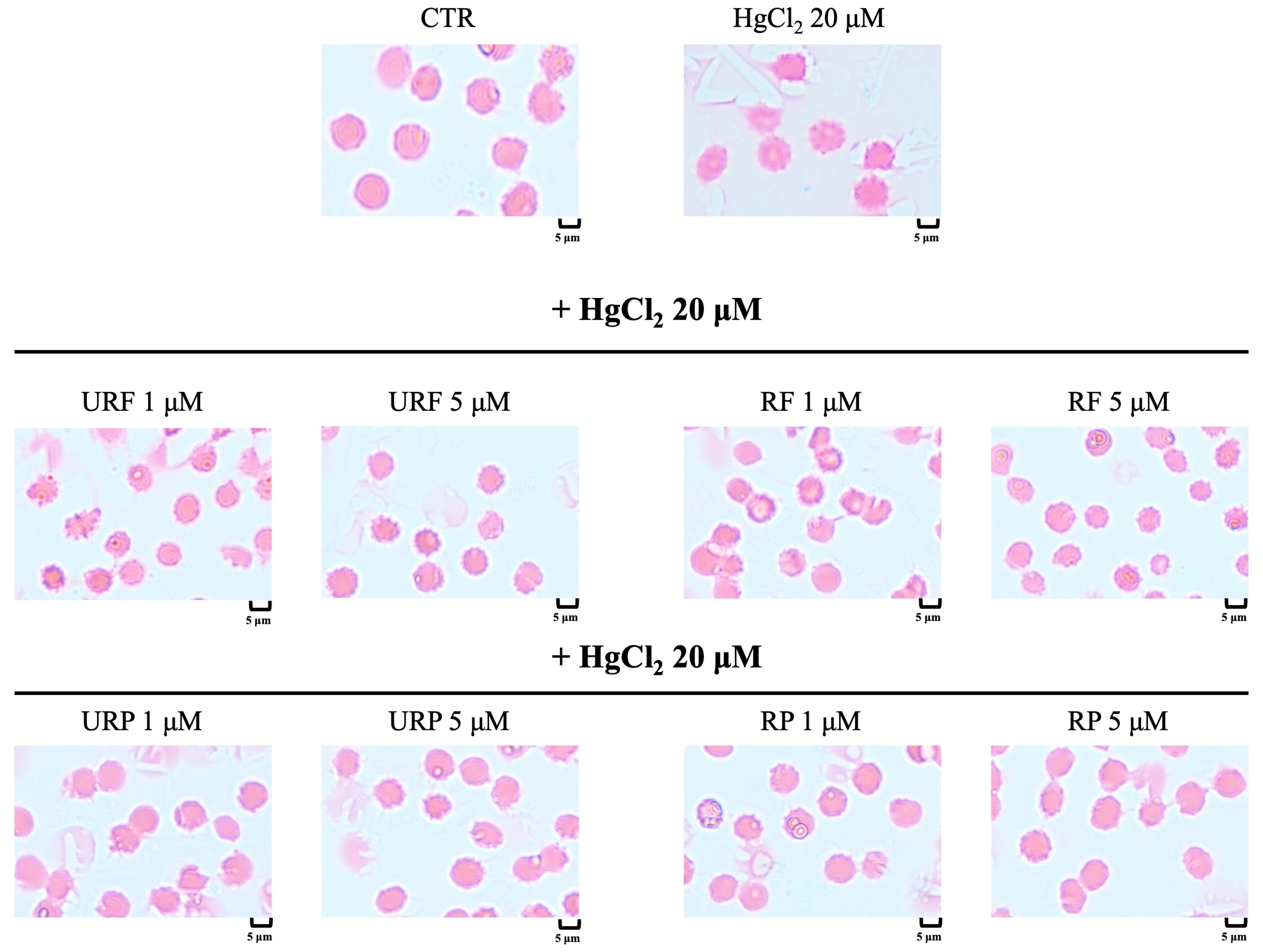
2.8. Microscopic Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions
4.2. Fruit Collection
4.3. Polyphenol Extraction
4.4. Preparation of RBC and Treatment with HgCl2 and Annurca Apple Extracts
4.5. Detection of Apoptotic RBC
4.6. Total Sulfhydryl Group Content
4.7. Determination of MetHb Levels
4.8. TBARS Level Measurement
4.9. ROS Determination
4.10. GSH Assay
4.11. Quantification of MV
4.12. Microscopic Analysis
4.13. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RBCs | Erythrocytes |
| GSH | Glutathione |
| Hg | Mercury |
| MetHb | Methemoglobin |
| MeHg | Methylmercury |
| MV | Microvesicles |
| ROS | Reactive oxygen species |
| SH | Sulfhydryl group |
| TBARS | Thiobarbituric acid reactive substances |
References
- Vuoso, D.; Porcelli, M.; Cacciapuoti, G.; D’Angelo, S. Biological Activity of MelAnnurca Flesh Apple Biophenols. Curr. Nutr. Food Sci. 2020, 16, 1149–1162. [Google Scholar] [CrossRef]
- D’Angelo, S.; Cimmino, A.; Raimo, M.; Salvatore, A.; Zappia, V.; Galletti, P. Effect of Reddening-Ripening on the Antioxidant Activity of Polyphenol Extracts from Cv. “Annurca” Apple Fruits. J. Agric. Food Chem. 2007, 55, 9977–9985. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S. Diet and Aging: The Role of Polyphenol-Rich Diets in Slow Down the Shortening of Telomeres: A Review. Antioxidants 2023, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; De Giulio, A.; Sada, A.; Nazzaro, F. Biochemical Characteristics and Biological Properties of Annurca Apple Cider. J. Med. Food 2012, 15, 18–23. [Google Scholar] [CrossRef]
- Vuoso, D.C.; D’Angelo, S.; Ferraro, R.; Caserta, S.; Guido, S.; Cammarota, M.; Porcelli, M.; Cacciapuoti, G. Annurca Apple Polyphenol Extract Promotes Mesenchymal-to-Epithelial Transition and Inhibits Migration in Triple-Negative Breast Cancer Cells through ROS/JNK Signaling. Sci. Rep. 2020, 10, 15921. [Google Scholar] [CrossRef]
- Boccellino, M.; Quagliuolo, L.; D’Angelo, S. Annurca Apple Biophenols’ Effects in Combination with Cisplatin on A549 Cells. Curr. Nutr. Food Sci. 2020, 17, 111–120. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-Oxidant and pro-Apoptotic Activity of Polyphenol Extract from Annurca Apple and Its Underlying Mechanisms in Human Breast Cancer Cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Cacciapuoti, G. Effects of Annurca Apple (Malus pumila cv Annurca) Polyphenols on Breast Cancer Cells. Curr. Nutr. Food Sci. 2019, 15, 745–751. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health. Nutrients 2025, 17, 948. [Google Scholar] [CrossRef]
- Perrone, P.; De Rosa, C.; D’Angelo, S. Mediterranean Diet and Agri-Food By-Products: A Possible Sustainable Approach for Breast Cancer Treatment. Antioxidants 2025, 14, 789. [Google Scholar] [CrossRef]
- Riccio, G.; Maisto, M.; Bottone, S.; Badolati, N.; Rossi, G.B.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. WNT Inhibitory Activity of Malus pumila Miller Cv Annurca and Malus domestica Cv Limoncella Apple Extracts on Human Colon-Rectal Cells Carrying Familial Adenomatous Polyposis Mutations. Nutrients 2017, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Aschner, M.; Carvalho, C. The Biochemistry of Mercury Toxicity. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129412. [Google Scholar] [CrossRef] [PubMed]
- Yorifuji, T.; Tsuda, T.; Kashima, S.; Takao, S.; Harada, M. Long-Term Exposure to Methylmercury and Its Effects on Hypertension in Minamata. Environ. Res. 2010, 110, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Renneberg, A.J.; Dudas, M.J. Transformations of Elemental Mercury to Inorganic and Organic Forms in Mercury and Hydrocarbon Co-Contaminated Soils. Chemosphere 2001, 45, 1103–1109. [Google Scholar] [CrossRef]
- Domínguez-Morueco, N.; Pedraza-Díaz, S.; González-Caballero, M.d.C.; Esteban-López, M.; de Alba-González, M.; Katsonouri, A.; Santonen, T.; Cañas-Portilla, A.; Castaño, A. Methylmercury Risk Assessment Based on European Human Biomonitoring Data. Toxics 2022, 10, 427. [Google Scholar] [CrossRef]
- Ye, B.-J.; Kim, B.-G.; Jeon, M.-J.; Kim, S.-Y.; Kim, H.-C.; Jang, T.-W.; Chae, H.-J.; Choi, W.-J.; Ha, M.-N.; Hong, Y.-S. Evaluation of Mercury Exposure Level, Clinical Diagnosis and Treatment for Mercury Intoxication. Ann. Occup. Environ. Med. 2016, 28, 5. [Google Scholar] [CrossRef]
- Robitaille, S.; Mailloux, R.J.; Chan, H.M. Methylmercury Alters Glutathione Homeostasis by Inhibiting Glutaredoxin 1 and Enhancing Glutathione Biosynthesis in Cultured Human Astrocytoma Cells. Toxicol. Lett. 2016, 256, 1–10. [Google Scholar] [CrossRef]
- Notariale, R.; Längst, E.; Perrone, P.; Crettaz, D.; Prudent, M.; Manna, C. Effect of Mercury on Membrane Proteins, Anionic Transport and Cell Morphology in Human Erythrocytes. Cell. Physiol. Biochem. 2022, 56, 500–513. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, X.; Luo, H.; Zhao, L.; Ji, X.; Qiao, X.; Jin, Y.; Liu, W. Acute Exposure of Mercury Chloride Stimulates the Tissue Regeneration Program and Reactive Oxygen Species Production in the Drosophila Midgut. Environ. Toxicol. Pharmacol. 2016, 41, 32–38. [Google Scholar] [CrossRef]
- Notariale, R.; Perrone, P.; Mele, L.; Lettieri, G.; Piscopo, M.; Manna, C. Olive Oil Phenols Prevent Mercury-Induced Phosphatidylserine Exposure and Morphological Changes in Human Erythrocytes Regardless of Their Different Scavenging Activity. Int. J. Mol. Sci. 2022, 23, 5693. [Google Scholar] [CrossRef]
- Ahmad, S.; Mahmood, R. Mercury Chloride Toxicity in Human Erythrocytes: Enhanced Generation of ROS and RNS, Hemoglobin Oxidation, Impaired Antioxidant Power, and Inhibition of Plasma Membrane Redox System. Environ. Sci. Pollut. Res. Int. 2019, 26, 5645–5657. [Google Scholar] [CrossRef]
- Massaccesi, L.; Galliera, E.; Corsi Romanelli, M.M. Erythrocytes as Markers of Oxidative Stress Related Pathologies. Mech. Ageing Dev. 2020, 191, 111333. [Google Scholar] [CrossRef]
- Perrone, P.; Palmieri, S.; Piscopo, M.; Lettieri, G.; Eugelio, F.; Fanti, F.; D’Angelo, S. Antioxidant Activity of Annurca Apple By-Products at Different Ripening Stages: A Sustainable Valorization Approach. Antioxidants 2025, 14, 941. [Google Scholar] [CrossRef]
- Perrone, P.; Spinelli, S.; Mantegna, G.; Notariale, R.; Straface, E.; Caruso, D.; Falliti, G.; Marino, A.; Manna, C.; Remigante, A.; et al. Mercury Chloride Affects Band 3 Protein-Mediated Anionic Transport in Red Blood Cells: Role of Oxidative Stress and Protective Effect of Olive Oil Polyphenols. Cells 2023, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Hare, G.M.T.; Tsui, A.K.Y.; Crawford, J.H.; Patel, R.P. Is Methemoglobin an Inert Bystander, Biomarker or a Mediator of Oxidative Stress--The Example of Anemia? Redox Biol. 2013, 1, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Aguilar Diaz De Leon, J.; Borges, C.R. Evaluation of Oxidative Stress in Biological Samples Using the Thiobarbituric Acid Reactive Substances Assay. J. Vis. Exp. 2020, 159. [Google Scholar] [CrossRef]
- Brieger, K.; Schiavone, S.; Miller, F.J.; Krause, K.-H. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Original Article Extra Virgin Olive Oil as a Functional Food for Athletes: Recovery, Health, and Performance. J. Phys. Educ. Sport 2025, 25, 370–381. [Google Scholar] [CrossRef]
- Hernández, L.E.; Sobrino-Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of Glutathione to the Control of Cellular Redox Homeostasis under Toxic Metal and Metalloid Stress. Available online: https://pubmed.ncbi.nlm.nih.gov/25750419/ (accessed on 18 June 2024).
- Moriello, C.; De Rosa, C.; D’Angelo, S.; Pasquale, P. Polyphenols and Chronic Myeloid Leukemia: Emerging Therapeutic Opportunities. Hemato 2025, 6, 28. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxidative Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Omanwar, S.; Fahim, M. Mercury Exposure and Endothelial Dysfunction: An Interplay Between Nitric Oxide and Oxidative Stress. Int. J. Toxicol. 2015, 34, 300–307. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2012, 10, 4–18. [Google Scholar] [CrossRef]
- Ad, T.; An, R.; Ml, H. Mercury Intoxication and Arterial Hypertension: Report of Two Patients and Review of the Literature. Pediatrics 2000, 105, e34. [Google Scholar] [CrossRef]
- Perrone, P.; Ortega-Luna, R.; Manna, C.; Álvarez-Ribelles, Á.; Collado-Diaz, V. Increased Adhesiveness of Blood Cells Induced by Mercury Chloride: Protective Effect of Hydroxytyrosol. Antioxidants 2024, 13, 1576. [Google Scholar] [CrossRef] [PubMed]
- Dhyani, N.; Tian, C.; Gao, L.; Rudebush, T.L.; Zucker, I.H. Nrf2-Keap1 in Cardiovascular Disease: Which Is the Cart and Which the Horse? Physiology 2024, 39, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, R.; Xu, R.; Chu, Y.; Gu, W. Procyanidin B2 Ameliorates Endothelial Dysfunction and Impaired Angiogenesis via the Nrf2/PPARγ/sFlt-1 Axis in Preeclampsia. Pharmacol. Res. 2022, 177, 106127. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal Chelation of Polyphenols. Methods Enzymol. 2001, 335, 190–203. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Bajic, V.P.; Van Neste, C.; Obradovic, M.; Zafirovic, S.; Radak, D.; Bajic, V.B.; Essack, M.; Isenovic, E.R. Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxidative Med. Cell. Longev. 2019, 2019, 5028181. [Google Scholar] [CrossRef]
- Daraghmeh, D.N.; Karaman, R. The Redox Process in Red Blood Cells: Balancing Oxidants and Antioxidants. Antioxidants 2024, 14, 36. [Google Scholar] [CrossRef]
- Özduran, G.; Becer, E.; Vatansever, H.S. The Role and Mechanisms of Action of Catechins in Neurodegenerative Diseases. J. Am. Nutr. Assoc. 2023, 42, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Romana, M.; Connes, P. The Red Blood Cell—Inflammation Vicious Circle in Sickle Cell Disease. Front. Immunol. 2020, 11, 454. [Google Scholar] [CrossRef]
- Spengler, M.I.; Svetaz, M.J.; Leroux, M.B.; Bertoluzzo, S.M.; Parente, F.M.; Bosch, P. Lipid Peroxidation Affects Red Blood Cells Membrane Properties in Patients with Systemic Lupus Erythematosus. Clin. Hemorheol. Microcirc. 2014, 58, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Fraga, C.G.; Oteiza, P.I. Interactions of Flavan-3-Ols and Procyanidins with Membranes: Mechanisms and the Physiological Relevance. Food Funct. 2015, 6, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Martinez Fernandez, A.; Casalnuovo, F.; Altomare, A.; Aldini, G.; Banfi, C. Lipid Peroxidation in Atherosclerotic Cardiovascular Diseases. Antioxid. Redox Signal 2021, 34, 49–98. [Google Scholar] [CrossRef]
- Mendonça, R.; Silveira, A.A.A.; Conran, N. Red Cell DAMPs and Inflammation. Inflamm. Res. 2016, 65, 665–678. [Google Scholar] [CrossRef]
- Sudnitsyna, J.; Skverchinskaya, E.; Dobrylko, I.; Nikitina, E.; Gambaryan, S.; Mindukshev, I. Microvesicle Formation Induced by Oxidative Stress in Human Erythrocytes. Antioxidants 2020, 9, 929. [Google Scholar] [CrossRef]
- Perrone, P.; Notariale, R.; Lettieri, G.; Mele, L.; La Pietra, V.; Piscopo, M.; Manna, C. Protective Effects of Olive Oil Antioxidant Phenols on Mercury-Induced Phosphatidylserine Externalization in Erythrocyte Membrane: Insights into Scramblase and Flippase Activity. Free Radic. Biol. Med. 2025, 227, 42–51. [Google Scholar] [CrossRef]
- Perrotta, S.; Gallagher, P.G.; Mohandas, N. Hereditary Spherocytosis. Lancet 2008, 372, 1411–1426. [Google Scholar] [CrossRef]
- Principe, D.R.; Reilly, P.; Dhavamani, S.; Rivers, A.; Molokie, R.; Hsu, L.L.; Ramasamy, J. Hereditary Spherocytosis with Mitochondrial Retention, Increased Oxidative Stress, and Alterations to Bioactive Membrane Lipids. J. Pediatr. Hematol. Oncol. 2024, 46, e457–e462. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Yang, J.; Sun, J.; Qin, G. Extracellular Vesicles in Cardiovascular Disease: Biological Functions and Therapeutic Implications. Pharmacol. Ther. 2022, 233, 108025. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, P.; Richards, R.S.; Bwititi, P.T.; Nwose, E.U. Association of Abnormal Erythrocyte Morphology with Oxidative Stress and Inflammation in Metabolic Syndrome. Blood Cells Mol. Dis. 2015, 54, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Notariale, R.; Moriello, C.; Alessio, N.; Del Vecchio, V.; Mele, L.; Perrone, P.; Manna, C. Protective Effect of Hydroxytyrosol against Hyperglycemia-Induced Phosphatidylserine Exposure in Human Erythrocytes: Focus on Dysregulation of Calcium Homeostasis and Redox Balance. Redox Biol. 2025, 85, 103783. [Google Scholar] [CrossRef]
- Loyola-Leyva, A.; Loyola-Rodríguez, J.P.; Atzori, M.; González, F.J. Morphological Changes in Erythrocytes of People with Type 2 Diabetes Mellitus Evaluated with Atomic Force Microscopy: A Brief Review. Micron 2018, 105, 11–17. [Google Scholar] [CrossRef]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, Flavonoid-Rich Foods, and Cardiovascular Risk: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Iqbal, A.; Naz, R.; Mubarak, M.S. Plant Polyphenols and Their Potential Benefits on Cardiovascular Health: A Review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Rodrigo, R.; Retamal, C.; Schupper, D.; Vergara-Hernández, D.; Saha, S.; Profumo, E.; Buttari, B.; Saso, L. Antioxidant Cardioprotection against Reperfusion Injury: Potential Therapeutic Roles of Resveratrol and Quercetin. Molecules 2022, 27, 2564. [Google Scholar] [CrossRef]
- Naoum, P.C.; Radispiel, J.; Moraes, M. da S. Spectrometric Measurement of Methemoglobin without Interference of Chemical or Enzymatic Reagents. Rev. Bras. Hematol. Hemoter. 2004, 26, 19–22. [Google Scholar] [CrossRef]
- Mendanha, S.A.; Anjos, J.L.V.; Silva, A.H.M.; Alonso, A. Electron Paramagnetic Resonance Study of Lipid and Protein Membrane Components of Erythrocytes Oxidized with Hydrogen Peroxide. Braz. J. Med. Biol. Res. 2012, 45, 473–481. [Google Scholar] [CrossRef]

| Annurca Apple Part | Polyphenolic Compounds |
|---|---|
| URF | Protocatecuic acid; tyrosol; caffeic acid; ferulic acid; epicatechin; catechin; clorogenic acid; quercetin hexoside; rutina. |
| RF | Protocatecuic acid; caffeic acid; ferulic acid; epicatechin; catechin; clorogenic acid; quercetin; quercetin hexoside; rutina. |
| URP | Caffeic acid; ferulic acid; epicatechin; catechin; clorogenic acid; quercetin hexoside; rutina. |
| RP | Protocatecuic acid; ferulic acid; epicatechin; clorogenic acid; quercetin hexoside; rutina. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, P.; Moriello, C.; Alessio, N.; Manna, C.; D’Angelo, S. Cytoprotective Potential of Annurca Apple Polyphenols on Mercury-Induced Oxidative Stress in Human Erythrocytes. Int. J. Mol. Sci. 2025, 26, 8826. https://doi.org/10.3390/ijms26188826
Perrone P, Moriello C, Alessio N, Manna C, D’Angelo S. Cytoprotective Potential of Annurca Apple Polyphenols on Mercury-Induced Oxidative Stress in Human Erythrocytes. International Journal of Molecular Sciences. 2025; 26(18):8826. https://doi.org/10.3390/ijms26188826
Chicago/Turabian StylePerrone, Pasquale, Claudia Moriello, Nicola Alessio, Caterina Manna, and Stefania D’Angelo. 2025. "Cytoprotective Potential of Annurca Apple Polyphenols on Mercury-Induced Oxidative Stress in Human Erythrocytes" International Journal of Molecular Sciences 26, no. 18: 8826. https://doi.org/10.3390/ijms26188826
APA StylePerrone, P., Moriello, C., Alessio, N., Manna, C., & D’Angelo, S. (2025). Cytoprotective Potential of Annurca Apple Polyphenols on Mercury-Induced Oxidative Stress in Human Erythrocytes. International Journal of Molecular Sciences, 26(18), 8826. https://doi.org/10.3390/ijms26188826








