Amelioration of Post-traumatic Osteoarthritis by Iontophoretic Liposomal Strontium Ranelate Collaborated with Low-Intensity Pulsed Ultrasound in Rats
Abstract
1. Introduction
2. Results
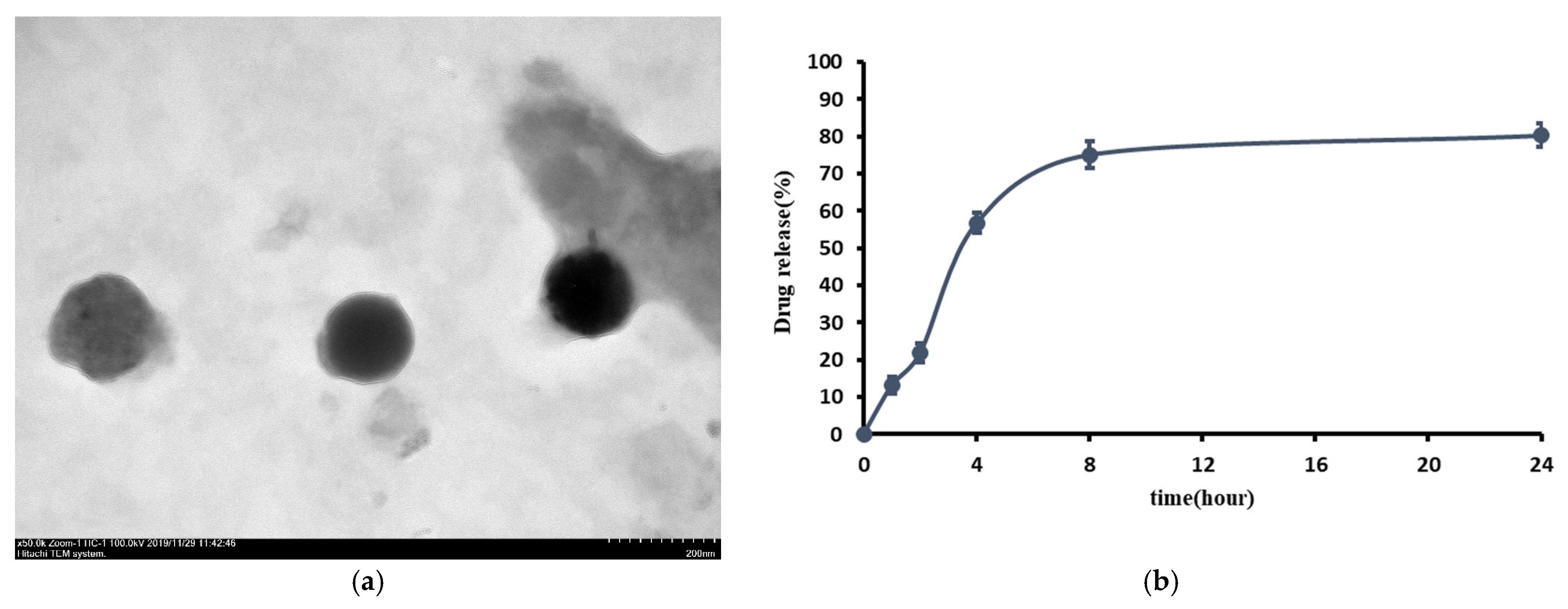
2.1. Observations of L-SR by Transmission Electron Microscopy (TEM)
2.2. Encapsulation Efficiency and In Vitro Drug Release
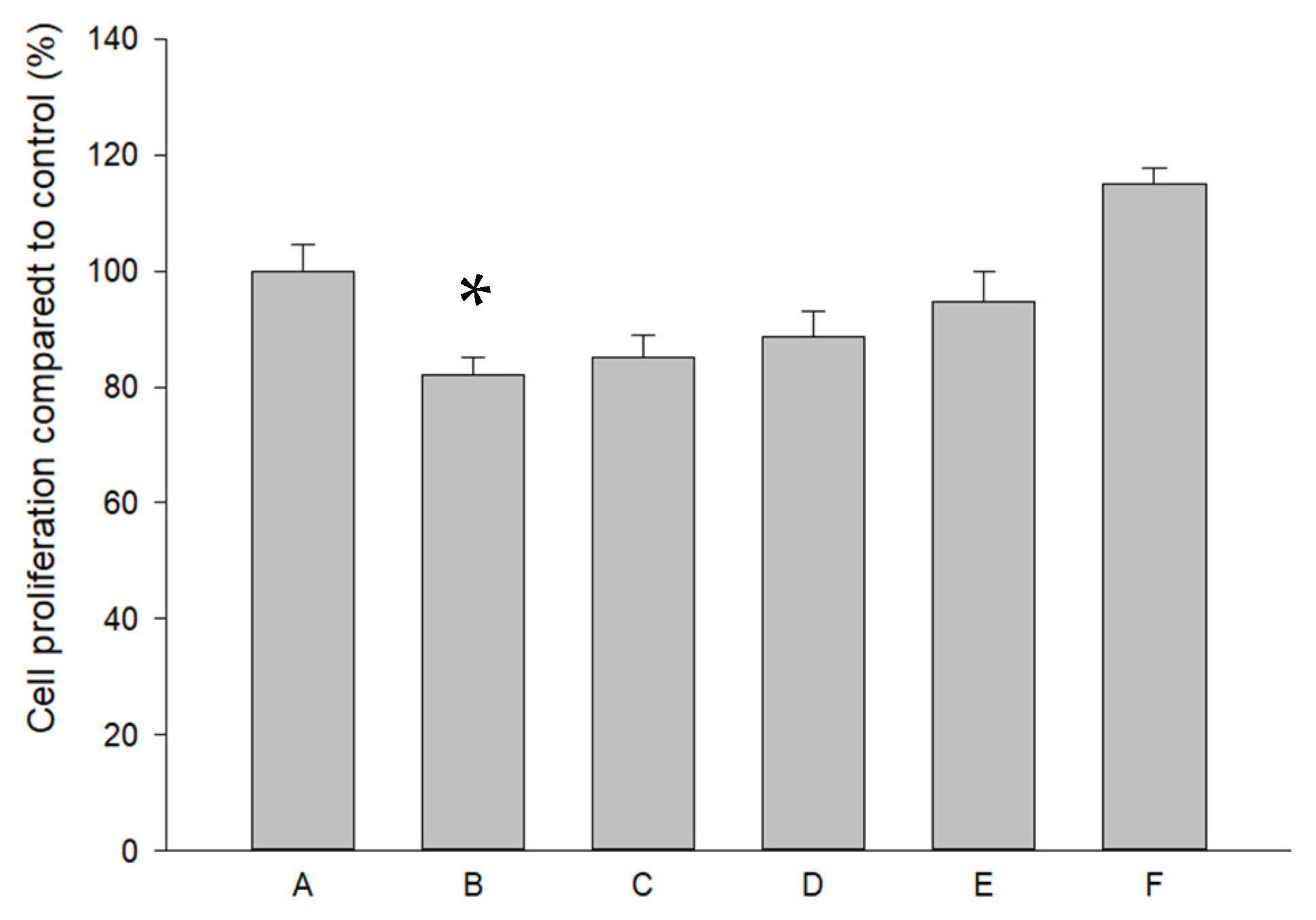
2.3. Effects of Pure SR and L-SR in the Presence or Absence of LIPUS on HOAC Proliferation
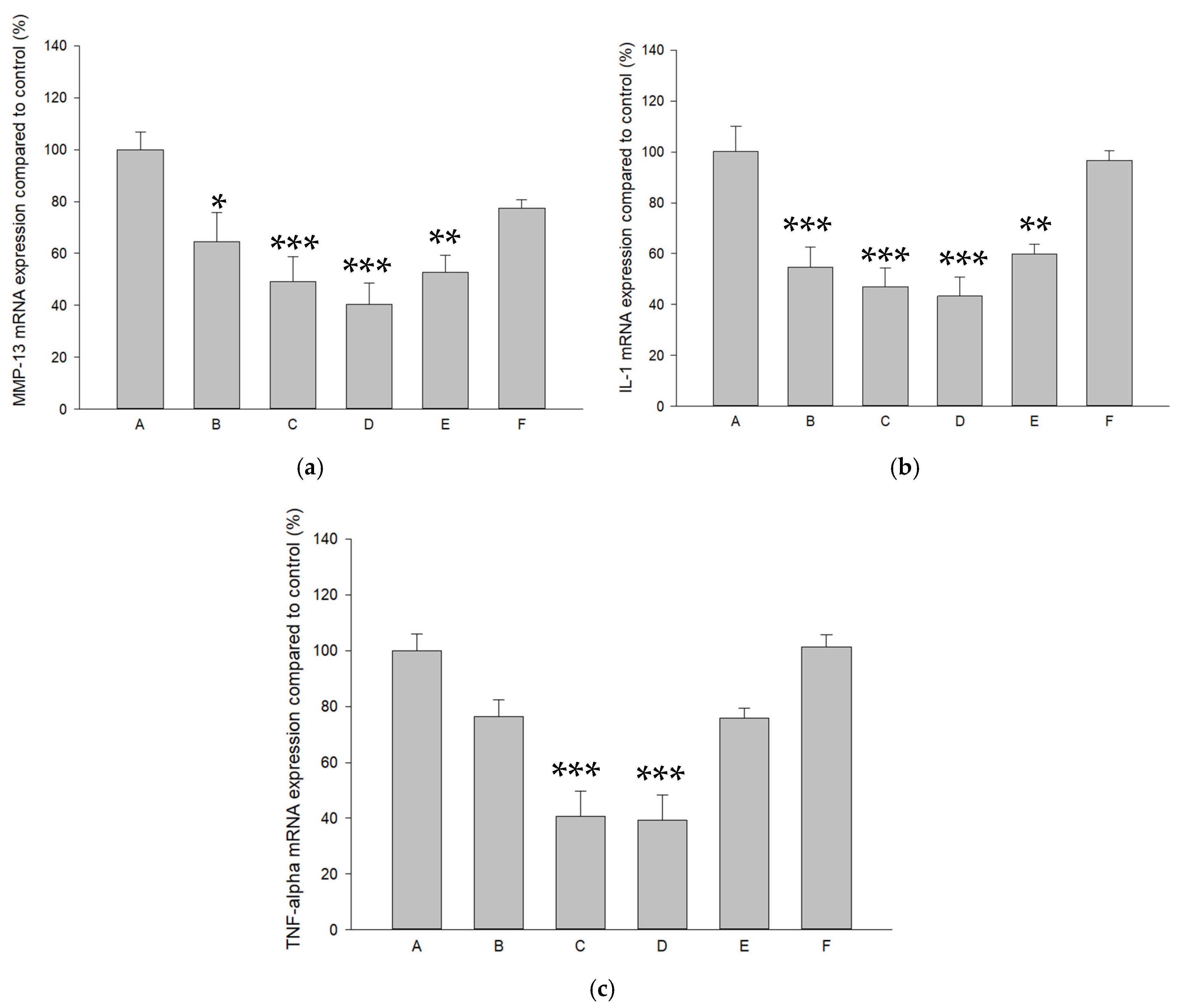
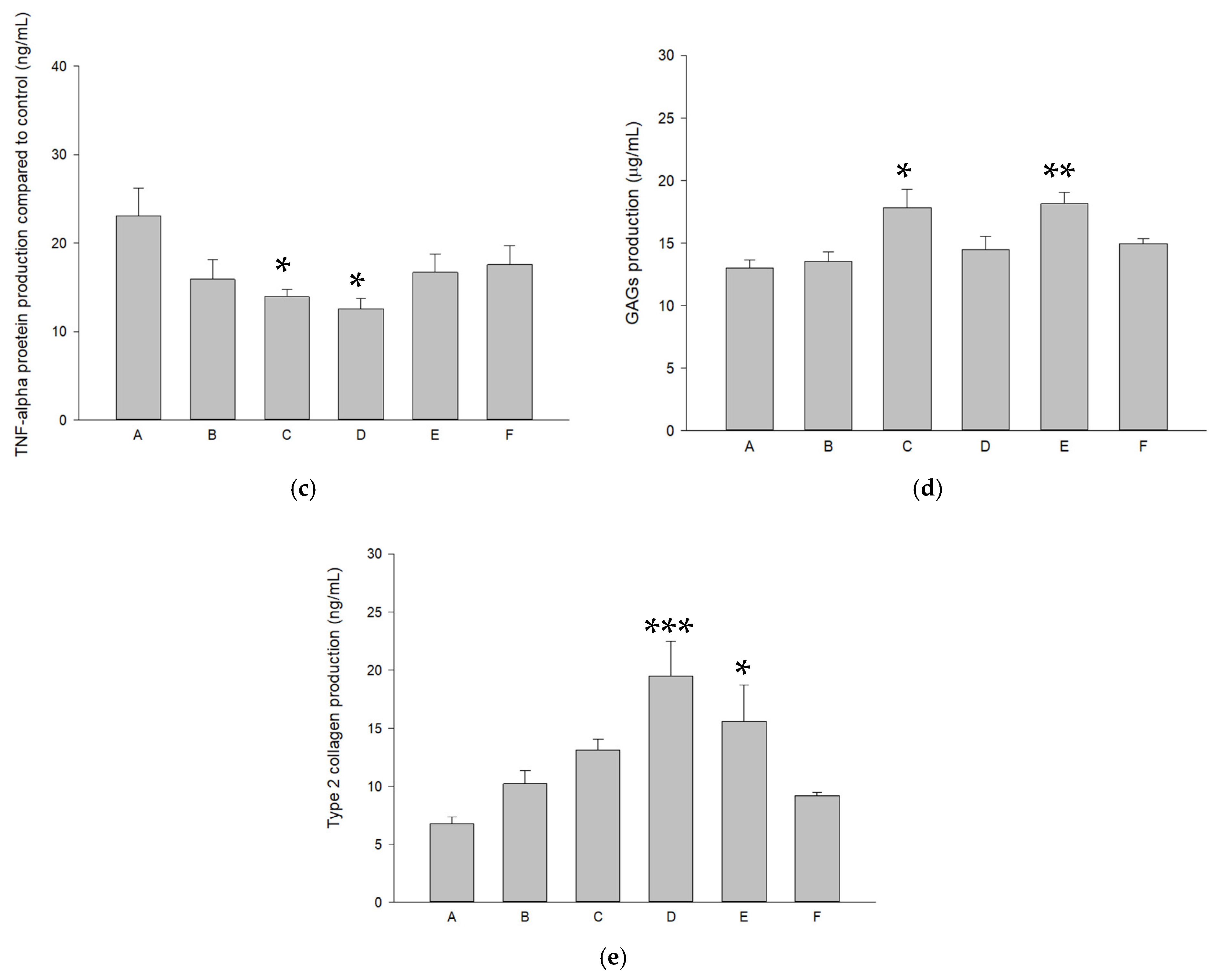
2.4. Modulations of Pure SR and L-SR with or Without LIPUS on MMP-13, IL-1β, and TNF-α mRNA and Proteins in HOACs
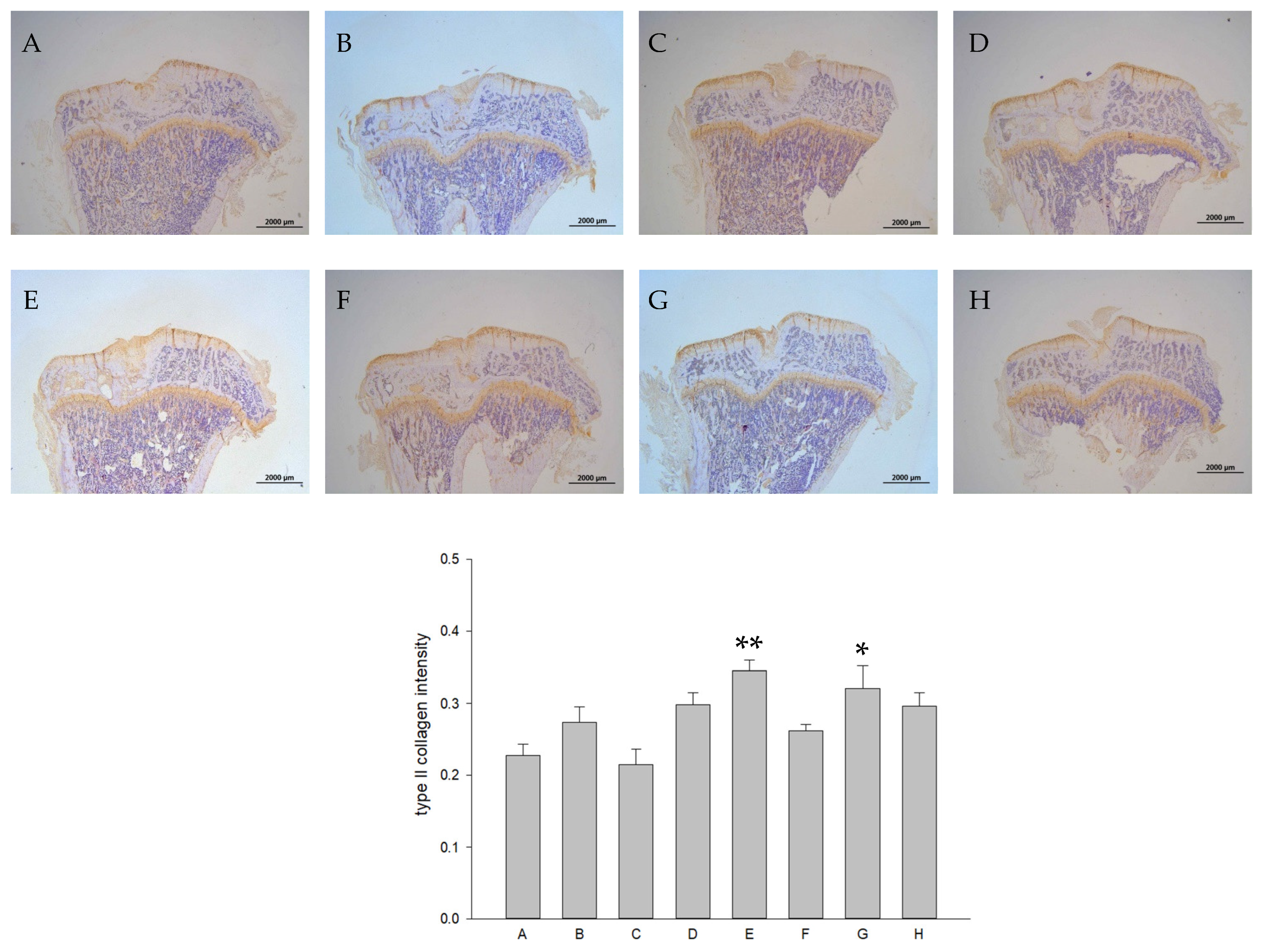
2.5. L-SR in Combination with LIPUS Increased GAGs and Type II Collagen in HOACs
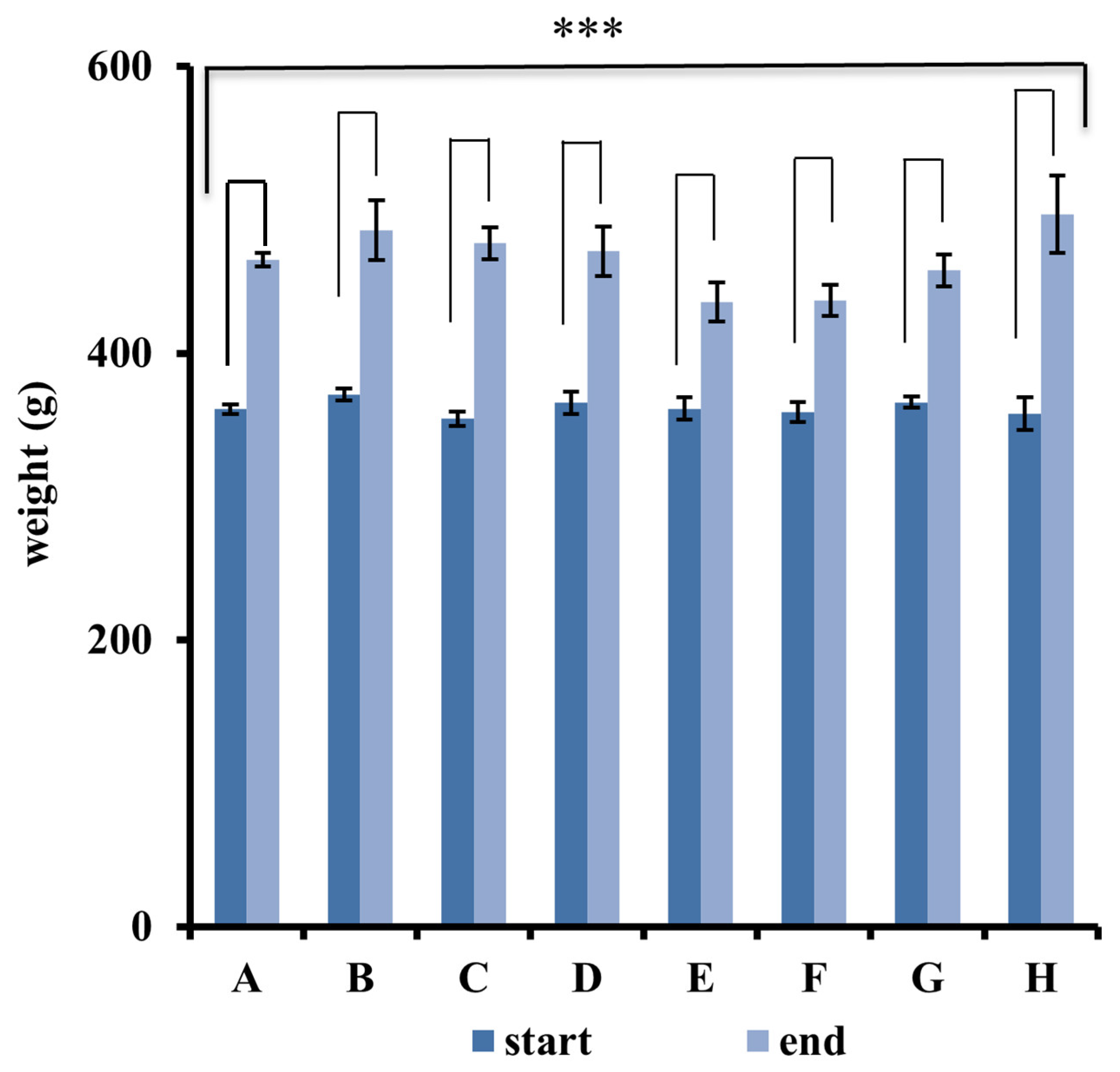
2.6. Measurement of Animal Weight
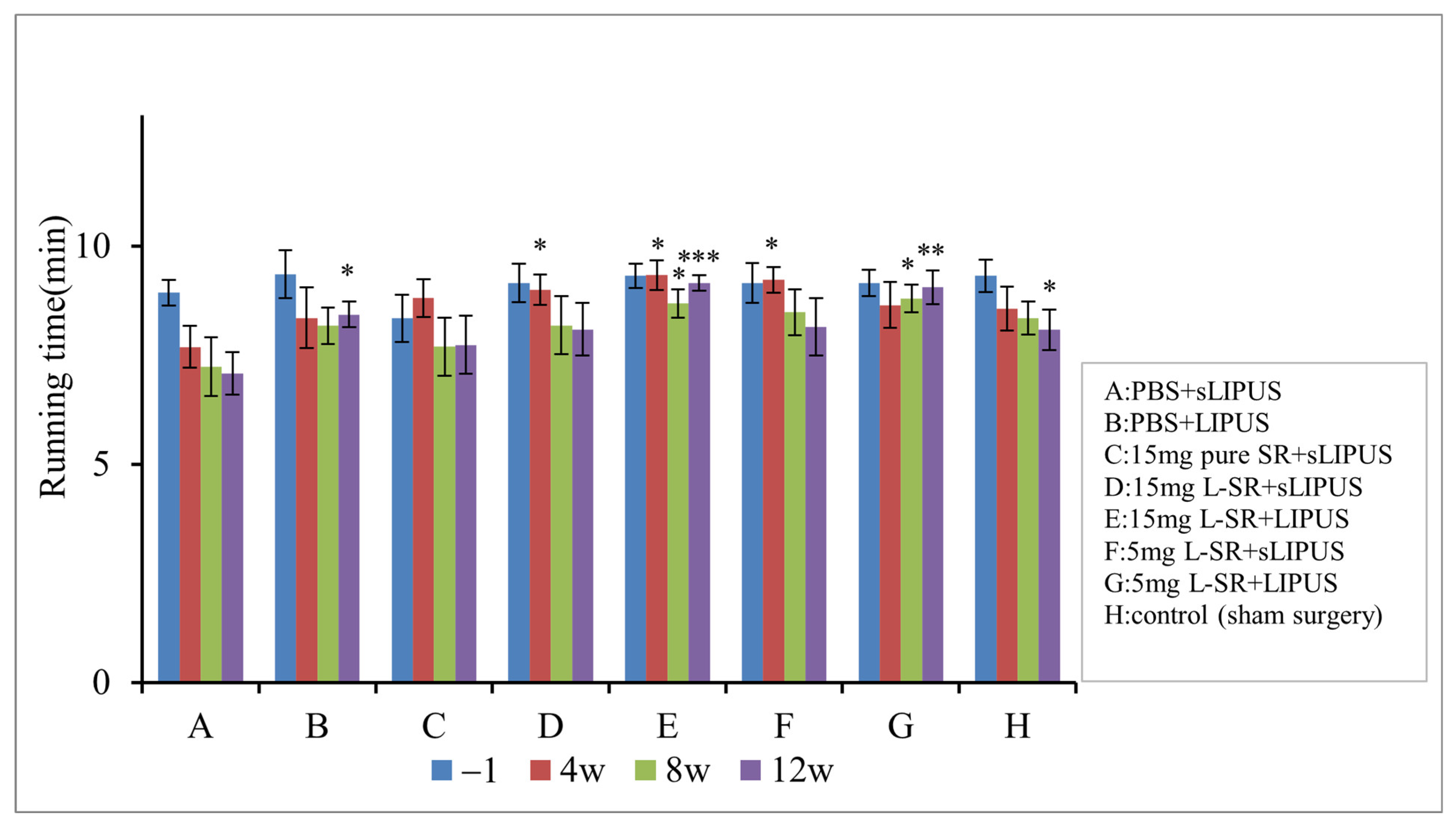
2.7. Iontophoretic L-SR Collaborated with LIPUS Improved Weight Bearing and Exercise Endurance in PTOA Rats
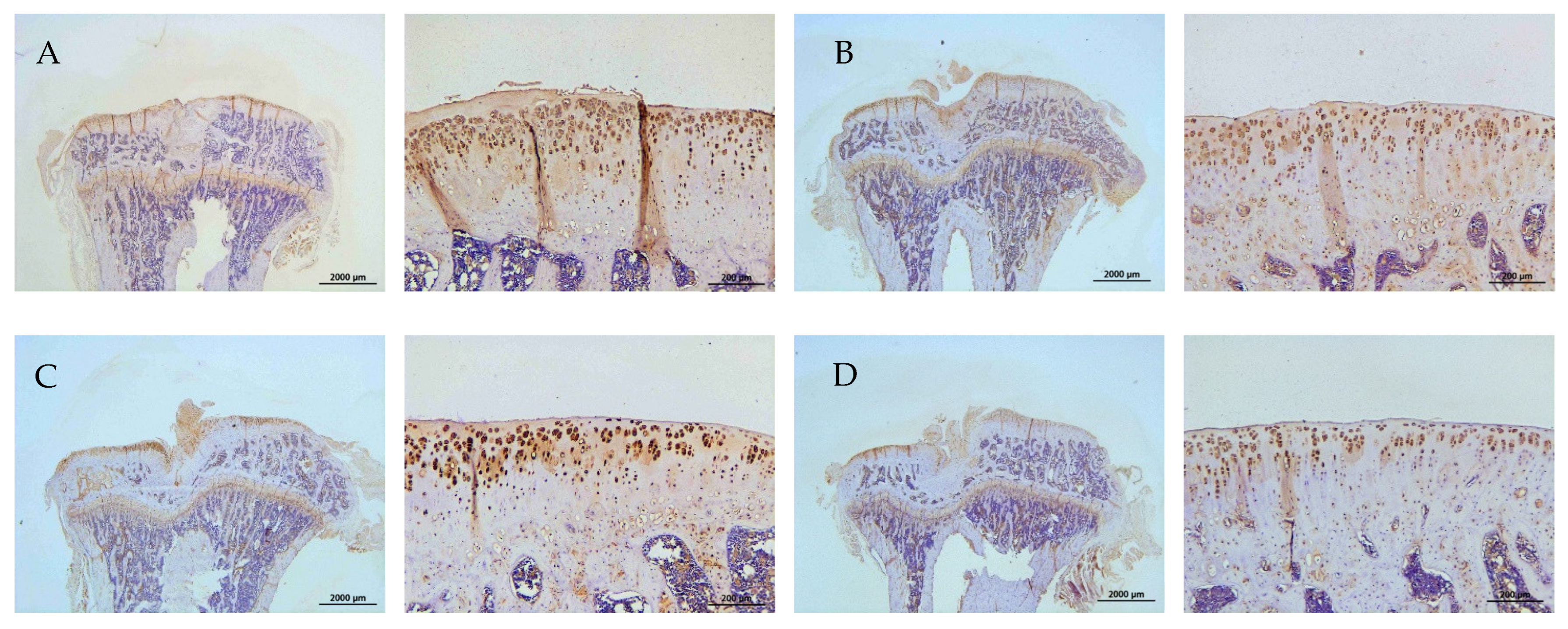
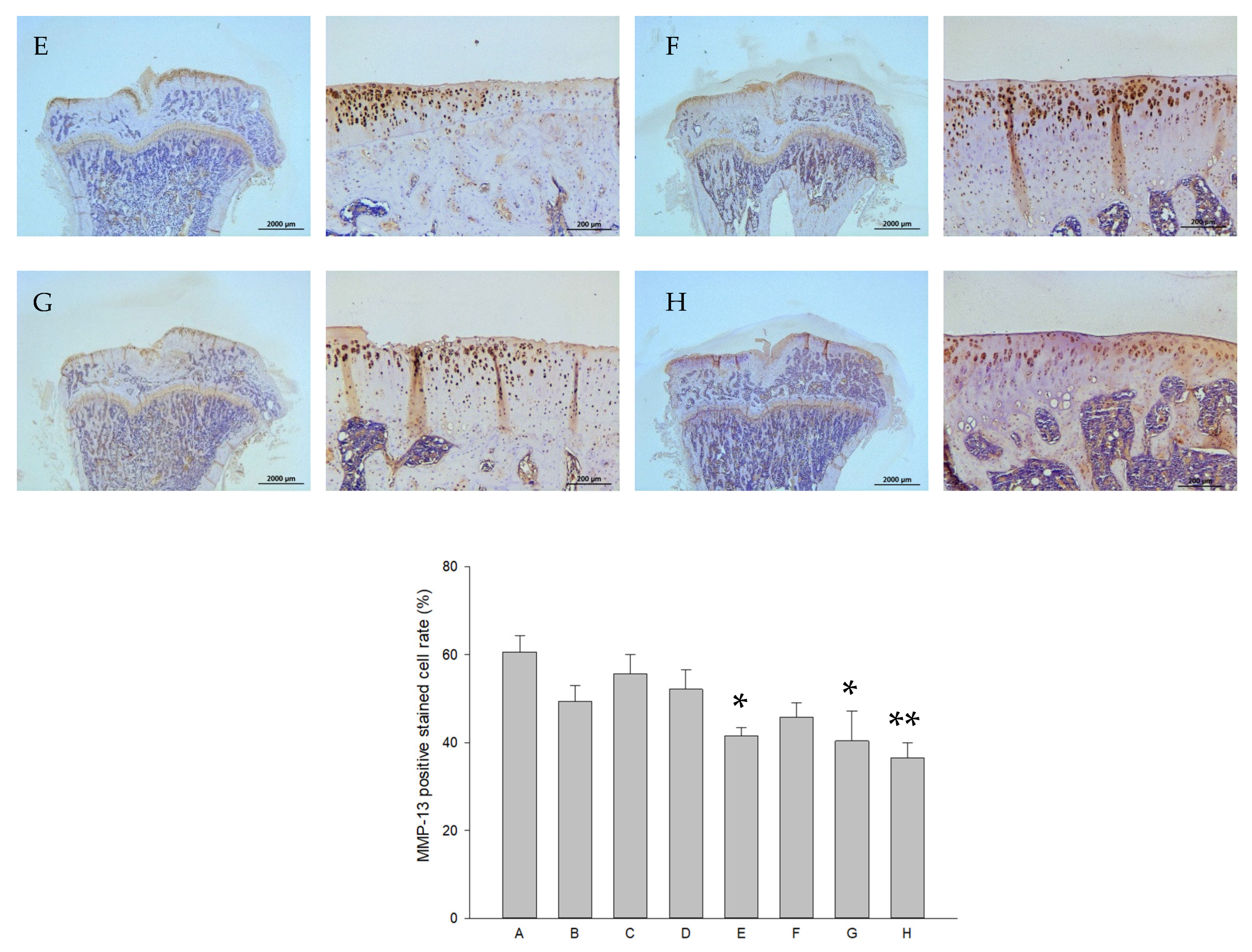
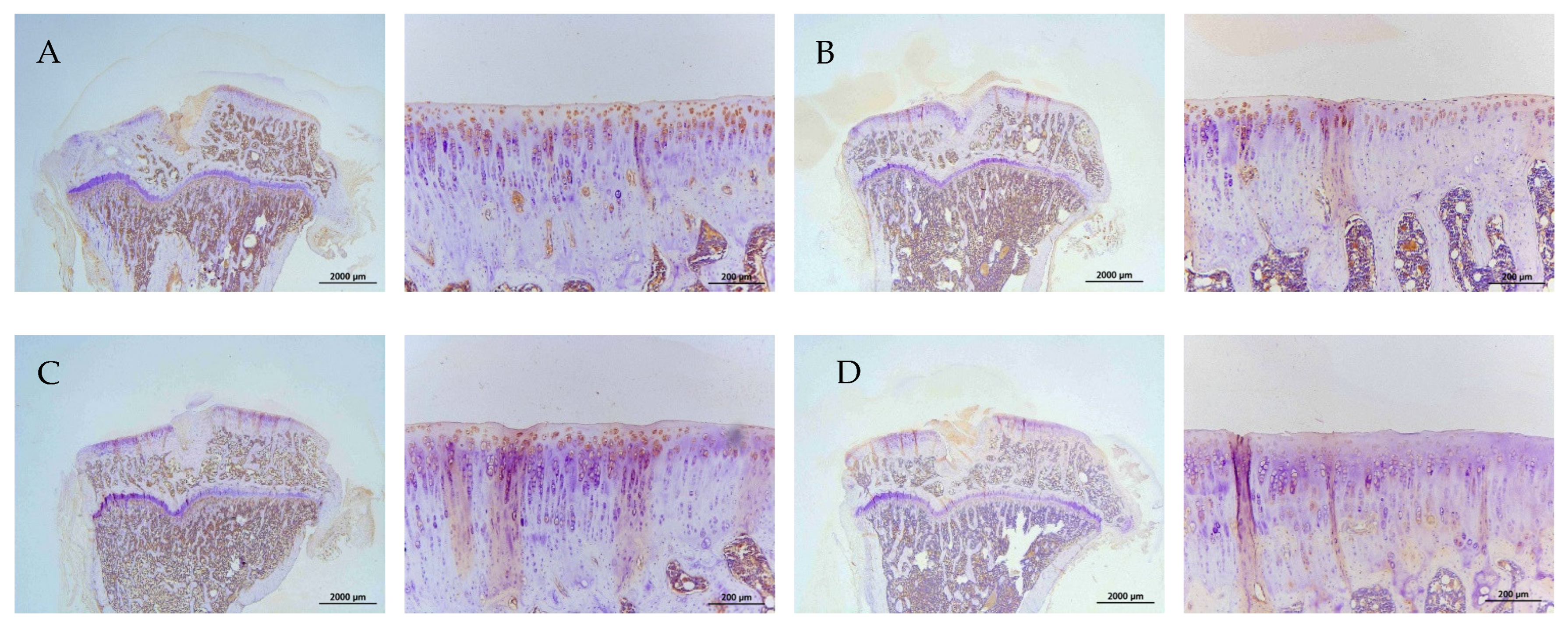
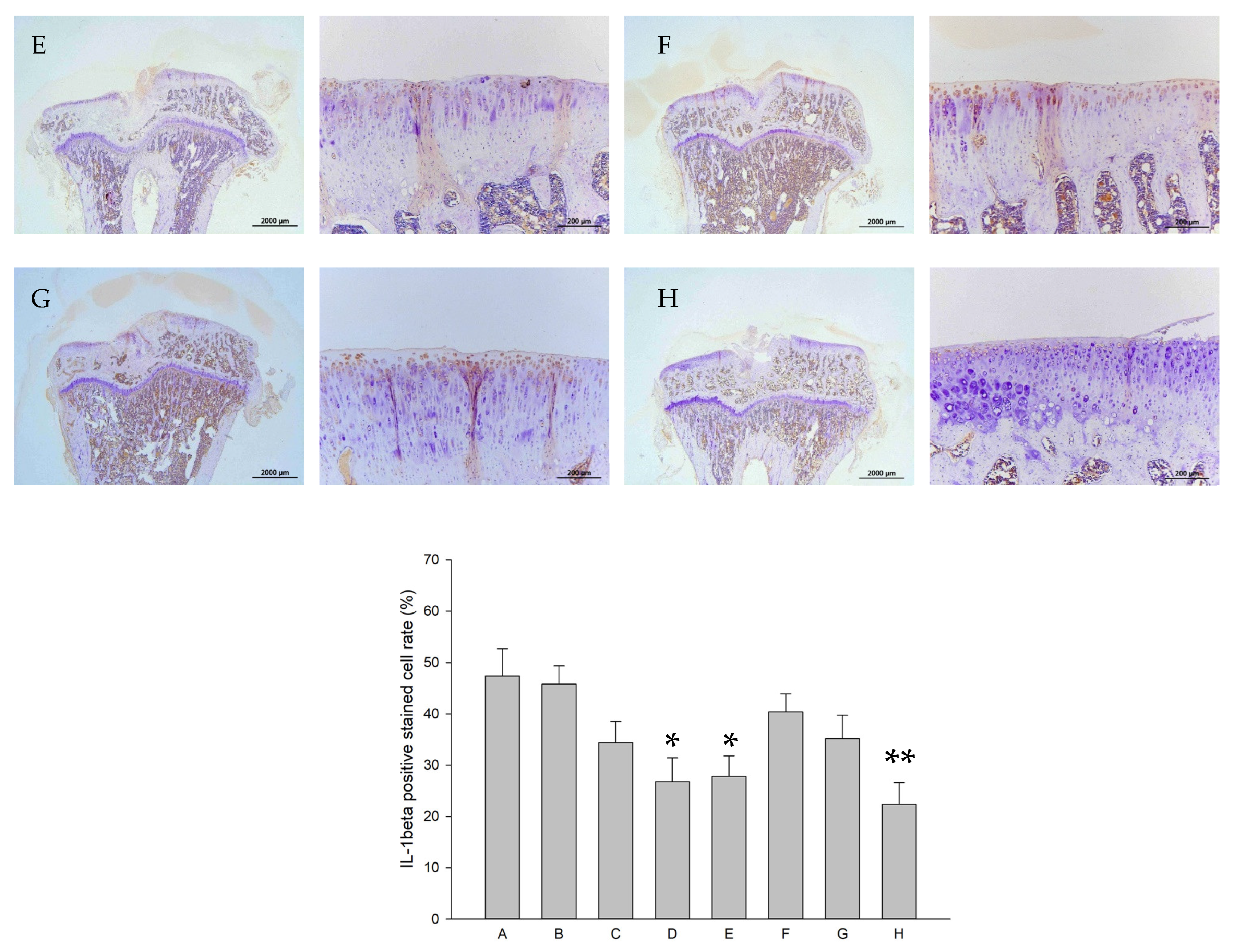
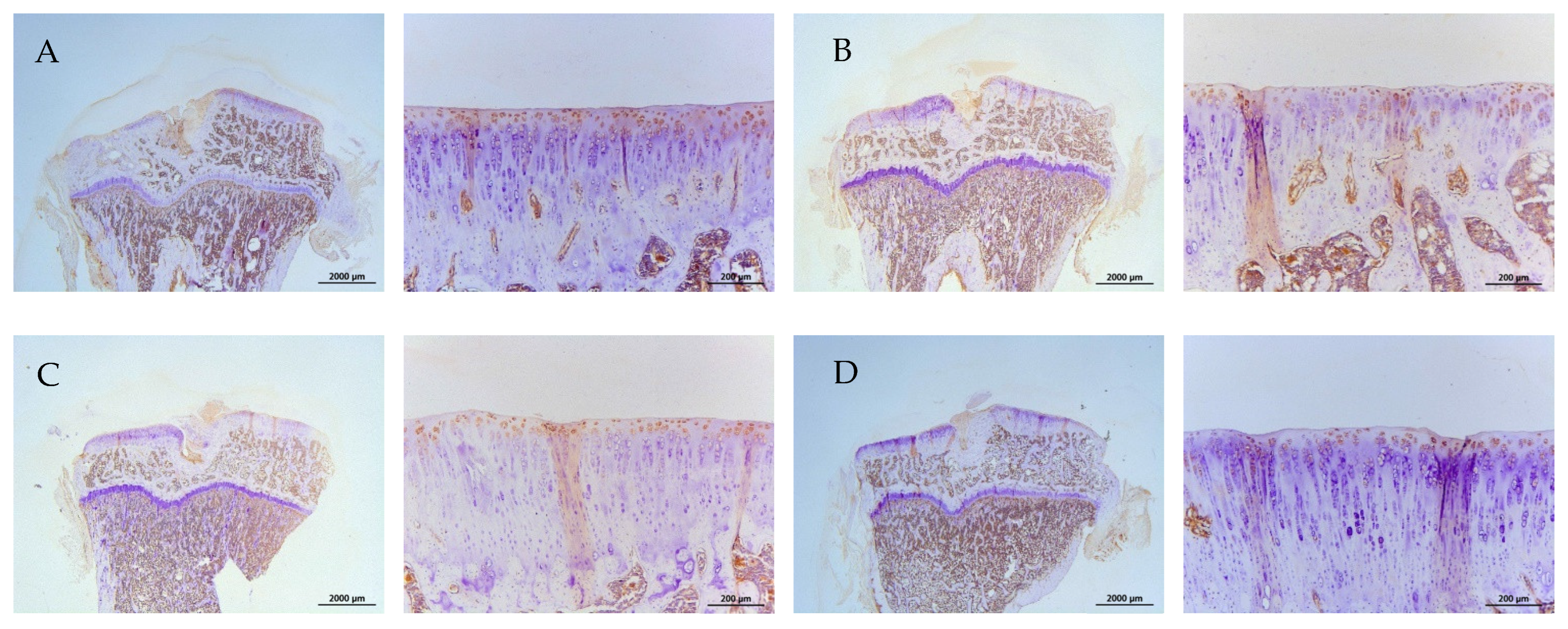
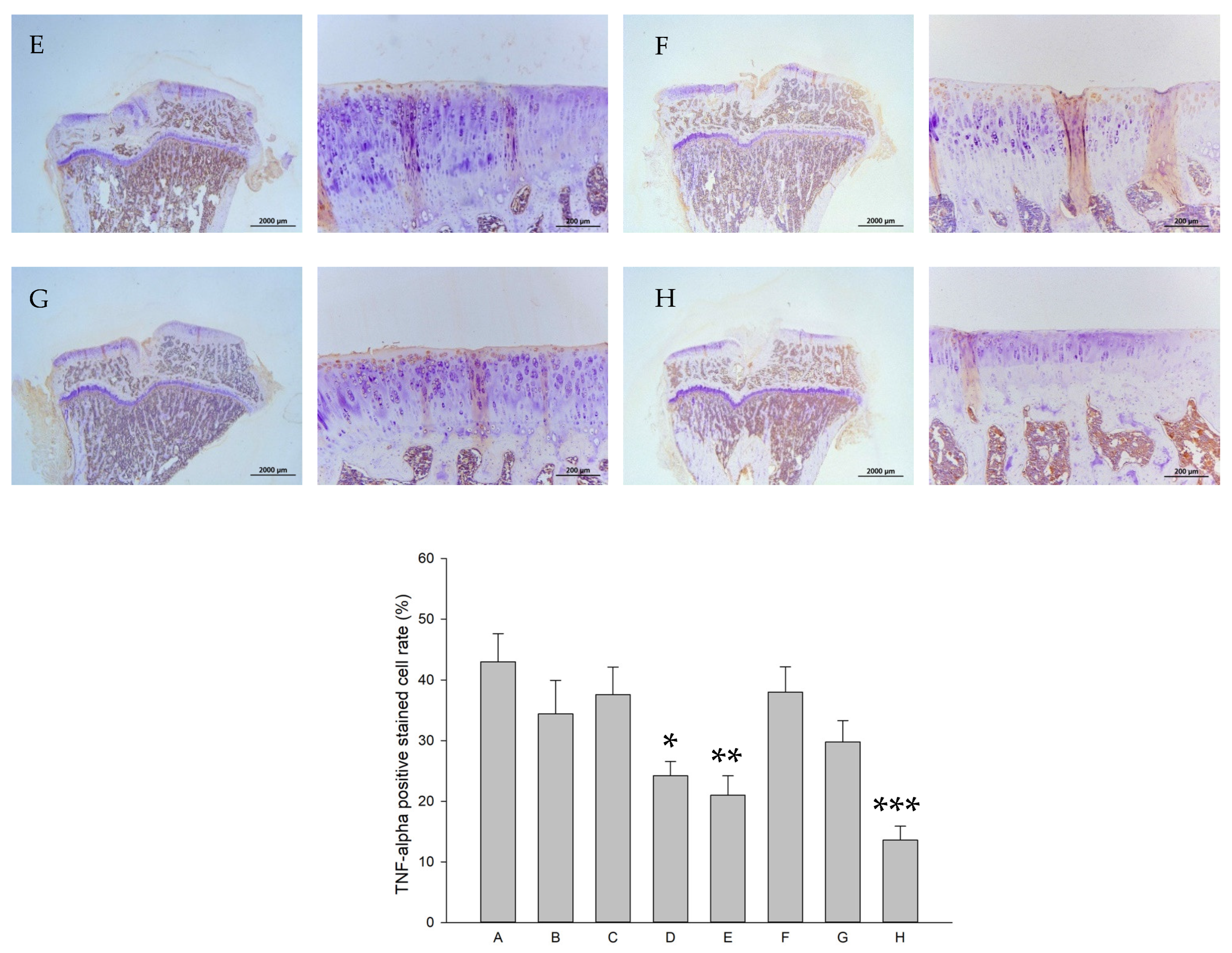
2.8. Histology and Immunohistochemistry (IHC)
2.9. Effects on RBC, WBC, Platelets, and Biochemistry
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Production of DSPC L-SR
4.3. Transmission Electron Microscopy (TEM)
4.4. Encapsulation Efficiency and In Vitro Drug Release
4.5. Cell Culture Using Alginate Beads and LIPUS Treatment In Vitro
4.6. Assessment of Effects of L-SR and LIPUS on Human OA Chondrocyte Proliferation
4.7. Quantitation of mRNA for MMP-13, IL-1β, and TNF-α
4.8. Assessment of GAGs and Type II Collagen Produced by HOACs
4.9. Determination of MMP-13, IL-1β, and TNF-α Proteins Produced by HOACs
4.10. Experimental Animals and Treatments
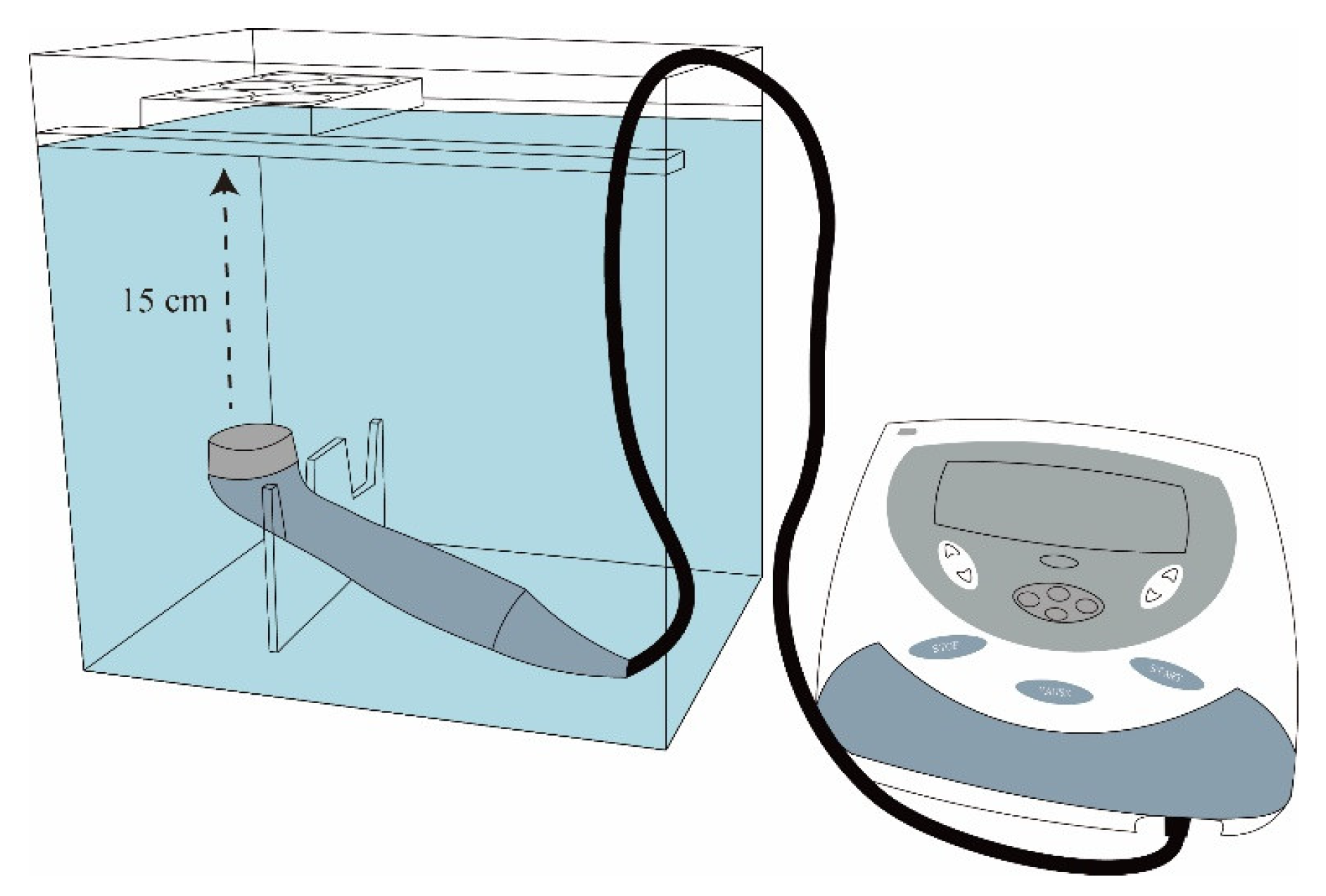
4.11. Iontophoresis
4.12. Assessment of Weight Bearing and Running Endurance
4.13. Histology and Immunohistochemistry In Vivo
4.14. Examination of WBC, RBC, Platelets, and Serum Biochemistry
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Yan, Z.P.; Chen, X.Y.; Ni, G.X. Infrapatellar Fat Pad and Knee Osteoarthritis. Aging Dis. 2020, 11, 1317–1328. [Google Scholar] [CrossRef]
- Thomas, A.C.; Hubbard-Turner, T.; Wikstrom, E.A.; Palmieri-Smith, R.M. Epidemiology of Posttraumatic Osteoarthritis. J. Athl. Train. 2017, 52, 491–496. [Google Scholar] [CrossRef]
- Carbone, A.; Rodeo, S. Review of current understanding of post-traumatic osteoarthritis resulting from sports injuries. J. Orthop. Res. 2017, 35, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Friel, N.A.; Chu, C.R. The role of ACL injury in the development of posttraumatic knee osteoarthritis. Clin. Sports Med. 2013, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef]
- Belluzzi, E.; Macchi, V.; Fontanella, C.G.; Carniel, E.L.; Olivotto, E.; Filardo, G.; Sarasin, G.; Porzionato, A.; Granzotto, M.; Pozzuoli, A.; et al. Infrapatellar Fat Pad Gene Expression and Protein Production in Patients with and without Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 6016. [Google Scholar] [CrossRef]
- Olivotto, E.; Belluzzi, E.; Pozzuoli, A.; Cigolotti, A.; Scioni, M.; Goldring, S.R.; Goldring, M.B.; Ruggieri, P.; Ramonda, R.; Grigolo, B.; et al. Do Synovial Inflammation and Meniscal Degeneration Impact Clinical Outcomes of Patients Undergoing Arthroscopic Partial Meniscectomy? A Histological Study. Int. J. Mol. Sci. 2022, 23, 3903. [Google Scholar] [CrossRef]
- Wang, L.J.; Zeng, N.; Yan, Z.P.; Li, J.T.; Ni, G.X. Post-traumatic osteoarthritis following ACL injury. Arthritis Res. Ther. 2020, 22, 57. [Google Scholar] [CrossRef]
- Mobasheri, A. The future of osteoarthritis therapeutics: Targeted pharmacological therapy. Curr. Rheumatol. Rep. 2013, 15, 364. [Google Scholar] [CrossRef]
- Pulsatelli, L.; Addimanda, O.; Brusi, V.; Pavloska, B.; Meliconi, R. New findings in osteoarthritis pathogenesis: Therapeutic implications. Ther. Adv. Chronic Dis. 2013, 4, 23–43. [Google Scholar] [CrossRef]
- Teichtahl, A.J.; Wluka, A.E.; Wijethilake, P.; Wang, Y.; Ghasem-Zadeh, A.; Cicuttini, F.M. Wolff’s law in action: A mechanism for early knee osteoarthritis. Arthritis Res. Ther. 2015, 17, 207. [Google Scholar] [CrossRef]
- Yu, D.G.; Ding, H.F.; Mao, Y.Q.; Liu, M.; Yu, B.; Zhao, X.; Wang, X.Q.; Li, Y.; Liu, G.W.; Nie, S.B.; et al. Strontium ranelate reduces cartilage degeneration and subchondral bone remodeling in rat osteoarthritis model. Acta Pharmacol. Sin. 2013, 34, 393–402. [Google Scholar] [CrossRef]
- Gallacher, S.J.; Dixon, T. Impact of treatments for postmenopausal osteoporosis (bisphosphonates, parathyroid hormone, strontium ranelate, and denosumab) on bone quality: A systematic review. Calcif. Tissue Int. 2010, 87, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Goker, F.; Ersanli, S.; Arisan, V.; Cevher, E.; Guzel, E.E.; Issever, H.; Omer, B.; Durmus Altun, G.; Morina, D.; Ekiz Yilmaz, T.; et al. Combined effect of parathyroid hormone and strontium ranelate on bone healing in ovariectomized rats. Oral Dis. 2018, 24, 1255–1269, Correction in Int. J. Nanomed. 2021, 16, 329–330. . [Google Scholar] [CrossRef] [PubMed]
- McGreevy, C.; Williams, D. Safety of drugs used in the treatment of osteoporosis. Ther. Adv. Drug Saf. 2011, 2, 159–172. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium ranelate: New insights into its dual mode of action. Bone 2007, 40 (Suppl. S1), S5–S8. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Yang, X.; He, J.; Zhang, F.; Zhong, Q.; Guo, X. Strontium ranelate promotes chondrogenesis through inhibition of the Wnt/beta-catenin pathway. Stem Cell Res. Ther. 2021, 12, 296. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Yang, X.; He, J.; Zhong, Q.; Guo, X. The anti-inflammation effect of strontium ranelate on rat chondrocytes with or without IL-1beta in vitro. Exp. Ther. Med. 2022, 23, 208. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shen, B.; Loor, J.J.; Jiang, Q.; Yuan, Y.; Kong, Y.; Tan, P.; Zeng, F.; Zhao, C.; Zhu, X.; et al. Strontium Regulates the Proliferation and Differentiation of Isolated Primary Bovine Chondrocytes via the TGFbeta/SMAD Pathway. Front. Pharmacol. 2022, 13, 925302. [Google Scholar] [CrossRef]
- Hu, P.; Du, J.; Zhang, S.; Wang, T.; Li, J.; Chen, G.; Zhou, G. Oral Administration of Strontium Gluconate Effectively Reduces Articular Cartilage Degeneration Through Enhanced Anabolic Activity of Chondrocytes and Chondrogenetic Differentiation of Mesenchymal Stromal Cells. Biol. Trace Elem. Res. 2020, 193, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.; Kapoor, M.; Fahmi, H.; Lajeunesse, D.; Blesius, A.; Maillet, J.; Martel-Pelletier, J. Strontium ranelate reduces the progression of experimental dog osteoarthritis by inhibiting the expression of key proteases in cartilage and of IL-1beta in the synovium. Ann. Rheum. Dis. 2013, 72, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, A.; Wang, X.; Zhang, L.; Yang, M.; Ma, J.; Deng, M.; Liu, H. Pharmacokinetics and bioequivalence of two strontium ranelate formulations after single oral administration in healthy Chinese subjects. Xenobiotica 2019, 49, 457–462. [Google Scholar] [CrossRef]
- Bruyere, O.; Reginster, J.Y.; Bellamy, N.; Chapurlat, R.; Richette, P.; Cooper, C.; Investigators, S. Clinically meaningful effect of strontium ranelate on symptoms in knee osteoarthritis: A responder analysis. Rheumatology 2014, 53, 1457–1464. [Google Scholar] [CrossRef]
- Roubille, C.; Martel-Pelletier, J.; Raynauld, J.P.; Abram, F.; Dorais, M.; Delorme, P.; Pelletier, J.P. Meniscal extrusion promotes knee osteoarthritis structural progression: Protective effect of strontium ranelate treatment in a phase III clinical trial. Arthritis Res. Ther. 2015, 17, 82. [Google Scholar] [CrossRef]
- Shashi, K.; Satinder, K.; Bharat, P. A complete review on: Liposomes. Int. Res. J. Pharm. 2012, 3, 10–16. [Google Scholar]
- Romberg, B.; Oussoren, C.; Snel, C.J.; Hennink, W.E.; Storm, G. Effect of liposome characteristics and dose on the pharmacokinetics of liposomes coated with poly(amino acid)s. Pharm. Res. 2007, 24, 2394–2401. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- Chen, C.H.; Kuo, S.M.; Tien, Y.C.; Shen, P.C.; Kuo, Y.W.; Huang, H.H. Steady Augmentation of Anti-Osteoarthritic Actions of Rapamycin by Liposome-Encapsulation in Collaboration with Low-Intensity Pulsed Ultrasound. Int. J. Nanomed. 2020, 15, 3771–3790, Correction in Int. J. Nanomed. 2021, 16, 329–330. [Google Scholar] [CrossRef]
- Xia, P.; Shen, S.; Lin, Q.; Cheng, K.; Ren, S.; Gao, M.; Li, X. Low-Intensity Pulsed Ultrasound Treatment at an Early Osteoarthritis Stage Protects Rabbit Cartilage From Damage via the Integrin/Focal Adhesion Kinase/Mitogen-Activated Protein Kinase Signaling Pathway. J. Ultrasound Med. 2015, 34, 1991–1999. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Cheng, K.; Lin, Q.; Wang, D.; Zhang, H.; An, H.; Gao, M.; Chen, A. Effect of low-intensity pulsed ultrasound on MMP-13 and MAPKs signaling pathway in rabbit knee osteoarthritis. Cell Biochem. Biophys. 2011, 61, 427–434. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Teong, B.; Kuo, S.M.; Tsai, W.H.; Ho, M.L.; Chen, C.H.; Huang, H.H. Liposomal Encapsulation for Systemic Delivery of Propranolol via Transdermal Iontophoresis Improves Bone Microarchitecture in Ovariectomized Rats. Int. J. Mol. Sci. 2017, 18, 822. [Google Scholar] [CrossRef]
- Pierre, M.B.; Dos Santos Miranda Costa, I. Liposomal systems as drug delivery vehicles for dermal and transdermal applications. Arch. Dermatol. Res. 2011, 303, 607–621. [Google Scholar] [CrossRef]
- Roman-Blas, J.A.; Bizzi, E.; Largo, R.; Migliore, A.; Herrero-Beaumont, G. An update on the up and coming therapies to treat osteoarthritis, a multifaceted disease. Expert Opin. Pharmacother. 2016, 17, 1745–1756. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Dickenson, A.H.; Baron, R. Osteoarthritis pain: Nociceptive or neuropathic? Nat. Rev. Rheumatol. 2014, 10, 374–380. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Delwatta, S.L.; Gunatilake, M.; Baumans, V.; Seneviratne, M.D.; Dissanayaka, M.L.B.; Batagoda, S.S.; Udagedara, A.H.; Walpola, P.B. Reference values for selected hematological, biochemical and physiological parameters of Sprague-Dawley rats at the Animal House, Faculty of Medicine, University of Colombo, Sri Lanka. Anim. Model. Exp. Med. 2018, 1, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Giknis, M.L.A.; Clifford, C.B. Clinical Laboratory Parameters for Crl:CD (SD) Rats; Charles River Laboratories: Wilmington, MA, USA, 2006; p. 18. [Google Scholar]
- Lennox, A.M.; Bauck, L. Basic anatomy, physiology, husbandry, and clinical techniques. In Ferrets, Rabbits and Rodents: Clinical Medicine and Surgery, 4th ed.; Quesenberry, K.E., Carpenter, J.W., Eds.; Elsevier/Saunders: St. Louis, MO, USA, 2022; pp. 339–352. [Google Scholar]
- Obeagu, E.I. Stress-induced hemostasis: Mechanisms and implications for health. Ann. Med. Surg. 2025, 87, 3300–3309. [Google Scholar] [CrossRef] [PubMed]
- Everds, N.E.; Ramaiah, L. The Labaratory Rat. In The Clinical Chemistry of Labaratory Animals, 3rd ed.; Kurtz, D.M., Travlos, G.S., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 33–78. [Google Scholar]
- Leifer, V.P.; Katz, J.N.; Losina, E. The burden of OA-health services and economics. Osteoarthr. Cartil. 2022, 30, 10–16. [Google Scholar] [CrossRef]
- Man, G.S.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 37–41. [Google Scholar]
- Sengprasert, P.; Kamenkit, O.; Tanavalee, A.; Reantragoon, R. The Immunological Facets Of Chondrocytes In Osteoarthritis: A Narrative Review. J. Rheumatol. 2023, 51, 13–24. [Google Scholar] [CrossRef]
- Guilak, F.; Fermor, B.; Keefe, F.J.; Kraus, V.B.; Olson, S.A.; Pisetsky, D.S.; Setton, L.A.; Weinberg, J.B. The role of biomechanics and inflammation in cartilage injury and repair. Clin. Orthop. Relat. Res. 2004, 423, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.L.; Wishnok, J.S.; Chai, D.H.; Grodzinsky, A.J.; Tannenbaum, S.R. A sodium dodecyl sulfate-polyacrylamide gel electrophoresis-liquid chromatography tandem mass spectrometry analysis of bovine cartilage tissue response to mechanical compression injury and the inflammatory cytokines tumor necrosis factor alpha and interleukin-1beta. Arthritis Rheum. 2008, 58, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Han, P.F.; Wei, L.; Duan, Z.Q.; Zhang, Z.L.; Chen, T.Y.; Lu, J.G.; Zhao, R.P.; Cao, X.M.; Li, P.C.; Lv, Z.; et al. Contribution of IL-1beta, 6 and TNF-alpha to the form of post-traumatic osteoarthritis induced by “idealized” anterior cruciate ligament reconstruction in a porcine model. Int. Immunopharmacol. 2018, 65, 212–220. [Google Scholar] [CrossRef]
- Chen, C.H.; Ho, M.L.; Chang, L.H.; Kang, L.; Lin, Y.S.; Lin, S.Y.; Wu, S.C.; Chang, J.K. Parathyroid hormone-(1-34) ameliorated knee osteoarthritis in rats via autophagy. J. Appl. Physiol. (1985) 2018, 124, 1177–1185. [Google Scholar] [CrossRef]
- Teong, B.; Kuo, S.M.; Chen, C.H.; Chen, Y.K.; Cheng, Z.J.; Huang, H.H. Characterization and human osteoblastic proliferation- and differentiation-stimulatory effects of phosphatidylcholine liposomes-encapsulated propranolol hydrochloride. Biomed. Mater. Eng. 2014, 24, 1875–1887. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Liu, L.; Shi, X.; Yin, L.; Zhang, Y.; Li, X.; Wang, Z.; Liu, G. Effects of strontium on collagen content and expression of related genes in rat chondrocytes cultured in vitro. Biol. Trace Elem. Res. 2013, 153, 212–219. [Google Scholar] [CrossRef] [PubMed]
- De Ceuninck, F.; Lesur, C.; Pastoureau, P.; Caliez, A.; Sabatini, M. Culture of chondrocytes in alginate beads. Methods Mol. Med. 2004, 100, 15–22. [Google Scholar] [CrossRef]
- Cakir, S.; Hepguler, S.; Ozturk, C.; Korkmaz, M.; Isleten, B.; Atamaz, F.C. Efficacy of therapeutic ultrasound for the management of knee osteoarthritis: A randomized, controlled, and double-blind study. Am. J. Phys. Med. Rehabil. 2014, 93, 405–412. [Google Scholar] [CrossRef]
- Tascioglu, F.; Kuzgun, S.; Armagan, O.; Ogutler, G. Short-term effectiveness of ultrasound therapy in knee osteoarthritis. J. Int. Med. Res. 2010, 38, 1233–1242. [Google Scholar] [CrossRef]
- Jia, L.; Wang, Y.; Chen, J.; Chen, W. Efficacy of focused low-intensity pulsed ultrasound therapy for the management of knee osteoarthritis: A randomized, double blind, placebo-controlled trial. Sci. Rep. 2016, 6, 35453. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Kwak, H.B.; Seo, K.W.; McGregor, R.A.; Yeo, J.Y.; Ko, T.H.; Bolorerdene, S.; Kim, N.; Ko, K.S.; et al. Voluntary stand-up physical activity enhances endurance exercise capacity in rats. Korean J. Physiol. Pharmacol. 2016, 20, 287–295. [Google Scholar] [CrossRef]
- Kovacs, B.; Kantor, L.K.; Croitoru, M.D.; Kelemen, E.K.; Obreja, M.; Nagy, E.E.; Szekely-Szentmiklosi, B.; Gyeresi, A. Reversed phase HPLC for strontium ranelate: Method development and validation applying experimental design. Acta Pharm. 2018, 68, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Kuo, S.M.; Wu, Y.J.; Su, J.H. Improvement and enhancement of antibladder carcinoma cell effects of heteronemin by the nanosized hyaluronan aggregation. Int. J. Nanomed. 2016, 11, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Alshenibr, W.; Tashkandi, M.M.; Alsaqer, S.F.; Alkheriji, Y.; Wise, A.; Fulzele, S.; Mehra, P.; Goldring, M.B.; Gerstenfeld, L.C.; Bais, M.V. Anabolic role of lysyl oxidase like-2 in cartilage of knee and temporomandibular joints with osteoarthritis. Arthritis Res. Ther. 2017, 19, 179. [Google Scholar] [CrossRef]
- Otero, M.; Favero, M.; Dragomir, C.; Hachem, K.E.; Hashimoto, K.; Plumb, D.A.; Goldring, M.B. Human chondrocyte cultures as models of cartilage-specific gene regulation. Methods Mol. Biol. 2012, 806, 301–336. [Google Scholar] [CrossRef] [PubMed]
- Chubinskaya, S.; Huch, K.; Schulze, M.; Otten, L.; Aydelotte, M.B.; Cole, A.A. Gene expression by human articular chondrocytes cultured in alginate beads. J. Histochem. Cytochem. 2001, 49, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Tien, Y.C.; Lin, S.D.; Chen, C.H.; Lu, C.C.; Su, S.J.; Chih, T.T. Effects of pulsed low-intensity ultrasound on human child chondrocytes. Ultrasound Med. Biol. 2008, 34, 1174–1181. [Google Scholar] [CrossRef]
- Shafiaa, S.; Shaha, A.Z.; Sofib, A.F. TNF-alpha, IL-1beta and IL-6 Cytokine Gene Expression in Synovial Fluid of Rheumatoid Arthritis and Osteoarthritis Patients and Their Relationship with Gene Polymorphisms. Rheumatology 2016, 6, 1. [Google Scholar]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.J.; Wei, Y.; Zhou, B.; Bernhard, J.; Robinson, S.; Burapachaisri, A.; Guo, X.E.; Vunjak-Novakovic, G. Recapitulation of physiological spatiotemporal signals promotes in vitro formation of phenotypically stable human articular cartilage. Proc. Natl. Acad. Sci. USA 2017, 114, 2556–2561. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lin, Y.S.; Fu, Y.C.; Wang, C.K.; Wu, S.C.; Wang, G.J.; Eswaramoorthy, R.; Wang, Y.H.; Wang, C.Z.; Wang, Y.H.; et al. Electromagnetic fields enhance chondrogenesis of human adipose-derived stem cells in a chondrogenic microenvironment in vitro. J. Appl. Physiol. (1985) 2013, 114, 647–655. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Laurin, J.; Pin-Barre, C.; Bernard, G.; Dousset, E.; Decherchi, P. Functional and Neuromuscular Changes after Anterior Cruciate Ligament Rupture in Rats. Med. Sci. Sports Exerc. 2016, 48, 1033–1043. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Chen, H.Y.; Yang, C.C. Early Intervention with Therapeutic Low-Intensity Pulsed Ultrasound in Halting the Progression of Post-traumatic Osteoarthritis in a Rat Model. Ultrasound Med. Biol. 2018, 44, 2637–2645. [Google Scholar] [CrossRef]
- Tashiro, Y.; Sami, M.; Shichibe, S.; Kato, Y.; Hayakawa, E.; Itoh, K. Effect of lipophilicity on in vivo iontophoretic delivery. II. Beta-blockers. Biol. Pharm. Bull. 2001, 24, 671–677. [Google Scholar] [CrossRef]



| RBC (106/μL) | WBC (103/μL) | Platelet (103/μL) | |
|---|---|---|---|
| control (sham surgery) | 8.14 ± 0.27 | 6743.71 ± 729.34 | 809.00 ± 90.40 |
| PBS + sLIPUS | 8.28 ± 0.12 | 7818.50 ± 747.73 | 911.17 ± 10.41 |
| PBS + LIPUS | 8.15 ± 1.50 | 7834.20 ± 1578.17 | 969.80 ± 184.21 |
| 15mg pure SR + sLIPUS | 8.13 ± 0.12 | 6190.00 ± 281.08 | 862.71 ± 149.00 |
| 15mg L-SR + sLIPUS | 8.18 ± 0.15 | 6953.33 ± 262.13 | 1061.00 ± 63.76 |
| 15mg L-SR + LIPUS | 8.76 ± 0.14 | 6157.71 ± 645.26 | 1066.57 ± 58.16 |
| 5mg L-SR + sLIPUS | 8.03 ± 0.19 | 6655.00 ± 480.26 | 895.33 ± 190.05 |
| 5mg L-SR + LIPUS | 8.30 ± 0.29 | 6150.40 ± 1328.68 | 883.40 ± 255.29 |
| AST (U/L) | ALT (U/L) | BUN (mg/dL) | Creatinine (mg/dL) | ||
|---|---|---|---|---|---|
| control (sham surgery) | 167.14 ± 22.04 | 65.14 ± 10.66 | 16.99 ± 0.81 | 0.36 ± 0.02 | |
| PBS + sLIPUS | 134.17 ± 9.30 | 51.83 ± 2.75 | 18.20 ± 0.69 | 0.35 ± 0.02 | |
| PBS + LIPUS | 179 ± 64.69 | 61.83 ± 8.19 | 18.78 ± 0.42 | 0.39 ± 0.01 | |
| 15mg pure SR + sLIPUS | 139.86 ± 6.17 | 59.71 ± 6.14 | 17.83 ± 0.46 | 0.36 ± 0.01 | |
| 15mg L-SR + sLIPUS | 145.83 ± 13.09 | 87.83 ± 3.28 | 17.82 ± 0.71 | 0.37 ± 0.03 | |
| 15mg L-SR + LIPUS | 150.57 ± 16.25 | 62.14 ± 5.63 | 16.40 ± 0.60 | 0.38 ± 0.03 | |
| 5mg L-SR + sLIPUS | 131.33 ± 5.19 | 52.00 ± 1.88 | 17.52 ± 0.70 | 0.36 ± 0.02 | |
| 5mg L-SR + LIPUS | 136.50 ± 23.45 | 61.83 ± 4.41 | 16.55 ± 0.71 | 0.39 ± 0.02 | |
| Na (mg/dL) | K (mg/dL) | Cl (mg/dL) | Ca (mg/dL) | P (mg/dL) | |
| control (sham surgery) | 139.93 ± 0.30 | 5.80 ± 0.18 | 99.07 ± 0.46 | 9.93 ± 0.08 | 6.56 ± 0.32 |
| PBS + sLIPUS | 140.78 ± 0.82 | 6.15 ± 0.25 | 99.75 ± 0.69 | 9.98 ± 0.14 | 6.40 ± 0.28 |
| PBS + LIPUS | 140.58 ± 0.56 | 6.45 ± 0.45 | 99.12 ± 0.33 | 9.88 ± 0.11 | 7.52 ± 0.53 |
| 15mg pure SR + sLIPUS | 140.69 ± 0.69 | 6.06 ± 0.11 | 99.93 ± 0.58 | 9.90 ± 0.08 | 7.16 ± 0.18 |
| 15mg L-SR + sLIPUS | 140.87 ± 0.98 | 6.13 ± 0.12 | 98.78 ± 0.90 | 9.93 ± 0.10 | 7.65 ± 0.73 |
| 15mg L-SR + LIPUS | 140.97 ± 0.99 | 6.17 ± 0.17 | 99.96 ± 1.02 | 10.00 ± 0.11 | 7.37 ± 0.38 |
| 5mg L-SR + sLIPUS | 141.90 ± 0.68 | 5.67 ± 0.47 | 100.72 ± 0.49 | 10.00 ± 0.06 | 6.97 ± 0.22 |
| 5mg L-SR + LIPUS | 140.67 ± 0.88 | 6.34 ± 0.38 | 99.02 ± 0.74 | 9.97 ± 0.18 | 7.03 ± 0.42 |
| Groups | Treatments (In Vitro) | Treatments (In Vivo) |
|---|---|---|
| A | control | Iontophoretic PBS + sLIPUS (ACLT) |
| B | 10−4 M pure SR | Iontophoretic PBS + LIPUS (ACLT) |
| C | 10−4 M L-SR | Iontophoretic 15 mg pure SR + sLIPUS (ACLT) |
| D | 10−4 M L-SR + LIPUS | Iontophoretic 15 mg L-SR +sLIPUS (ACLT) |
| E | 10−5 M L-SR + LIPUS | Iontophoretic 15 mg L-SR + LIPUS (ACLT) |
| F | LIPUS alone | Iontophoretic 5 mg L-SR + sLIPUS (ACLT) |
| G | - | Iontophoretic 5 mg L-SR + LIPUS (ACLT) |
| H | - | sham surgery (control) |
| Forward (5′-3′) | Reverse (5′-3′) | |
|---|---|---|
| MMP-13 | CTTCCCAACCGTATTGATGCT | CTGGTTTCCTGAGAACAGGAG |
| IL-1β | ATGATGGCTTATTACAGTGCAA | GTCGGAGATTCGTAGCTGGA |
| TNF-α | CCCAGGGACTCTCTCTAATC | ATGGGCTACAGGCTTGTCACT |
| GAPDH | TGTTGCCATCAATGACCCCTT | CTCCACGACGTACTCAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Lin, S.-L.; Kuo, S.M.; Lai, J.-M.; Shih, W.-L.; Shen, P.-C.; Kuo, Y.-W.; Huang, H.H. Amelioration of Post-traumatic Osteoarthritis by Iontophoretic Liposomal Strontium Ranelate Collaborated with Low-Intensity Pulsed Ultrasound in Rats. Int. J. Mol. Sci. 2025, 26, 8815. https://doi.org/10.3390/ijms26188815
Chen C-H, Lin S-L, Kuo SM, Lai J-M, Shih W-L, Shen P-C, Kuo Y-W, Huang HH. Amelioration of Post-traumatic Osteoarthritis by Iontophoretic Liposomal Strontium Ranelate Collaborated with Low-Intensity Pulsed Ultrasound in Rats. International Journal of Molecular Sciences. 2025; 26(18):8815. https://doi.org/10.3390/ijms26188815
Chicago/Turabian StyleChen, Chung-Hwan, Syu-Lun Lin, Shyh Ming Kuo, Jyh-Mirn Lai, Wen-Ling Shih, Po-Chih Shen, Yi-Wen Kuo, and Han Hsiang Huang. 2025. "Amelioration of Post-traumatic Osteoarthritis by Iontophoretic Liposomal Strontium Ranelate Collaborated with Low-Intensity Pulsed Ultrasound in Rats" International Journal of Molecular Sciences 26, no. 18: 8815. https://doi.org/10.3390/ijms26188815
APA StyleChen, C.-H., Lin, S.-L., Kuo, S. M., Lai, J.-M., Shih, W.-L., Shen, P.-C., Kuo, Y.-W., & Huang, H. H. (2025). Amelioration of Post-traumatic Osteoarthritis by Iontophoretic Liposomal Strontium Ranelate Collaborated with Low-Intensity Pulsed Ultrasound in Rats. International Journal of Molecular Sciences, 26(18), 8815. https://doi.org/10.3390/ijms26188815






