Development of a Comprehensive Lesion Severity Classification Model for Largemouth Bass (Micropterus salmoides) Ranavirus (LMBV) Based on Machine Vision
Abstract
1. Introduction
2. Results
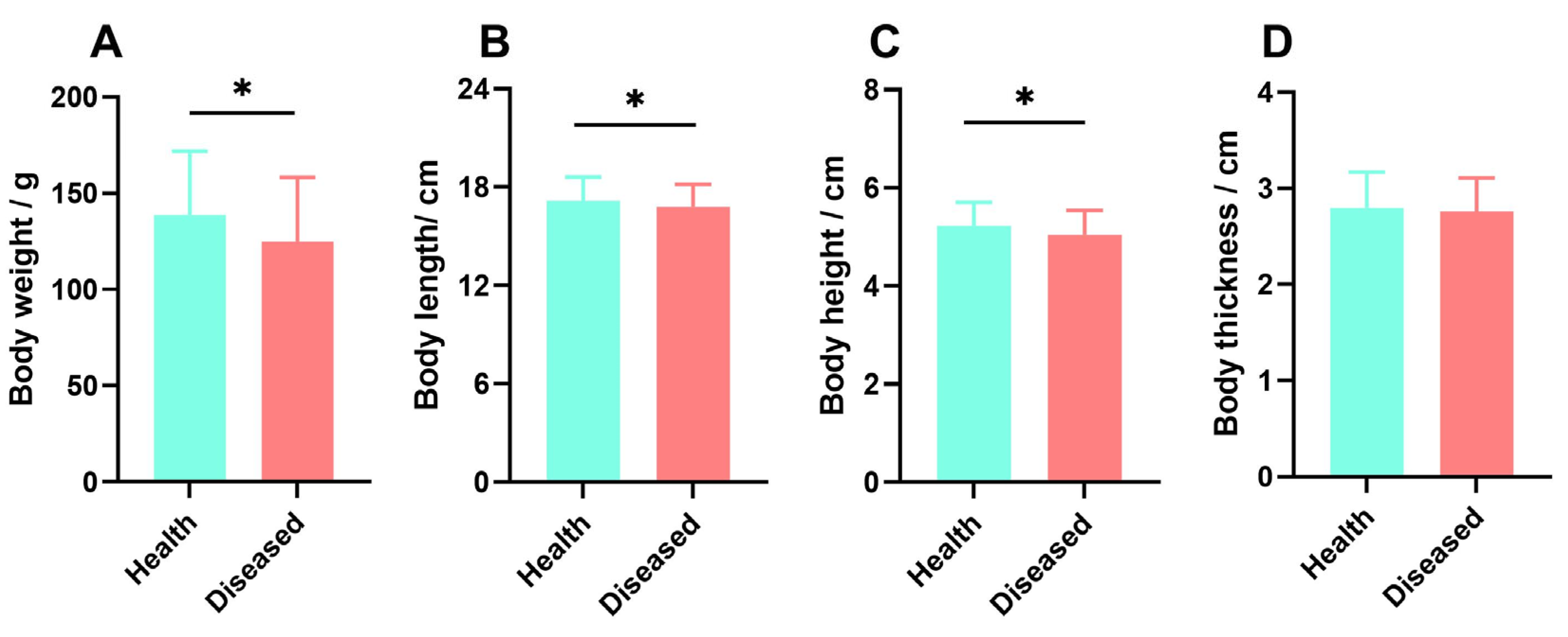
2.1. Measurement of Growth Indicators in Virus Infection Breeding Experiments
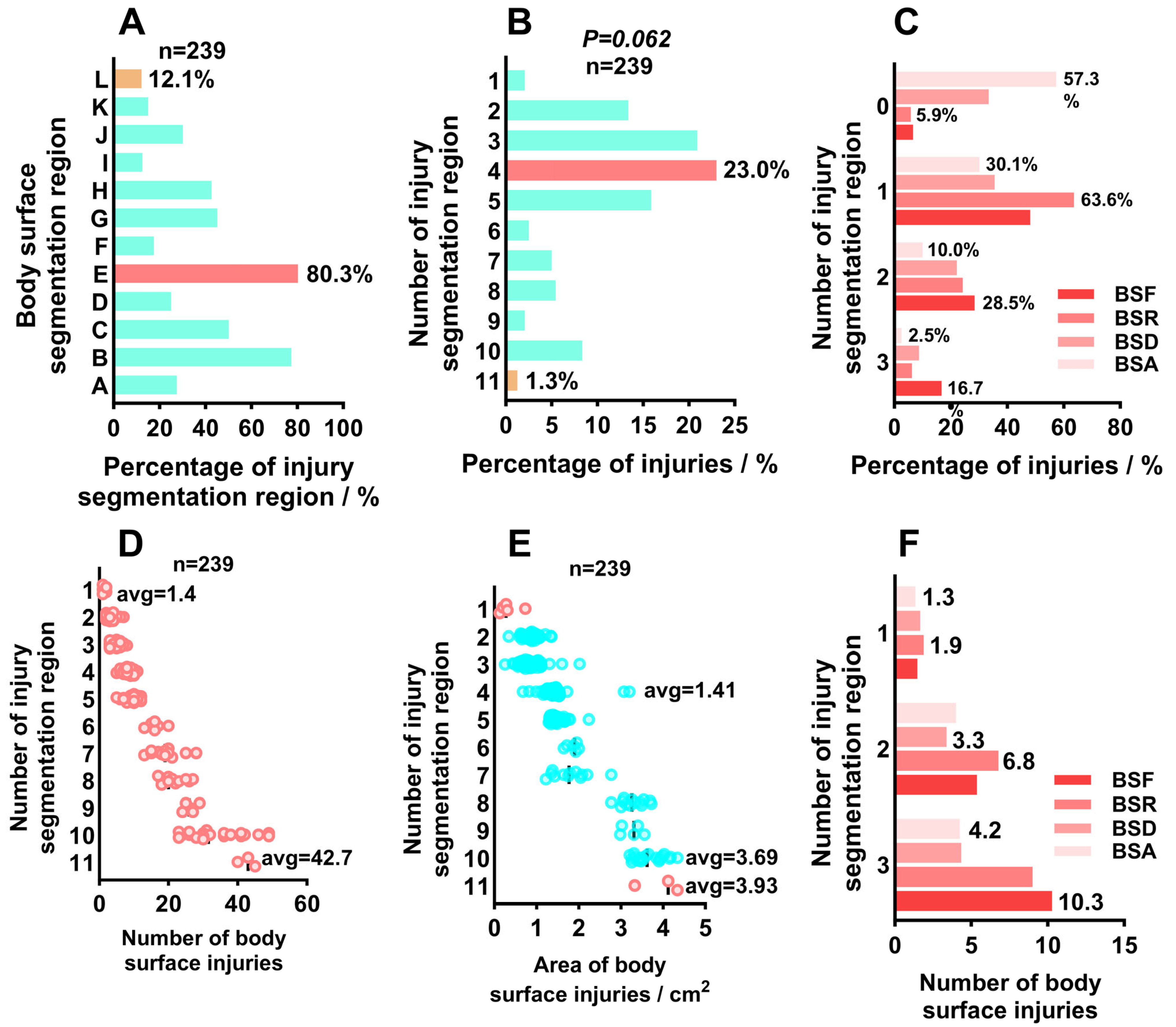
2.2. Collection and Analysis of Body Surface Injury Image Data
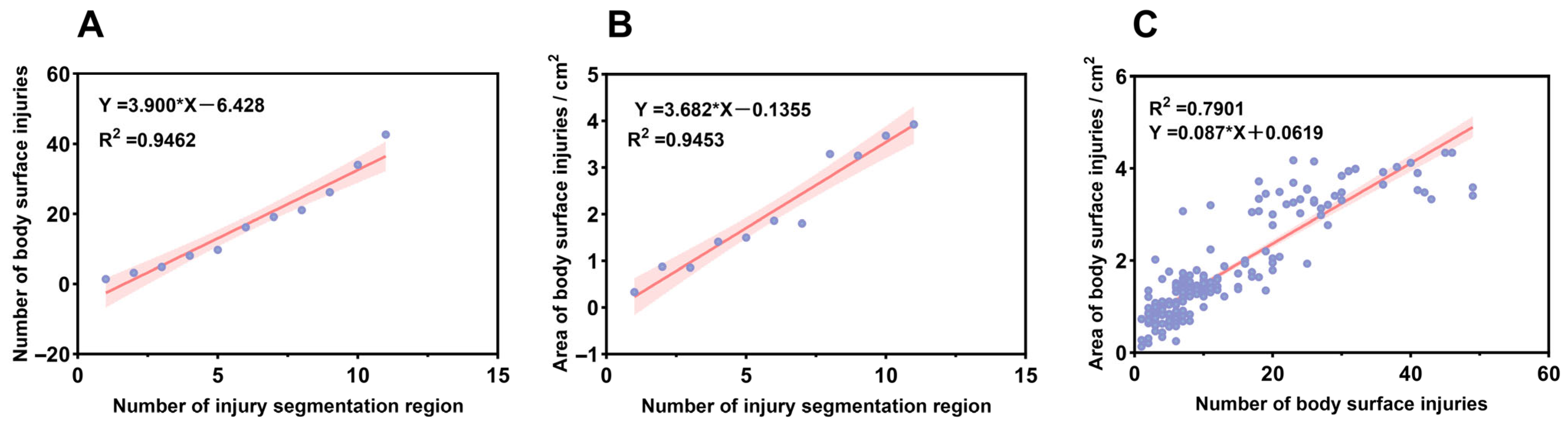
2.3. Analysis of Surface Injury Characteristics
2.4. Establishment of a Comprehensive Injury Scoring Model
- 0 < for minor injuries;
- 0.53 < for moderate injuries;
- 1.13 < for severe injuries.
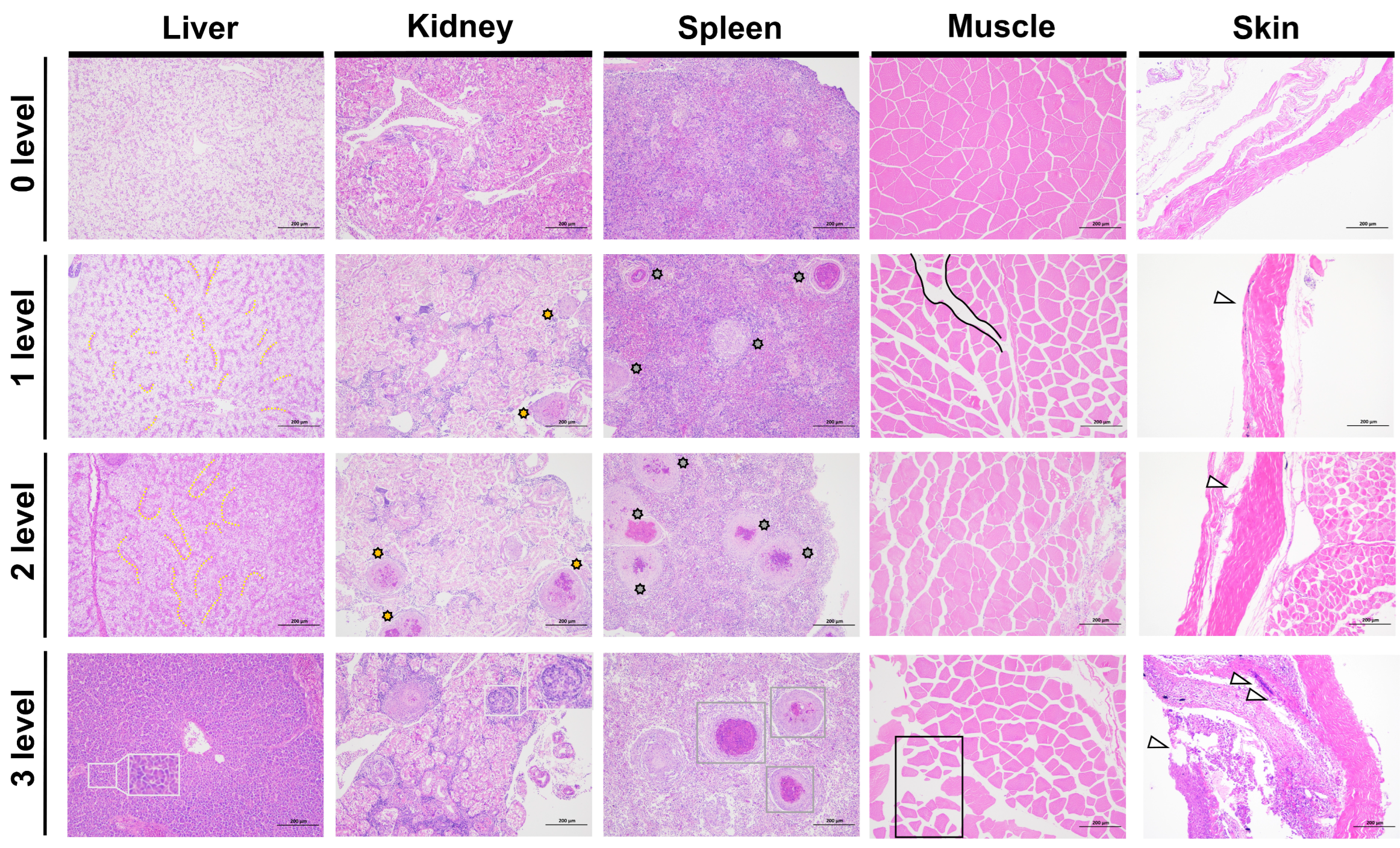
2.5. Histopathological Analysis
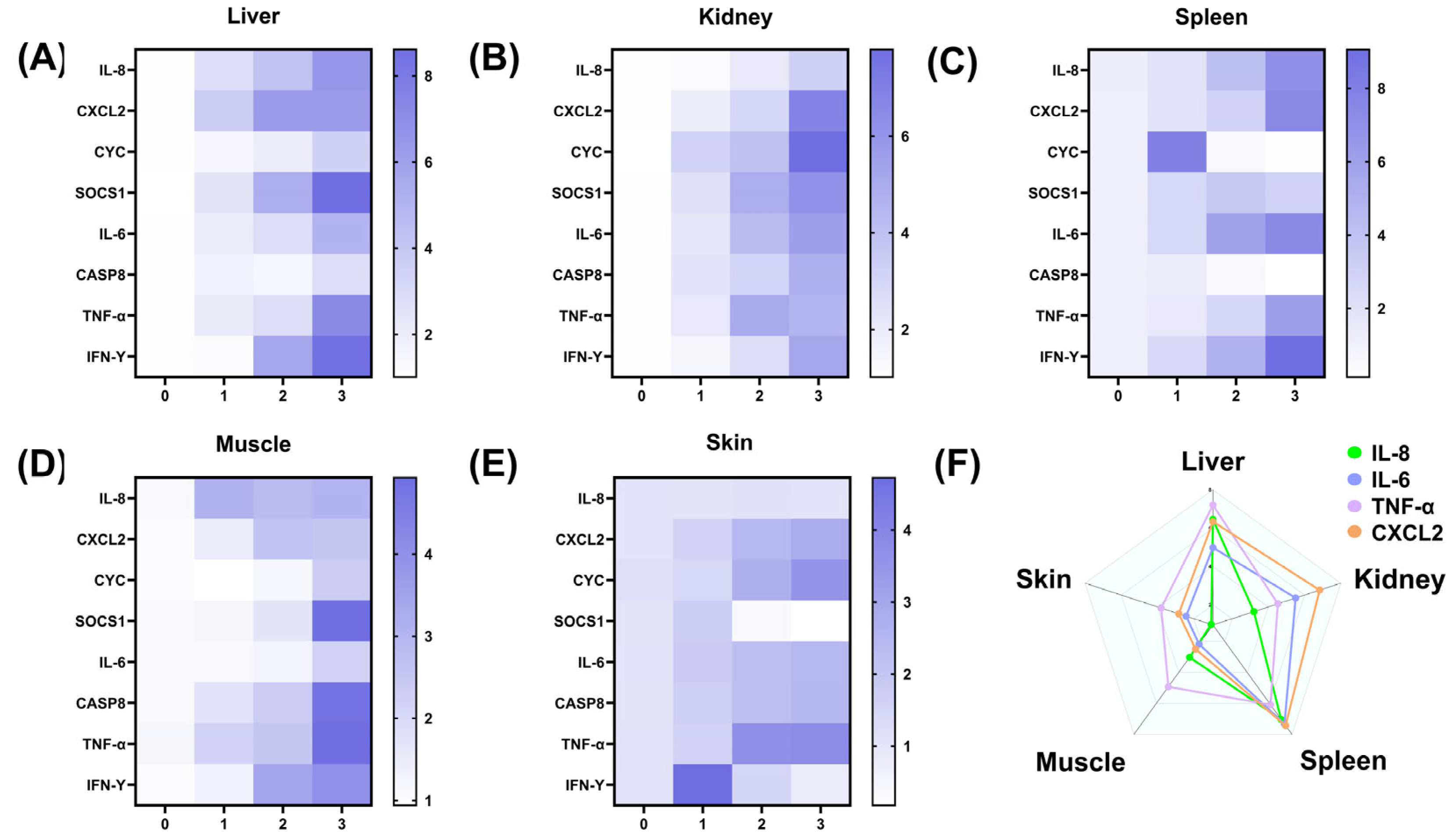
2.6. qRT-PCR Results
3. Discussion
3.1. Methodological Innovations in Machine Vision Technology for LMBV Diagnosis
3.2. Analysis of the Correlation Between Surface Lesion Characteristics and Immunopathology
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation and Culture of LMBV Strains
4.3. Viral Infection Farming Tests
4.4. Measurement of Growth Indicators
4.5. Machine Vision-Assisted Injury Data Collection
4.6. Analysis of Injury Data
4.7. Establishment of Criteria for Determining the Level of Injury
4.8. Histological Analysis
4.9. RNA Extraction, Reverse Transcription, and qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tacon, J.G.A.; Metian, M. Fish Matters: Importance of Aquatic Foods in Human Nutrition and Global Food Supply. Rev. Fish. Sci. 2013, 21, 22–38. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture. China Aquatic Products Report; U.S. Department of Agriculture: Washington, DC, USA, 2025.
- Hua, J.X.; Tao, Y.F.; Li, Y.; Wang, Q.C.; Chen, W.H.; Zhu, G.Y.; Zhang, M.Y.; Dong, Y.L.; Lu, S.Q.; Qiang, J.; et al. Development of fast-growth SNP screening and association analysis with growth traits based on RAD-seq for largemouth bass (Micropterus salmoides) breeding populations. J. Fish. Sci. China 2024, 31, 241–256. [Google Scholar] [CrossRef]
- Hua, X.J.; Li, C.; Xiao, Y.C.; Lu, Y.N.; Liu, X.Q. Oral administration of recombinant Lactococcus lactis expressing largemouth bass (Micropterus salmoides) IFNa3 protein enhances immune response against largemouth bass virus (LMBV) infection. Fish Shellfish Immunol. 2024, 154, 109875. [Google Scholar] [CrossRef]
- Fisheries Bureau of the Ministry of Agriculture and Rural Affairs PRC; National Fisheries Technology Extension Center; China Society of Fisheries. China Fishery Statistical Yearbook; Chinese Agriculture Press: Beijing, China, 2024. [Google Scholar]
- Zhang, X.; Zhang, Z.; Li, J.; Huang, X.; Wei, J.; Yang, J.; Guan, L.; Wen, X.; Wang, S.; Qin, Q. A novel sandwich ELASA based on aptamer for detection of largemouth bass virus (LMBV). Viruses 2022, 14, 945. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, Y.; Li, P.T.; Jiang, J.; Zeng, W.H.; Ye, K.; Wang, Y.L.; Zou, P.F. Genetic and Pathogenic Characterization of an Iridovirus from the Cultured Largemouth Bass Micropterus salmoides. Fishes 2024, 9, 314. [Google Scholar] [CrossRef]
- Lacaze, E.; Gendron, A.D.; Miller, J.L.; Colson, T.L.; Sherry, J.P.; Giraudo, M.; Marcogliese, D.J.; Houde, M. Cumulative effects of municipal effluent and parasite infection in yellow perch: A field study using high-throughput RNA-sequencing. Sci. Total Environ. 2019, 665, 797–809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Y.; Bai, S.J.; He, J.; Xiong, Q.X.; Zhong, Z.D.; Lu, C.W.; Kuang, L.F.; Jian, Z.R.; Gu, J.L.; Liu, M.Z.; et al. Pathogenicity, ultrastructure and genomics analysis of Nocardia seriolae isolated from largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2025, 205, 107715. [Google Scholar] [CrossRef]
- Fu, X.; Li, W.; Liu, C.; Luo, X.; Lin, Q.; Niu, Y.; Liang, H.; Ma, B.; Li, N. A naturaly attenuated largemouth bass ranavirus strain provided protection for Micropterus salmoides by immersion immunization. Fish Shellfish Immunol. 2024, 153, 109871. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ma, B.; Wu, Z.; Wang, Y.; Yang, S.; Ling, F.; Liu, T.; Xu, K.; Wang, G. CRISPR/CasRx: A novel antiviral approach to combat largemouth bass (Micropterus salmoides) Rhabdovirus infections. Aquaculture 2025, 599, 742189. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; An, N.; Li, D.C.; Huang, M.M.; Fei, H. Updates on infectious diseases of largemouth bass: A major review. Fish Shellfish Immunol. 2024, 154, 109976. [Google Scholar] [CrossRef]
- Deng, G.C.; Xie, J.; Li, S.J.; Bai, J.J.; Chen, K.C.; Ma, D.M.; Jiang, X.Y.; Lao, H.H. Isolation and preliminary identification of the pathogen from largemouth bass ulcera-tive syndrome. J. Fish. China 2009, 33, 871–877. [Google Scholar] [CrossRef]
- Wang, R.X.; Luo, X.; Li, N.Q.; Lin, Q.; Niu, Y.J.; Liang, H.R.; Fu, X.Z.; Lv, A.J. Preparation and application of polyclonal antibodies against the MCP protein of the rainbow virus of the black bass frog. J. Northwest AF Univ. (Nat. Sci. Ed.) 2024, 52, 21–27. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Tan, X.; Guo, M.; Kong, W.; An, Z. MicroRNA profiling yields immune response and metabolic changes in juvenile largemouth bass (Micropterus salmoides) infected with LMBV. Water Biol. Secur. 2025, 4, 100327. [Google Scholar] [CrossRef]
- Mao, J.; Wang, J.; Chinchar, G.D.; Chinchar, V.G. Molecular characterization of a ranavirus isolated from largemouth bass Micropterus salmoides. Dis. Aquat. Org. 1999, 30, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Geng, Y.; Qin, Z.; Wang, K.; Ouyang, P.; Chen, D.; Huang, X.; Zuo, Z.; He, C.; Guo, H.; et al. A new ranavirus of the Santee-Cooper group invades largemouth bass (Micropterus salmoides) culture in southwest China. Aquaculture 2020, 526, 735363. [Google Scholar] [CrossRef]
- Hui, S.; Hua, J.; Tao, Y.; He, J.; Wang, Q.; Dong, Y.; He, J.; Qiang, J. Disease Resistance and Genetic Characteristics of Genetically Selected Largemouth Bass (Micropterus salmoides) Revealed by Whole Genome Resequencing. Int. J. Mol. Sci. 2025, 165, 110558. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Rangsichol, A.; Dong, H.T.; Rodkhum, C.; Maita, M.; Katagiri, T.; Pirarat, N. Outbreaks of ulcerative disease associated with ranavirus infection in barcoo grunter, Scortum barcoo (McCulloch & Waite). Fish Dis. 2007, 40, 1341–1350. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Li, Y.; Zhang, Z.; Li, J.; Xu, C.; Du, R.; Li, D.; Duan, Q. Research progress of computer vision technology in abnormal fish detection. Aquac. Eng. 2023, 103, 102350. [Google Scholar] [CrossRef]
- Groocock, G.H.; Grimmett, S.G.; Getchell, R.G.; Wooster, G.A.; Bowser, P.R. A survey to determine the presence and distribution of largemouth bass virus in wild freshwater bass in New York state. Aquat. Anim. Health 2008, 20, 158–164. [Google Scholar] [CrossRef]
- Vo, T.T.E.; Ko, H.; Huh, J.H.; Kim, Y. Overview of Smart Aquaculture System: Focusing on Applications of Machine Learning and Computer Vision. Electronics 2021, 10, 2882. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, D.M.; Chen, L.; Zhang, S.; Sun, C.H.; Yang, X.T.; Wang, Y.B. Evaluation of fish feeding intensity in aquaculture using a convolutional neural network and machine vision. Aquaculture 2019, 507, 457–465. [Google Scholar] [CrossRef]
- Mutka, A.M.; Bart, R.S. Image-based phenotyping of plant disease symptoms. Front. Plant Sci. 2014, 1, 5734. [Google Scholar] [CrossRef]
- Li, D.L.; Du, L. Recent advances of deep learning algorithms for aquacultural machine vision systems with emphasis on fish. Artif. Intell. Rev. 2021, 55, 4077–4116. [Google Scholar] [CrossRef]
- Zhang, L.; Li, B.; Sun, X.; Hong, Q.; Duan, Q. Intelligent Fish Feeding Based on Machine Vision: A Review. Biosyst. Eng. 2023, 231, 133–164. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Aurpa, T.T.; Azad, M.A.K. Fish Disease Detection Using Image Based Machine Learning Technique in Aquaculture. J. King Saud Univ.-Comput. Inf. Sci. 2022, 34, 5170–5182. [Google Scholar] [CrossRef]
- Bai, Y.; Feng, M.; Zhao, J.; Wang, J.; Ke, Q.; Jiang, Z.; Jiang, P.; Chen, S.; Chen, L.; Liu, W.; et al. Machine vision-assisted genomic prediction and genome-wide association of spleen-related traits in large yellow croaker infected with visceral white-nodules disease. Fish Shellfish Immunol. 2024, 154, 109948. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Su, Y.; Li, W.; Yin, X.; Li, Z.; Ying, Y.; Wang, J.; Wu, J.; Miao, F.; et al. Abnormal Behavior Monitoring Method of Larimichthys crocea in Recirculating Aquaculture System Based on Computer Vision. Sensors 2023, 23, 2835. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, Y.; Xue, M.Y.; Zhu, S.M.; Zhao, J.; Cai, H.Y.; Ye, Z.Y. Lightweight nondestructive detection model of crucian carp disease based on machine vision and improved yolov5s. Acta Hydrobiol. Sin. 2024, 48, 1141–1148. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, D.; Wang, W.; Li, Y.; Li, Z.; He, H.; He, B.; Zhu, L.; Chu, P. Pathogenicity characterization, immune response mechanisms, and antiviral strategies analysis underlying a LMBV strain in largemouth bass (Micropterus salmoides). Aquac. Rep. 2024, 36, 102133. [Google Scholar] [CrossRef]
- Deng, G.; Li, S.; Xie, J.; Bai, J.; Chen, K.; Ma, D.; Jiang, X.; Lao, H.; Yu, L. Characterization of a ranavirus isolated from cultured largemouth bass (Micropterus salmoides) in China. Aquaculture 2011, 312, 198–204. [Google Scholar] [CrossRef]
- Zhao, L.; Zhong, Y.; Luo, M.; Zheng, G.; Huang, J.; Wang, G.; Geng, Y.; Qian, X. Largemouth bass ranavirus: Current status and research progression. Aquac. Rep. 2023, 32, 101706. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, Y.; Li, C.; Huang, Y.; Ouyang, P.; Chen, D.; Geng, Y.; Huang, X. The pathological characteristics at different stages and early diagnosis by duplex PCR of Nocardia seriolae disease in largemouth bass (Micropterus salmoides). Aquaculture 2025, 605, 742520. [Google Scholar] [CrossRef]
- Qin, K.L. Research on Skin Lesion Image Segmentation Algorithms Based on Deep Learning. Master’s Dissertation, Anhui University, Anhui, China, 2021. [Google Scholar]
- Zhang, Z.; Zou, B.S. A model for identifying fish feeding behaviour based on the fusion of visual and water quality characteristics. J. South China Agric. Univ. 2025, 46, 538–548. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, H.; Cui, Z.; Wang, L.; Li, H.; Qu, K.; Cui, H. Early warning system for nocardiosis in largemouth bass (Micropterus salmoides) based on multimodal information fusion. Comput. Electron. Agric. 2024, 226, 109393. [Google Scholar] [CrossRef]
- Wang, M.; Yang, B.; Ren, Z.; Liu, J.; Lu, C.; Jiang, H.; Ling, F.; Wang, G.; Liu, T. Inhibition of the largemouth bass virus replication by piperine demonstrates potential application in aquaculture. J. Fish Dis. 2023, 46, 261–271. [Google Scholar] [CrossRef]
- Kumar, N.; Chandan, N.K.; Gupta, S.K.; Bhushan, S.; Patole, P.B. Omega-3 Fatty Acids Effectively Modulate Growth Performance, Immune Response, and Disease Resistance in Fish against Multiple Stresses. Aquaculture 2021, 545, 737506. [Google Scholar] [CrossRef]
- Deng, Y.; Ding, C.; Yang, H.; Zhang, M.; Xiao, Y.; Wang, H.; Li, J.; Xiao, T.; Lv, Z. First In Vitro and In Vivo Evaluation of Recombinant IL-1β Protein as a Potential Immunomodulator against Viral Infection in Fish. Int. J. Biol. Macromol. 2023, 257, 128192. [Google Scholar] [CrossRef]
- He, M.; Ding, N.-Z.; He, C.-Q. Novirhabdoviruses versus Fish Innate Immunity: A Review. Virus Res. 2021, 302, 198525. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, M.; Fan, X.; Zhu, P.; Jiang, Z.; Li, F.; Yuan, W.; You, S.; Chen, J.; Li, Y.; et al. ROS Induced by Spring Viraemia of Carp Virus Activate the Inflammatory Response via the MAPK/AP-1 and PI3K Signaling Pathways. Dev. Comp. Immunol. 2021, 126, 104291. [Google Scholar] [CrossRef]
- He, Z.; Zhao, J.; Chen, X.; Liao, M.; Xue, Y.; Zhou, J.; Chen, H.; Chen, G.; Zhang, S.; Sun, C. The Molecular Mechanism of Hemocyte Immune Response in Marsupenaeus japonicus Infected with Decapod Iridescent Virus 1. Front. Microbiol. 2021, 12, 710845. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, M.; Chen, H.; Zhang, L.; Xu, Q.; Zhan, Z.; Xu, Z.; Liu, S.; Wu, S.; Zhang, X.; et al. Singapore Grouper Iridovirus VP122 Targets Grouper STING to Evade the Interferon Immune Response. Fish Shellfish Immunol. 2023, 142, 108990. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, S.; Guo, M.; Wang, Z.; Hu, B.; Zhou, B.; Chen, S. The Dynamic Immune Response of the Liver and Spleen in Leopard Coral Grouper (Plectropomus leopardus) to Vibrio harveyi Infection Based on Transcriptome Analysis. Front. Immunol. 2024, 15, 1457745. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, A.; Yang, C.; Yang, Y.; Akhtar, M.; Umar, T.; Khan, M.A.; Ahmad, S.; Deng, G.; Guo, M. MerTK Negatively Regulates Staphylococcus aureus Induced Inflammatory Response via SOCS1/SOCS3 and Mal. Immunobiology 2020, 225, 151960. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.; Costa, J.; Serrão, J.; Cruz, C.; Eiras, J.C. A histologybased fish health assessment of farmed seabass (Dicentrarchus labrax L.). Aquaculture 2015, 448, 375–381. [Google Scholar] [CrossRef]
- Raskovic, B.; Poleksic, V. Fish histopathology as biomarker in ecotoxicology. Trends Fish. Aquat. Anim. Health 2017, 27, 155–181. [Google Scholar] [CrossRef]
- Sirri, R.; Sarli, G.; Bianco, C.; Bonaldo, A.; Gatta, P.P.; Fontanillas, R.; De Vico, G.; Carella, F.; Brachelente, C.; Parma, L.; et al. Retrospective study of pathology-based investigative techniques for the assessment of diet-induced changes in liver and intestine of flatfish. Ital. J. Anim. Sci. 2018, 17, 518–529. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, H.M.; Kim, H.; Cho, J.H. Molecular cloning and functional analysis of deubiquitinase CYLD in rainbow trout, Oncorhynchus mykiss. Fish Shellfish Immunol. 2020, 101, 135–142. [Google Scholar] [CrossRef]
- Dai, C.; Zhu, Y.; Li, L.; Gu, Z.; Wang, J.; Yuan, J. Kaempferol attenuated largemouth bass virus caused inflammation via inhibiting NF-κB and ERK-MAPK signaling pathways. Aquaculture 2025, 607, 742626. [Google Scholar] [CrossRef]
- Huo, X.; Zhao, F.; Yang, C.; Su, J. Antioxidant anthocyanin synergistic immune enhancer nanopeptide C-I20 remarkably enhances the protective effect of largemouth bass against largemouth bass ranavirus. Fish Shellfish Immunol. 2024, 154, 109952. [Google Scholar] [CrossRef]
- Jiang, W.D.; Feng, L.; Qu, B.; Wu, P.; Kuang, S.Y.; Jiang, J.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; et al. Changes in integrity of the gill during histidine deficiency or excess due to depression of cellular anti-oxidative ability, induction of apoptosis, inflammation and impair of cell-cell tight junctions related to Nrf2, TOR and NF-kB signaling in fish. Fish Shellfish Immunol. 2016, 56, 111–122. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, H.; Mokrani, A.; Ji, K.; Yu, H.; Ge, X.; Ren, M.; Xie, J.; Pan, L.; Sun, A.; et al. Dietary histidine affects intestinal antioxidant enzyme activities, antioxidant gene expressions and inflammatory factors in juvenile blunt snout bream (Megalobrama amblycephala). Aquac. Nutr. 2019, 25, 249–259. [Google Scholar] [CrossRef]
- Fijan, N.; Sulimanović, D.; Bearzotti, M.; Muzinić, D.; Zwillenberg, L.O.; Chilmonczyk, S.; Vautherot, J.F.; de Kinkelin, P. Some properties of the Epithelioma papulosum cyprini (EPC) cell line from carp cyprinus carpio. Ann. L’Institut Pasteur/Virol. 1983, 134, 207–220. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Szeliski, R. Computer Vision: Algorithms and Applications; Springer: London, UK, 2011; Available online: http://szeliski.org/Book/ (accessed on 3 May 2025).
- Byadgi, O.; Chen, C.W.; Wang, P.C.; Tsai, M.A.; Chen, S.C. De Novo Transcriptome Analysis of Differential Functional Gene Expression in Largemouth Bass (Micropterus salmoides) after Challenge with Nocardia seriolae. Int. J. Mol. Sci. 2016, 17, 1315. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, Z.; Lai, F.; Yang, J.; Qin, Q.; Huang, Y.; Huang, X. Transcriptome analysis reveals the host immune response upon LMBV infection in largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2023, 137, 108753. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huo, X.; Wang, P.; Zhao, F.; Yuan, G.; Yang, C.; Su, J. Lactobacillus casei displaying MCP2α and FlaC delivered by PLA microspheres effectively enhances the immune protection of largemouth bass (Micropterus salmoides) against LMBV infection. Fish Shellfish Immunol. 2024, 153, 109870. [Google Scholar] [CrossRef] [PubMed]




| PCA | Variance Contribution Rate | Cumulative Variance Contribution Rate |
|---|---|---|
| PC1 | 72.30% | 72.30% |
| PC2 | 21.50% | 93.80% |
| PC3 | 6.20% | 100% |
| Variable | PC1 Loading | Interpretation |
|---|---|---|
| N1 | 0.59 | Injury spread range |
| N2 | 0.63 | Injury density |
| S | 0.68 | The severe injury of a single region |
| Gene Full Name | Primer Names | Sequence (5′-3′) |
|---|---|---|
| Cytochrome c | CYC-F | AGAAGTGTGCCCAATGCCATACTG |
| CYC-R | GCGTCCGAACAGACCCCAAAG | |
| C-X-C motif chemokine ligand 2 | CXCL2-F | ACACATTCTGCTGTCCTCGTTTCC |
| CXCL2-R | TCTACACCCAGGCTCCTCAAACTC | |
| Suppressor of cytokine signaling 1 | SOCS1-F | AGTGTGGTGGTTAGAGATGGGAGAG |
| SOCS1-R | GAGGATGACGATGATGACGATGAGC | |
| Interleukin-1 beta | IL-Iβ-F | CGTACATCCGTGCCAACAGI |
| IL-Iβ-R | ATGCTCTTTAACTCCTCCI | |
| Tumor necrosis factor-alpha | TNF-a-F | CTAGTGAAGAACCAGATTGT |
| TNF-a-R | AGGAGACTCTGAACGATG | |
| Interferon-gamma | IFN-Y-F | TCCCTCTGAAGATGAACAAA |
| IFN-Y-R | AACGCCACCCATAAACA | |
| Interleukin-8 | IL-8-F | GAGTTTGAGGAGCCTGGGTGT |
| IL-8-R | GGGTCCAGGCAAACCTCTTG | |
| Interleukin-6 | IL-6-F | GGGAGACTCGCTCTGACCTACTG |
| IL-6-R | TACCTCCTCCTTGTGGCGTTGG | |
| Beta-actin (reference gene) | β-ACTIN-F | CCACCACAGCCGAGAGGGAA |
| β-ACTIN-R | TCATGGTGGATGGGGCCAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Hua, J.; Tao, Y.; Yang, Z.; Zhu, T.; Lu, S.; Wang, W.; Dong, Y.; Zhang, L.; He, J.; et al. Development of a Comprehensive Lesion Severity Classification Model for Largemouth Bass (Micropterus salmoides) Ranavirus (LMBV) Based on Machine Vision. Int. J. Mol. Sci. 2025, 26, 8810. https://doi.org/10.3390/ijms26188810
Sun H, Hua J, Tao Y, Yang Z, Zhu T, Lu S, Wang W, Dong Y, Zhang L, He J, et al. Development of a Comprehensive Lesion Severity Classification Model for Largemouth Bass (Micropterus salmoides) Ranavirus (LMBV) Based on Machine Vision. International Journal of Molecular Sciences. 2025; 26(18):8810. https://doi.org/10.3390/ijms26188810
Chicago/Turabian StyleSun, Hui, Jixiang Hua, Yifan Tao, Ziying Yang, Taide Zhu, Siqi Lu, Wen Wang, Yalun Dong, Linbing Zhang, Jixiang He, and et al. 2025. "Development of a Comprehensive Lesion Severity Classification Model for Largemouth Bass (Micropterus salmoides) Ranavirus (LMBV) Based on Machine Vision" International Journal of Molecular Sciences 26, no. 18: 8810. https://doi.org/10.3390/ijms26188810
APA StyleSun, H., Hua, J., Tao, Y., Yang, Z., Zhu, T., Lu, S., Wang, W., Dong, Y., Zhang, L., He, J., He, J., & Qiang, J. (2025). Development of a Comprehensive Lesion Severity Classification Model for Largemouth Bass (Micropterus salmoides) Ranavirus (LMBV) Based on Machine Vision. International Journal of Molecular Sciences, 26(18), 8810. https://doi.org/10.3390/ijms26188810






