Dual Role of Transformer 2 Beta as Both a Developmental Necessity and a Disease Modulator
Abstract
1. Introduction
2. TRA2β Protein Domains, Isoforms and Its Relationship to Family Members
3. TRA2β in Embryogenesis and Systemic Development
3.1. Role in Embryogenesis and Sex-Specificity
3.2. Muscle Development
3.3. Neurological Development
3.3.1. Cerebral Cortex Development
3.3.2. The Visual System
4. Contribution to the Pathogenesis of Various Diseases
4.1. Neurological Disease
4.2. Cancers
4.3. Immune System Disorders
5. Therapeutic Potential and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takashima, S.; Sun, W.; Otten, A.B.C.; Cai, P.; Peng, S.I.; Tong, E.; Bui, J.; Mai, M.; Amarbayar, O.; Cheng, B.; et al. Alternative mRNA splicing events and regulators in epidermal differentiation. Cell Rep. 2024, 43, 113814. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, L. Alternative splicing: Human disease and quantitative analysis from high-throughput sequencing. Comput. Struct. Biotechnol. J. 2021, 19, 183–195. [Google Scholar] [CrossRef]
- Lejeune, F. Nonsense-Mediated mRNA Decay, a Finely Regulated Mechanism. Biomedicines 2022, 10, 141. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Q.; Wang, H.; Yang, X.; Mu, H. Alternative splicing and related RNA binding proteins in human health and disease. Signal Transduct. Target. Ther. 2024, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Perera, L.; Blackshear, P.J. An Ancient Family of RNA-Binding Proteins: Still Important! Trends Biochem. Sci. 2017, 42, 285–296. [Google Scholar] [CrossRef]
- Geuens, T.; Bouhy, D.; Timmerman, V. The hnRNP family: Insights into their role in health and disease. Hum. Genet. 2016, 135, 851–867. [Google Scholar] [CrossRef]
- Tacke, R.; Manley, J.L. Functions of SR and Tra2 proteins in pre-mRNA splicing regulation. Proc. Soc. Exp. Biol. Med. 1999, 220, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Dauwalder, B.; Amaya-Manzanares, F.; Mattox, W. A human homologue of the Drosophila sex determination factor transformer-2 has conserved splicing regulatory functions. Proc. Natl. Acad. Sci. USA 1996, 93, 9004–9009. [Google Scholar] [CrossRef]
- Burtis, K.C. The regulation of sex determination and sexually dimorphic differentiation in Drosophila. Curr. Opin. Cell Biol. 1993, 5, 1006–1014. [Google Scholar] [CrossRef]
- Xue, J.; Ma, T.; Zhang, X. TRA2: The dominant power of alternative splicing in tumors. Heliyon 2023, 9, e15516. [Google Scholar] [CrossRef]
- Elliott, D.J.; Best, A.; Dalgliesh, C.; Ehrmann, I.; Grellscheid, S. How does Tra2beta protein regulate tissue-specific RNA splicing? Biochem. Soc. Trans. 2012, 40, 784–788. [Google Scholar] [CrossRef]
- Tsuda, K.; Someya, T.; Kuwasako, K.; Takahashi, M.; He, F.; Unzai, S.; Inoue, M.; Harada, T.; Watanabe, S.; Terada, T.; et al. Structural basis for the dual RNA-recognition modes of human Tra2-beta RRM. Nucleic Acids Res. 2011, 39, 1538–1553. [Google Scholar] [CrossRef]
- Chen, G.; Chen, J.; Qi, L.; Yin, Y.; Lin, Z.; Wen, H.; Zhang, S.; Xiao, C.; Bello, S.F.; Zhang, X.; et al. Bulk and single-cell alternative splicing analyses reveal roles of TRA2B in myogenic differentiation. Cell Prolif. 2024, 57, e13545. [Google Scholar] [CrossRef]
- Shatokhina, O.; Kovalskaia, V.; Sparber, P.; Sharkova, I.; Mishina, I.; Kuznetsova, V.; Ryzhkova, O. TRA2B Gene Splice Variant Linked to Seizures and Neurodevelopmental Delay: A Second Case Study. Int. J. Mol. Sci. 2023, 24, 15572. [Google Scholar] [CrossRef]
- Kuwano, Y.; Nishida, K.; Kajita, K.; Satake, Y.; Akaike, Y.; Fujita, K.; Kano, S.; Masuda, K.; Rokutan, K. Transformer 2beta and miR-204 regulate apoptosis through competitive binding to 3’ UTR of BCL2 mRNA. Cell Death Differ. 2015, 22, 815–825. [Google Scholar] [CrossRef]
- Best, A.; James, K.; Dalgliesh, C.; Hong, E.; Kheirolahi-Kouhestani, M.; Curk, T.; Xu, Y.; Danilenko, M.; Hussain, R.; Keavney, B.; et al. Human Tra2 proteins jointly control a CHEK1 splicing switch among alternative and constitutive target exons. Nat. Commun. 2014, 5, 4760. [Google Scholar] [CrossRef]
- Ramond, F.; Dalgliesh, C.; Grimmel, M.; Wechsberg, O.; Vetro, A.; Guerrini, R.; FitzPatrick, D.; Poole, R.L.; Lebrun, M.; Bayat, A.; et al. Clustered variants in the 5’ coding region of TRA2B cause a distinctive neurodevelopmental syndrome. Genet. Med. 2023, 25, 100003. [Google Scholar] [CrossRef] [PubMed]
- Mende, Y.; Jakubik, M.; Riessland, M.; Schoenen, F.; Rossbach, K.; Kleinridders, A.; Kohler, C.; Buch, T.; Wirth, B. Deficiency of the splicing factor Sfrs10 results in early embryonic lethality in mice and has no impact on full-length SMN/Smn splicing. Hum. Mol. Genet. 2010, 19, 2154–2167. [Google Scholar] [CrossRef]
- Blencowe, B.J. Exonic splicing enhancers: Mechanism of action, diversity and role in human genetic diseases. Trends Biochem. Sci. 2000, 25, 106–110, Erratum in Trends Biochem. Sci. 2000, 25, 228. [Google Scholar] [CrossRef]
- Platt, C.; Calimano, M.; Nemet, J.; Bubenik, J.; Cochrane, A. Differential Effects of Tra2ss Isoforms on HIV-1 RNA Processing and Expression. PLoS ONE 2015, 10, e0125315. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Ennajdaoui, H.; Edmondson, C.; Wirth, B.; Sanford, J.R.; Chen, B. Splicing factor TRA2B is required for neural progenitor survival. J. Comp. Neurol. 2014, 522, 372–392. [Google Scholar] [CrossRef]
- Grellscheid, S.; Dalgliesh, C.; Storbeck, M.; Best, A.; Liu, Y.; Jakubik, M.; Mende, Y.; Ehrmann, I.; Curk, T.; Rossbach, K.; et al. Identification of evolutionarily conserved exons as regulated targets for the splicing activator tra2beta in development. PLoS Genet. 2011, 7, e1002390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Fu, P.; Chen, D.; Liu, R. Transformer 2beta regulates the alternative splicing of cell cycle regulatory genes to promote the malignant phenotype of ovarian cancer. Oncol. Res. 2023, 31, 769–785. [Google Scholar] [CrossRef]
- Dalgliesh, C.; Aldalaqan, S.; Atallah, C.; Best, A.; Scott, E.; Ehrmann, I.; Merces, G.; Mannion, J.; Badurova, B.; Sandher, R.; et al. An ultra-conserved poison exon in the Tra2b gene encoding a splicing activator is essential for male fertility and meiotic cell division. EMBO J. 2025, 44, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Dichmann, D.S.; Walentek, P.; Harland, R.M. The alternative splicing regulator Tra2b is required for somitogenesis and regulates splicing of an inhibitory Wnt11b isoform. Cell Rep. 2015, 10, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Reho, J.J.; Wirth, B.; Fisher, S.A. TRA2beta controls Mypt1 exon 24 splicing in the developmental maturation of mouse mesenteric artery smooth muscle. Am. J. Physiol. Cell Physiol. 2015, 308, C289–C296. [Google Scholar] [CrossRef]
- Fu, K.; Mende, Y.; Bhetwal, B.P.; Baker, S.; Perrino, B.A.; Wirth, B.; Fisher, S.A. Tra2beta protein is required for tissue-specific splicing of a smooth muscle myosin phosphatase targeting subunit alternative exon. J. Biol. Chem. 2012, 287, 16575–16585. [Google Scholar] [CrossRef]
- Shukla, S.; Fisher, S.A. Tra2beta as a novel mediator of vascular smooth muscle diversification. Circ. Res. 2008, 103, 485–492. [Google Scholar] [CrossRef]
- Storbeck, M.; Hupperich, K.; Gaspar, J.A.; Meganathan, K.; Martinez Carrera, L.; Wirth, R.; Sachinidis, A.; Wirth, B. Neuronal-specific deficiency of the splicing factor Tra2b causes apoptosis in neurogenic areas of the developing mouse brain. PLoS ONE 2014, 9, e89020. [Google Scholar] [CrossRef]
- Adesnik, H.; Naka, A. Cracking the Function of Layers in the Sensory Cortex. Neuron 2018, 100, 1028–1043. [Google Scholar] [CrossRef]
- Rakic, P. Specification of cerebral cortical areas. Science 1988, 241, 170–176. [Google Scholar] [CrossRef]
- Noctor, S.C.; Flint, A.C.; Weissman, T.A.; Dammerman, R.S.; Kriegstein, A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001, 409, 714–720. [Google Scholar] [CrossRef]
- Diao, Y.; Cui, L.; Chen, Y.; Burbridge, T.J.; Han, W.; Wirth, B.; Sestan, N.; Crair, M.C.; Zhang, J. Reciprocal Connections Between Cortex and Thalamus Contribute to Retinal Axon Targeting to Dorsal Lateral Geniculate Nucleus. Cereb. Cortex 2018, 28, 1168–1182. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Li, H.L.; Su, J.B.; Ding, F.H.; Zhao, J.J.; Chai, F.; Li, Y.X.; Cui, S.C.; Sun, F.Y.; Wu, Z.Y.; et al. Regulation of RAGE splicing by hnRNP A1 and Tra2beta-1 and its potential role in AD pathogenesis. J. Neurochem. 2015, 133, 187–198. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, J.G.; Jong, Y.J.; Liu, T.Y.; Yuo, C.Y. High expression level of Tra2-beta1 is responsible for increased SMN2 exon 7 inclusion in the testis of SMA mice. PLoS ONE 2015, 10, e0120721. [Google Scholar] [CrossRef]
- Dominguez, C.E.; Cunningham, D.; Venkataramany, A.S.; Chandler, D.S. Heat increases full-length SMN splicing: Promise for splice-augmenting therapies for SMA. Hum. Genet. 2022, 141, 239–256. [Google Scholar] [CrossRef]
- Fischer, D.C.; Noack, K.; Runnebaum, I.B.; Watermann, D.O.; Kieback, D.G.; Stamm, S.; Stickeler, E. Expression of splicing factors in human ovarian cancer. Oncol. Rep. 2004, 11, 1085–1090. [Google Scholar] [CrossRef]
- Leclair, N.K.; Brugiolo, M.; Park, S.; Devoucoux, M.; Urbanski, L.; Angarola, B.L.; Yurieva, M.; Anczukow, O. Antisense oligonucleotide-mediated TRA2beta poison exon inclusion induces the expression of a lncRNA with anti-tumor effects. Nat. Commun. 2025, 16, 1670. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, X.; Sun, X.; Ning, Y.; Song, X.; Song, G.; Guo, X.; Sun, R. Tra2beta exerts tumor-promoting effects via GSK3/beta-catenin signaling in oral squamous cell carcinoma. Oral Dis. 2024, 30, 4956–4970. [Google Scholar] [CrossRef]
- Esakki, A.; Pandi, A.; Girija, S.A.S.; Jayaseelan, V.P. Correlating the genetic alterations and expression profile of the TRA2B gene in HNSCC and LUSC. Folia Medica 2024, 66, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Polo-Generelo, S.; Rodriguez-Mateo, C.; Torres, B.; Pintor-Tortolero, J.; Guerrero-Martinez, J.A.; Konig, J.; Vazquez, J.; Bonzon-Kulichenco, E.; Padillo-Ruiz, J.; de la Portilla, F.; et al. Serpine1 mRNA confers mesenchymal characteristics to the cell and promotes CD8+ T cells exclusion from colon adenocarcinomas. Cell Death Discov. 2024, 10, 116. [Google Scholar] [CrossRef]
- Lee, A.R.; Tangiyan, A.; Singh, I.; Choi, P.S. Incomplete paralog compensation generates selective dependency on TRA2A in cancer. PLoS Genet. 2025, 21, e1011685. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Sim, D.Y.; Zhen, Y.; Tian, H.; Koh, J.; Roca, X. PRPF40A induces inclusion of exons in GC-rich regions important for human myeloid cell differentiation. Nucleic Acids Res. 2024, 52, 8800–8814. [Google Scholar] [CrossRef] [PubMed]
- Ozyerli-Goknar, E.; Kala, E.Y.; Aksu, A.C.; Bulut, I.; Cingoz, A.; Nizamuddin, S.; Biniossek, M.; Seker-Polat, F.; Morova, T.; Aztekin, C.; et al. Epigenetic-focused CRISPR/Cas9 screen identifies (absent, small, or homeotic)2-like protein (ASH2L) as a regulator of glioblastoma cell survival. Cell Commun. Signal. 2023, 21, 328. [Google Scholar] [CrossRef]
- Karginov, T.A.; Menoret, A.; Leclair, N.K.; Harrison, A.G.; Chandiran, K.; Suarez-Ramirez, J.E.; Yurieva, M.; Karlinsey, K.; Wang, P.; O’Neill, R.J.; et al. Autoregulated splicing of TRA2beta programs T cell fate in response to antigen-receptor stimulation. Science 2024, 385, eadj1979. [Google Scholar] [CrossRef] [PubMed]
- Nian, F.; Wang, Y.; Yang, M.; Zhang, B. Identification the role of necroptosis in rheumatoid arthritis by WGCNA network. Autoimmunity 2024, 57, 2358069. [Google Scholar] [CrossRef]
- Wu, C.; Yang, L.; Tucker, D.; Dong, Y.; Zhu, L.; Duan, R.; Liu, T.C.; Zhang, Q. Beneficial Effects of Exercise Pretreatment in a Sporadic Alzheimer’s Rat Model. Med. Sci. Sports Exerc. 2018, 50, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yang, L.; Li, Y.; Dong, Y.; Yang, B.; Tucker, L.D.; Zong, X.; Zhang, Q. Effects of Exercise Training on Anxious-Depressive-like Behavior in Alzheimer Rat. Med. Sci. Sports Exerc. 2020, 52, 1456–1469, Erratum in Med. Sci. Sports Exerc. 2025, 57, 447. [Google Scholar] [CrossRef]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef]
- Monani, U.R.; Lorson, C.L.; Parsons, D.W.; Prior, T.W.; Androphy, E.J.; Burghes, A.H.; McPherson, J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999, 8, 1177–1183. [Google Scholar] [CrossRef]
- Kashima, T.; Rao, N.; David, C.J.; Manley, J.L. hnRNP A1 functions with specificity in repression of SMN2 exon 7 splicing. Hum. Mol. Genet. 2007, 16, 3149–3159. [Google Scholar] [CrossRef]
- Avila, A.M.; Burnett, B.G.; Taye, A.A.; Gabanella, F.; Knight, M.A.; Hartenstein, P.; Cizman, Z.; Di Prospero, N.A.; Pellizzoni, L.; Fischbeck, K.H.; et al. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J. Clin. Investig. 2007, 117, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Brichta, L.; Hofmann, Y.; Hahnen, E.; Siebzehnrubl, F.A.; Raschke, H.; Blumcke, I.; Eyupoglu, I.Y.; Wirth, B. Valproic acid increases the SMN2 protein level: A well-known drug as a potential therapy for spinal muscular atrophy. Hum. Mol. Genet. 2003, 12, 2481–2489. [Google Scholar] [CrossRef] [PubMed]
- Best, A.; Dagliesh, C.; Ehrmann, I.; Kheirollahi-Kouhestani, M.; Tyson-Capper, A.; Elliott, D.J. Expression of Tra2 beta in Cancer Cells as a Potential Contributory Factor to Neoplasia and Metastasis. Int. J. Cell Biol. 2013, 2013, 843781. [Google Scholar] [CrossRef]
- Watermann, D.O.; Tang, Y.; Zur Hausen, A.; Jager, M.; Stamm, S.; Stickeler, E. Splicing factor Tra2-beta1 is specifically induced in breast cancer and regulates alternative splicing of the CD44 gene. Cancer Res. 2006, 66, 4774–4780. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef]
- Preusser, M.; de Ribaupierre, S.; Wohrer, A.; Erridge, S.C.; Hegi, M.; Weller, M.; Stupp, R. Current concepts and management of glioblastoma. Ann. Neurol. 2011, 70, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, O.; Selheim, F.; Hernandez-Valladares, M.; Reikvam, H. Monocytic Differentiation in Acute Myeloid Leukemia Cells: Diagnostic Criteria, Biological Heterogeneity, Mitochondrial Metabolism, Resistance to and Induction by Targeted Therapies. Int. J. Mol. Sci. 2024, 25, 6356. [Google Scholar] [CrossRef]
- Stoilov, P.; Daoud, R.; Nayler, O.; Stamm, S. Human tra2-beta1 autoregulates its protein concentration by influencing alternative splicing of its pre-mRNA. Hum. Mol. Genet. 2004, 13, 509–524. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Puri, R.; Liu, K.; Nunez, I.; Chen, L.; Zheng, S. Molecular profiling of individual FDA-approved clinical drugs identifies modulators of nonsense-mediated mRNA decay. Mol. Ther. Nucleic Acids 2022, 27, 304–318. [Google Scholar] [CrossRef]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
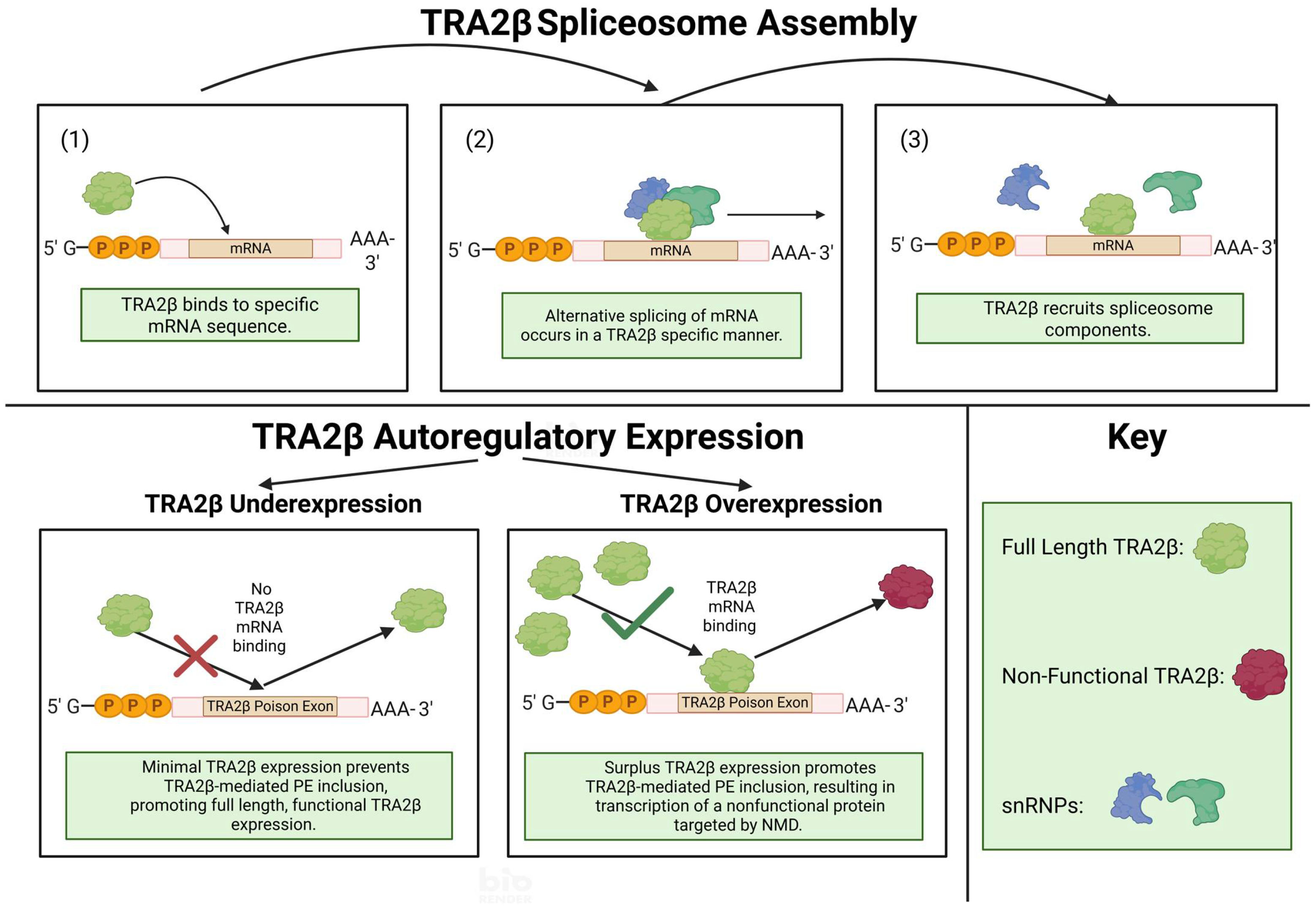
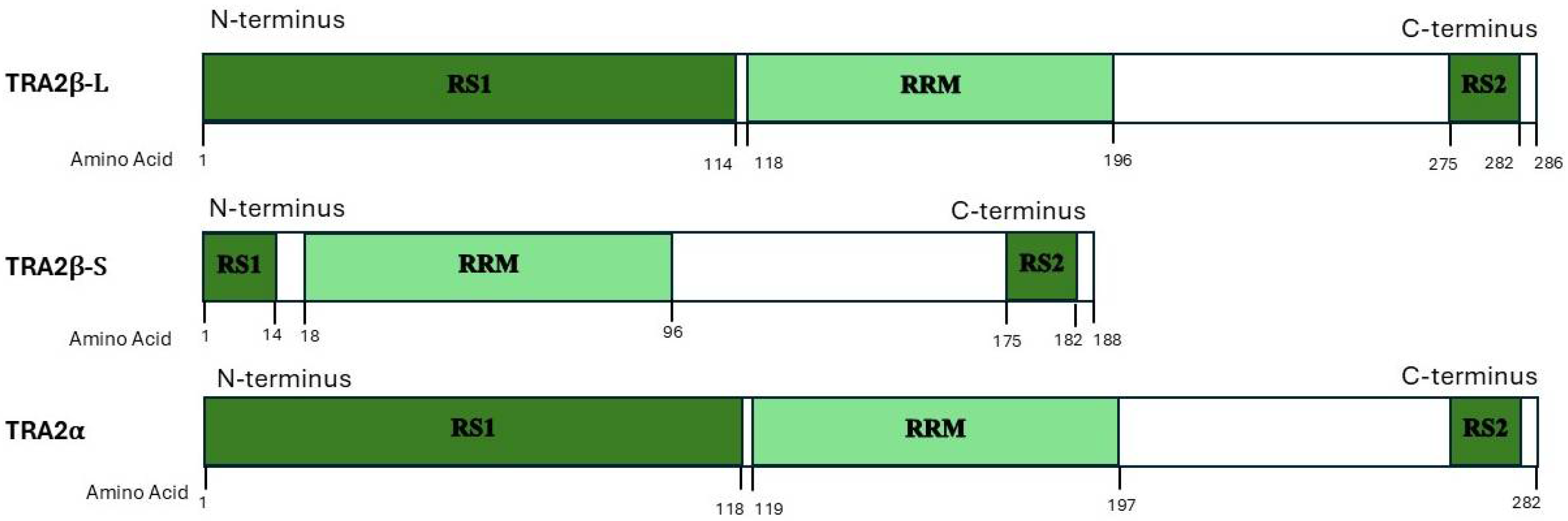
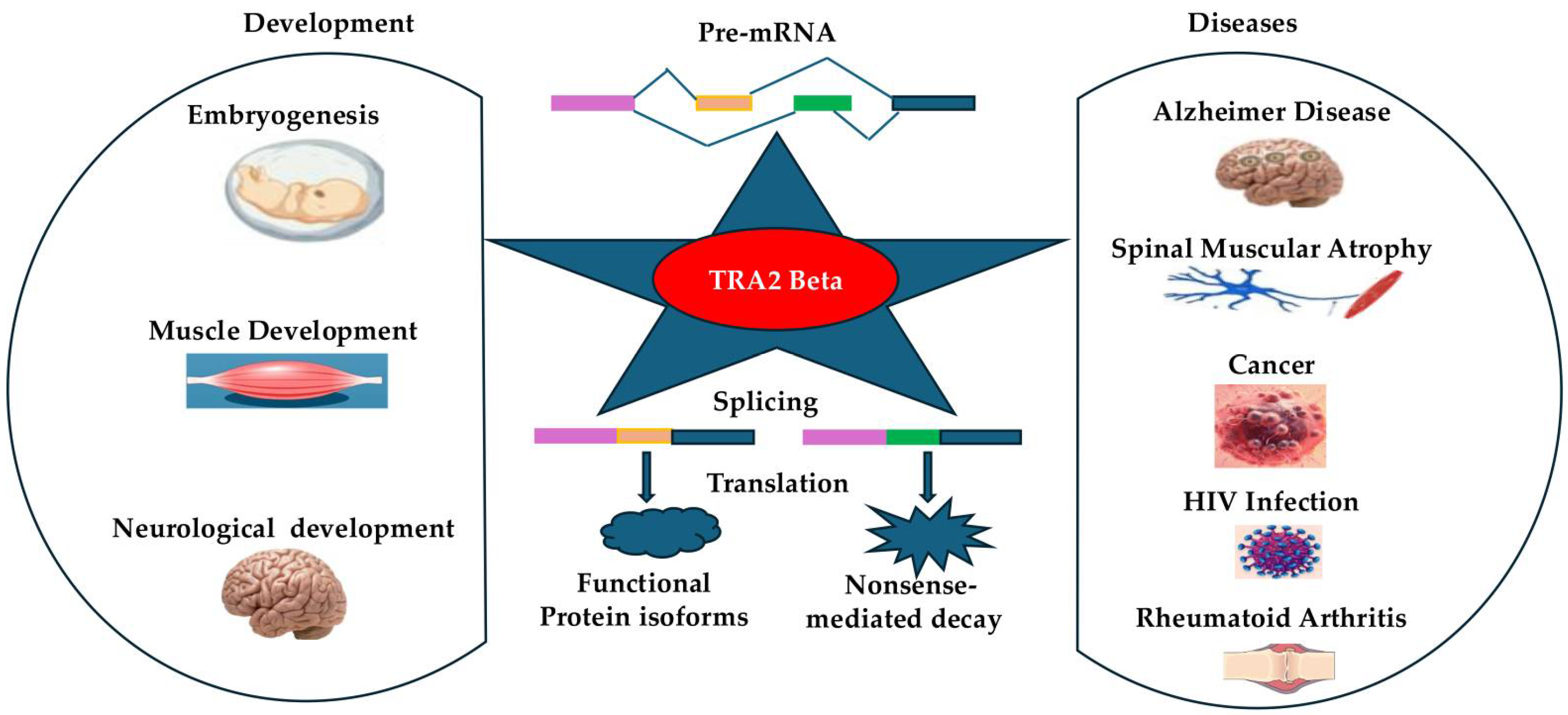
| Disease/Disorder | Organism/Cell Type | Relevant Mechanism | References |
|---|---|---|---|
| Neurodevelopmental Disease | Human-derived lymphoblastoid cells analyzed ex vivo. | Increased TRA2β-S and decreased TRA2β-L disrupts CHEK1 exon 3, resulting in developmental delay, epilepsy, seizures, and facial abnormalities. | [14,17] |
| Alzheimer’s Disease (AD) | Human neuroblastoma SH-SY5Y cells studied in vitro. | TRA2β reduces full-length mRAGE but increases truncated esRAGE, preventing Aβ-RAGE interaction, reducing AD pathogenesis. | [34] |
| Spinal Muscular Atrophy (SMA) | Mouse testis cell lines, GC-1 spermatogenia, SMN2 −/−; SMN2 +/+ spinal neuron cells and cortical neurons studied in vitro. | TRA2β promotes SMN2 exon 7 inclusion in vitro but shows limited in vivo effects unless highly overexpressed. | [35,36] |
| Ovarian Cancer (OC) | Human cervical carcinoma cell line HeLa (CCTCC CDC0009) studied in vitro. | TRA2β promotes progression by upregulating cell cycle/mitotic, and downregulates cell adhesion. | [23,37] |
| Squamous Cell Carcinoma (SCC) | Human MDA-MB231, T84, U87-MG, A375, NCI-H647, SK-OV-3, and 5637 cell lines studied in vitro. Additionally, human head and neck and lung squamous cell carcinoma from the Cancer Genome Atlas database studied in vivo | Upregulated TRA2β in SCC promotes tumor growth via splicing and proliferation and survival genes. | [38,39,40] |
| Adenocarcinoma | Normal mouse mammary gland epithelial cells and HEK293T, A549, LN229, and LN319 cell lines studied in vitro. | TRA2β influences splicing and tumor progression. It is suppressed by Serpine1. | [41,42] |
| Acute Myeloid Leukemia | Human HL-60 cells studied in vitro. | Competitively binds with PRPF40A, a known acute myeloid leukemia inhibitor. Relationship needs further characterization | [43] |
| Glioblastoma | U87MG, U373, and T98G human cell lines studied in vitro. | Reduced TRA2β leads to apoptosis and cell cycle arrest, reduced glioblastoma progression, | [44] |
| T Cell Differentiation | Mouse splenic OT-I cells in vivo/in vitro | TRA2β poison exon inclusion triggers T cell differentiation. | [45] |
| Rheumatoid Arthritis | Human rheumatoid arthritis patient immune cell from GEO database. | TRA2β promotes necroptosis and inflammation. | [46] |
| HIV Infection | HEK 293T cells transfected with pAdML dsx or pHxb R-/RII plasmids studied in vitro. | TRA2β suppresses HIV replication, reducing GAG and ENV protein levels. | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swarup, E.; Qiu, H. Dual Role of Transformer 2 Beta as Both a Developmental Necessity and a Disease Modulator. Int. J. Mol. Sci. 2025, 26, 8805. https://doi.org/10.3390/ijms26188805
Swarup E, Qiu H. Dual Role of Transformer 2 Beta as Both a Developmental Necessity and a Disease Modulator. International Journal of Molecular Sciences. 2025; 26(18):8805. https://doi.org/10.3390/ijms26188805
Chicago/Turabian StyleSwarup, Evan, and Hongyu Qiu. 2025. "Dual Role of Transformer 2 Beta as Both a Developmental Necessity and a Disease Modulator" International Journal of Molecular Sciences 26, no. 18: 8805. https://doi.org/10.3390/ijms26188805
APA StyleSwarup, E., & Qiu, H. (2025). Dual Role of Transformer 2 Beta as Both a Developmental Necessity and a Disease Modulator. International Journal of Molecular Sciences, 26(18), 8805. https://doi.org/10.3390/ijms26188805







