The Impact of Smoking-Associated Genetic Variants on Post-Exercise Heart Rate
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics and Lipid Profile
2.2. The Best-Fitting Genetic Models by SNPs
2.3. Association of Smoking-Related SNPs with Resting Heart Rate
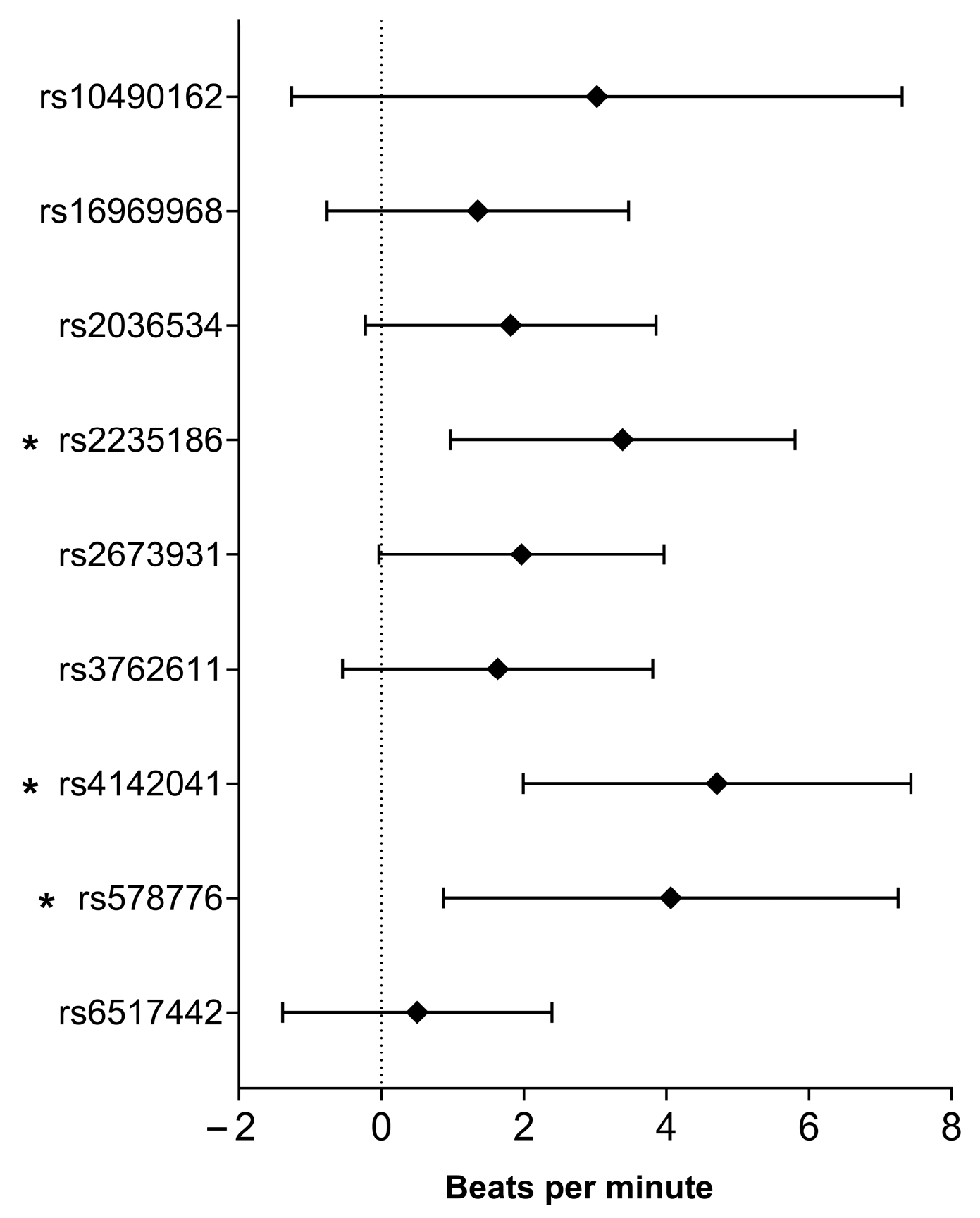
2.4. Association of Smoking-Related SNPs with Heart Rate After Exercise
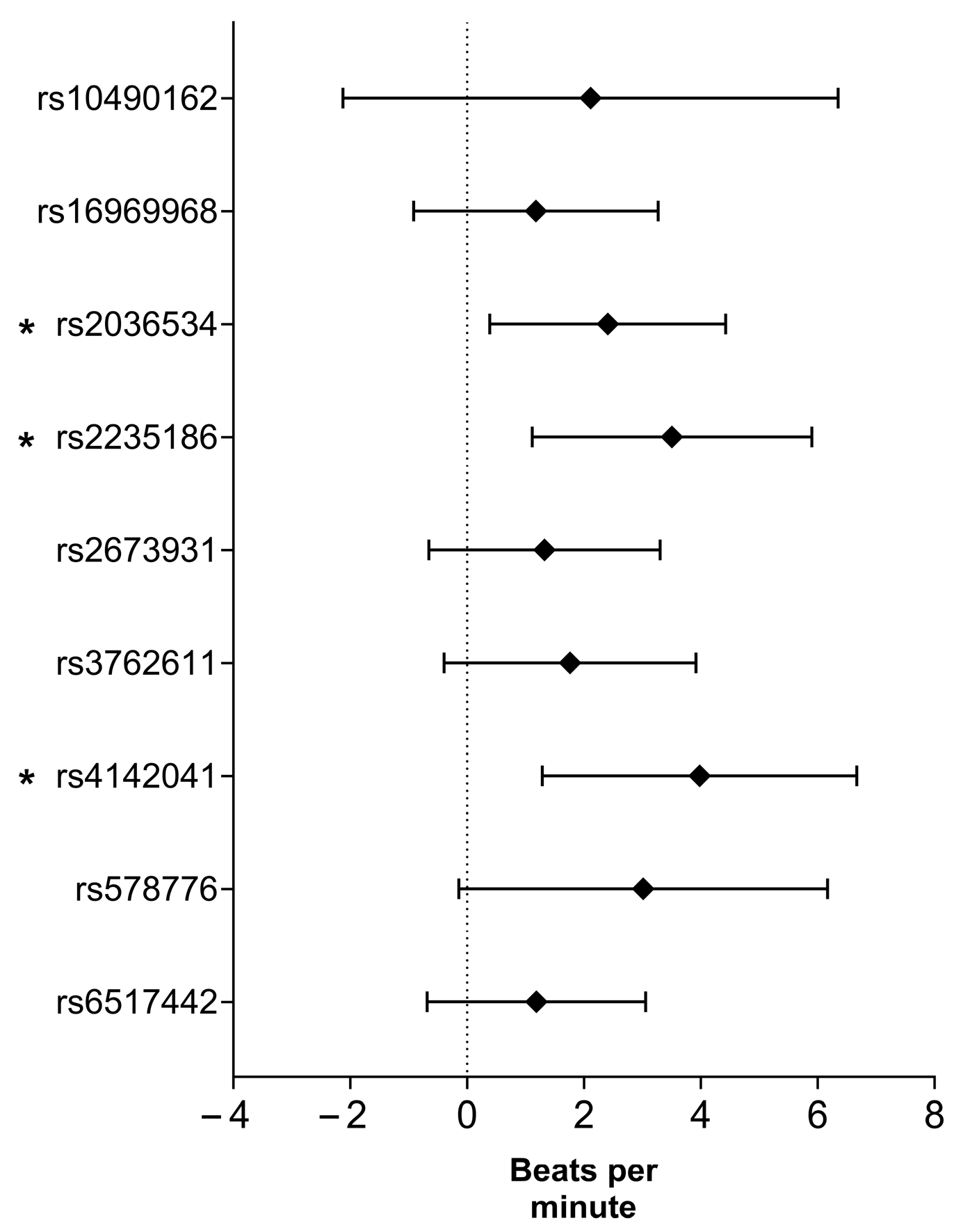
2.5. Association of Smoking-Related SNPs with Delta Heart Rate
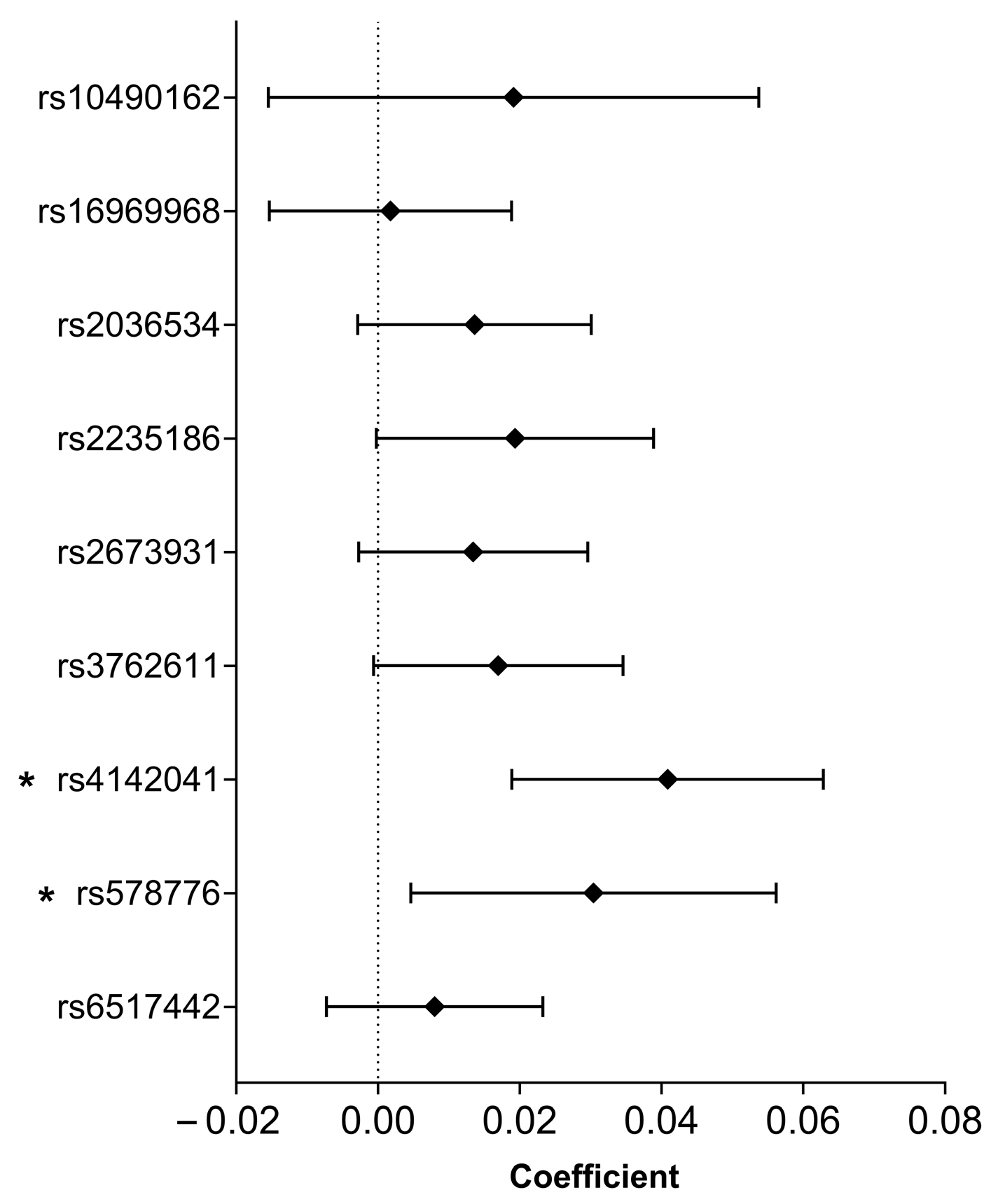
2.6. Association of Smoking-Related SNPs with Heart Rate Recovery Coefficient
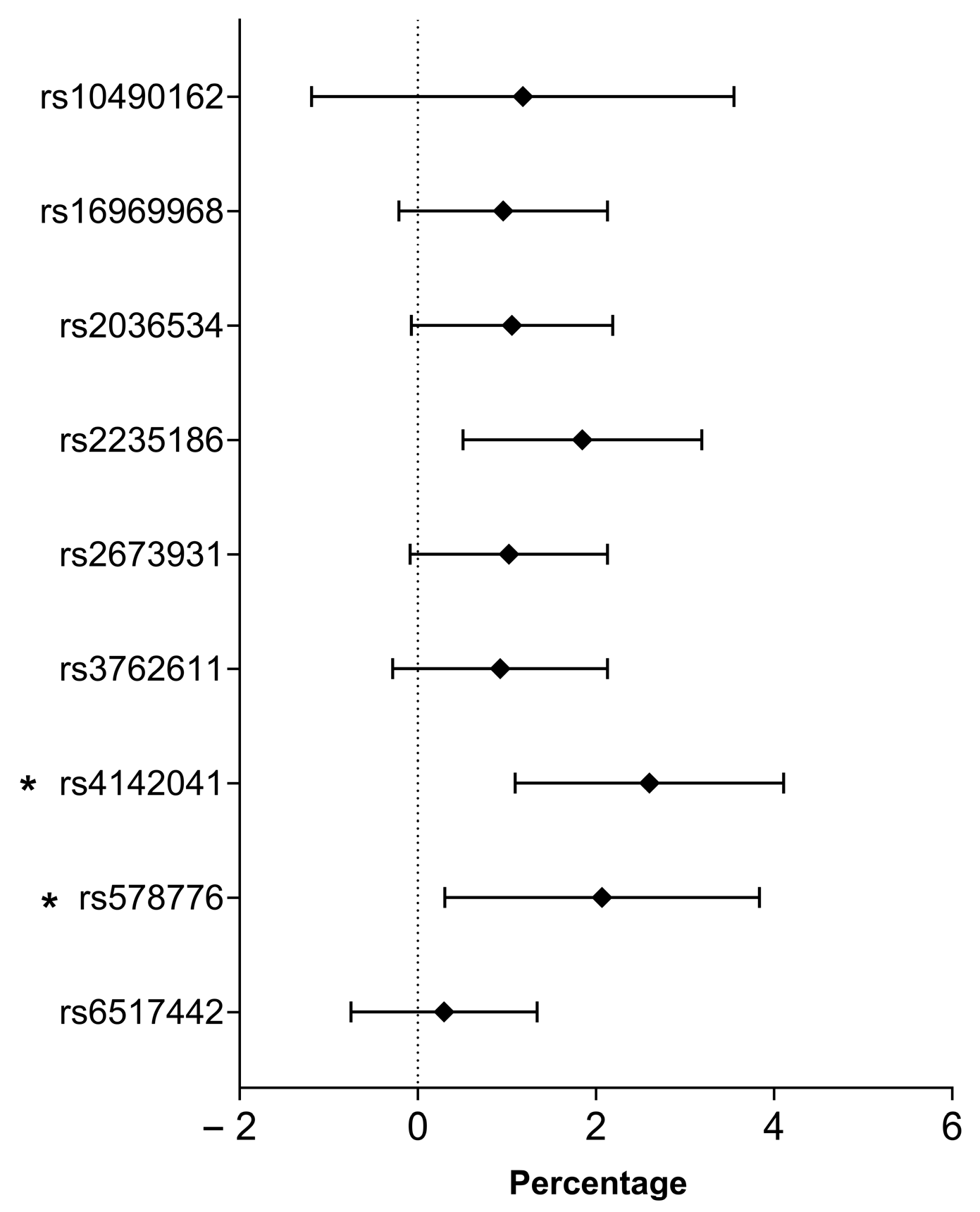
2.7. Association of Smoking-Related SNPs with the Percent of Predicted Maximum Heart Rate
2.8. Genetic Risk Score and Its Association with Heart Rate Change in Associated Parameters
3. Discussion
4. Materials and Methods
4.1. Study Design and Populations
4.2. DNA Extraction, SNP Selection, Testing Hardy–Weinberg Equilibrium, Linkage Disequilibrium, and Genotyping
4.3. Measurement of Heart Rate Responses to Physical Exertion
4.4. Calculation of the Individual Effect of SNPs and the Joint Effect Estimated by Genetic Risk Score
- (a)
- Codominant model: the homozygous genotype with the risk allele was coded as 2, the heterozygote as 1, and the homozygous genotype with no risk allele as 0.
- (b)
- Dominant model: genotypes with one or two risk alleles were coded as 2; those with no risk allele were coded as 0.
- (c)
- Recessive model: genotypes with two risk alleles were coded as 2; both the heterozygote and the homozygous genotype with no risk allele were coded as 0.
4.5. Statistical Analyses
4.6. Ethical Approval
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CAD | Coronary Artery Disease |
| CHRIS | Cooperative Health Research in South Tyrol |
| ΔHR | Delta Heart Rate |
| EDTA | Ethylenediaminetetraacetic Acid |
| GRS | Genetic Risk Score |
| GPMSSP | General Practitioners’ Morbidity Sentinel Stations Program |
| HDL | High-Density Lipoprotein |
| HOMA-IR | Homeostatic Model Assessment of Insulin Resistance |
| HR | Heart Rate |
| HRaft | Heart Rate after (exercise) |
| HRmax | Maximum Heart Rate |
| HRmax% | Percent of Predicted Maximum Heart Rate |
| HRR | Heart Rate Recovery |
| HRrest | Heart Rate at Rest |
| HRV | Heart Rate Variability |
| LDL | Low-Density Lipoprotein |
| MAF | Mutation Analysis Facility |
| SNP | Single Nucleotide Polymorphism |
| SPSS | Statistical Package for the Social Sciences |
References
- WHO. WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000–2025; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Rahman, M.; Alatiqi, M.; AL Jarallah, M.; Hussain, M.Y.; Monayem, A.; Panduranga, P.; Rajan, R. Cardiovascular Effects of Smoking and Smoking Cessation: A 2024 Update. Glob. Heart 2025, 20, 15. [Google Scholar] [CrossRef]
- World Health Organization. Tobacco Fact Sheet; World Health Organization: Geneva, Switzerland, 2025. [Google Scholar]
- WHO. Tobacco EURO—Regional Overview. Available online: https://www.who.int/europe/health-topics/tobacco (accessed on 17 July 2025).
- Ádány, R.; Pikó, P.; Fiatal, S.; Kósa, Z.; Sándor, J.; Bíró, É.; Kósa, K.; Paragh, G.; Bácsné Bába, É.; Veres-Balajti, I.; et al. Prevalence of Insulin Resistance in the Hungarian General and Roma Populations as Defined by Using Data Generated in a Complex Health (Interview and Examination) Survey. Int. J. Environ. Res. Public Health 2020, 17, 4833. [Google Scholar] [CrossRef]
- Kósa, Z.; Széles, G.; Kardos, L.; Kósa, K.; Németh, R.; Országh, S.; Fésüs, G.; McKee, M.; Adány, R.; Vokó, Z. A comparative health survey of the inhabitants of Roma settlements in Hungary. Am. J. Public Health 2007, 97, 853–859. [Google Scholar] [CrossRef]
- Ishida, M.; Sakai, C.; Kobayashi, Y.; Ishida, T. Cigarette Smoking and Atherosclerotic Cardiovascular Disease. J. Atheroscler. Thromb. 2024, 31, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Najem, B.; Houssière, A.; Pathak, A.; Janssen, C.; Lemogoum, D.; Xhaët, O.; Cuylits, N.; vad de Borne, P. Acute cardiovascular and sympathetic effects of nicotine replacement therapy. Hypertension 2006, 48, E23, Erratum in Hypertension 2006, 47, 1162. [Google Scholar] [CrossRef]
- Kucera, C.; Ramalingam, A.; Srivastava, S.; Bhatnagar, A.; Carll, A.P. Nicotine Formulation Influences the Autonomic and Arrhythmogenic Effects of Electronic Cigarettes. Nicotine Tob. Res. 2023, 26, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Murgia, F.; Melotti, R.; Foco, L.; Gögele, M.; Meraviglia, V.; Motta, B.; Steger, A.; Toifl, M.; Sinnecker, D.; Müller, A.; et al. Effects of smoking status, history and intensity on heart rate variability in the general population: The CHRIS study. PLoS ONE 2019, 14, e0215053. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Porchet, H.; Sheiner, L.; Jacob, P. Nicotine Absorption and Cardiovascular Effects with Smokeless Tobacco Use—Comparison with Cigarettes and Nicotine Gum. Clin. Pharmacol. Ther. 1988, 44, 23–28. [Google Scholar] [CrossRef]
- Barutcu, I.; Esen, A.M.; Kaya, D.; Turkmen, M.; Karakaya, O.; Melek, M.; Esen, O.B.; Basaran, Y. Cigarette smoking and heart rate variability: Dynamic influence of parasympathetic and sympathetic maneuvers. Ann. Noninvas Electro 2005, 10, 324–329. [Google Scholar] [CrossRef]
- Harte, C.B.; Meston, C.M. Effects of Smoking Cessation on Heart Rate Variability Among Long-Term Male Smokers. Int. J. Behav. Med. 2014, 21, 302–309. [Google Scholar] [CrossRef]
- Batra, V.; Patkar, A.A.; Berrettini, W.H.; Weinstein, S.P.; Leone, F.T. The genetic determinants of smoking. Chest 2003, 123, 1730–1739. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Liu, Q.; Li, M.; Yao, Y.H.; Qi, F.Y.; Xu, Y.; Lu, S.M.; Yang, Z.L.; Guan, Y.; Li, M.D.; et al. Determination of genetic correlation between tobacco smoking and coronary artery disease. Front. Psychiatry 2023, 14, 1279962. [Google Scholar] [CrossRef]
- van de Vegte, Y.J.; Tegegne, B.S.; Verweij, N.; Snieder, H.; van der Harst, P. Genetics and the heart rate response to exercise. Cell Mol. Life Sci. 2019, 76, 2391–2409. [Google Scholar] [CrossRef]
- Golosheykin, S.; Grant, J.D.; Novak, O.V.; Heath, A.C.; Anokhin, A.P. Genetic influences on heart rate variability. Int. J. Psychophysiol. 2017, 115, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Mason, A.M.; Bäck, M.; Klarin, D.; Damrauer, S.M.; Michaëlsson, K.; Burgess, S. Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur. Heart J. 2020, 41, 3304–3310. [Google Scholar] [CrossRef]
- Nolte, I.M.; Munoz, M.L.; Tragante, V.; Amare, A.T.; Jansen, R.; Vaez, A.; von der Heyde, B.; Avery, C.L.; Bis, J.C.; Dierckx, B.; et al. Genetic loci associated with heart rate variability and their effects on cardiac disease risk. Nat. Commun. 2017, 8, 16140, Erratum in Nat. Commun. 2017, 8, 15805. [Google Scholar] [CrossRef] [PubMed]
- Thom, C.S.; Ding, Z.R.; Levin, M.G.; Damrauer, S.M.; Lee, K.M.; Lynch, J.; Chang, K.M.; Tsao, P.S.; Cho, K.; Wilson, P.W.F.; et al. Genetic determinants of increased body mass index mediate the effect of smoking on increased risk for type 2 diabetes but not coronary artery disease. Hum. Mol. Genet. 2020, 29, 3327–3337. [Google Scholar] [CrossRef]
- Liu, M.Z.; Jiang, Y.; Wedow, R.; Li, Y.; Brazel, D.M.; Chen, F.; Datta, G.; Davila-Velderrain, J.; McGuire, D.; Tian, C.; et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 2019, 51, 237–244. [Google Scholar] [CrossRef]
- Ware, J.J.; van den Bree, M.; Munafò, M.R. From Men to Mice: Smoking Behavior and Disease. Nicotine Tob. Res. 2012, 14, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.N.; Wang, X.P.; Jia, J.Z.; Huang, T. Smoking Status and Type 2 Diabetes, and Cardiovascular Disease: A Comprehensive Analysis of Shared Genetic Etiology and Causal Relationship. Front. Endocrinol. 2022, 13, 809445. [Google Scholar] [CrossRef]
- Armani, C.; Landini, L.; Leone, A. Interactive Effect of Cigarette Smoking and Gene Variants for Predisposing to Cardiovascular Disease. Curr. Pharm. Design 2010, 16, 2531–2538. [Google Scholar] [CrossRef]
- Levin, M.G.; Klarin, D.; Assimes, T.L.; Freiberg, M.S.; Ingelsson, E.; Lynch, J.; Natarajan, P.; O’Donnell, C.; Rader, D.J.; Tsao, P.S.; et al. Genetics of Smoking and Risk of Atherosclerotic Cardiovascular Diseases A Mendelian Randomization Study. JAMA Netw. Open 2021, 4, e2034461. [Google Scholar] [CrossRef] [PubMed]
- Papathanasiou, G.; Georgakopoulos, D.; Georgoudis, G.; Spyropoulos, P.; Perrea, D.; Evangelou, A. Effects of chronic smoking on exercise tolerance and on heart rate-systolic blood pressure product in young healthy adults. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 646–652. [Google Scholar] [CrossRef]
- Dinas, P.C.; Koutedakis, Y.; Flouris, A.D. Effects of active and passive tobacco cigarette smoking on heart rate variability. Int. J. Cardiol. 2013, 163, 109–115. [Google Scholar] [CrossRef]
- den Hoed, M.; Eijgelsheim, M.; Esko, T.; Brundel, B.J.; Peal, D.S.; Evans, D.M.; Nolte, I.M.; Segrè, A.V.; Holm, H.; Handsaker, R.E.; et al. Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat. Genet. 2013, 45, 621–631. [Google Scholar] [CrossRef]
- Mason, D.A.; Moore, J.D.; Green, S.A.; Liggett, S.B. A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor. J. Biol. Chem. 1999, 274, 12670–12674. [Google Scholar] [CrossRef]
- Liu, X.H.; Liu, M.; Li, X.; Zhang, L.H.; Fan, R.; Wang, J. Prioritizing Genes Related to Nicotine Addiction Via a Multi-source-Based Approach. Mol. Neurobiol. 2015, 52, 442–455. [Google Scholar] [CrossRef]
- Sherva, R.; Wilhelmsen, K.; Pomerleau, C.S.; Chasse, S.A.; Rice, J.P.; Snedecor, S.M.; Bierut, L.J.; Neuman, R.J.; Pomerleau, O.F. Association of a single nucleotide polymorphism in neuronal acetylcholine receptor subunit alpha 5 (CHRNA5) with smoking status and with ‘pleasurable buzz’ during early experimentation with smoking. Addiction 2008, 103, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.E.; Bigdeli, T.B.; Ripke, S.; Bacanu, S.A.; Gejman, P.V.; Levinson, D.F.; Li, Q.S.; Rujescu, D.; Rietschel, M.; Weinberger, D.R.; et al. Genome-wide analyses of smoking behaviors in schizophrenia: Findings from the Psychiatric Genomics Consortium. J. Psychiatr. Res. 2021, 137, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Saccone, N.L.; Culverhouse, R.C.; Schwantes-An, T.H.; Cannon, D.S.; Chen, X.N.; Cichon, S.; Giegling, I.; Han, S.Z.; Han, Y.H.; Keskitalo-Vuokko, K.; et al. Multiple Independent Loci at Chromosome 15q25.1 Affect Smoking Quantity: A Meta-Analysis and Comparison with Lung Cancer and COPD. PLoS Genet. 2010, 6, e1001053. [Google Scholar] [CrossRef]
- Robinson, J.D.; Versace, F.; Lam, C.Y.; Minnix, J.A.; Engelmann, J.M.; Cui, Y.; Karam-Hage, M.; Shete, S.S.; Tomlinson, G.E.; Chen, T.T.; et al. The CHRNA3 rs578776 Variant is Associated with an Intrinsic Reward Sensitivity Deficit in Smokers. Front. Psychiatry 2013, 4, 114. [Google Scholar] [CrossRef]
- Armstrong, R.; Wheen, P.; Brandon, L.; Maree, A.; Kenny, R.A. Heart rate: Control mechanisms, pathophysiology and assessment of the neurocardiac system in health and disease. QJM-Int. J. Med. 2022, 115, 806–812. [Google Scholar] [CrossRef]
- Wen, L.; Jiang, K.; Yuan, W.; Cui, W.; Li, M.D. Contribution of Variants in CHRNA5/A3/B4 Gene Cluster on Chromosome 15 to Tobacco Smoking: From Genetic Association to Mechanism. Mol. Neurobiol. 2016, 53, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Higashikuni, Y. Self-DNA sensing in cigarette smoke-induced vascular inflammation: The role of mitochondrial DNA release in vascular endothelial cells. Hypertens. Res. 2024, 47, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Linneberg, A.; Jacobsen, R.K.; Skaaby, T.; Taylor, A.E.; Fluharty, M.E.; Jeppesen, J.L.; Bjorngaard, J.H.; Åsvold, B.O.; Gabrielsen, M.E.; Campbell, A.; et al. Effect of Smoking on Blood Pressure and Resting Heart Rate: A Mendelian Randomization Meta-Analysis in the CARTA Consortium. Circ. Cardiovasc. Genet. 2015, 8, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.F.; Yin, R.X.; Deng, J.L. Homocysteine, hyperhomocysteinemia, and H-type hypertension. Eur. J. Prev. Cardiol. 2024, 31, 1092–1103. [Google Scholar] [CrossRef]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Weinert, D.; Gubin, D. The Impact of Physical Activity on the Circadian System: Benefits for Health, Performance and Wellbeing. Appl. Sci. 2022, 12, 9220. [Google Scholar] [CrossRef]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac Parasympathetic Reactivation Following Exercise: Implications for Training Prescription. Sports Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef]
- Zhou, W.; Kanai, M.; Wu, K.H.H.; Rasheed, H.; Tsuo, K.; Hirbo, J.B.; Wang, Y.; Bhattacharya, A.; Zhao, H.L.; Namba, S.; et al. Global Biobank Meta-analysis Initiative: Powering genetic discovery across human disease. Cell Genom. 2022, 2, 100192. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Richmond, R.C.; Sanderson, E. Methods and practical considerations for performing Mendelian randomization. Int. J. Epidemiol. 2022, 51, 2031–2034. [Google Scholar] [CrossRef]
- Cao, S.; Zeng, Y.; Pang, K.; Chen, M.; Guo, R.; Wu, N.; Fang, C.; Deng, H.; Zhang, X.; Xie, X.; et al. Unraveling the causal impact of smoking and its DNA methylation signatures on cardiovascular disease: Mendelian randomization and colocalization analysis. Clin. Epigenet. 2025, 17, 1. [Google Scholar] [CrossRef]
- Ye, X.; Wu, Q.; Lv, Q.; Hou, X.; Yang, Y.; Yang, C.; Wang, S. Smoking, Alcohol Consumption, and Atrial Fibrillation: Mendelian Randomization Study. Cardiovasc. Toxicol. 2025, 25, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.I.; Mascarenhas, J. A smoking gun? Clonal expansion in response to cigarette exposure. Front. Oncol. 2023, 13, 1252643. [Google Scholar] [CrossRef]
- Piko, P.; Kosa, Z.; Sandor, J.; Seres, I.; Paragh, G.; Adany, R. The profile of HDL-C subfractions and their association with cardiovascular risk in the Hungarian general and Roma populations. Sci. Rep. 2022, 12, 10915. [Google Scholar] [CrossRef]
- Szeles, G.; Voko, Z.; Jenei, T.; Kardos, L.; Pocsai, Z.; Bajtay, A.; Papp, E.; Pasti, G.; Kosa, Z.; Molnar, I.; et al. A preliminary evaluation of a health monitoring programme in Hungary. Eur. J. Public Health 2005, 15, 26–32. [Google Scholar] [CrossRef]
- Fiatal, S.; Tóth, R.; Moravcsik-Kornyicki, A.; Kósa, Z.; Sándor, J.; McKee, M.; Adány, R. High Prevalence of Smoking in the Roma Population Seems to Have No Genetic Background. Nicotine Tob. Res. 2016, 18, 2260–2267. [Google Scholar] [CrossRef]
- Molina, G.E.; Fontana, K.E.; Porto, L.G.G.; Junqueira, L.F. Post-exercise heart-rate recovery correlates to resting heart-rate variability in healthy men. Clin. Auton. Res. 2016, 26, 415–421. [Google Scholar] [CrossRef]
- FOX III, S. Physical activity and the prevention of coronary heart disease. Ann. Clin. Res. 1971, 3, 404–432. [Google Scholar] [CrossRef]
- Salanti, G.; Zeggini, E.; Ioannidis, J.P.A. Re: “Underlying Genetic Models of Inheritance in Established Type 2 Diabetes Associations”—Three Authors Reply. Am. J. Epidemiol. 2010, 171, 1155–1156. [Google Scholar] [CrossRef][Green Version]
- Templeton, G.F.; Brian Pope, M.; Burney, L.L. The Usefulness of the Two-Step Normality Transformation in Retesting Existing Theories: Evidence on the Productivity Paradox. Data Base Adv. Inf. Sy 2021, 52, 53–64. [Google Scholar] [CrossRef]
- Pikó, P.; Al Ashkar, H.; Kovács, N.; Veres-Balajti, I.; Adány, R. Genetic Background of Acute Heart Rate Response to Exercise. Int. J. Mol. Sci. 2024, 25, 3238. [Google Scholar] [CrossRef] [PubMed]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Jouven, X.; Empana, J.P.; Schwartz, P.J.; Desnos, M.; Courbon, D.; Ducimetière, P. Heart-rate profile during exercise as a predictor of sudden death. N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef]

| Non-Smokers (n = 330) | Smokers (n = 331) | p-Value | ||
|---|---|---|---|---|
| Average (95% CI) | ||||
| Age (years) | 43.68 (42.29–45.08) | 42.49 (41.16–43.82) | 0.182 | |
| Waist circumference (cm) | 97.22 (95.55–98.88) | 92.71 (90.96–94.45) | <0.001 * | |
| BMI (kg/m2) | 27.96 (27.32–28.59) | 26.51 (25.83–27.19) | 0.001 * | |
| Systolic blood pressure (mmHg) | 126.24 (124.67–127.82) | 124.24 (122.26–126.21) | 0.048 * | |
| Diastolic blood pressure (mmHg) | 79.90 (78.99–80.82) | 78.62 (77.48–79.76) | 0.035 * | |
| Homa-IR | 4.37 (3.61–5.14) | 3.70 (3.10–4.31) | 0.046 * | |
| Domains of physical activity | Work (MET-min/week) | 4881.90 (4229.64–5534.16) | 4924.11 (4251.23–5596.99) | 0.516 |
| Transport (MET-min/week) | 1345.26 (1153.32–1537.20) | 1669.42 (1430.87–1907.98) | 0.007 * | |
| Domestic (MET-min/week) | 2930.61 (2616.53–3244.68) | 2976.23 (2654.54–3297.93) | 0.790 | |
| Leisure-time (MET-min/week) | 1340.36 (1137.50–1543.22) | 1007.11 (819.53–1194.69) | <0.001 * | |
| Sitting time (min/week) | 529.29 (479.46–579.11) | 413.44 (383.16–443.73) | 0.001 * | |
| Low-density lipoprotein cholesterol (mmol/L) | 3.11 (3.01–3.21) | 3.15 (3.04–3.25) | 0.517 | |
| Triglycerides (mmol/L) | 1.56 (1.44–1.67) | 1.55 (1.44–1.66) | 0.750 | |
| High-density lipoprotein cholesterol (mmol/L) | 1.37 (1.33–1.42) | 1.26 (1.22–1.30) | <0.001 * | |
| Resting heart rate (bpm) | 77.20 (76.13–78.27) | 77.61 (76.43–78.79) | 0.808 | |
| Heart rate after exercise (bpm) | 109.79 (107.38–112.21) | 112.25 (108.94 115.55) | 0.378 | |
| Delta heart rate (bpm) | 32.59 (30.30–34.88) | 34.64 (31.45–37.82) | 0.171 | |
| Heart rate after 5 min (bpm) | 91.92 (90.24–93.60) | 94.55 (92.59–96.51) | 0.332 | |
| Heart rate after 10 min (bpm) | 81.13 (79.95–82.31) | 82.85 (81.44–84.25) | 0.213 | |
| Heart rate recovery coefficient | 0.23 (0.21–0.26) | 0.23 (0.21–0.25) | 0.338 | |
| Maximum heart rate expressed as percentage | 62.60 (61.15–64.05) | 63.46 (61.57–65.36) | 0.446 | |
| Average prevalence in % (95% CI) | p-value | |||
| Women | 63.03 (57.73–68.11) | 68.81 (63.51–73.77) | 0.123 | |
| Roma | 32.12 (27.26–37.30) | 69.13 (63.84–74.07) | <0.001 * | |
| Financial status | Bad | 14.85 (11.33–18.98) | 25.72 (21.11–30.79) | <0.001 * |
| Average | 55.15 (49.76–60.45) | 56.59 (51.04–62.02) | ||
| Good | 30.00 (25.25–35.10) | 17.68 (13.75–22.21) | ||
| Education | Less than primary and primary | 36.36 (31.31–41.65) | 71.70 (66.51–76.49) | <0.001 * |
| Vocational and high school | 48.79 (43.43–54.17) | 24.76 (20.21–29.77) | ||
| College and university | 14.85 (11.33–18.98) | 3.54 (1.89–6.04) | ||
| Alcohol consumption | Less than 1 time per month | 49.09 (43.72–54.47) | 50.80 (45.26–56.33) | 0.641 |
| 1 time per month | 33.03 (28.12–38.24) | 34.08 (28.98–39.48) | ||
| More than 2 times per month | 17.88 (14.03–22.28) | 15.11 (11.46–19.41) | ||
| Anti-hypertensive medication | 31.82 (26.97–36.99) | 26.37 (21.70–31.47) | 0.129 | |
| Anti-diabetic medication | 7.27 (4.84–10.45) | 9.00 (6.20–12.56) | 0.423 | |
| Lipid-lowering medication | 9.39 (6.60–12.90) | 9.32 (6.47–12.93) | 0.976 | |
| SNP (Risk Allele) | Inheritance Model | B (95% CI) | p-Value | R2 |
|---|---|---|---|---|
| rs10490162 (C) | Recessive | 0.542 (−7.295–8.379) | 0.892 | 0.174 |
| Codominant | 1.820 (−2.426–6.065) | 0.370 | 0.175 | |
| Dominant | 1.080 (−1.285–3.446) | 0.400 | 0.175 | |
| rs16969968 (A) | Recessive | 0.797 (−2.102–3.696) | 0.589 | 0.175 |
| Codominant | 1.833 (−0.947–4.613) | 0.196 | 0.176 | |
| Dominant | 1.340 (−0.540–3.219) | 0.162 | 0.177 | |
| rs2036534 (C) | Recessive | 0.808 (−3.255–4.871) | 0.696 | 0.174 |
| Codominant | 2.600 (−0.518–5.717) | 0.102 | 0.178 | |
| Dominant | 1.765 (−0.140–3.670) | 0.069 | 0.179 | |
| rs2235186 (G) | Recessive | 1.522 (−0.393–3.437) | 0.119 | 0.177 |
| Codominant | 3.241 (0.830–5.652) | 0.008 | 0.184 | |
| Dominant | 4.059 (1.654–6.464) | 9.74 × 10−4 | 0.189 | |
| rs2673931 (T) | Recessive | 1.198 (−0.805–3.200 | 0.236 | 0.176 |
| Codominant | 1.650 (−1.084–4.385) | 0.241 | 0.176 | |
| Dominant | 0.870 (−1.602–3.341) | 0.490 | 0.175 | |
| rs3762611 (G) | Recessive | 2.408 (0.252–4.565) | 0.029 | 0.181 |
| Codominant | 3.479 (−0.134–7.092 | 0.059 | 0.179 | |
| Dominant | 0.441 (−4.798–5.679) | 0.869 | 0.174 | |
| rs4142041 (A) | Recessive | 0.497 (−1.410–2.404) | 0.609 | 0.175 |
| Codominant | 2.427 (−0.301–5.155) | 0.081 | 0.178 | |
| Dominant | 3.825 (1.110–6.541) | 0.006 | 0.185 | |
| rs578776 (G) | Recessive | −0.019 (−1.897–1.859) | 0.984 | 0.174 |
| Codominant | 1.229 (−1.460–3.919) | 0.370 | 0.175 | |
| Dominant | 2.661 (−0.109–5.431) | 0.060 | 0.179 | |
| rs6517442 (C) | Recessive | 1.265 (−1.836–4.366) | 0.423 | 0.175 |
| Codominant | 2.761 (−0.070–5.592) | 0.056 | 0.179 | |
| Dominant | 1.948 (0.079–3.817) | 0.041 | 0.180 |
| Genetic Risk Score | p for Trend | |||
|---|---|---|---|---|
| 0–2 (n = 41) | 4 (n = 219) | 6 (n = 381) | ||
| Average (95% CI) | ||||
| HRrest | 75.32 (72.69–77.95) | 77.03 (75.63–78.42) | 77.84 (76.81–78.87) | 0.253 |
| HRaft | 98.83 (91.72–105.94) | 105.75 (102.75–108.76) | 115.30 (112.53–118.07) | 1.47 × 10−8 ** |
| ΔHR | 23.51 (16.87–30.16) | 28.73 (26.02–31.43) | 37.46 (34.74–40.18) | 8.12 × 10−7 ** |
| HRR | 0.14 (0.10–0.19) | 0.19 (0.17–0.22) | 0.26 (0.24–0.29) | 6.19 × 10−8 ** |
| HRmax% | 55.74 (51.77–59.72) | 60.20 (58.37–62.02) | 65.42 (63.83–67.02) | 6.34 × 10−8 ** |
| B (95% CI) | p-Value | |
|---|---|---|
| HRrest | 0.301 (−0.322–0.925) | 0.343 |
| HRaft | 3.986 (2.486–5.486) | 2.47 × 10−7 ** |
| ΔHR | 1.640 (0.676–2.604) | 8.86 × 10−4 ** |
| HRR | 0.028 (0.016–0.040) | 5.42 × 10−6 ** |
| HRmax% | 2.193 (1.362–3.023) | 2.93 × 10−7 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Ashkar, H.; Kharrat Helu, N.; Kovacs, N.; Fiatal, S.; Adany, R.; Piko, P. The Impact of Smoking-Associated Genetic Variants on Post-Exercise Heart Rate. Int. J. Mol. Sci. 2025, 26, 8787. https://doi.org/10.3390/ijms26188787
Al Ashkar H, Kharrat Helu N, Kovacs N, Fiatal S, Adany R, Piko P. The Impact of Smoking-Associated Genetic Variants on Post-Exercise Heart Rate. International Journal of Molecular Sciences. 2025; 26(18):8787. https://doi.org/10.3390/ijms26188787
Chicago/Turabian StyleAl Ashkar, Habib, Nihad Kharrat Helu, Nora Kovacs, Szilvia Fiatal, Roza Adany, and Peter Piko. 2025. "The Impact of Smoking-Associated Genetic Variants on Post-Exercise Heart Rate" International Journal of Molecular Sciences 26, no. 18: 8787. https://doi.org/10.3390/ijms26188787
APA StyleAl Ashkar, H., Kharrat Helu, N., Kovacs, N., Fiatal, S., Adany, R., & Piko, P. (2025). The Impact of Smoking-Associated Genetic Variants on Post-Exercise Heart Rate. International Journal of Molecular Sciences, 26(18), 8787. https://doi.org/10.3390/ijms26188787







