Are Procoagulant Platelets an Emerging Therapeutic Target? A General Review with an Emphasis on Their Clinical Significance in Companion Animals
Abstract
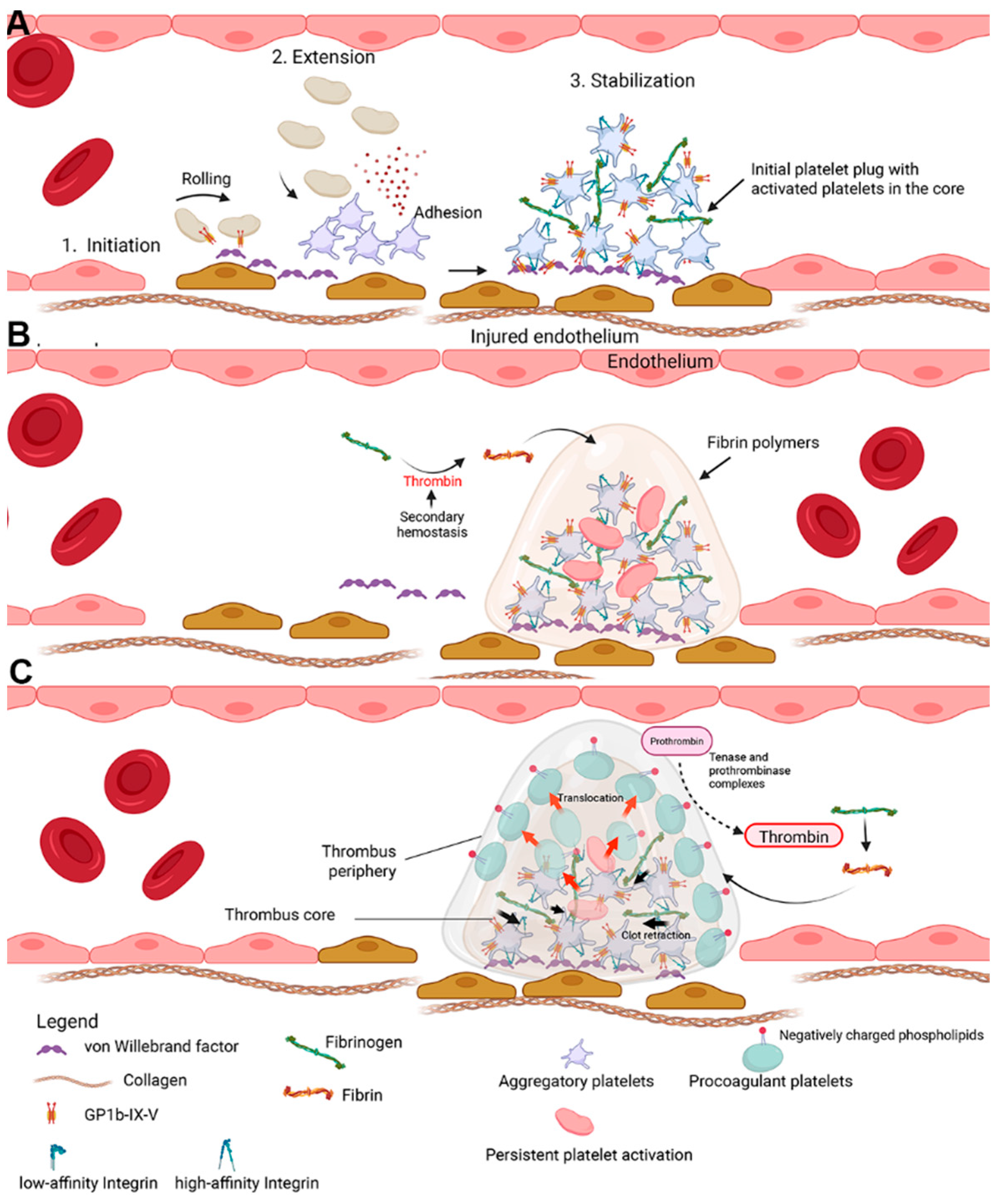
1. Revisiting the Role of Platelets in Thrombus Formation
Species Differences
2. History of Procoagulant Platelets
3. Mechanisms of Procoagulant Platelet Formation
3.1. Cytosolic Calcium Elevation via Both Store and Non-Store Operated Calcium Entry Mechanisms
3.2. Role of Mitochondria in Procoagulant Platelet Generation
4. Characteristics of Procoagulant Platelets
4.1. Affinity for Integrin αIIbβ3
4.2. Phosphatidylserine Exposure
4.3. Morphology
4.4. Agonist Stimulation
5. Clinical Relevance
5.1. Procoagulant Platelets in Cardiovascular Diseases
5.2. Procoagulant Platelets in Thromboinflammation
5.3. Procoagulant Platelets in Veterinary Medicine
5.4. From Being Procoagulant to Exhausted: The Spectrum of Platelet Activation
6. Laboratory and Clinical Assessment of Procoagulant Platelets
6.1. Procoagulant Platelets as Potential Biomarkers
6.2. P-Selectin
6.3. Phosphatidylserine Detection
6.4. Decreased αIIbβ3 Integrin Affinity
6.5. Alternative Markers for Studying Procoagulant Platelets
6.5.1. Inner Mitochondrial Membrane Potential (Δψm)
6.5.2. 4-[N-(S-Glutathionylacetyl)amino]phenylarsonous Acid (GSAO)
6.5.3. Fibrinogen Binding
6.5.4. Platelet-Derived Microvesicles
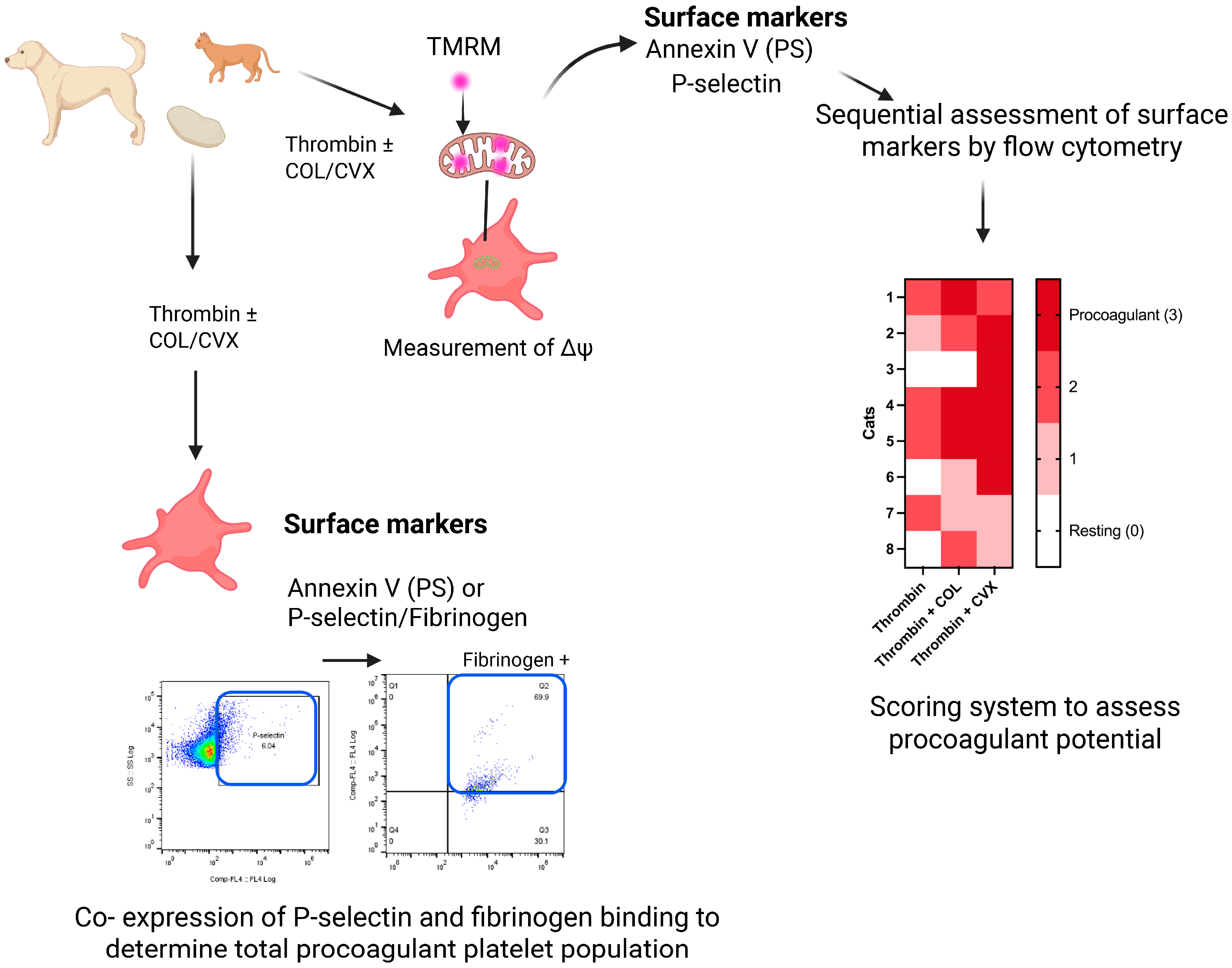
6.6. Laboratory Detection of Procoagulant Platelets in Veterinary Medicine
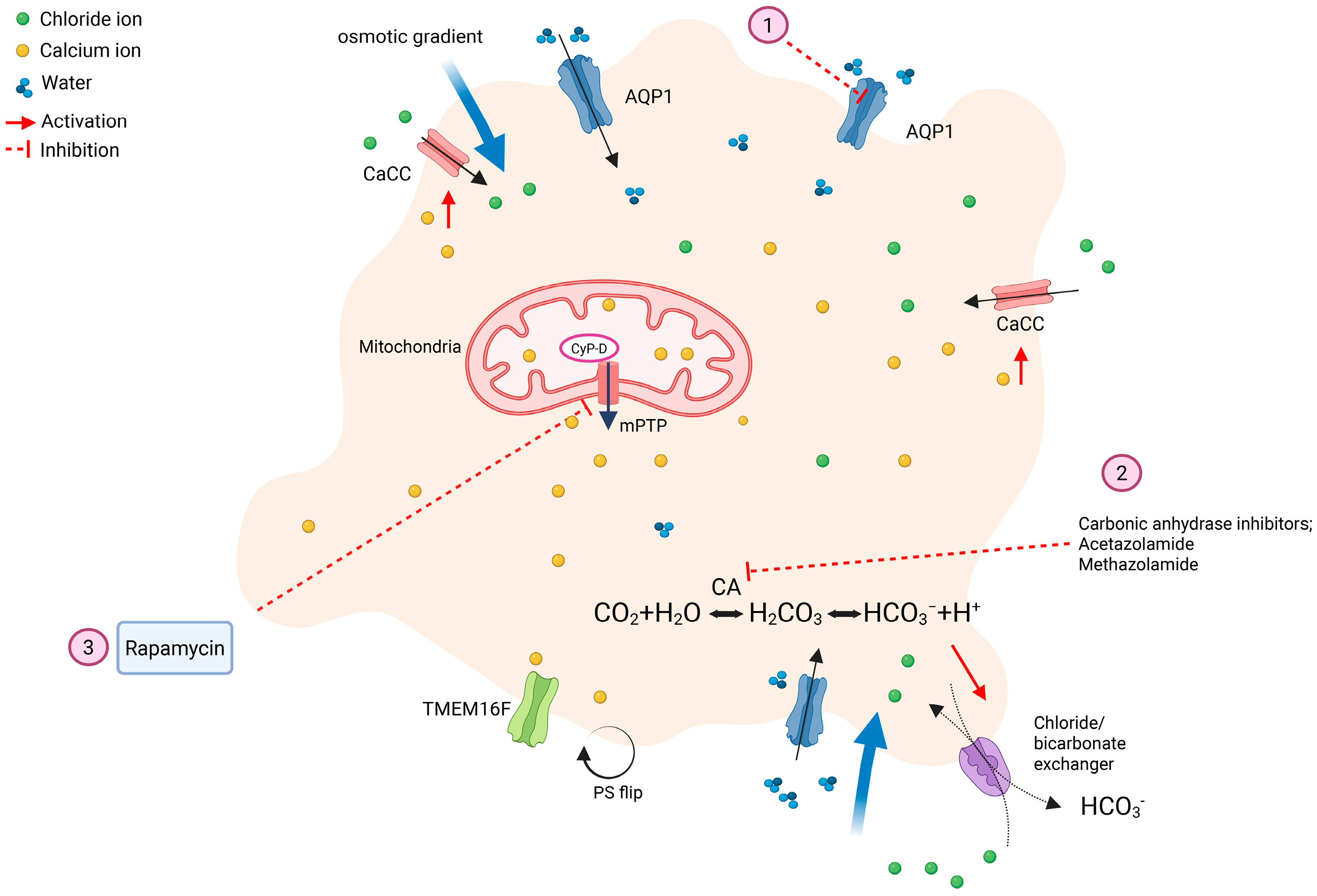
7. Novel Anticoagulant Therapies: Targeting Procoagulant Platelets
7.1. Carbonic Anhydrase Inhibitors
7.2. Aquaporin-1
7.3. Rapamycin
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 319–327. [Google Scholar] [PubMed]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Agbani, E.O.; Poole, A.W. Procoagulant platelets: Generation, function, and therapeutic targeting in thrombosis. Blood 2017, 130, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Monroe, D.M.; Hoffman, M.; Roberts, H.R. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1381–1389. [Google Scholar] [CrossRef]
- Nechipurenko, D.Y.; Receveur, N.; Yakimenko, A.O.; Shepelyuk, T.O.; Yakusheva, A.A.; Kerimov, R.R.; Obydennyy, S.I.; Eckly, A.; Léon, C.; Gachet, C.; et al. Clot Contraction Drives the Translocation of Procoagulant Platelets to Thrombus Surface. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 37–47. [Google Scholar] [CrossRef]
- Pasalic, L.; Wing-Lun, E.; Lau, J.K.; Campbell, H.; Pennings, G.J.; Lau, E.; Connor, D.; Liang, H.P.; Muller, D.; Kritharides, L.; et al. Novel assay demonstrates that coronary artery disease patients have heightened procoagulant platelet response. J. Thromb. Haemost. 2018, 16, 1198–1210. [Google Scholar] [CrossRef]
- Prodan, C.I.; Dale, G.L. Coated-platelets in ischemic stroke—Potential insight into the etiology of stroke subtypes. Int. J. Stroke 2008, 3, 249–250. [Google Scholar] [CrossRef]
- Prodan, C.I.; Joseph, P.M.; Vincent, A.S.; Dale, G.L. Coated-platelets in ischemic stroke: Differences between lacunar and cortical stroke. J. Thromb. Haemost. 2008, 6, 609–614. [Google Scholar] [CrossRef]
- Prodan, C.I.; Stoner, J.A.; Cowan, L.D.; Dale, G.L. Higher coated-platelet levels are associated with stroke recurrence following nonlacunar brain infarction. J. Cereb. Blood Flow. Metab. 2013, 33, 287–292. [Google Scholar] [CrossRef]
- Tablin, F.; Walker, N.J.; Klein, S.D.; Field, C.L.; Crowe, J.H. Animal models for studies on cold-induced platelet activation in human beings. J. Lab. Clin. Med. 2000, 135, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Oshinowo, O.; Copeland, R.; Sakurai, Y.; Fay, M.E.; Petrich, B.G.; Leong, T.; Brainard, B.; Lam, W.A. Significant differences in single-platelet biophysics exist across species but attenuate during clot formation. Blood Adv. 2021, 5, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Sophocleous, R.A.; Curtis, S.J.; Curtis, B.L.; Ooi, L.; Sluyter, R. P2Y1 and P2Y12 Receptors Mediate Aggregation of Dog and Cat Platelets: A Comparison to Human Platelets. Int. J. Mol. Sci. 2025, 26, 1206. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P.K.; Kim, S. Characterization of the distinct mechanism of agonist-induced canine platelet activation. J. Vet. Sci. 2019, 20, 10–15. [Google Scholar] [CrossRef]
- Cremer, S.E.; Catalfamo, J.L.; Goggs, R.; Seemann, S.E.; Kristensen, A.T.; Szklanna, P.B.; Maguire, P.B.; Brooks, M.B. The canine activated platelet secretome (CAPS): A translational model of thrombin-evoked platelet activation response. Res. Pract. Thromb. Haemost. 2021, 5, 55–68. [Google Scholar] [CrossRef]
- Bevers, E.M.; Comfurius, P.; van Rijn, J.L.; Hemker, H.C.; Zwaal, R.F. Generation of prothrombin-converting activity and the exposure of phosphatidylserine at the outer surface of platelets. Eur. J. Biochem. 1982, 122, 429–436. [Google Scholar] [CrossRef]
- Bevers, E.M.; Comfurius, P.; Zwaal, R.F. Changes in membrane phospholipid distribution during platelet activation. Biochim. Biophys. Acta 1983, 736, 57–66. [Google Scholar] [CrossRef]
- Coller, B.S. Historical perspective and future directions in platelet research. J. Thromb. Haemost. 2011, 9 (Suppl. 1), 374–395. [Google Scholar] [CrossRef]
- Zwaal, R.F.; Comfurius, P.; Bevers, E.M. Mechanism and function of changes in membrane-phospholipid asymmetry in platelets and erythrocytes. Biochem. Soc. Trans. 1993, 21, 248–253. [Google Scholar] [CrossRef]
- Brooks, M.B.; Catalfamo, J.L.; Brown, H.A.; Ivanova, P.; Lovaglio, J. A hereditary bleeding disorder of dogs caused by a lack of platelet procoagulant activity. Blood 2002, 99, 2434–2441. [Google Scholar] [CrossRef]
- Weiss, H.J.; Vicic, W.J.; Lages, B.A.; Rogers, J. Isolated deficiency of platelet procoagulant activity. Am. J. Med. 1979, 67, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Alberio, L.; Safa, O.; Clemetson, K.J.; Esmon, C.T.; Dale, G.L. Surface expression and functional characterization of alpha-granule factor V in human platelets: Effects of ionophore A23187, thrombin, collagen, and convulxin. Blood 2000, 95, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Jackson, S.P. Platelet factor XIII and calpain negatively regulate integrin alphaIIbbeta3 adhesive function and thrombus growth. J. Biol. Chem. 2004, 279, 30697–30706. [Google Scholar] [CrossRef] [PubMed]
- Dale, G.L. Coated-platelets: An emerging component of the procoagulant response. J. Thromb. Haemost. 2005, 3, 2185–2192. [Google Scholar] [CrossRef]
- Hess, M.W.; Siljander, P. Procoagulant platelet balloons: Evidence from cryopreparation and electron microscopy. Histochem. Cell Biol. 2001, 115, 439–443. [Google Scholar] [CrossRef]
- Prodan, C.I.; Vincent, A.S.; Dale, G.L. Coated-platelet levels are elevated in patients with transient ischemic attack. Transl. Res. 2011, 158, 71–75. [Google Scholar] [CrossRef]
- Hogan, D.F.; Fox, P.R.; Jacob, K.; Keene, B.; Laste, N.J.; Rosenthal, S.; Sederquist, K.; Weng, H.Y. Secondary prevention of cardiogenic arterial thromboembolism in the cat: The double-blind, randomized, positive-controlled feline arterial thromboembolism; clopidogrel vs. aspirin trial (FAT CAT). J. Vet. Cardiol. 2015, 17 (Suppl. 1), S306–S317. [Google Scholar] [CrossRef]
- Liu, D.; Xu, W.P.; Xu, H.; Zhao, L.; Jin, D.Q. Efficacy and safety of clopidogrel versus aspirin monotherapy for secondary prevention in patients with coronary artery disease: A meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1265983. [Google Scholar] [CrossRef]
- Millington-Burgess, S.L.; Harper, M.T. Cytosolic and mitochondrial Ca. Platelets 2021, 32, 855–862. [Google Scholar] [CrossRef]
- Abbasian, N.; Millington-Burgess, S.L.; Chabra, S.; Malcor, J.D.; Harper, M.T. Supramaximal calcium signaling triggers procoagulant platelet formation. Blood Adv. 2020, 4, 154–164. [Google Scholar] [CrossRef]
- Heemskerk, J.W.; Mattheij, N.J.; Cosemans, J.M. Platelet-based coagulation: Different populations, different functions. J. Thromb. Haemost. 2013, 11, 2–16. [Google Scholar] [CrossRef]
- Harper, M.T.; Londoño, J.E.; Quick, K.; Londoño, J.C.; Flockerzi, V.; Philipp, S.E.; Birnbaumer, L.; Freichel, M.; Poole, A.W. Transient receptor potential channels function as a coincidence signal detector mediating phosphatidylserine exposure. Sci. Signal 2013, 6, ra50. [Google Scholar] [CrossRef] [PubMed]
- Varga-Szabo, D.; Braun, A.; Kleinschnitz, C.; Bender, M.; Pleines, I.; Pham, M.; Renné, T.; Stoll, G.; Nieswandt, B. The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J. Exp. Med. 2008, 205, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.; Varga-Szabo, D.; Kleinschnitz, C.; Pleines, I.; Bender, M.; Austinat, M.; Bösl, M.; Stoll, G.; Nieswandt, B. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood 2009, 113, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Kholmukhamedov, A.; Janecke, R.; Choo, H.J.; Jobe, S.M. The mitochondrial calcium uniporter regulates procoagulant platelet formation. J. Thromb. Haemost. 2018, 16, 2315–2321. [Google Scholar] [CrossRef]
- Amanakis, G.; Murphy, E. Cyclophilin D: An Integrator of Mitochondrial Function. Front. Physiol. 2020, 11, 595. [Google Scholar] [CrossRef]
- Jobe, S.M.; Wilson, K.M.; Leo, L.; Raimondi, A.; Molkentin, J.D.; Lentz, S.R.; Di Paola, J. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood 2008, 111, 1257–1265. [Google Scholar] [CrossRef]
- Mattheij, N.J.; Gilio, K.; van Kruchten, R.; Jobe, S.M.; Wieschhaus, A.J.; Chishti, A.H.; Collins, P.; Heemskerk, J.W.; Cosemans, J.M. Dual mechanism of integrin αIIbβ3 closure in procoagulant platelets. J. Biol. Chem. 2013, 288, 13325–13336. [Google Scholar] [CrossRef]
- Heemskerk, J.W.; Vuist, W.M.; Feijge, M.A.; Reutelingsperger, C.P.; Lindhout, T. Collagen but not fibrinogen surfaces induce bleb formation, exposure of phosphatidylserine, and procoagulant activity of adherent platelets: Evidence for regulation by protein tyrosine kinase-dependent Ca2+ responses. Blood 1997, 90, 2615–2625. [Google Scholar] [CrossRef]
- Munnix, I.C.; Cosemans, J.M.; Auger, J.M.; Heemskerk, J.W. Platelet response heterogeneity in thrombus formation. Thromb. Haemost. 2009, 102, 1149–1156. [Google Scholar] [CrossRef]
- Schoenwaelder, S.M.; Yuan, Y.; Josefsson, E.C.; White, M.J.; Yao, Y.; Mason, K.D.; O’Reilly, L.A.; Henley, K.J.; Ono, A.; Hsiao, S.; et al. Two distinct pathways regulate platelet phosphatidylserine exposure and procoagulant function. Blood 2009, 114, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Topalov, N.N.; Yakimenko, A.O.; Canault, M.; Artemenko, E.O.; Zakharova, N.V.; Abaeva, A.A.; Loosveld, M.; Ataullakhanov, F.I.; Nurden, A.T.; Alessi, M.C.; et al. Two types of procoagulant platelets are formed upon physiological activation and are controlled by integrin α(IIb)β(3). Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2475–2483. [Google Scholar] [CrossRef] [PubMed]
- Agbani, E.O.; van den Bosch, M.T.; Brown, E.; Williams, C.M.; Mattheij, N.J.; Cosemans, J.M.; Collins, P.W.; Heemskerk, J.W.; Hers, I.; Poole, A.W. Coordinated Membrane Ballooning and Procoagulant Spreading in Human Platelets. Circulation 2015, 132, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Agbani, E.O.; Williams, C.M.; Li, Y.; van den Bosch, M.T.; Moore, S.F.; Mauroux, A.; Hodgson, L.; Verkman, A.S.; Hers, I.; Poole, A.W. Aquaporin-1 regulates platelet procoagulant membrane dynamics and in vivo thrombosis. JCI Insight 2018, 3, e99062. [Google Scholar] [CrossRef]
- Hua, V.M.; Abeynaike, L.; Glaros, E.; Campbell, H.; Pasalic, L.; Hogg, P.J.; Chen, V.M. Necrotic platelets provide a procoagulant surface during thrombosis. Blood 2015, 126, 2852–2862. [Google Scholar] [CrossRef]
- Shaverdian, M.; Nguyen, N.; Li, R.H.L. A novel technique to characterize procoagulant platelet formation and evaluate platelet procoagulant tendency in cats by flow cytometry. Front. Vet. Sci. 2024, 11, 1480756. [Google Scholar] [CrossRef]
- Khattab, M.H.; Prodan, C.I.; Vincent, A.S.; Xu, C.; Jones, K.R.; Thind, S.; Rabadi, M.; Mithilesh, S.; Mathews, E.; Guthery, L.; et al. Increased procoagulant platelet levels are predictive of death in COVID-19. Geroscience 2021, 43, 2055–2065. [Google Scholar] [CrossRef]
- Lee, C.S.M.; Selvadurai, M.V.; Pasalic, L.; Yeung, J.; Konda, M.; Kershaw, G.W.; Favaloro, E.J.; Chen, V.M. Measurement of procoagulant platelets provides mechanistic insight and diagnostic potential in heparin-induced thrombocytopenia. J. Thromb. Haemost. 2022, 20, 975–988. [Google Scholar] [CrossRef]
- Zeiger, F.; Stephan, S.; Hoheisel, G.; Pfeiffer, D.; Ruehlmann, C.; Koksch, M. P-Selectin expression, platelet aggregates, and platelet-derived microparticle formation are increased in peripheral arterial disease. Blood Coagul. Fibrinolysis 2000, 11, 723–728. [Google Scholar] [CrossRef]
- Kirkpatrick, A.C.; Stoner, J.A.; Dale, G.L.; Prodan, C.I. Elevated coated-platelets in symptomatic large-artery stenosis patients are associated with early stroke recurrence. Platelets 2014, 25, 93–96. [Google Scholar] [CrossRef]
- Prodan, C.I.; Stoner, J.A.; Dale, G.L. Lower Coated-Platelet Levels Are Associated With Increased Mortality After Spontaneous Intracerebral Hemorrhage. Stroke 2015, 46, 1819–1825. [Google Scholar] [CrossRef]
- Prodan, C.I.; Vincent, A.S.; Padmanabhan, R.; Dale, G.L. Coated-platelet levels are low in patients with spontaneous intracerebral hemorrhage. Stroke 2009, 40, 2578–2580. [Google Scholar] [CrossRef]
- Kirkpatrick, A.C.; Vincent, A.S.; Dale, G.L.; Prodan, C.I. Coated-platelets predict stroke at 30 days following TIA. Neurology 2017, 89, 125–128. [Google Scholar] [CrossRef]
- Guo, J.; Cui, B.; Zheng, J.; Yu, C.; Zheng, X.; Yi, L.; Zhang, S.; Wang, K. Platelet-derived microparticles and their cargos: The past, present and future. Asian J. Pharm. Sci. 2024, 19, 100907. [Google Scholar] [CrossRef]
- Fox, J.E.; Austin, C.D.; Reynolds, C.C.; Steffen, P.K. Evidence that agonist-induced activation of calpain causes the shedding of procoagulant-containing microvesicles from the membrane of aggregating platelets. J. Biol. Chem. 1991, 266, 13289–13295. [Google Scholar] [CrossRef] [PubMed]
- Kaplanski, G.; Marin, V.; Fabrigoule, M.; Boulay, V.; Benoliel, A.M.; Bongrand, P.; Kaplanski, S.; Farnarier, C. Thrombin-activated human endothelial cells support monocyte adhesion in vitro following expression of intercellular adhesion molecule-1 (ICAM-1; CD54) and vascular cell adhesion molecule-1 (VCAM-1; CD106). Blood 1998, 92, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Ueno, A.; Murakami, K.; Yamanouchi, K.; Watanabe, M.; Kondo, T. Thrombin stimulates production of interleukin-8 in human umbilical vein endothelial cells. Immunology 1996, 88, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Barry, O.P.; Praticò, D.; Savani, R.C.; FitzGerald, G.A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J. Clin. Investig. 1998, 102, 136–144. [Google Scholar] [CrossRef]
- Mause, S.F.; von Hundelshausen, P.; Zernecke, A.; Koenen, R.R.; Weber, C. Platelet microparticles: A transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1512–1518. [Google Scholar] [CrossRef]
- Celi, A.; Lorenzet, R.; Furie, B.C.; Furie, B. Microparticles and a P-selectin-mediated pathway of blood coagulation. Dis. Markers 2004, 20, 347–352. [Google Scholar] [CrossRef]
- Falati, S.; Liu, Q.; Gross, P.; Merrill-Skoloff, G.; Chou, J.; Vandendries, E.; Celi, A.; Croce, K.; Furie, B.C.; Furie, B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 2003, 197, 1585–1598. [Google Scholar] [CrossRef]
- Lukasik, M.; Rozalski, M.; Luzak, B.; Michalak, M.; Ambrosius, W.; Watala, C.; Kozubski, W. Enhanced platelet-derived microparticle formation is associated with carotid atherosclerosis in convalescent stroke patients. Platelets 2013, 24, 63–70. [Google Scholar] [CrossRef]
- Rectenwald, J.E.; Myers, D.D.; Hawley, A.E.; Longo, C.; Henke, P.K.; Guire, K.E.; Schmaier, A.H.; Wakefield, T.W. D-dimer, P-selectin, and microparticles: Novel markers to predict deep venous thrombosis. A pilot study. Thromb. Haemost. 2005, 94, 1312–1317. [Google Scholar] [CrossRef]
- van der Zee, P.M.; Biró, E.; Ko, Y.; de Winter, R.J.; Hack, C.E.; Sturk, A.; Nieuwland, R. P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin. Chem. 2006, 52, 657–664. [Google Scholar] [CrossRef]
- Visentin, G.P.; Ford, S.E.; Scott, J.P.; Aster, R.H. Antibodies from patients with heparin-induced thrombocytopenia/thrombosis are specific for platelet factor 4 complexed with heparin or bound to endothelial cells. J. Clin. Investig. 1994, 93, 81–88. [Google Scholar] [CrossRef]
- Makielski, K.M.; Fox, L.E.; Johannes, C.M.; Rendahl, A.K.; Schulte, A.J.; Kim, J.H.; Husbands, B.D.; Walz, J.Z.; Henson, M.S.; Modiano, J.F.; et al. Evaluation of coated platelets, a subset of highly procoagulant platelets, in healthy dogs and dogs with neoplasia. Am. J. Vet. Res. 2022, 83, ajvr.22.03.0042. [Google Scholar] [CrossRef]
- Zhao, L.; Bi, Y.; Kou, J.; Shi, J.; Piao, D. Phosphatidylserine exposing-platelets and microparticles promote procoagulant activity in colon cancer patients. J. Exp. Clin. Cancer Res. 2016, 35, 54. [Google Scholar] [CrossRef] [PubMed]
- Plantureux, L.; Mège, D.; Crescence, L.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Impacts of Cancer on Platelet Production, Activation and Education and Mechanisms of Cancer-Associated Thrombosis. Cancers 2018, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Guo, Y.; Chang, Z.; Zhang, D.; Zhang, S.; Pei, H.; Pang, J.; Zhao, Z.J.; Chen, Y. Bidirectional Interaction Between Cancer Cells and Platelets Provides Potential Strategies for Cancer Therapies. Front. Oncol. 2021, 11, 764119. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.; Li, R.H.L.; Simpson, L.; Smith, P.; Shaverdian, M. Investigating the role of neutrophil extracellular traps in procoagulant platelet formation using an in vitro model of immune-mediated hemolytic anemia in dogs. In Proceedings of the 2024 Annual Research Forum and Litwack Lecture, Abstract number 35. Raleigh, NC, USA, 25 August 2024. [Google Scholar]
- Li, R.H.; Stern, J.A.; Ho, V.; Tablin, F.; Harris, S.P. Platelet Activation and Clopidogrel Effects on ADP-Induced Platelet Activation in Cats with or without the A31P Mutation in MYBPC3. J. Vet. Intern. Med. 2016, 30, 1619–1629. [Google Scholar] [CrossRef]
- Tablin, F.; Schumacher, T.; Pombo, M.; Marion, C.T.; Huang, K.; Norris, J.W.; Jandrey, K.E.; Kittleson, M.D. Platelet activation in cats with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 2014, 28, 411–418. [Google Scholar] [CrossRef]
- Tan, A.W.K.; Li, R.H.L.; Ueda, Y.; Stern, J.A.; Hussain, M.; Haginoya, S.; Sharpe, A.N.; Gunther-Harrington, C.T.; Epstein, S.E.; Nguyen, N. Platelet Priming and Activation in Naturally Occurring Thermal Burn Injuries and Wildfire Smoke Exposure Is Associated With Intracardiac Thrombosis and Spontaneous Echocardiographic Contrast in Feline Survivors. Front. Vet. Sci. 2022, 9, 892377. [Google Scholar] [CrossRef]
- Uriel, N.; Pak, S.W.; Jorde, U.P.; Jude, B.; Susen, S.; Vincentelli, A.; Ennezat, P.V.; Cappleman, S.; Naka, Y.; Mancini, D. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J. Am. Coll. Cardiol. 2010, 56, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Roka-Moiia, Y.; Miller-Gutierrez, S.; Palomares, D.E.; Italiano, J.E.; Sheriff, J.; Bluestein, D.; Slepian, M.J. Platelet Dysfunction During Mechanical Circulatory Support: Elevated Shear Stress Promotes Downregulation of α. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1319–1336. [Google Scholar] [CrossRef] [PubMed]
- Baaten, C.C.F.M.; Sternkopf, M.; Henning, T.; Marx, N.; Jankowski, J.; Noels, H. Platelet Function in CKD: A Systematic Review and Meta-Analysis. J. Am. Soc. Nephrol. 2021, 32, 1583–1598. [Google Scholar] [CrossRef] [PubMed]
- Virk, H.U.H.; Escobar, J.; Rodriguez, M.; Bates, E.R.; Khalid, U.; Jneid, H.; Birnbaum, Y.; Levine, G.N.; Smith, S.C.; Krittanawong, C. Dual Antiplatelet Therapy: A Concise Review for Clinicians. Life 2023, 13, 1580. [Google Scholar] [CrossRef]
- Josefsson, E.C.; Ramström, S.; Thaler, J.; Lordkipanidzé, M.; COAGAPO study group. Consensus report on markers to distinguish procoagulant platelets from apoptotic platelets: Communication from the Scientific and Standardization Committee of the ISTH. J. Thromb. Haemost. 2023, 21, 2291–2299. [Google Scholar] [CrossRef]
- Yeo, E.L.; Gemmell, C.H.; Sutherland, D.R.; Sefton, M.V. Characterization of canine platelet P-selectin (CD 62) and its utility in flow cytometry platelet studies. Comp. Biochem. Physiol. B 1993, 105, 625–636. [Google Scholar] [CrossRef]
- Zhang, H.; Nimmer, P.M.; Tahir, S.K.; Chen, J.; Fryer, R.M.; Hahn, K.R.; Iciek, L.A.; Morgan, S.J.; Nasarre, M.C.; Nelson, R.; et al. Bcl-2 family proteins are essential for platelet survival. Cell Death Differ. 2007, 14, 943–951. [Google Scholar] [CrossRef]
- Newburg, D.S.; Peterson, J.A.; Ruiz-Palacios, G.M.; Matson, D.O.; Morrow, A.L.; Shults, J.; Guerrero, M.L.; Chaturvedi, P.; Newburg, S.O.; Scallan, C.D.; et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 1998, 351, 1160–1164. [Google Scholar] [CrossRef]
- Albanyan, A.M.; Murphy, M.F.; Rasmussen, J.T.; Heegaard, C.W.; Harrison, P. Measurement of phosphatidylserine exposure during storage of platelet concentrates using the novel probe lactadherin: A comparison study with annexin V. Transfusion 2009, 49, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, B.; Montana, V.; Wei, M.D.; Wuskell, J.P.; Loew, L.M. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys. J. 1988, 53, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Choo, H.J.; Kholmukhamedov, A.; Zhou, C.; Jobe, S. Inner Mitochondrial Membrane Disruption Links Apoptotic and Agonist-Initiated Phosphatidylserine Externalization in Platelets. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Bourcy, M.; Pasalic, L.; Chen, V.M. Flow Cytometry Assessment of Procoagulant Platelets Using a Dithiol-Reactive Probe. Methods Mol. Biol. 2019, 1967, 305–321. [Google Scholar] [CrossRef]
- Dale, G.L.; Friese, P.; Batar, P.; Hamilton, S.F.; Reed, G.L.; Jackson, K.W.; Clemetson, K.J.; Alberio, L. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature 2002, 415, 175–179. [Google Scholar] [CrossRef]
- Mattheij, N.J.; Swieringa, F.; Mastenbroek, T.G.; Berny-Lang, M.A.; May, F.; Baaten, C.C.; van der Meijden, P.E.; Henskens, Y.M.; Beckers, E.A.; Suylen, D.P.; et al. Coated platelets function in platelet-dependent fibrin formation via integrin αIIbβ3 and transglutaminase factor XIII. Haematologica 2016, 101, 427–436. [Google Scholar] [CrossRef]
- Dale, G.L.; Remenyi, G.; Friese, P. Quantitation of microparticles released from coated-platelets. J. Thromb. Haemost. 2005, 3, 2081–2088. [Google Scholar] [CrossRef]
- Robert, S.; Poncelet, P.; Lacroix, R.; Arnaud, L.; Giraudo, L.; Hauchard, A.; Sampol, J.; Dignat-George, F. Standardization of platelet-derived microparticle counting using calibrated beads and a Cytomics FC500 routine flow cytometer: A first step towards multicenter studies? J. Thromb. Haemost. 2009, 7, 190–197. [Google Scholar] [CrossRef]
- Demirin, H.; Ozhan, H.; Ucgun, T.; Celer, A.; Bulur, S.; Cil, H.; Gunes, C.; Yildirim, H.A. Normal range of mean platelet volume in healthy subjects: Insight from a large epidemiologic study. Thromb. Res. 2011, 128, 358–360. [Google Scholar] [CrossRef]
- Weiser, M.G.; Kociba, G.J. Platelet concentration and platelet volume distribution in healthy cats. Am. J. Vet. Res. 1984, 45, 518–522. [Google Scholar] [CrossRef]
- Boudreaux, M.K.; Ebbe, S. Comparison of platelet number, mean platelet volume and platelet mass in five mammalian species. Comp. Haematol. Int. 1998, 8, 16–20. [Google Scholar] [CrossRef]
- Handtke, S.; Thiele, T. Large and small platelets-(When) do they differ? J. Thromb. Haemost. 2020, 18, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Nurden, A.T.; Butcher, P.D.; Hawkey, C.M. Comparative studies on the glycoprotein composition of mammalian platelets. Comp. Biochem. Physiol. B 1977, 56, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Tablin, F.; Oliver, A.E.; Walker, N.J.; Crowe, L.M.; Crowe, J.H. Membrane phase transition of intact human platelets: Correlation with cold-induced activation. J. Cell Physiol. 1996, 168, 305–313. [Google Scholar] [CrossRef]
- Lo, S.T.; Li, R.H.L.; Georges, C.J.; Nguyen, N.; Chen, C.K.; Stuhlmann, C.; Oldach, M.S.; Rivas, V.N.; Fousse, S.; Harris, S.P.; et al. Synergistic inhibitory effects of clopidogrel and rivaroxaban on platelet function and platelet-dependent thrombin generation in cats. J. Vet. Intern. Med. 2023, 37, 1390–1400. [Google Scholar] [CrossRef]
- Paikin, J.S.; Wright, D.S.; Eikelboom, J.W. Effectiveness and safety of combined antiplatelet and anticoagulant therapy: A critical review of the evidence from randomized controlled trials. Blood Rev. 2011, 25, 123–129. [Google Scholar] [CrossRef]
- Agbani, E.O.; Zhao, X.; Williams, C.M.; Aungraheeta, R.; Hers, I.; Swenson, E.R.; Poole, A.W. Carbonic Anhydrase Inhibitors suppress platelet procoagulant responses and in vivo thrombosis. Platelets 2020, 31, 853–859. [Google Scholar] [CrossRef]
- Gao, J.; Wang, X.; Chang, Y.; Zhang, J.; Song, Q.; Yu, H.; Li, X. Acetazolamide inhibits osmotic water permeability by interaction with aquaporin-1. Anal. Biochem. 2006, 350, 165–170. [Google Scholar] [CrossRef]
- Zhang, J.; An, Y.; Gao, J.; Han, J.; Pan, X.; Pan, Y.; Tie, L.; Li, X. Aquaporin-1 translocation and degradation mediates the water transportation mechanism of acetazolamide. PLoS ONE 2012, 7, e45976. [Google Scholar] [CrossRef]
- Kaiser, R.; Dewender, R.; Mulkers, M.; Stermann, J.; Rossaro, D.; Di Fina, L.; Li, L.; Gold, C.; Schmid, M.; Kääb, L.; et al. Procoagulant platelet activation promotes venous thrombosis. Blood 2024, 144, 2546–2553. [Google Scholar] [CrossRef]
- Swenson, E.R. Safety of carbonic anhydrase inhibitors. Expert. Opin. Drug Saf. 2014, 13, 459–472. [Google Scholar] [CrossRef]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Aslan, J.E.; Tormoen, G.W.; Loren, C.P.; Pang, J.; McCarty, O.J. S6K1 and mTOR regulate Rac1-driven platelet activation and aggregation. Blood 2011, 118, 3129–3136. [Google Scholar] [CrossRef] [PubMed]
- Babinska, A.; Markell, M.S.; Salifu, M.O.; Akoad, M.; Ehrlich, Y.H.; Kornecki, E. Enhancement of human platelet aggregation and secretion induced by rapamycin. Nephrol. Dial. Transplant. 1998, 13, 3153–3159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Q.; Huang, K.S.; Chen, M.; Huang, D.J. Rapamycin enhances platelet aggregation induced by adenosine diphosphate in vitro. Platelets 2009, 20, 428–431. [Google Scholar] [CrossRef]
- Moore, S.F.; Hunter, R.W.; Hers, I. mTORC2 protein complex-mediated Akt (Protein Kinase B) Serine 473 Phosphorylation is not required for Akt1 activity in human platelets [corrected]. J. Biol. Chem. 2011, 286, 24553–24560. [Google Scholar] [CrossRef]
- Śledź, K.M.; Moore, S.F.; Durrant, T.N.; Blair, T.A.; Hunter, R.W.; Hers, I. Rapamycin restrains platelet procoagulant responses via FKBP-mediated protection of mitochondrial integrity. Biochem. Pharmacol. 2020, 177, 113975. [Google Scholar] [CrossRef]
- Abstracts from the International Veterinary Emergency and Critical Care Symposium and the European Veterinary Emergency and Critical Care Annual Congress 2024. J. Vet. Emerg. Crit. Care 2024, 34, S2–S47. [CrossRef]
- Gao, X.M.; Wong, G.; Wang, B.; Kiriazis, H.; Moore, X.L.; Su, Y.D.; Dart, A.; Du, X.J. Inhibition of mTOR reduces chronic pressure-overload cardiac hypertrophy and fibrosis. J. Hypertens. 2006, 24, 1663–1670. [Google Scholar] [CrossRef]
- Kaplan, J.L.; Rivas, V.N.; Walker, A.L.; Grubb, L.; Farrell, A.; Fitzgerald, S.; Kennedy, S.; Jauregui, C.E.; Crofton, A.E.; McLaughlin, C.; et al. Delayed-release rapamycin halts progression of left ventricular hypertrophy in subclinical feline hypertrophic cardiomyopathy: Results of the RAPACAT trial. J. Am. Vet. Med. Assoc. 2023, 261, 1628–1637. [Google Scholar] [CrossRef]
- McMullen, J.R.; Sherwood, M.C.; Tarnavski, O.; Zhang, L.; Dorfman, A.L.; Shioi, T.; Izumo, S. Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation 2004, 109, 3050–3055. [Google Scholar] [CrossRef]
- Paoletti, E.; Amidone, M.; Cassottana, P.; Gherzi, M.; Marsano, L.; Cannella, G. Effect of sirolimus on left ventricular hypertrophy in kidney transplant recipients: A 1-year nonrandomized controlled trial. Am. J. Kidney Dis. 2008, 52, 324–330. [Google Scholar] [CrossRef]

| Aggregatory Platelets | Procoagulant Platelets | Apoptotic Platelets | |
|---|---|---|---|
| Role in coagulation | Platelet adhesion, aggregation, and clot retraction | Providing a procoagulant membrane surface for clotting factor complex formation thrombin generation | Programmed cell death regulating platelet lifespan |
| αIIbβ3 integrin | In high affinity of state or “active” | Integrin in low-affinity state or “inactive” Likely decreased expression | Integrin increases thrombin-mediated platelet apoptosis |
| Morphology | Develop numerous filopodia and lamellipodia resulting in the subsequent spreading to increase surface areas | Balloon-shaped without pseudopodia along with their PS exposed | Formation of apoptotic bodies, reduction in cell size with membrane blebbing |
| Cytosolic calcium levels | Oscillatory cytosolic calcium concentrations | Sustained intracellular hypercalcemia through SOCE | Calcium independent |
| Agonist | Formed upon stimulation of various agonists such as ADP, thromboxane A2, thrombin, collagen, epinephrine and serotonin | Formed upon stimulation of strong agonists, notably co-stimulation of thrombin and collagen/convulxin | High concentrations of thrombin induce Bak/Bax activation via PAR1 in a caspase dependent process |
| ΔΨm | Negative on matrix side (hyperpolarized) | Depolarized | Depolarized |
| MPTP formation | Not required | Required | Required |
| Marker |
Possible Detection Method(s) | Procoagulant Platelets | Apoptotic Platelets | Aggregatory Platelets | Comments |
|---|---|---|---|---|---|
| PS | Flow cytometry: Annexin V or Lactadherin | Yes | Yes | No |
|
| P-selectin | Flow cytometry: CD62P conjugated antibodies | Yes | No | Yes |
|
| Δψm | Flow cytometry Fluorescence microscopy | Yes | Yes | No |
|
| Activated αIIbβ3 integrin | Flow cytometry: (PAC-1, JON/A) Western blot | No | No | Yes |
|
| GSAO | Flow cytometry Fluorescence microscopy | Yes | No | No |
|
| Fibrinogen binding | Flow cytometry Confocal microscopy Light transmission aggregometry | Yes | No | Yes |
|
| Platelet-derive (PDMVs) | Flow cytometry | Yes | Yes | No |
|
| Cytosolic calcium | Fluorescent dyes using microplate readers | Yes (sustained) | No | Yes (transient) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaverdian, M.; Viall, A.; Li, R.H.L. Are Procoagulant Platelets an Emerging Therapeutic Target? A General Review with an Emphasis on Their Clinical Significance in Companion Animals. Int. J. Mol. Sci. 2025, 26, 8776. https://doi.org/10.3390/ijms26188776
Shaverdian M, Viall A, Li RHL. Are Procoagulant Platelets an Emerging Therapeutic Target? A General Review with an Emphasis on Their Clinical Significance in Companion Animals. International Journal of Molecular Sciences. 2025; 26(18):8776. https://doi.org/10.3390/ijms26188776
Chicago/Turabian StyleShaverdian, Meg, Austin Viall, and Ronald H. L. Li. 2025. "Are Procoagulant Platelets an Emerging Therapeutic Target? A General Review with an Emphasis on Their Clinical Significance in Companion Animals" International Journal of Molecular Sciences 26, no. 18: 8776. https://doi.org/10.3390/ijms26188776
APA StyleShaverdian, M., Viall, A., & Li, R. H. L. (2025). Are Procoagulant Platelets an Emerging Therapeutic Target? A General Review with an Emphasis on Their Clinical Significance in Companion Animals. International Journal of Molecular Sciences, 26(18), 8776. https://doi.org/10.3390/ijms26188776





