Mitochondrial ATP Biosynthesis Is Negatively Associated with FFA in Cardiac and Skeletal Muscle During the Development of Obesity in a Rodent Model
Abstract
1. Introduction
2. Results
2.1. General Characteristics of Animals
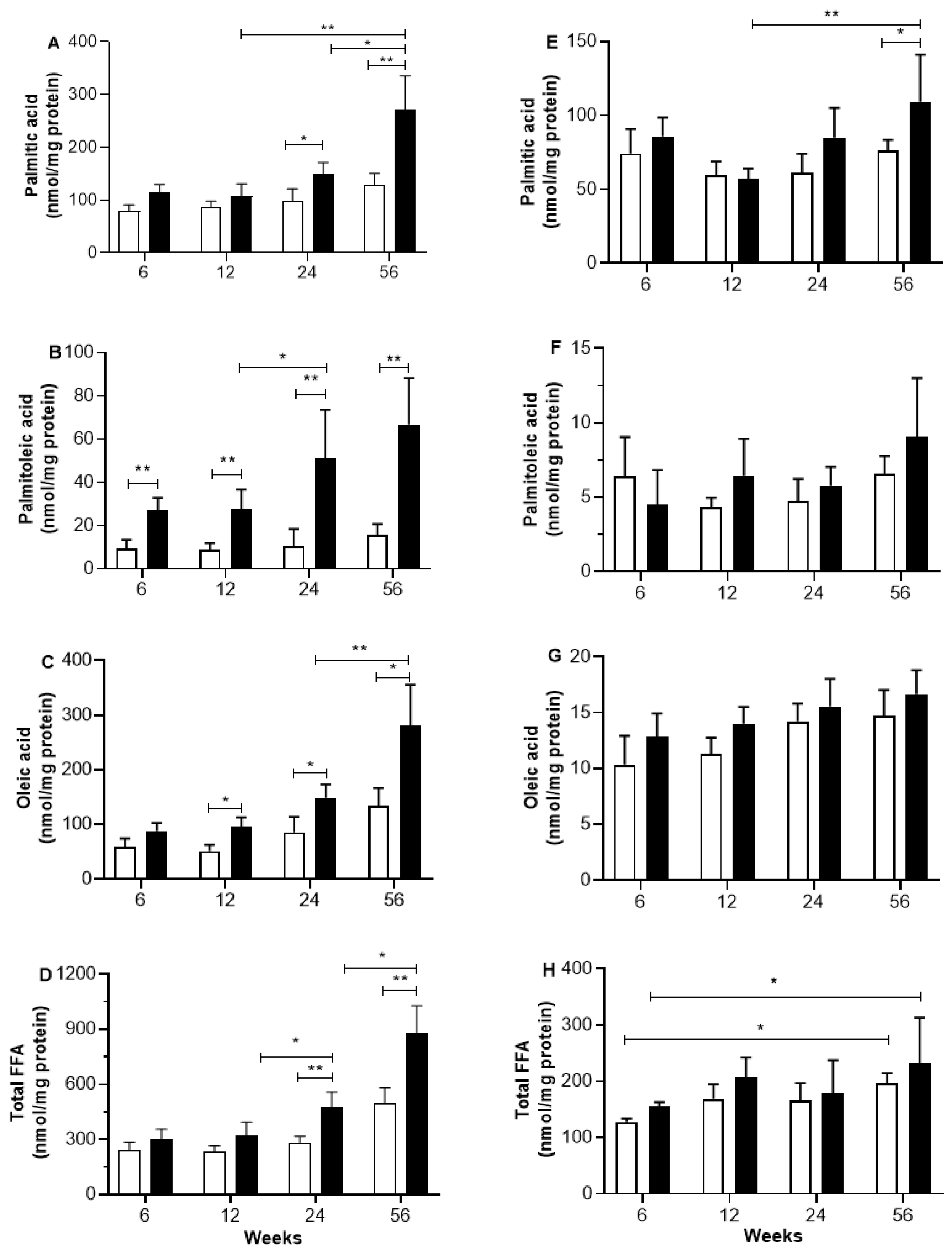
2.2. Skeletal and Heart Muscle FFA Content
2.3. Mitochondria Oxygen Consumption Rate (OCR)
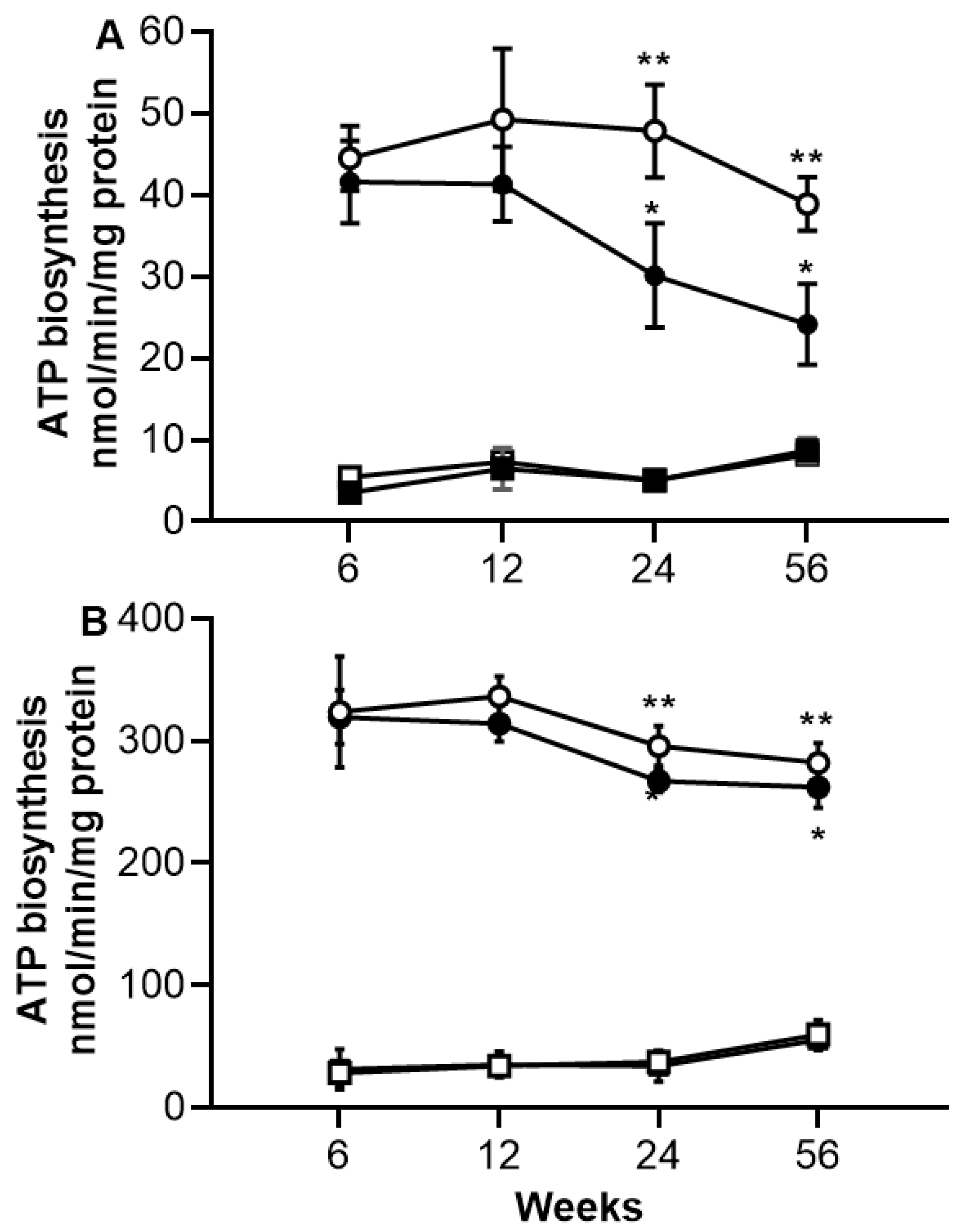
2.4. ATP Biosynthesis in Skeletal and Heart Muscles
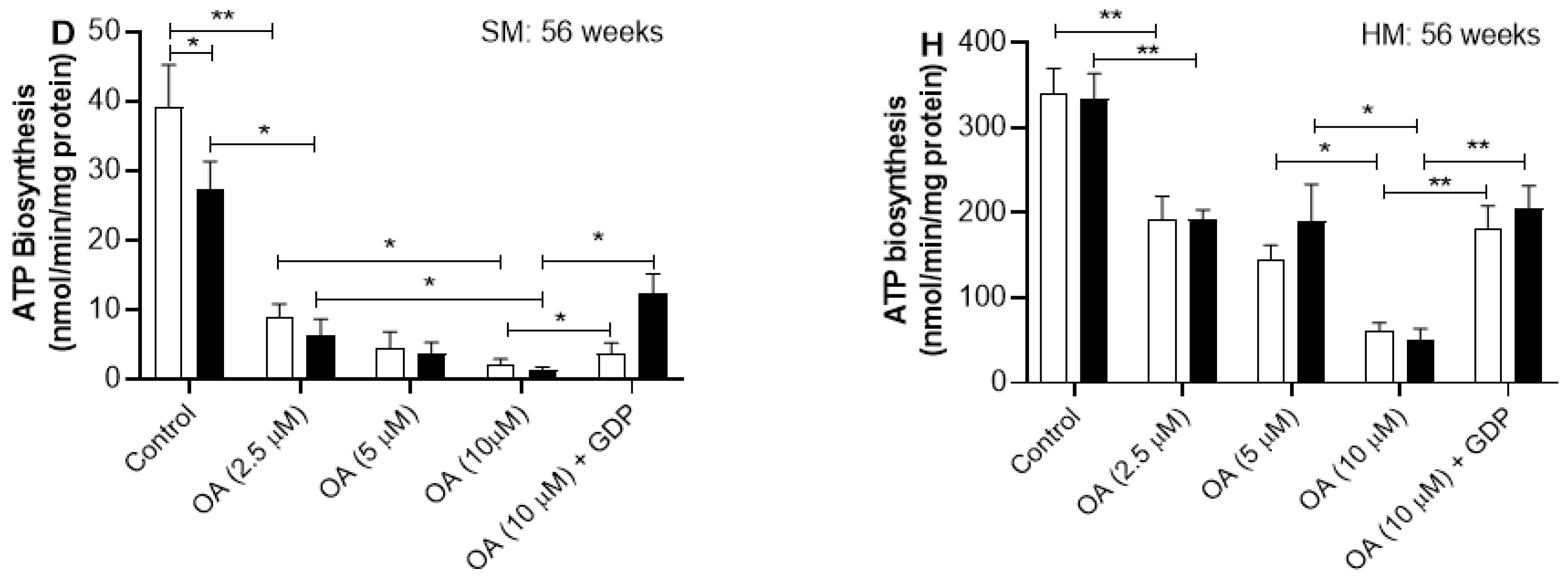
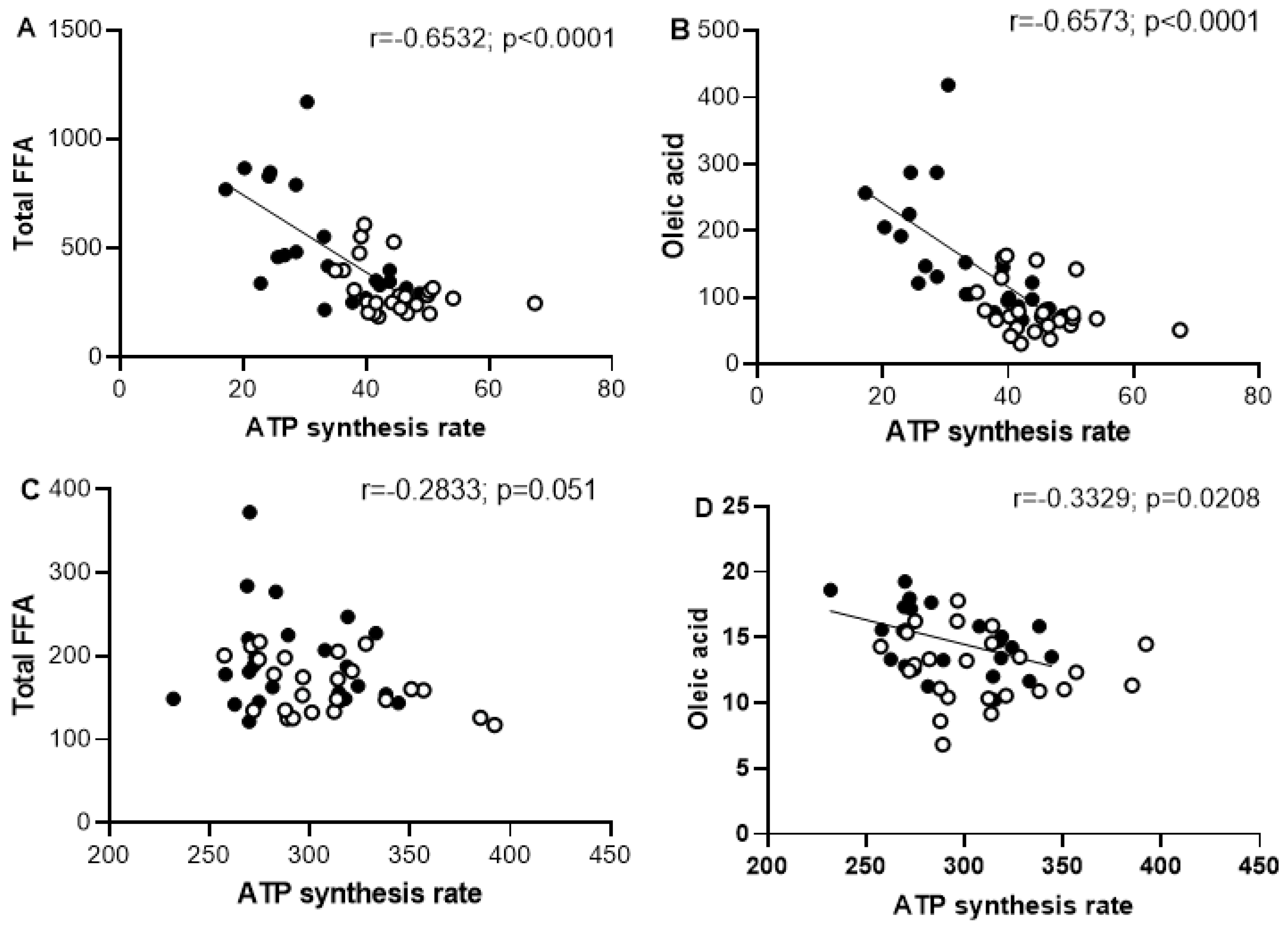
2.5. Effect of Oleic Acid on ATP Synthesis Rate in Skeletal and Heart Muscles
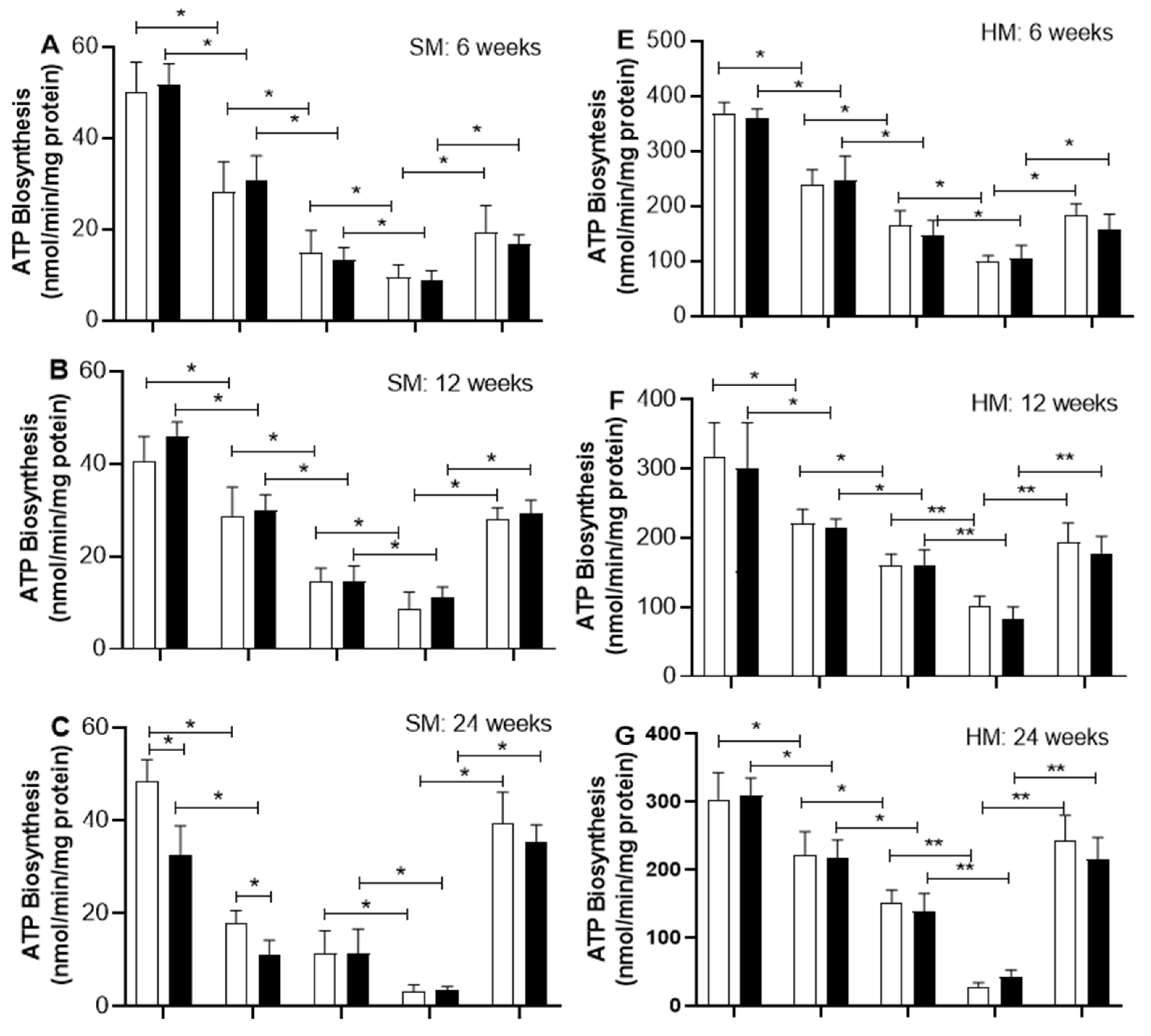
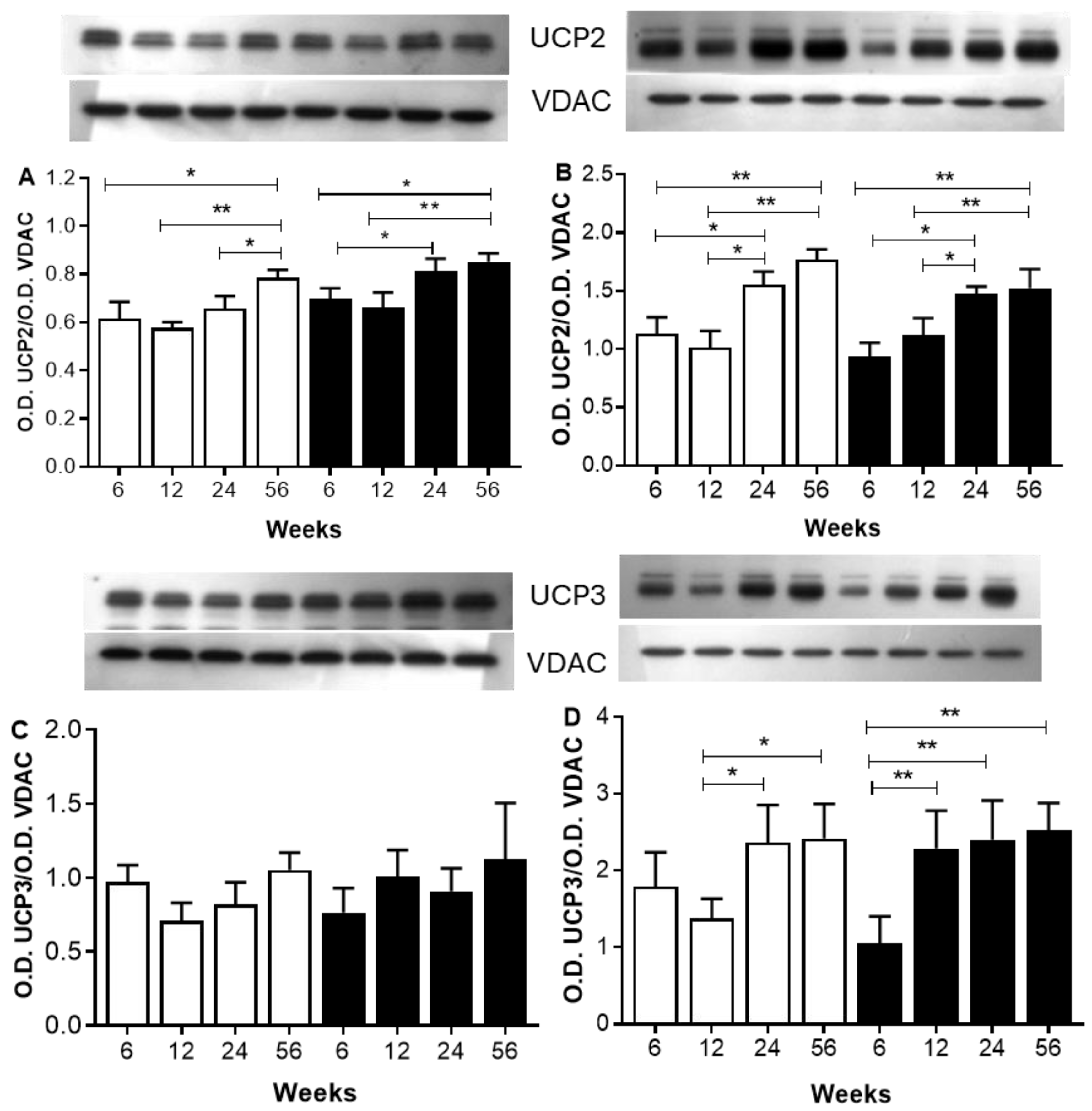
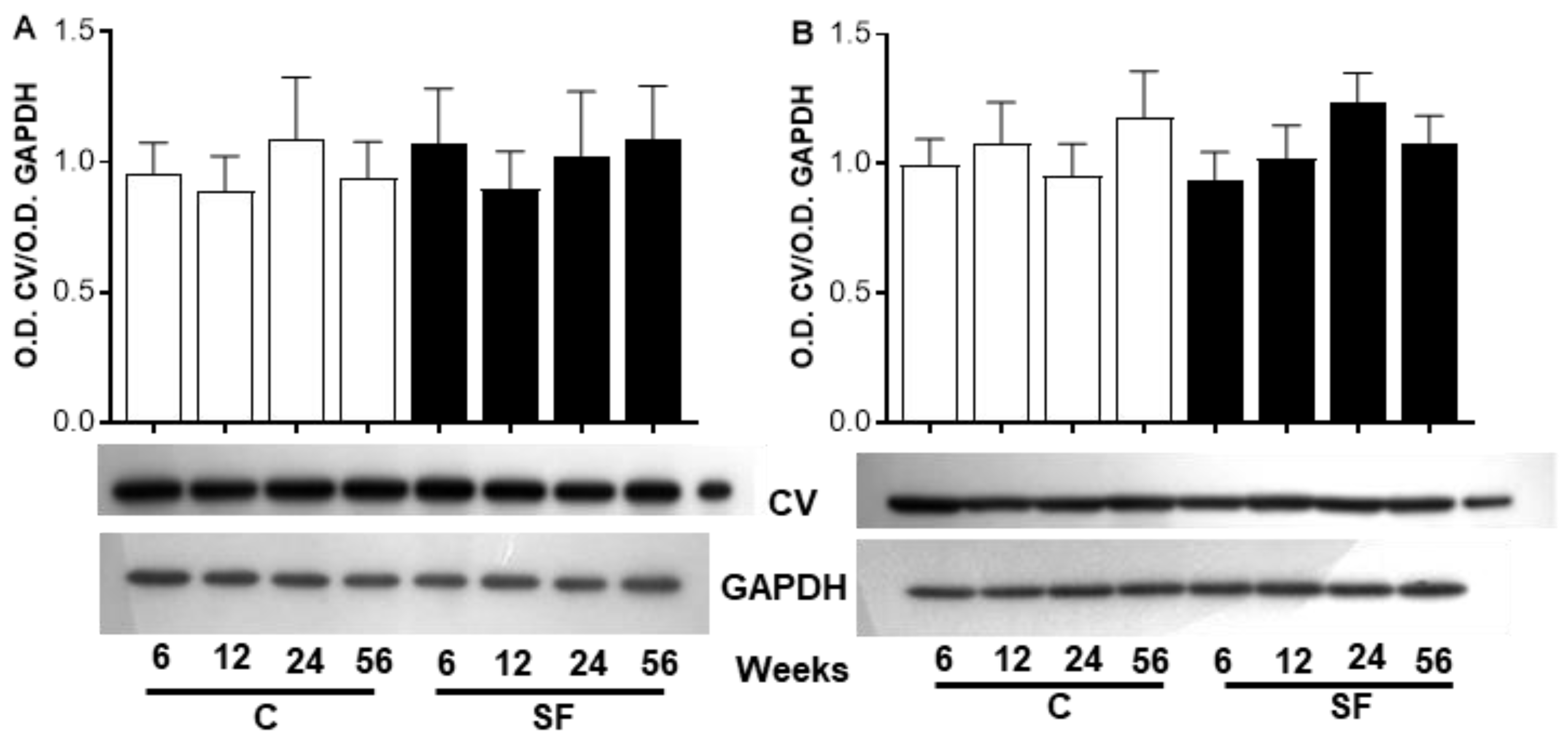
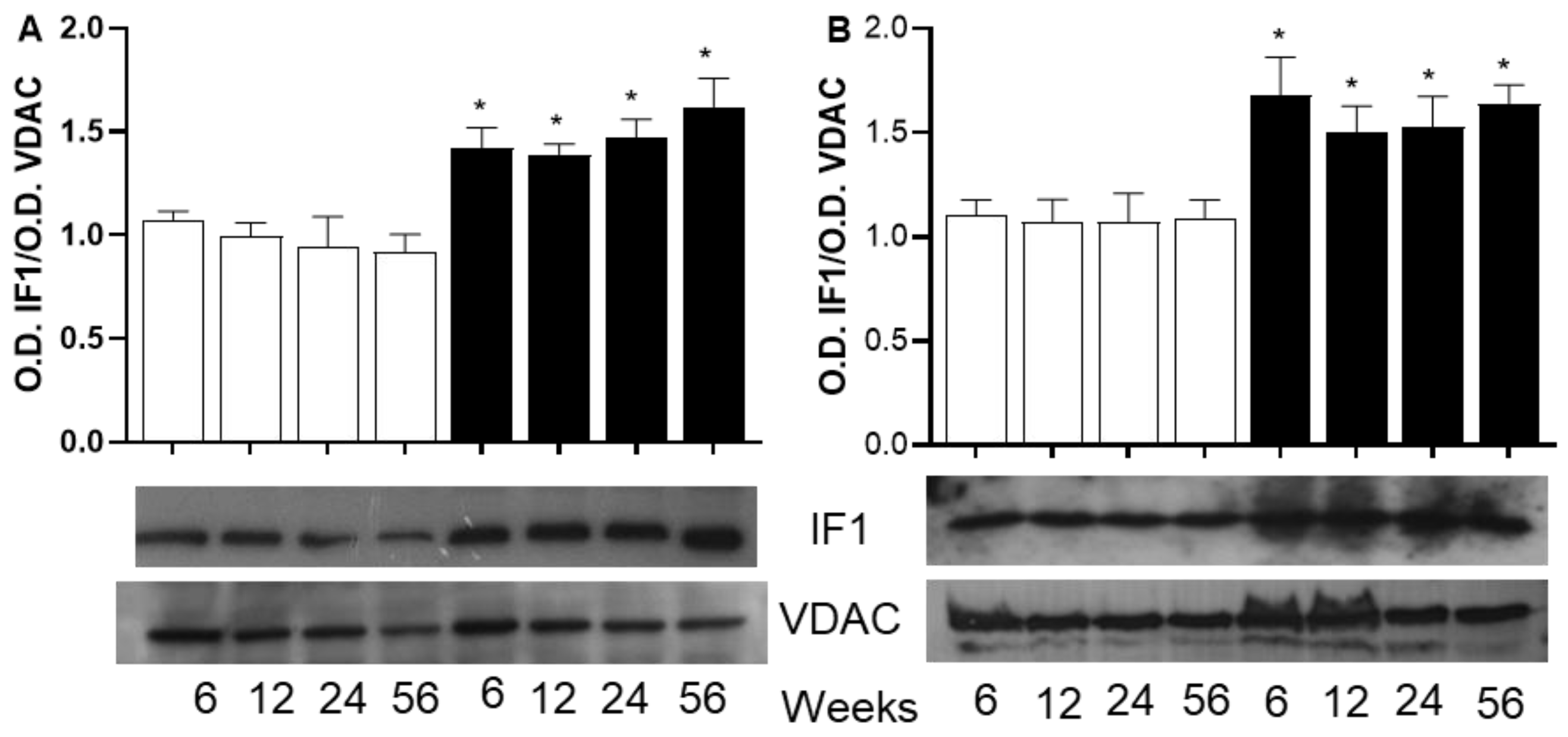
2.6. The Analysis Focuses on Proteins Involved in ATP Biosynthesis
3. Discussion
4. Experimental Design
4.1. Animals and Their Treatment
4.2. Measurement of Plasma Glucose, Cholesterol, and Triglycerides
4.3. FFA Extraction and Determination by Gas Chromatography (GC)
4.4. Mitochondria Preparation
4.5. Mitochondrial Oxygen Consumption Rate (OCR)
4.6. ATP Biosynthesis
4.7. Western Blot Analysis of UCPs, IF1, and Mitochondrial Respiratory Complex V
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, D.; Kolwicz, S.C.; Wang, P.; Roe, N.D.; Villet, O.; Nishi, K.; Hsu, Y.-W.A.; Flint, G.V.; Caudal, A.; Wang, W.; et al. Increasing Fatty Acid Oxidation Prevents High-Fat Diet–Induced Cardiomyopathy Through Regulating Parkin-Mediated Mitophagy. Circulation 2020, 142, 983–997. [Google Scholar] [CrossRef]
- Nesci, S.; Rubattu, S. UCP2, a Member of the Mitochondrial Uncoupling Proteins: An Overview from Physiological to Pathological Roles. Biomedicines 2024, 12, 1307. [Google Scholar] [CrossRef]
- Bertholet, A.M.; Natale, A.M.; Bisignano, P.; Suzuki, J.; Fedorenko, A.; Hamilton, J.; Brustovetsky, T.; Kazak, L.; Garrity, R.; Chouchani, E.T.; et al. Mitochondrial Uncouplers Induce Proton Leak by Activating AAC and UCP1. Nature 2022, 606, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Križančić Bombek, L.; Čater, M. Skeletal Muscle Uncoupling Proteins in Mice Models of Obesity. Metabolites 2022, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Čater, M.; Križančić Bombek, L.K. Protective Role of Mitochondrial Uncoupling Proteins against Age-Related Oxidative Stress in Type 2 Diabetes Mellitus. Antioxidants 2022, 11, 1473. [Google Scholar] [CrossRef]
- Della Guardia, L.; Luzi, L.; Codella, R. Muscle-UCP3 in the Regulation of Energy Metabolism. Mitochondrion 2024, 76, 101872. [Google Scholar] [CrossRef] [PubMed]
- Nabben, M.; Hoeks, J. Mitochondrial Uncoupling Protein 3 and Its Role in Cardiac- and Skeletal Muscle Metabolism. Physiol. Behav. 2008, 94, 259–269. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- Boudina, S.; Han, Y.H.; Pei, S.; Tidwell, T.J.; Henrie, B.; Tuinei, J.; Olsen, C.; Sena, S.; Abel, E.D. UCP3 Regulates Cardiac Efficiency and Mitochondrial Coupling in High Fat–Fed Mice but Not in Leptin-Deficient Mice. Diabetes 2012, 61, 3260–3269. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, M.; Zuo, Z. Palmitate Induces Mitochondrial Energy Metabolism Disorder and Cellular Damage via the PPAR Signaling Pathway in Diabetic Cardiomyopathy. Diabetes Metab. Syndr. Obes. 2022, 15, 2287–2299. [Google Scholar] [CrossRef]
- Haas de Mello, A.; Ferreira, G.K.; Rezin, G.T. Abnormal Mitochondrial Metabolism in Obesity and Insulin Resistance. In Clinical Bioenergetics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 83–92. [Google Scholar]
- Li, Y.; Xu, S.; Zhang, X.; Yi, Z.; Cichello, S. Skeletal Intramyocellular Lipid Metabolism and Insulin Resistance. Biophys. Rep. 2015, 1, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Berry, B.J.; Mjelde, E.; Carreno, F.; Gilham, K.; Hanson, E.J.; Na, E.; Kaeberlein, M. Preservation of Mitochondrial Membrane Potential Is Necessary for Lifespan Extension from Dietary Restriction. Geroscience 2023, 45, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Yang, W.Y.; Tan, Z.; Zhang, X.Y.; Shen, Y.L.; Guo, Q.W.; Su, G.M.; Chen, X.; Lin, J.; Fang, D.Z. Serum Levels of Free Fatty Acids in Obese Mice and Their Associations with Routine Lipid Profiles. Diabetes Metab. Syndr. Obes. 2022, 15, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Lemos, G.d.O.; Torrinhas, R.S.; Waitzberg, D.L. Nutrients, Physical Activity, and Mitochondrial Dysfunction in the Setting of Metabolic Syndrome. Nutrients 2023, 15, 1217. [Google Scholar] [CrossRef]
- Owesny, P.; Grune, T. The Link between Obesity and Aging—Insights into Cardiac Energy Metabolism. Mech. Ageing Dev. 2023, 216, 111870. [Google Scholar] [CrossRef]
- El Hafidi, M.; Cuéllar, A.; Ramírez, J.; Baños, G. Effect of Sucrose Addition to Drinking Water, That Induces Hypertension in the Rats, on Liver Microsomal Δ9 and Δ5-Desaturase Activities. J. Nutr. Biochem. 2001, 12, 396–403. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, A.; Chávez-Salgado, M.; Peñeda-Flores, J.A.; Zapata, E.; Masso, F.; El-Hafidi, M. High-Sucrose Diet Increases ROS Generation, FFA Accumulation, UCP2 Level, and Proton Leak in Liver Mitochondria. Am. J. Physiol.-Endocrinol. Metab. 2011, 301, E1198–E1207. [Google Scholar] [CrossRef]
- Drew, B.; Phaneuf, S.; Dirks, A.; Selman, C.; Gredilla, R.; Lezza, A.; Barja, G.; Leeuwenburgh, C. Effects of Aging and Caloric Restriction on Mitochondrial Energy Production in Gastrocnemius Muscle and Heart. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 284, R474–R480. [Google Scholar] [CrossRef]
- Murru, E.; Manca, C.; Carta, G.; Banni, S. Impact of Dietary Palmitic Acid on Lipid Metabolism. Front. Nutr. 2022, 9, 861664. [Google Scholar] [CrossRef]
- Blaak, E.E. Characterisation of Fatty Acid Metabolism in Different Insulin-Resistant Phenotypes by Means of Stable Isotopes. Proc. Nutr. Soc. 2017, 76, 419–424. [Google Scholar] [CrossRef]
- Ceja-Galicia, Z.A.; Cespedes-Acuña, C.L.A.; El-Hafidi, M. Protection Strategies Against Palmitic Acid-Induced Lipotoxicity in Metabolic Syndrome and Related Diseases. Int. J. Mol. Sci. 2025, 26, 788. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernández-Fernández, C.; Mouriño-Bayolo, D. Mitochondrial β-Oxidation of Saturated Fatty Acids in Humans. Mitochondrion 2019, 46, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Muller, F.L.; Liu, Y.; Chavez, A.O.; Balas, B.; Zuo, P.; Chang, Z.; Tripathy, D.; Jani, R.; Molina-Carrion, M.; et al. Deleterious Action of FA Metabolites on ATP Synthesis: Possible Link between Lipotoxicity, Mitochondrial Dysfunction, and Insulin Resistance. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E678–E685. [Google Scholar] [CrossRef] [PubMed]
- Rzheshevsky, A.V. Decrease in ATP Biosynthesis and Dysfunction of Biological Membranes. Two Possible Key Mechanisms of Phenoptosis. Biochemistry 2014, 79, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Zhang, X.; Sun, Q.; Liu, Y.; Liao, N.; Wu, H.; Wang, X.; Hai, C. Evolution of Metabolic Disorder in Rats Fed High Sucrose or High Fat Diet: Focus on Redox State and Mitochondrial Function. Gen. Comp. Endocrinol. 2017, 242, 92–100. [Google Scholar] [CrossRef]
- Schrauwen, P.; Hesselink, M. UCP2 and UCP3 in Muscle Controlling Body Metabolism. J. Exp. Biol. 2002, 205, 2275–2285. [Google Scholar] [CrossRef]
- Vaisy, M.; Szlufcik, K.; Maris, M.; De Bock, K.; Hesselink, M.K.C.; Eijnde, B.O.; Schrauwen, P.; Hespel, P. Hespel Hyperglycemic Diet and Training Alter Insulin Sensitivity, Intramyocellular Lipid Content but Not UCP3 Protein Expression in Rat Skeletal Muscles. Int. J. Mol. Med. 2010, 25, 905–913. [Google Scholar] [CrossRef]
- Pohl, E.E.; Rupprecht, A.; Macher, G.; Hilse, K.E. Important Trends in UCP3 Investigation. Front. Physiol. 2019, 10, 470. [Google Scholar] [CrossRef]
- He, Y.; Wang, N.; Shen, Y.; Zheng, Z.; Xu, X. Inhibition of High Glucose-Induced Apoptosis by Uncoupling Protein 2 in Human Umbilical Vein Endothelial Cells. Int. J. Mol. Med. 2014, 33, 1275–1281. [Google Scholar] [CrossRef]
- Pheiffer, C.; Jacobs, C.; Patel, O.; Ghoor, S.; Muller, C.; Louw, J. Expression of UCP2 in Wistar Rats Varies According to Age and the Severity of Obesity. J. Physiol. Biochem. 2016, 72, 25–32. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, A.; Barrios-Maya, M.; Quezada-Pablo, H.; López-Acosta, O.; El-Hafidi, M. Kidney Dysfunction Induced by a Sucrose-Rich Diet in Rat Involves Mitochondria ROS Generation, Cardiolipin Changes, and the Decline of Autophagy Protein Markers. Am. J. Physiol. Renal Physiol. 2020, 318, F53–F66. [Google Scholar] [CrossRef]
- Ruiz-Ramirez, A.; Lopez-Acosta, O.; Barrios-Maya, M.; El-Hafidi, M. Uncoupling Protein Overexpression in Metabolic Disease and the Risk of Uncontrolled Cell Proliferation and Tumorigenesis. Curr. Mol. Med. 2018, 17, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Hilse, K.E.; Rupprecht, A.; Egerbacher, M.; Bardakji, S.; Zimmermann, L.; Wulczyn, A.E.M.S.; Pohl, E.E. The Expression of Uncoupling Protein 3 Coincides with the Fatty Acid Oxidation Type of Metabolism in Adult Murine Heart. Front. Physiol. 2018, 9, 747. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P.; Hoeks, J.; Schaart, G.; Kornips, E.; Binas, B.; Vusse, G.J.; Bilsen, M.; Luiken, J.J.F.P.; Coort, S.L.M.; Glatz, J.F.C.; et al. Uncoupling Protein 3 as a Mitochondrial Fatty Acid Anion Exporter. FASEB J. 2003, 17, 2272–2274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Alder, N.N.; Wang, W.; Szeto, H.; Marcinek, D.J.; Rabinovitch, P.S. Reduction of Elevated Proton Leak Rejuvenates Mitochondria in the Aged Cardiomyocyte. eLife 2020, 9, e60827. [Google Scholar] [CrossRef]
- MacLellan, J.D.; Gerrits, M.F.; Gowing, A.; Smith, P.J.S.; Wheeler, M.B.; Harper, M.-E. Physiological Increases in Uncoupling Protein 3 Augment Fatty Acid Oxidation and Decrease Reactive Oxygen Species Production Without Uncoupling Respiration in Muscle Cells. Diabetes 2005, 54, 2343–2350. [Google Scholar] [CrossRef]
- Ciapaite, J.; Bakker, S.J.L.; Diamant, M.; Van Eikenhorst, G.; Heine, R.J.; Westerhoff, H.V.; Krab, K. Metabolic Control of Mitochondrial Properties by Adenine Nucleotide Translocator Determines Palmitoyl-CoA Effects. FEBS J. 2006, 273, 5288–5302. [Google Scholar] [CrossRef]
- Kobayashi, R.; Ueno, H.; Okazaki, K.; Noji, H. Molecular Mechanism on Forcible Ejection of ATPase Inhibitory Factor 1 from Mitochondrial ATP Synthase. Nat. Commun. 2023, 14, 1682. [Google Scholar] [CrossRef]
- Cardoso-Saldaña, G.C.; Antonio-Villa, N.E.; Martínez-Alvarado, M.d.R.; González-Salazar, M.d.C.; Posadas-Sánchez, R. Low HDL-C/ApoA-I Index Is Associated with Cardiometabolic Risk Factors and Coronary Artery Calcium: A Sub-Analysis of the Genetics of Atherosclerotic Disease (GEA) Study. BMC Endocr. Disord. 2024, 24, 110. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Tserng, K.Y.; Kliegman, R.M.; Miettinen, E.L.; Kalhan, S.C. A Rapid, Simple, and Sensitive Procedure for the Determination of Free Fatty Acids in Plasma Using Glass Capillary Column Gas-Liquid Chromatography. J. Lipid Res. 1981, 22, 852–858. [Google Scholar] [CrossRef]
- Hafidi, M.E.; Pérez, I.; Zamora, J.; Soto, V.; Carvajal-Sandoval, G.; Baños, G. Glycine Intake Decreases Plasma Free Fatty Acids, Adipose Cell Size, and Blood Pressure in Sucrose-Fed Rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R1387–R1393. [Google Scholar] [CrossRef]
- Vázquez-Memije, M.E.; Izquierdo-Reyes, V.; Delhumeau-Ongay, G. The Insensitivity to Uncouplers of Testis Mitochondrial ATPase. Arch. Biochem. Biophys. 1988, 260, 67–74. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- García, J.J.; Ogilvie, I.; Robinson, B.H.; Capaldi, R.A. Structure, Functioning, and Assembly of the ATP Synthase in Cells from Patients with the T8993G Mitochondrial DNA Mutation. J. Biol. Chem. 2000, 275, 11075–11081. [Google Scholar] [CrossRef]
| Animals | C | SF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Weeks | 6 | 12 | 24 | 56 | 6 | 12 | 24 | 56 | |
| Variables | |||||||||
| Body mass (g) | 284.8 ± 20.1 | 384.15 ± 2.7 | 552 ± 33.5 | 575.7 ± 46.4 | 259.1 ± 17.7 | 388 ± 35.3 | 572.9 ± 37.4 $ | 716.8 ± 50.3 *$ | |
| Intrabdominal fat (g) | 2.3 ± 0.9 | 4.3 ± 0.79 | 5.9 ± 1.2 | 8.6 ± 2.9 | 3.4 ± 0.7 | 8.6 ± 1.5 *$ | 14.9 ± 2.6 *$ | 25.6 ± 5.96 *$ | |
| Triglycerides (mM) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.1 * | 1.4 ± 0.2 * | 1.8 ± 0.3 *$ | 1.7 ± 0.2 *$ | |
| Total FFA (µM) | 243.2 ± 40.8 | 248.9 ± 38.9 | 312.8 ± 106.1 | 479.6 ± 113.2 | 353.7 ± 41.7 | 319.7 ± 74.4 | 461.1 ± 87.7 *$ | 830.2 ± 202.3 *$ | |
| Glucose (mM) | 6.2 ± 0.8 | 4.8 ± 1.9 | 4.9 ± 2.1 | 5.8 ± 0.7 | 5.5 ± 1.1 | 4.6 ± 1.8 | 4.9 ± 1.9 | 6.2 ± 0.7 | |
| Cholesterol (mM) | 1.3 ± 0.2 | 1.5 ± 0.1 | 1.4 ± 0.2 | 1.6 ± 0.2 | 1.7 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.2 | 1.6 ± 0.3 | |
| Animals | C | SF | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Weeks | 6 | 12 | 24 | 56 | 6 | 12 | 24 | 56 | |
| OCR | |||||||||
| Mitochondria from skeletal muscle | |||||||||
| State III | 121.0 ± 7.2 | 108.2 ± 16.0 * | 79.2 ± 7.1 * | 69.9 ± 10.8 ** | 115.6 ± 9.15 | 101.1 ± 13.6 | 70.9 ± 13.0 $ | 50.08 ± 7.1 $$ | |
| State IV | 22.6 ± 5.3 | 23.1 ± 6.5 | 20.8 ± 2.8 | 21.4 ± 3.2 | 22.1 ± 3.1 | 22.1 ± 4.4 | 26.1 ± 2.3 & | 27.4 ± 2.8 && | |
| Mitochondria from heart muscle | |||||||||
| State III | 118.8 ± 10.2 | 98.6 ± 9.2 | 89.5 ± 24.8 * | 61.7 ± 8.9 ** | 95.6 ± 14.8 | 92.1 ± 18.2 | 60.4 ± 9.7 $ | 50.8 ± 16.4 $$ | |
| State IV | 22.4 ± 3.3 | 21.7 ± 2.8 | 18.5 ± 3.4 | 19.3 ± 3.8 | 21.4 ± 4.6 | 22.8 ± 3.5 & | 21.3 ± 2.4 & | 23.0 ± 6.6 & | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Aguilar, V.; Ruiz-Ramirez, A.; Ceja-Galicia, Z.A.; de la Luz Hernandez Esquivel, M.; Cristobal Garcia, M.; Carbó Zabala, R.; Cardoso-Saldaña, G.-C.; El-Hafidi, M. Mitochondrial ATP Biosynthesis Is Negatively Associated with FFA in Cardiac and Skeletal Muscle During the Development of Obesity in a Rodent Model. Int. J. Mol. Sci. 2025, 26, 8768. https://doi.org/10.3390/ijms26188768
Nava-Aguilar V, Ruiz-Ramirez A, Ceja-Galicia ZA, de la Luz Hernandez Esquivel M, Cristobal Garcia M, Carbó Zabala R, Cardoso-Saldaña G-C, El-Hafidi M. Mitochondrial ATP Biosynthesis Is Negatively Associated with FFA in Cardiac and Skeletal Muscle During the Development of Obesity in a Rodent Model. International Journal of Molecular Sciences. 2025; 26(18):8768. https://doi.org/10.3390/ijms26188768
Chicago/Turabian StyleNava-Aguilar, Vianey, Angelica Ruiz-Ramirez, Zeltzin Alejandra Ceja-Galicia, Maria de la Luz Hernandez Esquivel, Magalena Cristobal Garcia, Roxana Carbó Zabala, Guillermo-Celestino Cardoso-Saldaña, and Mohammed El-Hafidi. 2025. "Mitochondrial ATP Biosynthesis Is Negatively Associated with FFA in Cardiac and Skeletal Muscle During the Development of Obesity in a Rodent Model" International Journal of Molecular Sciences 26, no. 18: 8768. https://doi.org/10.3390/ijms26188768
APA StyleNava-Aguilar, V., Ruiz-Ramirez, A., Ceja-Galicia, Z. A., de la Luz Hernandez Esquivel, M., Cristobal Garcia, M., Carbó Zabala, R., Cardoso-Saldaña, G.-C., & El-Hafidi, M. (2025). Mitochondrial ATP Biosynthesis Is Negatively Associated with FFA in Cardiac and Skeletal Muscle During the Development of Obesity in a Rodent Model. International Journal of Molecular Sciences, 26(18), 8768. https://doi.org/10.3390/ijms26188768








