Reduced Systemic Levels of Bile Acids in Individuals with Coronary Artery Disease: Insights from a Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Participants
2.5. Comparisons
2.6. Outcome (Assessment)
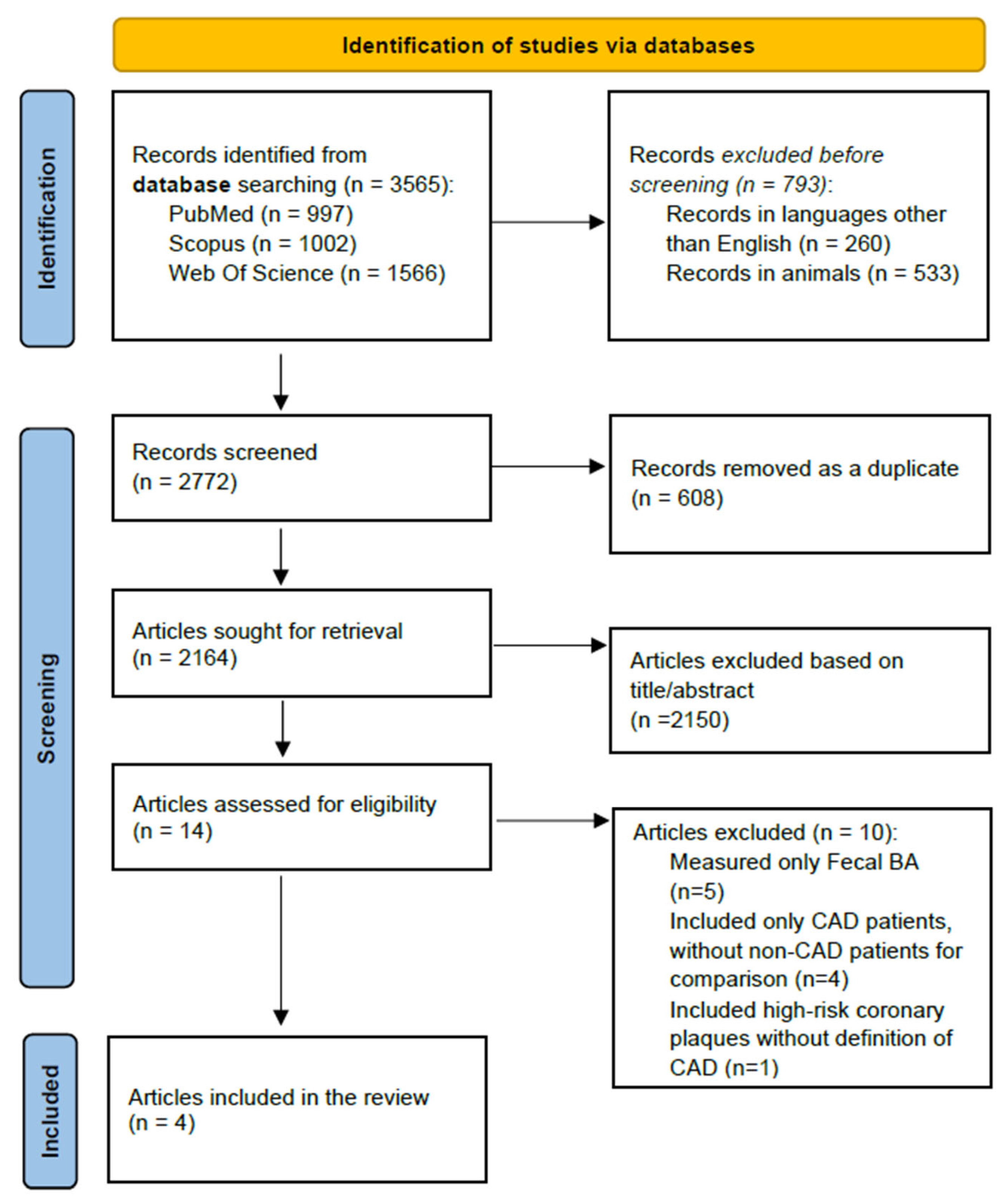
2.7. Study Identification and Selection
3. Results
4. Discussion
Limitations of the Studies Included in the Systematic Review
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndrome |
| BA | Bile Acid |
| BMI | Body Mass Index |
| CAD | Coronary Artery Disease |
| CYP27A1 | Sterol 27-Hydroxylase |
| CYP7A1 | Cholesterol 7α-Hydroxylase |
| DCA | Deoxycholic Acid |
| DM | Diabetes Mellitus |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FMO3 | Flavin-Containing Monooxygenase 3 |
| FXR | Farnesoid X Receptor |
| GLCA | Glycolithocholic Acid |
| GLP-1 | Glucagon-Like Peptide-1 |
| HPLC-MS/MS | High-Performance Liquid Chromatography–Tandem Mass Spectrometry |
| LCA | Lithocholic acid |
| LDL | Low-Density Lipoprotein |
| LPS | Lipopolysaccharides |
| MI | Myocardial Infarction |
| NCAD | Non-Coronary Artery Disease |
| NOS | Newcastle–Ottawa Scale |
| NR | Not Reported |
| OR | Odds Ratio |
| PXR | Pregnane X Receptor |
| T2D | Type 2 Diabetes |
| TBA | Total Bile Acids |
| TGR5 | Takeda G Protein-Coupled Receptor 5 |
| TMA | Trimethylamine |
| TMAO | Trimethylamine N-oxide |
| VDR | Vitamin D Receptor |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021, Correction in J. Am. Coll. Cardiol. 2021, 77, 1958–1959. [Google Scholar] [CrossRef]
- Azami, P.; Mohammadzadeh, S.; Seirafi, S.; Razeghian-Jahromi, I. A Review of Cutting-Edge Biomarkers for Diagnosing Coronary Artery Disease. Medicine 2025, 104, e41377. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, A.N.; Pletsch, M.; Chorbajian, A.; Zitser, D.; Rai, V.; Agrawal, D.K. Biomarkers to Monitor the Prognosis, Disease Severity, and Treatment Efficacy in Coronary Artery Disease. Expert Rev. Cardiovasc. Ther. 2023, 21, 675–692. [Google Scholar] [CrossRef] [PubMed]
- Canouï-Poitrine, F.; Luc, G.; Mallat, Z.; Machez, E.; Bingham, A.; Ferrieres, J.; Ruidavets, J.-B.; Montaye, M.; Yarnell, J.; Haas, B.; et al. Systemic Chemokine Levels, Coronary Heart Disease, and Ischemic Stroke Events. Neurology 2011, 77, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Chaulin, A.M. False-Positive Causes in Serum Cardiac Troponin Levels. J. Clin. Med. Res. 2022, 14, 80–87. [Google Scholar] [CrossRef]
- Hofmann, A.F. The Continuing Importance of Bile Acids in Liver and Intestinal Disease. Arch. Intern. Med. 1999, 159, 2647. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile Acids and the Gut Microbiota: Metabolic Interactions and Impacts on Disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Bile Acid Receptors and Gastrointestinal Functions. Liver Res. 2019, 3, 31–39. [Google Scholar] [CrossRef]
- Lefebvre, P.; Cariou, B.; Lien, F.; Kuipers, F.; Staels, B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol. Rev. 2009, 89, 147–191. [Google Scholar] [CrossRef]
- Nakahara, M.; Fujii, H.; Maloney, P.R.; Shimizu, M.; Sato, R. Bile Acids Enhance Low Density Lipoprotein Receptor Gene Expression via a MAPK Cascade-Mediated Stabilization of MRNA. J. Biol. Chem. 2002, 277, 37229–37234. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The Well-Built Clinical Question: A Key to Evidence-Based Decisions. ACP J. Club 1995, 123, A12. [Google Scholar] [CrossRef]
- Qi, L.; Chen, Y. Circulating Bile Acids as Biomarkers for Disease Diagnosis and Prevention. J. Clin. Endocrinol. Metab. 2023, 108, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.Ø.; Julienne, H.; Hyötyläinen, T.; Sen, P.; Fan, Y.; Pedersen, H.K.; Jäntti, S.; Hansen, T.H.; Nielsen, T.; Jørgensen, T.; et al. Conjugated C-6 Hydroxylated Bile Acids in Serum Relate to Human Metabolic Health and Gut Clostridia Species. Sci. Rep. 2021, 11, 13252. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L. Bile Acids: Regulation of Synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef]
- Martinot, E.; Sèdes, L.; Baptissart, M.; Lobaccaro, J.-M.; Caira, F.; Beaudoin, C.; Volle, D.H. Bile Acids and Their Receptors. Mol. Asp. Med. 2017, 56, 2–9. [Google Scholar] [CrossRef]
- Liu, T.T.; Wang, J.; Liang, Y.; Wu, X.Y.; Li, W.Q.; Wang, Y.H. The Level of Serum Total Bile Acid is Related to Atherosclerotic Lesions, Prognosis and Gut Lactobacillus in Acute Coronary Syndrome Patients. Ann. Med. 2023, 55, 2232369. [Google Scholar] [CrossRef]
- Wen, W.; Li, Q.; She, J.; Bai, X.; Zhang, L.; Li, R. Predictive Value of Serum TBA for 2-Year MACEs in ACS Patients Undergoing PCI: A Prospective Cohort Study. Sci. Rep. 2024, 14, 1733. [Google Scholar] [CrossRef]
- Zeng, X.C.; Jian, Z.J.; Li, S.S.; Xu, Y.; Li, B.L.; Ding, N.; Zhang, Y.Q.; Zhang, H.; Wu, Y.; Yang, J.; et al. The Association between Serum Total Bile Acid Level and Long-Term Prognosis in Patients with Coronary Chronic Total Occlusion Undergoing Percutaneous Coronary Intervention. Dis. Markers 2022, 2022, 1434111. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 15 February 2025).
- Li, W.; Shu, S.; Cheng, L.; Hao, X.; Wang, L.; Wu, Y.; Yuan, Z.; Zhou, J. Fasting Serum Total Bile Acid Level is Associated with Coronary Artery Disease, Myocardial Infarction and Severity of Coronary Lesions. Atherosclerosis 2020, 292, 193–200. [Google Scholar] [CrossRef]
- Chong Nguyen, C.; Duboc, D.; Rainteau, D.; Sokol, H.; Humbert, L.; Seksik, P.; Bellino, A.; Abdoul, H.; Bouazza, N.; Treluyer, J.M.; et al. Circulating Bile Acids Concentration is Predictive of Coronary Artery Disease in Human. Sci. Rep. 2021, 11, 22661. [Google Scholar] [CrossRef]
- Feng, X.; Zhai, G.; Yang, J.; Liu, Y.; Zhou, Y.; Guo, Q. Myocardial Infarction and Coronary Artery Disease in Menopausal Women with Type 2 Diabetes Mellitus Negatively Correlate with Total Serum Bile Acids. Front. Endocrinol. 2021, 12, 754006. [Google Scholar] [CrossRef]
- Bay, B.; Fuh, M.M.; Rohde, J.; Worthmann, A.; Goßling, A.; Arnold, N.; Koester, L.; Lorenz, T.; Blaum, C.; Kirchhof, P.; et al. Sex Differences in Lipidomic and Bile Acid Plasma Profiles in Patients with and without Coronary Artery Disease. Lipids Health Dis. 2024, 23, 197. [Google Scholar] [CrossRef] [PubMed]
- Prawitt, J.; Caron, S.; Staels, B. Bile Acid Metabolism and the Pathogenesis of Type 2 Diabetes. Curr. Diab. Rep. 2011, 11, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Duran-Sandoval, D.; Cariou, B.; Percevault, F.; Hennuyer, N.; Grefhorst, A.; van Dijk, T.H.; Gonzalez, F.J.; Fruchart, J.-C.; Kuipers, F.; Staels, B. The Farnesoid X Receptor Modulates Hepatic Carbohydrate Metabolism during the Fasting-Refeeding Transition. J. Biol. Chem. 2005, 280, 29971–29979. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.P.; Asgharpour, A.; Mirshahi, F.; Park, S.H.; Liu, S.; Imai, Y.; Nadler, J.L.; Grider, J.R.; Murthy, K.S.; Sanyal, A.J. Activation of Transmembrane Bile Acid Receptor TGR5 Modulates Pancreatic Islet α Cells to Promote Glucose Homeostasis. J. Biol. Chem. 2016, 291, 6626–6640. [Google Scholar] [CrossRef]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef]
- Bennion, L.J.; Grundy, S.M. Effects of diabetes mellitus on cholesterol metabolism in man. N. Engl. J. Med. 1977, 296, 1365–1371. [Google Scholar] [CrossRef]
- Abrams, J.J.; Ginsberg, H.; Grundy, S.M. Metabolism of cholesterol and plasma triglycerides in nonketotic diabetes mellitus. Diabetes 1982, 31, 903–910. [Google Scholar] [CrossRef]
- Suhre, K.; Meisinger, C.; Döring, A.; Altmaier, E.; Belcredi, P.; Gieger, C.; Chang, D.; Milburn, M.V.; Gall, W.E.; Weinberger, K.M.; et al. Metabolic Footprint of Diabetes: A Multiplatform Metabolomics Study in an Epidemiological Setting. PLoS ONE 2010, 5, e13953. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, J.; Wu, W.; Zhu, Y.; Liu, X. The Role of Bile Acids in Cardiovascular Diseases: From Mechanisms to Clinical Implications. Aging Dis. 2023, 14, 261–282. [Google Scholar] [CrossRef]
- Charach, G.; Grosskopf, I.; Rabinovich, A.; Shochat, M.; Weintraub, M. The Association of Bile Acid Excretion and Atherosclerotic Coronary Artery Disease. Therap. Adv. Gastroenterol. 2011, 4, 95–101. [Google Scholar] [CrossRef]
- Hansen, M.; Sonne, D.P.; Mikkelsen, K.H.; Gluud, L.L.; Vilsboll, T. Bile acid sequestrants for glycemic control in patients with type 2 diabetes: A systematic review with meta-analysis of randomized controlled trials. J. Diabetes Complicat. 2017, 31, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, A.; Renga, B.; Distrutti, E.; Fiorucci, S. Antiatherosclerotic effect of farnesoid X receptor. Am. J. Physiol.-Heart Circ. Physiol. 2009, 296, H272–H281. [Google Scholar] [CrossRef] [PubMed]
- Calmus, Y. Diferential Efects of Chenodeoxycholic and Ursodeoxycholic Acids on Interleukin 1, Interleukin 6 and Tumornecrosis Factor-Alpha Production by Monocytes. Hepatol. Baltim. 1992, 16, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Keitel, V.; Donner, M.; Winandy, S.; Kubitz, R.; Häussinger, D. Expression and function of the bile acid receptor TGR5 in Kupfer cells. Biochem. Biophys. Res. Commun. 2008, 372, 78–84. [Google Scholar] [CrossRef]
- Miyazaki-Anzai, S.; Masuda, M.; Kohno, S.; Levi, M.; Shiozaki, Y.; Keenan, A.L.; Miyazaki, M. Simultaneous Inhibition of FXR and TGR5 Exacerbates Atherosclerotic Formation. J. Lipid Res. 2018, 59, 1709–1713. [Google Scholar] [CrossRef]
- Hageman, J.; Herrema, H.; Groen, A.K.; Kuipers, F. A Role of the Bile Salt Receptor FXR in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1519–1528. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Lin, X.; Wang, Y.; Wu, X.; Yang, F.; Gao, W.; Zhang, Y.; Sun, J.; Jiang, C.; et al. DCA-TGR5 Signaling Activation Alleviates Inflammatory Response and Improves Cardiac Function in Myocardial Infarction. J. Mol. Cell. Cardiol. 2021, 151, 3–14. [Google Scholar] [CrossRef]
- Pols, T.W.H.; Nomura, M.; Harach, T.; Lo Sasso, G.; Oosterveer, M.H.; Thomas, C.; Rizzo, G.; Gioiello, A.; Adorini, L.; Pellicciari, R.; et al. TGR5 Activation Inhibits Atherosclerosis by Reducing Macrophage Inflammation and Lipid Loading. Cell Metab. 2011, 14, 747–757. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Zhang, Y.; Zuo, Y.; Huang, K.; Deng, L.; Liao, B.; Zhong, Y.; Feng, J. Takeda G Protein–Coupled Receptor 5 (TGR5): An Attractive Therapeutic Target for Aging-Related Cardiovascular Diseases. Front. Pharmacol. 2025, 16, 1493662. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.D.; Cui, J.Y.; Klaassen, C.D. Atorvastatin Induces Bile Acid-Synthetic Enzyme Cyp7a1 by Suppressing FXR Signaling in Both Liver and Intestine in Mice. J. Lipid Res. 2014, 55, 2576–2586. [Google Scholar] [CrossRef]
- Frank, A.T.H.; Zhao, B.; Jose, P.O.; Azar, K.M.J.; Fortmann, S.P.; Palaniappan, L.P. Racial/Ethnic Differences in Dyslipidemia Patterns. Circulation 2014, 129, 570–579. [Google Scholar] [CrossRef]
- Patton, M.E.; Kelekar, S.; Taylor, L.J.; Dean, A.E.; Zuo, Q.; Thakare, R.N.; Lee, S.H.; Gentry, E.; Panitchpakdi, M.; Dorrestein, P.; et al. Sex Differences in Bile Acid Homeostasis and Excretion Underlie the Disparity in Liver Cancer Incidence between Males and Females. bioRxiv 2020, 25, 172635. [Google Scholar] [CrossRef]
- Frommherz, L.; Bub, A.; Hummel, E.; Rist, M.J.; Roth, A.; Watzl, B.; Kulling, S.E. Age-Related Changes of Plasma Bile Acid Concentrations in Healthy Adults—Results from the Cross-Sectional KarMeN Study. PLoS ONE 2016, 11, e0153959. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Design | Aim | BA Measurement | Country | Statins Use (%) | Participants | Results |
|---|---|---|---|---|---|---|---|
| Li et al., 2019. [21] | Observational, cross-sectional | Investigate the association between TBA levels and the incidence of CAD, MI, and coronary lesions | TBA enzymatic assay | China | CAD = 97.8% NCAD = 94.5%. | CAD (n = 5853; 62 ± 10 yr.; 28% female), NCAD (n = 1585; 60 ± 10 yr.; 49% female) | Patients with CAD had 15% lower serum TBA levels than those with NCAD (3.4 vs. 4 µmol/L). Lower TBA levels were associated with CAD, MI, and the severity of coronary lesions in patients with suspected CAD |
| Nguyen et al., 2021. [22] | Observational, cross-sectional | Compare BA levels of CAD and NCAD patients | HPLC-MS/MS | France | NR | CAD (n = 45; 66 ± 1 yr.; 27% female) non-CAD (n = 35; 57 ± 2 yr.; 51% female) | Patients with CAD presented 53% lower serum TBA levels than those without NCAD. These differences were also significant for primary, secondary, and conjugate BAs, and glycochenodeoxycholic acid |
| Feng et al., 2021. [23] | Observational, cross-sectional | Explore the relationship between TBA levels and MI or CAD, with or without T2DM, in menopausal women | TBA enzymatic assay | China | CAD = 83.3% NCAD = 69.7% | CAD (n = 12,639; 65 [60–70] yr.) NCAD (n = 7616; 63 [58–68] yr.) CAD + T2D (n = 4701) NCAD + T2D (n = 1720) All patients were menopausal females | Women with CAD had 5.4% lower serum TBA levels than NCAD. Women with CAD + T2D had 10.3% lower levels than NCAD + T2D Lower TBA also correlated with MI in groups with and without T2D. |
| Bay et al., 2024. [24] | Observational, cross-sectional | Investigate the lipidomic and BA profile differences between CAD and NCAD patients | HPLC-MS/MS | Germany | CAD men = 20.5% CAD women = 27.3% NCAD men = 15.6% NCAD women = 22.7% | CAD (n = 88; 72 ± 2 yr.; 51% female) NCAD (n = 89; 68 ± 13 yr.; 51% female) | Men, but not women, CAD patients presented lower secondary glycolithocholic and lithocholic BA levels vs. NCAD patients |
| First Author, Year | Selection (0–4) | Comparability (0–2) | Outcome (0–3) | Total Score (0–9) | Quality |
|---|---|---|---|---|---|
| Li et al., 2019. [21] | ★★★★ | ★★ | ★★ | 8 | High |
| Nguyen et al., 2021. [22] | ★★★ | ★ | ★★ | 6 | Moderate |
| Feng et al., 2021. [23] | ★★★★ | ★★ | ★★★ | 9 | High |
| Bay et al., 2024. [24] | ★★★ | ★ | ★★ | 6 | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López Espinosa, V.M.; Amaro-Gahete, F.J.; Osuna-Prieto, F.J. Reduced Systemic Levels of Bile Acids in Individuals with Coronary Artery Disease: Insights from a Systematic Review. Int. J. Mol. Sci. 2025, 26, 8764. https://doi.org/10.3390/ijms26188764
López Espinosa VM, Amaro-Gahete FJ, Osuna-Prieto FJ. Reduced Systemic Levels of Bile Acids in Individuals with Coronary Artery Disease: Insights from a Systematic Review. International Journal of Molecular Sciences. 2025; 26(18):8764. https://doi.org/10.3390/ijms26188764
Chicago/Turabian StyleLópez Espinosa, Víctor Manuel, Francisco J. Amaro-Gahete, and Francisco J. Osuna-Prieto. 2025. "Reduced Systemic Levels of Bile Acids in Individuals with Coronary Artery Disease: Insights from a Systematic Review" International Journal of Molecular Sciences 26, no. 18: 8764. https://doi.org/10.3390/ijms26188764
APA StyleLópez Espinosa, V. M., Amaro-Gahete, F. J., & Osuna-Prieto, F. J. (2025). Reduced Systemic Levels of Bile Acids in Individuals with Coronary Artery Disease: Insights from a Systematic Review. International Journal of Molecular Sciences, 26(18), 8764. https://doi.org/10.3390/ijms26188764







