The Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid Enhance the Effects of Temozolomide Chemotherapy in Glioblastoma Cells
Abstract
1. Introduction
2. Results
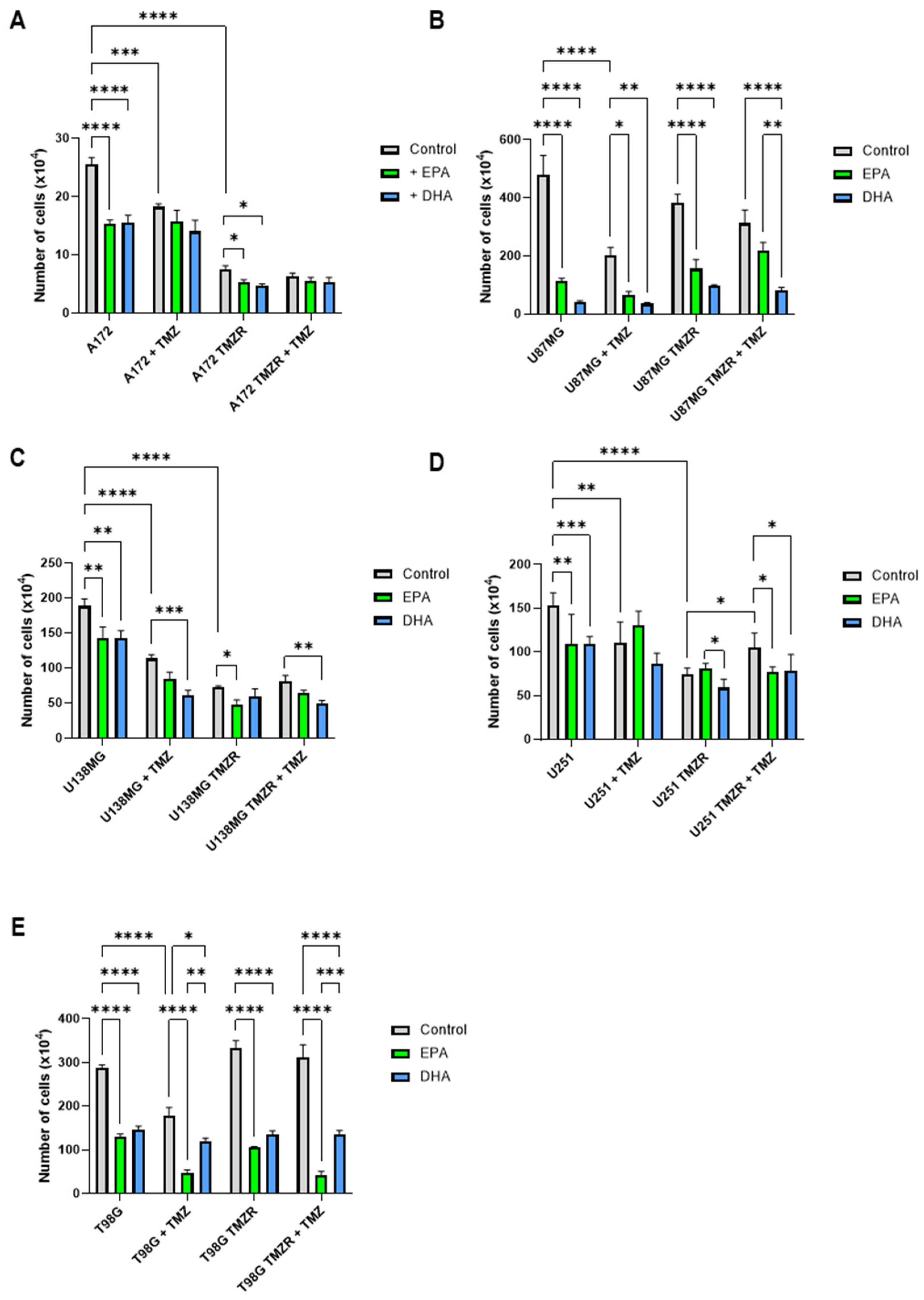
2.1. Influence of EPA or DHA on Cell Number
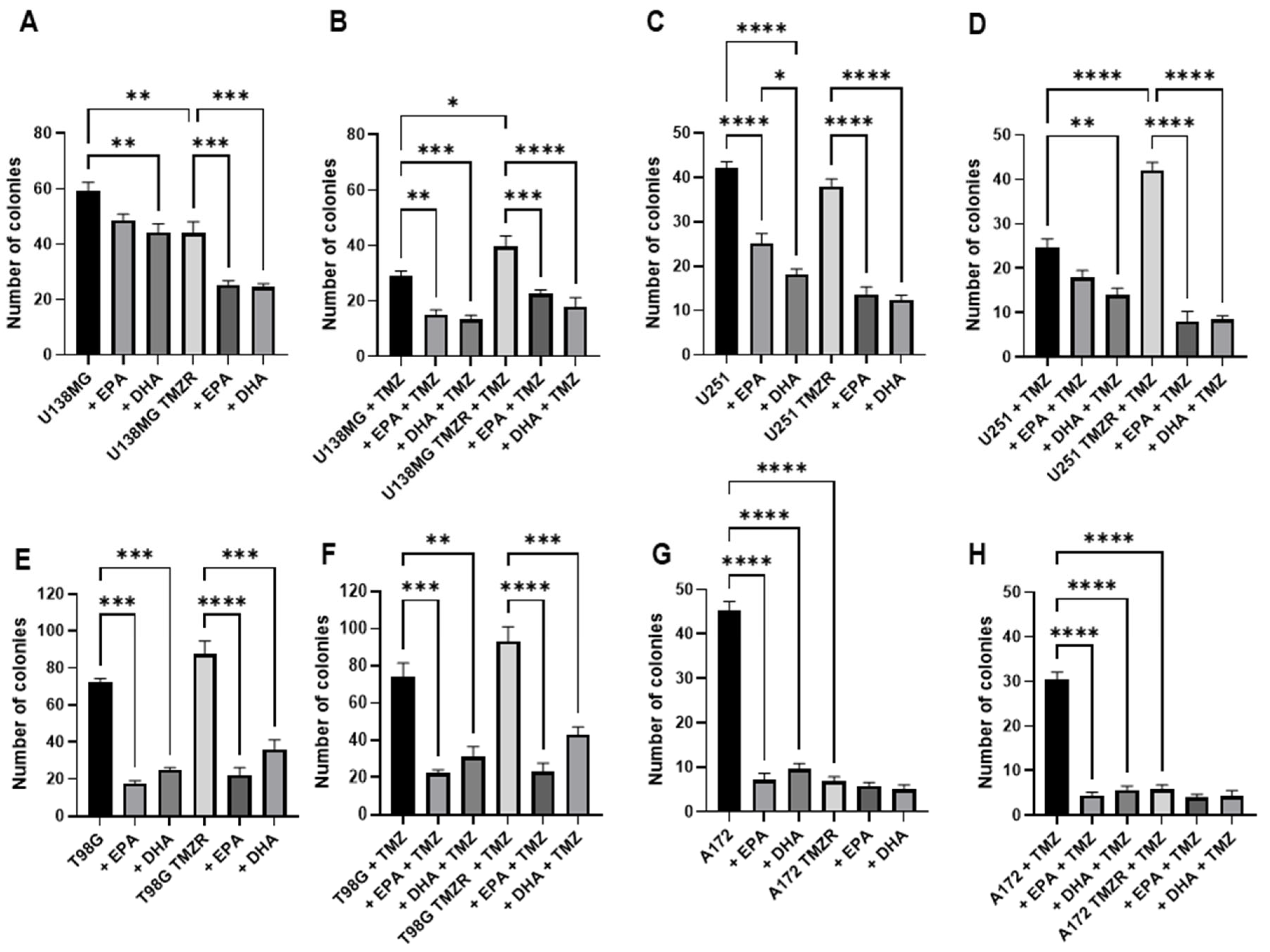
2.2. Influence of TMZ, EPA, or DHA on Clonogenic Capacity
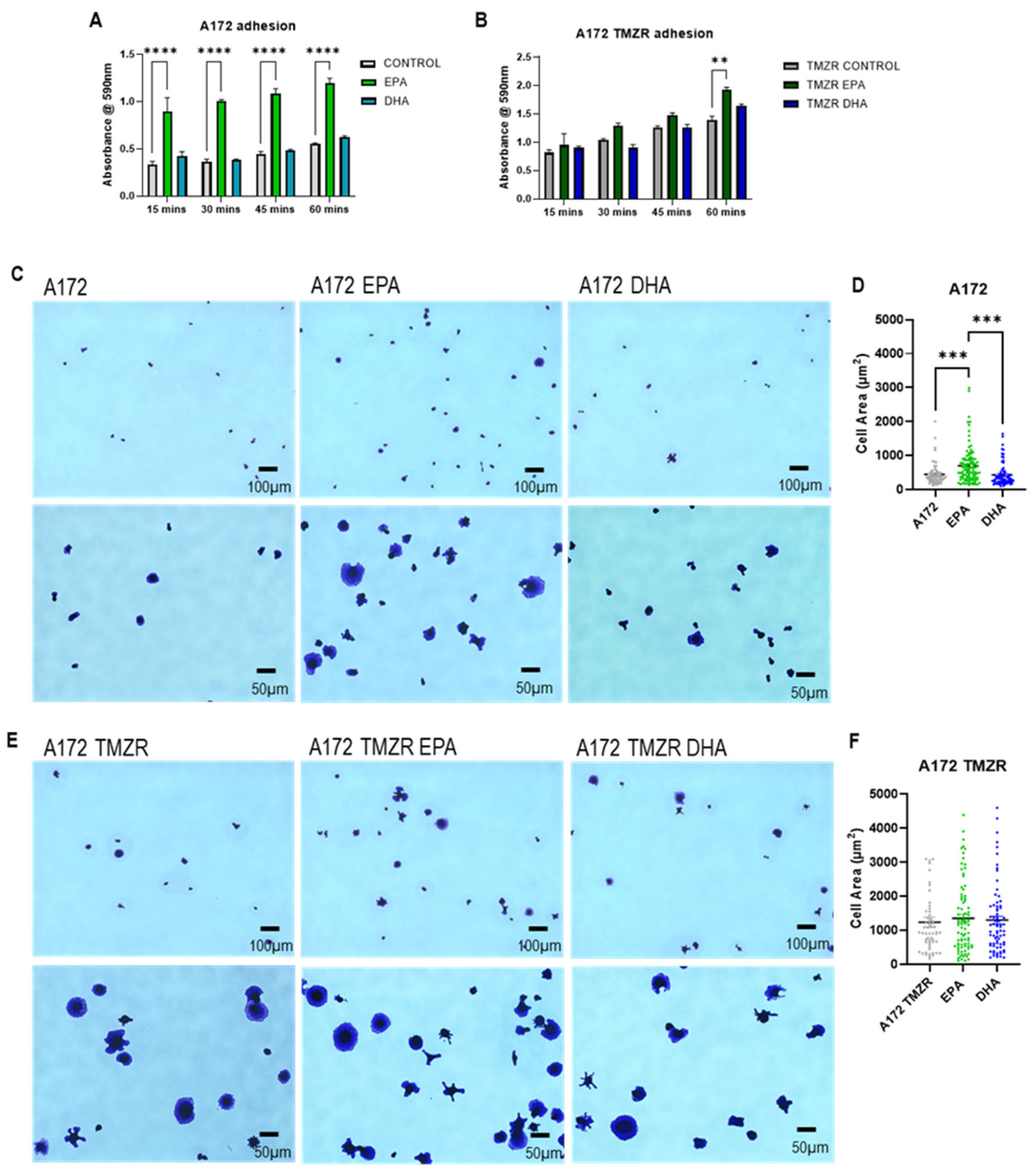
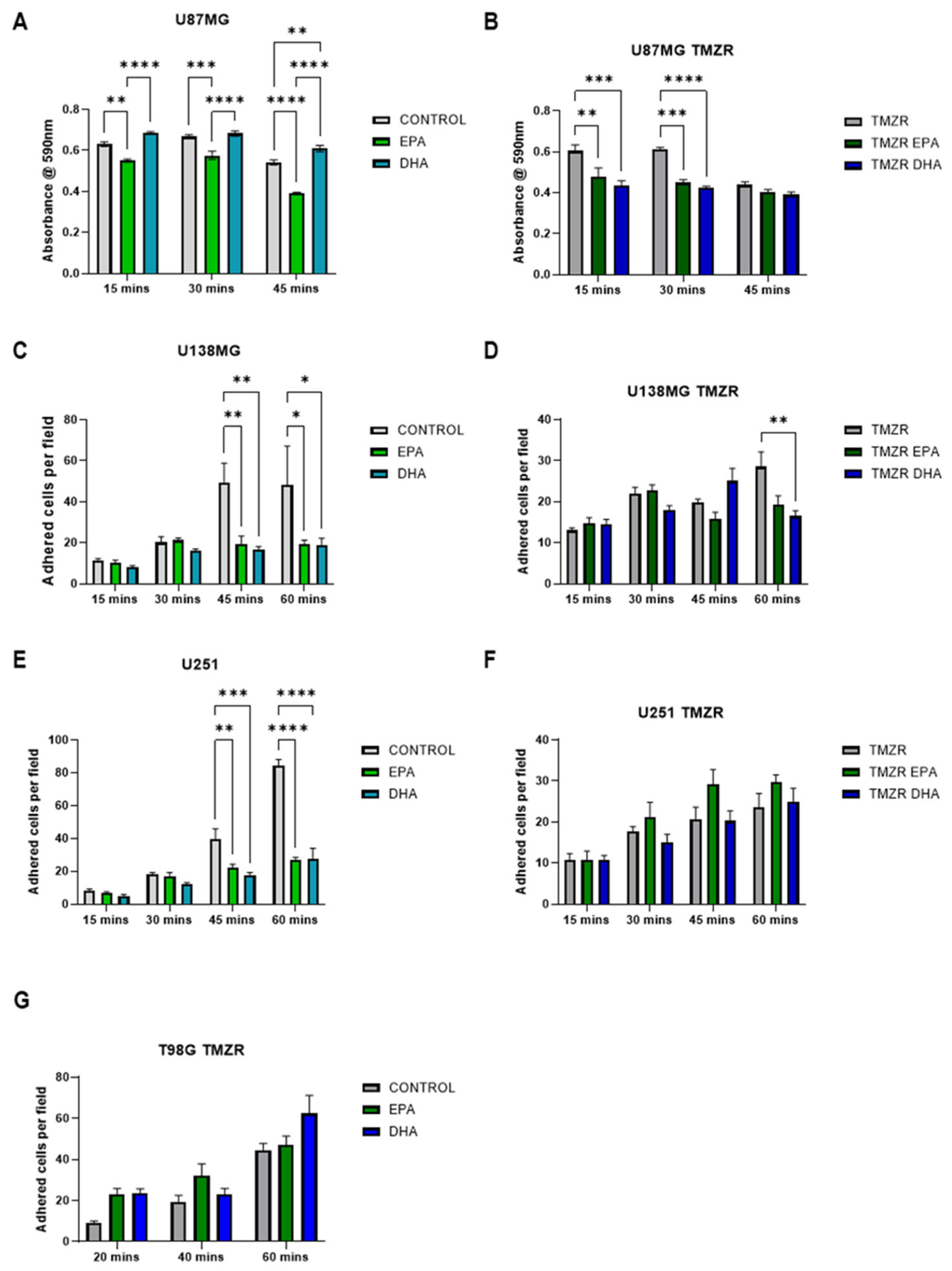
2.3. Influence of EPA or DHA on Cellular Adhesion and Spreading
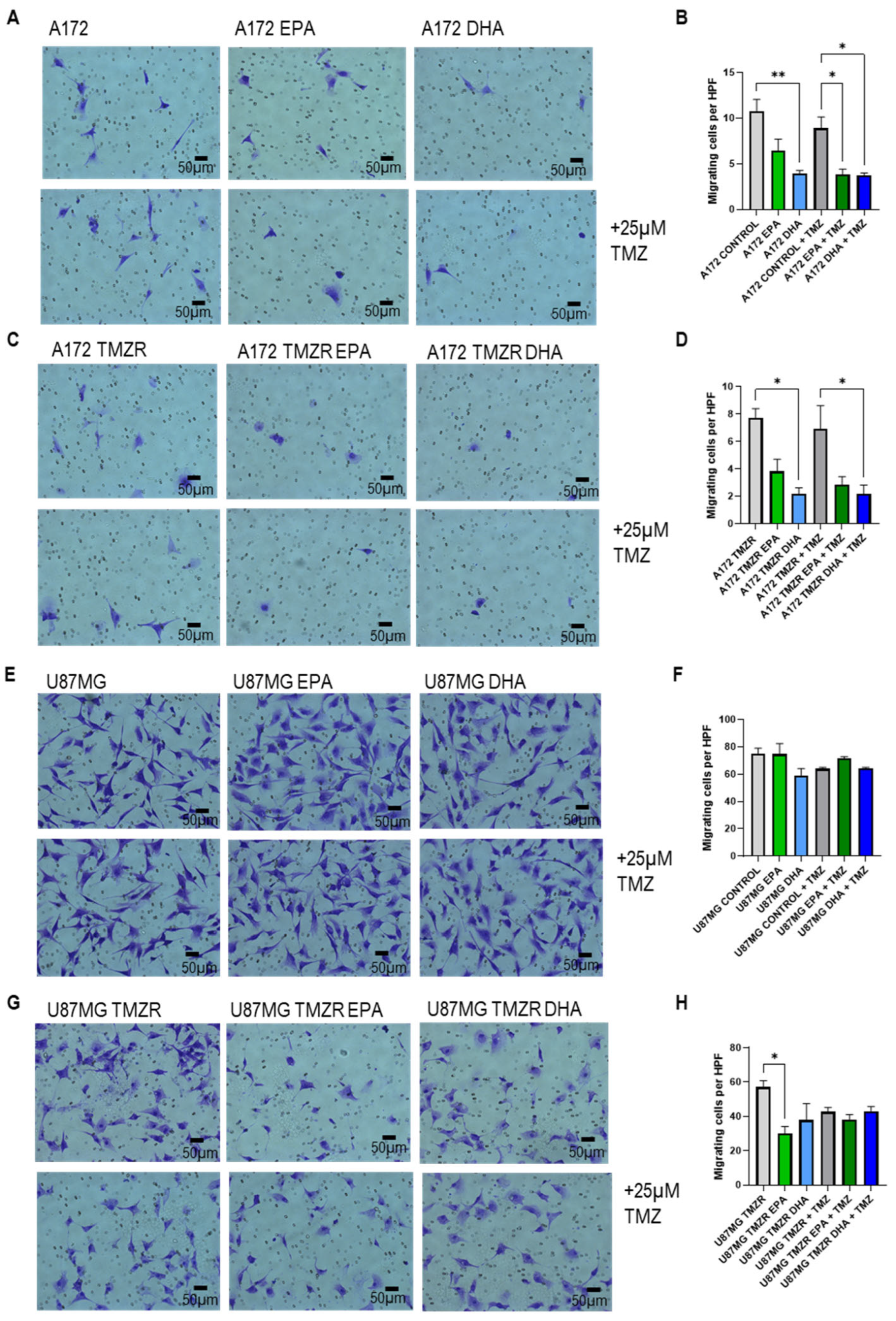
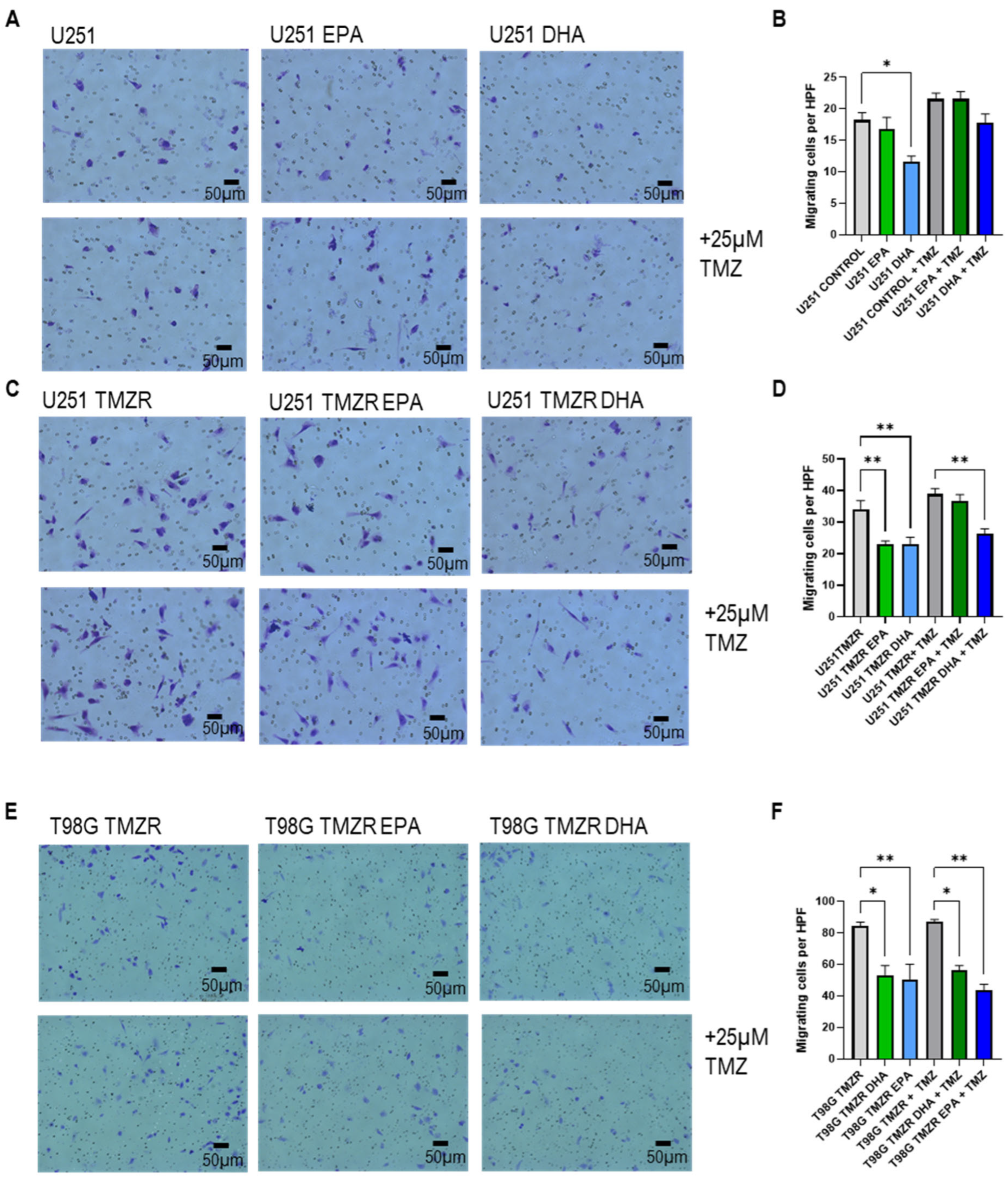
2.4. Influence of EPA or DHA on Glioma Cell Migration
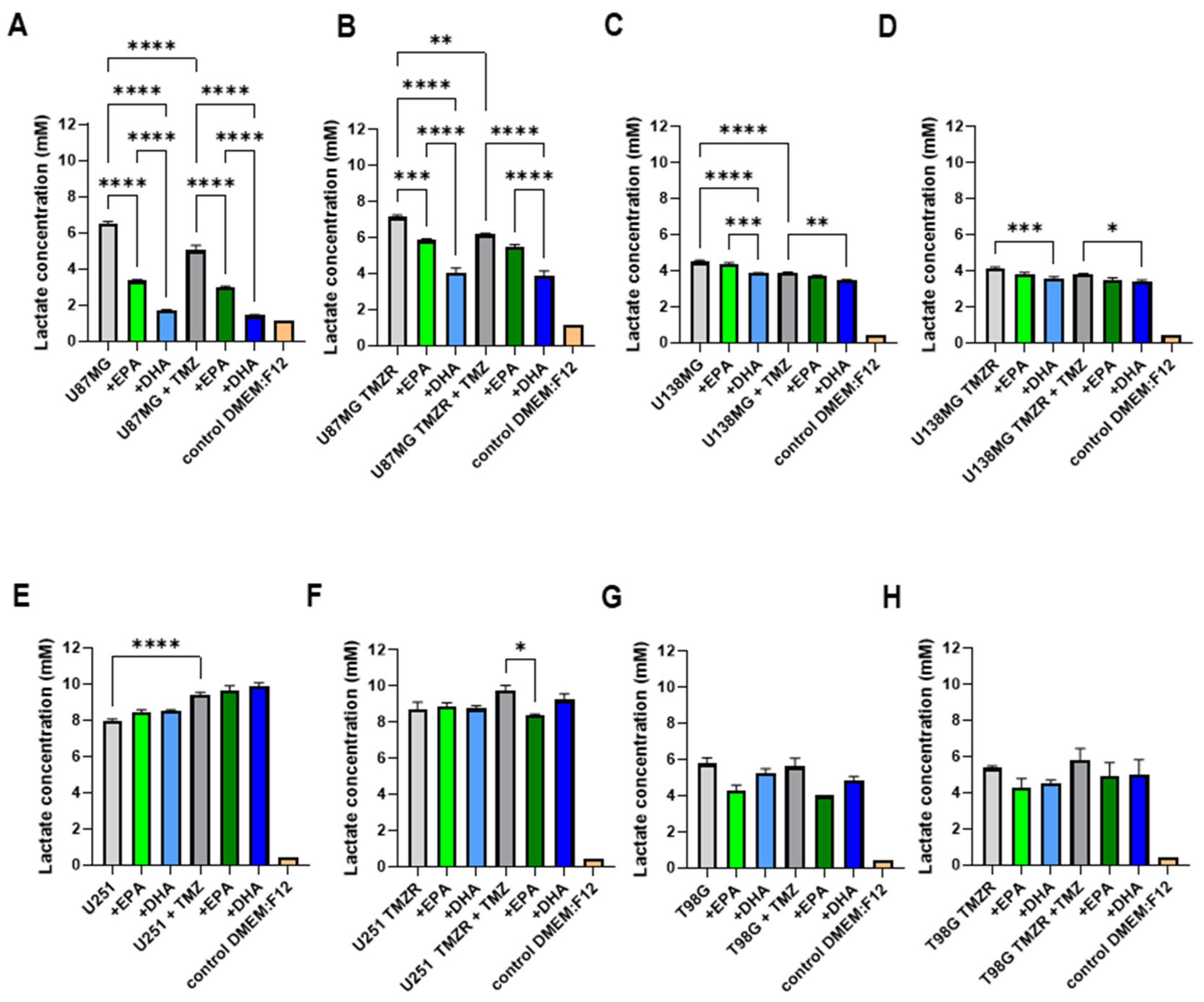
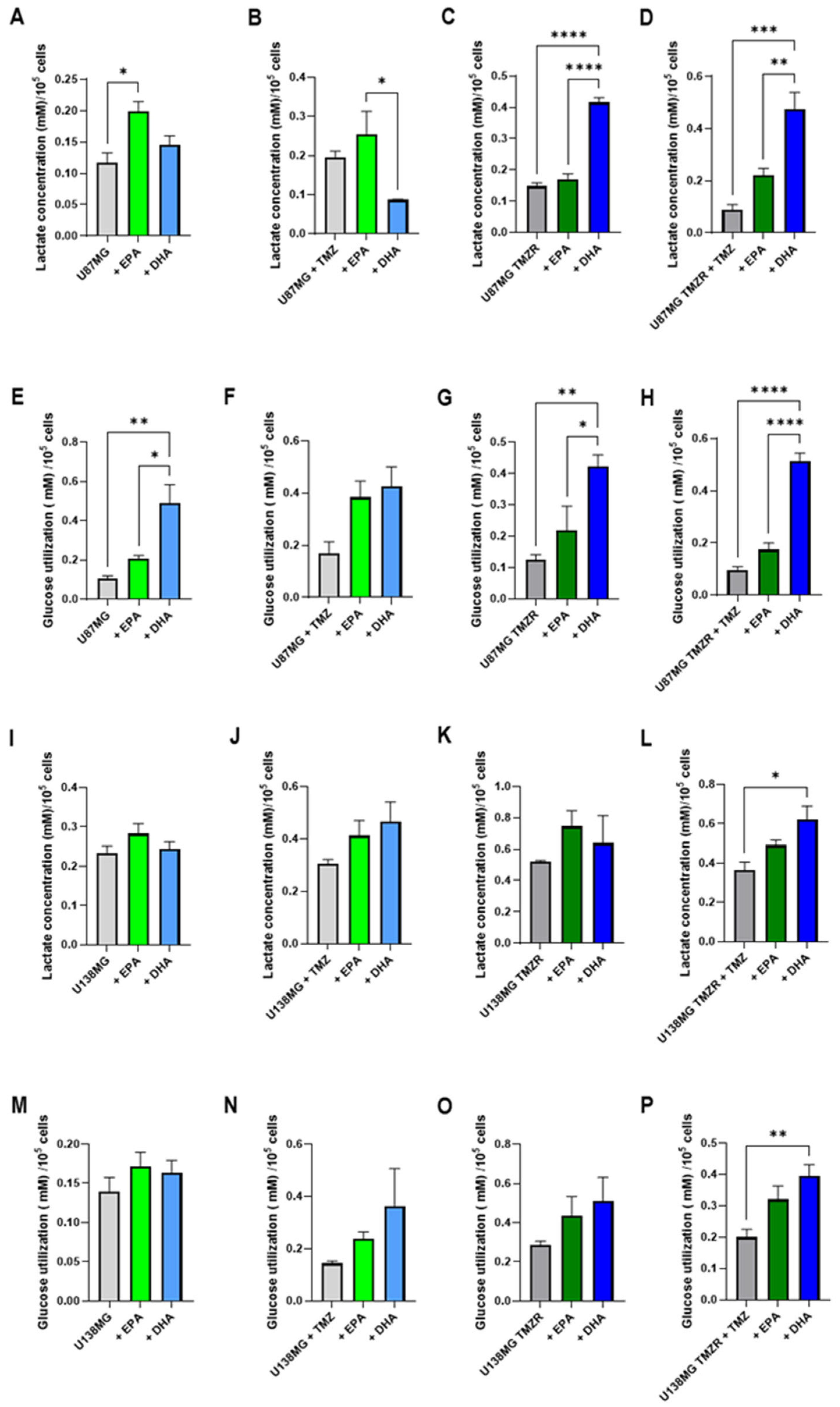
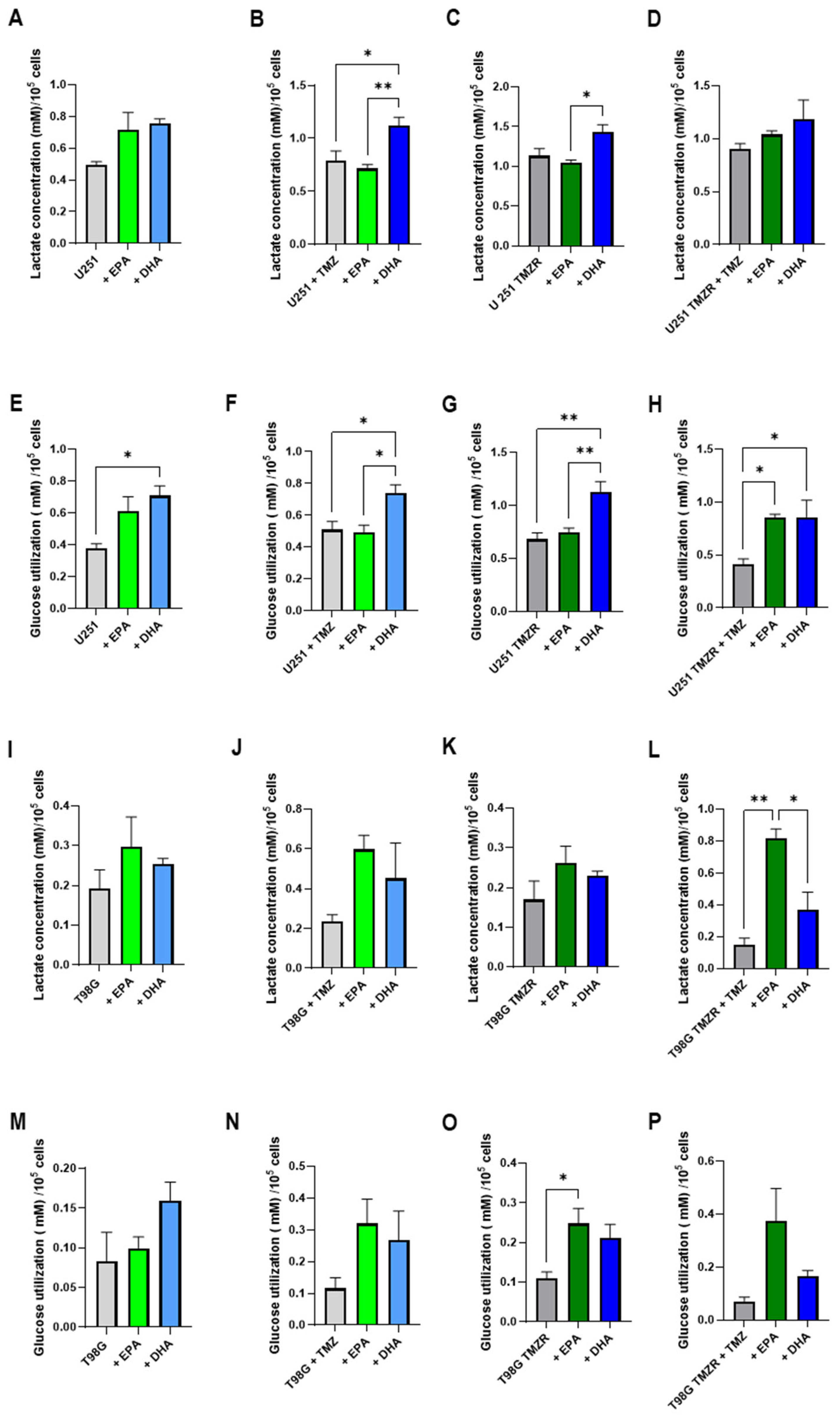
2.5. Alterations in Energy Metabolism Caused by EPA or DHA
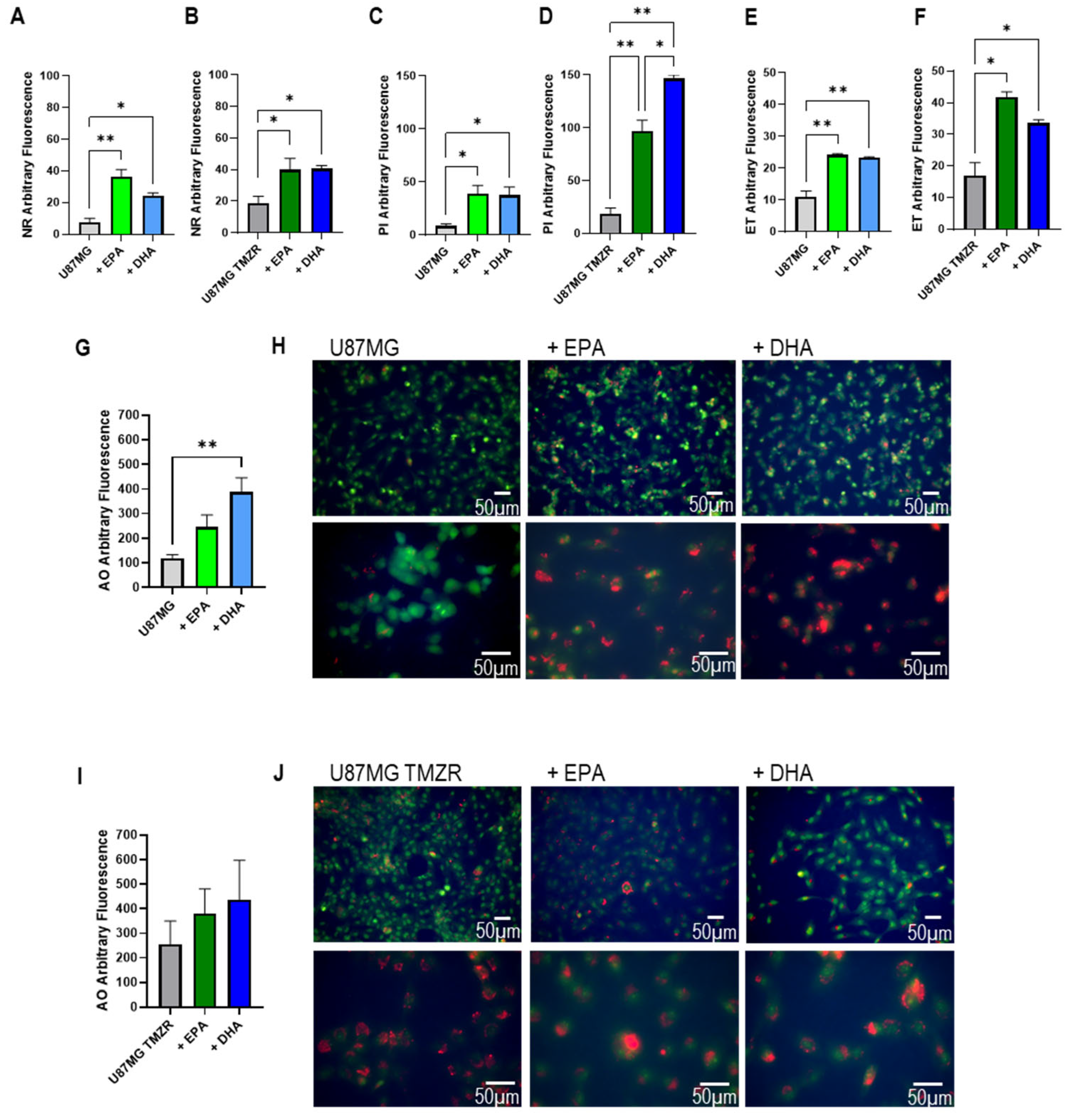
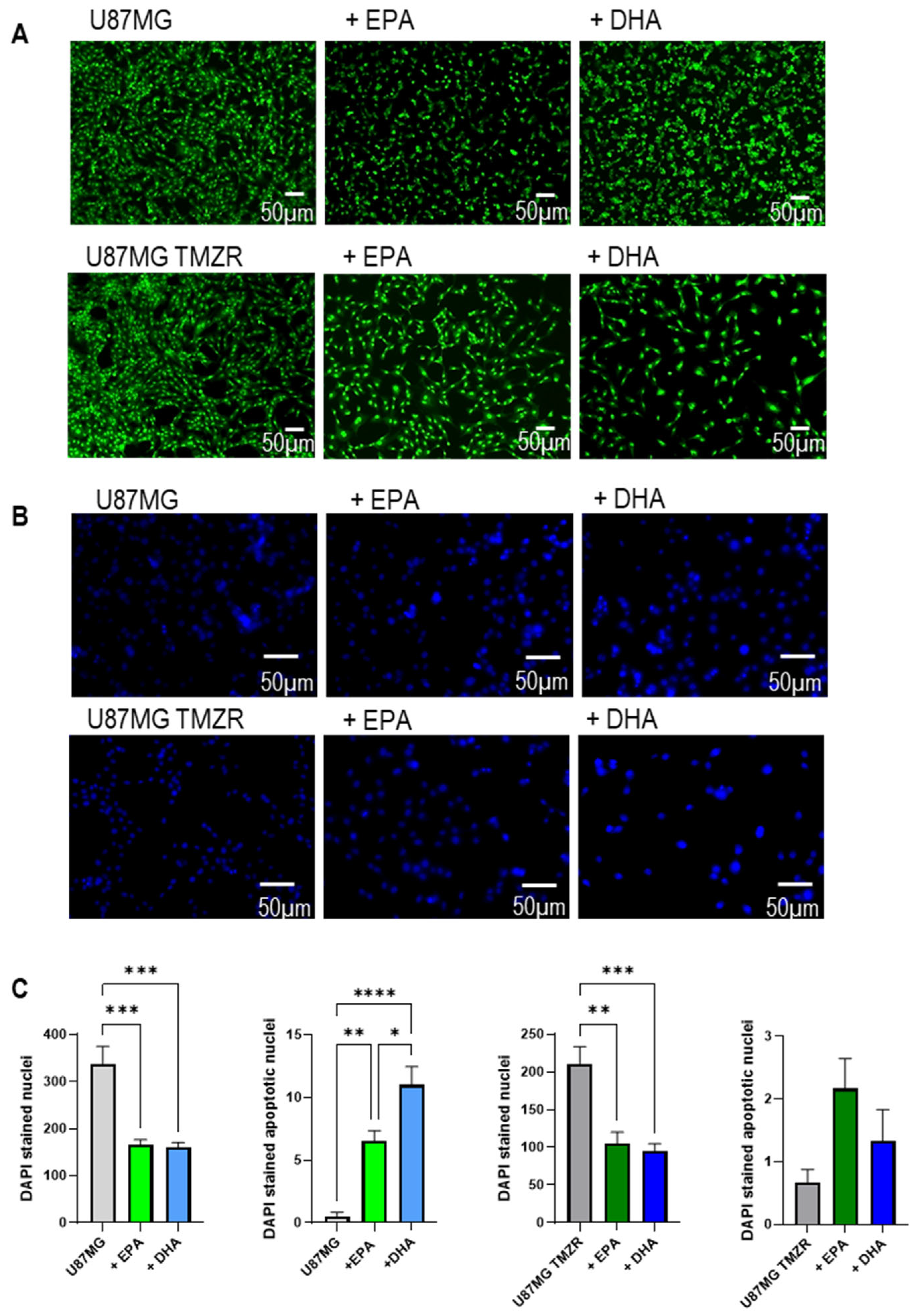
2.6. Lipid Accumulation, Cellular Damage, and Morphological Alterations Caused by EPA or DHA
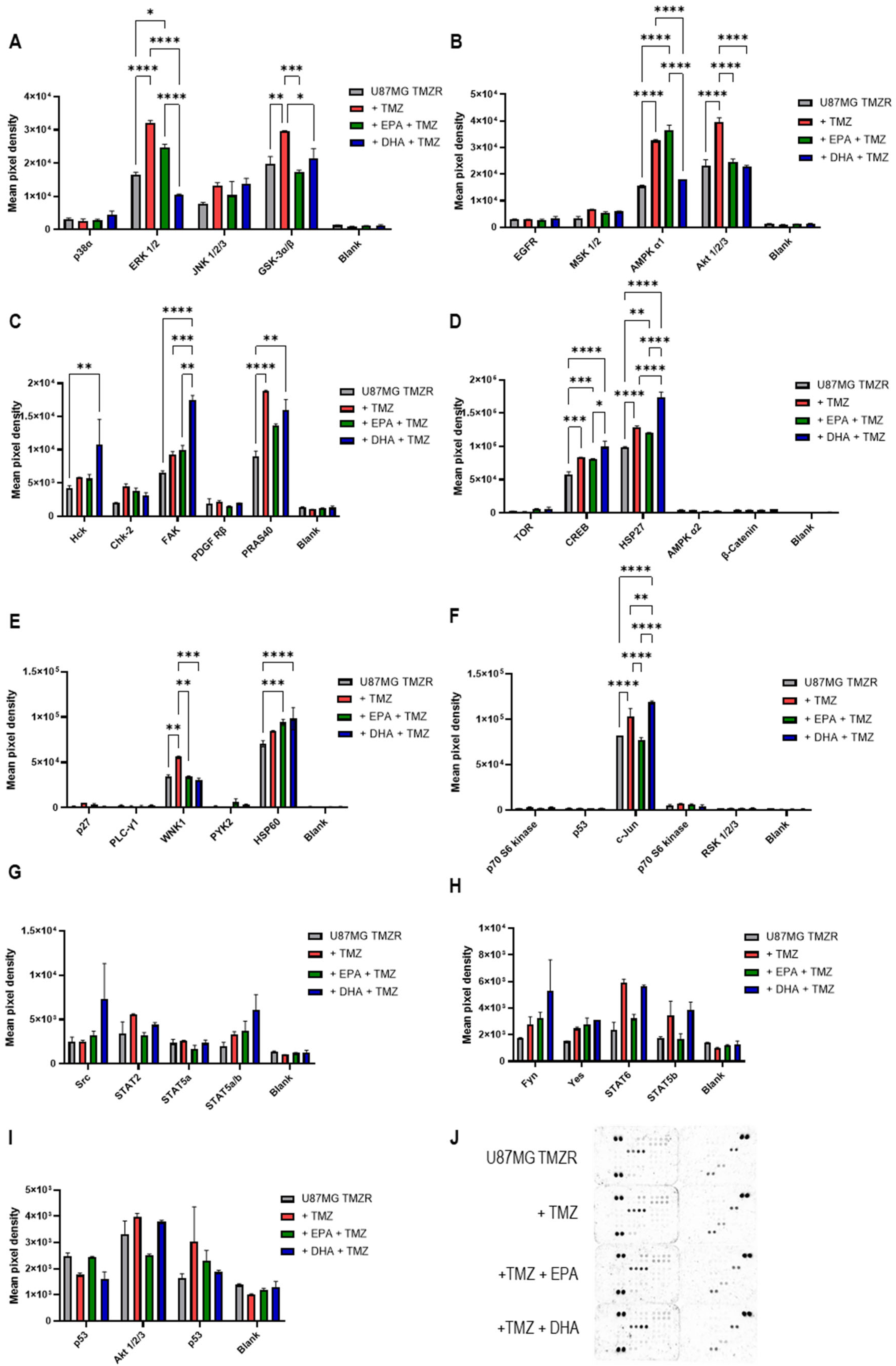
2.7. Human Phosphokinase Proteome Array
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Establishing the Temozolomide Drug-Resistant Cell Lines
4.3. Cell Counts Assay
4.4. Clonogenic Assay
4.5. Adhesion and Cell Spreading Assay
4.6. Transwell Migration Assay
4.7. Lactate and Glucose Assay
4.8. Nile Red Staining of Neutral Lipids
4.9. Propidium Iodide Staining
4.10. Dihydroethidium Staining
4.11. Acridine Orange Staining
4.12. DAPI Staining
4.13. Proteome Profiler Antibody Array
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| DHA | Docosahexaenoic acid |
| GLA | Gamma-linolenic acid |
| EPA | Eicosapentaenoic acid |
| GBM | Glioblastoma |
| IDH | Isocitrate dehydrogenase |
| MDR | Multiple drug resistance |
| MGMT | O6-methylguanine-DNA methyltransferase |
| PUFA | Polyunsaturated fatty acids |
| TMZ | Temozolomide |
| TMZR | Temozolomide resistant |
References
- Louis, D.N. (Ed.) WHO Classification of Tumors of the Central Nervous System, 5th ed.; IARC: Lyon, France, 2021. [Google Scholar]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23 (Suppl. S3), iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, A.; Singh, S.K.; Agnihotri, S.; Jalali, S.; Burrell, K.; Aldape, K.D.; Zadeh, G. GBM’s multifaceted landscape: Highlighting regional and microenvironmental heterogeneity. Neuro-Oncology 2014, 16, 1167–1175. [Google Scholar] [CrossRef]
- Giese, A.; Bjerkvig, R.; Berens, M.; Westphal, M. Cost of migration: Invasion of malignant gliomas and implications for treatment. J. Clin. Oncol. 2003, 21, 1624–1636. [Google Scholar] [CrossRef]
- Okolie, O.; Bago, J.R.; Schmid, R.S.; Irvin, D.M.; Bash, R.E.; Miller, C.R.; Hingtgen, S.D. Reactive astrocytes potentiate tumor aggressiveness in a murine glioma resection and recurrence model. Neuro-Oncology 2016, 18, 1622–1633. [Google Scholar] [CrossRef]
- Choi, C.H. ABC transporters as multidrug resistance mechanisms and the development of chemosensitizers for their reversal. Cancer Cell Int. 2005, 5, 30. [Google Scholar] [CrossRef][Green Version]
- Perazzoli, G.; Prados, J.; Ortiz, R.; Caba, O.; Cabeza, L.; Berdasco, M.; Gónzalez, B.; Melguizo, C.; Alonso, M.M. Temozolomide Resistance in Glioblastoma Cell Lines: Implication of MGMT, MMR, P-Glycoprotein and CD133 Expression. PLoS ONE 2015, 10, e0140131. [Google Scholar] [CrossRef]
- Stavrovskaya, A.A. Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry 2016, 81, 91–100. [Google Scholar][Green Version]
- Colquhoun, A. Mechanisms of action of eicosapentaenoic acid in bladder cancer cells in vitro: Alterations in mitochondrial metabolism, reactive oxygen species generation and apoptosis induction. J. Urol. 2009, 181, 1885–1893. [Google Scholar] [CrossRef]
- Colquhoun, A. Lipids, Mitochondria and Cell Death: Implications in Neuro-oncology. Mol. Neurobiol. 2010, 42, 76–88. [Google Scholar] [CrossRef]
- Das, M.; Das, S. Identification of cytotoxic mediators and their putative role in the signaling pathways during docosahexaenoic acid (DHA)-induced apoptosis of cancer cells. Apoptosis 2016, 21, 1408–1421. [Google Scholar] [CrossRef]
- Benadiba, M.; Miyake, J.A.; Colquhoun, A. Gamma-linolenic Acid Alters Ku80, E2F1, and Bax Expression and Induces Micronucleus Formation in C6 Glioma Cells In Vitro. IUBMB Life 2009, 61, 244–251. [Google Scholar] [CrossRef]
- Zahid, S.; El Dahan, M.S.; Iehl, F.; Fernandez-Varela, P.; Le Du, M.-H.; Ropars, V.; Charbonnier, J.B. The Multifaceted Roles of Ku70/80. Int. J. Mol. Sci. 2021, 22, 4134. [Google Scholar] [CrossRef]
- Yuan, Y.; Shah, N.; Almohaisin, M.I.; Saha, S.; Lu, F. Assessing fatty acid-induced lipotoxicity and its therapeutic potential in glioblastoma using stimulated Raman microscopy. Sci. Rep. 2021, 11, 7422. [Google Scholar] [CrossRef]
- Kim, S.; Jing, K.; Shin, S.; Jeong, S.; Han, S.-H.; Oh, H.; Yoo, Y.-S.; Han, J.; Jeon, Y.-J.; Heo, J.-Y.; et al. ω3-polyunsaturated fatty acids induce cell death through apoptosis and autophagy in glioblastoma cells: In vitro and in vivo. Oncol. Rep. 2018, 39, 239–246. [Google Scholar] [CrossRef]
- Faragó, N.; Fehér, L.Z.; Kitajka, K.; Das, U.N.; Puskás, L.G. MicroRNA profile of polyunsaturated fatty acid treated glioma cells reveal apoptosis-specific expression changes. Lipids Health Dis. 2011, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Miyake, J.A.; Benadiba, M.; Colquhoun, A. Gamma-linolenic acid inhibits both tumour cell cycle progression and angiogenesis in the orthotopic C6 glioma model through changes in VEGF, Flt1, ERK1/2, MMP2, cyclin D1, pRb, p53 and p27 protein expression. Lipids Health Dis. 2009, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Torvund, M.; Mandal, C.C. Omega-3 Fatty Acid Treatment Combined with Chemotherapy to Prevent Toxicity, Drug Resistance, and Metastasis in Cancer. Curr. Drug Targets 2022, 23, 574–596. [Google Scholar] [CrossRef]
- Wang, F.; Bhat, K.; Doucette, M.; Zhou, S.; Gu, Y.; Law, B.; Liu, X.; Wong, E.T.; Kang, J.X.; Hsieh, T.C.; et al. Docosahexaenoic Acid (DHA) Sensitizes Brain Tumor Cells to Etoposide-Induced Apoptosis. Curr. Mol. Med. 2011, 11, 503–511. [Google Scholar] [CrossRef]
- Harvey, K.A.; Xu, Z.; Saaddatzadeh, M.R.; Wang, H.; Pollok, K.; Cohen-Gadol, A.A.; Siddiqui, R.A. Enhanced anticancer properties of lomustine in conjunction with docosahexaenoic acid in glioblastoma cell lines. J. Neurosurg. 2015, 122, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Chen, X.; Liu, C.; Gao, Y.; Hu, W. Eicosapentaenoic acid enhances the sensitivity of glioma cell line U87 to temozolomide via inhibiting endoplasmic reticulum stress. Chin. J. Clin. Nutr. 2017, 25, 361–365. [Google Scholar]
- Silva, J.A.; Colquhoun, A. Effect of Polyunsaturated Fatty Acids on Temozolomide Drug-Sensitive and Drug-Resistant Glioblastoma Cells. Biomedicines 2023, 11, 779. [Google Scholar] [CrossRef]
- Rosso, L.; Brock, C.S.; Gallo, J.M.; Saleem, A.; Price, P.M.; Turkheimer, F.E.; Aboagye, E.O. A New model for prediction of drug distribution in tumor and normal tissues: Pharmacokinetics of temozolomide in glioma patients. Cancer Res. 2009, 69, 120–127. [Google Scholar] [CrossRef]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Temozolomide in Malignant Glioma Patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef]
- Beltzig, L.; Schwarzenbach, C.; Leukel, P.; Frauenknecht, K.B.M.; Sommer, C.; Tancredi, A.; Hegi, M.E.; Christmann, M.; Kaina, B. Senescence Is the Main Trait Induced by Temozolomide in Glioblastoma Cells. Cancers 2022, 14, 2233. [Google Scholar] [CrossRef]
- Colquhoun, A.; Schumacher, R.I. γ-Linolenic acid and eicosapentaenoic acid induce modifications in mitochondrial metabolism, reactive oxygen species generation, lipid peroxidation and apoptosis in Walker 256 rat carcinosarcoma cells. Biochim. Biophys. Acta 2001, 1533, 207–219. [Google Scholar] [CrossRef]
- Ramos, K.L.; Colquhoun, A. Protective role of glucose-6-phosphate dehydrogenase activity in the metabolic response of C6 rat glioma cells to polyunsaturated fatty acid exposure. Glia 2003, 43, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Lin, H.; Zhang, X.; Li, J. Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-κB-dependent pathway. Oncol. Rep. 2012, 27, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Braeckman, R.A.; Stirtan, W.G.; Soni, P.N. Pharmacokinetics of Eicosapentaenoic Acid in Plasma and Red Blood Cells After Multiple Oral Dosing With Icosapent Ethyl in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2013, 3, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Mortaz, E.; Asghari, M.H.; Adcock, I.M.; Redegeld, F.A.; Garssen, J. Effects of the polyunsaturated fatty acids, EPA and DHA, on hematological malignancies: A systematic review. Oncotarget 2018, 9, 11858–11875. [Google Scholar] [CrossRef]
- von Schacky, C. Importance of EPA and DHA Blood Levels in Brain Structure and Function. Nutrients 2021, 13, 1074. [Google Scholar] [CrossRef]
- Rapaport, S.; Ramadan, E.; Basselin, M. Docosahexaenoic acid (DHA) incorporation into the brain from plasma, as an in vivo biomarker of brain DHA metabolism and neurotransmission. Prostaglandins Other Lipid Mediat. 2011, 96, 109–113. [Google Scholar] [CrossRef]
- Nasrollahzadeh, J.; Siassi, F.; Doosti, M.; Eshraghian, M.R.; Shokri, F.; Modarressi, M.H.; Mohammadi-Asl, J.; Abdi, K.; Nikmanesh, A.; Karimian, S.M. The influence of feeding linoleic, gamma-linolenic and docosahexaenoic acid rich oils on rat brain tumor fatty acids composition and fatty acid binding protein 7 mRNA expression. Lipids Health Dis. 2008, 7, 45. [Google Scholar] [CrossRef]
- Yassine, H.N.; Croteau, E.; Rawat, V.; Hibbeln, J.R.; Rapoport, S.I.; Cunnane, S.C.; Umhau, J.C. DHA brain uptake and APOE4 status: A PET study with [1-11C]-DHA. Alzheimer’s Res. Ther. 2017, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.P.; Brown, R.E.; Zhang, P.-C.; Zhao, Y.-T.; Ju, X.-H.; Song, C. DHA, EPA and their combination at various ratios differently modulated Aβ25-35-induced neurotoxicity in SH-SY5Y cells. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 fatty acids eicosapentaenoic (EPA) and docosahexaenoic (DHA) as modulatory and anti-inflammatory agents in noncommunicable diet-related diseases—Reports from the last 10 years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Joardar, A.; Sen, A.K.; Das, S. Docosahexaenoic acid facilitates cell maturation and beta-adrenergic transmission in astrocytes. J. Lipid Res. 2006, 47, 571–581. [Google Scholar] [CrossRef]
- Champeil-Potokar, G.; Hennebelle, M.; Latour, A.; Vancassel, S.; Denis, I. Docosahexaenoic acid (DHA) prevents corticosterone-induced changes in astrocyte morphology and function. J. Neurochem. 2016, 136, 1155–1167. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Markiewicz, M.; Walczewska, A. Omega-3 PUFAs Suppress IL-1β-Induced Hyperactivity of Immunoproteasomes in Astrocytes. Int. J. Mol. Sci. 2021, 22, 5410. [Google Scholar] [CrossRef]
- Serafim, R.B.; da Silva, P.; Cardoso, C.; Di Cristofaro, L.F.M.; Netto, R.P.; de Almeida, R.; Navegante, G.; Storti, C.B.; de Sousa, J.F.; de Souza, F.C.; et al. Expression Profiling of Glioblastoma Cell Lines Reveals Novel Extracellular Matrix-Receptor Genes Correlated With the Responsiveness of Glioma Patients to Ionizing Radiation. Front. Oncol. 2021, 11, 668090. [Google Scholar] [CrossRef]
- Delamarre, E.; Taboubi, S.; Mathieu, S.; Bérenguer, C.; Rigot, V.; Lissitzky, J.-C.; Figarella-Branger, D.; Ouafik, L.; Luis, J. Expression of Integrin 6 1 Enhances Tumorigenesis in Glioma Cells. Am. J. Pathol. 2009, 175, 844–855. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Poleszak, K.; Pasierbinska, M.; Kaminska, B. Integrin Signaling in Glioma Pathogenesis: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 888. [Google Scholar] [CrossRef]
- Kubota, H.; Hirai, T.; Yamauchi, A.; Ogo, A.; Matsumoto, H.; Ueno, T. Inhibitory Effect of Eicosapentaenoic Acid on the Migration of the Esophageal Squamous Cell Carcinoma Cell Line TE-1. Anticancer Res. 2020, 40, 5043–5048. [Google Scholar] [CrossRef]
- Rahman, M.; Veigas, J.M.; Williams, P.J.; Fernandes, G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Res. Treat. 2013, 141, 341–352. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Li, X.; Tan, Y.-W.; Fang, Y.-J.; Liu, K.-Y.; Wang, Y.-F.; Ma, T.; Ou, Q.-J.; Zhang, C.-X. Docosahexaenoic acid inhibits the invasion and migration of colorectal cancer by reversing EMT through the TGF-β1/Smad signaling pathway. Food Funct. 2024, 15, 9420–9433. [Google Scholar] [CrossRef] [PubMed]
- Javadian, M.; Shekari, N.; Zangbar, M.S.S.; Mohammadi, A.; Mansoori, B.; Maralbashi, S.; Shanehbandi, D.; Baradaran, B.; Darabi, M.; Kazemi, T. Docosahexaenoic acid suppresses migration of triple-negative breast cancer cell through targeting metastasis-related genes and microRNA under normoxic and hypoxic conditions. J. Cell. Biochem. 2020, 121, 2416–2427. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Song, K.-S.; Shin, S.; Kim, S.; Heo, J.-Y.; Kweon, G.-R.; Wu, T.; Park, J.-I.; Lim, K. Docosahexaenoic acid suppresses breast cancer cell metastasis by targeting matrix-metalloproteinases. Oncotarget 2016, 7, 49961–49971. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Choi, W.-S.; Sun, X.; Godbout, R. Super resolution microscopy reveals DHA-dependent alterations in glioblastoma membrane remodelling and cell migration. Nanoscale 2021, 13, 9706–9722. [Google Scholar] [CrossRef]
- Kim, J.; Han, J.; Jang, Y.; Kim, S.J.; Lee, M.J.; Ryu, M.J.; Kweon, G.R.; Heo, J.Y. High-capacity glycolytic and mitochondrial oxidative metabolisms mediate the growth ability of glioblastoma. Int. J. Oncol. 2015, 47, 1009–1016. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Y.; Shingu, T.; Feng, L.; Chen, Z.; Ogasawara, M.; Keating, M.J.; Kondo, S.; Huang, P. Metabolic Alterations in Highly Tumorigenic Glioblastoma Cells. J. Biol. Chem. 2011, 286, 32843–32853. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Chen, N.-F.; Wen, Z.-H.; Yao, Z.-K.; Tsui, K.-H.; Kuo, H.-M.; Chen, W.-F. Glutathione S-Transferase M3 Is Associated with Glycolysis in Intrinsic Temozolomide-Resistant Glioblastoma Multiforme Cells. Int. J. Mol. Sci. 2021, 22, 7080. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; van der Kellen, D.; Gonçalves, L.G.; Serpa, J. Metabolic Profiles Point Out Metabolic Pathways Pivotal in Two Glioblastoma (GBM) Cell Lines, U251 and U-87MG. Biomedicines 2023, 11, 2041. [Google Scholar] [CrossRef] [PubMed]
- Caragher, S.; Miska, J.; Shireman, J.; Park, C.H.; Muroski, M.; Lesniak, M.S.; Ahmed, A.U. Temozolomide Treatment Increases Fatty Acid Uptake in Glioblastoma Stem Cells. Cancers 2020, 2, 3126. [Google Scholar] [CrossRef]
- Katsnelson, G.; Ceddia, R.B. Docosahexaenoic and eicosapentaenoic fatty acids differentially regulate glucose and fatty acid metabolism in L6 rat skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2020, 319, C1120–C1129. [Google Scholar] [CrossRef] [PubMed]
- Hari, A.; Vegi, N.; Das, U. Arachidonic and eicosapentaenoic acids induce oxidative stress to suppress proliferation of human glioma cells. Arch. Med. Sci. 2020, 16, 974–983. [Google Scholar] [CrossRef]
- Campos-Sandoval, J.A.; Gómez-García, M.C.; Santos-Jiménez, J.d.L.; Matés, J.M.; Alonso, F.J.; Márquez, J. Antioxidant responses related to temozolomide resistance in glioblastoma. Neurochem. Int. 2021, 149, 105136. [Google Scholar] [CrossRef]
- Clark, C.; Behrooz, A.B.; Cordani, M.; Shojaei, S.; Ghavami, S. Assessing Autophagy Flux in Glioblastoma Temozolomide Resistant Cells. Methods Mol. Biol. 2025, 2879, 225–238. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, Z.; Xu, N.; Liu, B.; Fu, Z.; Lian, C.; Guo, H. Connective tissue growth factor promotes temozolomide resistance in glioblastoma through TGF-β1-dependent activation of Smad/ERK signaling. Cell Death Dis. 2017, 8, e2885. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Wang, W.; Liang, J. Temozolomide inhibits cellular growth and motility via targeting ERK signaling in glioma C6 cells. Mol. Med. Rep. 2016, 14, 5732–5738. [Google Scholar] [CrossRef]
- Yi, G.Z.; Liu, Y.-W.; Xiang, W.; Wang, H.; Chen, Z.-Y.; Xie, S.-D.; Qi, S.-T. Akt and β-catenin contribute to TMZ resistance and EMT of MGMT negative malignant glioma cell line. J. Neurol. Sci. 2016, 367, 101–106. [Google Scholar] [CrossRef]
- Sun, S.; Wang, X.; Xu, X.; Di, H.; Du, J.; Xu, B.; Wang, Q.; Wang, J. MiR-433-3p suppresses cell growth and enhances chemosensitivity by targeting CREB in human glioma. Oncotarget 2017, 8, 5057–5068. [Google Scholar] [CrossRef] [PubMed]
- Kıyga, E.; Adıgüzel, Z.; Uçar, E.Ö. Temozolomide increases heat shock proteins in extracellular vesicles released from glioblastoma cells. Mol. Biol. Rep. 2022, 49, 8701–8713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Begum, G.; Pointer, K.; Clark, P.A.; Yang, S.-S.; Lin, S.-H.; Kahle, K.T.; Kuo, J.S.; Sun, D. WNK1-OSR1 kinase-mediated phospho-activation of Na+-K+-2Cl- cotransporter facilitates glioma migration. Mol. Cancer 2014, 13, 31. [Google Scholar] [CrossRef]
- Luo, L.; Wang, J.; Ding, D.; Hasan, N.; Yang, S.-S.; Lin, S.-H.; Schreppel, P.; Sun, B.; Yin, Y.; Erker, T.; et al. Role of NKCC1 Activity in Glioma K+ Homeostasis and Cell Growth: New Insights With the Bumetanide-Derivative STS66. Front. Physiol. 2020, 11, 911. [Google Scholar] [CrossRef]
- Munoz, J.L.; Rodriguez-Cruz, V.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Temozolomide resistance in glioblastoma cells occurs partly through epidermal growth factor receptor-mediated induction of connexin 43. Cell Death Dis. 2014, 5, e1145. [Google Scholar] [CrossRef]
- Miyake, J.A.; Benadiba, M.; Ribeiro, G.; Silva, D.D.; Colquhoun, A. Novel Ruthenium—Gamma-linolenic Acid Complex Inhibits C6 Rat Glioma Cell Proliferation In Vitro and in the Orthotopic C6 Model In Vivo After Osmotic Pump Infusion. Anticancer Res. 2014, 34, 1901–1912. [Google Scholar]
- Yoshino, A.; Ogino, A.; Yachi, K.; Ohta, T.; Fukushima, T.; Watanabe, T.; Katayama, Y.; Okamoto, Y.; Naruse, N.; Sano, E.; et al. Gene expression profiling predicts response to temozolomide in malignant gliomas. Int. J. Oncol. 2010, 36, 1367–1377. [Google Scholar] [CrossRef]
- Barazzuol, L.; Jena, R.; Burnet, N.G.; Meira, L.B.; Jeynes, J.C.G.; Kirkby, K.J.; Kirkby, N.F. Evaluation of poly (ADP-ribose) polymerase inhibitor ABT-888 combined with radiotherapy and temozolomide in glioblastoma. Radiat. Oncol. 2013, 8, 65. [Google Scholar] [CrossRef]
- Catanzaro, D.; Milani, G.; Bozza, A.; Bernardi, M.; Chieregato, K.; Menarin, M.; Merlo, A.; Celli, P.; Belli, R.; Peroni, D.; et al. Selective cell cycle arrest in glioblastoma cell lines by quantum molecular resonance alone or in combination with temozolomide. Br. J. Cancer 2022, 127, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.D.C.; Ferreira, M.T.; Colquhoun, A. Influence of Lipoxygenase Inhibition on Glioblastoma Cell Biology. Int. J. Mol. Sci. 2020, 21, 8395. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Miyake, J.A.; Gomes, R.N.; Feitoza, F.; Stevannato, P.B.; da Cunha, A.S.; Serachi, F.d.O.; Panagopoulos, A.T.; Colquhoun, A. Cyclooxygenase Inhibition Alters Proliferative, Migratory, and Invasive Properties of Human Glioblastoma Cells In Vitro. Int. J. Mol. Sci. 2021, 22, 4297. [Google Scholar] [CrossRef]
- Ribeiro, G.; Benadiba, M.; Silva, D.d.O.; Colquhoun, A. The novel ruthenium-γ-linolenic complex [Ru2(aGLA)4Cl] inhibits C6 rat glioma cell proliferation and induces changes in mitochondrial membrane potential, increased reactive oxygen species generation and apoptosis in vitro. Cell Biochem. Funct. 2010, 28, 15–23. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.A.; Colquhoun, A. The Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid Enhance the Effects of Temozolomide Chemotherapy in Glioblastoma Cells. Int. J. Mol. Sci. 2025, 26, 8759. https://doi.org/10.3390/ijms26188759
Silva JA, Colquhoun A. The Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid Enhance the Effects of Temozolomide Chemotherapy in Glioblastoma Cells. International Journal of Molecular Sciences. 2025; 26(18):8759. https://doi.org/10.3390/ijms26188759
Chicago/Turabian StyleSilva, Janaína Alessandra, and Alison Colquhoun. 2025. "The Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid Enhance the Effects of Temozolomide Chemotherapy in Glioblastoma Cells" International Journal of Molecular Sciences 26, no. 18: 8759. https://doi.org/10.3390/ijms26188759
APA StyleSilva, J. A., & Colquhoun, A. (2025). The Omega-3 Fatty Acids Eicosapentaenoic Acid and Docosahexaenoic Acid Enhance the Effects of Temozolomide Chemotherapy in Glioblastoma Cells. International Journal of Molecular Sciences, 26(18), 8759. https://doi.org/10.3390/ijms26188759





