Polysialylation of Glioblastoma Cells Is Regulated by Autophagy Under Nutrient Deprivation
Abstract
1. Introduction
2. Results
2.1. STs, NEUs, and CMAS Expression in GBM Cells
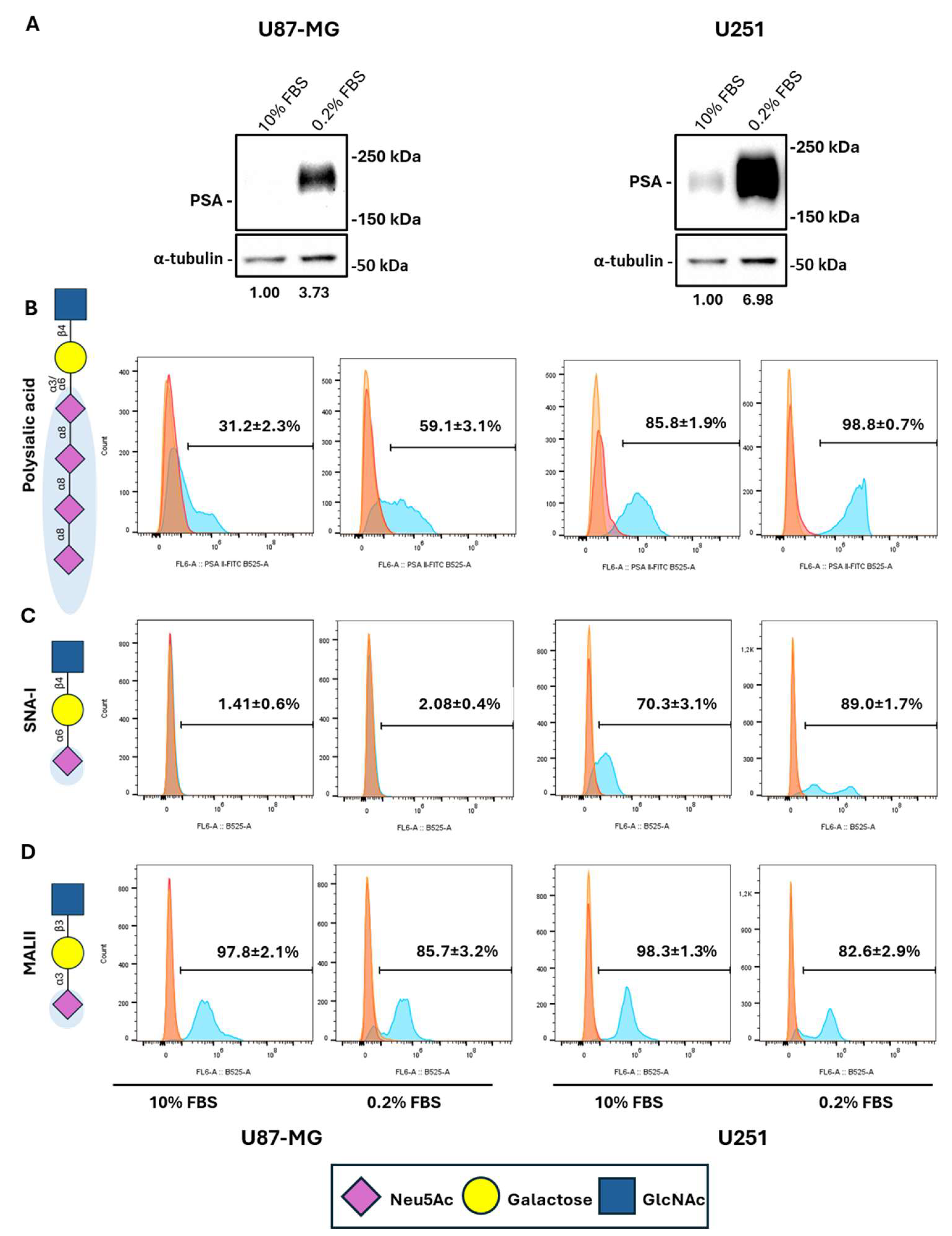
2.2. Serum Deprivation Induces PSA Levels in GBM Cells
2.3. Serum Starvation Preferentially Induces Cell Surface NCAM Polysialylation While Reducing Other Proteins’ Sialylation in GBM Cells
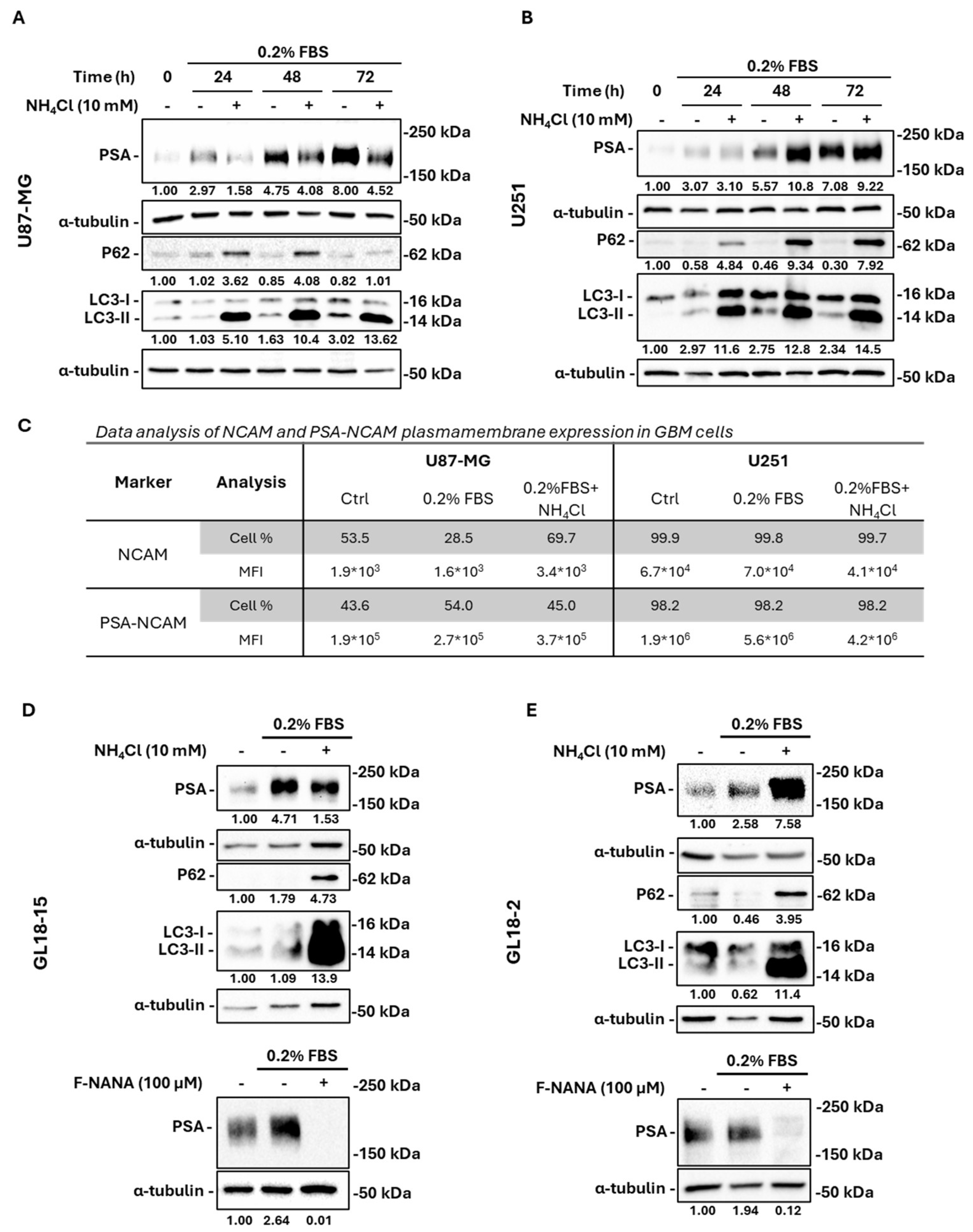
2.4. Nutrient Deprivation-Induced PSA Levels Are Regulated by Autophagy in GBM Cells
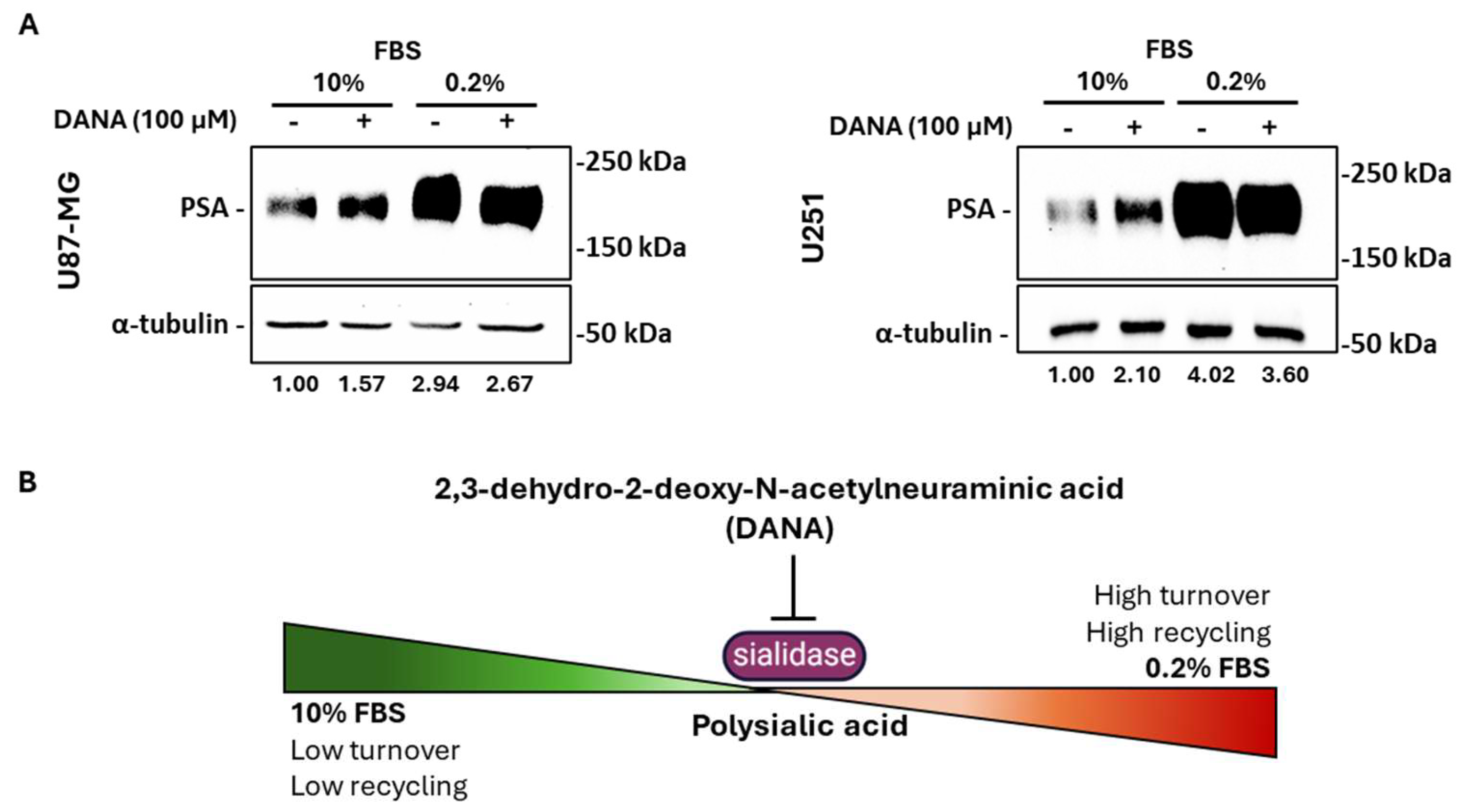
2.5. Neuraminidases Activity Is Fundamental for PSA Turnover in Serum-Deprived GBM Cells
2.6. PSA Expression Is Related to Autophagy Activation in GBM Tissues
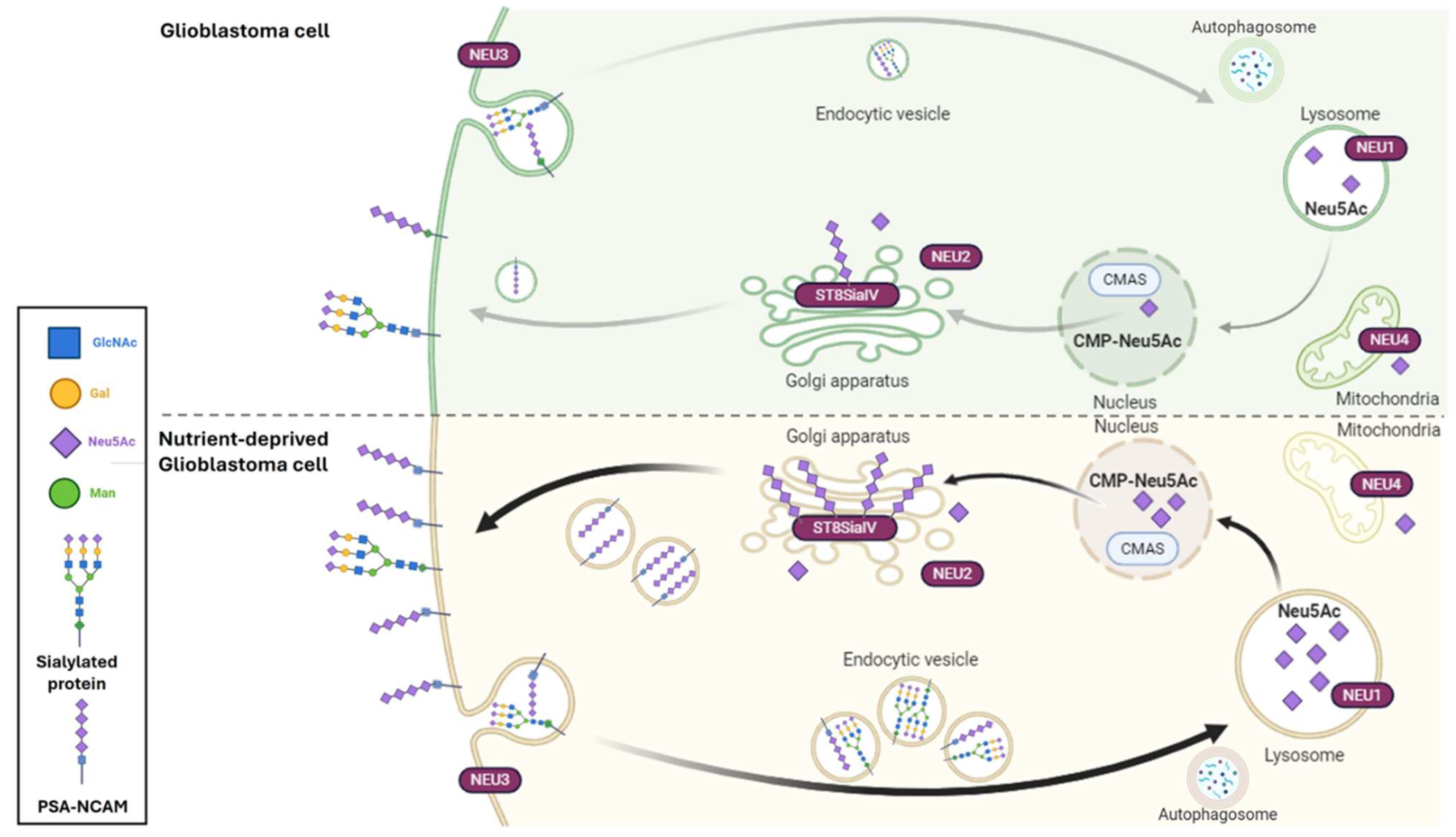
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Reagents
4.2. Cytofluorimetric Analysis
4.3. Western Blot Analysis
4.4. RNA Extraction and Real-Time PCR
4.5. Histological and Immunohistochemical Analyses
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, M.; De Gregorio, V.; Iorio, A.L.; Giunti, L.; Guidi, M.; de Martino, M.; Genitori, L.; Sardi, I. Glioblastoma Chemoresistance: The Double Play by Microenvironment and Blood-Brain Barrier. Int. J. Mol. Sci. 2018, 19, 2879. [Google Scholar] [CrossRef]
- Sharma, P.; Aaroe, A.; Liang, J.; Puduvalli, V.K. Tumor microenvironment in glioblastoma: Current and emerging concepts. Neuro-Oncol. Adv. 2023, 5, vdad009. [Google Scholar] [CrossRef]
- Tong, W.W.; Tong, G.H.; Liu, Y. Cancer stem cells and hypoxia-inducible factors (Review). Int. J. Oncol. 2018, 53, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Mennerich, D.; Kubaichuk, K.; Kietzmann, T. DUBs, Hypoxia, and Cancer. Trends Cancer 2019, 5, 632–653. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation (review). Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef]
- Miki, K.; Yagi, M.; Yoshimoto, K.; Kang, D.; Uchiumi, T. Mitochondrial dysfunction and impaired growth of glioblastoma cell lines caused by antimicrobial agents inducing ferroptosis under glucose starvation. Oncogenesis 2022, 11, 59. [Google Scholar] [CrossRef]
- Hwang, Y.K.; Lee, D.H.; Lee, E.C.; Oh, J.S. Importance of Autophagy Regulation in Glioblastoma with Temozolomide Resistance. Cells 2024, 13, 1332. [Google Scholar] [CrossRef]
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2021, 124, 76–90. [Google Scholar] [CrossRef]
- Kelm, S.; Schauer, R. Sialic acids in molecular and cellular interactions. Int. Rev. Cytol. 1997, 175, 137–240. [Google Scholar] [CrossRef]
- Barthel, L.; Hadamitzky, M.; Dammann, P.; Schedlowski, M.; Sure, U.; Thakur, B.K.; Hetze, S. Glioma: Molecular signature and crossroads with tumor microenvironment. Cancer Metastasis Rev. 2022, 41, 53–75. [Google Scholar] [CrossRef]
- Mühlenhoff, M.; Rollenhagen, M.; Werneburg, S.; Gerardy-Schahn, R.; Hildebrandt, H. Polysialic acid: Versatile modification of NCAM, SynCAM 1 and neuropilin-2. Neurochem. Res. 2013, 38, 1134–1143. [Google Scholar] [CrossRef]
- Kojima, N.; Kono, M.; Yoshida, Y.; Tachida, Y.; Nakafuku, M.; Tsuji, S. Biosynthesis and expression of polysialic acid on the neural cell adhesion molecule is predominantly directed by ST8Sia II/STX during in vitro neuronal differentiation. J. Biol. Chem. 1996, 271, 22058–22062. [Google Scholar] [CrossRef]
- Rutishauser, U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008, 9, 26–35. [Google Scholar] [CrossRef]
- Amoureux, M.C.; Coulibaly, B.; Chinot, O.; Loundou, A.; Metellus, P.; Rougon, G.; Figarella-Branger, D. Polysialic acid neural cell adhesion molecule (psa-ncam) is an adverse prognosis factor in glioblastoma, and regulates olig2 expression in glioma cell lines. BMC Cancer 2010, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Suzuki, M.; Nakayama, J.; Suzuki, A.; Angata, K.; Chen, S.; Sakai, K.; Hagihara, K.; Yamaguchi, Y.; Fukuda, M. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology 2005, 15, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Zeng, Y.N.; He, F.; Yang, X.M.; Guan, F. Enhanced expression of polysialic acid correlates with malignant phenotype in breast cancer cell lines and clinical tissue samples. Int. J. Mol. Med. 2016, 37, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Filipsky, F.; Läubli, H. Regulation of sialic acid metabolism in cancer. Carbohydr. Res. 2024, 539, 109123. [Google Scholar] [CrossRef]
- Jastrząb, P.; Narejko, K.; Car, H.; Wielgat, P. Cell Membrane Sialome: Sialic Acids as Therapeutic Targets and Regulators of Drug Resistance in Human Cancer Management. Cancers 2023, 15, 5103. [Google Scholar] [CrossRef]
- Khan, I.; Baig, M.H.; Mahfooz, S.; Rahim, M.; Karacam, B.; Elbasan, E.B.; Ulasov, I.; Dong, J.J.; Hatiboglu, M.A. Deciphering the role of autophagy in treatment of resistance mechanisms in glioblastoma. Int. J. Mol. Sci. 2021, 22, 1318. [Google Scholar] [CrossRef] [PubMed]
- Kanzawa, T.; Germano, I.M.; Komata, T.; Ito, H.; Kondo, Y.; Kondo, S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004, 11, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, S.K.; Mukhopadhyay, S.; Sinha, N.; Das, D.N.; Panda, P.K.; Patra, S.K.; Maiti, T.K.; Mandal, M.; Dent, P.; Wang, X.Y.; et al. Autophagy: Cancer’s friend or foe? In Advances in Cancer Research; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 118, pp. 61–95. [Google Scholar]
- He, M.; Zhou, X.; Wang, X. Glycosylation: Mechanisms, biological functions and clinical implications. Signal Transduct. Target. Ther. 2024, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta-Rev. Cancer 2021, 1875, 188464. [Google Scholar] [CrossRef]
- Huang, J.; Huang, J.; Zhang, G. Insights into the Role of Sialylation in Cancer Metastasis, Immunity, and Therapeutic Opportunity. Cancers 2022, 14, 5840. [Google Scholar] [CrossRef]
- Teoh, S.T.; Ogrodzinski, M.P.; Ross, C.; Hunter, K.W.; Lunt, S.Y. Sialic acid metabolism: A key player in breast cancer metastasis revealed by metabolomics. Front. Oncol. 2018, 8, 174. [Google Scholar] [CrossRef]
- Coccimiglio, M.; Chiodo, F.; van Kooyk, Y. The sialic acid–Siglec immune checkpoint: An opportunity to enhance immune responses and therapy effectiveness in melanoma. Br. J. Dermatol. 2024, 190, 627–635. [Google Scholar] [CrossRef]
- Elgohary, M.M.; Helmy, M.W.; Abdelfattah, E.Z.A.; Ragab, D.M.; Mortada, S.M.; Fang, J.Y.; Elzoghby, A.O. Targeting sialic acid residues on lung cancer cells by inhalable boronic acid-decorated albumin nanocomposites for combined chemo/herbal therapy. J. Control. Release 2018, 285, 230–243. [Google Scholar] [CrossRef]
- Rosa, P.; Scibetta, S.; Pepe, G.; Mangino, G.; Capocci, L.; Moons, S.J.; Boltje, T.J.; Fazi, F.; Petrozza, V.; Di Pardo, A.; et al. Polysialic Acid Sustains the Hypoxia-Induced Migration and Undifferentiated State of Human Glioblastoma Cells. Int. J. Mol. Sci. 2022, 23, 9563. [Google Scholar] [CrossRef]
- Mohamed, K.A.; Kruf, S.; Büll, C. Putting a cap on the glycome: Dissecting human sialyltransferase functions. Carbohydr. Res. 2024, 544, 109242. [Google Scholar] [CrossRef]
- Schildhauer, P.; Selke, P.; Staege, M.S.; Harder, A.; Scheller, C.; Strauss, C.; Horstkorte, R.; Scheer, M.; Leisz, S. Glycation Interferes with the Expression of Sialyltransferases and Leads to Increased Polysialylation in Glioblastoma Cells. Cells 2023, 12, 2758. [Google Scholar] [CrossRef]
- Selke, P.; Bork, K.; Zhang, T.; Wuhrer, M.; Strauss, C.; Horstkorte, R.; Scheer, M. Glycation interferes with the expression of sialyltransferases in meningiomas. Cells 2021, 10, 3298. [Google Scholar] [CrossRef]
- Al Saoud, R.; Hamrouni, A.; Idris, A.; Mousa, W.K.; Abu Izneid, T. Recent advances in the development of sialyltransferase inhibitors to control cancer metastasis: A comprehensive review. Biomed. Pharmacother. 2023, 165, 115091. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, J.; Ruan, Y.; Sun, L.; Xu, C.; Jiang, H. Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-β1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. 2018, 9, 1102. [Google Scholar] [CrossRef]
- Bos, P.D.; Zhang, X.H.F.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; Van De Vijver, M.J.; Gerald, W.L.; Foekens, J.A.; et al. Genes that mediate breast cancer metastasis to the brain. Nature 2009, 459, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, L.A.M.; Blanas, A.; van der Horst, J.C.; Kruijssen, L.; Zaal, A.; O’Toole, T.; Wiercx, L.; van Kooyk, Y.; van Vliet, S.J. Disruption of sialic acid metabolism drives tumor growth by augmenting CD8 + T cell apoptosis. Int. J. Cancer 2019, 144, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, A.; Illes, K.; Mazhab-Jafari, M.T.; Nagar, B. Structure of the immunoregulatory sialidase NEU1. Sci. Adv. 2023, 9, eadf8169. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shui, H.; Chen, R.; Dong, Y.; Xiao, C.; Hu, Y.; Wong, N.K. Neuraminidase-1 (NEU1): Biological Roles and Therapeutic Relevance in Human Disease. Curr. Issues Mol. Biol. 2024, 46, 8031–8052. [Google Scholar] [CrossRef]
- Sato, C.; Kitajima, K. Polysialylation and disease. Mol. Asp. Med. 2021, 79, 100892. [Google Scholar] [CrossRef]
- Li, F.; Ding, J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell 2019, 10, 550–565. [Google Scholar] [CrossRef]
- Nacher, J.; Guirado, R.; Varea, E.; Alonso-Llosa, G.; Röckle, I.; Hildebrandt, H. Divergent impact of the polysialyltransferases ST8SiaII and ST8SiaIV on polysialic acid expression in immature neurons and interneurons of the adult cerebral cortex. Neuroscience 2010, 167, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Oltmann-Norden, I.; Galuska, S.P.; Hildebrandt, H.; Geyer, R.; Gerardy-Schahn, R.; Geyer, H.; Mühlenhoff, M. Impact of the polysialyltransferases ST8SiaII and ST8SiaIV on polysialic acid synthesis during postnatal mouse brain development. J. Biol. Chem. 2008, 283, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Hane, M.; Niimi, Y.; Kitajima, K.; Sato, C. Different properties of polysialic acids synthesized by the polysialyltransferases ST8SIA2 and ST8SIA4. Glycobiology 2017, 27, 834–846. [Google Scholar] [CrossRef]
- Angata, K.; Fukuda, M. Roles of polysialic acid in Migration and differentiation of neural stem cells. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 479, pp. 25–36. [Google Scholar]
- Elkashef, S.M.; Allison, S.J.; Sadiq, M.; Basheer, H.A.; Ribeiro Morais, G.; Loadman, P.M.; Pors, K.; Falconer, R.A. Polysialic acid sustains cancer cell survival and migratory capacity in a hypoxic environment. Sci. Rep. 2016, 6, 33026. [Google Scholar] [CrossRef]
- Angata, K.; Huckaby, V.; Ranscht, B.; Terskikh, A.; Marth, J.D.; Fukuda, M. Polysialic Acid-Directed Migration and Differentiation of Neural Precursors Are Essential for Mouse Brain Development. Mol. Cell. Biol. 2007, 27, 6659–6668. [Google Scholar] [CrossRef]
- Büll, C.; Boltje, T.J.; Balneger, N.; Weischer, S.M.; Wassink, M.; Van Gemst, J.J.; Bloemendal, V.R.; Boon, L.; Van Der Vlag, J.; Heise, T.; et al. Sialic acid blockade suppresses tumor growth by enhancing t-cell-mediated tumor immunity. Cancer Res. 2018, 78, 3574–3588. [Google Scholar] [CrossRef]
- Büll, C.; Boltje, T.J.; Van Dinther, E.A.W.; Peters, T.; De Graaf, A.M.A.; Leusen, J.H.W.; Kreutz, M.; Figdor, C.G.; Den Brok, M.H.; Adema, G.J. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano 2015, 9, 733–745. [Google Scholar] [CrossRef]
- Shu, X.; Li, J.; Chan, U.I.; Su, S.M.; Shi, C.; Zhang, X.; An, T.; Xu, J.; Mo, L.; Liu, J.; et al. BRCA1 Insufficiency Induces a Hypersialylated Acidic Tumor Microenvironment That Promotes Metastasis and Immunotherapy Resistance. Cancer Res. 2023, 83, 2614–2633. [Google Scholar] [CrossRef]
- Lv, T.; Lv, H.; Fei, J.; Xie, Y.; Lian, D.; Hu, J.; Tang, L.; Shi, X.; Wang, J.; Zhang, S.; et al. p53-R273H promotes cancer cell migration via upregulation of neuraminidase-1. J. Cancer 2020, 11, 6874–6882. [Google Scholar] [CrossRef]
- Galavotti, S.; Bartesaghi, S.; Faccenda, D.; Shaked-Rabi, M.; Sanzone, S.; McEvoy, A.; Dinsdale, D.; Condorelli, F.; Brandner, S.; Campanella, M.; et al. The autophagy-associated factors DRAM1 and p62 regulate cell migration and invasion in glioblastoma stem cells. Oncogene 2013, 32, 699–712. [Google Scholar] [CrossRef]
- Coryell, P.R.; Goraya, S.K.; Griffin, K.A.; Redick, M.A.; Sisk, S.R.; Purvis, J.E. Autophagy regulates the localization and degradation of p16INK4a. Aging Cell 2020, 19, e13171. [Google Scholar] [CrossRef]
- Rahman, M.A.; Park, M.N.; Rahman, M.H.; Rashid, M.M.; Islam, R.; Uddin, M.J.; Hannan, M.A.; Kim, B. p53 Modulation of Autophagy Signaling in Cancer Therapies: Perspectives Mechanism and Therapeutic Targets. Front. Cell Dev. Biol. 2022, 10, 761080. [Google Scholar] [CrossRef]
- Shim, D.; Duan, L.; Maki, C.G. P53-regulated autophagy and its impact on drug resistance and cell fate. Cancer Drug Resist. 2021, 4, 85. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Weidberg, H.; Gonen, C.; Wilder, S.; Elazar, Z.; Oren, M. p53-dependent regulation of autophagy protein LC3 supports cancer cell survival under prolonged starvation. Proc. Natl. Acad. Sci. USA 2010, 107, 18511–18516. [Google Scholar] [CrossRef]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Criollo, A.; Kroemer, G. Crosstalk between apoptosis and autophagy within the Beclin 1 interactome. EMBO J. 2010, 29, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, E.; Maiuri, M.C.; Galluzzi, L.; Vitale, I.; Djavaheri-Mergny, M.; D’Amelio, M.; Criollo, A.; Morselli, E.; Zhu, C.; Harper, F.; et al. Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 2008, 10, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Reggiori, F.; Gabius, H.J.; Aureli, M.; Römer, W.; Sonnino, S.; Eskelinen, E.L. Glycans in autophagy, endocytosis and lysosomal functions. Glycoconj. J. 2021, 38, 625. [Google Scholar] [CrossRef]
- Yim, W.W.Y.; Mizushima, N. Lysosome biology in autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef]
- Itoh, K.; Tsukimoto, J. Lysosomal sialidase NEU1, its intracellular properties, deficiency, and use as a therapeutic agent. Glycoconj. J. 2023, 40, 611–619. [Google Scholar] [CrossRef]
- Huang, C.; Seino, J.; Wang, L.; Haga, Y.; Suzuki, T. Autophagy regulates the stability of sialin, a lysosomal sialic acid transporter. Biosci. Biotechnol. Biochem. 2015, 79, 553–557. [Google Scholar] [CrossRef]
- Lee, H.M.; Park, J.H.; Kim, T.H.; Kim, H.S.; Kim, D.E.; Lee, M.K.; You, J.; Lee, G.M.; Kim, Y.G. Effects of autophagy-inhibiting chemicals on sialylation of Fc-fusion glycoprotein in recombinant CHO cells. Appl. Microbiol. Biotechnol. 2024, 108, 224. [Google Scholar] [CrossRef]
- White, E.; Mehnert, J.M.; Chan, C.S. Autophagy, Metabolism, and Cancer. Clin. Cancer Res. 2015, 21, 5037–5046. [Google Scholar] [CrossRef]
- Badr, H.A.; AlSadek, D.M.M.; Mathew, M.P.; Li, C.Z.; Djansugurova, L.B.; Yarema, K.J.; Ahmed, H. Nutrient-deprived cancer cells preferentially use sialic acid to maintain cell surface glycosylation. Biomaterials 2015, 70, 23–36. [Google Scholar] [CrossRef]
- Minami, A.; Kurebayashi, Y.; Takahashi, T.; Otsubo, T.; Ikeda, K.; Suzuki, T. The function of sialidase revealed by sialidase activity imaging probe. Int. J. Mol. Sci. 2021, 22, 3187. [Google Scholar] [CrossRef]
- Kuliesiute, U.; Joseph, K.; Straehle, J.; Ravi, V.M.; Kueckelhaus, J.; Benotmane, J.K.; Zhang, J.; Vlachos, A.; Beck, J.; Schnell, O.; et al. Sialic acid metabolism orchestrates transcellular connectivity and signaling in glioblastoma. Neuro. Oncol. 2023, 25, 1963–1975. [Google Scholar] [CrossRef]
- Zhang, Y.; Albohy, A.; Zou, Y.; Smutova, V.; Pshezhetsky, A.V.; Cairo, C.W. Identification of selective inhibitors for human neuraminidase isoenzymes using C4,C7-modified 2-deoxy-2,3-didehydro-N-acetylneuraminic acid (DANA) analogues. J. Med. Chem. 2013, 56, 2948–2958. [Google Scholar] [CrossRef]
- Hyun, S.W.; Liu, A.; Liu, Z.; Cross, A.S.; Verceles, A.C.; Magesh, S.; Kommagalla, Y.; Kona, C.; Ando, H.; Luzina, I.G.; et al. The NEU1-selective sialidase inhibitor, C9-butyl-amide-DANA, blocks sialidase activity and NEU1-mediated bioactivities in human lung in vitro and murine lung in vivo. Glycobiology 2016, 26, 834–849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, S.; Guo, K.; Hu, Z.; Peng, J.; Li, J. Correlation and clinical significance of LC3, CD68+ microglia, CD4+ T lymphocytes, and CD8+ T lymphocytes in gliomas. Clin. Neurol. Neurosurg. 2018, 168, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Rosa, P.; De Falco, E.; Pacini, L.; Piazza, A.; Ciracì, P.; Ricciardi, L.; Fiorentino, F.; Trungu, S.; Miscusi, M.; Raco, A.; et al. Next-Generation Sequencing Comparative Analysis of DNA Mutations between Blood-Derived Extracellular Vesicles and Matched Cancer Tissue in Patients with Grade 4 Glioblastoma. Biomedicines 2022, 10, 2590. [Google Scholar] [CrossRef] [PubMed]
- Ponti, D.; Bastianelli, D.; Rosa, P.; Pacini, L.; Ibrahim, M.; Rendina, E.A.; Ragona, G.; Calogero, A. The expression of B23 and EGR1 proteins is functionally linked in tumor cells under stress conditions. BMC Cell Biol. 2015, 16, 27. [Google Scholar] [CrossRef]
- Scibetta, S.; Miceli, M.; Iuliano, M.; Stefanuto, L.; Carbone, E.; Piscopo, P.; Petrozza, V.; Romeo, G.; Mangino, G.; Calogero, A.; et al. In Vitro Evaluation of the Antioxidant Capacity of 3,3-Disubstituted-3H-benzofuran-2-one Derivatives in a Cellular Model of Neurodegeneration. Life 2024, 14, 422. [Google Scholar] [CrossRef]
- Rosa, P.; Zerbinati, C.; Crestini, A.; Canudas, A.M.; Ragona, G.; Confaloni, A.; Iuliano, L.; Calogero, A. Heme oxygenase-1 and brain oxysterols metabolism are linked to Egr-1 expression in aged mice cortex, but not in hippocampus. Front. Aging Neurosci. 2018, 10, 363. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scibetta, S.; Pepe, G.; Iuliano, M.; Iaiza, A.; Palazzo, E.; Quadri, M.; Boltje, T.J.; Fazi, F.; Petrozza, V.; Di Bartolomeo, S.; et al. Polysialylation of Glioblastoma Cells Is Regulated by Autophagy Under Nutrient Deprivation. Int. J. Mol. Sci. 2025, 26, 7625. https://doi.org/10.3390/ijms26157625
Scibetta S, Pepe G, Iuliano M, Iaiza A, Palazzo E, Quadri M, Boltje TJ, Fazi F, Petrozza V, Di Bartolomeo S, et al. Polysialylation of Glioblastoma Cells Is Regulated by Autophagy Under Nutrient Deprivation. International Journal of Molecular Sciences. 2025; 26(15):7625. https://doi.org/10.3390/ijms26157625
Chicago/Turabian StyleScibetta, Sofia, Giuseppe Pepe, Marco Iuliano, Alessia Iaiza, Elisabetta Palazzo, Marika Quadri, Thomas J. Boltje, Francesco Fazi, Vincenzo Petrozza, Sabrina Di Bartolomeo, and et al. 2025. "Polysialylation of Glioblastoma Cells Is Regulated by Autophagy Under Nutrient Deprivation" International Journal of Molecular Sciences 26, no. 15: 7625. https://doi.org/10.3390/ijms26157625
APA StyleScibetta, S., Pepe, G., Iuliano, M., Iaiza, A., Palazzo, E., Quadri, M., Boltje, T. J., Fazi, F., Petrozza, V., Di Bartolomeo, S., Di Pardo, A., Calogero, A., Mangino, G., Maglione, V., & Rosa, P. (2025). Polysialylation of Glioblastoma Cells Is Regulated by Autophagy Under Nutrient Deprivation. International Journal of Molecular Sciences, 26(15), 7625. https://doi.org/10.3390/ijms26157625










