Exploring the Prognostic Role of Neurofilaments and SEMA3A in Multiple Sclerosis Progression
Abstract
1. Introduction
2. Results
2.1. RRMS and SPMS Patient Group Characteristics: Description and Comparison
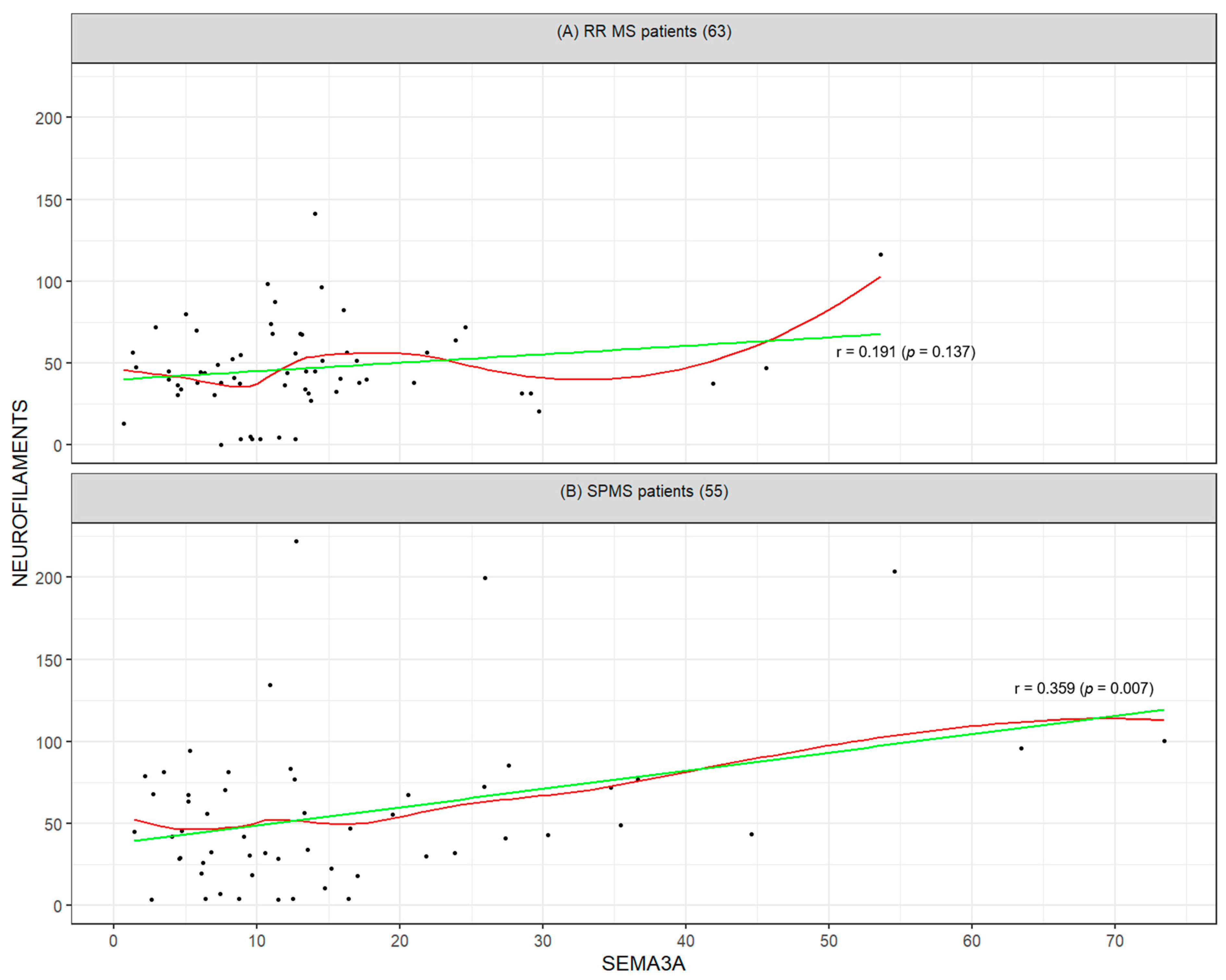
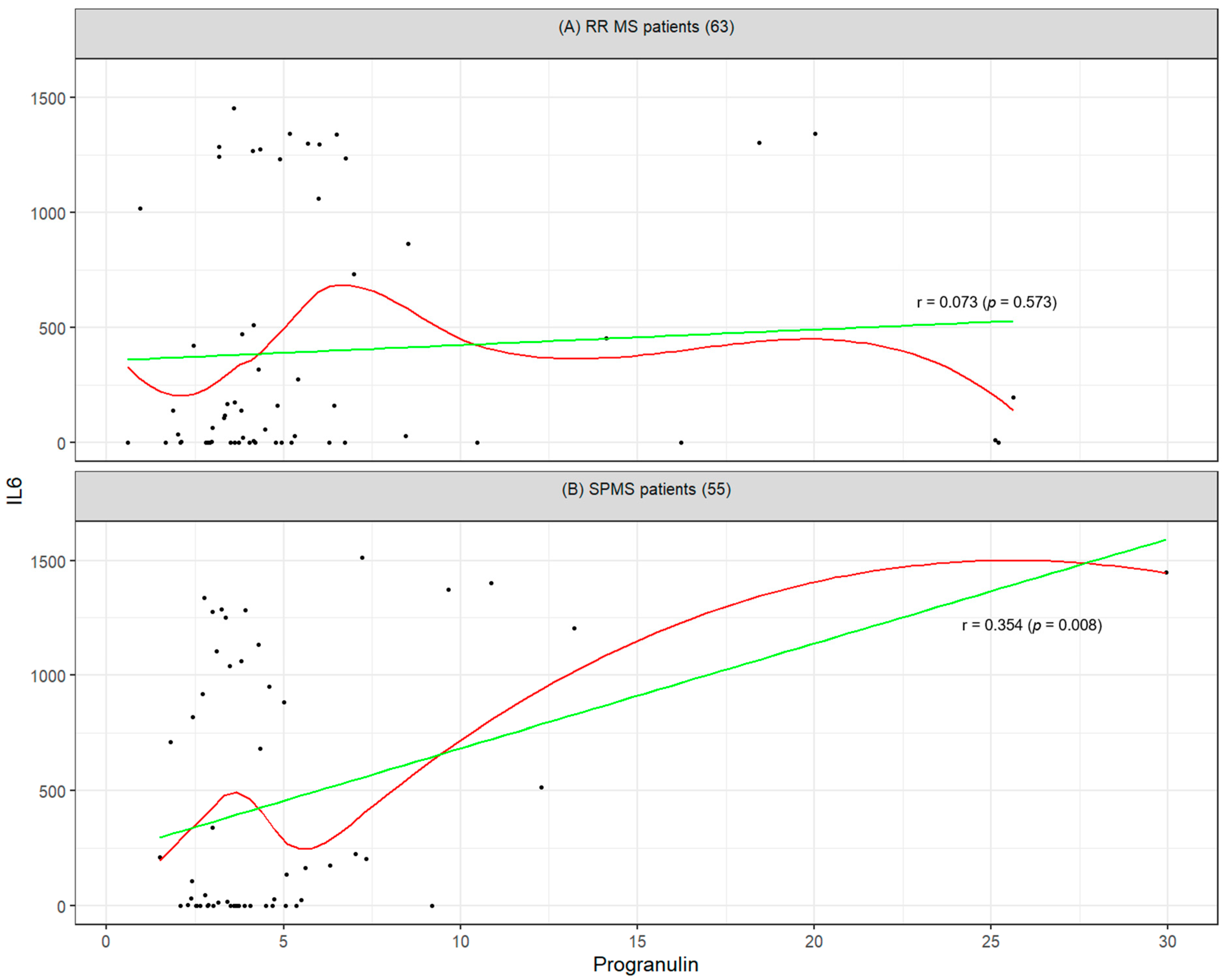
2.2. Mutual Correlation Between Biomarkers and Their Association with Clinical Disability (EDSS)
3. Discussion
3.1. Demographic and Clinical Differences Between RRMS and SPMS
3.2. Comparison of Absolute Biomarker Levels Between Groups
3.3. Intra-Group Correlations of Biomarkers in SPMS and RRMS
3.4. Relationship Between Biomarkers and Disability (EDSS)
3.5. Limitations and Future Directions
4. Materials and Methods
4.1. Study Population
4.2. Primary and Secondary Objectives and Endpoints
4.3. Determination of Serum Analytes
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMT | Disease-Modifying Therapy |
| EDSS | Expanded Disability Status Scale |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| IL-6 | Interleukin-6 |
| IVIG | Intravenous Immunoglobulin |
| IVMP | Intravenous Methylprednisolone |
| MRI | Magnetic Resonance Imaging |
| MS | Multiple Sclerosis |
| RR | Relapsing–Remitting |
| RRMS | Relapsing–Remitting Multiple Sclerosis |
| SEMA3A | Semaphorin 3A |
| SPMS | Secondary Progressive Multiple Sclerosis |
References
- Cree, B.A.C.; Arnold, D.L.; Chataway, J.; Chitnis, T.; Fox, R.J.; Ramajo, A.P.; Murphy, N.; Lassmann, H. Secondary Progressive Multiple Sclerosis: New Insights. Neurology 2021, 97, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.J.; Schorr, E.M.; Gadani, S.P.; Calabresi, P.A. Emerging Imaging and Liquid Biomarkers in Multiple Sclerosis. Eur. J. Immunol. 2023, 53, e2250228. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Qureshi, F.; Gehman, V.M.; Becich, M.; Bove, R.; Cree, B.A.C.; Gomez, R.; Hauser, S.L.; Henry, R.G.; Katrib, A.; et al. Inflammatory and Neurodegenerative Serum Protein Biomarkers Increase Sensitivity to Detect Clinical and Radiographic Disease Activity in Multiple Sclerosis. Nat. Commun. 2024, 15, 4297. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; O’Keeffe, J.; Pokryszko-Dragan, A. New Insights into Multiple Sclerosis Mechanisms: Lipids on the Track to Control Inflammation and Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 7319. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Gambino, C.M.; Ciaccio, A.M.; Masucci, A.; Vassallo, R.; Tamburello, M.; Scazzone, C.; Lo Sasso, B.; Ciaccio, M. Molecular Biomarkers of Neurodegenerative Disorders: A Practical Guide to Their Appropriate Use and Interpretation in Clinical Practice. Int. J. Mol. Sci. 2024, 25, 4323. [Google Scholar] [CrossRef] [PubMed]
- Srpova, B.; Uher, T.; Hrnciarova, T.; Barro, C.; Andelova, M.; Michalak, Z.; Vaneckova, M.; Krasensky, J.; Noskova, L.; Havrdova, E.K.; et al. Serum Neurofilament Light Chain Reflects Inflammation-Driven Neurodegeneration and Predicts Delayed Brain Volume Loss in Early Stage of Multiple Sclerosis. Mult. Scler. J. 2021, 27, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Good, P.F.; Alapat, D.; Hsu, A.; Chu, C.; Perl, D.; Wen, X.; Burstein, D.E.; Kohtz, D.S. A Role for Semaphorin 3A Signaling in the Degeneration of Hippocampal Neurons during Alzheimer’s Disease. J. Neurochem. 2004, 91, 716–736. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; da Silva, Á.P.; Araldi, R.P. The Impact of Interleukin-6 (IL-6) and Mesenchymal Stem Cell-Derived IL-6 on Neurological Conditions. Front. Immunol. 2024, 15, 1400533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Mendsaikhan, A.; Tooyama, I.; Walker, D.G. Microglial Progranulin: Involvement in Alzheimer’s Disease and Neurodegenerative Diseases. Cells 2019, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Smolinski, L.; Członkowska, A.; Akgun, K.; Antos, A.; Bembenek, J.; Kurkowska-Jastrzębska, I.; Przybyłkowski, A.; Skowrońska, M.; Redzia-Ogrodnik, B.; et al. Serum Neurofilament Light Chain and Initial Severity of Neurological Disease Predict the Early Neurological Deterioration in Wilson’s Disease. Acta Neurol. Belg. 2023, 123, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Ziemssen, T.; Arnold, D.L.; Alvarez, E.; Cross, A.H.; Willi, R.; Li, B.; Kukkaro, P.; Kropshofer, H.; Ramanathan, K.; Merschhemke, M.; et al. Prognostic Value of Serum Neurofilament Light Chain for Disease Activity and Worsening in Patients With Relapsing Multiple Sclerosis: Results From the Phase 3 ASCLEPIOS I and II Trials. Front. Immunol. 2022, 13, 852563. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C.; National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Defining the Clinical Course of Multiple Sclerosis: Results of an International Survey. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the Clinical Course of Multiple Sclerosis: The 2013 Revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall (118) | SPMS (55) | RR (63) | SPMS vs. RR | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Med [p25, p75] | Mean (SD) | Med [p25, p75] | Mean (SD) | Med [p25, p75] | p-Value | |
| Progranulin (ng/mL) | 5.59 (5.08) | 3.98 [2.99, 5.67] | 4.93 (4.26) | 3.66 [2.85, 5.07] | 6.16 (5.67) | 4.20 [3.24, 6.36] | 0.136 |
| IL-6 (pg/mL) | 421.01 (534.38) | 114.05 [0, 944.65] | 453.78 (547.09) | 136.10 [0.00, 996.25] | 392.40 (525.75) | 108.30 [0.00, 800.05] | 0.738 |
| SEMA3A (ng/mL) | 14.93 (12.79) | 11.73 [6.57, 17.02] | 16.46 (15.28) | 11.47 [6.29, 21.20] | 13.60 (10.08) | 11.93 [7.35, 15.94] | 0.802 |
| Neurofilaments (pg/mL) | 51.26 (38) | 44.25 [30.88, 68.05] | 56.15 (47.29) | 44.94 [28.63, 74.75] | 47.00 (27.15) | 44.15 [33.11, 56.69] | 0.658 |
| Current age | 53.02 (13.13) | 53.29 [44.58, 63.48] | 61.67 (7.60) | 63.04 [56.08, 67.56] | 45.47 (12.28) | 45.89 [34.94, 51.69] | <0.001 |
| Age | 52.24 (13.08) | 52.5 [44.0, 63.0] | 60.85 (7.59) | 62.00 [55.50, 66.50] | 44.71 (12.23) | 45.00 [34.00, 51.00] | <0.001 |
| EDSS | 4.83 (2.04) | 5.25 [3.0, 6.5] | 6.64 (0.61) | 6.50 [6.50, 7.00] | 3.25 (1.46) | 3.00 [2.00, 4.50] | <0.001 |
| MS duration (in years) | 16.38 (12.82) | 14.0 [4.0, 27.0] | 26.27 (9.68) | 26.00 [21.00, 32.50] | 7.75 (8.12) | 5.00 [2.00, 9.50] | <0.001 |
| CH/P duration (in years) | 6.2 (8.12) | 0.0 [0.0, 12.0] | 13.18 (6.92) | 13.00 [9.50, 17.00] | 0.00 (0.00) | 0.00 [0.00, 0.00] | <0.001 |
| Progranulin | IL6 | SEMA3A | NEUROFILAMENTS | EDSS | |
|---|---|---|---|---|---|
| Progranulin | 1 (0) | 0.07 (0.825) | −0.13 (0.635) | −0.06 (0.825) | −0.06 (0.825) |
| IL6 | 0.07 (0.573) | 1 (0) | 0.01 (0.945) | −0.13 (0.635) | −0.13 (0.635) |
| SEMA3A | −0.13 (0.302) | 0.01 (0.945) | 1 (0) | 0.19 (0.635) | −0.01 (0.945) |
| NEUROFILAMENTS | −0.06 (0.625) | −0.13 (0.308) | 0.19 (0.137) | 1 (0) | 0.2 (0.635) |
| EDSS | −0.06 (0.66) | −0.13 (0.318) | −0.01 (0.916) | 0.2 (0.12) | 1 (0) |
| Progranulin | IL6 | SEMA3A | NEUROFILAMENTS | EDSS | |
|---|---|---|---|---|---|
| Progranulin | 1 (0) | 0.35 (0.04) | 0.17 (0.417) | 0.24 (0.261) | −0.02 (0.91) |
| IL6 | 0.35 (0.008) | 1 (0) | 0.16 (0.417) | −0.04 (0.827) | −0.08 (0.723) |
| SEMA3A | 0.17 (0.225) | 0.16 (0.25) | 1 (0) | 0.36 (0.04) | −0.22 (0.272) |
| NEUROFILAMENTS | 0.24 (0.078) | −0.04 (0.745) | 0.36 (0.007) | 1 (0) | −0.11 (0.625) |
| EDSS | −0.02 (0.91) | −0.08 (0.578) | −0.22 (0.109) | −0.11 (0.437) | 1 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavelek, Z.; Souček, O.; Krejsek, J.; Součková, I.; Popovičová, A.; Matyáš, D.; Sobíšek, L.; Novotný, M. Exploring the Prognostic Role of Neurofilaments and SEMA3A in Multiple Sclerosis Progression. Int. J. Mol. Sci. 2025, 26, 8750. https://doi.org/10.3390/ijms26178750
Pavelek Z, Souček O, Krejsek J, Součková I, Popovičová A, Matyáš D, Sobíšek L, Novotný M. Exploring the Prognostic Role of Neurofilaments and SEMA3A in Multiple Sclerosis Progression. International Journal of Molecular Sciences. 2025; 26(17):8750. https://doi.org/10.3390/ijms26178750
Chicago/Turabian StylePavelek, Zbyšek, Ondřej Souček, Jan Krejsek, Ilona Součková, Andrea Popovičová, David Matyáš, Lukáš Sobíšek, and Michal Novotný. 2025. "Exploring the Prognostic Role of Neurofilaments and SEMA3A in Multiple Sclerosis Progression" International Journal of Molecular Sciences 26, no. 17: 8750. https://doi.org/10.3390/ijms26178750
APA StylePavelek, Z., Souček, O., Krejsek, J., Součková, I., Popovičová, A., Matyáš, D., Sobíšek, L., & Novotný, M. (2025). Exploring the Prognostic Role of Neurofilaments and SEMA3A in Multiple Sclerosis Progression. International Journal of Molecular Sciences, 26(17), 8750. https://doi.org/10.3390/ijms26178750








