Major Common Hallmarks and Potential Epigenetic Drivers of Wound Chronicity and Recurrence: Hypothesis and Reflections
Abstract
1. Introduction
2. Invariants of the Most Frequent Chronic Wounds
3. Brief Depiction of the Three Main Chronic Wounds
3.1. Diabetic Foot Ulcers
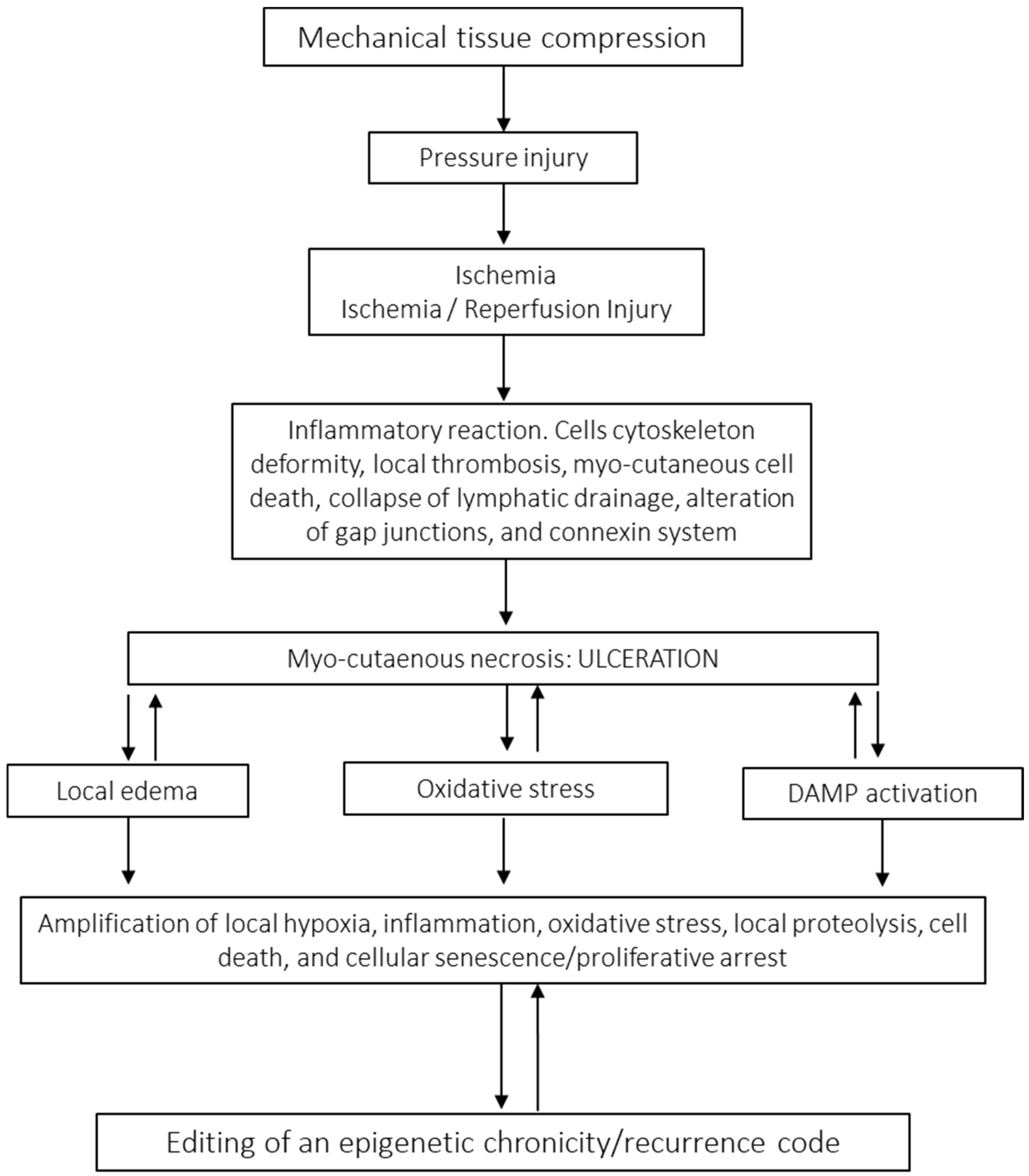
3.2. Pressure Injury
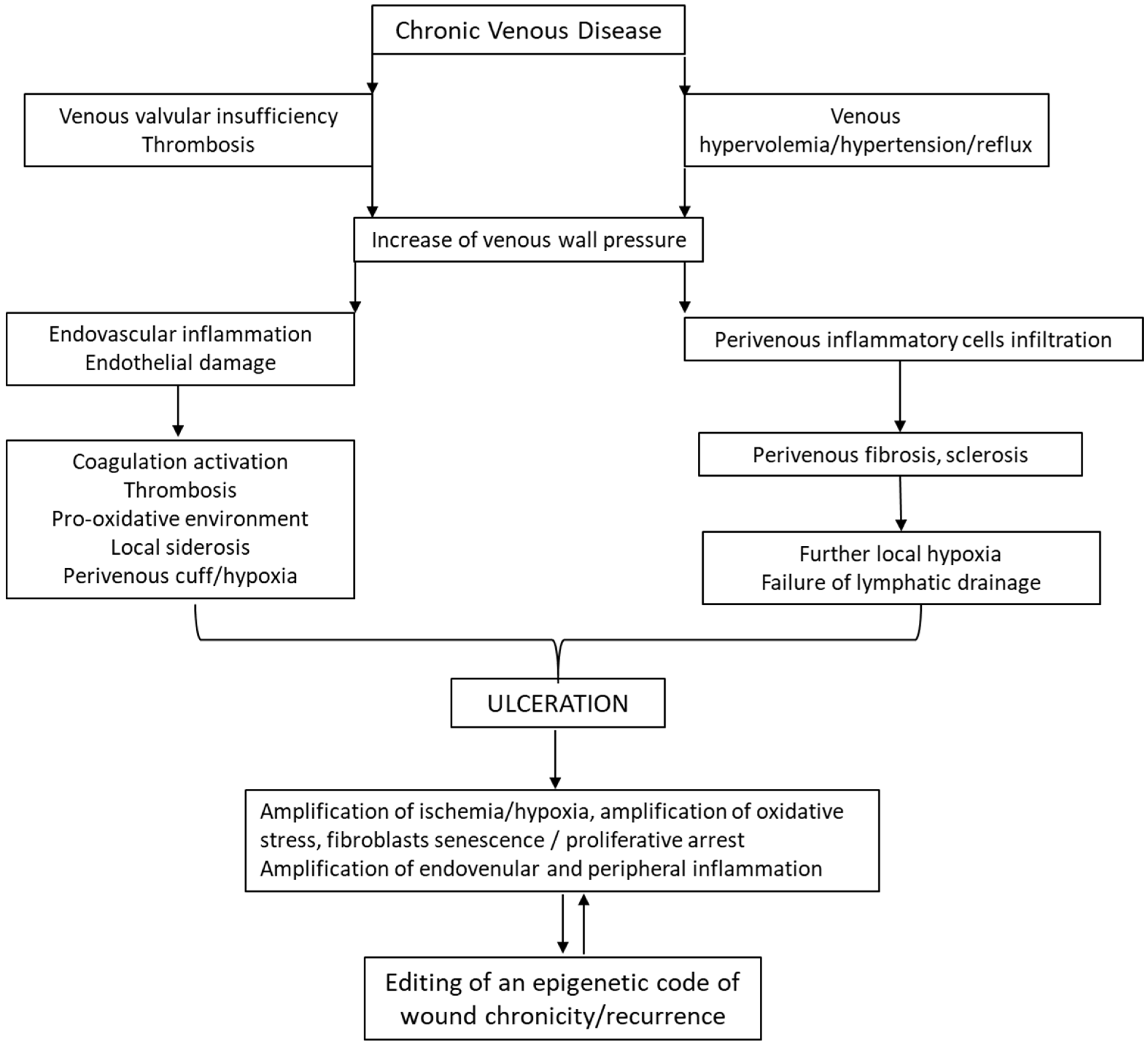
3.3. Venous Leg Ulcers
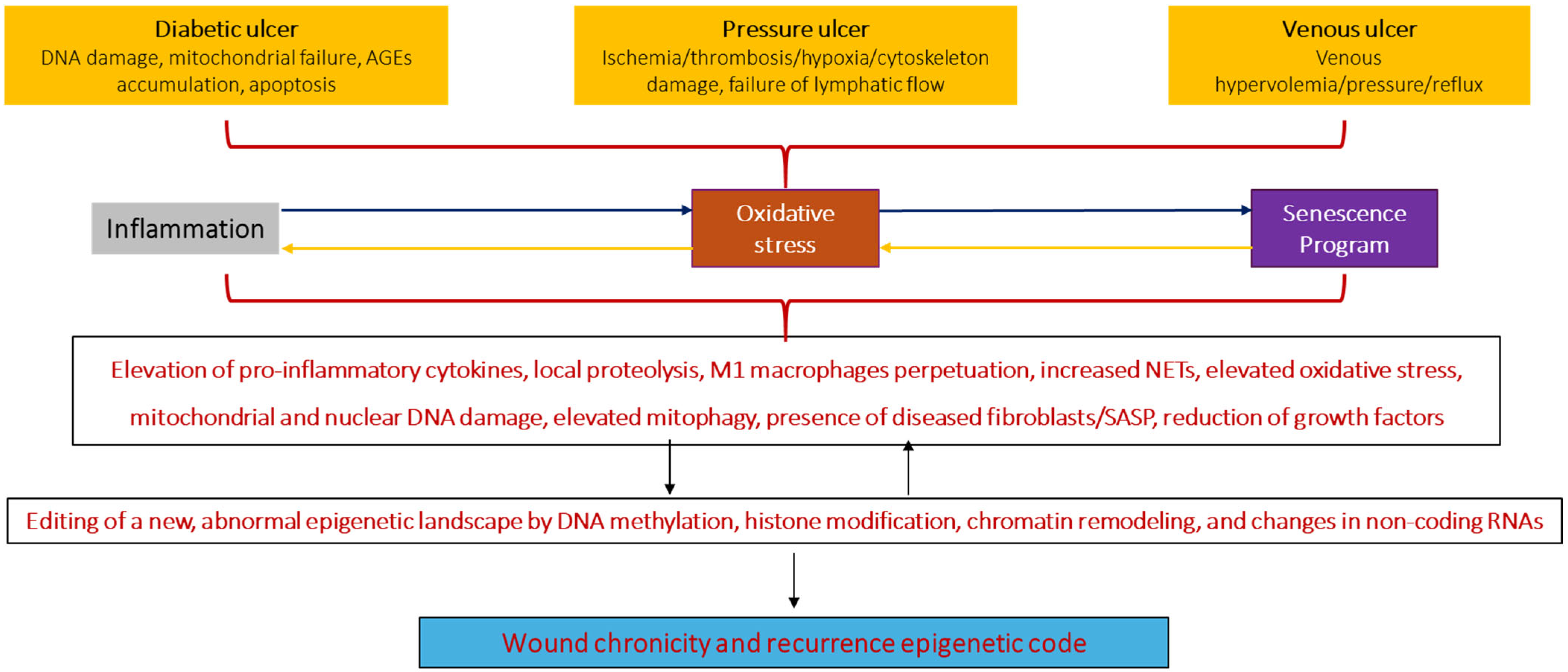
4. Common Pathologic Hallmarks in Chronic Wounds
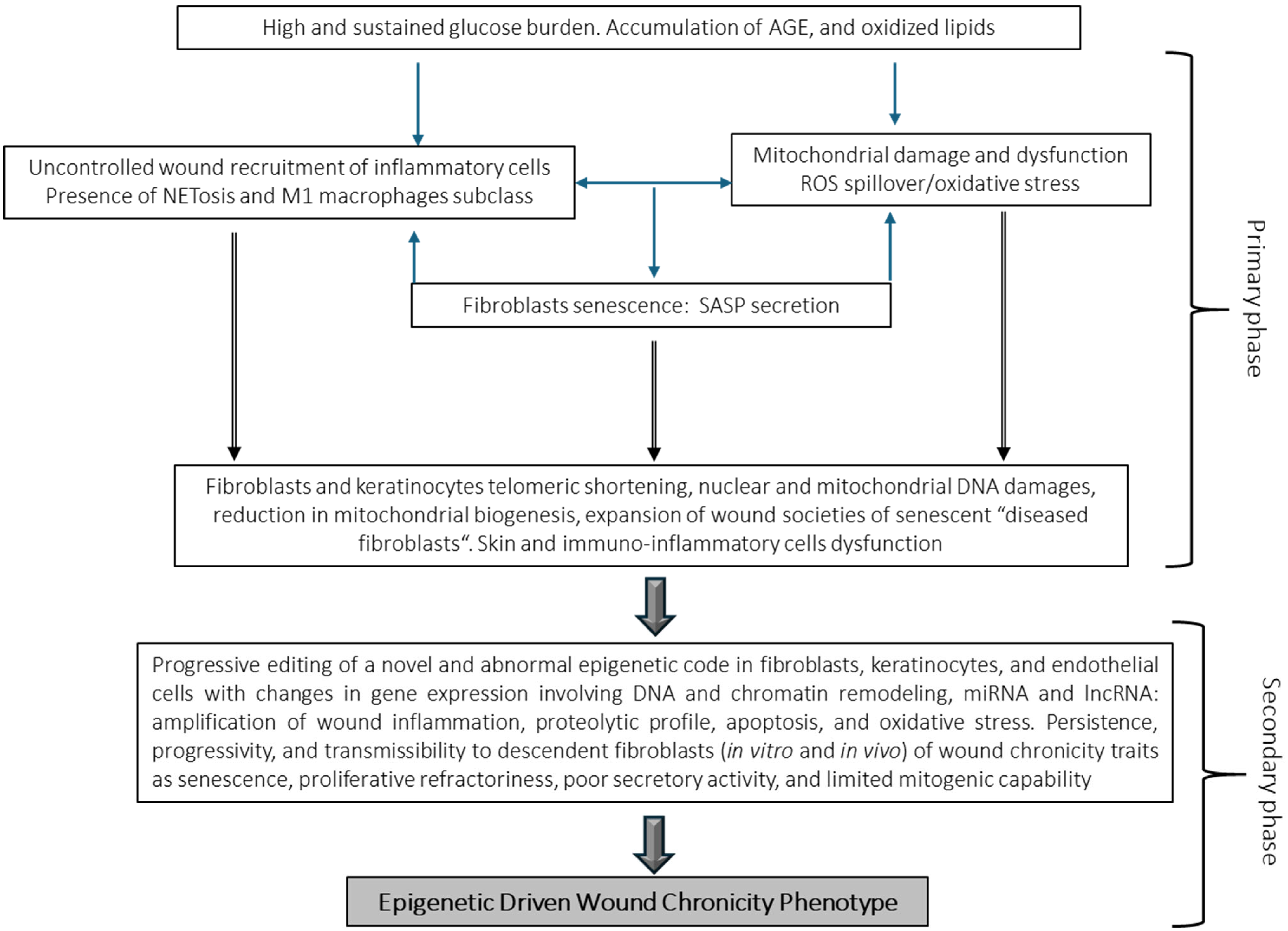
5. Chronic Wound Cells May Harbor a Chronicity–Recurrence Epigenetic Memory Code
5.1. In Vivo Transmissibility from Humans to Rats of Diabetic Wound Markers
5.2. Epigenetic Drivers in Wound Chronicity
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABI | ankle–brachial index |
| AGE | advanced glycation end-product |
| AKT1 | an isoform of the serine/threonine protein kinase B |
| BMP | bone morphogenetic protein |
| CEAP | Clinical–Etiological–Anatomical–Pathophysiological classification |
| CFF | cell-free filtrate |
| CVD | chronic venous disease |
| CircRNA | circular RNA |
| CW | chronic wound |
| CWF | chronic-wound-derived fibroblast |
| DAMPs | damage-associated molecular patterns |
| DFU | diabetic foot ulcer |
| DNMT | DNA methyltransferase |
| ECM | extracellular matrix |
| FGF | fibroblast growth factor |
| GT | granulation tissue |
| H/E | hematoxylin/eosin |
| HDAC | histone deacetylase |
| ICAM-1 | interstitial cell adhesion molecule 1 |
| IGF-I | insulin-like growth factor |
| JNK | c-Jun N-terminal kinase |
| lncRNA | long non-coding RNA |
| miRNA | microRNA |
| MMP | matrix metalloproteinase |
| NET | neutrophil extracellular trap |
| NF-κB | nuclear factor kappa B |
| NLRP3 | NOD-like receptor protein 3 |
| Nrf2 | nuclear respiratory factor 2 |
| OS | oxidative stress |
| PAMPs | pathogen-associated molecular patterns |
| PI | pressure injury |
| PI3 | phosphatidylinositol 3-kinase |
| PKC | protein kinase C |
| PTEN | phosphatase and tensin homolog |
| ROS | reactive oxygen species |
| SASP | senescence-associated secretory phenotype |
| TGF-β1 | transforming growth factor beta 1 |
| TNF-α | tumor necrosis factor alpha |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| VLU | venous leg ulcer |
References
- Kimura, S.; Tsuji, T. Mechanical and Immunological Regulation in Wound Healing and Skin Reconstruction. Int. J. Mol. Sci. 2021, 22, 5474. [Google Scholar] [CrossRef]
- Talbott, H.E.; Mascharak, S.; Griffin, M.; Wan, D.C.; Longaker, M.T. Wound healing, fibroblast heterogeneity, and fibrosis. Cell Stem Cell 2022, 29, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Enkhtaivan, E.; Lee, C.H. Role of Amine Neurotransmitters and Their Receptors in Skin Pigmentation: Therapeutic Implication. Int. J. Mol. Sci. 2021, 22, 8071. [Google Scholar] [CrossRef] [PubMed]
- Takaya, K.; Sunohara, A.; Sakai, S.; Aramaki-Hattori, N.; Okabe, K.; Kishi, K. Twist2 contributes to skin regeneration and hair follicle formation in mouse fetuses. Sci. Rep. 2024, 14, 10854. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kishen, A. Dysfunctional crosstalk between macrophages and fibroblasts under LPS-infected and hyperglycemic environment in diabetic wounds. Sci. Rep. 2025, 15, 17233. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, X.; Xue, B. New insights into the role of cellular senescence and chronic wounds. Front. Endocrinol. 2024, 15, 1400462. [Google Scholar] [CrossRef]
- Durant, F.; Whited, J.L. Finding Solutions for Fibrosis: Understanding the Innate Mechanisms Used by Super-Regenerator Vertebrates to Combat Scarring. Adv. Sci. 2021, 8, e2100407. [Google Scholar] [CrossRef]
- Kang, Y.; Liu, X.; Chen, X.; Duan, Y.; Wang, J.; Gao, C. Advances of Nanobiomaterials for Treating Skin Pathological Fibrosis. Adv. NanoBiomed Res. 2024, 4, 2400008. [Google Scholar] [CrossRef]
- Mascharak, S.; Talbott, H.E.; Januszyk, M.; Griffin, M.; Chen, K.; Davitt, M.F.; Demeter, J.; Henn, D.; Bonham, C.A.; Foster, D.S.; et al. Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell 2022, 29, 315–327.e6. [Google Scholar] [CrossRef]
- Zhu, X.; Olsson, M.M.; Bajpai, R.; Jarbrink, K.; Tang, W.E.; Car, J. Health-related quality of life and chronic wound characteristics among patients with chronic wounds treated in primary care: A cross-sectional study in Singapore. Int. Wound J. 2022, 19, 1121–1132. [Google Scholar] [CrossRef]
- Miteva, M.; Romanelli, P. Histopathology of Wounds. In Measurements in Wound Healing: Science and Practice; Mani, R., Romanelli, M., Shukla, V., Eds.; Springer: London, UK, 2013; pp. 155–173. [Google Scholar]
- Virador, G.M.; de Marcos, L.; Virador, V.M. Skin Wound Healing: Refractory Wounds and Novel Solutions. Methods Mol. Biol. 2019, 1879, 221–241. [Google Scholar]
- Sen, C.K. Human Wound and Its Burden: Updated 2020 Compendium of Estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Sharma, A.; Shankar, R.; Yadav, A.K.; Pratap, A.; Ansari, M.A.; Srivastava, V. Burden of Chronic Nonhealing Wounds: An Overview of the Worldwide Humanistic and Economic Burden to the Healthcare System. Int. J. Low Extrem. Wounds 2024, 15347346241246339. [Google Scholar] [CrossRef]
- Balikji, J.; Hoogbergen, M.M.; Garssen, J.; Verster, J.C. Mental Resilience, Mood, and Quality of Life in Young Adults with Self-Reported Impaired Wound Healing. Int. J. Environ. Res. Public Health 2022, 19, 2542. [Google Scholar] [CrossRef]
- Souza Nogueira, G.; Rodrigues Zanin, C.; Miyazaki, M.C.; Pereira de Godoy, J.M. Venous leg ulcers and emotional consequences. Int. J. Low Extrem. Wounds 2009, 8, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Wiechman, S.; Kalpakjian, C.Z.; Johnson, K.L. Measuring Depression in Adults With Burn Injury: A Systematic Review. J. Burn. Care Res. 2016, 37, e415–e426. [Google Scholar] [CrossRef]
- Zhou, K.; Jia, P. Depressive symptoms in patients with wounds: A cross-sectional study. Wound Repair Regen. 2016, 24, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef]
- Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Longaker, M.T.; Gurtner, G.C. Surgical approaches to create murine models of human wound healing. J. Biomed. Biotechnol. 2011, 2011, 969618. [Google Scholar] [CrossRef]
- Gazzaruso, C.; Gallotti, P.; Pujia, A.; Montalcini, T.; Giustina, A.; Coppola, A. Predictors of healing, ulcer recurrence and persistence, amputation and mortality in type 2 diabetic patients with diabetic foot: A 10-year retrospective cohort study. Endocrine 2021, 71, 59–68. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Mendoza-Mari, Y.; García Ojalvo, A.; Fernández Mayola, M.; Guillen, G. Epidermal Growth Factor Therapy Impact on Scar Tissue Resilience of Diabetic Lower Limbs Ulcers-An Enlightening Hypothesis. J. Diabetes Metab. 2018, 9, 798. [Google Scholar]
- Maheshwari, G. Chronic wounds: A rising public health concern. Wounds APAC 2024, 7, 6–11. [Google Scholar]
- Mendoza-Mari, Y.; Pérez, C.; Corrales, E.; Alba, J.; García Ojalvo, A.; Garcia del Barco, D.; Guillen, G.; Martinez, L.; Berlanga-Acosta, J. Histological and Transcriptional Expression differences between Diabetic Foot and Pressure Ulcers. Diabetes Metab. 2013, 4, 296. [Google Scholar]
- Liarte, S.; Bernabe-Garcia, A.; Nicolas, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ojalvo, A.; Berlanga Acosta, J.; Figueroa-Martinez, A.; Bequet-Romero, M.; Mendoza-Mari, Y.; Fernandez-Mayola, M.; Fabelo-Martinez, A.; Guillen-Nieto, G. Systemic translation of locally infiltrated epidermal growth factor in diabetic lower extremity wounds. Int. Wound J. 2019, 16, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.N.B.; Serna Gonzalez, C.V.; Borges, E.L.; Santos, V.; Rabeh, S.A.N.; Nogueira, P.C. Incidence of Recurrent Venous Ulcer in Patients Treated at an Outpatient Clinic: Historical Cohort. Int. J. Low Extrem. Wounds 2024, 23, 455–463. [Google Scholar] [CrossRef]
- Lin, C.; Liu, J.; Sun, H. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: A meta-analysis. PLoS ONE 2020, 15, e0239236. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; Garcia-Ojalvo, A.; Guillen-Nieto, G.; Ayala-Avila, M. Endogenous Biological Drivers in Diabetic Lower Limb Wounds Recurrence: Hypothetical Reflections. Int. J. Mol. Sci. 2023, 24, 10170. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef]
- Shah, P.; Inturi, R.; Anne, D.; Jadhav, D.; Viswambharan, V.; Khadilkar, R.; Dnyanmote, A.; Shahi, S. Wagner’s Classification as a Tool for Treating Diabetic Foot Ulcers: Our Observations at a Suburban Teaching Hospital. Cureus 2022, 14, e21501. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, C.X.; Xu, B.; Yu, Z. Diabetic foot ulcers: Classification, risk factors and management. World J. Diabetes 2022, 13, 1049–1065. [Google Scholar] [CrossRef]
- Ko, K.I.; Sculean, A.; Graves, D.T. Diabetic wound healing in soft and hard oral tissues. Transl. Res. 2021, 236, 72–86. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef]
- Wronka, M.; Krzeminska, J.; Mlynarska, E.; Rysz, J.; Franczyk, B. The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int. J. Mol. Sci. 2022, 23, 15743. [Google Scholar] [CrossRef]
- Swoboda, L.; Held, J. Impaired wound healing in diabetes. J. Wound Care 2022, 31, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; Garcia-Ojalvo, A.; Fernandez-Montequin, J.; Falcon-Cama, V.; Acosta-Rivero, N.; Guillen-Nieto, G.; Pujol-Ferrer, M.; Limonta-Fernandez, M.; Ayala-Avila, M.; Eriksson, E. Epidermal Growth Factor Intralesional Delivery in Chronic Wounds: The Pioneer and Standalone Technique for Reversing Wound Chronicity and Promoting Sustainable Healing. Int. J. Mol. Sci. 2024, 25, 10883. [Google Scholar] [CrossRef]
- Huang, F.; Lu, X.; Yang, Y.; Yang, Y.; Li, Y.; Kuai, L.; Li, B.; Dong, H.; Shi, J. Microenvironment-Based Diabetic Foot Ulcer Nanomedicine. Adv. Sci. 2023, 10, e2203308. [Google Scholar] [CrossRef]
- Zubair, M.; Ahmad, J. Role of growth factors and cytokines in diabetic foot ulcer healing: A detailed review. Rev. Endocr. Metab. Disord. 2019, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Halabitska, I.; Babinets, L.; Oksenych, V.; Kamyshnyi, O. Diabetes and Osteoarthritis: Exploring the Interactions and Therapeutic Implications of Insulin, Metformin, and GLP-1-Based Interventions. Biomedicines 2024, 12, 1630. [Google Scholar] [CrossRef]
- Soydas, T.; Sayitoglu, M.; Sarac, E.Y.; Cinar, S.; Solakoglu, S.; Tiryaki, T.; Sultuybek, G.K. Metformin reverses the effects of high glucose on human dermal fibroblasts of aged skin via downregulating RELA/p65 expression. J. Physiol. Biochem. 2021, 77, 443–450. [Google Scholar] [CrossRef]
- Ma, Z.; Ding, Y.; Ding, X.; Mou, H.; Mo, R.; Tan, Q. PDK4 rescues high-glucose-induced senescent fibroblasts and promotes diabetic wound healing through enhancing glycolysis and regulating YAP and JNK pathway. Cell Death Discov. 2023, 9, 424. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.A.; Guillen-Nieto, G.E.; Rodriguez-Rodriguez, N.; Mendoza-Mari, Y.; Bringas-Vega, M.L.; Berlanga-Saez, J.O.; Garcia Del Barco Herrera, D.; Martinez-Jimenez, I.; Hernandez-Gutierrez, S.; Valdes-Sosa, P.A. Cellular Senescence as the Pathogenic Hub of Diabetes-Related Wound Chronicity. Front. Endocrinol. 2020, 11, 573032. [Google Scholar] [CrossRef]
- da Silva, P.F.L.; Ogrodnik, M.; Kucheryavenko, O.; Glibert, J.; Miwa, S.; Cameron, K.; Ishaq, A.; Saretzki, G.; Nagaraja-Grellscheid, S.; Nelson, G.; et al. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 2019, 18, e12848. [Google Scholar] [CrossRef]
- Resnik, S.; Egger, A.; Abujamra, B.; Jozic, I. Clinical Implications of Cellular Senescence on Wound Healing. Curr. Dermatol. Rep. 2020, 9, 286–297. [Google Scholar] [CrossRef]
- Chen, Z.; Miao, F.; Braffett, B.H.; Lachin, J.M.; Zhang, L.; Wu, X.; Roshandel, D.; Carless, M.; Li, X.A.; Tompkins, J.D.; et al. DNA methylation mediates development of HbA1c-associated complications in type 1 diabetes. Nat. Metab. 2020, 2, 744–762. [Google Scholar] [CrossRef]
- Ahmed, S.A.H.; Ansari, S.A.; Mensah-Brown, E.P.K.; Emerald, B.S. The role of DNA methylation in the pathogenesis of type 2 diabetes mellitus. Clin. Epigenetics 2020, 12, 104. [Google Scholar] [CrossRef]
- Al-Rikabi, A.H.A.; Tobin, D.J.; Riches-Suman, K.; Thornton, M.J. Dermal fibroblasts cultured from donors with type 2 diabetes mellitus retain an epigenetic memory associated with poor wound healing responses. Sci. Rep. 2021, 11, 1474. [Google Scholar] [CrossRef] [PubMed]
- Pastar, I.; Marjanovic, J.; Liang, L.; Stone, R.C.; Kashpur, O.; Jozic, I.; Head, C.R.; Smith, A.; Gerami-Naini, B.; Garlick, J.A.; et al. Cellular reprogramming of diabetic foot ulcer fibroblasts triggers pro-healing miRNA-mediated epigenetic signature. Exp. Dermatol. 2021, 30, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; Fernandez-Mayola, M.; Mendoza-Mari, Y.; Garcia-Ojalvo, A.; Martinez-Jimenez, I.; Rodriguez-Rodriguez, N.; Garcia Del Barco Herrera, D.; Guillen-Nieto, G. Cell-Free Filtrates (CFF) as Vectors of a Transmissible Pathologic Tissue Memory Code: A Hypothetical and Narrative Review. Int. J. Mol. Sci. 2022, 23, 11575. [Google Scholar] [CrossRef] [PubMed]
- Berlanga-Acosta, J.; Fernandez-Mayola, M.; Mendoza-Mari, Y.; Garcia-Ojalvo, A.; Playford, R.J.; Guillen-Nieto, G. Intralesional Infiltrations of Cell-Free Filtrates Derived from Human Diabetic Tissues Delay the Healing Process and Recreate Diabetes Histopathological Changes in Healthy Rats. Front. Clin. Diabetes Healthc. 2021, 2, 617741. [Google Scholar] [CrossRef]
- Edsberg, L.; Black, J.; Goldberg, M.; McNichol, L.; Moore, L.; Seiggreen, M. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System Revised Pressure Injury Staging System. J. Wound Ostomy Cont. Nurs. 2016, 43, 585–597. [Google Scholar] [CrossRef]
- Nancy, G.A.; Kalpana, R.; Nandhini, S. A Study on Pressure Ulcer: Influencing Factors and Diagnostic Techniques. Int. J. Low Extrem. Wounds 2022, 21, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Edsberg, L.E.; Cutway, R.; Anain, S.; Natiella, J.R. Microstructural and mechanical characterization of human tissue at and adjacent to pressure ulcers. J. Rehabil. Res. Dev. 2000, 37, 463–471. [Google Scholar] [PubMed]
- Nasir, N.J.M.; Corrias, A.; Heemskerk, H.; Ang, E.T.; Jenkins, J.H.; Sebastin, S.J.; Tucker-Kellogg, L. The panniculus carnosus muscle: A missing link in the chronicity of heel pressure ulcers? J. R. Soc. Interface 2022, 19, 20210631. [Google Scholar] [CrossRef]
- Norman, G.; Wong, J.K.; Amin, K.; Dumville, J.C.; Pramod, S. Reconstructive surgery for treating pressure ulcers. Cochrane Database Syst. Rev. 2022, 10, CD012032. [Google Scholar] [CrossRef]
- Gould, L.J.; Bohn, G.; Bryant, R.; Paine, T.; Couch, K.; Cowan, L.; McFarland, F.; Simman, R. Pressure ulcer summit 2018: An interdisciplinary approach to improve our understanding of the risk of pressure-induced tissue damage. Wound Repair Regen. 2019, 27, 497–508. [Google Scholar] [CrossRef]
- Aldughayfiq, B.; Ashfaq, F.; Jhanjhi, N.Z.; Humayun, M. YOLO-Based Deep Learning Model for Pressure Ulcer Detection and Classification. Healthcare 2023, 11, 1222. [Google Scholar] [CrossRef]
- Marcusso, R.M.N.; Van Weyenbergh, J.; de Moura, J.V.L.; Dahy, F.E.; de Moura Brasil Matos, A.; Haziot, M.E.J.; Vidal, J.E.; Fonseca, L.A.M.; Smid, J.; Assone, T.; et al. Dichotomy in Fatal Outcomes in a Large Cohort of People Living with HTLV-1 in Sao Paulo, Brazil. Pathogens 2019, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, F.; Thalib, L.; Chaboyer, W. Global prevalence and incidence of pressure injuries in hospitalised adult patients: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2020, 105, 103546. [Google Scholar] [CrossRef]
- Gould, L.J.; Alderden, J.; Aslam, R.; Barbul, A.; Bogie, K.M.; El Masry, M.; Graves, L.Y.; White-Chu, E.F.; Ahmed, A.; Boanca, K.; et al. WHS guidelines for the treatment of pressure ulcers-2023 update. Wound Repair Regen. 2024, 32, 6–33. [Google Scholar] [CrossRef]
- Bennett, G.; Dealey, C.; Posnett, J. The cost of pressure ulcers in the UK. Age Ageing 2004, 33, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Mervis, J.S.; Phillips, T.J. Pressure ulcers: Pathophysiology, epidemiology, risk factors, and presentation. J. Am. Acad. Dermatol. 2019, 81, 881–890. [Google Scholar] [CrossRef]
- Milner, S.M.; Mathis, R. Pathogenesis of Pressure Injuries. Eplasty 2024, 24, ic16. [Google Scholar]
- Anders, J.; Heinemann, A.; Leffmann, C.; Leutenegger, M.; Profener, F.; von Renteln-Kruse, W. Decubitus ulcers: Pathophysiology and primary prevention. Dtsch. Arztebl. Int. 2010, 107, 371–381. [Google Scholar]
- Kottner, J.; Cuddigan, J.; Carville, K.; Balzer, K.; Berlowitz, D.; Law, S.; Litchford, M.; Mitchell, P.; Moore, Z.; Pittman, J.; et al. Pressure ulcer/injury classification today: An international perspective. J. Tissue Viability 2020, 29, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, S.M.; Louiselle, A.E.; Liechty, K.W.; Zgheib, C. Role of microRNAs in Pressure Ulcer Immune Response, Pathogenesis, and Treatment. Int. J. Mol. Sci. 2020, 22, 64. [Google Scholar] [CrossRef]
- Lowthian, P.T. Trauma and thrombosis in the pathogenesis of pressure ulcers. Clin. Dermatol. 2005, 23, 116–123. [Google Scholar] [CrossRef]
- Agrawal, K.; Chauhan, N. Pressure ulcers: Back to the basics. Indian J. Plast. Surg. 2012, 45, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A. The complex interplay between mechanical forces, tissue response and individual susceptibility to pressure ulcers. J. Wound Care 2024, 33, 620–628. [Google Scholar] [CrossRef]
- Morrow, M.M.; Hughes, L.C.; Collins, D.M.; Vos-Draper, T.L. Clinical Remote Monitoring of Individuals With Spinal Cord Injury at Risk for Pressure Injury Recurrence Using mHealth: Protocol for a Pilot, Pragmatic, Hybrid Implementation Trial. JMIR Res. Protoc. 2024, 13, e51849. [Google Scholar] [CrossRef] [PubMed]
- Oohashi, F.; Ogai, K.; Takahashi, N.; Arisandi, D.; Urai, T.; Sugama, J.; Oe, M. Increased temperature at the healed area detected by thermography predicts recurrent pressure ulcers. Wound Repair Regen. 2022, 30, 190–197. [Google Scholar] [CrossRef]
- Du, L.; Wang, N.; Pei, J.; Jiao, Y.; Xu, J.; Xu, X.; Wen, A.; Han, L.; Lv, L. Understanding recurrent pressure injuries: A scoping review of current research and risk factors. J. Tissue Viability 2025, 34, 100886. [Google Scholar] [CrossRef]
- Schryvers, O.I.; Stranc, M.F.; Nance, P.W. Surgical treatment of pressure ulcers: 20-year experience. Arch. Phys. Med. Rehabil. 2000, 81, 1556–1562. [Google Scholar] [CrossRef]
- Sirimaharaj, W.; Charoenvicha, C. Pressure Ulcers: Risk Stratification and Prognostic Factors That Promote Recurrence After Reconstructive Surgery. Int. J. Low Extrem. Wounds 2018, 17, 94–101. [Google Scholar] [CrossRef]
- Bamba, R.; Madden, J.J.; Hoffman, A.N.; Kim, J.S.; Thayer, W.P.; Nanney, L.B.; Spear, M.E. Flap Reconstruction for Pressure Ulcers: An Outcomes Analysis. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1187. [Google Scholar] [CrossRef] [PubMed]
- Keys, K.A.; Daniali, L.N.; Warner, K.J.; Mathes, D.W. Multivariate predictors of failure after flap coverage of pressure ulcers. Plast. Reconstr. Surg. 2010, 125, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.; Nixon, J.; Gorecki, C. A systematic review of risk factors for the development and recurrence of pressure ulcers in people with spinal cord injuries. Spinal Cord 2013, 51, 522–527. [Google Scholar] [CrossRef]
- Lustig, A.; Margi, R.; Orlov, A.; Orlova, D.; Azaria, L.; Gefen, A. The mechanobiology theory of the development of medical device-related pressure ulcers revealed through a cell-scale computational modeling framework. Biomech. Model. Mechanobiol. 2021, 20, 851–860. [Google Scholar] [CrossRef]
- Wurzer, P.; Winter, R.; Stemmer, S.O.; Ivancic, J.; Lebo, P.B.; Hundeshagen, G.; Cambiaso-Daniel, J.; Quehenberger, F.; Kamolz, L.P.; Lumenta, D.B. Risk factors for recurrence of pressure ulcers after defect reconstruction. Wound Repair Regen. 2018, 26, 64–68. [Google Scholar] [CrossRef]
- Hsu, W.J.; Minematsu, T.; Nakagami, G.; Koudounas, S.; Tomida, S.; Nakai, A.; Kunimitsu, M.; Nitta, S.; Sanada, H. Identification of microRNAs responsive to shear loading in rat skin. Int. Wound J. 2022, 19, 351–361. [Google Scholar] [CrossRef]
- Dini, V.; Janowska, A.; Oranges, T.; De Pascalis, A.; Iannone, M.; Romanelli, M. Surrounding skin management in venous leg ulcers: A systematic review. J. Tissue Viability 2020, 29, 169–175. [Google Scholar] [CrossRef]
- Xie, T.; Ye, J.; Rerkasem, K.; Mani, R. The venous ulcer continues to be a clinical challenge: An update. Burn. Trauma. 2018, 6, 18. [Google Scholar] [CrossRef]
- Maguire, C.; Carville, K.; Smith, J.; Richards, T.; Smith, K. The impact of venous leg ulcers on quality of life. Wound Pract. Res. 2023, 31, 164–173. [Google Scholar]
- Folguera-Alvarez, C.; Garrido-Elustondo, S.; Rico-Blazquez, M.; Verdu-Soriano, J. Factors Associated With the Quality of Life of Patients With Venous Leg Ulcers in Primary Care: Cross-Sectional Study. Int. J. Low Extrem. Wounds 2022, 21, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Cleman, J.; Xia, K.; Haider, M.; Nikooie, R.; Scierka, L.; Romain, G.; Attaran, R.R.; Grimshaw, A.; Mena-Hurtado, C.; Smolderen, K.G. A state-of-the-art review of quality-of-life assessment in venous disease. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101725. [Google Scholar] [CrossRef] [PubMed]
- Jun, D. Pathophysiology and Conservative Treatment of Venous Ulcers: A Review. J. Wound Manag. Res. 2022, 18, 161–169. [Google Scholar] [CrossRef]
- Raffetto, J.D.; Ligi, D.; Maniscalco, R.; Khalil, R.A.; Mannello, F. Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, Clinical Consequences, and Treatment. J. Clin. Med. 2020, 10, 29. [Google Scholar] [CrossRef]
- Krizanova, O.; Penesova, A.; Hokynkova, A.; Pokorna, A.; Samadian, A.; Babula, P. Chronic venous insufficiency and venous leg ulcers: Aetiology, on the pathophysiology-based treatment. Int. Wound J. 2023, 21, e14405. [Google Scholar] [CrossRef]
- Chandran Latha, K.; Sreekumar, A.; Beena, V.; Binil Raj, S.S.; Lakkappa, R.B.; Kalyani, R.; Nair, R.; Kalpana, S.R.; Kartha, C.C.; Surendran, S. Shear Stress Alterations Activate BMP4/pSMAD5 Signaling and Induce Endothelial Mesenchymal Transition in Varicose Veins. Cells 2021, 10, 3563. [Google Scholar] [CrossRef]
- Coelho, G.A.; Secretan, P.H.; Tortolano, L.; Charvet, L.; Yagoubi, N. Evolution of the Chronic Venous Leg Ulcer Microenvironment and Its Impact on Medical Devices and Wound Care Therapies. J. Clin. Med. 2023, 12, 5605. [Google Scholar] [CrossRef]
- Casili, G.; Lanza, M.; Campolo, M.; Messina, S.; Scuderi, S.; Ardizzone, A.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Therapeutic potential of flavonoids in the treatment of chronic venous insufficiency. Vasc. Pharmacol. 2021, 137, 106825. [Google Scholar] [CrossRef]
- Lyons, O.T.; Saha, P.; Smith, A. Redox dysregulation in the pathogenesis of chronic venous ulceration. Free Radic. Biol. Med. 2020, 149, 23–29. [Google Scholar] [CrossRef]
- He, B.; Shi, J.; Li, L.; Ma, Y.; Zhao, H.; Qin, P.; Ma, P. Prevention strategies for the recurrence of venous leg ulcers: A scoping review. Int. Wound J. 2024, 21, e14759. [Google Scholar] [CrossRef]
- Finlayson, K.; Wu, M.L.; Edwards, H.E. Identifying risk factors and protective factors for venous leg ulcer recurrence using a theoretical approach: A longitudinal study. Int. J. Nurs. Stud. 2015, 52, 1042–1051. [Google Scholar] [CrossRef]
- Li, X.; Lv, D.; Xie, J.; Ye, X.; Xia, C.; Liu, D. Screening and analysis of differentially expressed circRNAs and miRNAs in chronic diabetic extremity wounds. Front. Surg. 2022, 9, 1007312. [Google Scholar] [CrossRef]
- Ju, C.C.; Liu, X.X.; Liu, L.H.; Guo, N.; Guan, L.W.; Wu, J.X.; Liu, D.W. Epigenetic modification: A novel insight into diabetic wound healing. Heliyon 2024, 10, e28086. [Google Scholar] [CrossRef] [PubMed]
- Graves, L.Y.; Schwartz, K.R.; Shiff, J.; Chan, E.R.; Galea, M.; Henzel, M.K.; Olney, C.; Bogie, K.M. Genomic Biomarkers Can Provide a Deeper Understanding of Recurrent Pressure Injuries. Adv. Skin Wound Care 2023, 36, 534–539. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.R.; Yang, H.; Stechmiller, J.; Lyon, D.E. MicroRNA Expression in Chronic Venous Leg Ulcers and Implications for Wound Healing: A Scoping Review. Biol. Res. Nurs. 2025, 27, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qi, F.; Luo, H.; Xu, G.; Wang, D. Inflammatory Microenvironment of Skin Wounds. Front. Immunol. 2022, 13, 789274. [Google Scholar] [CrossRef]
- Geng, K.; Ma, X.; Jiang, Z.; Huang, W.; Gu, J.; Wang, P.; Luo, L.; Xu, Y.; Xu, Y. High glucose-induced STING activation inhibits diabetic wound healing through promoting M1 polarization of macrophages. Cell Death Discov. 2023, 9, 136. [Google Scholar] [CrossRef]
- Bender, E.C.; Tareq, H.S.; Suggs, L.J. Inflammation: A matter of immune cell life and death. npj Biomed. Innov. 2025, 2, 7. [Google Scholar] [CrossRef]
- Huang, W.; Jiao, J.; Liu, J.; Huang, M.; Hu, Y.; Ran, W.; Yan, L.; Xiong, Y.; Li, M.; Quan, Z.; et al. MFG-E8 accelerates wound healing in diabetes by regulating “NLRP3 inflammasome-neutrophil extracellular traps” axis. Cell Death Discov. 2020, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.; Nuermaimaiti, Y.; Maimaituxun, G.; Luo, X.; Maimaituxun, M.; Akbar, A.; Tuerxun, K.; Wu, Y. Neutrophil Extracellular Traps (NETs) Are Associated with Type 2 Diabetes and Diabetic Foot Ulcer Related Amputation: A Prospective Cohort Study. Diabetes Ther. 2024, 15, 1333–1348. [Google Scholar] [CrossRef]
- Zhu, S.; Yu, Y.; Ren, Y.; Xu, L.; Wang, H.; Ling, X.; Jin, L.; Hu, Y.; Zhang, H.; Miao, C.; et al. The emerging roles of neutrophil extracellular traps in wound healing. Cell Death Dis. 2021, 12, 984. [Google Scholar] [CrossRef]
- Worsley, A.L.; Lui, D.H.; Ntow-Boahene, W.; Song, W.; Good, L.; Tsui, J. The importance of inflammation control for the treatment of chronic diabetic wounds. Int. Wound J. 2023, 20, 2346–2359. [Google Scholar] [CrossRef]
- Larouche, J.; Sheoran, S.; Maruyama, K.; Martino, M. Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv. Wound Care 2018, 7, 209–231. [Google Scholar] [CrossRef]
- Nazari, M.; Taremi, S.; Elahi, R.; Mostanadi, P.; Esmeilzadeh, A. Therapeutic Properties of M2 Macrophages in Chronic Wounds: An Innovative Area of Biomaterial-Assisted M2 Macrophage Targeted Therapy. Stem Cell Rev. Rep. 2025, 21, 390–422. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.G.; Deinsberger, J.; Oszwald, A.; Weber, B. The Histopathology of Leg Ulcers. Dermatopathology 2024, 11, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Sindrilaru, A.; Peters, T.; Wieschalka, S.; Baican, C.; Baican, A.; Peter, H.; Hainzl, A.; Schatz, S.; Qi, Y.; Schlecht, A.; et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Investig. 2011, 121, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Burian, E.A.; Sabah, L.; Karlsmark, T.; Kirketerp-Moller, K.; Moffatt, C.J.; Thyssen, J.P.; Agren, M.S. Cytokines and Venous Leg Ulcer Healing-A Systematic Review. Int. J. Mol. Sci. 2022, 23, 6526. [Google Scholar] [CrossRef]
- Silva, K.C.N.; da Cruz Ballerini, A.P.A.; Flose, B.R.; Silva, M.D.; Pimenta, C.A.M.; Shio, M.T.; do Amaral, J.B.; Arruda, L.B.; Bachi, A.L.L.; França, C.N.; et al. Profile of expression of human endogenous retroviruses and their interplay on inflammatory status in patients with chronic venous disease. Human Gene 2025, 44, 201411. [Google Scholar] [CrossRef]
- Tavecchio, M.; Fanni, S.; Wu, X.; Petruk, G.; Puthia, M.; Schmidtchen, A. A murine pressure ulcer model for evaluating persistence and treatment of Staphylococcus aureus infection. Front. Med. 2025, 12, 1561732. [Google Scholar] [CrossRef]
- Tomic-Canic, M.; Burgess, J.L.; O’Neill, K.E.; Strbo, N.; Pastar, I. Skin Microbiota and its Interplay with Wound Healing. Am. J. Clin. Dermatol. 2020, 21 (Suppl. S1), 36–43. [Google Scholar] [CrossRef] [PubMed]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Versey, Z.; da Cruz Nizer, W.S.; Russell, E.; Zigic, S.; DeZeeuw, K.G.; Marek, J.E.; Overhage, J.; Cassol, E. Biofilm-Innate Immune Interface: Contribution to Chronic Wound Formation. Front. Immunol. 2021, 12, 648554. [Google Scholar] [CrossRef]
- Basu, P.; Kim, J.H.; Saeed, S.; Martins-Green, M. Using systems biology approaches to identify signalling pathways activated during chronic wound initiation. Wound Repair Regen. 2021, 29, 881–898. [Google Scholar] [CrossRef]
- Hunt, M.; Torres, M.; Bachar-Wikstrom, E.; Wikstrom, J.D. Cellular and molecular roles of reactive oxygen species in wound healing. Commun. Biol. 2024, 7, 1534. [Google Scholar] [CrossRef]
- Panayi, A.C.; Endo, Y.; Karvar, M.; Sensharma, P.; Haug, V.; Fu, S.; Mi, B.; An, Y.; Orgill, D.P. Low mortality oxidative stress murine chronic wound model. BMJ Open Diabetes Res. Care 2020, 8, e001221. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Jaganjac, M.; Milkovic, L.; Zarkovic, N.; Zarkovic, K. Oxidative stress and regeneration. Free Radic. Biol. Med. 2022, 181, 154–165. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxid. Med. Cell Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Wang, W.; Peng, M.; Zhang, X.Z. Free radicals for cancer theranostics. Biomaterials 2021, 266, 120474. [Google Scholar] [CrossRef]
- Johnson, J.B.; Broszczak, D.A.; Mani, J.S.; Anesi, J.; Naiker, M. A cut above the rest: Oxidative stress in chronic wounds and the potential role of polyphenols as therapeutics. J. Pharm. Pharmacol. 2022, 74, 485–502. [Google Scholar] [CrossRef]
- Maeso, L.; Antezana, P.E.; Hvozda Arana, A.G.; Evelson, P.A.; Orive, G.; Desimone, M.F. Progress in the Use of Hydrogels for Antioxidant Delivery in Skin Wounds. Pharmaceutics 2024, 16, 524. [Google Scholar] [CrossRef]
- Lopez, T.; Wendremaire, M.; Lagarde, J.; Duquet, O.; Alibert, L.; Paquette, B.; Garrido, C.; Lirussi, F. Wound Healing versus Metastasis: Role of Oxidative Stress. Biomedicines 2022, 10, 2784. [Google Scholar] [CrossRef] [PubMed]
- Harithpriya, K.; Kaussikaa, S.; Kavyashree, S.; Geetha, A.; Ramkumar, K.M. Pathological insights into cell death pathways in diabetic wound healing. Pathol. Res. Pract. 2024, 264, 155715. [Google Scholar] [CrossRef]
- Atayik, M.C.; Cakatay, U. Redox signaling in impaired cascades of wound healing: Promising approach. Mol. Biol. Rep. 2023, 50, 6927–6936. [Google Scholar] [CrossRef] [PubMed]
- Jurk, D.; Wilson, C.; Passos, J.F.; Oakley, F.; Correia-Melo, C.; Greaves, L.; Saretzki, G.; Fox, C.; Lawless, C.; Anderson, R.; et al. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun. 2014, 2, 4172. [Google Scholar] [CrossRef]
- D’Agostino, M.; Sileno, S.; Lulli, D.; De Luca, N.; Scarponi, C.; Teson, M.; Torcinaro, A.; De Santa, F.; Cirielli, C.; Furgiuele, S.; et al. miR-200c inhibition and catalase accelerate diabetic wound healing. J. Biomed. Sci. 2025, 32, 21. [Google Scholar] [CrossRef]
- Dham, D.; Roy, B.; Gowda, A.; Pan, G.; Sridhar, A.; Zeng, X.; Thandavarayan, R.A.; Palaniyandi, S.S. 4-Hydroxy-2-nonenal, a lipid peroxidation product, as a biomarker in diabetes and its complications: Challenges and opportunities. Free Radic. Res. 2021, 55, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Fang, D.; Chen, J.; Hu, S.; Chen, N.; Jiang, J.; Zeng, M.; Luo, M. LncRNAs associated with oxidative stress in diabetic wound healing: Regulatory mechanisms and application prospects. Theranostics 2023, 13, 3655–3674. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Lei, H.; Cai, Y.; Shen, J.; Zhu, P.; He, Q.; Zhao, M. The Nrf-2/HO-1 Signaling Axis: A Ray of Hope in Cardiovascular Diseases. Cardiol. Res. Pract. 2020, 2020, 5695723. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.L.; Stupar, D.; Croll, T.; Leavesley, D.; Upton, Z. Xanthine Oxidoreductase: A Novel Therapeutic Target for the Treatment of Chronic Wounds? Adv. Wound Care 2018, 7, 95–104. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Upton, Z.; Shooter, G.K. Uric acid and xanthine oxidoreductase in wound healing. Curr. Rheumatol. Rep. 2014, 16, 396. [Google Scholar] [CrossRef] [PubMed]
- Siedlar, A.M.; Seredenina, T.; Faivre, A.; Cambet, Y.; Stasia, M.J.; Andre-Levigne, D.; Bochaton-Piallat, M.L.; Pittet-Cuenod, B.; de Seigneux, S.; Krause, K.H.; et al. NADPH oxidase 4 is dispensable for skin myofibroblast differentiation and wound healing. Redox Biol. 2023, 60, 102609. [Google Scholar] [CrossRef]
- Chen, D.; Guo, Z.; Yao, L.; Sun, Y.; Dian, Y.; Zhao, D.; Ke, Y.; Zeng, F.; Zhang, C.; Deng, G.; et al. Targeting oxidative stress-mediated regulated cell death as a vulnerability in cancer. Redox Biol. 2025, 84, 103686. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef]
- Jin, S.; Li, K.; Zong, X.; Eun, S.; Morimoto, N.; Guo, S. Hallmarks of Skin Aging: Update. Aging Dis. 2023, 14, 2167–2176. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, C. Cellular senescence is a promising target for chronic wounds: A comprehensive review. Burn. Trauma 2020, 8, tkaa021. [Google Scholar] [CrossRef] [PubMed]
- Shakel, Z.; Costa Lima, S.A.; Reis, S. Strategies to make human skin models based on cellular senescence for ageing research. Ageing Res. Rev. 2024, 100, 102430. [Google Scholar] [CrossRef]
- Yu, G.T.; Gomez, P.T.; Prata, L.G.; Lehman, J.S.; Tchkonia, T.; Kirkland, J.L.; Meves, A.; Wyles, S.P. Clinicopathological and cellular senescence biomarkers in chronic stalled wounds. Int. J. Dermatol. 2024, 63, 1227–1235. [Google Scholar] [CrossRef]
- Wyles, S.P.; Dashti, P.; Pirtskhalava, T.; Tekin, B.; Inman, C.; Gomez, L.S.; Lagnado, A.B.; Prata, L.; Jurk, D.; Passos, J.F.; et al. A chronic wound model to investigate skin cellular senescence. Aging 2023, 15, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Kordinas, V.; Ioannidis, A.; Chatzipanagiotou, S. The Telomere/Telomerase System in Chronic Inflammatory Diseases. Cause Eff. Genes 2016, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.T.; Monie, D.D.; Khosla, S.; Tchkonia, T.; Kirkland, J.L.; Wyles, S.P. Mapping cellular senescence networks in human diabetic foot ulcers. Geroscience 2024, 46, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Baltzis, D.; Meimeti, E.; Grammatikopoulou, M.G.; Roustit, M.; Mavrogonatou, E.; Kletsas, D.; Efraimidou, S.; Manes, C.; Nikolouzakis, T.K.; Tsiaoussis, J.; et al. Assessment of telomerase activity in leukocytes of type 2 diabetes mellitus patients having or not foot ulcer: Possible correlation with other clinical parameters. Exp. Ther. Med. 2018, 15, 3420–3424. [Google Scholar]
- Franco, A.C.; Martini, H.; Victorelli, S.; Lagnado, A.B.; Wyles, S.P.; Rowsey, J.L.; Pirius, N.; Woo, S.H.; Costa, D.G.; Chaib, S.; et al. Senescent cell transplantation into the skin induces age-related peripheral dysfunction and cognitive decline. Aging Cell 2025, 24, e14340. [Google Scholar] [CrossRef]
- Chaithanya, V.; Kumar, J.; Leela, K.; Murugesan, R.; Angelin, M.; Satheesan, A. Impact of Telomere attrition on Diabetes Mellitus and its Complications. Diabetes Epidemiol. Manag. 2023, 12, 100174. [Google Scholar] [CrossRef]
- Lavarti, R.; Alvarez-Diaz, T.; Marti, K.; Kar, P.; Raju, R.P. The context-dependent effect of cellular senescence: From embryogenesis and wound healing to aging. Ageing Res. Rev. 2025, 109, 102760. [Google Scholar] [CrossRef]
- Lim, D.X.E.; Richards, T.; Kanapathy, M.; Sudhaharan, T.; Wright, G.D.; Phillips, A.R.J.; Becker, D.L. Extracellular matrix and cellular senescence in venous leg ulcers. Sci. Rep. 2021, 11, 20168. [Google Scholar] [CrossRef]
- Vande Berg, J.S.; Rose, M.A.; Haywood-Reid, P.L.; Rudolph, R.; Payne, W.G.; Robson, M.C. Cultured pressure ulcer fibroblasts show replicative senescence with elevated production of plasmin, plasminogen activator inhibitor-1, and transforming growth factor-beta1. Wound Repair Regen. 2005, 13, 76–83. [Google Scholar] [CrossRef]
- Wall, I.B.; Moseley, R.; Baird, D.M.; Kipling, D.; Giles, P.; Laffafian, I.; Price, P.E.; Thomas, D.W.; Stephens, P. Fibroblast dysfunction is a key factor in the non-healing of chronic venous leg ulcers. J. Investig. Dermatol. 2008, 128, 2526–2540. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Haba, D.; Takizawa, C.; Tomida, S.; Horinouchi, A.; Katagiri, M.; Nomura, S.; Nakagami, G. Candidate Biomarkers for Hard-to-Heal Wounds Revealed by Single-Cell RNA Sequencing of Wound Fluid in Murine Wound Models. Wound Repair Regen. 2025, 33, e70038. [Google Scholar] [CrossRef]
- Konstantinou, E.; Longange, E.; Kaya, G. Mechanisms of Senescence and Anti-Senescence Strategies in the Skin. Biology 2024, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Al Madhoun, A. Epigenetics and diabetic wound healing: Wilms tumor 1-associated protein as a therapeutic target. World J. Diabetes 2025, 16, 105615. [Google Scholar] [CrossRef]
- Singh, K.; Rustagi, Y.; Abouhashem, A.S.; Tabasum, S.; Verma, P.; Hernandez, E.; Pal, D.; Khona, D.K.; Mohanty, S.K.; Kumar, M.; et al. Genome-wide DNA hypermethylation opposes healing in patients with chronic wounds by impairing epithelial-mesenchymal transition. J. Clin. Investig. 2022, 132, e157279. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Sun, Y.; Nie, L.; Cui, A.; Zhao, P.; Leung, W.K.; Wang, Q. Metabolic memory: Mechanisms and diseases. Signal Transduct. Target. Ther. 2024, 9, 38. [Google Scholar] [CrossRef]
- Chen, Z.; Malek, V.; Natarajan, R. Update: The role of epigenetics in the metabolic memory of diabetic complications. Am. J. Physio.l Ren. Physiol. 2024, 327, F327–F339. [Google Scholar] [CrossRef]
- Dubey, R.; Prabhakar, P.K.; Gupta, J. Epigenetics: Key to improve delayed wound healing in type 2 diabetes. Mol. Cell Biochem. 2022, 477, 371–383. [Google Scholar] [CrossRef]
- Yang, Y.; Luan, Y.; Feng, Q.; Chen, X.; Qin, B.; Ren, K.D.; Luan, Y. Epigenetics and Beyond: Targeting Histone Methylation to Treat Type 2 Diabetes Mellitus. Front. Pharmacol. 2021, 12, 807413. [Google Scholar] [CrossRef]
- Villeneuve, L.M.; Reddy, M.A.; Natarajan, R. Epigenetics: Deciphering its role in diabetes and its chronic complications. Clin. Exp. Pharmacol. Physiol. 2011, 38, 451–459. [Google Scholar] [CrossRef]
- Keating, S.T.; El-Osta, A. Epigenetic changes in diabetes. Clin. Genet. 2013, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Mocharla, P.; Akhmedov, A.; Costantino, S.; Osto, E.; Volpe, M.; Luscher, T.F.; Cosentino, F. Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes. Circ. Res. 2012, 111, 278–289. [Google Scholar] [CrossRef]
- Davis, F.M.; Kimball, A.; Boniakowski, A.; Gallagher, K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr. Diab. Rep. 2018, 18, 2. [Google Scholar] [CrossRef]
- Porel, P.; Kaur, M.; Sharma, V.; Aran, K.R. Understanding molecular mechanism of diabetic wound healing: Addressing recent advancements in therapeutic managements. J. Diabetes Metab. Disord. 2025, 24, 76. [Google Scholar] [CrossRef] [PubMed]
- Hajj, J.; Sizemore, B.; Singh, K. Impact of Epigenetics, Diet, and Nutrition-Related Pathologies on Wound Healing. Int. J. Mol. Sci. 2024, 25, 10474. [Google Scholar] [CrossRef]
- Sun, X.; Joost, S.; Kasper, M. Plasticity of Epithelial Cells during Skin Wound Healing. Cold Spring Harb. Perspect. Biol. 2023, 15, a041232. [Google Scholar] [CrossRef] [PubMed]
- Besse, J.L.; Leemrijse, T.; Deleu, P.A. Diabetic foot: The orthopedic surgery angle. Orthop. Traumatol. Surg. Res. 2011, 97, 314–329. [Google Scholar] [CrossRef]
- desJardins-Park, H.E.; Foster, D.S.; Longaker, M.T. Fibroblasts and wound healing: An update. Regen. Med. 2018, 13, 491–495. [Google Scholar] [CrossRef]
- Kirk, T.; Ahmed, A.; Rognoni, E. Fibroblast Memory in Development, Homeostasis and Disease. Cells 2021, 10, 2840. [Google Scholar] [CrossRef]
- Loots, M.A.; Lamme, E.N.; Mekkes, J.R.; Bos, J.D.; Middelkoop, E. Cultured fibroblasts from chronic diabetic wounds on the lower extremity (non-insulin-dependent diabetes mellitus) show disturbed proliferation. Arch. Dermatol. Res. 1999, 291, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Mancini, E.; Xu, L.; Moore, A.; Jahanbani, F.; Hebestreit, K.; Srinivasan, R.; Li, X.; Devarajan, K.; Prelot, L.; et al. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature 2019, 574, 553–558. [Google Scholar] [CrossRef]
- Vande Berg, J.S.; Rudolph, R.; Hollan, C.; Haywood-Reid, P.L. Fibroblast senescence in pressure ulcers. Wound Repair Regen. 1998, 6, 38–49. [Google Scholar] [CrossRef]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.J.; Al-Amoudi, H.O.; Leverkus, M.; Park, H.Y. Effect of chronic wound fluid on fibroblasts. J. Wound Care 1998, 7, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.Y.; Wu, X.Q.; He, W.J.; Liao, X.; Tang, M.; Nie, X.Q. Targeting DNA methylation and demethylation in diabetic foot ulcers. J. Adv. Res. 2023, 54, 119–131. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, C.; Lin, Y.; Parviz, Y.; Sun, K.; Wang, W.; Ren, M.; Yan, L. Matrix metalloproteinase 9 induces keratinocyte apoptosis through FasL/Fas pathway in diabetic wound. Apoptosis 2019, 24, 542–551. [Google Scholar] [CrossRef]
- Zhou, L.; Ren, M.; Zeng, T.; Wang, W.; Wang, X.; Hu, M.; Su, S.; Sun, K.; Wang, C.; Liu, J.; et al. TET2-interacting long noncoding RNA promotes active DNA demethylation of the MMP-9 promoter in diabetic wound healing. Cell Death Dis. 2019, 10, 813. [Google Scholar] [CrossRef]
- Neary, R.; Watson, C.J.; Baugh, J.A. Epigenetics and the overhealing wound: The role of DNA methylation in fibrosis. Fibrogenesis Tissue Repair. 2015, 8, 18. [Google Scholar] [CrossRef]
- Lewis, C.J.; Stevenson, A.; Fear, M.W.; Wood, F.M. A review of epigenetic regulation in wound healing: Implications for the future of wound care. Wound Repair Regen. 2020, 28, 710–718. [Google Scholar] [CrossRef]
- Li, J.; Wei, M.; Liu, X.; Xiao, S.; Cai, Y.; Li, F.; Tian, J.; Qi, F.; Xu, G.; Deng, C. The progress, prospects, and challenges of the use of non-coding RNA for diabetic wounds. Mol. Ther. Nucleic Acids 2021, 24, 554–578. [Google Scholar] [CrossRef]
- Petkovic, M.; Sorensen, A.E.; Leal, E.C.; Carvalho, E.; Dalgaard, L.T. Mechanistic Actions of microRNAs in Diabetic Wound Healing. Cells 2020, 9, 2228. [Google Scholar] [CrossRef]
- Alvarez-Rafael, D.; de la Escosura-Muniz, A. MicroRNAs as emerging biomarkers for chronic wound monitoring: Analytical methods for the point-of-care detection. J. Pharm. Biomed. Anal. 2025, 266, 117110. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.; Santos, D.; Vilaca, M.; Carvalho, A.; Carvalho, R.; Jesus Dantas, M.; Pereira, M.G.; Carvalho, E. Impact of Psychological Distress on Physiological Indicators of Healing Prognosis in Patients with Chronic Diabetic Foot Ulcers: A Longitudinal Study. Adv. Wound Care 2024, 13, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, Y.; Jia, Z.; Zhao, X.; Chen, M. Decreased expression of miR-24 in peripheral plasma of type 2 diabetes mellitus patients associated with diabetic foot ulcer. Wound Repair Regen. 2020, 28, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.W.; Wang, Y.; Chen, L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551. [Google Scholar] [CrossRef]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genomics Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar]
- Pastar, I.; Marjanovic, J.; Stone, R.C.; Chen, V.; Burgess, J.L.; Mervis, J.S.; Tomic-Canic, M. Epigenetic regulation of cellular functions in wound healing. Exp. Dermatol. 2021, 30, 1073–1089. [Google Scholar] [CrossRef]
- Kretz, M.; Siprashvili, Z.; Chu, C.; Webster, D.E.; Zehnder, A.; Qu, K.; Lee, C.S.; Flockhart, R.J.; Groff, A.F.; Chow, J.; et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature 2013, 493, 231–235. [Google Scholar] [CrossRef]
- Cheng, X.W.; Chen, Z.F.; Wan, Y.F.; Zhou, Q.; Wang, H.; Zhu, H.Q. Long Non-coding RNA H19 Suppression Protects the Endothelium Against Hyperglycemic-Induced Inflammation via Inhibiting Expression of miR-29b Target Gene Vascular Endothelial Growth Factor a Through Activation of the Protein Kinase B/Endothelial Nitric Oxide Synthase Pathway. Front. Cell Dev. Biol. 2019, 7, 263. [Google Scholar]
- Hu, J.; Zhang, L.; Liechty, C.; Zgheib, C.; Hodges, M.M.; Liechty, K.W.; Xu, J. Long Noncoding RNA GAS5 Regulates Macrophage Polarization and Diabetic Wound Healing. J. Investig. Dermatol. 2020, 140, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wu, Y.; Yang, C.; Wang, X.; Wang, W.; Zhou, L.; Zeng, T.; Zhou, J.; Wang, C.; Lao, G.; et al. Novel Long Noncoding RNA lnc-URIDS Delays Diabetic Wound Healing by Targeting Plod1. Diabetes 2020, 69, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Guo, J.; Li, J.; Shi, X.; Xu, N.; Jiang, Y.; Chen, W.; Hu, Q. lncRNA-H19 in Fibroblasts Promotes Wound Healing in Diabetes. Diabetes 2022, 71, 1562–1578. [Google Scholar]
- Jayasuriya, R.; Dhamodharan, U.; Karan, A.N.; Anandharaj, A.; Rajesh, K.; Ramkumar, K.M. Role of Nrf2 in MALAT1/HIF-1alpha loop on the regulation of angiogenesis in diabetic foot ulcer. Free Radic. Biol. Med. 2020, 156, 168–175. [Google Scholar] [CrossRef]
- Herter, E.K.; Li, D.; Toma, M.A.; Vij, M.; Li, X.; Visscher, D.; Wang, A.; Chu, T.; Sommar, P.; Blomqvist, L.; et al. WAKMAR2, a Long Noncoding RNA Downregulated in Human Chronic Wounds, Modulates Keratinocyte Motility and Production of Inflammatory Chemokines. J. Investig. Dermatol. 2019, 139, 1373–1384. [Google Scholar] [CrossRef]
- Li, D.; Liu, Z.; Zhang, L.; Bian, X.; Wu, J.; Li, L.; Chen, Y.; Luo, L.; Pan, L.; Kong, L.; et al. The lncRNA SNHG26 drives the inflammatory-to-proliferative state transition of keratinocyte progenitor cells during wound healing. Nat. Commun. 2024, 15, 8637. [Google Scholar] [PubMed]
- Li, D.; Guo, J.; Ni, X.; Sun, G.; Bao, H. The progress and challenges of circRNA for diabetic foot ulcers: A mini-review. Front. Endocrinol. 2022, 13, 1019935. [Google Scholar] [CrossRef]
- Tang, T.; Chen, L.; Zhang, M.; Wang, C.; Du, X.; Ye, S.; Li, X.; Chen, H.; Hu, N. Exosomes derived from BMSCs enhance diabetic wound healing through circ-Snhg11 delivery. Diabetol. Metab. Syndr. 2024, 16, 37. [Google Scholar] [CrossRef]
- Chen, Z.J.; Shi, X.J.; Fu, L.J.; Liu, J.; Shi, K.; Zhang, W.B.; Su, P.K. Serum and exosomal hsa_circ_0000907 and hsa_circ_0057362 as novel biomarkers in the early diagnosis of diabetic foot ulcer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8117–8126. [Google Scholar] [PubMed]

| Wound | Clinical Hallmarks | Recurrence Rates | Relevant Epigenetic Driver |
|---|---|---|---|
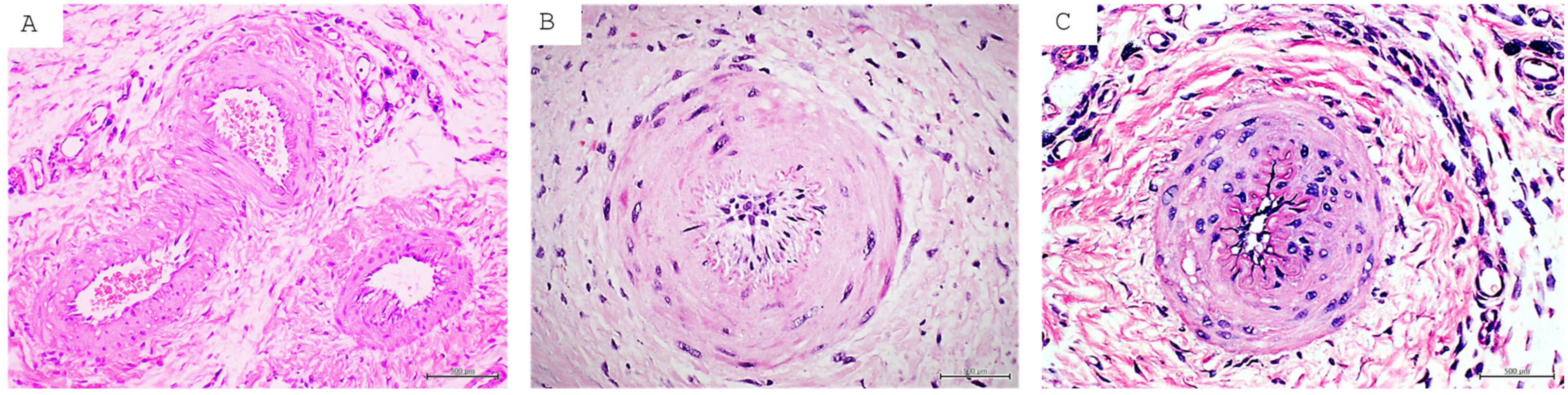
| DFU | Consequent to long-term hyperglycemia and cytotoxic by-products like AGEs and ROS. Ulcer onset by external factors like trauma, funguses, etc. Major cause of amputation and disability. Two main clinical classifications: ischemic and neuropathic. GT histopathology with large differences between the two clinical forms. The former with poor and abnormal angiogenesis, the latter with extensive nerve fiber edema and axonal degenerative changes. | About 40% of patients will experience recurrence within the first year of re-epithelialization. The wound is preferentially considered in “remission” instead of healed [31]. | Exhibits the broadest and deepest characterization of epigenetic abnormal codes, introduced by hyperglycemia, ROS, and AGEs. This epigenetic code is the foundation of metabolic memory which sustains the perpetuation of complications, including the poor healing response in skin and mucosa. The epigenetic anomalies supporting inflammation, senescence, and oxidative stress have been identified. They include changes in DNA methylation pattern, histone modifications, and non-coding RNA [97,98]. |
| PI | Largely prevailing wound in both elder and non-elder subjects mostly with spinal cord injury. Although with endogenous and external predisposing factors, the triggering one is prolonged pressure, friction, or shearing force over a bone prominence. It is a cause of multimorbidity and mortality in elder populations. At the histopathological level, the main pressure targets are large dermal vessels, leading to muscle necrosis and a cascade of damage concluding in full-thickness skin necrosis and adjacent vessel thrombosis. | The incidence of recurrence may span 5.4–73.6%. The most commonly reported site of recurrence is the ischium [74]. | Epigenetic studies on PI are scarce. To our understanding a major and recent contribution is the identification of genes related to recurrence. Upregulated activity in genes involved in metabolic pathways (ENOSF1) and pathways in biological senescence (TMEM158) and downregulated activity in the interleukin 17 signaling pathway are directly involved in antimicrobial protection in vivo. Conversely, persons without recurrent PIs have upregulated activity in genes related to bioprocesses such as regulators of vasodilation (TAC3) and downregulated activity in biological pathways related to addiction (FOS, JUN, FOSB) [99]. |
| VLU | Largely prevailing chronic wound consequent to long-term chronic venous disease. It imposes a poor quality of life. VLUs exhibit a complex and intricate pathophysiological mechanism, involving hemodynamic, cellular, and molecular alterations of macro- and microcirculation, which ultimately drives skin necrosis by venous ischemia, hypertension, and hypoxia. Histological examination reveals dilated veins, hemosiderin and fibrin deposition, sclerosis hemorrhage, and vascular fibrin cuffs. Acute phase lipodermatosclerosis shows lymphocytic and inflammatory cell infiltrate. | VLUs are characterized by repeated cycles of healing and recurrence in 60% to 70% of patients [28,94]. The median recurrence time is 42 weeks with a recurrence incidence of 22% within three months after re-epithelialization, 39% at the sixth month, and 57% by the twelfth month [95]. | Numerous upregulated miRNAs have been shown to participate in prolonging VLU inflammation and limiting angiogenesis. Concurrently, the involvement of upregulated miRNA inhibited keratinocyte proliferation, prolonged inflammation, and impaired the healing response [100]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamayo-Carbón, A.; García-Ojalvo, A.; Fernández-Montequín, J.; Savigne-Gutiérrez, W.; de Armas-López, G.; Carbonell-López, C.; Montero-Alvarez, S.; Casillas-Casanova, D.; Pino-Fernández, G.; Berlanga-Acosta, J. Major Common Hallmarks and Potential Epigenetic Drivers of Wound Chronicity and Recurrence: Hypothesis and Reflections. Int. J. Mol. Sci. 2025, 26, 8745. https://doi.org/10.3390/ijms26178745
Tamayo-Carbón A, García-Ojalvo A, Fernández-Montequín J, Savigne-Gutiérrez W, de Armas-López G, Carbonell-López C, Montero-Alvarez S, Casillas-Casanova D, Pino-Fernández G, Berlanga-Acosta J. Major Common Hallmarks and Potential Epigenetic Drivers of Wound Chronicity and Recurrence: Hypothesis and Reflections. International Journal of Molecular Sciences. 2025; 26(17):8745. https://doi.org/10.3390/ijms26178745
Chicago/Turabian StyleTamayo-Carbón, Alicia, Ariana García-Ojalvo, José Fernández-Montequín, William Savigne-Gutiérrez, Gretel de Armas-López, Cristina Carbonell-López, Sheila Montero-Alvarez, Dionne Casillas-Casanova, Gabriela Pino-Fernández, and Jorge Berlanga-Acosta. 2025. "Major Common Hallmarks and Potential Epigenetic Drivers of Wound Chronicity and Recurrence: Hypothesis and Reflections" International Journal of Molecular Sciences 26, no. 17: 8745. https://doi.org/10.3390/ijms26178745
APA StyleTamayo-Carbón, A., García-Ojalvo, A., Fernández-Montequín, J., Savigne-Gutiérrez, W., de Armas-López, G., Carbonell-López, C., Montero-Alvarez, S., Casillas-Casanova, D., Pino-Fernández, G., & Berlanga-Acosta, J. (2025). Major Common Hallmarks and Potential Epigenetic Drivers of Wound Chronicity and Recurrence: Hypothesis and Reflections. International Journal of Molecular Sciences, 26(17), 8745. https://doi.org/10.3390/ijms26178745






