Plant Extracts as Modulators of the Wound Healing Process—Preliminary Study
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Activity of Plant Extracts
2.2. Antioxidant Activity of Plant Extracts
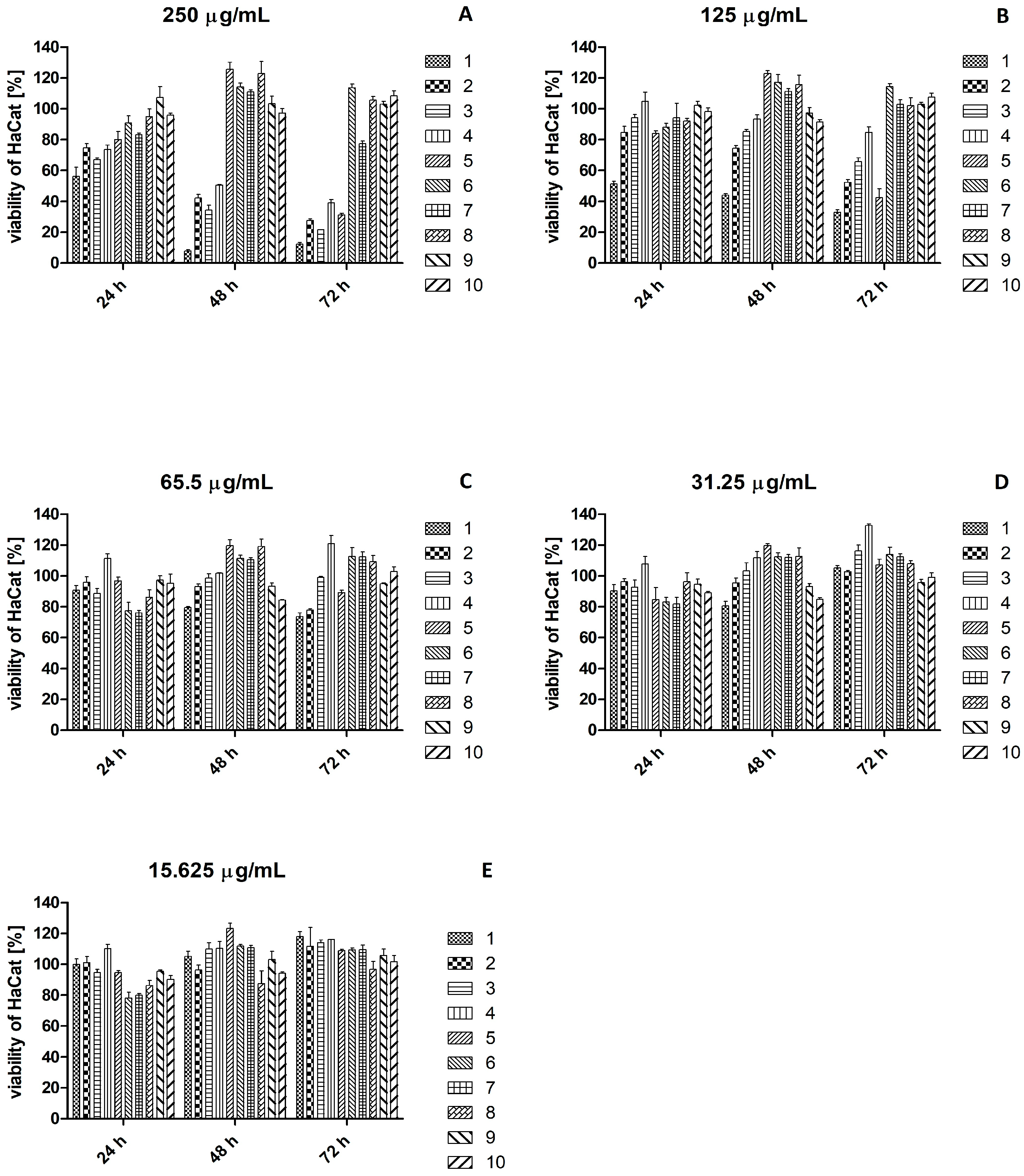
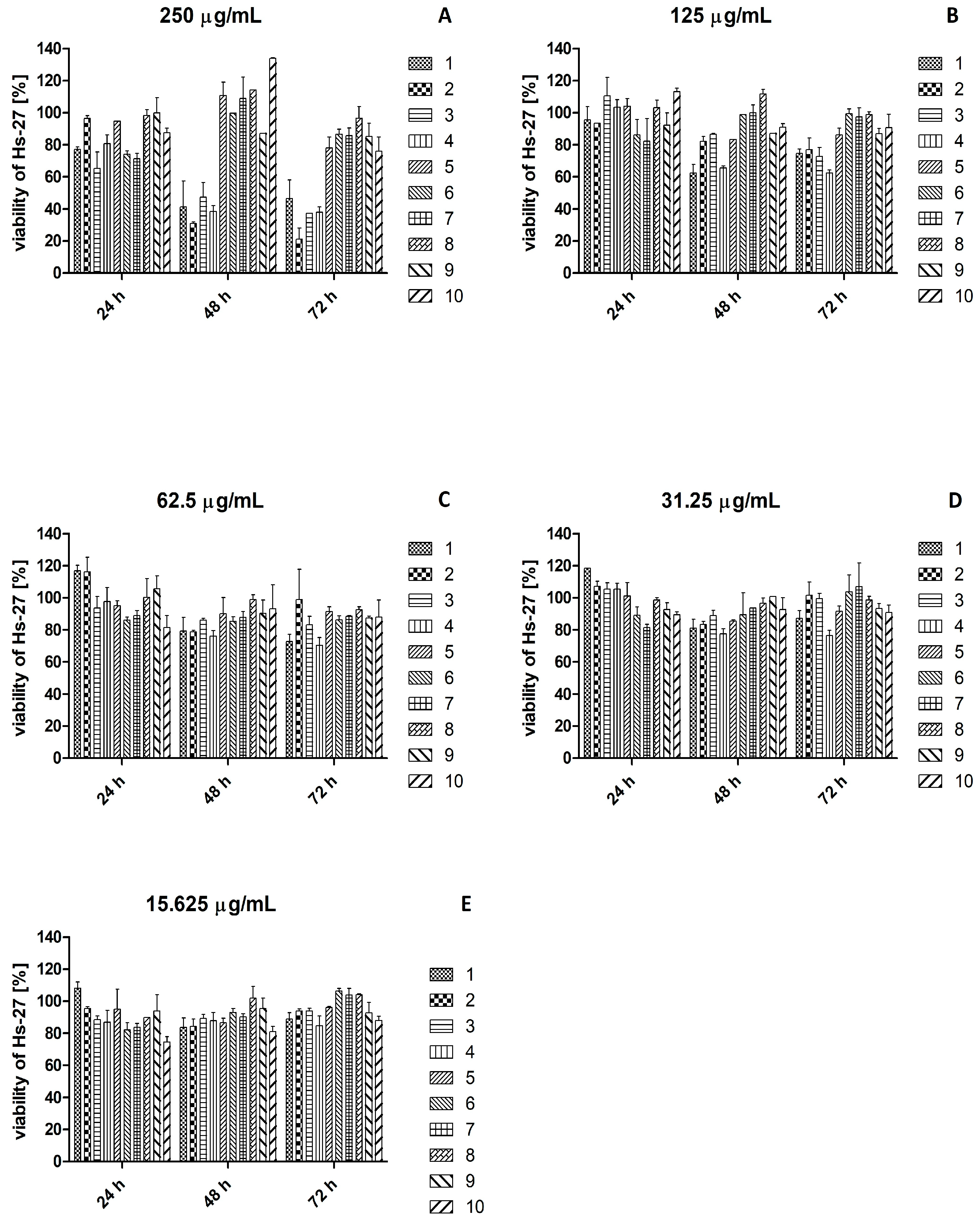
2.3. Effect of Plant Extracts on Viability of HaCaT and Hs27 Cell Lines
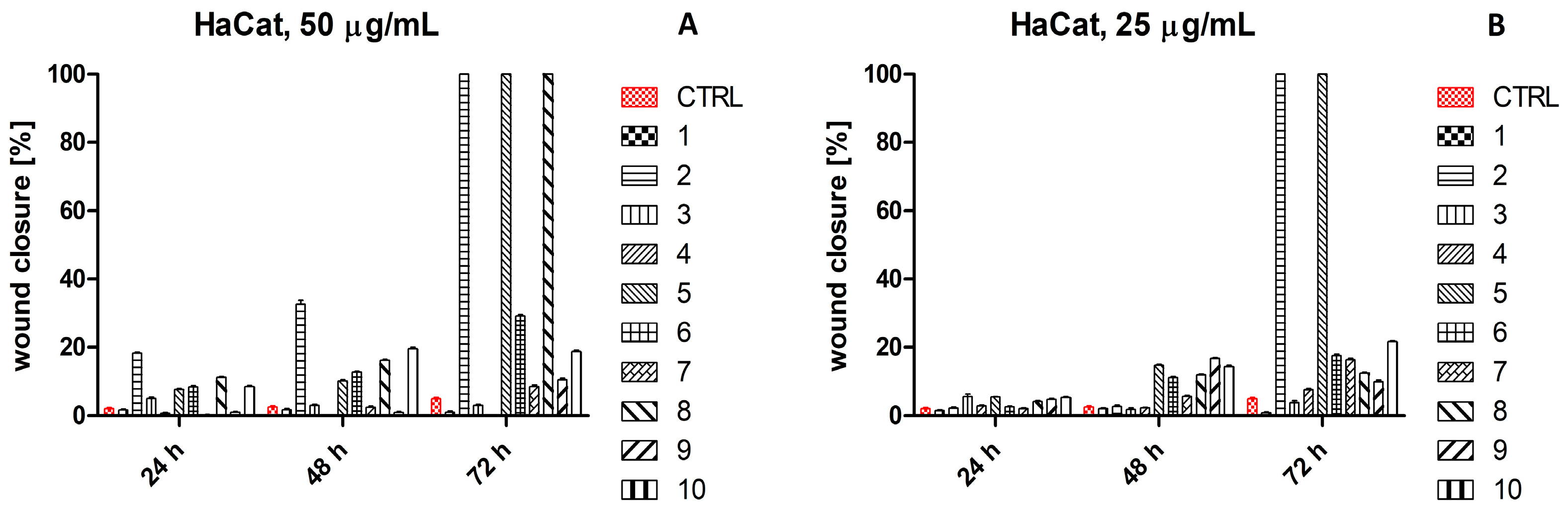
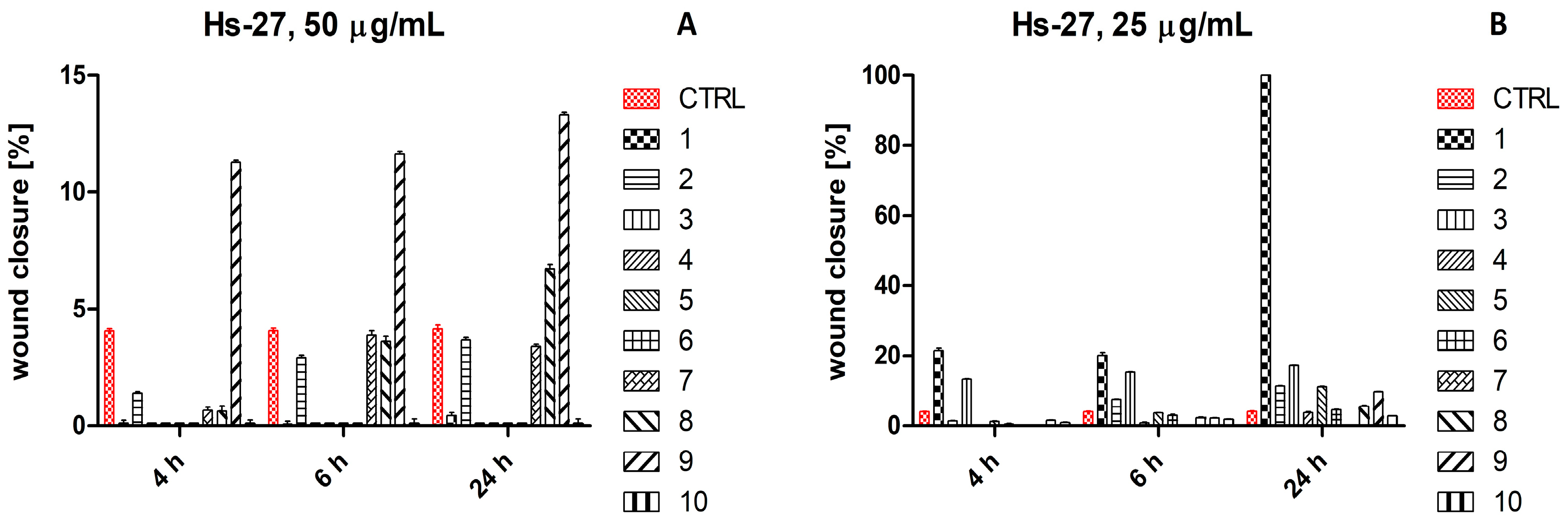
2.4. Scratch Wound Healing Assay
2.5. Collagen Assay
3. Discussion
4. Materials and Methods
4.1. Plant Extracts
4.2. Microorganisms
4.3. Cell Cultures
4.4. Determination of Minimum Inhibition Concentration (MIC), Minimum Bactericidal Concentration (MBC), and Minimum Fungicidal Concentration (MFC) of Plant Extracts by Broth Microdilution Methods
4.5. Determination of Antioxidant Activity of Plant Extracts—ABTS Assay
- % RSA—percent of radical scavenging activity
- Abs ABTS—absorbance of ABTS
- Abs Sample—absorbance of a sample
4.6. Determination of Antioxidant Activity of Plant Extracts—DPPH Assay
- % RSA—percent of radical scavenging activity
- Abs DPPH—absorbance of DPPH
- Abs Sample—absorbance of a sample
4.7. 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT)—Based Viability Assay
4.8. Scratch Wound Healing Assay
4.9. Collagen Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowan, M.P.; Cancio, L.C.; Elster, E.A.; Burmeister, D.M.; Rose, L.F.; Natesan, S.; Chan, R.K.; Christy, R.J.; Chung, K.K. Burn wound healing and treatment: Review and advancements. Crit. Care 2015, 19, 243. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic wound healing: A review of current management and treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Falanga, V. Wound healing and its impairment in the diabetic foot. Lancet 2005, 366, 1736–1743. [Google Scholar] [CrossRef]
- Verbanic, S.; Shen, Y.; Lee, J.; Deacon, J.M.; Chen, I.A. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiome 2020, 6, 21. [Google Scholar] [CrossRef]
- Bowers, S.; Franco, E. Chronic wounds: Evaluation and management. Am. Family Physic 2020, 101, 159–166. [Google Scholar]
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Gantwerker, E.A.; Hom, D.B. Skin: Histology and physiology of wound healing. Facial Plast. Surg. Clin. N. Am. 2011, 19, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, P.; Ciappellano, S.; Gardana, C.; Bramati, L.; Pietta, P. Procyanidins from Vitis vinifera seeds: In vivo effects on oxidative stress. J. Agric. Food Chem. 2002, 50, 6217–6221. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.D.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura-Carretero, A. Cocoa and grape seed byproducts as a source of antioxidant and anti-inflammatory proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef]
- Sochorova, L.; Prusova, B.; Jurikova, T.; Mlcek, J.; Adamkova, A.; Baron, M.; Sochor, J. The study of antioxidant components in grape seeds. Molecules 2020, 25, 3736. [Google Scholar] [CrossRef]
- Felhi, S.; Baccouch, N.; Ben Salah, H.; Smaoui, S.; Allouche, N.; Gharsallah, N.; Kadri, A. Nutritional constituents, phyto-chemical profiles, in vitro antioxidant and antimicrobial properties, and gas chromatography–mass spectrometry analysis of various solvent extracts from grape seeds (Vitis vinifera L.). Food Sci. Biotechnol. 2016, 25, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.H.; Duarte, M.P.; Andrade, M.A.; Mateus, A.R.; Vilarinho, F.; Fernando, A.L.; Silva, A.S. Exploring Cynara cardunculus L. by-products potential: Antioxidant and antimicrobial properties. Ind. Crops Prod. 2024, 222, 119559. [Google Scholar] [CrossRef]
- Nikolova, K.; Velikova, M.; Gentscheva, G.; Gerasimova, A.; Slavov, P.; Harbaliev, N.; Makedonski, L.; Buhalova, D.; Petkova, N.; Gavrilova, A. Chemical compositions, pharmacological properties and medicinal effects of genus Passiflora L.: A review. Plants 2024, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative study regarding the chemical composition and biological activity of pine (Pinus nigra and P. sylvestris) bark extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef]
- Levdanskiy, V.A.; Korol’kova, I.V.; Levdanskiy, A.V.; Kuznetsov, B.N. Isolation and study of proanthocyanidins from bark of pine Pinus sylvestris L. Russ. J. Bioorganic Chem. 2021, 47, 1445–1450. [Google Scholar] [CrossRef]
- Benmakhlouf, Z.; Benserradj, O.; Kellab, R. Identification of phytochemical constituents of Syzygium aromaticum L. using gas chromatography coupled with mass spectrometry and evaluation of antimicrobial activity. Biodivers. J. Biol. Divers. 2022, 23, 2586–2593. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L.(Myrtaceae): Traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Saunders, D.; Poppleton, D.; Struchkov, A.; Ireland, R. Analysis of five bioactive compounds from naturally occurring Rhodiola rosea in eastern Canada. Can. J. Plant Sci. 2014, 94, 741–748. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Jakstas, V.; Kopustinskiene, D.M. Phenolic compounds of Rhodiola rosea L. as the potential alternative therapy in the treatment of chronic diseases. Int. J. Mol. Sci. 2023, 24, 12293. [Google Scholar] [CrossRef]
- Dzięcioł, M.; Wala, K.; Wróblewska, A.; Janda-Milczarek, K. The effect of the extraction conditions on the antioxidant activity and bioactive compounds content in ethanolic extracts of Scutellaria baicalensis root. Molecules 2024, 29, 4153. [Google Scholar] [CrossRef]
- Tian, L.W.; Zhang, Z.; Long, H.L.; Zhang, Y.J. Steroidal saponins from the genus Smilax and their biological activities. Nat. Prod. Bioprospect 2017, 7, 283–298. [Google Scholar] [CrossRef]
- Peña, F.; Valencia, S.; Tereucán, G.; Nahuelcura, J.; Jiménez-Aspee, F.; Cornejo, P.; Ruiz, A. Bioactive compounds and antioxidant activity in the fruit of rosehip (Rosa canina L. and Rosa rubiginosa L.). Molecules 2023, 28, 3544. [Google Scholar] [CrossRef]
- Ammon, H.P.T. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 2020, 17, 862–867. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Herbal products in postsurgical wound healing—Incision, excision and dead space wound models. Planta Med. 2020, 86, 732–748. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Herbal products for treatment of burn wounds. J. Burn. Care Res. 2020, 41, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Herbal products and their active constituents for diabetic wound healing—Preclinical and clinical studies: A systematic review. Pharmaceutics 2023, 15, 281. [Google Scholar] [CrossRef] [PubMed]
- Manipal, S.; Fathima, L.; Hussain, S.T.; Venkat, R. Efficacy of antibacterial and antifungal action on four medicinal plants extract the A. arabica, T. chebula, A. indica, and V. vinifera against Streptococcus mutans and Candida albicans—An in vitro study. Int. J. Res. Pharm. Sci. 2019, 10, 3121–3126. [Google Scholar] [CrossRef]
- Radulescu, C.; Buruleanu, L.C.; Nicolescu, C.M.; Olteanu, R.L.; Bumbac, M.; Holban, G.C.; Simal-Gandara, J. Phytochem-ical profiles, antioxidant and antibacterial activities of grape (Vitis vinifera L.) seeds and skin from organic and conventional vineyards. Plants 2020, 9, 1470. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Filocamo, A.; Bisignamo, C.; Mandalari, G.; Navarra, M. In vitro antimicrobial activity and effect on biofilm production of a white grape juice (Vitis vinifera) extract. Evid. Based Complement. Alternat Med. 2015, 2015, 856243. [Google Scholar] [CrossRef]
- Gadelha, L.M.U.; Valadas, L.A.R.; de Mello Fiallos, N.; Peralta, S.L. Evaluation of the antifungal effect Vitis vinifera extract on Candida albicans. J. Young Pharmaciation 2018, 10, 164. [Google Scholar] [CrossRef]
- Simonetti, G.; D’Auria, F.D.; Mulinacci, N.; Innocenti, M.; Antonacci, D.; Angiolella, L.; Santamaria, A.R.; Valletta, A.; Donati, L.; Pasqua, G. Anti-dermatophyte and anti-Malassezia activity of extracts rich in polymeric flavan-3-ols obtained from Vitis vinifera seeds. Phytother. Res. 2017, 31, 124–131. [Google Scholar] [CrossRef]
- Grace Nirmala, J.; Evangeline Celsia, S.; Swaminathan, A.; Narendhirakannan, R.T.; Chatterjee, S. Cytotoxicity and apoptotic cell death induced by Vitis vinifera peel and seed extracts in A431 skin cancer cells. Cytotechnology 2018, 70, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.S.; Ramdath, D.D.; Marshall, J.R.; Isitor, G.N.; Eversley, M.; Xue, S.; Shi, J. Wound-healing activity of the skin of the common grape (Vitis Vinifera) variant, cabernet sauvignon. Phytother. Res. 2010, 24, 1151–1157. [Google Scholar] [CrossRef]
- Hemmati, A.A.; Aghel, N.; Rashidi, I.; Gholampur-Aghdami, A. Topical grape (Vitis vinifera) seed extract promotes repair of full thickness wound in rabbit. Int. Wound J. 2011, 8, 514–520. [Google Scholar] [CrossRef]
- Hammam, M.M.; Mansour, H.A.F.; Hassan, W.A. Grape seed extract promotes Staphyloccous aureus infected skin wound healing in diabetic rat model. Egypt. J. Med. Microbiol. 2022, 31, 15–23. [Google Scholar] [CrossRef]
- Tahir, R.; Nazir, A.; Qadir, M.B.; Khaliq, Z.; Hareem, F.; Arshad, S.N.; Aslam, M. Fabrication and physio-chemical char-acterization of biocompatible and antibacterial Vitis vinifera (grapes) loaded PVA nanomembranes for dermal applications. Mater. Today Commun. 2025, 42, 111178. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Zahran, E.M.; Selim, S.; Al-Sanea, M.M.; Ghoneim, M.M.; Maher, S.A.; Mostafa, Y.A.; Alsenani, F.; Elrehany, M.A.; Almuhayawi, M.S.; et al. Antioxidant and wound healing potential of Vitis vinifera seeds supported by phytochemical characterization and docking studies. Antioxidants 2022, 11, 881. [Google Scholar] [CrossRef]
- Moayeri, A.; Ramz, K.; Karimi, E.; Azizi, M.; Abbasi, N.; Aidy, A.; Bahmani, M. Therapeutic effects of Aloe vera (L.) Burm. f. and Vitis vinifera L. combination cream on wound healing in second-degree burn model in rats: Quantification of compounds and VEGF & TGFβ gene expression. Tradit. Integr. Med. 2022, 7, 52–63. [Google Scholar]
- Rajakumari, R.; Volova, T.; Oluwafemi, O.S.; Rajeshkumar, S.; Thomas, S.; Kalarikkal, N. Nano formulated proanthocy-anidins as an effective wound healing component. Mater. Sci. Eng. C 2020, 106, 110056. [Google Scholar] [CrossRef]
- Rovna, K.; Petrová, J.; Terentjeva, M.; Cerna, J.; Kacaniova, M. Antimicrobial activity of Rosa canina flowers against selected microorganisms. J. Microbiol. Biotech. Food Sci. 2015, 4, 62–64. [Google Scholar] [CrossRef]
- Mármol, I.; Sánchez-de-Diego, C.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M. Therapeutic applica-tions of rose hips from different Rosa species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef]
- Boyera, N.; Galey, I.; Bernard, B.A. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int. J. Cosmet. Sci. 1998, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Weeks, B.S.; Fu, R.; Zaidi, M. Vitamin C promotes wound healing: The use of in vitro scratch assays to assess re-epithelialization. In Cell Physiology—Annual Volume; Catala, A., Ed.; IntechOpen Books: London, UK, 2023. [Google Scholar]
- Lima, C.C.; Pereira, A.P.C.; Silva, J.R.F.; Oliveira, L.S.; Resck, M.C.C.; Grechi, C.O.; Bernardes, M.T.C.P.; Olímpio, F.M.P.; Santos, A.M.M.; Incerpi, E.K.; et al. Ascorbic acid for the healing of skin wounds in rats. Braz. J. Biol. 2009, 69, 1195–1201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rossi, M.; Rossello, S.; Sallustio, V.; Mandrone, M.; Cerchiara, T.; Chiocchio, I.; Chidichimo, G.; Protti, M.; Mercolini, L.; Luppi, B.; et al. GinExtraMed: Focus on Rosa canina L. extract encapsulated into glycethosomes and allanthosomes for accelerating skin wound healing. Pharmaceutics 2025, 17, 632. [Google Scholar] [CrossRef]
- Kosakowska, O.; Bączek, K.; Przybył, J.L.; Pióro-Jabrucka, E.; Czupa, W.; Synowiec, A.; Gniewosz, M.; Costa, R.; Mondello, L.; Węglarz, Z. Antioxidant and antibacterial activity of roseroot (Rhodiola rosea L.) dry extracts. Molecules 2018, 23, 1767. [Google Scholar] [CrossRef]
- Bany, J.; Zdanowska, D.; Skopińska-Różewska, E.; Sommer, E.; Siwicki, A.K.; Wasiutyński, A. The effect of Rhodiola rosea extracts on the bacterial infection in mice. Cent. Eur. J. Immunol. 2009, 34, 35–37. [Google Scholar]
- Ju, Y.; Luo, Y.; Li, R.; Zhang, W.; Ge, Y.; Tang, J. Multifunctional combined drug-loaded nanofibrous dressings with anti-inflammatory, antioxidant stress and microenvironment improvement for diabetic wounds. RSC Adv. 2024, 14, 29606–29623. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Fernandes, A.; Pereira, C.; Tzortzakis, N.; Vaz, J.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Bioactivities, chemical composition and nutritional value of Cynara cardunculus L. seeds. Food Chem. 2019, 289, 404–412. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Chaieb, K.; Karray-Bouraoui, N.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. C R. Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef]
- Velez, Z.; Campinho, M.A.; Guerra, Â.R.; García, L.; Ramos, P.; Guerreiro, O.; Felicio, L.; Schmitt, F.; Duarte, M. Biological characterization of Cynara cardunculus L. methanolic extracts: Antioxidant, anti-proliferative, anti-migratory and anti-angiogenic activities. Agriculture 2012, 2, 472–492. [Google Scholar] [CrossRef]
- Sukoyan, G.; Tsivtsivadze, E.; Golovach, V.; Kezeli, T.; Demina, N. Anti-aging effect of Cynara cardunculus L. var. Cynara scolymus L. extract in d-galactose-induced skin aging model in rats. Pharmacol. Pharm. 2018, 9, 428. [Google Scholar]
- Brás, T.; Rosa, D.; Gonçalves, A.C.; Gomes, A.C.; Alves, V.D.; Crespo, J.G.; Duarte, M.F.; Neves, L.A. Development of bi-oactive films based on chitosan and Cynara cardunculus leaves extracts for wound dressings. Int. J. Biol. Macromolec 2020, 163, 1707–1718. [Google Scholar] [CrossRef]
- EUCAST Broth Microdilution. Version 5.0 valid from January 2024. Available online: www.eucast.org (accessed on 1 January 2024).
- EUCAST Method for Susceptibility Testing of Yeasts. Version 7.4 Valid from October 2023. Available online: www.eucast.org (accessed on 13 October 2023).
- Olicheva, V.; Beloborodov, V.; Sharifi, S.; Dubrovskaya, A.; Zhevlakova, A.; Selivanova, I.; Ilyasov, I. Dihydroquercetin and related flavonoids in antioxidant formulations with α-tocopherol. Int. J. Mol. Sci. 2025, 26, 5659. [Google Scholar] [CrossRef]
- Ilyasov, I.; Beloborodov, V.; Antonov, D.; Dubrovskaya, A.; Terekhov, R.; Zhevlakova, A.; Saydasheva, A.; Evteev, V.; Selivanova, I. Flavonoids with glutathione antioxidant synergy: Influence of free radicals inflow. Antioxidants 2020, 9, 695. [Google Scholar] [CrossRef] [PubMed]
- Terekhov, R.P.; Ilyasov, I.R.; Beloborodov, V.L.; Zhevlakova, A.K.; Pankov, D.I.; Dzuban, A.V.; Bogdanov, A.G.; Davidovich, G.N.; Shilov, G.V.; Utenyshev, A.N.; et al. Solubility enhancement of dihydroquercetin via “green” phase modification. Int. J. Mol. Sci. 2022, 23, 15965. [Google Scholar] [CrossRef] [PubMed]


| Plant Extracts | P. aeruginosa | S. aureus | C. albicans | |||
|---|---|---|---|---|---|---|
| MIC [mg/mL] | MBC [mg/mL] | MIC [mg/mL] | MBC [mg/mL] | MIC [mg/mL] | MFC [mg/mL] | |
| V. vinifera | 3.13 | 6.25 | 1.56 | 3.13 | >50.00 | >50.00 |
| C. cardunculus | 25.00 | 50.00 | 3.13 | 6.25 | >50.00 | >50.00 |
| P. sylvestris | 3.13 | 6.25 | 3.13 | 6.25 | >50.00 | >50.00 |
| S. aromaticum | 0.39 | 0.78 | 0.39 | 0.78 | >50.00 | >50.00 |
| R. rosea | 6.25 | 12.50 | 6.25 | 12.50 | >50.00 | >50.00 |
| S. baicalensis | >50.00 | >50.00 | 25.00 | 50.00 | >50.00 | >50.00 |
| S. officinalis | >50.00 | >50.00 | 3.13 | 6.25 | >50.00 | >50.00 |
| R. canina | 0.20 | 0.39 | 6.25 | 12.50 | >50.00 | >50.00 |
| B. serrata | 0.78 | 1.56 | 1.56 | 3.13 | >50.00 | >50.00 |
| P. incarnata | 25.00 | 50.00 | 6.25 | 12.50 | >50.00 | >50.00 |
| Novobiocin | <0.01 | <0.01 | 0.31 | 0.66 | - | - |
| Cycloheximide | - | - | - | - | <0.01 | <0.01 |
| Plant Extract | ABTS | DPPH | ||||
|---|---|---|---|---|---|---|
| EC50 [mg/mL] | TEAC | mg TE/g | EC50 [mg/mL] | TEAC | mg TE/g | |
| V. vinifera | 0.078 | 2.216 | 2437 | 0.005 | 1.234 | 1538 |
| C. cardunculus | 2.275 | 0.076 | 78.62 | 0.300 | 0.019 | 48.55 |
| P. sylvestris | 0.309 | 0.559 | 535.4 | 0.008 | 0.739 | 848.5 |
| S. aromaticum | 0.264 | 0.654 | 874.8 | 0.015 | 0.383 | 434.8 |
| R. rosea | 1.194 | 0.145 | 164.8 | 0.038 | 0.152 | 140.0 |
| S. baicalensis | 19.602 | 0.009 | 7.895 | 0.016 | 0.353 | 36.68 |
| S. officinalis | 2.909 | 0.059 | 54.31 | 1.949 | 0.003 | 19.52 |
| R. canina | 0.121 | 1.425 | 1362 | 0.006 | 1.018 | 1052 |
| B. serrata | 15.700 | 0.011 | 10.68 | 6.092 | 0.001 | 0.965 |
| P. incarnata | 2.036 | 0.085 | 86.18 | 0.472 | 0.012 | 14.93 |
| Trolox | 0.173 | 1.000 | - | 0.006 | 1.000 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, A.; Leska, A.; Wińska, P.; Herman, A.P. Plant Extracts as Modulators of the Wound Healing Process—Preliminary Study. Int. J. Mol. Sci. 2025, 26, 7490. https://doi.org/10.3390/ijms26157490
Herman A, Leska A, Wińska P, Herman AP. Plant Extracts as Modulators of the Wound Healing Process—Preliminary Study. International Journal of Molecular Sciences. 2025; 26(15):7490. https://doi.org/10.3390/ijms26157490
Chicago/Turabian StyleHerman, Anna, Aleksandra Leska, Patrycja Wińska, and Andrzej Przemysław Herman. 2025. "Plant Extracts as Modulators of the Wound Healing Process—Preliminary Study" International Journal of Molecular Sciences 26, no. 15: 7490. https://doi.org/10.3390/ijms26157490
APA StyleHerman, A., Leska, A., Wińska, P., & Herman, A. P. (2025). Plant Extracts as Modulators of the Wound Healing Process—Preliminary Study. International Journal of Molecular Sciences, 26(15), 7490. https://doi.org/10.3390/ijms26157490






