Connecting the Dots: AMOG/β2 and Its Elusive Adhesion Partner in CNS
Abstract
1. Introduction
2. Background
2.1. Adhesion Molecules in CNS
2.1.1. Classic Cadherins
2.1.2. Protocadherins
2.1.3. Nectins
2.1.4. Nectin-like Molecules (Necls)
2.1.5. NCAM (Neural Cell Adhesion Molecule)
2.1.6. Integrins
2.1.7. NgCAM (Neuron–Glia Cell Adhesion Molecule)
2.1.8. Contactins
2.1.9. TAG-1 (Transient Axonal Glycoprotein-1)
2.1.10. SYG-1 and SYG-2
2.1.11. Sidekicks
2.1.12. Neuroligins and Neurexins
2.2. The Na+/K+-ATPase in Neuron–Astrocyte Interactions
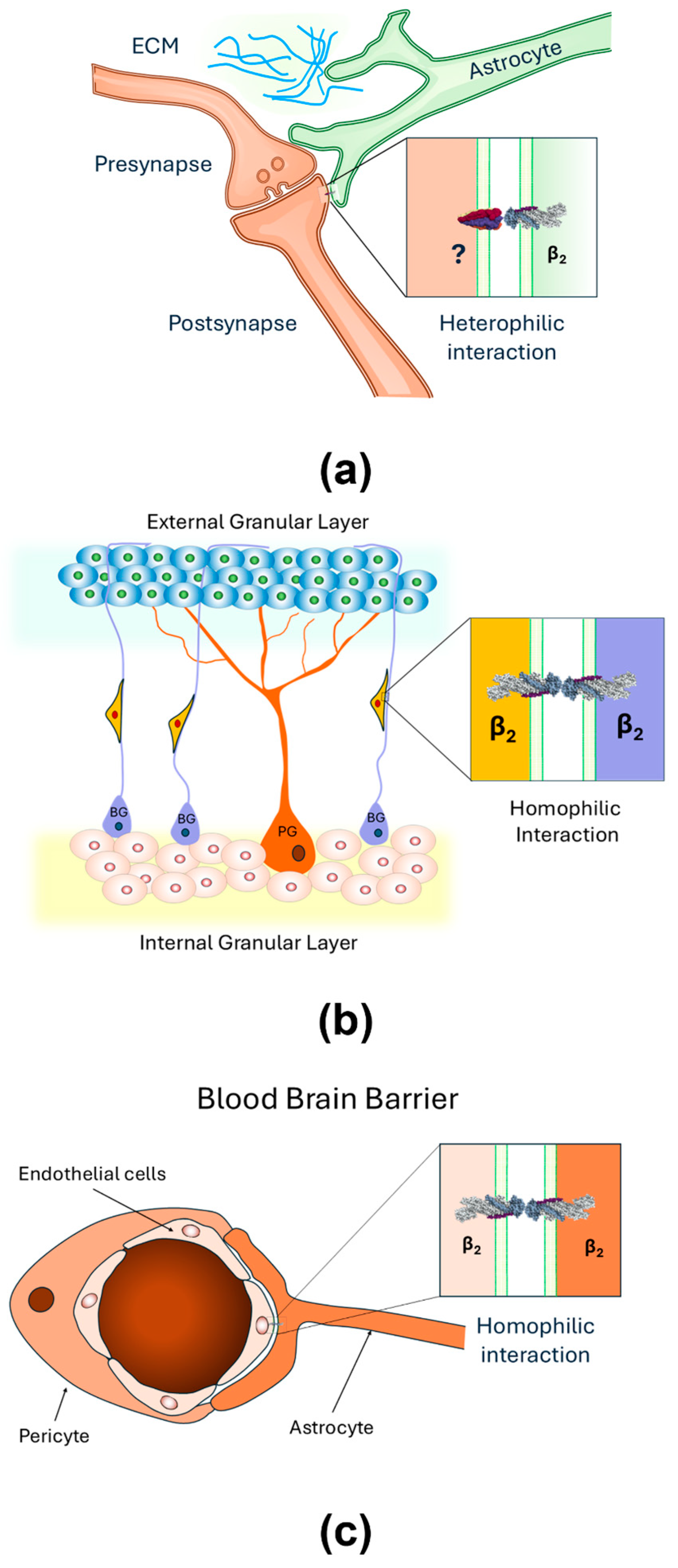
2.3. AMOG as a Heterophilic Adhesion Molecule
3. Current Understanding and Knowledge Gaps
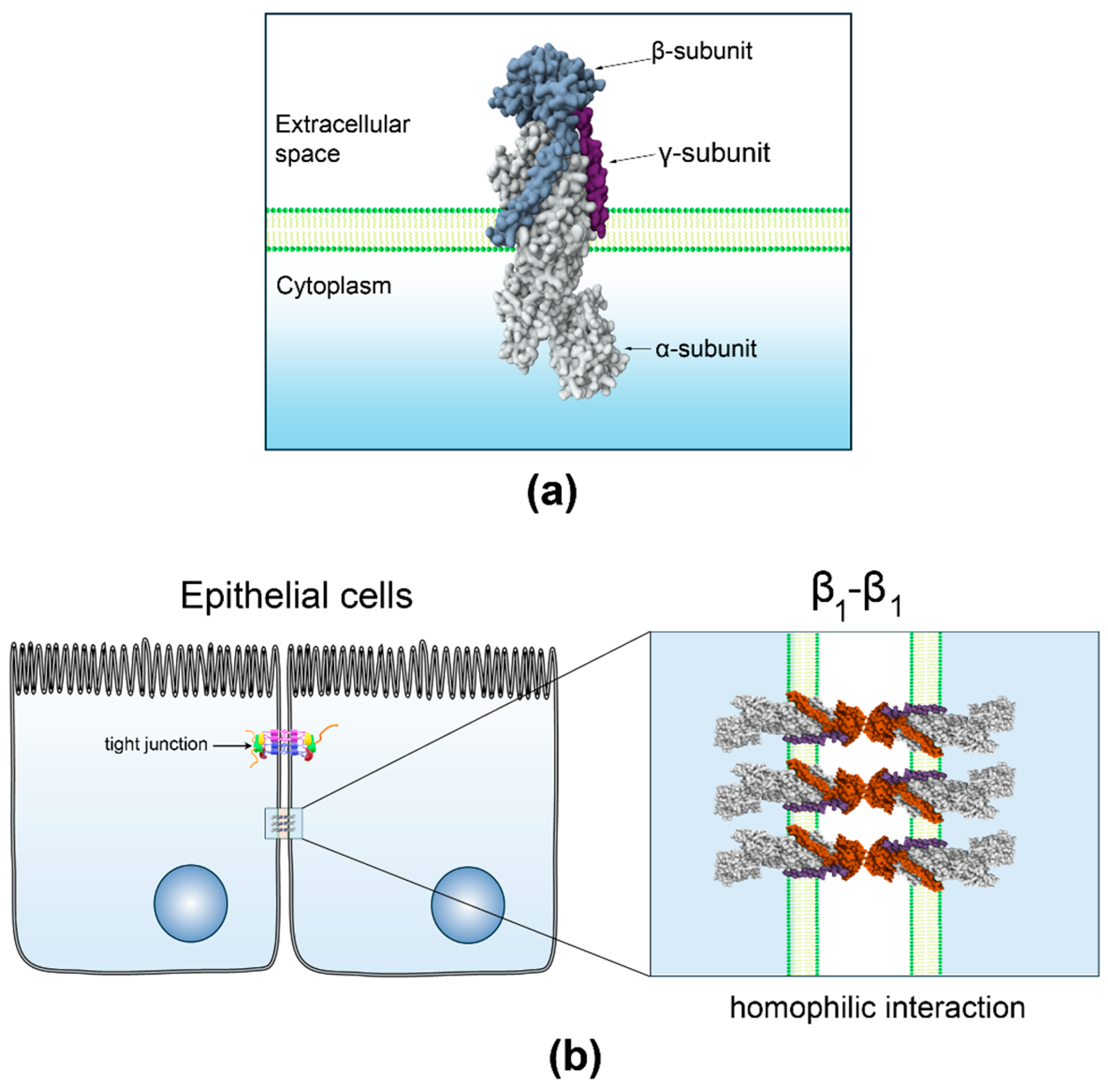
3.1. β1-Subunit as a Homophilic Adhesion Molecule in Epithelia
3.2. Gaps and Unresolved Questions
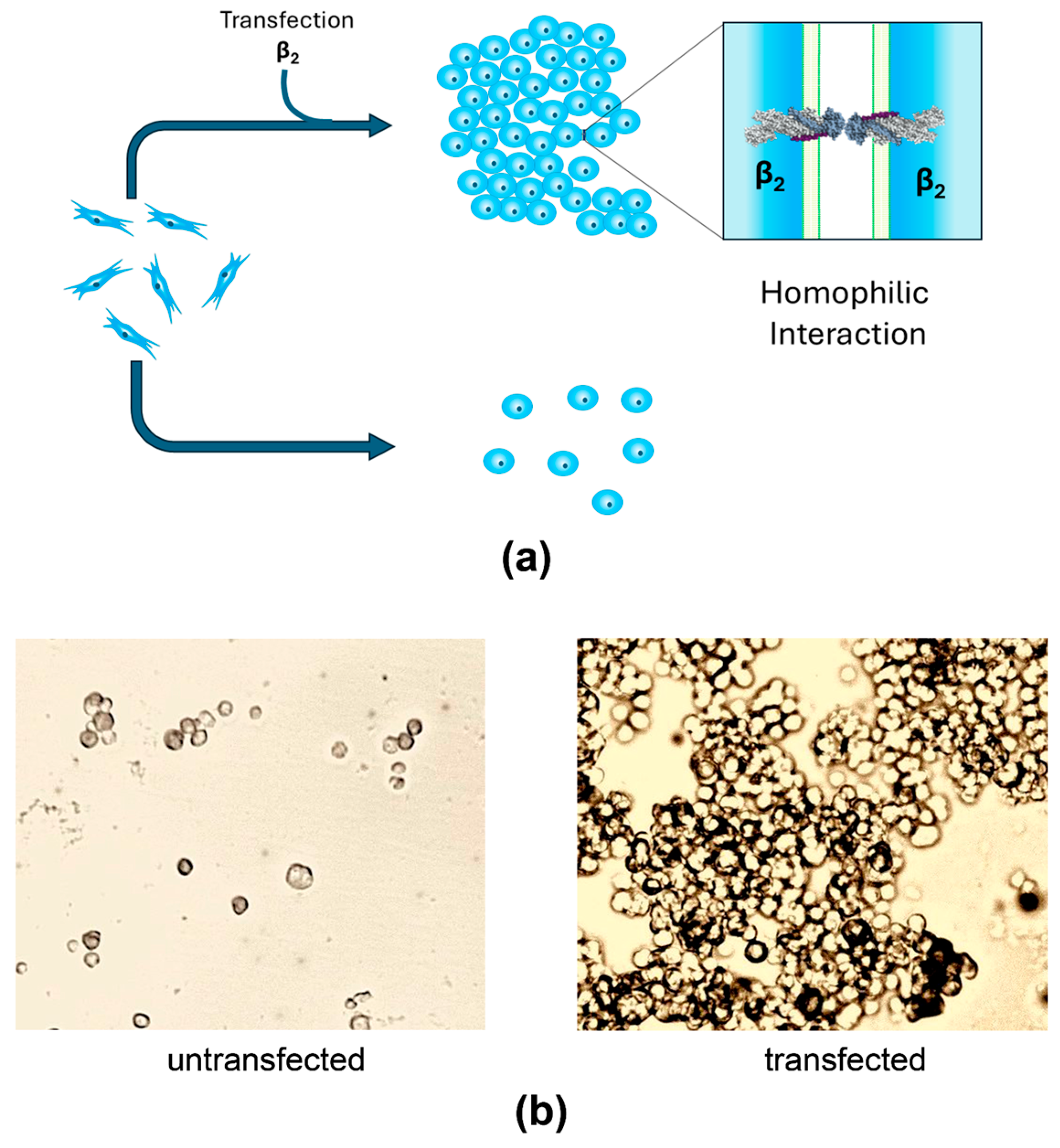
4. Recent Findings
5. Looking for the Partner
5.1. Experimental Strategies
5.2. Candidates for AMOG/β2 Receptor
- TSPAN31: As a member of the Tetraspanin family, TSPAN31 is notable for its role in organizing membrane microdomains and mediating lateral interactions between cell surface proteins. Tetraspanins act as molecular scaffolds, clustering adhesion molecules, integrins, and signaling receptors into functional complexes [105,106,107]. TSPAN31 has been implicated in cell adhesion, migration, and membrane signaling functions aligning closely with AMOG/β2 activities. TSPAN31 might associate in cis with a neuronal adhesion receptor, creating a complex that interacts in trans with AMOG/β2 on astrocytes. Alternatively, TSPAN31 might directly stabilize or present the neuronal partner required for AMOG/β2 recognition. Its potential involvement raises intriguing questions about how these microdomain organizations contribute to AMOG/β2 function.
- RTN4 (Nogo-A): Known for inhibiting neurite outgrowth, RTN4 has a complex topology and is present not only in the endoplasmic reticulum but also on the plasma membrane of axons and dendrites [108]. Its interactions with membrane proteins suggest it could serve as a scaffold or modulator for a receptor complex capable of interacting with AMOG/β2. If RTN4 is enriched in specific neuronal compartments, such as dendritic spines or axon terminals, its spatial distribution could explain the specificity and context-dependence of AMOG/β2-mediated adhesion during synaptogenesis or glial ensheathment. RTN4’s role in membrane dynamics and its interaction network make it a compelling candidate for further investigation.
6. Functional Implications in the CNS
7. Future Directions
7.1. Research Avenues and Implications
7.2. Therapeutic Targeting Potential
8. Summary
9. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMOG/β2 | Adhesion molecule on Glia |
| CNS | Central nervous system |
| TSPAN31 | Tetraspanin 31 |
| RTN4 | Reticulon 4 |
| CAMs | Cell adhesion molecules |
| IgCAMs | Inmunoglobulin superfamily cell adhesion molecules |
| CTLDs | C-type lectin-like domain proteins |
| Pcdh | Protocadherin |
| PAJs | Puncta adherentia junctions |
| Necls | Nectin-like molecules |
| NCAM | Neural Cell Adhesion molecule |
| FGFR | Fibroblast growth factor receptor |
| ECM | Extracellular matrix |
| NgCAM | Neuron–glia cell adhesion molecule |
| CNTNs | Contactins |
| TAG-1 | Transient Axonal Glycoprotein-1 |
| GABA | Gamma-aminobutyric acid |
| ATP | Adenosine triphosphate |
| BBB | Blood–brain barrier |
| EMT | Epithelial–mesenchymal transition |
| U87-MG | Uppsala 87 malignant glioma |
| YFP | Yellow fluorescence protein |
| CHO | Chinese hamster ovary cells |
| MDCK | Madin–Darby Canine Kidney |
| MD | Molecular dynamic |
| HEK | Human embryonic kidney |
| CRISPR | Clustered Regularly Interspaced short palindromic repeats |
| FRET | Förster resonance energy transfer |
| BiFC | Biomolecular Fluorescence Complementation |
References
- Kennedy, M.B. Synaptic Signaling in Learning and Memory. Cold Spring Harb. Perspect. Biol. 2016, 8, a016824. [Google Scholar] [CrossRef] [PubMed]
- Noriega-Prieto, J.A.; Araque, A. Sensing and Regulating Synaptic Activity by Astrocytes at Tripartite Synapse. Neurochem. Res. 2021, 46, 2580–2585. [Google Scholar] [CrossRef]
- Accogli, A.; Addour-Boudrahem, N.; Srour, M. Chapter 4—Neurogenesis, Neuronal Migration, and Axon Guidance. In Handbook of Clinical Neurology; Gallagher, A., Bulteau, C., Cohen, D., Michaud, J.L., Eds.; Neurocognitive Development: Normative Development; Elsevier: Amsterdam, The Netherlands, 2020; Volume 173, pp. 25–42. [Google Scholar]
- Affrald R, J.; Narayan, S. A Review: Oligodendrocytes in Neuronal Axonal Conduction and Methods for Enhancing Their Performance. Int. J. Neurosci. 2024, 1–22. [Google Scholar] [CrossRef]
- Norris, G.T.; Kipnis, J. Immune Cells and CNS Physiology: Microglia and Beyond. J. Exp. Med. 2018, 216, 60–70. [Google Scholar] [CrossRef]
- Thalhammer, A.; Cingolani, L.A. Cell Adhesion and Homeostatic Synaptic Plasticity. Neuropharmacology 2014, 78, 23–30. [Google Scholar] [CrossRef]
- Ben Achour, S.; Pascual, O. Glia: The Many Ways to Modulate Synaptic Plasticity. Neurochem. Int. 2010, 57, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Durkee, C.A.; Araque, A. Diversity and Specificity of Astrocyte–Neuron Communication. Neuroscience 2019, 396, 73–78. [Google Scholar] [CrossRef]
- Benarroch, E.E. Neuron-Astrocyte Interactions: Partnership for Normal Function and Disease in the Central Nervous System. Mayo Clin. Proc. 2005, 80, 1326–1338. [Google Scholar] [CrossRef]
- Antonicek, H.; Persohn, E.; Schachner, M. Biochemical and Functional Characterization of a Novel Neuron-Glia Adhesion Molecule That Is Involved in Neuronal Migration. J. Cell Biol. 1987, 104, 1587–1595. [Google Scholar] [CrossRef]
- Gloor, S.; Antonicek, H.; Sweadner, K.J.; Pagliusi, S.; Frank, R.; Moos, M.; Schachner, M. The Adhesion Molecule on Glia (AMOG) Is a Homologue of the Beta Subunit of the Na,K-ATPase. J. Cell Biol. 1990, 110, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Martin-Vasallo, P.; Dackowski, W.; Emanuel, J.R.; Levenson, R. Identification of a Putative Isoform of the Na,K-ATPase β Subunit: Primary Structure and Tissue-Specific Expression. J. Biol. Chem. 1989, 264, 4613–4618. [Google Scholar] [CrossRef]
- Contreras, R.G.; Torres-Carrillo, A.; Flores-Maldonado, C.; Shoshani, L.; Ponce, A. Na+/K+-ATPase: More than an Electrogenic Pump. Int. J. Mol. Sci. 2024, 25, 6122. [Google Scholar] [CrossRef]
- Lutsenko, S.; Kaplan, J.H. An Essential Role for the Extracellular Domain of the Sodium-Potassium-ATPase .Beta.-Subunit in Cation Occlusion. Biochemistry 1993, 32, 6737–6743. [Google Scholar] [CrossRef]
- Rajasekaran, S.A.; Palmer, L.G.; Moon, S.Y.; Peralta Soler, A.; Apodaca, G.L.; Harper, J.F.; Zheng, Y.; Rajasekaran, A.K. Na,K-ATPase Activity Is Required for Formation of Tight Junctions, Desmosomes, and Induction of Polarity in Epithelial Cells. Mol. Biol. Cell 2001, 12, 3717–3732. [Google Scholar] [CrossRef]
- Shoshani, L.; Contreras, R.G.; Roldán, M.L.; Moreno, J.; Lázaro, A.; Balda, M.S.; Matter, K.; Cereijido, M. The Polarized Expression of Na+, K+-ATPase in Epithelia Depends on the Association between β-Subunits Located in Neighboring Cells. Mol. Biol. Cell 2005, 16, 1071–1081. [Google Scholar] [CrossRef]
- Padilla-Benavides, T.; Roldán, M.L.; Larre, I.; Flores-Benitez, D.; Villegas-Sepúlveda, N.; Contreras, R.G.; Cereijido, M.; Shoshani, L. The Polarized Distribution of Na+, K+-ATPase: Role of the Interaction between β Subunits. Mol. Biol. Cell 2010, 21, 2217–2225. [Google Scholar] [CrossRef]
- Vagin, O.; Dada, L.A.; Tokhtaeva, E.; Sachs, G. The Na-K-ATPase α1β1 Heterodimer as a Cell Adhesion Molecule in Epithelia. Am. J. Physiol.-Cell Physiol. 2012, 302, C1271–C1281. [Google Scholar] [CrossRef]
- Vagin, O.; Tokhtaeva, E.; Sachs, G. The Role of the β1 Subunit of the Na,K-ATPase and Its Glycosylation in Cell-Cell Adhesion. J. Biol. Chem. 2006, 281, 39573–39587. [Google Scholar] [CrossRef]
- Tokhtaeva, E.; Sachs, G.; Sun, H.; Dada, L.A.; Sznajder, J.I.; Vagin, O. Identification of the Amino Acid Region Involved in the Intercellular Interaction between the β1 Subunits of Na+/K+-ATPase. J. Cell Sci. 2012, 125, 1605–1616. [Google Scholar] [CrossRef]
- Tokhtaeva, E.; Sachs, G.; Souda, P.; Bassilian, S.; Whitelegge, J.P.; Shoshani, L.; Vagin, O. Epithelial Junctions Depend on Intercellular Trans-Interactions between the Na,K-ATPase β1 Subunits. J. Biol. Chem. 2011, 286, 25801–25812. [Google Scholar] [CrossRef]
- Antonicek, H.; Schachner, M. The Adhesion Molecule on Glia (AMOG) Incorporated into Lipid Vesicles Binds to Subpopulations of Neurons. J. Neurosci. 1988, 8, 2961–2966. [Google Scholar] [CrossRef]
- Togashi, H.; Sakisaka, T.; Takai, Y. Cell Adhesion Molecules in the Central Nervous System. Cell Adhes. Migr. 2009, 3, 29–35. [Google Scholar] [CrossRef]
- Saint-Martin, M.; Goda, Y. Astrocyte–Synapse Interactions and Cell Adhesion Molecules. FEBS J. 2023, 290, 3512–3526. [Google Scholar] [CrossRef]
- Shapiro, L.; Love, J.; Colman, D.R. Adhesion Molecules in the Nervous System: Structural Insights into Function and Diversity. Annu. Rev. Neurosci. 2007, 30, 451–474. [Google Scholar] [CrossRef]
- Uchida, N.; Honjo, Y.; Johnson, K.R.; Wheelock, M.J.; Takeichi, M. The Catenin/Cadherin Adhesion System Is Localized in Synaptic Junctions Bordering Transmitter Release Zones. J. Cell Biol. 1996, 135, 767–779. [Google Scholar] [CrossRef]
- Hirano, S.; Takeichi, M. Cadherins in Brain Morphogenesis and Wiring. Physiol. Rev. 2012, 92, 597–634. [Google Scholar] [CrossRef]
- de Agustín-Durán, D.; Mateos-White, I.; Fabra-Beser, J.; Gil-Sanz, C. Stick around: Cell–Cell Adhesion Molecules during Neocortical Development. Cells 2021, 10, 118. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, Z. Wiring the Brain by Clustered Protocadherin Neural Codes. Neurosci. Bull. 2021, 37, 117–131. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Nakanishi, H.; Kimura, K.; Matsubara, K.; Ozaki-Kuroda, K.; Katata, T.; Honda, T.; Kiyohara, Y.; Heo, K.; Higashi, M.; et al. Nectin: An Adhesion Molecule Involved in Formation of Synapses. J. Cell Biol. 2002, 156, 555–565. [Google Scholar] [CrossRef]
- Mizutani, K.; Miyata, M.; Shiotani, H.; Kameyama, T.; Takai, Y. Nectins and Nectin-like Molecules in Synapse Formation and Involvement in Neurological Diseases. Mol. Cell. Neurosci. 2021, 115, 103653. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Müller, D. Contribution of the Neural Cell Adhesion Molecule to Neuronal and Synaptic Plasticity. Rev. Neurosci. 2001, 12, 297–310. [Google Scholar] [CrossRef]
- Walsh, F.S.; Doherty, P. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily: Role in Axon Growth and Guidance. Annu. Rev. Cell Dev. Biol. 1997, 13, 425–456. [Google Scholar] [CrossRef]
- Kerrisk, M.E.; Cingolani, L.A.; Koleske, A.J. Chapter 5—ECM Receptors in Neuronal Structure, Synaptic Plasticity, and Behavior. In Progress in Brain Research; Dityatev, A., Wehrle-Haller, B., Pitkänen, A., Eds.; Brain Extracellular Matrix in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; Volume 214, pp. 101–131. [Google Scholar]
- Zuko, A.; Kleijer, K.T.E.; Oguro-Ando, A.; Kas, M.J.H.; van Daalen, E.; van der Zwaag, B.; Burbach, J.P.H. Contactins in the Neurobiology of Autism. Eur. J. Pharmacol. 2013, 719, 63–74. [Google Scholar] [CrossRef]
- Masuda, T. Contactin-2/TAG-1, Active on the Front Line for Three Decades. Cell Adhes. Migr. 2017, 11, 524–531. [Google Scholar] [CrossRef]
- Shen, K.; Fetter, R.D.; Bargmann, C.I. Synaptic Specificity Is Generated by the Synaptic Guidepost Protein SYG-2 and Its Receptor, SYG-1. Cell 2004, 116, 869–881, Erratum in Cell 2004, 117, 553. [Google Scholar] [CrossRef]
- Shen, K.; Bargmann, C.I. The Immunoglobulin Superfamily Protein SYG-1 Determines the Location of Specific Synapses in C. elegans. Cell 2003, 112, 619–630. [Google Scholar] [CrossRef]
- Goodman, K.M.; Yamagata, M.; Jin, X.; Mannepalli, S.; Katsamba, P.S.; Ahlsén, G.; Sergeeva, A.P.; Honig, B.; Sanes, J.R.; Shapiro, L. Molecular Basis of Sidekick-Mediated Cell-Cell Adhesion and Specificity. eLife 2016, 5, e19058. [Google Scholar] [CrossRef]
- Craig, A.M.; Kang, Y. Neurexin–Neuroligin Signaling in Synapse Development. Curr. Opin. Neurobiol. 2007, 17, 43–52. [Google Scholar] [CrossRef]
- Bang, M.L.; Owczarek, S. A Matter of Balance: Role of Neurexin and Neuroligin at the Synapse. Neurochem. Res. 2013, 38, 1174–1189. [Google Scholar] [CrossRef]
- Kryvenko, V.; Vagin, O.; Dada, L.A.; Sznajder, J.I.; Vadász, I. Maturation of the Na,K-ATPase in the Endoplasmic Reticulum in Health and Disease. J. Membr. Biol. 2021, 254, 447–457. [Google Scholar] [CrossRef]
- Burrow, C.R.; Devuyst, O.; Li, X.; Gatti, L.; Wilson, P.D. Expression of the Beta2-Subunit and Apical Localization of Na+-K+-ATPase in Metanephric Kidney. Am. J. Physiol. 1999, 277, F391–F403. [Google Scholar] [CrossRef]
- Vagin, O.; Sachs, G.; Tokhtaeva, E. The Roles of the Na,K-ATPase Beta 1 Subunit in Pump Sorting and Epithelial Integrity. J. Bioenerg. Biomembr. 2007, 39, 367–372. [Google Scholar] [CrossRef]
- Arystarkhova, E.; Sweadner, K.J. Na,K-ATPase Expression Can Be Limited Post-Transcriptionally: A Test of the Role of the Beta Subunit, and a Review of Evidence. Int. J. Mol. Sci. 2024, 25, 7414. [Google Scholar] [CrossRef]
- Hilbers, F.; Kopec, W.; Isaksen, T.J.; Holm, T.H.; Lykke-Hartmann, K.; Nissen, P.; Khandelia, H.; Poulsen, H. Tuning of the Na,K-ATPase by the Beta Subunit. Sci. Rep. 2016, 6, 20442. [Google Scholar] [CrossRef]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Álvarez, J.A.L.; Murillo, T.d.C.L.; Nestor, C.A.V.; Gutierrez, M.L.R.; Gómez, O.P.; Shoshani, L. Epithelial Na+,K+-ATPase—A Sticky Pump. In Cell Biology—New Insights; IntechOpen: London, UK, 2016; ISBN 978-953-51-2242-5. [Google Scholar]
- Cereijido, M.; Contreras, R.G.; Shoshani, L.; Larre, I. The Na+-K+-ATPase as Self-Adhesion Molecule and Hormone Receptor. Am. J. Physiol.-Cell Physiol. 2012, 302, C473–C481. [Google Scholar] [CrossRef]
- Blanco, G. Na,K-ATPase Subunit Heterogeneity as a Mechanism for Tissue-Specific Ion Regulation. Semin. Nephrol. 2005, 25, 292–303. [Google Scholar] [CrossRef]
- Peng, L.; Martin-Vasallo, P.; Sweadner, K.J. Isoforms of Na,K-ATPase α and β Subunits in the Rat Cerebellum and in Granule Cell Cultures. J. Neurosci. 1997, 17, 3488–3502. [Google Scholar] [CrossRef]
- Levenson, R. Isoforms of the Na,K-ATPase: Family Members in Search of Function. In Reviews of Physiology, Biochemistry and Pharmacology, Volume 123; Springer: Berlin/Heidelberg, Germany, 1994; pp. 1–45. ISBN 978-3-540-48217-8. [Google Scholar]
- Lingrel, J.B. Na,K-ATPase: Isoform Structure, Function, and Expression. J. Bioenerg. Biomembr. 1992, 24, 263–270. [Google Scholar] [CrossRef]
- Lingrel, J.B.; Orlowski, J.; Shull, M.M.; Price, E.M. Molecular Genetics of Na,K-ATPase. In Progress in Nucleic Acid Research and Molecular Biology; Cohn, W.E., Moldave, K., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 38, pp. 37–89. [Google Scholar]
- Sweadner, K.J. Overlapping and Diverse Distribution of Na–K ATPase Isozymes in Neurons and Glia. Can. J. Physiol. Pharmacol. 1992, 70, S255–S259. [Google Scholar] [CrossRef]
- Brines, M.L.; Robbins, R.J. Cell-Type Specific Expression of Na+,K+-ATPase Catalytic Subunits in Cultured Neurons and Glia: Evidence for Polarized Distribution in Neurons. Brain Res. 1993, 631, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.; Klein, L.; Shyjan, A.W.; Rakic, P.; Levenson, R. Neurons and Astroglia Express Distinct Subsets of Na,K-ATPase α and β Subunits. Mol. Brain Res. 1994, 21, 333–343. [Google Scholar] [CrossRef]
- Fink, D.; Knapp, P.E.; Mata, M. Differential Expression of Na,K-ATPase Isoforms in Oligodendrocytes and Astrocytes. Dev. Neurosci. 1996, 18, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, R.K.; Arystarkhova, E.; Sweadner, K.J. Cellular and Subcellular Specification of Na,K-ATPase α and β Isoforms in the Postnatal Development of Mouse Retina. J. Neurosci. 1999, 19, 9878–9889. [Google Scholar] [CrossRef]
- Lavoie, L.; Levenson, R.; Martin-Vasallo, P.; Klip, A. The Molar Ratios of α and β Subunits of the Na+−K+-ATPase Differ in Distinct Subcellular Membranes from Rat Skeletal Muscle. Biochemistry 1997, 36, 7726–7732. [Google Scholar] [CrossRef]
- Shyjan, A.W.; Ceña, V.; Klein, D.C.; Levenson, R. Differential Expression and Enzymatic Properties of the Na+,K(+)-ATPase Alpha 3 Isoenzyme in Rat Pineal Glands. Proc. Natl. Acad. Sci. USA 1990, 87, 1178–1182. [Google Scholar] [CrossRef]
- Pagliusi, S.R.; Schachner, M.; Seeburg, P.H.; Shivers, B.D. The Adhesion Molecule on Glia (AMOG) Is Widely Expressed by Astrocytes in Developing and Adult Mouse Brain. Eur. J. Neurosci. 1990, 2, 471–480. [Google Scholar] [CrossRef]
- Malik, N.; Canfield, V.A.; Beckers, M.-C.; Gros, P.; Levenson, R. Identification of the Mammalian Na,K-ATPase β3 Subunit. J. Biol. Chem. 1996, 271, 22754–22758. [Google Scholar] [CrossRef]
- Arystarkhova, E.; Sweadner, K.J. Tissue-Specific Expression of the Na,K-ATPase β3 Subunit: The Presence of β3 in Lung and Liver Addresses the Problem of the Missing Subunit. J. Biol. Chem. 1997, 272, 22405–22408. [Google Scholar] [CrossRef]
- Book, C.B.; Wilson, R.P.; Ng, Y.C. Cardiac Hypertrophy in the Ferret Increases Expression of the Na(+)-K(+)-ATPase Alpha 1- but Not Alpha 3-Isoform. Am. J. Physiol.-Heart Circ. Physiol. 1994, 266, H1221–H1227. [Google Scholar] [CrossRef] [PubMed]
- Charlemagne, D.; Swynghedauw, B. Myocardial Phenotypic Changes in Na+, K+-ATPase in Left Ventricular Hypertrophy: Pharmacological Consequences. Eur. Heart J. 1995, 16, 20–23. [Google Scholar] [CrossRef]
- Charlemagne, D.; Orlowski, J.; Oliviero, P.; Rannou, F.; Sainte Beuve, C.; Swynghedauw, B.; Lane, L.K. Alteration of Na,K-ATPase Subunit mRNA and Protein Levels in Hypertrophied Rat Heart. J. Biol. Chem. 1994, 269, 1541–1547. [Google Scholar] [CrossRef]
- Ewart, H.S.; Klip, A. Hormonal Regulation of the Na(+)-K(+)-ATPase: Mechanisms Underlying Rapid and Sustained Changes in Pump Activity. Am. J. Physiol.-Cell Physiol. 1995, 269, C295–C311. [Google Scholar] [CrossRef] [PubMed]
- Zahler, R.; Gilmore-Hebert, M.; Sun, W.; Benz, E.J. Na,K-ATPase Isoform Gene Expression in Normal and Hypertrophied Dog Heart. Basic Res. Cardiol. 1996, 91, 256–266. [Google Scholar] [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G.; Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite Synapses: Glia, the Unacknowledged Partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef]
- Chung, W.-S.; Allen, N.J.; Eroglu, C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb. Perspect. Biol. 2015, 7, a020370. [Google Scholar] [CrossRef]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef]
- Baldwin, K.T.; Eroglu, C. Molecular Mechanisms of Astrocyte-Induced Synaptogenesis. Curr. Opin. Neurobiol. 2017, 45, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kilb, W.; Kirischuk, S. GABA Release from Astrocytes in Health and Disease. Int. J. Mol. Sci. 2022, 23, 15859. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Kamiya, T.; Tsuboi, T. Gliotransmitter Release from Astrocytes: Functional, Developmental and Pathological Implications in the Brain. Front. Neurosci. 2016, 9, 499. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte–Endothelial Interactions at the Blood–Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Pietrobon, D.; Conti, F. Astrocytic Na+, K+ ATPases in Physiology and Pathophysiology. Cell Calcium 2024, 118, 102851. [Google Scholar] [CrossRef]
- Pagliusi, S.; Antonicek, H.; Gloor, S.; Frank, R.; Moos, M.; Schachner, M. Identification of a cDNA Clone Specific for the Neural Cell Adhesion Molecule AMOG. J. Neurosci. Res. 1989, 22, 113–119. [Google Scholar] [CrossRef]
- Müller-Husmann, G.; Gloor, S.; Schachner, M. Functional Characterization of Beta Isoforms of Murine Na,K-ATPase. The Adhesion Molecule on Glia (AMOG/Beta 2), but Not Beta 1, Promotes Neurite Outgrowth. J. Biol. Chem. 1993, 268, 26260–26267. [Google Scholar] [CrossRef]
- Magyar, J.P.; Bartsch, U.; Wang, Z.Q.; Howells, N.; Aguzzi, A.; Wagner, E.F.; Schachner, M. Degeneration of Neural Cells in the Central Nervous System of Mice Deficient in the Gene for the Adhesion Molecule on Glia, the Beta 2 Subunit of Murine Na,K-ATPase. J. Cell Biol. 1994, 127, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.; Bartsch, U.; Schachner, M.; Montag, D. Na,K-ATPase Subunit β1 Knock-in Prevents Lethality of β2 Deficiency in Mice. J. Neurosci. 1998, 18, 9192–9203. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, S.; Molthagen, M.; Brandner, S.; Bartsch, U.; Kühne, G.; Magyar, J.P.; Sure, U.; Schachner, M.; Aguzzi, A. The AMOG/β2 Subunit of Na,K-ATPase Is Not Necessary for Long-Term Survival of Telencephalic Grafts. Glia 1995, 15, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Páez, O.; Martínez-Archundia, M.; Villegas-Sepúlveda, N.; Roldan, M.L.; Correa-Basurto, J.; Shoshani, L. A Model for the Homotypic Interaction between Na+,K+-ATPase β1 Subunits Reveals the Role of Extracellular Residues 221–229 in Its Ig-Like Domain. Int. J. Mol. Sci. 2019, 20, 4538. [Google Scholar] [CrossRef]
- Scheidenhelm, D.K.; Cresswell, J.; Haipek, C.A.; Fleming, T.P.; Mercer, R.W.; Gutmann, D.H. Akt-Dependent Cell Size Regulation by the Adhesion Molecule on Glia Occurs Independently of Phosphatidylinositol 3-Kinase and Rheb Signaling. Mol. Cell. Biol. 2005, 25, 3151–3162. [Google Scholar] [CrossRef]
- Barreto, N.; Caballero, M.; Bonfanti, A.P.; de Mato, F.C.P.; Munhoz, J.; da Rocha-e-Silva, T.A.A.; Sutti, R.; Vitorino-Araujo, J.L.; Verinaud, L.; Rapôso, C. Spider Venom Components Decrease Glioblastoma Cell Migration and Invasion through RhoA-ROCK and Na+/K+-ATPase β2: Potential Molecular Entities to Treat Invasive Brain Cancer. Cancer Cell Int. 2020, 20, 576. [Google Scholar] [CrossRef]
- Litan, A.; Li, Z.; Tokhtaeva, E.; Kelly, P.; Vagin, O.; Langhans, S.A. A Functional Interaction Between Na,K-ATPase β2-Subunit/AMOG and NF2/Merlin Regulates Growth Factor Signaling in Cerebellar Granule Cells. Mol. Neurobiol. 2019, 56, 7557–7571. [Google Scholar] [CrossRef]
- Kanai, R.; Ogawa, H.; Vilsen, B.; Cornelius, F.; Toyoshima, C. Crystal Structure of a Na+-Bound Na+,K+-ATPase Preceding the E1P State. Nature 2013, 502, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern Web App for 3D Visualization and Analysis of Large Biomolecular Structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B.V.; Mackic, J.B.; Wang, L.; McComb, J.G.; McDonough, A. Differential Expression of Na,K-ATPase Alpha and Beta Subunit Isoforms at the Blood-Brain Barrier and the Choroid Plexus. J. Biol. Chem. 1993, 268, 8019–8025. [Google Scholar] [CrossRef] [PubMed]
- Boer, K.; Spliet, W.G.M.; van Rijen, P.C.; Jansen, F.E.; Aronica, E. Expression Patterns of AMOG in Developing Human Cortex and Malformations of Cortical Development. Epilepsy Res. 2010, 91, 84–93. [Google Scholar] [CrossRef] [PubMed]
- McGrail, K.M.; Phillips, J.M.; Sweadner, K.J. Immunofluorescent Localization of Three Na,K-ATPase Isozymes in the Rat Central Nervous System: Both Neurons and Glia Can Express More than One Na,K-ATPase. J. Neurosci. 1991, 11, 381–391. [Google Scholar] [CrossRef]
- Watts, A.G.; Sanchez-Watts, G.; Emanuel, J.R.; Levenson, R. Cell-Specific Expression of mRNAs Encoding Na+,K(+)-ATPase Alpha- and Beta-Subunit Isoforms within the Rat Central Nervous System. Proc. Natl. Acad. Sci. USA 1991, 88, 7425–7429. [Google Scholar] [CrossRef]
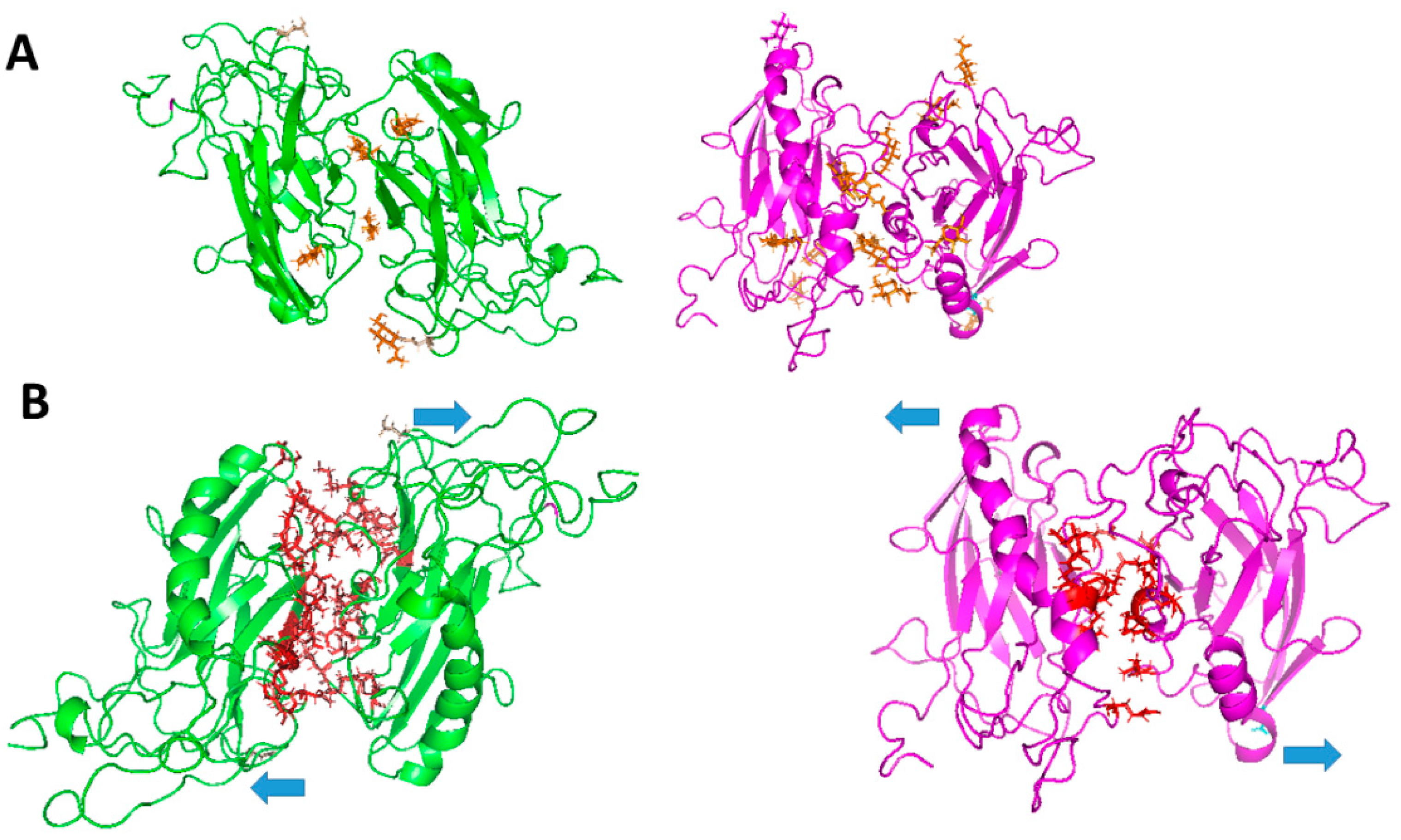
- Roldán, M.L.; Ramírez-Salinas, G.L.; Martinez-Archundia, M.; Cuellar-Perez, F.; Vilchis-Nestor, C.A.; Cancino-Diaz, J.C.; Shoshani, L. The β2-Subunit (AMOG) of Human Na+, K+-ATPase Is a Homophilic Adhesion Molecule. Int. J. Mol. Sci. 2022, 23, 7753. [Google Scholar] [CrossRef]
- Ramírez-Salinas, G.; Shoshani, L.; Rosas-Trigueros, J.L.; Huerta, C.S.; Martínez-Archundia, M. In Silico Studies Provide New Structural Insights into Trans-Dimerization of β1 and β2 Subunits of the Na+, K+-ATPase. PLoS ONE 2025, 20, e0321064. [Google Scholar] [CrossRef] [PubMed]
- Goulding, S.P.; Szumlinski, K.K.; Contet, C.; MacCoss, M.J.; Wu, C.C. A Mass Spectrometry-Based Proteomic Analysis of Homer2-Interacting Proteins in the Mouse Brain. J. Proteomics 2017, 166, 127–137. [Google Scholar] [CrossRef]
- Roux, K.J.; Kim, D.I.; Burke, B.; May, D.G. BioID: A Screen for Protein-Protein Interactions. Curr. Protoc. Protein Sci. 2018, 91, 19.23.1–19.23.15. [Google Scholar] [CrossRef]
- Mannix, K.M.; Starble, R.M.; Kaufman, R.S.; Cooley, L. Proximity Labeling Reveals Novel Interactomes in Live Drosophila Tissue. Development 2019, 146, dev176644. [Google Scholar] [CrossRef]
- Tsiami, F.; Lago, C.; Pozza, N.; Piccioni, F.; Zhao, X.; Lülsberg, F.; Root, D.E.; Tiberi, L.; Kool, M.; Schittenhelm, J.; et al. Genome-Wide CRISPR-Cas9 Knockout Screens Identify DNMT1 as a Druggable Dependency in Sonic Hedgehog Medulloblastoma. Acta Neuropathol. Commun. 2024, 12, 125. [Google Scholar] [CrossRef]
- Charrin, S.; Naour, F.L.; Oualid, M.; Billard, M.; Faure, G.; Hanash, S.M.; Boucheix, C.; Rubinstein, E. The Major CD9 and CD81 Molecular Partner: IDENTIFICATION AND CHARACTERIZATION OF THE COMPLEXES. J. Biol. Chem. 2001, 276, 14329–14337. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.; Cohen-Barak, E.; Koetsier, J.L.; Najor, N.A.; Arvanitis, C.; Sprecher, E.; Green, K.J.; Godsel, L.M. Proximity Ligation Assay for Detecting Protein-Protein Interactions and Protein Modifications in Cells and Tissues in Situ. Curr. Protoc. Cell Biol. 2020, 89, e115. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Martínez, P.; Coronel, R.; Sachse, M.; González-Sastre, R.; Maeso, L.; Rodriguez, M.J.; Terrón, M.C.; López-Alonso, V.; Liste, I. Human Cerebral Organoids: Cellular Composition and Subcellular Morphological Features. Front. Cell. Neurosci. 2024, 18, 1406839. [Google Scholar] [CrossRef] [PubMed]
- Rakotomamonjy, J.; Rylaarsdam, L.; Fares-Taie, L.; McDermott, S.; Davies, D.; Yang, G.; Fagbemi, F.; Epstein, M.; Fairbanks-Santana, M.; Rozet, J.-M.; et al. PCDH12 Loss Results in Premature Neuronal Differentiation and Impeded Migration in a Cortical Organoid Model. Cell Rep. 2023, 42, 112845. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Paulo, J.A.; Cannon, J.R.; Ting, L.; Baltier, K.; Colby, G.; Gebreab, F.; Gygi, M.P.; Parzen, H.; et al. Architecture of the Human Interactome Defines Protein Communities and Disease Networks. Nature 2017, 545, 505–509. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Navarrete-Perea, J.; Cannon, J.R.; Baltier, K.; Gebreab, F.; Gygi, M.P.; Thornock, A.; Zarraga, G.; Tam, S.; et al. Dual Proteome-Scale Networks Reveal Cell-Specific Remodeling of the Human Interactome. Cell 2021, 184, 3022–3040.e28. [Google Scholar] [CrossRef]
- Charrin, S.; Jouannet, S.; Boucheix, C.; Rubinstein, E. Tetraspanins at a Glance. J. Cell Sci. 2014, 127, 3641–3648. [Google Scholar] [CrossRef]
- Ovalle, S.; Gutiérrez-López, M.D.; Olmo, N.; Turnay, J.; Lizarbe, M.A.; Majano, P.; Molina-Jiménez, F.; López-Cabrera, M.; Yáñez-Mó, M.; Sánchez-Madrid, F.; et al. The Tetraspanin CD9 Inhibits the Proliferation and Tumorigenicity of Human Colon Carcinoma Cells. Int. J. Cancer 2007, 121, 2140–2152. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Li, D.; Sun, X.; Deng, Y.; Zhao, Q. TSPAN31 Is a Critical Regulator on Transduction of Survival and Apoptotic Signals in Hepatocellular Carcinoma Cells. FEBS Lett. 2017, 591, 2905–2918. [Google Scholar] [CrossRef]
- Chen, M.S.; Huber, A.B.; van der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.; Schwab, M.E. Nogo-A Is a Myelin-Associated Neurite Outgrowth Inhibitor and an Antigen for Monoclonal Antibody IN-1. Nature 2000, 403, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Kleene, R.; Loers, G.; Langer, J.; Frobert, Y.; Buck, F.; Schachner, M. Prion Protein Regulates Glutamate-Dependent Lactate Transport of Astrocytes. J. Neurosci. 2007, 27, 12331–12340. [Google Scholar] [CrossRef] [PubMed]
- Rotoli, D.; Cejas, M.-M.; Maeso, M.-C.; Pérez-Rodríguez, N.-D.; Morales, M.; Ávila, J.; Mobasheri, A.; Martín-Vasallo, P. The Na,K-ATPase β-Subunit Isoforms Expression in Glioblastoma Multiforme: Moonlighting Roles. Int. J. Mol. Sci. 2017, 18, 2369. [Google Scholar] [CrossRef]
- Sun, M.Z.; Kim, J.M.; Oh, M.C.; Safaee, M.; Kaur, G.; Clark, A.J.; Bloch, O.; Ivan, M.E.; Kaur, R.; Oh, T.; et al. Na+/K+-ATPase β2-Subunit (AMOG) Expression Abrogates Invasion of Glioblastoma-Derived Brain Tumor-Initiating Cells. Neuro-Oncol. 2013, 15, 1518–1531. [Google Scholar] [CrossRef]
- Lefranc, F.; Kiss, R. The Sodium Pump Alpha1 Subunit as a Potential Target to Combat Apoptosis-Resistant Glioblastomas. Neoplasia N. Y. 2008, 10, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Senner, V.; Schmidtpeter, S.; Braune, S.; Püttmann, S.; Thanos, S.; Bartsch, U.; Schachner, M.; Paulus, W. AMOG/β2 and Glioma Invasion: Does Loss of AMOG Make Tumour Cells Run Amok? Neuropathol. Appl. Neurobiol. 2003, 29, 370–377. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, J.; Yoon, B.-E. Neuron-Glia Interactions in Neurodevelopmental Disorders. Cells 2020, 9, 2176. [Google Scholar] [CrossRef]
- Phatnani, H.; Maniatis, T. Astrocytes in Neurodegenerative Disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a020628. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoshani, L.; Sosa Huerta, C.; Roldán, M.L.; Ponce, A.; Martínez-Archundia, M. Connecting the Dots: AMOG/β2 and Its Elusive Adhesion Partner in CNS. Int. J. Mol. Sci. 2025, 26, 8744. https://doi.org/10.3390/ijms26178744
Shoshani L, Sosa Huerta C, Roldán ML, Ponce A, Martínez-Archundia M. Connecting the Dots: AMOG/β2 and Its Elusive Adhesion Partner in CNS. International Journal of Molecular Sciences. 2025; 26(17):8744. https://doi.org/10.3390/ijms26178744
Chicago/Turabian StyleShoshani, Liora, Christian Sosa Huerta, María Luisa Roldán, Arturo Ponce, and Marlet Martínez-Archundia. 2025. "Connecting the Dots: AMOG/β2 and Its Elusive Adhesion Partner in CNS" International Journal of Molecular Sciences 26, no. 17: 8744. https://doi.org/10.3390/ijms26178744
APA StyleShoshani, L., Sosa Huerta, C., Roldán, M. L., Ponce, A., & Martínez-Archundia, M. (2025). Connecting the Dots: AMOG/β2 and Its Elusive Adhesion Partner in CNS. International Journal of Molecular Sciences, 26(17), 8744. https://doi.org/10.3390/ijms26178744




