Clock Gene Expression Modulation by Low- and High-Intensity Exercise Regimens in Aging Mice
Abstract
1. Introduction
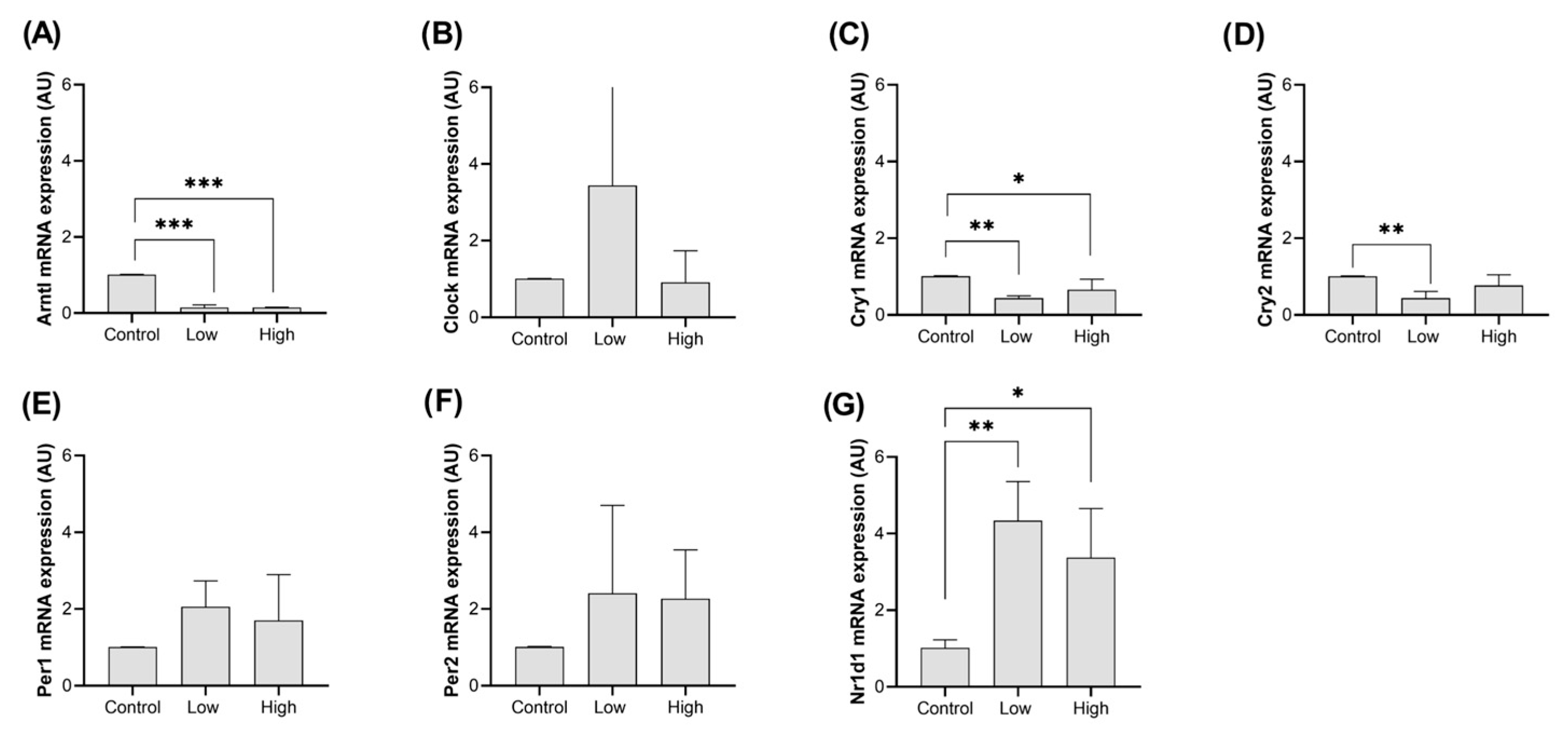
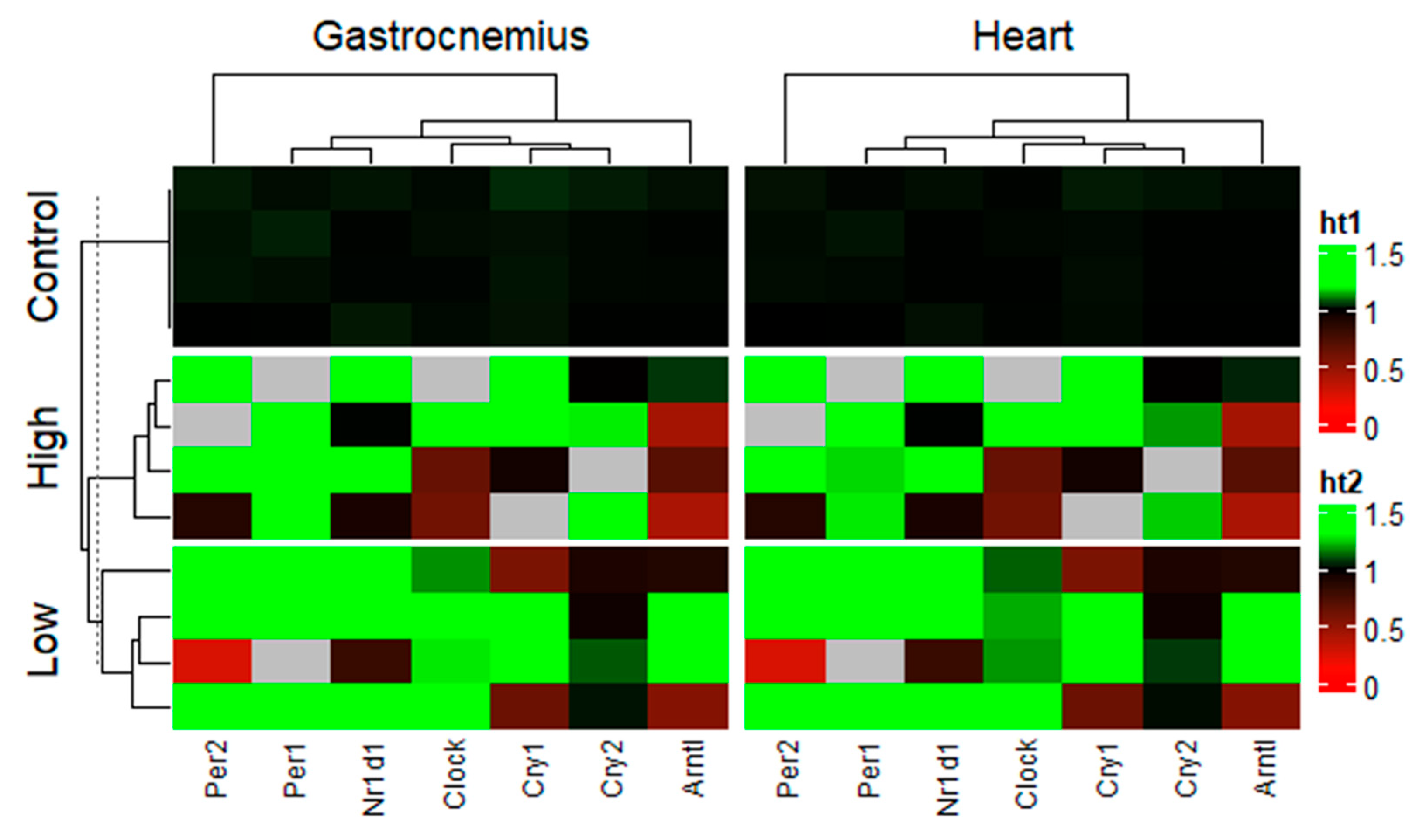
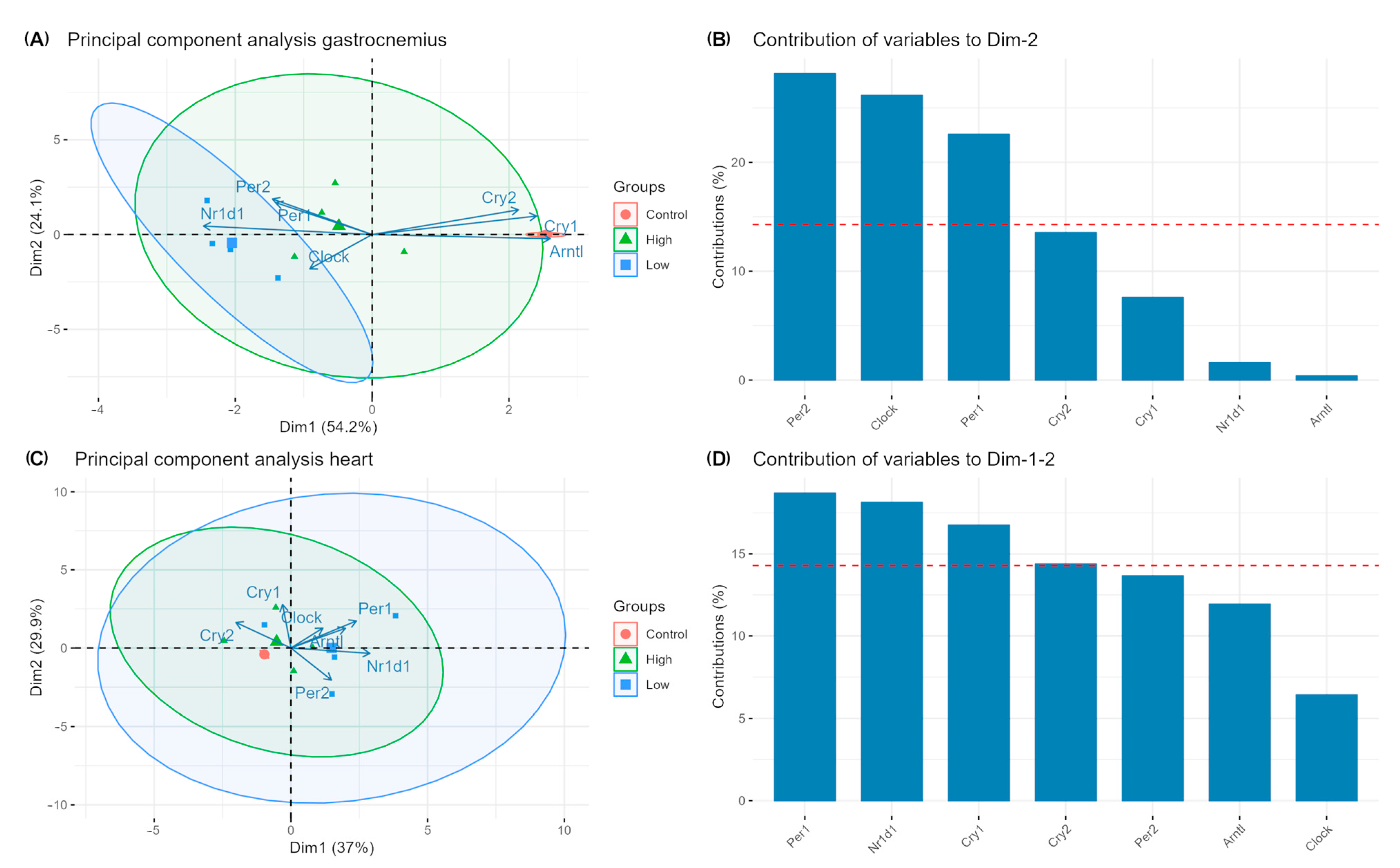
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piggins, H.D.; Guilding, C. The neural circadian system of mammals. Essays Biochem. 2011, 49, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; Van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464, Erratum in Nature 2012, 489, 590. [Google Scholar] [CrossRef]
- Inoue, K.; Araki, T.; Endo, M. Circadian clock during plant development. J. Plant Res. 2018, 131, 59–66. Available online: https://link.springer.com/article/10.1007/s10265-017-0991-8 (accessed on 3 December 2024). [CrossRef]
- Liu, W.; Xuan, J. Circadian Rhythms in Animals: Mechanisms and Functions. Int. J. Mol. Zool. 2024, 14, 166–181. Available online: http://animalscipublisher.com/index.php/ijmz/article/view/3799 (accessed on 3 December 2024). [CrossRef]
- Andreani, T.S.; Itoh, T.Q.; Yildirim, E.; Hwangbo, D.S.; Allada, R. Genetics of circadian rhythms. Sleep Med. Clin. 2015, 10, 413–421. [Google Scholar] [CrossRef]
- Ko, C.H.; Takahashi, J.S. Molecular components of the mammalian circadian clock. Hum. Mol. Genet. 2006, 15 (Suppl. S2), R271–R277. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef]
- Kumar Jha, P.; Challet, E.; Kalsbeek, A. Circadian rhythms in glucose and lipid metabolism in nocturnal and diurnal mammals. Mol. Cell Endocrinol. 2015, 418, 74–88. [Google Scholar] [CrossRef]
- Ko, M.L.; Shi, L.; Tsai, J.Y.; Young, M.E.; Neuendorff, N.; Earnest, D.J.; Ko, G.Y.-P.; Pan, M.; Xu, Y.; Zhao, Y.; et al. Cardiac-specific mutation of clock alters the quantitative measurements of physical activities without changing behavioral circadian rhythms. J. Biol. Rhythms 2011, 26, 412–422. [Google Scholar] [CrossRef]
- Pastore, S.; Hood, D.A. Endurance training ameliorates the metabolic and performance characteristics of circadian Clock mutant mice. J. Appl. Physiol. 2013, 114, 1076–1084. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; King, D.P.; Chang, A.-M.; Kornhauser, J.M.; Lowrey, P.L.; McDonald, J.D.; Dove, W.F.; Pinto, L.H.; Turek, F.W.; Takahashi, J.S. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science 1994, 264, 719–725. [Google Scholar] [CrossRef]
- Škrlec, I.; Milić, J.; Steiner, R. The Impact of the Circadian Genes CLOCK and ARNTL on Myocardial Infarction. J. Clin. Med. 2020, 9, 484. [Google Scholar] [CrossRef]
- Tsang, K.; Liu, H.; Yang, Y.; Charles, J.F.; Ermann, J. Defective circadian control in mesenchymal cells reduces adult bone mass in mice by promoting osteoclast function. Bone 2019, 121, 172–180. [Google Scholar] [CrossRef]
- Crislip, G.R.; Douma, L.G.; Masten, S.H.; Cheng, K.Y.; Lynch, I.J.; Johnston, J.G.; Barral, D.; Glasford, K.B.; Holzworth, M.R.; Verlander, J.W.; et al. Differences in renal BMAL1 contribution to Na+homeostasis and blood pressure control in male and female mice. Am. J. Physiol. Renal Physiol. 2020, 318, F1463–F1477. [Google Scholar] [CrossRef] [PubMed]
- Pati, P.; Valcin, J.A.; Zhang, D.; Neder, T.H.; Millender-Swain, T.; Allan, J.M.; Sedaka, R.; Jin, C.; Becker, B.K.; Pollock, D.M.; et al. Liver circadian clock disruption alters perivascular adipose tissue gene expression and aortic function in mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2021, 320, R960–R971. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Navez, B.; Brichard, S.M. Circadian clock dysfunction in human omental fat links obesity to metabolic inflammation. Nat. Commun. 2021, 12, 2388. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Wang, T.; Zhao, Y.; Yang, Y.; Sun, Q.; Yang, X.; Gao, Y.; Xu, X.; Zhang, J.; Jin, C.; et al. Period1 mediates rhythmic metabolism of toxins by interacting with CYP2E1. Cell Death Dis. 2021, 12, 76. [Google Scholar] [CrossRef]
- Hamada, K.; Ishii, Y.; Yoshida, Y.; Nakaya, M.; Sato, Y.; Kanai, M.; Kikuchi, Y.; Yamaguchi, T.; Iijima, N.; Sutherland, K.; et al. The analysis of Period1 gene expression in vivo and in vitro using a micro PMT system. Biochem. Biophys. Res. Commun. 2021, 577, 64–70. [Google Scholar] [CrossRef]
- Bolsius, Y.G.; Zurbriggen, M.D.; Kyoung Kim, J.; Kas, M.J.; Meerlo, P.; Aton, S.J.; Havekes, R.Y.G.; Zurbriggen, M.D.; Kyoung Kim, J.; Kas, M.J.; et al. The role of clock genes in sleep, stress and memory. Biochem. Pharmacol. 2021, 191, 114493. [Google Scholar] [CrossRef]
- Urban, M.W.; Lo, C.; Bodinayake, K.K.; Brunswick, C.A.; Murakami, S.; Heimann, A.C.; Kwapis, J.L. The circadian clock gene Per1 modulates context fear memory formation within the retrosplenial cortex in a sex-specific manner. Neurobiol. Learn. Mem. 2021, 185, 107535. [Google Scholar] [CrossRef]
- Saracino, P.G.; Rossetti, M.L.; Steiner, J.L.; Gordon, B.S. Hormonal regulation of core clock gene expression in skeletal muscle following acute aerobic exercise. Biochem. Biophys. Res. Commun. 2019, 508, 871–876. [Google Scholar] [CrossRef]
- Shafi, A.A.; McNair, C.M.; McCann, J.J.; Alshalalfa, M.; Shostak, A.; Severson, T.M.; Zhu, Y.; Bergman, A.; Gordon, N.; Mandigo, A.C.; et al. The circadian cryptochrome, CRY1, is a pro-tumorigenic factor that rhythmically modulates DNA repair. Nat. Commun. 2021, 12, 401. [Google Scholar] [CrossRef]
- Chan, A.B.; Parico, G.C.G.; Fribourgh, J.L.; Ibrahim, L.H.; Bollong, M.J.; Partch, C.L.; Lamia, K.A. CRY2 missense mutations suppress P53 and enhance cell growth. Proc. Natl. Acad. Sci. USA 2021, 118, e2101416118. [Google Scholar] [CrossRef]
- Sokolowska, E.; Viitanen, R.; Misiewicz, Z.; Mennesson, M.; Saarnio, S.; Kulesskaya, N.; Kängsep, S.; Liljenbäck, H.; Marjamäki, P.; Autio, A.; et al. The circadian gene Cryptochrome 2 influences stress-induced brain activity and depressive-like behavior in mice. Genes Brain Behav. 2021, 20, e12708. [Google Scholar] [CrossRef]
- Li, C.; Xiao, S.; Hao, J.; Liao, X.; Li, G. Cry1 deficiency leads to testicular dysfunction and altered expression of genes involved in cell communication, chromatin reorganization, spermatogenesis, and immune response in mouse testis. Mol. Reprod. Dev. 2018, 85, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, S.; Fontaine, C.; Fruchart, J.C.; Staels, B. The role of the orphan nuclear receptor Rev-Erbα in adipocyte differentiation and function. Biochimie 2005, 87, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wu, N.; Lazar, M.A. Nuclear receptor Rev-erbalpha: A heme receptor that coordinates circadian rhythm and metabolism. Nucl. Recept. Signal. 2010, 8, e001. [Google Scholar] [CrossRef] [PubMed]
- Vasu, V.T.; Cross, C.E.; Gohil, K. Nr1d1, an important circadian pathway regulatory gene, is suppressed by cigarette smoke in murine lungs. Integr. Cancer Ther. 2009, 8, 321–328. [Google Scholar] [CrossRef]
- Pariollaud, M.; Gibbs, J.E.; Hopwood, T.W.; Brown, S.; Begley, N.; Vonslow, R.; Poolman, T.; Guo, B.; Saer, B.; Jones, D.H.; et al. Circadian clock component REV-ERBa controls homeostatic regulation of pulmonary inflammation. J. Clin. Investig. 2018, 128, 2281–2296. [Google Scholar] [CrossRef]
- Wang, J.; Lazar, M.A. Bifunctional Role of Rev-erbα in Adipocyte Differentiation. Mol. Cell Biol. 2008, 28, 2213–2220. [Google Scholar] [CrossRef]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant nice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Delezie, J.; Dumont, S.; Dardente, H.; Oudart, H.; Gréchez-Cassiau, A.; Klosen, P.; Teboul, M.; Delaunay, F.; Pévet, P.; Challet, E.; et al. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012, 26, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.M.; Carter, A.M.; Grant, P.J. Association between polymorphisms in the Clock gene, obesity and the metabolic syndrome in man. Int. J. Obes. 2008, 32, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Escames, G.; Yang, Z.; Zhao, H.; Qian, L.; Xue, C.; Xu, D.; Acuña-Castroviejo, D.; Yang, Y.; et al. Deciphering clock genes as emerging targets against aging. Ageing Res. Rev. 2022, 81, 101725. Available online: https://pubmed.ncbi.nlm.nih.gov/36029999/ (accessed on 9 December 2024). [CrossRef]
- Khapre, R.V.; Samsa, W.E.; Kondratov, R.V. Circadian regulation of cell cycle: Molecular connections between aging and the circadian clock. Ann. Med. 2010, 42, 404–415. Available online: https://pubmed.ncbi.nlm.nih.gov/20568980/ (accessed on 9 December 2024). [CrossRef]
- Kunieda, T.; Minamino, T.; Katsuno, T.; Tateno, K.; Nishi, J.I.; Miyauchi, H.; Orimo, M.; Okada, S.; Komuro, I.; Shimizu, T.; et al. Cellular senescence impairs circadian expression of clock genes in vitro and in vivo. Circ. Res. 2006, 98, 532–539. Available online: https://pubmed.ncbi.nlm.nih.gov/16424366/ (accessed on 9 December 2024). [CrossRef]
- Wolff, G.; Esser, K.A. Scheduled exercise phase shifts the circadian clock in skeletal Muscle. Med. Sci. Sports Exerc. 2012, 44, 1663–1670. [Google Scholar] [CrossRef]
- Back, F.A.; Fortes, F.S.; Santos, E.H.R.; Tambelli, R.; Menna-Barreto, L.S.; Louzada, F.M. Non-photic synchronization: The effect of aerobic physical exercise. Rev. Bras. Med. Esporte 2007, 13, 138–142. [Google Scholar] [CrossRef]
- Van Reeth, O.; Sturis, J.; Byrne, M.M.; Blackman, J.D.; L’Hermite-Baleriaux, M.; Leproult, R.; Oliner, C.; Refetoff, S.; Turek, F.W.; Van Cauter, E.; et al. Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. Am. J. Physiol. Endocrinol. Metab. 1994, 266, 964–974. [Google Scholar] [CrossRef]
- Buxton, O.M.; Frank, S.A.; L’Hermite-Balériaux, M.; Leproult, R.; Turek, F.W.; Van Cauter, E. Roles of intensity and duration of nocturnal exercise in causing phase delays of human circadian rhythms. Am. J. Physiol. 1997, 273, 536–542. [Google Scholar] [CrossRef]
- Taylor, K.; Cronin, J.B.; Gill, N.; Chapman, D.W.; Sheppard, J.M. Warm-up affects diurnal variation in power output. Int. J. Sports Med. 2011, 32, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Hashimoto, S.; Masubuchi, S.; Honma, S.; Honma, K.I. Phase-advance shifts of human circadian pacemaker are accelerated by daytime physical exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Douma, L.G.; Crislip, G.R.; Cheng, K.Y.; Barral, D.; Masten, S.; Holzworth, M.R.; Roig, E.; Glasford, K.B.; Beguiristain, K.; Li, W.; et al. Knockout of the Circadian Clock Protein PER1 Results in Sex-Dependent Alterations of ET-1 Production in Mice in Response to a High Salt Diet plus Mineralocorticoid Treatment. Can. J. Physiol. Pharmacol. 2020, 98, 579. [Google Scholar] [CrossRef] [PubMed]
- Jesus, I.C.G.; Araújo, F.M.; Mesquita, T.; Júnior, N.N.S.; Silva, M.M.; Morgan, H.J.N.; Silva, K.S.C.; Silva, C.L.A.; Birbrair, A.; Amaral, F.A.; et al. Molecular basis of Period 1 regulation by adrenergic signaling in the heart. FASEB J. 2021, 35, e21886. Available online: https://pubmed.ncbi.nlm.nih.gov/34473369/ (accessed on 9 December 2024). [CrossRef]
- Guan, D.; Lazar, M.A. Interconnections between circadian clocks and metabolism. J. Clin. Investig. 2021, 131, e148278. [Google Scholar] [CrossRef]
- Ray, A.; Wen, J.; Yammine, L.; Culver, J.; Parida, I.S.; Garren, J.; Xue, L.; Hales, K.; Xiang, Q.; Birnbaum, M.J.; et al. Regulated dynamic subcellular GLUT4 localization revealed by proximal proteome mapping in human muscle cells. J. Cell Sci. 2023, 136, jcs261454. [Google Scholar] [CrossRef]
- Maier, G.; Delezie, J.; Westermark, P.O.; Santos, G.; Ritz, D.; Handschin, C. Transcriptomic, proteomic and phosphoproteomic underpinnings of daily exercise performance and zeitgeber activity of training in mouse muscle. J. Physiol. 2022, 600, 769–796. [Google Scholar] [CrossRef]
- Abdelmoez, A.M.; Puig, L.S.; Smith, J.A.B.; Gabriel, B.M.; Savikj, M.; Dollet, L.; Chibalin, A.V.; Krook, A.; Zierath, J.R.; Pillon, N.J.; et al. Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism. Am. J. Physiol. Cell Physiol. 2020, 318, C615–C626. Available online: https://pubmed.ncbi.nlm.nih.gov/31825657/ (accessed on 3 August 2025). [CrossRef]
- Moravčík, R.; Olejárová, S.; Zlacká, J.; Herichová, I. Effect of miR-34a on the expression of clock and clock-controlled genes in DLD1 and Lovo human cancer cells with different backgrounds with respect to p53 functionality and 17β-estradiol-mediated regulation. PLoS ONE 2023, 18, e0292880. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0292880 (accessed on 14 May 2025). [CrossRef]
- Wang, Z.; Wang, S.; Bi, Y.; Boiti, A.; Zhang, S.; Vallone, D.; Lan, X.; Foulkes, N.S.; Zhao, H.; Liu, Y.; et al. Light-regulated microRNAs shape dynamic gene expression in the zebrafish circadian clock. PLoS Genet. 2025, 21, e1011545. Available online: https://journals.plos.org/plosgenetics/article?id=10.1371/journal.pgen.1011545 (accessed on 14 May 2025). [CrossRef]
- Zacharewicz, E.; Lamon, S.; Russell, A.P. MicroRNAs in skeletal muscle and their regulation with exercise, ageing, and disease. Front. Physiol. 2013, 4, 266. [Google Scholar] [CrossRef]
- Robinson, M.M.; Dasari, S.; Konopka, A.R.; Johnson, M.L.; Manjunatha, S.; Esponda, R.R.; Carter, R.E.; Lanza, I.R.; Nair, K.S.; Glynn, E.L.; et al. Enhanced Protein Translation Underlies Improved Metabolic and Physical Adaptations to Different Exercise Training Modes in Young and Old Humans. Cell Metab. 2017, 25, 581. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC5423095/ (accessed on 14 May 2025). [CrossRef] [PubMed]
- Abreu, P.; Leal-Cardoso, J.H.; Ceccatto, V.M. Adaptação do músculo esquelético ao exercício físico: Considerações moleculares e energéticas. Rev. Bras. Med. Esporte 2017, 23, 60–65. Available online: https://www.scielo.br/j/rbme/a/nrVqz5ncsFTpzBrnvwhYfng/ (accessed on 14 May 2025). [CrossRef][Green Version]
- Schwanhüusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M.; Zidek, A.; Dehghani, F.; et al. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342, Erratum in Nature 2013, 495, 126–127. Available online: https://www.nature.com/articles/nature10098 (accessed on 3 August 2025). [CrossRef] [PubMed]
- Makhnovskii, P.A.; Zgoda, V.G.; Bokov, R.O.; Shagimardanova, E.I.; Gazizova, G.R.; Gusev, O.A.; Lysenko, E.A.; Kolpakov, F.A.; Vinogradova, O.L.; Popov, D.V.; et al. Regulation of Proteins in Human Skeletal Muscle: The Role of Transcription. Sci. Rep. 2020, 10, 3514. Available online: https://www.nature.com/articles/s41598-020-60578-2 (accessed on 3 August 2025). [CrossRef]
- Feng, L.; Li, B.; Xi, Y.; Cai, M.; Tian, Z. Aerobic exercise and resistance exercise alleviate skeletal muscle atrophy through IGF-1/IGF-1R-PI3K/Akt pathway in mice with myocardial infarction. Am. J. Physiol. Cell Physiol. 2022, 322, C164–C176. [Google Scholar] [CrossRef]
- Nishimura, Y.; Musa, I.; Holm, L.; Lai, Y.C. Recent advances in measuring and understanding the regulation of exercise-mediated protein degradation in skeletal muscle. Am. J. Physiol. Cell Physiol. 2021, 321, C276–C287. [Google Scholar] [CrossRef]
- Ji, F.; Lee, H.S.; Kim, J.H. Resistance exercise and skeletal muscle: Protein synthesis, degradation, and controversies. Eur. J. Appl. Physiol. 2025, 125, 1–30. Available online: https://link.springer.com/article/10.1007/s00421-025-05832-z (accessed on 3 August 2025). [CrossRef]
- Noble, E.G.; Milne, K.J.; Melling, C.W.J. Heat shock proteins and exercise: A primer. Appl. Physiol. Nutr. Metab. 2008, 33, 1050–1065. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Koukoulas, I. HSP72 gene expression progressively increases in human skeletal muscle during prolonged, exhaustive exercise. J. Appl. Physiol. 2000, 89, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Rebouças, E.d.L.; Costa, J.J.d.N.; Passos, M.J.; Passos, J.R.d.S.; van den Hurk, R.; Silva, J.R.V. Real time PCR and importance of housekeepings genes for normalization and quantification of mRNA expression in different tissues. Braz. Arch. Biol. Technol. 2013, 56, 143–154. Available online: https://www.scielo.br/j/babt/a/jMGrtmZrwnzfpwzXbpjhqLc/?lang=en (accessed on 3 August 2025). [CrossRef]
- Hostrup, M.; Lemminger, A.K.; Stocks, B.; Gonzalez-Franquesa, A.; Larsen, J.K.; Quesada, J.P.; Thomassen, M.; Weinert, B.T.; Bangsbo, J.; Deshmukh, A.S.; et al. High-intensity interval training remodels the proteome and acetylome of human skeletal muscle. Elife 2022, 11, e69802. [Google Scholar] [CrossRef]
- Tung, K.F.; Pan, C.Y.; Lin, W.C. Housekeeping protein-coding genes interrogated with tissue and individual variations. Sci. Rep. 2024, 14, 12454. Available online: https://www.nature.com/articles/s41598-024-63269-4 (accessed on 3 August 2025). [CrossRef]
- Sun, Y.; Shui, K.; Li, Q.; Liu, C.; Jin, W.; Ni, J.Q.; Lu, J.; Zhang, L. Upstream open reading frames dynamically modulate CLOCK protein translation to regulate circadian rhythms and sleep. PLoS Biol. 2025, 23, e3003173. Available online: https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3003173 (accessed on 3 August 2025). [CrossRef]
- Rovina, R.L.; da Rocha, A.L.; Marafon, B.B.; Pauli, J.R.; de Moura, L.P.; Cintra, D.E.; Ropelle, E.R.; da Silva, A.S.R. One Bout of Aerobic Exercise Can Enhance the Expression of Nr1d1 in Oxidative Skeletal Muscle Samples. Front. Physiol. 2021, 12, 626096. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ogata, H.; Kayaba, M.; Ando, A.; Park, I.; Yajima, K.; Araki, A.; Suzuki, C.; Osumi, H.; Zhang, S.; et al. Effect of a single bout of exercise on clock gene expression in human leukocyte. J. Appl. Physiol. 2020, 128, 847–854. [Google Scholar] [CrossRef]
- Yeung, C.Y.C.; Schjerling, P.; Heinemeier, K.M.; Boesen, A.P.; Dideriksen, K.; Kjær, M. Investigating circadian clock gene expression in human tendon biopsies from acute exercise and immobilization studies. Eur. J. Appl. Physiol. 2019, 119, 1387–1394. [Google Scholar] [CrossRef]
- Zambon, A.C.; McDearmon, E.L.; Salomonis, N.; Vranizan, K.M.; Johansen, K.L.; Adey, D.; Takahashi, J.S.; Schambelan, M.; Conklin, B.R. Time- and exercise-dependent gene regulation in human skeletal muscle. Genome Biol. 2003, 4, R61. [Google Scholar] [CrossRef]
- Murphy, B.A.; Wagner, A.L.; McGlynn, O.F.; Kharazyan, F.; Browne, J.A.; Elliott, J.A. Exercise influences circadian gene expression in equine skeletal muscle. Vet. J. 2014, 201, 39–45. [Google Scholar] [CrossRef]
- Bruns, D.R.; Yusifova, M.; Marcello, N.A.; Green, C.J.; Walker, W.J.; Schmitt, E.E. The peripheral circadian clock and exercise: Lessons from young and old mice. J. Circadian Rhythm. 2021, 18, 7. [Google Scholar] [CrossRef]
- Erickson, M.L.; Zhang, H.U.I.; Mey, J.T.; Kirwan, J.P. Exercise Training Impacts Skeletal Muscle Clock Machinery in Prediabetes. Med. Sci. Sports Exerc. 2020, 52, 2078–2085. [Google Scholar] [CrossRef]
- Weiss, J.; Hiltbrand, B. Functional compartmentation of glycolytic versus oxidative metabolism in isolated rabbit heart. J. Clin. Investig. 1985, 75, 436. [Google Scholar] [CrossRef]
- Allard, M.F.; Schonekess, B.O.; Henning, S.L.; English, D.R.; Lopaschuk, G.D. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am. J. Physiol. Heart Circ. Physiol. 1994, 267, H742–H750. [Google Scholar] [CrossRef] [PubMed]
- Crupi, A.N.; Nunnelee, J.S.; Taylor, D.J.; Thomas, A.; Vit, J.P.; Riera, C.E.; Gottlieb, R.A.; Goodridge, H.S. Oxidative muscles have better mitochondrial homeostasis than glycolytic muscles throughout life and maintain mitochondrial function during aging. Aging 2018, 10, 3327. [Google Scholar] [CrossRef] [PubMed]
- Jingting, S.; Qin, X.; Yanju, S.; Ming, Z.; Yunjie, T.; Gaige, J.; Zhongwei, S.; Jianmin, Z. Oxidative and glycolytic skeletal muscles show marked differences in gene expression profile in Chinese Qingyuan partridge chickens. PLoS ONE 2017, 12, e0183118. [Google Scholar] [CrossRef] [PubMed]
- Philippi, M.; Sillau, A.H. Oxidative capacity distribution in skeletal muscle fibers of the rat. J. Exp. Biol. 1994, 189, 1–11. [Google Scholar] [CrossRef]
- Park, S.Y.; Gifford, J.R.; Andtbacka, R.H.I.; Trinity, J.D.; Hyngstrom, J.R.; Garten, R.S.; Diakos, N.A.; Ives, S.J.; Dela, F.; Larsen, S.; et al. Cardiac, skeletal, and smooth muscle mitochondrial respiration: Are all mitochondria created equal? Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H346. [Google Scholar] [CrossRef]
- Pereira, B. Biogenêse mitocondrial e exercício físico: Hipótese do acoplamento elétrico-transcripcional. Rev. Bras. Educ. Física Esporte 2015, 29, 687–703. [Google Scholar] [CrossRef]
- Bizeau, M.E.; Willis, W.T.; Hazel, J.R. Differential responses to endurance training in subsarcolemmal and intermyofibrillar mitochondria. J. Appl. Physiol. 1998, 85, 1279–1284. [Google Scholar] [CrossRef]
- Mayeuf-Louchart, A.; Thorel, Q.; Delhaye, S.; Beauchamp, J.; Duhem, C.; Danckaert, A.; Lancel, S.; Pourcet, B.; Woldt, E.; Boulinguiez, A.; et al. Rev-erb-α regulates atrophy-related genes to control skeletal muscle mass. Sci. Rep. 2017, 7, 14383. [Google Scholar] [CrossRef] [PubMed]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.C.; Paquet, C.; Delhaye, S.; Shin, Y.; et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.A. Invited Review: Contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 2001, 90, 1137–1157. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Simões, H.G.; Denadai, B.S.; Baldissera, V.; Campbell, C.S.G.; Hill, D.W. Relationships and significance of lactate minimum, critical velocity, heart rate deflection and 3000 m track-tests for running. J. Sports Med. Phys. Fitness 2005, 45, 441–451. [Google Scholar]
- Gaesser, G.A.; Poole, D.C. The slow component of oxygen uptake kinetics in humans. Exerc. Sport Sci. Rev. 1996, 24, 35–71. [Google Scholar] [CrossRef]
- Simões, H.G.; Hiyane, W.C.; Benford, R.E.; Madrid, B.; Prada, F.A.; Moreira, S.R.; Oliveira, R.J.; Nakamura, F.Y.; Campbell, C.S.G.; Kushnick, M.R. Lactate threshold prediction by blood glucose and rating of perceived exertion in people with type 2 diabetes. Percept. Mot. Skills 2010, 111, 365–378. [Google Scholar] [CrossRef]
- Simões, H.G.; Moreira, S.R.; Moffatt, R.J.; Campbell, C.S.G. Methods to identify the anaerobic threshold for type-2 diabetic and non-diabetic subjects. Arq. Bras. Cardiol. 2010, 94, 71–78. [Google Scholar] [CrossRef]
- Asano, R.Y.; Browne, R.A.V.; Da Costa Sotero, R.; Sales, M.M.; De Moraes, J.F.V.N.; Campbell, C.S.G.; Simões, H.G. Cycling above rather than below lactate threshold is more effective for nitric oxide release and post-exercise blood pressure reduction in individuals with type-2 diabetes. Mot. Rev. Educ. Física 2013, 19, 633–640. [Google Scholar] [CrossRef][Green Version]
- Cunha, R.R.; de Carvalho Cunha, V.N.; Segundo, P.R.; Moreira, S.R.; Kokubun, E.; Campbell, C.S.G.; de Oliveira, R.J.; Simões, H.G. Determination of the lactate threshold and maximal blood lactate steady state intensity in aged rats. Cell Biochem. Funct. 2009, 27, 351–357. [Google Scholar] [CrossRef]
- Aylett, E.; Small, N.; Bower, P. Exercise in the treatment of clinical anxiety in general practice—A systematic review and meta-analysis. BMC Health Serv. Res. 2018, 18, 1–18. [Google Scholar] [CrossRef]
- Verbrugghe, J.; Agten, A.; Stevens, S.; Hansen, D.; Demoulin, C.; Eijnde, B.O.; Vandenabeele, F.; Timmermans, A. High Intensity Training to Treat Chronic Nonspecific Low Back Pain: Effectiveness of Various Exercise Modes. J. Clin. Med. 2020, 9, 2401. [Google Scholar] [CrossRef]
- Koch, C.E.; Leinweber, B.; Drengberg, B.C.; Blaum, C.; Oster, H. Interaction between circadian rhythms and stress. Neurobiol. Stress 2017, 6, 57. [Google Scholar] [CrossRef]
- Helfrich-Förster, C. Interactions between psychosocial stress and the circadian endogenous clock. Psych. J. 2017, 6, 277–289. [Google Scholar] [CrossRef]
- Endo, Y.; Shiraki, K. Behavior and body temperature in rats following chronic foot shock or psychological stress exposure. Physiol. Behav. 2000, 71, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Agorastos, A.; Nicolaides, N.C.; Bozikas, V.P.; Chrousos, G.P.; Pervanidou, P. Multilevel Interactions of Stress and Circadian System: Implications for Traumatic Stress. Front. Psychiatry 2020, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Perreau-Lenz, S.; Spanagel, R. Clock Genes × Stress × Reward Interactions in Alcohol and Substance Use Disorders. Alcohol 2015, 49, 351. [Google Scholar] [CrossRef]
- Miyazaki, K.; Itoh, N.; Ohyama, S.; Kadota, K.; Oishi, K. Continuous Exposure to a Novel Stressor Based on Water Aversion Induces Abnormal Circadian Locomotor Rhythms and Sleep-Wake Cycles in Mice. PLoS ONE 2013, 8, e55452. [Google Scholar] [CrossRef]
- Wang, M.; Yu, D.; Zheng, L.; Hong, B.; Li, H.; Hu, X.; Zhang, K.; Mou, Y.; Liu, Y.; Chen, Y.; et al. Mechanical Stress Affects Circadian Rhythm in Skeletal Muscle (C2C12 Myoblasts) by Reducing Per/Cry Gene Expression and Increasing Bmal1 Gene Expression. Med. Sci. Monit. 2021, 27, e928359-1–e928359-10. [Google Scholar] [CrossRef]
- de Alencar Silva, B.S.; Uzeloto, J.S.; Lira, F.S.; Pereira, T.; Coelho-E.-silva, M.J.; Caseiro, A. Exercise as a Peripheral Circadian Clock Resynchronizer in Vascular and Skeletal Muscle Aging. Int. J. Environ. Res. Public Health 2021, 18, 12949. [Google Scholar] [CrossRef]
- Tahara, Y.; Aoyama, S.; Shibata, S. The mammalian circadian clock and its entrainment by stress and exercise. J. Physiol. Sci. 2017, 67, 1–10. [Google Scholar] [CrossRef]
- Cunha, V.N.d.C.; Dos Santos Rosa, T.; Sales, M.M.; Sousa, C.V.; Da Silva Aguiar, S.; Deus, L.A.; Simões, H.G.; De Andrade, R.V. Training Performed above Lactate Threshold Decreases p53 and Shelterin Expression in Mice. Int. J. Sports Med. 2018, 39, 704–711. [Google Scholar] [CrossRef]
- Dalbram, E.; Basse, A.L.; Zierath, J.R.; Treebak, J.T. Voluntary wheel running in the late dark phase ameliorates diet-induced obesity in mice without altering insulin action. J. Appl. Physiol. 2019, 126, 993–1005. [Google Scholar] [CrossRef]
- Yasumoto, Y.; Nakao, R.; Oishi, K. Free access to a running-wheel advances the phase of behavioral and physiological circadian rhythms and peripheral molecular clocks in mice. PLoS ONE 2015, 10, e0116476, Erratum in PLoS ONE 2015, 10, e0125646. [Google Scholar]
- Small, L.; Altıntaş, A.; Laker, R.C.; Ehrlich, A.; Pattamaprapanont, P.; Villarroel, J.; Pillon, N.J.; Zierath, J.R.; Barrès, R.; Hawley, J.A.; et al. Contraction influences Per2 gene expression in skeletal muscle through a calcium-dependent pathway. J. Physiol. 2020, 598, 5739–5752. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Z.; Shi, G.; Xing, L.; Wang, X.; Gu, X.; Qu, Z.; Dong, Z.; Xiong, J.; Gao, X.; et al. The circadian clock influences heart performance. J. Biol. Rhythms 2011, 26, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.M.; Truong, D.; Loh, D.H.; Jordan, M.C.; Roos, K.P.; Colwell, C.S. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J. Physiol. 2012, 590, 6213–6226. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Monreal, M.A.; Harmsen, J.F.; Schrauwen, P.; Esser, K.A. Ticking for Metabolic Health: The Skeletal-Muscle Clocks. Obesity 2020, 28, S46–S54. [Google Scholar] [CrossRef]
- Mancilla, R.; Brouwers, B.; Schrauwen-Hinderling, V.B.; Hesselink, M.K.C.; Hoeks, J.; Schrauwen, P. Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Physiol Rep. 2021, 8, e14669. [Google Scholar] [CrossRef]
- Erickson, M.L.; Esser, K.A.; Kraus, W.E.; Buford, T.W.; Redman, L.M. A Role for Exercise to Counter Skeletal Muscle Clock Disruption. Exerc. Sport Sci. Rev. 2021, 49, 35–41. [Google Scholar] [CrossRef]
- Hood, S.; Amir, S. The aging clock: Circadian rhythms and later life. J. Clin. Investig. 2017, 127, 437. [Google Scholar] [CrossRef] [PubMed]
- Cunha, V.N.d.C. Efeitos da Intensidade do Treinamento Aeróbio Sobre o Comprimento do Telômero e Suas Proteínas de Proteção Durante o Envelhecimento; Universidade Católica de Brasília (UCB): Taguatinga, Brazil, 2015. [Google Scholar]
- Roy, R.R.; Hutchison, D.L.; Pierotti, D.J.; Hodgson, J.A.; Edgerton, V.R. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J. Appl. Physiol. 1991, 70, 2522–2529. [Google Scholar] [CrossRef]
- Bustin, S.A. Why the need for qPCR publication guidelines?—The case for MIQE. Methods 2010, 50, 217–226. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Gene | Accession No. | Sense Primer (5′-3′) | Antisense Primer (5′-3′) |
|---|---|---|---|
| 36B4 | NM_007475 | GAGGAATCAGATGAGGATATGGGA | AAGCAGGCTGACTTGGTTGC |
| Clock | NM_007715 | TTGCTCCACGGGAATCCTT | GGAGGGAAAGTGCTCTGTTGTAG |
| Arntl | NM_007489 | GGACTTGCGCTCTACCTGTTCA | AACCATGTGCGAGTGCAGGCGC |
| Nr1d1 | NM_145434 | GGTGCGCTTTGCATCGTT | GGTTGTGCGGCTCAGGAA |
| Per1 | NM_011065 | GCGGGTCTTCGGTTAAGGTT | AGGCTCAGCTGGGATTTGG |
| Per2 | NM_011066 | CCAACACAGACGACAGCATCA | CTTCAACACCGCCTGGAGAT |
| Cry1 | NM_007771 | AGATCTTGGTAAGAGATTTGCTTAATGTAA | TGACTCTCAAAACTCTTGAGATTTATATCA |
| Cry2 | NM_009963 | AGCCCAGGCCAAGAGGAA | GTTTTTCAGGCCCACTCTACCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitorino, M.C.T.; de Luca Corrêa, H.; de Carvalho Cunha, V.N.; de Souza, M.S.; Simões, H.G.; Rosa, T.d.S.; Vieira, E.; de Andrade, R.V. Clock Gene Expression Modulation by Low- and High-Intensity Exercise Regimens in Aging Mice. Int. J. Mol. Sci. 2025, 26, 8739. https://doi.org/10.3390/ijms26178739
Vitorino MCT, de Luca Corrêa H, de Carvalho Cunha VN, de Souza MS, Simões HG, Rosa TdS, Vieira E, de Andrade RV. Clock Gene Expression Modulation by Low- and High-Intensity Exercise Regimens in Aging Mice. International Journal of Molecular Sciences. 2025; 26(17):8739. https://doi.org/10.3390/ijms26178739
Chicago/Turabian StyleVitorino, Matheus Callak Teixeira, Hugo de Luca Corrêa, Verusca Najara de Carvalho Cunha, Mariana Saliba de Souza, Herbert Gustavo Simões, Thiago dos Santos Rosa, Elaine Vieira, and Rosângela Vieira de Andrade. 2025. "Clock Gene Expression Modulation by Low- and High-Intensity Exercise Regimens in Aging Mice" International Journal of Molecular Sciences 26, no. 17: 8739. https://doi.org/10.3390/ijms26178739
APA StyleVitorino, M. C. T., de Luca Corrêa, H., de Carvalho Cunha, V. N., de Souza, M. S., Simões, H. G., Rosa, T. d. S., Vieira, E., & de Andrade, R. V. (2025). Clock Gene Expression Modulation by Low- and High-Intensity Exercise Regimens in Aging Mice. International Journal of Molecular Sciences, 26(17), 8739. https://doi.org/10.3390/ijms26178739






