Exogenous Application of Glycine Betaine to Passiflora edulis Sims f. flavicarpa to Mitigate Drought Stress on Two Propagation Methods
Abstract
1. Introduction
2. Results
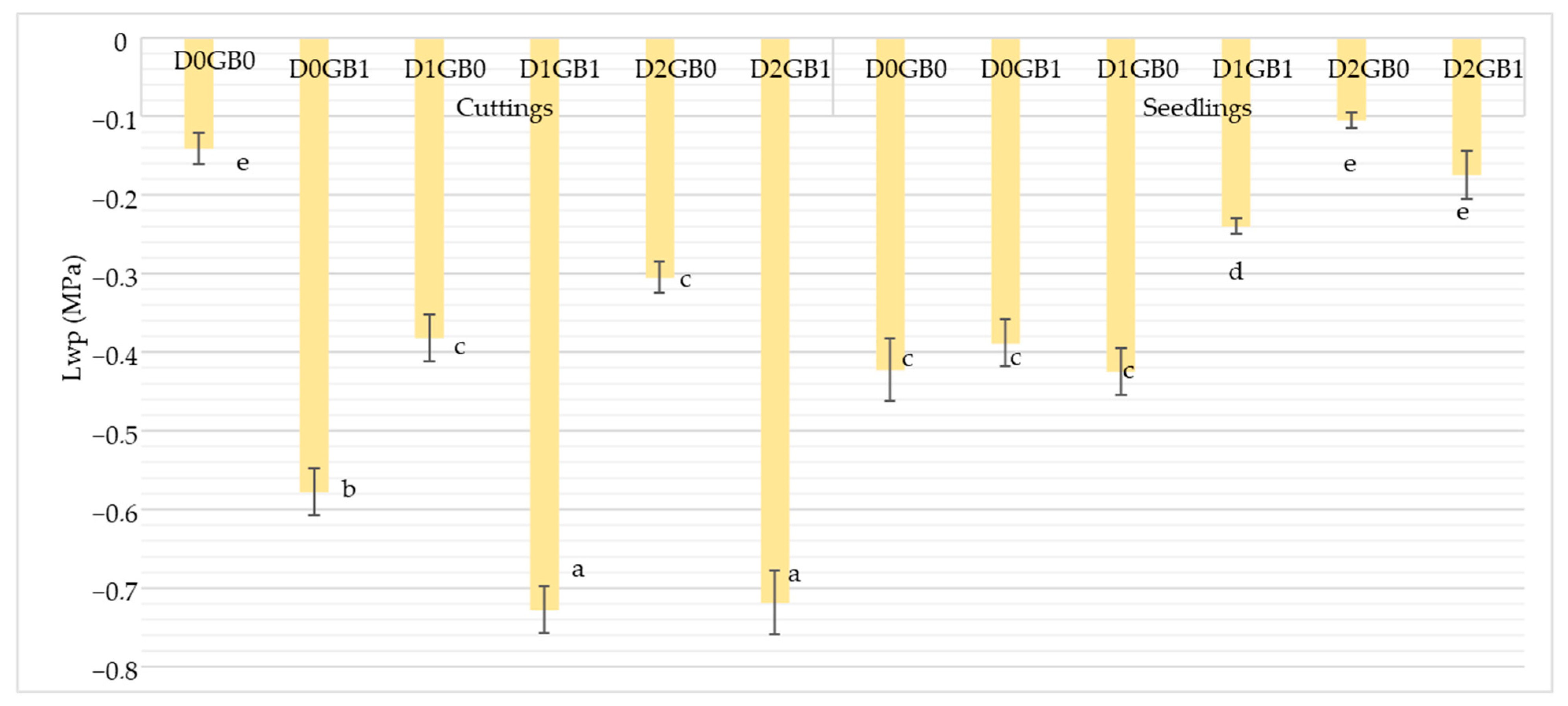
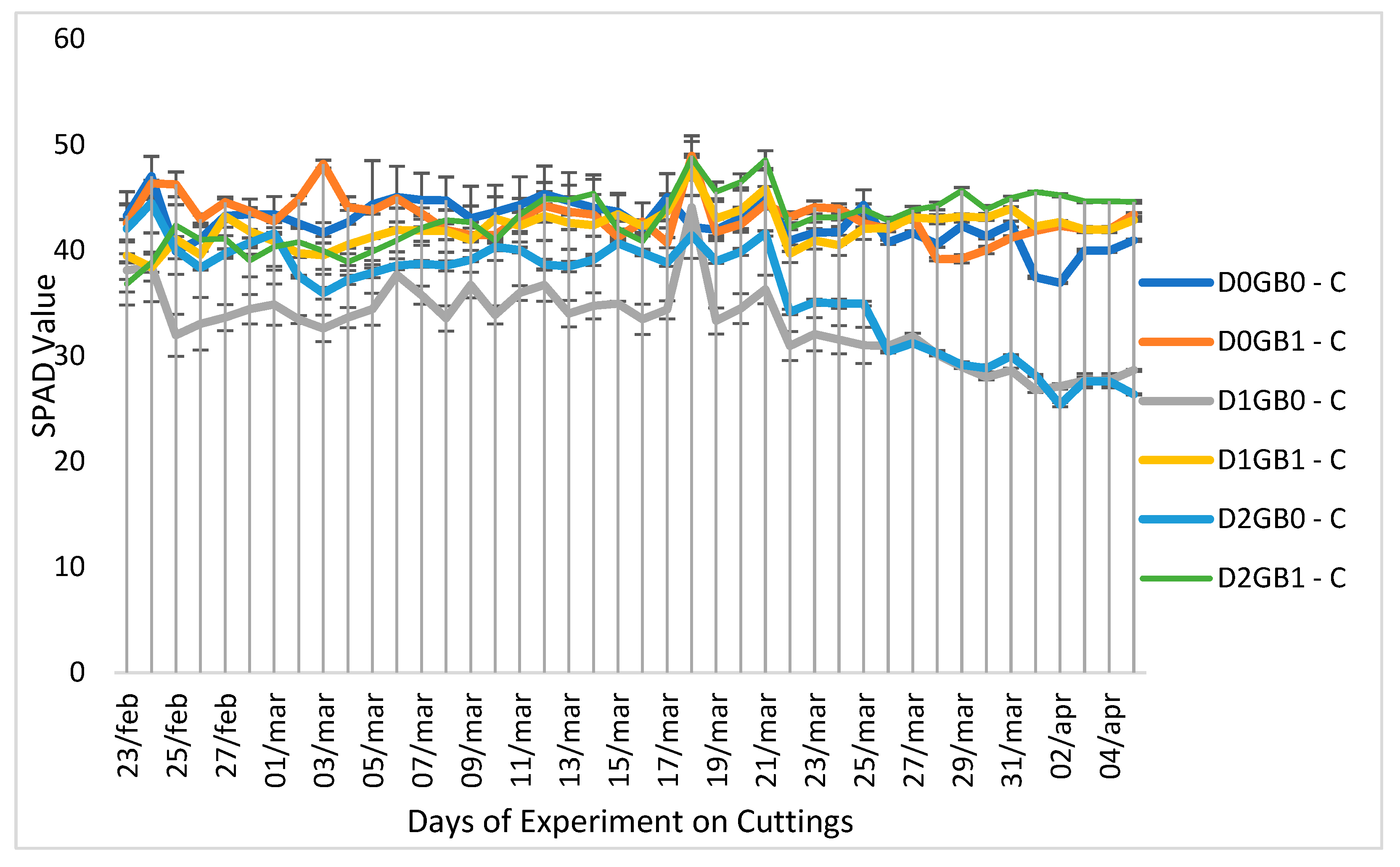
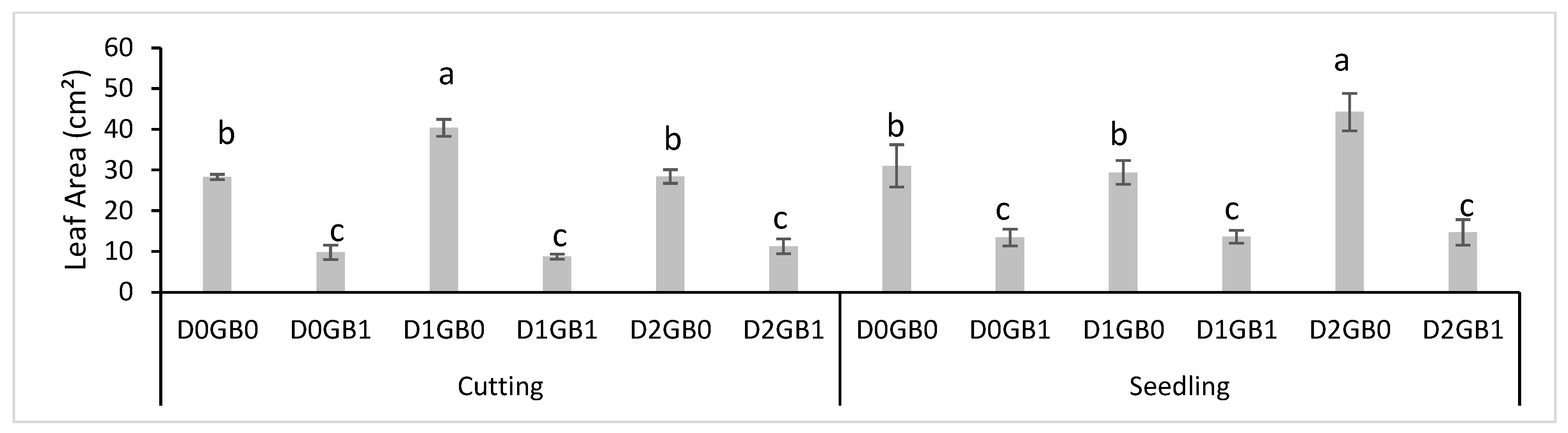
2.1. Effect of Glycine Betaine on Water Relations and Leaf Chlorophyll Content
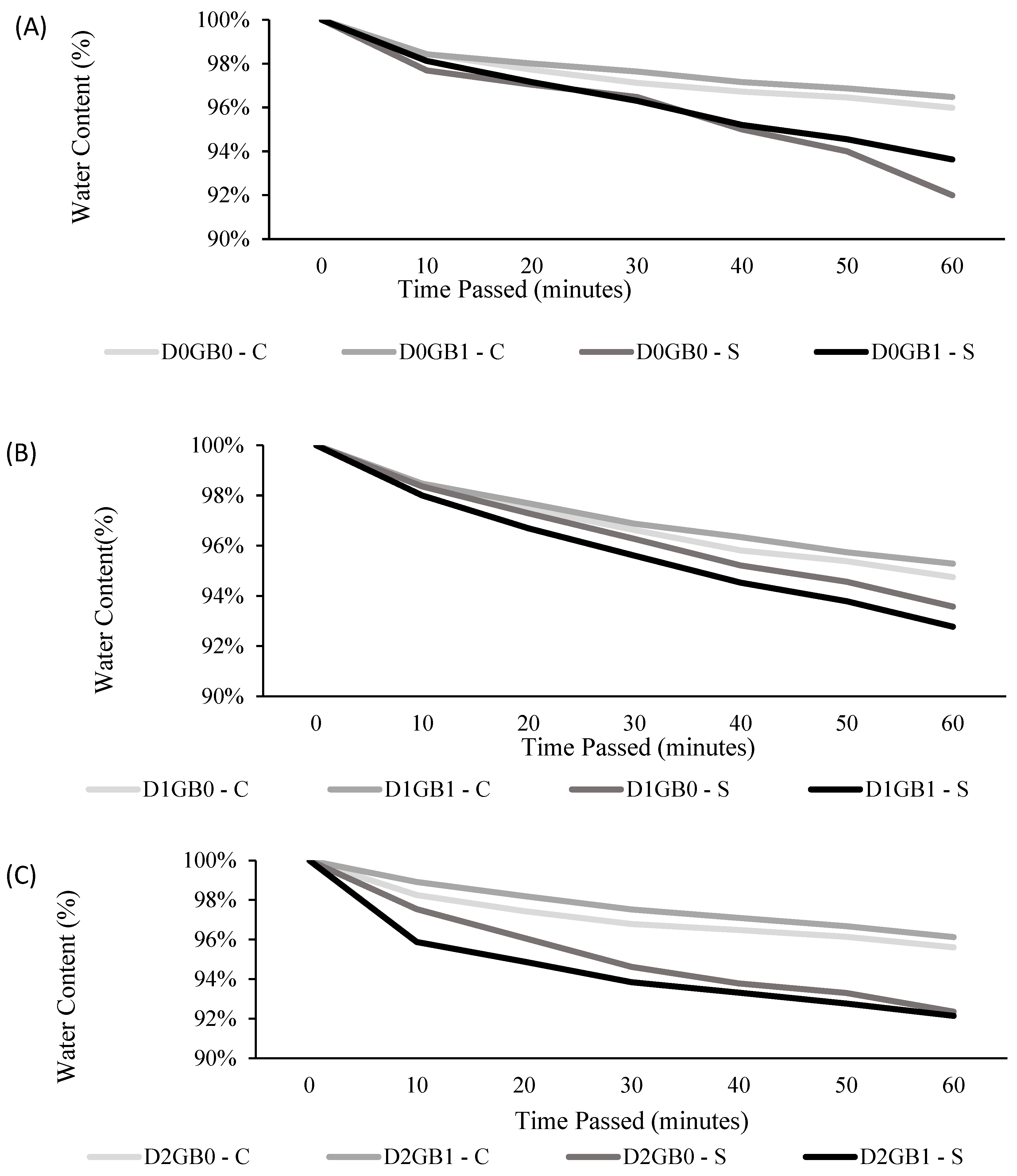
2.2. Effect of Glycine Betaine on Stomatal Behavior, Cuticle Thickness, and Transpiration
2.3. Adverse Effect of Glycine Betaine
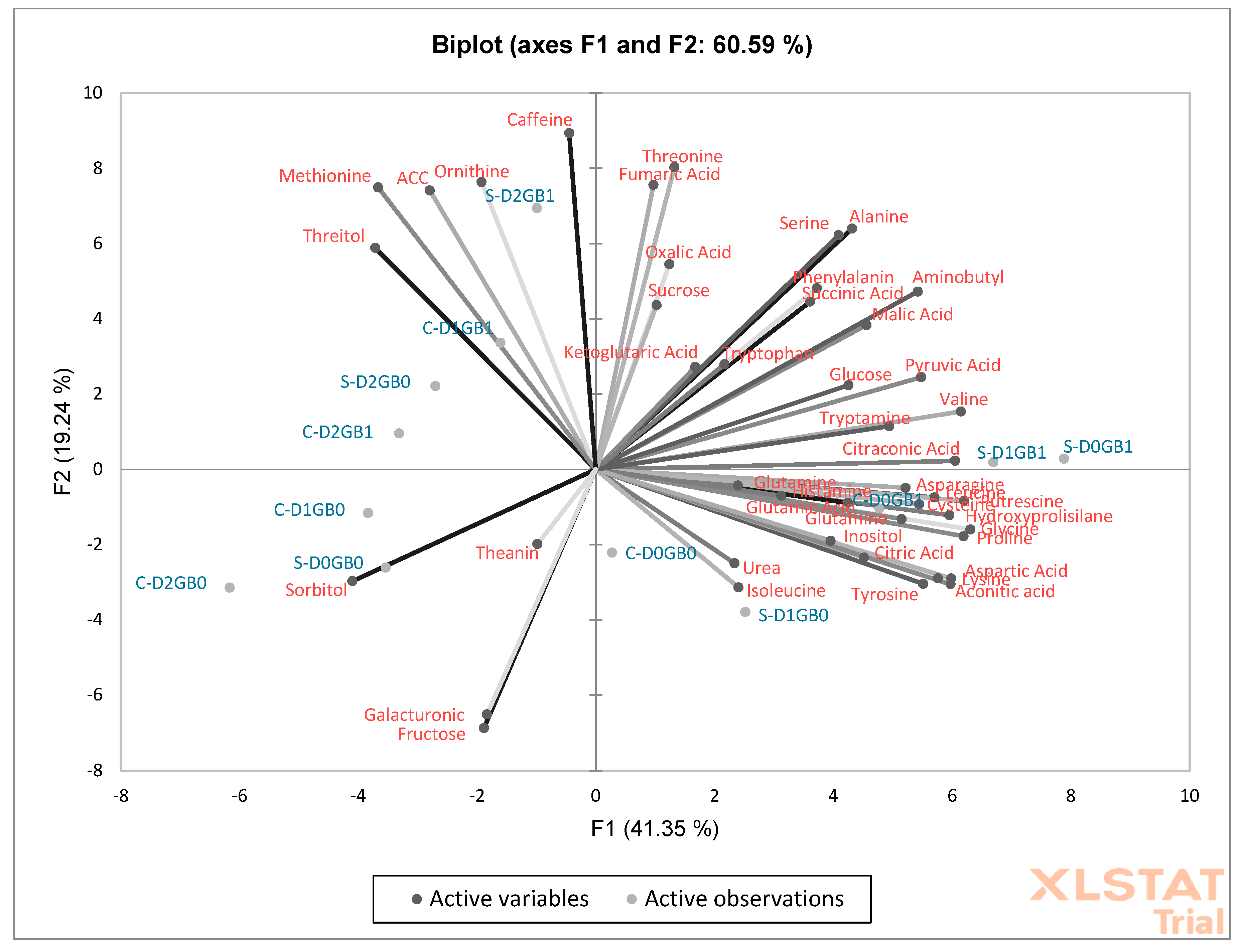
2.4. Metabolic Responses to Stress
3. Discussion
3.1. Role of Glycine Betaine in Water Relations and Leaf Chlorophyll Content
3.2. Stomatal Behavior, Cuticle Thickness, and Transpiration
3.3. Potential Phytotoxicity of Glycine Betaine
3.4. Amino Acids and Metabolic Responses to Stress
3.5. Organic Acids and Osmotic Defense
3.6. Sugar Alcohols and Non-Protein Amino Acids in Stress Responses
3.7. Citric Acid and Soluble Sugars in Plant Stress
3.8. Proline and Other Osmoprotectants
4. Materials and Methods
4.1. Experimental Design
4.2. Experimental Indexes
4.3. Experiment Management
4.4. Experiment Measurement
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GB | Glycine betaine |
| LWP | Leaf water potential |
| PCA | Principal component analysis |
| bZIPs | Basic leucine zipper transcription factors |
| BCAA | Branched-chain amino acids |
| GABA | Gamma-aminobutyric acid |
| MACl | Methoxyamine hydrochloride |
| MSTFA | N-Methyl-N-trimethylsilyltrifluoroacetamide |
| GCMS | Gas chromatograph–mass spectrometer |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| C | Cuttings |
| S | Seedlings |
| D0 | Control drought (no water deficit) |
| D1 | Mild drought |
| D2 | Severe drought |
| GB0 | No application of glycine betaine |
| GB1 | Application of 100 mM glycine betaine |
References
- Castillo, N.R.; Ambachew, D.; Melgarejo, L.M.; Blair, M.W. Morphological and agronomic variability among cultivars, landraces, and genebank accessions of purple passion fruit, Passiflora edulis f. edulis. HortScience 2022, 55, 768–777. [Google Scholar]
- Faleiro, F.G.; Junqueira, N.T.V.; Costa, A.M.; de Jesus, O.N.; Machado, C.D.F. Passion fruit. In Embrapa Cassava and Fruits—Technical Book (INFOTECA-E); Embrapa Mandioca e Fruticultura: Cruz das Almas, Brazil, 2017. (In Portuguese) [Google Scholar]
- IBGE (Brazilian Institute of Geography and Statistics). Municipal Agricultural Production; IBGE: Rio de Janeiro, Brazil, 2020. (In Portuguese) [Google Scholar]
- Carvalho, J.A.; Caldas, A.L.D.; Rezende, F.C.; Nakazone, M.V.; Faria, L.A. Production and quality of yellow passion fruit as a function of soil water tension. Rev. Eng. Agric. 2014, 22, 231–238. (In Portuguese) [Google Scholar] [CrossRef]
- Gomes, M.T.G.; Falqueto, A.R.; Silva, D.M.; Rossi, M.S.; Luz, C.A.; Bati-Tucci, M.D. FB100 and FB200 passion fruit cultivars (Passiflora edulis Sims.) differ in physiological responses to water stress. In XXVIII Reunión Argentina de Fisiología Vegetal; Sociedad Argentina de Fisiología Vegetal: La Plata, Argentina, 2010. [Google Scholar]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of Abiotic Stress on Crops. In Sustainable Crop Production; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef]
- Park, E.-J.; Jeknić, Z.; Pino, M.-T.; Murata, N.; Chen, T.H.H. Glycinebetaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. Plant Cell Environ. 2007, 30, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H. Role of glycine betaine in the thermotolerance of plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Khalil, L.; Alturki, S.M.; Sebaaly, Z.; Sajyan, T.K.; Sassine, Y.N. A trial to enhance cold tolerance of passion fruit (Passiflora edulis) crop in Lebanon through foliar application of the natural osmoprotectant glycine betaine. In Proceedings of the XXX International Horticultural Congress IHC2018: VII International Symposium on Tropical and Subtropical Fruits, Avocado II 1299, Istanbul, Turkey, 12–16 August 2018; pp. 25–34. [Google Scholar]
- Metwaly, E.S.E.; Al-Yasi, H.M.; Ali, E.F.; Farouk, H.A.; Farouk, S. Deteriorating harmful effects of drought in cucumber by spraying glycine betaine. Agriculture 2022, 12, 2166. [Google Scholar] [CrossRef]
- Doklega, S.M.A.; El-Afify, S.T.M.; Ibrahim, E.A. Effect of Glycine Betaine and Chitosan on Water Stress Tolerance of Summer Squash Plants. J. Plant Prod. 2022, 13, 675–681. [Google Scholar] [CrossRef]
- Nada, M.M. Effect of foliar application with potassium silicate and glycine betaine on growth and early yield quality of strawberry plants. J. Plant Prod. 2020, 11, 1295–1302. [Google Scholar] [CrossRef]
- Duan, Y.; Han, J.; Guo, B.; Zhao, W.; Zhou, S.; Zhou, C.; Zhang, L.; Li, X.; Han, D. MbICE1 Confers Drought and Cold Tolerance through Up-Regulating Antioxidant Capacity and Stress-Resistant Genes in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 16072. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Wang, Y.; Liang, X.; Han, J.; Han, D. MbMYBC1, a M. baccata MYB transcription factor, contribute to cold and drought stress tolerance in transgenic Arabidopsis. Front. Plant Sci. 2023, 14, 1141446. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, Z.; Li, L.; Li, Q.; Geng, Z.; Liu, W.; Hou, R.; Zhang, L.; Han, D. MbWRKY50 confers cold and drought tolerance through upregulating antioxidant capacity associated with ROS scavenging. J. Plant Physiol. 2025, 310, 154526. [Google Scholar] [CrossRef]
- Li, W.; Li, P.; Chen, H.; Zhong, J.; Liang, X.; Wei, Y.; Zhang, L.; Wang, H.; Han, D. Overexpression of a Fragaria vesca 1R-MYB Transcription Factor Gene (FvMYB114) Increases Salt and Cold Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 5261. [Google Scholar] [CrossRef]
- Li, W.; Wei, Y.; Zhang, L.; Wang, Y.; Song, P.; Li, X.; Han, D. FvMYB44, a Strawberry R2R3-MYB Transcription Factor, Improved Salt and Cold Stress Tolerance in Transgenic Arabidopsis. Agronomy 2023, 13, 1051. [Google Scholar] [CrossRef]
- Ren, C.; Li, Z.; Song, P.; Wang, Y.; Liu, W.; Zhang, L.; Li, X.; Li, W.; Han, D. Overexpression of a Grape MYB Transcription Factor Gene VhMYB2 Increases Salinity and Drought Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 10743. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, L.; Dai, J.; Li, Z.; Zhang, A.; Wang, T.; Liu, W.; Li, X.; Han, D. Overexpression of a Grape WRKY Transcription Factor VhWRKY44 Improves the Resistance to Cold and Salt of Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 7437. [Google Scholar] [CrossRef]
- Bajer, D. Cuttings vs. Seeds. Forest City Plants. Available online: https://forestcityplants.com/cuttings-vs-seeds-propagation/ (accessed on 8 April 2018).
- Wagner Júnior, A.; Santos, C.E.M.; Silva, J.O.C.; Pimentel, L.D.; Bruckner, C.H.; Mazaro, S.M. Seed density of three passion fruit species in seedling emergence and initial development. Rev. Bras. Agrociência 2011, 17, 359–364. (In Portuguese) [Google Scholar]
- Blake, T.J.; Filho, W.S. Drought tolerance, growth partitioning and vigor in eucalypt seedlings and rooted cuttings. Tree Physiol. 1988, 4, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Hernández, J.A.; Aguilar, A.B.; Portillo, B.; López-Gómez, E.; Beneyto, J.M.; García-Legaz, M.F. The effect of calcium on the antioxidant enzymes from salt-treated loquat and anger plants. Funct. Plant Biol. 2003, 30, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.B.; Ferraris, J.D. Intracellular organic osmolytes: Function and regulation. J. Biol. Chem. 2008, 283, 7309–7313. [Google Scholar] [CrossRef]
- Lardos, M.; Marmagne, A.; Bonadé Bottino, N.; Caris, P.; Béal, D.; Chardon, F.; Masclaux-Daubresse, C. Discovery of the biostimulant effect of asparagine and glutamine on leaf area expansion in the presence of nitrate supply. Front. Plant Sci. 2024, 14, 1281495. [Google Scholar] [CrossRef]
- Rajendra Kumar, C.S.; Suryanarayana, T.; Reddy, A.R. DNA helix destabilization by proline and betaine: Possible role in the salinity tolerance process. FEBS Lett. 1997, 410, 201–205. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, X. Reprogramming of Plant Central Metabolism in Response to Abiotic Stresses: A Metabolomics View. Int. J. Mol. Sci. 2022, 23, 5716. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Morizono, H. Effects of drought stress on flower number in ’Summer Queen’ passion fruit. Hortic. J. 2022, 91, 448–452. [Google Scholar] [CrossRef]
- Diniz, G.L.; Nobre, R.G.; Lima, G.S.; Soares, L.A.; Gheyi, H.R. Irrigation with saline water and silicate fertilization in the cultivation of ’Gigante Amarelo’ passion fruit. Rev. Caatinga 2021, 34, 199–207. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rackova, L.; Paganová, V.; Swoczyna, T.; Rusinowski, S.; Sitko, K. Can chlorophyll-a fluorescence parameters be used as bio-indicators to distinguish between drought and salinity stress in Tilia cordata Mill? Environ. Exp. Bot. 2018, 152, 149–157. [Google Scholar] [CrossRef]
- Zhu, J.K.; Hasegawa, P.M.; Bressan, R.A. Molecular aspects of osmotic stress. Crit. Rev. Plant Sci. 1997, 16, 253–277. [Google Scholar] [CrossRef]
- Raza, M.A.S.; Saleem, M.F.; Shah, G.M.; Khan, I.H.; Raza, A. Exogenous application of glycine betaine and potassium for improving water relations and grain yield of wheat under drought. J. Soil Sci. Plant Nutr. 2014, 14, 348–364. [Google Scholar] [CrossRef]
- Fisher, A. The Impact of Glycine Betaine Applications on Drought Response in Wild Blueberries. Bachelor’s Thesis, University of Maine, Orono, ME, USA, 2022. Available online: https://digitalcommons.library.umaine.edu/honors/775 (accessed on 2 February 2025).
- Chungloo, D.; Tisarum, R.; Samphumphuang, T.; Sotesaritkul, T.; Singh, H.P.; Takabe, T.; Cha-Um, S. Mitigation of water-deficit stress, physio-morphological adaptation, and elevation of andrographolide in Andrographis paniculata using foliar glycine betaine. J. Plant Growth Regul. 2023, 42, 6273–6285. [Google Scholar] [CrossRef]
- Buckley, T.N. How do stomata respond to water status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Riederer, M.; Schreiber, L. Protecting against water loss: Analysis of cuticular transpiration. J. Exp. Bot. 2001, 52, 2023–2032. [Google Scholar] [CrossRef]
- Schönherr, J. Characterization of aqueous pores in plant cuticles and permeation of ionic solutes. J. Exp. Bot. 2006, 57, 2471–2479. [Google Scholar] [CrossRef]
- Chen, T.-H.H.; Murata, N. Glycine betaine: An effective protectant against abiotic stress in plants. Trends Plant Sci. 2008, 13, 499–505. [Google Scholar] [CrossRef]
- León, J.; Gayubas, B.; Castillo, M.C. Valine-glutamine proteins in plant responses to oxygen and nitric oxide. Front. Plant Sci. 2021, 11, 632678. [Google Scholar] [CrossRef]
- Yu, Y.; Qian, Y.; Jiang, M.; Xu, J.; Yang, J.; Zhang, T.; Pi, E. Regulation mechanisms of plant basic leucine zippers to various abiotic stresses. Front. Plant Sci. 2020, 11, 1258. [Google Scholar] [CrossRef] [PubMed]
- Ganie, S.A. Amino acids other than proline and their participation in abiotic stress tolerance. In Compatible Solutes Engineering for Crop Plants Facing Climate Change; Springer: Berlin/Heidelberg, Germany, 2021; pp. 47–96. [Google Scholar] [CrossRef]
- Shu, L.; Lou, Q.; Ma, C.; Ding, W.; Zhou, J.; Wu, J. Genetic, proteomic and metabolic analysis of the regulation of energy storage in rice seedlings in response to drought. Proteomics 2011, 11, 4122–4138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Zhou, R.; Wang, X.; Dossa, K.; Wang, L. Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in the rapid adaptive response to salt stress. BMC Plant Biol. 2019, 19, 66. [Google Scholar] [CrossRef]
- Aledo, J.C. Methionine in proteins: The Cinderella of the proteinogenic amino acids. Protein Sci. 2019, 28, 1785–1796. [Google Scholar] [CrossRef]
- Niu, T.; Zhang, J.; Li, J.; Gao, X.; Ma, H.; Gao, Y.; Xie, J. Effects of exogenous glycine betaine and cycloleucine on photosynthetic capacity, amino acid composition, and hormone metabolism in Solanum melongena L. Sci. Rep. 2023, 13, 7626. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Du, S.; Zhang, X.; Jiang, J.; Wang, G. Analysis of amino acids in the roots of Tamarix ramosissima by application of exogenous potassium (K⁺) under NaCl stress. Int. J. Mol. Sci. 2022, 23, 9331. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Li, S.; Chen, J.; Amin, A.S.; Wang, G.; Xiaojun, S.; Zain, M.; Gao, Y. Linking exogenous foliar application of glycine betaine and stomatal characteristics with salinity stress tolerance in cotton (Gossypium hirsutum L.) seedlings. BMC Plant Biol. 2021, 21, 146. [Google Scholar] [CrossRef]
- Heinemann, B.; Hildebrandt, T.M. The role of amino acid metabolism in signaling and metabolic adaptation to stress-induced energy deficiency in plants. J. Exp. Bot. 2021, 72, 4634–4645. [Google Scholar] [CrossRef]
- Naya, L.; Ladreda, R.; Ramos, J.; Gonzáles, E.M.; Arrese-Igor, C.; Minchin, F.R.; Becana, M. The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and the subsequent recovery of plants. Plant Physiol. 2007, 144, 1104–1114. [Google Scholar] [CrossRef]
- Steuer, R.; Nesi, A.N.; Fernie, A.R.; Gross, T.; Blasius, B.; Selbig, J. From structure to dynamics of metabolic pathways: Application to the plant mitochondrial TCA cycle. Bioinformatics 2007, 23, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Han, G.; Meng, Z.; Lin, L.; Sui, N. Roles of malic enzymes in plant development and stress responses. Plant Signal. Behav. 2019, 14, e1644596. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative metabolomic profiling in the roots and leaves in contrasting genotypes reveals complex mechanisms involved in post-anthesis drought tolerance in wheat. PLoS ONE 2019, 14, e0213502. [Google Scholar] [CrossRef]
- Fernández de Simón, B.; Cadahía, E.; Aranda, I. Aerial and underground organs display specific metabolic strategies to cope with water stress under rising atmospheric CO2; in Fagus sylvatica L. Physiol. Plant. 2022, 174, e13711. [Google Scholar] [CrossRef]
- Li, Z.; Huang, T.; Tang, M.; Cheng, B.; Peng, Y.; Zhang, X. iTRAQ-based proteomics reveals the key role of γ-aminobutyric acid (GABA) in regulating drought tolerance in perennial creeping bentgrass (Agrostis stolonifera). Plant Physiol. Biochem. 2019, 145, 216–226. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Seyyedi, S.M.; Ebrahimian, E.; Moghaddam, S.S.; Damalas, C.A. Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa L.). Ind. Crops Prod. 2018, 112, 741–748. [Google Scholar] [CrossRef]
- Guo, L.X.; Shi, C.Y.; Liu, X.; Ning, D.Y.; Jing, L.F.; Yang, H.; Liu, Y.Z. Citrate accumulation-related gene expression and/or enzyme activity analysis combined with metabolomics provides a novel insight for an orange mutant. Sci. Rep. 2016, 6, 29343. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010, 5, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Yamada, N.; Cha-um, S. Foliar application of glycine betaine regulates soluble sugars and maintains physiological adaptation in sweet potato (Ipomoea batatas L.) under water-deficit conditions. Plant Prod. Sci. 2020, 23, 482–494. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, M.; Shahid, M.A.; Babar, M.D.A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Carrow, R.N.; Duncan, R.R. Identification of new soluble sugars accumulated in a halophytic turfgrass (seashore paspalum) under salinity stress. Environ. Exp. Bot. 2008, 63, 198–206. [Google Scholar]


| Metabolite Concentration (μmol/g) | |||||||
|---|---|---|---|---|---|---|---|
| Leucine | Valine | Succinic Acid | Malic Acid | Threitol | |||
| Treatments | Cutting | D0GB0 | 1.04 ± 0.09 b | 0.81 ± 0.07 b | 0.35 ± 0.01 e | 65.51 ± 1.64 f | 0.90 ± 0.02 c |
| D0GB1 | 1.15 ± 0.11 a | 0.91 ± 0.05 b | 1.14 ± 0.11 a | 133.70 ± 11.72 c | 0.55 ± 0.04 f | ||
| D1GB0 | 0.08 ± 0.02 e | 0.03 ± 0.02 g | 0.81 ± 0.08 b | 93.57 ± 3.83 e | 0.66 ± 0.05 d | ||
| D1GB1 | 0.67 ± 0.05 c | 0.50 ± 0.05 d | 0.48 ± 0.04 e | 164.09 ± 5 b | 1.16 ± 0.06 b | ||
| D2GB0 | 0.01 ± 0.00 e | 0 ± 0 g | 0.33 ± 0.05 f | 65.02 ± 14.06 f | 1.29 ± 0.05 a | ||
| D2GB1 | 0.34 ± 0.03 d | 0.21 ± 0.04 f | 0.54 ± 0.05 d | 108.27 ± 6.82 d | 1.08 ± 0.13 b | ||
| Seedling | D0GB0 | 0.96 ± 0.04 b | 0.30 ± 0.06 e | 0.33 ± 0.01 f | 113.71 ± 3.9 d | 0.45 ± 0.02 f | |
| D0GB1 | 1.32 ± 0.02 a | 1.17 ± 0.02 a | 0.80 ± 0.08 b | 172.97 ± 3.83 b | 0.61 ± 0.05 e | ||
| D1GB0 | 1.05 ± 0.08 a | 0.63 ± 0.02 c | 0.64 ± 0.11c | 175.81 ± 7.74 b | 0.42 ± 0.06 f | ||
| D1GB1 | 1.41 ± 0.11 a | 0.93 ± 0.03 a | 0.87 ± 0.06 a | 231.75 ± 2.63 a | 0.37 ± 0.05 f | ||
| D2GB0 | 0.64 ± 0.12 c | 0.62 ± 0.05 c | 0.58 ± 0.02 c | 89.5 ± 7.60 e | 0.94 ± 0.09 c | ||
| D2GB1 | 0.52 ± 0.08 d | 0.64 ± 0.08 c | 0.99 ± 0.11 a | 187.22 ± 6.91 b | 1.57 ± 0.10 a | ||
| Aminobutyl | Citric Acid | Glucose | Proline | ||||
| Cutting | D0GB0 | 2.27 ± 0.36 e | 11.89 ± 0.83 d | 84.01 ± 0.99 c | 14.35 ± 1.46 c | ||
| D0GB1 | 6.64 ± 0.45 b | 58.51 ± 4.27 a | 72.24 ± 4.19 d | 20.09 ± 1.38 b | |||
| D1GB0 | 0.32 ± 0.14 e | 21.16 ± 1.59 c | 87.53 ± 10.18 b | 0 ± 0 e | |||
| D1GB1 | 4.94 ± 0.55 c | 9.69 ± 0.42 e | 99.43 ± 6.93 b | 0 ± 0 e | |||
| D2GB0 | 0 ± 0 f | 0.51 ± 0.06 f | 91.06 ± 0.99 b | 0 ± 0 e | |||
| D2GB1 | 5.19 ± 0.64 c | 16.20 ± 1.13 d | 65.44 ± 4.85 e | 0 ± 0 e | |||
| Seedling | D0GB0 | 2.24 ± 0.13 e | 27.41 ± 1.37 b | 60.67 ± 3.41 f | 0 ± 0 e | ||
| D0GB1 | 9.41 ± 0.14 a | 33.29 ± 1.59 b | 145.24 ± 10.18 a | 31.42 ± 2 a | |||
| D1GB0 | 5.90 ± 0.38 b | 49.14 ± 3.37 a | 107.00 ± 3.66 b | 11.84 ± 2.34 c | |||
| D1GB1 | 8.87 ± 0.31 a | 32.89 ± 3.46 b | 142.00 ± 5.79 a | 30.57 ± 2.90 a | |||
| D2GB0 | 4.50 ± 0.55 d | 11.56 ± 0.45 d | 69.25 ± 2.75 d | 7.15 ± 0.9 d | |||
| D2GB1 | 7.74 ± 0.40 a | 20.46 ± 3.81 c | 110.42 ± 8.28 b | 0 e | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, L.d.A.; Nguyen, N.T.T.; Habibi, N.; Dabirimirhosseinloo, M.; Terada, N.; Sanada, A.; Koshio, K. Exogenous Application of Glycine Betaine to Passiflora edulis Sims f. flavicarpa to Mitigate Drought Stress on Two Propagation Methods. Int. J. Mol. Sci. 2025, 26, 8734. https://doi.org/10.3390/ijms26178734
Oliveira LdA, Nguyen NTT, Habibi N, Dabirimirhosseinloo M, Terada N, Sanada A, Koshio K. Exogenous Application of Glycine Betaine to Passiflora edulis Sims f. flavicarpa to Mitigate Drought Stress on Two Propagation Methods. International Journal of Molecular Sciences. 2025; 26(17):8734. https://doi.org/10.3390/ijms26178734
Chicago/Turabian StyleOliveira, Leonardo de Almeida, Nga Thi Thu Nguyen, Nasratullah Habibi, Maryam Dabirimirhosseinloo, Naoki Terada, Atsushi Sanada, and Kaihei Koshio. 2025. "Exogenous Application of Glycine Betaine to Passiflora edulis Sims f. flavicarpa to Mitigate Drought Stress on Two Propagation Methods" International Journal of Molecular Sciences 26, no. 17: 8734. https://doi.org/10.3390/ijms26178734
APA StyleOliveira, L. d. A., Nguyen, N. T. T., Habibi, N., Dabirimirhosseinloo, M., Terada, N., Sanada, A., & Koshio, K. (2025). Exogenous Application of Glycine Betaine to Passiflora edulis Sims f. flavicarpa to Mitigate Drought Stress on Two Propagation Methods. International Journal of Molecular Sciences, 26(17), 8734. https://doi.org/10.3390/ijms26178734






