Polynucleotides Enhance Collagen Synthesis via Modulating Phosphoenolpyruvate Carboxykinase 1 in Senescent Macrophages: Experimental Evidence
Abstract
1. Introduction
2. Results
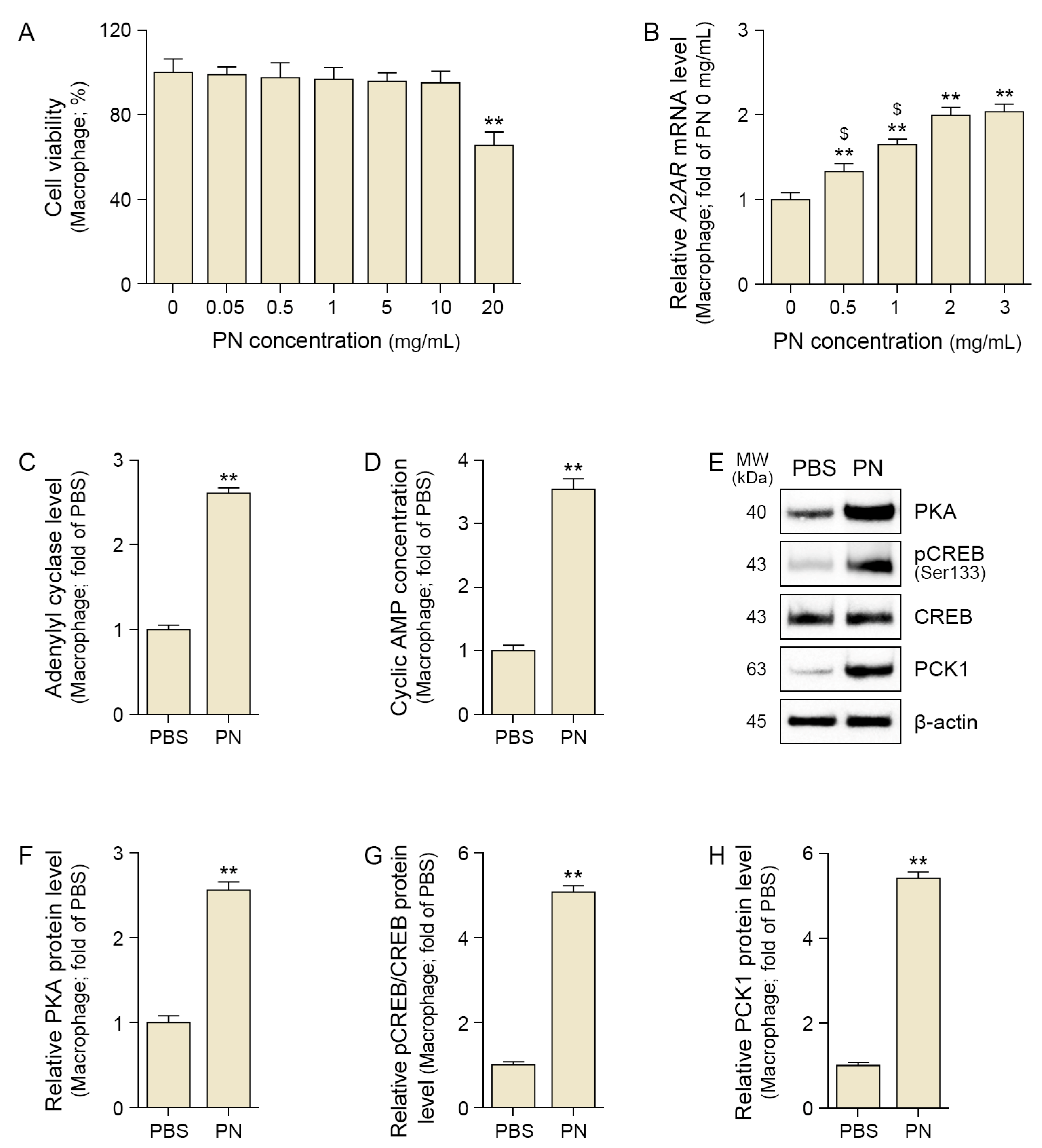
2.1. PN Increased A2AR, CREB, and PCK1 in the Senescent Macrophage
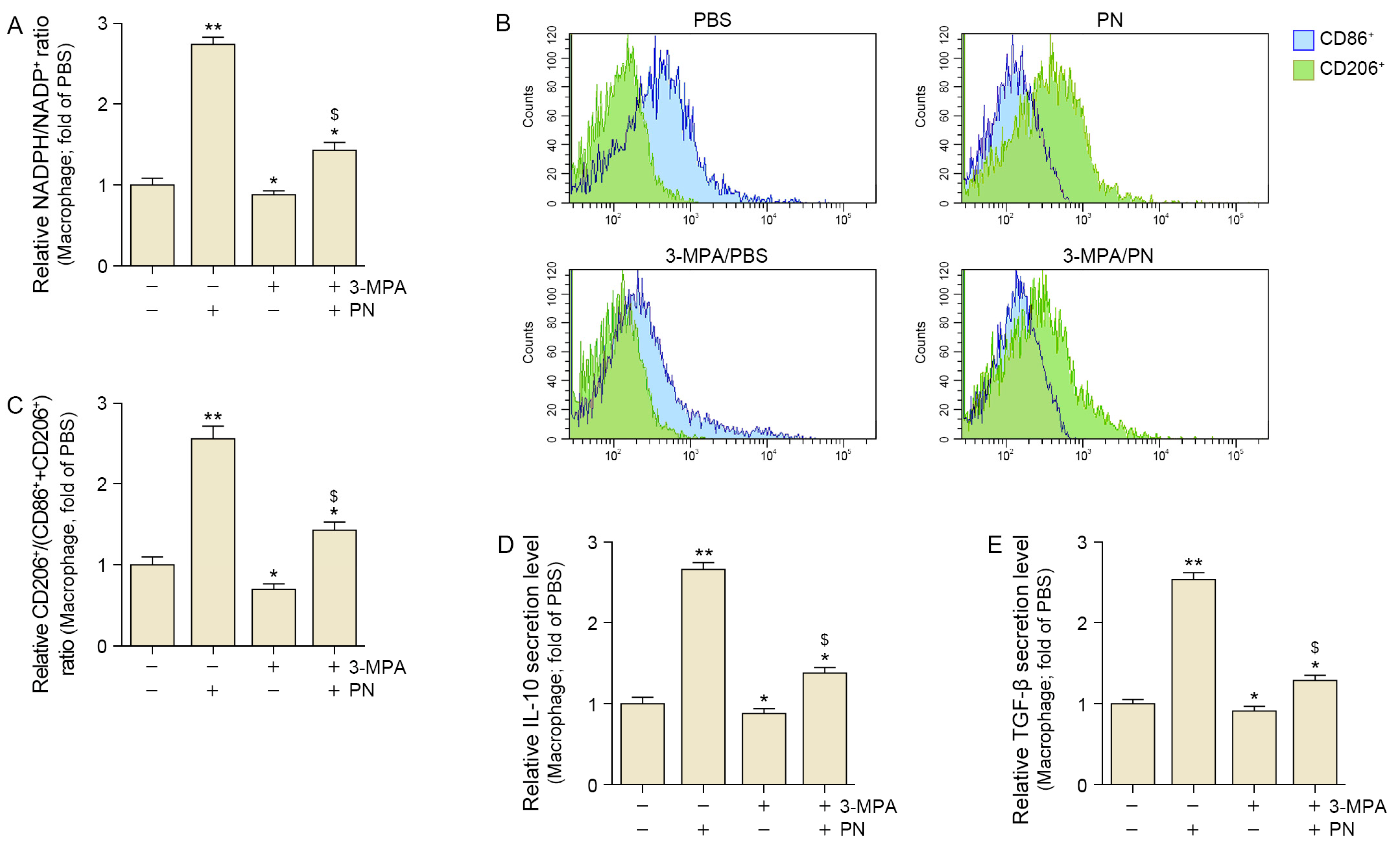
2.2. PN Decreased Oxidative Stress and Increased M2 Polarization in the Senescent Macrophages
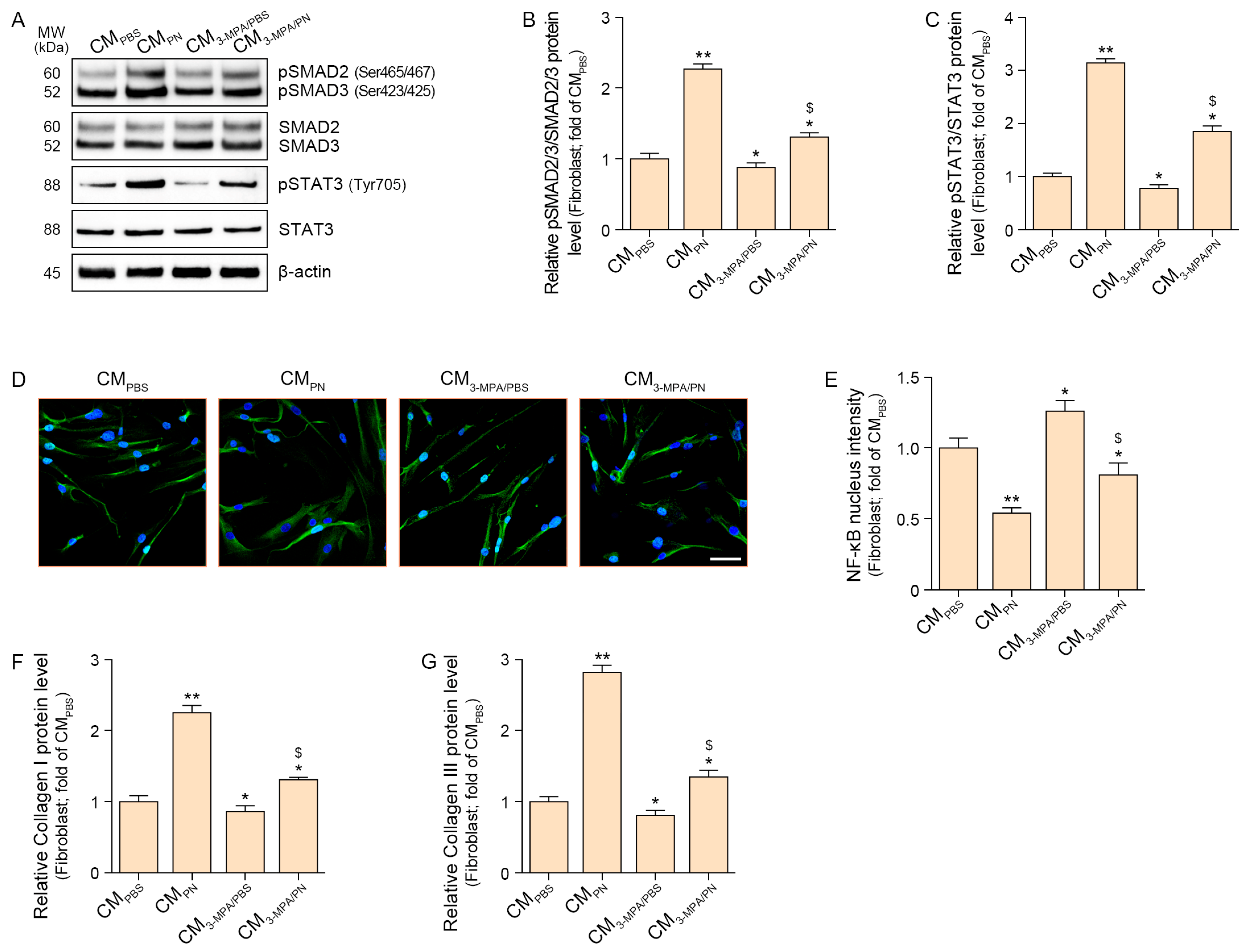
2.3. PN Increased SMAD2/3 and STAT3 Which Increased Collagen in the Fibroblasts
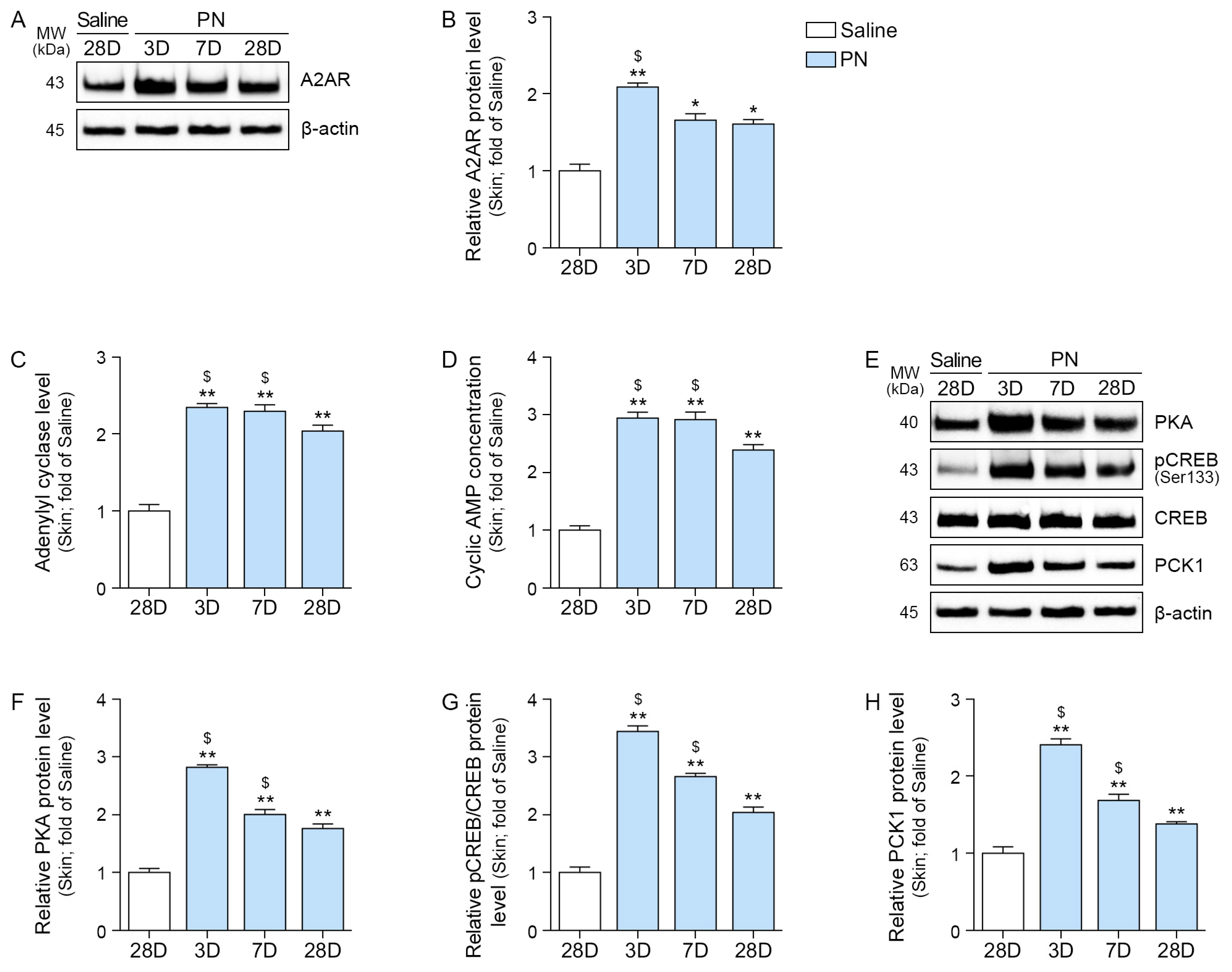
2.4. PN Increased A2AR, AC, PKA, CREB, and PCK1 in the Aged Skin
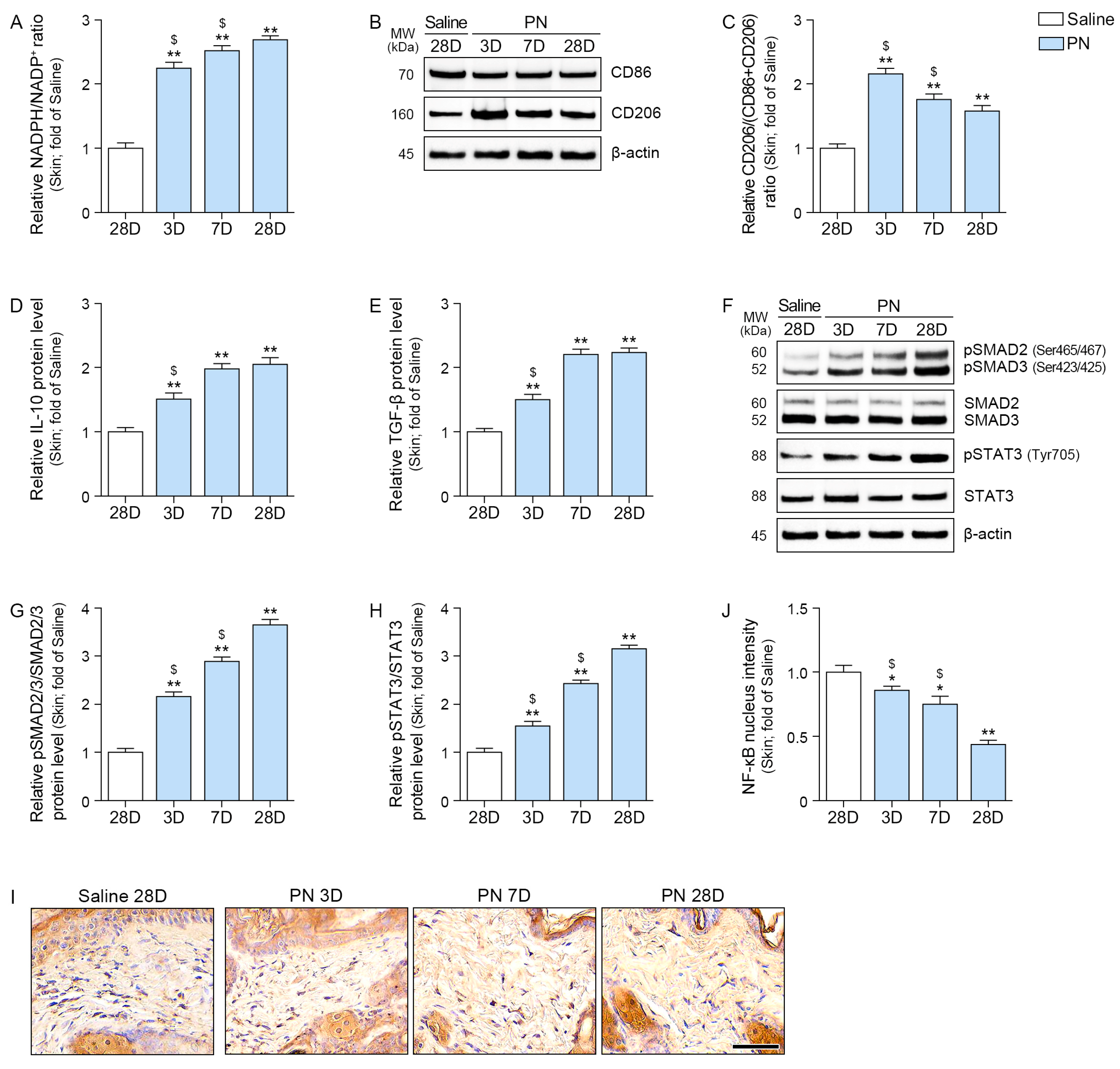
2.5. PN Decreased Oxidative Stress and Increased M2, IL-10, TGF-β, SMAD2/3, and STAT3 in the Aged Skin
2.6. PN Increased Collagen Density in the Aged Skin
3. Discussion
4. Materials and Methods
4.1. PN Preparation
4.2. In Vitro Experiments
4.2.1. Cell Culture
4.2.2. Experimental Design for PN Treatment
4.2.3. PCK1 Inhibition
4.3. Cell Viability Assessment
4.4. Quantitative Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.5. Flow Cytometry
4.6. In Vivo Experiments
4.6.1. Mouse Model and Maintenance
4.6.2. Experimental Design for PN Injections
4.6.3. Skin Elasticity
4.7. Sample Preparation
4.7.1. Protein Isolation and Concentration Quantitation
4.7.2. Paraffin-Embedded Skin Tissue Blocks
4.8. Enzyme-Linked Immunosorbent Assay
4.9. Western Blotting
4.10. Staining
4.10.1. Immunocytochemistry
4.10.2. Immunohistochemistry
4.10.3. Masson Trichrome Staining
4.10.4. Herovici Staining
4.11. Quantitative and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharmacol. 2017, 8, 224, Corrigendum in Front. Pharmacol. 2022, 13, 1073510. [Google Scholar] [CrossRef]
- Kim, T.H.; Heo, S.Y.; Oh, G.W.; Heo, S.J.; Jung, W.K. Applications of Marine Organism-Derived Polydeoxyribonucleotide: Its Potential in Biomedical Engineering. Mar. Drugs 2021, 19, 296. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Porcello, A.; Cerrano, M.; Hadjab, F.; Chemali, M.; Lourenço, K.; Hadjab, B.; Raffoul, W.; Applegate, L.A.; Laurent, A.E. From Polydeoxyribonucleotides (PDRNs) to Polynucleotides (PNs): Bridging the Gap Between Scientific Definitions, Molecular Insights, and Clinical Applications of Multifunctional Biomolecules. Biomolecules 2025, 15, 148. [Google Scholar] [CrossRef]
- Lee, K.W.A.; Chan, K.W.L.; Lee, A.; Lee, C.H.; Wan, J.; Wong, S.; Yi, K.-H. Polynucleotides in Aesthetic Medicine: A Review of Current Practices and Perceived Effectiveness. Int. J. Mol. Sci. 2024, 25, 8224. [Google Scholar] [CrossRef]
- Montesinos, M.C.; Gadangi, P.; Longaker, M.; Sung, J.; Levine, J.; Nilsen, D.; Reibman, J.; Li, M.; Jiang, C.K.; Hirschhorn, R.; et al. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J. Exp. Med. 1997, 186, 1615–1620. [Google Scholar] [CrossRef]
- Ko, I.G.; Jin, J.J.; Hwang, L.; Kim, S.H.; Kim, C.J.; Jeon, J.W.; Chung, J.Y.; Han, J.H. Adenosine A2A receptor agonist polydeoxyribonucleotide ameliorates short-term memory impairment by suppressing cerebral ischemia-induced inflammation via MAPK pathway. PLoS ONE 2021, 16, e0248689. [Google Scholar] [CrossRef]
- Hwang, L.; Jin, J.J.; Ko, I.G.; Kim, S.; Cho, Y.A.; Sung, J.S.; Choi, C.W.; Chang, B.S. Polydeoxyribonucleotide Attenuates Airway Inflammation Through A2AR Signaling Pathway in PM10-Exposed Mice. Int. Neurourol. J. 2021, 25, S19–S26. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. Polydeoxyribonucleotide regulation of inflammation. Adv. Wound Care 2020, 9, 576–589. [Google Scholar] [CrossRef]
- Castellini, C.; Belletti, S.; Govoni, P.; Guizzardi, S. Anti inflammatory property of PDRN—An in vitro study on cultured macrophages. Adv. Biosci. Biotechnol. 2017, 8, 13–26. [Google Scholar] [CrossRef]
- Imai, E.; Miner, J.N.; Mitchell, J.A.; Yamamoto, K.R.; Granner, D.K. Glucocorticoid receptor-cAMP response element-binding protein interaction and the response of the phosphoenolpyruvate carboxykinase gene to glucocorticoids. J. Biol. Chem. 1993, 268, 5353–5356. [Google Scholar] [CrossRef]
- Janah, L.; Kjeldsen, S.; Galsgaard, K.D.; Winther-Sørensen, M.; Stojanovska, E.; Pedersen, J.; Knop, F.K.; Holst, J.J.; Albrechtsen, N.J.W. Glucagon Receptor Signaling and Glucagon Resistance. Int. J. Mol. Sci. 2019, 20, 3314. [Google Scholar] [CrossRef]
- Tuo, L.; Xiang, J.; Pan, X.; Gao, Q.; Zhang, G.; Yang, Y.; Liang, L.; Xia, J.; Wang, K.; Tang, N. PCK1 Downregulation Promotes TXNRD1 Expression and Hepatoma Cell Growth via the Nrf2/Keap1 Pathway. Front. Oncol. 2018, 8, 611. [Google Scholar] [CrossRef]
- Ko, C.W.; Counihan, D.; Wu, J.; Hatzoglou, M.; Puchowicz, M.A.; Croniger, C.M. Macrophages with a deletion of the phosphoenolpyruvate carboxykinase 1 (Pck1) gene have a more proinflammatory phenotype. J. Biol. Chem. 2018, 293, 3399–3409. [Google Scholar] [CrossRef]
- Mia, S.; Warnecke, A.; Zhang, X.M.; Malmström, V.; Harris, R.A. An optimized protocol for human M2 macrophages using M-CSF and IL-4/IL-10/TGF-β yields a dominant immunosuppressive phenotype. Scand. J. Immunol. 2014, 79, 305–314. [Google Scholar] [CrossRef]
- Verrecchia, F.; Chu, M.L.; Mauviel, A. Identification of novel TGF-beta /Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem. 2001, 276, 17058–17062. [Google Scholar] [CrossRef]
- Wang, P.; Wu, P.; Siegel, M.I.; Egan, R.W.; Billah, M.M. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J. Biol. Chem. 1995, 270, 9558–9563. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Nishinakamura, H.; Minoda, Y.; Saeki, K.; Koga, K.; Takaesu, G.; Onodera, M.; Yoshimura, A.; Kobayashi, T. An RNA-binding protein alphaCP-1 is involved in the STAT3-mediated suppression of NF-kappaB transcriptional activity. Int. Immunol. 2007, 19, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Bond, M.; Baker, A.H.; Newby, A.C. Nuclear factor kappaB activity is essential for matrix metalloproteinase-1 and-3 upregulation in rabbit dermal fibroblasts. Biochem. Biophys. Res. Commun. 1999, 264, 561–567. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Oh, S.; Rho, N.K.; Byun, K.A.; Yang, J.Y.; Sun, H.J.; Jang, M.; Kang, D.; Son, K.H.; Byun, K. Combined Treatment of Monopolar and Bipolar Radiofrequency Increases Skin Elasticity by Decreasing the Accumulation of Advanced Glycated End Products in Aged Animal Skin. Int. J. Mol. Sci. 2022, 23, 2993. [Google Scholar] [CrossRef]
- Wiegand, C.; Raschke, C.; Elsner, P. Skin Aging: A Brief Summary of Characteristic Changes. In Textbook of Aging Skin; Springer: Berlin/Heidelberg, Germany, 2017; pp. 55–65. [Google Scholar]
- Khan, A.; Wang, G.; Zhou, F.; Gong, L.; Zhang, J.; Qi, L.; Cui, H. Polydeoxyribonucleotide: A promising skin anti-aging agent. Chin. J. Plast. Reconstr. Surg. 2022, 4, 187–193. [Google Scholar] [CrossRef]
- Yi, K.H.; Winayanuwattikun, W.; Kim, S.Y.; Wan, J.; Vachatimanont, V.; Putri, A.I.; Hidajat, I.J.; Yogya, Y.; Pamela, R. Skin boosters: Definitions and varied classifications. Skin. Res. Technol. 2024, 30, e13627. [Google Scholar] [CrossRef]
- Koju, N.; Qin, Z.H.; Sheng, R. Reduced nicotinamide adenine dinucleotide phosphate in redox balance and diseases: A friend or foe? Acta Pharmacol. Sin. 2022, 43, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Herovici, C. Picropolychrome: Histological staining technic intended for the study of normal and pathological connective tissue. Rev. Fr. D’etudes Clin. Biol. 1963, 8, 88–89. [Google Scholar]
- Anthony, P.P. Manual of histological demonstration techniques. J. Clin. Pathol. 1975, 28, 339. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.L.; Nguyen, V.B. Recent advances on polydeoxyribonucleotide extraction and its novel application in cosmeceuticals. Int. J. Biol. Macromol. 2024, 282, 137051. [Google Scholar] [CrossRef]
- Zorina, A.; Zorin, V.; Isaev, A.; Kudlay, D.; Vasileva, M.; Kopnin, P. Dermal Fibroblasts as the Main Target for Skin Anti-Age Correction Using a Combination of Regenerative Medicine Methods. Curr. Issues Mol. Biol. 2023, 45, 3829–3847. [Google Scholar] [CrossRef] [PubMed]
- Boraldi, F.; Lofaro, F.D.; Bonacorsi, S.; Mazzilli, A.; Garcia-Fernandez, M.; Quaglino, D. The Role of Fibroblasts in Skin Homeostasis and Repair. Biomedicines 2024, 12, 1586. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Wu, Y.T.; Ru, Z.Q.; Peng, Y.; Fu, Z.; Jia, Q.Y.; Kang, Z.J.; Li, Y.S.; Huang, Y.B.; Yin, S.G.; Guo, K.; et al. Peptide Cy RL-QN15 accelerates hair regeneration in diabetic mice by binding to the Frizzled-7 receptor. Zool. Res. 2024, 45, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, Y.; Fu, Z.; Ji, Y.; Yan, J.; Kong, Y.; Peng, Y.; Ru, Z.; Huang, Y.; Li, Y.; et al. The direct binding of bioactive peptide Andersonin-W1 to TLR4 expedites the healing of diabetic skin wounds. Cell Mol. Biol. Lett. 2024, 29, 24. [Google Scholar] [CrossRef]
- Jia, Q.; Fu, Z.; Li, Y.; Kang, Z.; Wu, Y.; Ru, Z.; Peng, Y.; Huang, Y.; Luo, Y.; Li, W.; et al. Hydrogel Loaded with Peptide-Containing Nanocomplexes: Symphonic Cooperation of Photothermal Antimicrobial Nanoparticles and Prohealing Peptides for the Treatment of Infected Wounds. ACS Appl. Mater. Interfaces 2024, 16, 13422–13438. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, Q.; Liu, N.; Yin, S.; Wang, J.; Ding, Y.; Yang, Y.; Peng, Y.; Ru, Z.; Zhang, S.; et al. Peptide RL-QN15 Regulates Functions of Epidermal Stem Cells to Accelerate Skin Wound Regeneration via the FZD8/β-Catenin Axis. Exploration 2025. [Google Scholar] [CrossRef]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calò, M.; Cascio, P.L.; D’ALcontres, F.S.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair. Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The role of the transcription factor CREB in immune function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef]
- Luan, B.; Yoon, Y.S.; Le Lay, J.; Kaestner, K.H.; Hedrick, S.; Montminy, M. CREB pathway links PGE2 signaling with macrophage polarization. Proc. Natl. Acad. Sci. USA 2015, 112, 15642–15647. [Google Scholar] [CrossRef]
- Bitto, A.; Polito, F.; Irrera, N.; D’AScola, A.; Avenoso, A.; Nastasi, G.; Campo, G.M.; Micali, A.; Bagnato, G.; Minutoli, L.; et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A2A receptor. Arthritis Rheum. 2011, 63, 3364–3371. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef]
- Li, R.; Feng, D.; Han, S.; Zhai, X.; Yu, X.; Fu, Y.; Jin, F. Macrophages and fibroblasts in foreign body reactions: How mechanical cues drive cell functions? Mater. Today Bio 2023, 22, 100783. [Google Scholar] [CrossRef]
- Kelly, A.; Gunaltay, S.; McEntee, C.P.; Shuttleworth, E.E.; Smedley, C.; Houston, S.A.; Fenton, T.M.; Levison, S.; Mann, E.R.; Travis, M.A. Human monocytes and macrophages regulate immune tolerance via integrin αvβ8-mediated TGFβ activation. J. Exp. Med. 2018, 215, 2725–2736. [Google Scholar] [CrossRef]
- Perez-Aso, M.; Fernandez, P.; Mediero, A.; Chan, E.S.; Cronstein, B.N. Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 2014, 28, 802–812. [Google Scholar] [CrossRef]
- Pacheco, N.C.S.; de Almeida, A.P.C.; de Siqueira, K.C.; de Lima, F.M.; Reis, S.R.D.L.; Latorraca, M.Q.; Stoppiglia, L.F. Nutritional recovery with a soybean diet impaired the glucagon response but did not alter liver gluconeogenesis in the adult offspring of rats deprived of protein during pregnancy and lactation. Appl. Physiol. Nutr. Metab. 2019, 44, 13–21. [Google Scholar] [CrossRef]
- Kwon, S.M.; Hong, S.M.; Lee, Y.K.; Min, S.; Yoon, G. Metabolic features and regulation in cell senescence. BMB Rep. 2019, 52, 5–12. [Google Scholar] [CrossRef]
- Liu, M.X.; Jin, L.; Sun, S.J.; Liu, P.; Feng, X.; Cheng, Z.L.; Liu, W.R.; Guan, K.L.; Shi, Y.H.; Yuan, H.X.; et al. Metabolic reprogramming by PCK1 promotes TCA cataplerosis, oxidative stress and apoptosis in liver cancer cells and suppresses hepatocellular carcinoma. Oncogene 2018, 37, 1637–1653. [Google Scholar] [CrossRef]
- Oh, S.; Jeon, H.D.; Rho, N.K.; Son, K.H.; Byun, K. Polynucleotide Mixture Attenuates Ultraviolet B-Induced Skin Pigmentation. Int. J. Mol. Sci. 2025, 26, 6399. [Google Scholar] [CrossRef] [PubMed]
- Phuangbubpha, P.; Thara, S.; Sriboonaied, P.; Saetan, P.; Tumnoi, W.; Charoenpanich, A. Optimizing THP-1 Macrophage Culture for an Immune-Responsive Human Intestinal Model. Cells 2023, 12, 1427. [Google Scholar] [CrossRef]
- Hu, S.; Li, R.; Gong, D.; Hu, P.; Xu, J.; Ai, Y.; Zhao, X.; Hu, C.; Xu, M.; Liu, C.; et al. Atf3-mediated metabolic reprogramming in hepatic macrophage orchestrates metabolic dysfunction-associated steatohepatitis. Sci. Adv. 2024, 10, eado3141. [Google Scholar] [CrossRef] [PubMed]
- Brearley, M.C.; Daniel, Z.C.T.R.; Loughna, P.T.; Parr, T.; Brameld, J.M. The phosphoenolpyruvate carboxykinase (PEPCK) inhibitor, 3-mercaptopicolinic acid (3-MPA), induces myogenic differentiation in C2C12 cells. Sci. Rep. 2020, 10, 22177. [Google Scholar] [CrossRef] [PubMed]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Baidoo, N.; Crawley, E.; Knowles, C.H.; Sanger, G.J.; Belai, A. Total collagen content and distribution is increased in human colon during advancing age. PLoS ONE 2022, 17, e0269689. [Google Scholar] [CrossRef] [PubMed]
- Umair, Z.; Baek, M.O.; Song, J.; An, S.; Chon, S.J.; Yoon, M.S. MicroRNA-4516 in Urinary Exosomes as a Biomarker of Premature Ovarian Insufficiency. Cells 2022, 11, 2797. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Q.; Xu, C.B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by ImageJ software. Int. J. Clin. Exp. Med. 2017, 10, 14904–14910. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Byun, K.-A.; Park, H.J.; Oh, S.; Son, K.H.; Byun, K. Polynucleotides Enhance Collagen Synthesis via Modulating Phosphoenolpyruvate Carboxykinase 1 in Senescent Macrophages: Experimental Evidence. Int. J. Mol. Sci. 2025, 26, 8720. https://doi.org/10.3390/ijms26178720
Byun K-A, Park HJ, Oh S, Son KH, Byun K. Polynucleotides Enhance Collagen Synthesis via Modulating Phosphoenolpyruvate Carboxykinase 1 in Senescent Macrophages: Experimental Evidence. International Journal of Molecular Sciences. 2025; 26(17):8720. https://doi.org/10.3390/ijms26178720
Chicago/Turabian StyleByun, Kyung-A, Hyun Jun Park, Seyeon Oh, Kuk Hui Son, and Kyunghee Byun. 2025. "Polynucleotides Enhance Collagen Synthesis via Modulating Phosphoenolpyruvate Carboxykinase 1 in Senescent Macrophages: Experimental Evidence" International Journal of Molecular Sciences 26, no. 17: 8720. https://doi.org/10.3390/ijms26178720
APA StyleByun, K.-A., Park, H. J., Oh, S., Son, K. H., & Byun, K. (2025). Polynucleotides Enhance Collagen Synthesis via Modulating Phosphoenolpyruvate Carboxykinase 1 in Senescent Macrophages: Experimental Evidence. International Journal of Molecular Sciences, 26(17), 8720. https://doi.org/10.3390/ijms26178720





