DNA Vaccines in the Post-mRNA Era: Engineering, Applications, and Emerging Innovations
Abstract
1. Introduction
2. Key Technological Advancements
3. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haelle, T. The Staggering Success of Vaccines. Nature 2024, 634, S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838, Erratum in Nat. Rev. Drug Discov. 2021, 20, 880. [Google Scholar] [CrossRef]
- Uddin, M.N.; Roni, M.A. Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines 2021, 9, 1033. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247 Pt 1, 1465–1468. [Google Scholar] [CrossRef]
- Agarwal, V.; Kelley, D.R. The genetic and biochemical determinants of mRNA degradation rates in mammals. Genome Biol. 2022, 23, 245. [Google Scholar] [CrossRef]
- Lu, B.; Lim, J.M.; Yu, B.; Song, S.; Neeli, P.; Sobhani, N.; Pavithra, K.; Bonam, S.R.; Kurapati, R.; Zheng, J.; et al. The next-generation DNA vaccine platforms and delivery systems: Advances, challenges and prospects. Front. Immunol. 2024, 15, 1332939. [Google Scholar] [CrossRef] [PubMed]
- Il’in, K.V. The antigenic properties of DNA. Bull. Exp. Biol. Med. 1966, 61, 567–569. [Google Scholar] [CrossRef]
- Ito, Y. A tumor-producing factor extracted by phenol from papillomatous tissue (Shope) of cottontail rabbits. Virology 1960, 12, 596–601. [Google Scholar] [CrossRef]
- Yankauckas, M.A.; Morrow, J.E.; Parker, S.E.; Abai, A.; Rhodes, G.H.; Dwarki, V.J.; Gromkowski, S.H. Long-term anti-nucleoprotein cellular and humoral immunity is induced by intramuscular injection of plasmid DNA containing NP gene. DNA Cell Biol. 1993, 12, 771–776. [Google Scholar] [CrossRef]
- MacGregor, R.R.; Boyer, J.D.; Ugen, K.E.; Lacy, K.E.; Gluckman, S.J.; Bagarazzi, M.L.; Chattergoon, M.A.; Baine, Y.; Higgins, T.J.; Ciccarelli, R.B. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: Safety and host response. J. Infect. Dis. 1998, 178, 92–100. [Google Scholar] [CrossRef]
- Wang, R.; Doolan, D.L.; Le, T.P.; Hedstrom, R.C.; Coonan, K.M.; Charoenvit, Y.; Jones, T.R.; Hobart, P.; Margalith, M.; Ng, J. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science 1998, 282, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Fomsgaard, A.; Liu, M.A. The key role of nucleic acid vaccines for one health. Viruses 2021, 13, 258. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Hu, Y.; Liang, Q.; Wei, M.; Zhu, F. Ebola vaccines in clinical trial: The promising candidates. Hum. Vaccines Immunother. 2017, 13, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Tebas, P.; Roberts, C.C.; Muthumani, K.; Reuschel, E.L.; Kudchodkar, S.B.; Zaidi, F.I.; White, S.; Khan, A.S.; Racine, T.; Choi, H. Safety and immunogenicity of an anti–Zika virus DNA vaccine. N. Engl. J. Med. 2021, 385, e35. [Google Scholar] [CrossRef]
- Yoon, I.-K.; Kim, J.H. First clinical trial of a MERS coronavirus DNA vaccine. Lancet Infect. Dis. 2019, 19, 924–925. [Google Scholar] [CrossRef]
- Sheridan, C. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat. Biotechnol. 2021, 39, 1479–1482. [Google Scholar] [CrossRef]
- Stenler, S.; Blomberg, P.; Smith, C.I. Safety and efficacy of DNA vaccines: Plasmids vs. minicircles. Hum. Vaccines Immunother. 2014, 10, 1306–1308. [Google Scholar] [CrossRef]
- Nitika; Wei, J.; Hui, A.M. The Delivery of mRNA Vaccines for Therapeutics. Life 2022, 12, 1254. [Google Scholar] [CrossRef]
- Gary, E.N.; Weiner, D.B. DNA vaccines: Prime time is now. Curr. Opin. Immunol. 2020, 65, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Swingle, K.L.; Hamilton, A.G.; Mitchell, M.J. Lipid Nanoparticle-Mediated Delivery of mRNA Therapeutics and Vaccines. Trends Mol. Med. 2021, 27, 616–617. [Google Scholar] [CrossRef]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Kozak, M.; Hu, J. DNA Vaccines: Their Formulations, Engineering and Delivery. Vaccines 2024, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Asakura, Y.; Liu, L.J.; Shono, N.; Hinkula, J.; Kjerrström, A.; Aoki, I.; Okuda, K.; Wahren, B.; Fukushima, J. Th1-biased immune responses induced by DNA-based immunizations are mediated via action on professional antigen-presenting cells to up-regulate IL-12 production. Clin. Exp. Immunol. 2000, 119, 130–139. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- Neeli, P.; Chai, D.; Wang, X.; Sobhani, N.; Udeani, G.; Li, Y. Comparison of DNA vaccines with AddaS03 as an adjuvant and an mRNA vaccine against SARS-CoV-2. iScience 2024, 27, 110969. [Google Scholar] [CrossRef]
- Xie, C.; Yao, R.; Xia, X. The advances of adjuvants in mRNA vaccines. npj Vaccines 2023, 8, 162. [Google Scholar] [CrossRef]
- Li, L.; Petrovsky, N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Neeli, P.; Maza, P.; Chai, D.; Zhao, D.; Hoi, X.P.; Chan, K.S.; Young, K.H.; Li, Y. DNA vaccines against GPRC5D synergize with PD-1 blockade to treat multiple myeloma. npj Vaccines 2024, 9, 180. [Google Scholar] [CrossRef]
- Liao, H.-C.; Liu, S.-J. Advances in nucleic acid-based cancer vaccines. J. Biomed. Sci. 2025, 32, 10. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Dean, D.A. Electroporation-mediated gene delivery. Adv. Genet. 2015, 89, 49–88. [Google Scholar] [CrossRef]
- Rols, M.P. Electropermeabilization, a physical method for the delivery of therapeutic molecules into cells. Biochim. Biophys. Acta 2006, 1758, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Hannaman, D. Electroporation Based TriGrid™ Delivery System (TDS) for DNA Vaccine Administration. In Gene Vaccines; Thalhamer, J., Weiss, R., Scheiblhofer, S., Eds.; Springer: Vienna, Austria, 2012; pp. 163–181. [Google Scholar]
- Gharia, A.A.; Bradfield, C.J.; Jenkins, E.P.W.; Fraser, I.D.C.; Malliaras, G.G. Efficient electroporation in primary cells with PEDOT:PSS electrodes. Sci. Adv. 2024, 10, eado5042. [Google Scholar] [CrossRef] [PubMed]
- Mau, T.; Amin, M.R.; Belafsky, P.C.; Best, S.R.; Friedman, A.D.; Klein, A.M.; Lott, D.G.; Paniello, R.C.; Pransky, S.M.; Saba, N.F.; et al. Interim Results of a Phase 1/2 Open-Label Study of INO-3107 for HPV-6 and/or HPV-11-Associated Recurrent Respiratory Papillomatosis. Laryngoscope 2023, 133, 3087–3093. [Google Scholar] [CrossRef]
- Vasan, S.; Hurley, A.; Schlesinger, S.J.; Hannaman, D.; Gardiner, D.F.; Dugin, D.P.; Boente-Carrera, M.; Vittorino, R.; Caskey, M.; Andersen, J.; et al. In Vivo Electroporation Enhances the Immunogenicity of an HIV-1 DNA Vaccine Candidate in Healthy Volunteers. PLoS ONE 2011, 6, e19252. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Bilsel, P.; del Guercio, M.F.; Stewart, S.; Marinkovic-Petrovic, A.; Southwood, S.; Crimi, C.; Vang, L.; Walker, L.; Ishioka, G.; et al. Universal influenza DNA vaccine encoding conserved CD4+ T cell epitopes protects against lethal viral challenge in HLA-DR transgenic mice. Vaccine 2010, 28, 664–672. [Google Scholar] [CrossRef]
- Generotti, A.; Contreras, R.; Zounes, B.; Schade, E.; Kemme, A.; Rane, Y.; Liu, X.; Elwood, D.; Schultheis, K.; Marston, J.; et al. Intradermal DNA vaccine delivery using vacuum-controlled, needle-free electroporation. Mol. Ther. Nucleic Acids 2023, 34, 102070. [Google Scholar] [CrossRef]
- Diehl, M.C.; Lee, J.C.; Daniels, S.E.; Tebas, P.; Khan, A.S.; Giffear, M.; Sardesai, N.Y.; Bagarazzi, M.L. Tolerability of intramuscular and intradermal delivery by CELLECTRA(®) adaptive constant current electroporation device in healthy volunteers. Hum. Vaccines Immunother. 2013, 9, 2246–2252. [Google Scholar] [CrossRef]
- Fusco, R.; Di Bernardo, E.; D’Alessio, V.; Salati, S.; Cadossi, M. Reduction of muscle contraction and pain in electroporation-based treatments: An overview. World J. Clin. Oncol. 2021, 12, 367–381. [Google Scholar] [CrossRef]
- Tzeng, T.T.; Chai, K.M.; Shen, K.Y.; Yu, C.Y.; Yang, S.J.; Huang, W.C.; Liao, H.C.; Chiu, F.F.; Dou, H.Y.; Liao, C.L.; et al. A DNA vaccine candidate delivered by an electroacupuncture machine provides protective immunity against SARS-CoV-2 infection. npj Vaccines 2022, 7, 60. [Google Scholar] [CrossRef]
- Todorova, B.; Adam, L.; Culina, S.; Boisgard, R.; Martinon, F.; Cosma, A.; Ustav, M.; Kortulewski, T.; Le Grand, R.; Chapon, C. Electroporation as a vaccine delivery system and a natural adjuvant to intradermal administration of plasmid DNA in macaques. Sci. Rep. 2017, 7, 4122. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, G. Current status and future perspectives of DNA vaccine delivery by attenuated intracellular bacteria. Arch. Immunol. Ther. Exp. 2000, 48, 177–182. [Google Scholar] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Chai, D.; Wang, J.; Fan, C.; Lim, J.M.; Wang, X.; Neeli, P.; Yu, X.; Young, K.H.; Li, Y. Remodeling of anti-tumor immunity with antibodies targeting a p53 mutant. J. Hematol. Oncol. 2024, 17, 45. [Google Scholar] [CrossRef]
- Chai, D.; Wang, J.; Lim, J.M.; Xie, X.; Yu, X.; Zhao, D.; Maza, P.A.M.; Wang, Y.; Cyril-Remirez, D.; Young, K.H.; et al. Lipid nanoparticles deliver DNA-encoded biologics and induce potent protective immunity. Mol. Cancer 2025, 24, 12. [Google Scholar] [CrossRef]
- Guimaraes, L.C.; Costa, P.A.C.; Scalzo Júnior, S.R.A.; Ferreira, H.A.S.; Braga, A.C.S.; de Oliveira, L.C.; Figueiredo, M.M.; Shepherd, S.; Hamilton, A.; Queiroz-Junior, C.M. Nanoparticle-based DNA vaccine protects against SARS-CoV-2 variants in female preclinical models. Nat. Commun. 2024, 15, 590. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kon, E.; Sharma, P.; Peer, D. Endosomal escape: A bottleneck for LNP-mediated therapeutics. Proc. Natl. Acad. Sci. USA 2024, 121, e2307800120. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, R.; Vuong, I.; Reynolds, R.A.; Shears, M.J.; Yao, Z.-C.; Hu, Y.; Cho, W.J.; Kong, J.; Reddy, S.K.; et al. Multi-step screening of DNA/lipid nanoparticles and co-delivery with siRNA to enhance and prolong gene expression. Nat. Commun. 2022, 13, 4282. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Renzi, S.; Quagliarini, E.; Digiacomo, L.; Amenitsch, H.; Masuelli, L.; Bei, R.; Ferri, G.; Cardarelli, F.; Wang, J.; et al. Efficient Delivery of DNA Using Lipid Nanoparticles. Pharmaceutics 2022, 14, 1698. [Google Scholar] [CrossRef]
- Park, S.; Kim, M.; Lee, J.W. Optimizing nucleic acid delivery systems through barcode technology. ACS Synth. Biol. 2024, 13, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.N.; Tiwari, S.; Wang, Y.; O’Neill, S.; Wu, J.; Omo-Lamai, S.; Espy, C.; Chase, L.S.; Majumder, A.; Hoffman, E.; et al. Safer non-viral DNA delivery using lipid nanoparticles loaded with endogenous anti-inflammatory lipids. Nat. Biotechnol. 2025, 43, 1–11. [Google Scholar] [CrossRef]
- Liu, W.; Alameh, M.G.; Yang, J.F.; Xu, J.R.; Lin, P.J.C.; Tam, Y.K.; Weissman, D.; You, J. Lipid Nanoparticles Delivering Constitutively Active STING mRNA to Stimulate Antitumor Immunity. Int. J. Mol. Sci. 2022, 23, 14504. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Muñoz-López, M.; García-Pérez, J.L. DNA transposons: Nature and applications in genomics. Curr. Genom. 2010, 11, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Antonello, R.M.; Giacomelli, A.; Riccardi, N. Tularemia for clinicians: An up-to-date review on epidemiology, diagnosis, prevention and treatment. Eur. J. Intern. Med. 2025, 135, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kawula, T.H.; Hall, J.D.; Fuller, J.R.; Craven, R.R. Use of Transposon-Transposase Complexes To Create Stable Insertion Mutant Strains of Francisella tularensis LVS. Appl. Environ. Microbiol. 2004, 70, 6901–6904. [Google Scholar] [CrossRef]
- Chiang, S.L.; Mekalanos, J.J. Construction of a Vibrio cholerae Vaccine Candidate Using Transposon Delivery and FLP Recombinase-Mediated Excision. Infect. Immun. 2000, 68, 6391–6397. [Google Scholar] [CrossRef]
- Skeate, J.G.; Pomeroy, E.J.; Slipek, N.J.; Jones, B.J.; Wick, B.J.; Chang, J.W.; Lahr, W.S.; Stelljes, E.M.; Patrinostro, X.; Barnes, B.; et al. Evolution of the clinical-stage hyperactive TcBuster transposase as a platform for robust non-viral production of adoptive cellular therapies. Mol. Ther. 2024, 32, 1817–1834. [Google Scholar] [CrossRef]
- Gurney, M.; O’Reilly, E.; Corcoran, S.; Brophy, S.; Krawczyk, J.; Otto, N.M.; Hermanson, D.L.; Childs, R.W.; Szegezdi, E.; O’Dwyer, M.E. Concurrent transposon engineering and CRISPR/Cas9 genome editing of primary CLL-1 chimeric antigen receptor–natural killer cells. Cytotherapy 2022, 24, 1087–1094. [Google Scholar] [CrossRef]
- Moretti, A.; Ponzo, M.; Nicolette, C.A.; Tcherepanova, I.Y.; Biondi, A.; Magnani, C.F. The Past, Present, and Future of Non-Viral CAR T Cells. Front. Immunol. 2022, 13, 867013. [Google Scholar] [CrossRef]
- Skipper, K.A.; Andersen, P.R.; Sharma, N.; Mikkelsen, J.G. DNA transposon-based gene vehicles-scenes from an evolutionary drive. J. Biomed. Sci. 2013, 20, 92. [Google Scholar] [CrossRef]
- Wilson, M.H.; Coates, C.J.; George, A.L. PiggyBac Transposon-mediated Gene Transfer in Human Cells. Mol. Ther. 2007, 15, 139–145. [Google Scholar] [CrossRef]
- Liljeström, P.; Garoff, H. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Bio/Technology 1991, 9, 1356–1361. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Wang, C.; Huo, Y.-X.; Lu, Y. Comparative Function Analysis of Self-Amplifying mRNA and Self-Amplifying DNA. ChemBioChem 2025, 26, e202500110. [Google Scholar] [CrossRef]
- Jefferies, W.A.; Choi, K.B.; Ribeca, P.; Kari, S.; Young, J.; Hui, E.; Qi, S.Y.; Garrosvillas, E.; Lincez, P.; Welch, T.; et al. A Binary RNA and DNA Self-Amplifying Platform for Next Generation Vaccines and Therapeutics. bioRxiv, 2023. [Google Scholar] [CrossRef]
- Brito, L.A.; Kommareddy, S.; Maione, D.; Uematsu, Y.; Giovani, C.; Scorza, F.B.; Otten, G.R.; Yu, D.; Mandl, C.W.; Mason, P.W. Self-amplifying mRNA vaccines. Adv. Genet. 2015, 89, 179–233. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, Q.; Chen, J.L.; Jiang, N.; Wang, Y.J.; Deng, Q.; Hu, B.; Guo, R.Q. Enhanced effect of nuclear localization signal peptide during ultrasound-targeted microbubble destruction-mediated gene transfection. Mol. Med. Rep. 2017, 16, 565–572. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Moor, W.R.J.; Regnard, G.L.; Rybicki, E.P.; Williamson, A.L. Characterization of a dynamic self-replicating mammalian expression vector based on the circular ssDNA genome of beak and feather disease virus. J. Gen. Virol. 2022, 103, 001746. [Google Scholar] [CrossRef] [PubMed]
- Roig-Merino, A.; Urban, M.; Bozza, M.; Peterson, J.D.; Bullen, L.; Büchler-Schäff, M.; Stäble, S.; van der Hoeven, F.; Müller-Decker, K.; McKay, T.R.; et al. An episomal DNA vector platform for the persistent genetic modification of pluripotent stem cells and their differentiated progeny. Stem Cell Rep. 2022, 17, 143–158. [Google Scholar] [CrossRef]
- Dailey, G.P.; Crosby, E.J.; Hartman, Z.C. Cancer vaccine strategies using self-replicating RNA viral platforms. Cancer Gene Ther. 2023, 30, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Application of DNA Replicons in Gene Therapy and Vaccine Development. Pharmaceutics 2023, 15, 947. [Google Scholar] [CrossRef]
- Spearman, P.; Lally, M.A.; Elizaga, M.; Montefiori, D.; Tomaras, G.D.; McElrath, M.J.; Hural, J.; De Rosa, S.C.; Sato, A.; Huang, Y.; et al. A trimeric, V2-deleted HIV-1 envelope glycoprotein vaccine elicits potent neutralizing antibodies but limited breadth of neutralization in human volunteers. J. Infect. Dis. 2011, 203, 1165–1173. [Google Scholar] [CrossRef]
- Szurgot, I.; Hanke, L.; Sheward, D.J.; Vidakovics, L.P.; Murrell, B.; McInerney, G.M.; Liljeström, P. DNA-launched RNA replicon vaccines induce potent anti-SARS-CoV-2 immune responses in mice. Sci. Rep. 2021, 11, 3125. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Plasmid DNA-Based Alphavirus Vaccines. Vaccines 2019, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Shariati, A.; Khezrpour, A.; Shariati, F.; Afkhami, H.; Yarahmadi, A.; Alavimanesh, S.; Kamrani, S.; Modarressi, M.H.; Khani, P. DNA vaccines as promising immuno-therapeutics against cancer: A new insight. Front. Immunol. 2025, 15, 1498431. [Google Scholar] [CrossRef]
- Bordoloi, D.; Xiao, P.; Choi, H.; Ho, M.; Perales-Puchalt, A.; Khoshnejad, M.; Kim, J.J.; Humeau, L.; Srinivasan, A.; Weiner, D.B.; et al. Immunotherapy of prostate cancer using novel synthetic DNA vaccines targeting multiple tumor antigens. Genes Cancer 2021, 12, 51–64. [Google Scholar] [CrossRef]
- Williams, J.A. Vector Design for Improved DNA Vaccine Efficacy, Safety and Production. Vaccines 2013, 1, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Garmory, H.S.; Brown, K.A.; Titball, R.W. DNA vaccines: Improving expression of antigens. Genet. Vaccines Ther. 2003, 1, 2. [Google Scholar] [CrossRef]
- Teixeira, F.M.; Teixeira, H.C.; Ferreira, A.P.; Rodrigues, M.F.; Azevedo, V.; Macedo, G.C.; Oliveira, S.C. DNA Vaccine Using Mycobacterium bovis Ag85B Antigen Induces Partial Protection against Experimental Infection in BALB/c Mice. Clin. Vaccine Immunol. 2006, 13, 930–935. [Google Scholar] [CrossRef]
- Chowdhury, T.; Saha, A.; Saha, A.; Chakraborty, A.; Das, N. NeuralCodOpt: Codon optimization for the development of DNA vaccines. Comput. Biol. Chem. 2025, 116, 108377. [Google Scholar] [CrossRef]
- Deans, T.L.; Singh, A.; Gibson, M.; Elisseeff, J.H. Regulating synthetic gene networks in 3D materials. Proc. Natl. Acad. Sci. USA 2012, 109, 15217–15222. [Google Scholar] [CrossRef]
- Deans, T.L.; Cantor, C.R.; Collins, J.J. A tunable genetic switch based on RNAi and repressor proteins for regulating gene expression in mammalian cells. Cell 2007, 130, 363–372. [Google Scholar] [CrossRef]
- Smanski, M.J.; Bhatia, S.; Zhao, D.; Park, Y.; BA Woodruff, L.; Giannoukos, G.; Ciulla, D.; Busby, M.; Calderon, J.; Nicol, R. Functional optimization of gene clusters by combinatorial design and assembly. Nat. Biotechnol. 2014, 32, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.A.; Voigt, C.A. Principles of genetic circuit design. Nat. Methods 2014, 11, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Slusarczyk, A.L.; Lin, A.; Weiss, R. Foundations for the design and implementation of synthetic genetic circuits. Nat. Rev. Genet. 2012, 13, 406–420. [Google Scholar] [CrossRef]
- Andries, O.; Kitada, T.; Bodner, K.; Sanders, N.N.; Weiss, R. Synthetic biology devices and circuits for RNA-based ‘smart vaccines’: A propositional review. Expert Rev. Vaccines 2015, 14, 313–331. [Google Scholar] [CrossRef]
- Sedlmayer, F.; Aubel, D.; Fussenegger, M. Synthetic gene circuits for the detection, elimination and prevention of disease. Nat. Biomed. Eng. 2018, 2, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, D.C.; Rocha, V.P.C.; Damasceno, P.K.F.; Barbosa, J.D.V.; Soares, M.B.P. Therapeutic applications of synthetic gene/genetic circuits: A patent review. Front. Bioeng. Biotechnol. 2024, 12, 1425529. [Google Scholar] [CrossRef]
- Barbier, I.; Kusumawardhani, H.; Chauhan, L.; Harlapur, P.V.; Jolly, M.K.; Schaerli, Y. Synthetic Gene Circuits Combining CRISPR Interference and CRISPR Activation in E coli: Importance of Equal Guide RNA Binding Affinities to Avoid Context-Dependent Effects. ACS Synth. Biol. 2023, 12, 3064–3071. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Wang, B. Customizing cellular signal processing by synthetic multi-level regulatory circuits. Nat. Commun. 2023, 14, 8415. [Google Scholar] [CrossRef]
- Rai, K.; Wang, Y.; O’Connell, R.W.; Patel, A.B.; Bashor, C.J. Using machine learning to enhance and accelerate synthetic biology. Curr. Opin. Biomed. Eng. 2024, 31, 100553. [Google Scholar] [CrossRef]
- Kelly, C.L.; Harris, A.W.K.; Steel, H.; Hancock, E.J.; Heap, J.T.; Papachristodoulou, A. Synthetic negative feedback circuits using engineered small RNAs. Nucleic Acids Res. 2018, 46, 9875–9889. [Google Scholar] [CrossRef] [PubMed]
- Perales-Puchalt, A.; Duperret, E.K.; Muthumani, K.; Weiner, D.B. Simplifying checkpoint inhibitor delivery through in vivo generation of synthetic DNA-encoded monoclonal antibodies (DMAbs). Oncotarget 2019, 10, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Andrews, C.D.; Luo, Y.; Sun, M.; Yu, J.; Goff, A.J.; Glass, P.J.; Padte, N.N.; Huang, Y.; Ho, D.D. In vivo production of monoclonal antibodies by gene transfer via electroporation protects against lethal influenza and Ebola infections. Mol. Ther. Methods Clin. Dev. 2017, 7, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Duperret, E.K.; Trautz, A.; Stoltz, R.; Patel, A.; Wise, M.C.; Perales-Puchalt, A.; Smith, T.; Broderick, K.E.; Masteller, E.; Kim, J.J. Synthetic DNA-encoded monoclonal antibody delivery of Anti-CTLA-4 antibodies induces tumor shrinkage in vivo. Cancer Res. 2018, 78, 6363–6370. [Google Scholar] [CrossRef]
- Przepiorka, D.; Ko, C.W.; Deisseroth, A.; Yancey, C.L.; Candau-Chacon, R.; Chiu, H.J.; Gehrke, B.J.; Gomez-Broughton, C.; Kane, R.C.; Kirshner, S.; et al. FDA Approval: Blinatumomab. Clin. Cancer Res. 2015, 21, 4035–4039. [Google Scholar] [CrossRef]
- Perales-Puchalt, A.; Duperret, E.K.; Yang, X.; Hernandez, P.; Wojtak, K.; Zhu, X.; Jung, S.-H.; Tello-Ruiz, E.; Wise, M.C.; Montaner, L.J. DNA-encoded bispecific T cell engagers and antibodies present long-term antitumor activity. JCI Insight 2019, 4, e126086. [Google Scholar] [CrossRef]
- Muthumani, K.; Flingai, S.; Wise, M.; Tingey, C.; Ugen, K.E.; Weiner, D.B. Optimized and enhanced DNA plasmid vector based in vivo construction of a neutralizing anti-HIV-1 envelope glycoprotein Fab. Hum. Vaccines Immunother. 2013, 9, 2253–2262. [Google Scholar] [CrossRef][Green Version]
- Xu, Z.; Wise, M.C.; Choi, H.; Perales-Puchalt, A.; Patel, A.; Tello-Ruiz, E.; Chu, J.D.; Muthumani, K.; Weiner, D.B. Synthetic DNA delivery by electroporation promotes robust in vivo sulfation of broadly neutralizing anti-HIV immunoadhesin eCD4-Ig. EBioMedicine 2018, 35, 97–105. [Google Scholar] [CrossRef]
- Wise, M.C.; Xu, Z.; Tello-Ruiz, E.; Beck, C.; Trautz, A.; Patel, A.; Elliott, S.T.; Chokkalingam, N.; Kim, S.; Kerkau, M.G. In vivo delivery of synthetic DNA-encoded antibodies induces broad HIV-1-neutralizing activity. J. Clin. Investig. 2020, 130, 827–837. [Google Scholar] [CrossRef]
- Elliott, S.T.; Kallewaard, N.L.; Benjamin, E.; Wachter-Rosati, L.; McAuliffe, J.M.; Patel, A.; Smith, T.R.; Schultheis, K.; Park, D.H.; Flingai, S. DMAb inoculation of synthetic cross reactive antibodies protects against lethal influenza A and B infections. npj Vaccines 2017, 2, 18. [Google Scholar] [CrossRef]
- Flingai, S.; Plummer, E.M.; Patel, A.; Shresta, S.; Mendoza, J.M.; Broderick, K.E.; Sardesai, N.Y.; Muthumani, K.; Weiner, D.B. Protection against dengue disease by synthetic nucleic acid antibody prophylaxis/immunotherapy. Sci. Rep. 2015, 5, 12616. [Google Scholar] [CrossRef]
- Muthumani, K.; Block, P.; Flingai, S.; Muruganantham, N.; Chaaithanya, I.K.; Tingey, C.; Wise, M.; Reuschel, E.L.; Chung, C.; Muthumani, A. Rapid and long-term immunity elicited by DNA-encoded antibody prophylaxis and DNA vaccination against chikungunya virus. J. Infect. Dis. 2016, 214, 369–378. [Google Scholar] [CrossRef]
- Esquivel, R.N.; Patel, A.; Kudchodkar, S.B.; Park, D.H.; Stettler, K.; Beltramello, M.; Allen, J.W.; Mendoza, J.; Ramos, S.; Choi, H. In vivo delivery of a DNA-encoded monoclonal antibody protects non-human primates against Zika virus. Mol. Ther. 2019, 27, 974–985. [Google Scholar] [CrossRef]
- Patel, A.; Park, D.H.; Davis, C.W.; Smith, T.R.; Leung, A.; Tierney, K.; Bryan, A.; Davidson, E.; Yu, X.; Racine, T. In vivo delivery of synthetic human DNA-encoded monoclonal antibodies protect against ebolavirus infection in a mouse model. Cell Rep. 2018, 25, 1982–1993.e4. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Bah, M.A.; Weiner, D.B. In Vivo Delivery of Nucleic Acid-Encoded Monoclonal Antibodies. BioDrugs 2020, 34, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; DiGiandomenico, A.; Keller, A.E.; Smith, T.R.; Park, D.H.; Ramos, S.; Schultheis, K.; Elliott, S.T.; Mendoza, J.; Broderick, K.E. An engineered bispecific DNA-encoded IgG antibody protects against Pseudomonas aeruginosa in a pneumonia challenge model. Nat. Commun. 2017, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Esquivel, R.; Flingai, S.; Schiller, Z.A.; Kern, A.; Agarwal, S.; Chu, J.; Patel, A.; Sullivan, K.; Wise, M.C. Anti-OspA DNA-encoded monoclonal antibody prevents transmission of spirochetes in tick challenge providing sterilizing immunity in mice. J. Infect. Dis. 2019, 219, 1146–1150. [Google Scholar] [CrossRef]
- Patel, A.; Rosenke, K.; Parzych, E.M.; Feldmann, F.; Bharti, S.; Griffin, A.J.; Schouest, B.; Lewis, M.; Choi, J.; Chokkalingam, N.; et al. In vivo delivery of engineered synthetic DNA-encoded SARS-CoV-2 monoclonal antibodies for pre-exposure prophylaxis in non-human primates. Emerg. Microbes Infect. 2024, 13, 2294860. [Google Scholar] [CrossRef]
- Cho, H.; Gonzales-Wartz, K.K.; Huang, D.; Yuan, M.; Peterson, M.; Liang, J.; Beutler, N.; Torres, J.L.; Cong, Y.; Postnikova, E.; et al. Bispecific antibodies targeting distinct regions of the spike protein potently neutralize SARS-CoV-2 variants of concern. Sci. Transl. Med. 2021, 13, eabj5413. [Google Scholar] [CrossRef]
- Bhojnagarwala, P.S.; O’Connell, R.P.; Park, D.; Liaw, K.; Ali, A.R.; Bordoloi, D.; Cassel, J.; Tursi, N.J.; Gary, E.; Weiner, D.B. In vivo DNA-launched bispecific T cell engager targeting IL-13Rα2 controls tumor growth in an animal model of glioblastoma multiforme. Mol. Ther. Oncolytics 2022, 26, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, N.; Langroudi, L.; Hajimoradi, M.; Hassan, Z.M. Co-administration of GP96 and Her2/neu DNA vaccine in a Her2 breast cancer model. Cell Stress Chaperones 2010, 15, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Pfeifle, A.; Thulasi Raman, S.N.; Lansdell, C.; Zhang, W.; Tamming, L.; Cecillon, J.; Laryea, E.; Patel, D.; Wu, J.; Gravel, C.; et al. DNA lipid nanoparticle vaccine targeting outer surface protein C affords protection against homologous Borrelia burgdorferi needle challenge in mice. Front. Immunol. 2023, 14, 1020134. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Huang, D.; Miller, M.; Ashok, B.; Jain, S.; Peppas, N.A. CRISPR/Cas systems to overcome challenges in developing the next generation of T cells for cancer therapy. Adv. Drug Deliv. Rev. 2020, 158, 17–35. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Wang, J.; Chen, J.; Guo, Z.; Liu, Y.; Hua, H. Exploiting RIG-I-like receptor pathway for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 8. [Google Scholar] [CrossRef]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. TLR agonists as vaccine adjuvants in the prevention of viral infections: An overview. Front. Microbiol. 2023, 14, 1249718. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Enhancing Vaccine Efficacy and Stability: A Review of the Utilization of Nanoparticles in mRNA Vaccines. Biomolecules 2024, 14, 1036. [Google Scholar] [CrossRef]
- Abraham, N.; Spruin, S.; Rossi, T.; Fireman, B.; Zafack, J.; Blaser, C.; Shaw, A.; Hutchings, K.; Ogunnaike-Cooke, S. Myocarditis and/or pericarditis risk after mRNA COVID-19 vaccination: A Canadian head to head comparison of BNT162b2 and mRNA-1273 vaccines. Vaccine 2022, 40, 4663–4671. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-M.; Yan, C.; Feng, Y.-M. Nanomedicine for the treatment of diabetes-associated cardiovascular diseases and fibrosis. Adv. Drug Deliv. Rev. 2021, 172, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Kim, S.H.; Subedi, L.; Mujahid, K.; Kim, Y.; Cho, Y.-C.; Shim, J.-H.; Kim, K.-T.; Cho, S.-S.; Choi, J.U.; et al. Oral lymphatic delivery of alpha-galactosylceramide and ovalbumin evokes anti-cancer immunization. J. Control. Release 2023, 356, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tang, H.; Li, L.; Wang, X.; Yu, Z.; Li, J. Peptide-based therapeutic cancer vaccine: Current trends in clinical application. Cell Prolif. 2021, 54, e13025. [Google Scholar] [CrossRef]
- Smith, C.C.; Selitsky, S.R.; Chai, S.; Armistead, P.M.; Vincent, B.G.; Serody, J.S. Alternative tumour-specific antigens. Nat. Rev. Cancer 2019, 19, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Redman, J.M.; Gulley, J.L. Combining vaccines and immune checkpoint inhibitors to prime, expand, and facilitate effective tumor immunotherapy. Expert Rev. Vaccines 2018, 17, 697–705. [Google Scholar] [CrossRef]
- McNeel, D.G.; Dunphy, E.J.; Davies, J.G.; Frye, T.P.; Johnson, L.E.; Staab, M.J.; Horvath, D.L.; Straus, J.; Alberti, D.; Marnocha, R. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. J. Clin. Oncol. 2009, 27, 4047–4054. [Google Scholar] [CrossRef]
- McNeel, D.G.; Eickhoff, J.C.; Johnson, L.E.; Roth, A.R.; Perk, T.G.; Fong, L.; Antonarakis, E.S.; Wargowski, E.; Jeraj, R.; Liu, G. Phase II trial of a DNA vaccine encoding prostatic acid phosphatase (pTVG-HP [MVI-816]) in patients with progressive, nonmetastatic, castration-sensitive prostate cancer. J. Clin. Oncol. 2019, 37, 3507–3517. [Google Scholar] [CrossRef]
- McNeel, D.G.; Emamekhoo, H.; Eickhoff, J.C.; Kyriakopoulos, C.E.; Wargowski, E.; Tonelli, T.P.; Johnson, L.E.; Liu, G. Phase 2 trial of a DNA vaccine (pTVG-HP) and nivolumab in patients with castration-sensitive non-metastatic (M0) prostate cancer. J. Immunother. Cancer 2023, 11, e008067. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.E.; Eickhoff, J.C.; Ferrari, A.C.; Schweizer, M.T.; Wargowski, E.; Olson, B.M.; McNeel, D.G. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in patients with metastatic prostate cancer. Clin. Cancer Res. 2020, 26, 5162–5171. [Google Scholar] [CrossRef]
- Gamat-Huber, M.; Jeon, D.; Johnson, L.E.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; Rastogi, I.; Wargowski, E.; Zahm, C.D.; McNeel, D.G. Treatment Combinations with DNA Vaccines for the Treatment of Metastatic Castration-Resistant Prostate Cancer (mCRPC). Cancers 2020, 12, 2831. [Google Scholar] [CrossRef]
- Childs, J.; Higgins, D.; DeShong, K.; Heckman-Stoddard, B.; Wojtowicz, M.; Stanton, S.; Bailey, H.; Wisinski, K.; Disis, M. Abstract OT3-01-03: A phase I trial of the safety and immunogenicity of a DNA plasmid based vaccine (WOKVAC) encoding epitopes derived from three breast cancer antigens (IGFBP2, HER2, and IGF1R) in patients with breast cancer. Cancer Res. 2017, 77, OT3-01-03. [Google Scholar] [CrossRef]
- Gwin, W.R.; Kuano, K.; Childs, J.; Symonds, L.K.; Coveler, A.L.; Liao, J.B.; Vinayak, S.; Disis, M.L. A phase II study of concurrent WOKVAC vaccination with neoadjuvant chemotherapy and HER2-targeted monoclonal antibody therapy. J. Clin. Oncol. 2023, 41, TPS636. [Google Scholar] [CrossRef]
- Kim, T.J.; Jin, H.-T.; Hur, S.-Y.; Yang, H.G.; Seo, Y.B.; Hong, S.R.; Lee, C.-W.; Kim, S.; Woo, J.-W.; Park, K.S. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014, 5, 5317. [Google Scholar] [CrossRef]
- Higgins, D.; Childs, J.; Salazar, L.; Disis, M. Abstract OT1-01-01: A phase I trial of the safety and immunogenicity of a multiple antigen vaccine (STEMVAC) in HER2 negative advanced stage breast cancer patients. Cancer Res. 2016, 76, OT1-01-01. [Google Scholar] [CrossRef]
- Wang, W.; Sawleshwarkar, S.; Piraveenan, M. Computational approaches of modelling human papillomavirus transmission and prevention strategies: A systematic review. J. Biol. Dyn. 2025, 19, 2436376. [Google Scholar] [CrossRef] [PubMed]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef]
- Bhuyan, P.K.; Dallas, M.; Kraynyak, K.; Herring, T.; Morrow, M.; Boyer, J.; Duff, S.; Kim, J.; Weiner, D.B. Durability of response to VGX-3100 treatment of HPV16/18 positive cervical HSIL. Hum. Vaccines Immunother. 2021, 17, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.W.; Hur, S.Y.; Woo, J.W.; Kim, Y.M.; Lim, M.C.; Park, S.Y.; Seo, S.S.; No, J.H.; Kim, B.G.; Lee, J.K.; et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: Interim results of a single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1653–1660. [Google Scholar] [CrossRef]
- Butterfield, L.H. Lessons learned from cancer vaccine trials and target antigen choice. Cancer Immunol. Immunother. 2016, 65, 805–812. [Google Scholar] [CrossRef]
- Lang, F.; Schrörs, B.; Löwer, M.; Türeci, Ö.; Sahin, U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat. Rev. Drug Discov. 2022, 21, 261–282. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Wang, X.; Kim, S.W.; Herndon, J.M.; Becker-Hapak, M.K.; Carreno, B.M.; Myers, N.B.; Sturmoski, M.A.; McLellan, M.D. Optimized polyepitope neoantigen DNA vaccines elicit neoantigen-specific immune responses in preclinical models and in clinical translation. Genome Med. 2021, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Duperret, E.K.; Perales-Puchalt, A.; Stoltz, R.; GH, H.; Mandloi, N.; Barlow, J.; Chaudhuri, A.; Sardesai, N.Y.; Weiner, D.B. A synthetic DNA, multi-neoantigen vaccine drives predominately MHC class I CD8+ T-cell responses, impacting tumor challenge. Cancer Immunol. Res. 2019, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Pilla, L.; Ferrone, S.; Maccalli, C. Methods for improving the immunogenicity and efficacy of cancer vaccines. Expert Opin. Biol. Ther. 2018, 18, 765–784. [Google Scholar] [CrossRef]
- Zhao, Y.; Baldin, A.V.; Isayev, O.; Werner, J.; Zamyatnin, A.A., Jr.; Bazhin, A.V. Cancer vaccines: Antigen selection strategy. Vaccines 2021, 9, 85. [Google Scholar] [CrossRef] [PubMed]
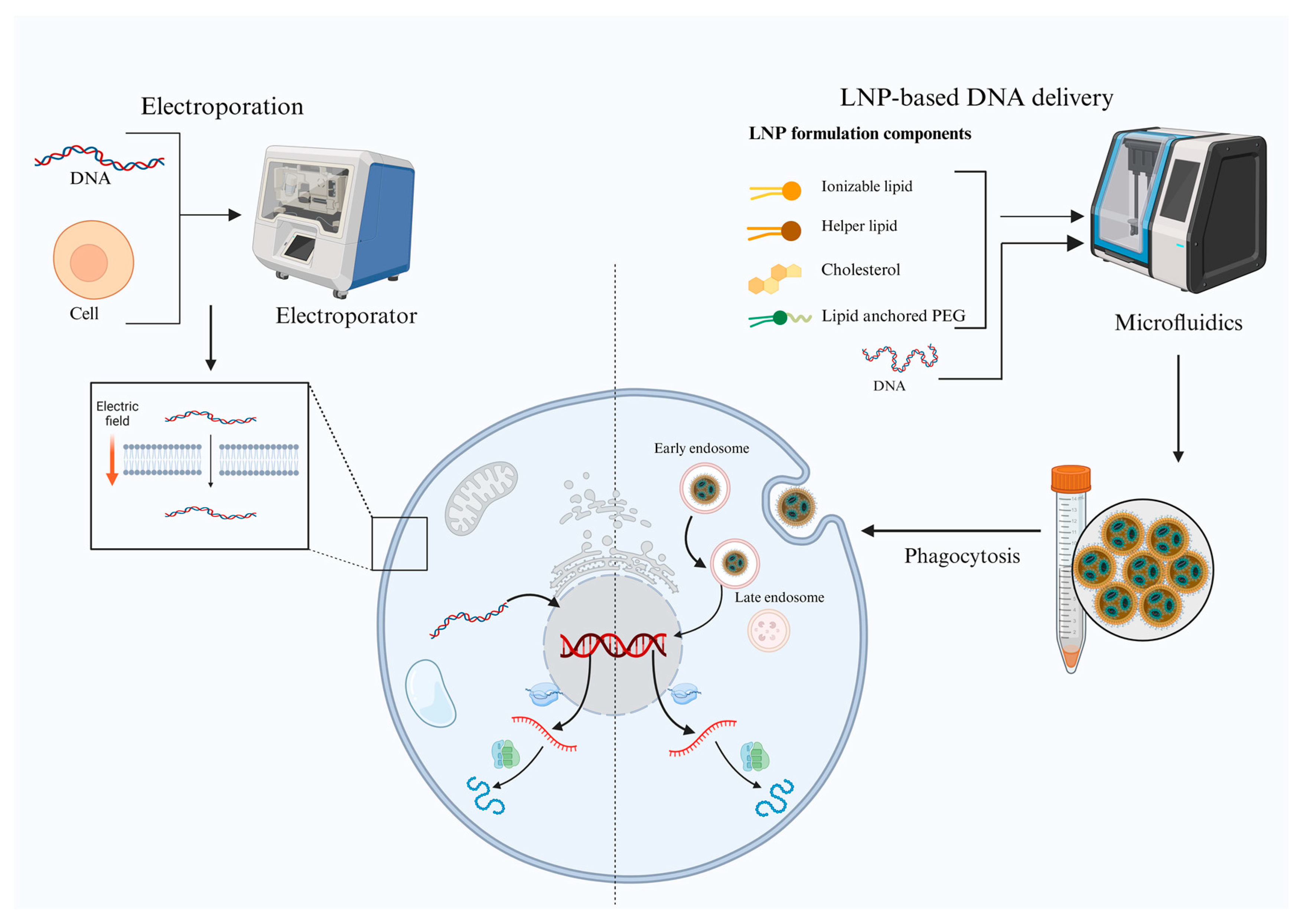

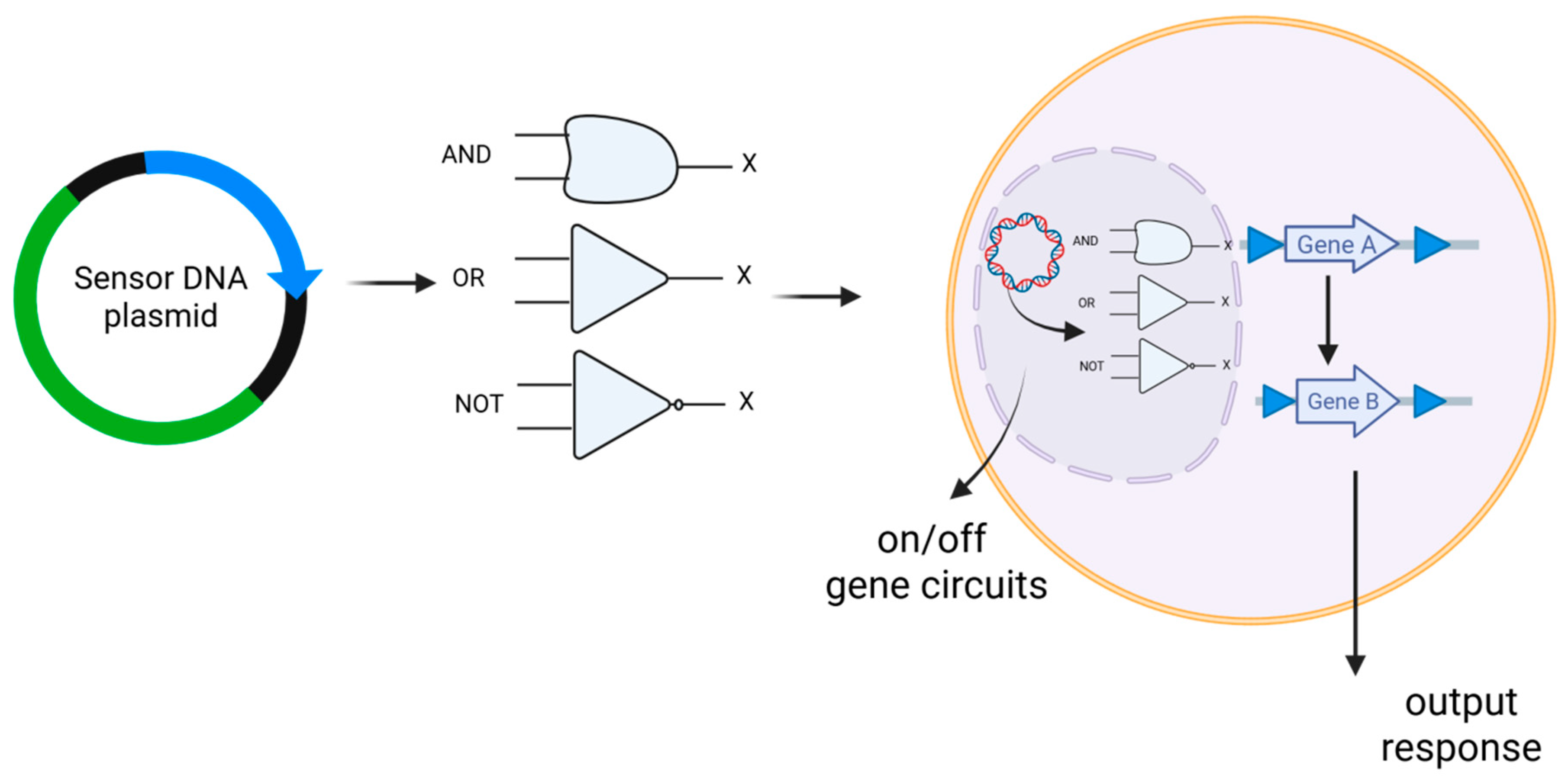
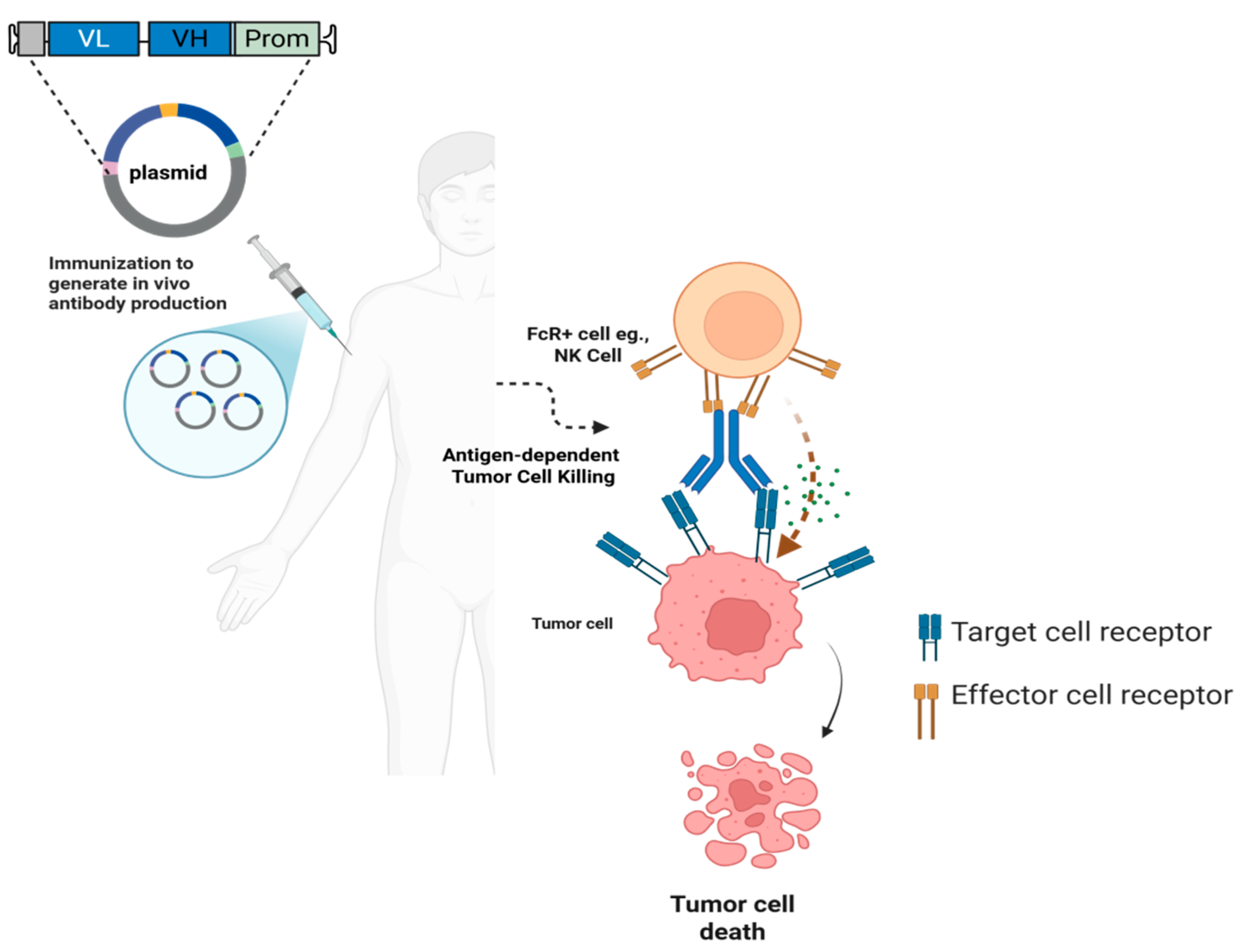
| Feature | DNA Vaccines | mRNA Vaccines |
|---|---|---|
| Stability | Stable at 2–8 °C; can be lyophilized for easier transport and longer shelf life [17] | Requires ultracold storage (−20 °C to −70 °C), complicating logistics [3] |
| Delivery target | Requires entry into the nucleus for transcription [6] | Requires only cytoplasmic delivery for translation [18] |
| Delivery systems | Typically delivered via electroporation, gene gun, or viral vectors [19] | Often delivered in lipid nanoparticles (LNPs) for enhanced stability and cell uptake [20] |
| Production | Relatively inexpensive; scalable with bacterial fermentation systems [19] | Fast production, initially higher cost, but increasingly optimized for large-scale manufacturing [21] |
| Mechanism of action | Transcribed into mRNA in the nucleus, then translated into an antigenic protein in the cytoplasm [22] | Direct translation of mRNA into protein in the cytoplasm [21] |
| Immune response | Induces both cellular and humoral responses; often Th1-biased [23] | Strong inducer of both humoral and cellular immunity, particularly CD8+ T cell responses [24] |
| Adjuvant requirement | Often requires co-delivery with adjuvants to boost immunogenicity [25] | May not need separate adjuvants due to innate immunostimulatory properties of RNA and LNPs [26] |
| Safety profile | Very low risk of genomic integration, especially with improved non-integrating plasmid vectors [27] | No integration risk: mRNA is transient and degraded by normal cellular processes [28] |
| Disease/Target | Vector Format | Dose (μg of DNA) | Key Findings | References |
|---|---|---|---|---|
| Tumor Neoantigens + PSMA | SFV replicon DNA plasmid | 20 | Induced tumor regressions and long-term tumor-free survival in murine models | [71] |
| HPV E6/E7 | SFV replicon DNA plasmid | 25 | Strong CTL responses and tumor control in HPV-positive mouse tumor model | [71] |
| HIV Env, Gag-Pol-Nef | SFV replicon DNA plasmid | 50 | Robust antibody and CTL responses, enhanced by protein boost | [72,73] |
| SARS-CoV-2 Spike | DREP-S (SFV-based replicon DNA) | 10 | High IgG and neutralizing antibody titers, strong T cell immunity in mice | [74] |
| Influenza HA | CMV/SFV replicon DNA plasmid | 5 | Enhanced immune responses vs. conventional DNA vaccine at lower doses | [64,75] |
| Target | Disease Etiology | Biologic Class | Key Findings | References |
|---|---|---|---|---|
| SARS-CoV-2 virus | Infectious | DMAb | Phase 1 trial in humans shows durable (72-week) expression, stable levels, and tolerable safety profile | [111] |
| SARS-CoV-2 virus | Infectious | DNA-encoded bispecific | BNT142 RNA-encoded T-cell engager shows preclinical efficacy | [112] |
| IL-13Rα2 (glioblastoma) | Malignancy | DNA-encoded bispecific T-cell engager (dBTE) | In vivo DNA-launched bispecific engage T cells, control heterogeneous GBM | [113] |
| ufgHER2 | Malignancy | Her2/GP96 vaccine | Anti-HER2 DMAb alone, or bispecific HER2/CD3, controls ovarian tumors and extends survival in mice | [114] |
| Chikungunya virus | Infectious | DMAb | Single injections protect mice; combo DNA + DMAb offers rapid + durable protection | [105] |
| Pseudomonas aeruginosa | Infectious | DMAb | DMAb protects from lethal pneumonia and works synergistically with antibiotics | [109] |
| Zika virus | Infectious | DMAb | In vivo plasmid delivery generates neutralizing ZIKV antibodies; protects mice from lethal challenge | [106] |
| Borrelia burgdorferi | Infectious | DMAb | OspA-targeting DMAb blocks tick-borne Lyme transmission in mice | [115] |
| HIV-1 | Infectious | Broadly neutralizing Abs | Multiple bNAbs expressed in mice/NHPs at functional levels | [100,102] |
| Influenza A/B | Infectious | DMAb | Single-dose DMAbs protect mice from lethal influenza challenge | [95,103] |
| Ebola virus | Infectious | DMAb | DNA-encoded EBOV mAbs confer full protection in mouse models | [107] |
| Dengue virus | Infectious | DMAb | Multivalent DMAb delivery neutralizes all DENV serotypes; blocks ADE | [104] |
| PD-1 | Malignancy | DMAb | Anti-PD-1 DMAb expressed rapidly, sustained in serum; enhances checkpoint blockade in mice | [96] |
| CTLA-4 | Malignancy | DMAb | DNA-encoded anti-CTLA-4 induces tumor shrinkage in mouse models | [97] |
| HIV-1 | Infectious | F(ab) | DNA-encoded VRC01-like F(ab) yields rapid in vivo expression post-electroporation | [100] |
| HIV-1 | Infectious | Ig-like molecule | Proof-of-concept study for DNA-based delivery of anti-HIV immunoadhesins and in vivo modulation of protein function | [101] |
| Sponsor/Collaborator | Vaccine (Brand) | Encoded Antigens | Delivery + Combination | Cancer Type and Phase (NCT) | Start | Status/Key Results |
|---|---|---|---|---|---|---|
| Dana-Farber/Yale | Personalized DNA neoantigen vaccine | Patient-specific neoantigens | IM + EP; monotherapy | Advanced kidney, phase I (NCT Pending) | 2025 | Remission in 9/9 patients ≥3 yrs |
| Washington Univ (TNBC) | Personalized polyepitope DNA vaccine | 4–20 patient neoantigens | IM + EP; monotherapy | TNBC, phase I (NCT02348320) | 2024 | 87.5% RFS at 36 mo |
| Nature ’24 RCC study | Neoantigen-targeting DNA vaccine | RCC driver mutations | IM + EP; monotherapy | Renal cell carcinoma, phase I | 2024 | Strong T-cell responses |
| WUSTL | Personalized neoantigen | Neoantigens | IM EP | Pediatric brain tumor, phase I (NCT03988283) | 2024 | Not yet recruiting |
| Wash U (GBM) | INO-5410 | LAMP1, IE-1, pp65, gB | IM + EP | Glioblastoma, phase I (NCT05698199) | 2023 | Recruiting |
| Wash U (GBM) | Personalized neoantigen DNA w/retifanlimab | Patient-specific neoantigens | IM + EP; + retifanlimab | Unmethylated GBM, phase I (NCT05743595) | 2023 | Recruiting |
| University of Washington | STEMVAC | CD105, YB-1, SOX2, CDH3, MDM2 | / | Lung NSCLC, phase II (NCT05242965) | 2023 | Recruiting |
| Immunomic Therapeutics | ITI-1001 | IE-1, pp65, gB | LAMP1, IM EP | Glioblastoma, phase I (NCT05698199) | 2023 | Recruiting |
| WUSTL | Personalized neoantigen | Neoantigens | Retifanlimab, IM EP | Glioblastoma, phase I (NCT05743595) | 2023 | Recruiting |
| Wash U (SCLC) | Personalized neoantigen DNA + durvalumab | Patient-specific neoantigens | IM + EP; + durvalumab | Small-cell lung cancer, phase II (NCT04397003) | 2022 | Recruiting |
| University of Washington | WOKVAC | IGFBP2, HER2, IGF1R | Paclitaxel, Trastuzumab, Pertuzumab, ID | Breast cancer, phase II (NCT04329065) | 2022 | Recruiting |
| University of Washington | STEMVAC | CD105, YB-1, SOX2, CDH3, MDM2 | rhu GM-CSF, ID | Breast cancer, phase II (NCT05455658) | 2022 | Recruiting |
| WUSTL | Personalized neoantigen | Neoantigens | Durvalumab, IM EP | SCLC, phase II (NCT04397003) | 2022 | Recruiting |
| University of Wisconsin, Madison | pTGV-AR | AR LBD | Degarelix, Nivolumab, ID | Prostate cancer, phase I/II (NCT04989946) | 2021 | Recruiting |
| NCI | pNGVL4a CRTE6E7L2 | HPV E6/E7/L2 | EP | HPV-16 positive cervical neoplasia, phase I (NCT04131413) | 2020 | Recruiting |
| WUSTL | Personalized neoantigen | Neoantigens | INO-9012, IM EP | Glioblastoma, phase I (NCT04015700) | 2020 | Active, not recruiting |
| Geneos Therapeutics | GNOS-PV02 | Personalized neoantigen | INO-9012, Pembrolizumab, ID EP | HCC, phase I/II (NCT04251117) | 2020 | Active, not recruiting |
| University of Wisconsin, Madison | pTGV-HP + pTGV-AR | PAP + AR LBD | Pembrolizumab (a-PD1 Ab), ID | Castration-resistant prostate cancer, phase II (NCT04090528) | 2019 | Recruiting |
| INOVIO | VGX-3100 | HPV E6/E7 | / | Cervical HSIL, phase III (NCT03721978) | 2019 | Completed |
| WUSTL | Neoantigens | Neoantigens | Durvalumab, IM EP | TNBC, phase I (NCT03199040) | 2019 | Terminated |
| WUSTL | Neoantigens | Neoantigens | Darvalumab, Tremelimumab, IM EP | Renal cell carcinoma, phase II (NCT03598816) | 2019 | Withdrawn |
| INOVIO | INO-5401 + INO-9012 | WT1, PSMA, hTERT | IM + EP; + cemiplimab, RT | Glioblastoma, phase I/II (NCT03491683) | 2018 | OS 18–32 mo; immune active |
| University of Wisconsin, Madison | pTGV-HP | PAP | Nivolumab (a-PD1 Ab), GM-CSF, ID | Prostate cancer, phase II (NCT03600350) | 2018 | Active |
| INOVIO | INO-5410 | WT1, PSMA, hTERT | Cemiplimab, radiation, chemo, INO-9012, IM EP | Glioblastoma, phase I/II (NCT03491683) | 2018 | Active |
| INOVIO | INO-9012 | WT1, PSMA, hTERT | INO-9012, Atezolizumab, IM EP | Urothelial carcinoma, phase I/II (NCT03502785) | 2018 | Active |
| INOVIO | VGX-3100 | HPV E6/E7 | / | Anal neoplasm, phase II (NCT03499795) | 2018 | Completed |
| INOVIO | VGX-3100 | HPV E6/E7 | / | CIN 2/3, phase II (NCT01304524) | 2018 | Completed |
| NCI | MEDI0457 (INO-3112) | HPV E6/E7 | INO-9012, Durvalumab, IM EP | HPV-16/18 cancers, phase II (NCT03439085) | 2018 | Active, not recruiting |
| Genexine | GX-188E | HPV E6/E7 | Pembrolizumab, IM EP | Cervical cancer, phase I/II (NCT03444376) | 2018 | Completed |
| BMS/Bavarian Nordic | Neoantigens | Neoantigens | Nivolumab, Ipilimumab, Prostvac, IM EP | Metastatic prostate cancer, phase I (NCT03532217) | 2018 | Completed |
| NCI | pING vector | Neoantigens + mesothelin | Chemotherapy, IM EP | Pancreatic cancer, phase I (NCT03122106) | 2018 | Terminated |
| University of Wisconsin | VGX-3100 | HPV E6/E7 | IM + EP; monotherapy | Cervical/Anal (NCT03185013, etc.) | 2017 | Completed |
| University of Washington | WOKVAC: pUMVC3-IGFBP2-HER2-IGF1R | IGFBP2, HER2, IGF1R | Carboplatin, Paclitaxel, ID | Ovarian cancer, phase II (NCT03029611) | 2017 | Terminated |
| INOVIO | VGX-3100 | HPV E6/E7 | / | Cervical cancer, phase III (NCT03185013) | 2017 | Completed |
| Genexine | GX-188E | HPV E6/E7 | GX-I7, Imiquimod, IM | Cervical cancer, phase/(NCT03206138) | 2017 | Unknown |
| University of Washington | WOKVAC | IGFBP2, HER2, IGF1R | rhu GM-CSF, ID | Breast cancer, phase I (NCT02780401) | 2016 | Active |
| Washington Univ (Breast) | Mam-A DNA vaccine | Mammaglobin-A | IM + EP | Breast cancer, phase I (NCT02204098) | 2015 | Recruiting |
| University of Wisconsin, Madison | pTGV-AR | Androgen Receptor LBD | GM-CSF, ID | Prostate cancer, phase I (NCT02411786) | 2015 | Completed |
| University of Washington | STEMVAC | CD105, YB-1, SOX2, CDH3, MDM2 | / | Breast cancer, phase I (NCT02157051) | 2015 | Active, not recruiting |
| Genexine | GX-188E | HPV E6/E7 | / | Cervical cancer, phase II (NCT02596243) | 2015 | Unknown |
| WUSTL | Personalized polyepitopes | / | IM EP | TNBC, phase I (NCT02348320) | 2015 | Completed |
| University of Wisconsin, Madison | pTGV-HP | Prostatic acid phosphatase (PAP) | rhGM-CSF, ID | Prostate cancer, phase II (NCT01341652) | 2011 | Completed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neeli, P.; Chai, D.; Roy, D.; Prajapati, S.; Bonam, S.R. DNA Vaccines in the Post-mRNA Era: Engineering, Applications, and Emerging Innovations. Int. J. Mol. Sci. 2025, 26, 8716. https://doi.org/10.3390/ijms26178716
Neeli P, Chai D, Roy D, Prajapati S, Bonam SR. DNA Vaccines in the Post-mRNA Era: Engineering, Applications, and Emerging Innovations. International Journal of Molecular Sciences. 2025; 26(17):8716. https://doi.org/10.3390/ijms26178716
Chicago/Turabian StyleNeeli, Praveen, Dafei Chai, Debanjana Roy, Shivank Prajapati, and Srinivasa Reddy Bonam. 2025. "DNA Vaccines in the Post-mRNA Era: Engineering, Applications, and Emerging Innovations" International Journal of Molecular Sciences 26, no. 17: 8716. https://doi.org/10.3390/ijms26178716
APA StyleNeeli, P., Chai, D., Roy, D., Prajapati, S., & Bonam, S. R. (2025). DNA Vaccines in the Post-mRNA Era: Engineering, Applications, and Emerging Innovations. International Journal of Molecular Sciences, 26(17), 8716. https://doi.org/10.3390/ijms26178716







