Bacteriophage-Based Approach Against Biofilm Infections Associated with Medical Devices: A Narrative Review of ESKAPE Pathogens
Abstract
1. Introduction
2. Bacterial Biofilm
2.1. Characteristics of Bacterial Biofilm and Biofilm Structure
2.2. Mechanism of Biofilm Formation
2.3. Role of Biofilm in Pathogens
2.4. Risk Factors of Biofilm Formation
2.5. ESKAPE as Examples of Pathogens Forming Biofilm on Medical Devices
3. Bacteriophages as a Tool in Biofilm Control
3.1. Mechanism of Bacteriophage Action, Including Phages’ Ability to Penetrate and Degrade Biofilms
3.2. Different Approaches to Using Phages for Biofilm Elimination
3.2.1. Phage-Derived Lytic Enzymes
3.2.2. Single-Phage Therapy
3.2.3. Phage Cocktails
3.2.4. Phage and Antibiotic Combination
3.2.5. Genetically Engineered Phages
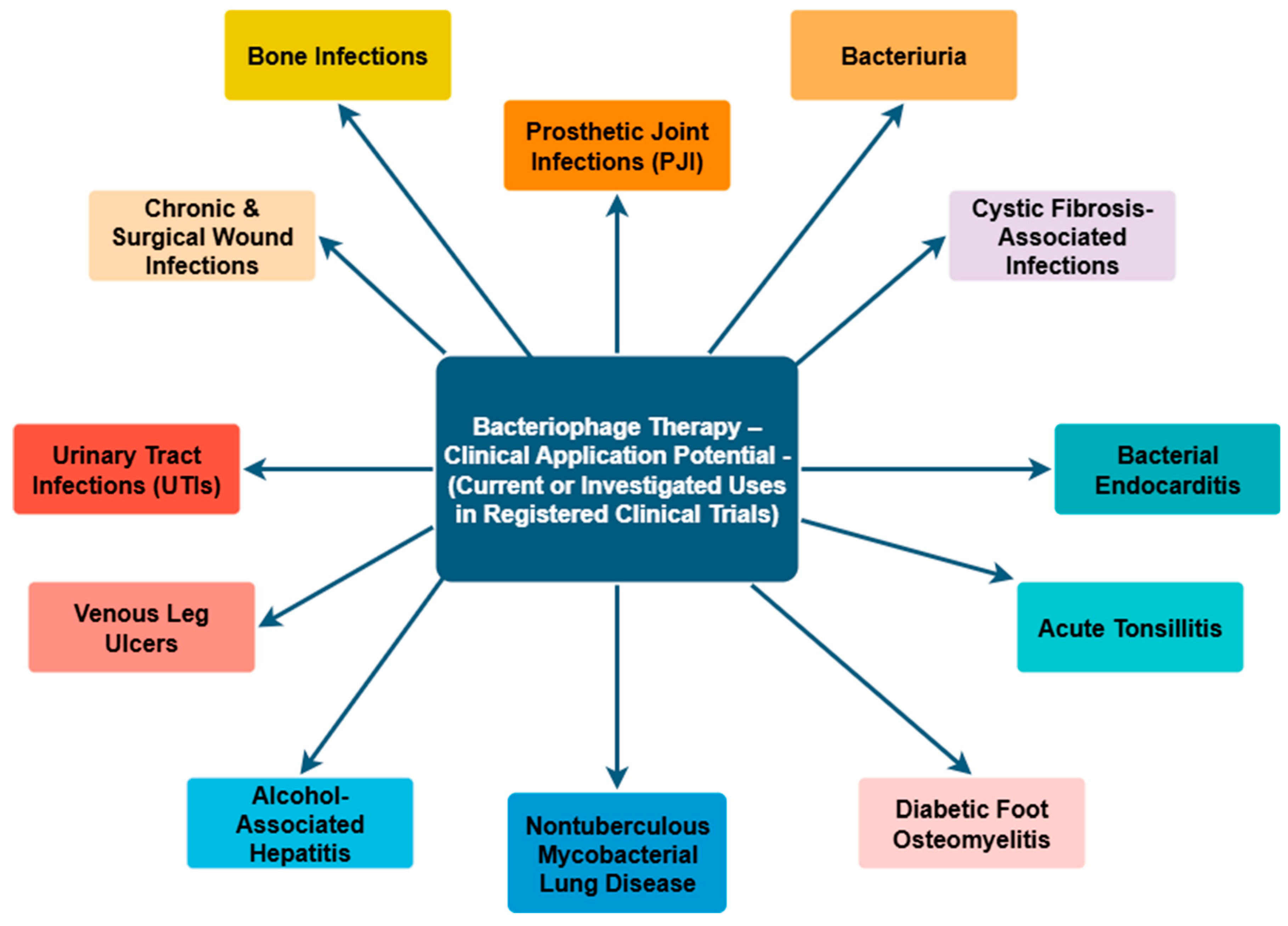
3.3. Clinical Applications of Phage Therapy and Its Relevance to ESKAPE-Related Infections
4. Limitations and Implications
5. Future Perspectives and Research Directions
6. Materials and Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide Clean Care Is Safer Care; World Health Organization: Geneva, Switzerland, 2011.
- World Health Organization. Global Report on Infection Prevention and Control 2024 Executive Summary; World Health Organization: Geneva, Switzerland, 2024. [CrossRef]
- Bryers, J.D. Medical Biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef]
- Van Epps, J.S.; Younger, J.G. Implantable Device Related Infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef]
- Wyss, U.P. Improving the Quality of Life of Patients with Medical Devices by a Timely Analysis of Adverse Events. Front. Med. 2019, 6, 56. [Google Scholar] [CrossRef]
- Yasir, M.; Willcox, M.D.P.; Dutta, D. Action of Antimicrobial Peptides against Bacterial Biofilms. Materials 2018, 11, 2468. [Google Scholar] [CrossRef] [PubMed]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Biofilm-Forming Microorganisms Causing Hospital-Acquired Infections from Intravenous Catheter: A Systematic Review. Curr. Res. Microb. Sci. 2022, 3, 100175. [Google Scholar] [CrossRef] [PubMed]
- Bouhrour, N.; Nibbering, P.H.; Bendali, F. Medical Device-Associated Biofilm Infections and Multidrug-Resistant Pathogens. Pathogens 2024, 13, 393. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Reffuveille, F.; Dghoughi, Y.; Colin, M.; Torres, M.D.T.; de la Fuente-Nunez, C. Antibiofilm Approaches as a New Paradigm for Treating Infections. Prog. Biomed. Eng. 2024, 6, 023001. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614, Erratum in Microorganisms 2024, 12, 1961. [Google Scholar] [CrossRef]
- Chen, M.; Yu, Q.; Sun, H. Novel Strategies for the Prevention and Treatment of Biofilm Related Infections. Int. J. Mol. Sci. 2013, 14, 18488–18501. [Google Scholar] [CrossRef]
- Agarwal, A.; Kelkar, A.; Agarwal, A.G.; Jayaswal, D.; Schultz, C.; Jayaswal, A.; Goel, V.K.; Agarwal, A.K.; Gidvani, S. Novel Approaches to Combat Medical Device-Associated BioFilms. Coatings 2021, 11, 294. [Google Scholar] [CrossRef]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017.
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Werneburg, G.T.; Hettel, D.; Goldman, H.B.; Vasavada, S.P.; Miller, A.W. Indwelling Urological Device Biofilm Composition and Characteristics in the Presence and Absence of Infection. Urology 2025, 196, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial Biofilm Formation on Implantable Devices and Approaches to Its Treatment and Prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.A.; Lodise, T.P.; Tamma, P.D.; Knisely, J.M.; Alexander, J.; Aslam, S.; Barton, K.D.; Bizzell, E.; Totten, K.M.C.; Campbell, J.L.; et al. Considerations for the Use of Phage Therapy in Clinical Practice. Antimicrob. Agents Chemother. 2022, 66, e02071-21. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.R. Going beyond the Control of Quorum-Sensing to Combat Biofilm Infections. Antibiotics 2016, 5, 3. [Google Scholar] [CrossRef]
- Gebreyohannes, G.; Nyerere, A.; Bii, C.; Sbhatu, D.B. Challenges of Intervention, Treatment, and Antibiotic Resistance of Biofilm-Forming Microorganisms. Heliyon 2019, 5, e02192. [Google Scholar] [CrossRef]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of Biofilm Formation by Quorum Quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef]
- Harper, D.R.; Parracho, H.M.R.T.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S. Bacteriophages and Biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- Pires, D.P.; Cleto, S.; Sillankorva, S.; Azeredo, J.; Lu, T.K. Genetically Engineered Phages: A Review of Advances over the Last Decade. Microbiol. Mol. Biol. Rev. 2016, 80, 523–543. [Google Scholar] [CrossRef]
- Allesen-Holm, M.; Barken, K.B.; Yang, L.; Klausen, M.; Webb, J.S.; Kjelleberg, S.; Molin, S.; Givskov, M.; Tolker-Nielsen, T. A Characterization of DNA Release in Pseudomonas aeruginosa Cultures and Biofilms. Mol. Microbiol. 2006, 59, 1114–1128. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lembre, P.; Lorentz, C.; Di, P. Exopolysaccharides of the Biofilm Matrix: A Complex Biophysical World. In The Complex World of Polysaccharides; InTech: London, UK, 2012. [Google Scholar]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.E.; Rice, K.C.; Boles, B.R.; Endres, J.L.; Ranjit, D.; Chandramohan, L.; Tsang, L.H.; Smeltzer, M.S.; Horswill, A.R.; Bayles, K.W. Modulation of EDNA Release and Degradation Affects Staphylococcus Aureus Biofilm Maturation. PLoS ONE 2009, 4, e5822. [Google Scholar] [CrossRef]
- Okshevsky, M.; Meyer, R.L. The Role of Extracellular DNA in the Establishment, Maintenance and Perpetuation of Bacterial Biofilms. Crit. Rev. Microbiol. 2015, 41, 341–352. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic Di-GMP: The First 25 Years of a Universal Bacterial Second Messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal Infections: Mechanisms of Biofilm Maturation and Detachment as Critical Determinants of Pathogenicity. Annu. Rev. Med. 2013, 64, 175–188. [Google Scholar] [CrossRef]
- Lebeaux, D.; Ghigo, J.-M.; Beloin, C. Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol. Mol. Biol. Rev. 2014, 78, 510–543. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus Biofilm: A Complex Developmental Organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal Biofilms. Microbiol. Spectr. 2018, 6, 4. [Google Scholar] [CrossRef]
- Archer, N.K.; Mazaitis, M.J.; William Costerton, J.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus Aureus Biofilms: Properties, Regulation and Roles in Human Disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef]
- Wei, Q.; Ma, L.Z. Biofilm Matrix and Its Regulation in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2013, 14, 20983–21005. [Google Scholar] [CrossRef]
- Ghafoor, A.; Hay, I.D.; Rehm, B.H.A. Role of Exopolysaccharides in Pseudomonas aeruginosa Biofilm Formation and Architecture. Appl. Environ. Microbiol. 2011, 77, 5238–5246. [Google Scholar] [CrossRef]
- Desai, S.; Sanghrajka, K.; Gajjar, D. High Adhesion and Increased Cell Death Contribute to Strong Biofilm Formation in Klebsiella pneumoniae. Pathogens 2019, 8, 277. [Google Scholar] [CrossRef]
- Schroll, C.; Barken, K.B.; Krogfelt, K.A.; Struve, C. Role of Type 1 and Type 3 Fimbriae in Klebsiella pneumoniae Biofilm Formation. BMC Microbiol. 2010, 10, 179. [Google Scholar] [CrossRef]
- Gedefie, A.; Demsiss, W.; Belete, M.A.; Kassa, Y.; Tesfaye, M.; Tilahun, M.; Bisetegn, H.; Sahle, Z. Acinetobacter baumannii Biofilm Formation and Its Role in Disease Pathogenesis: A Review. Infect. Drug Resist. 2021, 14, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vivas, J.; Chapartegui-González, I.; Fernández-Martínez, M.; González-Rico, C.; Fortún, J.; Escudero, R.; Marco, F.; Linares, L.; Montejo, M.; Aranzamendi, M.; et al. Biofilm Formation by Multidrug Resistant Enterobacteriaceae Strains Isolated from Solid Organ Transplant Recipients. Sci. Rep. 2019, 9, 8928. [Google Scholar] [CrossRef] [PubMed]
- Cieślik, M.; Wójcicki, M.; Migdał, P.; Grygiel, I.; Bajrak, O.; Orwat, F.; Górski, A.; Jończyk-Matysiak, E. Fighting Biofilm: Bacteriophages Eliminate Biofilm Formed by Multidrug-Resistant Enterobacter Hormaechei on Urological Catheters. Med. Microbiol. Immunol. 2025, 214, 33. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S. Mechanisms of Antibiotic Resistance in Bacterial Biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular Mechanisms of Biofilm-Based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Lewis, K. Persister Cells, Dormancy and Infectious Disease. Nat. Rev. Microbiol. 2007, 5, 48–56. [Google Scholar] [CrossRef]
- Zhang, Y. Persisters, Persistent Infections and the Yin-Yang Model. Emerg. Microbes Infect. 2014, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grooters, K.E.; Ku, J.C.; Richter, D.M.; Krinock, M.J.; Minor, A.; Li, P.; Kim, A.; Sawyer, R.; Li, Y. Strategies for Combating Antibiotic Resistance in Bacterial Biofilms. Front. Cell. Infect. Microbiol. 2024, 14, 1352273. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Armbruster, C.R.; Parsek, M.R. New Insight into the Early Stages of Biofilm Formation. Proc. Natl. Acad. Sci. USA 2018, 115, 4317–4319. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental Factors That Shape Biofilm Formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Mishra, A.; Aggarwal, A.; Khan, F. Medical Device-Associated Infections Caused by Biofilm-Forming Microbial Pathogens and Controlling Strategies. Antibiotics 2024, 13, 623. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Zhang, L.; Mah, T.F. Involvement of a Novel Efflux System in Biofilm-Specific Resistance to Antibiotics. J. Bacteriol. 2008, 190, 4447–4452. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Tasneem, U.; Hussain, T.; Andleeb, S. Bacterial Biofilm: Its Composition, Formation and Role in Human Infections. Res. Rev. J. Microbiol. Biotechnol. 2015, 4, 1–14. [Google Scholar]
- Rather, M.A.; Gupta, K.; Bardhan, P.; Borah, M.; Sarkar, A.; Eldiehy, K.S.H.; Bhuyan, S.; Mandal, M. Microbial Biofilm: A Matter of Grave Concern for Human Health and Food Industry. J. Basic Microbiol. 2021, 61, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Beatson, S.A.; Walker, M.J. Tracking Antibiotic Resistance. Science (1979) 2014, 345, 1454–1455. [Google Scholar] [CrossRef]
- Tiwari, V. ESKAPE Biofilm: Challenges and Solutions. Front. Cell. Infect. Microbiol. 2023, 13, 1253439. [Google Scholar] [CrossRef]
- Manandhar, S.; Karn, D.; Shrestha, M.R.; Shakya, J.; Singh, A. Biofilm Formation, Methicillin Resistance and SCCmec Types among Staphylococcus Aureus Isolated from Clinical Samples from a Tertiary Care Hospital, in Nepal. BMC Infect. Dis. 2025, 25, 534. [Google Scholar] [CrossRef]
- McCarthy, H.; Rudkin, J.K.; Black, N.S.; Gallagher, L.; O’Neill, E.; O’Gara, J.P. Methicillin Resistance and the Biofilm Phenotype in Staphylococcus Aureus. Front. Cell. Infect. Microbiol. 2015, 5, 1. [Google Scholar] [CrossRef]
- Lodes, M.J.; Cong, Y.; Elson, C.O.; Mohamath, R.; Landers, C.J.; Targan, S.R.; Fort, M.; Hershberg, R.M. Bacterial Flagellin Is a Dominant Antigen in Crohn Disease. J. Clin. Investig. 2004, 113, 1296–1306. [Google Scholar] [CrossRef]
- Rice, K.C.; Bayles, K.W. Molecular Control of Bacterial Death and Lysis. Microbiol. Mol. Biol. Rev. 2008, 72, 85–109. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science (1979) 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic Resistance in Pseudomonas aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Chegini, Z.; Khoshbayan, A.; Taati Moghadam, M.; Farahani, I.; Jazireian, P.; Shariati, A. Bacteriophage Therapy against Pseudomonas aeruginosa Biofilms: A Review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J.; Williamson, K.S.; Folsom, J.P.; Boegli, L.; James, G.A. Contribution of Stress Responses to Antibiotic Tolerance in Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2015, 59, 3838–3847. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Tolker-Nielsen, T. Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial Agents-How P. aeruginosa Can Escape Antibiotics. Front. Microbiol. 2019, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Billón, M.; Llambías-Cabot, A.E.; Jordana-Lluch, E.; Oliver, A.; Macià, M.D. Mechanisms of Antibiotic Resistance in Pseudomonas aeruginosa Biofilms. Biofilm 2023, 5, 100129. [Google Scholar] [CrossRef]
- Zadeh, R.G.; Kalani, B.S.; Ari, M.M.; Talebi, M.; Razavi, S.; Jazi, F.M. Isolation of Persister Cells within the Biofilm and Relative Gene Expression Analysis of Type II Toxin/Antitoxin System in Pseudomonas aeruginosa Isolates in Exponential and Stationary Phases. J. Glob. Antimicrob. Resist. 2022, 28, 30–37. [Google Scholar] [CrossRef]
- Stahlhut, S.G.; Struve, C.; Krogfelt, K.A.; Reisner, A. Biofilm Formation of Klebsiella pneumoniae on Urethral Catheters Requires Either Type 1 or Type 3 Fimbriae. FEMS Immunol. Med. Microbiol. 2012, 65, 350–359. [Google Scholar] [CrossRef]
- Rifaat, R.M.; Ghaima, K.K. The Effect of Antimicrobial Peptide LL-37 on Biofilm Formation of Klebsiella pneumoniae Isolated from Catheter-Associated Urinary Tract Infection Patients. Iraqi J. Biotechnol. 2023, 22, 193–199. [Google Scholar]
- Zapotoczna, M.; McCarthy, H.; Rudkin, J.K.; O’Gara, J.P.; O’Neill, E. An Essential Role for Coagulase in Staphylococcus aureus Biofilm Development Reveals New Therapeutic Possibilities for Device-Related Infections. J. Infect. Dis. 2015, 212, 1883–1893. [Google Scholar] [CrossRef]
- Tart, A.H.; Wozniak, D.J. Shifting Paradigms in Pseudomonas aeruginosa Biofilm Research. In Bacterial Biofilms; Springer: Berlin/Heidelberg, Germany, 2008; pp. 193–206. [Google Scholar]
- Brunke, M.S.; Konrat, K.; Schaudinn, C.; Piening, B.; Pfeifer, Y.; Becker, L.; Schwebke, I.; Arvand, M. Tolerance of Biofilm of a Carbapenem-Resistant Klebsiella pneumoniae Involved in a Duodenoscopy-Associated Outbreak to the Disinfectant Used in Reprocessing. Antimicrob. Resist. Infect. Control. 2022, 11, 81. [Google Scholar] [CrossRef]
- Donelli, G.; Guaglianone, E. Emerging Role of Enterococcus spp. in Catheter-Related Infections: Biofilm Formation and Novel Mechanisms of Antibiotic Resistance. J. Vasc. Access 2004, 5, 3–9. [Google Scholar] [CrossRef]
- Busch, A.W.U.; Montgomery, B.L. The Tryptophan-Rich Sensory Protein (TSPO) Is Involved in Stress-Related and Light-Dependent Processes in the Cyanobacterium Fremyella Diplosiphon. Front. Microbiol. 2015, 6, 1393. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Z.; Elbaz, A.; Ni, S.Q. Synergistic Action of Phages and Lytic Proteins with Antibiotics: A Combination Strategy to Target Bacteria and Biofilms. BMC Microbiol. 2023, 23, 149. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-Encoded Endolysins. Antibiotics 2021, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Mayorga-Ramos, A.; Carrera-Pacheco, S.E.; Barba-Ostria, C.; Guamán, L.P. Bacteriophage-Mediated Approaches for Biofilm Control. Front. Cell. Infect. Microbiol. 2024, 14, 1428637. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, M.; Zhang, D. Potential of Phage Depolymerase for the Treatment of Bacterial Biofilms. Virulence 2023, 14, 2273567. [Google Scholar] [CrossRef]
- Dicks, L.M.T.; Vermeulen, W. Bacteriophage-Host Interactions and the Therapeutic Potential of Bacteriophages. Viruses 2024, 16, 478. [Google Scholar] [CrossRef]
- Liu, H.; Wei, X.; Wang, Z.; Huang, X.; Li, M.; Hu, Z.; Zhang, K.; Hu, Q.; Peng, H.; Shang, W.; et al. LysSYL: A Broad-Spectrum Phage Endolysin Targeting Staphylococcus Species and Eradicating S. Aureus Biofilms. Microb. Cell Fact. 2024, 23, 89. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Li, M.; Yang, Y.; Lu, S.; Rao, X. Therapeutic Potential of Bacteriophage Endolysins for Infections Caused by Gram-Positive Bacteria. J. Biomed. Sci. 2023, 30, 29. [Google Scholar] [CrossRef]
- Azeredo, J.; García, P.; Drulis-Kawa, Z. Targeting Biofilms Using Phages and Their Enzymes. Curr. Opin. Biotechnol. 2021, 68, 251–261. [Google Scholar] [CrossRef]
- Topka-Bielecka, G.; Dydecka, A.; Necel, A.; Bloch, S.; Nejman-Faleńczyk, B.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-Derived Depolymerases against Bacterial Biofilm. Antibiotics 2021, 10, 175. [Google Scholar] [CrossRef]
- Wang, J.; Liang, S.; Lu, X.; Xu, Q.; Zhu, Y.; Yu, S.; Zhang, W.; Liu, S.; Xie, F. Bacteriophage Endolysin Ply113 as a Potent Antibacterial Agent against Polymicrobial Biofilms Formed by Enterococci and Staphylococcus Aureus. Front. Microbiol. 2023, 14, 1304932. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Liu, B.; Wu, L.; Bao, H.; García, P.; Wang, Y.; Zhou, Y.; Zhang, H. A Broad-Spectrum Phage Endolysin (LysCP28) Able to Remove Biofilms and Inactivate Clostridium Perfringens Strains. Foods 2023, 12, 411. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Sousa, L.G.V.; França, Â.; Podpera Tisakova, L.; Corsini, L.; Cerca, N. Exploiting the Anti-Biofilm Effect of the Engineered Phage Endolysin PM-477 to Disrupt In Vitro Single- and Dual-Species Biofilms of Vaginal Pathogens Associated with Bacterial Vaginosis. Antibiotics 2022, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Briers, Y.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Role of the Pre-Neck Appendage Protein (Dpo7) from Phage VB_SepiS-PhiIPLA7 as an Anti-Biofilm Agent in Staphylococcal Species. Front. Microbiol. 2015, 6, 1315. [Google Scholar] [CrossRef]
- Shahed-Al-Mahmud, M.; Roy, R.; Sugiokto, F.G.; Islam, M.N.; Lin, M.-D.; Lin, L.-C.; Lin, N.-T. Phage ΦAB6-Borne Depolymerase Combats Acinetobacter baumannii Biofilm Formation and Infection. Antibiotics 2021, 10, 279. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, R.; Xu, M.; Liu, Y.; Zhu, X.; Qiu, J.; Liu, Q.; He, P.; Li, Q. A Novel Polysaccharide Depolymerase Encoded by the Phage SH-KP152226 Confers Specific Activity Against Multidrug-Resistant Klebsiella pneumoniae via Biofilm Degradation. Front. Microbiol. 2019, 10, 2768. [Google Scholar] [CrossRef]
- Amankwah, S.; Abdella, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review On The Recent Use of Phage-Based Strategies With Other Antibiofilm Agents. Nanotechnol. Sci. Appl. 2021, 14, 161–177. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Ananthasubramanian, M. Characterization and Lytic Activity of Pseudomonas Fluorescens Phages from Sewage. Braz. J. Microbiol. 2012, 43, 356–362. [Google Scholar] [CrossRef][Green Version]
- Alexyuk, P.; Bogoyavlenskiy, A.; Alexyuk, M.; Akanova, K.; Moldakhanov, Y.; Berezin, V. Isolation and Characterization of Lytic Bacteriophages Active against Clinical Strains of E. Coli and Development of a Phage Antimicrobial Cocktail. Viruses 2022, 14, 2381. [Google Scholar] [CrossRef]
- Kutter, E.; De Vos, D.; Gvasalia, G.; Alavidze, Z.; Gogokhia, L.; Kuhl, S.; Abedon, S. Phage Therapy in Clinical Practice: Treatment of Human Infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef]
- Paranos, P.; Pournaras, S.; Meletiadis, J. Detection of Phage’s Lytic Activity Against Carbapenemase-Producing Klebsiella pneumoniae Isolates Using a High-Throughput Microbroth Growth Inhibition Assay. Infect. Dis. Ther. 2025, 14, 217–228. [Google Scholar] [CrossRef]
- Hietala, V.; Horsma-Heikkinen, J.; Carron, A.; Skurnik, M.; Kiljunen, S. The Removal of Endo- and Enterotoxins From Bacteriophage Preparations. Front. Microbiol. 2019, 10, 1674. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.; Suh, G.A. The Safety and Toxicity of Phage Therapy: A Review of Pre-Clinical and Clinical Studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef] [PubMed]
- Taha, O.A.; Connerton, P.L.; Connerton, I.F.; El-Shibiny, A. Bacteriophage ZCKP1: A Potential Treatment for Klebsiella pneumoniae Isolated From Diabetic Foot Patients. Front. Microbiol. 2018, 9, 2127. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, N.M.; Devequi Gomes Nunes, D.; Shiach, J.; Valeria Saraiva Hodel, K.; Dantas Viana Barbosa, J.; Alencar Pereira Rodrigues, L.; Coler, B.S.; Botelho Pereira Soares, M.; Badaró, R. Current Clinical Landscape and Global Potential of Bacteriophage Therapy. Viruses 2023, 15, 1020. [Google Scholar] [CrossRef]
- Febvre, H.P.; Rao, S.; Gindin, M.; Goodwin, N.D.M.; Finer, E.; Vivanco, J.S.; Lu, S.; Manter, D.K.; Wallace, T.C.; Weir, T.L. PHAGE Study: Effects of Supplemental Bacteriophage Intake on Inflammation and Gut Microbiota in Healthy Adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef]
- Mu, A.; McDonald, D.; Jarmusch, A.K.; Martino, C.; Brennan, C.; Bryant, M.; Humphrey, G.C.; Toronczak, J.; Schwartz, T.; Nguyen, D.; et al. Assessment of the Microbiome during Bacteriophage Therapy in Combination with Systemic Antibiotics to Treat a Case of Staphylococcal Device Infection. Microbiome 2021, 9, 92. [Google Scholar] [CrossRef]
- Torkashvand, N.; Kamyab, H.; Aarabi, P.; Shahverdi, A.R.; Torshizi, M.A.K.; Khoshayand, M.R.; Sepehrizadeh, Z. Evaluating the Effectiveness and Safety of a Novel Phage Cocktail as a Biocontrol of Salmonella in Biofilm, Food Products, and Broiler Chicken. Front. Microbiol. 2024, 15, 1505805. [Google Scholar] [CrossRef]
- Bull, J.J.; Wichman, H.A.; Krone, S.M.; Molineux, I.J. Controlling Recombination to Evolve Bacteriophages. Cells 2024, 13, 585. [Google Scholar] [CrossRef]
- Bull, J.J.; Levin, B.R.; Molineux, I.J. Promises and Pitfalls of In Vivo Evolution to Improve Phage Therapy. Viruses 2019, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Bozidis, P.; Markou, E.; Gouni, A.; Gartzonika, K. Does Phage Therapy Need a Pan-Phage? Pathogens 2024, 13, 522. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lu, H.; Zhang, S.; Shi, Y.; Chen, Q. Phages against Pathogenic Bacterial Biofilms and Biofilm-Based Infections: A Review. Pharmaceutics 2022, 14, 427. [Google Scholar] [CrossRef]
- Brás, A.; Braz, M.; Martinho, I.; Duarte, J.; Pereira, C.; Almeida, A. Effect of Bacteriophages against Biofilms of Escherichia Coli on Food Processing Surfaces. Microorganisms 2024, 12, 366. [Google Scholar] [CrossRef]
- Tabassum, R.; Shafique, M.; Khawaja, K.A.; Alvi, I.A.; Rehman, Y.; Sheik, C.S.; Abbas, Z.; Rehman, S.u. Complete Genome Analysis of a Siphoviridae Phage TSK1 Showing Biofilm Removal Potential against Klebsiella pneumoniae. Sci. Rep. 2018, 8, 17904. [Google Scholar] [CrossRef]
- Abdelghafar, A.; El-Ganiny, A.; Shaker, G.; Askoura, M. A Novel Lytic Phage Exhibiting a Remarkable in Vivo Therapeutic Potential and Higher Antibiofilm Activity against Pseudomonas aeruginosa. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 42, 1207–1234. [Google Scholar] [CrossRef]
- Adnan, M.; Ali Shah, M.R.; Jamal, M.; Jalil, F.; Andleeb, S.; Nawaz, M.A.; Pervez, S.; Hussain, T.; Shah, I.; Imran, M.; et al. Isolation and Characterization of Bacteriophage to Control Multidrug-Resistant Pseudomonas aeruginosa Planktonic Cells and Biofilm. Biologicals 2020, 63, 89–96. [Google Scholar] [CrossRef]
- Hakim, T.A.; Zaki, B.M.; Mohamed, D.A.; Blasdel, B.; Gad, M.A.; Fayez, M.S.; El-Shibiny, A. Novel Strategies for Vancomycin-Resistant Enterococcus Faecalis Biofilm Control: Bacteriophage (VB_EfaS_ZC1), Propolis, and Their Combined Effects in an Ex Vivo Endodontic Model. Ann. Clin. Microbiol. Antimicrob. 2025, 24, 24. [Google Scholar] [CrossRef]
- Singh, A.; Padmesh, S.; Dwivedi, M.; Kostova, I. How Good Are Bacteriophages as an Alternative Therapy to Mitigate Biofilms of Nosocomial Infections. Infect. Drug Resist. 2022, 15, 503–532. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Y.; Galgano, S.; Houdijk, J.; Xie, W.; Jin, Y.; Lin, J.; Song, W.; Fu, Y.; Li, X.; et al. Recent Progress in Phage Therapy to Modulate Multidrug-Resistant Acinetobacter baumannii, Including in Human and Poultry. Antibiotics 2022, 11, 1406. [Google Scholar] [CrossRef] [PubMed]
- Ponce Benavente, L.; Wagemans, J.; Hinkel, D.; Aguerri Lajusticia, A.; Lavigne, R.; Trampuz, A.; Gonzalez Moreno, M. Targeted Enhancement of Bacteriophage Activity against Antibiotic-Resistant Staphylococcus Aureus Biofilms through an Evolutionary Assay. Front. Microbiol. 2024, 15, 1372325. [Google Scholar] [CrossRef] [PubMed]
- Królikowska, D.; Szymańska, M.; Krzyżaniak, M.; Guziński, A.; Matusiak, R.; Kajdanek, A.; Kaczorek-Łukowska, E.; Maszewska, A.; Wójcik, E.A.; Dastych, J. A New Approach for Phage Cocktail Design in the Example of Anti-Mastitis Solution. Pathogens 2024, 13, 839. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Mahbub, N.U.; Shin, W.S.; Oh, M.H. Phage-Encoded Depolymerases as a Strategy for Combating Multidrug-Resistant Acinetobacter baumannii. Front. Cell. Infect. Microbiol. 2024, 14, 1462620. [Google Scholar] [CrossRef]
- Mgomi, F.C.; Yuan, L.; Chen, C.; Zhang, Y.; Yang, Z. Bacteriophages: A Weapon against Mixed-Species Biofilms in the Food Processing Environment. J. Appl. Microbiol. 2022, 133, 2107–2121. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Borin, J.M.; Lee, J.J.; Gerbino, K.R.; Meyer, J.R. Comparison of Bacterial Suppression by Phage Cocktails, Dual-receptor Generalists, and Coevolutionarily Trained Phages. Evol. Appl. 2023, 16, 152–162. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, W.; Zhong, Q.; Chen, Q.; He, X.; Baker, J.L.; Xiong, K.; Jin, X.; Wang, J.; Hu, F.; et al. Development of a Bacteriophage Cocktail to Constrain the Emergence of Phage-Resistant Pseudomonas aeruginosa. Front. Microbiol. 2020, 11, 327. [Google Scholar] [CrossRef]
- Kunisch, F.; Campobasso, C.; Wagemans, J.; Yildirim, S.; Chan, B.K.; Schaudinn, C.; Lavigne, R.; Turner, P.E.; Raschke, M.J.; Trampuz, A.; et al. Targeting Pseudomonas aeruginosa Biofilm with an Evolutionary Trained Bacteriophage Cocktail Exploiting Phage Resistance Trade-Offs. Nat. Commun. 2024, 15, 8572. [Google Scholar] [CrossRef]
- Gao, D.; Ji, H.; Wang, L.; Li, X.; Hu, D.; Zhao, J.; Wang, S.; Tao, P.; Li, X.; Qian, P. Fitness Trade-Offs in Phage Cocktail-Resistant Salmonella Enterica Serovar Enteritidis Results in Increased Antibiotic Susceptibility and Reduced Virulence. Microbiol. Spectr. 2022, 10, e0291422. [Google Scholar] [CrossRef]
- Malik, S.; Nehra, K.; Rana, J.S. Bacteriophage Cocktail and Phage Antibiotic Synergism as Promising Alternatives to Conventional Antibiotics for the Control of Multi-Drug-Resistant Uropathogenic Escherichia Coli. Virus Res. 2021, 302, 198496. [Google Scholar] [CrossRef]
- Vashisth, M.; Jaglan, A.B.; Yashveer, S.; Sharma, P.; Bardajatya, P.; Virmani, N.; Bera, B.C.; Vaid, R.K.; Anand, T. Development and Evaluation of Bacteriophage Cocktail to Eradicate Biofilms Formed by an Extensively Drug-Resistant (XDR) Pseudomonas aeruginosa. Viruses 2023, 15, 427. [Google Scholar] [CrossRef]
- Moura de Sousa, J.A.; Pfeifer, E.; Touchon, M.; Rocha, E.P.C. Causes and Consequences of Bacteriophage Diversification via Genetic Exchanges across Lifestyles and Bacterial Taxa. Mol. Biol. Evol. 2021, 38, 2497–2512. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F.; Piccardi, P.; Mancini, S.; Gabard, J.; Moreillon, P.; Entenza, J.M.; Resch, G.; Que, Y.-A. Synergistic Interaction between Phage Therapy and Antibiotics Clears Pseudomonas aeruginosa Infection in Endocarditis and Reduces Virulence. J. Infect. Dis. 2016, 215, jiw632. [Google Scholar] [CrossRef] [PubMed]
- Górniak, M.; Zalewska, A.; Jurczak-Kurek, A. Recombination Events in Putative Tail Fibre Gene in Litunavirus Phages Infecting Pseudomonas aeruginosa and Their Phylogenetic Consequences. Viruses 2022, 14, 2669. [Google Scholar] [CrossRef] [PubMed]
- Blasco, L.; Bleriot, I.; González de Aledo, M.; Fernández-García, L.; Pacios, O.; Oliveira, H.; López, M.; Ortiz-Cartagena, C.; Fernández-Cuenca, F.; Pascual, Á.; et al. Development of an Anti-Acinetobacter baumannii Biofilm Phage Cocktail: Genomic Adaptation to the Host. Antimicrob. Agents Chemother. 2022, 66, e0192321. [Google Scholar] [CrossRef]
- Yang, Q.; Le, S.; Zhu, T.; Wu, N. Regulations of Phage Therapy across the World. Front. Microbiol. 2023, 14, 1250848. [Google Scholar] [CrossRef]
- Al-Anany, A.M.; Fatima, R.; Hynes, A.P. Temperate Phage-Antibiotic Synergy Eradicates Bacteria through Depletion of Lysogens. Cell Rep. 2021, 35, 109172. [Google Scholar] [CrossRef]
- Glen, K.A.; Lamont, I.L. Penicillin-Binding Protein 3 Sequence Variations Reduce Susceptibility of Pseudomonas aeruginosa to β-Lactams but Inhibit Cell Division. J. Antimicrob. Chemother. 2024, 79, 2170–2178. [Google Scholar] [CrossRef]
- Ogino, H.; Teramoto, H.; Inui, M.; Yukawa, H. DivS, a Novel SOS-inducible Cell-division Suppressor in Corynebacterium glutamicum. Mol. Microbiol. 2008, 67, 597–608. [Google Scholar] [CrossRef]
- Ojkic, N.; Lilja, E.; Direito, S.; Dawson, A.; Allen, R.J.; Waclaw, B. A Roadblock-and-Kill Mechanism of Action Model for the DNA-Targeting Antibiotic Ciprofloxacin. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Possoz, C.; Newmark, J.; Sorto, N.; Sherratt, D.J.; Tolmasky, M.E. Sublethal Concentrations of the Aminoglycoside Amikacin Interfere with Cell Division without Affecting Chromosome Dynamics. Antimicrob. Agents Chemother. 2007, 51, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Supina, B.S.I.; Dennis, J.J. The Current Landscape of Phage–Antibiotic Synergistic (PAS) Interactions. Antibiotics 2025, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Bulssico, J.; PapukashvilI, I.; Espinosa, L.; Gandon, S.; Ansaldi, M. Phage-Antibiotic Synergy: Cell Filamentation Is a Key Driver of Successful Phage Predation. PLoS Pathog. 2023, 19, e1011602. [Google Scholar] [CrossRef]
- Zhao, M.; Li, H.; Gan, D.; Wang, M.; Deng, H.; Yang, Q.E. Antibacterial Effect of Phage Cocktails and Phage-Antibiotic Synergy against Pathogenic Klebsiella Pneumoniae. mSystems 2024, 9, e0060724. [Google Scholar] [CrossRef]
- Gu Liu, C.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplan, H.B.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry. mBio 2020, 11, e01462-20. [Google Scholar] [CrossRef]
- Morris, T.C.; Reyneke, B.; Khan, S.; Khan, W. Phage-Antibiotic Synergy to Combat Multidrug Resistant Strains of Gram-Negative ESKAPE Pathogens. Sci. Rep. 2025, 15, 17235. [Google Scholar] [CrossRef]
- Karthika, C.; Malligarjunan, N.; Hari Prasath, N.; Karutha Pandian, S.; Gowrishankar, S. Phage (Cocktail)-Antibiotic Synergism: A New Frontier in Addressing Klebsiella pneumoniae Resistance. Front. Microbiol. 2025, 16, 1588472. [Google Scholar] [CrossRef]
- Lin, L.-C.; Tsai, Y.-C.; Lin, N.-T. Phage–Antibiotic Synergy Enhances Biofilm Eradication and Survival in a Zebrafish Model of Pseudomonas aeruginosa Infection. Int. J. Mol. Sci. 2025, 26, 5337. [Google Scholar] [CrossRef]
- De Soir, D.S.; Parée, H.; Kumarudin, N.H.N.; Waegemans, J.; Lavigne, P.R.; Braem, P.A.; Merabishvilli, M.; De Vos, D.; Pirnay, J.-P.; Van Bambeke, P.F. Exploring Phage-Antibiotic Synergies in The Context of Biofilm-Related Infectious Diseases. Int. J. Infect. Dis. 2025, 152, 107590. [Google Scholar] [CrossRef]
- Vashisth, M.; Yashveer, S.; Jaglan, A.B.; Virmani, N.; Bera, B.C.; Vaid, R.K.; Anand, T. Synergy of a Virulent Phage (ΦAB182) with Antibiotics Leading to Successful Elimination of Biofilms Formed by MDR Acinetobacter baumannii. Can. J. Microbiol. 2022, 68, 731–746. [Google Scholar] [CrossRef]
- Kunz Coyne, A.J.; Stamper, K.; Bleick, C.; Kebriaei, R.; Lehman, S.M.; Rybak, M.J. Synergistic Bactericidal Effects of Phage-Enhanced Antibiotic Therapy against MRSA Biofilms. Microbiol. Spectr. 2024, 12, e0321223. [Google Scholar] [CrossRef] [PubMed]
- Lev, K.; Kunz Coyne, A.J.; Kebriaei, R.; Morrisette, T.; Stamper, K.; Holger, D.J.; Canfield, G.S.; Duerkop, B.A.; Arias, C.A.; Rybak, M.J. Evaluation of Bacteriophage-Antibiotic Combination Therapy for Biofilm-Embedded MDR Enterococcus Faecium. Antibiotics 2022, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Shen, L.; Zhang, B.-L.; Yu, J.; Wei, F.; Sun, Y.; Chen, W.; Wang, S. Advance on Engineering of Bacteriophages by Synthetic Biology. Infect. Drug Resist. 2023, 16, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Mion, S.; Rémy, B.; Plener, L.; Brégeon, F.; Chabrière, E.; Daudé, D. Quorum Quenching Lactonase Strengthens Bacteriophage and Antibiotic Arsenal Against Pseudomonas aeruginosa Clinical Isolates. Front. Microbiol. 2019, 10, 2049. [Google Scholar] [CrossRef]
- Tinoco, J.M.; Buttaro, B.; Zhang, H.; Liss, N.; Sassone, L.; Stevens, R. Effect of a Genetically Engineered Bacteriophage on Enterococcus Faecalis Biofilms. Arch. Oral. Biol. 2016, 71, 80–86. [Google Scholar] [CrossRef]
- Łobocka, M.; Dąbrowska, K.; Górski, A. Engineered Bacteriophage Therapeutics: Rationale, Challenges and Future. BioDrugs 2021, 35, 255–280. [Google Scholar] [CrossRef]
- Steczynska, J.P.; Kerr, S.J.; Kelly, M.L.; Whittle, M.J.; Bilverstone, T.W.; Minton, N.P. A Tale of Two Phage Tails: Engineering the Host Range of Bacteriophages Infecting Clostridioides difficile. bioRxiv 2023. bioRxiv:2023.10.16.562632. [Google Scholar] [CrossRef]
- Mapes, A.C.; Trautner, B.W.; Liao, K.S.; Ramig, R.F. Development of Expanded Host Range Phage Active on Biofilms of Multi-Drug Resistant Pseudomonas aeruginosa. Bacteriophage 2016, 6, e1096995. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Bai, C.; Leung, S.S.Y. Translating Bacteriophage-Derived Depolymerases into Antibacterial Therapeutics: Challenges and Prospects. Acta Pharm. Sin. B 2024, 14, 155–169. [Google Scholar] [CrossRef]
- Khan, F.M.; Chen, J.-H.; Zhang, R.; Liu, B. A Comprehensive Review of the Applications of Bacteriophage-Derived Endolysins for Foodborne Bacterial Pathogens and Food Safety: Recent Advances, Challenges, and Future Perspective. Front. Microbiol. 2023, 14, 1259210. [Google Scholar] [CrossRef]
- Chung, K.M.; Liau, X.L.; Tang, S.S. Bacteriophages and Their Host Range in Multidrug-Resistant Bacterial Disease Treatment. Pharmaceuticals 2023, 16, 1467. [Google Scholar] [CrossRef]
- Fatima, R.; Hynes, A.P. Phage-Antibiotic Combinations for Pseudomonas: Successes in the Clinic and In Vitro Tenuously Connected. Microb. Biotechnol. 2025, 18, e70193. [Google Scholar] [CrossRef]
- Kakkar, A.; Kandwal, G.; Nayak, T.; Jaiswal, L.K.; Srivastava, A.; Gupta, A. Engineered Bacteriophages: A Panacea against Pathogenic and Drug Resistant Bacteria. Heliyon 2024, 10, e34333. [Google Scholar] [CrossRef]
- Ruedelsheim, P.L.J.; Smets, G.; Van Der Meulen, K.; Van Rooij, P. Advances in the Application of GM Phages in Research, Development and Industry, Final COGEM Report CGM 2024-02 2024. Available online: https://cogem.net/en/publication/advances-in-the-application-of-gm-phages-in-research-development-and-industry/ (accessed on 24 July 2025).
- ClinicalTrials.Gov. Search Results for “Bacteriophage Therapy”; Identifier List Accessed via ClinicalTrials.Gov. Available online: Https://Clinicaltrials.Gov/Search?Intr=Bacteriophage%20Therapy&page=3 (accessed on 24 July 2025).
- Latka, A.; Drulis-Kawa, Z. Advantages and Limitations of Microtiter Biofilm Assays in the Model of Antibiofilm Activity of Klebsiella Phage KP34 and Its Depolymerase. Sci. Rep. 2020, 10, 20338. [Google Scholar] [CrossRef]
- Zurabov, F.; Zhilenkov, E. Characterization of Four Virulent Klebsiella pneumoniae Bacteriophages, and Evaluation of Their Potential Use in Complex Phage Preparation. Virol. J. 2021, 18, 9. [Google Scholar] [CrossRef]
- Asghar, S.; Ahmed, A.; Khan, S.; Lail, A.; Shakeel, M. Genomic Characterization of Lytic Bacteriophages A¥L and A¥M Infecting ESBL K. Pneumoniae and Its Therapeutic Potential on Biofilm Dispersal and in-Vivo Bacterial Clearance. Microbiol. Res. 2022, 262, 127104. [Google Scholar] [CrossRef] [PubMed]
- Wintachai, P.; Surachat, K.; Chaimaha, G.; Septama, A.W.; Smith, D.R. Isolation and Characterization of a Phapecoctavirus Infecting Multidrug-Resistant Acinetobacter baumannii in A549 Alveolar Epithelial Cells. Viruses 2022, 14, 2561. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, N.K.D.; Muthuirulandi Sethuvel, D.P.; Gopikrishnan, M.; Dwarakanathan, H.T.; Murugan, D.; Biswas, I.; Bakthavachalam, Y.D.; Murugesan, M.; George Priya Doss, C.; Monk, P.N.; et al. Phage-Based Therapy against Biofilm Producers in Gram-Negative ESKAPE Pathogens. Microb. Pathog. 2023, 178, 106064. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.J.; Rapp, E.M.; Rankin, W.R.; McKenzie, S.M.; Brasko, B.K.; Hebert, K.E.; Bachert, B.A.; Kick, A.R.; Burpo, F.J.; Barnhill, J.C. Combinations of Bacteriophage Are Efficacious against Multidrug-Resistant Pseudomonas aeruginosa and Enhance Sensitivity to Carbapenem Antibiotics. Viruses 2024, 16, 1000. [Google Scholar] [CrossRef]
- Guła, G.; Majkowska-Skrobek, G.; Misterkiewicz, A.; Salwińska, W.; Piasecki, T.; Drulis-Kawa, Z. Klebsiella Lytic Phages Induce Pseudomonas aeruginosa PAO1 Biofilm Formation. Viruses 2025, 17, 615. [Google Scholar] [CrossRef]
- NCT06942624; Orthopaedic Innovation Centre Phage Therapy for the Treatment of a Chronic Enterococcus Faecium Periprosthetic Joint Infection. Adaptive Phage Therapeutics, Inc.: Gaithersburg, MD, USA, 2025.
- NCT06456424; University of Calgary Bacteriophage Therapy for Methicillin-Sensitive Staphylococcus Aureus Prosthetic Joint Infection. Adaptive Phage Therapeutics, Inc.: Gaithersburg, MD, USA, 2024.
- NCT06798168; Ottawa Hospital Research Institute Bacteriophage Clinical Trial for Periprosthetic Joint Infection of Multidrug Resistant Pseudomonas aeruginosa. Adaptive Phage Therapeutics, Inc.: Gaithersburg, MD, USA, 2025.
- NCT06605651; Phaxiam Therapeutics A Phase II Proof of Concept Multicenter, Randomized, Double-Blind Study to Assess the Safety and Efficacy of Phage Therapy in Patients with Hip or Knee Prosthetic Joint Infection due to Staphylococcus Aureus Treated by DAIR. Adaptive Phage Therapeutics, Inc.: Gaithersburg, MD, USA, 2025.
- NCT04787250; Randomized Open Label, Parallel Group, Controlled Study to Evaluate the Safety and Surgery Sparing Effect of Phage Therapy with Antibiotics for Patients with Prosthetic Joint Infections Who Are Candidates for Two Stage Exchange Arthroplasty. Adaptive Phage Therapeutics, Inc.: Gaithersburg, MD, USA, 2022.
- NCT05269121; An Open-Label Multicenter Study to Evaluate the Safety and Efficacy of PhageBankTM Phage Therapy in Conjunction With Debridement, Antibiotics, and Implant Retention (DAIR) for Patients with First Time Culture Proven Chronic Prosthetic Joint Infection. Adaptive Phage Therapeutics, Inc.: Gaithersburg, MD, USA, 2022.
- NCT05269134; A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of APT Phage Bank Phage Therapy Versus Placebo in Conjunction with Debridement, Antibiotics, and Implant Retention (DAIR) in Subjects with Chronic Prosthetic Joint Infection (PJI). Adaptive Phage Therapeutics, Inc.: Gaithersburg, MD, USA, 2023.
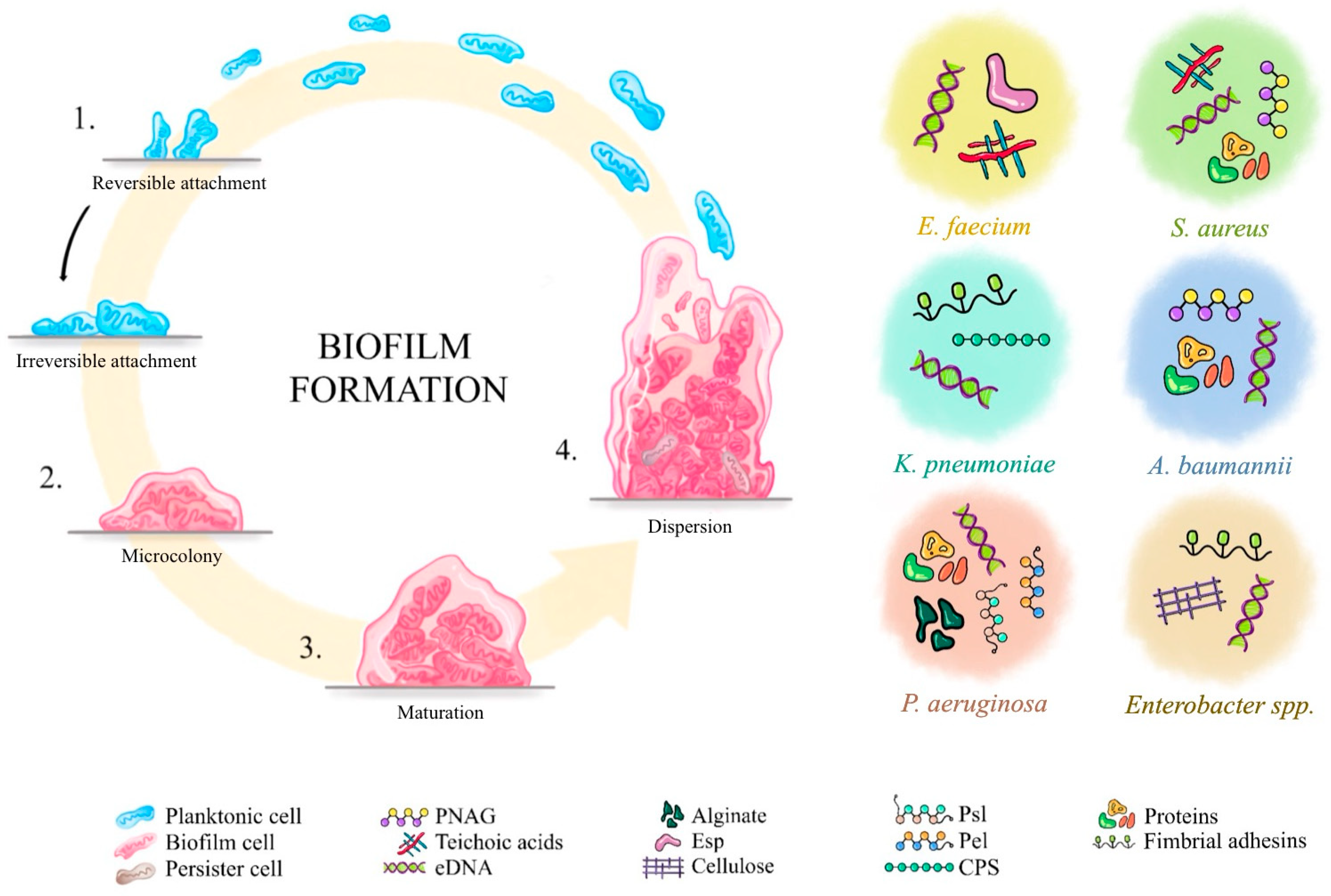
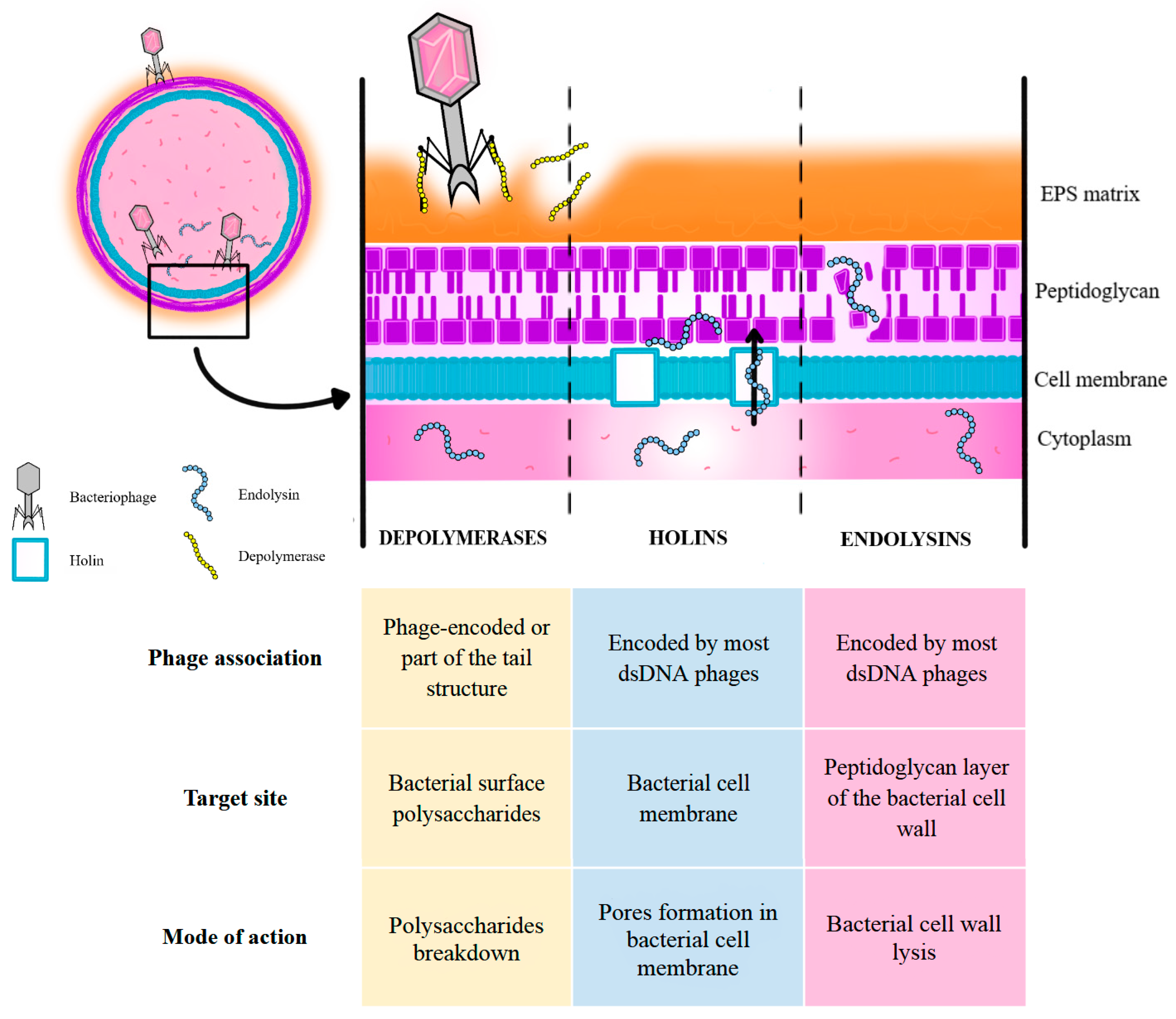
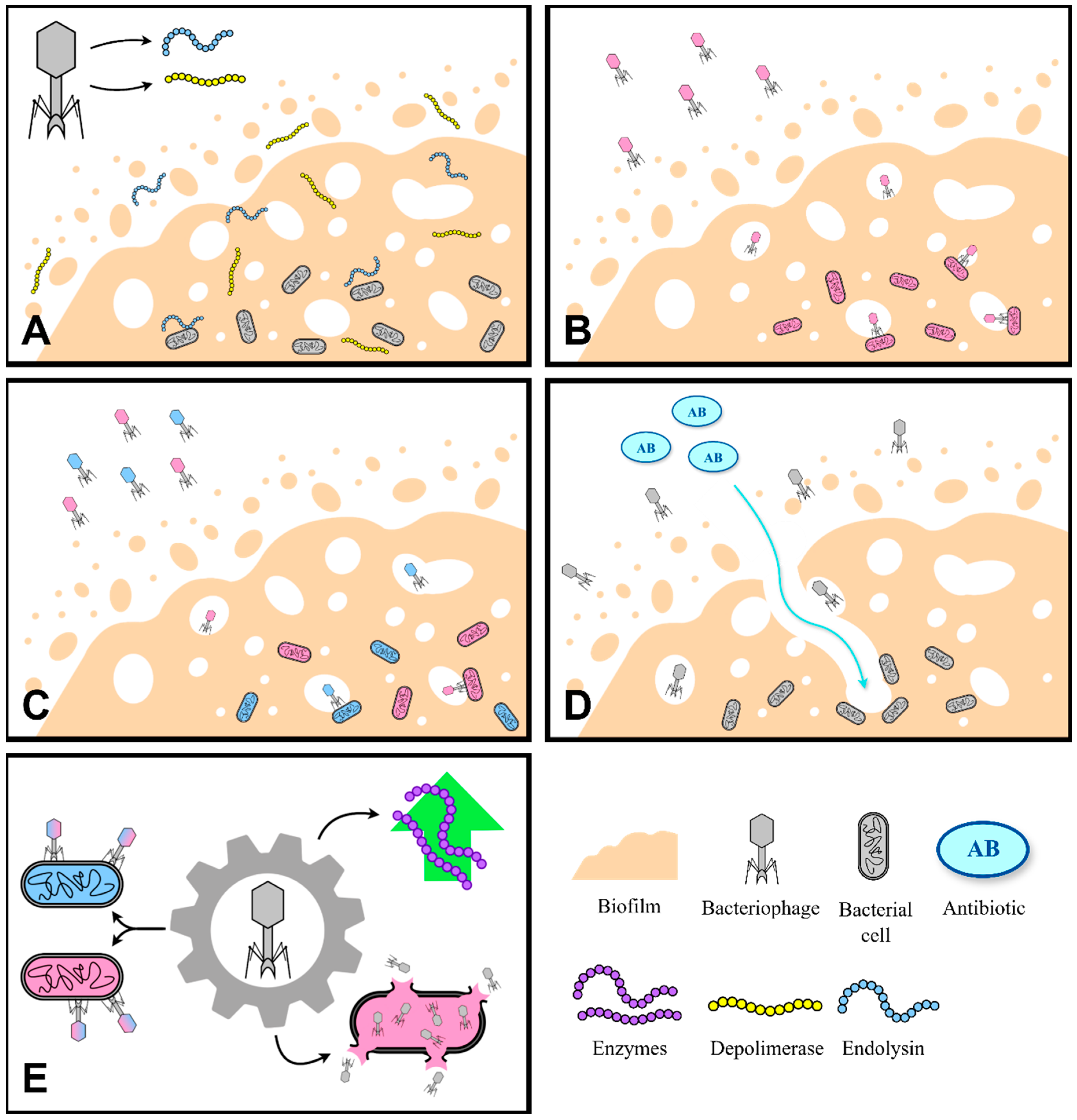
| Pathogen | EPS Components | References |
|---|---|---|
| Staphylococcus aureus | - PIA/PNAG - Teichoic acids - eDNA - Proteins: coagulase, adhesins, proteases | [25,26,27] |
| Pseudomonas aeruginosa | - Alginate - Pel - Psl - eDNA via autolysis - Proteins: lectins LecA, LecB | [24,25] |
| Klebsiella pneumoniae | - CPS - Colanic acid-like EPS - Fimbrial adhesins type 1 & 3 - eDNA | [25,26,27,38,39] |
| Enterococcus faecium | - Polysaccharide antigen - Esp - Lipoteichoic acids - eDNA | [25,26,27,29,32] |
| Acinetobacter baumannii | - PNAG - Capsule polysaccharides - eDNA - Proteins | [25,26,27,29,40] |
| Enterobacter spp. | - Colanic acid-like polysaccharides - Cellulose - Fimbrial adhesins - eDNA | [25,27,41,42] |
| Pathogen | Risk Factors | References |
|---|---|---|
| Staphylococcus aureus | - Catheters, mechanical heart valves, hip prosthesis - Chronic wounds, severe cutaneous infections, and skin diseases - Protein recycling - Matrix proteins enhancing flexibility, adaptation, and mixed species of biofilm - SCVs—diverse phenotype within biofilms | [9,56] |
| Pseudomonas aeruginosa | - Efflux pumps - OprD oprin - Wounds, cystic fibrosis, and other chronic infections - Endotracheal tubes, contact lenses - Protective response to stress - Neutralizing enzymes (cephalosporinase AmpC) - Slow growth rate inside colony - SCVs—diverse phenotype within biofilms - Matrix acidification | [9,56,57] |
| Klebsiella pneumoniae | - Temperature from 35 °C to 40 °C - Consistent growth on abiotic surfaces - Mixed strains of Klebsiella penumonaiae - Infectious urinary stones | [9,56] |
| Enterococcus faecium | - Intestinal infections - Hip prosthesis - Local environment | [56,57] |
| Acinetobacter baumannii | - Temperature, osmolarity, ferrous iron concentration - Nutrients and glucose availability - Ambient acidic conditions - Hydrophobicity and oxygen content | [40] |
| Enterobacter spp. | - SCVs—diverse phenotype within biofilms - Urological catheters - Also risk of biofilm formation on: dental materials, nasogastric and orogastric enteral feeding tubes, prostheses, and other medical devices - Body temperature (37 °C) > room temperature (24 °C) | [9,42] |
| Priority | Name of Bacteria from ESKAPE |
|---|---|
| 1: CRITICAL | Acinetobacter baumannii, carbapenem-resistant; Pseudomonas aeruginosa, carbapenem-resistant; Enterobacteriaceae, carbapenem-resistant, 3rd generation cephalosporin-resistant |
| 2: HIGH | Enterococcus faecium, vancomycin-resistant; Staphylococcus aureus, methicillin-resistant, vancomycin intermediate and -resistant |
| Feature | Staphylococcus aureus | Pseudomonas aeruginosa | Klebsiella pneumoniae | Enterococcus faecium | Acinetobacter baumannii | Enterobacter spp. |
|---|---|---|---|---|---|---|
| Gram-stain and shape | G (+) cocci | G (−) rod | G (−) rod | G (+) cocci | G (−) rod | G (−) rod |
| Antibiotic resistance | MRSA | Carbapenem–resistant P. aeruginosa | ESBL | VREs | Carbapenem–resistant A. baumannii | ESBL, carbapenem– resistant Enterobacter spp. |
| Adhesion mechanism | Fibronectin binding proteins A, B, clumping factors, A and B, and collagen binding proteins (MSCRAMMs) | Type IV pili, alginate | Type III fimbriae fim (homolog of enterococcal ebp), type I fimbriae, type VI protein secretion system | ESP, MSCRAMMAce, aggregation substance, capsule | Capsule (cell-to-cell adhesion) | Type VI secretion system, enterobactin |
| Biofilm formation | Aggregation substance | Type IV pili | Capsular polysaccharide, type III fimbriae | Capsule, cell wall polysaccharide, aggregation substance | Capsular polysaccharide | Capsule |
| Device adhesion | Prosthetic joints, pacemakers, vascular catheters | Ventilator tubing, urinary catheters, central lines | Endotracheal tubes, duodenoscopes, urinary catheters | Central venous catheters, prosthetic valves | Ventilators, central lines, urinary catheters | Urinary catheters, transplant–related devices |
| Infections | Wound infections, multiple soft tissue infections, infective carditis, bacteremia, fatal pneumonia | Immunocompromised patients, isolated from CF and burn patients. Nosocomial infections–ventilator-associated pneumonia, urinary tract infections, central line bloodstream infections, surgical infections | Community–acquired pneumonia, urinary tract, blood stream, and brain infections | Catheter–associated urinary tract infections, surgical site infections, bloostream infections | Critically ill patients who are severely immunocompromised, hospital-acquired respiratory infections and urinary tract, wound infections | Bacteremia, urinary tract infections, surgical site infections, device-related infections |
| References | [33,34,35,60,61,63,73] | [36,37,62,63,64,65,66,67,68,69,70,74] | [38,39,71,72,75] | [76] | [40] | [41,42] |
| Type of Phage Therapy | Characteristic | Advantages | Disadvantages | Effectiveness (In Vitro/In Vivo) |
|---|---|---|---|---|
| Phage-derived lytic enzymes | Direct influence on the biofilm matrix components without the use of phage [85,86] | No use of viral vectors, quick action onset, low risk of resistance [84,155,156] | Limited effectiveness on G(−) bacteria [85,86] | High especially on G(+) bacteria [85,86] |
| Single-phage therapy | Influence on a specific strain of the pathogen and its biofilm [157] | High specificity, low risk of dysbiosis, lower risk of genetic recombination [103,107] | Narrow spectrum, risk of resistance development [66,108,109] | High on specific pathogen strains [100,110,111,112,113,114,115,116] |
| Phage cocktails | Influence on heterogenic-strain pathogen and its biofilm or multi-species infections and biofilms; better biofilm penetration [80,117,119,120,121] | Wide spectrum, low risk of resistance development [122,123,124,125] | Interphage genetic recombination risk [105,128,129,130,131] | High [104,126,127] |
| PAS | Increased penetration of antibiotics, increased susceptibility to phages [80,133,134,135,136,137,138,139] | Lower medication doses, effectiveness on MDR biofilms [140,141,142,143] | Lack of standardized medication schematics [158] | High, synergistic effect [143,144,145] |
| Engineered phages | Increased production of biofilm-degrading enzymes, modifications of lytic/lysogenic character, host range modifications [149] | enhanced biofilm degradation, wider host range | High costs, regulation problems, uncertain genomic stability [159,160] | Very high [121,149,150,151] |
| Target Pathogen | Phage(s) Used | Key Findings | Study Authors and Date |
|---|---|---|---|
| MDR Klebsiella pneumoniae biofilm | KP34 (depolymerase-producing), KP15 (non-depolymerase), recombinant KP34p57 enzyme | Phage KP34 achieved a ~3-log biofilm reduction, further enhanced to 4 logs when combined with KP15. The triple combination with ciprofloxacin led to a 5.7-log decrease. While KP34p57 depolymerase alone showed minimal effect, it significantly boosted phage efficacy, highlighting its role as a supportive agent. | Latka et al. (2020) [162] |
| MDR K. pneumoniae biofilm | vB_KpnS_FZ10 vB_KpnS_FZ41, vB_KpnP_FZ12 vB_KpnM_FZ14 | Three phages showed halo zones linked to depolymerase activity, helping break down capsules and biofilms. The fourth, vB_KpnS_FZ41, lacked these enzymes and had a narrower host range. A cocktail of all four phages lysed all tested K. pneumoniae strains, showing the benefit of combining phages with and without depolymerases to broaden effectiveness. | Zurabov et al. (2021) [163] |
| K. pneumoniae biofilm | A¥L and A¥M which belonged to Myoviridae and Siphoviridae family | When applied individually or in combination, they achieved 50–70% reduction of mature (48 h old) K. pneumoniae biofilms in vitro. Significant biofilm disruption and bacterial killing were further confirmed through live/dead fluorescence staining and scanning electron microscopy. | Asghar et al. (2022) [164] |
| MDR Acinetobacter baumannii biofilm | vB_AbaM_ABPW7 | Phage vABPW7 significantly reduced biofilm biomass and successfully eradicated preformed biofilms. In an A549 human alveolar epithelial cell model, it effectively decreased both planktonic bacterial load and bacterial adhesion, without inducing any detectable cytotoxic effects. | Wintachai et al. (2022) [165] |
| MDR and XDR isolates of A. baumannii, K. pneumoniae, and Pseudomonas aeruginosa biofilm | Phage cocktails | From 81 hospital wastewater samples, 31 phages targeting MDR bacteria were isolated. Phage cocktails showed the best results, fully eradicating A. baumannii biofilms with colistin at just 1–2 µg/mL. In P. aeruginosa, strong phage–antibiotic synergy was observed, with MBECs reduced up to 64-fold. While effects in K. pneumoniae were less consistent, two strains also responded better to combined phage–colistin treatment. | Ragupathi et al. (2023) [166] |
| P. aeruginosa strain PAO1 biofilm | PaPC1, PaWP1, and PaWP2 | Each phage in the in vitro study significantly reduced 24 h old P. aeruginosa biofilms on polystyrene—PaPC1 by 66.7%, PaWP1 by 39.1%, and PaWP2 by 62.9%. When combined, the phage cocktail achieved over 75% reduction, showing clear synergistic effects. | Kovacs et al. (2024) [167] |
| ClinicalTrials. Gov ID and Research Status | Pathogen | Conditions | Intervention/Treatmentclassification | Intervention/ Treatment–Informations | Reaserch Phase |
|---|---|---|---|---|---|
| NCT06942624 [169]; Not yet recruiting | A methicillin-susceptible Enterococcus faecium | PJI of the hip | Biological: Phage Therapy | Lytic phages (saline-magnesium buffer)–intravenous and intra-articular administration, twice daily for 14 days; combined with standard antibiotic therapy | I, II |
| NCT06456424 [170]; Active, not recruiting | A methicillin-susceptible Staphylococcus aureus | PJI of the hip | Biological: Phage therapy | A bacteriophage cocktail composed of phages BP13 and J1P3 is administered intra-articularly on day 1, followed by intravenous dosing twice daily from day 1 through day 14. | I/II |
| NCT06798168 [171]; Available | Pseudomonas aeruginosa MDR | Chronic PJI | Biological: Combining bacteriophage therapy with antibiotics for a case with hip PJI | Biological: Bacteriophage + Antibiotic Therapy Treatment includes weekly intra-articular injections of a personalized phage cocktail (QDP-PSA-011) for 3 consecutive weeks, combined with a 6-week course of antibiotics. | - |
| NCT06605651 [172]; Not yet recruiting | Staphylococcus aureus | PJI of the hip, Knee Prosthesis Infection | Biological: Anti-Staphylococcus aureus Bacteriophages (PP1493 and PP1815) intra-articular injection with 0.9% NaCl solution Drug: 0.9% NaCl solution | Experimental Arm (Active): Anti-Staphylococcus aureus bacteriophages (PP1493 and PP1815) administered via intra-articular injection in a 0.9% NaCl solution. Placebo Comparator (Control Arm): Intra-articular injection of 0.9% NaCl solution only. | II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłuszkiewicz, K.; Busłowicz, T.; Korgiel, M.; Faltus, A.; Kucharczyk, E.; Porębska, B.; Pochciał, P.; Kucharczyk, N.; Paluch, E. Bacteriophage-Based Approach Against Biofilm Infections Associated with Medical Devices: A Narrative Review of ESKAPE Pathogens. Int. J. Mol. Sci. 2025, 26, 8699. https://doi.org/10.3390/ijms26178699
Pawłuszkiewicz K, Busłowicz T, Korgiel M, Faltus A, Kucharczyk E, Porębska B, Pochciał P, Kucharczyk N, Paluch E. Bacteriophage-Based Approach Against Biofilm Infections Associated with Medical Devices: A Narrative Review of ESKAPE Pathogens. International Journal of Molecular Sciences. 2025; 26(17):8699. https://doi.org/10.3390/ijms26178699
Chicago/Turabian StylePawłuszkiewicz, Karolina, Tomasz Busłowicz, Matylda Korgiel, Anita Faltus, Emilia Kucharczyk, Barbara Porębska, Paweł Pochciał, Natalia Kucharczyk, and Emil Paluch. 2025. "Bacteriophage-Based Approach Against Biofilm Infections Associated with Medical Devices: A Narrative Review of ESKAPE Pathogens" International Journal of Molecular Sciences 26, no. 17: 8699. https://doi.org/10.3390/ijms26178699
APA StylePawłuszkiewicz, K., Busłowicz, T., Korgiel, M., Faltus, A., Kucharczyk, E., Porębska, B., Pochciał, P., Kucharczyk, N., & Paluch, E. (2025). Bacteriophage-Based Approach Against Biofilm Infections Associated with Medical Devices: A Narrative Review of ESKAPE Pathogens. International Journal of Molecular Sciences, 26(17), 8699. https://doi.org/10.3390/ijms26178699








