1,3-Dipolar Cycloaddition of Nitrile Imines and Nitrile Oxides to Exocyclic C=N Bonds—An Approach to Spiro-N-Heterocycles
Abstract
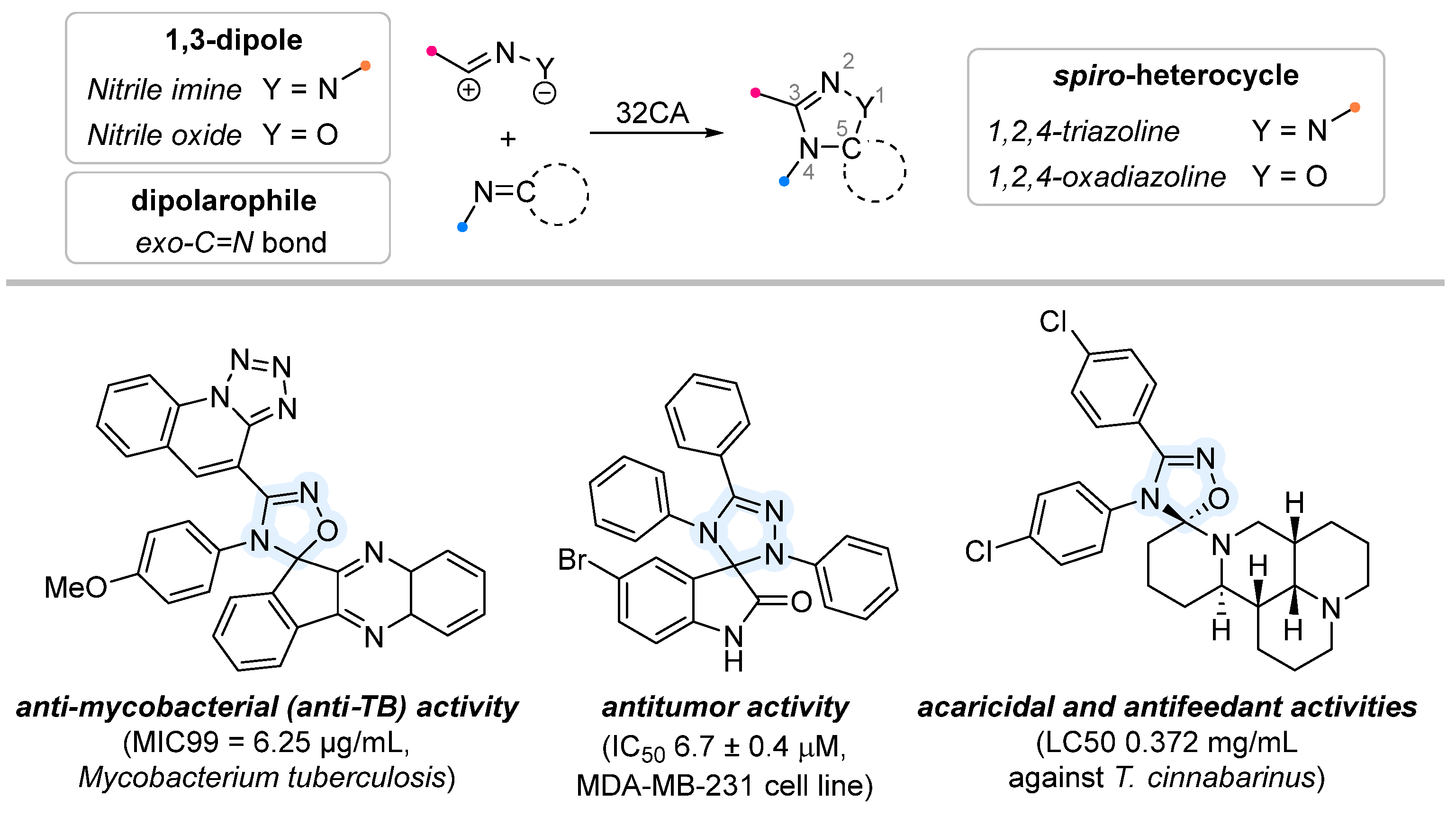
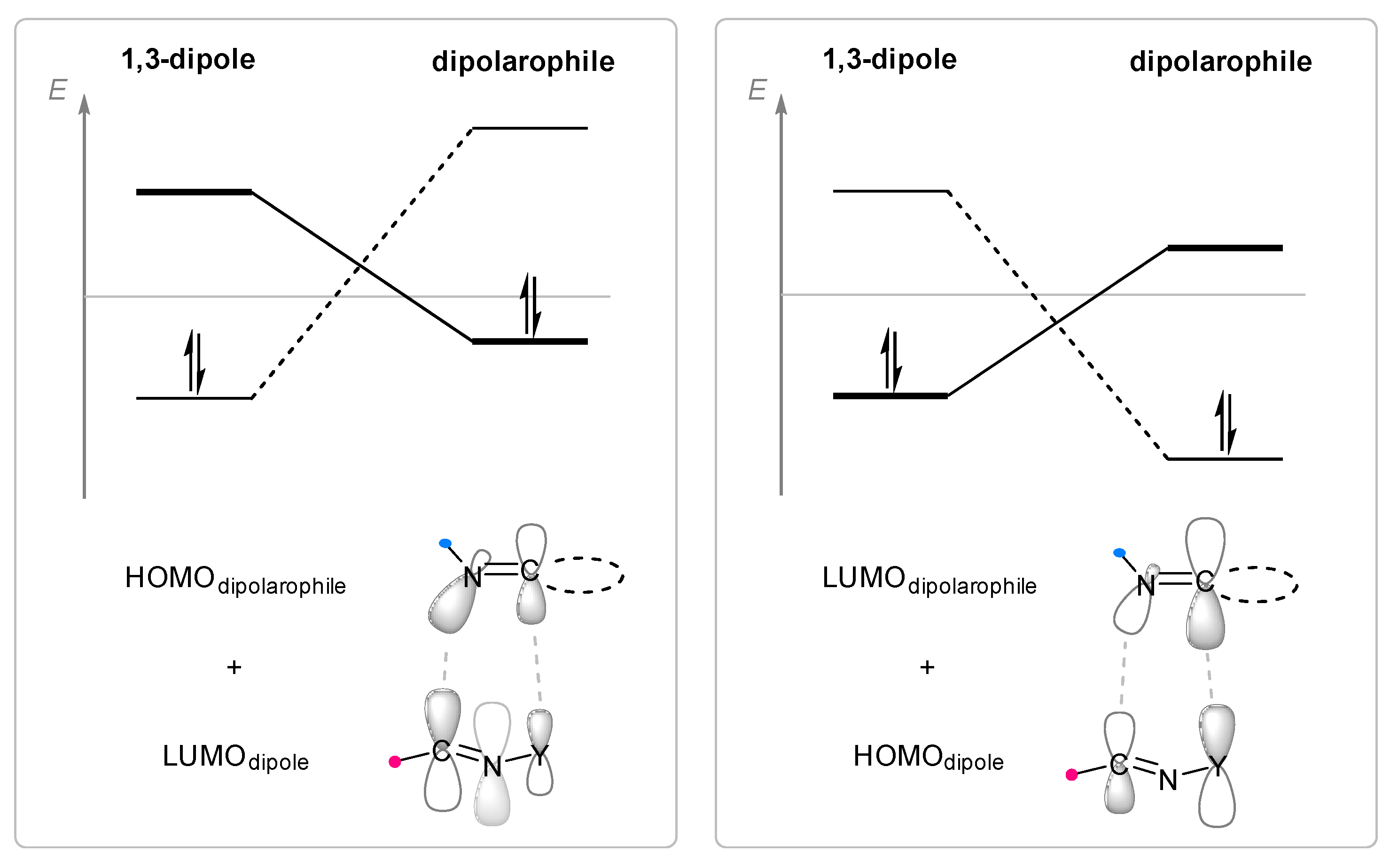
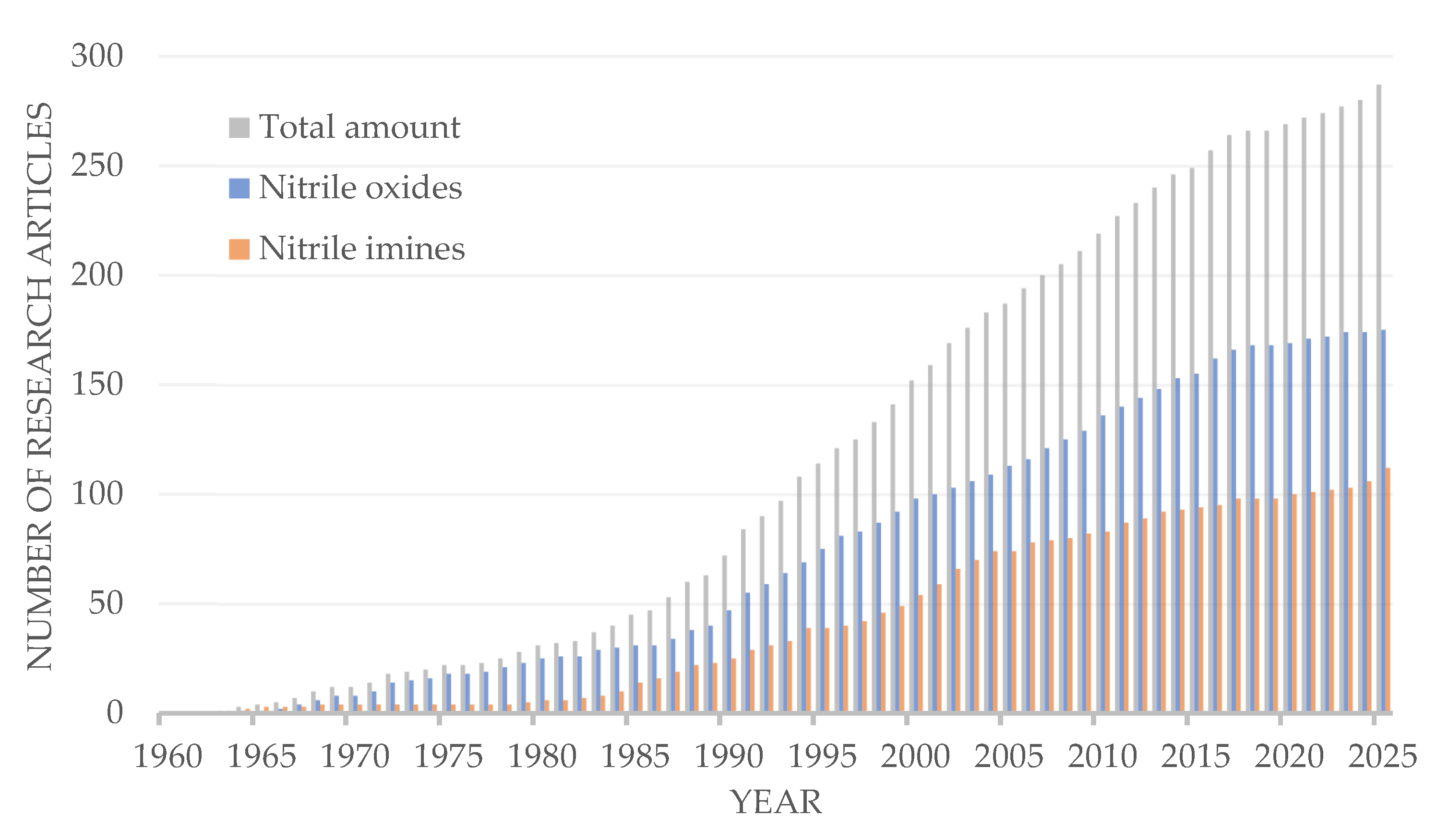
1. Introduction
2. Cycloaddition to Exocyclic C=N Bonds
2.1. Saturated Carbocycles and Piperidine Derivatives with exo-C=N-Bonds in Reactions with Nitrile Imines and Nitrile Oxides
2.2. Unsaturated Cycles with an Exocyclic C=N Bond as Dipolarophiles in the Reactions with Nitrile Imines and Nitrile Oxides
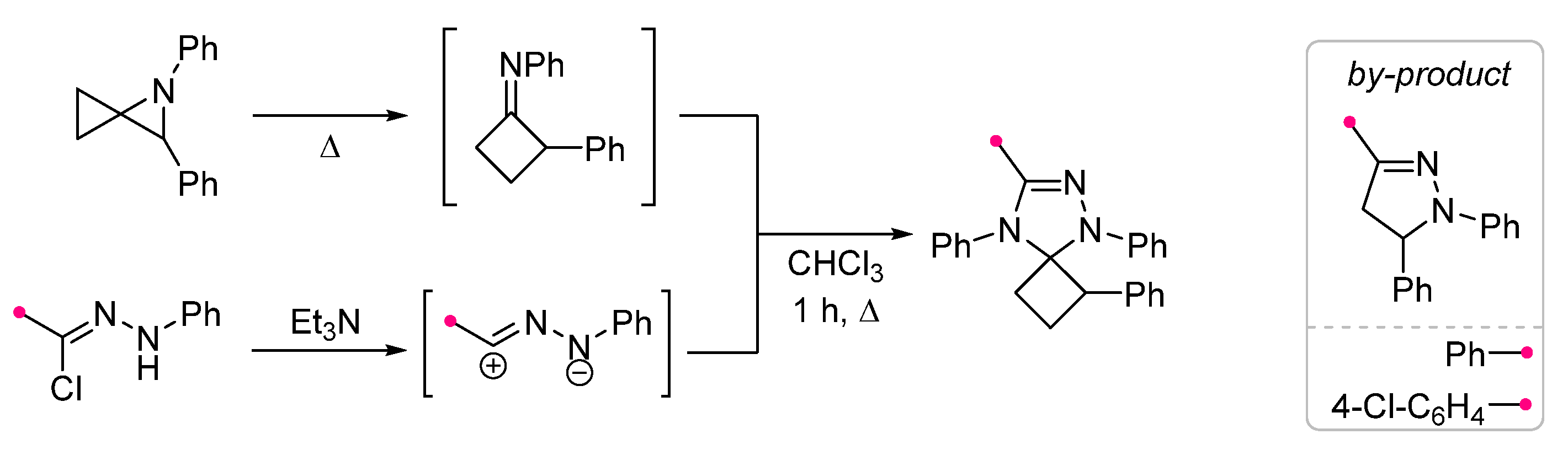
2.2.1. Reactions of Nitrile Imines and Nitrile Oxides with exo-C=N-Bonds of Monocyclic Compounds
2.2.2. Addition of Nitrile Oxides and Nitrile Imines at the Exocyclic C=N Bond of Fused Carbocycles
2.2.3. Reactions of Nitrile Oxides and Nitrile Imines at Exocyclic C=N Bonds of Fused Heterocycles
2.3. Addition Reactions of Nitrile Imines and Nitrile Oxides to the Exocyclic C=N Bonds of Cycles with Two Heteroatoms
2.4. Nitrile Imine and Nitrile Oxide Reactions at Exocyclic C=N Bonds of Heterocycles with Three Heteroatoms
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 32CA | (3+2)-cycloaddition |
| DPNI | diphenyl nitrile imine |
| THF | tetrahydrofuran |
| DCM | dichloromethane |
| DMF | N,N-dimethylformamide |
| DMA | N,N-dimethylacetamide |
| NCS | N-chlorosuccinimide |
References
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Jamieson, C.; Livingstone, K. The Nitrile Imine 1,3-Dipole; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-43480-9. [Google Scholar]
- Esipenko, A.A.; Samarai, L.I. Cycloaddition of Nitrile Oxides to Multiple Bonds Containing a Heteroatom. Russ. Chem. Rev. 1993, 62, 1097–1105. [Google Scholar] [CrossRef]
- Belen’kii, L.I. Nitrile Oxides. In Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 1–127. [Google Scholar]
- Kanchrana, M.; Gamidi, R.K.; Kumari, J.; Sriram, D.; Basavoju, S. Design, Synthesis, Anti-Mycobacterial Activity, Molecular Docking and ADME Analysis of Spiroquinoxaline-1,2,4-Oxadiazoles via [3 + 2] Cycloaddition Reaction under Ultrasound Irradiation. Mol. Divers. 2024, 28, 3979–3991. [Google Scholar] [CrossRef]
- Ribeiro, C.J.A.; Nunes, R.C.; Amaral, J.D.; Gonçalves, L.M.; Rodrigues, C.M.P.; Moreira, R.; Santos, M.M.M. Spirotriazoline Oxindoles: A Novel Chemical Scaffold with In Vitro Anticancer Properties. Eur. J. Med. Chem. 2017, 140, 494–509. [Google Scholar] [CrossRef]
- Lv, M.; Ma, Q.; Zhang, S.; Xu, H. Construction of Spiro-1,2,4-Oxadiazoline-Fused Matrine-Type Alkaloids as Pesticidal Agents. Bioorg. Med. Chem. Lett. 2021, 51, 128356. [Google Scholar] [CrossRef]
- Ess, D.H.; Houk, K.N. Theory of 1,3-Dipolar Cycloadditions: Distortion/Interaction and Frontier Molecular Orbital Models. J. Am. Chem. Soc. 2008, 130, 10187–10198. [Google Scholar] [CrossRef]
- Houk, K.N.; Sims, J.; Duke, R.E.; Strozier, R.W.; George, J.K. Frontier molecular orbitals of 1,3 dipoles and dipolarophiles. J. Am. Chem. Soc. 1973, 95, 7287–7301. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Kurdyukov, A.I.; Moskva, V.V. Phosphorylnitrile oxides. 6. 1,3-cycloaddition with azomethines and nitriles. Chem. Heterocycl. Compd. 1994, 30, 1095–1100. [Google Scholar] [CrossRef]
- Huisgen, R. Proceedings of the Chemical Society. October 1961. Proc. Chem. Soc. 1961, 357–396. [Google Scholar] [CrossRef]
- Sharp, J.T. Nitrile Ylides and Nitrile Imines. In Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Padwa, A., Pearson, W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; Volume 59, pp. 473–537. [Google Scholar] [CrossRef]
- Jäger, V.; Colinas, P.A. Nitrile Oxides. In Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Padwa, A., Pearson, W., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2002; Volume 59, pp. 361–472. [Google Scholar] [CrossRef]
- Grundmann, C. The Chemistry of Nitrile Oxides. In Organische Chemie; Springer: Berlin/Heidelberg, Germany, 2006; pp. 62–127. [Google Scholar]
- Michael Paton, R. 3.20-N-Substituted Nitriles and Other Heteroanalogues of Nitriles of the Type RCZ. In Comprehensive Organic Functional Group Transformations; Katritzky, A.R., Meth-Cohn, O., Rees, C.W., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1995; pp. 677–692. [Google Scholar] [CrossRef]
- Kumar, A.K.; Kumar, K.A.; Govindaraju, M.; Kumar, V.G. Nitrile Imines: Versatile Intermediates in the Synthesis of Five Membered Heterocycles. Int. J. Res. Pharm. Chem. 2013, 3, 140–152. [Google Scholar]
- Quilico, A. Advances in Nitrile Oxides Chemistry. Experientia 1970, 26, 1169–1183. [Google Scholar] [CrossRef]
- Grundmann, C. Synthesis of Heterocyclic Compounds with the Aid of Nitrile Oxides. Synthesis 1970, 1970, 344–359. [Google Scholar] [CrossRef]
- Grundmann, C.; Grünanger, P. Addition Reactions Leading to Cyclic Structures: 1,3-Dipolar Cycloadditions. In The Nitrile Oxides; Organische Chemie in Einzeldarstellungen; Springer: Berlin/Heidelberg, Germany, 1971; Volume 59, pp. 85–139. [Google Scholar] [CrossRef]
- Deepthi, A.; Acharjee, N.; Sruthi, S.L.; Meenakshy, C.B. An Overview of Nitrile Imine Based [3+2] Cycloadditions over Half a Decade. Tetrahedron 2022, 116, 132812. [Google Scholar] [CrossRef]
- Molteni, G.; Silvani, A. Spiro-2-oxindoles via 1,3-dipolar Cycloadditions. A Decade Update. Eur. J. Org. Chem. 2021, 2021, 1653–1675. [Google Scholar] [CrossRef]
- Kadaba, P.K. 1,2,4-Triazolines. Adv. Heterocycl. Chem. 1989, 46, 169–281. [Google Scholar] [CrossRef]
- Shawali, A.S. Chemoselectivity in 1,3-Dipolar Cycloaddition Reactions of Nitrilimines with Multifunctionalized Dipolarophiles. Curr. Org. Chem. 2014, 18, 598–614. [Google Scholar] [CrossRef]
- Ferwanah, A.-R.S.; Awadallah, A.M. Reaction of Nitrilimines and Nitrile Oxides with Hydrazines, Hydrazones and Oximes. Molecules 2005, 10, 492–507. [Google Scholar] [CrossRef]
- Tsuge, O.; Watanabe, H.; Kiryu, Y. The Reactions of 1,2-Diphenyl-1-Azaspiro[2,2]Pentane and 2-Phenyl-1-Azaspiro[2.2]Pent-1-Ene with C,N-Diarylnitrilimines. Bull. Chem. Soc. Jpn. 1979, 52, 3654–3658. [Google Scholar] [CrossRef]
- Dalloul, H.M. Heterocyclic synthesis using Nitrile imines. 4. synthesis of 3-substituted 1-aryl-1,2,4-triazaspiroalk-2-enes. Chem. Heterocycl. Compd. 2004, 40, 1402–1407. [Google Scholar] [CrossRef]
- Dalloul, H.M.; Boyle, P.H. Heterocyclic Synthesis Using Nitrilimines: Part 7. Synthesis of Some New Substituted 1,2,4,8-Tetraazaspiro[4.5]Dec-2-Enes. Heterocycl. Commun. 2007, 13, 155–160. [Google Scholar] [CrossRef]
- Dalloul, H.M.; Samaha, A.S.A. Synthesis of Nitrogen-Containing Dispiroheterocycles Using Nitrilimines (II). J. Serbian Chem. Soc. 2010, 75, 1473–1479. [Google Scholar] [CrossRef]
- Dalloul Peter, H.; Boyle, H.M.; Dalloul, H.M.; Boyle, P.H. Heterocyclic Synthesis Using Nitrilimines: Part 5. Synthesis of Some Novel Spiro Heterocycles. Turk. J. Chem. 2006, 30, 119–124. [Google Scholar]
- Awadallah, A.M. Synthesis of Schiff Bases, Oximes and Hydrazones of 1,2,4-Oxadiazole and 1,2,4-Triazoles. Asian J. Chem. 2006, 18, 2151–2158. [Google Scholar]
- Liu, B.; Li, X.F.; Liu, H.C.; Yu, X.Y. Unexpected Nitrilimine Cycloaddition of Thiazolo[3,2-a]Pyrimidine Derivatives. Tetrahedron Lett. 2013, 54, 6952–6954. [Google Scholar] [CrossRef]
- Li, X.; Zheng, A.; Liu, B.; Yu, X.; Yi, P. Synthesis of [1,2,4]Oxadiazolo[4,5-a]Thiazolo[2,3-b]Pyrimidin-9(10H)-ones via 1,3-Dipolar Cycloaddition of Nitrile Oxide to Thiazolo[3,2-a]Pyrimidin-3-one Derivatives. Chin. J. Chem. 2010, 28, 977–980. [Google Scholar] [CrossRef]
- Gandolfi, R.; Toma, L. Site specificity in cycloadditions of diphenylnitrile imine with 8-substituted-8-azaheptafulvenes and tricarbonyl (8-azaheptafulvene) iron complexes. Tetrahedron 1980, 36, 935–941. [Google Scholar] [CrossRef]
- Freccero, M.; Gandolfi, R.; Amade’, M.S. Reactions of O,O’-Disubstituted Benzonitrile Oxides with 8-Azaheptafulvenes. Heterocycles 1998, 47, 453–466. [Google Scholar] [CrossRef]
- Gandolfi, R.; Gamba, A.; Grünanger, P. 1,3-Dipolar Cycloaddition of Nitrile Oxides to 8-Azaheptafulvenes. Heterocycles 1995, 40, 619–638. [Google Scholar] [CrossRef]
- Saito, K.; Ito, K.; Takahashi, K. 1,3-Dipolar Cycloaddition Reactions of N-Aryl-2,4,6-Cycloheptarien-1-Imines with p-Substituted Benzonitrile Oxides: Formations of 1,2,4-Oxadiazaspiro[4.6]Undeca-6,8,10-Trienes. Heterocycles 1993, 36, 21–24. [Google Scholar] [CrossRef]
- Ito, K.; Saito, K. 1,3-Dipolar Cycloaddition Reactions of 2,4,6-Cycloheptatrien-1-Imines with Benzonitrile Oxides Leading to 1,2,4-Oxadiazaspiro[4.6]Undeca-2,6,8,10-Tetraenes. Bull. Chem. Soc. Jpn. 1995, 68, 3539–3547. [Google Scholar] [CrossRef]
- Freccero, M.; Gandolfi, R.; Sarzi-Amade’, M.; Bovio, B. Reactions between Triazolinediones and Equilibrating Forms of Cycloheptatriene Derivatives Featuring 7,7-Spiro and 1,7-Fused Heterocyclic Rings. Eur. J. Org. Chem. 2002, 2002, 569–579. [Google Scholar] [CrossRef]
- Burdisso, M.; Gandolfi, R.; Toma, L.; Oberti, R. Hexafluoroisopropanol as a Suitable Solvent for Rearrangements via Zwitterionic Intermediates. Tetrahedron 1991, 47, 6725–6736. [Google Scholar] [CrossRef]
- Enders, D.; Meyer, I.; Runsink, J.; Raabe, G. Diastereo- and Enantioselective Synthesis of ∆2-1,2,4-Oxadiazolines by 1,3-Dipolar Cycloaddition of Nitrile Oxides with Chiral Hydrazones. Heterocycles 1999, 50, 995–1024. [Google Scholar] [CrossRef]
- Beltrame, P.; Cadoni, E.; Floris, C.; Gelli, G.; Lai, A. Reactions of a Stable Benzonitrile Oxide with Aminopyridines. Heterocycles 2000, 53, 1915–1938. [Google Scholar]
- Awad, W.I.; Sobhy, M. 1,3-Dipolar Cycloaddition of Nitrile Oxides. II. Reactions with o-Quinoid Structures. Can. J. Chem. 1969, 47, 1473–1477. [Google Scholar] [CrossRef]
- Awad, W.I.; Mohayed, S.; Rahman Omran, S.M.A. Studies of Quinoid Structures. VII.1 Reaction of Benzonitrile Oxide with o-Quinones. J. Org. Chem. 1966, 31, 331–333. [Google Scholar] [CrossRef]
- Bazian, A.; Taheri, M.; Alavi, H. Synthesis of 4′-[3-Methyl-5-Thioxo-1H-1,2,4-Triazol-4(5H)-Yl]-2′,5′-Diphenyl-2′,4′-Dihydro Spiro[Indolin-3,3′[1,2,4]Triazol]-2-One Derivatives. Russ. J. Gen. Chem. 2014, 84, 586–592. [Google Scholar] [CrossRef]
- Souzangarzadeh, S.; Bazian, A.; Anaraki-Ardakani, H. A Facile Synthesis of Novel Spiro Indoline-Based Heterocycles through 1,3-Dipolar Cycloaddition Reactions. J. Chem. Res. 2012, 36, 94–95. [Google Scholar] [CrossRef]
- Azizian, J.; Soozangarzadeh, S.; Jadidi, K. Microwave-Induced One-Pot Synthesis of Some New Spiro[3H-Indole-3,5′(4′H)-[1,2,4]Triazoline]-2-Ones. Synth. Commun. 2001, 31, 1069–1073. [Google Scholar] [CrossRef]
- Franke, A. Spirocyclische 2-Indolinone Durch 1,3-dipolare Cycloaddition. Liebigs Ann. Chem. 1978, 1978, 717–725. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Mohan, G.; Ramesh, P.; Rao, E. Synthesis of Some Novel Isoxazolyl-Spiro-[3H-Indole-3,2′-Thiazolidine]-2,4′-(1H)-Diones, [3H-Indole-3,4′-Azetidine]-2,2′-(1H)-Diones and [3H-Indole-3,5′-3′-Phenyl-1′,2′,4′-Oxadiazoline]-2(1H)-Ones. Heterocycl. Commun. 2006, 12, 431–436. [Google Scholar] [CrossRef]
- Ribeiro, C.J.A.; Amaral, J.D.; Rodrigues, C.M.P.; Moreira, R.; Santos, M.M.M. Spirooxadiazoline Oxindoles with Promising in Vitro Antitumor Activities. MedChemComm 2016, 7, 420–425. [Google Scholar] [CrossRef]
- de Azevedo, L.D.; Leite, D.I.; de Oliveira, A.P.; Junior, F.P.S.; Dantas, R.F.; Bastos, M.M.; Boechat, N.; Pimentel, L.C.F. Spirooxadiazoline-oxindoles Derived from Imatinib Show Antimyeloproliferative Potential in K562 Cells. Arch. Pharm. 2024, 357, e2400029. [Google Scholar] [CrossRef] [PubMed]
- Azizian, J.; Sarrafi, Y.; Mehrdad, M. Synthesis of Some New I-Methyl-3′, 3′-Dichlorospiro[Indol-3, 4′-Azetidine]-2(3H), 2′-Diones and Bis[1-Methyl-3′, 3′-Dichlorospiro(Indole-3, 4′-Azetidine)-2(3H), 2′-Diones]. Indian J. Chem.-B 2000, 39, 304–307. [Google Scholar]
- Souzangarzadeh, S. 1,3-Dipolar Cycloaddition Reaction of Nitrile Oxides to Isatin Imines. Iran. J. Chem. Chem. Eng. 2016, 35, 31–35. [Google Scholar]
- Shi, G.; He, X.; Shang, Y.; Xiang, L.; Yang, C.; Han, G.; Du, B. Synthesis of 3′,4′-Diaryl-4′H-Spiro[Indoline-3,5′-[1′,2′,4′]Oxadiazol]-2-Ones via DMAP-Catalyzed Domino Reactions and Their Antibacterial Activity. Chin. J. Chem. 2016, 34, 901–909. [Google Scholar] [CrossRef]
- Kanchrana, M.; Allaka, B.S.; Krishna, G.R.; Basavoju, S. An Ultrasound Assisted Synthesis of Spirooxindolo-1,2,4-Oxadiazoles via [3+2] Cycloaddition Reaction and Their Anti-Cancer Activity. Arkivoc 2023, 2023, 202211940. [Google Scholar] [CrossRef]
- Azizian, J.; Mohammadizadeh, M.R.; Javadi, M.; Mohammadi, A.A.; Karimi, A.R. One-Pot Synthesis of Some New Spiro 6H-Indolo[b-1,2]Quinazoline-12-One-[1,2,4]Triazolines. J. Chem. Res. 2004, 2004, 125–126. [Google Scholar] [CrossRef]
- Azizian, J.; Madani, M.; Souzangarzadeh, S. One-Pot Synthesis of Novel Derivatives of Spiro-[6H-Indolo [2,1-b]Quinazoline-6,3′-[1,2,4]Oxadiazoline]. Synth. Commun. 2005, 35, 765–768. [Google Scholar] [CrossRef]
- Dalloul, H.M. Synthesis of Spiro-Heterocycles with the Thiazolidinone Moiety from Nitrilimines. J. Chin. Chem. Soc. 2009, 56, 196–201. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Raju, S.; Reddy, A.S.R. Synthesis of Novel Isoxazolyl 1,6-Dithia-4,9-Diazaspiro[4,4]Nonane-3,8-Diones and 1-Oxa-6-Thia-2,4,9-Triazaspiro[4,4]Non-2-Ene-8-Ones. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 3139–3148. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Rao, E.K.; Shaik, F.P.; Reddy, M.N.; Srinivas, M. Design, Synthesis, Antibacterial and Antifungal Activity of Novel Spiro-Isoxazolyl Bis-[5,5′]Thiazolidin-4-Ones and Spiro-Isoxazolyl Thiazolidin-4-One-[5,5′]-1,2-4 Oxdiazolines. J. Sulfur Chem. 2010, 31, 263–274. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Reddy, A.S.R.; Reddy, K.G.; Raju, S. Synthesis and Antimicrobial Activity of Novel Spiro-isoxazolyl Oxazolidin-4-one-[5,51]-1,2,4-oxadiazolines and Thiazolidin-4-one-[5,51]-1,2,4-oxadiazolines. Indian J. Chem. B Org. Chem. Incl. Med. Chem. 2011, 50, 1800–1806. [Google Scholar] [CrossRef]
- Vodela, S.; Reddy Mekala, R.; Gadegoni, H. Synthesis, Characterization and Biological Evaluation of Some Novel 9-Benzooxazol-4-Yl-3,4-Diphenyl-1-Oxa-6-Thia-2,4,9-Triaza-Spiro[4.4]Non-2-En-8-Ones. Int. J. Biomed. Res. 2013, 5, 5–12. [Google Scholar] [CrossRef][Green Version]
- Vodela, S.; Reddy Mekala, R.; Reddy Modugu, N.; Vannada, J. Synthesis and Antimicrobial Evaluation of Some Novel Spirothiazolidin-Based Derivatives. World J. Pharm. Pharm. Sci. 2014, 3, 800–810. [Google Scholar][Green Version]
- Kishore, B.; Brahmeshwari, G. Synthesis and Antimicrobial Screening Of Novel 4-(1H-Benzo[D] Imidazol-2-Yl)-8,9-Diaryl-1,6-Dioxa-4,7,9-Triazaspiro[4,5]Dec-7-En-3-Ones. Heterocycl. Lett. 2018, 8, 631–639. [Google Scholar][Green Version]
- Kuznetsova, J.V.; Tkachenko, V.T.; Petrovskaya, L.M.; Filkina, M.E.; Shybanov, D.E.; Grishin, Y.K.; Roznyatovsky, V.A.; Tafeenko, V.A.; Pestretsova, A.S.; Yakovleva, V.A.; et al. [3+2]-Cycloaddition of Nitrile Imines to Parabanic Acid Derivatives—An Approach to Novel Spiroimidazolidinediones. Int. J. Mol. Sci. 2023, 25, 18. [Google Scholar] [CrossRef]
- Petrova, J.V.; Tkachenko, V.T.; Tafeenko, V.A.; Pestretsova, A.S.; Pokrovsky, V.S.; Kukushkin, M.E.; Beloglazkina, E.K. Facile Synthesis of Hydantoin/1,2,4-Oxadiazoline Spiro-Compounds via 1,3-Dipolar Cycloaddition of Nitrile Oxides to 5-Iminohydantoins. Beilstein J. Org. Chem. 2025, 21, 1552–1560. [Google Scholar] [CrossRef]
- Jia, P.; Lin, Z.; Luo, C.; Liang, J.; Lai, R.; Guo, L.; Yao, Y.; Wu, Y. Synthesis of Spirotriazolines and Spirooxadiazolinesvia Light-Induced 1,3-Dipolar [3+2] Cycloadditions. New J. Chem. 2023, 47, 20248–20252. [Google Scholar] [CrossRef]
- Huisgen, R.; Grashey, R.; Kunz, R.; Wallbillich, G.; Aufderhaar, E. 1.3-Dipolare Cycloadditionen, XVII: BisaddukteAusNitriliminen Und Carbodiimiden. Chem. Berichte 1965, 98, 2174–2184. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Liu, X.; Huang, D.; Wang, K.-H.; Wang, J.; Hu, Y. Synthesis of Di/Trifluoromethyl Bis(1,2,4-Triazoline)Spiranes and 1,2,4-Triazoles via 1,3-Dipolar Cycloaddition of Nitrilimines and Carbodiimides. Org. Biomol. Chem. 2025, 23, 2662–2671. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R.; Aufderhaar, E. 1.3-Dipolare Cycloadditionen, XVIII: Die UmsetzungDes Phenyl-cyanamidsMit C-Äthoxycarbonyl-N-phenyl-nitrilimin. Chem. Berichte 1965, 98, 2185–2192. [Google Scholar] [CrossRef]
- Yen, W.; Kung, F.; Wong, F.F. 1,3-Dipolar Cycloaddition of Carbodiimides and Nitrilimines: Synthesis and Mechanistic Study of 5-Amino-1,2,4-triazoles. Eur. J. Org. Chem. 2016, 2016, 2328–2335. [Google Scholar] [CrossRef]
- Grundmann, C.; Richter, R. Die Bortrifluorid Katalysierte Addition von Nitriloxiden an Cn-Doppelbindungssysteme. Tetrahedron Lett. 1968, 9, 963–966. [Google Scholar] [CrossRef]
- Sharma, P.; RangaPrabhath, M.R.; Wong, D.; Ampem-Lassen, M.A.; Bhat, S.V.; Williams, L.; Carvalho, T.G. Synthesis of Biologically Active Heterospirocycles through Iterative 1,3-Dipolar Cycloaddition Pathways. J. Org. Chem. 2021, 86, 1223–1230. [Google Scholar] [CrossRef]
- Kesornpun, C.; Aree, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Water-Assisted Nitrile Oxide Cycloadditions: Synthesis of Isoxazoles and Stereoselective Syntheses of Isoxazolines and 1,2,4-Oxadiazoles. Angew. Chem. Int. Ed. 2016, 55, 3997–4001. [Google Scholar] [CrossRef] [PubMed]
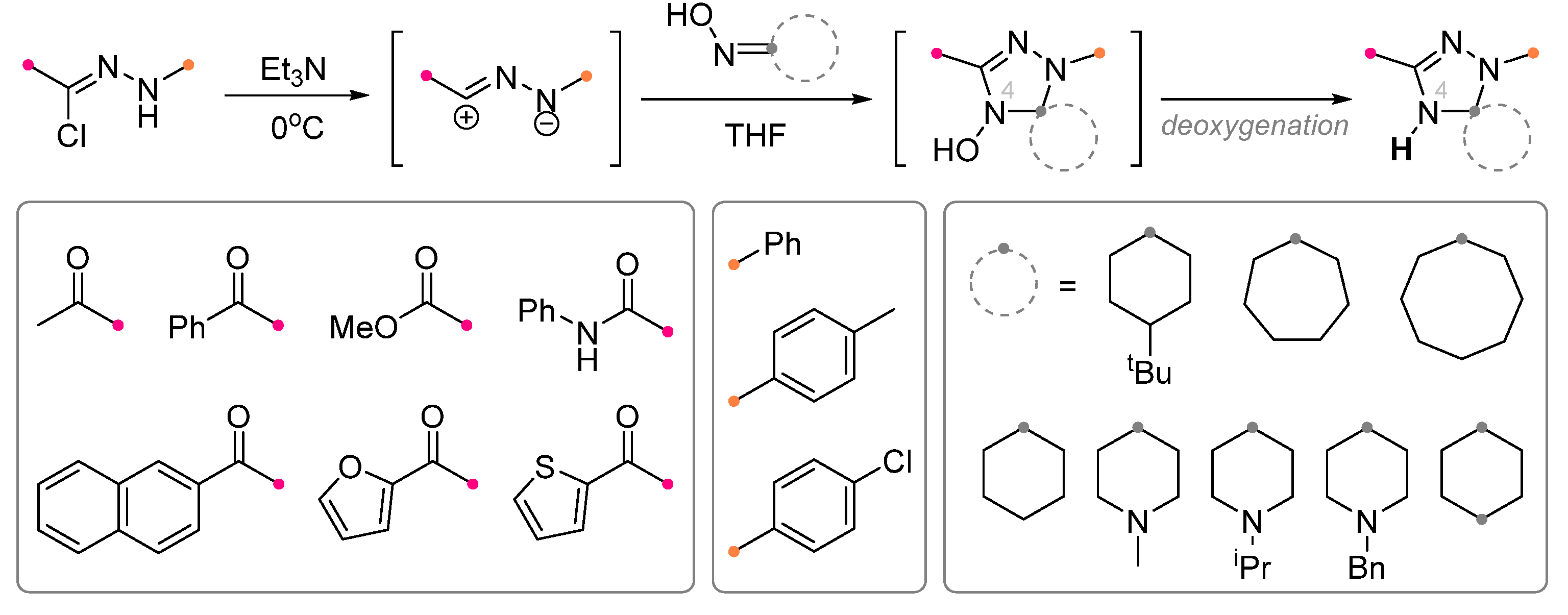
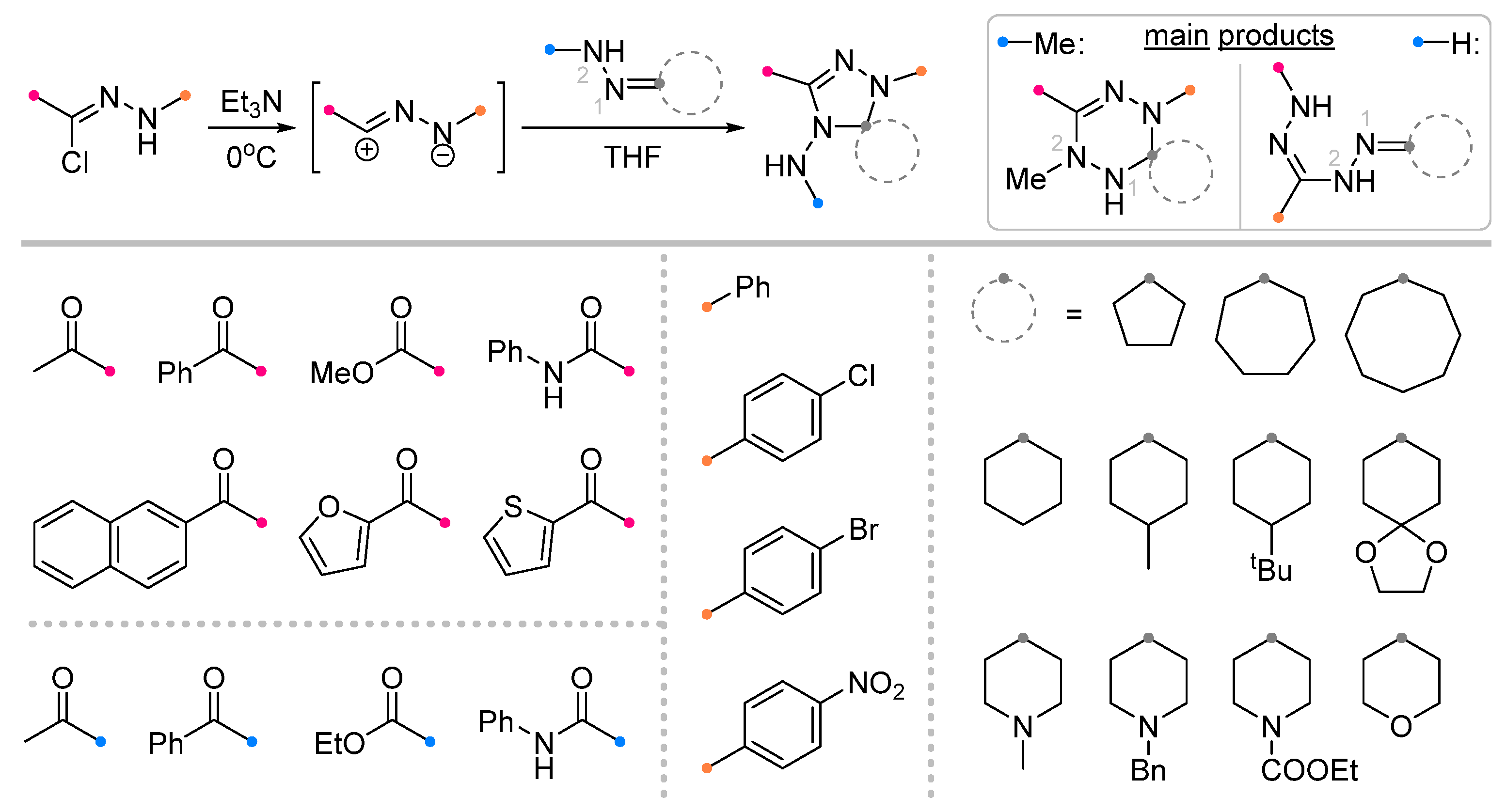
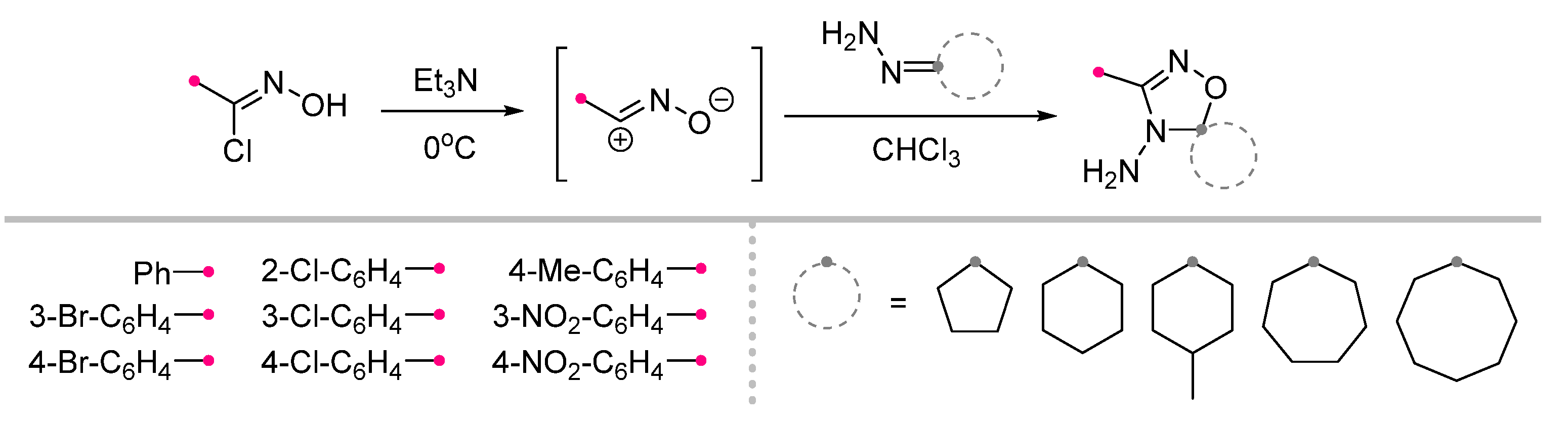
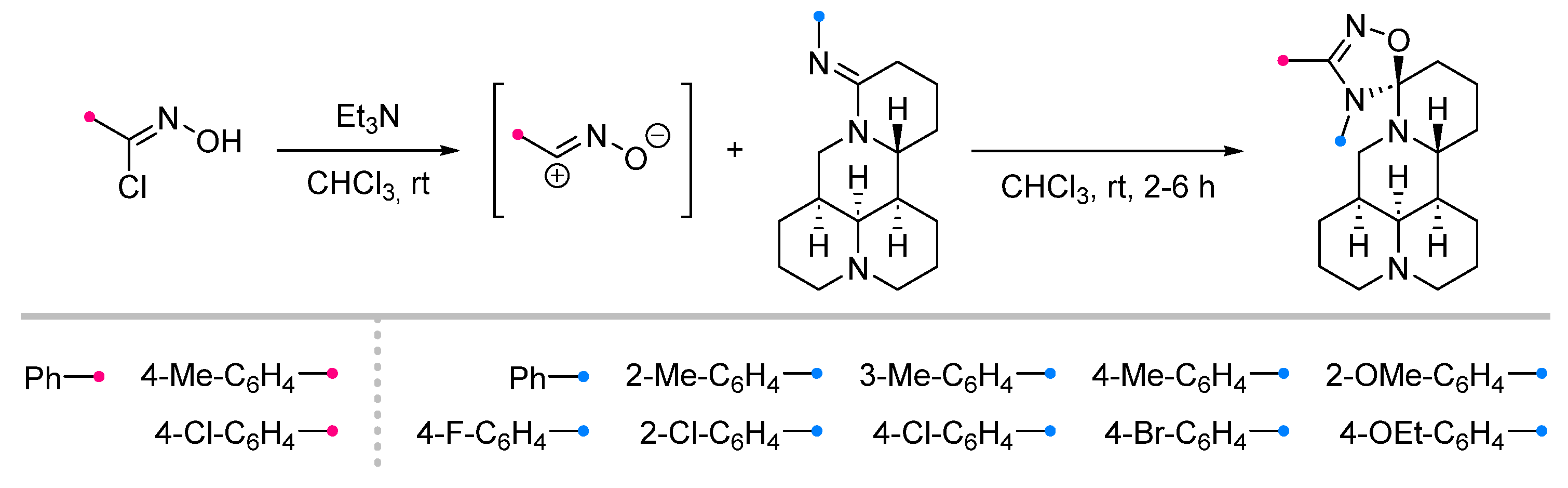




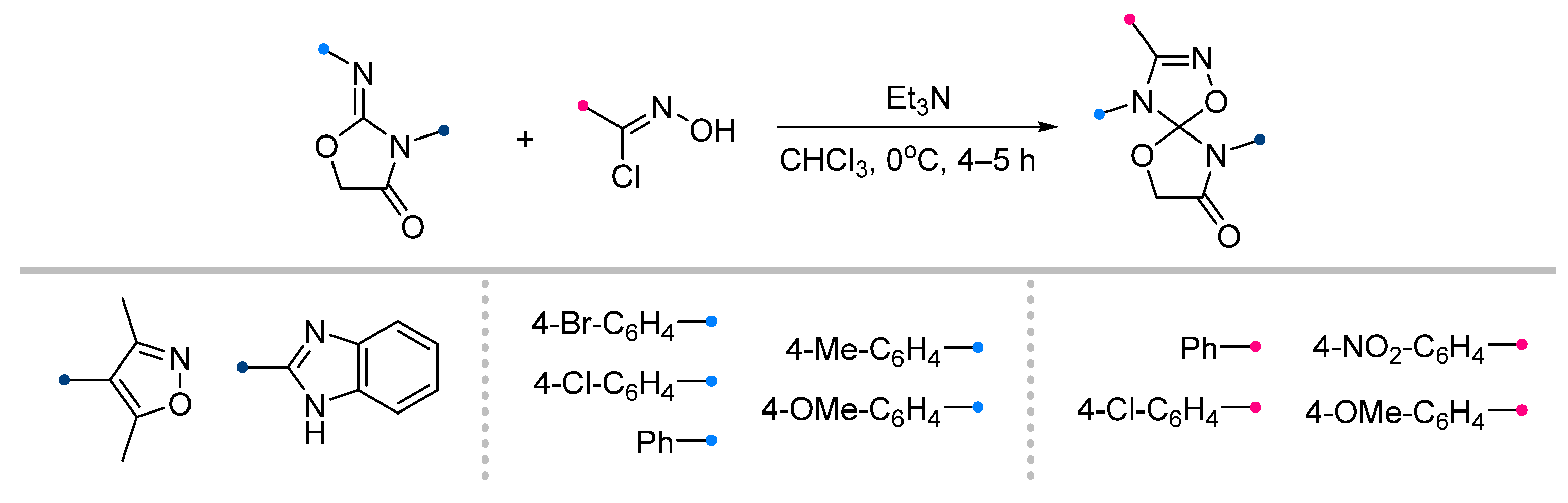
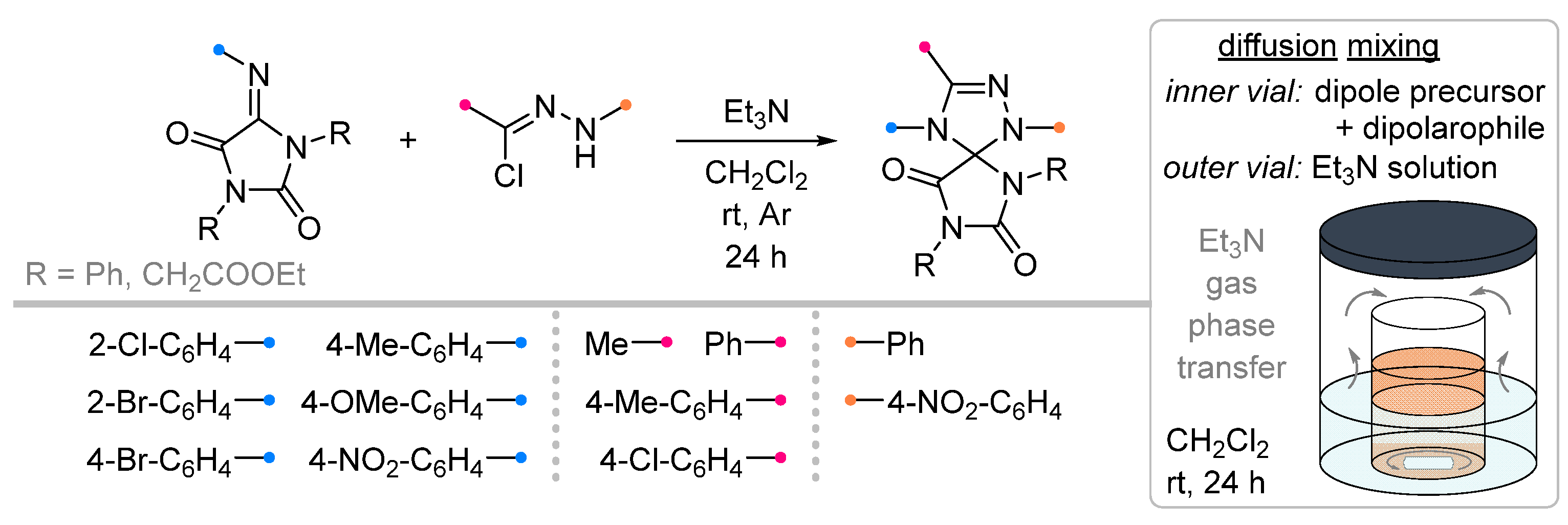
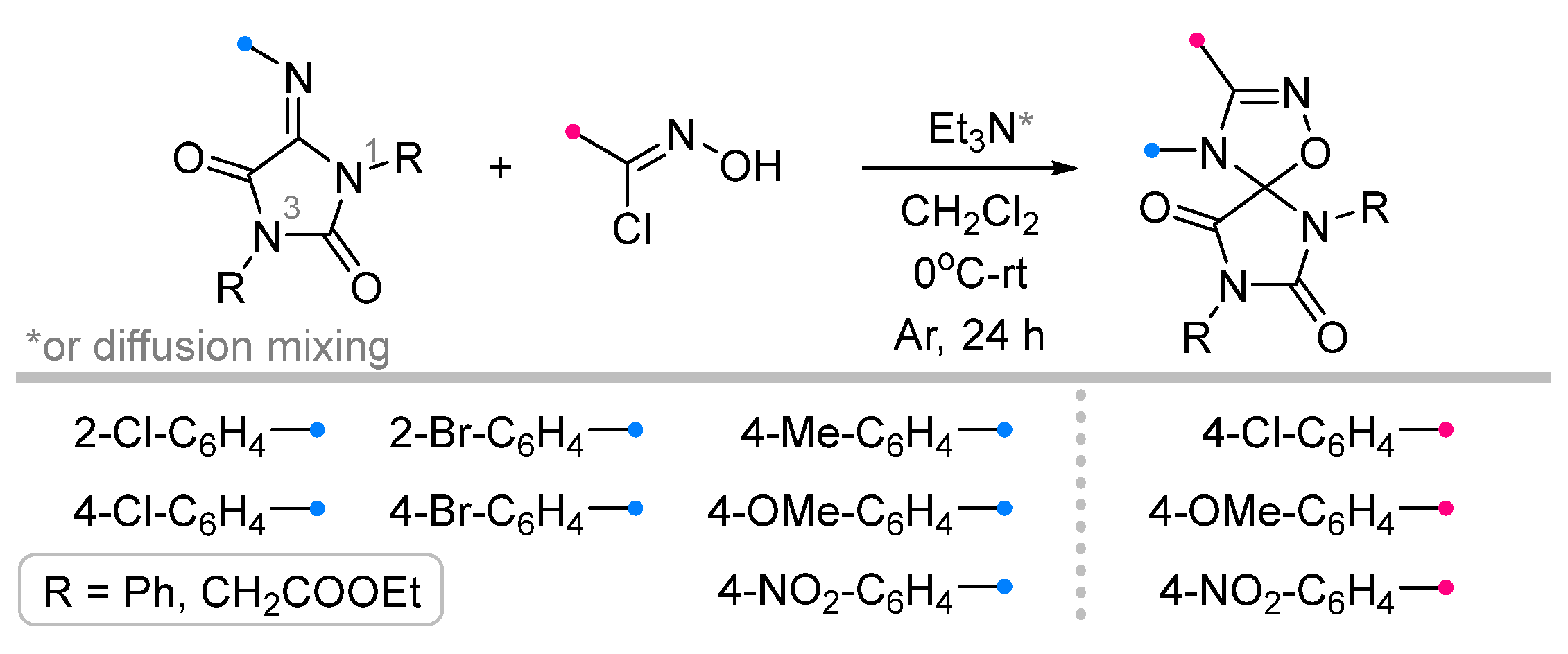
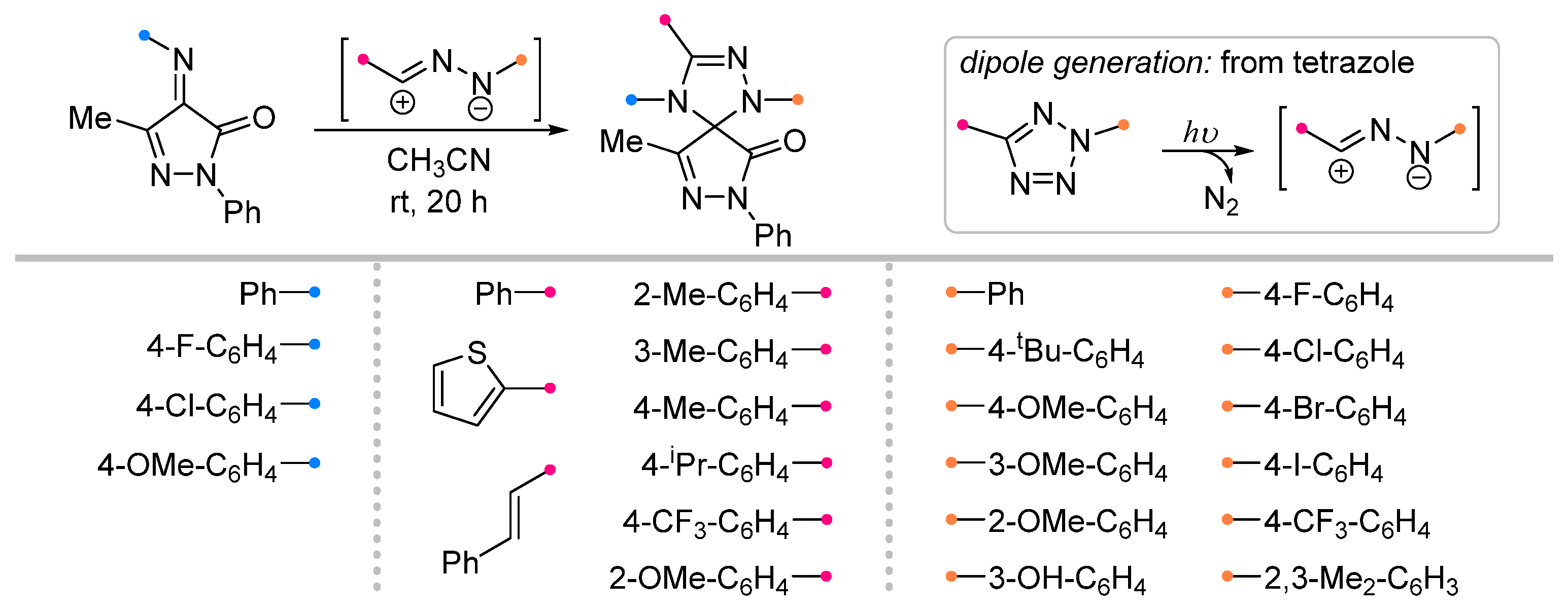
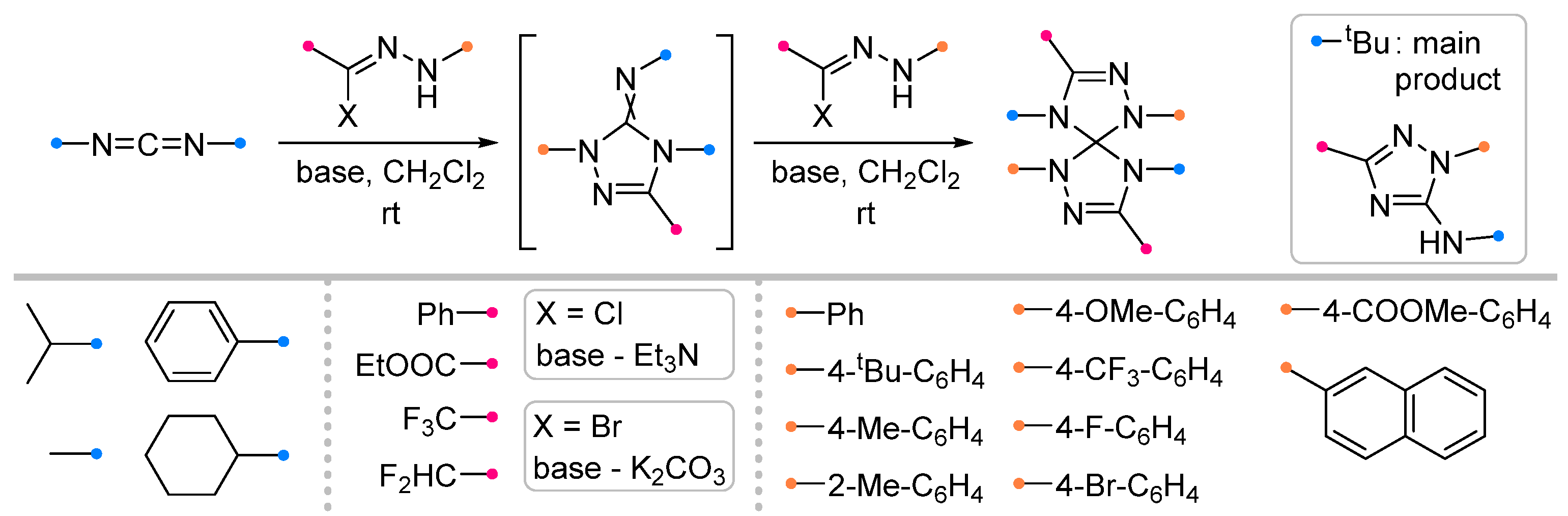

| Substrate | Dipole | Reagents Ratio a | Time | Yields, % | Note | Refs. |
|---|---|---|---|---|---|---|
 |  | 1/1/5 | 1 h | 54 | Reactions were conducted in CHCl3 under reflux, dipole precursor was added dropwise to the mixture of dipolarophile precursor and Et3N | [25] |
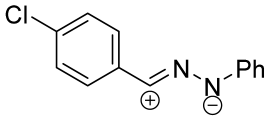 | 1/2/10 | 4 h | 38 | |||
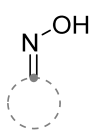 | 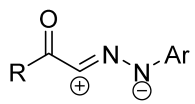 | 3/1/6 | 12 h | 52–72 | R = methyl, methoxy, aryl, anilino b | [27] |
| 2.5/1/5 | 12 h | 48–62 | R = anilino, heteroaryl; 1,4-Dioxane was used as the solvent b | [26] | ||
| 3/1.5/5 | overnight | 40–58 | R = methyl, aryl | [24] | ||
| 1/2/2 | overnight | 45–54 | R = methyl, methoxy, aryl, anilino; Dipolarophile was 1,4-cyclohexanedione dioxime | [28] | ||
 | 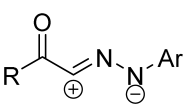 | 3/1.5/5 | overnight | 30–87 | R = methyl | [24] |
| 2/1.5/5 | overnight | 25–87 | R = methyl, methoxy | |||
| 1/1/5 | 4–6 h | 50–86 | R = aryl | |||
| 2/1/5 | overnight | 70–90 | R = anilino, aryl, heteroaryl b | [29] | ||
 | 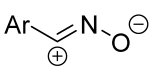 | 2.5/1/2.5 | 2 h | 38–80 | Reactions were conducted in CHCl3, dipole precursor was added dropwise to the mixture of dipolarophile and Et3N. | [24] [30] |
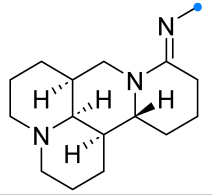 | 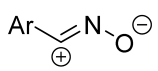 | 1/1/2 | 2–6 h | 25–82 | Reactions in CHCl3 b | [7] |
| Substrate | Dipole | Reagents Ratio a | Conditions | Total Yields and Selectivity b, % | Note | Refs. |
|---|---|---|---|---|---|---|
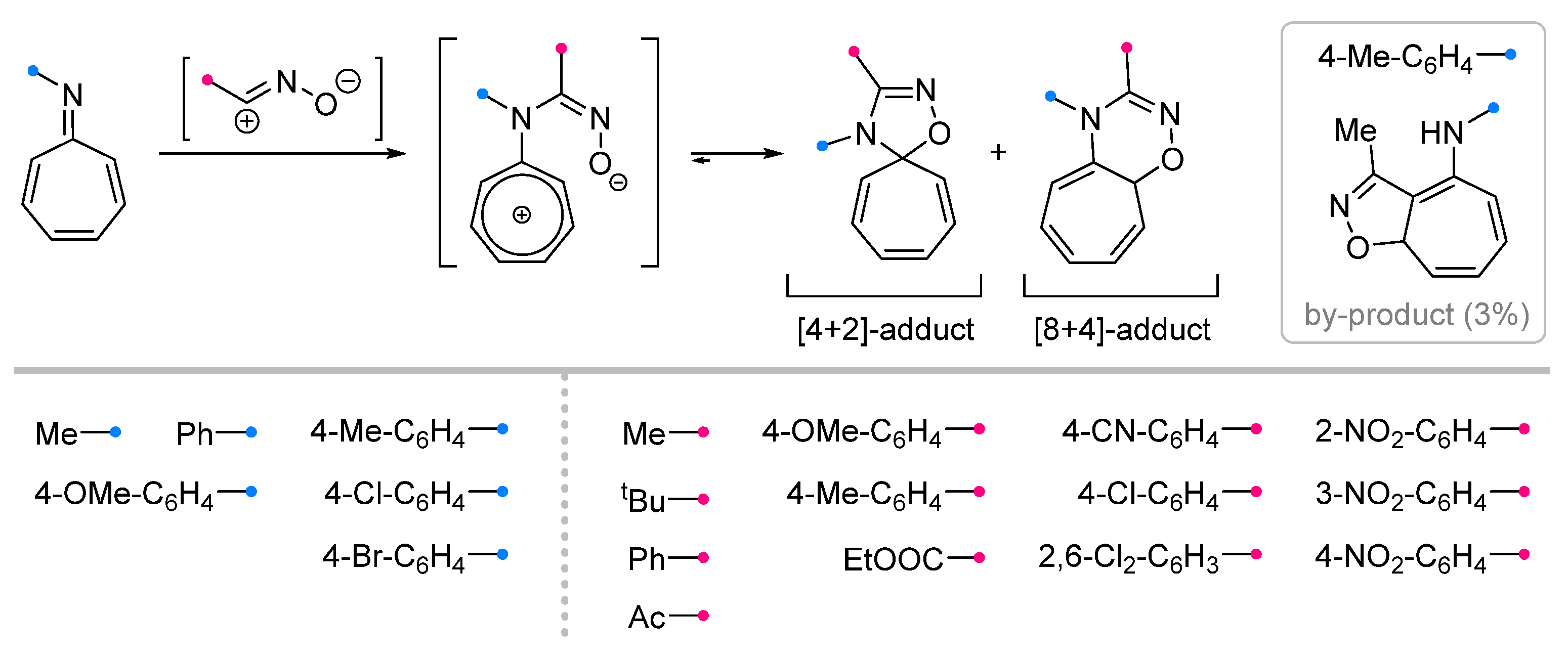
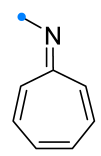 | 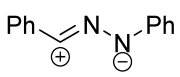 | 1/1/3 | CH3CN, rt, 5 h | >90 (100) | Only [8+4]-adducts were obtained. None of the reagents were added gradually. | [33] |
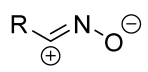 | 1/1.1/1.25 | C6H6, rt, ~12 h c | 50–90 (0–100) | R = alkyl, aryl. Substituents in both reagents had an impact on the preference between [4+2]- and [8+4]-products. | [35] | |
| 1/1.2 | CH3OH, rt, 1–15 h, Ar | 85–90 (100) | Only spiro products were obtained. Benzonitrile oxides were used themselves. | [34] | ||
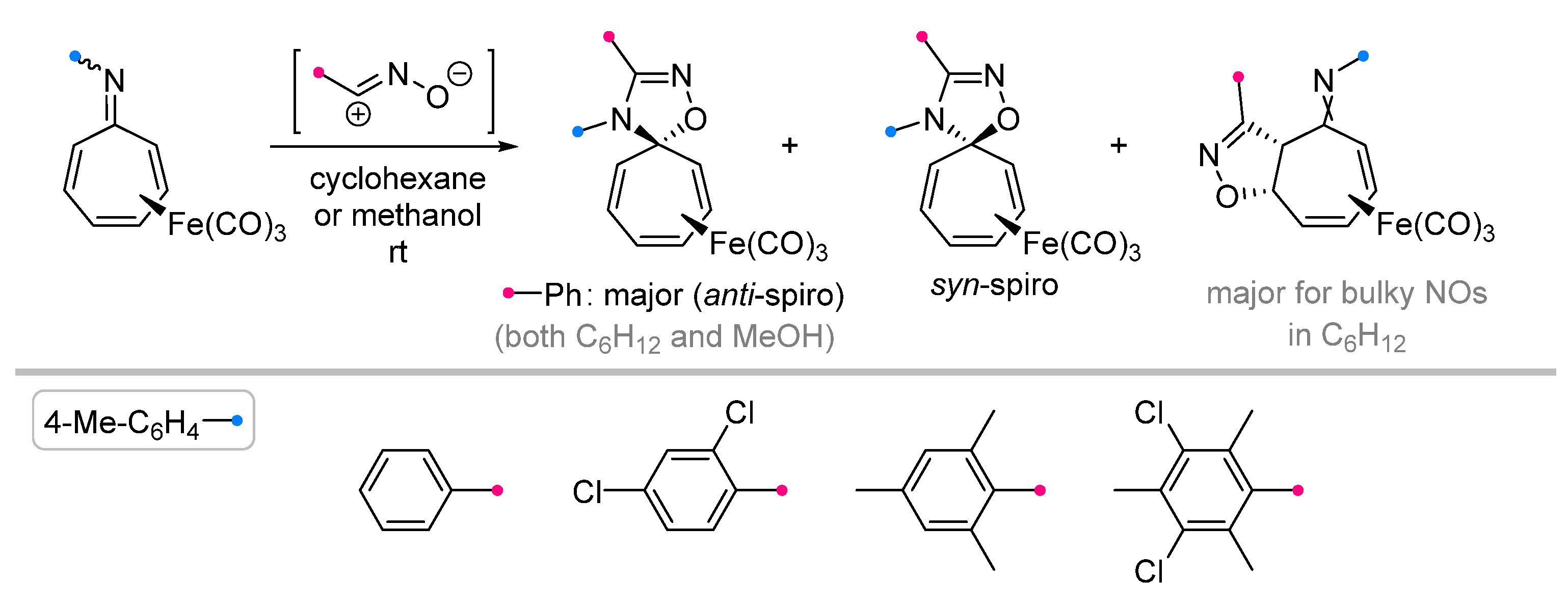
| 1/2 | rt, 30 min d | 77–98 (100) | [36] [37] | |||
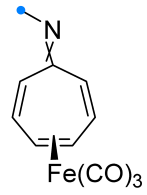 | 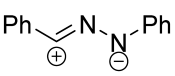 | 1/1/3 | CH3CN, rt, 6 h | >80 (100) | Only spiro products were obtained. None of the reagents were added gradually. | [33] |
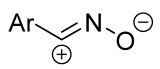 | no data | CH3OH, rt e | ≥95 (65–100) | Major [4+2]-product is spiro compound (mixture of syn- and anti-), minor is fused (C=C). | [34] | |
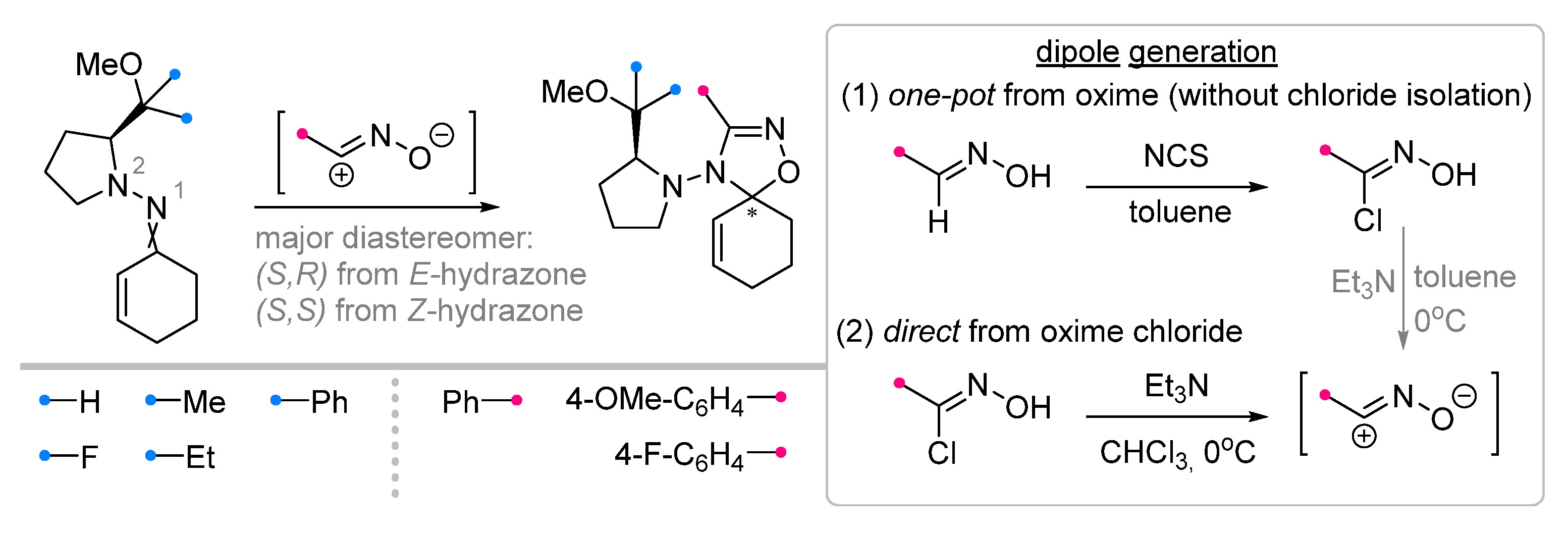
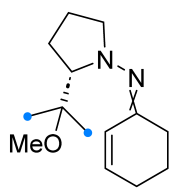 | 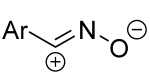 | 1/2/2 | CHCl3, 0 °C to rt, 6–8 h | 49–87 (100) | Only spiro products were obtained as two diastereomers: (S,R) from E-hydrazone (de = 28–86%), (S,S) from Z-hydrazone (de = 5–98%). The dipole precursor was gradually added to the dipolarophile and Et3N. | [40] |
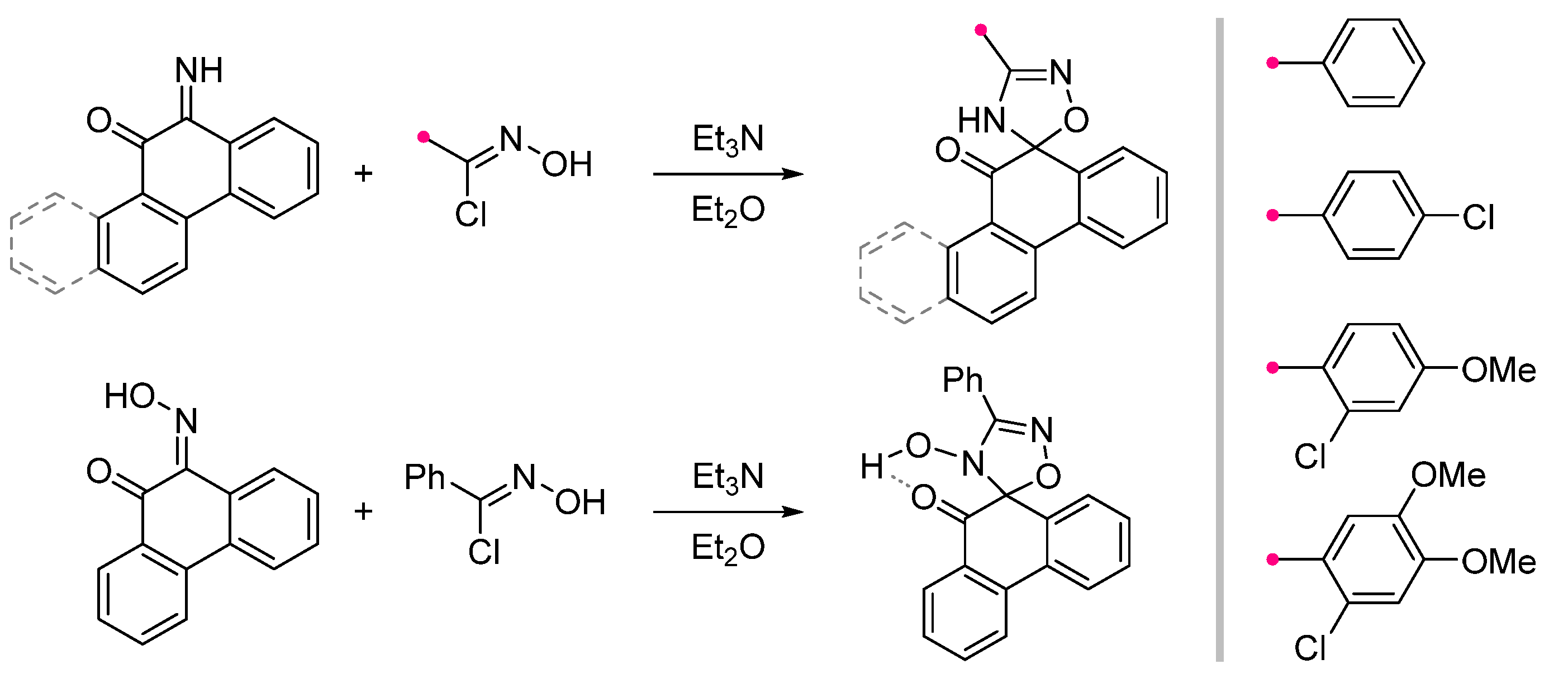
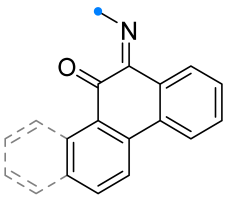 | 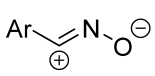 | see Note column | Et2O, reflux, 2 h | 55–82 (100) | Only masses of reagents were given: 1 g of imine and 10 g of hydroxybenzimidoyl chloride [42], 1 g of imine and 7 g of hydroxyimidoyl chloride [43], 1 g of oxime and 5 g of hydroxyimidoyl chloride [43]. | [42] [43] |
| Substrate | Dipole | Reagents Ratio a | Yields, % | Conditions | Note | Refs. |
|---|---|---|---|---|---|---|
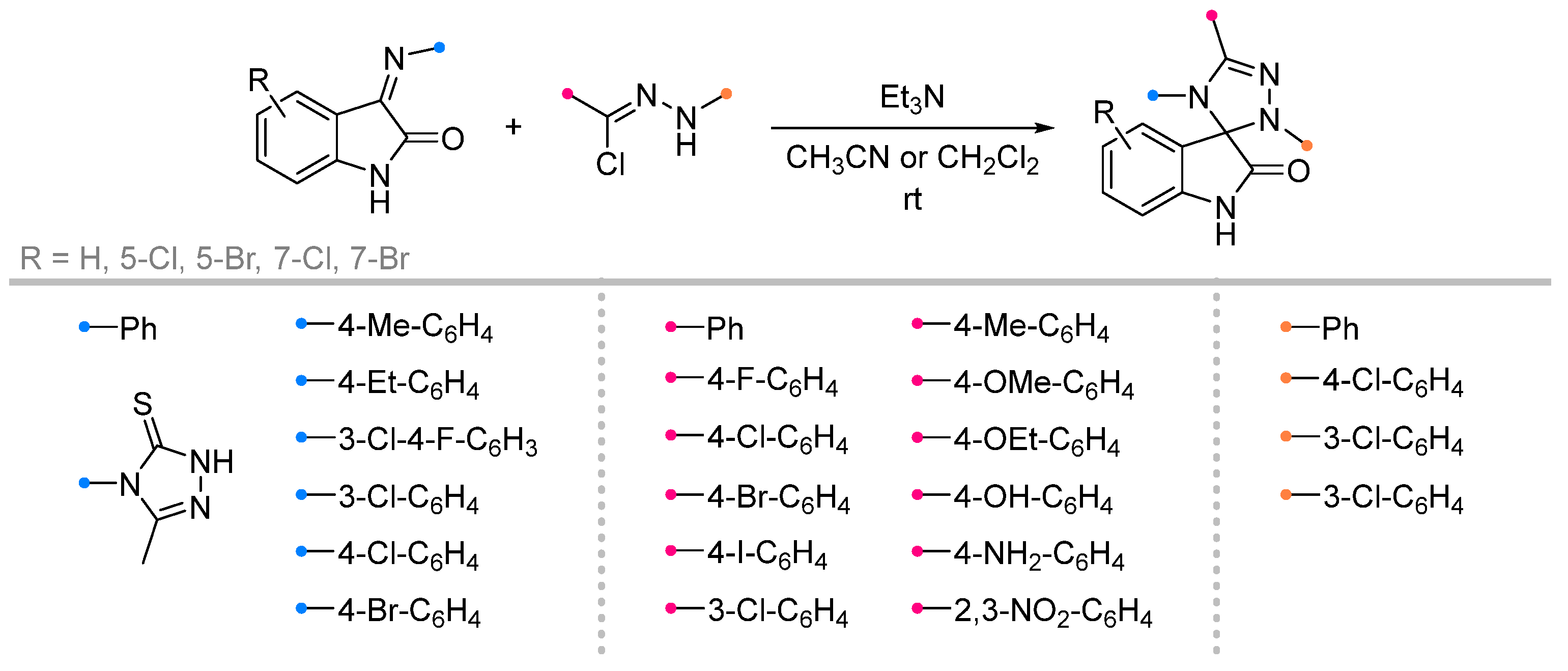
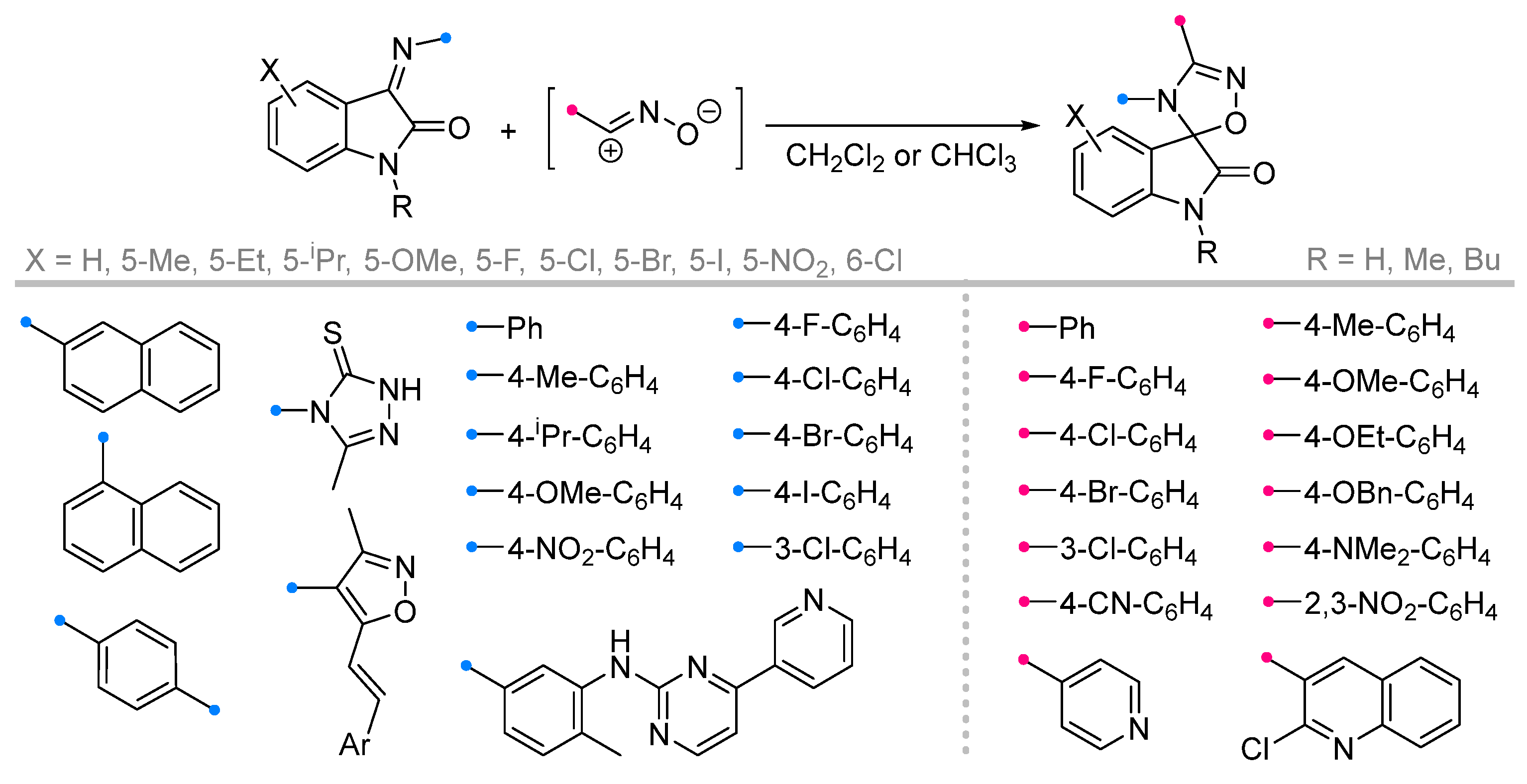
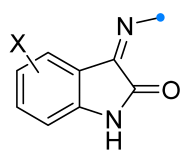 | 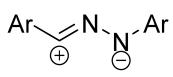 | 1/2/2 | 60–95 | DCM b, rt, 24 h, N2 | Dipolarophiles with substituents in indoline aryl fragment (X) were investigated. | [6] |
| 1/1/24 | 75–95 | CH3CN, rt, 18–21 h | X = H. None of the reagents were added gradually. | [44] | ||
| 1/1/1 | 70–90 | CH3CN, rt, 19–30 h | X = H. Triethylamine was added gradually. | [45] [46] | ||
| 1/1/1 | 78–95 | DMF b or DMA b, MW, 3–5 min | X = H. None of the reagents were added gradually. Reaction mixture was irradiated at 360 W in DMF [45] or at 200 W in DMA [46]. | |||
 | 1/2.2/2.6 | 62–93 | Et2O/THF (1/1), 0 °C to rt, 3 h | Dipolarophiles with substituents in indoline aryl fragment (X) were investigated. Et3N solution was added gradually. | [47] | |
| 1/2/2 | 54–87 | DCM, rt, 5–12 h, N2 | [49] | |||
| 1/2/1 | 64–86 | DCM/acetone, rt, 24 h | Dipolarophiles with substituents in indoline aryl fragment (X) were investigated. None of the reagents were added gradually. | [50] | ||
| 1/1/1 | 77–97 | CHCl3, 0 °C to rt, 4.5 h | X = H. Dipolarophile with 3-methyl-5-styryl-4-isoxazolylimino-group was investigated. Only C=N-cycloaddition product was obtained (not C=C of styryl). Et3N was added gradually upon cooling. | [48] | ||
| 1/1/15 c | 75–92 | DCM, 20 to 40 °C, 1–3 h | X = H. One-pot strategy: dipole precursor was synthesized oxime, NCS b and pyridine in DCM. Then dipolarophile (in one portion) and Et3N solution (gradually) were added. | [51] | ||
| 1/1/7 | 67–87 | [52] | ||||
| 1/1/1 | 89–96 | DMSO, 80 °C, MW, 3 min | X = H. Reaction mixture was irradiated at 400 W. | [52] | ||
| 1/1/- | 78–95 | EtOH, rt, 3 + 3 h | Dipolarophiles with substituents (X) were investigated. One-pot strategy (domino): dipolarophile was generated via condensation, then dipole precursor and DMAP (10 mol %) were added. | [53] | ||
| 1/1.1/2 | 80–85 | CHCl3, rt, ultrasonication, 20–30 min | Dipolarophiles with substituents in indoline aryl fragment (X). Et3N solution was added dropwise. | [54] | ||
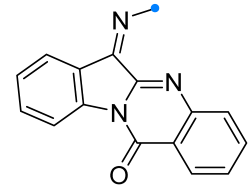 |  | 2.2/2.2/2.5 | 70–93 | CH3CN, rt, 30 h | Et3N solution was added dropwise. | [55] |
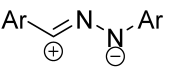
 | 1/1/15 c | 76–89 | DCM, 20 to 40 °C, 15 h | One-pot strategy: dipole precursor was synthesized oxime, NCS b and pyridine in DCM. Then dipolarophile (in one portion) and Et3N solution (gradually) were added. | [56] | |
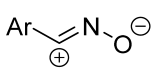
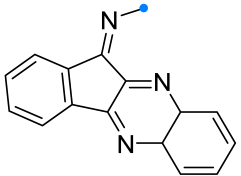 | 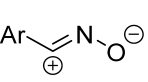 | 1/1.1/2 | 80–86 | DCM, rt, 15–25 min ultrasonication | Et3N solution was added dropwise. | [5] |
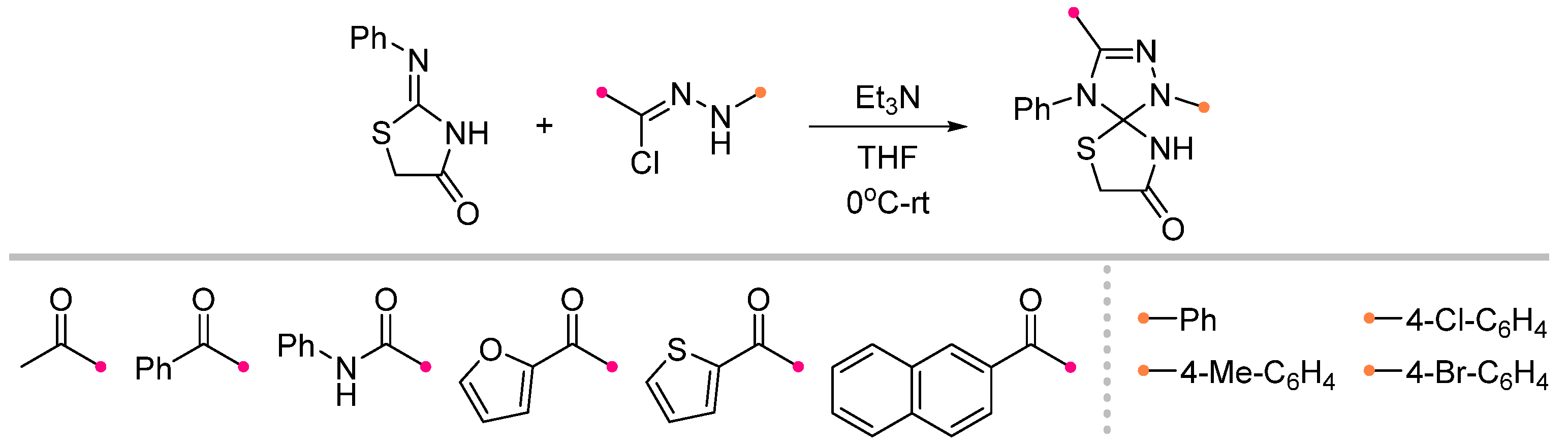
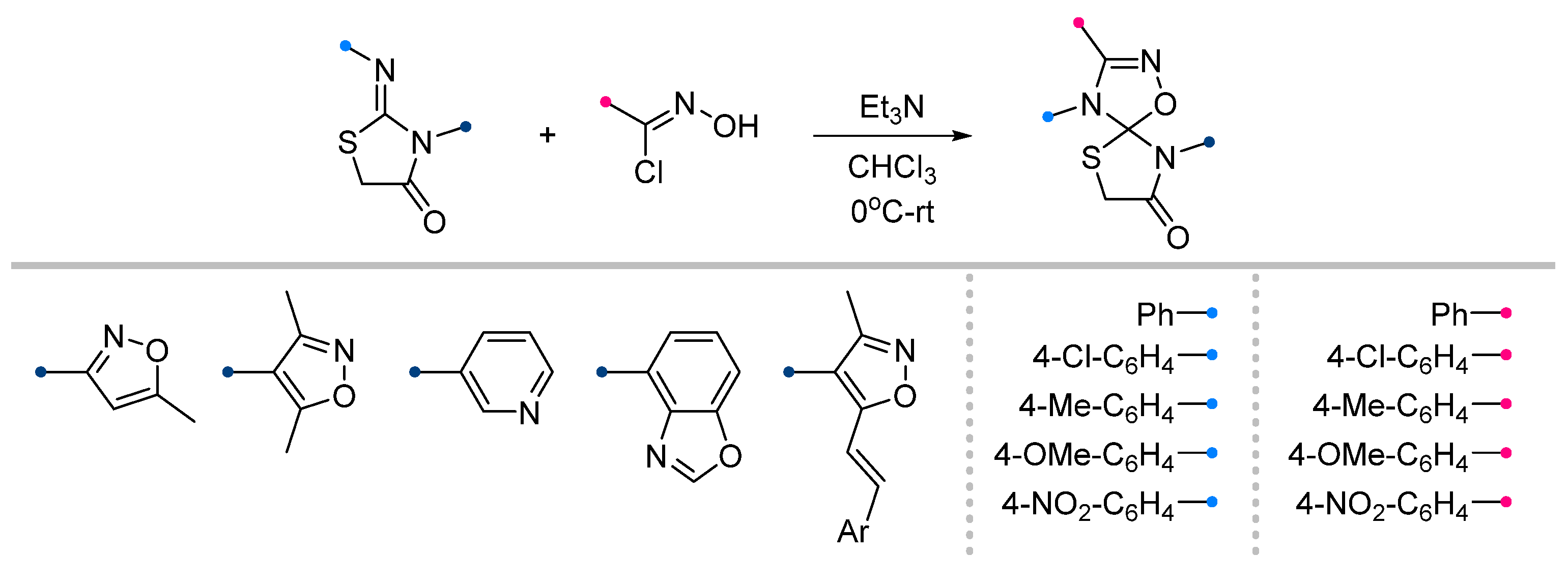
| Substrate | Dipole | Reagents Ratio a | Yields, % | Conditions | Note | Refs. |
|---|---|---|---|---|---|---|
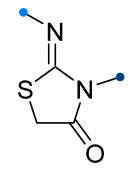 |  | 1/1/5 | 60–87 | THF, 0 °C to rt, overnight | Et3N solution was added gradually. Only C=N cycloaddition products were formed, C=O bond was inert. | [57] |
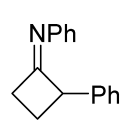
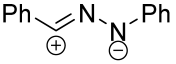
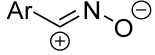 | 1/1/1 | 65–85 | CHCl3, 0 °C, 4–5 h | The same as above. | [58,59,60,61,62,63] | |
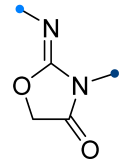 | 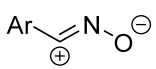 | 1/1/1 | 70–82 | CHCl3, 0 °C, 4–5 h | The same as above. | [60,63] |
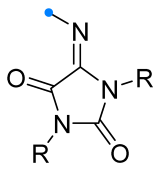 | 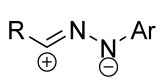 | 1/1.1/1.2 | 50–93 | DCM, rt, 24 h, Ar | R = methyl, aryl. Et3N was added dropwise. Low yield of spiro compound (13%) obtained from p-nitrophenyl substituted dipolarophile. | [64] |
| 1/1.1 | 52–90 | DCM, rt, 24 h | R = methyl, aryl. Diffusion mixing. Low yield of spiro compound (40%) obtained from p-nitrophenyl substituted dipolarophile. | |||
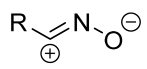 | 1/1.1/1.2 | 46–93 | DCM, 0 °C to rt, 24 h, Ar | R = aryl. No product when R = COOEt. Et3N solution was added dropwise. | [65] | |
| 1/1.1 | 34–88 | DCM, rt, 24 h | R = aryl. No product when R = COOEt. Diffusion mixing. | |||
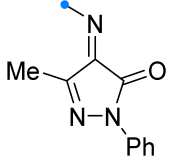 | 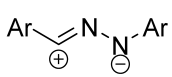 | 1/1.5 | 70–97 | CH3CN, rt, 20 h, light | Light-induced reaction (ultraviolet high-pressure Hg lamp, 365 nm): nitrile imine was generated from tetrazole. Gram scale experiments were conducted. | [66] |
| Substrate | Dipole | Reagents Ratio a | Yields, % | Conditions | Note | Refs. |
|---|---|---|---|---|---|---|
 | 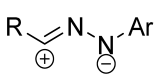 | 5 b/1/2.9 | 19–69 | C6H6, rt, 3 d, N2 | R = Ph, COOEt. Hydrazonoyl chloride was used as a dipole precursor, Et3N was used as a base. Large scale reactions. | [67] |
| 1 b/2.5/2.5 | 57–88 | DCM, rt, 38 h | R = CHF2, CF3. Hydrazonoyl bromide and K2CO3 were utilized. No reaction for arylated carbodiimide. Gram scale experiments were conducted. | [68] | ||
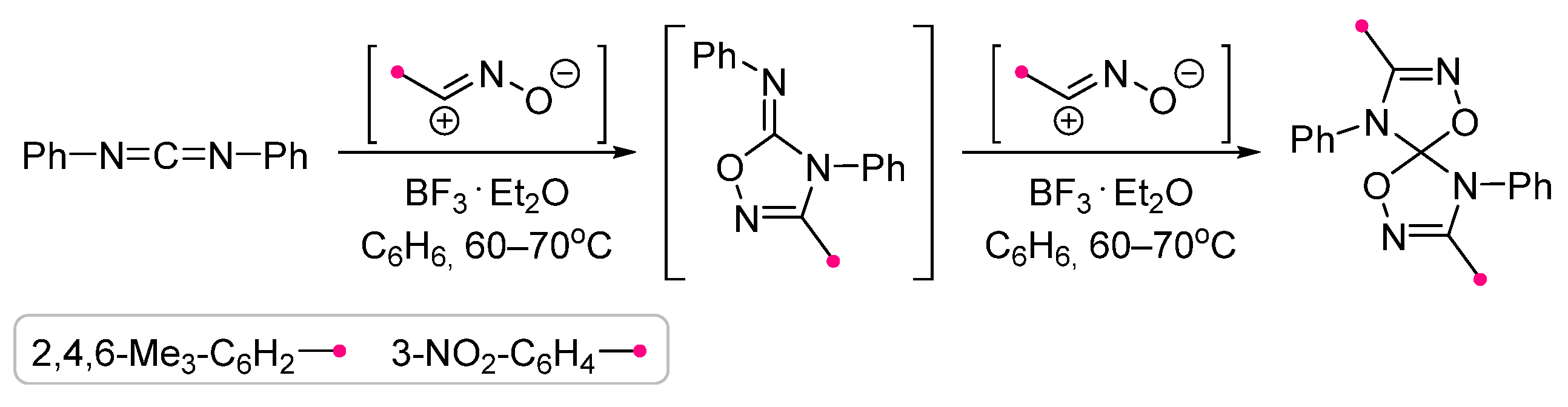
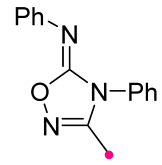 | 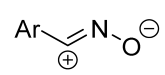 | 1 b/2 or 1 c/1 | 50–75 | C6H6, rt to 60–70 °C, 20–30 min | BF3⋅Et2O catalyzed reactions (equal amount relative to dipole). Benzonitrile oxides were used themselves. The method is suitable for carbodiimides as well as independently synthesized substrates. | [71] |
 | 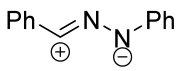 | 1/1.25/1.3 | 61–75 | THF, rt, 24 h | Hydrazonoyl and hydroxyimidoyl chlorides were used as the dipole precursors, Et3N was used as a base (gradually added). Gram scale experiments were conducted. | [72] |
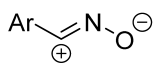 | 1/1.25/1.3 | 68–83 | THF, rt, 2 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, J.V.; Kukushkin, M.E.; Beloglazkina, E.K. 1,3-Dipolar Cycloaddition of Nitrile Imines and Nitrile Oxides to Exocyclic C=N Bonds—An Approach to Spiro-N-Heterocycles. Int. J. Mol. Sci. 2025, 26, 8673. https://doi.org/10.3390/ijms26178673
Petrova JV, Kukushkin ME, Beloglazkina EK. 1,3-Dipolar Cycloaddition of Nitrile Imines and Nitrile Oxides to Exocyclic C=N Bonds—An Approach to Spiro-N-Heterocycles. International Journal of Molecular Sciences. 2025; 26(17):8673. https://doi.org/10.3390/ijms26178673
Chicago/Turabian StylePetrova, Juliana V., Maxim E. Kukushkin, and Elena K. Beloglazkina. 2025. "1,3-Dipolar Cycloaddition of Nitrile Imines and Nitrile Oxides to Exocyclic C=N Bonds—An Approach to Spiro-N-Heterocycles" International Journal of Molecular Sciences 26, no. 17: 8673. https://doi.org/10.3390/ijms26178673
APA StylePetrova, J. V., Kukushkin, M. E., & Beloglazkina, E. K. (2025). 1,3-Dipolar Cycloaddition of Nitrile Imines and Nitrile Oxides to Exocyclic C=N Bonds—An Approach to Spiro-N-Heterocycles. International Journal of Molecular Sciences, 26(17), 8673. https://doi.org/10.3390/ijms26178673







