Targeting EGFR/IGF-IR Functional Crosstalk in 2D and 3D Triple-Negative Breast Cancer Models to Evaluate Tumor Progression
Abstract
1. Introduction
2. Results
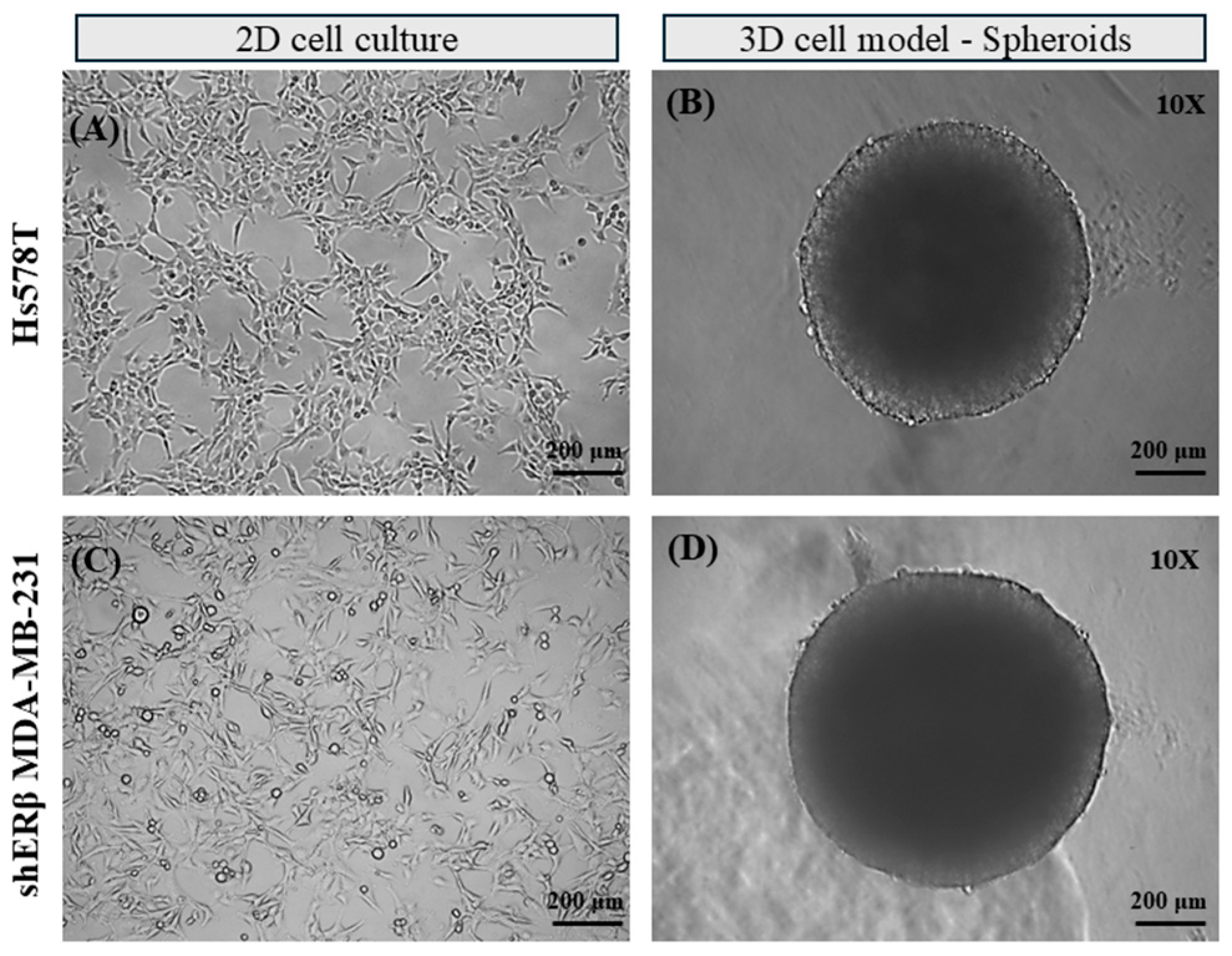
2.1. Development of 3D TNBC Cell-Derived Spheroids
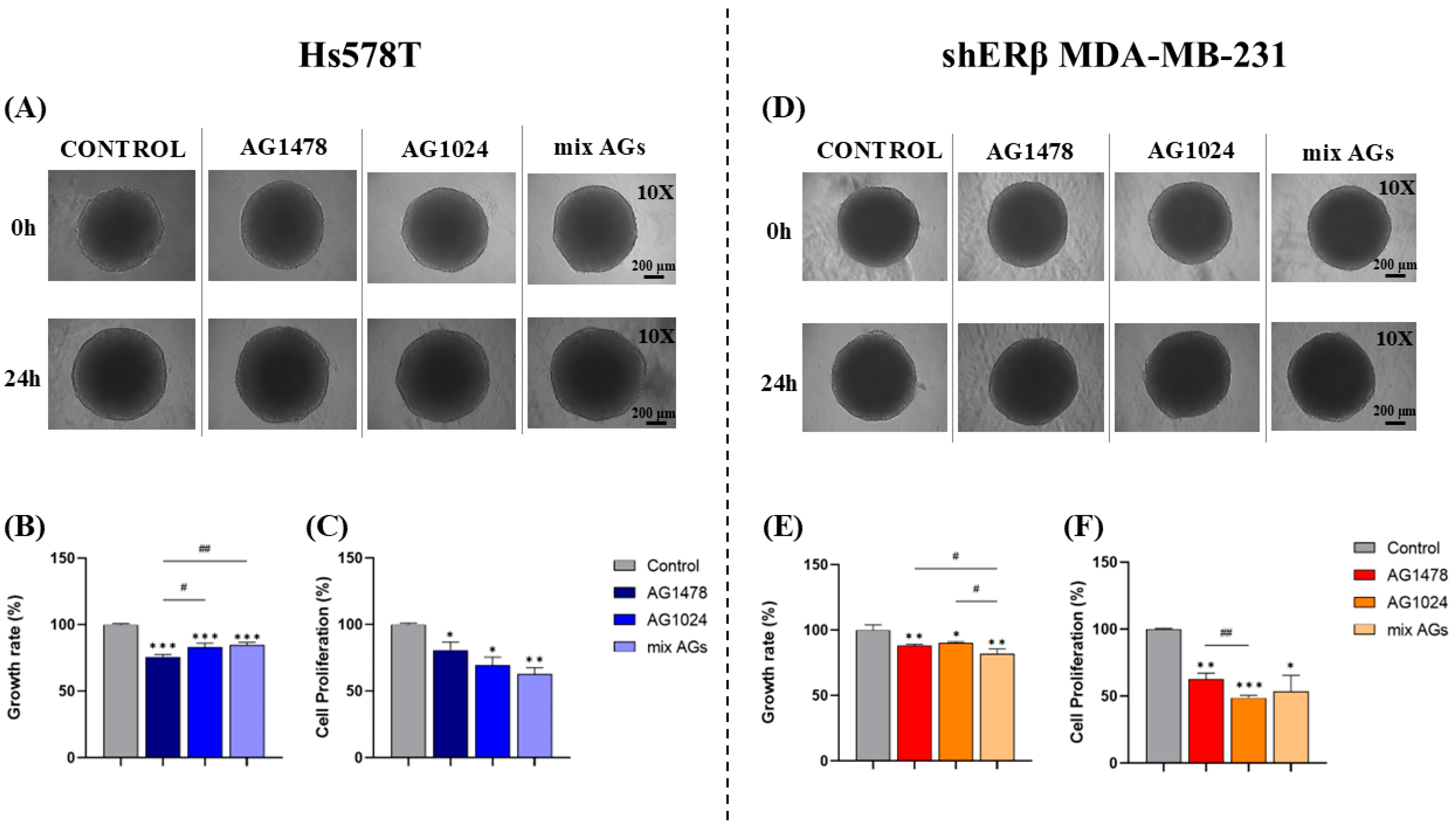
2.2. Effect of EGFR/IGF-IR Inhibition on Spheroid Growth Rate
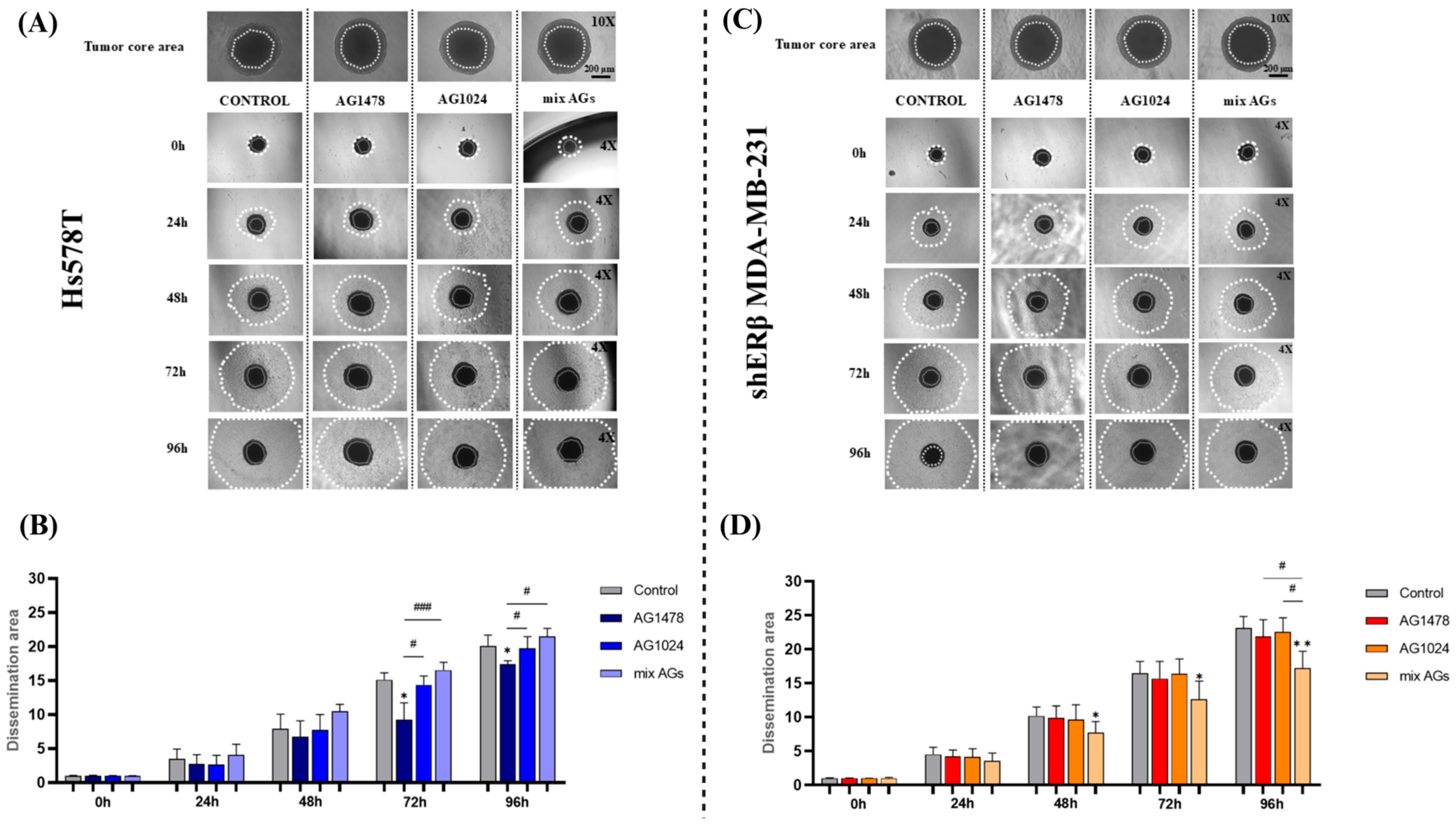
2.3. Effect of EGFR/IGF-IR Inhibition on Spheroid Dissemination
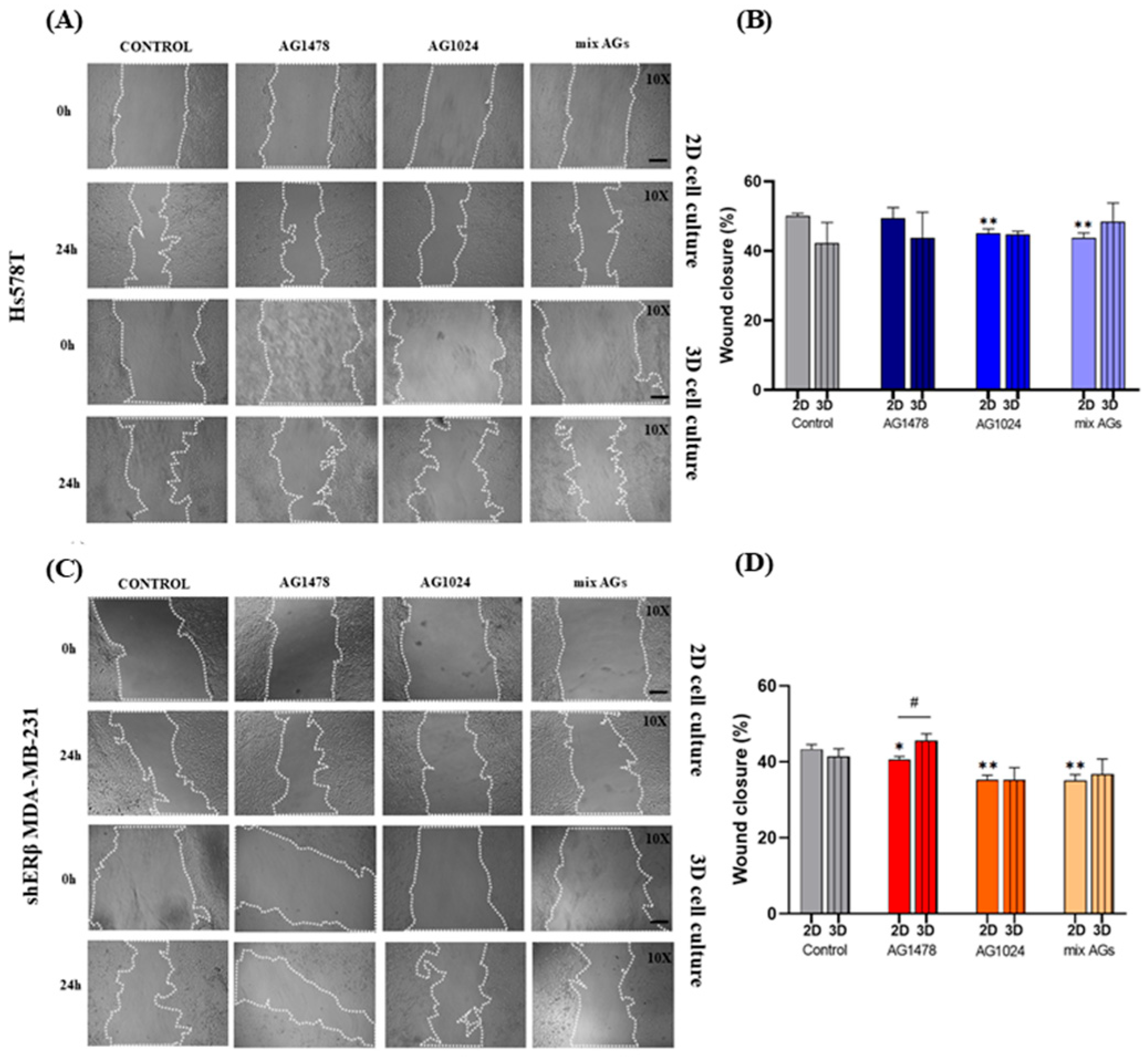
2.4. Evaluation of the Effect of EGFR/IGF-IR Inhibition on the Migratory Capacity of Spheroid-Derived Cells vs. 2D Cell Cultures
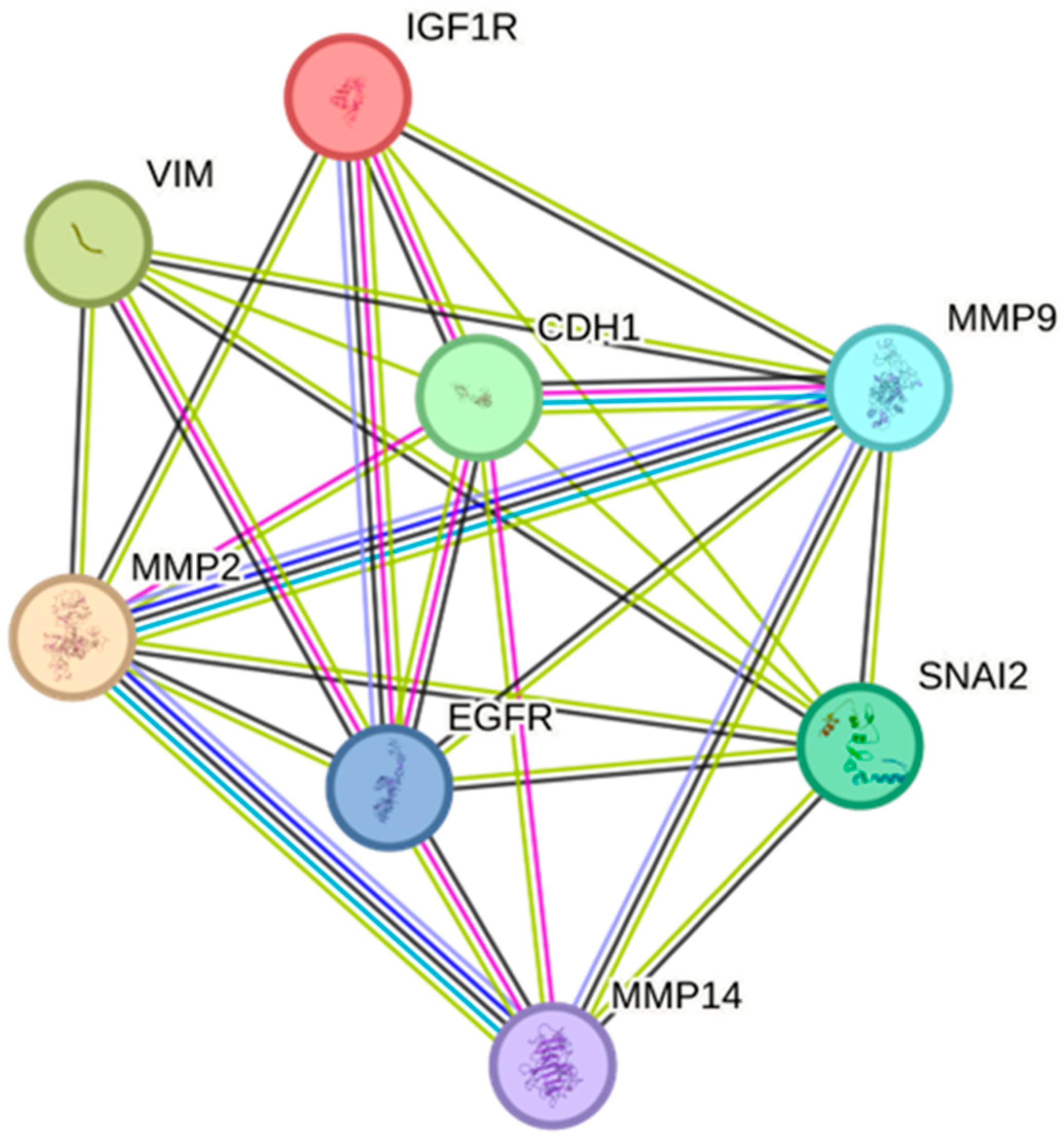
2.5. Protein–Protein Interactions of EGFR/IGF-IR with EMT Markers and Matrix Remodeling Enzymes
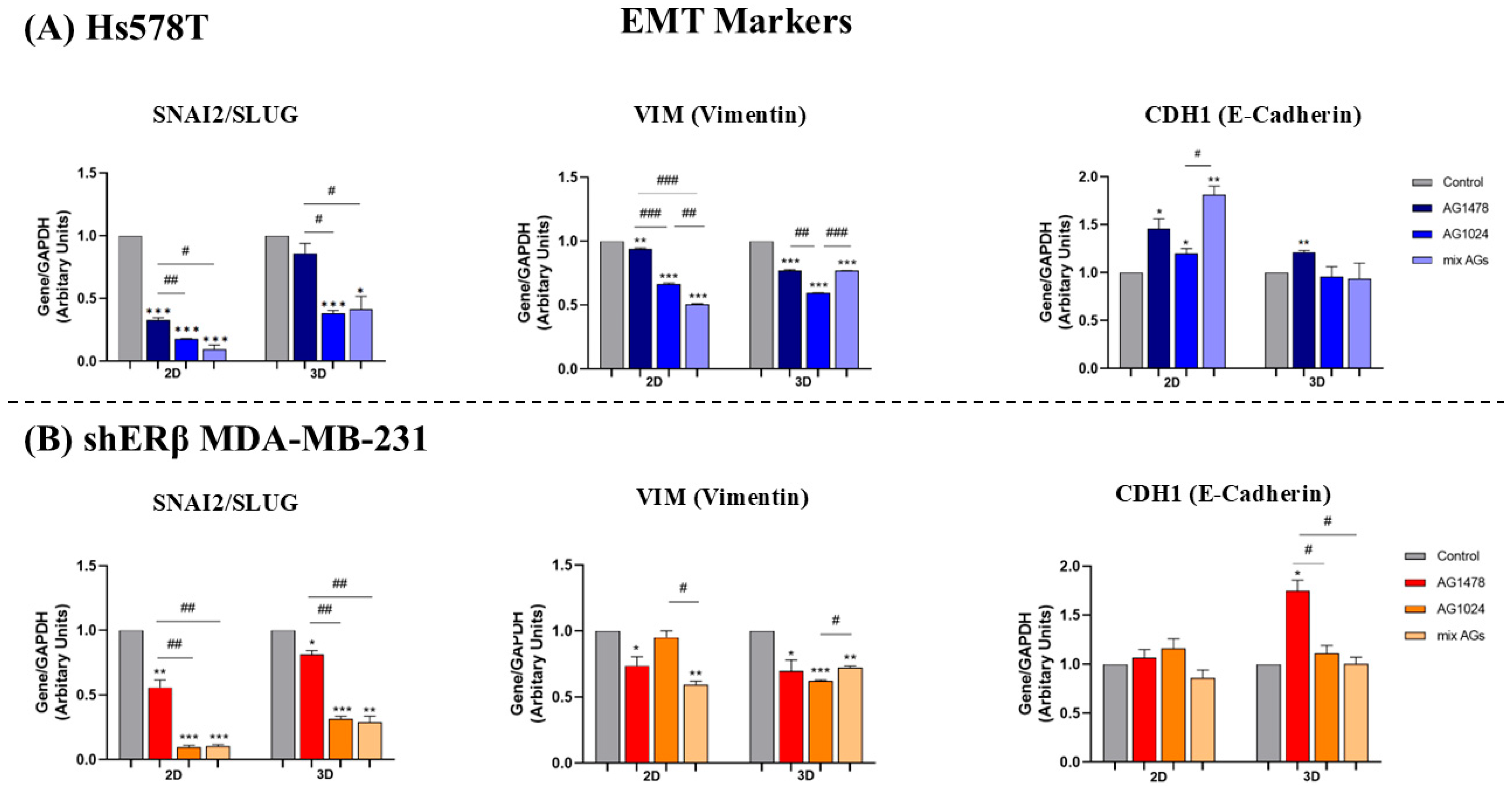
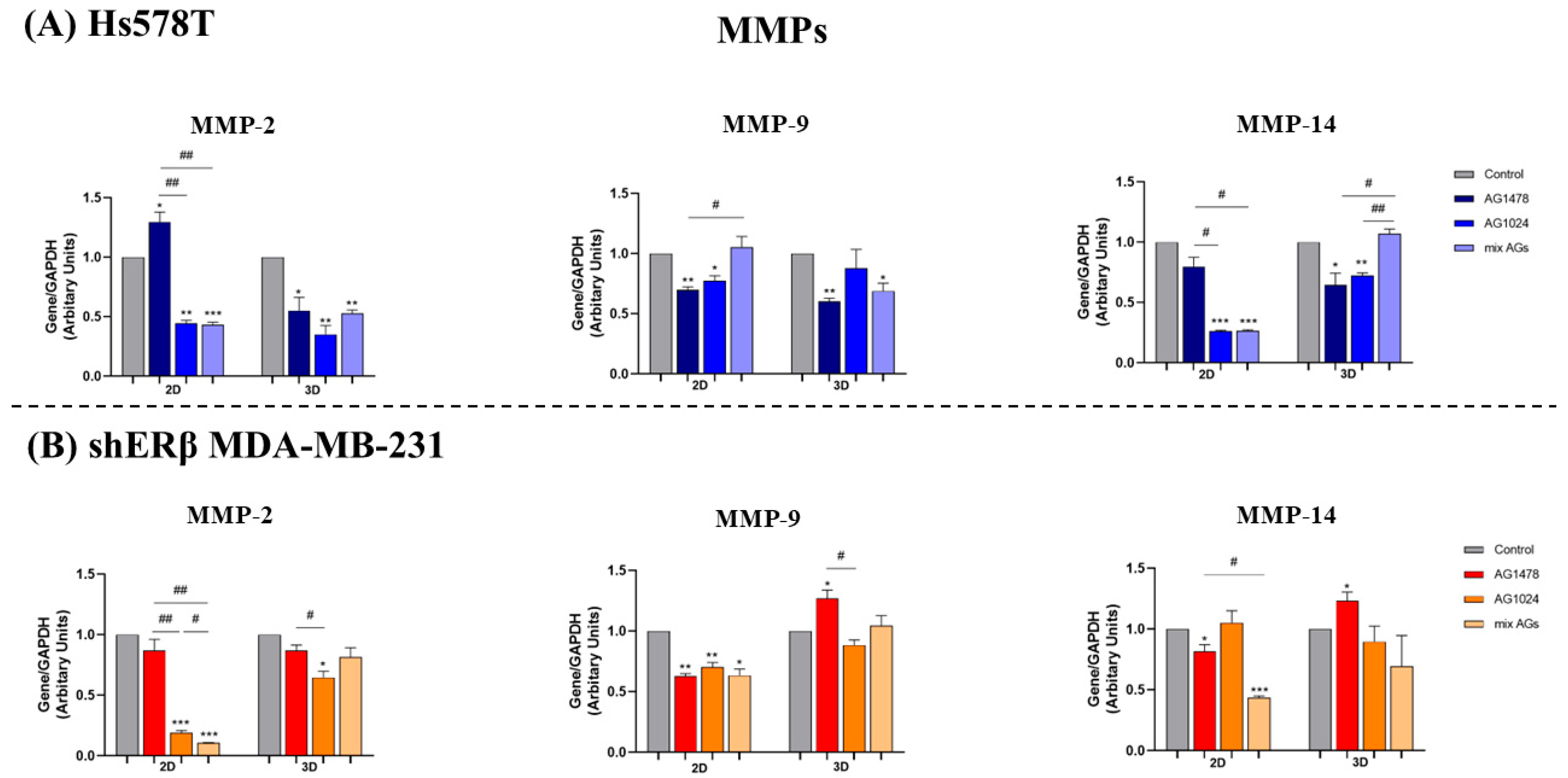
2.6. EGFR/IGF-IR Inhibition Effects on Gene Expression of EMT Markers and MMPs in 2D and 3D TNBC Cell Cultures
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Reagents
4.2. Development of 3D Spheroid Cultures
4.3. Spheroid Growth and Proliferation
4.4. Spheroid Dissemination Assay
4.5. Wound Healing Assays in 2D and 3D Cell Cultures
4.6. STRING Database
4.7. RNA Isolation, Complementary DNA (cDNA) Synthesis and Real-Time qPCR Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D/3D | Two-/three-dimensional |
| BRCA1/2 | Breast cancer gene 1/2 |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-to-mesenchymal transition |
| ERα/β | Estrogen receptor alpha/beta |
| HER2 | Human epidermal receptor 2 |
| IGF-IR | Insulin-like growth factor Ι receptor |
| MMPs | Matrix metalloproteinases |
| PR | Progesterone receptor |
| RTKs | Receptor tyrosine kinases |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Eliyatkin, N.; Yalcin, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J. Breast Health 2015, 11, 59–66. [Google Scholar] [CrossRef]
- Charan, M.; Verma, A.K.; Hussain, S.; Misri, S.; Mishra, S.; Majumder, S.; Ramaswamy, B.; Ahirwar, D.; Ganju, R.K. Molecular and Cellular Factors Associated with Racial Disparity in Breast Cancer. Int. J. Mol. Sci. 2020, 21, 5936. [Google Scholar] [CrossRef]
- Mota, A.M.; Mendes, J.; Matela, N. Breast Cancer Molecular Subtype Prediction: A Mammography-Based AI Approach. Biomedicines 2024, 12, 1371. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Wu, H.; Yu, Z. Advancements and Challenges in Triple-Negative Breast Cancer: A Comprehensive Review of Therapeutic and Diagnostic Strategies. Front. Oncol. 2024, 14, 1405491. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; Siewertsz Van Reesema, L.L.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies. Cancers 2020, 12, 2392. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent Advances in Therapeutic Strategies for Triple-Negative Breast Cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Biray Avci, C.; Goker Bagca, B.; Nikanfar, M.; Takanlou, L.S.; Takanlou, M.S.; Nourazarian, A. Tumor Microenvironment and Cancer Metastasis: Molecular Mechanisms and Therapeutic Implications. Front. Pharmacol. 2024, 15, 1442888. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A Guide to the Composition and Functions of the Extracellular Matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Insua-Rodríguez, J.; Oskarsson, T. The Extracellular Matrix in Breast Cancer. Adv. Drug Deliv. Rev. 2016, 97, 41–55. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Wan, D.; Zhou, L.; Zheng, S.; Lin, S.; Qiao, Y. Extracellular Matrix and Its Therapeutic Potential for Cancer Treatment. Signal Transduct. Target. Ther. 2021, 6, 153. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Zhang, G.; Chai, X.-X.; Ren, L.; Yin, D.-C.; Zhang, C.-Y. Recent Progress on the Effect of Extracellular Matrix on Occurrence and Progression of Breast Cancer. Life Sci. 2023, 332, 122084. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, X.; Zheng, Y.; Chen, Y.; Fei, W.; Wang, F.; Zheng, C. Extracellular Matrix: Emerging Roles and Potential Therapeutic Targets for Breast Cancer. Front. Oncol. 2021, 11, 650453. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Y. Receptor Tyrosine Kinases: Biological Functions and Anticancer Targeted Therapy. MedComm 2023, 4, e446. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lovly, C.M. Mechanisms of Receptor Tyrosine Kinase Activation in Cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Saraon, P.; Pathmanathan, S.; Snider, J.; Lyakisheva, A.; Wong, V.; Stagljar, I. Receptor Tyrosine Kinases and Cancer: Oncogenic Mechanisms and Therapeutic Approaches. Oncogene 2021, 40, 4079–4093. [Google Scholar] [CrossRef]
- Sigismund, S.; Avanzato, D.; Lanzetti, L. Emerging Functions of the EGFR in Cancer. Mol. Oncol. 2018, 12, 3–20. [Google Scholar] [CrossRef]
- Soni, U.K.; Jenny, L.; Hegde, R.S. IGF-1R Targeting in Cancer—Does Sub-Cellular Localization Matter? J. Exp. Clin. Cancer Res. 2023, 42, 273. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Tocheny, C.E.; Shaw, L.M. The Insulin-like Growth Factor Signaling Pathway in Breast Cancer: An Elusive Therapeutic Target. Life 2022, 12, 1992. [Google Scholar] [CrossRef]
- Ali, R.; Wendt, M.K. The Paradoxical Functions of EGFR during Breast Cancer Progression. Signal Transduct. Target. Ther. 2017, 2, 16042. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Fardi, E.; Ghaderi, H.; Palizdar, S.; Khorram, R.; Vafadar, R.; Ghanaatian, M.; Rezaei-Tazangi, F.; Baziyar, P.; Ahmadi, A.; et al. Receptor Tyrosine Kinase Inhibitors in Cancer. Cell. Mol. Life Sci. 2023, 80, 104. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Patel, A.P.; Debs, L.H.; Nguyen, D.; Patel, K.; Grati, M.; Mittal, J.; Yan, D.; Chapagain, P.; Liu, X.Z. Intricate Functions of Matrix Metalloproteinases in Physiological and Pathological Conditions. J. Cell. Physiol. 2016, 231, 2599–2621. [Google Scholar] [CrossRef]
- Khalili-Tanha, G.; Radisky, E.S.; Radisky, D.C.; Shoari, A. Matrix Metalloproteinase-Driven Epithelial-Mesenchymal Transition: Implications in Health and Disease. J. Transl. Med. 2025, 23, 436. [Google Scholar] [CrossRef]
- Kollet, O.; Das, A.; Karamanos, N.; Auf Dem Keller, U.; Sagi, I. Redefining Metalloproteases Specificity through Network Proteolysis. Trends Mol. Med. 2024, 30, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Koran, S.; AlOmair, L. Insights Into the Role of Matrix Metalloproteinases in Cancer and Its Various Therapeutic Aspects: A Review. Front. Mol. Biosci. 2022, 9, 896099. [Google Scholar] [CrossRef] [PubMed]
- Gobin, E.; Bagwell, K.; Wagner, J.; Mysona, D.; Sandirasegarane, S.; Smith, N.; Bai, S.; Sharma, A.; Schleifer, R.; She, J.-X. A Pan-Cancer Perspective of Matrix Metalloproteases (MMP) Gene Expression Profile and Their Diagnostic/Prognostic Potential. BMC Cancer 2019, 19, 581. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Koo, I.-S.; Hwang, H.J.; Lee, D.W. In Vitro Three-Dimensional (3D) Cell Culture Tools for Spheroid and Organoid Models. SLAS Discov. 2023, 28, 119–137. [Google Scholar] [CrossRef]
- Lai, X.; Lu, T.; Zhang, F.; Khan, A.; Zhao, Y.; Li, X.; Xiang, S.; Lin, K. Lysosome-Targeted Theranostics: Integration of Real-Time Fluorescence Imaging and Controlled Drug Delivery via Zn(II)-Schiff Base Complexes. J. Inorg. Biochem. 2025, 272, 113015. [Google Scholar] [CrossRef]
- Mangani, S.; Kremmydas, S.; Karamanos, N.K. Mimicking the Complexity of Solid Tumors: How Spheroids Could Advance Cancer Preclinical Transformative Approaches. Cancers 2025, 17, 1161. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Koutsakis, C.; Piperigkou, Z.; Karamanos, N.K. Recreating the Extracellular Matrix: Novel 3D Cell Culture Platforms in Cancer Research. FEBS J. 2023, 290, 5238–5247. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Singh, S.; Mittal, A.; Desai, N.; Khatri, D.K.; Gugulothu, D.; Lather, V.; Pandita, D.; Vora, L.K. Spheroids in Cancer Research: Recent Advances and Opportunities. J. Drug Deliv. Sci. Technol. 2024, 100, 106033. [Google Scholar] [CrossRef]
- Benelli, R.; Zocchi, M.R.; Poggi, A. Three-Dimensional (3D) Culture Models in Cancer Investigation, Drug Testing and Immune Response Evaluation. Int. J. Mol. Sci. 2020, 22, 150. [Google Scholar] [CrossRef]
- Abbas, Z.N.; Al-Saffar, A.Z.; Jasim, S.M.; Sulaiman, G.M. Comparative Analysis between 2D and 3D Colorectal Cancer Culture Models for Insights into Cellular Morphological and Transcriptomic Variations. Sci. Rep. 2023, 13, 18380. [Google Scholar] [CrossRef]
- Thakuri, P.S.; Gupta, M.; Plaster, M.; Tavana, H. Quantitative Size-Based Analysis of Tumor Spheroids and Responses to Therapeutics. ASSAY Drug Dev. Technol. 2019, 17, 140–149. [Google Scholar] [CrossRef]
- Sant, S.; Johnston, P.A. The Production of 3D Tumor Spheroids for Cancer Drug Discovery. Drug Discov. Today Technol. 2017, 23, 27–36. [Google Scholar] [CrossRef]
- Lal-Nag, M.; McGee, L.; Titus, S.A.; Brimacombe, K.; Michael, S.; Sittampalam, G.; Ferrer, M. Exploring Drug Dosing Regimens In Vitro Using Real-Time 3D Spheroid Tumor Growth Assays. SLAS Discov. 2017, 22, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Ferrari-Amorotti, G.; Chiodoni, C.; Shen, F.; Cattelani, S.; Soliera, A.R.; Manzotti, G.; Grisendi, G.; Dominici, M.; Rivasi, F.; Colombo, M.P.; et al. Suppression of Invasion and Metastasis of Triple-Negative Breast Cancer Lines by Pharmacological or Genetic Inhibition of Slug Activity. Neoplasia 2014, 16, 1047–1058. [Google Scholar] [CrossRef]
- Winter, M.; Meignan, S.; Völkel, P.; Angrand, P.-O.; Chopin, V.; Bidan, N.; Toillon, R.-A.; Adriaenssens, E.; Lagadec, C.; Le Bourhis, X. Vimentin Promotes the Aggressiveness of Triple Negative Breast Cancer Cells Surviving Chemotherapeutic Treatment. Cells 2021, 10, 1504. [Google Scholar] [CrossRef]
- Kwon, M.J. Matrix Metalloproteinases as Therapeutic Targets in Breast Cancer. Front. Oncol. 2023, 12, 1108695. [Google Scholar] [CrossRef]
- Ling, B.; Watt, K.; Banerjee, S.; Newsted, D.; Truesdell, P.; Adams, J.; Sidhu, S.S.; Craig, A.W.B. A Novel Immunotherapy Targeting MMP-14 Limits Hypoxia, Immune Suppression and Metastasis in Triple-Negative Breast Cancer Models. Oncotarget 2017, 8, 58372–58385. [Google Scholar] [CrossRef]
- Jiang, H.; Li, H. Prognostic Values of Tumoral MMP2 and MMP9 Overexpression in Breast Cancer: A Systematic Review and Meta-Analysis. BMC Cancer 2021, 21, 149. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Kefali, E.; Piperigkou, Z.; Riethmüller, C.; Greve, B.; Franchi, M.; Götte, M.; Karamanos, N.K. EGFR Is a Pivotal Player of the E2/ERβ—Mediated Functional Properties, Aggressiveness, and Stemness in Triple-negative Breast Cancer Cells. FEBS J. 2022, 289, 1552–1574. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Biswas, M.; Sadhukhan, S.; Sonawane, A. Role of EGFR and FASN in Breast Cancer Progression. J. Cell Commun. Signal. 2023, 17, 1249–1282. [Google Scholar] [CrossRef]
- Kleiman, L.B.; Maiwald, T.; Conzelmann, H.; Lauffenburger, D.A.; Sorger, P.K. Rapid Phospho-Turnover by Receptor Tyrosine Kinases Impacts Downstream Signaling and Drug Binding. Mol. Cell 2011, 43, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cai, L.; Wang, X.; Yu, Y.; Jian, W.; Bao, G.; Gao, Z.; Guo, J.; Zhang, J.; Li, C.; et al. RHBDD1 Promotes Proliferation, Migration, Invasion and EMT in Renal Cell Carcinoma via the EGFR/AKT Signaling Pathway. Mol. Med. Rep. 2021, 24, 826. [Google Scholar] [CrossRef]
- Zhang, D.; LaFortune, T.A.; Krishnamurthy, S.; Esteva, F.J.; Cristofanilli, M.; Liu, P.; Lucci, A.; Singh, B.; Hung, M.-C.; Hortobagyi, G.N.; et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Reverses Mesenchymal to Epithelial Phenotype and Inhibits Metastasis in Inflammatory Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 6639–6648. [Google Scholar] [CrossRef] [PubMed]
- Gourdoupi, C.; Kremmydas, S.; Mangani, S.; Ioannou, P.; Afratis, N.A.; Piperigkou, Z.; Karamanos, N.K. From Structure to Function: The Impact of EGFR and IGF-IR in 3D Breast Cancer Spheroids. Cancers 2025, 17, 2606. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Veldwijk, M.R.; Zhang, Q.; Li, Z.; Xu, W.; Fu, S. Co-Inhibition of Epidermal Growth Factor Receptor and Insulin-like Growth Factor Receptor 1 Enhances Radiosensitivity in Human Breast Cancer Cells. BMC Cancer 2013, 13, 297. [Google Scholar] [CrossRef]
- Tsonis, A.I.; Afratis, N.; Gialeli, C.; Ellina, M.; Piperigkou, Z.; Skandalis, S.S.; Theocharis, A.D.; Tzanakakis, G.N.; Karamanos, N.K. Evaluation of the Coordinated Actions of Estrogen Receptors with Epidermal Growth Factor Receptor and Insulin-like Growth Factor Receptor in the Expression of Cell Surface Heparan Sulfate Proteoglycans and Cell Motility in Breast Cancer Cells. FEBS J. 2013, 280, 2248–2259. [Google Scholar] [CrossRef]
- Butti, R.; Das, S.; Gunasekaran, V.P.; Yadav, A.S.; Kumar, D.; Kundu, G.C. Receptor Tyrosine Kinases (RTKs) in Breast Cancer: Signaling, Therapeutic Implications and Challenges. Mol. Cancer 2018, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D Cell Cultures—A Comparison of Different Types of Cancer Cell Cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Sabetta, S.; Vecchiotti, D.; Clementi, L.; Di Vito Nolfi, M.; Zazzeroni, F.; Angelucci, A. Comparative Analysis of Dasatinib Effect between 2D and 3D Tumor Cell Cultures. Pharmaceutics 2023, 15, 372. [Google Scholar] [CrossRef]
- Shome, R.; Ghosh, S.S. Tweaking EMT and MDR Dynamics to Constrain Triple-Negative Breast Cancer Invasiveness by EGFR and Wnt/β-Catenin Signaling Regulation. Cell. Oncol. 2021, 44, 405–422. [Google Scholar] [CrossRef]
- Wei, J.; Lei, D.; Chen, M.; Ran, P.; Li, X. Engineering HepG2 Spheroids with Injectable Fiber Fragments as Predictable Models for Drug Metabolism and Tumor Infiltration. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3331–3344. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.; Meehan, J.; Mullen, P.; Supuran, C.; Dixon, J.M.; Thomas, J.S.; Winum, J.-Y.; Lambin, P.; Dubois, L.; Pavathaneni, N.-K.; et al. Evaluation of Carbonic Anhydrase IX as a Therapeutic Target for Inhibition of Breast Cancer Invasion and Metastasis Using a Series of in Vitro Breast Cancer Models. Oncotarget 2015, 6, 24856–24870. [Google Scholar] [CrossRef]
- Godet, I.; Doctorman, S.; Wu, F.; Gilkes, D.M. Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances. Cells 2022, 11, 686. [Google Scholar] [CrossRef]
- Borcherding, N.; Cole, K.; Kluz, P.; Jorgensen, M.; Kolb, R.; Bellizzi, A.; Zhang, W. Re-Evaluating E-Cadherin and β-Catenin. Am. J. Pathol. 2018, 188, 1910–1920. [Google Scholar] [CrossRef]
- Taliaferro-Smith, L.; Oberlick, E.; Liu, T.; McGlothen, T.; Alcaide, T.; Tobin, R.; Donnelly, S.; Commander, R.; Kline, E.; Nagaraju, G.P.; et al. FAK Activation Is Required for IGF1R-Mediated Regulation of EMT, Migration, and Invasion in Mesenchymal Triple Negative Breast Cancer Cells. Oncotarget 2015, 6, 4757–4772. [Google Scholar] [CrossRef]
- Radisky, E.S. Matrix Metalloproteinases as Drivers and Therapeutic Targets in Breast Cancer. Front. Biosci. 2015, 20, 1144–1163. [Google Scholar] [CrossRef]
- Knapinska, A.M.; Fields, G.B. The Expanding Role of MT1-MMP in Cancer Progression. Pharmaceuticals 2019, 12, 77. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielińska, W.; Krajewski, A.; Hałas-Wiśniewska, M.; Mikołajczyk, K.; Gagat, M.; Grzanka, A. Downregulation of MMP-9 Enhances the Anti-Migratory Effect of Cyclophosphamide in MDA-MB-231 and MCF-7 Breast Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 12783. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein–Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Muguruma, M.; Teraoka, S.; Miyahara, K.; Ueda, A.; Asaoka, M.; Okazaki, M.; Kawate, T.; Kuroda, M.; Miyagi, Y.; Ishikawa, T. Differences in Drug Sensitivity between Two-Dimensional and Three-Dimensional Culture Systems in Triple-Negative Breast Cancer Cell Lines. Biochem. Biophys. Res. Commun. 2020, 533, 268–274. [Google Scholar] [CrossRef]
- Kaur, G.; Doroshow, J.H.; Teicher, B.A. Format (2D vs. 3D) and Media Effect Target Expression and Response of Patient-Derived and Standard NSCLC Lines to EGFR Inhibitors. Cancer Treat. Res. Commun. 2021, 29, 100463. [Google Scholar] [CrossRef]
- Florio, A.; Johnson, S.; Salvatori, R.; Vasmatzis, G. Monolayer Culture Alters EGFR Inhibitor Response through Abrogation of microRNA-Mediated Feedback Regulation. Sci. Rep. 2024, 14, 7303. [Google Scholar] [CrossRef] [PubMed]
- El Harane, S.; Zidi, B.; El Harane, N.; Krause, K.-H.; Matthes, T.; Preynat-Seauve, O. Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells 2023, 12, 1001. [Google Scholar] [CrossRef]
- Hofmann, S.; Cohen-Harazi, R.; Maizels, Y.; Koman, I. Patient-Derived Tumor Spheroid Cultures as a Promising Tool to Assist Personalized Therapeutic Decisions in Breast Cancer. Transl. Cancer Res. 2022, 11, 134–147. [Google Scholar] [CrossRef] [PubMed]

| Target Gene | Primers Sequences (5′–3′) | Annealing Τ (°C) | |
|---|---|---|---|
| SNAI2 | F | AGACCCTGGTTGCTTCAAGGA | 60 |
| R | CTCAGATTTGACCTGTCTGCAAA | ||
| VIM | F | GGCTCGTCACCTTCGTGAAT | 60 |
| R | GAGAAATCCTGCTCTCCTCGC | ||
| CDH1 | F | TACGCCTGGGACTCCACCTA | 60 |
| R | CCAGAAACGGAGGCCTGAT | ||
| MMP-2 | F | CGTCTGTCCCAGGATGACATC | 62 |
| R | ATGTCAGGAGAGGCCCCATA | ||
| MMP-9 | F | TTCCAGTACCGAGAGAAAGCCTAT | 62 |
| R | GGTCACGTAGCCCACTTGGT | ||
| MMP-14 | F | CATGGGCAGCGATGAAGTCT | 60 |
| R | CCAGTATTTGTTCCCCTTGTAGAAGTA | ||
| GAPDH | F | AGGCTGTTGTCATACTTCTCAT | 60 |
| R | GGAGTCCACTGGCGTCTT | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kremmydas, S.; Gourdoupi, C.; Piperigkou, Z.; Karamanos, N.K. Targeting EGFR/IGF-IR Functional Crosstalk in 2D and 3D Triple-Negative Breast Cancer Models to Evaluate Tumor Progression. Int. J. Mol. Sci. 2025, 26, 8665. https://doi.org/10.3390/ijms26178665
Kremmydas S, Gourdoupi C, Piperigkou Z, Karamanos NK. Targeting EGFR/IGF-IR Functional Crosstalk in 2D and 3D Triple-Negative Breast Cancer Models to Evaluate Tumor Progression. International Journal of Molecular Sciences. 2025; 26(17):8665. https://doi.org/10.3390/ijms26178665
Chicago/Turabian StyleKremmydas, Spyros, Chrisavgi Gourdoupi, Zoi Piperigkou, and Nikos K. Karamanos. 2025. "Targeting EGFR/IGF-IR Functional Crosstalk in 2D and 3D Triple-Negative Breast Cancer Models to Evaluate Tumor Progression" International Journal of Molecular Sciences 26, no. 17: 8665. https://doi.org/10.3390/ijms26178665
APA StyleKremmydas, S., Gourdoupi, C., Piperigkou, Z., & Karamanos, N. K. (2025). Targeting EGFR/IGF-IR Functional Crosstalk in 2D and 3D Triple-Negative Breast Cancer Models to Evaluate Tumor Progression. International Journal of Molecular Sciences, 26(17), 8665. https://doi.org/10.3390/ijms26178665









