Tenebrio molitor Meal-Induced Changes in Rat Gut Microbiota: Microbiological and Metagenomic Findings
Abstract
1. Introduction
2. Results
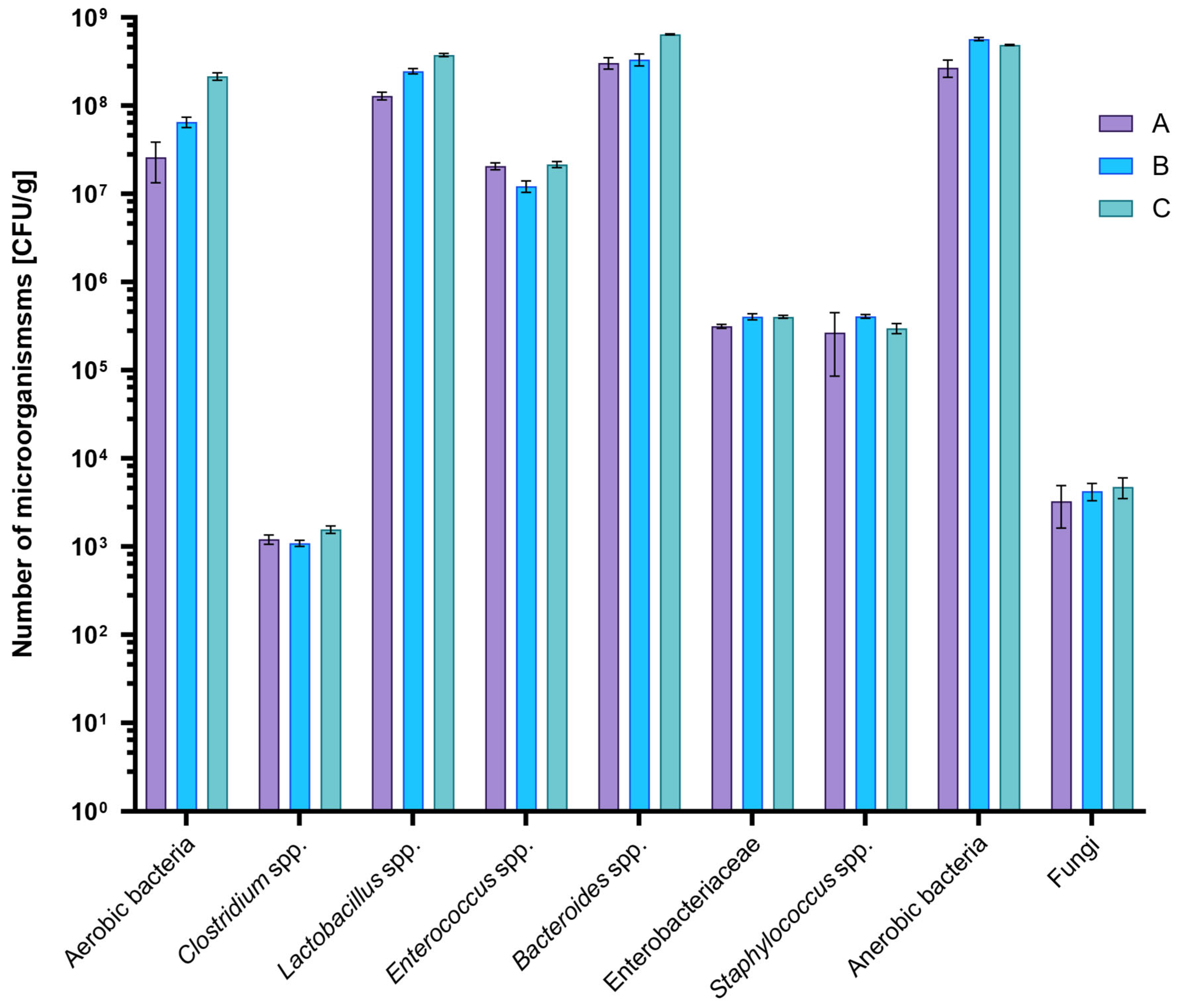
2.1. Microbiological Analysis of Rats Feces
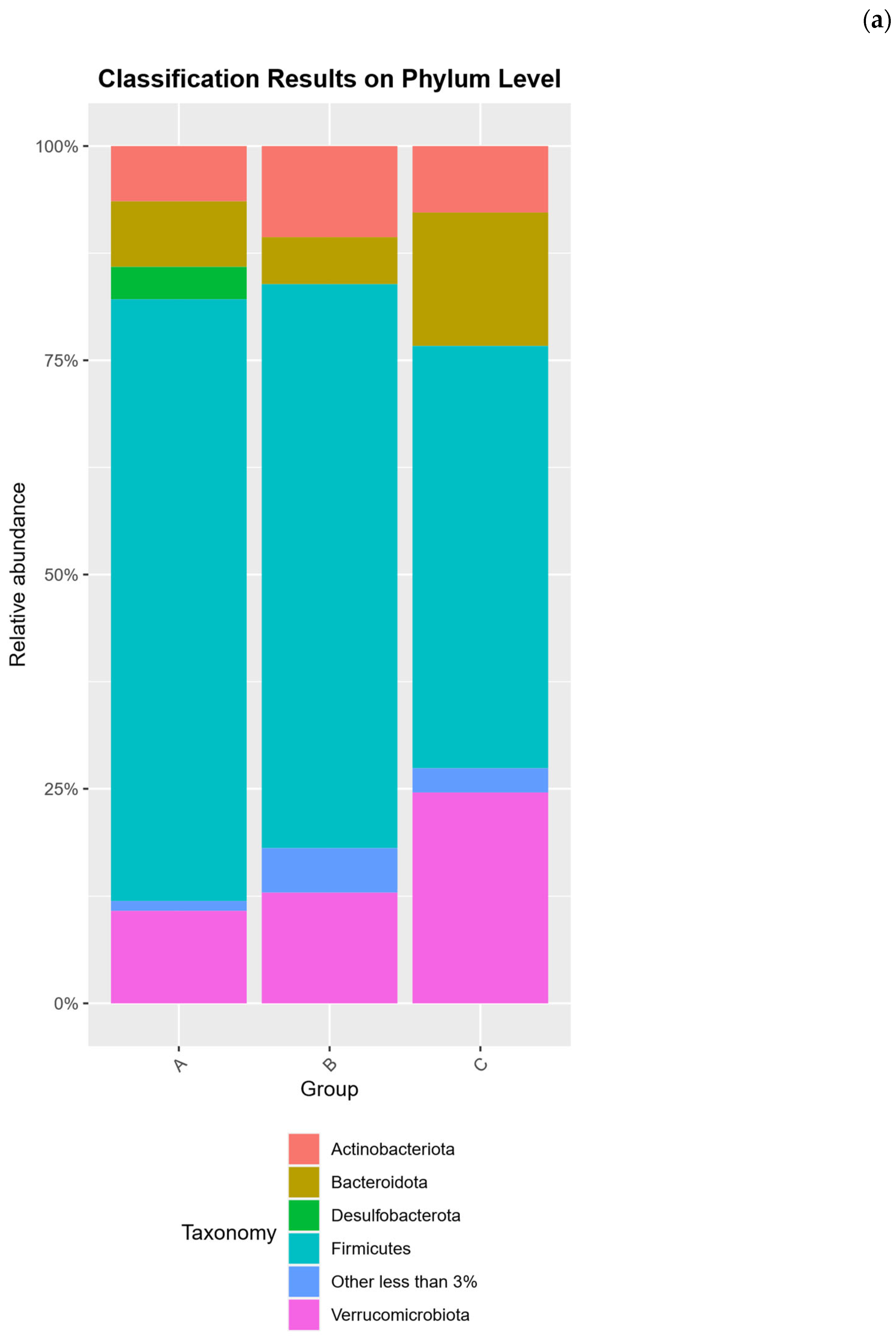
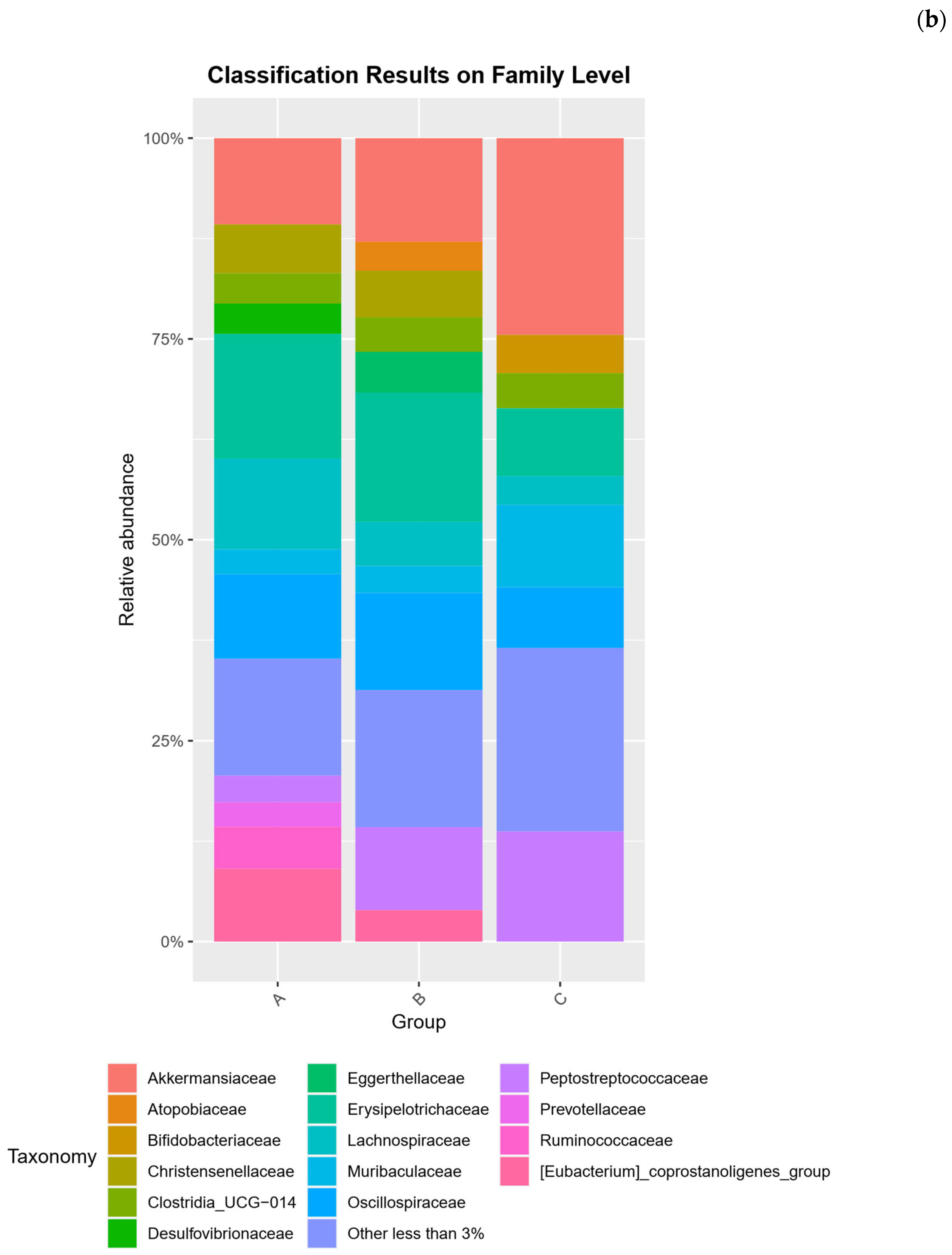
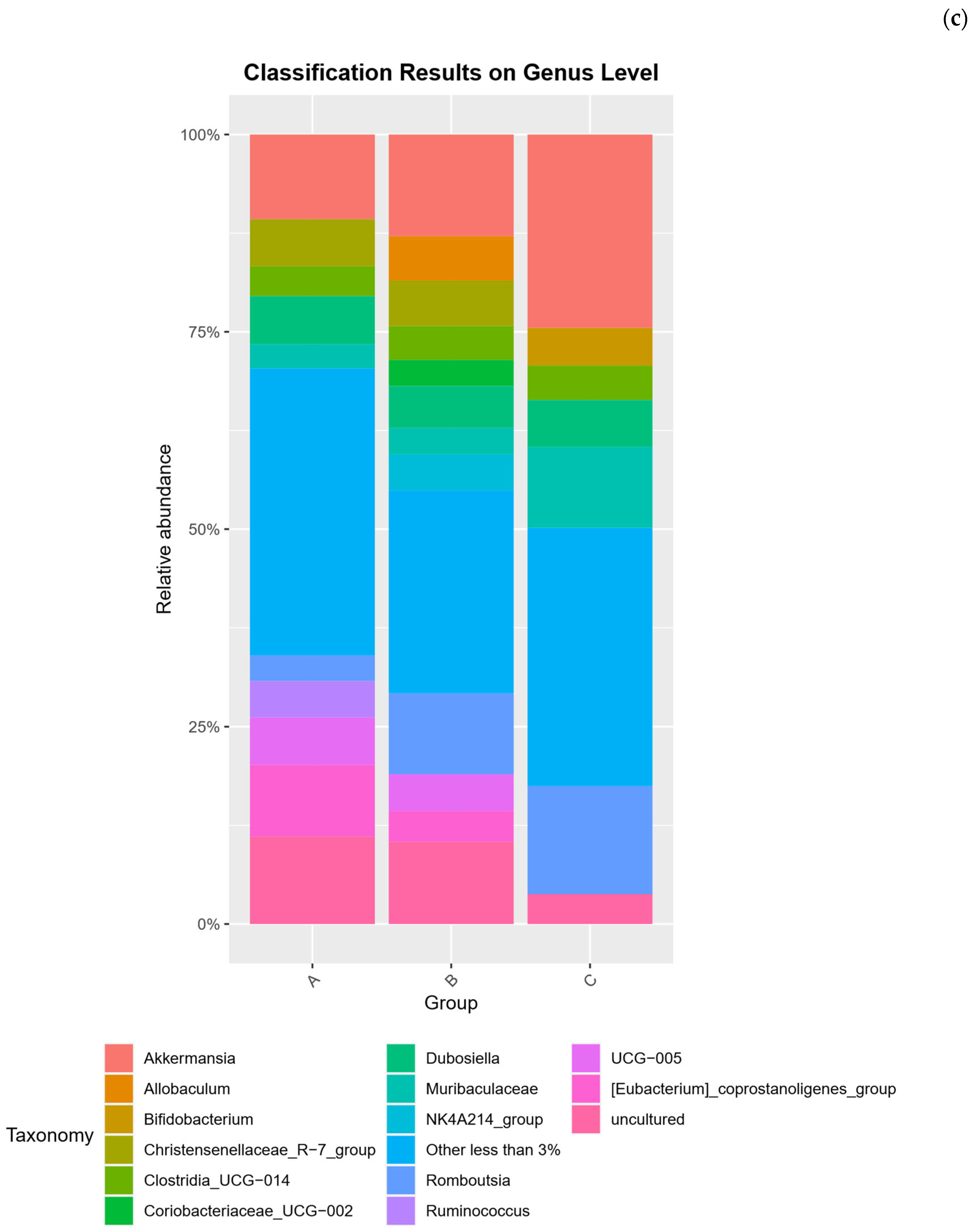
2.2. Microbial Community Compositional Analysis
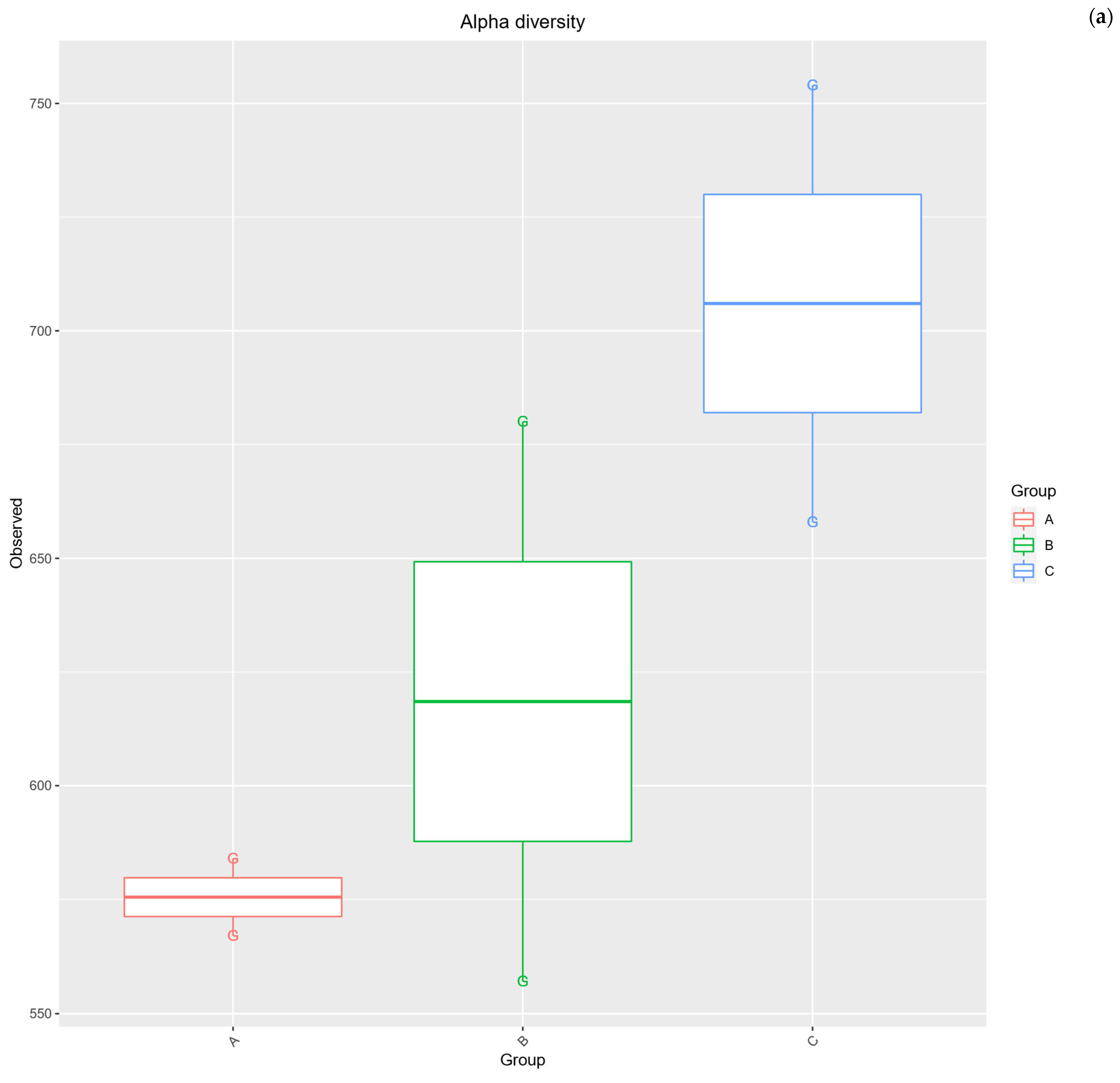
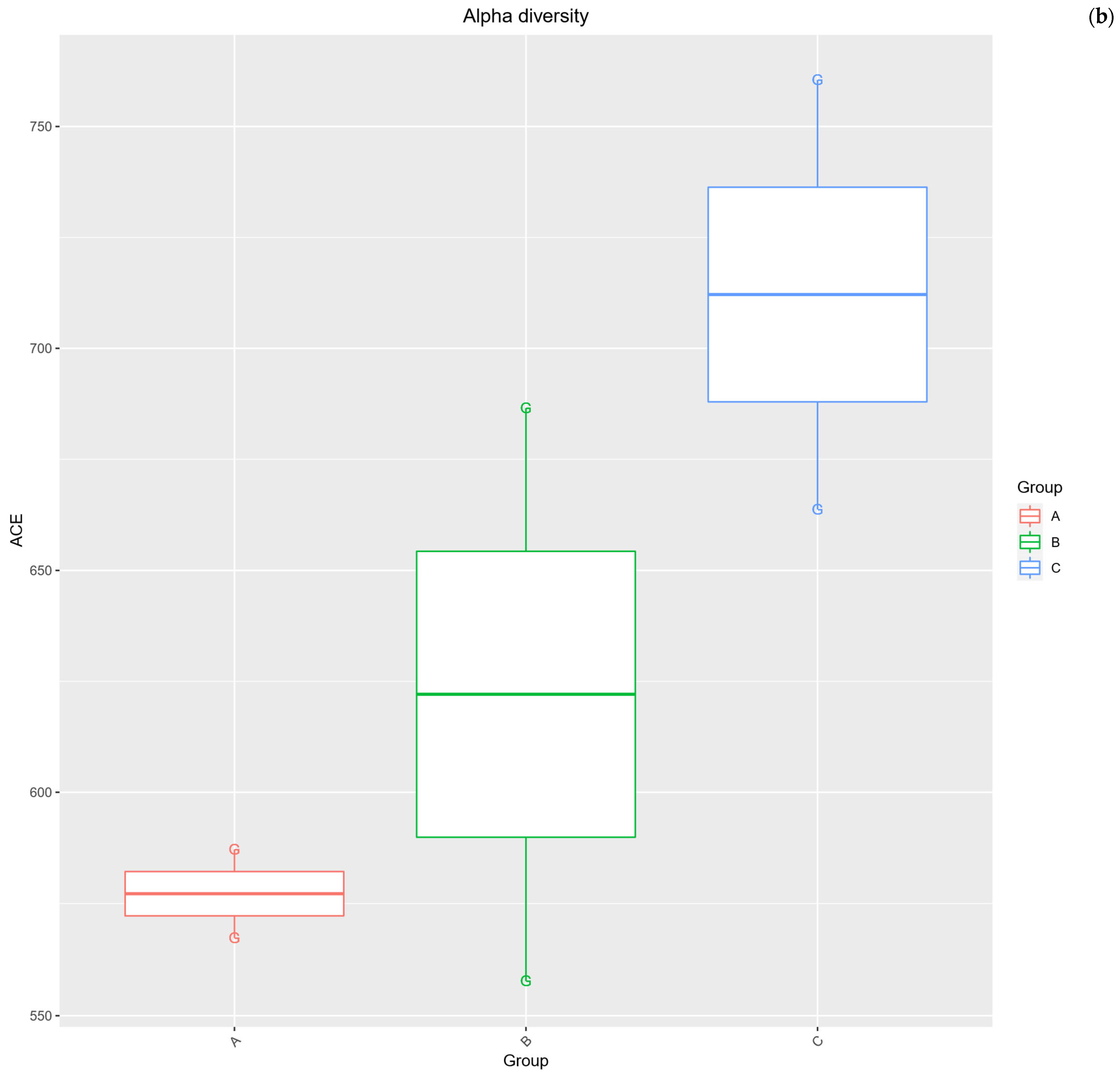
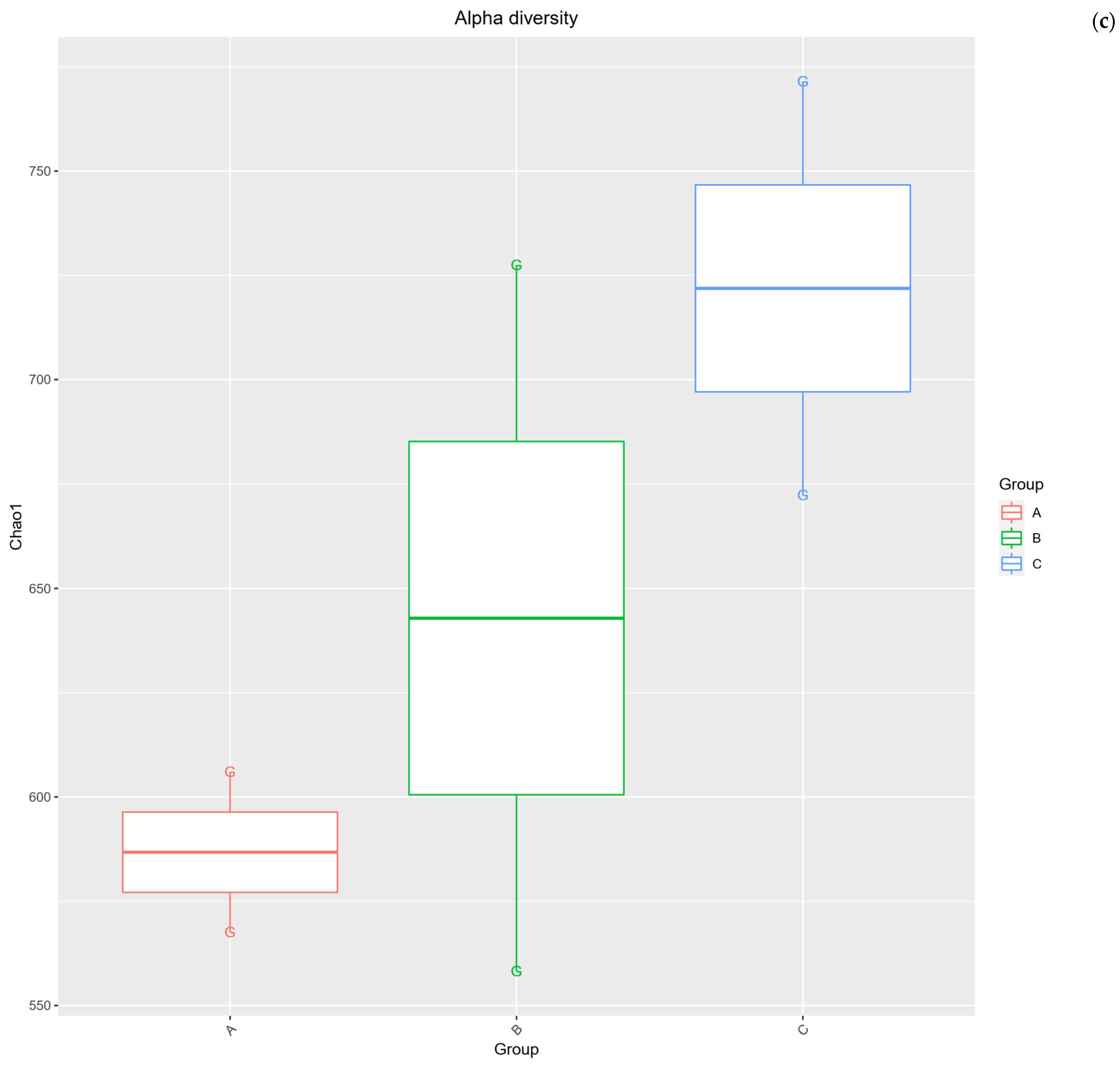
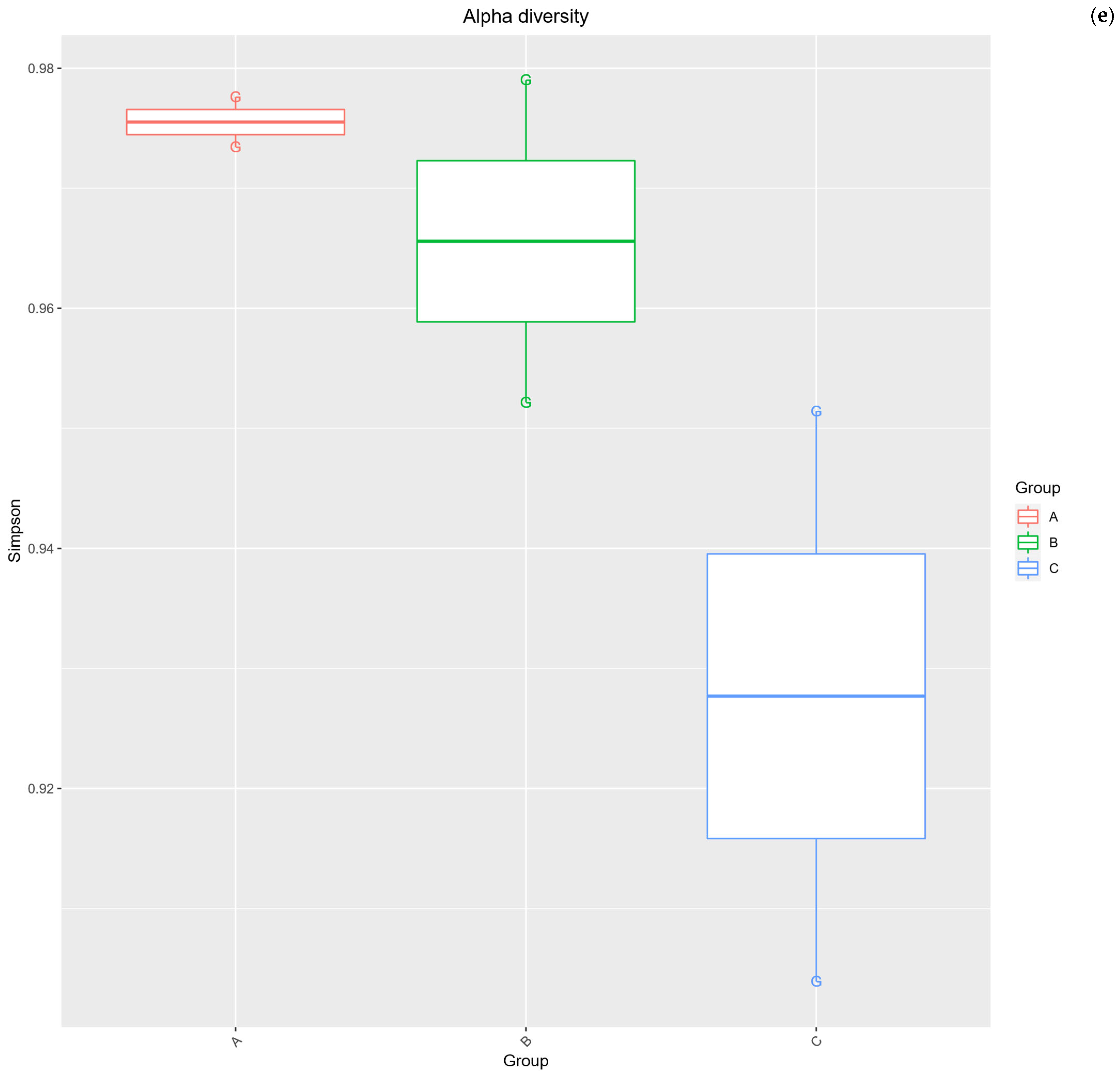
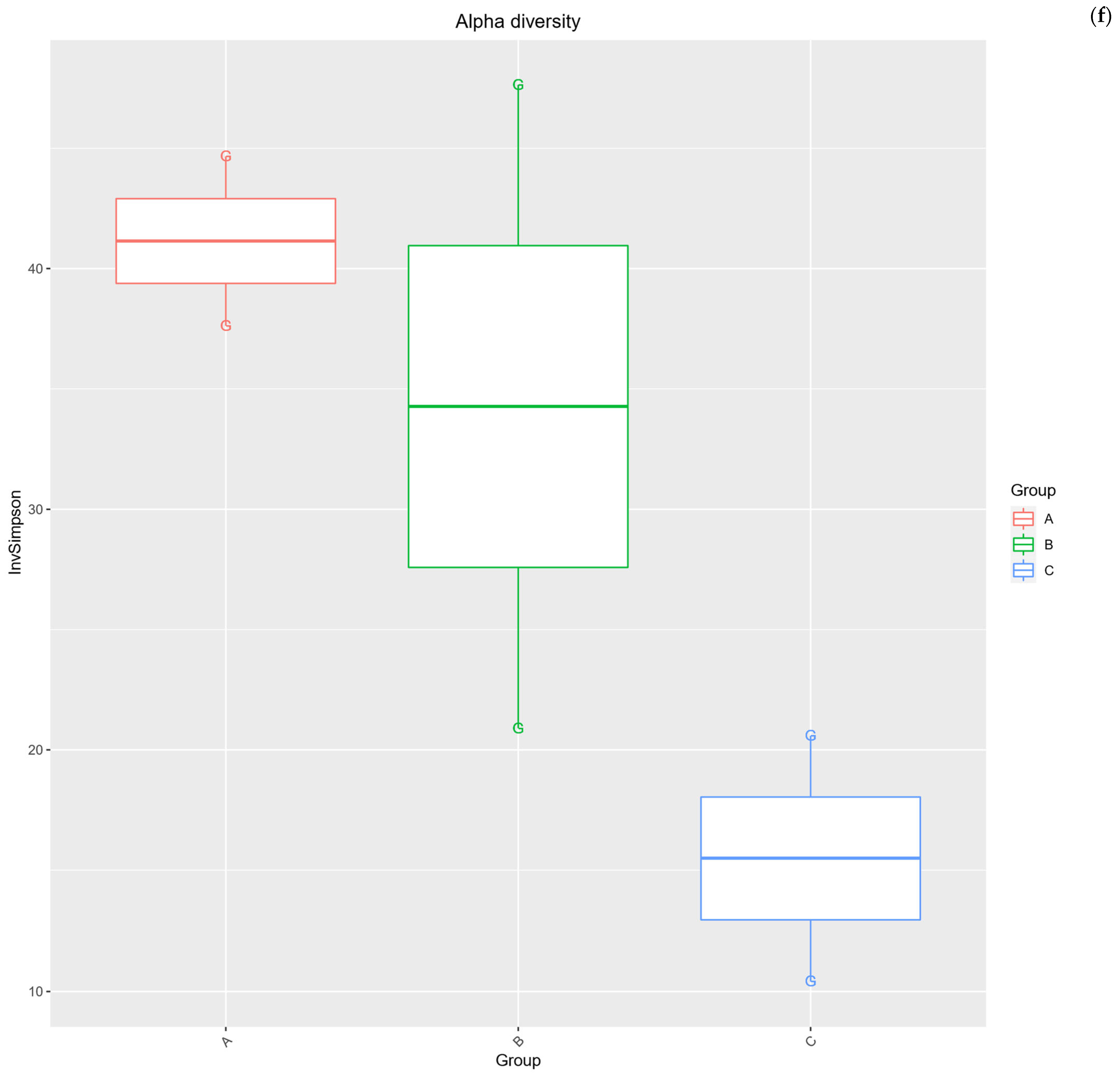
2.3. Alpha Diversity Analysis
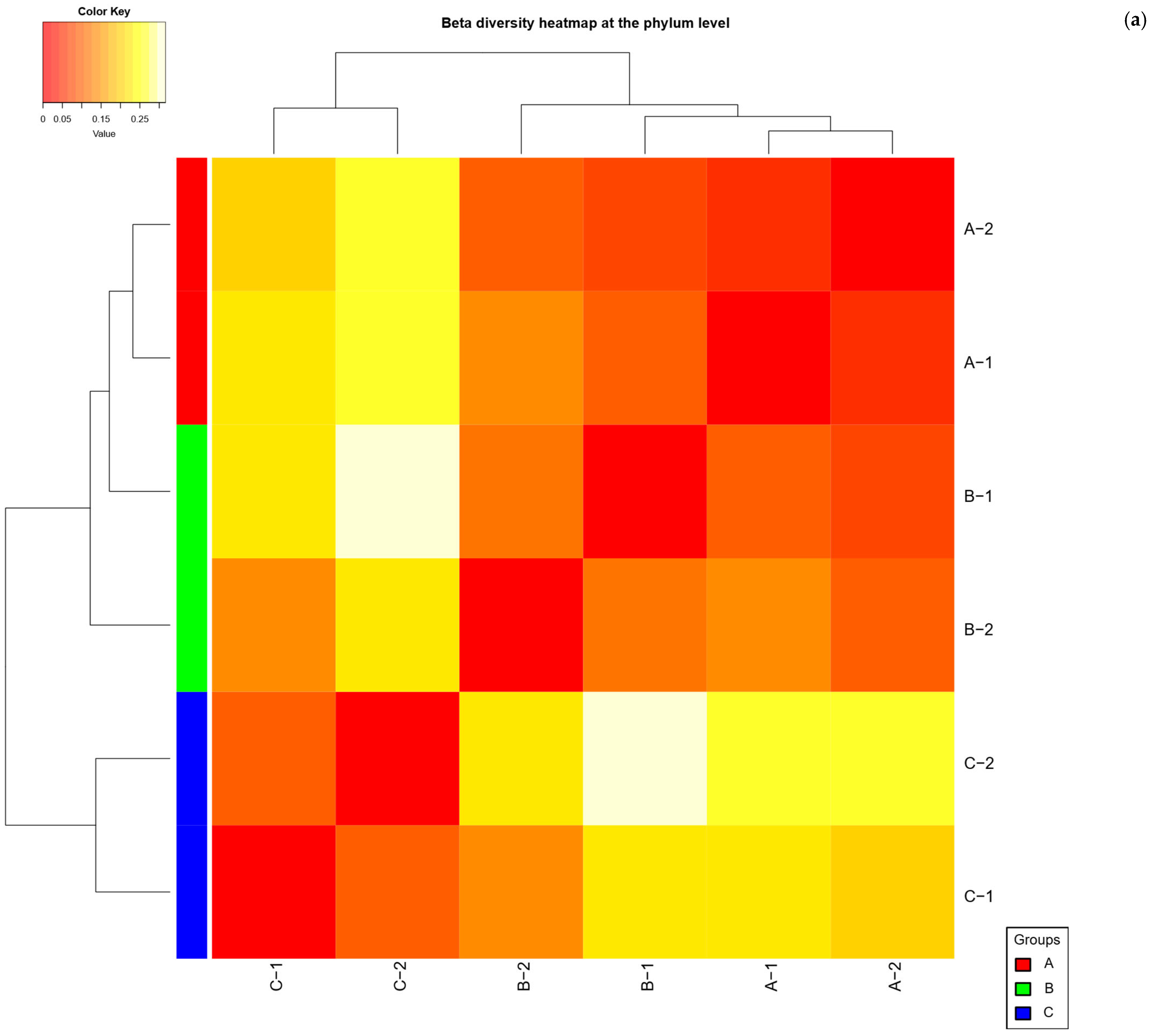
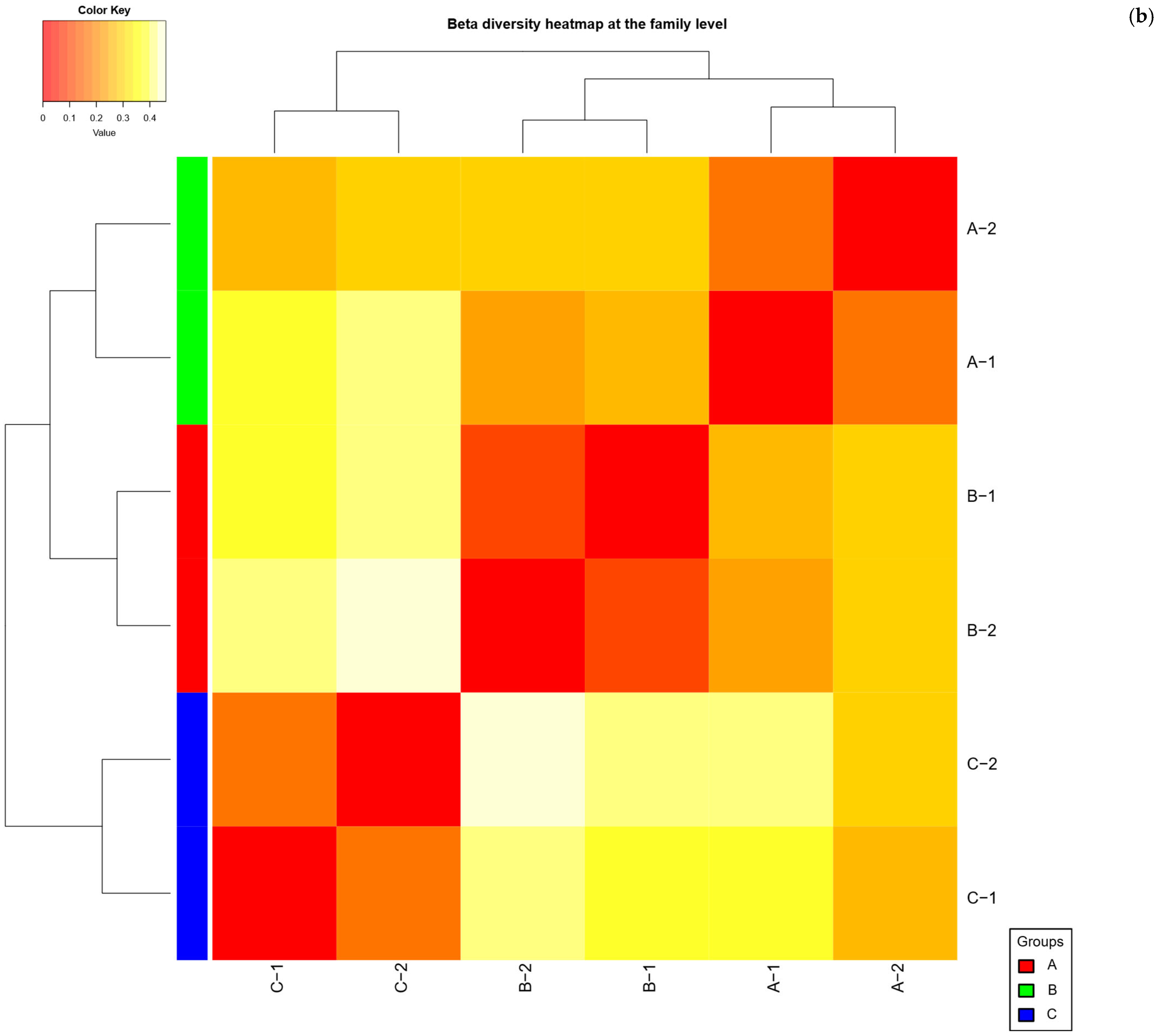
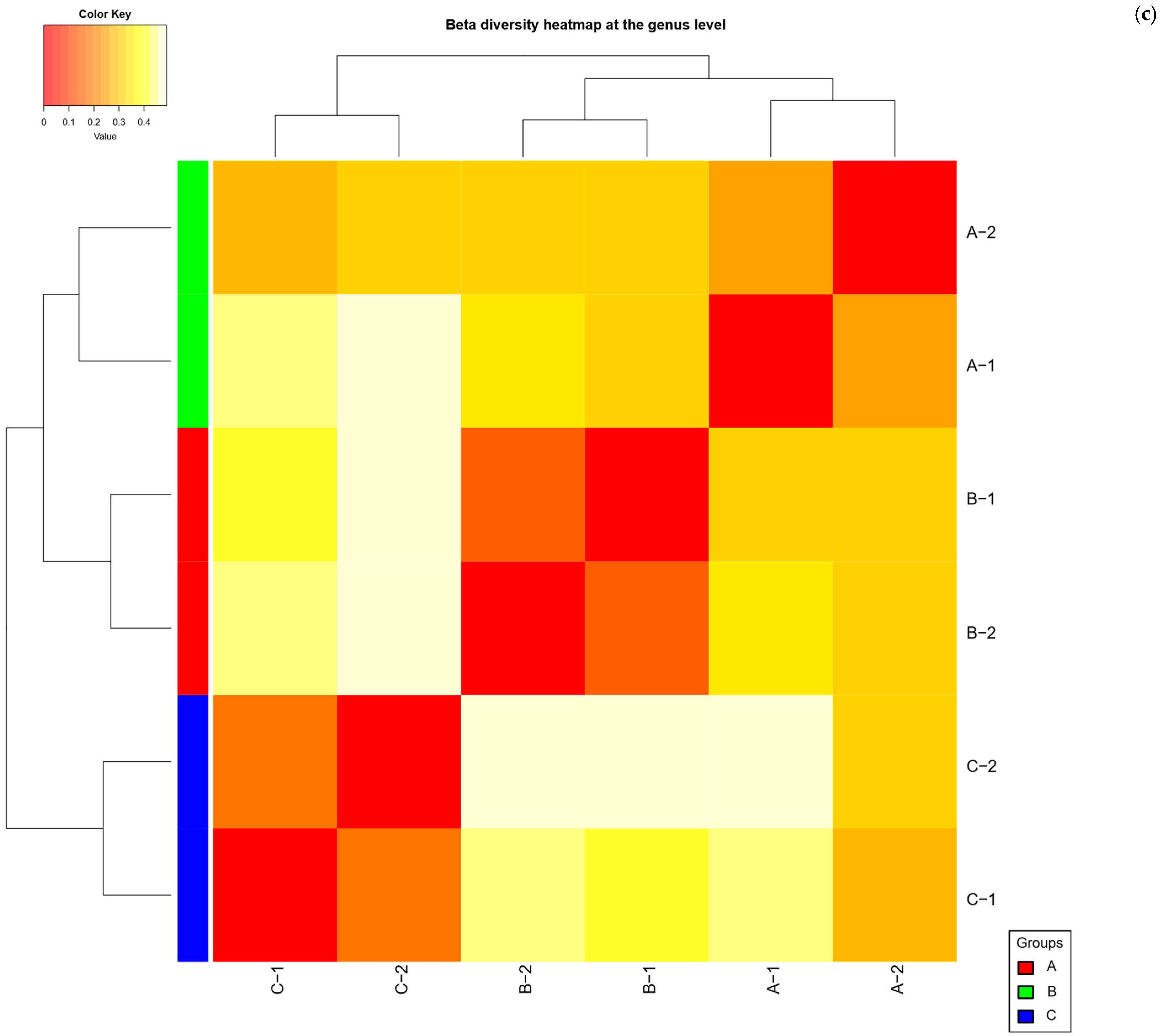
2.4. Beta Diversity Analysis
2.5. PCA and PCoA Ordination
2.6. Heat Tree Visualization
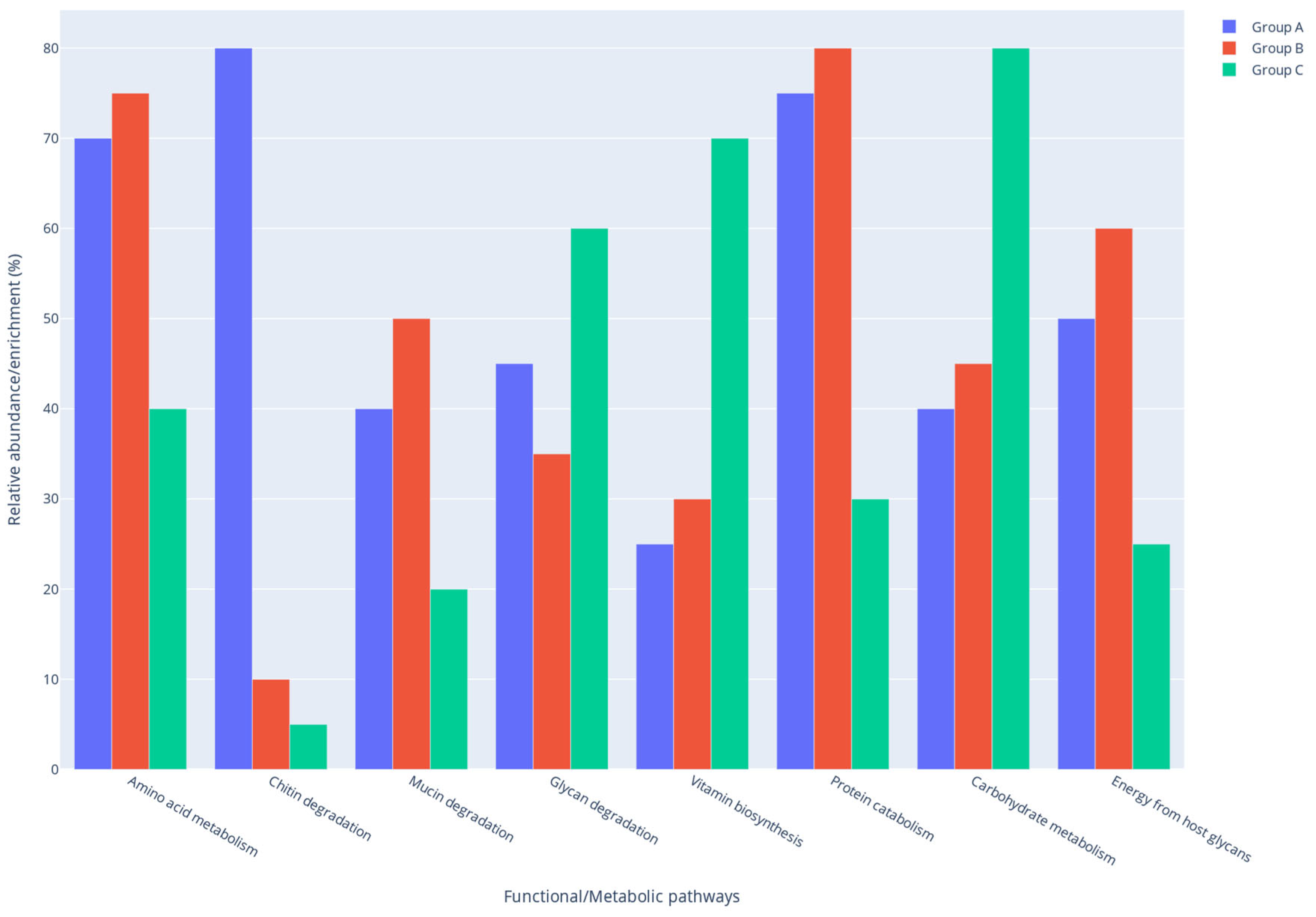
2.7. Predicted Functional Shifts in the Gut Microbiome of Rats
3. Discussion
4. Materials and Methods
4.1. Feed Preparation
4.2. Laboratory Animals and Experimental Procedure
4.3. Microbiological Analysis of Rats Feces
4.4. Statistical Analysis of Microbiological Counts
4.5. Microbiological Biodiversity of Rat Feces on the Basis of High-Throughput Sequencing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Feng, Y.; Chen, X.M.; Zhao, M.; He, Z.; Sun, L.; Wang, C.Y.; Ding, W.F. Edible insects in China: Utilization and prospects. Insect Sci. 2018, 25, 184–198. [Google Scholar] [CrossRef]
- Lanng, S.K.; Zhang, Y.; Christensen, K.R.; Hansen, A.K.; Nielsen, D.S.; Kot, W.; Bertram, H.C. Partial substitution of meat with insect (Alphitobius diaperinus) in a carnivore diet changes the gut microbiome and metabolome of healthy rats. Foods 2021, 10, 1814. [Google Scholar] [CrossRef]
- Lange, K.W.; Nakamura, Y. Edible insects as future food: Chances and challenges. J. Future Foods 2021, 1, 38–46. [Google Scholar] [CrossRef]
- Van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef]
- Oonincx, D.G.; Van Itterbeeck, J.; Heetkamp, M.J.; Van Den Brand, H.; Van Loon, J.J.; Van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed]
- Omuse, E.R.; Tonnang, H.E.; Yusuf, A.A.; Machekano, H.; Egonyu, J.P.; Kimathi, E.; Mohamed, S.F.; Kassie, M.; Subramanian, S.; Onditi, J. The global atlas of edible insects: Analysis of diversity and commonality contributing to food systems and sustainability. Sci. Rep. 2024, 14, 5045. [Google Scholar] [CrossRef]
- Elhassan, M.; Wendin, K.; Olsson, V.; Langton, M. Quality aspects of insects as food—Nutritional, sensory, and related concepts. Foods 2019, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A. Edible insects: Challenges and prospects. Entomol. Res. 2022, 52, 161–177. [Google Scholar] [CrossRef]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr. 2022, 62, 3499–3508. [Google Scholar] [CrossRef]
- Mancini, S.; Sogari, G.; Espinosa Diaz, S.; Menozzi, D.; Paci, G.; Moruzzo, R. Exploring the future of edible insects in Europe. Foods 2022, 11, 455. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Pinckaers, P.J.; van Loon, J.J.; van Loon, L.J. Consideration of insects as a source of dietary protein for human consumption. Nutr. Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Moruzzo, R.; Mancini, S.; Guidi, A. Edible insects and sustainable development goals. Insects 2021, 12, 557. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Rist, V.; Weiss, E.; Eklund, M.; Mosenthin, R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: A review. Animal 2013, 7, 1067–1078. [Google Scholar] [CrossRef]
- Illiano, P.; Brambilla, R.; Parolini, C. The mutual interplay of gut microbiota, diet and human disease. FEBS J. 2020, 287, 833–855. [Google Scholar] [CrossRef]
- Palomba, A.; Tanca, A.; Abbondio, M.; Sau, R.; Serra, M.; Marongiu, F.; Fraumene, C.; Pagnozzi, D.; Laconi, E.; Uzzau, S. Time-restricted feeding induces Lactobacillus-and Akkermansia-specific functional changes in the rat fecal microbiota. NPJ Biofilms Microbiomes 2021, 7, 85. [Google Scholar] [CrossRef]
- Klunder, H.; Wolkers-Rooijackers, J.; Korpela, J.M.; Nout, M.R. Microbiological aspects of processing and storage of edible insects. Food Control 2012, 26, 628–631. [Google Scholar] [CrossRef]
- Garofalo, C.; Osimani, A.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Clementi, F. The microbiota of marketed processed edible insects as revealed by high-throughput sequencing. Food Microbiol. 2017, 62, 15–22. [Google Scholar] [CrossRef]
- Garofalo, C.; Milanović, V.; Cardinali, F.; Aquilanti, L.; Clementi, F.; Osimani, A. Current knowledge on the microbiota of edible insects intended for human consumption: A state-of-the-art review. Food Res. Int. 2019, 125, 108527. [Google Scholar] [CrossRef]
- Meijer, N.; Safitri, R.; Tao, W.; Hoek-Van den Hil, E. European Union legislation and regulatory framework for edible insect production–Safety issues. Animal 2025, 101468. [Google Scholar] [CrossRef]
- Gałęcki, R.; Bakuła, T.; Gołaszewski, J. Foodborne diseases in the edible insect industry in Europe—New challenges and old problems. Foods 2023, 12, 770. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef]
- Pinckaers, P.J.; Weijzen, M.E.; Houben, L.H.; Zorenc, A.H.; Kouw, I.W.; de Groot, L.C.; Verdijk, L.B.; Snijders, T.; van Loon, L.J. The muscle protein synthetic response following ingestion of corn protein, milk protein and their protein blend in young males. Curr. Dev. Nutr. 2020, 4, nzaa049_044. [Google Scholar] [CrossRef]
- Hermans, W.J.; Senden, J.M.; Churchward-Venne, T.A.; Paulussen, K.J.; Fuchs, C.J.; Smeets, J.S.; van Loon, J.J.; Verdijk, L.B.; van Loon, L.J. Insects are a viable protein source for human consumption: From insect protein digestion to postprandial muscle protein synthesis in vivo in humans: A double-blind randomized trial. Am. J. Clin. Nutr. 2021, 114, 934–944. [Google Scholar] [CrossRef]
- Meyer, S.; Gessner, D.K.; Maheshwari, G.; Röhrig, J.; Friedhoff, T.; Most, E.; Zorn, H.; Ringseis, R.; Eder, K. Tenebrio molitor larvae meal affects the cecal microbiota of growing pigs. Animals 2020, 10, 1151. [Google Scholar] [CrossRef]
- Gałęcki, R.; Hanuszewska-Dominiak, M.; Kaczmar, E. Edible insects as a source of dietary protein for companion animals with food responsive enteropathies–perspectives and possibilities. Pol. J. Vet. Sci. 2024, 27, 309–318. [Google Scholar] [CrossRef]
- Kar, S.K.; Schokker, D.; Harms, A.C.; Kruijt, L.; Smits, M.A.; Jansman, A.J. Local intestinal microbiota response and systemic effects of feeding black soldier fly larvae to replace soybean meal in growing pigs. Sci. Rep. 2021, 11, 15088. [Google Scholar] [CrossRef]
- Glowacki, R.W.; Martens, E.C. If you eat it or secrete it, they will grow: The expanding list of nutrients utilized by human gut bacteria. J. Bacteriol. 2021, 203, e00481-20. [Google Scholar] [CrossRef]
- Koboziev, I.; Webb, C.R.; Furr, K.L.; Grisham, M.B. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic. Biol. Med. 2014, 68, 122–133. [Google Scholar] [CrossRef]
- Blaut, M.; Clavel, T. Metabolic diversity of the intestinal microbiota: Implications for health and disease. J. Nutr. 2007, 137, 751S–755S. [Google Scholar] [CrossRef]
- Śliżewska, K.; Libudzisz, Z.; Barczyńska, R.; Kapuśniak, J.; Zduńczyk, Z.; Juśkiewicz, J. Dietary resistant dextrins positively modulate fecal and cecal microbiota composition in young rats. Acta Biochim. Pol. 2015, 62, 677–681. [Google Scholar] [CrossRef]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.; Sears, M.; HayGlass, K.; Mandhane, P.; Turvey, S.; Subbarao, P.; Becker, A. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef]
- Brooks, S.P.; McAllister, M.; Sandoz, M.; Kalmokoff, M. Culture-independent phylogenetic analysis of the faecal flora of the rat. Can. J. Microbiol. 2003, 49, 589–601. [Google Scholar] [CrossRef]
- Chettaoui, R.; Mayot, G.; De Almeida, L.; Di Martino, P. Cranberry (Vaccinium macrocarpon) dietary supplementation and fecal microbiota of Wistar rats. AIMS Microbiol. 2021, 7, 257. [Google Scholar] [CrossRef]
- Layton, B.A.; Walters, S.; Boehm, A. Distribution and diversity of the enterococcal surface protein (esp) gene in animal hosts and the Pacific coast environment. J. Appl. Microbiol. 2009, 106, 1521–1531. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Haberer, P.; Snel, J.; Schillinger, U.; Huis in’t Veld, J.H. Overview of gut flora and probiotics. Int. J. Food Microbiol. 1998, 41, 85–101. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180, Erratum in Nature 2014, 27, 506–516. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; Tian, G.; Huang, Z. Fungi in gastrointestinal tracts of human and mice: From community to functions. Microb. Ecol. 2018, 75, 821–829. [Google Scholar] [CrossRef]
- Scupham, A.J.; Presley, L.L.; Wei, B.; Bent, E.; Griffith, N.; McPherson, M.; Zhu, F.; Oluwadara, O.; Rao, N.; Braun, J. Abundant and diverse fungal microbiota in the murine intestine. Appl. Environ. Microbiol. 2006, 72, 793–801. [Google Scholar] [CrossRef]
- Karcher, N.; Nigro, E.; Punčochář, M.; Blanco-Míguez, A.; Ciciani, M.; Manghi, P.; Zolfo, M.; Cumbo, F.; Manara, S.; Golzato, D. Genomic diversity and ecology of human-associated Akkermansia species in the gut microbiome revealed by extensive metagenomic assembly. Genome Biol. 2021, 22, 209. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, M.; Everard, A.; Gómez-Valadés, A.; Matamoros, S.; Ramírez, S.; Delzenne, N.; Gomis, R.; Claret, M.; Cani, P. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef]
- Gerritsen, J.; Fuentes, S.; Grievink, W.; van Niftrik, L.; Tindall, B.J.; Timmerman, H.M.; Rijkers, G.T.; Smidt, H. Characterization of Romboutsia ilealis gen. nov., sp. nov., isolated from the gastro-intestinal tract of a rat, and proposal for the reclassification of five closely related members of the genus Clostridium into the genera Romboutsia gen. nov., Intestinibacter gen. nov., Terrisporobacter gen. nov. and Asaccharospora gen. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 1600–1616. [Google Scholar] [CrossRef]
- Gerritsen, J.; Hornung, B.; Ritari, J.; Paulin, L.; Rijkers, G.T.; Schaap, P.J.; de Vos, W.M.; Smidt, H. A comparative and functional genomics analysis of the genus Romboutsia provides insight into adaptation to an intestinal lifestyle. bioRxiv 2019, 845511. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, X.; Li, H.; Li, Y.; Shi, X.; Zhao, F.; Xu, X.; Li, C.; Zhou, G. Intake of meat proteins substantially increased the relative abundance of genus Lactobacillus in rat feces. PLoS ONE 2016, 11, e0152678. [Google Scholar] [CrossRef]
- Arora, T.; Anastasovska, J.; Gibson, G.; Tuohy, K.; Sharma, R.K.; Bell, J.; Frost, G. Effect of Lactobacillus acidophilus NCDC 13 supplementation on the progression of obesity in diet-induced obese mice. Br. J. Nutr. 2012, 108, 1382–1389. [Google Scholar] [CrossRef]
- Li, L.; Buhman, K.K.; Hartman, P.A.; Beitz, D.C. Hypocholesterolemic effect of Eubacterium coprostanoligenes ATCC 51222 in rabbits. Lett. Appl. Microbiol. 1995, 20, 137–140. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-H.; Zhao, L.; Zhang, C.-Y.; Li, X.-Y.; Wu, T.-L.; Dai, Y.-Y.; Sheng, Y.-Y.; Ren, Y.-L.; Xue, Y.-Z. Gut microbial evidence chain in high-salt diet exacerbates intestinal aging process. Front. Nutr. 2022, 9, 1046833. [Google Scholar] [CrossRef] [PubMed]
- Vojinovic, D.; Radjabzadeh, D.; Kurilshikov, A.; Amin, N.; Wijmenga, C.; Franke, L.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J. Relationship between gut microbiota and circulating metabolites in population-based cohorts. Nat. Commun. 2019, 10, 5813. [Google Scholar] [CrossRef]
- Terova, G.; Gini, E.; Gasco, L.; Moroni, F.; Antonini, M.; Rimoldi, S. Effects of full replacement of dietary fishmeal with insect meal from Tenebrio molitor on rainbow trout gut and skin microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Habte-Tsion, H.-M.; Hawkyard, M.; Sealey, W.M.; Bradshaw, D.; Meesala, K.-M.; Bouchard, D.A. Effects of fishmeal substitution with mealworm meals (Tenebrio molitor and Alphitobius diaperinus) on the growth, physiobiochemical response, digesta microbiome, and immune genes expression of Atlantic salmon (Salmo salar). Aquac. Nutr. 2024, 6618117. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Grego, E.; Dabbou, S.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Gut microbiota and mucin composition in female broiler chickens fed diets including yellow mealworm (Tenebrio molitor, L.). Animals 2019, 9, 213. [Google Scholar] [CrossRef]
- Józefiak, A.; Benzertiha, A.; Kierończyk, B.; Łukomska, A.; Wesołowska, I.; Rawski, M. Improvement of cecal commensal microbiome following the insect additive into chicken diet. Animals 2020, 10, 577. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Grego, E.; Dabbou, S.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Yellow mealworm inclusion in diets for heavy-size broiler chickens: Implications for intestinal microbiota and mucin dynamics. Animals 2020, 10, 1909. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Hermetia illucens larvae as a potential dietary protein source altered the microbiota and modulated mucosal immune status in the colon of finishing pigs. J. Anim. Sci. Biotechnol. 2019, 10, 50. [Google Scholar] [CrossRef]
- Meyer, S.; Gessner, D.K.; Braune, M.S.; Friedhoff, T.; Most, E.; Höring, M.; Liebisch, G.; Zorn, H.; Eder, K.; Ringseis, R. Comprehensive evaluation of the metabolic effects of insect meal from Tenebrio molitor L. in growing pigs by transcriptomics, metabolomics and lipidomics. J. Anim. Sci. Biotechnol. 2020, 11, 20. [Google Scholar] [CrossRef]
- Saeb, A.; Grundmann, S.M.; Gessner, D.K.; Schuchardt, S.; Most, E.; Wen, G.; Eder, K.; Ringseis, R. Feeding of cuticles from Tenebrio molitor larvae modulates the gut microbiota and attenuates hepatic steatosis in obese Zucker rats. Food Funct. 2022, 13, 1421–1436. [Google Scholar] [CrossRef]
- Kwon, G.T.; Yuk, H.-G.; Lee, S.J.; Chung, Y.H.; Jang, H.S.; Yoo, J.-S.; Cho, K.-H.; Kong, H.; Shin, D. Mealworm larvae (Tenebrio molitor L.) exuviae as a novel prebiotic material for BALB/c mouse gut microbiota. Food Sci. Biotechnol. 2020, 29, 531–537. [Google Scholar] [CrossRef]
- Kang, Y.; Oba, P.M.; Gaulke, C.A.; Sánchez-Sánchez, L.; Swanson, K.S. Dietary inclusion of Yellow mealworms (T. molitor) and lesser mealworms (A. diaperinus) modifies intestinal microbiota populations of Diet-Induced obesity mice. J. Nutr. 2023, 153, 3220–3236. [Google Scholar] [CrossRef] [PubMed]
- Soglia, D.; Viola, I.; Nery, J.; Maione, S.; Sartore, S.; Lasagna, E.; Perini, F.; Gariglio, M.; Bongiorno, V.; Moretti, R.; et al. Nutrigenomics in animal feeding: Digital gene expression analysis in poultry fed Tenebrio molitor larvae meal. Poultry 2022, 1, 14–29. [Google Scholar] [CrossRef]
- Malematja, E.; Manyelo, T.; Sebola, N.; Mabelebele, M. The role of insects in promoting the health and gut status of poultry. Comp. Clin. Pathol. 2023, 32, 501–513. [Google Scholar] [CrossRef]
- Stull, V.J.; Weir, T.L. Chitin and omega-3 fatty acids in edible insects have underexplored benefits for the gut microbiome and human health. Nat. Food 2023, 4, 283–287. [Google Scholar] [CrossRef]
- Biasato, I.; Gasco, L.; Schiavone, A.; Capucchio, M.T.; Ferrocino, I. Gut microbiota changes in insect-fed monogastric species: State-of-the-art and future perspectives. Anim. Front. 2023, 13, 72–80. [Google Scholar] [CrossRef]
- Butowski, C.F.; Dixit, Y.; Reis, M.M.; Mu, C. Metatranscriptomics for understanding the microbiome in food and nutrition science. Metabolites 2025, 15, 185. [Google Scholar] [CrossRef]
- Teullet, S.; Tilak, M.-K.; Magdeleine, A.; Schaub, R.; Weyer, N.M.; Panaino, W.; Fuller, A.; Loughry, W.; Avenant, N.L.; de Thoisy, B. Metagenomics uncovers dietary adaptations for chitin digestion in the gut microbiota of convergent myrmecophagous mammals. mSystems 2023, 8, e00388-23. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, J.; Agostinetto, G.; Galimberti, A.; De Mattia, F.; Labra, M.; Bruno, A. Tasting the differences: Microbiota analysis of different insect-based novel food. Food Res. Int. 2020, 137, 109426. [Google Scholar] [CrossRef]
- Pöllinger-Zierler, B.; Lienhard, A.; Mayer, C.; Berner, S.; Rehorska, R.; Schöpfer, A.; Grasser, M. Tenebrio molitor (Linnaeus, 1758): Microbiological screening of feed for a safe food choice. Foods 2023, 12, 2139. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Pandey, D.; Næss, G.; Cabrita, A.R.; Fonseca, A.J.; Maia, M.R.; Timilsina, B.; Veldkamp, T.; Sapkota, R.; Overrein, H. Yellow mealworms (Tenebrio molitor) as an alternative animal feed source: A comprehensive characterization of nutritional values and the larval gut microbiome. J. Clean. Prod. 2023, 389, 136104. [Google Scholar] [CrossRef]
- EN ISO 9001:2015; Quality Management Systems—Requirements. European Committee for Standardization: Brussels, Belgium, 2015.
- Gałęcki, R.; Pszczółkowski, B.; Zielonka, Ł. Experiences in formulating insect-based feeds: Selected physicochemical properties of dog food containing yellow mealworm meal. Animals 2025, 15, 2087. [Google Scholar] [CrossRef] [PubMed]
- Flecknell, P. Replacement, reduction, refinement. ALTEX 2002, 19, 73–78. [Google Scholar]
- National Research Council (NRC). Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gałęcki, R.; Nowak, A.; Szulc, J. Tenebrio molitor Meal-Induced Changes in Rat Gut Microbiota: Microbiological and Metagenomic Findings. Int. J. Mol. Sci. 2025, 26, 8663. https://doi.org/10.3390/ijms26178663
Gałęcki R, Nowak A, Szulc J. Tenebrio molitor Meal-Induced Changes in Rat Gut Microbiota: Microbiological and Metagenomic Findings. International Journal of Molecular Sciences. 2025; 26(17):8663. https://doi.org/10.3390/ijms26178663
Chicago/Turabian StyleGałęcki, Remigiusz, Adriana Nowak, and Justyna Szulc. 2025. "Tenebrio molitor Meal-Induced Changes in Rat Gut Microbiota: Microbiological and Metagenomic Findings" International Journal of Molecular Sciences 26, no. 17: 8663. https://doi.org/10.3390/ijms26178663
APA StyleGałęcki, R., Nowak, A., & Szulc, J. (2025). Tenebrio molitor Meal-Induced Changes in Rat Gut Microbiota: Microbiological and Metagenomic Findings. International Journal of Molecular Sciences, 26(17), 8663. https://doi.org/10.3390/ijms26178663






