1. Epigenetics at the Host–Microbiota Interface
All multicellular organisms have co-evolved with diverse communities of microorganisms that are vital for host physiology. Host-microbiome interactions have shaped animal evolution and diversification, particularly by enabling nutrient breakdown and absorption [
1,
2]. To coordinate these interactions, most organisms have developed sophisticated epigenetic mechanisms that reversibly regulate gene expression in response to environmental cues, thereby supporting phenotypic plasticity and adaptation. Epigenetics broadly refers to heritable phenotypic changes that occur without alterations to the DNA sequence. Major classes of epigenetic mechanisms include DNA and RNA methylation [
3], histone modifications driving chromatin remodeling [
4], and the actions of non-coding RNAs [
5]. Although distinct, these processes often function together, adding layers of regulatory complexity that influence gene expression, genome stability, development, and cellular differentiation, function, and identity.
The gut microbiome—comprising bacteria, viruses, fungi, and other microorganisms inhabiting the human gastrointestinal tract (GI)—is most densely concentrated in the colon, particularly the cecum, where microbial communities replicate and interact with the host [
6,
7]. This ecosystem contributes to numerous physiological functions, including nutrient release, vitamin production, digestion, energy balance, and regulation of carbohydrate and lipid metabolism. Anaerobic fermentation produces short-chain fatty acids (SCFAs) and other metabolites that sustain intestinal and systemic health. In addition, the gut microbiome primes and modulates host immune and neuroimmune responses [
8].
In return, the host provides a stable environment and dietary substrates that sustain microbial metabolism, fostering a dynamic and mutually beneficial relationship. Quorum-sensing molecules and other bioactive compounds mediate this bidirectional crosstalk, shaping host defense and preventing pathogen overgrowth [
9,
10]. Despite extensive research underscoring the microbiome’s role in health, the precise mechanisms by which microorganisms modulate host epigenetic landscapes remain incompletely defined and require further study.
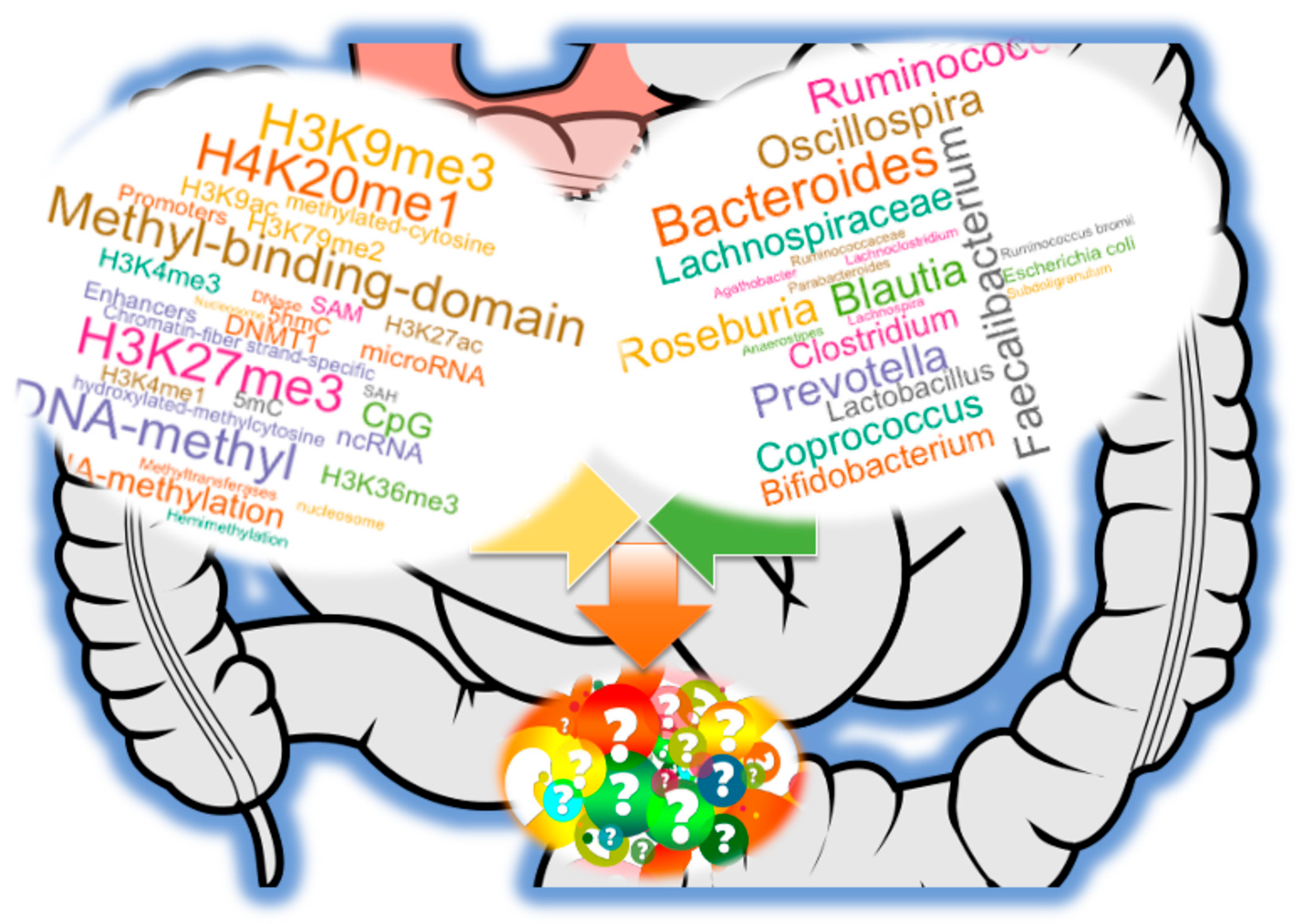
To date, accumulating evidence supports a bidirectional relationship: host genetics and epigenetic states can shape the composition and function of the microbiota, while microbial communities, in turn, influence host epigenetic regulation through diet- and environment-dependent mechanisms (
Figure 1). This reciprocity highlights the gut microbiome as both a target and driver of molecular adaptation. By modulating chromatin accessibility and transcriptional programs, microbial metabolites and signaling molecules may reprogram host cell states in ways that affect immunity, metabolism, neurobiology, and disease susceptibility. Conversely, epigenetic regulation in host tissues can impose selective pressures that favor particular microbial taxa, suggesting that the microbiome is an active participant in evolutionary and developmental trajectories rather than a passive bystander.
Clarifying this interplay carries profound implications. Understanding how microbial factors reshape host epigenetic landscapes could reveal new therapeutic avenues for alleviating genetic and epigenetic constraints on health, while also informing microbiome-targeted strategies such as dietary interventions, probiotics, and metabolite-based therapeutics. At the same time, dissecting how host epigenetic states influence microbial ecology may uncover principles for designing precision approaches to maintain or restore microbial balance in chronic disease.
Herein, we review emerging evidence of how the gut microbiome modulates host epigenetic processes, with particular emphasis on DNA and RNA methylation. We highlight key cellular pathways and mediator molecules through which this regulation occurs, and we discuss future strategies to disentangle these complex, reciprocal interactions in health and disease. Framed within the era of precision medicine, these insights underscore the potential of microbiome–epigenome crosstalk as a foundation for next-generation diagnostics and therapeutics.
2. Evolutionary Origins and Comparative Mechanisms of Methylation
Understanding the evolution of epigenetic regulation is key to contextualizing how humans respond to microbial signals at the molecular level. While this review centers on the human gut microbiome and its impact on host methylation, insights from yeast, plants, and model animals reveal conserved enzymatic pathways, distinct DNA and RNA methylation patterns, and adaptive strategies that underlie epigenomic plasticity. Such comparative perspectives enhance our interpretation of microbiome–epigenome interactions and guide the development of cross-kingdom models and bioengineered systems.
2.1. Overview of DNA and RNA Methylation Across Domains
Epigenetic regulation is conserved across most domains of life, with DNA and RNA methylation systems essential for genome defense, transcriptional control, and developmental plasticity. DNA methylation involves the covalent addition of a methyl group to the fifth carbon of the cytosine ring, generating 5-methylcytosine (5mC). In vertebrates, this modification occurs predominantly at 5′-CG-3′ (CpG) dinucleotides, while in other taxa it can also appear in non-CpG contexts, though to varying degrees. RNA modifications such as N6-methyladenosine (m6A) have likewise emerged as key regulators of gene expression, influencing RNA stability, splicing, and translation. These marks are dynamically controlled by writer, reader, and eraser proteins, many of which are evolutionarily conserved and act concertedly in a context-dependent manner [
11,
12,
13,
14,
15,
16,
17,
18,
19] (
Table 1).
2.2. DNA Methylation and Small RNA Regulatory Functions in Microbiota
In bacteria, post-replicative DNA methylation commonly involves three modifications: 5-methylcytosine (5mC), N6-adenine methylation (6mA), and N4-methylcytosine (4mC) [
20,
21]. Most research has focused on 5mC, which functions within restriction-modification (RM) systems that protect bacterial genomes from foreign DNA. These RM systems, comprising endonucleases and methyltransferases, are classified into four main types (I–IV) based on their genetic organization, biochemical properties, and recognition mechanisms.
Bacteria have become valuable models for studying epigenetics, providing tools such as isoschizomer restriction enzymes (e.g.,
MspI and
HpaII) for surveying genome-wide DNA methylation states [
38,
39]. Bacterial DNA methylation influences clinically relevant traits, including host colonization, biofilm formation, sporulation, and virulence [
40]. In addition, bacterial small RNAs (sRNAs)—typically 50 to 500 nucleotides in length—act as versatile regulators by base-pairing with target mRNAs to modulate gene expression [
41,
42,
43], adding another layer of regulatory complexity. For example, certain RNA methyltransferases uniquely modify adenosine residues at specific carbon positions. Mutations disrupting the RNA methyltransferase
KsgA have been linked to antibiotic resistance in
Escherichia coli, highlighting the physiological importance of RNA methylation in bacterial adaptation [
22].
2.3. Yeast and Fungal DNA Methylation
Historically, yeast such as
Saccharomyces cerevisiae were considered to lack DNA methylation [
44]; however, low levels of 5mC and noncanonical methylation systems have been reported in some fungal species, including
Candida albicans and
Neurospora crassa. In
N. crassa, DNA methylation occurs via DIM-2 methyltransferase and plays a role in silencing transposable elements [
45]. RNA-based mechanisms such as small interfering RNAs (siRNAs) and heterochromatic silencing also contribute to epigenetic control in fungi, providing a functional parallel to mammalian systems.
Fungal DNA methylation plays diverse biological roles, including regulation of reproductive development, phase transitions, and phenotypic variation. In the human gut, common fungal genera include
Aspergillus,
Candida,
Debaryomyces,
Penicillium,
Pichia, and
Saccharomyces [
45]. Among these,
Candida albicans exhibits DNA methylation associated with key processes such as its dimorphic switch and iron metabolism [
24]. Fungi encode distinct DNA methyltransferases (DNMTs), such as Masc1, which mediates methylation-induced premeiotic mutations, and DNMT5, which targets repetitive elements. Interestingly, some model yeasts like
Saccharomyces cerevisiae and
Schizosaccharomyces pombe display little to no detectable DNA methylation. However, RNA-directed DNA methylation (RdDM)-like pathways [
30], functionally analogous to those in plants (described below), have been identified in members of the Basidiomycota phylum [
24], suggesting conserved epigenetic regulatory mechanisms across fungal lineages.
2.4. Plant DNA Methylation
Plants possess a highly structured and complex DNA methylation system that plays critical roles in development, transposon silencing, and responses to environmental stress. Cytosine methylation in plants occurs not only in the CG context, but also in CHG and CHH contexts (where H = A, T, or C), each regulated by distinct enzymatic pathways. CG methylation is maintained by DNA methyltransferase 1 (MET1), while chromomethylases such as CMT2 and CMT3 maintain methylation at CHG sites. CHH methylation, which is more dynamic, is established
de novo by DRM2 or CMT2 [
46]. Demethylation in plants is an active process involving DNA glycosylases, including Repressor of Silencing 1 (ROS1) and members of the demeter-like family [
27,
28,
29]. One of the most distinctive features of plant epigenetics is RdDM, a potent gene silencing mechanism that lacks a direct mammalian counterpart but offers a conceptual framework for understanding small RNA-mediated chromatin regulation. In this process, RNA polymerase IV synthesizes single-stranded RNAs, which are converted into double-stranded RNAs and processed into siRNAs [
43]. Concurrently, RNA polymerase V transcribes non-coding RNAs [
47] that guide the siRNA–Argonaute complex [
48,
49] to specific genomic loci, recruiting
de novo methyltransferases such as DRM2 [
47,
50,
51] to deposit new methylation marks and reinforce silencing.
2.5. DNA Methylation and RNA Regulatory Activity in Animals
In animals, DNA methylation is primarily established by DNMT3A/3B and maintained by DNMT1. These marks are vital for X-inactivation, genomic imprinting, and transposon suppression. Dynamic demethylation is facilitated by the TET family of enzymes, which oxidize 5mC to 5hmC and further intermediates [
52]. RNA methylation, particularly m6A, is recognized by YTH domain-containing proteins and has emerged as a major layer of post-transcriptional gene regulation [
53,
54,
55]. These pathways are responsive to environmental cues and metabolites—including those derived from the microbiota—making them central to the microbiome–host epigenome crosstalk.
Cytosine methylation at the position 5 is the predominant modification in most animal genomes [
52], although 6mA has been reported in some lineages [
25,
55]. Comparative methylome analyses show that while most animal genomes contain DNA methylation, certain animal model organisms such as
Drosophila melanogaster and
Caenorhabditis elegans exhibit minimal to non-detectable DNA methylation [
24]. In humans and rodents, DNA methylation is a dynamic, tissue-specific process influenced by age, environment, lifestyle [
56,
57,
58], and disease status [
59]. Indeed, we observed evolutionary conservation of tissue-specific DNA methylation patterns as a common feature in regulating cellular plasticity via alternative promoter usage and RNA splicing [
57,
58]. The human genome encodes three principal DNA methyltransferases (DNMTs): DNMT1, which maintains existing methylation patterns during DNA replication, and DNMT3A and DNMT3B, which catalyze
de novo methylation. In contrast, active DNA demethylation is mediated by the ten-eleven translocation (TET) family of dioxygenases (TET1–TET3), which sequentially oxidizes 5mC to generate 5hmC and other oxidized derivatives.
In parallel, RNA methylation—most notably m6A—represents one of the most abundant post-transcriptional RNA modifications. It regulates multiple aspects of RNA metabolism, including stability, alternative splicing, translation efficiency, and RNA–protein interactions, and plays essential roles in development and disease [
3,
32,
37,
54]. This dynamic mark is regulated by “writers” (e.g., METTL3, METTL14, WTAP), “erasers” (e.g., FTO, ALKBH5), and “readers” (e.g., YTH domain protein and IGF2BP family) [
33,
35]. Methylation affects diverse RNA species other than mRNA, including circular RNAs, tRNAs, rRNAs, and multiple classes of small RNAs (miRNAs, siRNAs, piRNAs, snoRNAs, tRFs) [
60]. The case of DNMT2, the most highly conserved methyltransferase across eukaryotes, underscores the evolutionary flexibility of epigenetic enzymes. Although originally classified as a DNA methyltransferase, DNMT2 primarily catalyzes cytosine-5 methylation of tRNAs, with a strong preference for tRNA
Asp; remarkably, DNMT2 from one species can functionally complement
Dnmt2-deficient models in others, restoring tRNA
Asp methylation in mice,
Arabidopsis, and
Drosophila [
36,
37]. Collectively, these modifications shape cellular regulatory networks and may also underlie evolutionarily conserved host–microbiome interactions.
3. Mechanistic Insights into Microbiome-Induced Host Epigenetic Regulation
The gut microbiome plays an increasingly recognized role in modulating the host epigenome by influencing DNA and RNA methylation though diverse mechanisms outlined in
Table 2. Herein, we summarize key pathways through which microorganisms impact host methylation processes, focusing on microbial presence, one-carbon metabolism, fermentation products, extracellular vesicles (EVs), and inflammatory modulation within intestinal epithelial cells (IECs).
3.1. Microbial Presence Shapes Host DNA and RNA Methylation Profiles
The presence or absence of gut microbiota can profoundly influence the expression and activity of the host’s methylation machinery. Comparative studies between germ-free (GF) mice and conventionally raised (CR) or specific pathogen-free (SPF) mice have demonstrated that microbial colonization significantly reshapes the host methylome.
For instance, GF mice exhibit disorganized lymphoid architecture and impaired immune responses, in contrast to SPF mice with normal microbiota colonization [
72]. In one study, Reduced Representation Bisulfite Sequencing (RRBS) combined with transcriptomic analysis of IECs identified over 100 genomic regions with microbiota-dependent differences in DNA methylation and gene expression. Notably, microbial presence influenced the expression of key epigenetic regulators, including DNMT3A and TET3 [
73]. Another investigation revealed that CR mice displayed lower levels of global promoter DNA methylation in IECs compared to GF mice, alongside enhanced regulatory activity and elevated TET3 expression. Genetic knockout of TET2 and TET3 in this context led to increased methylation and decreased expression of genes located in hypermethylated regions. These epigenetic changes were accompanied by the enrichment of histone-modifying regulatory elements, suggesting a direct mechanistic connection between microbial colonization and host chromatin remodeling [
74]. Together, these studies underscore the critical role of gut microbiota in shaping the host epigenetic landscape, particularly during immune system maturation and intestinal development.
Emerging evidence also indicates that the gut microbiome may influence RNA modifications, particularly m6A, a key epitranscriptomic mark that regulates RNA stability, splicing, and translation. Liquid chromatography–mass spectrometry (LC-MS) analyses have revealed distinct m6A methylation profiles in multiple organs—including the brain, liver, and intestines—of SPF mice compared to their GF counterparts [
75]. These findings suggest that microbial colonization exerts systemic effects on RNA methylation across diverse tissues. Specifically, certain gut microbes such as
Akkermansia muciniphila and
Lactiplantibacillus plantarum have been shown to modulate host m6A marks in genes involved in cell growth, differentiation, and metabolic regulation [
76].
These microbial influences likely occur through signaling pathways and metabolite-mediated mechanisms that regulate the expression or activity of m6A “writers,” “erasers,” and “readers”—including METTL3/14 (methyltransferases), FTO/ALKBH5 (demethylases), and YTH-domain proteins. Although still an emerging area of study, these findings point to an underexplored layer of host–microbiome interaction that operates at the level of post-transcriptional gene regulation.
3.2. Role of Microbial One-Carbon Metabolism
One-carbon metabolism serves as a critical biochemical nexus linking nutritional status, microbial activity, and epigenetic regulation. This pathway generates S-adenosylmethionine (SAM), the universal methyl donor required for DNA, RNA, and histone methylation processes [
62]. Central to one-carbon metabolism are the folate cycle, methionine cycle, and trans-sulfuration pathway, all of which depend on essential micronutrients—namely folate (vitamin B9), riboflavin (vitamin B2), pyridoxine (vitamin B6), and cobalamin (vitamin B12) [
77,
78,
79]. Because humans cannot synthesize these cofactors endogenously, they must obtain them through dietary intake and gut microbial biosynthesis [
80].
Gut-resident microorganisms—particularly taxa within the Bacteroidetes, Fusobacteria, and Proteobacteria phyla—play a key role in producing or metabolizing these vitamins [
61], thereby modulating the host’s methyl group bioavailability. Dysbiosis or shifts in microbial composition can impair this balance. For example, an overrepresentation of choline-degrading bacteria may simulate dietary choline deficiency, reducing SAM levels and contributing to global hypomethylation and associated metabolic dysfunctions [
81]. Conversely, Lactobacillus [
62] and Bifidobacterium [
63] species are known to synthesize folate and contribute to maintaining epigenetic homeostasis by supporting adequate methyl donor supply.
3.3. Intersection of One-Carbon and Polyamine Metabolism
Polyamines (PAs)—including putrescine, spermidine, and spermine—are small, positively charged molecules that play essential roles in cell proliferation, gene regulation, stress responses, and epigenetic maintenance [
82]. These bioactive compounds are acquired through dietary intake and are also synthesized
de novo by both host cells and the gut microbiota [
83]. Within the lower GI [
84], specific microbial taxa, including
Lactobacillus spp. [
64] and
Shewanella xiamenensis [
65], convert dietary arginine into ornithine, and subsequently into putrescine via ornithine decarboxylase (ODC) activity. Additional enzymatic steps then generate spermidine and spermine, utilizing aminopropyl groups derived from SAM.
Importantly, polyamine metabolism is tightly interlinked with one-carbon metabolism, as it consumes and regulates levels of SAM and its decarboxylated form (dcSAM). For example, elevated intracellular concentrations of spermine can inhibit both ODC and adenosylmethionine decarboxylase (AdoMetDC), resulting in increased SAM availability and consequently enhanced DNMT activity [
85]. Through this regulatory feedback, polyamines indirectly modulate DNA methylation patterns, contribute to genomic stability, and influence immune homeostasis and inflammatory responses.
3.4. Impact of Microbial Fermentation Products
Microbial fermentation of dietary carbohydrates in the colon leads to the production of SCFAs—primarily acetate, propionate, and butyrate—which serve not only as energy sources but also as potent signaling molecules within the intestinal epithelium. Among their diverse biological effects, SCFAs are best known for their role as histone deacetylase (HDAC) inhibitors, thereby promoting a more open chromatin configuration and enhancing gene expression [
66,
68]. In addition to histone modifications, SCFAs can indirectly influence DNA methylation by modulating substrate availability for methylation pathways and altering the expression of DNMTs. Notably, in GF mouse models, SCFA supplementation can partially restore methylation and chromatin modification patterns that are otherwise dependent on the presence of gut microbiota [
79].
Beyond SCFAs, other microbiota-derived metabolites significantly impact epigenetic regulation. For example, intermediates of the tricarboxylic acid (TCA) cycle such as succinate and fumarate can act as competitive inhibitors of α-ketoglutarate-dependent dioxygenases, including TET enzymes, thereby suppressing active DNA and histone demethylation processes. Furthermore, certain dietary glucosinolates, when metabolized by gut bacteria like
Bacteroides thetaiotaomicron [
69], are converted into bioactive isothiocyanates (e.g., sulforaphane), which have been shown to inhibit DNMT activity and alter host DNA methylation patterns [
70]. Collectively, these microbial metabolites serve as epigenetic modulators, linking diet, microbial metabolism, and host gene regulation through both direct enzymatic inhibition and substrate competition in chromatin remodeling pathways.
3.5. Extracellular Vesicles as Epigenetic Messengers
Microbial EVs—especially outer membrane vesicles (OMVs) secreted by Gram-negative bacteria—are increasingly recognized as potent mediators of inter-kingdom communication. These nanoscale vesicles encapsulate nucleic acids, proteins, metabolites, and lipids, and can traverse epithelial barriers to deliver their cargo into host cells, including epithelial, immune, and stromal tissues [
86,
87]. Once internalized, microbial EVs have the capacity to modulate host signaling cascades, epigenetic machinery, and gene expression profiles at local and distant sites.
A striking example of such interaction is provided by
Pseudomonas aeruginosa, whose OMVs were shown to induce widespread hypomethylation in human lung macrophages, thereby enhancing the accessibility of distal regulatory elements linked to immune defense genes [
71]. These changes not only affect transcriptional outputs but may also prime immune cells for subsequent inflammatory or tolerogenic responses. Similar effects have been observed in intestinal systems, where OMVs derived from
Bacteroides fragilis carry PSA and influence Foxp3 regulatory T cell expression, potentially through epigenetic remodeling of enhancer regions, further discussed below.
3.6. Epigenetic Modulation of the Inflammatory Response
The gut microbiome plays a critical role in maintaining intestinal homeostasis, in part by shaping the epigenetic responsiveness of IECs to microbial stimuli. One well-characterized example involves the epigenetic repression of Toll-like receptor 4 (TLR4)—a key pattern recognition receptor involved in detecting lipopolysaccharide (LPS) from Gram-negative bacteria. In IECs, TLR4 expression is attenuated through a combination of DNA hypermethylation and histone deacetylation at its promoter region. This process is mediated by the zinc finger protein ZNF160, which recruits the co-repressor KAP1 (KRAB-associated protein 1) to establish a repressive chromatin state [
88]. As a result, IECs exhibit reduced sensitivity to LPS, thereby mitigating excessive inflammatory responses to the dense microbial populations in the gut lumen. This so called “epigenetic buffering” of microbial reactivity is crucial for mucosal tolerance, helping to prevent inappropriate immune activation while preserving host defense. It also underscores the tissue-specific tuning of host epigenetic programs in response to the local microbial environment.
Collectively, the interconnected pathways discussed—including nutrient provisioning for methylation reactions, production of bioactive metabolites (e.g., SCFAs, polyamines, isothiocyanates), extracellular vesicle-mediated communication, and immune receptor regulation—highlight the multifaceted ways in which the gut microbiota modulates host DNA and RNA methylation (
Figure 1). Nevertheless, the precise molecular mechanisms by which microbial signals interface with host chromatin regulators—such as DNMTs, TET enzymes, and histone modifiers—remain incompletely defined and represent an urgent area for future research.
4. Host Epigenetic Landscape Shapes Microbiome Composition and Function
The relationship between the gut microbiome and the host epigenome is fundamentally bidirectional. While microbial communities exert significant influence on host DNA and RNA methylation, accumulating evidence indicates that the host’s genetic and epigenetic landscape actively shape the composition, diversity, and metabolic function of the microbiota [
89,
90]. This reciprocal regulation plays a central role in maintaining physiological homeostasis and responding to environmental stimuli, with important implications for precision medicine.
Recent studies suggest that host genomic variants, particularly in genes related to immunity [
91,
92], epithelial integrity, and metabolism [
93], can influence microbial colonization patterns and niche selection. Additionally, host epigenetic modifications—including methylation of genes involved in mucosal barrier function, antimicrobial peptide expression, and glycosylation—can create a selective environment that favors the expansion or suppression of specific microbial taxa [
94].
Beyond genetic and epigenetic determinants, extrinsic host factors such as diet, aging, and sociocultural context significantly modulate microbiota structure and function [
95,
96,
97,
98,
99,
100,
101,
102,
103]. These influences interaction with host regulatory pathways to shape the dynamic ecosystem of the gut. This section summarizes emerging evidence on how host-intrinsic factors and environmentally responsive epigenetic programs contribute to shaping the microbiota, highlighting their role in reinforcing the feedback loop between host and microbial signals.
4.1. Host Genetic Determinants Influence Microbial Composition
Host genetic variation, particularly in genes governing immune function, plays a pivotal role in shaping the structure and diversity of the gut microbiota. For instance, mutations in the MEFV gene, which encodes the inflammasome-associated protein pyrin, are linked to familial Mediterranean fever (FMF) and have been shown to significantly alter microbial profiles. Affected individuals often exhibit reduced microbial diversity and distinct shifts in bacterial composition, which tend to correlate with disease flares and inflammatory status [
96,
97]. Such mutations are associated with reduced microbial diversity and compositional shifts that often parallel disease activity.
Twin studies further underscore the heritability of microbial traits, revealing that certain microbial taxa—such as members of the
Christensenellaceae family—demonstrate high concordance among monozygotic twins, even when raised in different environments [
95]. This genetic control is reinforced by genome-wide association studies (GWAS) that have identified single nucleotide polymorphisms (SNPs) associated with the relative abundance of key bacterial groups, including
Bifidobacterium,
Lactobacillus, and
Faecalibacterium.
Additionally, in vivo evidence from animal gene knockout models lends further support: targeted deletion of host genes involved in immune signaling, epithelial barrier function, and metabolic regulation leads to reproducible changes in microbial community structure. Indeed, at least 30 distinct genomic loci have been implicated in regulating microbiota composition in such models [
98], indicating a functional, genotype-dependent axis of host–microbiota interaction. Together, these findings highlight the importance of host genetics in establishing and maintaining a stable and health-promoting microbial ecosystem.
4.2. Nutritional Modulation of the Gut Microbiota
Diet is one of the most powerful and modifiable environmental factors influencing gut microbial diversity, abundance, and metabolic output. Dietary components not only determine nutrient availability for microbial fermentation but also modulate the production of epigenetically active metabolites, such as SCFAs, polyamines, and vitamins essential for one-carbon metabolism.
One widely studied dietary intervention is the low-FODMAP diet, which restricts fermentable oligosaccharides, disaccharides, monosaccharides, and polyols. This approach has proven to be clinically effective in managing irritable bowel syndrome (IBS) symptoms, in part by restructuring the gut microbiota [
99]. Multi-center trials have consistently demonstrated that a low-FODMAP diet reduces populations of fermentative and SCFA-producing bacteria—such as certain
Clostridium and
Bacteroides species—while promoting the growth of beneficial taxa, including
Bifidobacterium longum and
Anaerostipes propionicum [
100]. These microbiota shifts correlate with improved gastrointestinal function, reduced inflammation, and alleviation of IBS symptom severity.
Beyond gastrointestinal disorders, diet–microbiome–epigenome interactions are increasingly recognized as modulators of systemic immunity and neurodevelopment. For instance, ketogenic diet interventions in children with autism spectrum disorder (ASD) have been associated with favorable shifts in the gut microbiome including an increase in butyrate-producing bacteria, reduction in proinflammatory cytokines, and changes in the expression of microRNAs linked to brain-derived neurotrophic factor (BDNF) signaling [
101]. These findings suggest a mechanistic link between diet-induced microbial changes and epigenetically regulated neural and immune pathways.
Together, these examples highlight the potential of dietary strategies to modulate the microbiota and, through it, the host epigenome, opening novel avenues for personalized nutrition and therapeutic interventions.
4.3. Aging, Psychosocial Stress, and the Microbiome–Epigenome Axis
Aging is accompanied by progressive changes in both the epigenome and the gut microbiome, which together shape immune function, metabolic health, and disease susceptibility. Epigenetic clocks—which estimate biological age based on DNA methylation patterns at specific CpG sites—have become valuable tools for assessing age-related physiological decline [
56,
92,
102]. These clocks often reveal discrepancies between chronological and biological age, particularly in populations exposed to chronic stress or environmental insults. In studies involving Native Hawaiian and Pacific Islander (NHPI) cohorts, accelerated epigenetic aging was found to correlate with declines in beneficial taxa such as
Actinobacteria and
Bacteroides, and increases in proinflammatory taxa, including
Deferribacteres and
Proteobacteria [
103]. These microbial shifts mirror age-related immune dysregulation and systemic inflammation, commonly referred to as inflammaging.
Beyond aging, mental health and psychosocial stress are increasingly recognized as modulators of the microbiome–epigenome axis. Individuals experiencing lower self-esteem and elevated psychological stress often exhibit increased circulating levels of proinflammatory cytokines such as IL-8 and TNF-α. These cytokine profiles are associated with distinct gut microbial configurations and differential DNA methylation patterns in peripheral immune cells [
92]. Such epigenetic changes may mediate the biological imprint of chronic psychosocial stress, linking environmental adversity to immune dysfunction and microbial dysbiosis.
These effects are particularly pronounced in underserved populations such as NHPIs, where socioeconomic disparities contribute to both microbial imbalances and epigenetic alterations, compounding health risks across generations. Understanding these interconnected dynamics is crucial for designing equitable interventions that account for social determinants of health.
4.4. An Integrated View
Collectively, these findings underscore the dynamic and reciprocal nature of host–microbiome interactions. Host genetic architecture, dietary patterns, lifespan-related epigenetic remodeling, and the broader sociocultural environment play pivotal roles in shaping the composition, diversity, and functional capacity of the gut microbiome. In turn, microbial communities influence host physiology by modulating epigenetic regulators, including DNA and RNA methylation, histone modifications, and non-coding RNA networks that concertedly act to shape health trajectories.
This bidirectional feedback loop enables the epigenome to act as both a sensor and effector, translating environmental and microbial signals into heritable changes in gene expression. Through this framework, the host epigenome contributes to microbiota stability, adaptability, and resilience, which are essential for maintaining immune homeostasis, metabolic balance, and neurobehavioral integrity. Disruption of this delicate interplay—via genetic predisposition, dietary shifts, psychosocial stress, or aging—can predispose individuals to a wide spectrum of diseases, from inflammatory and metabolic disorders to neurodevelopmental and psychiatric conditions.
A systems-level understanding of these host–microbiome–epigenome interactions is critical for developing precision medicine strategies that account for individual variability and sociocultural context.
5. Dissecting Cellular Mechanisms of Microbiome–Epigenome Crosstalk: Mediator Molecules and Pathways
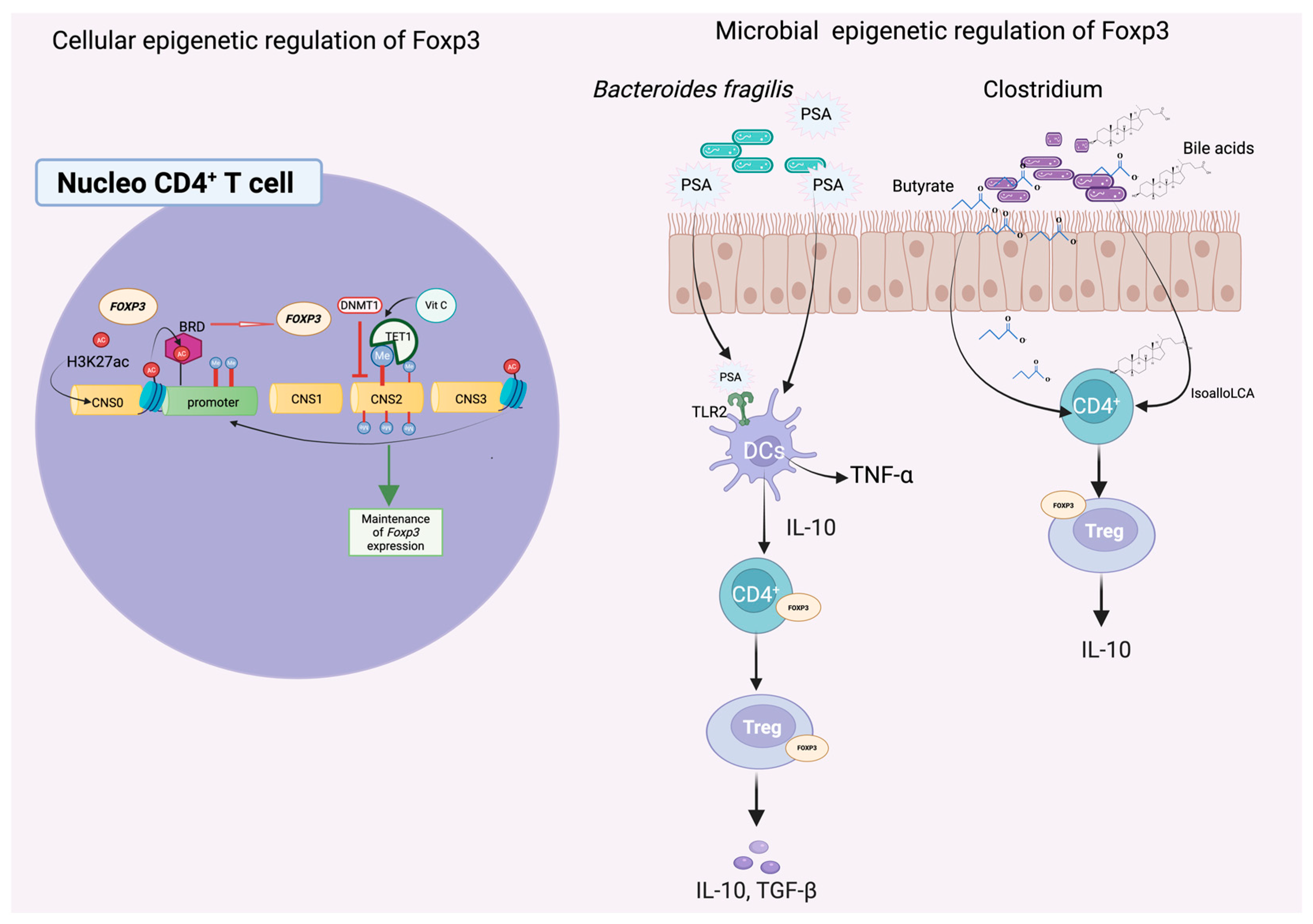
Although substantial progress has been made in linking the gut microbiome to host epigenetic regulation, the precise cellular signaling pathways and molecular mediators that orchestrate this dialog remain incompletely understood. Recent advances in multi-omics, gnotobiotic models, and chromatin profiling have begun to elucidate the complex interplay between microbial signals and host transcriptional regulators, offering promising avenues for therapeutic intervention. A particularly well-characterized example illustrating this intricate cross-kingdom communication involves the epigenetic regulation of the forkhead box P3 (FOXP3) transcription factor by gut microbiota-derived metabolites and signaling molecules (
Figure 2).
5.1. Epigenetic Regulation of FOXP3 by the Gut Microbiome
The intestinal mucosal immune system requires a finely tuned balance between tolerance and defense to maintain homeostasis in the presence of a vast and dynamic microbial ecosystem [
104]. Central to this regulation are regulatory T cells (T
reg), which express the FOXP3 transcription factor and play critical roles in suppressing inflammatory responses, promoting tolerance to commensals, and preserving epithelial integrity [
105,
106].
The stable expression of FOXP3 is epigenetically governed through both DNA methylation and histone modifications. Specifically, demethylation of the conserved non-coding sequence 2 (CNS2) region within the FOXP3 locus is essential for transcriptional activation and heritable T
reg lineage stability [
107]. This demethylation is facilitated by TET family enzymes (TET1–3), whose activity is enhanced by ascorbic acid (vitamin C), a cofactor that promotes chromatin accessibility and facilitates transcription factor binding [
108].
Mouse models have demonstrated that T
reg differentiation and FOXP3 expression are profoundly shaped by the gut microbiota. GF or microbiota-depleted mice exhibit impaired T
reg development and abnormal immune activation. Notably, Foxp3-deficient mice display both elevated intestinal inflammation and altered gut microbial composition, including increased Firmicutes and reduced abundance of beneficial Clostridia and Lachnospiraceae [
106,
109,
110]. Reintroduction of specific Clostridium strains restored the immunoregulatory microenvironment by inducing transforming growth factor-beta (TGF-β) production and facilitating FOXP3
+ T
reg differentiation in the colon [
110]. These findings are echoed in human immunodeficiency syndromes, such as IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked), where FOXP3 loss-of-function mutations lead to severe autoimmunity and disrupted microbiota homeostasis [
111].
Among the most well-characterized microbial influences on FOXP3 regulation are microbial metabolites that act through epigenetic and immunomodulatory mechanisms. Butyrate, a short-chain fatty acid produced by
Clostridium species, functions as an HDAC inhibitor, thereby enhancing histone acetylation at the FOXP3 promoter and CNS3 enhancer regions. This increase in acetylation fosters a more transcriptionally permissive chromatin state, facilitating sustained FOXP3 expression and stable T
reg differentiation [
106,
112]. In addition to SCFAs, secondary bile acid metabolites, such as isoalloLCA, have also been shown to stimulate FOXP3 transcription. This effect is mediated through the induction of mitochondrial reactive oxygen species (ROS), which can influence local chromatin accessibility and support the epigenetic stability of the T
reg lineage [
113]. Another key microbial product is the capsular polysaccharide A (PSA) produced by
Bacteroides fragilis, which enhances the function of FOXP3
+ T
reg cells by upregulating CD39 and stimulating the secretion of interleukin-10 (IL-10) and interferon-gamma (IFN-γ), thereby reinforcing immunosuppressive pathways. In germ-free mice, the absence of PSA-producing strains results in diminished FOXP3
+ T
reg populations and impaired mucosal tolerance—deficits that can be restored by colonization with PSA-expressing
B. fragilis strains [
72].
At the chromatin level, the interplay between DNA demethylation and histone acetylation establishes an open transcriptional environment. This epigenetic landscape allows bromodomain-containing (BRD) proteins to bind acetylated lysines and sustain FOXP3 expression. Pharmacological inhibition of BRD proteins using small molecules like JQ1 disrupts this interaction and has been proposed as a therapeutic strategy to modulate T
reg function in inflammatory and autoimmune diseases [
105].
5.2. Broader Implications
The example of FOXP3 epigenetic regulation underscores a broader paradigm in which microbial-derived metabolites, surface polysaccharides, and molecular signals function as potent mediators of host–microbiome crosstalk, particularly within the immune compartment [
105,
106,
107]. While FOXP3 remains among the most thoroughly studied targets of microbial modulation, emerging evidence suggests that a wide array of host regulatory genes and signaling pathways—including those involved in T helper cell differentiation, antigen presentation, and cytokine production—may be similarly influenced by microbiome-dependent epigenetic mechanisms [
104].
For instance, butyrate and other short-chain fatty acids have been shown to modulate histone acetylation and methylation in dendritic cells and macrophages, altering their capacity to shape adaptive immune responses [
68,
79]. Similarly, microbial-derived indole derivatives [
114], bile acid metabolites [
113], and extracellular vesicles [
86,
87] can affect the expression of genes involved in barrier integrity and inflammation by altering local chromatin states.
These observations suggest that the microbiota contributes not only to immune education during development but also to dynamic epigenetic reprogramming in response to environmental cues throughout life. Understanding these broader interactions provides a conceptual framework for developing precision therapeutics aimed at restoring immunological tolerance or dampening pathological inflammation through microbiota-informed epigenetic targeting.
6. Future Research Strategies to Study the Microbiome–Epigenome Axis
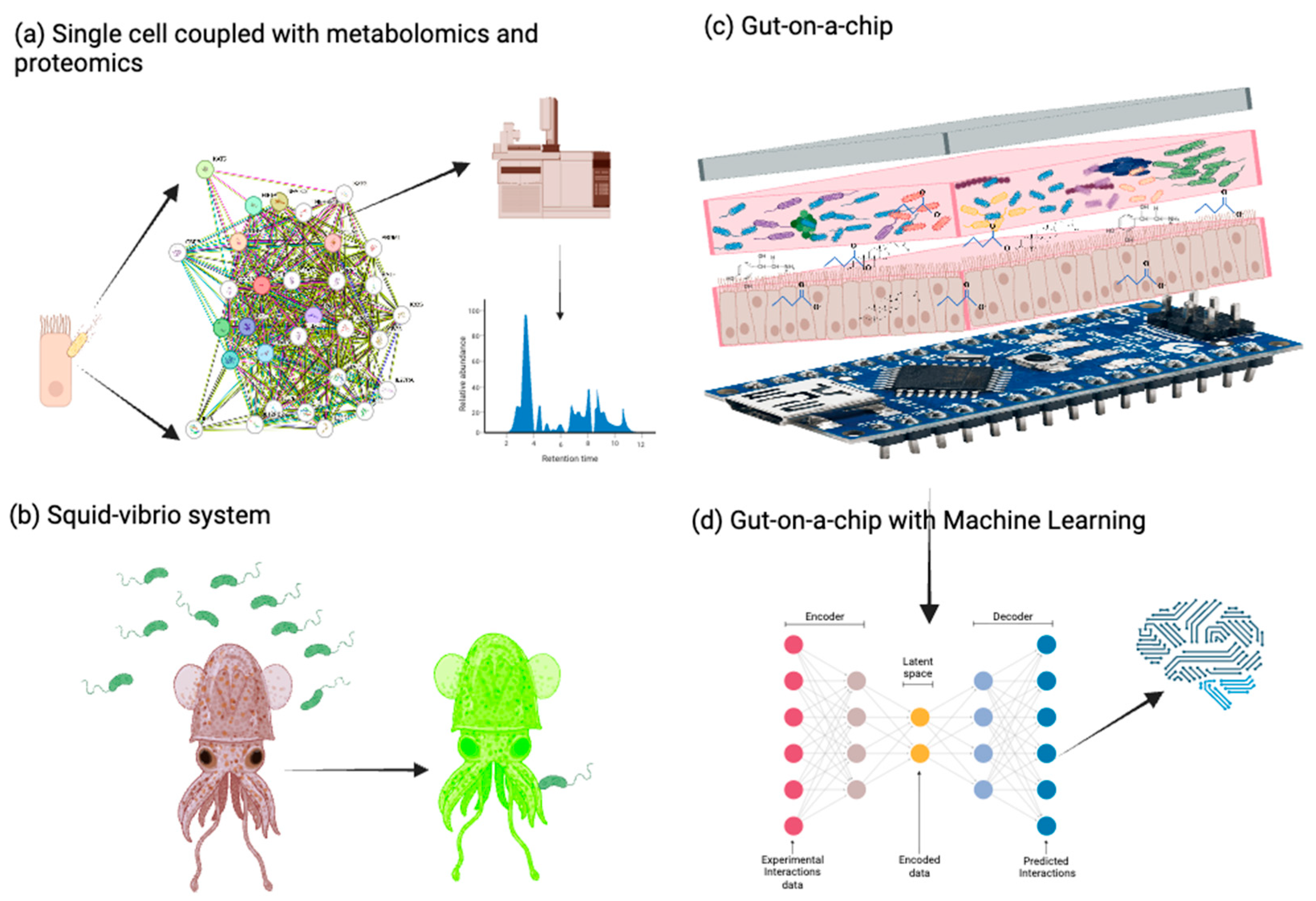
Despite recent insights into how microbial communities modulate host DNA and RNA methylation through defined and evolutionarily conserved biochemical pathways, our ability to interrogate these processes at high resolution and translate them into clinically meaningful outcomes remains limited. Advancing this field will require the convergence of cutting-edge methylome profiling tools, longitudinal multi-omics studies, machine learning-based integrative models, and context-aware experimental systems. Moreover, aligning mechanistic discoveries with therapeutic development, biomarker validation, and equitable data governance will be critical for moving from bench to bedside. The following section outlines the most promising strategies and tools shaping this next phase of microbiome–epigenome research (
Figure 3).
6.1. Mapping Methylation Landscapes with High-Throughput Technologies
Modern epigenomic research benefits from increasingly sensitive tools for profiling DNA and RNA methylation marks across diverse organisms. Early assumptions that many model organisms lacked significant methylation (e.g.,
Drosophila melanogaster) have been revised though the application of HPLC, LC-MS/MS [
115], whole-genome bisulfite sequencing (WGBS), RRBS, and emerging single-molecule real-time (SMRT) sequencing platforms, which allow direct detection of methylated cytosine and adenine residues with ever higher sensitivity and resolution.
These technologies are now widely applied to bacteria, fungi, plants, and animals to uncover lineage-specific methylation patterns and discover novel methyltransferases [
116,
117]. When coupled with RNA-Seq, proteomics, and chromatin accessibility assays (ChIP-Seq, ATAC-Seq, etc.), they enable researchers to link methylation states with transcriptional activity, protein expression, and functional phenotypes. In microbial contexts, phase-variable restriction-modification systems, such as those characterized in
Helicobacter pylori [
117],
Campylobacter jejuni [
118], and
Neisseria species [
119], illustrate how epigenetic switches can drive rapid changes in gene expression, virulence, and immune evasion [
120]. Resolving whether these microbial modifications can, in turn, reshape host epigenetic programs remains a compelling area for further study. Multi-omics techniques—including joint profiling of transcriptomics, methylomics, proteomics, and metabolomics in single cells [
121], are becoming increasingly valuable for resolving cell-type-specific regulatory networks in response to microbial stimuli.
6.2. Systems-Level Integration Using Longitudinal Multi-Omics and AI
To fully elucidate microbiome–epigenome interactions, there is a growing need to move beyond static or reductionist approaches and toward dynamic, systems-level models. One promising direction involves the integration of high-dimensional multi-omics platforms across longitudinal cohorts. Studies such as the Survey of the Health of Wisconsin (SHOW) and the Qatar-based Omouma cohort have employed time-series analyses of host methylation, microbial taxa, and metabolic outputs to identify predictive biomarkers of disease susceptibility and progression [
122,
123]. Such designs can support causal inference and provide critical insight into temporal relationships between microbial community dynamics and host epigenetic states.
On the computational front, deep learning frameworks such as MethylNet [
124] and DeepCpG [
125] have shown promise in predicting cell-type-specific methylation patterns by integrating genomic, microbial, and environmental features. Meanwhile, spatial–temporal modeling tools like MEFISTO enable the integration of diverse data types (e.g., transcriptome, methylome, and metabolome) while preserving biological context [
126]. While these tools will require greater interpretability and validation in diverse real-world populations, applying them in simplified experimental models may enable new insights into the mechanisms underlying microbial–epigenomic interactions.
6.3. Emerging Experimental Models to Study Microbial–Epigenomic Interactions
Despite methodological progress, dissecting the causal pathways that link microbial signaling molecules and the host epigenetic machinery is inherently challenging. Many studies rely on reductionist models that do not capture the full ecological complexity of gut microbial communities or the physiological state of host tissues. For example, germ-free or mono-colonized mice remain standard models but have limited translational value for recapitulating human–microorganism interactions [
127]. Additionally, phase-variable restriction–modification systems and microbial methyltransferases can respond dynamically to environmental signals, horizontal gene transfers, and phage exposure, adding further complexity to their regulatory roles.
To address these limitations, innovative ex vivo and in vitro models are being developed. For instance, three-dimensional (3D) organoid cultures [
128,
129,
130] and gut-on-a-chip [
131,
132,
133] systems allow for co-culturing human IECs with microbial communities under controlled, physiologically relevant conditions. These systems can replicate nutrient gradients, oxygen level, and epithelial integrity, facilitating real-time monitoring of host–microorganism interactions [
128]. The well-studied
Euprymna scolopes (squid)—
Vibrio fischeri (bacteria) symbiosis represents a tractable animal model for investigating how colonization can modulate host gene expression, given the interactions of a single bacterial species with its host on regulating squid development, health, and behavior [
134,
135,
136]. Although DNA methylation has been documented in the squid [
137], the epigenetic basis of this symbiosis remains underexplored and presents a highly simplified experimental system to more precisely explore the functional relationships between the endogenous host epigenetic processes and microbial symbiont factors that together shape host phenotypes.
6.4. From Mechanism to Translation: Clinical and Therapeutic Frontiers
A critical objective for the field is to translate mechanistic insights into actionable clinical interventions that harness microbiome–epigenome interactions. Live biotherapeutic products (LBPs), which include engineered or naturally occurring microbial strains capable of secreting beneficial metabolites such as butyrate, inosine, or PSA, are actively being evaluated in early-phase clinical trials for a range of immune-mediated diseases, including IBD, atopic dermatitis, and autoimmune arthritis [
138]. These microbes exert immunomodulatory effects in part through epigenetic reprogramming of intestinal and immune cells, such as the induction of histone acetylation in regulatory T cells or suppression of DNA methyltransferase activity in macrophages [
91].
Beyond targeted LBPs, broader microbiome-modulating strategies such as fecal microbiota transplantation (FMT) have gained prominence, particularly in the treatment of recurrent
Clostridioides difficile infections [
139,
140,
141]. However, their utility in treating extraintestinal conditions such as metabolic syndrome, autism spectrum disorders, and even cancer remains investigational. Studies suggest that FMT can induce durable shifts in host methylation profiles, including in genes involved in lipid metabolism, neurodevelopment, and inflammatory cascades—highlighting the need to decode the epigenetic consequences of engraftment and donor–recipient compatibility [
142]. The long-term success of such interventions may depend on epigenomic “readiness” of host tissues to respond to microbial stimuli, a concept that remains poorly understood.
In oncology, the intersection between microbial metabolites and cancer epigenetics is beginning to draw attention. For instance, microbial production of SCFAs can modulate HDAC activity in tumor cells, potentially influencing cancer progression and response to epigenetic drugs [
143]. Tumor-associated dysbiosis has also been linked to altered methylation patterns in colorectal and hepatocellular carcinomas, raising the possibility that microbiome-based interventions could re-sensitize tumors to epigenetic therapies [
144].
To achieve safe and effective clinical translation, stratification of patients based on combined microbiome and epigenome signatures will be essential. Biomarkers of therapeutic responsiveness may include DNA methylation patterns in key regulatory genes, chromatin accessibility in immune cell populations, or metabolite signatures indicative of microbial activity. Initiatives like the PRISM consortium and the Human Microbiome Project Phase 2 (Integrative Human Microbiome Project) are beginning to integrate multi-omic data at scale to define such predictive profiles [
145].
However, this progress must also contend with confounding biological and social variables. Factors such as age, biological sex, dietary habits, geographic location, and ancestry shape both microbial communities and epigenetic landscapes in non-linear ways [
146]. Adaptive clinical trial designs, including basket trials and real-world registry-based studies, have been proposed as promising frameworks to accommodate biological heterogeneity in personalized medicine and may be adaptable to microbiome–epigenome research [
147].
Ultimately, realizing the therapeutic potential of microbiome–epigenome research will require not only mechanistic precision, but also the development of delivery systems, dosing regimens, and regulatory frameworks that account for the dynamic and individualized nature of host epigenetic–microbial crosstalk.
6.5. Standards, Equity, and Open Data Stewardship
The reproducibility and translational impact of microbiome–epigenome research hinges on rigorous technical and ethical standardization. Key experimental variables—including reference DNA, spike-in controls, methylation quantification protocols, and harmonized procedures for biospecimen collection and stabilization—must be systematically benchmarked to minimize batch effects and ensure cross-cohort comparability. This is particularly crucial for global multi-center consortia where analytical variability can obscure genuine biological signals.
Several efforts are actively addressing these challenges. The International Human Epigenome Consortium (IHEC) [
148] and the National Institute of Standards and Technology (NIST) [
149] have released standardized protocols for bisulfite sequencing and quality control guidelines for epigenomic data generation, helping to reduce inter-laboratory discrepancies. Similarly, the Human Microbiome Project (HMP) [
145] and MetaSUB (Metagenomics and Metadesign of the Subways and Urban Biomes) [
150] have adopted platform-independent microbial metagenomic workflows that could be adapted for integrated epigenomic applications.
Beyond laboratory and analytical pipelines, robust metadata annotation and data stewardship practices are essential. The FAIR Data Principles—Findable, Accessible, Interoperable, and Reusable—have become foundational in microbiome and omics research, promoting transparency, reusability, and machine-readability of data [
151]. Public platforms such as MG-RAST, EBI Metagenomics, and GEO (Gene Expression Omnibus) increasingly require detailed metadata about sample origin, processing conditions, and participant demographics, improving data quality for meta-analyses.
However, as microbiome–epigenome studies expand into diverse human populations, attention to equity and justice in data governance becomes equally essential. The CARE principles—Collective Benefit, Authority to Control, Responsibility, and Ethics—developed by the Global Indigenous Data Alliance (GIDA), emphasize that open science must also serve the interests of historically marginalized and sovereign indigenous communities [
152]. These principles are not opposed to FAIR but serve as a necessary complement, especially in contexts where data extraction risks undermining community autonomy [
153], which is particular important for Indigenous Peoples.
Recent examples of CARE implementation include the Silent Genomes Project in Canada, which developed governance models for genomic data in indigenous populations, and Te Mana Raraunga in Aotearoa New Zealand, which works to embed Māori values into national data infrastructures [
154,
155,
156,
157,
158]. These frameworks provide vital blueprints for integrating ethical considerations into microbiome–epigenome consortia, particularly those involving ancestrally diverse or vulnerable populations.
7. Conclusions and Future Directions
The human gut microbiome represents a metabolically active and ecologically dynamic system that not only shapes, but is shaped by, the host’s epigenetic architecture. Mounting evidence now implicates gut microorganisms as modulators of host DNA and RNA methylation through multiple mechanistically distinct but converging routes—including nutrient provisioning for one-carbon metabolism, polyamine biosynthesis, SCFA production, and the release of extracellular vesicles loaded with regulatory molecules. In turn, host genomic and epigenomic contexts, including genetic variants, immune regulation, dietary inputs, and aging, exert selective pressures on microbial composition, metabolic output, and ecological stability.
Despite notable advances in deciphering these bidirectional relationships, many mechanistic and translational questions remain open. Key challenges include disentangling species- and tissue-specific effects, modeling microorganisms–host interactions under physiologically relevant conditions, and establishing causality in complex, polymicrobial settings. Reductionist models such as germ-free or mono-colonized mice, or even emerging models such as the squid-Vibrio fischeri, have yielded foundational insights; yet their translational relevance to human health remains limited. To bridge this gap, high-resolution platforms—including single-cell and spatial omics, 3D intestinal organoids, and gut-on-a-chip systems—are now enabling a more granular understanding of host-microbiota interactions within intact tissue contexts.
Simultaneously, the integration of longitudinal multi-omics studies with machine learning frameworks is opening new avenues for predictive modeling of epigenetic reprogramming and disease trajectories. These computational approaches, when paired with population-scale datasets, hold promise for identifying actionable biomarkers, therapeutic targets, and individualized intervention strategies. Interventions such as LBPs and FMT are already advancing into early-phase clinical trials for immune, metabolic, and neurological disorders, though their success will depend heavily on stratifying patients by microbial and epigenomic profiles. Innovative trial designs—including basket and registry-based studies—may be better suited to address population heterogeneity and improve translational fidelity.
In parallel, ensuring scientific rigor and equity in this emerging field will require robust standards for biospecimen handling, sequencing, metadata annotation, and data sharing. The adoption of FAIR principles has greatly enhanced reproducibility and data interoperability across microbiome and epigenome research. Complementary ethical frameworks such as the CARE principles are also essential to safeguard sovereignty and justice for underrepresented communities, especially in global and Indigenous genomics collaborations.
Ultimately, decoding the microbiome–epigenome interface offers unprecedented opportunities for precision medicine. This includes not only targeted epigenetic or microbial therapies, but also anticipatory approaches such as microbiome-informed nutrition and early disease risk stratification. Realizing this potential will demand sustained interdisciplinary collaboration across microbiology, genetics, epigenetics, computational biology, and clinical sciences, grounded in both methodological rigor and social responsibility.
Author Contributions
N.C.R., A.T. and A.K.M. conceived and designed the content for this review. N.C.R. and A.T. compiled, analyzed, and curated the data from the current literature as of the time of this publication. N.C.R. and A.T. prepared the figures and wrote the initial draft of the manuscript that was then edited by A.K.M. All authors approved the final version of the manuscript.
Funding
This review was supported in part by the National Institute on Minority Health and Health Disparities (NIMHD) of the National Institutes of Health (NIH) under grants R01MD016593 and R56MD014630. The contents herein are solely the responsibility of the authors and do not represent the official view of NIH or NIMHD. These funders had no role in the preparation, review, or approval of the manuscript nor in the decision to submit the manuscript for publication.
Acknowledgments
We thank members of the Maunakea laboratory at the University of Hawaii, including past members whose work contributes to this topic, for their valuable input during discussions of the topic, and for reviewing earlier drafts of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used herein:
| AdoMetDC | Adenosylmethionine decarboxylase |
| ASD | Autism spectrum disorder |
| ApT | Adenine followed by thymine |
| ATAC-Seq | Assay for transposase-accessible chromatin using sequencing |
| BDNF | Brain-derived neurotrophic factor |
| BRD | Bromodomain |
| CARE | Collective benefit, authority to control, responsibility, and ethics |
| CMT | Chromomethylases |
| CNS | Conserved noncoding sequence |
| CR | Conventionally raised mouse |
| ChIP-Seq | Chromatin immunoprecipitation followed by sequencing |
| dcSAM | Decarboxylated s-adenosylmethionine |
| DNMT | DNA methyltransferase |
| EV | Extracellular vesicle |
| FAIRE | Findable, accessible, interoperable, and reusable |
| FMT | Fecal microbiota transplantation |
| FODMAP | Fermentable oligosaccharides, disaccharides, monosaccharides and polyol |
| FOXP3 | Forkhead box P3 |
| GIDA | Global indigenous data alliance |
| GF | Germ-free mouse |
| HDAC | Histone deacetylase |
| IBS | Irritable bowel syndrome |
| IEC | Intestinal epithelial cells |
| IHEC | International human epigenome consortium |
| iHMP | Integrative human microbiome project |
| LBPs | Live biotherapeutic products |
| LPS | Lipopolysaccharide |
| NIST | National Institute of Standards and Technology |
| m6A | N6-methyl-adenosine |
| MetaSUB | Metagenomics and Metadesign of the Subways and Urban Biomes |
| ODC | Ornithine decarboxylase |
| OMV | Outer membrane vesicles |
| PA | Polyamines |
| PRISM | Psychiatric rating using intermediate stratified markers |
| PSA | Polysaccharide A |
| RdDM | RNA-directed DNA methylation |
| RNA-Seq | RNA sequencing; assay for transcriptome profiling |
| RM | Restriction-modification system |
| ROS1 | Repressor of silencing 1 protein |
| RRBS | Reduced representation bisulfite sequencing |
| SAM | S-adenosylmethionine |
| SCFA | Short-chain fatty acid |
| siRNA | Small interfering |
| SMRT | Single-molecule real time |
| SNP | Single nucleotide polymorphism |
| SPF | Specific pathogen-free model |
| sRNA | Small RNA |
| TET | Ten-eleven translocation |
| TLR4 | Toll-like receptor 4 |
| Treg | Regulatory T cell Lymphocyte |
| WGBS | Whole-genome bisulfite sequencing |
| 4mC | N4-methylcytosine |
| 5mC | N5-methylcytosine |
| 6mA | N6-methyl-adenosine |
References
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.; Carey, H.V.; Domazet-Loso, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.R.; Green, R.S.; Holzman, I.R.; Lin, J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zhao, X.; Wu, Y.S.; Li, M.M.; Wang, X.J.; Yang, Y.G. N6-methyl-adenosine (m6A) in RNA: An old modification with a novel epigenetic function. Genom. Proteom. Bioinform. 2013, 11, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Maunakea, A.K.; Chepelev, I.; Zhao, K. Epigenome mapping in normal and disease States. Circ. Res. 2010, 107, 327–339. [Google Scholar] [CrossRef]
- Mangiavacchi, A.; Morelli, G.; Orlando, V. Behind the scenes: How RNA orchestrates the epigenetic regulation of gene expression. Front. Cell Dev. Biol. 2023, 11, 1123975. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Jadhav, A.; Bajaj, A.; Xiao, Y.; Markandey, M.; Ahuja, V.; Kashyap, P.C. Role of Diet-Microbiome Interaction in Gastrointestinal Disorders and Strategies to Modulate Them with Microbiome-Targeted Therapies. Annu. Rev. Nutr. 2023, 43, 355–383. [Google Scholar] [CrossRef]
- Krishnan, S.; Alden, N.; Lee, K. Pathways and functions of gut microbiota metabolism impacting host physiology. Curr. Opin. Biotechnol. 2015, 36, 137–145. [Google Scholar] [CrossRef]
- Aravin, A.A.; Sachidanandam, R.; Girard, A.; Fejes-Toth, K.; Hannon, G.J. Developmentally Regulated piRNA Clusters Implicate MILI in Transposon Control. Science 2007, 316, 744–747. [Google Scholar] [CrossRef]
- Bourc’his, D.; Xu, G.-L.; Lin, C.-S.; Bollman, B.; Bestor, T.H. Dnmt3L and the Establishment of Maternal Genomic Imprints. Science 2001, 294, 2536–2539. [Google Scholar] [CrossRef]
- Carmell, M.A.; Girard, A.; van de Kant, H.J.G.; Bourc’his, D.; Bestor, T.H.; de Rooij, D.G.; Hannon, G.J. MIWI2 Is Essential for Spermatogenesis and Repression of Transposons in the Mouse Male Germline. Dev. Cell 2007, 12, 503–514. [Google Scholar] [CrossRef] [PubMed]
- He, X.-J.; Chen, T.; Zhu, J.-K. Regulation and function of DNA methylation in plants and animals. Cell Res. 2011, 21, 442–465. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, H.; Page, A.W.; Weier, H.-U.; Bestor, T.H. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell 1992, 71, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA Methyltransferases Dnmt3a and Dnmt3b Are Essential for De Novo Methylation and Mammalian Development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Vagin, V.V.; Wohlschlegel, J.; Qu, J.; Jonsson, Z.; Huang, X.; Chuma, S.; Girard, A.; Sachidanandam, R.; Hannon, G.J.; Aravin, A.A. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev. 2009, 23, 1749–1762. [Google Scholar] [CrossRef]
- Wu, S.C.; Zhang, Y. Active DNA demethylation: Many roads lead to Rome. Nat. Rev. Mol. Cell Biol. 2010, 11, 607–620. [Google Scholar] [CrossRef]
- Edwards, J.R.; Yarychkivska, O.; Boulard, M.; Bestor, T.H. DNA methylation and DNA methyltransferases. Epigenet. Chromatin 2017, 10, 23. [Google Scholar] [CrossRef]
- Rolando, M.; Silvestre, C.D.; Gomez-Valero, L.; Buchrieser, C. Bacterial methyltransferases: From targeting bacterial genomes to host epigenetics. Microlife 2022, 3, uqac014. [Google Scholar]
- Beaulaurier, J.; Schadt, E.E.; Fang, G. Deciphering bacterial epigenomes using modern sequencing technologies. Nat. Rev. Genet. 2019, 20, 157–172. [Google Scholar] [CrossRef]
- Stojkovic, V.; Fujimori, D.G. Mutations in RNA methylating enzymes in disease. Curr. Opin. Chem. Biol. 2017, 41, 20–27. [Google Scholar] [CrossRef]
- Chernov, A.V.; Vollmayr, P.; Walter, J.; Trautner, T.A. Masc2, a C5-DNA-methyltransferase from Ascobolus immersus with similarity to methyltransferases of higher organisms. Biol. Chem. 1997, 378, 1467–1473. [Google Scholar] [CrossRef]
- Nai, Y.S.; Huang, Y.C.; Yen, M.R.; Chen, P.Y. Diversity of Fungal DNA Methyltransferases and Their Association With DNA Methylation Patterns. Front. Microbiol. 2020, 11, 616922. [Google Scholar] [CrossRef] [PubMed]
- Mondo, S.J.; Dannebaum, R.O.; Kuo, R.C.; Louie, K.B.; Bewick, A.J.; LaButti, K.; Haridas, S.; Kuo, A.; Salamov, A.; Ahrendt, S.R.; et al. Widespread adenine N6-methylation of active genes in fungi. Nat. Genet. 2017, 49, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA Methylation. PLoS Genet. 2020, 16, e1009034. [Google Scholar] [CrossRef] [PubMed]
- Law, J.A.; Jacobsen, S.E. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 2010, 11, 204–220. [Google Scholar] [CrossRef]
- Gallego-Bartolomé, J. DNA methylation in plants: Mechanisms and tools for targeted manipulation. New Phytol. 2020, 227, 38–44. [Google Scholar] [CrossRef]
- Lucibelli, F.; Valoroso, M.C.; Aceto, S. Plant DNA Methylation: An Epigenetic Mark in Development, Environmental Interactions, and Evolution. Int. J. Mol. Sci. 2022, 23, 8299. [Google Scholar] [CrossRef]
- Matzke, M.A.; Mosher, R.A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef]
- Pastor, W.A.; Aravind, L.; Rao, A. TETonic shift: Biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 2013, 14, 341–356. [Google Scholar] [CrossRef]
- Motorin, Y.; Helm, M. RNA nucleotide methylation. WIREs RNA 2011, 2, 611–631. [Google Scholar] [CrossRef] [PubMed]
- Satterwhite, E.R.; Mansfield, K.D. RNA methyltransferase METTL16: Targets and function. Wiley Interdiscip. Rev. RNA 2022, 13, e1681. [Google Scholar] [CrossRef] [PubMed]
- Woo, V.; Alenghat, T. Epigenetic regulation by gut microbiota. Gut Microbes 2022, 14, 2022407. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wu, R.; Ming, L. The role of m6A RNA methylation in cancer. Biomed. Pharmacother. 2019, 112, 108613. [Google Scholar] [CrossRef]
- Goll, M.G.; Bestor, T.H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 2005, 74, 481–514. [Google Scholar] [CrossRef]
- Goll, M.G.; Kirpekar, F.; Maggert, K.A.; Yoder, J.A.; Hsieh, C.-L.; Zhang, X.; Golic, K.G.; Jacobsen, S.E.; Bestor, T.H. Methylation of tRNAAsp by the DNA Methyltransferase Homolog Dnmt2. Science 2006, 311, 395–398. [Google Scholar] [CrossRef]
- Meissner, A.; Gnirke, A.; Bell, G.W.; Ramsahoye, B.; Lander, E.S.; Jaenisch, R. Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation analysis. Nucleic Acids Res. 2005, 33, 5868–5877. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Li, L.; Gong, D.; Yao, Y.; Luo, H.; Lu, H.; Yi, N.; Wu, H.; Zhang, X.; et al. Double restriction-enzyme digestion improves the coverage and accuracy of genome-wide CpG methylation profiling by reduced representation bisulfite sequencing. BMC Genom. 2013, 14, 11. [Google Scholar] [CrossRef]
- Oliveira, P.H.; Fang, G. Conserved DNA Methyltransferases: A Window into Fundamental Mechanisms of Epigenetic Regulation in Bacteria. Trends Microbiol. 2021, 29, 28–40. [Google Scholar] [CrossRef]
- Gottesman, S.; Storz, G. Bacterial small RNA regulators: Versatile roles and rapidly evolving variations. Cold Spring Harb. Perspect. Biol. 2011, 3, a003798. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; da Cunha, A.P.; Rezende, R.M.; Cialic, R.; Wei, Z.; Bry, L.; Comstock, L.E.; Gandhi, R.; Weiner, H.L. The Host Shapes the Gut Microbiota via Fecal MicroRNA. Cell Host Microbe 2016, 19, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Behrouzi, A.; Ashrafian, F.; Mazaheri, H.; Lari, A.; Nouri, M.; Riazi Rad, F.; Hoseini Tavassol, Z.; Siadat, S.D. The importance of interaction between MicroRNAs and gut microbiota in several pathways. Microb. Pathog. 2020, 144, 104200. [Google Scholar] [CrossRef] [PubMed]
- Capuano, F.; Mulleder, M.; Kok, R.; Blom, H.J.; Ralser, M. Cytosine DNA methylation is found in Drosophila melanogaster but absent in Saccharomyces cerevisiae, Schizosaccharomyces pombe, and other yeast species. Anal. Chem. 2014, 86, 3697–3702. [Google Scholar] [CrossRef]
- Raimondi, S.; Amaretti, A.; Gozzoli, C.; Simone, M.; Righini, L.; Candeliere, F.; Brun, P.; Ardizzoni, A.; Colombari, B.; Paulone, S.; et al. Longitudinal Survey of Fungi in the Human Gut: ITS Profiling, Phenotyping, and Colonization. Front. Microbiol. 2019, 10, 1575. [Google Scholar] [CrossRef]
- Zhong, X.; Du, J.; Hale, C.J.; Gallego-Bartolome, J.; Feng, S.; Vashisht, A.A.; Chory, J.; Wohlschlegel, J.A.; Patel, D.J.; Jacobsen, S.E. Molecular mechanism of action of plant DRM de novo DNA methyltransferases. Cell 2014, 157, 1050–1060. [Google Scholar] [CrossRef]
- Wierzbicki, A.T.; Haag, J.R.; Pikaard, C.S. Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 2008, 135, 635–648. [Google Scholar] [CrossRef]
- He, X.J.; Hsu, Y.F.; Zhu, S.; Wierzbicki, A.T.; Pontes, O.; Pikaard, C.S.; Liu, H.L.; Wang, C.S.; Jin, H.; Zhu, J.K. An effector of RNA-directed DNA methylation in arabidopsis is an ARGONAUTE 4- and RNA-binding protein. Cell 2009, 137, 498–508. [Google Scholar] [CrossRef]
- Wierzbicki, A.T.; Ream, T.S.; Haag, J.R.; Pikaard, C.S. RNA polymerase V transcription guides ARGONAUTE4 to chromatin. Nat. Genet. 2009, 41, 630–634. [Google Scholar] [CrossRef]
- Zilberman, D.; Cao, X.; Jacobsen, S.E. ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 2003, 299, 716–719. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, J.; Kapoor, A.; Zhu, J.K. Role of Arabidopsis AGO6 in siRNA accumulation, DNA methylation and transcriptional gene silencing. EMBO J. 2007, 26, 1691–1701. [Google Scholar] [CrossRef]
- Klughammer, J.; Romanovskaia, D.; Nemc, A.; Posautz, A.; Seid, C.A.; Schuster, L.C.; Keinath, M.C.; Lugo Ramos, J.S.; Kosack, L.; Evankow, A.; et al. Comparative analysis of genome-scale, base-resolution DNA methylation profiles across 580 animal species. Nat. Commun. 2023, 14, 232. [Google Scholar] [CrossRef]
- Wang, S.; Lv, W.; Li, T.; Zhang, S.; Wang, H.; Li, X.; Wang, L.; Ma, D.; Zang, Y.; Shen, J.; et al. Dynamic regulation and functions of mRNA m6A modification. Cancer Cell Int. 2022, 22, 48. [Google Scholar] [CrossRef]
- Wang, X.; He, C. Reading RNA methylation codes through methyl-specific binding proteins. RNA Biol. 2014, 11, 669–672. [Google Scholar] [CrossRef] [PubMed]
- O’Brown, Z.K.; Greer, E.L. N6-Methyladenine: A Conserved and Dynamic DNA Mark. Adv. Exp. Med. Biol. 2016, 945, 213–246. [Google Scholar] [PubMed]
- Maunakea, A.K.; Phankitnirundorn, K.; Peres, R.; Dye, C.; Juarez, R.; Walsh, C.; Slavens, C.; Park, S.L.; Wilkens, L.R.; Le Marchand, L. Socioeconomic Status, Lifestyle, and DNA Methylation Age Among Racially and Ethnically Diverse Adults: NIMHD Social Epigenomics Program. JAMA Netw. Open 2024, 7, e2421889. [Google Scholar] [CrossRef] [PubMed]
- Maunakea, A.K.; Nagarajan, R.P.; Bilenky, M.; Ballinger, T.J.; D’Souza, C.; Fouse, S.D.; Johnson, B.E.; Hong, C.; Nielsen, C.; Zhao, Y.; et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature 2010, 466, 253–257. [Google Scholar] [CrossRef]
- Maunakea, A.K.; Chepelev, I.; Cui, K.; Zhao, K. Intragenic DNA methylation modulates alternative splicing by recruiting MeCP2 to promote exon recognition. Cell Res. 2013, 23, 1256–1269. [Google Scholar] [CrossRef]
- Ohgane, J.; Yagi, S.; Shiota, K. Epigenetics: The DNA Methylation Profile of Tissue-Dependent and Differentially Methylated Regions in Cells. Placenta 2008, 29, 29–35. [Google Scholar] [CrossRef]
- Cappannini, A.; Ray, A.; Purta, E.; Mukherjee, S.; Boccaletto, P.; Moafinejad, S.N.; Lechner, A.; Barchet, C.; Klaholz, B.P.; Stefaniak, F.; et al. MODOMICS: A database of RNA modifications and related information. 2023 update. Nucleic Acids Res. 2023, 52, D239–D244. [Google Scholar] [CrossRef]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B. Biomarkers of Nutrient Exposure and Status in One-Carbon (Methyl) Metabolism. J. Nutr. 2003, 133, 941S–947S. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Amaretti, A.; Raimondi, S. Folate production by probiotic bacteria. Nutrients 2011, 3, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Chun, B.H.; Kim, J.; Jeon, C.O. Identification of biogenic amine-producing microbes during fermentation of ganjang, a Korean traditional soy sauce, through metagenomic and metatranscriptomic analyses. Food Control 2021, 121, 107681. [Google Scholar] [CrossRef]
- Liu, C.; Wang, G.; Han, X.; Cao, L.; Wang, K.; Lin, H.; Sui, J. Heterologous expression and activity verification of ornithine decarboxylase from a wild strain of Shewanella xiamenensis. Front. Microbiol. 2022, 13, 1100889. [Google Scholar] [CrossRef]
- Xiao, M.; Yang, H.; Xu, W.; Ma, S.; Lin, H.; Zhu, H.; Liu, L.; Liu, Y.; Yang, C.; Xu, Y.; et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012, 26, 1326–1338. [Google Scholar] [CrossRef]
- King, A.; Selak, M.A.; Gottlieb, E. Succinate dehydrogenase and fumarate hydratase: Linking mitochondrial dysfunction and cancer. Oncogene 2006, 25, 4675–4682. [Google Scholar] [CrossRef]
- Letouzé, E.; Martinelli, C.; Loriot, C.; Burnichon, N.; Abermil, N.; Ottolenghi, C.; Janin, M.; Menara, M.; Nguyen, A.T.; Benit, P.; et al. SDH Mutations Establish a Hypermethylator Phenotype in Paraganglioma. Cancer Cell 2013, 23, 739–752. [Google Scholar] [CrossRef]
- Elfoul, L.; Rabot, S.; Khelifa, N.; Quinsac, A.; Duguay, A.; Rimbault, A. Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol. Lett. 2001, 197, 99–103. [Google Scholar] [CrossRef]
- Liu, Y.; Chakravarty, S.; Dey, M. Phenethylisothiocyanate alters site- and promoter-specific histone tail modifications in cancer cells. PLoS ONE 2013, 8, e64535. [Google Scholar] [CrossRef]
- Kyung Lee, M.; Armstrong, D.A.; Hazlett, H.F.; Dessaint, J.A.; Mellinger, D.L.; Aridgides, D.S.; Christensen, B.C.; Ashare, A. Exposure to extracellular vesicles from Pseudomonas aeruginosa result in loss of DNA methylation at enhancer and DNase hypersensitive site regions in lung macrophages. Epigenetics 2021, 16, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Telesford, K.M.; Yan, W.; Ochoa-Reparaz, J.; Pant, A.; Kircher, C.; Christy, M.A.; Begum-Haque, S.; Kasper, D.L.; Kasper, L.H. A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39(+)Foxp3(+) T cells and Treg function. Gut Microbes 2015, 6, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.H.; Sommer, F.; Falk-Paulsen, M.; Ulas, T.; Best, L.; Fazio, A.; Kachroo, P.; Luzius, A.; Jentzsch, M.; Rehman, A.; et al. Exposure to the gut microbiota drives distinct methylome and transcriptome changes in intestinal epithelial cells during postnatal development. Genome Med. 2018, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Ansari, I.; Raddatz, G.; Gutekunst, J.; Ridnik, M.; Cohen, D.; Abu-Remaileh, M.; Tuganbaev, T.; Shapiro, H.; Pikarsky, E.; Elinav, E.; et al. The microbiota programs DNA methylation to control intestinal homeostasis and inflammation. Nat. Microbiol. 2020, 5, 610–619. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Chen, W.; Shi, H.; Eren, A.M.; Morozov, A.; He, C.; Luo, G.Z.; Pan, T. Transcriptome-wide reprogramming of N(6)-methyladenosine modification by the mouse microbiome. Cell Res. 2019, 29, 167–170. [Google Scholar] [CrossRef]
- Jabs, S.; Biton, A.; Bécavin, C.; Nahori, M.-A.; Ghozlane, A.; Pagliuso, A.; Spanò, G.; Guérineau, V.; Touboul, D.; Giai Gianetto, Q.; et al. Impact of the gut microbiota on the m6A epitranscriptome of mouse cecum and liver. Nat. Commun. 2020, 11, 1344. [Google Scholar] [CrossRef]
- Clare, C.E.; Brassington, A.H.; Kwong, W.Y.; Sinclair, K.D. One-Carbon Metabolism: Linking Nutritional Biochemistry to Epigenetic Programming of Long-Term Development. Annu. Rev. Anim. Biosci. 2019, 7, 263–287. [Google Scholar] [CrossRef]
- Gurwara, S.; Ajami, N.J.; Jang, A.; Hessel, F.C.; Chen, L.; Plew, S.; Wang, Z.; Graham, D.Y.; Hair, C.; White, D.L.; et al. Dietary Nutrients Involved in One-Carbon Metabolism and Colonic Mucosa-Associated Gut Microbiome in Individuals with an Endoscopically Normal Colon. Nutrients 2019, 11, 613. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Rey, F.E.; Denu, J.M. Chemical signaling between gut microbiota and host chromatin: What is your gut really saying? J. Biol. Chem. 2017, 292, 8582–8593. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Beland, F.A. DNA hypomethylation in the origin and pathogenesis of human diseases. Cell. Mol. Life Sci. 2009, 66, 2249–2261. [Google Scholar] [CrossRef]
- Romano, K.A.; Martinez-Del Campo, A.; Kasahara, K.; Chittim, C.L.; Vivas, E.I.; Amador-Noguez, D.; Balskus, E.P.; Rey, F.E. Metabolic, Epigenetic, and Transgenerational Effects of Gut Bacterial Choline Consumption. Cell Host Microbe 2017, 22, 279–290.e7. [Google Scholar] [CrossRef]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44. [Google Scholar] [CrossRef]
- Matsumoto, M.; Benno, Y. The relationship between microbiota and polyamine concentration in the human intestine: A pilot study. Microbiol. Immunol. 2007, 51, 25–35. [Google Scholar] [CrossRef]
- Kibe, R.; Kurihara, S.; Sakai, Y.; Suzuki, H.; Ooga, T.; Sawaki, E.; Muramatsu, K.; Nakamura, A.; Yamashita, A.; Kitada, Y.; et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014, 4, 4548. [Google Scholar] [CrossRef]
- Fukui, T.; Soda, K.; Takao, K.; Rikiyama, T. Extracellular Spermine Activates DNA Methyltransferase 3A and 3B. Int. J. Mol. Sci. 2019, 20, 1254. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Catchpole, R.; Forterre, P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiol. Rev. 2018, 43, 273–303. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Bartold, P.M.; Salomon, C.; Ivanovski, S. Salivary Outer Membrane Vesicles and DNA Methylation of Small Extracellular Vesicles as Biomarkers for Periodontal Status: A Pilot Study. Int. J. Mol. Sci. 2021, 22, 2423. [Google Scholar] [CrossRef]
- Takahashi, K.; Sugi, Y.; Hosono, A.; Kaminogawa, S. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J. Immunol. 2009, 183, 6522–6529. [Google Scholar] [CrossRef] [PubMed]
- Pepke, M.L.; Hansen, S.B.; Limborg, M.T. Unraveling host regulation of gut microbiota through the epigenome–microbiome axis. Trends Microbiol. 2024, 32, 1229–1240. [Google Scholar] [CrossRef]
- Henry, L.P.; Bruijning, M.; Forsberg, S.K.G.; Ayroles, J.F. The microbiome extends host evolutionary potential. Nat. Commun. 2021, 12, 5141. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Becerra, C.Y.; Wells, R.K.; Kunihiro, B.P.; Lee, R.H.; Umeda, L.; Allan, N.P.; Rubas, N.C.; McCracken, T.A.; Nunokawa, C.K.L.; Lee, M.H.; et al. Examining the immunoepigenetic-gut microbiome axis in the context of self-esteem among Native Hawaiians and other Pacific Islanders. Front. Genet. 2023, 14, 1125217. [Google Scholar] [CrossRef] [PubMed]
- Rubas, N.C.; Peres, R.; Kunihiro, B.P.; Allan, N.P.; Phankitnirundorn, K.; Wells, R.K.; McCracken, T.; Lee, R.H.; Umeda, L.; Conching, A.; et al. HMGB1 mediates microbiome-immune axis dysregulation underlying reduced neutralization capacity in obesity-related post-acute sequelae of SARS-CoV-2. Sci. Rep. 2024, 14, 355. [Google Scholar]
- Blekhman, R.; Goodrich, J.K.; Huang, K.; Sun, Q.; Bukowski, R.; Bell, J.T.; Spector, T.D.; Keinan, A.; Ley, R.E.; Gevers, D.; et al. Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 2015, 16, 191. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Davenport, E.R.; Beaumont, M.; Jackson, M.A.; Knight, R.; Ober, C.; Spector, T.D.; Bell, J.T.; Clark, A.G.; Ley, R.E. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe 2016, 19, 731–743. [Google Scholar] [CrossRef]
- Khachatryan, Z.A.; Ktsoyan, Z.A.; Manukyan, G.P.; Kelly, D.; Ghazaryan, K.A.; Aminov, R.I. Predominant Role of Host Genetics in Controlling the Composition of Gut Microbiota. PLoS ONE 2008, 3, e3064. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Stella, A.; Bonfrate, L.; Wang, D.Q.H.; Portincasa, P. Gut Microbiota between Environment and Genetic Background in Familial Mediterranean Fever (FMF). Genes 2020, 11, 1041. [Google Scholar] [CrossRef]
- Bubier, J.A.; Chesler, E.J.; Weinstock, G.M. Host genetic control of gut microbiome composition. Mamm. Genome 2021, 32, 263–281. [Google Scholar] [CrossRef]
- van Lanen, A.S.; de Bree, A.; Greyling, A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: A systematic review and meta-analysis. Eur. J. Nutr. 2021, 60, 3505–3522. [Google Scholar] [CrossRef]
- Khoo, X.H.; Chong, C.W.; Talha, A.M.; Philip, K.; Teh, C.S.; Isa, A.M.; Wong, M.S.; Chew, D.C.; Wong, Z.; Jusoh, N.S.; et al. The impact of diet and ethnicity on gut microbiota variation in irritable bowel syndrome: A multi-center study. J. Gastroenterol. Hepatol. 2023, 38, 1259–1268. [Google Scholar] [CrossRef]
- Allan, N.P.; Yamamoto, B.Y.; Kunihiro, B.P.; Nunokawa, C.K.L.; Rubas, N.C.; Wells, R.K.; Umeda, L.; Phankitnirundorn, K.; Torres, A.; Peres, R.; et al. Ketogenic Diet Induced Shifts in the Gut Microbiome Associate with Changes to Inflammatory Cytokines and Brain-Related miRNAs in Children with Autism Spectrum Disorder. Nutrients 2024, 16, 1401. [Google Scholar] [CrossRef]
- Ciccarone, F.; Tagliatesta, S.; Caiafa, P.; Zampieri, M. DNA methylation dynamics in aging: How far are we from understanding the mechanisms? Mech. Ageing Dev. 2018, 174, 3–17. [Google Scholar] [CrossRef]
- Wells, R.K.; Kunihiro, B.P.; Phankitnirundorn, K.; Peres, R.; McCracken, T.A.; Umeda, L.; Lee, R.H.; Kim, D.Y.; Juarez, R.; Maunakea, A.K. Gut microbial indicators of metabolic health underlie age-related differences in obesity and diabetes risk among Native Hawaiians and Pacific Islanders. Front. Cell. Infect. Microbiol. 2022, 12, 1035641. [Google Scholar] [CrossRef]
- Ostman, S.; Rask, C.; Wold, A.E.; Hultkrantz, S.; Telemo, E. Impaired regulatory T cell function in germ-free mice. Eur. J. Immunol. 2006, 36, 2336–2346. [Google Scholar] [CrossRef]
- Li, J.; Xu, B.; He, M.; Zong, X.; Cunningham, T.; Sha, C.; Fan, Y.; Cross, R.; Hanna, J.H.; Feng, Y. Control of Foxp3 induction and maintenance by sequential histone acetylation and DNA demethylation. Cell Rep. 2021, 37, 110124. [Google Scholar] [CrossRef]
- Koshida, K.; Ito, M.; Yakabe, K.; Takahashi, Y.; Tai, Y.; Akasako, R.; Kimizuka, T.; Takano, S.; Sakamoto, N.; Haniuda, K.; et al. Dysfunction of Foxp3(+) Regulatory T Cells Induces Dysbiosis of Gut Microbiota via Aberrant Binding of Immunoglobulins to Microbes in the Intestinal Lumen. Int. J. Mol. Sci. 2023, 24, 8549. [Google Scholar] [CrossRef] [PubMed]
- Toker, A.; Engelbert, D.; Garg, G.; Polansky, J.K.; Floess, S.; Miyao, T.; Baron, U.; Düber, S.; Geffers, R.; Giehr, P.; et al. Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J. Immunol. 2013, 190, 3180–3188. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan Nair, V.; Song, M.H.; Oh, K.I. Vitamin C Facilitates Demethylation of the Foxp3 Enhancer in a Tet-Dependent Manner. J. Immunol. 2016, 196, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Kehrmann, J.; Effenberg, L.; Wilk, C.; Schoemer, D.; Ngo Thi Phuong, N.; Adamczyk, A.; Pastille, E.; Scholtysik, R.; Klein-Hitpass, L.; Klopfleisch, R.; et al. Depletion of Foxp3(+) regulatory T cells is accompanied by an increase in the relative abundance of Firmicutes in the murine gut microbiome. Immunology 2020, 159, 344–353. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Li, Z.; Li, D.; Tsun, A.; Li, B. FOXP3+ regulatory T cells and their functional regulation. Cell. Mol. Immunol. 2015, 12, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Hang, S.; Paik, D.; Yao, L.; Kim, E.; Trinath, J.; Lu, J.; Ha, S.; Nelson, B.N.; Kelly, S.P.; Wu, L.; et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature 2019, 576, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Li, H.; Anjum, K.; Zhong, X.; Miao, S.; Zheng, G.; Liu, W.; Li, L. Dual Role of Indoles Derived From Intestinal Microbiota on Human Health. Front. Immunol. 2022, 13, 903526. [Google Scholar] [CrossRef]
- Lyko, F.; Ramsahoye, B.H.; Jaenisch, R. DNA methylation in Drosophila melanogaster. Nature 2000, 408, 538–540. [Google Scholar] [CrossRef]
- De Ste Croix, M.; Vacca, I.; Kwun, M.J.; Ralph, J.D.; Bentley, S.D.; Haigh, R.; Croucher, N.J.; Oggioni, M.R. Phase-variable methylation and epigenetic regulation by type I restriction-modification systems. FEMS Microbiol. Rev. 2017, 41 (Suppl. S1), S3–S15. [Google Scholar] [CrossRef]
- Phillips, Z.N.; Husna, A.U.; Jennings, M.P.; Seib, K.L.; Atack, J.M. Phasevarions of bacterial pathogens–phase-variable epigenetic regulators evolving from restriction-modification systems. Microbiology 2019, 165, 917–928. [Google Scholar] [CrossRef]
- Anjum, A.; Brathwaite, K.J.; Aidley, J.; Connerton, P.L.; Cummings, N.J.; Parkhill, J.; Connerton, I.; Bayliss, C.D. Phase variation of a Type IIG restriction-modification enzyme alters site-specific methylation patterns and gene expression in Campylobacter jejuni strain NCTC11168. Nucleic Acids Res. 2016, 44, 4581–4594. [Google Scholar] [CrossRef]
- Fox, K.L.; Srikhanta, Y.N.; Jennings, M.P. Phase variable type III restriction-modification systems of host-adapted bacterial pathogens. Mol. Microbiol. 2007, 65, 1375–1379. [Google Scholar] [CrossRef]
- Seib, K.L.; Srikhanta, Y.N.; Atack, J.M.; Jennings, M.P. Epigenetic Regulation of Virulence and Immunoevasion by Phase-Variable Restriction-Modification Systems in Bacterial Pathogens. Annu. Rev. Microbiol. 2020, 74, 655–671. [Google Scholar] [CrossRef]
- Sharma, P.V.; Thaiss, C.A. Host-Microbiome Interactions in the Era of Single-Cell Biology. Front. Cell. Infect. Microbiol. 2020, 10, 569070. [Google Scholar] [CrossRef]
- Malecki, K.M.C.; Nikodemova, M.; Schultz, A.A.; LeCaire, T.J.; Bersch, A.J.; Cadmus-Bertram, L.; Engelman, C.D.; Hagen, E.; Palta, M.; Sethi, A.K.; et al. The Survey of the Health of Wisconsin (SHOW) Program: An infrastructure for Advancing Population Health Sciences. Front. Public Health 2022, 10, 818777. [Google Scholar] [CrossRef]
- Kumar, M.; Saadaoui, M.; Elhag, D.A.; Murugesan, S.; Al Abduljabbar, S.; Fagier, Y.; Ortashi, O.; Abdullahi, H.; Ibrahim, I.; Alberry, M.; et al. Omouma: A prospective mother and child cohort aiming to identify early biomarkers of pregnancy complications in women living in Qatar. BMC Pregnancy Childbirth 2021, 21, 570. [Google Scholar] [CrossRef]
- Levy, J.J.; Titus, A.J.; Petersen, C.L.; Chen, Y.; Salas, L.A.; Christensen, B.C. MethylNet: An automated and modular deep learning approach for DNA methylation analysis. BMC Bioinform. 2020, 21, 108. [Google Scholar] [CrossRef]
- Angermueller, C.; Lee, H.J.; Reik, W.; Stegle, O. DeepCpG: Accurate prediction of single-cell DNA methylation states using deep learning. Genome Biol. 2017, 18, 67. [Google Scholar]
- Velten, B.; Braunger, J.M.; Argelaguet, R.; Arnol, D.; Wirbel, J.; Bredikhin, D.; Zeller, G.; Stegle, O. Identifying temporal and spatial patterns of variation from multimodal data using MEFISTO. Nat. Methods 2022, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell. Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Barrila, J.; Crabbé, A.; Yang, J.; Franco, K.; Nydam, S.D.; Forsyth, R.J.; Davis, R.R.; Gangaraju, S.; Ott, C.M.; Coyne, C.B.; et al. Modeling Host-Pathogen Interactions in the Context of the Microenvironment: Three-Dimensional Cell Culture Comes of Age. Infect. Immun. 2018, 86, e00282-18. [Google Scholar] [CrossRef]
- Jalili-Firoozinezhad, S.; Gazzaniga, F.S.; Calamari, E.L.; Camacho, D.M.; Fadel, C.W.; Bein, A.; Swenor, B.; Nestor, B.; Cronce, M.J.; Tovaglieri, A.; et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 2019, 3, 520–531. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.; Choi, J.H.; Bahinski, A.; Ingber, D.E. Co-culture of Living Microbiome with Microengineered Human Intestinal Villi in a Gut-on-a-Chip Microfluidic Device. J. Vis. Exp. 2016, 114, 54344. [Google Scholar]
- Xiang, Y.; Wen, H.; Yu, Y.; Li, M.; Fu, X.; Huang, S. Gut-on-chip: Recreating human intestine in vitro. J. Tissue Eng. 2020, 11, 2041731420965318. [Google Scholar] [CrossRef] [PubMed]
- Valiei, A.; Aminian-Dehkordi, J.; Mofrad, M.R.K. Gut-on-a-chip models for dissecting the gut microbiology and physiology. APL Bioeng. 2023, 7, 011502. [Google Scholar] [CrossRef] [PubMed]
- Marrero, D.; Pujol-Vila, F.; Vera, D.; Gabriel, G.; Illa, X.; Elizalde-Torrent, A.; Alvarez, M.; Villa, R. Gut-on-a-chip: Mimicking and monitoring the human intestine. Biosens. Bioelectron. 2021, 181, 113156. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, S.V.; Deplancke, B.; Gaskins, H.R.; Apicella, M.A.; McFall-Ngai, M.J. Roles of Vibrio fischeri and Nonsymbiotic Bacteria in the Dynamics of Mucus Secretion during Symbiont Colonization of the Euprymna scolopes Light Organ. Appl. Environ. Microbiol. 2002, 68, 5113–5122. [Google Scholar] [CrossRef]
- Koropatnick, T.A.; Kimbell, J.R.; McFall-Ngai, M.J. Responses of host hemocytes during the initiation of the squid-Vibrio symbiosis. Biol. Bull. 2007, 212, 29–39. [Google Scholar] [CrossRef]
- Rader, B.A.; Nyholm, S.V. Host/microbe interactions revealed through “omics” in the symbiosis between the Hawaiian bobtail squid Euprymna scolopes and the bioluminescent bacterium Vibrio fischeri. Biol. Bull. 2012, 223, 103–111. [Google Scholar] [CrossRef]
- Macchi, F.; Edsinger, E.; Sadler, K.C. Epigenetic machinery is functionally conserved in cephalopods. BMC Biol. 2022, 20, 202. [Google Scholar] [CrossRef]
- Calvez, V.; Puca, P.; Di Vincenzo, F.; Del Gaudio, A.; Bartocci, B.; Murgiano, M.; Iaccarino, J.; Parand, E.; Napolitano, D.; Pugliese, D.; et al. Novel Insights into the Pathogenesis of Inflammatory Bowel Diseases. Biomedicines 2025, 13, 305. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Minkoff, N.Z.; Aslam, S.; Medina, M.; Tanner-Smith, E.E.; Zackular, J.P.; Acra, S.; Nicholson, M.R.; Imdad, A. Fecal microbiota transplantation for the treatment of recurrent Clostridioides difficile (Clostridium difficile). Cochrane Database Syst. Rev. 2023, 4, Cd013871. [Google Scholar]
- Drekonja, D.M.; Shaukat, A.; Huang, Y.; Zhang, J.H.; Reinink, A.R.; Nugent, S.; Dominitz, J.A.; Davis-Karim, A.; Gerding, D.N.; Kyriakides, T.C. A Randomized Controlled Trial of Efficacy and Safety of Fecal Microbiota Transplant for Preventing Recurrent Clostridioides difficile Infection. Clin. Infect. Dis. 2024, 80, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Visekruna, A.; Luu, M. The Role of Short-Chain Fatty Acids and Bile Acids in Intestinal and Liver Function, Inflammation, and Carcinogenesis. Front. Cell Dev. Biol. 2021, 9, 703218. [Google Scholar] [CrossRef] [PubMed]
- Sanapareddy, N.; Legge, R.M.; Jovov, B.; McCoy, A.; Burcal, L.; Araujo-Perez, F.; Randall, T.A.; Galanko, J.; Benson, A.; Sandler, R.S.; et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012, 6, 1858–1868. [Google Scholar] [CrossRef]
- iHMP. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef]
- Knights, D.; Ward, T.L.; McKinlay, C.E.; Miller, H.; Gonzalez, A.; McDonald, D.; Knight, R. Rethinking “Enterotypes”. Cell Host Microbe 2014, 16, 433–437. [Google Scholar] [CrossRef]
- Pallmann, P.; Bedding, A.W.; Choodari-Oskooei, B.; Dimairo, M.; Flight, L.; Hampson, L.V.; Holmes, J.; Mander, A.P.; Odondi, L.O.; Sydes, M.R.; et al. Adaptive designs in clinical trials: Why use them, and how to run and report them. BMC Med. 2018, 16, 29. [Google Scholar] [CrossRef]
- Stunnenberg, H.G.; Abrignani, S.; Adams, D.; de Almeida, M.; Altucci, L.; Amin, V.; Amit, I.; Antonarakis, S.E.; Aparicio, S.; Arima, T.; et al. The International Human Epigenome Consortium: A Blueprint for Scientific Collaboration and Discovery. Cell 2016, 167, 1145–1149. [Google Scholar] [CrossRef]
- Barker, P. Epigenetics in Cancer Prevention: Early Detection and Risk Assessment. In Cancer Biomarker Validation: Standards and Process—Roles for the National Institute of Standards and Technology (NIST); Wiley: Bethesda, MD, USA, 2003; Available online: https://quicksearch.lib.iastate.edu/discovery/fulldisplay?vid=01IASU_INST:01IASU&docid=alma990014466610102756&lang=en&context=L&adaptor=Local%20Search%20Engine (accessed on 19 July 2025).
- Mason, C.; Afshinnekoo, E.; Ahsannudin, S.; Ghedin, E.; Read, T.; Fraser, C.; Dudley, J.; Hernandez, M.; Bowler, C.; Stolovitzky, G.; et al. The Metagenomics and Metadesign of the Subways and Urban Biomes (MetaSUB) International Consortium inaugural meeting report. Microbiome 2016, 4, 24. [Google Scholar]
- Wilkinson, M.D.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef]
- Carroll, S.R.; Garba, I.; Figueroa-Rodriguez, O.L.; Holbrook, J.; Lovett, R.; Materechera, S.; Parsons, M.; Raseroka, K.; Rodruiguez-Lonebear, D.; Rowe, R.; et al. The CARE principles for Indigenous data governance. Data Sci. J. 2020, 19, 43. [Google Scholar] [CrossRef]
- Schriml, L.M.; Chuvochina, M.; Davies, N.; Eloe-Fadrosh, E.A.; Finn, R.D.; Hugenholtz, P.; Hunter, C.I.; Hurwitz, B.L.; Kyrpides, N.C.; Meyer, F.; et al. COVID-19 pandemic reveals the peril of ignoring metadata standards. Sci. Data 2020, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.; Bartholomew, K.; Sandiford, P.; Crengle, S. Te Oranga Pūkahukahu research programme: Intentional steps towards a national equity-focused lung cancer screening programme in Aotearoa New Zealand. J. R. Soc. N. Z. 2025, 55, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Silk, E.T.; Bayer, S.B.; Foster, M.; Roy, N.C.; Taylor, M.W.; Vatanen, T.; Gearry, R.B. Advancing microbiome research in Māori populations: Insights from recent literature exploring the gut microbiomes of underrepresented and Indigenous peoples. mSystems 2024, 9, e0090924. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Coe, R.R.; Lesueur, R.; Kenny, R.; Price, R.; Makela, N.; Birch, P.H. Indigenous Peoples and genomics: Starting a conversation. J. Genet. Couns. 2019, 28, 407–418. [Google Scholar] [CrossRef]
- McWhirter, R.E.; Mununggirritj, D.; Marika, D.; Dickinson, J.L.; Condon, J.R. Ethical genetic research in Indigenous communities: Challenges and successful approaches. Trends Mol. Med. 2012, 18, 702–708. [Google Scholar] [CrossRef]
- Valiani, A.A. Frontiers of Bio-Decolonization: Indigenous Data Sovereignty as a Possible Model for Community-Based Participatory Genomic Health Research for Racialized Peoples in Postgenomic Canada. Genealogy 2022, 6, 68. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).