Cannabinoid Receptor 2 (CB2) in Macrophages: A Promising Clinical Target for Immune Disorders
Abstract
1. Introduction
2. Macrophages in Health and Disease
2.1. General Roles and Functions of Macrophages
2.2. Macrophage Polarisation
2.3. Dysfunction and Disruption of Macrophages in Disease
3. Cannabinoid Receptor 2 (CB2) Molecular Pharmacology
3.1. Endocannabinoid System
3.2. Exogenous Cannabinoids
| Compound Name(s) | Description (Compounds Are Synthetic, Unless Indicated) | CB2 Affinity ▲ (or Functional Potency ◆) | CB2 > CB1 Binding ■ (or Functional ◆) Fold Selectivity |
|---|---|---|---|
| Anandamide (AEA) | Endocannabinoid non-selective partial agonist | 250 nM | 0.6 ° |
| 2-Arachidonoylglycerol (2-AG) | Endocannabinoid non-selective agonist | 400 nM | 2.0 ° |
| Δ9-Tetrahydrocannabinol (THC) | Cannabis-derived non-selective partial agonist | 20 nM | 0.7 ° |
| Cannabidiol (CBD) | Cannabis-derived non-selective context-dependent (allosteric? [116]) partial agonist or inverse agonist | 3770 nM [117] | 1.8 ° [117] |
| AM1241 (Racemic mix [Rac.] vs. R/S enantiomer often unspecified) | CB2-selective partial (protean? [118]) agonist | Rac. 10 nM [R] 7.9 nM [S] 250 nM [118] | Rac. 32–350 [R] 38–330 [S] >15–150 ° [119,120] |
| β-(E/trans)-Caryophyllene (BCP) | CB2-selective agonist | 160 nM [121] | >63 [121] |
| CP55,940 | Non-selective agonist | 2.0 nM | 1.5 ° |
| GP1a | CB2-selective agonist | 0.037 nM m [122] | 9800 m [122] |
| GW405833 | CB2-selective partial agonist | 3.9 nM [123] | 1200 [123] |
| GW833972A | CB2-selective agonist | 50 nM ◆ [124] | 630 ◆ [124] |
| HU308 | CB2-selective agonist | 32 nM | >360 |
| JWH-015 | Slightly CB2-selective agonist | 13 nM | 28 ° |
| JWH-133 | CB2-selective agonist | 16 nM | 130 ° |
| MDA7 (NTRX-07) (racemic mix) | Slightly CB2-selective agonist | 500 nM [125] | >24 [125] |
| NESS400 (GP2a) | Slightly CB2-selective agonist | 7.6 nM m [122] | 73 m [122] |
| O-1966 (0-1966[-A]) | CB2-selective agonist | 23 nM [126] | 220 [126]. |
| O-2137 (racemic mix of O-1966 and O-1967) | CB2-selective agonist | 10 nM [127] | 240 [127] |
| WIN55,212-2 | Non-selective agonist | 1.3 nM | 8.9 ° |
| AM630 | CB2-selective (protean? [128]) inverse agonist | 32 nM | 120 ° |
| SMM-189 | Slightly CB2-selective (non-competitive? [129]) inverse agonist | 120 nM [129] | 39 [129] |
| SR144528 (SR2) | CB2-selective inverse agonist | 7.9 nM | >960 |
3.3. Cannabinoid Receptor 2 (CB2) Activation and Signalling
3.4. Beyond Canonical Signalling: Biased Signalling and Allosteric Modulators
4. Regulation of CB2 Expression in Macrophages During Immune Responses
4.1. Regulation of CB2 Expression in Response to Inflammatory Stimuli
4.2. Regulation of CB2 Expression in Inflammatory Diseases
5. CB2-Mediated Modulation of Inflammatory Response in Macrophages
5.1. Phagocytosis and Antigen Presentation
5.2. Inflammasome and Autophagy
5.3. Migration
5.4. Cytokine Secretion
5.5. Differentiation and Polarisation
6. Therapeutic Effects Mediated by CB2 Activation on Macrophages
6.1. Infectious Diseases
6.2. Neuropathic Pain and Nerve Injury
6.3. Neuroinflammatory and Neurodegenerative Disorders
6.4. Autoimmune Diseases
6.5. Non-Neuronal Tissue Injury and Chronic Inflammation
6.6. Cancer and Tumour Microenvironment
7. Clinical Evaluation of Cannabinoids
7.1. Cannabis-Derived Compounds
| Drug Name | Description | Approved Uses | Earliest Approval Year |
|---|---|---|---|
| Dronabinol (Marinol®) | Synthetic THC (tetrahydrocannabinol) | Chemotherapy-induced nausea and vomiting (CINV), HIV/AIDS-induced anorexia | 1985 (CINV), 1992 (HIV/AIDS) |
| Nabilone (Cesamet®) | Synthetic cannabinoid, THC analogue | Chemotherapy-induced nausea and vomiting | 1985 |
| Nabiximols (Sativex®) | Cannabis-derived THC and CBD combination | Multiple sclerosis (MS)-related muscle spasticity and neuropathic pain | 2010 |
| Syndros® | Oral solution form of Dronabinol | Chemotherapy-induced nausea and vomiting, HIV/AIDS-induced anorexia | 2016 |
| Epidiolex® | Purified CBD oral solution | Epilepsy (Lennox–Gastaut syndrome, Dravet syndrome, tuberous sclerosis complex) | 2018 |
7.2. CB2-Selective Compounds
| Drug Name ° | CB2 > CB1 Selectivity [113,115] ■ | Indication | Phase Complete | Reported Outcome | Year Reported | Sponsor |
|---|---|---|---|---|---|---|
| Lenabasum * | 12 Δ | Neuropathic Pain | 2 | Significant reduction in pain score vs. placebo [337]. | 2003 | Atlantic Tech./Indevus Pharm. |
| Cannabinor (PRS-211,375) | ~320 ◆ | Nociceptive Pain | 2 | Reduced pain vs. placebo at lowest but not two higher doses [338]. | 2007 | Pharmos |
| GW842166 | >270 ◆ | Osteoarthritis Pain | 2 | No significant pain relief compared to placebo [339]. | 2008 | Glaxo-SmithKline |
| S-777469 | ~130 | Atopic Dermatitis | 2 | No significant effect on physician’s global assessment compared to placebo [340,341]. | 2009 | Shionogi |
| Tedalinab (GRC 10693) | >4630 ◆ | Neuropathic/Inflammatory Pain | 1 | No significant or serious adverse events [342]. | 2009 | Glenmark Pharmaceuticals |
| GW842166 | >270 ◆ | Post-surgical Pain | 2 | No significant pain relief compared to placebo [343]. | 2011 | Glaxo-SmithKline |
| AZD-1940 (ART-27.13) | 13 ⯌ | Post-surgical/Acute Pain | 2 | No effects on pain scores in two trials. Adverse events consistent with CNS activity [344,345]. | 2013 | AstraZeneca |
| KHK6188 | (not disclosed) | Neuropathic Pain | 2 | Highest dose effective in relieving postherpetic neuralgia [346]. | 2015 | Kyowa Hakko Kirin |
| LY2828360 | 32 ◆ | Osteoarthritis Pain | 2 | No significant change in primary pain score. Potential effects on two exploratory pain models [347,348]. | 2020 | Eli Lilly |
| Etrinabdione (EHP-101, VCE-004.8) | >230 Δ | Multiple (MS) and Systemic Sclerosis (SSc) | 1 (2) | Two phase 2 trials initiated but suspended for commercial reasons [349,350]. | 2020 | Emerald Health |
| Lenabasum * | 0.1–33 Δ,⯌ [351] | Cystic Fibrosis | 2 | 2a: No change in forced expiratory volume, but reduced inflammatory markers at highest dose [352]. 2b: Pulmonary exacerbation primary/secondary endpoints not met [353,354]. | 2021 | Corbus Pharmaceuticals |
| Lenabasum * | 0.1–33 Δ,⯌ [351] | Systemic Lupus Erythematosus (SLE) | 2 | No significant differences in pain or disease activity outcome measures [355]. | 2022 | National Inst. Allergy & Infectious Diseases |
| Lenabasum * | 0.1–33 Δ,⯌ [351] | Dermatomyositis | 3 | Did not meet primary or secondary endpoints, but decreased inflammatory markers [356,357]. Improvement in muscle strength and rash in subgroup analysis. | 2023 | Corbus Pharmaceuticals |
| Lenabasum * | 0.1–33 Δ,⯌ [351] | Systemic Sclerosis (SSc) | 3 | With background immunosuppressive treatment (IST), no significant effect on CRISS score. Trend toward improvement vs. placebo without IST [358]. | 2023 | Corbus Pharmaceuticals |
| Olorinab (APD371) | >1200 [359] | Crohn’s Disease (CD), Irritable Bowel Syndrome (IBS) | 2 | 2a (CD): Reduced abdominal pain over time (not placebo controlled) [360]. 2b (IBS): Primary endpoint not met. Subgroup with pain score improvement vs. placebo [361]. | 2023 | Arena Pharmaceuticals |
| NTRX-07 | 27 | Alzheimer’s Disease (AD) | 1 (2) | No dose-limiting or serious adverse events. Trend toward improved cognitive scores in AD patients [362,363]. Phase 2 recruiting [364]. | 2023 | NeuroTherapia |
| CNTX-6016 | >15,000 [365] | Diabetic Neuropathy | 1 | 1a completed 2019, 1b completed 2023; results not disclosed [366,367]. | (2023) | Centrexion Therapeutics |
| TT-816 ° | >380 [368] | Cancer (solid tumours) | (1 + 2) | Phase 1/2 trial initiated but suspended for commercial reasons [369]. | (2023) | Teon Therapeutics |
| Vicasinabin (RG7774) | >195 ◆ | Diabetic Retinopathy | 2 | No significant effect on retinopathy progression vs. placebo [370]. | 2024 | Hoffmann-La Roche |
| AZD-1940 | 13 ⯌ | Cancer Anorexia | (1 + 2) | Trial recruiting [371]. | (2025) | Artelo Biosciences |
| EHP-101 | >230 Δ | Arterial Disease | (2) | Upcoming trial registered [372]. | (2025) | VivaCell Biotechnology España |
8. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| 7KC | 7-ketocholesterol |
| AD | Alzheimer’s Disease |
| ad lib. | Ad libitum |
| AEA | Anandamide |
| AJA | Ajulemic acid |
| ALS | Amyotrophic lateral sclerosis |
| ALT | Alanine aminotransferase |
| APP | Amyloid precursor protein |
| Arg1 | Arginase 1 |
| aq. | Aqua (in water) |
| ASC | Apoptosis-associated Speck-like protein containing a Caspase recruitment domain |
| ATP | Adenosine triphosphate |
| Aβ | AD-associated amyloid β |
| BBB | Blood–brain barrier |
| BCP | β-(E/trans)-Caryophyllene |
| BDNF | Brain-derived neurotrophic factor |
| BLM | Bleomycin |
| BMDMs | Bone marrow-derived macrophages |
| cAMP | Cyclic adenosine monophosphate |
| CAR-M | Chimeric antigen receptor-macrophage |
| CATB | Cathepsin B |
| CB1 | Cannabinoid receptor 1 |
| CB2 | Cannabinoid receptor 2 |
| CBD | Cannabidiol |
| CCI | Controlled cortical impact |
| CD | Crohn’s disease |
| CF | Cystic fibrosis |
| CFA | Complete Freund’s adjuvant |
| APP/PS1 | Chimeric mouse/human APP with mutant human presenilin 1 |
| CINV | Chemotherapy-induced nausea and vomiting |
| CLP | Cecal ligation puncture |
| CNS | Central nervous system |
| COX | Cyclooxygenase |
| CRISS | Combined Response Index in diffuse cutaneous systemic sclerosis |
| DAMP | Danger-associated molecular pattern |
| DNFB | 1-fluoro-2,4-dinitrobenzene |
| EAE | Experimental autoimmune encephalomyelitis |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-to-mesenchymal transition |
| EP2 | PGE2 receptor 2 |
| ERK | Extracellular signal-regulated kinase |
| FDA | Food and Drug Administration |
| fMLP | Formyl-methionyl-leucine-phenylalanine |
| GABA | γ-aminobutyric acid |
| GC | Glucocorticoid |
| GDP | Guanosine diphosphate |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GPCR | G protein-coupled receptor |
| GRK | GPCR kinase |
| GRP | Gastrin-releasing peptide |
| GTP | Guanosine triphosphate |
| HAND | HIV-associated neurocognitive disorders |
| HD | Huntington’s disease |
| HIV-1 | Human immunodeficiency virus type 1 |
| HIVE | HIV encephalitis |
| iBCG | Irradiated Mycobacterium bovis-BCG |
| IBD | Inflammatory bowel disease |
| IBS | Irritable Bowel Syndrome |
| ICH | Intracerebral haemorrhage |
| ICTF | Trifluoro-icaritin |
| IFN-γ | Interferon-γ |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| i.p. | Intraperitoneally |
| IST | Immunosuppressive treatment |
| JAK/STAT | Janus-activated kinases-signal transducer and activator of transcription |
| JNK | C-Jun N-terminal kinase |
| KO | Knockout |
| LPS | Lipopolysaccharide |
| MAGL | Monoacylglycerol lipase |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1, CCL2 |
| M-CSF | Macrophage colony-stimulating factor |
| MDM | Monocyte-derived macrophage |
| MDMA | 3,4-Methylenedioxymethamphetamine |
| MHC | Major histocompatibility complex |
| MMF | Mycophenolate mofetil |
| Mrc1 | Mannose receptor 1 |
| MS | Multiple sclerosis |
| MTB | Mycobacterium tuberculosis |
| NFT | Neurofibrillary tangle |
| NF-κB | Nuclear Factor kappa B |
| NK | Natural killer |
| NLR | NOD-like receptor |
| NO | Nitric oxide |
| NRF2 | Nuclear factor erythroid 2-related factor |
| oxLDL | Oxidised LDL |
| PAMP | Pathogen-associated molecular pattern |
| PBMC | Peripheral blood mononuclear cells |
| PD | Parkinson’s disease |
| PEA | Palmitoylethanolamide |
| PERK | Protein kinase R-like endoplasmic reticulum kinase |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| PMA | Phorbol-12-myristate-13-acetate |
| p.o. | Orally |
| PPAR | Peroxisome proliferator–activated receptor |
| PRR | Pattern recognition receptor |
| PVR | Proliferative vitreoretinopathy |
| QA | Quinolinic acid |
| RA | Rheumatoid arthritis |
| RANTES | Regulated on activation, normal T-cell expressed and secreted, CCL5 |
| ROS | Reactive oxygen species |
| SBP | Spontaneous bacterial peritonitis |
| SIM | Spontaneously immortalised microglia |
| SLE | Systemic lupus erythematosus |
| SNP | Single nucleotide polymorphism |
| SOCS3 | Suppressor of cytokine signalling 3 |
| SR1 | SR141716A |
| SR2 | SR144528 |
| SSc | Systemic sclerosis |
| TAM | Tumour-associated macrophage |
| Tat | Trans-activating protein |
| TBI | Traumatic brain injury |
| TCA | Tricarboxylic acid |
| TGF-β | Transforming growth factor-β |
| Th1 | Type 1 T helper |
| THC | Δ9-tetrahydrocannabinol |
| TLR | Toll-like receptor |
| TMEV | Theiler’s murine encephalomyelitis virus |
| TNF | Tumour necrosis factor |
| TRPV1 | Transient receptor potential vanilloid 1 |
| UC | Ulcerative colitis |
| VEGF | Vascular endothelial growth factor |
References
- Duque, G.A.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Martinez-Pomares, L. Physiological roles of macrophages. Pflug. Arch. Eur. J. Physiol. 2017, 469, 365–374. [Google Scholar] [CrossRef]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and functions. Chemtexts 2022, 8, 12. [Google Scholar] [CrossRef]
- Zhou, P.; Tan, Y.; Wang, H.; Li, T.; He, T.; Yu, Y.; Zhang, J.; Zhang, D. Cytoprotective effect of autophagy on phagocytosis of apoptotic cells by macrophages. Exp. Cell Res. 2016, 348, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Moretti, J.; Blander, J.M. Insights into phagocytosis-coupled activation of Pattern Recognition Receptors and Inflammasomes. Curr. Opin. Immunol. 2014, 26, 100–110. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Khan, A.; Zhang, K.; Singh, V.K.; Mishra, A.; Kachroo, P.; Bing, T.; Won, J.H.; Mani, A.; Papanna, R.; Mann, L.K.; et al. Human M1 macrophages express unique innate immune response genes after mycobacterial infection to defend against tuberculosis. Commun. Biol. 2022, 5, 480. [Google Scholar] [CrossRef] [PubMed]
- Hortová-Kohoutková, M.; Tidu, F.; De Zuani, M.; Šrámek, V.; Helán, M.; Frič, J. Phagocytosis–Inflammation Crosstalk in Sepsis: New Avenues for Therapeutic Intervention. Shock 2020, 54, 606–614. [Google Scholar] [CrossRef]
- Karki, R.; Kanneganti, T.-D. The ‘cytokine storm’: Molecular mechanisms and therapeutic prospects. Trends Immunol. 2021, 42, 681–705. [Google Scholar] [CrossRef]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.E17. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Xie, D.; Bhatia, T.N.; Leak, R.K.; Hu, X.; Jiang, X. Microglial/Macrophage polarization and function in brain injury and repair after stroke. CNS Neurosci. Ther. 2021, 27, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Hamidzadeh, K.; Belew, A.T.; El-Sayed, N.M.; Mosser, D.M. The transition of M-CSF–derived human macrophages to a growth-promoting phenotype. Blood Adv. 2020, 4, 5460–5472. [Google Scholar] [CrossRef]
- Mosser, D.M.; Hamidzadeh, K.; Goncalves, R. Macrophages and the maintenance of homeostasis. Cell. Mol. Immunol. 2021, 18, 579–587. [Google Scholar] [CrossRef]
- Hamidzadeh, K.; Christensen, S.M.; Dalby, E.; Chandrasekaran, P.; Mosser, D.M. Macrophages and the Recovery from Acute and Chronic Inflammation. Annu. Rev. Physiol. 2017, 79, 567–592. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Glass, C.K.; Natoli, G. Molecular control of activation and priming in macrophages. Nat. Immunol. 2016, 17, 26–33. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, S.; de Vries, T.J.; Everts, V. Monocyte Heterogeneity: Consequences for Monocyte-Derived Immune Cells. J. Immunol. Res. 2016, 2016, 1475435. [Google Scholar] [CrossRef]
- Hickman, E.; Smyth, T.; Cobos-Uribe, C.; Immormino, R.; Rebuli, M.E.; Moran, T.; Alexis, N.E.; Jaspers, I. Expanded characterization of in vitro polarized M0, M1, and M2 human monocyte-derived macrophages: Bioenergetic and secreted mediator profiles. PLoS ONE 2023, 18, e0279037. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Orekhova, V.A.; Nikiforov, N.G.; Myasoedova, V.A.; Grechko, A.V.; Romanenko, E.B.; Zhang, D.; Chistiakov, D.A. Monocyte differentiation and macrophage polarization. Vessel Plus 2019, 3, 10. [Google Scholar] [CrossRef]
- Hamilton, J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008, 8, 533–544. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Fleetwood, A.J.; Lawrence, T.; Hamilton, J.A.; Cook, A.D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: Implications for CSF blockade in inflammation. J. Immunol. 2007, 178, 5245–5252. [Google Scholar] [CrossRef]
- Schulz, D.; Severin, Y.; Zanotelli, V.R.T.; Bodenmiller, B. In-Depth Characterization of Monocyte-Derived Macrophages using a Mass Cytometry-Based Phagocytosis Assay. Sci. Rep. 2019, 9, 1925. [Google Scholar] [CrossRef]
- Lacey, D.C.; Achuthan, A.; Fleetwood, A.J.; Dinh, H.; Roiniotis, J.; Scholz, G.M.; Chang, M.W.; Beckman, S.K.; Cook, A.D.; Hamilton, J.A. Defining GM-CSF– and Macrophage-CSF–Dependent Macrophage Responses by In Vitro Models. J. Immunol. 2012, 188, 5752–5765. [Google Scholar] [CrossRef]
- Farzam-kia, N.; Moratalla, A.C.; Lemaître, F.; Levert, A.; Da Cal, S.; Margarido, C.; Carpentier Solorio, Y.; Arbour, N. GM-CSF distinctly impacts human monocytes and macrophages via ERK1/2-dependent pathways. Immunol. Lett. 2023, 261, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wicks, I.P.; Roberts, A.W. Targeting GM-CSF in inflammatory diseases. Nat. Rev. Rheumatol. 2016, 12, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Chaintreuil, P.; Kerreneur, E.; Bourgoin, M.; Savy, C.; Favreau, C.; Robert, G.; Jacquel, A.; Auberger, P. The generation, activation, and polarization of monocyte-derived macrophages in human malignancies. Front. Immunol. 2023, 14, 1178337. [Google Scholar] [CrossRef]
- Lescoat, A.; Ballerie, A.; Augagneur, Y.; Morzadec, C.; Vernhet, L.; Fardel, O.; Jégo, P.; Jouneau, S.; Lecureur, V. Distinct Properties of Human M-CSF and GM-CSF Monocyte-Derived Macrophages to Simulate Pathological Lung Conditions In Vitro: Application to Systemic and Inflammatory Disorders with Pulmonary Involvement. Int. J. Mol. Sci. 2018, 19, 894. [Google Scholar] [CrossRef]
- Li, M.; Wang, M.; Wen, Y.; Zhang, H.; Zhao, G.-N.; Gao, Q. Signaling pathways in macrophages: Molecular mechanisms and therapeutic targets. MedComm 2023, 4, e349. [Google Scholar] [CrossRef]
- Ambarus, C.A.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.R.D.J.; Reedquist, K.A.; Tak, P.P.; Baeten, D.L.P. Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J. Immunol. Methods 2012, 375, 196–206. [Google Scholar] [CrossRef]
- Vogel, D.Y.S.; Glim, J.E.; Stavenuiter, A.W.D.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H.J. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, F.; Lehmbecker, A.; Raddatz, B.; Kegler, K.; Tipold, A.; Stein, V.; Kalkuhl, A.; Deschl, U.; Baumgärtner, W.; Ulrich, R.; et al. Morphologic, phenotypic, and transcriptomic characterization of classically and alternatively activated canine blood-derived macrophages in vitro. PLoS ONE 2017, 12, e0183572. [Google Scholar] [CrossRef]
- Caroff, M.; Novikov, A. Lipopolysaccharides: Structure, function and bacterial identifications. OCL 2020, 27, 31. [Google Scholar] [CrossRef]
- Lee, C.-H.; Choi, E.Y. Macrophages and Inflammation. J. Rheum. Dis. 2018, 25, 11–18. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Erratum in Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, S.; Jeon, R.; Vuckovic, I.; Jiang, X.; Lerman, A.; Folmes, C.D.; Dzeja, P.D.; Herrmann, J. Interferon Gamma Induces Reversible Metabolic Reprogramming of M1 Macrophages to Sustain Cell Viability and Pro-Inflammatory Activity. EBioMedicine 2018, 30, 303–316. [Google Scholar] [CrossRef]
- Lauterbach, M.A.; Hanke, J.E.; Serefidou, M.; Mangan, M.S.J.; Kolbe, C.-C.; Hess, T.; Rothe, M.; Kaiser, R.; Hoss, F.; Gehlen, J.; et al. Toll-like Receptor Signaling Rewires Macrophage Metabolism and Promotes Histone Acetylation via ATP-Citrate Lyase. Immunity 2019, 51, 997–1011.E7. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Wculek, S.K.; Dunphy, G.; Heras-Murillo, I.; Mastrangelo, A.; Sancho, D. Metabolism of tissue macrophages in homeostasis and pathology. Cell. Mol. Immunol. 2022, 19, 384–408. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Deng, F.; Yan, J.; Lu, J.; Luo, M.; Xia, P.; Liu, S.; Wang, X.; Zhi, F.; Liu, D. M2 Macrophage-Derived Exosomal miR-590-3p Attenuates DSS-Induced Mucosal Damage and Promotes Epithelial Repair via the LATS1/YAP/ β-Catenin Signalling Axis. J. Crohn’s Colitis 2021, 15, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, H.; Yao, Y.; Tao, Z.; Chen, W.; Huang, F.; Chen, X. Macrophage, a potential targeted therapeutic immune cell for cardiomyopathy. Front. Cell Dev. Biol. 2022, 10, 908790. [Google Scholar] [CrossRef]
- Purcu, D.U.; Korkmaz, A.; Gunalp, S.; Helvaci, D.G.; Erdal, Y.; Dogan, Y.; Suner, A.; Wingender, G.; Sag, D. Effect of stimulation time on the expression of human macrophage polarization markers. PLoS ONE 2022, 17, e0265196. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Kenealy, S.; Creagh, E.M. Autoinflammatory Diseases: Consequences of Uncontrolled Inflammasome Activation. EMJ Allergy Immunol. 2018, 3, 106–113. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Bonaguro, L.; Gemünd, I.; Reusch, N.; Saglam, A.; Hinkley, E.R.; Schultze, J.L. Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front. Immunol. 2019, 10, 2035. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Ma, Y.; Chen, L.; Huang, H.; Huang, S.; Zhang, H.; He, Y.; Tan, C.; He, Y.; et al. Macrophage autophagy deficiency-induced CEBPB accumulation alleviates atopic dermatitis via impairing M2 polarization. Cell Rep. 2023, 42, 113430. [Google Scholar] [CrossRef]
- Luque-Martin, R.; Angell, D.C.; Kalxdorf, M.; Bernard, S.; Thompson, W.; Eberl, H.C.; Ashby, C.; Freudenberg, J.; Sharp, C.; Van den Bossche, J.; et al. IFN-γ Drives Human Monocyte Differentiation into Highly Proinflammatory Macrophages That Resemble a Phenotype Relevant to Psoriasis. J. Immunol. 2021, 207, 555–568. [Google Scholar] [CrossRef]
- Tang, Y.; Shi, Y.; Gao, Y.; Xu, X.; Han, T.; Li, J.; Liu, C. Oxytocin system alleviates intestinal inflammation by regulating macrophages polarization in experimental colitis. Clin. Sci. 2019, 133, 1977–1992. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-T.; Gao, F.; Gu, K.; Chen, D.-K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2019, 10, 1140. [Google Scholar] [CrossRef]
- Labonte, A.C.; Kegerreis, B.; Geraci, N.S.; Bachali, P.; Madamanchi, S.; Robl, R.; Catalina, M.D.; Lipsky, P.E.; Grammer, A.C. Identification of alterations in macrophage activation associated with disease activity in systemic lupus erythematosus. PLoS ONE 2018, 13, e0208132. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, M.; Jia, S. Macrophage: Key player in the pathogenesis of autoimmune diseases. Front. Immunol. 2023, 14, 1080310. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ivashkiv, L.B. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity 2009, 31, 539–550. [Google Scholar] [CrossRef]
- Green, D.S.; Young, H.A.; Valencia, J.C. Current prospects of type II interferon γ signaling and autoimmunity. J. Biol. Chem. 2017, 292, 13925–13933. [Google Scholar] [CrossRef]
- Simpson, D.S.; Pang, J.; Weir, A.; Kong, I.Y.; Fritsch, M.; Rashidi, M.; Cooney, J.P.; Davidson, K.C.; Speir, M.; Djajawi, T.M.; et al. Interferon-γ primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway. Immunity 2022, 55, 423–441.E9. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Bachu, M.; Park, S.H.; Kang, K.; Bae, S.; Park-Min, K.-H.; Ivashkiv, L.B. IFN-γ selectively suppresses a subset of TLR4-activated genes and enhancers to potentiate macrophage activation. Nat. Commun. 2019, 10, 3320. [Google Scholar] [CrossRef] [PubMed]
- Kerneur, C.; Cano, C.E.; Olive, D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 2022, 13, 1026954. [Google Scholar] [CrossRef]
- Neamatallah, T. Mitogen-Activated Protein Kinase Pathway: A Critical Regulator in Tumor-associated Macrophage Polarization. J. Microsc. Ultrastruct. 2019, 7, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef]
- Rosenblum, M.D.; Gratz, I.K.; Paw, J.S.; Abbas, A.K. Treating Human Autoimmunity: Current Practice and Future Prospects. Sci. Transl. Med. 2012, 4, 125sr1. [Google Scholar] [CrossRef]
- Guan, F.; Wang, R.; Yi, Z.; Luo, P.; Liu, W.; Xie, Y.; Liu, Z.; Xia, Z.; Zhang, H.; Cheng, Q. Tissue macrophages: Origin, heterogenity, biological functions, diseases and therapeutic targets. Signal Transduct. Target. Ther. 2025, 10, 93. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Pu, X.; Liu, G.; Qin, H.; Wan, W.; Wang, Y.; Zhu, Y.; Yang, J. Macrophage-related therapeutic strategies: Regulation of phenotypic switching and construction of drug delivery systems. Pharmacol. Res. 2024, 199, 107022. [Google Scholar] [CrossRef]
- Bie, B.; Wu, J.; Foss, J.F.; Naguib, M. An overview of the cannabinoid type 2 (CB2) receptor system and its therapeutic potential. Curr. Opin. Anaesthesiol. 2018, 31, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Stella, N. Endocannabinoid signaling in microglial cells. Neuropharmacology 2009, 56 (Suppl. 1), 244–253. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Maccarrone, M.; De Petrocellis, L.; Bari, M.; Fezza, F.; Salvati, S.; Di Marzo, V.; Finazzi-Agrò, A. Lipopolysaccharide Downregulates Fatty Acid Amide Hydrolase Expression and Increases Anandamide Levels in Human Peripheral Lymphocytes. Arch. Biochem. Biophys. 2001, 393, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Panikashvili, D.; Simeonidou, C.; Ben-Shabat, S.; Hanuš, L.; Breuer, A.; Mechoulam, R.; Shohami, E. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature 2001, 413, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Mousawy, K.; Nagarkatti, M.; Nagarkatti, P. Endocannabinoids and immune regulation. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2009, 60, 85–92. [Google Scholar] [CrossRef]
- Eljaschewitsch, E.; Witting, A.; Mawrin, C.; Lee, T.; Schmidt, P.M.; Wolf, S.; Hoertnagl, H.; Raine, C.S.; Schneider-Stock, R.; Nitsch, R.; et al. The Endocannabinoid Anandamide Protects Neurons During CNS Inflammation by Induction of MKP-1 in Microglial Cells. Neuron 2006, 49, 67–79. [Google Scholar] [CrossRef]
- Garcia-Ovejero, D.; Arevalo-Martin, A.; Petrosino, S.; Docagne, F.; Hagen, C.; Bisogno, T.; Watanabe, M.; Guaza, C.; Di Marzo, V.; Molina-Holgado, E. The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiol. Dis. 2009, 33, 57–71. [Google Scholar] [CrossRef]
- Arevalo-Martin, A.; Garcia-Ovejero, D.; Sierra-Palomares, Y.; Paniagua-Torija, B.; Gonzalez-Gil, I.; Ortega-Gutierrez, S.; Molina-Holgado, E. Early Endogenous Activation of CB1 and CB2 Receptors after Spinal Cord Injury Is a Protective Response Involved in Spontaneous Recovery. PLoS ONE 2012, 7, e49057. [Google Scholar] [CrossRef]
- An, D.; Peigneur, S.; Hendrickx, L.; Tytgat, J. Targeting Cannabinoid Receptors: Current Status and Prospects of Natural Products. Int. J. Mol. Sci. 2020, 21, 5064. [Google Scholar] [CrossRef]
- Patel, S.; Hillard, C.J. Endocannabinoids as Modulators of Synaptic Signaling. In The Cannabinoid Receptors; Reggio, P.H., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 281–308. ISBN 978-1-59745-503-9. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Simard, M.; Rakotoarivelo, V.; Di Marzo, V.; Flamand, N. Expression and Functions of the CB2 Receptor in Human Leukocytes. Front. Pharmacol. 2022, 13, 826400. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; LE Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Graham, E.S.; Angel, C.E.; Schwarcz, L.E.; Dunbar, P.R.; Glass, M. Detailed Characterisation of CB2 Receptor Protein Expression in Peripheral Blood Immune Cells from Healthy Human Volunteers Using Flow Cytometry. Int. J. Immunopathol. Pharmacol. 2010, 23, 25–34. [Google Scholar] [CrossRef]
- Castaneda, J.T.; Harui, A.; Kiertscher, S.M.; Roth, J.D.; Roth, M.D. Differential expression of intracellular and extracellular CB(2) cannabinoid receptor protein by human peripheral blood leukocytes. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2013, 8, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Kleyer, J.; Nicolussi, S.; Taylor, P.; Simonelli, D.; Furger, E.; Anderle, P.; Gertsch, J. Cannabinoid receptor trafficking in peripheral cells is dynamically regulated by a binary biochemical switch. Biochem. Pharmacol. 2012, 83, 1393–1412. [Google Scholar] [CrossRef] [PubMed]
- Oyagawa, C.R.; Woodhouse, B.; Wood, K.C.; Glass, M.; Grimsey, N.L. Insights into Cannabinoid Receptor 2 (CB2) anterograde trafficking and pharmacological chaperoning. bioRxiv 2025. [Google Scholar] [CrossRef]
- Carrier, E.J.; Kearn, C.S.; Barkmeier, A.J.; Breese, N.M.; Yang, W.; Nithipatikom, K.; Pfister, S.L.; Campbell, W.B.; Hillard, C.J. Cultured Rat Microglial Cells Synthesize the Endocannabinoid 2-Arachidonylglycerol, Which Increases Proliferation via a CB2 Receptor-Dependent Mechanism. Mol. Pharmacol. 2004, 65, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.D.; Castaneda, J.T.; Kiertscher, S.M. Exposure to Δ9-Tetrahydrocannabinol Impairs the Differentiation of Human Monocyte-derived Dendritic Cells and their Capacity for T cell Activation. J. Neuroimmune Pharmacol. 2015, 10, 333–343. [Google Scholar] [CrossRef]
- Buckley, N.E.; McCoy, K.L.; Mezey, É.; Bonner, T.; Zimmer, A.; Felder, C.C.; Glass, M.; Zimmer, A. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur. J. Pharmacol. 2000, 396, 141–149. [Google Scholar] [CrossRef]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.-W.; Kim, Y.; Kang, J.-H.; Kang, S.-S.; Ahn, Y.K.; Park, C.-S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef]
- Jean-Gilles, L.; Braitch, M.; Latif, M.L.; Aram, J.; Fahey, A.J.; Edwards, L.J.; Robins, R.A.; Tanasescu, R.; Tighe, P.J.; Gran, B.; et al. Effects of pro-inflammatory cytokines on cannabinoid CB1 and CB2 receptors in immune cells. Acta Physiol. 2015, 214, 63–74. [Google Scholar] [CrossRef]
- Kaplan, B.L.F. The role of CB1 in immune modulation by cannabinoids. Pharmacol. Ther. 2013, 137, 365–374. [Google Scholar] [CrossRef]
- Tanasescu, R.; Constantinescu, C.S. Cannabinoids and the immune system: An overview. Immunobiology 2010, 215, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, W.; Yang, L.; Chang, N.; Fan, X.; Ji, X.; Xie, J.; Yang, L.; Li, L. Cannabinoid Receptor 1 Participates in Liver Inflammation by Promoting M1 Macrophage Polarization via RhoA/NF-κB p65 and ERK1/2 Pathways, Respectively, in Mouse Liver Fibrogenesis. Front. Immunol. 2017, 8, 1214. [Google Scholar] [CrossRef] [PubMed]
- Kibret, B.G.; Ishiguro, H.; Horiuchi, Y.; Onaivi, E.S. New Insights and Potential Therapeutic Targeting of CB2 Cannabinoid Receptors in CNS Disorders. Int. J. Mol. Sci. 2022, 23, 975. [Google Scholar] [CrossRef]
- Núñez, E.; Benito, C.; Pazos, M.R.; Barbachano, A.; Fajardo, O.; González, S.; Tolón, R.M.; Romero, J. Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: An immunohistochemical study. Synapse 2004, 53, 208–213. [Google Scholar] [CrossRef]
- Morcuende, A.; García-Gutiérrez, M.S.; Tambaro, S.; Nieto, E.; Manzanares, J.; Femenia, T. Immunomodulatory Role of CB2 Receptors in Emotional and Cognitive Disorders. Front. Psychiatry 2022, 13, 866052. [Google Scholar] [CrossRef]
- Aziz, A.; Nguyen, L.C.; Oumeslakht, L.; Bensussan, A.; Ben Mkaddem, S. Cannabinoids as Immune System Modulators: Cannabidiol Potential Therapeutic Approaches and Limitations. Cannabis Cannabinoid Res. 2023, 8, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Irving, A.; Abdulrazzaq, G.; Chan, S.L.F.; Penman, J.; Harvey, J.; Alexander, S.P.H. Chapter Seven—Cannabinoid Receptor-Related Orphan G Protein-Coupled Receptors. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Academic Press: New York, NY, USA, 2017; Volume 80, pp. 223–247. [Google Scholar] [CrossRef]
- Pietr, M.; Kozela, E.; Levy, R.; Rimmerman, N.; Lin, Y.H.; Stella, N.; Vogel, Z.; Juknat, A. Differential changes in GPR55 during microglial cell activation. FEBS Lett. 2009, 583, 2071–2076. [Google Scholar] [CrossRef]
- Zhou, J.; Burkovskiy, I.; Yang, H.; Sardinha, J.; Lehmann, C. CB2 and GPR55 Receptors as Therapeutic Targets for Systemic Immune Dysregulation. Front. Pharmacol. 2016, 7, 264. [Google Scholar] [CrossRef]
- Morales, P.; Lago-Fernandez, A.; Hurst, D.P.; Sotudeh, N.; Brailoiu, E.; Reggio, P.H.; Abood, M.E.; Jagerovic, N. Therapeutic Exploitation of GPR18: Beyond the Cannabinoids? J. Med. Chem. 2020, 63, 14216–14227. [Google Scholar] [CrossRef]
- Honkisz-Orzechowska, E.; Łażewska, D.; Baran, G.; Kieć-Kononowicz, K. Uncovering the Power of GPR18 Signalling: How RvD2 and Other Ligands Could Have the Potential to Modulate and Resolve Inflammation in Various Health Disorders. Molecules 2024, 29, 1258. [Google Scholar] [CrossRef] [PubMed]
- Leuti, A.; Fava, M.; Forte, G.; Pellegrini, N.; Oddi, S.; Scipioni, L.; Gomez, E.A.; Dalli, J.; Maccarrone, M. The endocannabinoid anandamide activates pro-resolving pathways in human primary macrophages by engaging both CB2 and GPR18 receptors. FASEB J. 2024, 38, e23675. [Google Scholar] [CrossRef]
- Oyagawa, C.R.M.; Grimsey, N.L. Cannabinoid receptor CB1 and CB2 interacting proteins: Techniques, progress and perspectives. In Methods in Cell Biology; Shukla, A.K., Ed.; Academic Press: New York, NY, USA, 2021; Volume 166, pp. 83–132. [Google Scholar] [CrossRef]
- Reyes-Resina, I.; Navarro, G.; Aguinaga, D.; Canela, E.I.; Schoeder, C.T.; Załuski, M.; Kieć-Kononowicz, K.; Saura, C.A.; Müller, C.E.; Franco, R. Molecular and functional interaction between GPR18 and cannabinoid CB2 G-protein-coupled receptors. Relevance in neurodegenerative diseases. Biochem. Pharmacol. 2018, 157, 169–179. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Chayasirisobhon, S. Mechanisms of Action and Pharmacokinetics of Cannabis. Perm. J. 2020, 25, 19.200. [Google Scholar] [CrossRef]
- Crocq, M.-A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Whiting, Z.M.; Yin, J.; De La Harpe, S.M.; Vernall, A.J.; Grimsey, N.L. Developing the Cannabinoid Receptor 2 (CB2) pharmacopoeia: Past, present, and future. Trends Pharmacol. Sci. 2022, 43, 754–771. [Google Scholar] [CrossRef]
- Vuic, B.; Milos, T.; Tudor, L.; Konjevod, M.; Nikolac Perkovic, M.; Jazvinscak Jembrek, M.; Nedic Erjavec, G.; Svob Strac, D. Cannabinoid CB2 Receptors in Neurodegenerative Proteinopathies: New Insights and Therapeutic Potential. Biomedicines 2022, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, R.A.; Painuli, R.; Akhter, Z.; Singh, P.P. Cannabinoid receptor 2 (CB2) modulators: A patent review (2016–2024). Bioorganic Chem. 2024, 153, 107775. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pinilla, E.; Varani, K.; Reyes-Resina, I.; Angelats, E.; Vincenzi, F.; Ferreiro-Vera, C.; Oyarzabal, J.; Canela, E.I.; Lanciego, J.L.; Nadal, X.; et al. Binding and Signaling Studies Disclose a Potential Allosteric Site for Cannabidiol in Cannabinoid CB2 Receptors. Front. Pharmacol. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carreiro, S.; Gómez-Cañas, M.; Lubrini, F.; Gonzalo-Consuegra, C.; Winkler, M.; Caprioglio, D.; Appendino, G.; García, C.; Morales, P.; Jagerovic, N.; et al. Investigation in the CB1 and CB2 receptor binding profile and intrinsic activity of (−) and (+)-enantiomers of some naturally occurring phytocannabinoids or synthetic derivatives. Eur. J. Med. Chem. Rep. 2025, 14, 100262. [Google Scholar] [CrossRef]
- Carruthers, E.R.; Grimsey, N.L. Cannabinoid CB2 receptor orthologues; in vitro function and perspectives for preclinical to clinical translation. Br. J. Pharmacol. 2024, 181, 2247–2269. [Google Scholar] [CrossRef]
- Bingham, B.; Jones, P.G.; Uveges, A.J.; Kotnis, S.; Lu, P.; Smith, V.A.; Sun, S.-C.; Resnick, L.; Chlenov, M.; He, Y.; et al. Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br. J. Pharmacol. 2007, 151, 1061–1070. [Google Scholar] [CrossRef]
- Soethoudt, M.; Grether, U.; Fingerle, J.; Grim, T.W.; Fezza, F.; de Petrocellis, L.; Ullmer, C.; Rothenhäusler, B.; Perret, C.; van Gils, N.; et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat. Commun. 2017, 8, 13958. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Murineddu, G.; Lazzari, P.; Ruiu, S.; Sanna, A.; Loriga, G.; Manca, I.; Falzoi, M.; Dessì, C.; Curzu, M.M.; Chelucci, G.; et al. Tricyclic Pyrazoles. 4. Synthesis and Biological Evaluation of Analogues of the Robust and Selective CB2 Cannabinoid Ligand 1-(2′,4′-Dichlorophenyl)-6-methyl-N-piperidin-1-yl- 1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide. J. Med. Chem. 2006, 49, 7502–7512. [Google Scholar] [CrossRef]
- Valenzano, K.J.; Tafesse, L.; Lee, G.; Harrison, J.E.; Boulet, J.M.; Gottshall, S.L.; Mark, L.; Pearson, M.S.; Miller, W.; Shan, S.; et al. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology 2005, 48, 658–672. [Google Scholar] [CrossRef]
- Belvisi, M.G.; Patel, H.J.; Freund-Michel, V.; Hele, D.J.; Crispino, N.; Birrell, M.A. Inhibitory activity of the novel CB2 receptor agonist, GW833972A, on guinea-pig and human sensory nerve function in the airways. Br. J. Pharmacol. 2008, 155, 547–557. [Google Scholar] [CrossRef]
- Naguib, M.; Diaz, P.; Xu, J.J.; Astruc-Diaz, F.; Craig, S.; Vivas-Mejia, P.; Brown, D.L. MDA7: A novel selective agonist for CB2 receptors that prevents allodynia in rat neuropathic pain models. Br. J. Pharmacol. 2008, 155, 1104–1116. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.L.; Beletskaya, I.D.; Ng, E.W.; Dai, Z.; Crocker, P.J.; Mahadevan, A.; Razdan, R.K.; Martin, B.R. Resorcinol Derivatives: A Novel Template for the Development of Cannabinoid CB1/CB2 and CB2-Selective Agonists. J. Pharmacol. Exp. Ther. 2002, 301, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Razdan, R. Cannabinoids. U.S. Patent US20050165259A1, 23 October 2007. Available online: https://patents.google.com/patent/US20050165259A1/en (accessed on 2 July 2025).
- Bolognini, D.; Cascio, M.G.; Parolaro, D.; Pertwee, R.G. AM630 behaves as a protean ligand at the human cannabinoid CB2 receptor. Br. J. Pharmacol. 2012, 165, 2561–2574. [Google Scholar] [CrossRef]
- Presley, C.; Abidi, A.; Suryawanshi, S.; Mustafa, S.; Meibohm, B.; Moore II, B.M. Preclinical evaluation of SMM-189, a cannabinoid receptor 2-specific inverse agonist. Pharmacol. Res. Perspect. 2015, 3, e00159. [Google Scholar] [CrossRef]
- Baratta, F.; Pignata, I.; Ravetto Enri, L.; Brusa, P. Cannabis for Medical Use: Analysis of Recent Clinical Trials in View of Current Legislation. Front. Pharmacol. 2022, 13, 888903. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, T.; Lu, X.; Lan, X.; Chen, Z.; Lu, S. G protein-coupled receptors (GPCRs): Advances in structures, mechanisms and drug discovery. Signal Transduct. Target. Ther. 2024, 9, 88. [Google Scholar] [CrossRef]
- Saroz, Y.; Kho, D.T.; Glass, M.; Graham, E.S.; Grimsey, N.L. Cannabinoid Receptor 2 (CB2) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes. ACS Pharmacol. Transl. Sci. 2019, 2, 414–428. [Google Scholar] [CrossRef]
- Tao, Y.; Li, L.; Jiang, B.; Feng, Z.; Yang, L.; Tang, J.; Chen, Q.; Zhang, J.; Tan, Q.; Feng, H.; et al. Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav. Immun. 2016, 58, 118–129. [Google Scholar] [CrossRef]
- Dhopeshwarkar, A.; Mackie, K. Functional Selectivity of CB2 Cannabinoid Receptor Ligands at a Canonical and Noncanonical Pathway. J. Pharmacol. Exp. Ther. 2016, 358, 342–351. [Google Scholar] [CrossRef]
- Gasperi, V.; Guzzo, T.; Topai, A.; Gambacorta, N.; Ciriaco, F.; Nicolotti, O.; Maccarrone, M. Recent Advances on Type-2 Cannabinoid (CB2) Receptor Agonists and Their Therapeutic Potential. Curr. Med. Chem. 2023, 30, 1420–1457. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, G.C.; Deliu, E.; Marcu, J.; Hoffman, N.E.; Console-Bram, L.; Zhao, P.; Madesh, M.; Abood, M.E.; Brailoiu, E. Differential Activation of Intracellular versus Plasmalemmal CB2 Cannabinoid Receptors. Biochemistry 2014, 53, 4990–4999. [Google Scholar] [CrossRef] [PubMed]
- Nogueras-Ortiz, C.; Roman-Vendrell, C.; Mateo-Semidey, G.E.; Liao, Y.-H.; Kendall, D.A.; Yudowski, G.A. Retromer stops beta-arrestin 1–mediated signaling from internalized cannabinoid 2 receptors. Mol. Biol. Cell 2017, 28, 3554–3561. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, M.S.; Finlay, D.B.; Patel, M.; Javitch, J.A.; Glass, M.; Grimsey, N.L. Cannabinoid CB1 and CB2 Receptor-Mediated Arrestin Translocation: Species, Subtype, and Agonist-Dependence. Front. Pharmacol. 2019, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Matti, C.; Grimsey, N.L.; Legler, D.F.; Javitch, J.A.; Finlay, D.B.; Glass, M. Delineating the interactions between the cannabinoid CB2 receptor and its regulatory effectors; β-arrestins and GPCR kinases. Br. J. Pharmacol. 2022, 179, 2223–2239. [Google Scholar] [CrossRef]
- Grimsey, N.L.; Goodfellow, C.E.; Dragunow, M.; Glass, M. Cannabinoid receptor 2 undergoes Rab5-mediated internalization and recycles via a Rab11-dependent pathway. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2011, 1813, 1554–1560. [Google Scholar] [CrossRef]
- Foyzun, T.; Whiting, M.; Velasco, K.K.; Jacobsen, J.C.; Connor, M.; Grimsey, N.L. Single nucleotide polymorphisms in the cannabinoid CB2 receptor: Molecular pharmacology and disease associations. Br. J. Pharmacol. 2024, 181, 2391–2412. [Google Scholar] [CrossRef]
- Kahsai, A.W.; Shah, K.S.; Shim, P.J.; Lee, M.A.; Shreiber, B.N.; Schwalb, A.M.; Zhang, X.; Kwon, H.Y.; Huang, L.-Y.; Soderblom, E.J.; et al. Signal transduction at GPCRs: Allosteric activation of the ERK MAPK by β-arrestin. Proc. Natl. Acad. Sci. USA 2023, 120, e2303794120. [Google Scholar] [CrossRef]
- Rankovic, Z.; Brust, T.F.; Bohn, L.M. Biased agonism: An emerging paradigm in GPCR drug discovery. Bioorganic Med. Chem. Lett. 2016, 26, 241–250. [Google Scholar] [CrossRef]
- Seyedabadi, M.; Ghahremani, M.H.; Albert, P.R. Biased signaling of G protein coupled receptors (GPCRs): Molecular determinants of GPCR/transducer selectivity and therapeutic potential. Pharmacol. Ther. 2019, 200, 148–178. [Google Scholar] [CrossRef]
- Smith, J.S.; Lefkowitz, R.J.; Rajagopal, S. Biased signalling: From simple switches to allosteric microprocessors. Nat. Rev. Drug Discov. 2018, 17, 243–260. [Google Scholar] [CrossRef]
- Grisanti, L.A.; Schumacher, S.M.; Tilley, D.G.; Koch, W.J. Designer Approaches for G Protein–Coupled Receptor Modulation for Cardiovascular Disease. JACC Basic Transl. Sci. 2018, 3, 550–562. [Google Scholar] [CrossRef]
- Oyagawa, C.R.M.; de la Harpe, S.M.; Saroz, Y.; Glass, M.; Vernall, A.J.; Grimsey, N.L. Erratum in Cannabinoid Receptor 2 Signalling Bias Elicited by 2,4,6-Trisubstituted 1,3,5-Triazines. Front. Pharmacol. 2018, 9, 1202. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Grimsey, N.L.; Banister, S.D.; Finlay, D.B.; Glass, M. Evaluating signaling bias for synthetic cannabinoid receptor agonists at the cannabinoid CB2 receptor. Pharmacol. Res. Perspect. 2023, 11, e01157. [Google Scholar] [CrossRef]
- Lin, X.; Dhopeshwarkar, A.S.; Huibregtse, M.; Mackie, K.; Hohmann, A.G. Slowly Signaling G Protein–Biased CB2 Cannabinoid Receptor Agonist LY2828360 Suppresses Neuropathic Pain with Sustained Efficacy and Attenuates Morphine Tolerance and Dependence. Mol. Pharmacol. 2018, 93, 49–62. [Google Scholar] [CrossRef]
- Mlost, J.; Kostrzewa, M.; Borczyk, M.; Bryk, M.; Chwastek, J.; Korostyński, M.; Starowicz, K. CB2 agonism controls pain and subchondral bone degeneration induced by mono-iodoacetate: Implications GPCR functional bias and tolerance development. Biomed. Pharmacother. 2021, 136, 111283. [Google Scholar] [CrossRef]
- Soethoudt, M.; Hoorens, M.W.H.; Doelman, W.; Martella, A.; van der Stelt, M.; Heitman, L.H. Structure-kinetic relationship studies of cannabinoid CB2 receptor agonists reveal substituent-specific lipophilic effects on residence time. Biochem. Pharmacol. 2018, 152, 129–142. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, S.; Whiting, Z.M.; Molitor, M.; Vernall, A.J.; Grimsey, N.L. Novel Cannabinoid Receptor 2 (CB2) Low Lipophilicity Agonists Produce Distinct cAMP and Arrestin Signalling Kinetics without Bias. Int. J. Mol. Sci. 2023, 24, 6406. [Google Scholar] [CrossRef]
- Li, A.-L.; Lin, X.; Dhopeshwarkar, A.S.; Thomaz, A.C.; Carey, L.M.; Liu, Y.; Nikas, S.P.; Makriyannis, A.; Mackie, K.; Hohmann, A.G. Cannabinoid CB2 Agonist AM1710 Differentially Suppresses Distinct Pathological Pain States and Attenuates Morphine Tolerance and Withdrawal. Mol. Pharmacol. 2019, 95, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Alcaraz, A.; Garcia-Rojas, E.Y.L.; Bond, R.A.; McConnell, B.K.; Reyes-Alcaraz, A.; Garcia-Rojas, E.Y.L.; Bond, R.A.; McConnell, B.K. Allosteric Modulators for GPCRs as a Therapeutic Alternative with High Potential in Drug Discovery. In Molecular Pharmacology; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Green, H.M.; Manning, J.J.; Greig, I.R.; Ross, R.A.; Finlay, D.B.; Glass, M. Positive allosteric modulation of the cannabinoid CB1 receptor potentiates endocannabinoid signalling and changes ERK1/2 phosphorylation kinetics. Br. J. Pharmacol. 2024, 181, 3642–3662. [Google Scholar] [CrossRef] [PubMed]
- Gado, F.; Di Cesare Mannelli, L.; Lucarini, E.; Bertini, S.; Cappelli, E.; Digiacomo, M.; Stevenson, L.A.; Macchia, M.; Tuccinardi, T.; Ghelardini, C.; et al. Identification of the First Synthetic Allosteric Modulator of the CB2 Receptors and Evidence of Its Efficacy for Neuropathic Pain Relief. J. Med. Chem. 2019, 62, 276–287. [Google Scholar] [CrossRef]
- Tham, M.; Yilmaz, O.; Alaverdashvili, M.; Kelly, M.E.M.; Denovan-Wright, E.M.; Laprairie, R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 2019, 176, 1455–1469. [Google Scholar] [CrossRef]
- Qi, A.; Han, X.; Quitalig, M.; Wu, J.; Christov, P.P.; Jeon, K.; Jana, S.; Kim, K.; Engers, D.W.; Lindsley, C.W.; et al. The cannabinoid CB2 receptor positive allosteric modulator EC21a exhibits complicated pharmacology in vitro. J. Recept. Signal Transduct. 2024, 44, 151–159. [Google Scholar] [CrossRef]
- Farooq, Z.; Delre, P.; Iliadis, S.; Mangiatordi, G.F.; Contino, M.; Howell, L.A.; McCormick, P.J. Identification of a Cannabinoid Receptor 2 Allosteric Site Using Computational Modeling and Pharmacological Analysis. ACS Pharmacol. Transl. Sci. 2025, 8, 423–434. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Gonsiorek, W.; Hesk, D.; Chen, S.-C.; Kinsley, D.; Fine, J.S.; Jackson, J.V.; Bober, L.A.; Deno, G.; Bian, H.; Fossetta, J.; et al. Characterization of Peripheral Human Cannabinoid Receptor (hCB2) Expression and Pharmacology Using a Novel Radioligand, [35S]Sch225336. J. Biol. Chem. 2006, 281, 28143–28151. [Google Scholar] [CrossRef] [PubMed]
- Ehrhart, J.; Obregon, D.; Mori, T.; Hou, H.; Sun, N.; Bai, Y.; Klein, T.; Fernandez, F.; Tan, J.; Shytle, R.D. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J. Neuroinflamm. 2005, 2, 29. [Google Scholar] [CrossRef]
- Carlisle, S.J.; Marciano-Cabral, F.; Staab, A.; Ludwick, C.; Cabral, G.A. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int. Immunopharmacol. 2002, 2, 69–82. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Das, S.; Williams, E.A.; Moore, D.; Jones, J.D.; Zahm, D.S.; Ndengele, M.M.; Lechner, A.J.; Howlett, A.C. Lipopolysaccharide and cyclic AMP regulation of CB2 cannabinoid receptor levels in rat brain and mouse RAW 264.7 macrophages. J. Neuroimmunol. 2006, 181, 82–92. [Google Scholar] [CrossRef]
- Malek, N.; Popiolek-Barczyk, K.; Mika, J.; Przewlocka, B.; Starowicz, K. Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast. 2015, 2015, e130639. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Carrillo-Salinas, F.J.; Rueda-Zubiaurre, A.; Ortega-Gutiérrez, S.; de Sola, R.G.; Guaza, C. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav. Immun. 2015, 49, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, V.; Muñoz-Luque, J.; Ros, J.; Casals, G.; Navasa, M.; Fernández-Varo, G.; Morales-Ruiz, M.; Jiménez, W. Bacterial lipopolyshaccaride inhibits CB2 receptor expression in human monocytic cells. Gut 2013, 62, 1089–1091. [Google Scholar] [CrossRef]
- Grabon, W.; Ruiz, A.; Gasmi, N.; Degletagne, C.; Georges, B.; Belmeguenai, A.; Bodennec, J.; Rheims, S.; Marcy, G.; Bezin, L. CB2 expression in mouse brain: From mapping to regulation in microglia under inflammatory conditions. J. Neuroinflamm. 2024, 21, 206. [Google Scholar] [CrossRef]
- Young, A.P.; Denovan-Wright, E.M. Synthetic cannabinoids reduce the inflammatory activity of microglia and subsequently improve neuronal survival in vitro. Brain Behav. Immun. 2022, 105, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Pu, J.; Han, Z.; Hu, L.; He, B. Role of activated endocannabinoid system in regulation of cellular cholesterol metabolism in macrophages. Cardiovasc. Res. 2009, 81, 805–813. [Google Scholar] [CrossRef]
- Heinemann, J.C.; Duerr, G.D.; Keppel, K.; Breitbach, M.; Fleischmann, B.K.; Zimmer, A.; Wehner, S.; Welz, A.; Dewald, O. CB2 receptor-mediated effects of pro-inflammatory macrophages influence survival of cardiomyocytes. Life Sci. 2015, 138, 18–28. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Q.; Guo, J.; Yang, L.; Tao, Y.; Li, L.; Miao, H.; Feng, H.; Chen, Z.; Zhu, G. Minocycline Attenuates Neonatal Germinal-Matrix-Hemorrhage-Induced Neuroinflammation and Brain Edema by Activating Cannabinoid Receptor 2. Mol. Neurobiol. 2016, 53, 1935–1948. [Google Scholar] [CrossRef]
- Galán-Ganga, M.; del Río, R.; Jiménez-Moreno, N.; Díaz-Guerra, M.; Lastres-Becker, I. Cannabinoid CB2 Receptor Modulation by the Transcription Factor NRF2 is Specific in Microglial Cells. Cell. Mol. Neurobiol. 2020, 40, 167–177. [Google Scholar] [CrossRef]
- Yiangou, Y.; Facer, P.; Durrenberger, P.; Chessell, I.P.; Naylor, A.; Bountra, C.; Banati, R.R.; Anand, P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 2006, 6, 12. [Google Scholar] [CrossRef]
- Benito, C.; Romero, J.P.; Tolón, R.M.; Clemente, D.; Docagne, F.; Hillard, C.J.; Guaza, C.; Romero, J. Cannabinoid CB1 and CB2 Receptors and Fatty Acid Amide Hydrolase Are Specific Markers of Plaque Cell Subtypes in Human Multiple Sclerosis. J. Neurosci. 2007, 27, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hilton, D.A.; Hanemann, C.O.; Zajicek, J. Cannabinoid Receptor and N-acyl Phosphatidylethanolamine Phospholipase D—Evidence for Altered Expression in Multiple Sclerosis. Brain Pathol. 2011, 21, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Palazuelos, J.; Davoust, N.; Julien, B.; Hatterer, E.; Aguado, T.; Mechoulam, R.; Benito, C.; Romero, J.; Silva, A.; Guzmán, M.; et al. The CB2 Cannabinoid Receptor Controls Myeloid Progenitor Trafficking: INVOLVEMENT IN THE PATHOGENESIS OF AN ANIMAL MODEL OF MULTIPLE SCLEROSIS. J. Biol. Chem. 2008, 283, 13320–13329. [Google Scholar] [CrossRef] [PubMed]
- Loría, F.; Petrosino, S.; Mestre, L.; Spagnolo, A.; Correa, F.; Hernangómez, M.; Guaza, C.; Di Marzo, V.; Docagne, F. Study of the regulation of the endocannabinoid system in a virus model of multiple sclerosis reveals a therapeutic effect of palmitoylethanolamide. Eur. J. Neurosci. 2008, 28, 633–641. [Google Scholar] [CrossRef]
- Palazuelos, J.; Aguado, T.; Pazos, M.R.; Julien, B.; Carrasco, C.; Resel, E.; Sagredo, O.; Benito, C.; Romero, J.; Azcoitia, I.; et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain 2009, 132, 3152–3164. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Z.; Zhou, L.; Ye, D.; Zhu, Y.; Huang, K.; Weng, Y.; Xiong, X.; Zhan, R.; Shen, J. Systemic immune responses after ischemic stroke: From the center to the periphery. Front. Immunol. 2022, 13, 911661. [Google Scholar] [CrossRef]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, C.; Chen, A.; Wang, H.; Zhou, Y.; Li, Y.; Hu, B. Immune Cells in the BBB Disruption After Acute Ischemic Stroke: Targets for Immune Therapy? Front. Immunol. 2021, 12, 678744. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Zanaboni, A.; Tumelero, E.; Elisa, C.; Persico, A.; Morotti, A.; Amantea, D.; Tassorelli, C. Characterization of CB2 Receptor Expression in Peripheral Blood Monocytes of Acute Ischemic Stroke Patients. Transl. Stroke Res. 2021, 12, 550–558. [Google Scholar] [CrossRef]
- Ashton, J.C.; Rahman, R.M.A.; Nair, S.M.; Sutherland, B.A.; Glass, M.; Appleton, I. Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci. Lett. 2007, 412, 114–117. [Google Scholar] [CrossRef]
- He, X.; Yang, X.; Li, G.; Zhao, Y.; Luo, J.; Xu, J.; Zheng, H.; Zhang, L.; Hu, X. Physical Exercise Improves the Neuronal Function in Ischemic Stroke Via Microglial CB2R/P2Y12 Signaling. Mol. Neurobiol. 2025, 62, 2039–2057. [Google Scholar] [CrossRef]
- Zarruk, J.G.; Fernández-López, D.; García-Yébenes, I.; García-Gutiérrez, M.S.; Vivancos, J.; Nombela, F.; Torres, M.; Burguete, M.C.; Manzanares, J.; Lizasoain, I.; et al. Cannabinoid Type 2 Receptor Activation Downregulates Stroke-Induced Classic and Alternative Brain Macrophage/Microglial Activation Concomitant to Neuroprotection. Stroke 2012, 43, 211–219. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Reichenbach, N.L.; Fan, S.; Rom, S.; Merkel, S.F.; Wang, X.; Ho, W.; Persidsky, Y. Attenuation of HIV-1 replication in macrophages by cannabinoid receptor 2 agonists. J. Leukoc. Biol. 2013, 93, 801–810. [Google Scholar] [CrossRef]
- Gómez-Gálvez, Y.; Palomo-Garo, C.; Fernández-Ruiz, J.; García, C. Potential of the cannabinoid CB2 receptor as a pharmacological target against inflammation in Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 200–208. [Google Scholar] [CrossRef]
- Sobue, A.; Komine, O.; Endo, F.; Kakimi, C.; Miyoshi, Y.; Kawade, N.; Watanabe, S.; Saito, Y.; Murayama, S.; Saido, T.C.; et al. Microglial cannabinoid receptor type II stimulation improves cognitive impairment and neuroinflammation in Alzheimer’s disease mice by controlling astrocyte activation. Cell Death Dis. 2024, 15, 858. [Google Scholar] [CrossRef]
- Benito, C.; Núñez, E.; Tolón, R.M.; Carrier, E.J.; Rábano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2 Receptors and Fatty Acid Amide Hydrolase Are Selectively Overexpressed in Neuritic Plaque-Associated Glia in Alzheimer’s Disease Brains. J. Neurosci. 2003, 23, 11136–11141. [Google Scholar] [CrossRef] [PubMed]
- Evens, N.; Vandeputte, C.; Coolen, C.; Janssen, P.; Sciot, R.; Baekelandt, V.; Verbruggen, A.M.; Debyser, Z.; Van Laere, K.; Bormans, G.M. Preclinical evaluation of [11C]NE40, a type 2 cannabinoid receptor PET tracer. Nucl. Med. Biol. 2012, 39, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Iga, Y.; Nakamura, M.; Takizawa, C.; Fukumoto, D.; Kakiuchi, T.; Nishiyama, S.; Ohba, H.; Tsukada, H.; Sato, K.; et al. Upregulation of cannabinoid receptor type 2, but not TSPO, in senescence-accelerated neuroinflammation in mice: A positron emission tomography study. J. Neuroinflamm. 2019, 16, 208. [Google Scholar] [CrossRef]
- Vandeputte, C.; Casteels, C.; Struys, T.; Koole, M.; van Veghel, D.; Evens, N.; Gerits, A.; Dresselaers, T.; Lambrichts, I.; Himmelreich, U.; et al. Small-animal PET imaging of the type 1 and type 2 cannabinoid receptors in a photothrombotic stroke model. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Horti, A.G.; Gao, Y.; Ravert, H.T.; Finley, P.; Valentine, H.; Wong, D.F.; Endres, C.J.; Savonenko, A.V.; Dannals, R.F. Synthesis and biodistribution of [11C]A-836339, a new potential radioligand for PET imaging of cannabinoid type 2 receptors (CB2). Bioorganic Med. Chem. 2010, 18, 5202–5207. [Google Scholar] [CrossRef]
- Ahmed, W.; Katz, S. Therapeutic Use of Cannabis in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016, 12, 668. [Google Scholar]
- Creoli, M.; Di Paola, A.; Tarallo, A.; Aziz, S.; Miele, E.; Martinelli, M.; Casertano, M.; Colucci, A.; Cenni, S.; Marrapodi, M.M.; et al. Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Inflammatory Bowel Disease. Int. J. Mol. Sci. 2025, 26, 3720. [Google Scholar] [CrossRef]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Shiratsuchi, A.; Watanabe, I.; Yoshida, H.; Nakanishi, Y. Involvement of cannabinoid receptor CB2 in dectin-1-mediated macrophage phagocytosis. Immunol. Cell Biol. 2008, 86, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Gokoh, M.; Kishimoto, S.; Oka, S.; Sugiura, T. 2-Arachidonoylglycerol enhances the phagocytosis of opsonized zymosan by HL-60 cells differentiated into macrophage-like cells. Biol. Pharm. Bull. 2007, 30, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Silvin, A.; Qian, J.; Ginhoux, F. Brain macrophage development, diversity and dysregulation in health and disease. Cell. Mol. Immunol. 2023, 20, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Ots, H.D.; Tracz, J.A.; Vinokuroff, K.E.; Musto, A.E. CD40–CD40L in Neurological Disease. Int. J. Mol. Sci. 2022, 23, 4115. [Google Scholar] [CrossRef]
- Tarique, A.A.; Evron, T.; Zhang, G.; Tepper, M.A.; Morshed, M.M.; Andersen, I.S.G.; Begum, N.; Sly, P.D.; Fantino, E. Anti-inflammatory effects of lenabasum, a cannabinoid receptor type 2 agonist, on macrophages from cystic fibrosis. J. Cyst. Fibros. 2020, 19, 823–829. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Hsieh, S.-L.; Lee, P.-C.; Yeh, Y.-C.; Lee, K.-C.; Hsieh, Y.-C.; Wang, Y.-W.; Lee, T.-Y.; Huang, Y.-H.; Chan, C.-C.; et al. Correction in Long-term cannabinoid type 2 receptor agonist therapy decreases bacterial translocation in rats with cirrhosis and ascites. J. Hepatol. 2014, 61, 1004–1013. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, Y.; Huang, X.; Yuan, A.; Shao, Q.; Pu, J.; He, B. Selective activation of CB2 receptor improves efferocytosis in cultured macrophages. Life Sci. 2016, 161, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Shao, Q.; Wang, X.; Ma, K.; Chen, N.; Yuan, Y. Correction in CB2 receptor activation inhibits the phagocytic function of microglia through activating ERK/AKT-Nurr1 signal pathways. Acta Pharmacol. Sin. 2022, 43, 2253–2266. [Google Scholar] [CrossRef]
- Mai, P.; Tian, L.; Yang, L.; Wang, L.; Yang, L.; Li, L. Cannabinoid receptor 1 but not 2 mediates macrophage phagocytosis by G(α)i/o/RhoA/ROCK signaling pathway. J. Cell. Physiol. 2015, 230, 1640–1650. [Google Scholar] [CrossRef]
- McCoy, K.L.; Matveyeva, M.; Carlisle, S.J.; Cabral, G.A. Cannabinoid Inhibition of the Processing of Intact Lysozyme by Macrophages: Evidence for CB2 Receptor Participation1. J. Pharmacol. Exp. Ther. 1999, 289, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Matveyeva, M.; Hartmann, C.B.; Harrison, M.T.; Cabral, G.A.; McCoy, K.L. Delta9-tetrahydrocannabinol selectively increases aspartyl cathepsin D proteolytic activity and impairs lysozyme processing by macrophages. Int. J. Immunopharmacol. 2000, 22, 373–381. [Google Scholar] [CrossRef]
- McCoy, K.L.; Gainey, D.; Cabral, G.A. delta 9-Tetrahydrocannabinol modulates antigen processing by macrophages. J. Pharmacol. Exp. Ther. 1995, 273, 1216–1223. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, F.; Liu, A.; Li, Z.; Zheng, F.; Liu, Q.; Yang, L.; Chen, K.; Wang, Y.; Zhang, Z.; et al. Activation of CB2 receptor inhibits pyroptosis and subsequently ameliorates cecal ligation and puncture-induced sepsis. Int. Immunopharmacol. 2021, 99, 108038. [Google Scholar] [CrossRef]
- Liu, A.P.; Yuan, Q.H.; Zhang, B.; Yang, L.; He, Q.W.; Chen, K.; Liu, Q.S.; Li, Z.; Zhan, J. Erratum in Cannabinoid receptor 2 activation alleviates septic lung injury by promoting autophagy via inhibition of inflammatory mediator release. Cell. Signal. 2020, 69, 109556. [Google Scholar] [CrossRef]
- Ke, P.; Shao, B.-Z.; Xu, Z.-Q.; Wei, W.; Han, B.-Z.; Chen, X.-W.; Su, D.-F.; Liu, C. Activation of Cannabinoid Receptor 2 Ameliorates DSS-Induced Colitis through Inhibiting NLRP3 Inflammasome in Macrophages. PLoS ONE 2016, 11, e0155076. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Wei, W.; Ke, P.; Xu, Z.-Q.; Zhou, J.-X.; Liu, C. Activating cannabinoid receptor 2 alleviates pathogenesis of experimental autoimmune encephalomyelitis via activation of autophagy and inhibiting NLRP3 inflammasome. CNS Neurosci. Ther. 2014, 20, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Tong, F.; Li, K.; Wang, Q.; Ding, J.; Wang, X. Cannabinoid receptor 2 agonist AM1241 alleviates epileptic seizures and epilepsy-associated depression via inhibiting neuroinflammation in a pilocarpine-induced chronic epilepsy mouse model. Mol. Cell. Neurosci. 2024, 130, 103958. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Liu, D.; Li, X.; He, L.; Pan, J.; Shen, Q.; Peng, Y. CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice. Brain Behav. Immun. 2023, 110, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Denaës, T.; Lodder, J.; Chobert, M.-N.; Ruiz, I.; Pawlotsky, J.-M.; Lotersztajn, S.; Teixeira-Clerc, F. The Cannabinoid Receptor 2 Protects Against Alcoholic Liver Disease via a Macrophage Autophagy-Dependent Pathway. Sci. Rep. 2016, 6, 28806. [Google Scholar] [CrossRef] [PubMed]
- Suryavanshi, S.V.; Zaiachuk, M.; Pryimak, N.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids Alleviate the LPS-Induced Cytokine Storm via Attenuating NLRP3 Inflammasome Signaling and TYK2-Mediated STAT3 Signaling Pathways In Vitro. Cells 2022, 11, 1391. [Google Scholar] [CrossRef]
- Marrs, W.R.; Blankman, J.L.; Horne, E.A.; Thomazeau, A.; Lin, Y.H.; Coy, J.; Bodor, A.L.; Muccioli, G.G.; Hu, S.S.-J.; Woodruff, G.; et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat. Neurosci. 2010, 13, 951–957. [Google Scholar] [CrossRef]
- Kishimoto, S.; Gokoh, M.; Oka, S.; Muramatsu, M.; Kajiwara, T.; Waku, K.; Sugiura, T. 2-Arachidonoylglycerol Induces the Migration of HL-60 Cells Differentiated into Macrophage-like Cells and Human Peripheral Blood Monocytes Through the Cannabinoid CB2 Receptor-dependent Mechanism. J. Biol. Chem. 2003, 278, 24469–24475. [Google Scholar] [CrossRef]
- Walter, L.; Franklin, A.; Witting, A.; Wade, C.; Xie, Y.; Kunos, G.; Mackie, K.; Stella, N. Nonpsychotropic Cannabinoid Receptors Regulate Microglial Cell Migration. J. Neurosci. 2003, 23, 1398–1405. [Google Scholar] [CrossRef]
- Gokoh, M.; Kishimoto, S.; Oka, S.; Mori, M.; Waku, K.; Ishima, Y.; Sugiura, T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces rapid actin polymerization in HL-60 cells differentiated into macrophage-like cells. Biochem. J. 2005, 386, 583–589. [Google Scholar] [CrossRef]
- Kishimoto, S.; Kobayashi, Y.; Oka, S.; Gokoh, M.; Waku, K.; Sugiura, T. 2-Arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces accelerated production of chemokines in HL-60 cells. J. Biochem. 2004, 135, 517–524. [Google Scholar] [CrossRef]
- Raborn, E.S.; Marciano-Cabral, F.; Buckley, N.E.; Martin, B.R.; Cabral, G.A. The Cannabinoid Delta-9-tetrahydrocannabinol Mediates Inhibition of Macrophage Chemotaxis to RANTES/CCL5: Linkage to the CB2 Receptor. J. Neuroimmune Pharmacol. 2008, 3, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Sacerdote, P.; Massi, P.; Panerai, A.E.; Parolaro, D. In vivo and in vitro treatment with the synthetic cannabinoid CP55,940 decreases the in vitro migration of macrophages in the rat: Involvement of both CB1 and CB2 receptors. J. Neuroimmunol. 2000, 109, 155–163. [Google Scholar] [CrossRef]
- Sacerdote, P.; Martucci, C.; Vaccani, A.; Bariselli, F.; Panerai, A.E.; Colombo, A.; Parolaro, D.; Massi, P. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J. Neuroimmunol. 2005, 159, 97–105. [Google Scholar] [CrossRef]
- Montecucco, F.; Burger, F.; Mach, F.; Steffens, S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1145–H1155. [Google Scholar] [CrossRef] [PubMed]
- Raborn, E.S.; Cabral, G.A. Cannabinoid Inhibition of Macrophage Migration to the Trans-Activating (Tat) Protein of HIV-1 Is Linked to the CB2 Cannabinoid Receptor. J. Pharmacol. Exp. Ther. 2010, 333, 319–327. [Google Scholar] [CrossRef]
- Fraga, D.; Raborn, E.S.; Ferreira, G.A.; Cabral, G.A. Cannabinoids Inhibit Migration of Microglial-like Cells to the HIV Protein Tat. J. Neuroimmune Pharmacol. 2011, 6, 566–577. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Z.; Liu, Y.; Xue, J.; Tian, Y.; Liu, W.; Zhang, W.; Shen, Y.; Xu, W.; Liang, X.; et al. Activation of Cannabinoid CB2 Receptor Ameliorates Atherosclerosis Associated with Suppression of Adhesion Molecules. J. Cardiovasc. Pharmacol. 2010, 55, 292. [Google Scholar] [CrossRef]
- Steffens, S.; Veillard, N.R.; Arnaud, C.; Pelli, G.; Burger, F.; Staub, C.; Zimmer, A.; Frossard, J.-L.; Mach, F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 2005, 434, 782–786. [Google Scholar] [CrossRef]
- Molica, F.; Matter, C.M.; Burger, F.; Pelli, G.; Lenglet, S.; Zimmer, A.; Pacher, P.; Steffens, S. Cannabinoid receptor CB2 protects against balloon-induced neointima formation. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1064–H1074. [Google Scholar] [CrossRef]
- Braun, M.; Khan, Z.T.; Khan, M.B.; Kumar, M.; Ward, A.; Achyut, B.R.; Arbab, A.S.; Hess, D.C.; Hoda, M.d.N.; Baban, B.; et al. Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain Behav. Immun. 2018, 68, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Schmöle, A.-C.; Lundt, R.; Ternes, S.; Albayram, Ö.; Ulas, T.; Schultze, J.L.; Bano, D.; Nicotera, P.; Alferink, J.; Zimmer, A. Cannabinoid receptor 2 deficiency results in reduced neuroinflammation in an Alzheimer’s disease mouse model. Neurobiol. Aging 2015, 36, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, T.; Corsten, C.; Beier, J.P.; Kim, B.-S. The immunosuppressive effect of the endocannabinoid system on the inflammatory phenotypes of macrophages and mesenchymal stromal cells: A comparative study. Pharmacol. Rep. 2021, 73, 143–153. [Google Scholar] [CrossRef]
- Persidsky, Y.; Fan, S.; Dykstra, H.; Reichenbach, N.L.; Rom, S.; Ramirez, S.H. Activation of Cannabinoid Type Two Receptors (CB2) Diminish Inflammatory Responses in Macrophages and Brain Endothelium. J. Neuroimmune Pharmacol. 2015, 10, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.; Mestre, L.; Docagne, F.; Guaza, C. Activation of cannabinoid CB2 receptor negatively regulates IL-12p40 production in murine macrophages: Role of IL-10 and ERK1/2 kinase signaling. Br. J. Pharmacol. 2005, 145, 441–448. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, R.; Long, C.; Shu, Y.; He, P.; Zhou, Y.; Xiang, Y.; Wang, Y. The protective effect of cannabinoid type II receptor agonist AM1241 on ConA-induced liver injury in mice via mitogen-activated protein kinase signalling pathway. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211035251. [Google Scholar] [CrossRef]
- Correa, F.; Hernangómez, M.; Mestre, L.; Loría, F.; Spagnolo, A.; Docagne, F.; Di Marzo, V.; Guaza, C. Anandamide enhances IL-10 production in activated microglia by targeting CB2 receptors: Roles of ERK1/2, JNK, and NF-κB. Glia 2010, 58, 135–147. [Google Scholar] [CrossRef]
- Correa, F.; Docagne, F.; Mestre, L.; Clemente, D.; Hernangómez, M.; Loría, F.; Guaza, C. A role for CB2 receptors in anandamide signalling pathways involved in the regulation of IL-12 and IL-23 in microglial cells. Biochem. Pharmacol. 2009, 77, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Gutierrez-Lopez, M.D.; Borcel, E.; Peraile, I.; Mayado, A.; O’Shea, E.; Colado, M.I. Evidence that MDMA (‘ecstasy’) increases cannabinoid CB2 receptor expression in microglial cells: Role in the neuroinflammatory response in rat brain. J. Neurochem. 2010, 113, 67–78. [Google Scholar] [CrossRef]
- Pérez-Diego, M.; Angelina, A.; Martín-Cruz, L.; de la Rocha-Muñoz, A.; Maldonado, A.; Sevilla-Ortega, C.; Palomares, O. Cannabinoid WIN55,212-2 reprograms monocytes and macrophages to inhibit LPS-induced inflammation. Front. Immunol. 2023, 14, 1147520. [Google Scholar] [CrossRef]
- Puffenbarger, R.A.; Boothe, A.C.; Cabral, G.A. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia 2000, 29, 58–69. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Lee, S.T.; Lin, W.-W. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: Involvement of eicosanoids. J. Cell. Biochem. 2001, 81, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Batkai, S.; Osei-Hyiaman, D.; Pan, H.; El-Assal, O.; Rajesh, M.; Mukhopadhyay, P.; Hong, F.; Harvey-White, J.; Jafri, A.; Haskó, G.; et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 2007, 21, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Mou, X.; Huang, J.; Xiong, N.; Li, H. Trans-Caryophyllene Suppresses Hypoxia-Induced Neuroinflammatory Responses by Inhibiting NF-κB Activation in Microglia. J. Mol. Neurosci. 2014, 54, 41–48. [Google Scholar] [CrossRef]
- Valeriano, J.D.P.; Andrade-Silva, M.; Pereira-Dutra, F.; Seito, L.N.; Bozza, P.T.; Rosas, E.C.; Souza Costa, M.F.; Henriques, M.G. Cannabinoid receptor type 2 agonist GP1a attenuates macrophage activation induced by M. bovis-BCG by inhibiting NF-κB signaling. J. Leukoc. Biol. 2025, 117, qiae246. [Google Scholar] [CrossRef] [PubMed]
- Correa, F.; Hernangómez-Herrero, M.; Mestre, L.; Loría, F.; Docagne, F.; Guaza, C. The endocannabinoid anandamide downregulates IL-23 and IL-12 subunits in a viral model of multiple sclerosis: Evidence for a cross-talk between IL-12p70/IL-23 axis and IL-10 in microglial cells. Brain Behav. Immun. 2011, 25, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.; Weigert, A.; Hériché, J.-K.; Mille-Baker, B.; Janssen, R.A.J.; Brüne, B. RNAi screen in apoptotic cancer cell-stimulated human macrophages reveals co-regulation of IL-6/IL-10 expression. Immunobiology 2013, 218, 40–51. [Google Scholar] [CrossRef]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.-N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology 2011, 54, 1217–1226. [Google Scholar] [CrossRef]
- Steib, C.J.; Gmelin, L.; Pfeiler, S.; Schewe, J.; Brand, S.; Göke, B.; Gerbes, A.L. Functional relevance of the cannabinoid receptor 2—heme oxygenase pathway: A novel target for the attenuation of portal hypertension. Life Sci. 2013, 93, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.; Hamdaoui, N.; Bizy, A.; Zoltani, K.; Souktani, R.; Zafrani, E.-S.; Mallat, A.; Lotersztajn, S.; Lafdil, F. Cannabinoid receptor 2 counteracts interleukin-17-induced immune and fibrogenic responses in mouse liver. Hepatology 2014, 59, 296–306. [Google Scholar] [CrossRef]
- Tomar, S.; Zumbrun, E.E.; Nagarkatti, M.; Nagarkatti, P.S. Protective Role of Cannabinoid Receptor 2 Activation in Galactosamine/Lipopolysaccharide-Induced Acute Liver Failure through Regulation of Macrophage Polarization and MicroRNAs. J. Pharmacol. Exp. Ther. 2015, 353, 369–379. [Google Scholar] [CrossRef]
- Du, Y.; Ren, P.; Wang, Q.; Jiang, S.-K.; Zhang, M.; Li, J.-Y.; Wang, L.-L.; Guan, D.-W. Cannabinoid 2 receptor attenuates inflammation during skin wound healing by inhibiting M1 macrophages rather than activating M2 macrophages. J. Inflamm. 2018, 15, 25. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, L.; Zhang, M.; Zhang, M.; Wang, C.; Zhao, R.; Guan, D. Cannabinoid type 2 receptor manipulates skeletal muscle regeneration partly by regulating macrophage M1/M2 polarization in IR injury in mice. Life Sci. 2020, 256, 117989. [Google Scholar] [CrossRef]
- Tortora, C.; Di Paola, A.; Argenziano, M.; Creoli, M.; Marrapodi, M.M.; Cenni, S.; Tolone, C.; Rossi, F.; Strisciuglio, C. Effects of CB2 Receptor Modulation on Macrophage Polarization in Pediatric Celiac Disease. Biomedicines 2022, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Staiano, R.I.; Loffredo, S.; Borriello, F.; Iannotti, F.A.; Piscitelli, F.; Orlando, P.; Secondo, A.; Granata, F.; Lepore, M.T.; Fiorelli, A.; et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J. Leukoc. Biol. 2016, 99, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Niu, W.; Lv, J.; Jia, J.; Zhu, M.; Yang, S. PGC-1α-Mediated Mitochondrial Biogenesis is Involved in Cannabinoid Receptor 2 Agonist AM1241-Induced Microglial Phenotype Amelioration. Cell. Mol. Neurobiol. 2018, 38, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shi, J.; Wang, B.; Li, J.; Jia, H. CB2 cannabinoid receptor agonist ameliorates novel object recognition but not spatial memory in transgenic APP/PS1 mice. Neurosci. Lett. 2019, 707, 134286. [Google Scholar] [CrossRef]
- Askari, V.R.; Baradaran Rahimi, V.; Tabatabaee, S.A.; Shafiee-Nick, R. Combination of Imipramine, a sphingomyelinase inhibitor, and β-caryophyllene improve their therapeutic effects on experimental autoimmune encephalomyelitis (EAE). Int. Immunopharmacol. 2019, 77, 105923. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Machín, I.; Cordero, C.; Carrillo-Salinas, F.; Mestre, L.; Hernández-Torres, G.; Ortega-Gutiérrez, S.; López-Rodríguez, M.L.; de Castro, F.; et al. 2-AG limits Theiler’s virus induced acute neuroinflammation by modulating microglia and promoting MDSCs. Glia 2018, 66, 1447–1463. [Google Scholar] [CrossRef]
- Li, L.; Luo, Q.; Shang, B.; Yang, X.; Zhang, Y.; Pan, Q.; Wu, N.; Tang, W.; Du, D.; Sun, X.; et al. Selective activation of cannabinoid receptor-2 reduces white matter injury via PERK signaling in a rat model of traumatic brain injury. Exp. Neurol. 2022, 347, 113899. [Google Scholar] [CrossRef]
- Reiner, A.; Heldt, S.A.; Presley, C.S.; Guley, N.H.; Elberger, A.J.; Deng, Y.; D’Surney, L.; Rogers, J.T.; Ferrell, J.; Bu, W.; et al. Motor, Visual and Emotional Deficits in Mice after Closed-Head Mild Traumatic Brain Injury Are Alleviated by the Novel CB2 Inverse Agonist SMM-189. Int. J. Mol. Sci. 2015, 16, 758–787. [Google Scholar] [CrossRef]
- Xiang, W.; Shi, R.; Kang, X.; Zhang, X.; Chen, P.; Zhang, L.; Hou, A.; Wang, R.; Zhao, Y.; Zhao, K.; et al. Monoacylglycerol lipase regulates cannabinoid receptor 2-dependent macrophage activation and cancer progression. Nat. Commun. 2018, 9, 2574. [Google Scholar] [CrossRef]
- Duan, J.; Chen, J.; Lin, Y.; Lin, S.L.; Wu, J. Endocannabinoid Receptor 2 Function is Associated with Tumor-Associated Macrophage Accumulation and Increases in T Cell Number to Initiate a Potent Antitumor Response in a Syngeneic Murine Model of Glioblastoma. Cannabis Cannabinoid Res. 2024, 9, 1524–1536. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Lanuti, M.; Catanzaro, G.; Fezza, F.; Rapino, C.; Maccarrone, M. Detailed characterization of the endocannabinoid system in human macrophages and foam cells, and anti-inflammatory role of type-2 cannabinoid receptor. Atherosclerosis 2014, 233, 55–63. [Google Scholar] [CrossRef]
- Williams, J.C.; Appelberg, S.; Goldberger, B.A.; Klein, T.W.; Sleasman, J.W.; Goodenow, M.M. Δ9-Tetrahydrocannabinol Treatment During Human Monocyte Differentiation Reduces Macrophage Susceptibility to HIV-1 Infection. J. Neuroimmune Pharmacol. 2014, 9, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cabral, G.A.; Martin, B.R.; Peterson, P.K. WIN55,212-2-Mediated Inhibition of HIV-1 Expression in Microglial Cells: Involvement of Cannabinoid Receptors. J. Neuroimmune Pharmacol. 2007, 2, 178–183. [Google Scholar] [CrossRef]
- Cosenza-Nashat, M.A.; Bauman, A.; Zhao, M.-L.; Morgello, S.; Suh, H.-S.; Lee, S.C. Cannabinoid receptor expression in HIV encephalitis and HIV-associated neuropathologic comorbidities. Neuropathol. Appl. Neurobiol. 2011, 37, 464–483. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Y.; Zhou, Y.; Yu, J.; Liang, M.; Qin, L.; Zhou, Y. Win55,212-2 improves neural injury induced by HIV-1 glycoprotein 120 in rats by exciting CB2R. Brain Res. Bull. 2022, 182, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Sheng, W.S.; Rock, R.B. CB2 Receptor Agonists Protect Human Dopaminergic Neurons Against Damage from HIV-1 gp120. PLoS ONE 2013, 8, e77577. [Google Scholar] [CrossRef]
- Rosario-Rodríguez, L.J.; Gerena, Y.; García-Requena, L.A.; Cartagena-Isern, L.J.; Cuadrado-Ruiz, J.C.; Borges-Vélez, G.; Meléndez, L.M. Cannabinoid receptor type 2 agonist JWH-133 decreases cathepsin B secretion and neurotoxicity from HIV-infected macrophages. Sci. Rep. 2022, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Rodríguez, L.J.; Cantres-Rosario, Y.M.; Carrasquillo-Carrión, K.; Rodríguez-De Jesús, A.E.; Cartagena-Isern, L.J.; García-Requena, L.A.; Roche-Lima, A.; Meléndez, L.M. Quantitative Proteomics Reveal That CB2R Agonist JWH-133 Downregulates NF-κB Activation, Oxidative Stress, and Lysosomal Exocytosis from HIV-Infected Macrophages. Int. J. Mol. Sci. 2024, 25, 3246. [Google Scholar] [CrossRef]
- Alferink, J.; Specht, S.; Arends, H.; Schumak, B.; Schmidt, K.; Ruland, C.; Lundt, R.; Kemter, A.; Dlugos, A.; Kuepper, J.M.; et al. Cannabinoid Receptor 2 Modulates Susceptibility to Experimental Cerebral Malaria through a CCL17-dependent Mechanism. J. Biol. Chem. 2016, 291, 19517–19531. [Google Scholar] [CrossRef]
- Klegeris, A.; Bissonnette, C.J.; McGeer, P.L. Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid-type CB2 receptor. Br. J. Pharmacol. 2003, 139, 775–786. [Google Scholar] [CrossRef]
- Ribeiro, R.; Wen, J.; Li, S.; Zhang, Y. Involvement of ERK1/2, cPLA2 and NF-κB in microglia suppression by cannabinoid receptor agonists and antagonists. Prostaglandins Other Lipid Mediat. 2013, 100–101, 1–14. [Google Scholar] [CrossRef]
- Racz, I.; Nadal, X.; Alferink, J.; Baños, J.E.; Rehnelt, J.; Martín, M.; Pintado, B.; Gutierrez-Adan, A.; Sanguino, E.; Manzanares, J.; et al. Crucial Role of CB2 Cannabinoid Receptor in the Regulation of Central Immune Responses during Neuropathic Pain. J. Neurosci. 2008, 28, 12125–12135. [Google Scholar] [CrossRef]
- Nent, E.; Nozaki, C.; Schmöle, A.-C.; Otte, D.; Zimmer, A. CB2 receptor deletion on myeloid cells enhanced mechanical allodynia in a mouse model of neuropathic pain. Sci. Rep. 2019, 9, 7468. [Google Scholar] [CrossRef]
- Racz, I.; Nadal, X.; Alferink, J.; Baños, J.E.; Rehnelt, J.; Martín, M.; Pintado, B.; Gutierrez-Adan, A.; Sanguino, E.; Bellora, N.; et al. Interferon-gamma is a critical modulator of CB2 cannabinoid receptor signaling during neuropathic pain. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 12136–12145. [Google Scholar] [CrossRef] [PubMed]
- Luongo, L.; Palazzo, E.; Tambaro, S.; Giordano, C.; Gatta, L.; Scafuro, M.A.; Rossi, F.; Lazzari, P.; Pani, L.; de Novellis, V.; et al. 1-(2′,4′-dichlorophenyl)-6-methyl-N-cyclohexylamine-1,4-dihydroindeno[1,2-c]pyrazole-3-carboxamide, a novel CB2 agonist, alleviates neuropathic pain through functional microglial changes in mice. Neurobiol. Dis. 2010, 37, 177–185. [Google Scholar] [CrossRef]
- Romero-Sandoval, A.; Nutile-McMenemy, N.; DeLeo, J.A. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology 2008, 108, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hocevar, M.; Bie, B.; Foss, J.F.; Naguib, M. Cannabinoid Type 2 Receptor System Modulates Paclitaxel-Induced Microglial Dysregulation and Central Sensitization in Rats. J. Pain 2019, 20, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jia, D.; Li, W.; Huang, Z.; Shan, R.; Huang, C. Trifluoro-Icaritin Ameliorates Neuroinflammation Against Complete Freund’s Adjuvant-Induced Microglial Activation by Improving CB2 Receptor-Mediated IL-10/β-endorphin Signaling in the Spinal Cord of Rats. J. Neuroimmune Pharmacol. 2024, 19, 53. [Google Scholar] [CrossRef]
- Li, L.; Tao, Y.; Tang, J.; Chen, Q.; Yang, Y.; Feng, Z.; Chen, Y.; Yang, L.; Yang, Y.; Zhu, G.; et al. A Cannabinoid Receptor 2 Agonist Prevents Thrombin-Induced Blood–Brain Barrier Damage via the Inhibition of Microglial Activation and Matrix Metalloproteinase Expression in Rats. Transl. Stroke Res. 2015, 6, 467–477. [Google Scholar] [CrossRef]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Elliott, M.B. A cannabinoid type 2 receptor agonist attenuates blood–brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012, 90, 2293–2305. [Google Scholar] [CrossRef]
- Bu, W.; Ren, H.; Deng, Y.; Del Mar, N.; Guley, N.M.; Moore, B.M.; Honig, M.G.; Reiner, A. Mild Traumatic Brain Injury Produces Neuron Loss That Can Be Rescued by Modulating Microglial Activation Using a CB2 Receptor Inverse Agonist. Front. Neurosci. 2016, 10, 449. [Google Scholar] [CrossRef]
- Yu, Y.; Li, L.; Nguyen, D.T.; Mustafa, S.M.; Moore, B.M.; Jiang, J. Inverse Agonism of Cannabinoid Receptor Type 2 Confers Anti-inflammatory and Neuroprotective Effects Following Status Epileptics. Mol. Neurobiol. 2020, 57, 2830–2845. [Google Scholar] [CrossRef]
- Ma, S.; Nakamura, Y.; Uemoto, S.; Yamamoto, K.; Hisaoka-Nakashima, K.; Morioka, N. Intranasal Treatment with Cannabinoid 2 Receptor Agonist HU-308 Ameliorates Cold Sensitivity in Mice with Traumatic Trigeminal Neuropathic Pain. Cells 2024, 13, 1943. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.; Zeng, Q.; Liu, Y.; Shan, L.; Ji, L.; Wu, Y.; Wu, J.; Chen, Y.; Li, Y.; et al. Cannabinoid CB2 receptor controls chronic itch by regulating spinal microglial activation and synaptic transmission. Cell Rep. 2025, 44, 115559. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, L.; Burnham, S.C.; Dell’Agnello, G.; Dowsett, S.A.; Epelbaum, S. Diagnostic Biomarkers of Amyloid and Tau Pathology in Alzheimer’s Disease: An Overview of Tests for Clinical Practice in the United States and Europe. J. Prev. Alzheimer’s Dis. 2023, 10, 426–442. [Google Scholar] [CrossRef] [PubMed]
- Tolón, R.M.; Núñez, E.; Pazos, M.R.; Benito, C.; Castillo, A.I.; Martínez-Orgado, J.A.; Romero, J. The activation of cannabinoid CB2 receptors stimulates in situ and in vitro beta-amyloid removal by human macrophages. Brain Res. 2009, 1283, 148–154. [Google Scholar] [CrossRef]
- Martín-Moreno, A.M.; Brera, B.; Spuch, C.; Carro, E.; García-García, L.; Delgado, M.; Pozo, M.A.; Innamorato, N.G.; Cuadrado, A.; de Ceballos, M.L. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers β-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J. Neuroinflamm. 2012, 9, 8. [Google Scholar] [CrossRef]
- Wu, J.; Bie, B.; Yang, H.; Xu, J.J.; Brown, D.L.; Naguib, M. Activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol. Aging 2013, 34, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Koppel, J.; Vingtdeux, V.; Marambaud, P.; D’abramo, C.; Jimenez, H.; Stauber, M.; Friedman, R.; Davies, P. CB2 Receptor Deficiency Increases Amyloid Pathology and Alters Tau Processing in a Transgenic Mouse Model of Alzheimer’s Disease. Mol. Med. 2013, 19, 29–36. [Google Scholar] [CrossRef]
- Schmöle, A.-C.; Lundt, R.; Toporowski, G.; Hansen, J.N.; Beins, E.; Halle, A.; Zimmer, A. Cannabinoid Receptor 2-Deficiency Ameliorates Disease Symptoms in a Mouse Model with Alzheimer’s Disease-Like Pathology. J. Alzheimer’s Dis. 2018, 64, 379–392. [Google Scholar] [CrossRef]
- Komorowska-Müller, J.A.; Rana, T.; Olabiyi, B.F.; Zimmer, A.; Schmöle, A.-C. Cannabinoid Receptor 2 Alters Social Memory and Microglial Activity in an Age-Dependent Manner. Molecules 2021, 26, 5984. [Google Scholar] [CrossRef] [PubMed]
- Olabiyi, B.F.; Schmoele, A.-C.; Beins, E.C.; Zimmer, A. Pharmacological blockade of cannabinoid receptor 2 signaling does not affect LPS/IFN-γ-induced microglial activation. Sci. Rep. 2023, 13, 11105. [Google Scholar] [CrossRef]
- Reusch, N.; Ravichandran, K.A.; Olabiyi, B.F.; Komorowska-Müller, J.A.; Hansen, J.N.; Ulas, T.; Beyer, M.; Zimmer, A.; Schmöle, A.-C. Cannabinoid receptor 2 is necessary to induce toll-like receptor-mediated microglial activation. Glia 2022, 70, 71–88. [Google Scholar] [CrossRef]
- Chung, Y.C.; Shin, W.-H.; Baek, J.Y.; Cho, E.J.; Baik, H.H.; Kim, S.R.; Won, S.-Y.; Jin, B.K. CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2016, 48, e205. [Google Scholar] [CrossRef]
- Shoemaker, J.L.; Seely, K.A.; Reed, R.L.; Crow, J.P.; Prather, P.L. The CB2 cannabinoid agonist AM-1241 prolongs survival in a transgenic mouse model of amyotrophic lateral sclerosis when initiated at symptom onset. J. Neurochem. 2007, 101, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Espejo-Porras, F.; García-Toscano, L.; Rodríguez-Cueto, C.; Santos-García, I.; de Lago, E.; Fernandez-Ruiz, J. Targeting glial cannabinoid CB2 receptors to delay the progression of the pathological phenotype in TDP-43 (A315T) transgenic mice, a model of amyotrophic lateral sclerosis. Br. J. Pharmacol. 2019, 176, 1585–1600. [Google Scholar] [CrossRef]
- Ouali Alami, N.; Schurr, C.; Olde Heuvel, F.; Tang, L.; Li, Q.; Tasdogan, A.; Kimbara, A.; Nettekoven, M.; Ottaviani, G.; Raposo, C.; et al. NF-κB activation in astrocytes drives a stage-specific beneficial neuroimmunological response in ALS. EMBO J. 2018, 37, e98697. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zeng, Y.; Wu, J. The CB2 Receptor as a Novel Therapeutic Target for Epilepsy Treatment. Int. J. Mol. Sci. 2021, 22, 8961. [Google Scholar] [CrossRef]
- Shapiro, L.; Wong, J.C.; Escayg, A. Reduced cannabinoid 2 receptor activity increases susceptibility to induced seizures in mice. Epilepsia 2019, 60, 2359–2369. [Google Scholar] [CrossRef]
- Huizenga, M.N.; Wicker, E.; Beck, V.C.; Forcelli, P.A. Anticonvulsant effect of cannabinoid receptor agonists in models of seizures in developing rats. Epilepsia 2017, 58, 1593–1602. [Google Scholar] [CrossRef]
- Alberti, T.B.; Barbosa, W.L.R.; Vieira, J.L.F.; Raposo, N.R.B.; Dutra, R.C. (−)-β-Caryophyllene, a CB2 Receptor-Selective Phytocannabinoid, Suppresses Motor Paralysis and Neuroinflammation in a Murine Model of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 691. [Google Scholar] [CrossRef]
- Navarrete, C.; Carrillo-Salinas, F.; Palomares, B.; Mecha, M.; Jiménez-Jiménez, C.; Mestre, L.; Feliú, A.; Bellido, M.L.; Fiebich, B.L.; Appendino, G.; et al. Hypoxia mimetic activity of VCE-004.8, a cannabidiol quinone derivative: Implications for multiple sclerosis therapy. J. Neuroinflamm. 2018, 15, 64. [Google Scholar] [CrossRef]
- Ortega-Gutiérrez, S.; Molina-Holgado, E.; Arévalo-Martín, Á.; Correa, F.; Viso, A.; López-Rodríguez, M.L.; Di Marzo, V.; Guaza, C. Activation of the endocannabinoid system as a therapeutic approach in a murine model of multiple sclerosis. FASEB J. 2005, 19, 1338–1340. [Google Scholar] [CrossRef]
- Wen, J.; Ribeiro, R.; Tanaka, M.; Zhang, Y. Activation of CB2 receptor is required for the therapeutic effect of ABHD6 inhibition in experimental autoimmune encephalomyelitis. Neuropharmacology 2015, 99, 196–209. [Google Scholar] [CrossRef] [PubMed]
- del Río, C.; Navarrete, C.; Collado, J.A.; Bellido, M.L.; Gómez-Cañas, M.; Pazos, M.R.; Fernández-Ruiz, J.; Pollastro, F.; Appendino, G.; Calzado, M.A.; et al. The cannabinoid quinol VCE-004.8 alleviates bleomycin-induced scleroderma and exerts potent antifibrotic effects through peroxisome proliferator-activated receptor-γ and CB2 pathways. Sci. Rep. 2016, 6, 21703. [Google Scholar] [CrossRef]
- García-Martín, A.; Garrido-Rodríguez, M.; Navarrete, C.; del Río, C.; Bellido, M.L.; Appendino, G.; Calzado, M.A.; Muñoz, E. EHP-101, an oral formulation of the cannabidiol aminoquinone VCE-004.8, alleviates bleomycin-induced skin and lung fibrosis. Biochem. Pharmacol. 2018, 157, 304–313. [Google Scholar] [CrossRef]
- García-Martín, A.; Garrido-Rodríguez, M.; Navarrete, C.; Caprioglio, D.; Palomares, B.; DeMesa, J.; Rollland, A.; Appendino, G.; Muñoz, E. Cannabinoid derivatives acting as dual PPARγ/CB2 agonists as therapeutic agents for systemic sclerosis. Biochem. Pharmacol. 2019, 163, 321–334. [Google Scholar] [CrossRef]
- Wu, Y.; Long, C.; Shu, Y.; He, P.; Zhou, Y.; Gu, J.; Yang, L.; Wang, Y. Cannabinoid receptor 2 deletion promotes proliferation and activation of hepatic macrophages in mice with acute liver injury induced by concanavalin A. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi Chin. J. Cell. Mol. Immunol. 2019, 35, 13–18. [Google Scholar] [CrossRef]
- Varga, Z.V.; Matyas, C.; Erdelyi, K.; Cinar, R.; Nieri, D.; Chicca, A.; Nemeth, B.T.; Paloczi, J.; Lajtos, T.; Corey, L.; et al. β-Caryophyllene protects against alcoholic steatohepatitis by attenuating inflammation and metabolic dysregulation in mice. Br. J. Pharmacol. 2018, 175, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Szczesniak, A.-M.; Porter, R.F.; Toguri, J.T.; Borowska-Fielding, J.; Gebremeskel, S.; Siwakoti, A.; Johnston, B.; Lehmann, C.; Kelly, M.E.M. Cannabinoid 2 receptor is a novel anti-inflammatory target in experimental proliferative vitreoretinopathy. Neuropharmacology 2017, 113, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Freeman-Anderson, N.E.; Pickle, T.G.; Netherland, C.D.; Bales, A.; Buckley, N.E.; Thewke, D.P. Cannabinoid (CB2) receptor deficiency reduces the susceptibility of macrophages to oxidized LDL/oxysterol-induced apoptosis. J. Lipid Res. 2008, 49, 2338–2346. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-K.; Zhang, M.; Tian, Z.-L.; Wang, M.; Zhao, R.; Wang, L.-L.; Li, S.-S.; Liu, M.; Li, J.-Y.; Zhang, M.-Z.; et al. The monoacylglycerol lipase inhibitor JZL184 decreases inflammatory response in skeletal muscle contusion in rats. Eur. J. Pharmacol. 2015, 761, 1–10. [Google Scholar] [CrossRef]
- Pennant, N.M.; Hinton, C.V. The Evolution of Cannabinoid Receptors in Cancer. WIREs Mech. Dis. 2023, 15, e1602. [Google Scholar] [CrossRef]
- Ravi, J.; Elbaz, M.; Wani, N.A.; Nasser, M.W.; Ganju, R.K. Cannabinoid receptor-2 agonist inhibits macrophage induced EMT in non-small cell lung cancer by downregulation of EGFR pathway. Mol. Carcinog. 2016, 55, 2063–2076. [Google Scholar] [CrossRef]
- Mounessa, J.S.; Siegel, J.A.; Dunnick, C.A.; Dellavalle, R.P. The role of cannabinoids in dermatology. J. Am. Acad. Dermatol. 2017, 77, 188–190. [Google Scholar] [CrossRef]
- Cabral, G.A.; Griffin-Thomas, L. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef]
- Ashton, J.C.; Glass, M. The Cannabinoid CB2 Receptor as a Target for Inflammation-Dependent Neurodegeneration. Curr. Neuropharmacol. 2007, 5, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Borgelt, L.M.; Franson, K.L.; Nussbaum, A.M.; Wang, G.S. The Pharmacologic and Clinical Effects of Medical Cannabis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.A.; Whalley, B.J. The proposed mechanisms of action of CBD in epilepsy. Epileptic Disord. 2020, 22, S10–S15. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef]
- Esposito, G.; Filippis, D.D.; Cirillo, C.; Iuvone, T.; Capoccia, E.; Scuderi, C.; Steardo, A.; Cuomo, R.; Steardo, L. Cannabidiol in inflammatory bowel diseases: A brief overview. Phytother. Res. 2013, 27, 633–636. [Google Scholar] [CrossRef]
- Mishra, R.; Dhawan, P.; Srivastava, A.S.; Singh, A.B. Inflammatory bowel disease: Therapeutic limitations and prospective of the stem cell therapy. World J. Stem Cells 2020, 12, 1050–1066. [Google Scholar] [CrossRef]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef]
- Nemeth, Z.H.; Bogdanovski, D.A.; Barratt-Stopper, P.; Paglinco, S.R.; Antonioli, L.; Rolandelli, R.H. Crohn’s Disease and Ulcerative Colitis Show Unique Cytokine Profiles. Cureus 2017, 9, e1177. [Google Scholar] [CrossRef]
- Vinci, A.; Ingravalle, F.; Bardhi, D.; Cesaro, N.; Frassino, S.; Licata, F.; Valvano, M. Cannabinoid Therapeutic Effects in Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biomedicines 2022, 10, 2439. [Google Scholar] [CrossRef]
- Popp, M.; Latta, S.; Nguyen, B.; Vincenzi, C.; Tosti, A. Cannabinoids for the Treatment of Hair, Scalp, and Skin Disorders: A Systematic Review. Cosmetics 2023, 10, 129. [Google Scholar] [CrossRef]
- Lu, C.-H.; Lai, C.-Y.; Yeh, D.-W.; Liu, Y.-L.; Su, Y.-W.; Hsu, L.-C.; Chang, C.-H.; Catherine Jin, S.-L.; Chuang, T.-H. Involvement of M1 Macrophage Polarization in Endosomal Toll-Like Receptors Activated Psoriatic Inflammation. Mediat. Inflamm. 2018, 2018, 3523642. [Google Scholar] [CrossRef] [PubMed]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Laurino, B.; Vadalà, M. A therapeutic effect of cbd-enriched ointment in inflammatory skin diseases and cutaneous scars. Clin. Ter. 2019, 170, e93–e99. [Google Scholar] [CrossRef]
- Kosar, M.; Mach, L.; Carreira, C.M.; Nazaré, M.; Pacher, P.; Grether, U. Patent review of cannabinoid receptor type 2 (CB2R) modulators (2016–present). Expert Opin. Ther. Pat. 2024, 34, 665–700. [Google Scholar] [CrossRef]
- Karst, M.; Salim, K.; Burstein, S.; Conrad, I.; Hoy, L.; Schneider, U. Analgesic Effect of the Synthetic Cannabinoid CT-3 on Chronic Neuropathic PainA Randomized Controlled Trial. JAMA 2003, 290, 1757–1762. [Google Scholar] [CrossRef]
- Pharmos Corporation—Pharmos Announces Clinical Data from Phase 2a Trial of Cannabinor for Nociceptive Pain in Third Molar Dental Extraction Model. Available online: https://web.archive.org/web/20070921045558/http:/investors.pharmoscorp.com/releasedetail.cfm?ReleaseID=239149 (accessed on 4 June 2025).
- GSK—CBA109389 Synopsis Redacted v2, July 2008. Available online: https://web.archive.org/web/20250814040308/https://s3.amazonaws.com/ctr-gsk-7381/CBA109389/ef325b9c-b4ff-483f-8cf4-fc9931e89b8b/d00a9e6c-096f-4734-bf93-0ee65a75a4f5/gsk-109389-synopsis-redact-v2.pdf (accessed on 14 August 2025).
- Shionogi & Co. Ltd. 1st Quarter of Fiscal 2009 Conference Call. Available online: https://web.archive.org/web/20220131131511/https:/www.shionogi.com/content/dam/shionogi/global/investors/pdf/e_p090803.pdf (accessed on 20 June 2025).
- Shionogi. A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Efficacy and Safety Study of 2 Doses of S-777469 (400 mg BID and 800 mg BID) in Patients with Atopic Dermatitis (Clinical Trial Registration No. NCT00703573). clinicaltrials.gov. 2018. Available online: https://clinicaltrials.gov/study/NCT00703573 (accessed on 30 July 2025).
- Glenmark Completes Phase I Trial of Neuropathic Drug. Available online: https://web.archive.org/web/20250523043251/https:/www.business-standard.com/article/companies/glenmark-completes-phase-i-trial-of-neuropathic-drug-109041300141_1.html (accessed on 20 June 2025).
- Ostenfeld, T.; Price, J.; Albanese, M.; Bullman, J.; Guillard, F.; Meyer, I.; Leeson, R.; Costantin, C.; Ziviani, L.; Nocini, P.F.; et al. A Randomized, Controlled Study to Investigate the Analgesic Efficacy of Single Doses of the Cannabinoid Receptor-2 Agonist GW842166, Ibuprofen or Placebo in Patients with Acute Pain Following Third Molar Tooth Extraction. Clin. J. Pain 2011, 27, 668. [Google Scholar] [CrossRef]
- Kalliomäki, J.; Segerdahl, M.; Webster, L.; Reimfelt, A.; Huizar, K.; Annas, P.; Karlsten, R.; Quiding, H. Evaluation of the analgesic efficacy of AZD1940, a novel cannabinoid agonist, on post-operative pain after lower third molar surgical removal. Scand. J. Pain 2013, 4, 17–22. [Google Scholar] [CrossRef]
- Kalliomäki, J.; Annas, P.; Huizar, K.; Clarke, C.; Zettergren, A.; Karlsten, R.; Segerdahl, M. Evaluation of the analgesic efficacy and psychoactive effects of AZD1940, a novel peripherally acting cannabinoid agonist, in human capsaicin-induced pain and hyperalgesia. Clin. Exp. Pharmacol. Physiol. 2013, 40, 212–218. [Google Scholar] [CrossRef]
- Tsukii, K.; Takebe, T.; Hyodo, Y.; Ohtsu, Y.; Morishita, T.; Taoka, S. A Phase 2a, Double-blind, Placebo-controlled, Crossover Study of Cannabinoid CB2 Receptor Agonist KHK6188 in Japanese Patients with Postherpetic Neuralgia. Neurotherapeutics 2015, 12, 678–687. [Google Scholar] [CrossRef]
- Eli Lilly and Company. A Proof of Concept Study of the Effects of LY2828360 in the Treatment of Patients With Osteoarthritic Knee Pain. (Clinical Trial Registration No. NCT01319929). clinicaltrials.gov. 2020. Available online: https://clinicaltrials.gov/study/NCT01319929 (accessed on 23 May 2025).
- Pereira, A.; Chappell, A.; Dethy, J.; Hoeck, H.; Arendt-Nielsen, L.; Verfaille, S.; Boulanger, B.; Jullion, A.; Johnson, M.; McNearney, T. A proof-of-concept (POC) study including experimental pain models (EPMs) to assess the effects of a CB2 agonist (LY2828360) in the treatment of patients with osteoarthritic (OA) knee pain: Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics, ASCPT. Clin. Pharmacol. Ther. 2013, 93, S56–S57. [Google Scholar] [CrossRef]
- Emerald Health Pharmaceuticals. A Phase IIa, Double-Blind, Randomised, Intracohort Placebo-Controlled, Multicentre Study to Evaluate the Safety, Tolerability and Preliminary Efficacy of EHP-101 in Patients with Diffuse Cutaneous Systemic Sclerosis (Clinical Trial Registration No. NCT04166552). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/study/NCT04166552 (accessed on 23 May 2025).
- Emerald Health Pharmaceuticals. A Phase IIa, Open-label, Multicentre Dose-Finding Trial in Patients with Relapsing Forms of Multiple Sclerosis (RMS) to Evaluate the Safety, Tolerability and Preliminary Efficacy of EHP-101 (Clinical Trial Registration No. NCT04909502). clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/study/NCT04909502 (accessed on 23 May 2025).
- Tepper, M.A.; Zurier, R.B.; Burstein, S.H. Ultrapure ajulemic acid has improved CB2 selectivity with reduced CB1 activity. Bioorganic Med. Chem. 2014, 22, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Chmiel, J.F.; Flume, P.; Downey, D.G.; Dozor, A.J.; Colombo, C.; Mazurek, H.; Sapiejka, E.; Rachel, M.; Constantine, S.; Conley, B.; et al. Safety and efficacy of lenabasum in a phase 2 randomized, placebo-controlled trial in adults with cystic fibrosis. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2021, 20, 78–85. [Google Scholar] [CrossRef]
- West, N.E.; Konstan, M.W.; Flume, P.A.; VanDevanter, D.R.; Magaret, A.; Mazurek, H.; Amelina, E.L.; Laki, I.; Chiron, R.; Sutharsan, S.; et al. Cannabinoid receptor 2 agonist, lenabasum, for the treatment of pulmonary exacerbations in cystic fibrosis. J. Cyst. Fibros. 2025, 24, 691–697. [Google Scholar] [CrossRef]
- Corbus Pharmaceuticals Inc. Trial to Evaluate Efficacy and Safety of Lenabasum in Cystic Fibrosis (Clinical Trial Registration No. NCT03451045). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/study/NCT03451045 (accessed on 25 May 2025).
- National Institute of Allergy and Infectious Diseases (NIAID). A Phase 2, Double-blind, Randomized, Placebo-Controlled Multicenter Study to Evaluate Efficacy, Safety, and Tolerability of JBT-101 in Systemic Lupus Erythematosus (Clinical Trial Registration No. NCT03093402). clinicaltrials.gov. 2022. Available online: https://clinicaltrials.gov/study/NCT03093402 (accessed on 23 May 2025).
- Vazquez, T.; Sharma, M.; Feng, R.; Diaz, D.; Kodali, N.; Dan, J.; Grinnell, M.; Keyes, E.; Sprow, G.; White, B.; et al. Lenabasum Reduces IFNγ and pIRF3 in Dermatomyositis Skin: Biomarker Results from a Double-Blind Phase 3 International Randomized Controlled Trial. Arthritis Rheumatol. 2022, 74, S9. Available online: https://acrabstracts.org/abstract/lenabasum-reduces-ifnγ-and-pirf3-in-dermatomyositis-skin-biomarker-results-from-a-double-blind-phase-3-international-randomized-controlled-trial/ (accessed on 23 May 2025). [CrossRef]
- Werth, V.; White, B.; Concha, J.; Dan, J.; Dgetluck, N.; Hally, K.; Constantine, S.; Aggarwal, R.; Fiorentino, D.; Lundberg, I.; et al. Cutaneous Manifestations, Clinical Trials, Safety Efficacy and Safety of Lenabasum in the Phase 3 DeterMine Trial in Dermatomyositis. Arthritis Rheumatol. 2022, 74, S9. Available online: https://acrabstracts.org/abstract/cutaneous-manifestations-clinical-trials-safety-efficacy-and-safety-of-lenabasum-in-the-phase-3-determine-trial-in-dermatomyositis/ (accessed on 23 May 2025).
- Spiera, R.; Kuwana, M.; Khanna, D.; Hummers, L.; Frech, T.M.; Stevens, W.; Matucci-Cerinic, M.; Kafaja, S.; Distler, O.; Jun, J.-B.; et al. Efficacy and Safety of Lenabasum, a Cannabinoid Type 2 Receptor Agonist, in a Phase 3 Randomized Trial in Diffuse Cutaneous Systemic Sclerosis. Arthritis Rheumatol. 2023, 75, 1608–1618. [Google Scholar] [CrossRef]
- Han, S.; Thoresen, L.; Jung, J.-K.; Zhu, X.; Thatte, J.; Solomon, M.; Gaidarov, I.; Unett, D.J.; Yoon, W.H.; Barden, J.; et al. Discovery of APD371: Identification of a Highly Potent and Selective CB2 Agonist for the Treatment of Chronic Pain. ACS Med. Chem. Lett. 2017, 8, 1309–1313. [Google Scholar] [CrossRef]
- Yacyshyn, B.R.; Hanauer, S.; Klassen, P.; English, B.A.; Stauber, K.; Barish, C.F.; Gilder, K.; Turner, S.; Higgins, P.D.R. Safety, Pharmacokinetics, and Efficacy of Olorinab, a Peripherally Acting, Highly Selective, Full Agonist of the Cannabinoid Receptor 2, in a Phase 2a Study of Patients with Chronic Abdominal Pain Associated with Crohn’s Disease. Crohn’s Colitis 360 2021, 3, otaa089. [Google Scholar] [CrossRef]
- Chang, L.; Cash, B.D.; Lembo, A.; Kunkel, D.C.; English, B.A.; Lindstrom, B.; Gu, G.; Skare, S.; Gilder, K.; Turner, S.; et al. Efficacy and safety of olorinab, a full agonist of the cannabinoid receptor 2, for the treatment of abdominal pain in patients with irritable bowel syndrome: Results from a phase 2b randomized placebo-controlled trial (CAPTIVATE). Neurogastroenterol. Motil. 2023, 35, e14539. [Google Scholar] [CrossRef] [PubMed]
- Foss, J.; Naguib, M.; Giordano, T. A phase I single ascending dose safety study of NTRX-07 in normal volunteers. Alzheimer’s Dement. 2020, 16, e039150. [Google Scholar] [CrossRef]
- NeuroTherapia Presents Clinical Data from Phase 1b Clinical Trial of NTRX-07 for the Treatment of Alzheimer’s Disease—BioSpace. Available online: https://web.archive.org/web/20250523051013/https:/www.biospace.com/neurotherapia-presents-clinical-data-from-phase-1b-clinical-trial-of-ntrx-07-for-the-treatment-of-alzheimer-s-disease (accessed on 23 May 2025).
- Neurotherapia Inc. A Multicenter, Randomized, Double-Masked, Placebo-Controlled Study to Evaluate the Safety, Tolerability, Pharmacokinetics (PK) and Pharmacodynamics (PD) Effects of NTRX-07 in Subjects with Mild Cognitive Impairment (MCI) or Mild to Moderate Alzheimer’s Disease (AD) (SPPN-AD) (Clinical Trial Registration No. NTRX-07-C201). 2025. Available online: https://euclinicaltrials.eu/search-for-clinical-trials/?lang=en&EUCT=2024-517957-29-00 (accessed on 23 May 2025).
- Stevens, R.M.; Nicholson, J.R.; Sauer, A.; Nolte, T.; Corradini, L. 103 Evaluation of the Selective Oral CB2 Agonist CNTX-6016 for the Treatment of Neuropathic Pain: Pharmacokinetic, Efficacy, and Safety Findings from Preclinical Studies. Postgrad. Med. 2019, 131, 82–83. [Google Scholar] [CrossRef]
- Centrexion Therapeutics. A Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of CNTX-6016 in Healthy Subjects and a Single Cohort of Subjects with Painful Diabetic Neuropathy (Clinical Trial Registration No. NCT04857957). clinicaltrials.gov. 2023. Available online: https://clinicaltrials.gov/study/NCT04857957 (accessed on 25 May 2025).
- Centrexion Therapeutics. A Study to Evaluate the Safety and Pharmacokinetics of CNTX-6016 in Healthy Subjects (Clinical Trial Registration No. NCT04154501). clinicaltrials.gov. 2019. Available online: https://clinicaltrials.gov/study/NCT04154501 (accessed on 25 May 2025).
- Fan, P.; Elzein, E.; Yao, L. Effect of TT-816, a novel immune response modifier targeting cannabinoid CB 2 receptor, on antitumor immunity and cancer growth. J. Clin. Oncol. 2024, 42, e14595. [Google Scholar] [CrossRef]
- Teon Therapeutics Inc. TT-816 As Monotherapy or in Combination with a PD-1 Inhibitor in Patients with Advanced Cancers (SEABEAM) (MK3475-E88) (Clinical Trial Registration No. NCT05525455). Available online: https://clinicaltrials.gov/study/NCT05525455 (accessed on 25 May 2025).
- Hoffmann-La Roche. A Randomized, Double-Masked, 48-Week, Parallel-Group, Placebo-Controlled, Proof-of-Concept Study to Investigate the Efficacy and Safety of RG7774 in Patients with Diabetes Mellitus Type 1 or Type 2 with Treatment-Naive Diabetic Retinopathy (Clinical Trial Registration No. NCT04265261). clinicaltrials.gov. 2024. Available online: https://clinicaltrials.gov/study/NCT04265261 (accessed on 25 May 2025).
- Artelo Biosciences Limited. A Phase 1/2 Trial of the Synthetic Cannabinoid ART27.13 in Patients with Cancer Anorexia and Weight Loss (Clinical Trial Registration No. ART27.13-100). 2021. Available online: https://euclinicaltrials.eu/search-for-clinical-trials/?lang=en&EUCT=2024-518177-33-00 (accessed on 25 May 2025).
- VivaCell Biotechnology España. A Phase IIa Open Label, Dose-Finding, Clinical Trial to Evaluate Safety, Tolerability and Preliminary Efficacy of Etrinabdione in Patients with Peripheral Arterial Disease (PAD) (Clinical Trial Registration No. NCT06774040). clinicaltrials.gov. 2025. Available online: https://clinicaltrials.gov/study/NCT06774040 (accessed on 23 May 2025).
- McCray, A.T.; Ide, N.C. Design and implementation of a national clinical trials registry. J. Am. Med. Inform. Assoc. JAMIA 2000, 7, 313–323. [Google Scholar] [CrossRef]
- Namiot, E.D.; Smirnovová, D.; Sokolov, A.V.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. The international clinical trials registry platform (ICTRP): Data integrity and the trends in clinical trials, diseases, and drugs. Front. Pharmacol. 2023, 14, 1228148. [Google Scholar] [CrossRef]
- Fan, P.; Elzein, E.; Yao, L. TT-816, a novel small molecule immune checkpoint inhibitor targeting cannabinoid CB2 receptor, stimulates innate and adaptive immunity for cancer therapy. J. Immunother. Cancer 2022, 10, A1001. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Sun, Y.; Bennett, A.J.; Randall, M.D.; Kendall, D.A. Time-dependent vascular actions of cannabidiol in the rat aorta. Eur. J. Pharmacol. 2009, 612, 61–68. [Google Scholar] [CrossRef]
- Burstein, S.H. The cannabinoid acids, analogs and endogenous counterparts. Bioorganic Med. Chem. 2014, 22, 2830–2843. [Google Scholar] [CrossRef]
- Dyson, A.; Peacock, M.; Chen, A.; Courade, J.-P.; Yaqoob, M.; Groarke, A.; Brain, C.; Loong, Y.; Fox, A. Antihyperalgesic properties of the cannabinoid CT-3 in chronic neuropathic and inflammatory pain states in the rat. Pain 2005, 116, 129. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Burstein, S.H.; Zurier, R.B.; Chen, J.D. Activation and Binding of Peroxisome Proliferator-Activated Receptor γ by Synthetic Cannabinoid Ajulemic Acid. Mol. Pharmacol. 2003, 63, 983–992. [Google Scholar] [CrossRef]
- Turton, K.B.; Ingram, R.J.; Valvano, M.A. Macrophage dysfunction in cystic fibrosis: Nature or nurture? J. Leukoc. Biol. 2021, 109, 573–582. [Google Scholar] [CrossRef]
- Balfour-Lynn, I.M.; Welch, K.; Smith, S. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst. Rev. 2019, CD001915. [Google Scholar] [CrossRef]
- Dinwiddie, R. Anti-inflammatory therapy in cystic fibrosis. J. Cyst. Fibros. 2005, 4, 45–48. [Google Scholar] [CrossRef]
- Gensler, L.S. Glucocorticoids. Neurohospitalist 2013, 3, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Toledo, D.M.; Pioli, P.A. Macrophages in Systemic Sclerosis: Novel Insights and Therapeutic Implications. Curr. Rheumatol. Rep. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Spiera, R.; Hummers, L.; Chung, L.; Frech, T.M.; Domsic, R.; Hsu, V.; Furst, D.E.; Gordon, J.; Mayes, M.; Simms, R.; et al. Safety and Efficacy of Lenabasum in a Phase II, Randomized, Placebo-Controlled Trial in Adults with Systemic Sclerosis. Arthritis Rheumatol. 2020, 72, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Kodali, N.; Diaz, D.; Dhiman, R.; Vazquez, T.; Feng, R.; Patel, J.; Dan, J.; Sprow, G.; Kleitsch, J.; Sharma, M.; et al. Diverse Lenabasum pathway activation in dermatomyositis patients’ blood. Sci. Rep. 2025, 15, 17232. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.J.; Ahuja, G.; Gomes, L.L.A.; Poroye, J.; Faden, D.F.; Xie, L.; Feng, R.; White, B.; Werth, V.P. Long-Term Safety and Efficacy of Lenabasum, a Cannabinoid Receptor Type 2 Agonist, in Patients with Dermatomyositis with Refractory Skin Disease: Follow-Up Data from a 3-Year Open-Label Extension Study. JID Innov. 2025, 5, 100311. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Garcia-Caraballo, S.; Maddern, J.; Schober, G.; Lumsden, A.; Harrington, A.; Schmiel, S.; Lindstrom, B.; Adams, J.; Brierley, S.M. Olorinab (APD371), a peripherally acting, highly selective, full agonist of the cannabinoid receptor 2, reduces colitis-induced acute and chronic visceral hypersensitivity in rodents. Pain 2022, 163, e72–e86. [Google Scholar] [CrossRef]
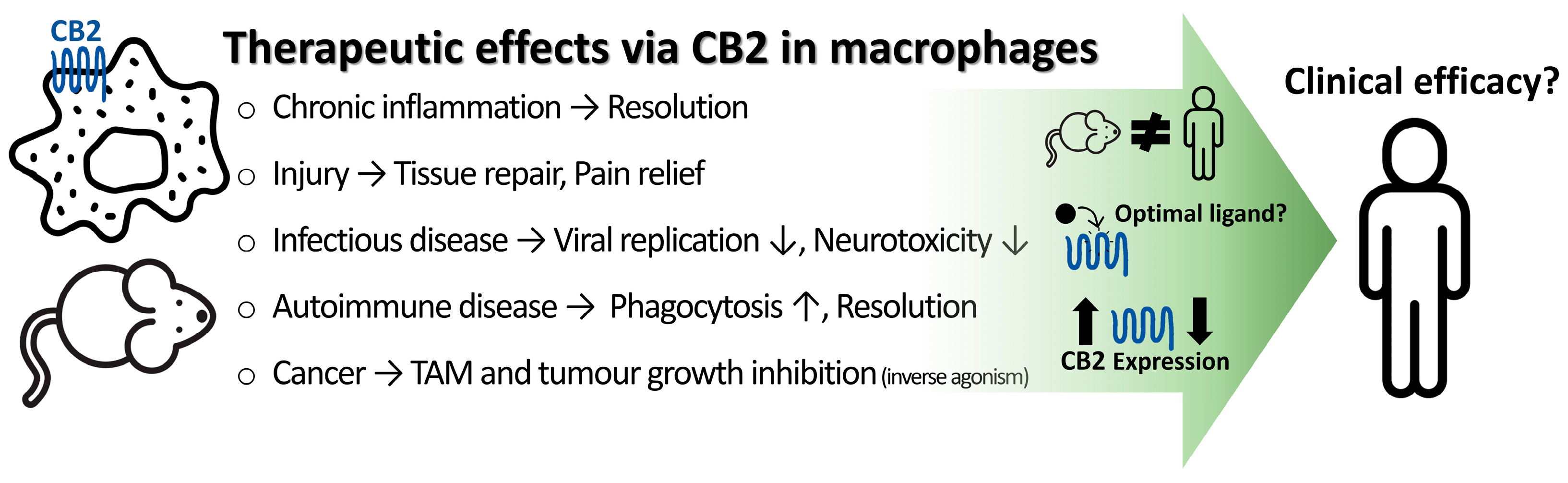
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, H.H.; Grimsey, N.L. Cannabinoid Receptor 2 (CB2) in Macrophages: A Promising Clinical Target for Immune Disorders. Int. J. Mol. Sci. 2025, 26, 8657. https://doi.org/10.3390/ijms26178657
Yoon HH, Grimsey NL. Cannabinoid Receptor 2 (CB2) in Macrophages: A Promising Clinical Target for Immune Disorders. International Journal of Molecular Sciences. 2025; 26(17):8657. https://doi.org/10.3390/ijms26178657
Chicago/Turabian StyleYoon, Hyeyoung Hailey, and Natasha Lillia Grimsey. 2025. "Cannabinoid Receptor 2 (CB2) in Macrophages: A Promising Clinical Target for Immune Disorders" International Journal of Molecular Sciences 26, no. 17: 8657. https://doi.org/10.3390/ijms26178657
APA StyleYoon, H. H., & Grimsey, N. L. (2025). Cannabinoid Receptor 2 (CB2) in Macrophages: A Promising Clinical Target for Immune Disorders. International Journal of Molecular Sciences, 26(17), 8657. https://doi.org/10.3390/ijms26178657








