Chronic Escherichia coli ST648 Infections in Patients with Cystic Fibrosis: The In Vitro Effects of an Antivirulence Agent
Abstract
1. Introduction
2. Results
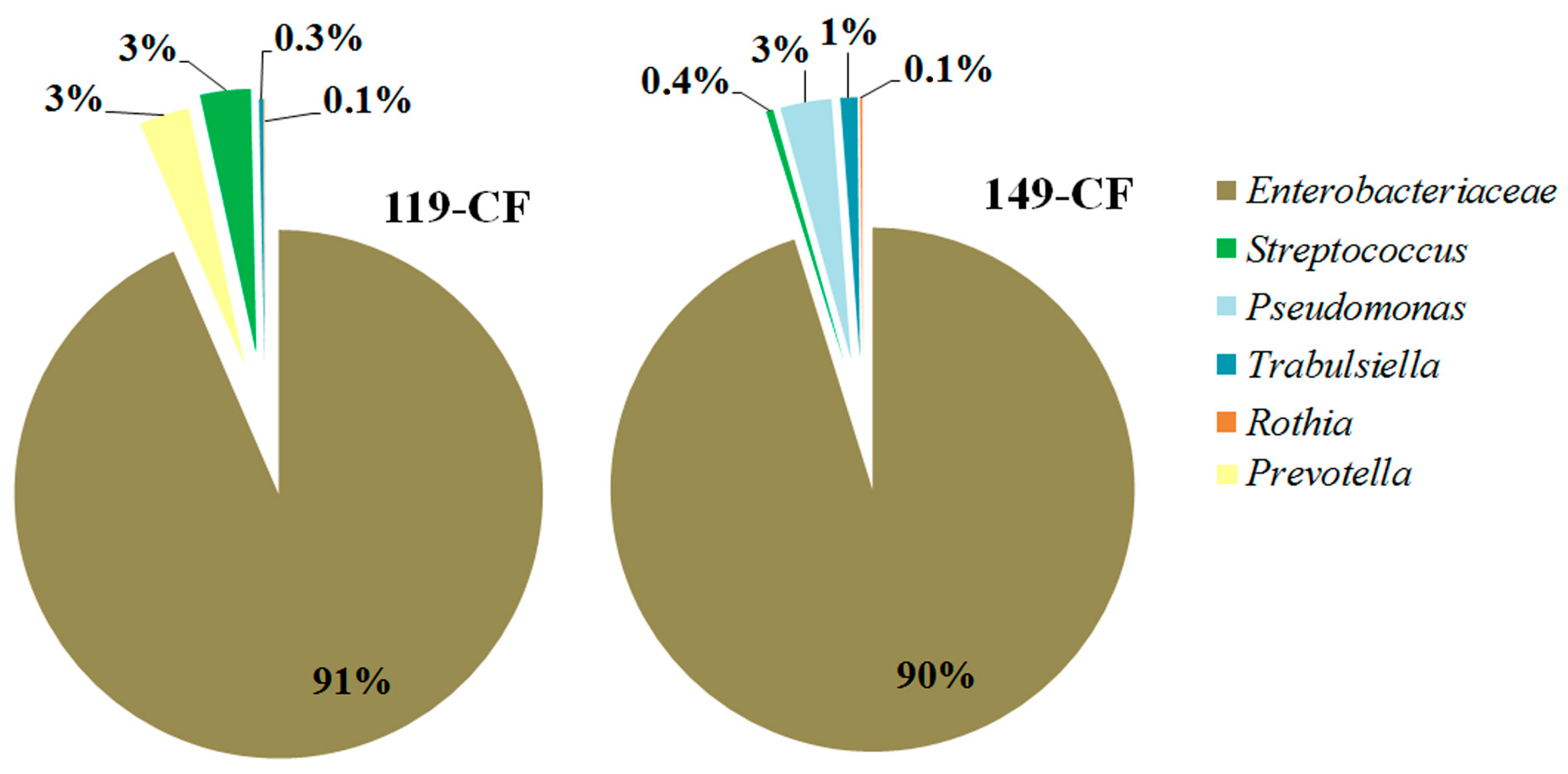
2.1. Microbiome of the Sputum Samples
2.2. Features of the Genomes of E. coli Isolates
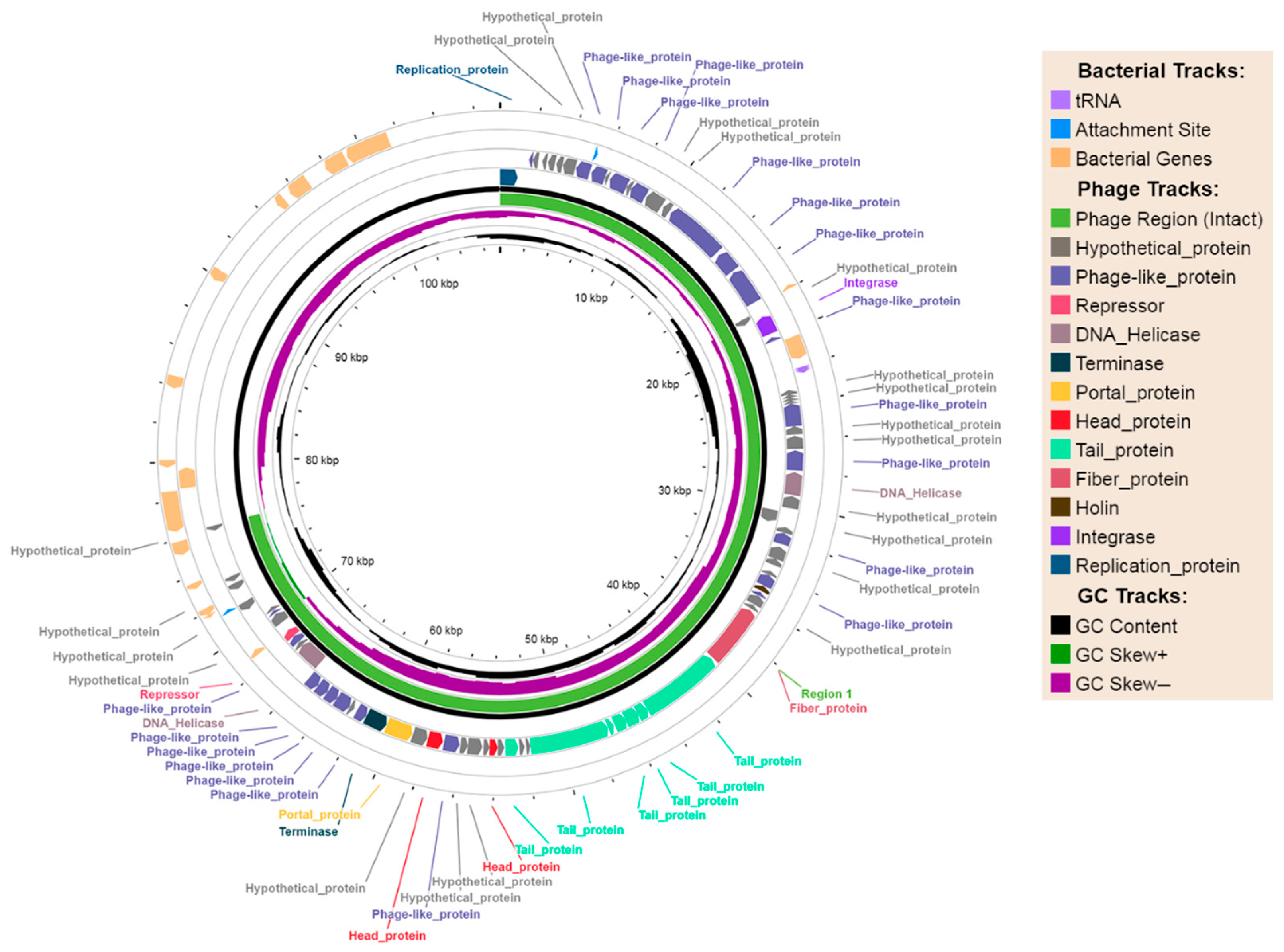
2.2.1. Phage-Plasmids
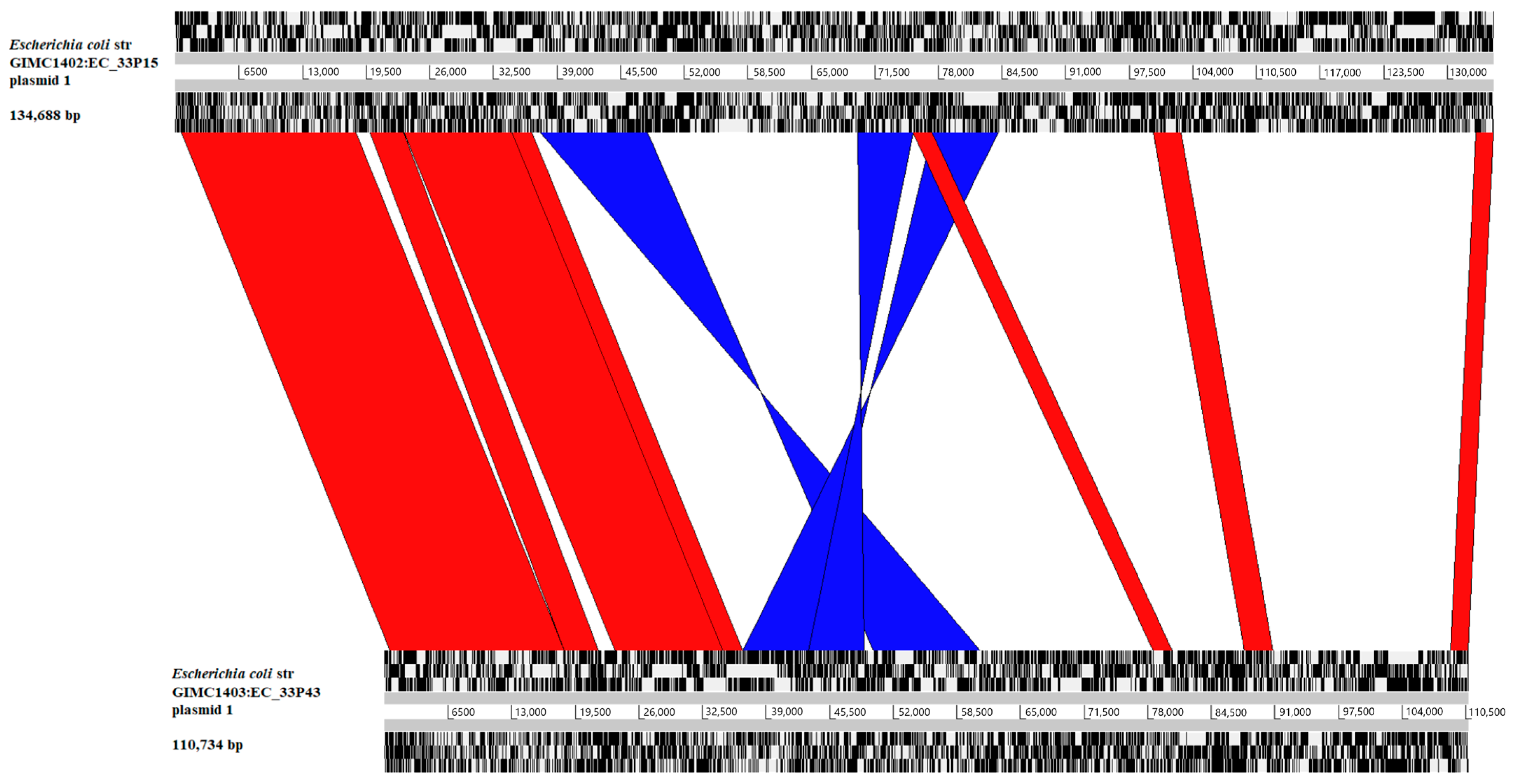
2.2.2. The Large Plasmids pEC_33P15-1 and pEC_33P43-1
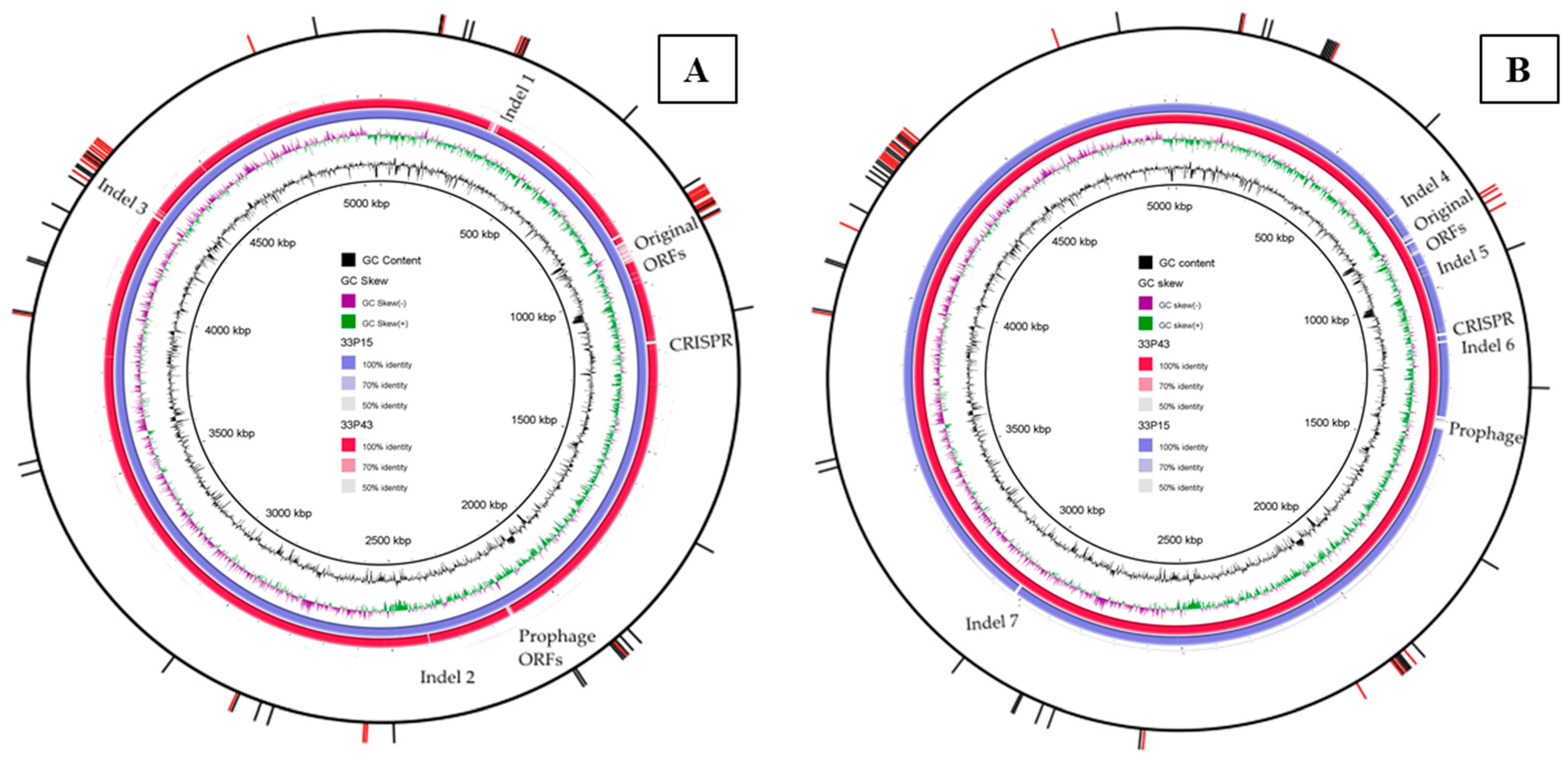
2.2.3. Comparative Analysis of Chromosomes
2.2.4. The Place of CF Isolates in the Population Structure of E. coli ST648
2.2.5. Adaptation to the Long-Term Chronic Infection
2.3. In Vitro Experiments
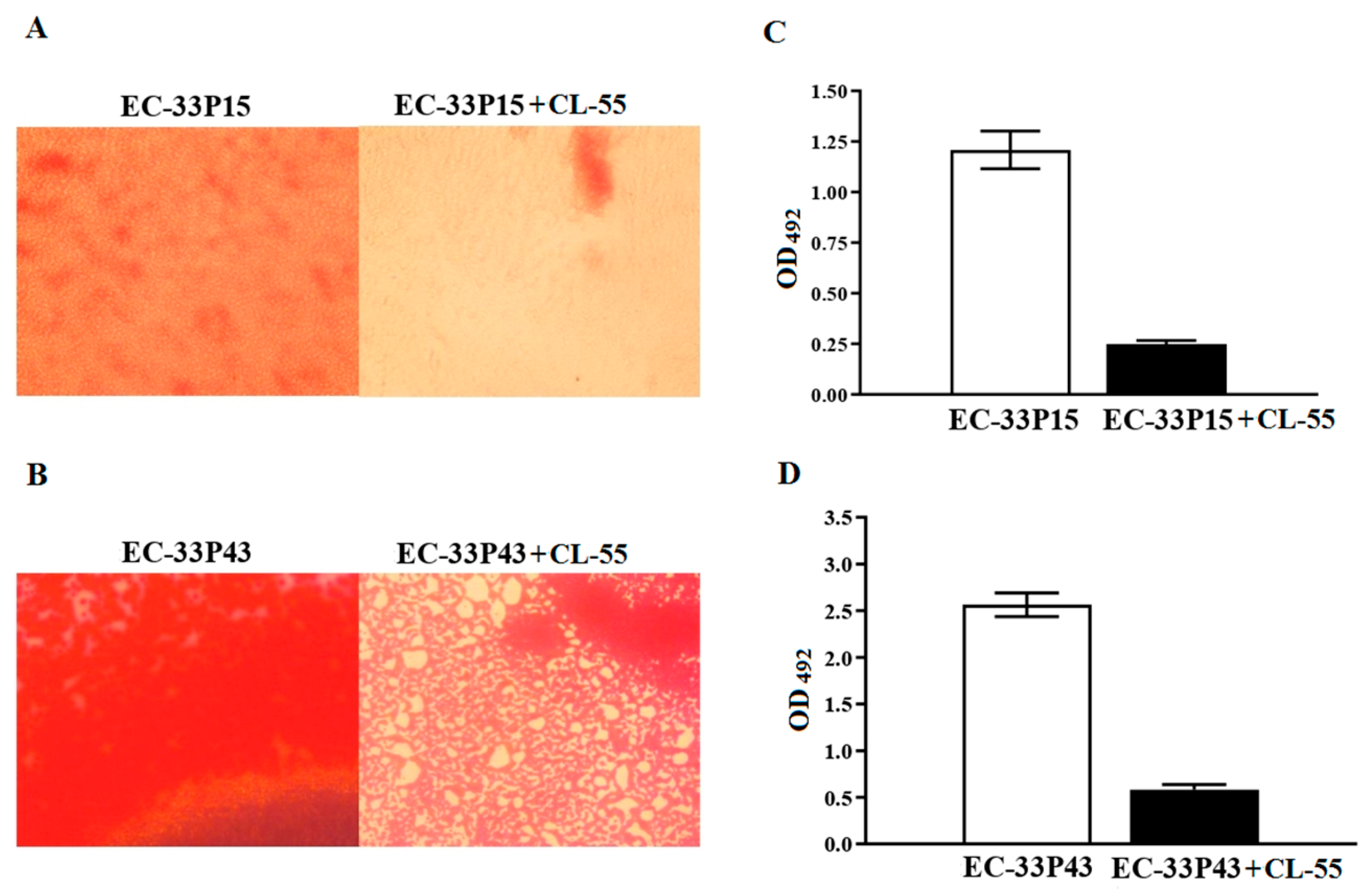
2.3.1. Biofilm Formation
2.3.2. Macrophage Internalization and Survival
2.3.3. CL-55 Does Not Inhibit Bacterial Viability
2.3.4. Effect of the Active Pharmaceutical Ingredient CL-55 on Biofilm Formation and Intracellular Survival of CF Isolates
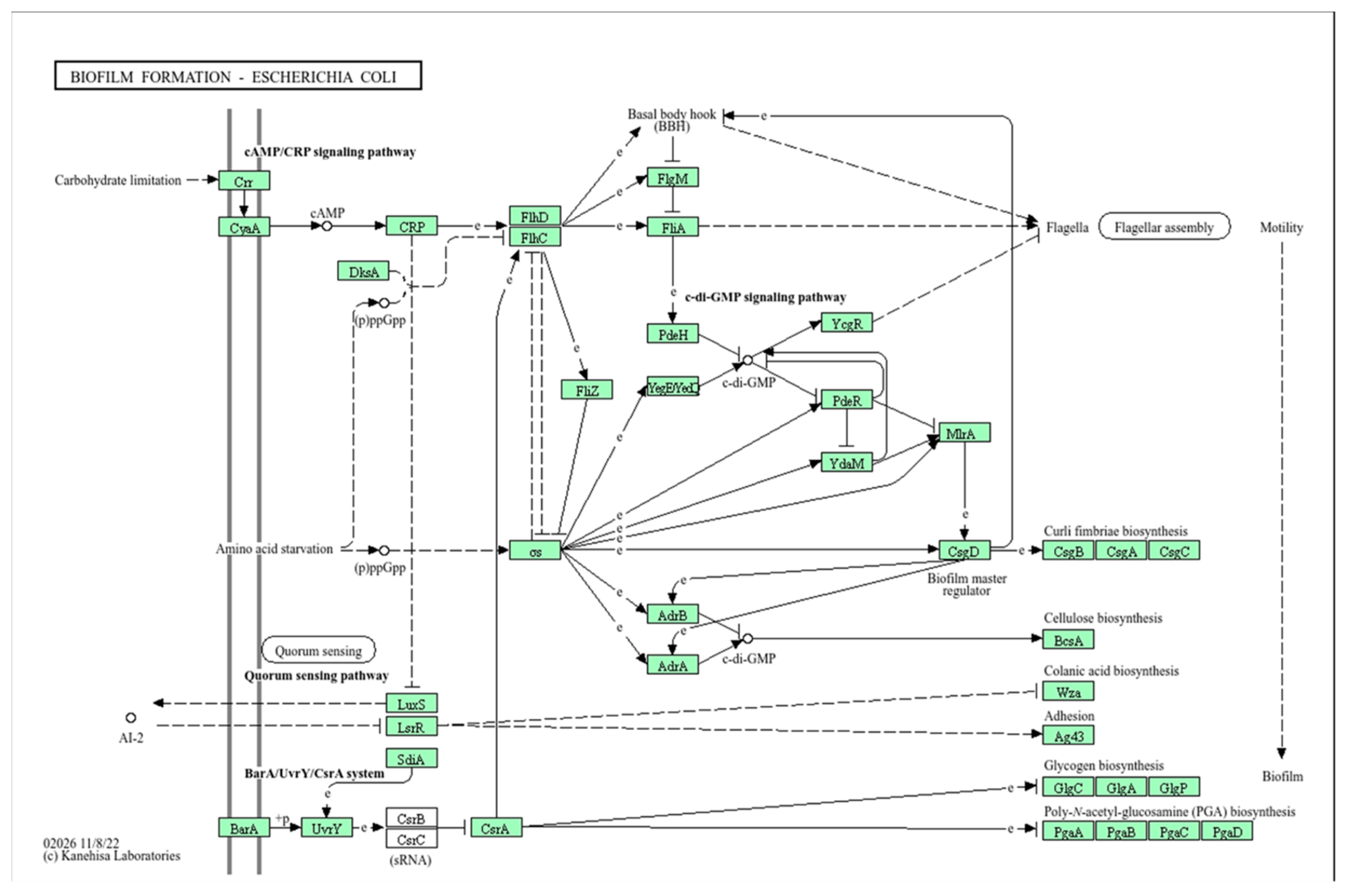
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
- Bacteria isolation and cultivation
- Isolate identification
- Microbiome analysis
- Whole-genome sequencing
- Genome analysis
- Phylogenetic analysis
- Evaluation of the antibacterial effect of CL-55
- Biofilm assay
- Macrophage cell culture and growth conditions
- Bacterial intracellular survival in RAW264.7 macrophages
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANI | Average nucleotide identity |
| AP | Alignment percentage |
| CA | Colanic acid |
| CAP | Community-acquired pneumonia |
| CDS | Coding DNA sequence |
| CF | Cystic fibrosis |
| CL-55 | The active pharmaceutical ingredient of Fluorthiazinone |
| CPS | Capsular polysaccharide |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| ECF | Energy-coupling factor |
| ESBL | Extended-spectrum beta-lactamase |
| ETT2 | E. coli type 3 secretion system 2 |
| ExPEC | Extraintestinal pathogenic E. coli |
| FT | Fluorthiazinone |
| MOI | Multiplicity of infection (the ratio of the number of bacterial cells to the number of host cells) |
| NP | Nosocomial pneumonia |
| ORF | Open reading frame |
| P-P | Phage-plasmid |
| T3SS | Type 3 secretion system |
| T6SS | Type 6 secretion system |
| TRAP | Tripartite ATP-independent periplasmic transporter |
| VAP | Ventilator-associated pneumonia |
References
- Marrie, T.J.; Fine, M.J.; Obrosky, D.S.; Coley, C.; Singer, D.E.; Kapoor, W.N. Community-acquired pneumonia due to Escherichia coli. Clin. Microbiol. Infect. 1998, 4, 717–723. [Google Scholar] [CrossRef]
- La Combe, B.; Clermont, O.; Messika, J.; Eveillard, M.; Kouatchet, A.; Lasocki, S.; Corvec, S.; Lakhal, K.; Billard-Pomares, T.; Fernandes, R.; et al. Pneumonia-specific Escherichia coli with distinct phylogenetic and virulence profiles, France, 2012–2014. Emerg. Infect. Dis. 2019, 25, 710–718. [Google Scholar] [CrossRef]
- John, T.M.; Deshpande, A.; Brizendine, K.; Yu, P.C.; Rothberg, M.B. Epidemiology and outcomes of community-acquired Escherichia coli pneumonia. Open Forum. Infect. Dis. 2021, 9, ofab597. [Google Scholar] [CrossRef]
- Messika, J.; Magdoud, F.; Clermont, O.; Margetis, D.; Gaudry, S.; Roux, D.; Branger, C.; Dreyfuss, D.; Denamur, E.; Ricard, J.D. Pathophysiology of Escherichia coli ventilator-associated pneumonia: Implication of highly virulent extraintestinal pathogenic strains. Intensive Care Med. 2012, 38, 2007–2016. [Google Scholar] [CrossRef]
- Koulenti, D.; Tsigou, E.; Rello, J. Nosocomial pneumonia in 27 ICUs in Europe: Perspectives from the EU-VAP/CAP study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1999–2006. [Google Scholar] [CrossRef]
- Stoesser, N.; Sheppard, A.E.; Pankhurst, L.; De Maio, N.; Moore, C.E.; Sebra, R.; Turner, P.; Anson, L.W.; Kasarskis, A.; Batty, E.M.; et al. Evolutionary history of the global emergence of the Escherichia coli epidemic clone ST131. mBio 2016, 22, e02162. [Google Scholar] [CrossRef] [PubMed]
- Schaufler, K.; Semmler, T.; Wieler, L.H.; Trott, D.J.; Pitout, J.; Peirano, G.; Bonnedahl, J.; Dolejska, M.; Literak, I.; Fuchs, S.; et al. Genomic and functional analysis of emerging virulent and multidrug-resistant Escherichia coli lineage Sequence Type 648. Antimicrob. Agents Chemother. 2019, 63, e00243-19. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Sherchan, J.B.; Hayakawa, K.; Miyoshi-Akiyama, T.; Ohmagari, N.; Kirikae, T.; Nagamatsu, M.; Tojo, M.; Ohara, H.; Sherchand, J.B.; Tandukar, S. Clinical epidemiology and molecular analysis of extended-spectrum-β-lactamase-producing Escherichia coli in Nepal: Characteristics of sequence types 131 and 648. Antimicrob. Agents Chemother. 2015, 59, 3424–3432. [Google Scholar] [CrossRef]
- Piekar, M.; Álvarez, V.E.; Knecht, C.; Leguina, C.; García Allende, N.; Carrera Páez, L.; Gambino, A.S.; González Machuca, A.; Campos, J.; Fox, B.; et al. Genomic data reveals the emergence of the co-occurrence of blaKPC-2 and blaCTX-M-15 in an Escherichia coli ST648 strain isolated from rectal swab within the framework of hospital surveillance. J. Glob. Antimicrob. Resist. 2023, 32, 108–112. [Google Scholar] [CrossRef]
- He, X.; Xu, L.; Dai, H.; Ge, M.; Zhu, J.; Fu, H.; Zhu, S.; Shao, J. Genomic characteristics of a multidrug-resistant ST648 Escherichia coli isolate co-carrying blaKPC-2 and blaCTX-M-15 genes recovered from a respiratory infection in China. Infect. Drug Resist. 2023, 16, 3535–3540. [Google Scholar] [CrossRef]
- Eger, E.; Heiden, S.E.; Korolew, K.; Bayingana, C.; Ndoli, J.M.; Sendegeya, A.; Gahutu, J.B.; Kurz, M.S.E.; Mockenhaupt, F.P.; Müller, J.; et al. Circulation of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli of pandemic Sequence Types 131, 648, and 410 among hospitalized patients, Caregivers, and the community in Rwanda. Front. Microbiol. 2021, 12, 662575. [Google Scholar] [CrossRef] [PubMed]
- Fuga, B.; Cerdeira, L.; Moura, Q.; Fontana, H.; Fuentes-Castillo, D.; Carvalho, A.C.; Lincopan, N. Genomic data reveals the emergence of an IncQ1 small plasmid carrying blaKPC-2 in Escherichia coli of the pandemic sequence type 648. J. Glob. Antimicrob. Resist. 2021, 25, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Sidjabat, H.E.; Paterson, D.L.; Adams-Haduch, J.M.; Ewan, L.; Pasculle, A.W.; Muto, C.A.; Tian, G.B.; Doi, Y. Molecular epidemiology of CTX-M-producing Escherichia coli isolates at a tertiary medical center in western Pennsylvania. Antimicrob. Agents Chemother. 2009, 53, 4733–4739. [Google Scholar] [CrossRef]
- Mshana, S.E.; Imirzalioglu, C.; Hain, T.; Domann, E.; Lyamuya, E.F.; Chakraborty, T. Multiple ST clonal complexes, with a predominance of ST131, of Escherichia coli harbouring blaCTX-M-15 in a tertiary hospital in Tanzania. Clin. Microbiol. Infect. 2011, 17, 1279–1282. [Google Scholar] [CrossRef]
- Zong, Z.; Yu, R. Escherichia coli carrying the blaCTX-M-15 gene of ST648. J. Med. Microbiol. 2010, 59, 1536–1537. [Google Scholar] [CrossRef][Green Version]
- Ewers, C.; Bethe, A.; Semmler, T.; Guenther, S.; Wieler, L.H. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: A global perspective. Clin. Microbiol. Infect. 2012, 18, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.S.; Feng, Y.; Lv, X.Y.; Duan, J.H.; Chen, J.; Fang, L.X.; Xia, J.; Liao, X.; Sun, J.; Liu, Y.H. Emergence of NDM-5- and MCR-1-Producing Escherichia coli clones ST648 and ST156 from a single muscovy duck (Cairina moschata). Antimicrob. Agents Chemother. 2016, 60, 6899–6902. [Google Scholar] [CrossRef]
- Dantas Palmeira, J.; Ferreira, H.; Madec, J.Y.; Haenni, M. Pandemic Escherichia coli ST648 isolate harbouring fosA3 and blaCTX-M-8 on an IncI1/ST113 plasmid: A new successful combination for the spread of fosfomycin resistance? J. Glob. Antimicrob. Resist. 2018, 15, 254–255. [Google Scholar] [CrossRef]
- Ewbank, A.C.; Fuentes-Castillo, D.; Sacristán, C.; Esposito, F.; Fuga, B.; Cardoso, B.; Godoy, S.N.; Zamana, R.R.; Gattamorta, M.A.; Catão-Dias, J.L.; et al. World Health Organization critical priority Escherichia coli clone ST648 in magnificent frigatebird (Fregata magnificens) of an uninhabited insular environment. Front. Microbiol. 2022, 13, 940600. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Zemanick, E.T.; Hoffman, L.R. Cystic Fibrosis: Microbiology and Host Response. Pediatr. Clin. N. Am. 2016, 63, 617–636. [Google Scholar] [CrossRef] [PubMed]
- Barillova, P.; Tchesnokova, V.; Dübbers, A.; Küster, P.; Peters, G.; Dobrindt, U.; Sokurenko, E.V.; Kahl, B.C. Prevalence and persistence of Escherichia coli in the airways of cystic fibrosis patients—An unrecognized CF pathogen? Int. J. Med. Microbiol. 2014, 304, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Edwards, B.D.; Somayaji, R.; Greysson-Wong, J.; Izydorczyk, C.; Waddell, B.; Storey, D.G.; Rabin, H.R.; Surette, M.G.; Parkins, M.D. Clinical outcomes associated with Escherichia coli infections in adults with cystic fibrosis: A cohort study. Open Forum Infect. Dis. 2019, 7, ofz476. [Google Scholar] [CrossRef]
- Izydorczyk, C.; Waddell, B.; Edwards, B.D.; Greysson-Wong, J.; Surette, M.G.; Somayaji, R.; Rabin, H.R.; Conly, J.M.; Church, D.L.; Parkins, M.D. Epidemiology of E. coli in cystic fibrosis airways demonstrates the capacity for persistent infection but not patient-patient transmission. Front. Microbiol. 2020, 11, 475. [Google Scholar] [CrossRef]
- Amelina, E.L.; Kashirskaya, N.Y.; Kondratyeva, E.I.; Krasovskiy, S.A.; Starinova, M.A.; Voronkova, A.Y.; Ginter, E.K. (Eds.) Registry of Patients with Cystic Fibrosis in the Russian Federation 2023; Publishing House “MEDPRACTIKA-M”: Moscow, Russia, 2025. [Google Scholar]
- Whitfield, C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006, 75, 39–68. [Google Scholar] [CrossRef]
- Xue, P.; Corbett, D.; Goldrick, M.; Naylor, C.; Roberts, I.S. Regulation of expression of the region 3 promoter of the Escherichia coli K5 capsule gene cluster involves H-NS, SlyA, and a large 5′ untranslated region. J. Bacteriol. 2009, 191, 1838–1846. [Google Scholar] [CrossRef]
- Harris, S.; Piotrowska, M.J.; Goldstone, R.J.; Qi, R.; Foster, G.; Dobrindt, U.; Madec, J.Y.; Valat, C.; Rao, F.V.; Smith, D.G.E. Variant O89 O-antigen of E. coli is associated with group 1 capsule loci and multidrug resistance. Front. Microbiol. 2018, 9, 2026. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Chen, Y.S.; Wu, C.Y.; Chang, H.Y.; Lai, Y.C.; Peng, H.L. RmpA regulation of capsular polysaccharide biosynthesis in Klebsiella pneumoniae CG43. J. Bacteriol. 2010, 192, 3144–3158. [Google Scholar] [CrossRef] [PubMed]
- Neumann, B.; Lippmann, N.; Wendt, S.; Karlas, T.; Lübbert, C.; Werner, G.; Pfeifer, Y.; Schuster, C.F. Recurrent bacteremia with a hypermucoviscous Escherichia coli isolated from a patient with perihilar cholangiocarcinoma: Insights from a comprehensive genome-based analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 28. [Google Scholar] [CrossRef]
- Lin, Y.T.; Jeng, Y.Y.; Chen, T.L.; Fung, C.P. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: Clinical and microbiological characteristics in Taiwan, 2001–2008. BMC Infect. Dis. 2010, 10, 307. [Google Scholar] [CrossRef]
- Han, B.; Li, M.; Xu, Y.; Islam, D.; Khang, J.; Del Sorbo, L.; Lee, W.; Szaszi, K.; Zhong, N.; Slutsky, A.S.; et al. Tsr Chemoreceptor interacts with IL-8 provoking E. coli transmigration across human lung epithelial cells. Sci. Rep. 2016, 6, 31087. [Google Scholar] [CrossRef] [PubMed]
- Grubwieser, P.; Hoffmann, A.; Hilbe, R.; Seifert, M.; Sonnweber, T.; Böck, N.; Theurl, I.; Weiss, G.; Nairz, M. Airway epithelial cells differentially adapt their iron metabolism to infection with Klebsiella pneumoniae and Escherichia coli in vitro. Front. Cell Infect. Microbiol. 2022, 12, 875543. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, X.; Sun, Y.; Jiang, M.; Wang, J.; Tang, F.; Xue, F.; Ren, J.; Zhu, W.; Dai, J. Acetate metabolic requirement of avian pathogenic Escherichia coli promotes its intracellular proliferation within macrophage. Vet. Res. 2019, 50, 31. [Google Scholar] [CrossRef]
- Zhuge, X.; Sun, Y.; Xue, F.; Tang, F.; Ren, J.; Li, D.; Wang, J.; Jiang, M.; Dai, J. A Novel PhoP/PhoQ regulation pathway modulates the survival of extraintestinal pathogenic Escherichia coli in macrophages. Front. Immunol. 2018, 9, 788. [Google Scholar] [CrossRef]
- Nazareth, H.; Genagon, S.A.; Russo, T.A. Extraintestinal pathogenic Escherichia coli survives within neutrophils. Infect. Immun. 2007, 75, 2776–2785. [Google Scholar] [CrossRef]
- Zigangirova, N.A.; Zayakin, E.S.; Kapotina, L.N.; Kost, E.A.; Didenko, L.V.; Davydova, D.Y.; Rumyanceva, J.P.; Gintsburg, A.L. Development of chlamydial Type III Secretion System inhibitors for suppression of acute and chronic forms of chlamydial infection. Acta Naturae 2012, 4, 87–97. [Google Scholar] [CrossRef][Green Version]
- Zigangirova, N.A.; Nesterenko, L.N.; Sheremet, A.B.; Soloveva, A.V.; Luyksaar, S.I.; Zayakin, E.S.; Balunets, D.V.; Gintsburg, A.L. Fluorothiazinon, a small-molecular inhibitor of T3SS, suppresses salmonella oral infection in mice. J. Antibiot. 2021, 74, 244–254. [Google Scholar] [CrossRef]
- Sheremet, A.B.; Zigangirova, N.A.; Zayakin, E.S.; Luyksaar, S.I.; Kapotina, L.N.; Nesterenko, L.N.; Kobets, N.V.; Gintsburg, A.L. Small Molecule Inhibitor of Type Three Secretion System Belonging to a Class 2,4-disubstituted-4H-[1,3,4]-thiadiazine-5-ones Improves Survival and Decreases Bacterial Loads in an Airway Pseudomonas aeruginosa Infection in Mice. Biomed. Res. Int. 2018, 2018, 5810767. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, E.A.; Soloveva, A.V.; Morgunova, E.Y.; Kapotina, L.N.; Luyksaar, S.I.; Luyksaar, S.V.; Bondareva, N.E.; Nelubina, S.A.; Lubenec, N.L.; Zigangirova, N.A.; et al. Fluorothiazinon inhibits the virulence factors of uropathogenic Escherichia coli involved in the development of urinary tract infection. J. Antibiot. 2023, 76, 279–290. [Google Scholar] [CrossRef]
- Zigangirova, N.A.; Lubenec, N.L.; Beloborodov, V.B.; Sheremet, A.B.; Nelyubina, S.A.; Bondareva, N.E.; Zakharov, K.A.; Luyksaar, S.I.; Zolotov, S.A.; Levchenko, E.U.; et al. A New “non-traditional” antibacterial drug Fluorothiazinone-clinical research in patients with complicated urinary tract infections. Antibiotics 2024, 13, 476. [Google Scholar] [CrossRef]
- Høiby, N. A short history of microbial biofilms and biofilm infections. APMIS 2017, 125, 272–275. [Google Scholar] [CrossRef]
- Pfeifer, E.; Moura de Sousa, J.A.; Touchon, M.; Rocha, E.P.C. Bacteria have numerous distinctive groups of phage-plasmids with conserved phage and variable plasmid gene repertoires. Nucleic Acids Res. 2021, 49, 2655–2673. [Google Scholar] [CrossRef]
- Boyd, J.M.; Sondelski, J.L.; Downs, D.M. Bacterial ApbC protein has two biochemical activities that are required for in vivo function. J. Biol. Chem. 2009, 284, 110–118. [Google Scholar] [CrossRef]
- Jurėnas, D.; Fraikin, N.; Goormaghtigh, F.; Van Melderen, L. Biology and evolution of bacterial toxin–antitoxin systems. Nat. Rev. Microbiol. 2022, 20, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.A.; Turner, D.P. The role of bacterial ATP-binding cassette (ABC) transporters in pathogenesis and virulence: Therapeutic and vaccine potential. Microb. Pathog. 2022, 171, 105734. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.; Léveillé, S.; Dozois, C.M. A SitABCD homologue from an avian pathogenic Escherichia coli strain mediates transport of iron and manganese and resistance to hydrogen peroxide. Microbiology 2006, 152, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Hrovat, K.; Zupančič, J.Č.; Seme, K.; Avguštin, J.A. QAC resistance genes in ESBL-producing E. coli isolated from patients with lower respiratory tract infections in the central Slovenia region—A 21-Year Survey. Trop. Med. Infect. Dis. 2023, 8, 273. [Google Scholar] [CrossRef]
- Virolle, C.; Goldlust, K.; Djermoun, S.; Bigot, S.; Lesterlin, C. Plasmid transfer by conjugation in gram-negative bacteria: From the cellular to the community level. Genes 2020, 11, 1239. [Google Scholar] [CrossRef]
- Ghigo, J.M. Natural conjugative plasmids induce bacterial biofilm development. Nature 2001, 412, 442–445. [Google Scholar] [CrossRef]
- Barrios, A.F.; Zuo, R.; Ren, D.; Wood, T.K. Hha, YbaJ, and OmpA regulate Escherichia coli K12 biofilm formation and conjugation plasmids abolish motility. Biotechnol. Bioeng. 2006, 93, 188–200. [Google Scholar] [CrossRef]
- Okshevsky, M.; Meyer, R.L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 2015, 41, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Goormaghtigh, F.; Fraikin, N.; Putrinš, M.; Hallaert, T.; Hauryliuk, V.; Garcia-Pino, A.; Sjödin, A.; Kasvandik, S.; Udekwu, K.; Tenson, T.; et al. Reassessing the Role of Type II Toxin-Antitoxin Systems in Formation of Escherichia coli Type II Persister. Cells. mBio. 2018, 9, e00640-18. [Google Scholar] [CrossRef]
- Boos, W. Binding protein-dependent ABC transport system for glycerol 3-phosphate of Escherichia coli. Methods Enzymol. 1998, 292, 40–51. [Google Scholar] [CrossRef]
- Karimova, G.; Davi, M.; Ladant, D. The β-lactam resistance protein Blr, a small membrane polypeptide, is a component of the Escherichia coli cell division machinery. J. Bacteriol. 2012, 194, 5576–5588. [Google Scholar] [CrossRef]
- Wong, R.S.; McMurry, L.M.; Levy, S.B. ‘Intergenic’ blr gene in Escherichia coli encodes a 41-residue membrane protein affecting intrinsic susceptibility to certain inhibitors of peptidoglycan synthesis. Mol. Microbiol. 2000, 37, 364–370. [Google Scholar] [CrossRef]
- Davies, J.S.; Currie, M.J.; North, R.A.; Scalise, M.; Wright, J.D.; Copping, J.M.; Remus, D.M.; Gulati, A.; Morado, D.R.; Jamieson, S.A.; et al. Structure and mechanism of a tripartite ATP-independent periplasmic TRAP transporter. Nat. Commun. 2023, 14, 1120. [Google Scholar] [CrossRef] [PubMed]
- Domka, J.; Lee, J.; Wood, T.K. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl. Environ. Microbiol. 2006, 72, 2449–2459. [Google Scholar] [CrossRef]
- Guiral, E.; Mendez-Arancibia, E.; Soto, S.M.; Salvador, P.; Fabrega, A.; Gascon, J.; Vila, J. CTX-M-15-producing enteroaggregative Escherichia coli as cause of travelers’ diarrhea. Emerg. Infect. Dis. 2011, 17, 1950–1953. [Google Scholar] [CrossRef]
- Chen, J.; Lee, S.M.; Mao, Y. Protective effect of exopolysaccharide colanic acid of Escherichia coli O157:H7 to osmotic and oxidative stress. Int. J. Food Microbiol. 2004, 93, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Makino, K.; Ohnishi, M.; Kurokawa, K.; Ishii, K.; Yokoyama, K.; Han, C.G.; Ohtsubo, E.; Nakayama, K.; Murata, T.; et al. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001, 8, 11–22. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, C.H.; Kharadi, R.R.; Yuan, X.; Sundin, G.W.; Triplett, L.; Wang, J.; Zeng, Q. Cell-length heterogeneity: A population-level solution to growth/virulence trade-offs in the plant pathogen Dickeya dadantii. PLoS Pathog. 2019, 15, e1007703. [Google Scholar] [CrossRef]
- Arredondo-Alonso, S.; Blundell-Hunter, G.; Fu, Z.; Gladstone, R.A.; Fillol-Salom, A.; Loraine, J.; Cloutman-Green, E.; Johnsen, P.J.; Samuelsen, Ø.; Pöntinen, A.K.; et al. Evolutionary and functional history of the Escherichia coli K1 capsule. Nat. Commun. 2023, 14, 3294. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.L.; Jann, B.; Jann, K. Structure and serological characteristics of the capsular K4 antigen of Escherichia coli O5:K4:H4, a fructose-containing polysaccharide with a chondroitin backbone. Eur. J. Biochem. 1988, 177, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Pea, F. Intracellular Pharmacokinetics of Antibacterials and Their Clinical Implications. Clin. Pharmacokinet. 2018, 57, 177–189. [Google Scholar] [CrossRef]
- Pea, F.; Viale, P.; Furlanut, M. Antimicrobial therapy in critically ill patients: A review of pathophysiological conditions responsible for altered disposition and pharmacokinetic variability. Clin. Pharmacokinet. 2005, 44, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Savitskii, M.V.; Moskaleva, N.E.; Brito, A.; Markin, P.A.; Zigangirova, N.A.; Soloveva, A.V.; Sheremet, A.B.; Bondareva, N.E.; Lubenec, N.L.; Tagliaro, F.; et al. Pharmacokinetics, tissue distribution, bioavailability and excretion of the anti-virulence drug Fluorothiazinon in rats and rabbits. J. Antibiot. 2024, 77, 382–388. [Google Scholar] [CrossRef]
- Garcia-Medina, R.; Dunne, W.M.; Singh, P.K.; Brody, S.L. Pseudomonas aeruginosa acquires biofilm-like properties within airway epithelial cells. Infect. Immun. 2005, 73, 8298–8305. [Google Scholar] [CrossRef]
- Hoiby, N.; Axelsen, N.H. Identification and quantitation of precipitins against Pseudomonas aeruginosa in patients with cystic fibrosis by means of crossed immunoelectrophoresis with intermediate gel. Acta Pathol. Microbiol. Scand. B. Microbiol. Immunol. 1973, 81, 298–308. [Google Scholar] [CrossRef]
- Burmølle, M.; Thomsen, T.R.; Fazli, M.; Dige, I.; Christensen, L.; Homøe, P.; Tvede, M.; Nyvad, B.; Tolker-Nielsen, T.; Givskov, M.; et al. Biofilms in chronic infections—A matter of opportunity—Monospecies biofilms in multispecies infections. FEMS Immunol. Med. Microbiol. 2010, 59, 324–336. [Google Scholar] [CrossRef]
- Staudinger, B.J.; Muller, J.F.; Halldórsson, S.; Boles, B.; Angermeyer, A.; Nguyen, D.; Rosen, H.; Baldursson, O.; Gottfreðsson, M.; Guðmundsson, G.H.; et al. Conditions associated with the cystic fibrosis defect promote chronic Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2014, 189, 812–824. [Google Scholar] [CrossRef]
- Voronina, O.L.; Kunda, M.S.; Ryzhova, N.N.; Aksenova, E.I.; Semenov, A.N.; Lasareva, A.V.; Amelina, E.L.; Chuchalin, A.G.; Lunin, V.G.; Gintsburg, A.L. The Variability of the Order Burkholderiales Representatives in the Healthcare Units. Biomed. Res. Int. 2015, 2015, 680210. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.; Ochman, H.; et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Voronina, O.L.; Kunda, M.S.; Ryzhova, N.N.; Aksenova, E.I.; Sharapova, N.E.; Semenov, A.N.; Amelina, E.L.; Chuchalin, A.G.; Gintsburg, A.L. On Burkholderiales order microorganisms and cystic fibrosis in Russia. BMC Genom. 2018, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef]
- Li, W.; O’Neill, K.R.; Haft, D.H.; DiCuccio, M.; Chetvernin, V.; Badretdin, A. RefSeq: Expanding the Prokaryotic Genome Annotation Pipeline reach with protein family model curation. Nucleic Acids Res. 2021, 49, D1020–D1028. [Google Scholar] [CrossRef]
- Liu, B.; Zheng, D.; Zhou, S.; Chen, L.; Yang, J. VFDB 2022: A general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022, 50, D912–D917. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Olson, R.D.; Assaf, R.; Brettin, T.; Conrad, N.; Cucinell, C.; Davis, J.J. Introducing the Bacterial and Viral Bioinformatics Resource Center (BV-BRC): A resource combining PATRIC, IRD and ViPR. Nucleic Acids Res. 2023, 51, D678–D689. [Google Scholar] [CrossRef]
- Naas, T.; Oueslati, S.; Bonnin, R.A.; Dabos, M.L.; Zavala, A.; Dortet, L.; Retailleau, P.; Iorga, B.I. Beta-lactamase database (BLDB)—Structure and function. J. Enzyme Inhib. Med. Chem. 2017, 32, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.; Varani, A.M.; Snesrud, E.; Huang, H.; Alvarenga, D.O.; Zhang, J.; Wu, C.; McGann, P.; Chandler, M. TnCentral: A Prokaryotic Transposable Element Database and Web Portal for Transposon Analysis. mBio 2021, 12, e0206021. [Google Scholar] [CrossRef]
- Johansson, M.H.K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J. Antimicrob. Chemother. 2021, 76, 101–109. [Google Scholar] [CrossRef]
- Carattoli, A.; Zankari, E.; García-Fernández, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef]
- Wishart, D.S.; Han, S.; Saha, S.; Oler, E.; Peters, H.; Grant, J.R.; Stothard, P.; Gautam, V. PHASTEST: Faster than PHASTER, better than PHAST. Nucleic Acids Res. 2023, 51, W443–W450. [Google Scholar] [CrossRef]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, W246–W251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alikhan, N.F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.J.; Rutherford, K.M.; Berriman, M.; Rajandream, M.A.; Barrell, B.G.; Parkhill, J. ACT: The Artemis Comparison Tool. Bioinformatics 2005, 21, 3422–3423. [Google Scholar] [CrossRef]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylogroups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]



| Features | GIMC1402:EC_33P15 | GIMC1403:EC_33P43 |
|---|---|---|
| Chromosome, bp. | 5,115,804 | 5,032,495 |
| Genes (total) | 5292 | 5154 |
| CDSs (total) | 5171 | 5032 |
| Genes (RNA) | 121 | 122 |
| tRNAs | 94 | 95 |
| Pseudo Genes (total) | 206 | 202 |
| CRISPR Arrays | 2 | 2 |
| Plasmid | pEC_33P15-1 (134,688 bp) | pEC_33P43-1 (110,734 bp) |
| pEC_33P15-2 (4237 bp) | pEC_33P43-2 (7176 bp) | |
| pEC_33P15-3 (4072 bp) | pEC_33P43-3 (2091 bp) | |
| pEC_33P43-4 (1459 bp) | ||
| Phage-Plasmid | p-pEC_33P15 (108,306 bp) | p-pEC_33P43 (107,320 bp) |
| Category | Function | GIMC1402:EC_33P15, pl1 | GIMC1403:EC_33P43, pl1 | |
|---|---|---|---|---|
| Product (Genome Position) | Product (Genome Position) | |||
| Resistance | Tetracycline resistance | Tetracycline efflux MFS transporter Tet(B) (50012..51217) | – | |
| Transcriptional repressor TetR(B) (51299..51922) | – | |||
| Chloramphenicol resistance | CatA1, chloramphenicol O-acetyltransferase (54209..54868) | – | ||
| Macrolide resistance | Mph(A), family macrolide 2′-phosphotransferase (59909..60814) | – | ||
| Mrx(A), macrolide resistance MFS transporter (60811..62049) | – | |||
| Sulfonamide resistance | Sul1 sulfonamide-resistant dihydropteroate synthase (66125..66964) | – | ||
| Aminoglycoside resistance | AadA5, ANT(3′)-Ia family aminoglycoside nucleotidyltransferase (67511..68299) | – | ||
| Trimethoprim resistance | Dft17, trimethoprim-resistant dihydrofolate reductase (68430..68903) | – | ||
| Quaternary ammonium compound resistance | QacE, QAC efflux SMR transporter (66958..67305) | – | ||
| Virulence factors | Protection against the macroorganism’s complement system; participation in the biofilm formation | F-type transfer system (24233–34796; 79117–84085) | F-type transfer system (22132–41597) | |
| Colonization and survival under conditions of Fe2+, Pb2+, Zn2+, and Mn2+ deficiency | iucABCD, iutA, aerobactin (110608..119908) | – | ||
| Fe2+ ABC-transporter (38945..43430) | Fe2+ ABC-transporter (59262..54777) | |||
| Fe2+/Pb2+ ILT-transporter (43534..45988) | Fe2+/Pb2+ ILT-transporter (54673..52237) | |||
| SitABCD ABC-transporter (105156..108605) | – | |||
| – | TonB-dependent transport system (68082..71288; 81969..83939) | |||
| – | YncE protein (71357..72532) | |||
| Toxin–antitoxin systems (TA) | Selective advantage of a clone in a bacterial population, formation of a persistent cell population | Type I * | Mok/Hok TA (20002..20218) | Mok/Hok TA (18537..18753) |
| Hok/Gef TA (77769..77903) | Hok/Gef TA (42811..42945) | |||
| Type II ** | – | Phd_YefM/Fic_DOC TA (98859..99460) | ||
| VapB/VapC TA (123886..124529; 128749..129392) | – | |||
| CcdA/CcdB TA (133356..133881) | CcdA/CcdB TA (109402..109927) | |||
| PemL/PemK TA (71821..72412) | PemL/PemK TA (46343..46934) | |||
| Region | GIMC1402:EC_33P15 | GIMC1403:EC_33P43 | ||
|---|---|---|---|---|
| Gene | Product | Gene | Product | |
| 1 | 351708..350878 | TEM-1 | ||
| 351852..352556 | IS6-Tnp | 331206..331280 | IS6-Tnp-pseudo | |
| 352700..353254 | AAC(6′)-Ib-cr | 331424..331978 | AAC(6′)-Ib-cr | |
| 353385..354215 | OXA-1 | 332109..332939 | OXA-1 | |
| 354353..354793 | CatB3-pseudo | 333077..333517 | CatB3-pseudo | |
| complement (354847..355551) | IS6-Tnp | complement (333571..334275) | IS6-Tnp | |
| 355658..356518 | AAC(3)-IIa | |||
| 356531..357073 | tmrB | |||
| 357165..358213 | IS3-Tnp | |||
| complement (358267..358971) | IS6-Tnp | |||
| 2 | complement (359039..361267) | Tn3-Tnp-pseudo | complement (334343..336571) | Tn3-Tnp-pseudo |
| complement (361672..362547) | CTX-M-15 | complement (336976..337851) | CTX-M-15 | |
| complement (362803..364065) | IS1380-Tnp | complement (338107..339369) | IS1380-Tnp | |
| Indel | GIMC1402:EC_33P15 | GIMC1403:EC_33P43 |
|---|---|---|
| Indel 1 | the operon for ABC transporter complex UgpBAEC | no |
| Indel 2 | 4 ORFs, including the ORF of the small-membrane protein Blr | no |
| Indel 3 | duplication of ORFs for the type IV toxin–antitoxin system | no |
| Indel 4 | the 3rd operon for the tripartite ATP-independent periplasmic (TRAP) transporter | no |
| Indel 5 | no | ORFs of the energy-coupling factor (ECF)–ABC transporter for cobalt transport |
| Indel 6 | no | the genes for some metabolic pathways and an additional GntP family transporter (gluconate:H+ symporter) |
| Indel 7 | no | 11 ORFs, and the most important are the mdtH gene encoding the multidrug efflux MFS transporter, and the biofilm formation regulator BssS |
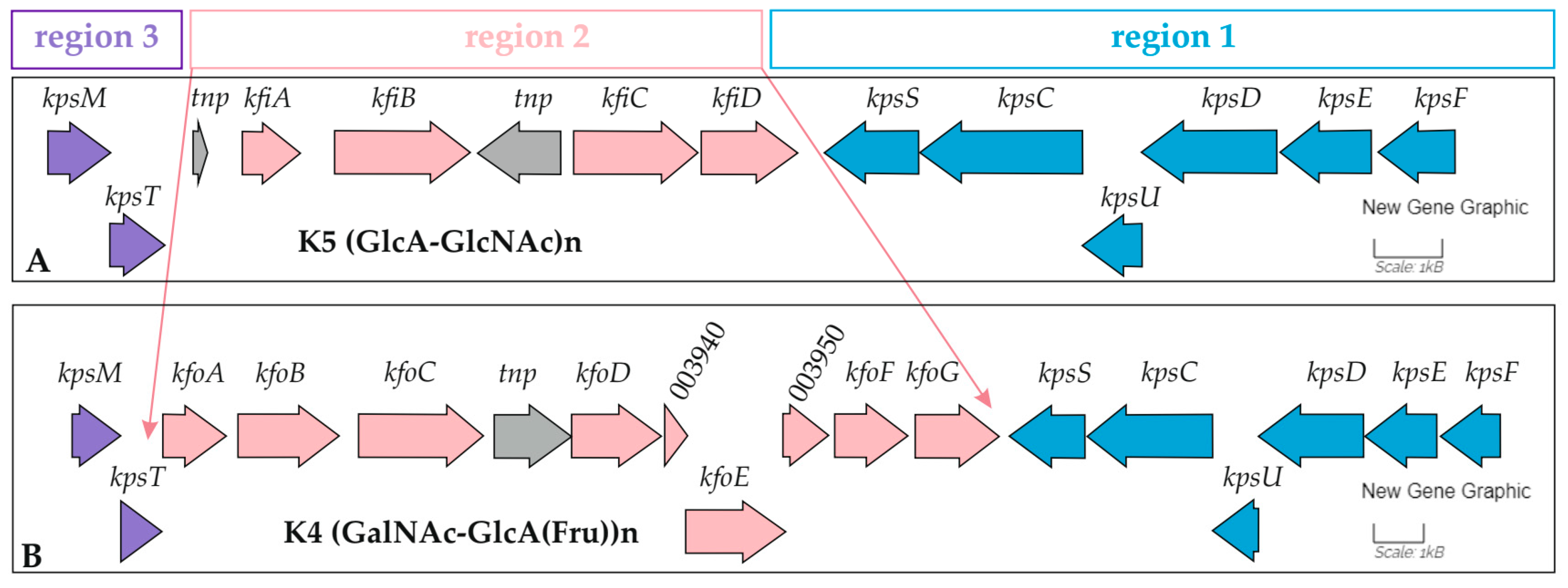
| Region of original ORFs | the K5 capsular gene cluster | the K4 capsular gene cluster |
| ORFs for the metabolosome (bacterial microcompartment) organization and propanediol utilization | no |
| Position | CRISPR Length | Consensus_Repeat | Repeat ID (CRISPRdb) | Spacers Nb | Evidence Level |
|---|---|---|---|---|---|
| GIMC1402:EC_33P15 | |||||
| 1154150…1155279 | 1129 | GTGTTCCCCGCGCCAGCGGGGATAAACCG | R6121 | 18 | 4 |
| 1180826…1181648 | 822 | GAGTTCCCCGCGCCAGCGGGGATAAACCG | R3946 | 13 | 4 |
| GIMC1403:EC_33P43 | |||||
| 1115575…1116213 | 638 | GAGTTCCCCGCGCCAGCGGGGATAAACCG | R3946 | 10 | 4 |
| 1143351…1143925 | 574 | GTGTTCCCCGCGCCAGCGGGGATAAA | Unknown | 9 | 4 |
| Strain, Accession number | GIMC1402:EC_33P15, CP181181.1 | GIMC1403:EC_33P43, CP181392.1 | NA023, JSXK000000000.1 | 32–2823 ED, DABAXP000000000.1 | VB 962116, DABAMI000000000.1 | F_30_1_R8, PIIR000000000.1 |
|---|---|---|---|---|---|---|
| clade | 1 | 2 | 3 | 4 | ||
| GIMC1402:EC_33P15 | - | 99.1 | 99.49 | 99.54 | 98.92 | 99.08 |
| GIMC1403:EC_33P43 | 95.65 | - | 99.39 | 99.36 | 98.84 | 98.98 |
| NA023, clade 1 | 92.45 | 91.54 | - | 99.51 | 98.98 | 98.98 |
| 32–2823 ED, clade 2 | 89.32 | 89.96 | 90.07 | - | 99.33 | 99.43 |
| VB 962116, clade 3 | 88.07 | 87.82 | 98.98 | 90.01 | - | 99.72 |
| F_30_1_R8, clade 4 | 90.63 | 89.98 | 89.95 | 89.76 | 90.87 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronina, O.L.; Kunda, M.S.; Ryzhova, N.N.; Ermolova, E.I.; Goncharova, E.R.; Koroleva, E.A.; Kapotina, L.N.; Morgunova, E.Y.; Amelina, E.L.; Zigangirova, N.A. Chronic Escherichia coli ST648 Infections in Patients with Cystic Fibrosis: The In Vitro Effects of an Antivirulence Agent. Int. J. Mol. Sci. 2025, 26, 8650. https://doi.org/10.3390/ijms26178650
Voronina OL, Kunda MS, Ryzhova NN, Ermolova EI, Goncharova ER, Koroleva EA, Kapotina LN, Morgunova EY, Amelina EL, Zigangirova NA. Chronic Escherichia coli ST648 Infections in Patients with Cystic Fibrosis: The In Vitro Effects of an Antivirulence Agent. International Journal of Molecular Sciences. 2025; 26(17):8650. https://doi.org/10.3390/ijms26178650
Chicago/Turabian StyleVoronina, Olga L., Marina S. Kunda, Natalia N. Ryzhova, Ekaterina I. Ermolova, Elizaveta R. Goncharova, Ekaterina A. Koroleva, Lidia N. Kapotina, Elena Yu. Morgunova, Elena L. Amelina, and Nailya A. Zigangirova. 2025. "Chronic Escherichia coli ST648 Infections in Patients with Cystic Fibrosis: The In Vitro Effects of an Antivirulence Agent" International Journal of Molecular Sciences 26, no. 17: 8650. https://doi.org/10.3390/ijms26178650
APA StyleVoronina, O. L., Kunda, M. S., Ryzhova, N. N., Ermolova, E. I., Goncharova, E. R., Koroleva, E. A., Kapotina, L. N., Morgunova, E. Y., Amelina, E. L., & Zigangirova, N. A. (2025). Chronic Escherichia coli ST648 Infections in Patients with Cystic Fibrosis: The In Vitro Effects of an Antivirulence Agent. International Journal of Molecular Sciences, 26(17), 8650. https://doi.org/10.3390/ijms26178650






