SOD-1/2 Involvement in the Antioxidant Molecular Events Occurring upon Complex Magnetic Fields Application in an In Vitro H2O2 Oxidative Stress-Induced Endothelial Cell Model
Abstract
1. Introduction
2. Results
2.1. Effect of CMFs Application on H2O2-Mediated ROS Production, Metabolic Activity and Cytotoxic Response in EA.hy926 Cells
2.2. Effect of CMFs on SOD1/2 Protein Expression
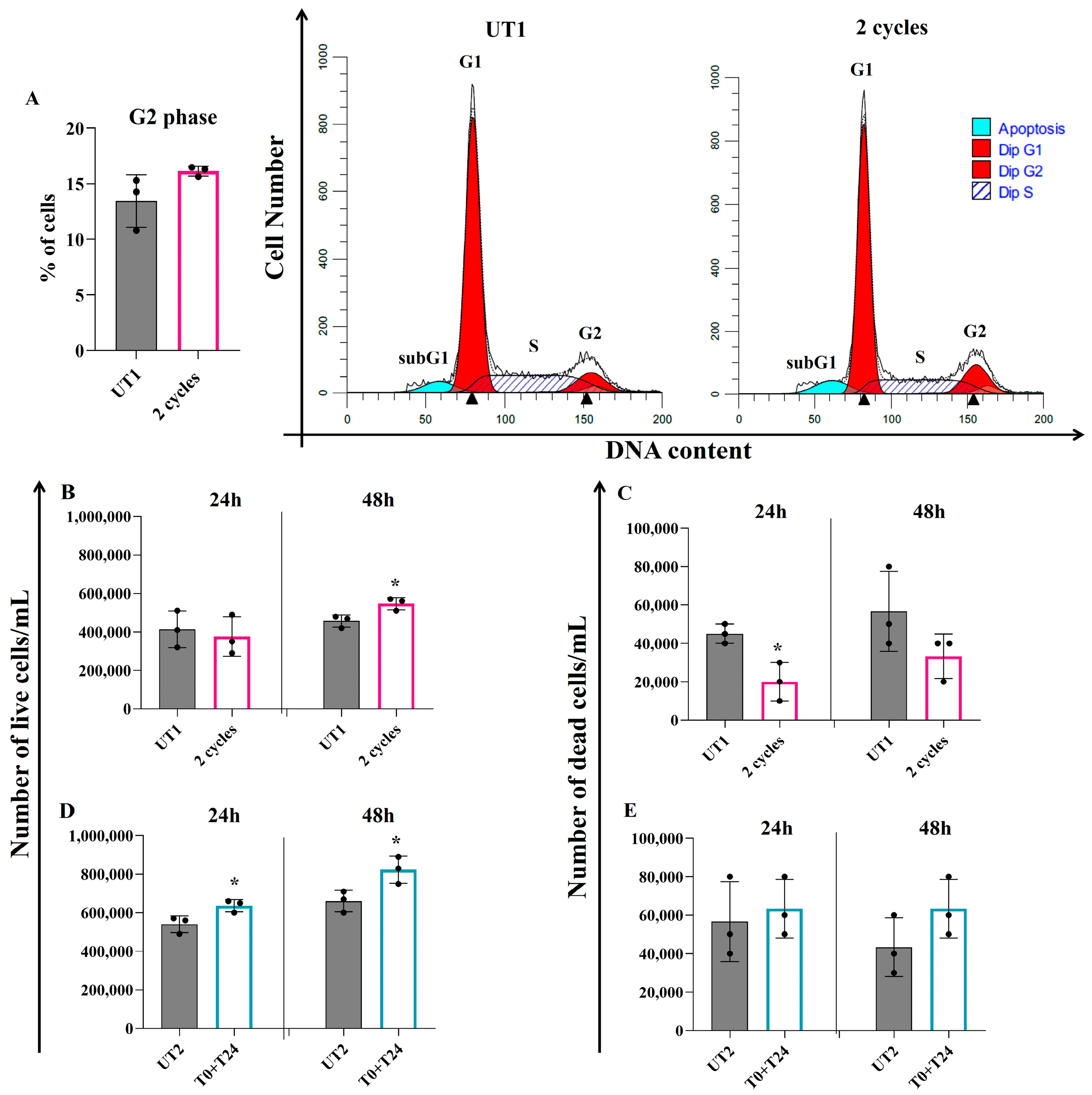
2.3. Effect of CMFs on EC Turnover Stimulation upon H2O2-Induced Oxidative Stress
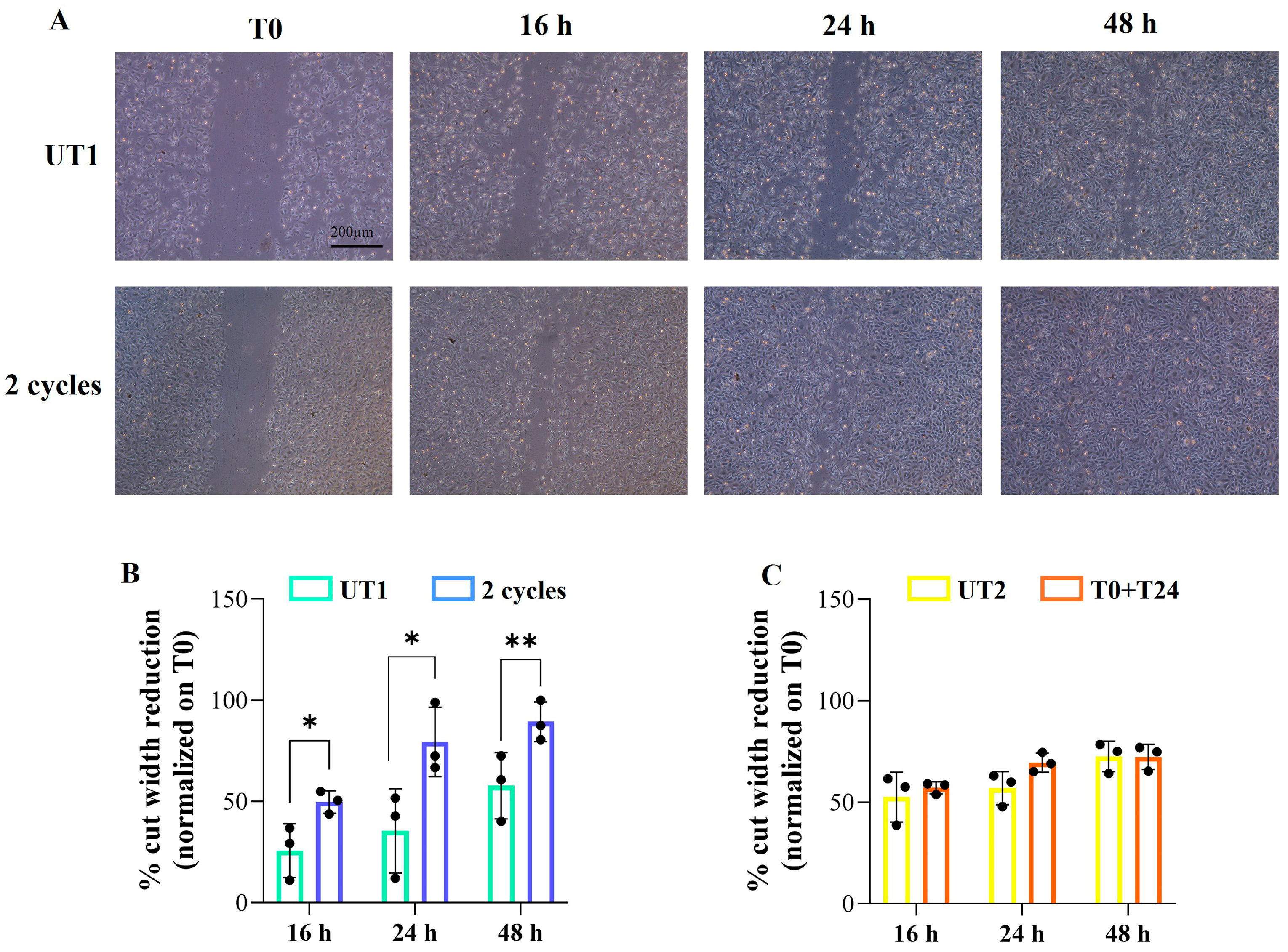
2.4. Effect of CMFs on H2O2-Stimulated EC Migration
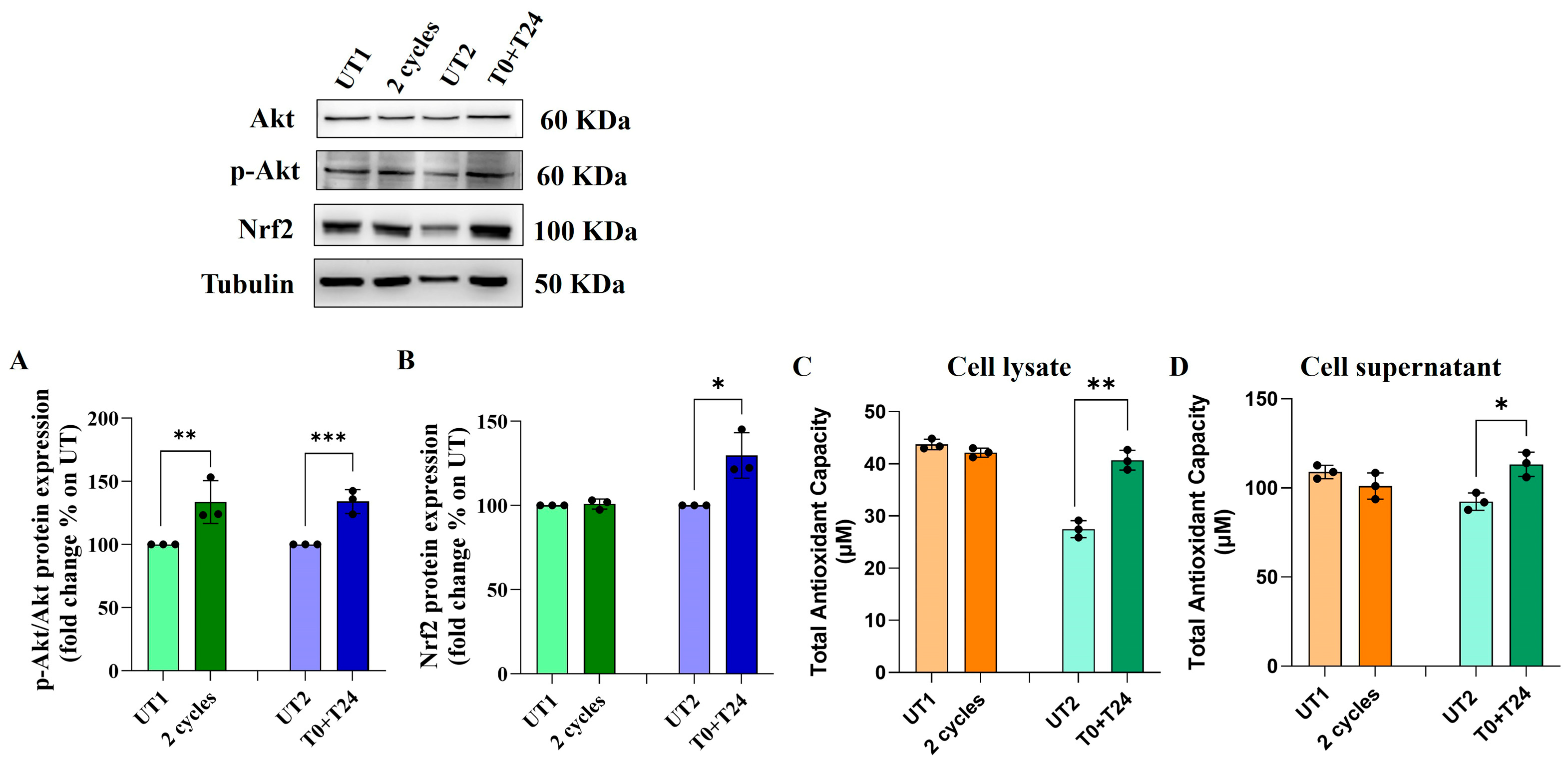
2.5. Effect of CMFs on the Antioxidant Factors Expression in H2O2-Stimulated ECs
3. Discussion
4. Materials and Methods
4.1. Cell Culture
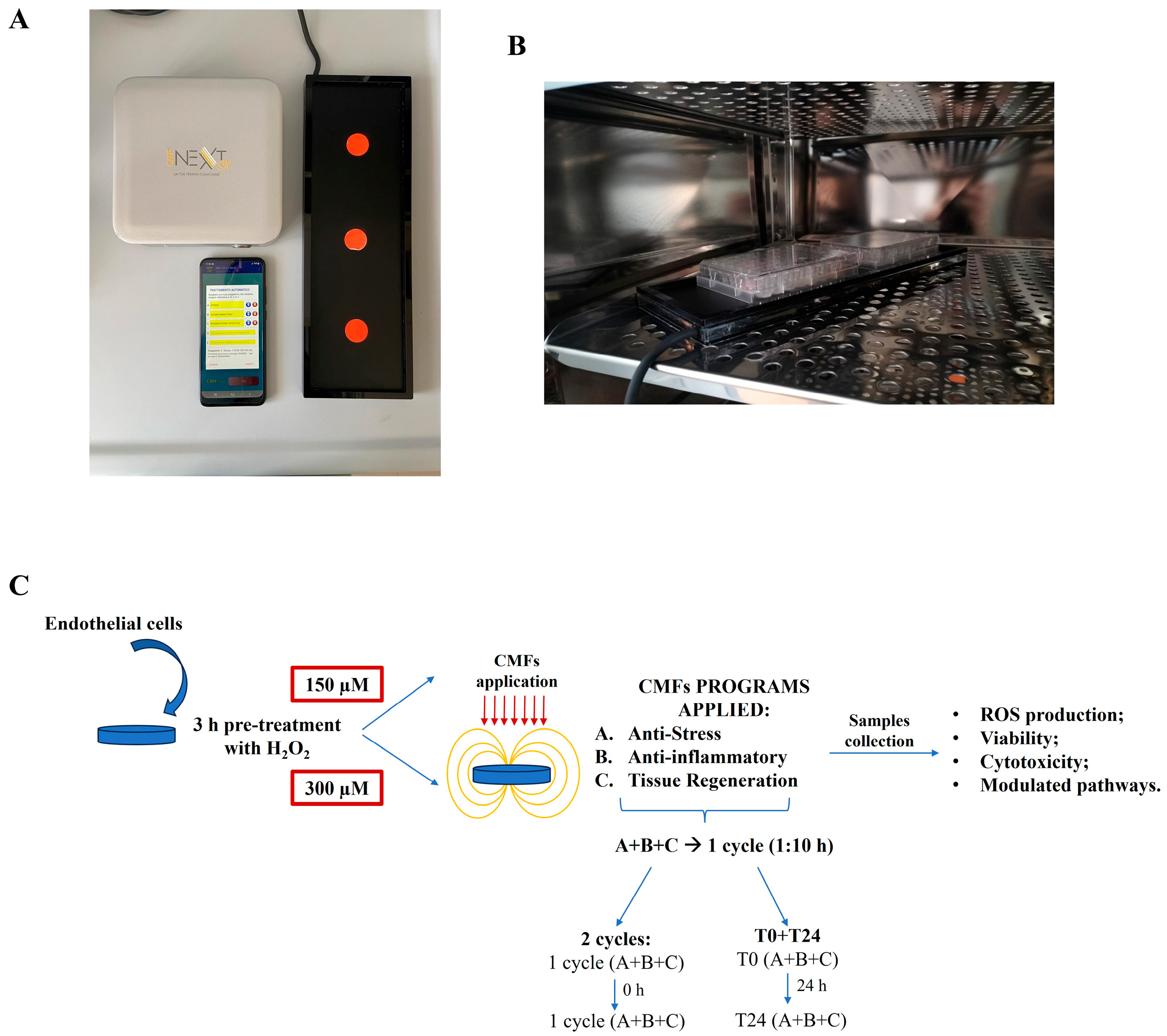
4.2. Cell Treatment and Stimulation
4.3. Flow Cytometry Analysis of ROS Production
4.4. Cell Metabolic Activity Assay (MTT)
4.5. Lactate Dehydrogenase (LDH) Assay
4.6. Western Blot Analysis
4.7. Detection of Apoptosis and Necrosis by Flow Cytometry
4.8. Trypan Blue Dye Exclusion Test
4.9. Cell Cycle Analysis
4.10. Wound Healing
4.11. Total Antioxidant Capacity (TAC) Assay
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Augustin, H.G.; Koh, G.Y. Organotypic vasculature: From descriptive heterogeneity to functional pathophysiology. Science 2017, 357, eaal2379. [Google Scholar] [CrossRef]
- Rivera, F.J.; Silva, M.E.; Aigner, L. Editorial: The Vascular Niche in Tissue Repair: A Therapeutic Target for Regeneration. Front. Cell Dev. Biol. 2017, 5, 88. [Google Scholar] [CrossRef]
- Shaito, A.; Aramouni, K.; Assaf, R.; Parenti, A.; Orekhov, A.; Yazbi, A.E.; Pintus, G.; Eid, A.H. Oxidative Stress-Induced Endothelial Dysfunction in Cardiovascular Diseases. Front. Biosci. 2022, 27, 105. [Google Scholar] [CrossRef]
- Camargo, L.L.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. Reactive oxygen species in hypertension. Nat. Rev. Cardiol. 2025, 22, 20–37. [Google Scholar] [CrossRef]
- Pang, B.; Dong, G.; Pang, T.; Sun, X.; Liu, X.; Nie, Y.; Chang, X. Emerging insights into the pathogenesis and therapeutic strategies for vascular endothelial injury-associated diseases: Focus on mitochondrial dysfunction. Angiogenesis 2024, 27, 623–639. [Google Scholar] [CrossRef]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide Dismutase Administration: A Review of Proposed Human Uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Jana, S.; Manna, A.; Jana, K. Apoptosis and Human Health: Understanding Mechanistic and Therapeutic Potential, 1st ed.; Springer: Singapore, 2024; pp. 141–153. [Google Scholar]
- Mustafa, M.; Ahmad, R.; Tantry, I.Q.; Ahmad, W.; Siddiqui, S.; Alam, M.; Abbas, K.; Moinuddin; Hassan, M.I.; Habib, S.; et al. Apoptosis: A Comprehensive Overview of Signaling Pathways, Morphological Changes, and Physiological Significance and Therapeutic Implications. Cells 2024, 13, 1838. [Google Scholar] [CrossRef] [PubMed]
- Rath-Deschner, B.; Nogueira, A.V.B.; Memmert, S.; Nokhbehsaim, M.; Augusto Cirelli, J.; Eick, S.; Miosge, N.; Kirschneck, C.; Kesting, M.; Deschner, J.; et al. Regulation of Anti-Apoptotic SOD2 and BIRC3 in Periodontal Cells and Tissues. Int. J. Mol. Sci. 2021, 22, 591. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wu, Q.; Liu, Y. Eucommia ulmoides Oliv. leaves flavonoids attenuate methylglyoxal-induced endothelial cell apoptosis in vitro and in vivo by upregulating AKT-Nrf2 signaling and downregulating oxidative stress. Food Sci. Nutr. 2024, 12, 7938–7953. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Song, J.; Li, S.; Zhang, B.; Wu, J.; Zhong, A. Quercetin protects human coronary artery endothelial cells against hypoxia/reoxygenation-induced mitochondrial apoptosis via the Nrf2/HO-1 axis. Biomed. Res. 2024, 45, 197–207. [Google Scholar] [CrossRef]
- Bontor, K.; Gabryel, B. Sulodexide protects endothelial cells against 4-hydroxynonenal-induced oxidative stress and glutathione-dependent redox imbalance by modulation of sestrin2/nuclear factor erythroid 2-related factor 2 pathway. J. Physiol. Pharmacol. 2024, 75, 373–387. [Google Scholar]
- Ismail, M.B.; Rajendran, P.; AbuZahra, H.M.; Veeraraghavan, V.P. Mangiferin Inhibits Apoptosis in Doxorubicin-Induced Vascular Endothelial Cells via the Nrf2 Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 4259. [Google Scholar] [CrossRef]
- Zanotti, F.; Trentini, M.; Zanolla, I.; Tiengo, E.; Mantarro, C.; Dalla Paola, L.; Tremoli, E.; Sambataro, M.; Sambado, L.; Picari, M.; et al. Playing with Biophysics: How a Symphony of Different Electromagnetic Fields Acts to Reduce the Inflammation in Diabetic Derived Cells. Int. J. Mol. Sci. 2023, 24, 1754. [Google Scholar] [CrossRef]
- Gallorini, M.; Mencarelli, N.; Di Pietro, N.; di Giacomo, V.; Zara, S.; Ricci, A.; Rapino, M.; Piattelli, A.; Cipollina, A.; Cataldi, A. The Immunophenotype and the Odontogenic Commitment of Dental Pulp Stem Cells Co-Cultured with Macrophages Under Inflammatory Conditions Is Modulated by Complex Magnetic Fields. Int. J. Mol. Sci. 2024, 26, 48. [Google Scholar] [CrossRef] [PubMed]
- Di Lodovico, S.; Petrini, M.; D’Amico, E.; Di Fermo, P.; Diban, F.; D’Arcangelo, S.; Piattelli, A.; Cellini, L.; Iezzi, G.; Di Giulio, M.; et al. Complex magnetic fields represent an eco-sustainable technology to counteract the resistant Candida albicans growth without affecting the human gingival fibroblasts. Sci. Rep. 2023, 13, 22067. [Google Scholar] [CrossRef]
- Ricci, A.; Cataldi, A.; Gallorini, M.; di Giacomo, V.; Rapino, M.; Di Pietro, N.; Mantarro, M.; Piattelli, A.; Zara, S. Angiogenic Events Positively Modulated by Complex Magnetic Fields in an In Vitro Endothelial Cell Model. Cells 2025, 14, 332. [Google Scholar] [CrossRef] [PubMed]
- Tota, M.; Jonderko, L.; Witek, J.; Novickij, V.; Kulbacka, J. Cellular and Molecular Effects of Magnetic Fields. Int. J. Mol. Sci. 2024, 25, 8973. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Q.; Yao, R.; Liu, X.; Qu, Z. 7, 8-Dihydroxyflavone Protects an Endothelial Cell Line from H2O2 Damage. PLoS ONE 2015, 10, e0135345. [Google Scholar] [CrossRef][Green Version]
- Zhu, M.; Li, J.; Wang, K.; Hao, X.; Ge, R.; Li, Q. Isoquercitrin Inhibits Hydrogen Peroxide-Induced Apoptosis of EA.hy926 Cells via the PI3K/Akt/GSK3β Signaling Pathway. Molecules 2016, 21, 356. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Mou, L.; Ge, H. Protective effects of ginsenoside Rb1 on H2O2-induced oxidative injury in human endothelial cell line (EA.hy926) via miR-210. Int. J. Immunopathol. Pharmacol. 2019, 35, 20587384211040399. [Google Scholar] [CrossRef]
- Amjad, M.D.; Marramiero, L.; Romasco, T.; Cipollina, A.; Hossein, H.H.S.; Mantarro, M.; Piattelli, A.; Fulle, S.; Di Pietro, N.; Mancinelli, R. Complex Magnetic Fields: Harnessing the Electromagnetic Symphony for Possible Applications in Regenerative Medicine and Antifungal Properties. Int. Wound J. 2025, 22, e70679. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Li, P.F. Mutual regulation of lactate dehydrogenase and redox robustness. Front. Physiol. 2022, 13, 1038421. [Google Scholar] [CrossRef]
- Hollenberg, A.M.; Huber, A.; Smith, C.O.; Eliseev, R.A. Electromagnetic stimulation increases mitochondrial function in osteogenic cells and promotes bone fracture repair. Sci. Rep. 2021, 11, 19114. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.C.; Krishna, L.; Pannir Selvan, R.M.; Tai, Y.K.; Kit Wong, C.J.; Yin, J.N.; Toh, S.J.; Torta, F.; Triebl, A.; Fröhlich, J.; et al. Magnetic field therapy enhances muscle mitochondrial bioenergetics and attenuates systemic ceramide levels following ACL reconstruction: Southeast Asian randomized-controlled pilot trial. J. Orthop. Translat. 2022, 35, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Sun, Q.; Li, N.; Chen, Z.; Wu, H.; Chen, Z.; Guo, X.; Fang, F. Superoxide dismutase 2 scavenges ROS to promote osteogenic differentiation of human periodontal ligament stem cells by regulating Smad3 in alveolar bone-defective rats. J. Periodontol. 2024, 95, 469–482. [Google Scholar] [CrossRef]
- Trist, B.G.; Hilton, J.B.; Hare, D.J.; Crouch, P.J.; Double, K.L. Superoxide Dismutase 1 in Health and Disease: How a Frontline Antioxidant Becomes Neurotoxic. Angew. Chem. Int. Ed. 2021, 60, 9215–9246. [Google Scholar] [CrossRef]
- Tsang, C.K.; Liu, Y.; Thomas, J.; Zhang, Y.; Zheng, X.F. Superoxide dismutase 1 acts as a nuclear transcription factor to regulate oxidative stress resistance. Nat. Commun. 2014, 5, 3446. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zheng, H.; Fu, Y.; Gu, Y.; Zou, H.; Yuan, Y.; Gu, J.; Liu, Z.; Bian, J. Role of PI3K/Akt-Mediated Nrf2/HO-1 Signaling Pathway in Resveratrol Alleviation of Zearalenone-Induced Oxidative Stress and Apoptosis in TM4 Cells. Toxins 2022, 14, 733. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Zara, S.; Carta, F.; Di Valerio, V.; Sancilio, S.; Cataldi, A.; Selleri, S.; Supuran, C.T.; Carradori, S.; Gallorini, M. 2-Substituted-4,7-dihydro-4-ethylpyrazolo[1,5-a]pyrimidin-7-ones alleviate LPS-induced inflammation by modulating cell metabolism via CD73 upon macrophage polarization. Mol. Immunol. 2024, 170, 99–109. [Google Scholar] [CrossRef]
- Yiran, D.; Jiayi, W.; Lifang, M. Harnessing Antioxidants to Counter Oxidative Stress-Induced Apoptosis. In The Power of Antioxidants-Unleashing Nature’s Defense Against Oxidative Stress; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Ricci, A.; Gallorini, M.; Feghali, N.; Sampò, S.; Cataldi, A.; Zara, S. Snail Slime Extracted by a Cruelty Free Method Preserves Viability and Controls Inflammation Occurrence: A Focus on Fibroblasts. Molecules 2023, 28, 1222. [Google Scholar] [CrossRef]



| A | |
| Type of Programs | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| B | |
| Program-Related CMF Parameters | Characteristics |
| Frequencies | 1–250 Hz |
| Intensities | 1–250 µT |
| Interval Times | 1–4 min each step (not exceeding 30 min/program) |
| Type of waveform with harmonic enrichment | square, sinusoidal, impulsive, triangular, trapezoidal |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, A.; Zara, S.; di Giacomo, V.; Gallorini, M.; Rapino, M.; Di Pietro, N.; Cipollina, A.; Piattelli, A.; Cataldi, A. SOD-1/2 Involvement in the Antioxidant Molecular Events Occurring upon Complex Magnetic Fields Application in an In Vitro H2O2 Oxidative Stress-Induced Endothelial Cell Model. Int. J. Mol. Sci. 2025, 26, 8600. https://doi.org/10.3390/ijms26178600
Ricci A, Zara S, di Giacomo V, Gallorini M, Rapino M, Di Pietro N, Cipollina A, Piattelli A, Cataldi A. SOD-1/2 Involvement in the Antioxidant Molecular Events Occurring upon Complex Magnetic Fields Application in an In Vitro H2O2 Oxidative Stress-Induced Endothelial Cell Model. International Journal of Molecular Sciences. 2025; 26(17):8600. https://doi.org/10.3390/ijms26178600
Chicago/Turabian StyleRicci, Alessia, Susi Zara, Viviana di Giacomo, Marialucia Gallorini, Monica Rapino, Natalia Di Pietro, Alessandro Cipollina, Adriano Piattelli, and Amelia Cataldi. 2025. "SOD-1/2 Involvement in the Antioxidant Molecular Events Occurring upon Complex Magnetic Fields Application in an In Vitro H2O2 Oxidative Stress-Induced Endothelial Cell Model" International Journal of Molecular Sciences 26, no. 17: 8600. https://doi.org/10.3390/ijms26178600
APA StyleRicci, A., Zara, S., di Giacomo, V., Gallorini, M., Rapino, M., Di Pietro, N., Cipollina, A., Piattelli, A., & Cataldi, A. (2025). SOD-1/2 Involvement in the Antioxidant Molecular Events Occurring upon Complex Magnetic Fields Application in an In Vitro H2O2 Oxidative Stress-Induced Endothelial Cell Model. International Journal of Molecular Sciences, 26(17), 8600. https://doi.org/10.3390/ijms26178600










