Molecular Underpinning of Treatment-Resistant Schizophrenia: A Putative Different Neurobiology from Treatment-Responsive Schizophrenia
Abstract
1. Introduction
2. Putative Neurotransmitter Basis of TRS
2.1. Dopamine and Glutamate Hypotheses in TRS
2.2. D-Amino Acid and Glycine Dynamics
2.3. Excitatory/Inhibitory Imbalance and GABA Alterations in TRS
2.4. Cholinergic Modulation of Dopaminergic and Glutamatergic Circuits in Treatment-Resistant Schizophrenia: From Nicotine to Muscarinic Agonists
2.5. The Noradrenergic System in Treatment-Resistant Schizophrenia: A Neglected Neurotransmitter Pathway?
3. Insights from “Omics” Studies (Genomics, Proteomics, Metabolomics) in TRS
3.1. Genomics Studies on TRS
3.2. Proteomics and Metabolomics
3.3. Animal Models of TRS
3.4. Postmortem Evidence of Neurochemical Alterations in TRS
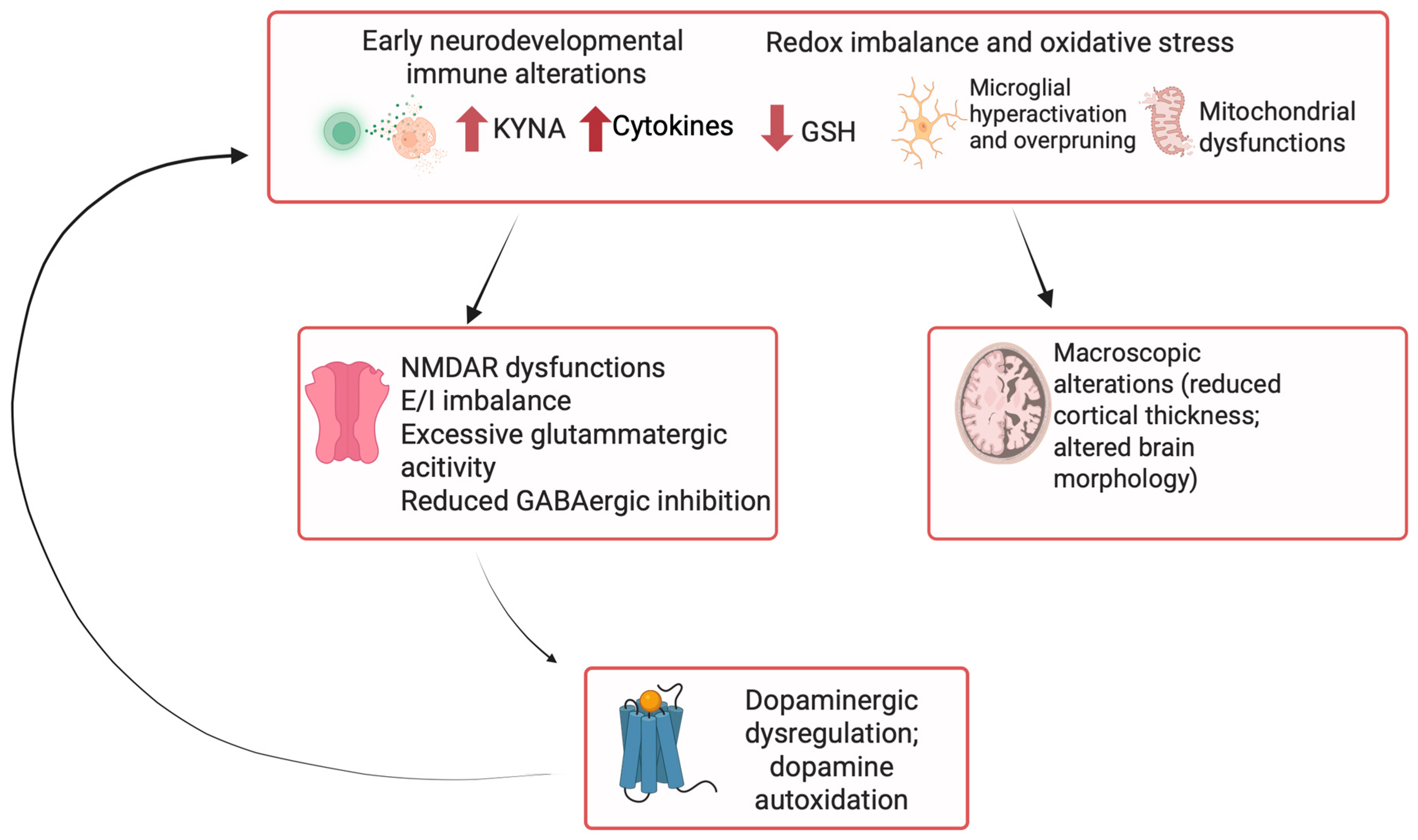
4. Inflammation and Immune System Involvement in TRS Pathophysiology
4.1. The Role of Glial–Neuronal Interactions and Neurochemical Inflammation in TRS
4.2. Mitochondrial Dysfunction and Oxidative Stress in TRS
4.3. Endoplasmic Reticulum Stress and Cellular Dysfunction in Schizophrenia: Relevance for Treatment Resistance
5. Structural Neuroimaging Biomarkers of Treatment Response and Resistance in Psychotic Disorders
5.1. Gray Matter Alterations and Antipsychotic Responsiveness
5.2. White Matter Integrity and Treatment Response in Psychotic Disorders: Insights from Diffusion Tensor Imaging
5.3. Alterations in Functional Connectivity and Network Parameters in Antipsychotic-Refractory Patients: Insights from fMRI Studies
5.3.1. Alterations in the Functional Connectivity of Cortical Regions as Predictors of Antipsychotic Resistance: Evidence from Resting State fMRI
5.3.2. Striatal Connectivity and Treatment Response Prediction
5.3.3. Functional Connectivity Alterations in Cognitive Task fMRI Studies
5.4. Metabolic Activity in Treatment-Resistant Schizophrenia: Evidence Collected from 18F-Deoxyglucose Positron Emission Tomography
6. Discussion
| TRS | SCZ | |
|---|---|---|
| Dopamine | -Poor clinical response with APS treatment despite adequate D2R occupancy; -Possibly lower PRL levels, suggesting non-dopaminergic mechanisms; | -Central dopaminergic dysfunction with striatal hyperdopaminergia; -Symptoms generally improve with D2 blockade; -Prolactin elevation consistent with dopaminergic antagonism; |
| Glutamate/GABA | -Stronger glutammatergic dysfunctions; -elevated Glu/Glx in ACC; -NMDA hypofunction; -E/I imbalance with reduced GABAergic inhibition | -Cortical glutamatergic dysfunction (↑ Glu/Glx in striatum and medial temporal lobe); -subtle NMDA hypofunction; -mild GABAergic alterations; |
| Inflammation and immunity | -↑ IL-6, IL-1RA, activated monocytes; -↓ anti-inflammatory proteins (e.g., CC16); -in UTRS: ↑ IL-6, TNF-α, IFNγ (Th17 pathway) | -Inflammatory alterations less pronounced; -Occasional ↑ IL-6 or ↑ CRP; -Microglial alterations occasionally reported; |
| Oxidative stress | -Stronger evidence of oxidative stress; -↓ GSH, mitochondrial dysfunction | -Evidence of oxidative stress (↑ lipid peroxidation, ↓ antioxidant defenses); -GSH levels often preserved; |
| Genomics/Pharmacogenomics | -Higher impact of metabolic polymorphisms (CYP2D6, CYP1A2, CYP3A4, COMT Val158Met, transporters ABCB1, ABCC2); -Higher association with glutamatergic and GABAergic genes variants; -Higher cumulative PRS burden than SCZ; -CNVs enriched in TRS; | – Enrichment for common SCZ risk variants (e.g., DRD2, COMT Val158Met, GRM3); – PRS indicates increased liability to psychosis compared with general population; – Rare but high risk CNVs (e.g., 22q11.2, 15q13.3, NRXN1 delections) contribute to SCZ risk; |
| Neuroimaging | -Greater cortical thinning; -↑ Glu in anterior cingulate cortex; -More marked connectivity abnormalities | -Cortical thinning and connectivity alterations present but less severe; -Abnormal striatal dopamine synthesis |
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| TRS | treatment-resistant schizophrenia |
| PANSS | positive and negative symptoms scale |
| TRRIP | treatment response and resistance in psychosis |
| DSP | dopamine supersensitivity psychosis |
| UTRS | ultra-treatment-resistant schizophrenia |
| ECT | electroconvulsive therapy |
| D2R | dopamine D2 receptor |
| D3R | dopamine D3 receptor |
| NMDAR | N-methyl-D-aspartate receptor |
| 1H-MRS | proton magnetic resonance spectroscopy |
| Glx | glutamate+glutamine |
| SPECT | single-photon emission computed tomography |
| GlyT1 | glycine transporter 1 |
| GABA | γ-aminobutyric acid |
| GABA-A | γ-aminobutyric acid receptor A |
| GABA-B | γ-aminobutyric acid receptor B |
| DAO | D-amino acid oxidase |
| E/I | excitatory/inhibitory |
| TMS | transcranial magnetic stimulation |
| SICI | short-interval intracortical inhibition |
| EMG | electromyography |
| nAChRs | nicotinic acetylcholine receptors |
| mAChRs | muscarinic acetylcholine receptors |
| CHRNA7 | cholinergic receptor nicotinic alpha 7 subunit |
| PET | positron emission tomography |
| TSPO | 18 kDa translocator protein |
| AMPAR | alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| PAMs | positive allosteric modulators |
| Ca2+ | Calcium |
| M1 | Muscarinic receptor 1 |
| M2 | Muscarinic receptor 2 |
| M3 | Muscarinic receptor 3 |
| M4 | Muscarinic receptor 4 |
| M5 | Muscarinic receptor 5 |
| BBB | blood–brain barrier |
| US | United States |
| FDA | Food and Drug Administration |
| CSF | cerebrospinal fluid |
| MHPG | 3-methoxy-4-hydroxyphenylglycol |
| α1 | alpha-1 adrenergic receptor |
| VTA | ventral tegmental area |
| GWAS | genome-wide association study |
| DDC | aromatic L-amino acid decarboxylase |
| L-DOPA | 3,4-dihydroxyphenylalanine |
| 5-HTP | 5-hydroxytryptophan |
| GRM3 | metabotropic glutamate receptor 3 gene |
| mGluR3 | metabotropic glutamate receptor 3 |
| GAD1 | glutamate decarboxylase 1 |
| GABBR2 | gamma-aminobutyric acid type B receptor subunit 2 |
| DRD1 | dopamine D1 receptor gene |
| DRD2 | dopamine D2 receptor gene |
| OXT | oxytocin |
| BDNF | brain-derived neurotrophic factor |
| SNP | single-nucleotide polymorphism |
| HTR2A | 5-hydroxytryptamine receptor 2A gene |
| HTR2C | 5-hydroxytryptamine receptor 2C gene |
| SLC6A4 | solute carrier family 6 member 4 |
| 5-HTTLPR | serotonin-transporter-linked promoter region |
| CYP2D6 | Cytochrome P450 2D6 |
| CYP1A2 | Cytochrome P450 1A2 |
| CYP3A4 | Cytochrome P450 3A4 |
| CYP2C9 | Cytochrome P450 2C49 |
| COMT | catechol-O-methyltransferase |
| SLC22A1 | Solute Carrier Family 22 Member 1 |
| OCT | organic cation transporter 1 |
| ABCB1 | ATP-binding cassette subfamily B member 1 |
| MRP2 | multidrug resistance-associated protein 2 |
| ABCC2 | ATP-binding cassette subfamily C member 2 protein |
| APOC3 | Apolipoprotein C-III |
| CNVs | Copy Number Variations |
| PRS | polygenic risk score |
| PWAS | proteome-wide association studies |
| CPT2 | carnitine palmitoyl transferases 2 |
| ApoL2 | apolipoprotein L2 |
| PRDX1 | Peroxiredoxin 1 |
| KP | kynurenine pathway |
| KYNA | Kynurenic acid |
| QUIN | quinolinic acid |
| ROS | Reactive oxygen species |
| GAD | glutamic acid decarboxylase |
| SHMT2 | serine hydroxymethyltransferase |
| PCP | phencyclidine |
| DAT | dopamine transporter |
| NR1 | N-methyl-D-aspartate (NMDA) receptor 1 subunit |
| vGLUT1 | vesicular glutamate transporter 1 |
| ACC | anterior cingulate cortex |
| MIA | maternal immune activation |
| TNF-α | tumor necrosis factor |
| sIL-6R | IL-6 soluble receptor |
| IL-1β | interleukins1β |
| IL-6 | interleukins6 |
| IL-6-R | Interleukin-6 receptor |
| CC16 | Clara cell protein 16 |
| IFN-γ | interferon-γ |
| IL-1RA | IL-1 receptor antagonist |
| IL-12/IL-23p40 | Interleukin-12/Interleukin-23 subunit p40 |
| IL-17A | Interleukin-17A |
| IL-10 | Interleukin-10 |
| B2M | beta-2 microglobulin |
| CRP | C-reactive protein |
| IL-8 | interleukins8 |
| Th17 | T helper 17 cells |
| ASC | apoptosis-associated speck-like protein containing a CARD (an adaptor protein essential for inflammasome assembly) |
| NLRP3 | NOD-like receptor family, pyrin domain containing 3 |
| IL-8 | interleukins18 |
| LTP | long-term potentiation |
| PI3K | Phosphoinositide 3-kinases |
| RNS | reactive nitrosative species |
| HPA | hypothalamic–pituitary–adrenal |
| CAT | catalase |
| SOD | superoxide dismutase |
| MDA | malondialdehyde |
| MAPK | mitogen-activated protein kinase |
| AP-1 | Activator Protein-1 |
| iNOS | inducible nitric oxide synthase |
| COX-2 | cyclooxygenase-2 |
| NKA | Na+/K+-ATPase |
| CNS | central nervous system |
| PGRMC1 | progesterone receptor membrane component 1 |
| GLP-1R | glucagon peptide-1 receptor |
| Mfn2 | mitofusin 2 |
| AMP | adenosine monophosphate |
| AMPK-ACC-CPT1 pathway | AMP-activated protein kinase-acetyl CoA carboxylase-carnitine palmitoyl transferase 1 pathway |
| WARS2 | mitochondrial tryptophanyl-tRNA synthetase |
| GSH | glutathione |
| GO | gene ontology |
| mtDNA | mitochondrial DNA |
| PV+ | parvalbumin positive |
| ATP | adenosine triphosphate |
| ER | endoplasmic reticulum |
| UPR | unfolded protein response |
| ERAD | ER-associated degradation |
| XBP-1 | X-box-binding protein 1 |
| 4-PBA | 4-phenylbutyric acid |
| PERK | protein kinase RNA-like endoplasmic reticulum kinase |
| ATF6 | activating transcription factor 6 |
| IRE1 | Inositol-requiring enzyme 1 |
| BiP/GRP78 | binding immunoglobulin protein/glucose-regulated protein 78 |
| FEP | first-episode psychosis |
| VBM | voxel-based morphometry |
| CT | computed tomography |
| PSP | prefrontal sulcal prominence |
| 18FDG-PET | 18F-deoxyglucose positron emission tomography |
| MRI | magnetic resonance imaging |
| ICC | intrinsic cortical curvature |
| DTI | diffusion tensor imaging |
| FA | fractional anisotropy |
| BOLD-fMRI | blood-oxygenation-level-dependent functional magnetic resonance imaging |
| DMN | default-mode network |
| AUC | area under the curve |
| fMRI | functional magnetic resonance imaging |
| DBS | deep brain stimulation |
| AI | artificial intelligence |
| MRS | magnetic resonance spectroscopy |
References
- Correll, C.U.; Ismail, Z.; McIntyre, R.S.; Rafeyan, R.; Thase, M.E. Patient Functioning, Life Engagement, and Treatment Goals in Schizophrenia. J. Clin. Psychiatry 2022, 83, Lu21112ah2. [Google Scholar] [CrossRef]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448. [Google Scholar] [CrossRef]
- Iasevoli, F.; Giordano, S.; Balletta, R.; Latte, G.; Formato, M.V.; Prinzivalli, E.; De Berardis, D.; Tomasetti, C.; de Bartolomeis, A. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 34–48. [Google Scholar] [CrossRef]
- Carbon, M.; Correll, C.U. Thinking and acting beyond the positive: The role of the cognitive and negative symptoms in schizophrenia. CNS Spectr. 2014, 19 (Suppl. 1), 35–53. [Google Scholar] [CrossRef]
- Mayoral-van Son, J.; de la Foz, V.O.; Martinez-Garcia, O.; Moreno, T.; Parrilla-Escobar, M.; Valdizan, E.M.; Crespo-Facorro, B. Clinical outcome after antipsychotic treatment discontinuation in functionally recovered first-episode nonaffective psychosis individuals: A 3-year naturalistic follow-up study. J. Clin. Psychiatry 2016, 77, 492–500. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R.; Agid, O.; de Bartolomeis, A.; van Beveren, N.J.; Birnbaum, M.L.; Bloomfield, M.A.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T.; et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.S.; Carvalho, D.R.; Ribeiro, M.D.T.; Diniz, E.J.B.; Rêgo, A.F.N. Prevalence and predictors of treatment-resistant schizophrenia in a tertiary hospital in Northeast Brazil. Trends Psychiatry Psychother. 2021, 43, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, F.C., Jr.; Woznica, E.; Lee, B.J.; Cascella, N.; Sawa, A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol. Dis. 2019, 131, 104257. [Google Scholar] [CrossRef] [PubMed]
- Demjaha, A.; Egerton, A.; Murray, R.M.; Kapur, S.; Howes, O.D.; Stone, J.M.; McGuire, P.K. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol. Psychiatry 2014, 75, e11–e13. [Google Scholar] [CrossRef]
- Diniz, E.; Fonseca, L.; Rocha, D.; Trevizol, A.; Cerqueira, R.; Ortiz, B.; Brunoni, A.R.; Bressan, R.; Correll, C.U.; Gadelha, A. Treatment resistance in schizophrenia: A meta-analysis of prevalence and correlates. Braz. J. Psychiatry 2023, 45, 448–458. [Google Scholar] [CrossRef]
- Velligan, D.I.; Brain, C.; Bouérat Duvold, L.; Agid, O. Caregiver Burdens Associated with Treatment-Resistant Schizophrenia: A Quantitative Caregiver Survey of Experiences, Attitudes, and Perceptions. Front. Psychiatry 2019, 10, 584. [Google Scholar] [CrossRef]
- Iasevoli, F.; Razzino, E.; Altavilla, B.; Avagliano, C.; Barone, A.; Ciccarelli, M.; D’Ambrosio, L.; Matrone, M.; Milandri, F.; Notar Francesco, D.; et al. Relationships between early age at onset of psychotic symptoms and treatment resistant schizophrenia. Early Interv. Psychiatry 2022, 16, 352–362. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Vellucci, L.; Barone, A.; Manchia, M.; De Luca, V.; Iasevoli, F.; Correll, C.U. Clozapine’s multiple cellular mechanisms: What do we know after more than fifty years? A systematic review and critical assessment of translational mechanisms relevant for innovative strategies in treatment-resistant schizophrenia. Pharmacol. Ther. 2022, 236, 108236. [Google Scholar] [CrossRef]
- Bachmann, C.J.; Aagaard, L.; Bernardo, M.; Brandt, L.; Cartabia, M.; Clavenna, A.; Coma Fusté, A.; Furu, K.; Garuoliené, K.; Hoffmann, F.; et al. International trends in clozapine use: A study in 17 countries. Acta Psychiatr. Scand. 2017, 136, 37–51. [Google Scholar] [CrossRef]
- Moreno-Sancho, L.; Juncal-Ruiz, M.; Vázquez-Bourgon, J.; Ortiz-Garcia de la Foz, V.; Mayoral-van Son, J.; Tordesillas-Gutierrez, D.; Setien-Suero, E.; Ayesa-Arriola, R.; Crespo-Facorro, B. Naturalistic study on the use of clozapine in the early phases of non-affective psychosis: A 10-year follow-up study in the PAFIP-10 cohort. J. Psychiatr. Res. 2022, 153, 292–299. [Google Scholar] [CrossRef]
- Üçok, A.; Çikrikçili, U.; Karabulut, S.; Salaj, A.; Öztürk, M.; Tabak, Ö.; Durak, R. Delayed initiation of clozapine may be related to poor response in treatment-resistant schizophrenia. Int. Clin. Psychopharmacol. 2015, 30, 290–295. [Google Scholar] [CrossRef]
- Siafis, S.; Wu, H.; Wang, D.; Burschinski, A.; Nomura, N.; Takeuchi, H.; Schneider-Thoma, J.; Davis, J.M.; Leucht, S. Antipsychotic dose, dopamine D2 receptor occupancy and extrapyramidal side-effects: A systematic review and dose-response meta-analysis. Mol. Psychiatry 2023, 28, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Takeuchi, H.; Graff-Guerrero, A.; Suzuki, T.; Watanabe, K.; Mamo, D.C. Dopamine D2 receptor occupancy and clinical effects: A systematic review and pooled analysis. J. Clin. Psychopharmacol. 2011, 31, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, T.; Bies, R.R.; Suzuki, T.; Mamo, D.C.; Pollock, B.G.; Graff-Guerrero, A.; Mimura, M.; Uchida, H. Hyperprolactinemia and estimated dopamine D2 receptor occupancy in patients with schizophrenia: Analysis of the CATIE data. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 45, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Johnston, M.; Bureau, Y.; Shah, N. Baseline serum prolactin in drug-naive, first-episode schizophrenia and outcome at five years: Is it a predictive factor? Innov. Clin. Neurosci. 2012, 9, 17–21. [Google Scholar]
- Potkin, S.G.; Kane, J.M.; Correll, C.U.; Lindenmayer, J.P.; Agid, O.; Marder, S.R.; Olfson, M.; Howes, O.D. The neurobiology of treatment-resistant schizophrenia: Paths to antipsychotic resistance and a roadmap for future research. npj Schizophr. 2020, 6, 1. [Google Scholar] [CrossRef]
- Chouinard, G.; Samaha, A.N.; Chouinard, V.A.; Peretti, C.S.; Kanahara, N.; Takase, M.; Iyo, M. Antipsychotic-Induced Dopamine Supersensitivity Psychosis: Pharmacology, Criteria, and Therapy. Psychother. Psychosom. 2017, 86, 189–219. [Google Scholar] [CrossRef]
- Iyo, M.; Tadokoro, S.; Kanahara, N.; Hashimoto, T.; Niitsu, T.; Watanabe, H.; Hashimoto, K. Optimal extent of dopamine D2 receptor occupancy by antipsychotics for treatment of dopamine supersensitivity psychosis and late-onset psychosis. J. Clin. Psychopharmacol. 2013, 33, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci. Ther. 2011, 17, 118–132. [Google Scholar] [CrossRef]
- Iasevoli, F.; Avagliano, C.; D’Ambrosio, L.; Barone, A.; Ciccarelli, M.; De Simone, G.; Mazza, B.; Vellucci, L.; de Bartolomeis, A. Dopamine Dynamics and Neurobiology of Non-Response to Antipsychotics, Relevance for Treatment Resistant Schizophrenia: A Systematic Review and Critical Appraisal. Biomedicines 2023, 11, 895. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Weinshenker, D.; Quirion, R.; Srivastava, L.K.; Bhardwaj, S.K.; Grandy, D.K.; Premont, R.T.; Sotnikova, T.D.; Boksa, P.; El-Ghundi, M.; et al. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc. Natl. Acad. Sci. USA 2005, 102, 3513–3518. [Google Scholar] [CrossRef]
- Connolly, A.; Wallman, P.; Dzahini, O.; Howes, O.; Taylor, D. Meta-analysis and systematic review of vesicular monoamine transporter (VMAT-2) inhibitors in schizophrenia and psychosis. Psychopharmacology 2024, 241, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.V.; Grace, A.A. Beyond Dopamine Receptor Antagonism: New Targets for Schizophrenia Treatment and Prevention. Int. J. Mol. Sci. 2021, 22, 4467. [Google Scholar] [CrossRef]
- Tachibana, M.; Niitsu, T.; Watanabe, M.; Hashimoto, T.; Kanahara, N.; Ishikawa, M.; Iyo, M. Effectiveness of blonanserin for patients with drug treatment-resistant schizophrenia and dopamine supersensitivity: A retrospective analysis. Asian J. Psychiatr. 2016, 24, 28–32. [Google Scholar] [CrossRef]
- Kim, E.; Howes, O.D.; Veronese, M.; Beck, K.; Seo, S.; Park, J.W.; Lee, J.S.; Lee, Y.S.; Kwon, J.S. Presynaptic Dopamine Capacity in Patients with Treatment-Resistant Schizophrenia Taking Clozapine: An [(18)F]DOPA PET Study. Neuropsychopharmacology 2017, 42, 941–950. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Krystal, J.H.; Howes, O.D. Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry 2020, 19, 15–33. [Google Scholar] [CrossRef]
- Kumar, V.; Manchegowda, S.; Jacob, A.; Rao, N.P. Glutamate metabolites in treatment resistant schizophrenia: A meta-analysis and systematic review of (1)H-MRS studies. Psychiatry Res. Neuroimaging 2020, 300, 111080. [Google Scholar] [CrossRef]
- Iwata, Y.; Nakajima, S.; Plitman, E.; Caravaggio, F.; Kim, J.; Shah, P.; Mar, W.; Chavez, S.; De Luca, V.; Mimura, M.; et al. Glutamatergic Neurometabolite Levels in Patients With Ultra-Treatment-Resistant Schizophrenia: A Cross-Sectional 3T Proton Magnetic Resonance Spectroscopy Study. Biol. Psychiatry 2019, 85, 596–605. [Google Scholar] [CrossRef]
- Tarumi, R.; Tsugawa, S.; Noda, Y.; Plitman, E.; Honda, S.; Matsushita, K.; Chavez, S.; Sawada, K.; Wada, M.; Matsui, M.; et al. Levels of glutamatergic neurometabolites in patients with severe treatment-resistant schizophrenia: A proton magnetic resonance spectroscopy study. Neuropsychopharmacology 2020, 45, 632–640. [Google Scholar] [CrossRef]
- Ochi, R.; Plitman, E.; Patel, R.; Tarumi, R.; Iwata, Y.; Tsugawa, S.; Kim, J.; Honda, S.; Noda, Y.; Uchida, H.; et al. Investigating structural subdivisions of the anterior cingulate cortex in schizophrenia, with implications for treatment resistance and glutamatergic levels. J. Psychiatry Neurosci. 2022, 47, E1–E10. [Google Scholar] [CrossRef]
- Matrone, M.; Kotzalidis, G.D.; Romano, A.; Bozzao, A.; Cuomo, I.; Valente, F.; Gabaglio, C.; Lombardozzi, G.; Trovini, G.; Amici, E.; et al. Treatment-resistant schizophrenia: Addressing white matter integrity, intracortical glutamate levels, clinical and cognitive profiles between early- and adult-onset patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 114, 110493. [Google Scholar] [CrossRef]
- Kegeles, L.S.; Abi-Dargham, A.; Zea-Ponce, Y.; Rodenhiser-Hill, J.; Mann, J.J.; Van Heertum, R.L.; Cooper, T.B.; Carlsson, A.; Laruelle, M. Modulation of amphetamine-induced striatal dopamine release by ketamine in humans: Implications for schizophrenia. Biol. Psychiatry 2000, 48, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Parellada, E.; Gassó, P. Glutamate and microglia activation as a driver of dendritic apoptosis: A core pathophysiological mechanism to understand schizophrenia. Transl. Psychiatry 2021, 11, 271. [Google Scholar] [CrossRef]
- Hashimoto, K.; Malchow, B.; Falkai, P.; Schmitt, A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 367–377. [Google Scholar] [CrossRef] [PubMed]
- de Bartolomeis, A.; Manchia, M.; Marmo, F.; Vellucci, L.; Iasevoli, F.; Barone, A. Glycine Signaling in the Framework of Dopamine-Glutamate Interaction and Postsynaptic Density. Implications for Treatment-Resistant Schizophrenia. Front. Psychiatry 2020, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- de Bartolomeis, A.; Vellucci, L.; Austin, M.C.; De Simone, G.; Barone, A. Rational and Translational Implications of D-Amino Acids for Treatment-Resistant Schizophrenia: From Neurobiology to the Clinics. Biomolecules 2022, 12, 909. [Google Scholar] [CrossRef]
- El-Tallawy, H.N.; Saleem, T.H.; El-Ebidi, A.M.; Hassan, M.H.; Gabra, R.H.; Farghaly, W.M.; Abo El-Maali, N.; Sherkawy, H.S. Clinical and biochemical study of d-serine metabolism among schizophrenia patients. Neuropsychiatr. Dis. Treat. 2017, 13, 1057–1063. [Google Scholar] [CrossRef]
- Garofalo, M.; De Simone, G.; Motta, Z.; Nuzzo, T.; De Grandis, E.; Bruno, C.; Boeri, S.; Riccio, M.P.; Pastore, L.; Bravaccio, C.; et al. Decreased free D-aspartate levels in the blood serum of patients with schizophrenia. Front. Psychiatry 2024, 15, 1408175. [Google Scholar] [CrossRef]
- Rampino, A.; Garofalo, M.; Nuzzo, T.; Favia, M.; Saltarelli, S.; Masellis, R.; Asselti, M.G.; Pennacchio, T.C.; Bruzzese, D.; Errico, F.; et al. Variations of blood D-serine and D-aspartate homeostasis track psychosis stages. Schizophrenia 2024, 10, 115. [Google Scholar] [CrossRef]
- Tsai, G.E.; Lin, P.Y. Strategies to enhance N-methyl-D-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr. Pharm. Des. 2010, 16, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Heresco-Levy, U.; Javitt, D.C.; Ebstein, R.; Vass, A.; Lichtenberg, P.; Bar, G.; Catinari, S.; Ermilov, M. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol. Psychiatry 2005, 57, 577–585. [Google Scholar] [CrossRef]
- Kantrowitz, J.T.; Malhotra, A.K.; Cornblatt, B.; Silipo, G.; Balla, A.; Suckow, R.F.; D’Souza, C.; Saksa, J.; Woods, S.W.; Javitt, D.C. High dose D-serine in the treatment of schizophrenia. Schizophr. Res. 2010, 121, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Yamamori, H.; Hashimoto, R.; Fujita, Y.; Numata, S.; Yasuda, Y.; Fujimoto, M.; Ohi, K.; Umeda-Yano, S.; Ito, A.; Ohmori, T.; et al. Changes in plasma D-serine, L-serine, and glycine levels in treatment-resistant schizophrenia before and after clozapine treatment. Neurosci. Lett. 2014, 582, 93–98. [Google Scholar] [CrossRef]
- Lányi, O.; Koleszár, B.; Schulze Wenning, A.; Balogh, D.; Engh, M.A.; Horváth, A.A.; Fehérvari, P.; Hegyi, P.; Molnár, Z.; Unoka, Z.; et al. Excitation/inhibition imbalance in schizophrenia: A meta-analysis of inhibitory and excitatory TMS-EMG paradigms. Schizophrenia 2024, 10, 56. [Google Scholar] [CrossRef]
- Barone, A.; Senerchia, G.; De Simone, G.; Manzo, M.; Ciccarelli, M.; Tozza, S.; Iuzzolino, V.V.; Spisto, M.; Dubbioso, R.; Iasevoli, F.; et al. In vivo assessment of GABAergic inhibition and glutamate facilitation in treatment-resistant schizophrenia: A TMS study integrating clinical, cognitive, and neurophysiological evaluations. Schizophrenia 2025, 11, 90. [Google Scholar] [CrossRef]
- Egerton, A.; Griffiths, K.; Casetta, C.; Deakin, B.; Drake, R.; Howes, O.D.; Kassoumeri, L.; Khan, S.; Lankshear, S.; Lees, J.; et al. Anterior cingulate glutamate metabolites as a predictor of antipsychotic response in first episode psychosis: Data from the STRATA collaboration. Neuropsychopharmacology 2023, 48, 567–575. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Meglio, M.; Cioni, B.; Tamburrini, G.; Tonali, P.; Rothwell, J.C. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin. Neurophysiol. 2000, 111, 794–799. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Orekhov, Y.; Ziemann, U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res. 2006, 173, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.J.; Bryant, Z.K.; Conn, P.J. Targeting muscarinic receptors to treat schizophrenia. Behav. Brain Res. 2021, 405, 113201. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.K.; Byun, N.; Bubser, M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology 2012, 37, 16–42. [Google Scholar] [CrossRef] [PubMed]
- Masterson, E.; O’Shea, B. Smoking and malignancy in schizophrenia. Br. J. Psychiatry 1984, 145, 429–432. [Google Scholar] [CrossRef]
- Hughes, J.R.; Hatsukami, D.K.; Mitchell, J.E.; Dahlgren, L.A. Prevalence of smoking among psychiatric outpatients. Am. J. Psychiatry 1986, 143, 993–997. [Google Scholar] [CrossRef]
- Goff, D.C.; Henderson, D.C.; Amico, E. Cigarette smoking in schizophrenia: Relationship to psychopathology and medication side effects. Am. J. Psychiatry 1992, 149, 1189–1194. [Google Scholar] [CrossRef]
- Diwan, A.; Castine, M.; Pomerleau, C.S.; Meador-Woodruff, J.H.; Dalack, G.W. Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr. Res. 1998, 33, 113–118. [Google Scholar] [CrossRef]
- Kelly, C.; McCreadie, R.G. Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am. J. Psychiatry 1999, 156, 1751–1757. [Google Scholar] [CrossRef]
- Gallagher, S.M.; Penn, P.E.; Schindler, E.; Layne, W. A comparison of smoking cessation treatments for persons with schizophrenia and other serious mental illnesses. J. Psychoact. Drugs 2007, 39, 487–497. [Google Scholar] [CrossRef] [PubMed]
- de Leon, J.; Dadvand, M.; Canuso, C.; White, A.O.; Stanilla, J.K.; Simpson, G.M. Schizophrenia and smoking: An epidemiological survey in a state hospital. Am. J. Psychiatry 1995, 152, 453–455. [Google Scholar] [CrossRef]
- Sacco, K.A.; Termine, A.; Seyal, A.; Dudas, M.M.; Vessicchio, J.C.; Krishnan-Sarin, S.; Jatlow, P.I.; Wexler, B.E.; George, T.P. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: Involvement of nicotinic receptor mechanisms. Arch. Gen. Psychiatry 2005, 62, 649–659. [Google Scholar] [CrossRef]
- Wing, V.C.; Wass, C.E.; Soh, D.W.; George, T.P. A review of neurobiological vulnerability factors and treatment implications for comorbid tobacco dependence in schizophrenia. Ann. N. Y. Acad. Sci. 2012, 1248, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Iasevoli, F.; Balletta, R.; Gilardi, V.; Giordano, S.; de Bartolomeis, A. Tobacco smoking in treatment-resistant schizophrenia patients is associated with impaired cognitive functioning, more severe negative symptoms, and poorer social adjustment. Neuropsychiatr. Dis. Treat. 2013, 9, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Verbois, S.L.; Sullivan, P.G.; Scheff, S.W.; Pauly, J.R. Traumatic brain injury reduces hippocampal alpha7 nicotinic cholinergic receptor binding. J. Neurotrauma 2000, 17, 1001–1011. [Google Scholar] [CrossRef]
- Woodruff-Pak, D.S.; Gould, T.J. Neuronal nicotinic acetylcholine receptors: Involvement in Alzheimer’s disease and schizophrenia. Behav. Cogn. Neurosci. Rev. 2002, 1, 5–20. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Pereira, E.F.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef]
- D’Hoedt, D.; Bertrand, D. Nicotinic acetylcholine receptors: An overview on drug discovery. Expert. Opin. Ther. Targets 2009, 13, 395–411. [Google Scholar] [CrossRef]
- Philip, N.S.; Carpenter, L.L.; Tyrka, A.R.; Price, L.H. Nicotinic acetylcholine receptors and depression: A review of the preclinical and clinical literature. Psychopharmacology 2010, 212, 1–12. [Google Scholar] [CrossRef]
- Hoffmeister, P.G.; Donat, C.K.; Schuhmann, M.U.; Voigt, C.; Walter, B.; Nieber, K.; Meixensberger, J.; Bauer, R.; Brust, P. Traumatic brain injury elicits similar alterations in α7 nicotinic receptor density in two different experimental models. Neuromol. Med. 2011, 13, 44–53. [Google Scholar] [CrossRef]
- Ishikawa, M.; Hashimoto, K. α7 nicotinic acetylcholine receptor as a potential therapeutic target for schizophrenia. Curr. Pharm. Des. 2011, 17, 121–129. [Google Scholar] [CrossRef]
- Stephens, S.H.; Logel, J.; Barton, A.; Franks, A.; Schultz, J.; Short, M.; Dickenson, J.; James, B.; Fingerlin, T.E.; Wagner, B.; et al. Association of the 5’-upstream regulatory region of the alpha7 nicotinic acetylcholine receptor subunit gene (CHRNA7) with schizophrenia. Schizophr. Res. 2009, 109, 102–112. [Google Scholar] [CrossRef]
- Nagamoto, H.T.; Adler, L.E.; Waldo, M.C.; Freedman, R. Sensory gating in schizophrenics and normal controls: Effects of changing stimulation interval. Biol. Psychiatry 1989, 25, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Saint-Georges, Z.; MacDonald, J.; Al-Khalili, R.; Hamati, R.; Solmi, M.; Keshavan, M.S.; Tuominen, L.; Guimond, S. Cholinergic system in schizophrenia: A systematic review and meta-analysis. Mol. Psychiatry 2025, 30, 3301–3315. [Google Scholar] [CrossRef]
- Coughlin, J.; Du, Y.; Crawford, J.L.; Rubin, L.H.; Behnam Azad, B.; Lesniak, W.G.; Horti, A.G.; Schretlen, D.J.; Sawa, A.; Pomper, M.G. The availability of the α7 nicotinic acetylcholine receptor in recent-onset psychosis: A study using (18)F-ASEM PET. J. Nucl. Med. 2018, 60, 241–243. [Google Scholar] [CrossRef]
- Wong, D.F.; Kuwabara, H.; Horti, A.G.; Roberts, J.M.; Nandi, A.; Cascella, N.; Brasic, J.; Weerts, E.M.; Kitzmiller, K.; Phan, J.A.; et al. Brain PET Imaging of α7-nAChR with [18F]ASEM: Reproducibility, Occupancy, Receptor Density, and Changes in Schizophrenia. Int. J. Neuropsychopharmacol. 2018, 21, 656–667. [Google Scholar] [CrossRef]
- Wong, N.R.; Rubin, L.H.; Harrington, C.K.; Jenkins, K.R.; Shinehouse, L.K.; Yoon, M.; Kilgore, J.J.; Soule, A.R.; Lesniak, W.G.; Rowe, S.P.; et al. Hippocampal Availability of the α7 Nicotinic Acetylcholine Receptor in Recent-Onset Psychosis. JAMA Netw. Open 2024, 7, e2427163. [Google Scholar] [CrossRef] [PubMed]
- Wakuda, T.; Yokokura, M.; Magata, Y.; Suzuki, C.; Murayama, C.; Goto, T.; Tamayama, T.; Kameno, Y.; Iwabuchi, T.; Isobe, T.; et al. α7 nicotinic acetylcholine receptor, activated glia, and cognitive impairment in schizophrenia: A dual-tracer PET study. Mol. Psychiatry 2025. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Weyn, A.; Mikkelsen, J.D. Hippocampal α7 nicotinic acetylcholine receptor levels in patients with schizophrenia, bipolar disorder, or major depressive disorder. Bipolar Disord. 2011, 13, 701–707. [Google Scholar] [CrossRef]
- Guan, Z.Z.; Zhang, X.; Blennow, K.; Nordberg, A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport 1999, 10, 1779–1782. [Google Scholar] [CrossRef] [PubMed]
- Court, J.; Spurden, D.; Lloyd, S.; McKeith, I.; Ballard, C.; Cairns, N.; Kerwin, R.; Perry, R.; Perry, E. Neuronal Nicotinic Receptors in Dementia with Lewy Bodies and Schizophrenia: α-Bungarotoxin and Nicotine Binding in the Thalamus. J. Neurochem. 1999, 73, 1590–1597. [Google Scholar] [CrossRef]
- Rousseau, S.J.; Jones, I.W.; Pullar, I.A.; Wonnacott, S. Presynaptic alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate [3H]d-aspartate release from rat frontal cortex in vitro. Neuropharmacology 2005, 49, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lippi, G.; Carlson, D.M.; Berg, D.K. Activation of α7-containing nicotinic receptors on astrocytes triggers AMPA receptor recruitment to glutamatergic synapses. J. Neurochem. 2013, 127, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Toyohara, J.; Hashimoto, K. α7 Nicotinic Receptor Agonists: Potential Therapeutic Drugs for Treatment of Cognitive Impairments in Schizophrenia and Alzheimer’s Disease. Open Med. Chem. J. 2010, 4, 37–56. [Google Scholar] [CrossRef][Green Version]
- Radek, R.J.; Robb, H.M.; Stevens, K.E.; Gopalakrishnan, M.; Bitner, R.S. Effects of the Novel α7 Nicotinic Acetylcholine Receptor Agonist ABT-107 on Sensory Gating in DBA/2 Mice: Pharmacodynamic Characterization. J. Pharmacol. Exp. Ther. 2012, 343, 736–745. [Google Scholar] [CrossRef]
- Hulme, E.C.; Birdsall, N.J.; Buckley, N.J. Muscarinic receptor subtypes. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 633–673. [Google Scholar] [CrossRef]
- Osterholm, R.K.; Camoriano, J.K. Transdermal scopolamine psychosis. JAMA 1982, 247, 3081. [Google Scholar] [CrossRef]
- Tandon, R.; Shipley, J.E.; Greden, J.F.; Mann, N.A.; Eisner, W.H.; Goodson, J.A. Muscarinic cholinergic hyperactivity in schizophrenia. Relationship to positive and negative symptoms. Schizophr. Res. 1991, 4, 23–30. [Google Scholar] [CrossRef]
- Bodick, N.C.; Offen, W.W.; Levey, A.I.; Cutler, N.R.; Gauthier, S.G.; Satlin, A.; Shannon, H.E.; Tollefson, G.D.; Rasmussen, K.; Bymaster, F.P.; et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch. Neurol. 1997, 54, 465–473. [Google Scholar] [CrossRef]
- Bodick, N.C.; Offen, W.W.; Shannon, H.E.; Satterwhite, J.; Lucas, R.; van Lier, R.; Paul, S.M. The selective muscarinic agonist xanomeline improves both the cognitive deficits and behavioral symptoms of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1997, 11 (Suppl. 4), S16–S22. [Google Scholar]
- Shekhar, A.; Potter, W.Z.; Lightfoot, J.; Lienemann, J.; Dubé, S.; Mallinckrodt, C.; Bymaster, F.P.; McKinzie, D.L.; Felder, C.C. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 2008, 165, 1033–1039. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Carter, P.A.; Yamada, M.; Gomeza, J.; Wess, J.; Hamilton, S.E.; Nathanson, N.M.; McKinzie, D.L.; Felder, C.C. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur. J. Neurosci. 2003, 17, 1403–1410. [Google Scholar] [CrossRef]
- Halaska, M.; Ralph, G.; Wiedemann, A.; Primus, G.; Ballering-Brühl, B.; Höfner, K.; Jonas, U. Controlled, double-blind, multicentre clinical trial to investigate long-term tolerability and efficacy of trospium chloride in patients with detrusor instability. World J. Urol. 2003, 20, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Todorova, A.; Vonderheid-Guth, B.; Dimpfel, W. Effects of tolterodine, trospium chloride, and oxybutynin on the central nervous system. J. Clin. Pharmacol. 2001, 41, 636–644. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA OKs First-in-Class Antipsychotic for Schizophrenia. 2024. Available online: https://www.medscape.com/viewarticle/fda-oks-first-class-antipsychotic-schizophrenia-2024a1000hno (accessed on 1 September 2025).
- FDA Approves Drug with New Mechanism of Action for Treatment of Schizophrenia. 2024. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-drug-new-mechanism-action-treatment-schizophrenia (accessed on 1 September 2025).
- Krystal, J.H.; Kane, J.M.; Correll, C.U.; Walling, D.P.; Leoni, M.; Duvvuri, S.; Patel, S.; Chang, I.; Iredale, P.; Frohlich, L.; et al. Emraclidine, a novel positive allosteric modulator of cholinergic M4 receptors, for the treatment of schizophrenia: A two-part, randomised, double-blind, placebo-controlled, phase 1b trial. Lancet 2022, 400, 2210–2220. [Google Scholar] [CrossRef]
- Weiner, D.M.; Meltzer, H.Y.; Veinbergs, I.; Donohue, E.M.; Spalding, T.A.; Smith, T.T.; Mohell, N.; Harvey, S.C.; Lameh, J.; Nash, N.; et al. The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical effects of clozapine. Psychopharmacology 2004, 177, 207–216. [Google Scholar] [CrossRef]
- Mäki-Marttunen, V.; Andreassen, O.A.; Espeseth, T. The role of norepinephrine in the pathophysiology of schizophrenia. Neurosci. Biobehav. Rev. 2020, 118, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Hornykiewicz, O. Proposal for a noradrenaline hypothesis of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 913–922. [Google Scholar] [CrossRef]
- Nagamine, T. Role of Norepinephrine in Schizophrenia: An Old Theory Applied to a New Case in Emergency Medicine. Innov. Clin. Neurosci. 2020, 17, 8–9. [Google Scholar] [PubMed]
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef] [PubMed]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef] [PubMed]
- Grueschow, M.; Kleim, B.; Ruff, C.C. Role of the locus coeruleus arousal system in cognitive control. J. Neuroendocrinol. 2020, 32, e12890. [Google Scholar] [CrossRef] [PubMed]
- Pelegrino, A.; Guimaraes, A.L.; Sena, W.; Emele, N.; Scoriels, L.; Panizzutti, R. Dysregulated noradrenergic response is associated with symptom severity in individuals with schizophrenia. Front. Psychiatry 2023, 14, 1190329. [Google Scholar] [CrossRef]
- Breier, A.; Wolkowitz, O.M.; Roy, A.; Potter, W.Z.; Pickar, D. Plasma norepinephrine in chronic schizophrenia. Am. J. Psychiatry 1990, 147, 1467–1470. [Google Scholar] [CrossRef]
- Pickar, D.; Breier, A.; Hsiao, J.K.; Doran, A.R.; Wolkowitz, O.M.; Pato, C.N.; Konicki, P.E.; Potter, W.Z. Cerebrospinal fluid and plasma monoamine metabolites and their relation to psychosis. Implications for regional brain dysfunction in schizophrenia. Arch. Gen. Psychiatry 1990, 47, 641–648. [Google Scholar] [CrossRef]
- Light, G.; Greenwood, T.A.; Swerdlow, N.R.; Calkins, M.E.; Freedman, R.; Green, M.F.; Gur, R.E.; Gur, R.C.; Lazzeroni, L.C.; Nuechterlein, K.H.; et al. Comparison of the heritability of schizophrenia and endophenotypes in the COGS-1 family study. Schizophr. Bull. 2014, 40, 1404–1411. [Google Scholar] [CrossRef]
- Consortium, S.W.G.o.t.P.G. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Trubetskoy, V.; Pardiñas, A.F.; Qi, T.; Panagiotaropoulou, G.; Awasthi, S.; Bigdeli, T.B.; Bryois, J.; Chen, C.Y.; Dennison, C.A.; Hall, L.S.; et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 2022, 604, 502–508. [Google Scholar] [CrossRef]
- Li, J.; Meltzer, H.Y. A genetic locus in 7p12.2 associated with treatment resistant schizophrenia. Schizophr. Res. 2014, 159, 333–339. [Google Scholar] [CrossRef]
- Bishop, J.R.; Miller del, D.; Ellingrod, V.L.; Holman, T. Association between type-three metabotropic glutamate receptor gene (GRM3) variants and symptom presentation in treatment refractory schizophrenia. Hum. Psychopharmacol. 2011, 26, 28–34. [Google Scholar] [CrossRef]
- Okubo, R.; Okada, M.; Motomura, E. Dysfunction of the NMDA Receptor in the Pathophysiology of Schizophrenia and/or the Pathomechanisms of Treatment-Resistant Schizophrenia. Biomolecules 2024, 14, 1128. [Google Scholar] [CrossRef]
- Miyazawa, A.; Kanahara, N.; Kogure, M.; Otsuka, I.; Okazaki, S.; Watanabe, Y.; Yamasaki, F.; Nakata, Y.; Oda, Y.; Hishimoto, A.; et al. A preliminary genetic association study of GAD1 and GABAB receptor genes in patients with treatment-resistant schizophrenia. Mol. Biol. Rep. 2022, 49, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Zazueta, A.; Castillo, T.; Cavieres, Á.; González, R.; Abarca, M.; Nieto, R.R.; Deneken, J.; Araneda, C.; Moya, P.R.; Bustamante, M.L. Polymorphisms in Schizophrenia-Related Genes Are Potential Predictors of Antipsychotic Treatment Resistance and Refractoriness. Int. J. Neuropsychopharmacol. 2022, 25, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.; Shinkai, T.; De Luca, V.; Ni, X.; Potkin, S.G.; Lieberman, J.A.; Meltzer, H.Y.; Kennedy, J.L. Association study of four dopamine D1 receptor gene polymorphisms and clozapine treatment response. J. Psychopharmacol. 2007, 21, 718–727. [Google Scholar] [CrossRef]
- Ota, V.K.; Spíndola, L.N.; Gadelha, A.; dos Santos Filho, A.F.; Santoro, M.L.; Christofolini, D.M.; Bellucco, F.T.; Ribeiro-dos-Santos Â, K.; Santos, S.; Mari Jde, J.; et al. DRD1 rs4532 polymorphism: A potential pharmacogenomic marker for treatment response to antipsychotic drugs. Schizophr. Res. 2012, 142, 206–208. [Google Scholar] [CrossRef]
- Zhang, J.P.; Lencz, T.; Geisler, S.; DeRosse, P.; Bromet, E.J.; Malhotra, A.K. Genetic variation in BDNF is associated with antipsychotic treatment resistance in patients with schizophrenia. Schizophr. Res. 2013, 146, 285–288. [Google Scholar] [CrossRef]
- Liberona, A.; Jones, N.; Zúñiga, K.; Garrido, V.; Zelada, M.I.; Silva, H.; Nieto, R.R. Brain-Derived Neurotrophic Factor (BDNF) as a Predictor of Treatment Response in Schizophrenia and Bipolar Disorder: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1204. [Google Scholar] [CrossRef]
- Vandewalle, A.; Lelongt, B.; Geniteau-Legendre, M.; Baudouin, B.; Antoine, M.; Estrade, S.; Chatelet, F.; Verroust, P.; Cassingena, R.; Ronco, P. Maintenance of proximal and distal cell functions in SV40-transformed tubular cell lines derived from rabbit kidney cortex. J. Cell. Physiol. 1989, 141, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Del Casale, A.; Gentile, G.; Lardani, S.; Modesti, M.N.; Arena, J.F.; Zocchi, C.; De Luca, O.; Parmigiani, G.; Angeletti, G.; Ferracuti, S.; et al. Investigating DRD2 and HTR2A polymorphisms in treatment-resistant schizophrenia: A comparative analysis with other treatment-resistant mental disorders and the healthy state. Eur. Arch. Psychiatry Clin. Neurosci. 2025. [Google Scholar] [CrossRef]
- Wu, S.; Xing, Q.; Gao, R.; Li, X.; Gu, N.; Feng, G.; He, L. Response to chlorpromazine treatment may be associated with polymorphisms of the DRD2 gene in Chinese schizophrenic patients. Neurosci. Lett. 2005, 376, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Lencz, T.; Robinson, D.G.; Xu, K.; Ekholm, J.; Sevy, S.; Gunduz-Bruce, H.; Woerner, M.G.; Kane, J.M.; Goldman, D.; Malhotra, A.K. DRD2 promoter region variation as a predictor of sustained response to antipsychotic medication in first-episode schizophrenia patients. Am. J. Psychiatry 2006, 163, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Joober, R.; Benkelfat, C.; Brisebois, K.; Toulouse, A.; Turecki, G.; Lal, S.; Bloom, D.; Labelle, A.; Lalonde, P.; Fortin, D.; et al. T102C polymorphism in the 5HT2A gene and schizophrenia: Relation to phenotype and drug response variability. J. Psychiatry Neurosci. 1999, 24, 141–146. [Google Scholar]
- Li, J.; Hashimoto, H.; Meltzer, H.Y. Association of Serotonin(2c) Receptor Polymorphisms with Antipsychotic Drug Response in Schizophrenia. Front. Psychiatry 2019, 10, 58. [Google Scholar] [CrossRef]
- Masellis, M.; Basile, V.; Meltzer, H.Y.; Lieberman, J.A.; Sevy, S.; Macciardi, F.M.; Cola, P.; Howard, A.; Badri, F.; Nöthen, M.M.; et al. Serotonin subtype 2 receptor genes and clinical response to clozapine in schizophrenia patients. Neuropsychopharmacology 1998, 19, 123–132. [Google Scholar] [CrossRef]
- Nakamura, M.; Ueno, S.; Sano, A.; Tanabe, H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol. Psychiatry 2000, 5, 32–38. [Google Scholar] [CrossRef]
- Vázquez-Bourgon, J.; Arranz, M.J.; Mata, I.; Pelayo-Terán, J.M.; Pérez-Iglesias, R.; Medina-González, L.; Carrasco-Marín, E.; Vázquez-Barquero, J.L.; Crespo-Facorro, B. Serotonin transporter polymorphisms and early response to antipsychotic treatment in first episode of psychosis. Psychiatry Res. 2010, 175, 189–194. [Google Scholar] [CrossRef]
- Wang, L.; Yu, L.; He, G.; Zhang, J.; Zhang, A.P.; Du, J.; Tang, R.Q.; Zhao, X.Z.; Ma, J.; Xuan, J.K.; et al. Response of risperidone treatment may be associated with polymorphisms of HTT gene in Chinese schizophrenia patients. Neurosci. Lett. 2007, 414, 1–4. [Google Scholar] [CrossRef]
- Arranz, M.J.; Bolonna, A.A.; Munro, J.; Curtis, C.J.; Collier, D.A.; Kerwin, R.W. The serotonin transporter and clozapine response. Mol. Psychiatry 2000, 5, 124–125. [Google Scholar] [CrossRef][Green Version]
- Mouchlianitis, E.; Bloomfield, M.A.; Law, V.; Beck, K.; Selvaraj, S.; Rasquinha, N.; Waldman, A.; Turkheimer, F.E.; Egerton, A.; Stone, J.; et al. Treatment-Resistant Schizophrenia Patients Show Elevated Anterior Cingulate Cortex Glutamate Compared to Treatment-Responsive. Schizophr. Bull. 2016, 42, 744–752. [Google Scholar] [CrossRef]
- Taylor, S.F.; Tso, I.F. GABA abnormalities in schizophrenia: A methodological review of in vivo studies. Schizophr. Res. 2015, 167, 84–90. [Google Scholar] [CrossRef]
- Zhang, J.P.; Malhotra, A.K. Pharmacogenetics and antipsychotics: Therapeutic efficacy and side effects prediction. Expert. Opin. Drug Metab. Toxicol. 2011, 7, 9–37. [Google Scholar] [CrossRef]
- Laika, B.; Leucht, S.; Heres, S.; Steimer, W. Intermediate metabolizer: Increased side effects in psychoactive drug therapy. The key to cost-effectiveness of pretreatment CYP2D6 screening? Pharmacogenomics J. 2009, 9, 395–403. [Google Scholar] [CrossRef]
- Zubiaur, P.; Soria-Chacartegui, P.; Koller, D.; Navares-Gómez, M.; Ochoa, D.; Almenara, S.; Saiz-Rodríguez, M.; Mejía-Abril, G.; Villapalos-García, G.; Román, M.; et al. Impact of polymorphisms in transporter and metabolizing enzyme genes on olanzapine pharmacokinetics and safety in healthy volunteers. Biomed. Pharmacother. 2021, 133, 111087. [Google Scholar] [CrossRef] [PubMed]
- van der Weide, K.; van der Weide, J. The Influence of the CYP3A4*22 Polymorphism and CYP2D6 Polymorphisms on Serum Concentrations of Aripiprazole, Haloperidol, Pimozide, and Risperidone in Psychiatric Patients. J. Clin. Psychopharmacol. 2015, 35, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro, T.; López-Rodríguez, R.; Román, M.; Ochoa, D.; Novalbos, J.; Borobia, A.; Carcas, A.; Abad-Santos, F. Pharmacogenetics of quetiapine in healthy volunteers: Association with pharmacokinetics, pharmacodynamics, and adverse effects. Int. Clin. Psychopharmacol. 2015, 30, 82–88. [Google Scholar] [CrossRef]
- Escamilla, R.; Camarena, B.; Saracco-Alvarez, R.; Fresán, A.; Hernández, S.; Aguilar-García, A. Association study between COMT, DRD2, and DRD3 gene variants and antipsychotic treatment response in Mexican patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2018, 14, 2981–2987. [Google Scholar] [CrossRef]
- Gupta, M.; Bhatnagar, P.; Grover, S.; Kaur, H.; Baghel, R.; Bhasin, Y.; Chauhan, C.; Verma, B.; Manduva, V.; Mukherjee, O.; et al. Association studies of catechol-O-methyltransferase (COMT) gene with schizophrenia and response to antipsychotic treatment. Pharmacogenomics 2009, 10, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Hajj, A.; Obeid, S.; Sahyoun, S.; Haddad, C.; Azar, J.; Rabbaa Khabbaz, L.; Hallit, S. Clinical and Genetic Factors Associated with Resistance to Treatment in Patients with Schizophrenia: A Case-Control Study. Int. J. Mol. Sci. 2019, 20, 4753. [Google Scholar] [CrossRef]
- Ozdemir, F.; Oz, M.D.; Tok, K.C.; Dural, E.; Kır, Y.; Gumustas, M.; Baskak, B.; Suzen, H.S. The effects of UGT1A4 and ABCB1 polymorphisms on clozapine and N- desmethyl clozapine plasma levels in Turkish schizophrenia patients. Toxicol. Appl. Pharmacol. 2025, 495, 117219. [Google Scholar] [CrossRef] [PubMed]
- Piatkov, I.; Caetano, D.; Assur, Y.; Lau, S.L.; Jones, T.; Boyages, S.C.; McLean, M. ABCB1 and ABCC1 single-nucleotide polymorphisms in patients treated with clozapine. Pharmgenomics Pers. Med. 2017, 10, 235–242. [Google Scholar] [CrossRef]
- Martin, A.K.; Mowry, B. Increased rare duplication burden genomewide in patients with treatment-resistant schizophrenia. Psychol. Med. 2016, 46, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, A.W.; Dhindsa, R.S.; Goldberg, T.E.; Mehralizade, A.; Motelow, J.E.; Wang, X.; Alkelai, A.; Harms, M.B.; Lieberman, J.A.; Markx, S.; et al. High-impact rare genetic variants in severe schizophrenia. Proc. Natl. Acad. Sci. USA 2021, 118, e2112560118. [Google Scholar] [CrossRef]
- Marín Serrano, E.; Duque Alcorta, M. Diagnosis of a cystic pancreatic neuroendocrine tumor: Is cystic fluid chromogranin A useful? Rev. Esp. Enferm. Dig. 2023, 115, 526–527. [Google Scholar] [CrossRef]
- Nascimento, J.M.; Martins-de-Souza, D. The proteome of schizophrenia. npj Schizophr. 2015, 1, 14003. [Google Scholar] [CrossRef]
- Föcking, M.; Lopez, L.M.; English, J.A.; Dicker, P.; Wolff, A.; Brindley, E.; Wynne, K.; Cagney, G.; Cotter, D.R. Proteomic and genomic evidence implicates the postsynaptic density in schizophrenia. Mol. Psychiatry 2015, 20, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Martins-de-Souza, D.; Guest, P.C.; Rahmoune, H.; Bahn, S. Proteomic approaches to unravel the complexity of schizophrenia. Expert. Rev. Proteom. 2012, 9, 97–108. [Google Scholar] [CrossRef]
- Resch, H.; Pietschmann, P.; Kromer, K.; Krexner, E.; Bernecker, P.; Willvonseder, R. Peripheral and axial bone mass in Austrian free climbers. Klin. Wochenschr. 1991, 69, 303–306. [Google Scholar] [CrossRef]
- Wei, W.; Zhang, H.; Cheng, B.; Qin, X.; He, D.; Zhang, N.; Zhao, Y.; Cai, Q.; Shi, S.; Chu, X.; et al. Identification of novel functional brain proteins for treatment-resistant schizophrenia: Based on a proteome-wide association study. Eur. Psychiatry 2023, 66, e33. [Google Scholar] [CrossRef]
- Galindo-Moreno, J.; Iurlaro, R.; El Mjiyad, N.; Díez-Pérez, J.; Gabaldón, T.; Muñoz-Pinedo, C. Apolipoprotein L2 contains a BH3-like domain but it does not behave as a BH3-only protein. Cell Death Dis. 2014, 5, e1275. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Park, S.H.; Shin, D.I.; Hwang, J.Y.; Park, B.; Park, Y.J.; Lee, T.H.; Chae, H.Z.; Jin, B.K.; Oh, T.H.; et al. Oxidative modification of peroxiredoxin is associated with drug-induced apoptotic signaling in experimental models of Parkinson disease. J. Biol. Chem. 2008, 283, 9986–9998. [Google Scholar] [CrossRef]
- Yang, G.Q.; Huang, J.C.; Yuan, J.J.; Zhang, Q.; Gong, C.X.; Chen, Q.; Xie, Q.; Xie, L.X.; Chen, R.; Qiu, Z.M.; et al. Prdx1 Reduces Intracerebral Hemorrhage-Induced Brain Injury via Targeting Inflammation- and Apoptosis-Related mRNA Stability. Front. Neurosci. 2020, 14, 181. [Google Scholar] [CrossRef]
- Berry, T.; Abohamza, E.; Moustafa, A.A. Treatment-resistant schizophrenia: Focus on the transsulfuration pathway. Rev. Neurosci. 2020, 31, 219–232. [Google Scholar] [CrossRef]
- Almulla, A.F.; Vasupanrajit, A.; Tunvirachaisakul, C.; Al-Hakeim, H.K.; Solmi, M.; Verkerk, R.; Maes, M. The tryptophan catabolite or kynurenine pathway in schizophrenia: Meta-analysis reveals dissociations between central, serum, and plasma compartments. Mol. Psychiatry 2022, 27, 3679–3691. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Barone, A.; Vellucci, L.; Mazza, B.; Austin, M.C.; Iasevoli, F.; Ciccarelli, M. Linking Inflammation, Aberrant Glutamate-Dopamine Interaction, and Post-synaptic Changes: Translational Relevance for Schizophrenia and Antipsychotic Treatment: A Systematic Review. Mol. Neurobiol. 2022, 59, 6460–6501. [Google Scholar] [CrossRef]
- Chen, W.; Tian, Y.; Gou, M.; Wang, L.; Tong, J.; Zhou, Y.; Feng, W.; Li, Y.; Chen, S.; Liu, Y.; et al. Role of the immune-kynurenine pathway in treatment-resistant schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 130, 110926. [Google Scholar] [CrossRef]
- Sapienza, J.; Agostoni, G.; Dall’Acqua, S.; Sut, S.; Nasini, S.; Martini, F.; Marchesi, A.; Bechi, M.; Buonocore, M.; Cocchi, F.; et al. The kynurenine pathway in treatment-resistant schizophrenia at the crossroads between pathophysiology and pharmacotherapy. Schizophr. Res. 2024, 264, 71–80. [Google Scholar] [CrossRef]
- Huang, J.; Tong, J.; Zhang, P.; Zhou, Y.; Li, Y.; Tan, S.; Wang, Z.; Yang, F.; Kochunov, P.; Chiappelli, J.; et al. Elevated salivary kynurenic acid levels related to enlarged choroid plexus and severity of clinical phenotypes in treatment-resistant schizophrenia. Brain Behav. Immun. 2022, 106, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, P.; Zhou, Y.; Tong, J.; Cui, Y.; Tan, S.; Wang, Z.; Yang, F.; Kochunov, P.; Tian, B.; et al. Serum kynurenine metabolites might not be associated with risk factors of treatment-resistant schizophrenia. J. Psychiatr. Res. 2022, 145, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Myint, A.M.; Schwarz, M.J. Kynurenine pathway in schizophrenia: Pathophysiological and therapeutic aspects. Curr. Pharm. Des. 2011, 17, 130–136. [Google Scholar] [CrossRef]
- Kessler, M.; Terramani, T.; Lynch, G.; Baudry, M. A glycine site associated with N-methyl-D-aspartic acid receptors: Characterization and identification of a new class of antagonists. J. Neurochem. 1989, 52, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Hilmas, C.; Pereira, E.F.; Alkondon, M.; Rassoulpour, A.; Schwarcz, R.; Albuquerque, E.X. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: Physiopathological implications. J. Neurosci. 2001, 21, 7463–7473. [Google Scholar] [CrossRef]
- Kruyer, A.; Parrilla-Carrero, J.; Powell, C.; Brandt, L.; Gutwinski, S.; Angelis, A.; Chalhoub, R.M.; Jhou, T.C.; Kalivas, P.W.; Amato, D. Accumbens D2-MSN hyperactivity drives antipsychotic-induced behavioral supersensitivity. Mol. Psychiatry 2021, 26, 6159–6169. [Google Scholar] [CrossRef]
- Yin, J.; Barr, A.M.; Ramos-Miguel, A.; Procyshyn, R.M. Antipsychotic Induced Dopamine Supersensitivity Psychosis: A Comprehensive Review. Curr. Neuropharmacol. 2017, 15, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Fujita, Y.; Oishi, K.; Nakata, Y.; Takase, M.; Niitsu, T.; Kanahara, N.; Shirayama, Y.; Hashimoto, K.; Iyo, M. Alterations in glutamatergic signaling in the brain of dopamine supersensitivity psychosis and non-supersensitivity psychosis model rats. Psychopharmacology 2017, 234, 3027–3036. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, S.; Okamura, N.; Sekine, Y.; Kanahara, N.; Hashimoto, K.; Iyo, M. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr. Bull. 2012, 38, 1012–1020. [Google Scholar] [CrossRef]
- Heidbreder, C.A.; Weiss, I.C.; Domeney, A.M.; Pryce, C.; Homberg, J.; Hedou, G.; Feldon, J.; Moran, M.C.; Nelson, P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 2000, 100, 749–768. [Google Scholar] [CrossRef]
- Marsden, C.A.; King, M.V.; Fone, K.C. Influence of social isolation in the rat on serotonergic function and memory--relevance to models of schizophrenia and the role of 5-HT6 receptors. Neuropharmacology 2011, 61, 400–407. [Google Scholar] [CrossRef]
- Gauvin, M.G.; Riddle, D.L.; Rothstein, J.M. Reliability of clinical measurements of forward bending using the modified fingertip-to-floor method. Phys. Ther. 1990, 70, 443–447. [Google Scholar] [CrossRef]
- Amitai, N.; Semenova, S.; Markou, A. Clozapine attenuates disruptions in response inhibition and task efficiency induced by repeated phencyclidine administration in the intracranial self-stimulation procedure. Eur. J. Pharmacol. 2009, 602, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Noda, Y.; Kamei, H.; Nagai, T.; Furukawa, H.; Miura, H.; Kayukawa, Y.; Ohta, T.; Nabeshima, T. Clozapine, but not haloperidol, reverses social behavior deficit in mice during withdrawal from chronic phencyclidine treatment. Neuroreport 2001, 12, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Purves-Tyson, T.D.; Owens, S.J.; Rothmond, D.A.; Halliday, G.M.; Double, K.L.; Stevens, J.; McCrossin, T.; Shannon Weickert, C. Putative presynaptic dopamine dysregulation in schizophrenia is supported by molecular evidence from post-mortem human midbrain. Transl. Psychiatry 2017, 7, e1003. [Google Scholar] [CrossRef]
- Kornhuber, J.; Mack-Burkhardt, F.; Kornhuber, M.E.; Riederer, P. [3H]MK-801 binding sites in post-mortem human frontal cortex. Eur. J. Pharmacol. 1989, 162, 483–490. [Google Scholar] [CrossRef]
- Barksdale, K.A.; Lahti, A.C.; Roberts, R.C. Synaptic proteins in the postmortem anterior cingulate cortex in schizophrenia: Relationship to treatment and treatment response. Neuropsychopharmacology 2014, 39, 2095–2103. [Google Scholar] [CrossRef]
- García-Mesa, Y.; García-Piqueras, J.; Cuendias, P.; Cobo, R.; Martín-Cruces, J.; Feito, J.; García-Suarez, O.; Biedma, B.M.; Vega, J.A. Synaptophysin is a selective marker for axons in human cutaneous end organ complexes. Ann. Anat. 2022, 243, 151955. [Google Scholar] [CrossRef]
- Wu, X.S.; Zhang, Z.; Zhao, W.D.; Wang, D.; Luo, F.; Wu, L.G. Calcineurin is universally involved in vesicle endocytosis at neuronal and nonneuronal secretory cells. Cell Rep. 2014, 7, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Somerville, S.M.; Lahti, A.C.; Conley, R.R.; Roberts, R.C. Mitochondria in the striatum of subjects with schizophrenia: Relationship to treatment response. Synapse 2011, 65, 215–224. [Google Scholar] [CrossRef]
- Misiak, B.; Stańczykiewicz, B.; Kotowicz, K.; Rybakowski, J.K.; Samochowiec, J.; Frydecka, D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: A systematic review. Schizophr. Res. 2018, 192, 16–29. [Google Scholar] [CrossRef]
- Goldsmith, D.R.; Haroon, E.; Miller, A.H.; Strauss, G.P.; Buckley, P.F.; Miller, B.J. TNF-α and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophr. Res. 2018, 199, 281–284. [Google Scholar] [CrossRef]
- Lin, A.; Kenis, G.; Bignotti, S.; Tura, G.J.; De Jong, R.; Bosmans, E.; Pioli, R.; Altamura, C.; Scharpé, S.; Maes, M. The inflammatory response system in treatment-resistant schizophrenia: Increased serum interleukin-6. Schizophr. Res. 1998, 32, 9–15. [Google Scholar] [CrossRef]
- Gillespie, A.L.; Samanaite, R.; Mill, J.; Egerton, A.; MacCabe, J.H. Is treatment-resistant schizophrenia categorically distinct from treatment-responsive schizophrenia? a systematic review. BMC Psychiatry 2017, 17, 12. [Google Scholar] [CrossRef]
- Dierynck, I.; Bernard, A.; Roels, H.; De Ley, M. Potent inhibition of both human interferon-gamma production and biologic activity by the Clara cell protein CC16. Am. J. Respir. Cell Mol. Biol. 1995, 12, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Bocchio Chiavetto, L.; Bignotti, S.; Battisa Tura, G.; Pioli, R.; Boin, F.; Kenis, G.; Bosmans, E.; de Jongh, R.; Lin, A.; et al. Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur. Neuropsychopharmacol. 2000, 10, 119–124. [Google Scholar] [CrossRef]
- Müller, N. Inflammation in Schizophrenia: Pathogenetic Aspects and Therapeutic Considerations. Schizophr. Bull. 2018, 44, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Derkits, E.J. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef]
- Meyer, U. Developmental neuroinflammation and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Simoes, L.R.; Quevedo, J.; Zhang, X.Y. Microglial Activation and Psychotic Disorders: Evidence from Pre-clinical and Clinical Studies. Curr. Top. Behav. Neurosci. 2020, 44, 161–205. [Google Scholar] [CrossRef]
- Boksa, P. Effects of prenatal infection on brain development and behavior: A review of findings from animal models. Brain Behav. Immun. 2010, 24, 881–897. [Google Scholar] [CrossRef]
- Kwon, D.J.; Ju, S.M.; Youn, G.S.; Choi, S.Y.; Park, J. Suppression of iNOS and COX-2 expression by flavokawain A via blockade of NF-κB and AP-1 activation in RAW 264.7 macrophages. Food Chem. Toxicol. 2013, 58, 479–486. [Google Scholar] [CrossRef]
- Mattei, D.; Ivanov, A.; Ferrai, C.; Jordan, P.; Guneykaya, D.; Buonfiglioli, A.; Schaafsma, W.; Przanowski, P.; Deuther-Conrad, W.; Brust, P.; et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl. Psychiatry 2017, 7, e1120. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Borges, M.C.; Horta, B.L.; Bowden, J.; Davey Smith, G. Inflammatory Biomarkers and Risk of Schizophrenia: A 2-Sample Mendelian Randomization Study. JAMA Psychiatry 2017, 74, 1226–1233. [Google Scholar] [CrossRef]
- Al-Dujaili, A.H.; Mousa, R.F.; Al-Hakeim, H.K.; Maes, M. High Mobility Group Protein 1 and Dickkopf-Related Protein 1 in Schizophrenia and Treatment-Resistant Schizophrenia: Associations With Interleukin-6, Symptom Domains, and Neurocognitive Impairments. Schizophr. Bull. 2021, 47, 530–541. [Google Scholar] [CrossRef]
- Leboyer, M.; Godin, O.; Terro, E.; Boukouaci, W.; Lu, C.L.; Andre, M.; Aouizerate, B.; Berna, F.; Barau, C.; Capdevielle, D.; et al. Immune Signatures of Treatment-Resistant Schizophrenia: A FondaMental Academic Centers of Expertise for Schizophrenia (FACE-SZ) Study. Schizophr. Bull. Open 2021, 2, sgab012. [Google Scholar] [CrossRef]
- Moustafa, S.R.; Al-Rawi, K.F.; Stoyanov, D.; Al-Dujaili, A.H.; Supasitthumrong, T.; Al-Hakeim, H.K.; Maes, M. The Endogenous Opioid System in Schizophrenia and Treatment Resistant Schizophrenia: Increased Plasma Endomorphin 2, and κ and μ Opioid Receptors Are Associated with Interleukin-6. Diagnostics 2020, 10, 633. [Google Scholar] [CrossRef]
- He, J.; Wei, Y.; Li, J.; Tang, Y.; Liu, J.; He, Z.; Zhou, R.; He, X.; Ren, H.; Liao, Y.; et al. Sex differences in the association of treatment-resistant schizophrenia and serum interleukin-6 levels. BMC Psychiatry 2023, 23, 470. [Google Scholar] [CrossRef]
- Capuzzi, E.; Bartoli, F.; Crocamo, C.; Clerici, M.; Carrà, G. Acute variations of cytokine levels after antipsychotic treatment in drug-naïve subjects with a first-episode psychosis: A meta-analysis. Neurosci. Biobehav. Rev. 2017, 77, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Bosmans, E.; Ranjan, R.; Vandoolaeghe, E.; Meltzer, H.Y.; De Ley, M.; Berghmans, R.; Stans, G.; Desnyder, R. Lower plasma CC16, a natural anti-inflammatory protein, and increased plasma interleukin-1 receptor antagonist in schizophrenia: Effects of antipsychotic drugs. Schizophr. Res. 1996, 21, 39–50. [Google Scholar] [CrossRef]
- Dayer, J.M.; Burger, D. Interleukin-1, tumor necrosis factor and their specific inhibitors. Eur. Cytokine Netw. 1994, 5, 563–571. [Google Scholar] [PubMed]
- Maes, M.; Bosmans, E.; Kenis, G.; De Jong, R.; Smith, R.S.; Meltzer, H.Y. In vivo immunomodulatory effects of clozapine in schizophrenia. Schizophr. Res. 1997, 26, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, W.; Wang, Y. Lights and shadows of clozapine on the immune system in schizophrenia: A narrative literature review. Metab. Brain Dis. 2025, 40, 128. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhu, M.; Li, Y.; Liu, N.; Wang, X.; Yang, B.; Li, S.; Li, Z. Association of cytokines levels, psychopathology and cognition among CR-TRS patients with metabolic syndrome. Schizophrenia 2024, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.Y.; Constantine, J.M.; Bonventre, J.V. Regulation of cytosolic free calcium concentration in cultured renal epithelial cells. Am. J. Physiol. 1986, 251, F690–F701. [Google Scholar] [CrossRef]
- Horsdal, H.T.; Wimberley, T.; Benros, M.E.; Gasse, C. C-reactive protein levels and treatment resistance in schizophrenia-A Danish population-based cohort study. Hum. Psychopharmacol. 2017, 32, e2632. [Google Scholar] [CrossRef] [PubMed]
- Enache, D.; Nikkheslat, N.; Fathalla, D.; Morgan, B.P.; Lewis, S.; Drake, R.; Deakin, B.; Walters, J.; Lawrie, S.M.; Egerton, A.; et al. Peripheral immune markers and antipsychotic non-response in psychosis. Schizophr. Res. 2021, 230, 1–8. [Google Scholar] [CrossRef]
- Squassina, A.; Manchia, M.; Pisanu, C.; Ardau, R.; Arzedi, C.; Bocchetta, A.; Caria, P.; Cocco, C.; Congiu, D.; Cossu, E.; et al. Telomere attrition and inflammatory load in severe psychiatric disorders and in response to psychotropic medications. Neuropsychopharmacology 2020, 45, 2229–2238. [Google Scholar] [CrossRef]
- Pisanu, C.; Severino, G.; Minelli, A.; Dierssen, M.; Potier, M.C.; Fabbri, C.; Serretti, A.; Gennarelli, M.; Baune, B.T.; Squassina, A. Biomarkers of treatment-resistant schizophrenia: A systematic review. Neurosci. Appl. 2024, 3, 104059. [Google Scholar] [CrossRef]
- Debnath, M.; Berk, M. Th17 pathway-mediated immunopathogenesis of schizophrenia: Mechanisms and implications. Schizophr. Bull. 2014, 40, 1412–1421. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: A reconceptualization. Transl. Psychiatry 2017, 7, e1024. [Google Scholar] [CrossRef]
- Osimo, E.F.; Beck, K.; Reis Marques, T.; Howes, O.D. Synaptic loss in schizophrenia: A meta-analysis and systematic review of synaptic protein and mRNA measures. Mol. Psychiatry 2019, 24, 549–561. [Google Scholar] [CrossRef]
- Laugeray, A.; Launay, J.M.; Callebert, J.; Surget, A.; Belzung, C.; Barone, P.R. Peripheral and cerebral metabolic abnormalities of the tryptophan-kynurenine pathway in a murine model of major depression. Behav. Brain Res. 2010, 210, 84–91. [Google Scholar] [CrossRef]
- Laugeray, A.; Launay, J.M.; Callebert, J.; Surget, A.; Belzung, C.; Barone, P.R. Evidence for a key role of the peripheral kynurenine pathway in the modulation of anxiety- and depression-like behaviours in mice: Focus on individual differences. Pharmacol. Biochem. Behav. 2011, 98, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Marques, T.R.; Ashok, A.H.; Pillinger, T.; Veronese, M.; Turkheimer, F.E.; Dazzan, P.; Sommer, I.E.C.; Howes, O.D. Neuroinflammation in schizophrenia: Meta-analysis of in vivo microglial imaging studies. Psychol. Med. 2019, 49, 2186–2196. [Google Scholar] [CrossRef]
- Di Biase, M.A.; Zalesky, A.; O’Keefe, G.; Laskaris, L.; Baune, B.T.; Weickert, C.S.; Olver, J.; McGorry, P.D.; Amminger, G.P.; Nelson, B.; et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl. Psychiatry 2017, 7, e1225. [Google Scholar] [CrossRef] [PubMed]
- Plavén-Sigray, P.; Matheson, G.J.; Coughlin, J.M.; Hafizi, S.; Laurikainen, H.; Ottoy, J.; De Picker, L.; Rusjan, P.; Hietala, J.; Howes, O.D.; et al. Meta-analysis of the Glial Marker TSPO in Psychosis Revisited: Reconciling Inconclusive Findings of Patient-Control Differences. Biol. Psychiatry 2021, 89, e5–e8. [Google Scholar] [CrossRef]
- Kraguljac, N.V.; McDonald, W.M.; Widge, A.S.; Rodriguez, C.I.; Tohen, M.; Nemeroff, C.B. Neuroimaging Biomarkers in Schizophrenia. Am. J. Psychiatry 2021, 178, 509–521. [Google Scholar] [CrossRef]
- Conen, S.; Gregory, C.J.; Hinz, R.; Smallman, R.; Corsi-Zuelli, F.; Deakin, B.; Talbot, P.S. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: Implications for immune pathogenesis. Mol. Psychiatry 2021, 26, 5398–5406. [Google Scholar] [CrossRef] [PubMed]
- Laricchiuta, D.; Papi, M.; Decandia, D.; Panuccio, A.; Cutuli, D.; Peciccia, M.; Mazzeschi, C.; Petrosini, L. The role of glial cells in mental illness: A systematic review on astroglia and microglia as potential players in schizophrenia and its cognitive and emotional aspects. Front. Cell. Neurosci. 2024, 18, 1358450. [Google Scholar] [CrossRef]
- Cannon, T.D. How Schizophrenia Develops: Cognitive and Brain Mechanisms Underlying Onset of Psychosis. Trends Cogn. Sci. 2015, 19, 744–756. [Google Scholar] [CrossRef]
- Glausier, J.R.; Lewis, D.A. Dendritic spine pathology in schizophrenia. Neuroscience 2013, 251, 90–107. [Google Scholar] [CrossRef]
- Sellgren, C.M.; Gracias, J.; Watmuff, B.; Biag, J.D.; Thanos, J.M.; Whittredge, P.B.; Fu, T.; Worringer, K.; Brown, H.E.; Wang, J.; et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 2019, 22, 374–385. [Google Scholar] [CrossRef]
- Hall, J.; Trent, S.; Thomas, K.L.; O’Donovan, M.C.; Owen, M.J. Genetic risk for schizophrenia: Convergence on synaptic pathways involved in plasticity. Biol. Psychiatry 2015, 77, 52–58. [Google Scholar] [CrossRef]
- Lewis, D.A. Cortical circuit dysfunction and cognitive deficits in schizophrenia--implications for preemptive interventions. Eur. J. Neurosci. 2012, 35, 1871–1878. [Google Scholar] [CrossRef]
- Lisman, J.E.; Coyle, J.T.; Green, R.W.; Javitt, D.C.; Benes, F.M.; Heckers, S.; Grace, A.A. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008, 31, 234–242. [Google Scholar] [CrossRef]
- Cannon, T.D. Microglial Activation and the Onset of Psychosis. Am. J. Psychiatry 2016, 173, 3–4. [Google Scholar] [CrossRef]
- Howes, O.D.; Onwordi, E.C. The synaptic hypothesis of schizophrenia version III: A master mechanism. Mol. Psychiatry 2023, 28, 1843–1856. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, H.; Zhang, D.; Yang, S.; Qian, L.; Wu, H.M.; Chen, P.S.; Wilson, B.; Gao, H.M.; Lu, R.B.; et al. Clozapine protects dopaminergic neurons from inflammation-induced damage by inhibiting microglial overactivation. J. Neuroimmune Pharmacol. 2012, 7, 187–201. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Schizophrenia: Linking prenatal infection to cytokines, the tryptophan catabolite (TRYCAT) pathway, NMDA receptor hypofunction, neurodevelopment and neuroprogression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Shibakawa, Y.S.; Sasaki, Y.; Goshima, Y.; Echigo, N.; Kamiya, Y.; Kurahashi, K.; Yamada, Y.; Andoh, T. Effects of ketamine and propofol on inflammatory responses of primary glial cell cultures stimulated with lipopolysaccharide. Br. J. Anaesth. 2005, 95, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Brinholi, F.F.; Farias, C.C.; Bonifácio, K.L.; Higachi, L.; Casagrande, R.; Moreira, E.G.; Barbosa, D.S. Clozapine and olanzapine are better antioxidants than haloperidol, quetiapine, risperidone and ziprasidone in in vitro models. Biomed. Pharmacother. 2016, 81, 411–415. [Google Scholar] [CrossRef]
- Gross, A.; Joffe, G.; Joutsiniemi, S.L.; Nyberg, P.; Rimón, R.; Appelberg, B. Decreased production of reactive oxygen species by blood monocytes caused by clozapine correlates with EEG slowing in schizophrenic patients. Neuropsychobiology 2003, 47, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Lin, A.; Kenis, G.; Bosmans, E.; Maes, M. Immunosuppressive effects of clozapine and haloperidol: Enhanced production of the interleukin-1 receptor antagonist. Schizophr. Res. 2000, 42, 157–164. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; De Simone, G.; Ciccarelli, M.; Castiello, A.; Mazza, B.; Vellucci, L.; Barone, A. Antipsychotics-Induced Changes in Synaptic Architecture and Functional Connectivity: Translational Implications for Treatment Response and Resistance. Biomedicines 2022, 10, 3183. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Avagliano, C.; Vellucci, L.; D’Ambrosio, L.; Manchia, M.; D’Urso, G.; Buonaguro, E.F.; Iasevoli, F. Translating preclinical findings in clinically relevant new antipsychotic targets: Focus on the glutamatergic postsynaptic density. Implications for treatment resistant schizophrenia. Neurosci. Biobehav. Rev. 2019, 107, 795–827. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Barone, A.; Buonaguro, E.F.; Tomasetti, C.; Vellucci, L.; Iasevoli, F. The Homer1 family of proteins at the crossroad of dopamine-glutamate signaling: An emerging molecular “Lego” in the pathophysiology of psychiatric disorders. A systematic review and translational insight. Neurosci. Biobehav. Rev. 2022, 136, 104596. [Google Scholar] [CrossRef]
- de Bartolomeis, A.; Vellucci, L.; De Simone, G.; Mazza, B.; Barone, A.; Ciccarelli, M. Dysregulated Signaling at Postsynaptic Density: A Systematic Review and Translational Appraisal for the Pathophysiology, Clinics, and Antipsychotics’ Treatment of Schizophrenia. Cells 2023, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; De Prisco, M.; Altavilla, B.; Avagliano, C.; Balletta, R.; Buonaguro, E.F.; Ciccarelli, M.; D’Ambrosio, L.; Giordano, S.; Latte, G.; et al. Disorganization domain as a putative predictor of Treatment Resistant Schizophrenia (TRS) diagnosis: A machine learning approach. J. Psychiatr. Res. 2022, 155, 572–578. [Google Scholar] [CrossRef]
- Vellucci, L.; Barone, A.; Buonaguro, E.F.; Ciccarelli, M.; De Simone, G.; Iannotta, F.; Matrone, M.; Mazza, B.; Vitelli, R.; de Bartolomeis, A.; et al. Severity of autism-related symptoms in treatment-resistant schizophrenia: Associations with cognitive performance, psychosocial functioning, and neurological soft signs—Clinical evidence and ROC analysis. J. Psychiatr. Res. 2025, 185, 119–129. [Google Scholar] [CrossRef]
- Buosi, P.; Borghi, F.A.; Lopes, A.M.; Facincani, I.D.S.; Fernandes-Ferreira, R.; Oliveira-Brancati, C.I.F.; do Carmo, T.S.; Souza, D.R.S.; da Silva, D.G.H.; de Almeida, E.A.; et al. Oxidative stress biomarkers in treatment-responsive and treatment-resistant schizophrenia patients. Trends Psychiatry Psychother. 2021, 43, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, C.; Madrigal, J.L.; García-Bueno, B.; Pradillo, J.M.; Moro, M.A.; Lizasoain, I.; Lorenzo, P.; Scavone, C.; Leza, J.C. TNF-alpha accounts for short-term persistence of oxidative status in rat brain after two weeks of repeated stress. Eur. J. Neurosci. 2004, 20, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, L.; Treccani, G.; Popoli, M. Functional and structural remodeling of glutamate synapses in prefrontal and frontal cortex induced by behavioral stress. Front. Psychiatry 2015, 6, 60. [Google Scholar] [CrossRef]
- Perez-Cruz, C.; Müller-Keuker, J.I.; Heilbronner, U.; Fuchs, E.; Flügge, G. Morphology of pyramidal neurons in the rat prefrontal cortex: Lateralized dendritic remodeling by chronic stress. Neural Plast. 2007, 2007, 46276. [Google Scholar] [CrossRef]
- Altamura, A.C.; Boin, F.; Maes, M. HPA axis and cytokines dysregulation in schizophrenia: Potential implications for the antipsychotic treatment. Eur. Neuropsychopharmacol. 1999, 10, 1–4. [Google Scholar] [CrossRef]
- Ding, J.B.; Hu, K. Cigarette Smoking and Schizophrenia: Etiology, Clinical, Pharmacological, and Treatment Implications. Schizophr. Res. Treat. 2021, 2021, 7698030. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, W.; Zhu, C.; Lin, G.N.; Cheng, Y.; Zou, J.; Cui, D. Use of tobacco in schizophrenia: A double-edged sword. Brain Behav. 2019, 9, e01433. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Yang, M.; Xu, L.; Chen, W.; Sun, Y.; Zhang, X. Catalase and interleukin-6 serum elevation in a prediction of treatment-resistance in male schizophrenia patients. Asian J. Psychiatr. 2023, 79, 103400. [Google Scholar] [CrossRef]
- Olmos, G.; Lladó, J. Tumor necrosis factor alpha: A link between neuroinflammation and excitotoxicity. Mediat. Inflamm. 2014, 2014, 861231. [Google Scholar] [CrossRef] [PubMed]
- Novaes, L.S.; Dos Santos, N.B.; Dragunas, G.; Perfetto, J.G.; Leza, J.C.; Scavone, C.; Munhoz, C.D. Repeated Restraint Stress Decreases Na,K-ATPase Activity via Oxidative and Nitrosative Damage in the Frontal Cortex of Rats. Neuroscience 2018, 393, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Meade, A.J.; Meloni, B.P.; Cross, J.; Bakker, A.J.; Fear, M.W.; Mastaglia, F.L.; Watt, P.M.; Knuckey, N.W. AP-1 inhibitory peptides are neuroprotective following acute glutamate excitotoxicity in primary cortical neuronal cultures. J. Neurochem. 2010, 112, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Allan, S.M.; Rothwell, N.J. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2001, 2, 734–744. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Palmieri, E.M.; Castegna, A. Clinical implications from proteomic studies in neurodegenerative diseases: Lessons from mitochondrial proteins. Expert. Rev. Proteom. 2016, 13, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Clausen, M.V.; Hilbers, F.; Poulsen, H. The Structure and Function of the Na,K-ATPase Isoforms in Health and Disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Xuereb, J.H.; Crepaldi, L.; Corsi, M.; Michielin, F.; Ferraguti, F. Altered levels of glutamatergic receptors and Na+/K+ ATPase-α1 in the prefrontal cortex of subjects with schizophrenia. Schizophr. Res. 2011, 128, 7–14. [Google Scholar] [CrossRef]
- McEwen, B.S.; Morrison, J.H. The brain on stress: Vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013, 79, 16–29. [Google Scholar] [CrossRef]
- Tan, H.Y.; Callicott, J.H.; Weinberger, D.R. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb. Cortex 2007, 17 (Suppl. 1), i171–i181. [Google Scholar] [CrossRef]
- De Simone, G.; Mazza, B.; Vellucci, L.; Barone, A.; Ciccarelli, M.; de Bartolomeis, A. Schizophrenia Synaptic Pathology and Antipsychotic Treatment in the Framework of Oxidative and Mitochondrial Dysfunction: Translational Highlights for the Clinics and Treatment. Antioxidants 2023, 12, 975. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Ma, R.; Zhang, B.; Yang, P.; Cao, T.; Jiao, S.; Chen, H.; Lin, C.; Cai, H. Insight into mitochondrial dysfunction mediated by clozapine-induced inhibition of PGRMC1 in PC12 cells. Toxicology 2023, 491, 153515. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, Z.; Han, X.; Wu, H.; Yu, Y.; Wu, J.; Liu, S.; Hou, Y. Progesterone attenuates Aβ(25-35)-induced neuronal toxicity via JNK inactivation and progesterone receptor membrane component 1-dependent inhibition of mitochondrial apoptotic pathway. J. Steroid Biochem. Mol. Biol. 2015, 154, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Timper, K.; Del Río-Martín, A.; Cremer, A.L.; Bremser, S.; Alber, J.; Giavalisco, P.; Varela, L.; Heilinger, C.; Nolte, H.; Trifunovic, A.; et al. GLP-1 Receptor Signaling in Astrocytes Regulates Fatty Acid Oxidation, Mitochondrial Integrity, and Function. Cell Metab. 2020, 31, 1189–1205.e1113. [Google Scholar] [CrossRef]
- Bach, D.; Pich, S.; Soriano, F.X.; Vega, N.; Baumgartner, B.; Oriola, J.; Daugaard, J.R.; Lloberas, J.; Camps, M.; Zierath, J.R.; et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J. Biol. Chem. 2003, 278, 17190–17197. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, S.H.; Yu, H.S.; Park, H.G.; Kang, U.G.; Ahn, Y.M.; Kim, Y.S. The effect of clozapine on the AMPK-ACC-CPT1 pathway in the rat frontal cortex. Int. J. Neuropsychopharmacol. 2012, 15, 907–917. [Google Scholar] [CrossRef]
- Narayan, S.; Head, S.R.; Gilmartin, T.J.; Dean, B.; Thomas, E.A. Evidence for disruption of sphingolipid metabolism in schizophrenia. J. Neurosci. Res. 2009, 87, 278–288. [Google Scholar] [CrossRef]
- Pardiñas, A.F.; Owen, M.J.; Walters, J.T.R. Pharmacogenomics: A road ahead for precision medicine in psychiatry. Neuron 2021, 109, 3914–3929. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Timal, S.; Venselaar, H.; Wintjes, L.T.; Kopajtich, R.; Feichtinger, R.G.; Onnekink, C.; Mühlmeister, M.; Brandt, U.; Smeitink, J.A.; et al. Biallelic variants in WARS2 encoding mitochondrial tryptophanyl-tRNA synthase in six individuals with mitochondrial encephalopathy. Hum. Mutat. 2017, 38, 1786–1795. [Google Scholar] [CrossRef]
- Contreras-Shannon, V.; Heart, D.L.; Paredes, R.M.; Navaira, E.; Catano, G.; Maffi, S.K.; Walss-Bass, C. Clozapine-induced mitochondria alterations and inflammation in brain and insulin-responsive cells. PLoS ONE 2013, 8, e59012. [Google Scholar] [CrossRef]
- Barron, H.; Hafizi, S.; Andreazza, A.C.; Mizrahi, R. Neuroinflammation and Oxidative Stress in Psychosis and Psychosis Risk. Int. J. Mol. Sci. 2017, 18, 651. [Google Scholar] [CrossRef]
- Blandino, G.; Fiorani, M.; Canonico, B.; De Matteis, R.; Guidarelli, A.; Montanari, M.; Buffi, G.; Coppo, L.; Arnér, E.S.J.; Cantoni, O. Clozapine suppresses NADPH oxidase activation, counteracts cytosolic H2O2, and triggers early onset mitochondrial dysfunction during adipogenesis of human liposarcoma SW872cells. Redox Biol. 2023, 67, 102915. [Google Scholar] [CrossRef]
- Scaini, G.; Quevedo, J.; Velligan, D.; Roberts, D.L.; Raventos, H.; Walss-Bass, C. Second generation antipsychotic-induced mitochondrial alterations: Implications for increased risk of metabolic syndrome in patients with schizophrenia. Eur. Neuropsychopharmacol. 2018, 28, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Martins-de-Souza, D.; Schiltz, K.; Sarnyai, Z.; Westphal, S.; Isermann, B.; Dobrowolny, H.; Turck, C.W.; Bogerts, B.; Bernstein, H.G.; et al. Clozapine promotes glycolysis and myelin lipid synthesis in cultured oligodendrocytes. Front. Cell. Neurosci. 2014, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Whitehurst, T.; Howes, O. The role of mitochondria in the pathophysiology of schizophrenia: A critical review of the evidence focusing on mitochondrial complex one. Neurosci. Biobehav. Rev. 2022, 132, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, S.; Swatton, J.E.; Ryan, M.M.; Huffaker, S.J.; Huang, J.T.; Griffin, J.L.; Wayland, M.; Freeman, T.; Dudbridge, F.; Lilley, K.S.; et al. Mitochondrial dysfunction in schizophrenia: Evidence for compromised brain metabolism and oxidative stress. Mol. Psychiatry 2004, 9, 684–697, 643. [Google Scholar] [CrossRef]
- Marchbanks, R.M.; Ryan, M.; Day, I.N.; Owen, M.; McGuffin, P.; Whatley, S.A. A mitochondrial DNA sequence variant associated with schizophrenia and oxidative stress. Schizophr. Res. 2003, 65, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ricci, J.E.; Gottlieb, R.A.; Green, D.R. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J. Cell Biol. 2003, 160, 65–75. [Google Scholar] [CrossRef]
- Murray, A.J.; Rogers, J.C.; Katshu, M.Z.U.H.; Liddle, P.F.; Upthegrove, R. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front. Psychiatry 2021, 12, 703452. [Google Scholar] [CrossRef]
- Mira, R.G.; Cerpa, W. Building a Bridge Between NMDAR-Mediated Excitotoxicity and Mitochondrial Dysfunction in Chronic and Acute Diseases. Cell. Mol. Neurobiol. 2021, 41, 1413–1430. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.; Berk, M.; Dean, O.; Bush, A.I. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008, 11, 851–876. [Google Scholar] [CrossRef]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A molecular web: Endoplasmic reticulum stress, inflammation, and oxidative stress. Front. Cell. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2006, 38, 317–332. [Google Scholar] [CrossRef]
- Hayashi-Takagi, A.; Vawter, M.P.; Iwamoto, K. Peripheral biomarkers revisited: Integrative profiling of peripheral samples for psychiatric research. Biol. Psychiatry 2014, 75, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.X.; Tanaka, L.Y.; Wosniak, J.; Laurindo, F.R. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: Roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid. Redox Signal 2009, 11, 2409–2427. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Mazzio, E.A.; Soliman, K.F. Glioma cell antioxidant capacity relative to reactive oxygen species produced by dopamine. J. Appl. Toxicol. 2004, 24, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Celik, C.; Lee, S.Y.T.; Yap, W.S.; Thibault, G. Endoplasmic reticulum stress and lipids in health and diseases. Prog. Lipid Res. 2023, 89, 101198. [Google Scholar] [CrossRef]
- Fehsel, K.; Bouvier, M.L.; Capobianco, L.; Lunetti, P.; Klein, B.; Oldiges, M.; Majora, M.; Löffler, S. Neuroreceptor Inhibition by Clozapine Triggers Mitohormesis and Metabolic Reprogramming in Human Blood Cells. Cells 2024, 13, 762. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Duan, S.; Zhou, J.; Sun, Y.; Zheng, Y.; Gu, N.; Feng, G.; He, L. A case-control study provides evidence of association for a functional polymorphism-197C/G in XBP1 to schizophrenia and suggests a sex-dependent effect. Biochem. Biophys. Res. Commun. 2004, 319, 866–870. [Google Scholar] [CrossRef]
- Kakiuchi, C.; Ishiwata, M.; Umekage, T.; Tochigi, M.; Kohda, K.; Sasaki, T.; Kato, T. Association of the XBP1-116C/G polymorphism with schizophrenia in the Japanese population. Psychiatry Clin. Neurosci. 2004, 58, 438–440. [Google Scholar] [CrossRef]
- He, M.; Huang, X.F.; Gao, G.; Zhou, T.; Li, W.; Hu, J.; Chen, J.; Li, J.; Sun, T. Olanzapine-induced endoplasmic reticulum stress and inflammation in the hypothalamus were inhibited by an ER stress inhibitor 4-phenylbutyrate. Psychoneuroendocrinology 2019, 104, 286–299. [Google Scholar] [CrossRef]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef]
- Kroeger, H.; Chiang, W.C.; Felden, J.; Nguyen, A.; Lin, J.H. ER stress and unfolded protein response in ocular health and disease. Febs J. 2019, 286, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.; Gouveia, A.M.; Almeida, H. ER Stress Response in Human Cellular Models of Senescence. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Kang, T.I.; So, J.S. Roles of XBP1s in Transcriptional Regulation of Target Genes. Biomedicines 2021, 9, 791. [Google Scholar] [CrossRef]
- Kim, P.; Scott, M.R.; Meador-Woodruff, J.H. Dysregulation of the unfolded protein response (UPR) in the dorsolateral prefrontal cortex in elderly patients with schizophrenia. Mol. Psychiatry 2021, 26, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Dazzan, P. Neuroimaging biomarkers to predict treatment response in schizophrenia: The end of 30 years of solitude? Dialogues Clin. Neurosci. 2014, 16, 491–503. [Google Scholar] [CrossRef]
- Zipursky, R.B.; Zhang-Wong, J.; Lambe, E.K.; Bean, G.; Beiser, M. MRI correlates of treatment response in first episode psychosis. Schizophr. Res. 1998, 30, 81–90. [Google Scholar] [CrossRef]
- Bodnar, M.; Harvey, P.O.; Malla, A.K.; Joober, R.; Lepage, M. The parahippocampal gyrus as a neural marker of early remission in first-episode psychosis: A voxel-based morphometry study. Clin. Schizophr. Relat. Psychoses 2011, 4, 217–228. [Google Scholar] [CrossRef]
- Molina, V.; Martín, C.; Ballesteros, A.; de Herrera, A.G.; Hernández-Tamames, J.A. Optimized voxel brain morphometry: Association between brain volumes and the response to atypical antipsychotics. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 407–416. [Google Scholar] [CrossRef]
- Palaniyappan, L.; Marques, T.R.; Taylor, H.; Handley, R.; Mondelli, V.; Bonaccorso, S.; Giordano, A.; McQueen, G.; DiForti, M.; Simmons, A.; et al. Cortical folding defects as markers of poor treatment response in first-episode psychosis. JAMA Psychiatry 2013, 70, 1031–1040. [Google Scholar] [CrossRef]
- Honer, W.G.; Smith, G.N.; Lapointe, J.S.; MacEwan, G.W.; Kopala, L.; Altman, S. Regional cortical anatomy and clozapine response in refractory schizophrenia. Neuropsychopharmacology 1995, 13, 85–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friedman, L.; Knutson, L.; Shurell, M.; Meltzer, H.Y. Prefrontal sulcal prominence is inversely related to response to clozapine in schizophrenia. Biol. Psychiatry 1991, 29, 865–877. [Google Scholar] [CrossRef]
- Arango, C.; Breier, A.; McMahon, R.; Carpenter, W.T., Jr.; Buchanan, R.W. The relationship of clozapine and haloperidol treatment response to prefrontal, hippocampal, and caudate brain volumes. Am. J. Psychiatry 2003, 160, 1421–1427. [Google Scholar] [CrossRef]
- Molina, V.; Reig, S.; Sarramea, F.; Sanz, J.; Francisco Artaloytia, J.; Luque, R.; Aragüés, M.; Pascau, J.; Benito, C.; Palomo, T.; et al. Anatomical and functional brain variables associated with clozapine response in treatment-resistant schizophrenia. Psychiatry Res. 2003, 124, 153–161. [Google Scholar] [CrossRef]
- Anderson, V.M.; Goldstein, M.E.; Kydd, R.R.; Russell, B.R. Extensive gray matter volume reduction in treatment-resistant schizophrenia. Int. J. Neuropsychopharmacol. 2015, 18, pyv016. [Google Scholar] [CrossRef]
- Ahmed, M.; Cannon, D.M.; Scanlon, C.; Holleran, L.; Schmidt, H.; McFarland, J.; Langan, C.; McCarthy, P.; Barker, G.J.; Hallahan, B.; et al. Progressive Brain Atrophy and Cortical Thinning in Schizophrenia after Commencing Clozapine Treatment. Neuropsychopharmacology 2015, 40, 2409–2417. [Google Scholar] [CrossRef]
- Pang, T.S.W.; Chun, J.S.W.; Wong, T.Y.; Chu, S.T.; Ma, C.F.; Honer, W.G.; Chan, S.K.W. A systematic review of neuroimaging studies of clozapine-resistant schizophrenia. Schizophrenia 2023, 9, 65. [Google Scholar] [CrossRef]
- Torres-Carmona, E.; Ueno, F.; Iwata, Y.; Nakajima, S.; Song, J.; Mar, W.; Abdolizadeh, A.; Agarwal, S.M.; de Luca, V.; Remington, G.; et al. Elevated intrinsic cortical curvature in treatment-resistant schizophrenia: Evidence of structural deformation in functional connectivity areas and comparison with alternate indices of structure. Schizophr. Res. 2024, 269, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Garver, D.L.; Holcomb, J.A.; Christensen, J.D. Compromised myelin integrity during psychosis with repair during remission in drug-responding schizophrenia. Int. J. Neuropsychopharmacol. 2008, 11, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Luck, D.; Buchy, L.; Czechowska, Y.; Bodnar, M.; Pike, G.B.; Campbell, J.S.; Achim, A.; Malla, A.; Joober, R.; Lepage, M. Fronto-temporal disconnectivity and clinical short-term outcome in first episode psychosis: A DTI-tractography study. J. Psychiatr. Res. 2011, 45, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, S.; Zhang, B.; Zhao, G.; Zhang, Z.; Li, S.; Li, H.; Yu, X.; Deng, H.; Cao, H. Baseline symptom-related white matter tracts predict individualized treatment response to 12-week antipsychotic monotherapies in first-episode schizophrenia. Transl. Psychiatry 2024, 14, 23. [Google Scholar] [CrossRef]
- Reis Marques, T.; Taylor, H.; Chaddock, C.; Dell’acqua, F.; Handley, R.; Reinders, A.A.; Mondelli, V.; Bonaccorso, S.; Diforti, M.; Simmons, A.; et al. White matter integrity as a predictor of response to treatment in first episode psychosis. Brain 2014, 137, 172–182. [Google Scholar] [CrossRef]
- McNabb, C.B.; McIlwain, M.E.; Anderson, V.M.; Kydd, R.R.; Sundram, F.; Russell, B.R. Aberrant white matter microstructure in treatment-resistant schizophrenia. Psychiatry Res. Neuroimaging 2020, 305, 111198. [Google Scholar] [CrossRef]
- Molent, C.; Olivo, D.; Wolf, R.C.; Balestrieri, M.; Sambataro, F. Functional neuroimaging in treatment resistant schizophrenia: A systematic review. Neurosci. Biobehav. Rev. 2019, 104, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Dominicus, L.S.; van Rijn, L.; van der A, J.; van der Spek, R.; Podzimek, D.; Begemann, M.; de Haan, L.; van der Pluijm, M.; Otte, W.M.; Cahn, W.; et al. fMRI connectivity as a biomarker of antipsychotic treatment response: A systematic review. Neuroimage Clin. 2023, 40, 103515. [Google Scholar] [CrossRef]
- Vercammen, A.; Knegtering, H.; den Boer, J.A.; Liemburg, E.J.; Aleman, A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biol. Psychiatry 2010, 67, 912–918. [Google Scholar] [CrossRef]
- Wolf, N.D.; Sambataro, F.; Vasic, N.; Frasch, K.; Schmid, M.; Schönfeldt-Lecuona, C.; Thomann, P.A.; Wolf, R.C. Dysconnectivity of multiple resting-state networks in patients with schizophrenia who have persistent auditory verbal hallucinations. J. Psychiatry Neurosci. 2011, 36, 366–374. [Google Scholar] [CrossRef]
- Alonso-Solís, A.; Vives-Gilabert, Y.; Grasa, E.; Portella, M.J.; Rabella, M.; Sauras, R.B.; Roldán, A.; Núñez-Marín, F.; Gómez-Ansón, B.; Pérez, V.; et al. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr. Res. 2015, 161, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; Qin, X.; Zheng, F.; Wang, H. Altered functional connectivity in anterior cingulate cortex subregions in treatment-resistant schizophrenia patients. Neurosci. Lett. 2023, 814, 137445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, S.; Liang, C.; Bustillo, J.; Kochunov, P.; Turner, J.A.; Calhoun, V.D.; Wu, L.; Fu, Z.; Jiang, R.; et al. Consistent frontal-limbic-occipital connections in distinguishing treatment-resistant and non-treatment-resistant schizophrenia. Neuroimage Clin. 2025, 45, 103726. [Google Scholar] [CrossRef] [PubMed]
- Ganella, E.P.; Bartholomeusz, C.F.; Seguin, C.; Whittle, S.; Bousman, C.; Phassouliotis, C.; Everall, I.; Pantelis, C.; Zalesky, A. Functional brain networks in treatment-resistant schizophrenia. Schizophr. Res. 2017, 184, 73–81. [Google Scholar] [CrossRef]
- McNabb, C.B.; Tait, R.J.; McIlwain, M.E.; Anderson, V.M.; Suckling, J.; Kydd, R.R.; Russell, B.R. Functional network dysconnectivity as a biomarker of treatment resistance in schizophrenia. Schizophr. Res. 2018, 195, 160–167. [Google Scholar] [CrossRef]
- Hadley, J.A.; Kraguljac, N.V.; White, D.M.; Ver Hoef, L.; Tabora, J.; Lahti, A.C. Change in brain network topology as a function of treatment response in schizophrenia: A longitudinal resting-state fMRI study using graph theory. npj Schizophr. 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.X.; Guo, F.; Zhu, Y.Q.; Wang, H.N.; Liu, W.M.; Li, C.; Wang, X.R.; Cui, L.B.; Xi, Y.B.; Yin, H. Effect of second-generation antipsychotics on brain network topology in first-episode schizophrenia: A longitudinal rs-fMRI study. Schizophr. Res. 2019, 208, 160–166. [Google Scholar] [CrossRef]
- Zong, X.; Hu, M.; Pantazatos, S.P.; Mann, J.J.; Wang, G.; Liao, Y.; Liu, Z.C.; Liao, W.; Yao, T.; Li, Z.; et al. A Dissociation in Effects of Risperidone Monotherapy on Functional and Anatomical Connectivity Within the Default Mode Network. Schizophr. Bull. 2019, 45, 1309–1318. [Google Scholar] [CrossRef]
- Blessing, E.M.; Murty, V.P.; Zeng, B.; Wang, J.; Davachi, L.; Goff, D.C. Anterior Hippocampal-Cortical Functional Connectivity Distinguishes Antipsychotic Naïve First-Episode Psychosis Patients From Controls and May Predict Response to Second-Generation Antipsychotic Treatment. Schizophr. Bull. 2020, 46, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Skouras, S.; Kleinert, M.L.; Lee, E.H.M.; Hui, C.L.M.; Suen, Y.N.; Camchong, J.; Chong, C.S.Y.; Chang, W.C.; Chan, S.K.W.; Lo, W.T.L.; et al. Aberrant connectivity in the hippocampus, bilateral insula and temporal poles precedes treatment resistance in first-episode psychosis: A prospective resting-state functional magnetic resonance imaging study with connectivity concordance mapping. Brain Commun. 2024, 6, fcae094. [Google Scholar] [CrossRef]
- McNabb, C.B.; Sundram, F.; Soosay, I.; Kydd, R.R.; Russell, B.R. Increased sensorimotor network connectivity associated with clozapine eligibility in people with schizophrenia. Psychiatry Res. Neuroimaging 2018, 275, 36–42. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Hu, Y.; Wu, J.; Li, C.; Wang, J.; Yang, Z. Functional Connectivity Between Sensory-Motor Subnetworks Reflects the Duration of Untreated Psychosis and Predicts Treatment Outcome of First-Episode Drug-Naïve Schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 697–705. [Google Scholar] [CrossRef]
- Sarpal, D.K.; Argyelan, M.; Robinson, D.G.; Szeszko, P.R.; Karlsgodt, K.H.; John, M.; Weissman, N.; Gallego, J.A.; Kane, J.M.; Lencz, T.; et al. Baseline Striatal Functional Connectivity as a Predictor of Response to Antipsychotic Drug Treatment. Am. J. Psychiatry 2016, 173, 69–77. [Google Scholar] [CrossRef]
- Li, A.; Zalesky, A.; Yue, W.; Howes, O.; Yan, H.; Liu, Y.; Fan, L.; Whitaker, K.J.; Xu, K.; Rao, G.; et al. A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat. Med. 2020, 26, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Becker, B.; Duan, X.; Cui, Q.; Xin, F.; Zong, X.; Hu, M.; Yang, M.; Li, R.; Yu, Y.; et al. Distinct striatum pathways connected to salience network predict symptoms improvement and resilient functioning in schizophrenia following risperidone monotherapy. Schizophr. Res. 2020, 215, 89–96. [Google Scholar] [CrossRef]
- Nelson, E.A.; Kraguljac, N.V.; Maximo, J.O.; Armstrong, W.; Lahti, A.C. Dorsal striatial hypoconnectivity predicts antipsychotic medication treatment response in first-episode psychosis and unmedicated patients with schizophrenia. Brain Behav. 2022, 12, e2625. [Google Scholar] [CrossRef]
- White, T.P.; Wigton, R.; Joyce, D.W.; Collier, T.; Fornito, A.; Shergill, S.S. Dysfunctional Striatal Systems in Treatment-Resistant Schizophrenia. Neuropsychopharmacology 2016, 41, 1274–1285. [Google Scholar] [CrossRef]
- Blazer, A.; Chengappa, K.N.R.; Foran, W.; Parr, A.C.; Kahn, C.E.; Luna, B.; Sarpal, D.K. Changes in corticostriatal connectivity and striatal tissue iron associated with efficacy of clozapine for treatment-resistant schizophrenia. Psychopharmacology 2022, 239, 2503–2514. [Google Scholar] [CrossRef]
- van Veelen, N.M.; Vink, M.; Ramsey, N.F.; van Buuren, M.; Hoogendam, J.M.; Kahn, R.S. Prefrontal lobe dysfunction predicts treatment response in medication-naive first-episode schizophrenia. Schizophr. Res. 2011, 129, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Bourque, J.; Lakis, N.; Champagne, J.; Stip, E.; Lalonde, P.; Lipp, O.; Mendrek, A. Clozapine and visuospatial processing in treatment-resistant schizophrenia. Cogn. Neuropsychiatry 2013, 18, 615–630. [Google Scholar] [CrossRef] [PubMed]
- Potvin, S.; Tikàsz, A.; Lungu, O.; Dumais, A.; Stip, E.; Mendrek, A. Emotion processing in treatment-resistant schizophrenia patients treated with clozapine: An fMRI study. Schizophr. Res. 2015, 168, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Cadena, E.J.; White, D.M.; Kraguljac, N.V.; Reid, M.A.; Jindal, R.; Pixley, R.M.; Lahti, A.C. Cognitive control network dysconnectivity and response to antipsychotic treatment in schizophrenia. Schizophr. Res. 2019, 204, 262–270. [Google Scholar] [CrossRef]
- Roldán, A.; Portella, M.J.; Sampedro, F.; Alonso-Solís, A.; Sarró, S.; Rabella, M.; Grasa, E.M.; Álvarez, E.; Rodríguez, R.; Camacho, V.; et al. Brain metabolic changes in patients with treatment resistant schizophrenia treated with deep brain stimulation: A series of cases. J. Psychiatr. Res. 2020, 127, 57–61. [Google Scholar] [CrossRef]
- Iasevoli, F.; D’Ambrosio, L.; Ciccarelli, M.; Barone, A.; Gaudieri, V.; Cocozza, S.; Pontillo, G.; Brunetti, A.; Cuocolo, A.; de Bartolomeis, A.; et al. Altered Patterns of Brain Glucose Metabolism Involve More Extensive and Discrete Cortical Areas in Treatment-resistant Schizophrenia Patients Compared to Responder Patients and Controls: Results From a Head-to-Head 2-[18F]-FDG-PET Study. Schizophr. Bull. 2023, 49, 474–485. [Google Scholar] [CrossRef]
- De Simone, G.; Iasevoli, F.; Barone, A.; Gaudieri, V.; Cuocolo, A.; Ciccarelli, M.; Pappatà, S.; de Bartolomeis, A. Addressing brain metabolic connectivity in treatment-resistant schizophrenia: A novel graph theory-driven application of (18)F-FDG-PET with antipsychotic dose correction. Schizophrenia 2024, 10, 116. [Google Scholar] [CrossRef]
- Veronese, M.; Santangelo, B.; Jauhar, S.; D’Ambrosio, E.; Demjaha, A.; Salimbeni, H.; Huajie, J.; McCrone, P.; Turkheimer, F.; Howes, O. A potential biomarker for treatment stratification in psychosis: Evaluation of an [(18)F] FDOPA PET imaging approach. Neuropsychopharmacology 2021, 46, 1122–1132. [Google Scholar] [CrossRef]
- Chestnykh, D.A.; Amato, D.; Kornhuber, J.; Müller, C.P. Pharmacotherapy of schizophrenia: Mechanisms of antipsychotic accumulation, therapeutic action and failure. Behav. Brain Res. 2021, 403, 113144. [Google Scholar] [CrossRef]
- Kristensen, M.W.P.; Biuk, B.; Nielsen, J.; Bojesen, K.B.; Nielsen, M. Glutamate, GABA and NAA in treatment-resistant schizophrenia: A systematic review of the effect of clozapine and group differences between clozapine-responders and non-responders. Behav. Brain Res. 2025, 479, 115338. [Google Scholar] [CrossRef]
- Lopes, J.J.; Carruthers, S.P.; Meyer, D.; Dean, B.; Rossell, S.L. Glutamatergic neurotransmission in schizophrenia: A systematic review and quantitative synthesis of proton magnetic resonance spectroscopy studies across schizophrenia spectrum disorders. Aust. N. Z. J. Psychiatry 2024, 58, 930–951. [Google Scholar] [CrossRef]
- Torres-Carmona, E.; Nakajima, S.; Iwata, Y.; Ueno, F.; Stefan, C.; Song, J.; Abdolizadeh, A.; Koizumi, M.T.; Kambari, Y.; Amaev, A.; et al. Clozapine treatment and astrocyte activity in treatment resistant schizophrenia: A proton magnetic resonance spectroscopy study. Schizophr. Res. 2024, 270, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Dienel, G.A.; Behar, K.L.; Rothman, D.L. Cellular Origin of [(18)F]FDG-PET Imaging Signals During Ceftriaxone-Stimulated Glutamate Uptake: Astrocytes and Neurons. Neuroscientist 2018, 24, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.H.; Kim, Y.K.; Choi, I.G.; Kim, H.J.; Son, Y.D.; Kim, H.K.; Cumming, P.; Kim, J.H. In vivo glucose metabolism and glutamate levels in mGluR5 knockout mice: A multimodal neuroimaging study using [(18)F]FDG microPET and MRS. EJNMMI Res. 2020, 10, 116. [Google Scholar] [CrossRef]
- Magistretti, P.J.; Pellerin, L. The contribution of astrocytes to the 18F-2-deoxyglucose signal in PET activation studies. Mol. Psychiatry 1996, 1, 445–452. [Google Scholar] [PubMed]
- Naidoo, C.; Jialal, I.; Joubert, S.M. Is there a defect in the hepatic extraction of insulin in patients with NIDDY? Diabetes Care 1986, 9, 96–97. [Google Scholar] [CrossRef]
- Almulla, A.F.; Maes, M. Peripheral Immune-Inflammatory Pathways in Major Depressive Disorder, Bipolar Disorder, and Schizophrenia: Exploring Their Potential as Treatment Targets. CNS Drugs 2025, 39, 739–762. [Google Scholar] [CrossRef]
- Liu, Y.D.; Chang, Y.H.; Xie, X.T.; Wang, X.Y.; Ma, H.Y.; Liu, M.C.; Zhang, H.M. PET Imaging Unveils Neuroinflammatory Mechanisms in Psychiatric Disorders: From Microglial Activation to Therapeutic Innovation. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Šafářová, N.; Kolenič, M.; Španiel, F. Beyond dopamine: Exploring anti-inflammatory mechanisms of antipsychotics. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 141, 111459. [Google Scholar] [CrossRef]
- Sendi, M.S.E.; Itkyal, V.S.; Edwards-Swart, S.J.; Chun, J.Y.; Mathalon, D.H.; Ford, J.M.; Preda, A.; van Erp, T.G.M.; Pearlson, G.D.; Turner, J.A.; et al. Visualizing functional network connectivity differences using an explainable machine-learning method. Physiol. Meas. 2025, 46, 045009. [Google Scholar] [CrossRef]
- Moumgiakmas, S.S.; Vrochidou, E.; Papakostas, G.A. Mapping the brain: AI-driven radiomic approaches to mental disorders. Artif. Intell. Med. 2025, 168, 103219. [Google Scholar] [CrossRef] [PubMed]
- Omlor, W.; Rabe, F.; Fuchs, S.; Surbeck, W.; Cecere, G.; Huang, G.Y.; Homan, S.; Kallen, N.; Georgiadis, F.; Spiller, T.; et al. Estimating Multimodal Structural Brain Variability in Schizophrenia Spectrum Disorders: A Worldwide ENIGMA Study. Am. J. Psychiatry 2025, 182, 373–388. [Google Scholar] [CrossRef] [PubMed]
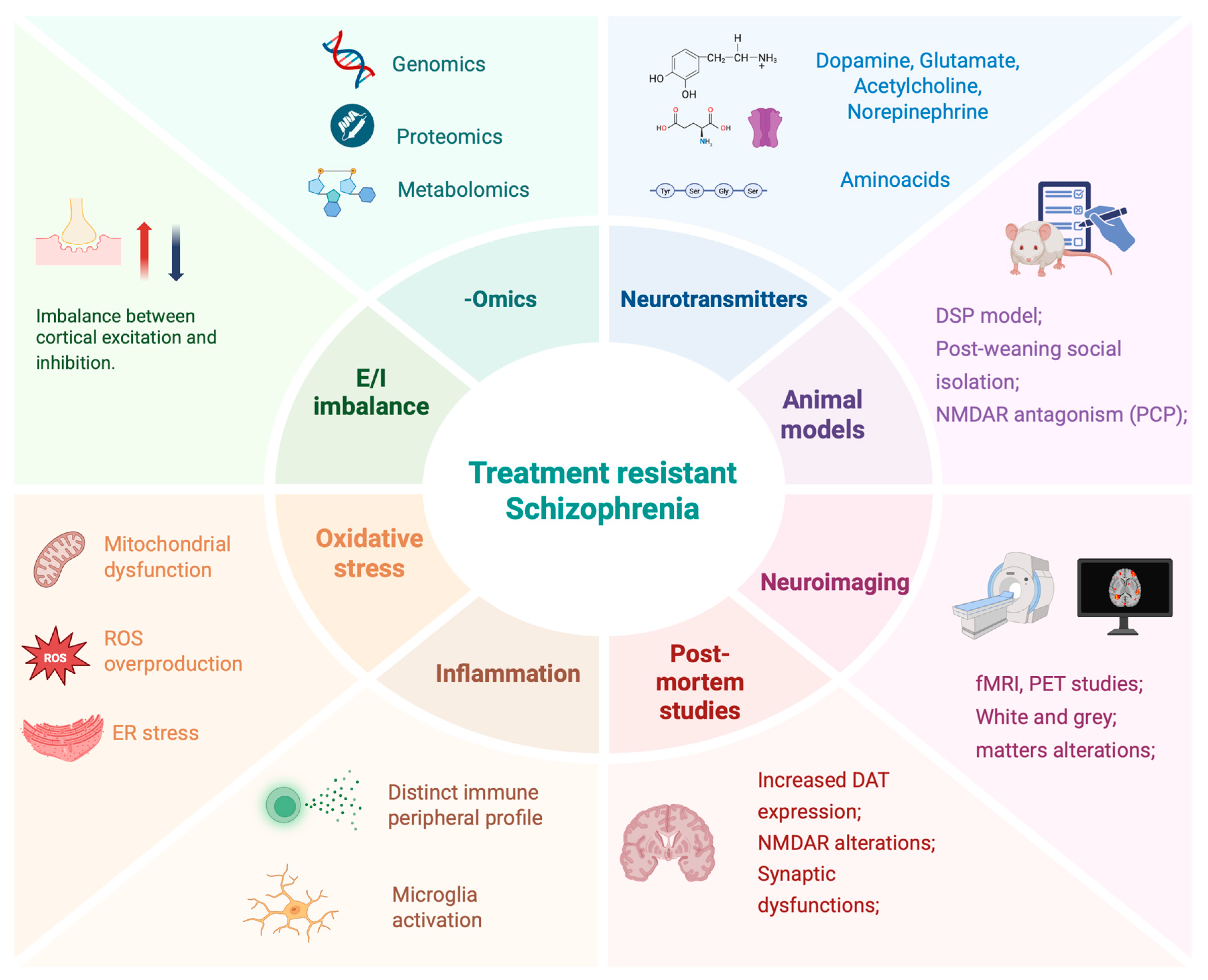
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, A.; Vellucci, L.; Ciccarelli, M.; Matrone, M.; De Simone, G.; Iannotta, F.; Iasevoli, F.; de Bartolomeis, A. Molecular Underpinning of Treatment-Resistant Schizophrenia: A Putative Different Neurobiology from Treatment-Responsive Schizophrenia. Int. J. Mol. Sci. 2025, 26, 8598. https://doi.org/10.3390/ijms26178598
Barone A, Vellucci L, Ciccarelli M, Matrone M, De Simone G, Iannotta F, Iasevoli F, de Bartolomeis A. Molecular Underpinning of Treatment-Resistant Schizophrenia: A Putative Different Neurobiology from Treatment-Responsive Schizophrenia. International Journal of Molecular Sciences. 2025; 26(17):8598. https://doi.org/10.3390/ijms26178598
Chicago/Turabian StyleBarone, Annarita, Licia Vellucci, Mariateresa Ciccarelli, Marta Matrone, Giuseppe De Simone, Federica Iannotta, Felice Iasevoli, and Andrea de Bartolomeis. 2025. "Molecular Underpinning of Treatment-Resistant Schizophrenia: A Putative Different Neurobiology from Treatment-Responsive Schizophrenia" International Journal of Molecular Sciences 26, no. 17: 8598. https://doi.org/10.3390/ijms26178598
APA StyleBarone, A., Vellucci, L., Ciccarelli, M., Matrone, M., De Simone, G., Iannotta, F., Iasevoli, F., & de Bartolomeis, A. (2025). Molecular Underpinning of Treatment-Resistant Schizophrenia: A Putative Different Neurobiology from Treatment-Responsive Schizophrenia. International Journal of Molecular Sciences, 26(17), 8598. https://doi.org/10.3390/ijms26178598










