Changes in Placentas of Pregnant Women Infected with COVID-19
Abstract
1. Introduction
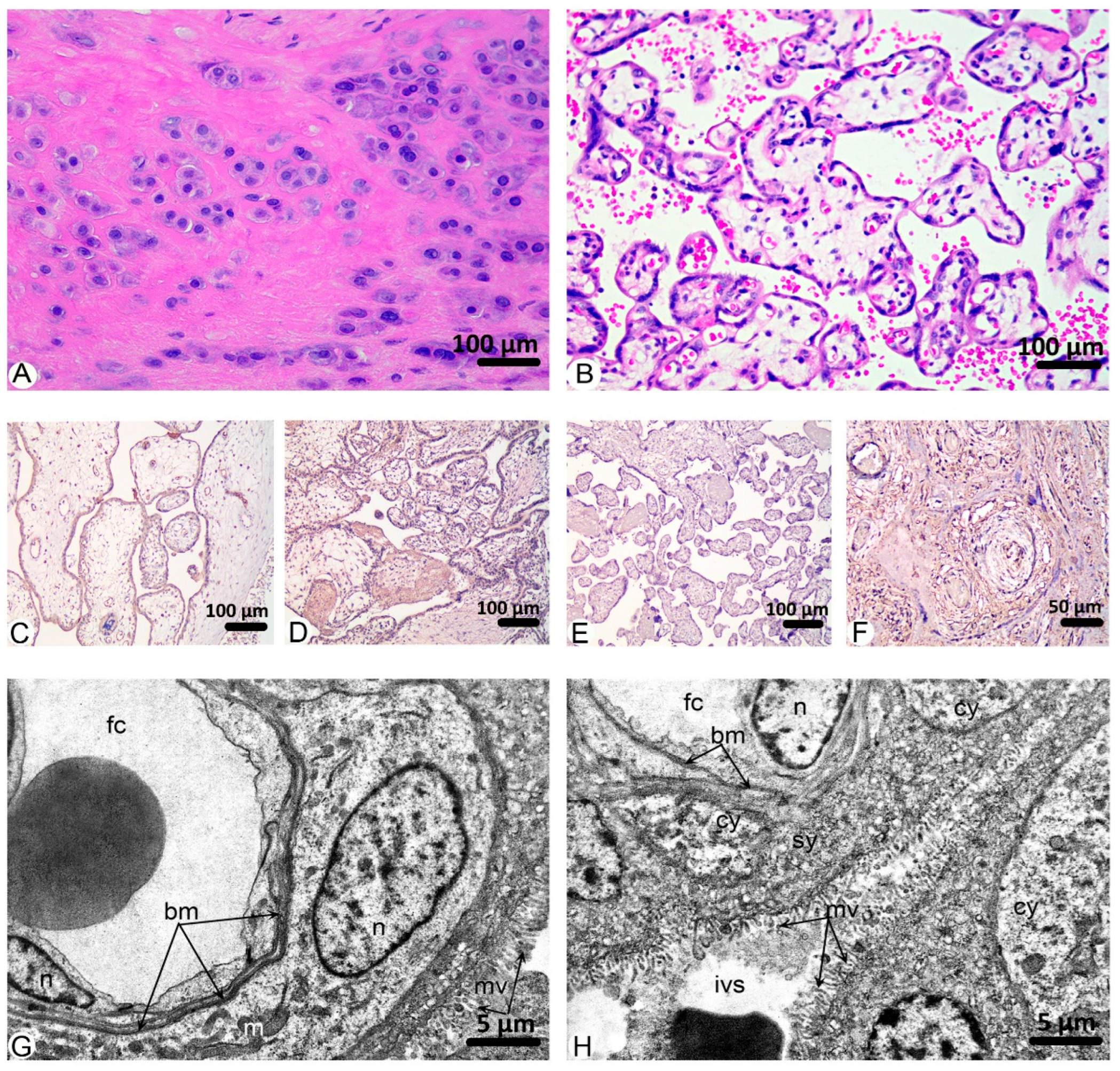
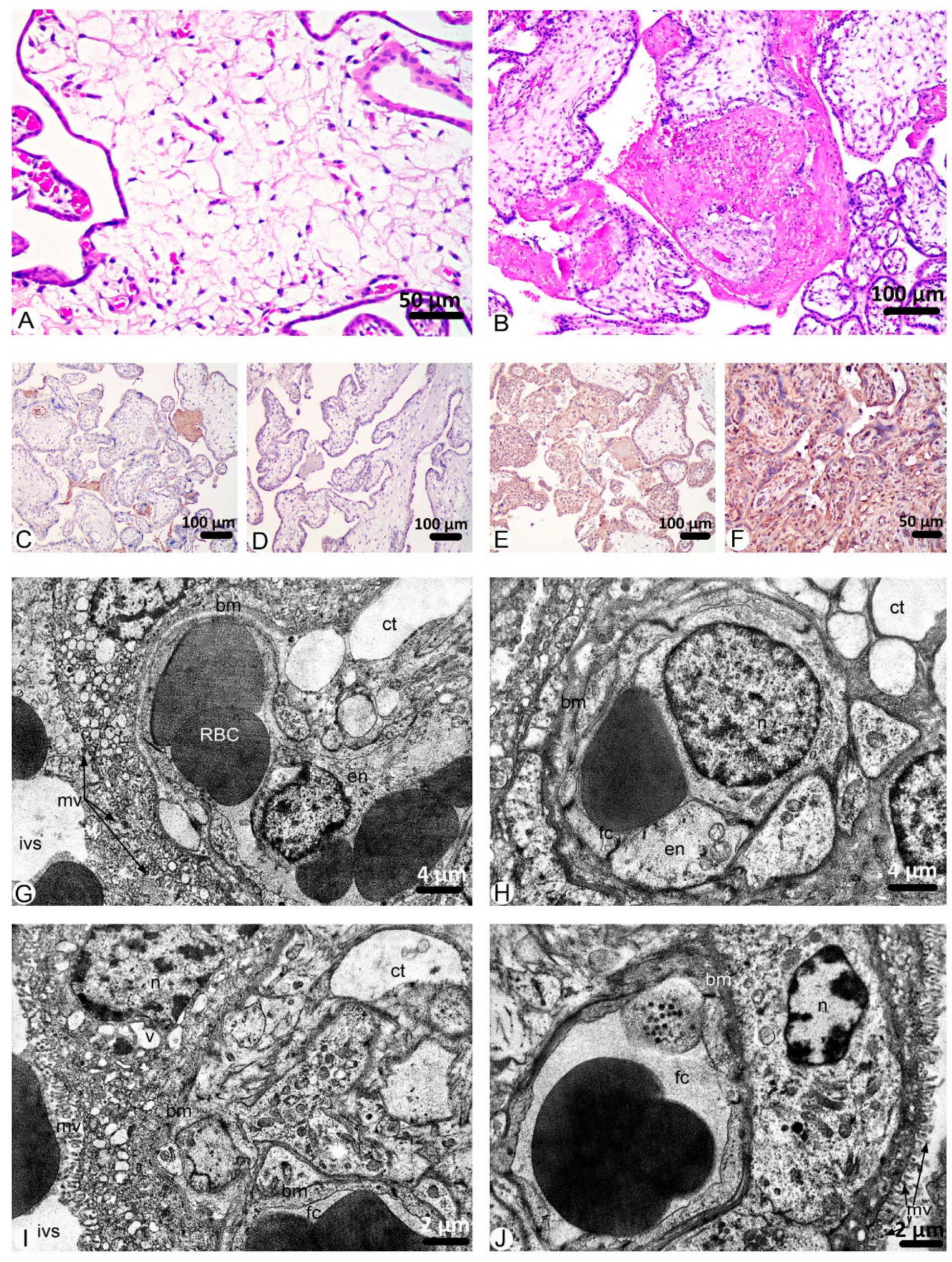
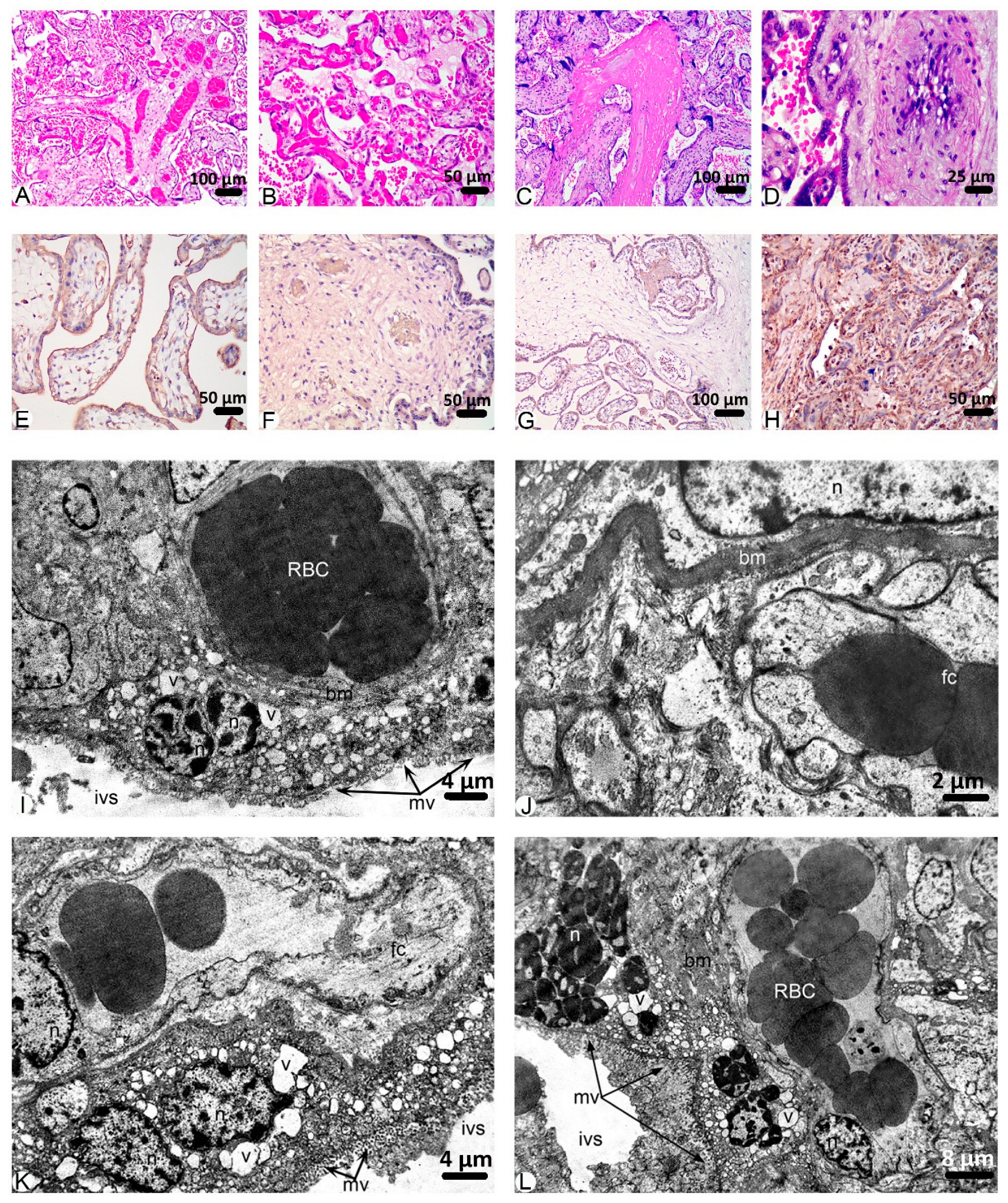
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Histology
4.3. Immunohistochemistry
4.4. Electron Microscopy
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babajani, A.; Moeinabadi-Bidgoli, K.; Niknejad, F.; Rismanchi, H.; Shafiee, S.; Shariatzadeh, S.; Jamshidi, E.; Farjoo, M.H.; Niknejad, H. Human placenta-derived amniotic epithelial cells as a new therapeutic hope for COVID-19-associated acute respiratory distress syndrome (ARDS) and systemic inflammation. Stem Cell Res. Ther. 2022, 13, 126. [Google Scholar] [CrossRef]
- Kumar, D.; Verma, S.; Mysorekar, I.U. COVID-19 and pregnancy: Clinical outcomes; mechanisms, and vaccine efficacy. Transl. Res. 2023, 251, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, A.; Szczepański, A.; Barreto-Duran, E.; Lie, L.K.; Pyrc, K. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Systemic Infection. Clin. Microbiol. Rev. 2021, 34, e00133-20. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Petrolini, C.; Corradini, E.; Campanini, N.; Esposito, S.; Perrone, S. COVID-19 in pregnancy: Placental pathological patterns and effect on perinatal outcome in five cases. Diagn. Pathol. 2021, 16, 88. [Google Scholar] [CrossRef]
- WHO Data. WHO COVID-19 Dashboard. 2024. Available online: https://data.who.int/dashboards/covid19/deaths?n=o/ (accessed on 24 December 2024).
- Seyed Alinaghi, S.; Afsahi, A.M.; Mohsseni Pour, M.; Behnezhad, F.; Salehi, M.A.; Barzegary, A.; Mirzapour, P.; Mehraeen, E.; Dadras, O. Late Complications of COVID-19; a Systematic Review of Current Evidence. Arch. Acad. Emerg. Med. 2021, 9, e14. [Google Scholar] [CrossRef]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J. COVID-19 and Pregnancy. Infect. Dis. Clin. N. Am. 2022, 36, 423–433. [Google Scholar] [CrossRef]
- Brioschi Dos Santos, A.P.; Vicente, C.R.; Cola, J.P.; Tanaka, L.F.; Garbin, J.R.T.; Dell’Antonio, L.S.; Dell’Antonio, C.S.D.S.; Miranda, A.E. The impact of COVID-19 on maternal death and fetal death, a cohort study in Brazil. PLoS ONE 2023, 18, e0290343. [Google Scholar] [CrossRef]
- Zaigham, M.; Gisselsson, D.; Sand, A.; Wikström, A.K.; von Wowern, E.; Schwartz, D.A.; Iorizzo, L.; Nelander, M.; Blomberg, M.; Papadogiannakis, N.; et al. Clinical-pathological features in placentas of pregnancies with SARS-CoV-2 infection and adverse outcome: Case series with and without congenital transmission. BJOG Int. J. Obstet. Gynaecol. 2022, 129, 1361–1374. [Google Scholar] [CrossRef]
- Magnus, M.C.; Örtqvist, A.K.; Urhoj, S.K.; Aabakke, A.; Mortensen, L.H.; Gjessing, H.; Nybo Andersen, A.-M.; Stephansson, O.; Håberg, S.E. Infection with SARS-CoV-2 during pregnancy and risk of stillbirth: A Scandinavian registry study. BMJ Public Health 2023, 1, e000314. [Google Scholar] [CrossRef] [PubMed]
- DeSisto, C.L.; Wallace, B.; Simeone, R.M.; Polen, K.; Ko, J.Y.; Meaney-Delman, D.; Ellington, S.R. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States. Morb. Mortal. Wkly. Rep. 2020, 70, 1640–1645. [Google Scholar] [CrossRef]
- Cromb, D.; Hall, M.; Story, L.; Shangaris, P.; Al-Adnani, M.; Rutherford, M.A.; Fox, G.F.; Gupta, N. Clinical value of placental examination for paediatricians. Arch. Dis. Child. Fetal Neonatal Ed. 2024, 109, 362–370. [Google Scholar] [CrossRef]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32. [Google Scholar] [CrossRef]
- Schwartz, D.A. Stillbirth after COVID-19 in Unvaccinated Mothers Can Result from SARS-CoV-2 Placentitis, Placental Insufficiency, and Hypoxic Ischemic Fetal Demise, Not Direct Fetal Infection: Potential Role of Maternal Vaccination in Pregnancy. Viruses 2022, 14, 458. [Google Scholar] [CrossRef]
- Kummer, J.; Ameli, G.; Jebens, A.; Königbauer, J.; Mihajlov, V.; Nacke, A.K. Covid-19 during Pregnancy—Histopathological Lesions of the Placenta. Z. Geburtshilfe Neonatol. 2024, 228, 49–56. [Google Scholar] [CrossRef]
- Dubucs, C.; Groussolles, M.; Ousselin, J.; Sartor, A.; Van Acker, N.; Vayssière, C.; Ville, Y. Severe placental lesions due to maternal SARS-CoV-2 infection associated to intrauterine fetal death. Hum. Pathol. 2022, 121, 46–55. [Google Scholar] [CrossRef]
- Hecht, J.L.; Quade, B.; Deshpande, V.; Mino-Kenudson, M.; Ting, D.T.; Desai, N.; Dygulska, B.; Heyman, T.; Salafia, C.; Shen, D.; et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: A series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020, 33, 2092–2103. [Google Scholar] [CrossRef] [PubMed]
- Smithgall, M.C.; Liu-Jarin, X.; Hamele-Bena, D.; Cimic, A.; Mourad, M.; Debelenko, L.; Chen, X. Third-trimester placentas of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive women: Histomorphology, including viral immunohistochemistry and in-situ hybridization. Histopathology 2020, 77, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ren, J.; Xu, L.; Ke, X.; Xiong, L.; Tian, X.; Fan, C.; Yan, H.; Yuan, J. Placental pathology of the third trimester pregnant women from COVID-19. Diagn. Pathol. 2021, 16, 8. [Google Scholar] [CrossRef]
- Hsu, A.L.; Guan, M.; Johannesen, E.; Stephens, A.J.; Khaleel, N.; Kagan, N.; Tuhlei, B.C.; Wan, X.-F. Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease. J. Med. Virol. 2021, 93, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.K.; Lee, S.Y.; Park, S.; Ryoo, N.H.; Hwang, I.; Park, J.M.; Bae, J.-G. Pregnancy outcome, antibodies, and placental pathology in SARS-CoV-2 infection during early pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 5709. [Google Scholar] [CrossRef]
- Menter, T.; Mertz, K.D.; Jiang, S.; Chen, H.; Monod, C.; Tzankov, A.; Waldvogel, S.; Schulzke, S.M.; Hösli, I.; Bruder, E. Placental pathology findings during and after SARS-CoV-2 infection: Features of villitis and malperfusion. Pathobiology 2021, 88, 69–77. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Baldewijns, M.; Benachi, A.; Bugatti, M.; Collins, R.R.; De Luca, D.; Facchetti, F.; Linn, R.L.; Marcelis, L.; Morotti, D.; et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (COVID-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission in live-born and stillborn infants. Arch. Pathol. Lab. Med. 2021, 145, 517–528. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Avvad-Portari, E.; Babál, P.; Baldewijns, M.; Blomberg, M.; Bouachba, A.; Camacho, J.; Collardeau-Frachon, S.; Colson, A.; Dehaene, I.; et al. Placental tissue destruction and insufficiency from COVID-19 causes stillbirth and neonatal death from hypoxic-ischemic injury: A study of 68 cases with SARS-CoV-2 placentitis from 12 countries. Arch. Pathol. Lab. Med. 2022, 146, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Heeralall, C.; Ibrahim, U.H.; Lazarus, L.; Gathiram, P.; Mackraj, I. The effects of COVID-19 on placental morphology. Placenta 2023, 138, 88–96. [Google Scholar] [CrossRef]
- Facchetti, F.; Bugatti, M.; Drera, E.; Tripodo, C.; Sartori, E.; Cancila, V.; Papaccio, M.; Castellani, R.; Casola, S.; Boniotti, M.B.; et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. eBioMedicine 2020, 59, 102951. [Google Scholar] [CrossRef] [PubMed]
- Rakheja, D.; Treat, K.; Timmons, C.F.; Carrillo, D.; Miller, S.E.; Stroberg, E.; Barton, L.M.; Duval, E.J.; Mukhopadhyay, S. SARS-CoV-2 Immunohistochemistry in Placenta. Int. J. Surg. Pathol. 2022, 30, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Parcial, A.L.N.; Salomão, N.G.; Portari, E.A.; Arruda, L.V.; de Carvalho, J.J.; de Matos Guedes, H.L.; Conde, T.C.; Moreira, M.E.; Batista, M.M.; Paes, M.V.; et al. SARS-CoV-2 Is Persistent in Placenta and Causes Macroscopic, Histopathological, and Ultrastructural Changes. Viruses 2022, 14, 1885. [Google Scholar] [CrossRef]
- Komine-Aizawa, S.; Takada, K.; Hayakawa, S. Placental barrier against COVID-19. Placenta 2020, 99, 45–49. [Google Scholar] [CrossRef]
- Wastnedge, E.A.; Reynolds, R.M.; van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318. [Google Scholar] [CrossRef]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.F.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS–CoV-2 infection of the placenta. J. Clin. Investig. 2020, 130, 4947–4953. [Google Scholar] [CrossRef]
- Prochaska, E.; Jang, M.; Burd, I. COVID-19 in pregnancy: Placental and neonatal involvement. Am. J. Reprod. Immunol. 2020, 84, e13306. [Google Scholar] [CrossRef] [PubMed]
- Vari, S.G.; Shevchuk, O.; Boychuk, A.; Kramar, S.; Nebesna, Z.; Yakymchuk, Y.; Kobylinska, L.; Chernyshenko, V.; Korolova, D.; Gaspar-Suranyi, A.; et al. Common mechanisms of placental dysfunction in preeclampsia, gestational diabetes, and COVID-19 in pregnant women. Ukr. Biochem. J. 2023, 95, 5–11. [Google Scholar] [CrossRef]
- Burton, G.J.; Charnock-Jones, D.S.; Jauniaux, E. Regulation of vascular growth and function in the human placenta. Reproduction 2009, 138, 895–902. [Google Scholar] [CrossRef]
- Zhou, Y.; McMaster, M.; Woo, K.; Janatpour, M.; Perry, J.; Karpanen, T.; Alitalo, K.; Damsky, C.; Fisher, S.J. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am. J. Pathol. 2002, 160, 1405–1423. [Google Scholar] [CrossRef]
- Chen, D.B.; Zheng, J. Regulation of placental angiogenesis. Microcirculation 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Yazihan, N.; Tanacan, A.; Erol, S.A.; Anuk, A.T.; Sinaci, S.; Biriken, D.; Keskin, H.L.; Moraloglu, O.T.; Sahin, D. Comparison of VEGF-A values between pregnant women with COVID-19 and healthy pregnancies and its association with composite adverse outcomes. J. Med. Virol. 2021, 93, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226 (Suppl. S2), S1019–S1034. [Google Scholar] [CrossRef]
- De Falco, S. The discovery of placenta growth factor and its biological activity. Exp. Mol. Med. 2012, 44, 1–9. [Google Scholar] [CrossRef]
- Chau, K.; Hennessy, A.; Makris, A. Placental growth factor and pre-eclampsia. J. Hum. Hypertens. 2017, 31, 782–786. [Google Scholar] [CrossRef]
- Wang, X.; Yip, K.C.; He, A.; Tang, J.; Liu, S.; Yan, R.; Zhang, Q.; Li, R. Plasma Olink Proteomics Identifies CCL20 as a Novel Predictive and Diagnostic Inflammatory Marker for Preeclampsia. J. Proteome Res. 2022, 21, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Chen, Y. Effect of PLGF and EZH2 Expression in Placenta Tissue on GDM Placental Structure and Pregnancy Outcome. Altern. Ther. Health Med. 2024, 30, 206–211. [Google Scholar]
- Smadja, D.M.; Philippe, A.; Bory, O.; Gendron, N.; Beauvais, A.; Gruest, M.; Peron, N.; Khider, L.; Guérin, C.L.; Goudot, G.; et al. Placental growth factor level in plasma predicts COVID-19 severity and in-hospital mortality. J. Thromb. Haemost. 2021, 19, 1823–1830. [Google Scholar] [CrossRef]
- Kosinska-Kaczynska, K.; Malicka, E.; Szymusik, I.; Dera, N.; Pruc, M.; Feduniw, S.; Rafique, Z.; Szarpak, Ł. The sFlt-1/PlGF Ratio in Pregnant Patients Affected by COVID-19. J. Clin. Med. 2023, 12, 1059. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Mui, L.; Martin, C.M.; Tschirhart, B.J.; Feng, Q. Therapeutic Potential of Annexins in Sepsis and COVID-19. Front. Pharmacol. 2021, 12, 735472. [Google Scholar] [CrossRef]
- Liu, W.; Hajjar, K.A. The Annexin A2 System and Angiogenesis. Biol. Chem. 2016, 397, 1005–1016. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Jiang, M.; Dai, L.; Zhang, W.; Wu, D.; Ruan, C. Expression of Annexin II and Its Role in the Fibrinolytic Activity in Acute Promyelocytic Leukemia. Leuk. Res. 2011, 35, 879–884. [Google Scholar] [CrossRef]
- Fassel, H.; Chen, H.; Ruisi, M.; Kumar, N.; DeSancho, M.; Hajjar, K.A. Reduced Expression of Annexin A2 Is Associated with Impaired Cell Surface Fibrinolysis and Venous Thromboembolism. Blood 2021, 137, 2221–2230. [Google Scholar] [CrossRef]
- Patil, P.; Shetty, P.; Kuriakose, N.; Gollapalli, P.; Shetty, S.; Bhandary, R.; Vishwanatha, J.K.; Ghate, S.D. Molecular Insights on the Possible Role of Annexin A2 in COVID-19 Pathogenesis and Post-Infection Complications. Int. J. Mol. Sci. 2021, 22, 11028. [Google Scholar] [CrossRef]
- Balemans, W.; Ebeling, M.; Patel, N.; Van Hul, E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; Van Den Ende, J.; Willems, P.J.; et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef]
- Costa, A.G.; Cremers, S.; Bilezikian, J.P. Sclerostin measurement in human disease: Validity and current limitations. Bone 2017, 96, 24–28. [Google Scholar] [CrossRef]
- Catalano, A.; Bellone, F.; Morabito, N.; Corica, F. Sclerostin and Vascular Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4779. [Google Scholar] [CrossRef]
- Tobias, J.H. Sclerostin and Cardiovascular Disease. Curr. Osteoporos. Rep. 2023, 21, 519–526. [Google Scholar] [CrossRef]
- Bruzzese, A.; Lacquaniti, A.; Cernaro, V.; Ricciardi, C.A.; Loddo, S.; Romeo, A.; Montalto, G.; Costantino, G.; Torre, F.; Pettinato, G.; et al. Sclerostin levels in uremic patients: A link between bone and vascular disease. Ren. Fail. 2016, 38, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Thymara, E.; Maratou, E.; Kanellopoulos, G.; Papaevangelou, V.; Kalantaridou, S.; Kanellakis, S.; Triantafyllidou, P.; Valsamakis, G.; Mastorakos, G. Human Placental LRP5 and Sclerostin are Increased in Gestational Diabetes Mellitus Pregnancies. J. Clin. Endocrinol. Metab. 2023, 108, 2666–2675. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K.; Dovjak, P.; Butylina, M.; Rainer, A.; Mayr, B.; Röggla, V.; Haslacher, H.; Weber, M.; Jordakieva, G.; Pietschmann, P. Moderate COVID-19 Disease Is Associated with Reduced Bone Turnover. J. Bone Miner. Res. 2023, 38, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Castro, J.; Toledano, J.M.; Sanchez-Romero, J.; Aguilar, A.C.; Martín-Alvarez, E.; Puche-Juarez, M.; Moreno-Fernández, J.; Pinar-González, M.; Prados, S.; Carrillo, M.P.; et al. COVID-19 and Pregnancy: A Dangerous Mix for Bone Turnover and Metabolism Biomarkers in Placenta and Colostrum. J. Clin. Med. 2024, 13, 2124. [Google Scholar] [CrossRef] [PubMed]


| Group | Characteristics | Number of Patients |
|---|---|---|
| Control | pregnant COVID-19-negative women with a physiological course of pregnancy | 10 |
| Group I | patients who were diagnosed with COVID-19 during the 1st trimester of pregnancy; miscarriage | 10 |
| Group II | patients who were diagnosed with COVID-19 during the 2nd trimester of pregnancy | 15 |
| Group III | patients who were diagnosed with COVID-19 during the 3rd trimester of pregnancy | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramar, S.; Nebesna, Z.; Yakymchuk, Y.; Boychuk, A.; Shevchuk, O.; Korda, M.; Vari, S.G. Changes in Placentas of Pregnant Women Infected with COVID-19. Int. J. Mol. Sci. 2025, 26, 8596. https://doi.org/10.3390/ijms26178596
Kramar S, Nebesna Z, Yakymchuk Y, Boychuk A, Shevchuk O, Korda M, Vari SG. Changes in Placentas of Pregnant Women Infected with COVID-19. International Journal of Molecular Sciences. 2025; 26(17):8596. https://doi.org/10.3390/ijms26178596
Chicago/Turabian StyleKramar, Solomiia, Zoia Nebesna, Yuliia Yakymchuk, Alla Boychuk, Oksana Shevchuk, Mykhaylo Korda, and Sandor George Vari. 2025. "Changes in Placentas of Pregnant Women Infected with COVID-19" International Journal of Molecular Sciences 26, no. 17: 8596. https://doi.org/10.3390/ijms26178596
APA StyleKramar, S., Nebesna, Z., Yakymchuk, Y., Boychuk, A., Shevchuk, O., Korda, M., & Vari, S. G. (2025). Changes in Placentas of Pregnant Women Infected with COVID-19. International Journal of Molecular Sciences, 26(17), 8596. https://doi.org/10.3390/ijms26178596






