Nup153 and TPR/Megator Interact with TREX-2 Subunits and Are Essential for TREX-2-Dependent Nuclear Export of hsp70 mRNA in Drosophila
Abstract
1. Introduction
2. Results
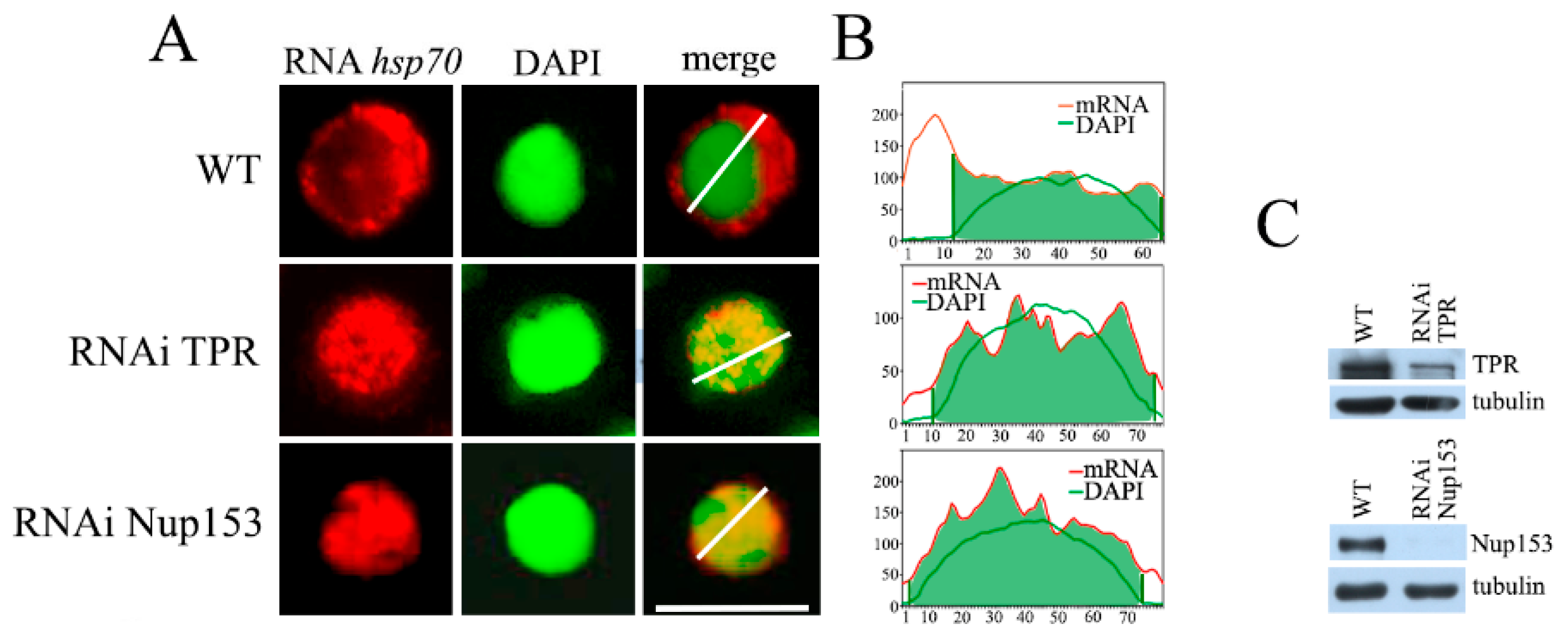
2.1. TPR and Nup153 Are Required for TREX-2-Dependent Export of hsp70 mRNA
2.2. Nup153 Knockdown Alters TPR Localization
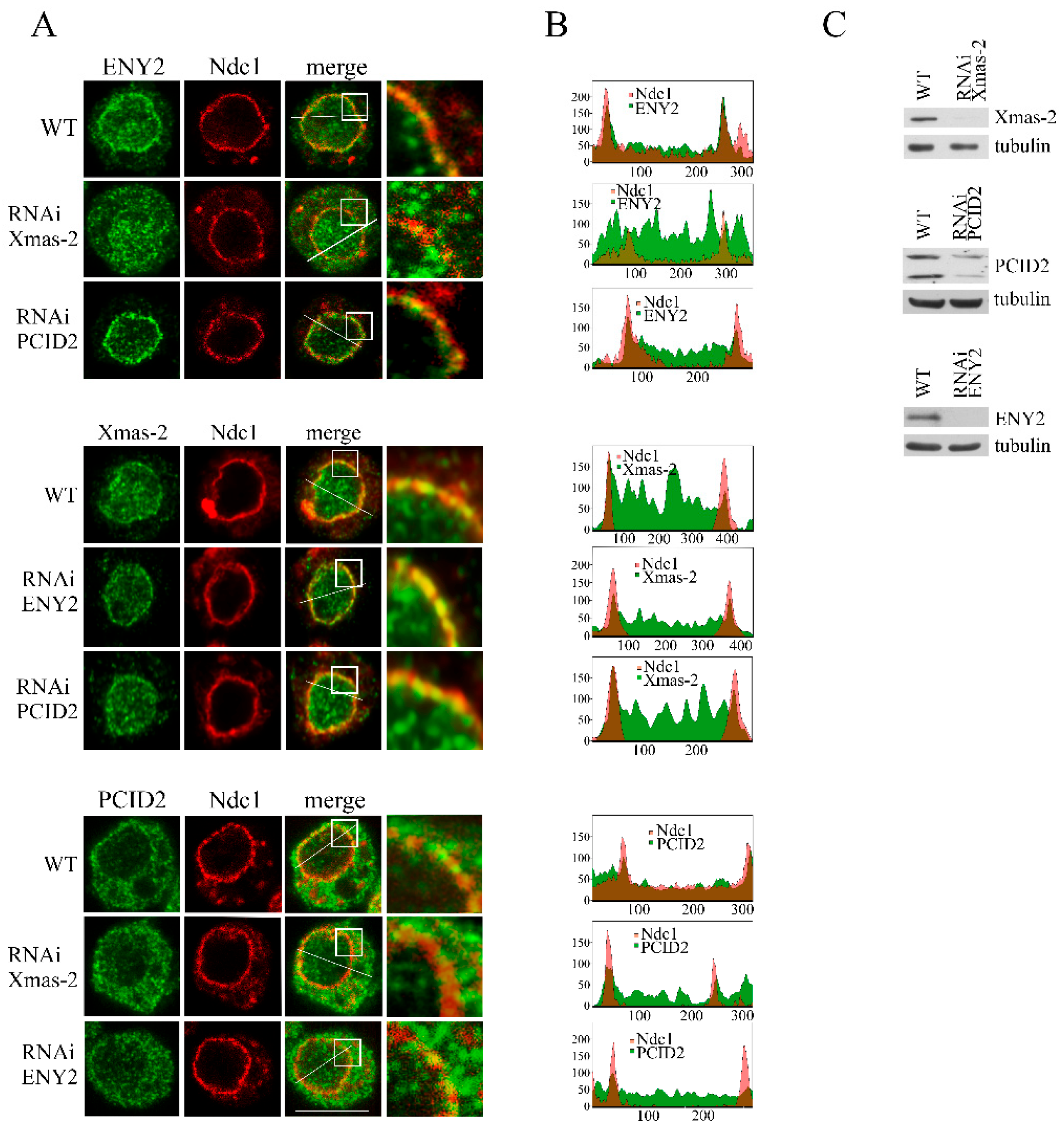
2.3. TPR and Nup153 Knockdown Disrupts the Localization of TREX-2 Subunits in the Cell
2.4. TREX-2 Has Multiple Contacts with the Nuclear Pore
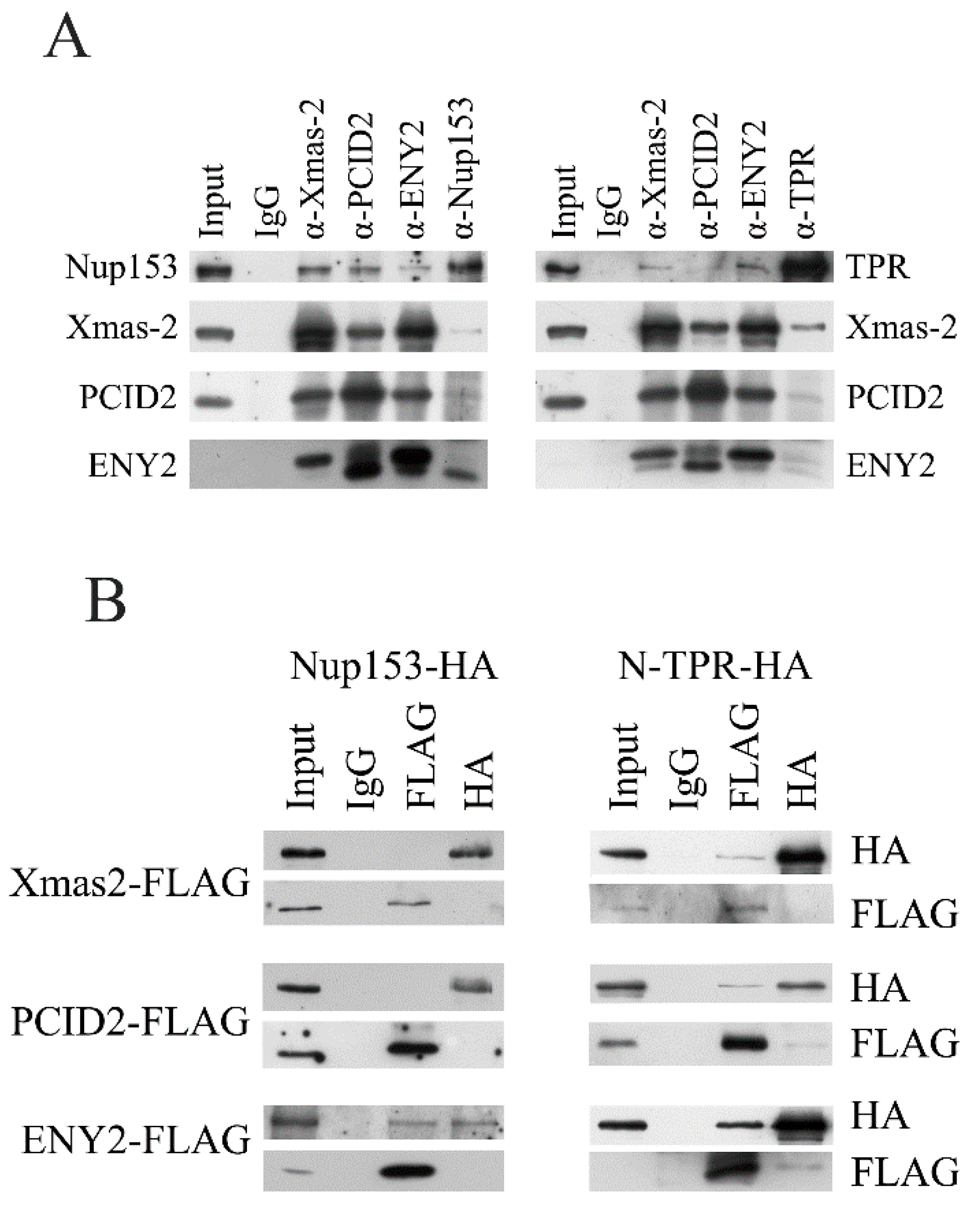
2.5. TREX-2 Subunits Interact with TPR и Nup153
3. Discussion
4. Materials and Methods
4.1. Expression Vectors
4.2. Antibodies
4.3. Drosophila Cultured Cell Lines
4.4. Drosophila Cell Culture Extract
4.5. Drosophila Embryonic Nuclear Extract
4.6. RNAi Knockdown in S2 Cells and Transfection Experiments
4.7. RNA FISH
4.8. Immunostaining Experiments and Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fischer, T.; Strasser, K.; Racz, A.; Rodriguez-Navarro, S.; Oppizzi, M.; Ihrig, P.; Lechner, J.; Hurt, E. The mRNA export machinery requires the novel Sac3p-Thp1p complex to dock at the nucleoplasmic entrance of the nuclear pores. EMBO J. 2002, 21, 5843–5852. [Google Scholar] [CrossRef] [PubMed]
- Jani, D.; Lutz, S.; Hurt, E.; Laskey, R.A.; Stewart, M.; Wickramasinghe, V.O. Functional and structural characterization of the mammalian TREX-2 complex that links transcription with nuclear messenger RNA export. Nucleic Acids Res. 2012, 40, 4562–4573. [Google Scholar] [CrossRef]
- Kurshakova, M.M.; Krasnov, A.N.; Kopytova, D.V.; Shidlovskii, Y.V.; Nikolenko, J.V.; Nabirochkina, E.N.; Spehner, D.; Schultz, P.; Tora, L.; Georgieva, S.G. SAGA and a novel Drosophila export complex anchor efficient transcription and mRNA export to NPC. EMBO J. 2007, 26, 4956–4965. [Google Scholar] [CrossRef]
- Lu, Q.; Tang, X.; Tian, G.; Wang, F.; Liu, K.; Nguyen, V.; Kohalmi, S.E.; Keller, W.A.; Tsang, E.W.T.; Harada, J.J.; et al. Arabidopsis homolog of the yeast TREX-2 mRNA export complex: Components and anchoring nucleoporin. Plant J. Cell Mol. Biol. 2010, 61, 259–270. [Google Scholar] [CrossRef]
- Luna, R.; Gonzalez-Aguilera, C.; Aguilera, A. Transcription at the proximity of the nuclear pore: A role for the THP1-SAC3-SUS1-CDC31 (THSC) complex. RNA Biol. 2009, 6, 145–148. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Navarro, S.; Fischer, T.; Luo, M.-J.; Antúnez, O.; Brettschneider, S.; Lechner, J.; Pérez-Ortín, J.E.; Reed, R.; Hurt, E. Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell 2004, 116, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, V.O.; Stewart, M.; Laskey, R.A. GANP enhances the efficiency of mRNA nuclear export in mammalian cells. Nucleus 2010, 1, 393–396. [Google Scholar] [CrossRef]
- Cabal, G.G.; Genovesio, A.; Rodriguez-Navarro, S.; Zimmer, C.; Gadal, O.; Lesne, A.; Buc, H.; Feuerbach-Fournier, F.; Olivo-Marin, J.-C.; Hurt, E.C.; et al. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature 2006, 441, 770–773. [Google Scholar] [CrossRef]
- Chekanova, J.A.; Abruzzi, K.C.; Rosbash, M.; Belostotsky, D.A. Sus1, Sac3, and Thp1 mediate post-transcriptional tethering of active genes to the nuclear rim as well as to non-nascent mRNP. RNA 2008, 14, 66–77. [Google Scholar] [CrossRef]
- Kopytova, D.; Popova, V.; Kurshakova, M.; Shidlovskii, Y.; Nabirochkina, E.; Brechalov, A.; Georgiev, G.; Georgieva, S. ORC interacts with THSC/TREX-2 and its subunits promote Nxf1 association with mRNP and mRNA export in Drosophila. Nucleic Acids Res. 2016, 44, 4920–4933. [Google Scholar] [CrossRef] [PubMed]
- Ellisdon, A.M.; Dimitrova, L.; Hurt, E.; Stewart, M. Structural basis for the assembly and nucleic acid binding of the TREX-2 transcription-export complex. Nat. Struct. Mol. Biol. 2012, 19, 328–336. [Google Scholar] [CrossRef]
- Garcia-Oliver, E.; Garcia-Molinero, V.; Rodriguez-Navarro, S. mRNA export and gene expression: The SAGA-TREX-2 connection. Biochim. Biophys. Acta 2012, 1819, 555–565. [Google Scholar] [CrossRef]
- Bhat, P.; Aksenova, V.; Gazzara, M.; Rex, E.A.; Aslam, S.; Haddad, C.; Gao, S.; Esparza, M.; Cagatay, T.; Batten, K.; et al. Influenza virus mRNAs encode determinants for nuclear export via the cellular TREX-2 complex. Nat. Commun. 2023, 14, 2304. [Google Scholar] [CrossRef]
- Schneider, M.; Hellerschmied, D.; Schubert, T.; Amlacher, S.; Vinayachandran, V.; Reja, R.; Pugh, B.F.; Clausen, T.; Kohler, A. The Nuclear Pore-Associated TREX-2 Complex Employs Mediator to Regulate Gene Expression. Cell 2015, 162, 1016–1028. [Google Scholar] [CrossRef]
- Wickramasinghe, V.O.; Andrews, R.; Ellis, P.; Langford, C.; Gurdon, J.B.; Stewart, M.; Venkitaraman, A.R.; Laskey, R.A. Selective nuclear export of specific classes of mRNA from mammalian nuclei is promoted by GANP. Nucleic Acids Res. 2014, 42, 5059–5071. [Google Scholar] [CrossRef] [PubMed]
- Umlauf, D.; Bonnet, J.; Waharte, F.; Fournier, M.; Stierle, M.; Fischer, B.; Brino, L.; Devys, D.; Tora, L. The human TREX-2 complex is stably associated with the nuclear pore basket. J. Cell Sci. 2013, 126, 2656–2667. [Google Scholar] [CrossRef] [PubMed]
- Glukhova, A.A.; Kurshakova, M.M.; Nabirochkina, E.N.; Georgieva, S.G.; Kopytova, D.V. PCID2, a subunit of the Drosophila TREX-2 nuclear export complex, is essential for both mRNA nuclear export and its subsequent cytoplasmic trafficking. RNA Biol. 2021, 18, 1969–1980. [Google Scholar] [CrossRef]
- Farny, N.G.; Hurt, J.A.; Silver, P.A. Definition of global and transcript-specific mRNA export pathways in metazoans. Genes Dev. 2008, 22, 66–78. [Google Scholar] [CrossRef]
- Jani, D.; Valkov, E.; Stewart, M. Structural basis for binding the TREX2 complex to nuclear pores, GAL1 localisation and mRNA export. Nucleic Acids Res. 2014, 42, 6686–6697. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Soni, N.; Hutchings, J.; Echeverria, I.; Shaikh, F.; Duquette, M.; Suslov, S.; Li, Z.; van Eeuwen, T.; Molloy, K.; et al. The molecular architecture of the nuclear basket. Cell 2024, 187, 5267–5281.e13. [Google Scholar] [CrossRef]
- Liang, Y.; Hetzer, M.W. Functional interactions between nucleoporins and chromatin. Curr. Opin. Cell Biol. 2011, 23, 65–70. [Google Scholar] [CrossRef]
- Vaquerizas, J.M.; Suyama, R.; Kind, J.; Miura, K.; Luscombe, N.M.; Akhtar, A. Nuclear pore proteins nup153 and megator define transcriptionally active regions in the Drosophila genome. PLoS Genet. 2010, 6, e1000846. [Google Scholar] [CrossRef]
- Aksenova, V.; Smith, A.; Lee, H.; Bhat, P.; Esnault, C.; Chen, S.; Iben, J.; Kaufhold, R.; Yau, K.C.; Echeverria, C.; et al. Nucleoporin TPR is an integral component of the TREX-2 mRNA export pathway. Nat. Commun. 2020, 11, 4577. [Google Scholar] [CrossRef] [PubMed]
- Jani, D.; Lutz, S.; Marshall, N.J.; Fischer, T.; Kohler, A.; Ellisdon, A.M.; Hurt, E.; Stewart, M. Sus1, Cdc31, and the Sac3 CID region form a conserved interaction platform that promotes nuclear pore association and mRNA export. Mol. Cell 2009, 33, 727–737. [Google Scholar] [CrossRef]
- Duheron, V.; Chatel, G.; Sauder, U.; Oliveri, V.; Fahrenkrog, B. Structural characterization of altered nucleoporin Nup153 expression in human cells by thin-section electron microscopy. Nucleus 2014, 5, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Hase, M.E.; Cordes, V.C. Direct interaction with nup153 mediates binding of Tpr to the periphery of the nuclear pore complex. Mol. Biol. Cell 2003, 14, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Kamenova, I.; Mukherjee, P.; Conic, S.; Mueller, F.; El-Saafin, F.; Bardot, P.; Garnier, J.-M.; Dembele, D.; Capponi, S.; Timmers, H.T.M.; et al. Co-translational assembly of mammalian nuclear multisubunit complexes. Nat. Commun. 2019, 10, 1740. [Google Scholar] [CrossRef]
- Rajanala, K.; Nandicoori, V.K. Localization of nucleoporin Tpr to the nuclear pore complex is essential for Tpr-mediated regulation of the export of unspliced RNA. PLoS ONE 2012, 7, e29921. [Google Scholar] [CrossRef]
- Georgieva, S.; Nabirochkina, E.; Dilworth, F.J.; Eickhoff, H.; Becker, P.; Tora, L.; Georgiev, P.; Soldatov, A. The novel transcription factor e(y)2 interacts with TAF(II)40 and potentiates transcription activation on chromatin templates. Mol. Cell. Biol. 2001, 21, 5223–5231. [Google Scholar] [CrossRef]
- Clemens, J.C.; Worby, C.A.; Simonson-Leff, N.; Muda, M.; Maehama, T.; Hemmings, B.A.; Dixon, J.E. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc. Natl. Acad. Sci. USA 2000, 97, 6499–6503. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vdovina, Y.; Nikolenko, J.; Orlova, A.; Glukhova, A.; Kurshakova, M.; Fet, S.; Tvorogova, A.; Tyurin-Kuzmin, P.; Golovnin, A.; Georgieva, S.; et al. Nup153 and TPR/Megator Interact with TREX-2 Subunits and Are Essential for TREX-2-Dependent Nuclear Export of hsp70 mRNA in Drosophila. Int. J. Mol. Sci. 2025, 26, 8595. https://doi.org/10.3390/ijms26178595
Vdovina Y, Nikolenko J, Orlova A, Glukhova A, Kurshakova M, Fet S, Tvorogova A, Tyurin-Kuzmin P, Golovnin A, Georgieva S, et al. Nup153 and TPR/Megator Interact with TREX-2 Subunits and Are Essential for TREX-2-Dependent Nuclear Export of hsp70 mRNA in Drosophila. International Journal of Molecular Sciences. 2025; 26(17):8595. https://doi.org/10.3390/ijms26178595
Chicago/Turabian StyleVdovina, Yulia, Julia Nikolenko, Anastasia Orlova, Anna Glukhova, Maria Kurshakova, Savva Fet, Anna Tvorogova, Pyotr Tyurin-Kuzmin, Anton Golovnin, Sofia Georgieva, and et al. 2025. "Nup153 and TPR/Megator Interact with TREX-2 Subunits and Are Essential for TREX-2-Dependent Nuclear Export of hsp70 mRNA in Drosophila" International Journal of Molecular Sciences 26, no. 17: 8595. https://doi.org/10.3390/ijms26178595
APA StyleVdovina, Y., Nikolenko, J., Orlova, A., Glukhova, A., Kurshakova, M., Fet, S., Tvorogova, A., Tyurin-Kuzmin, P., Golovnin, A., Georgieva, S., & Kopytova, D. (2025). Nup153 and TPR/Megator Interact with TREX-2 Subunits and Are Essential for TREX-2-Dependent Nuclear Export of hsp70 mRNA in Drosophila. International Journal of Molecular Sciences, 26(17), 8595. https://doi.org/10.3390/ijms26178595






