Neuroinflammatory Signature of Post-Traumatic Confusional State: The Role of Cytokines in Moderate-to-Severe Traumatic Brain Injury
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Characteristics of Study Subjects
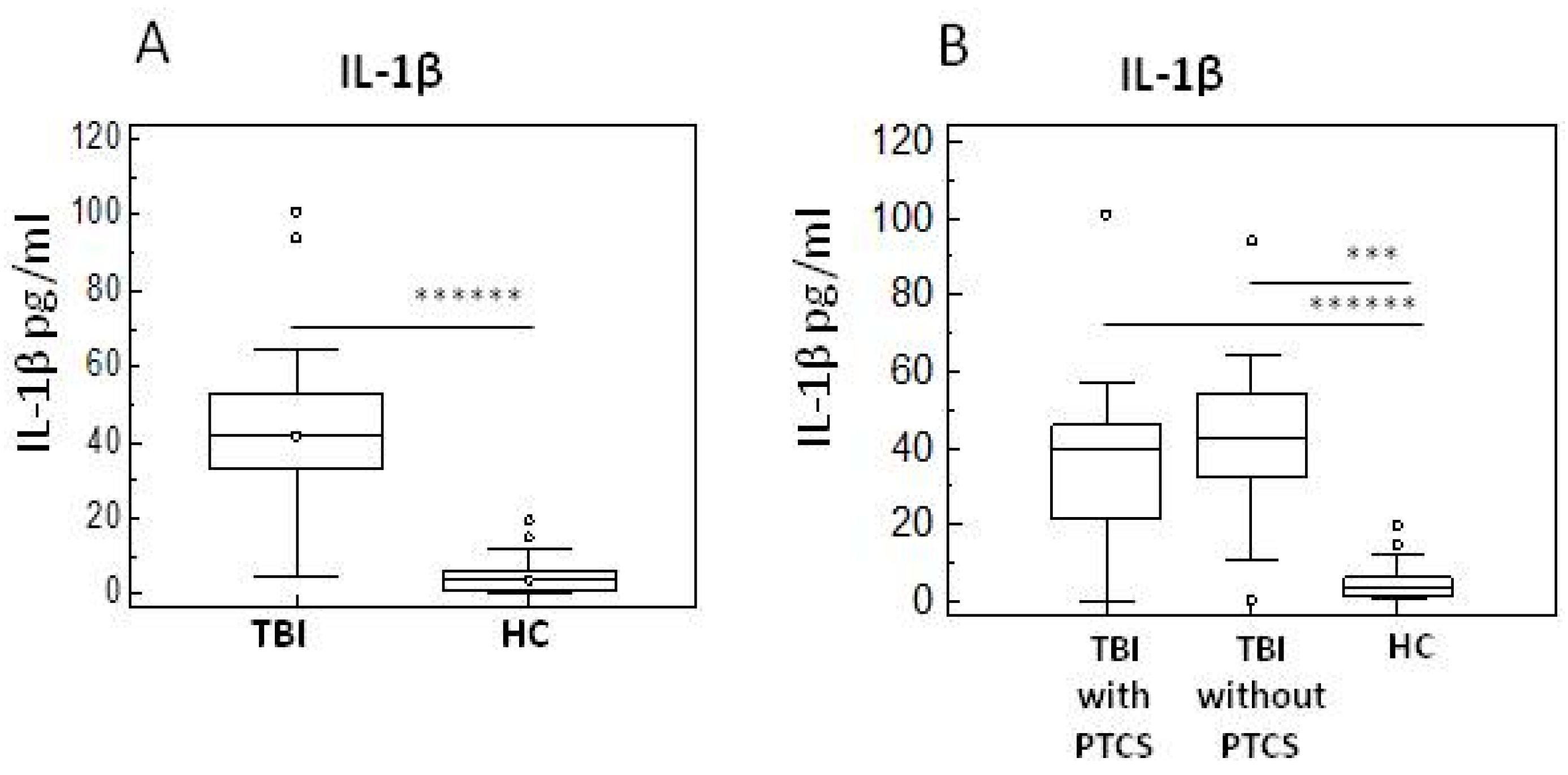
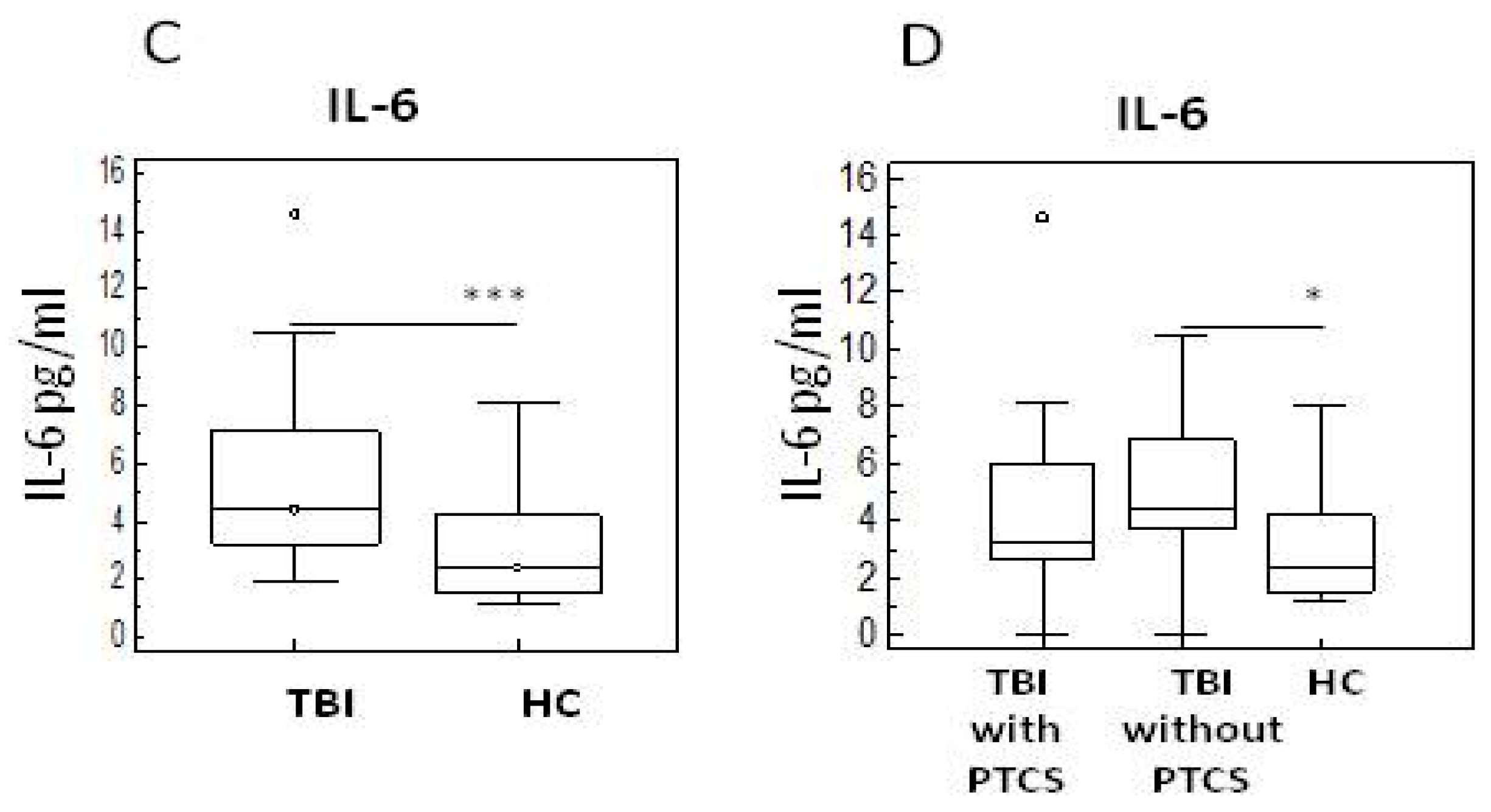
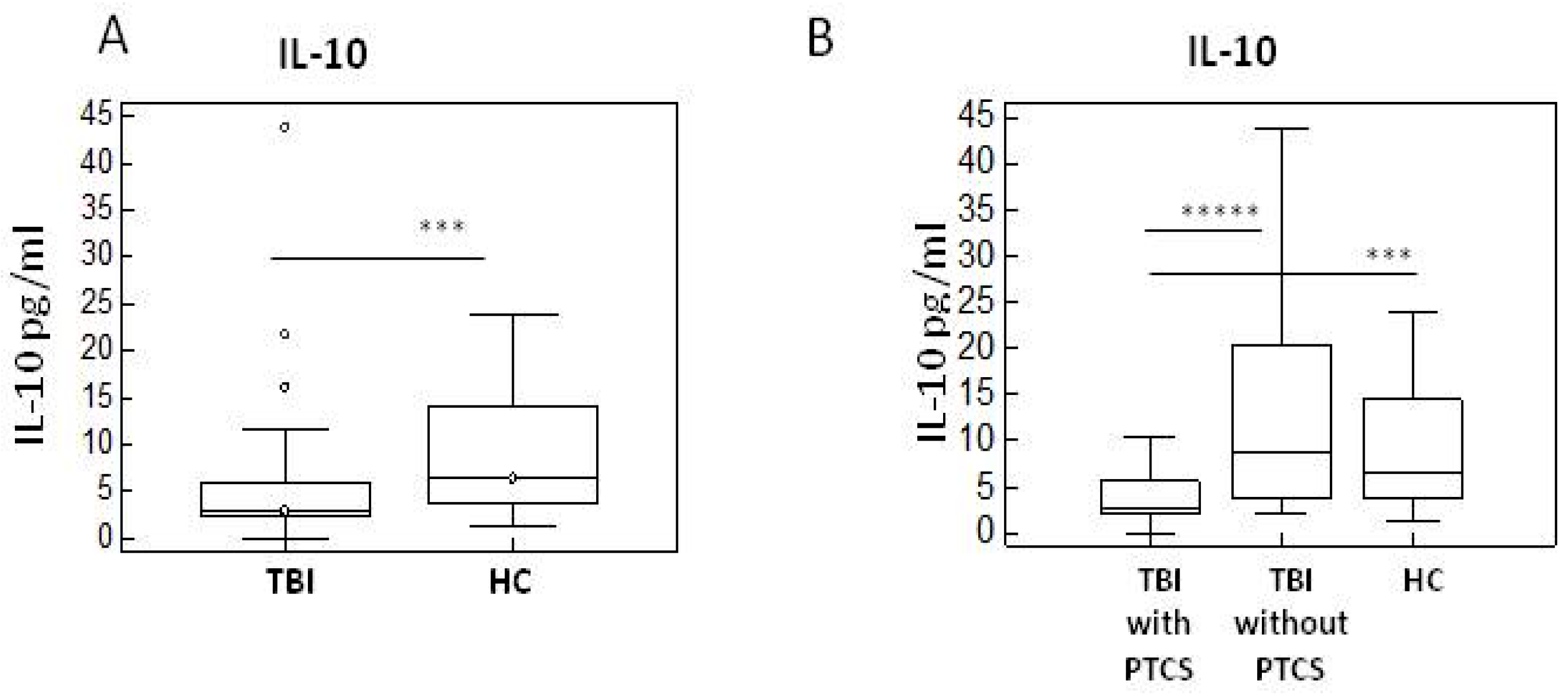
2.2. Pro- and Anti-Inflammatory Cytokines in TBI Patients with and Without PTCS and in Healthy Controls
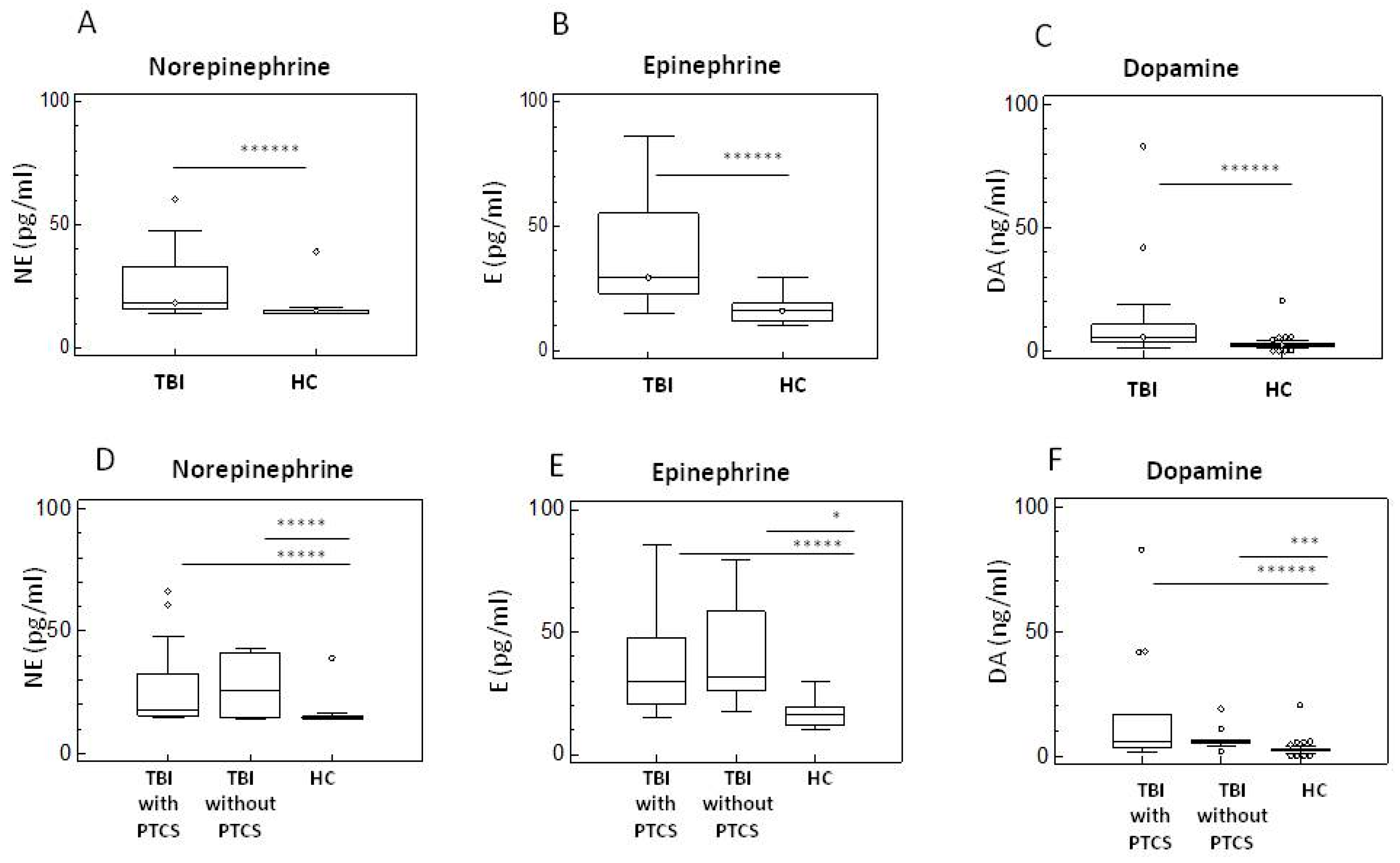
2.3. Neurotransmitters in TBI Patients with and Without PTCS and in Healthy Controls
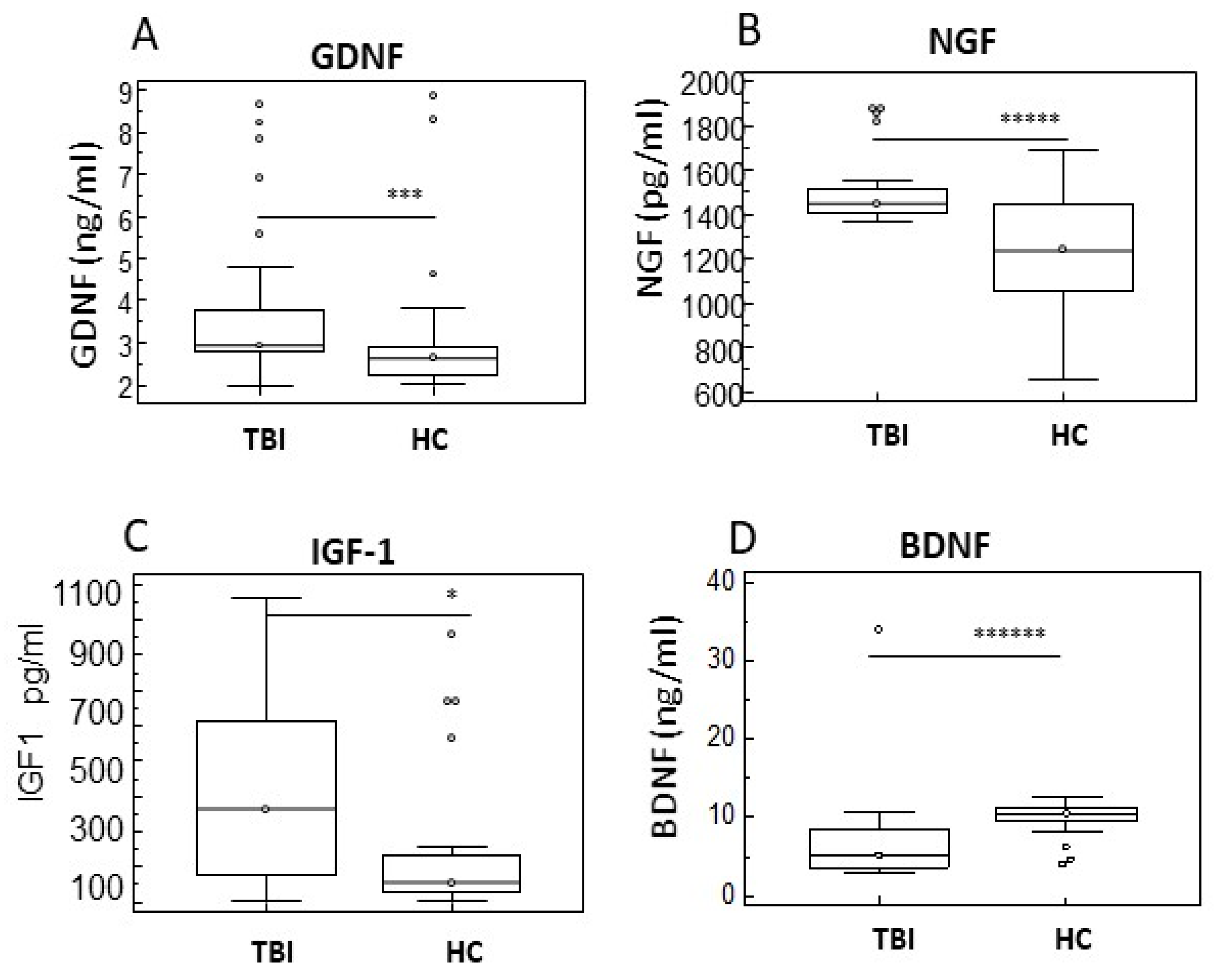
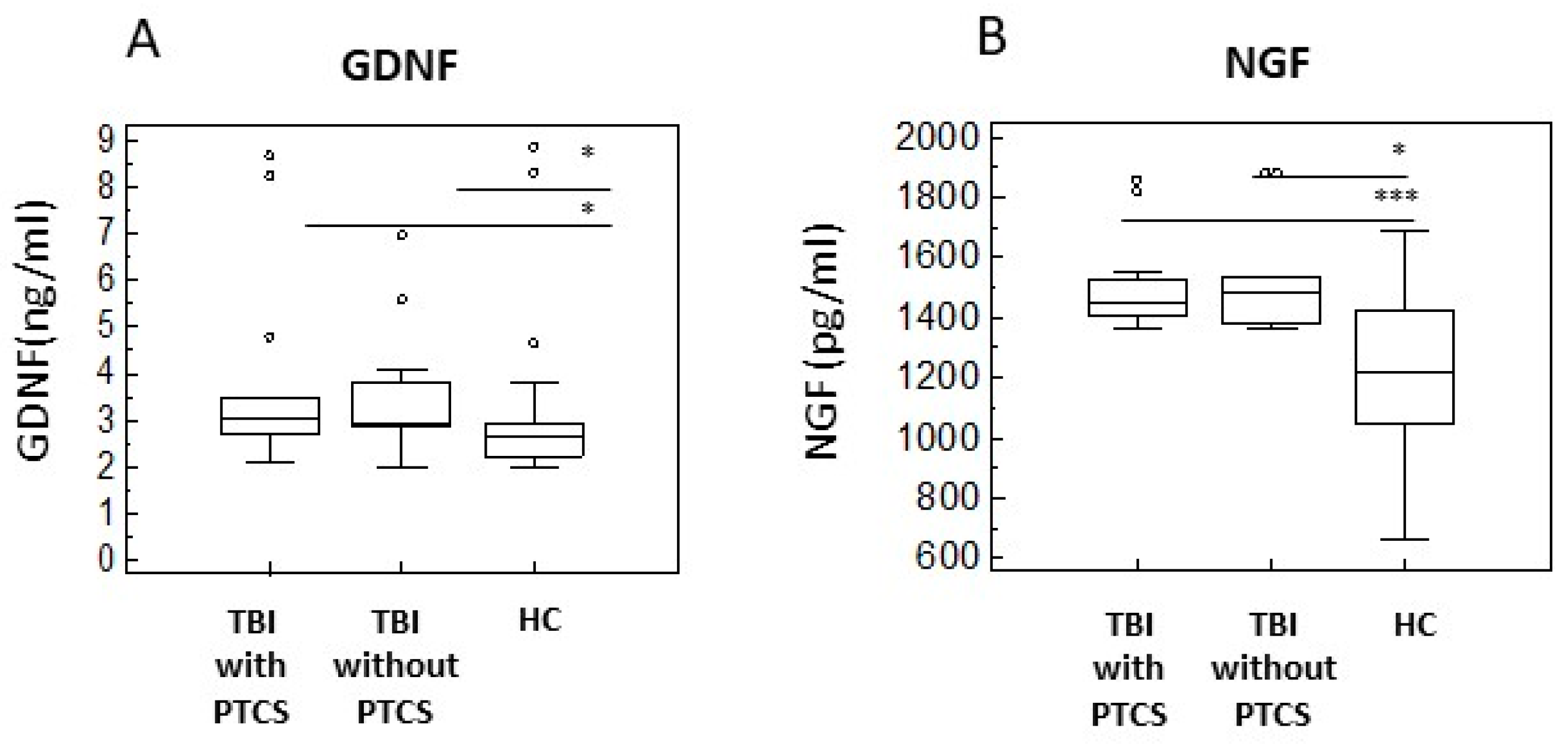
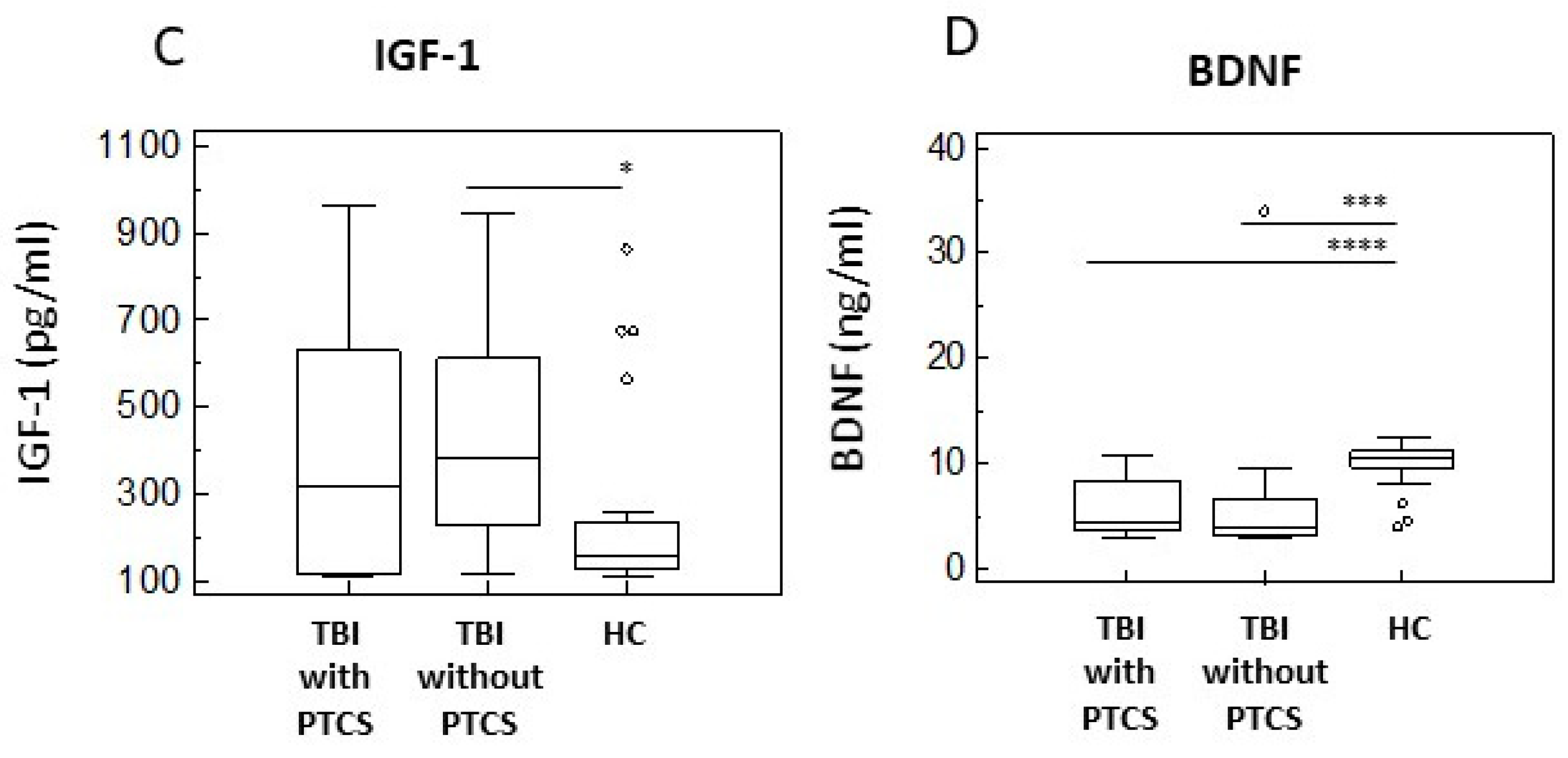
2.4. Neurotrophins of in TBI Patients with and Without PTCS and in Healthy Controls
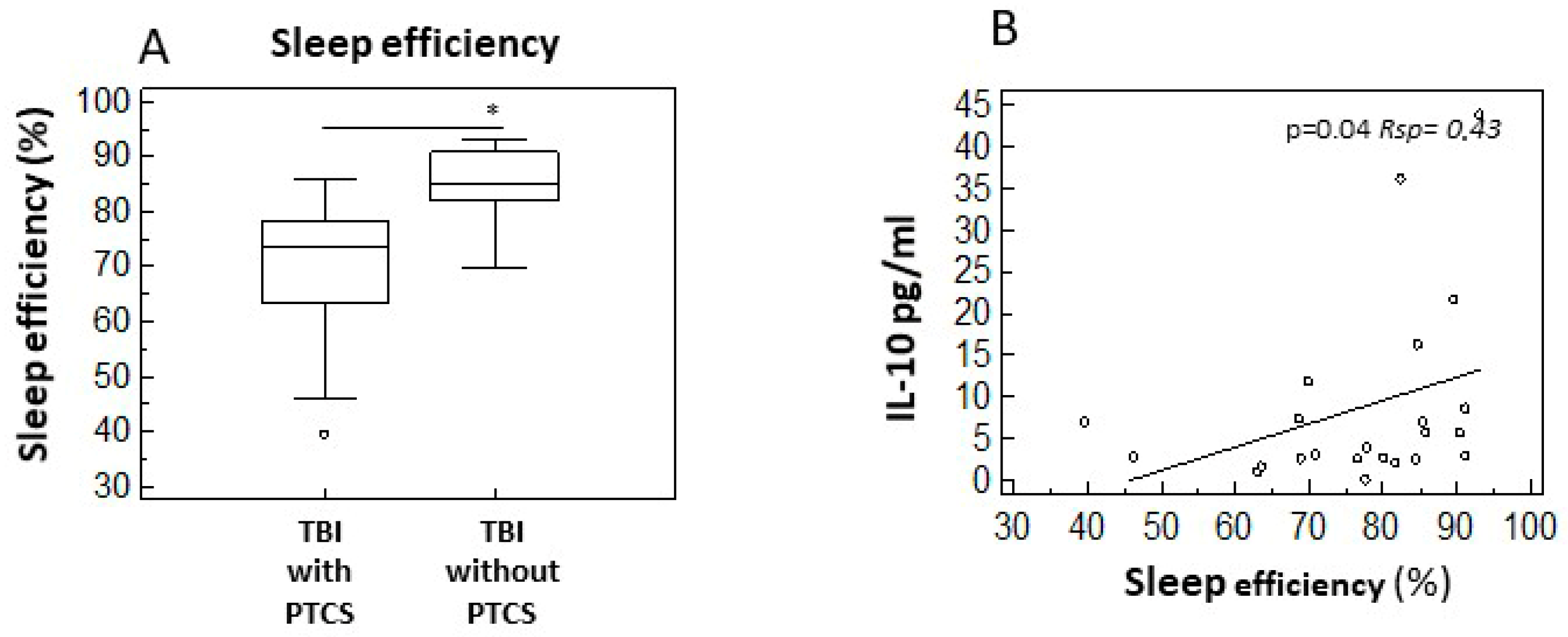
2.5. Sleep–Wake Disturbances and Cytokine Profiles
3. Discussion
4. Materials and Methods
4.1. Patients Enrolled in the Study
4.2. Ethics Approval
4.3. Clinical Evaluation of Post-Traumatic Confusional State
4.4. Actigraphic Evaluation of Sleep
4.5. Serum Sample Collection
4.6. ELISA
4.6.1. Cytokines
4.6.2. Neurotransmitters
4.6.3. Neurotrophins
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TBI | Traumatic brain injury |
| PTCS | Post-traumatic confusional state |
| IRU | Rehabilitation unit |
| ELISA | Enzyme-linked immunosorbent assay |
| HC | Healthy control |
| IL | Interleukin |
| NE | Norepinephrine |
| E | Epinephrine |
| DA | Dopamine |
| GDNF | Glial cell line-derived neurotrophic factor |
| NGF | Nerve growth factor |
| IGF-1 | Insuline-like growth factor1 |
| BDNF | Brain derived growth factor |
| DAMPs | Damage associated molecular patterns |
| PRRs | Patterns recognition receptors |
| CNS | Central nervous system |
| LPS | Lipopolysaccharide |
| DCX | Neuronal migration protein doublecortin |
| PSA-NCAM | Polysialic acid- neural cell adhesion molecule (PSA-NCAM) |
| NCS | Neuronal/stem cells |
| NT | Neurotrophin |
| SE | Sleep efficiency |
| CAP | Confusion assessment protocol |
| GOAT | Galveston Orientation and Amnesia Test |
| OD | Optical density |
| S | Sensivity |
| AR | Assay range |
| RSP | Spermean’s rank correlation coefficient |
| DRS | Disability rating scale |
| M | Male |
| F | Female |
| PTA | Post-traumatic amnesia |
References
- Sherer, M.; Katz, D.I.; Bodien, Y.G.; Arciniegas, D.B.; Block, C.; Blum, S.; Doiron, M.; Frey, K.; Giacino, J.T.; Graf, M.J.P.; et al. Post-traumatic Confusional State: A Case Definition and Diagnostic Criteria. Arch. Phys. Med. Rehabil. 2020, 101, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Mele, C.; Pingue, V.; Caputo, M.; Zavattaro, M.; Pagano, L.; Prodam, F.; Nardone, A.; Aimaretti, G.; Marzullo, P. Neuroinflammation and Hypothalamo-Pituitary Dysfunction: Focus of Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 2686. [Google Scholar] [CrossRef]
- Wu, J.; Ren, R.; Chen, T.; Su, L.D.; Tang, T. Neuroimmune and neuroinflammation response for traumatic brain injury. Brain Res. Bull. 2024, 217, 111066. [Google Scholar] [CrossRef] [PubMed]
- Kokiko-Cochran, O.N.; Godbout, J.P. The Inflammatory Continuum of Traumatic Brain Injury and Alzheimer’s Disease. Front. Immunol. 2018, 9, 672. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.S.N.; Zimmerman, K.A.; Moro, F.; Heslegrave, A.; Maillard, S.A.; Bernini, A.; Miroz, J.-P.; Donat, C.K.; Lopez, M.Y.; Bourke, N.; et al. Axonal marker neurofilament light predicts long-term outcomes and progressive neurodegeneration after traumatic brain injury. Sci. Transl. Med. 2021, 13, eabg9922. [Google Scholar] [CrossRef]
- Ozen, I.; Ruscher, K.; Nilsson, R.; Flygt, J.; Clausen, F.; Marklund, N. Interleukin-1 Beta Neutralization Attenuates Traumatic Brain Injury-Induced Microglia Activation and Neuronal Changes in the Globus Pallidus. Int. J. Mol. Sci. 2020, 21, 387. [Google Scholar] [CrossRef]
- Clausen, F.; Hånell, A.; Björk, M.; Hillered, L.; Mir, A.K.; Gram, H.; Marklund, N. Neutralization of interleukin-1beta modifies the inflammatory response and improves histological and cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 2009, 30, 385–396. [Google Scholar] [CrossRef]
- Clausen, F.; Hånell, A.; Israelsson, C.; Hedin, J.; Ebendal, T.; Mir, A.K.; Gram, H.; Marklund, N. Neutralization of interleukin-1β reduces cerebral edema and tissue loss and improves late cognitive outcome following traumatic brain injury in mice. Eur. J. Neurosci. 2011, 34, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, N.; Minami, K.; Shirakawa, F.; Uezono, Y.; Kobayashi, H.; Eto, S.; Izumi, F. Stimulatory effect of IL-1 beta on catecholamine secretion from cultured bovine adrenal medullary cells. Biochem. Biophys. Res. Commun. 1994, 198, 81–87. [Google Scholar] [CrossRef]
- Johnson, J.D.; Campisi, J.; Sharkey, C.M.; Kennedy, S.L.; Nickerson, M.; Greenwood, B.N.; Fleshner, M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 2005, 135, 1295–1307. [Google Scholar] [CrossRef]
- Rizoli, S.B.; Jaja, B.N.; Di Battista, A.P.; Rhind, S.G.; Neto, A.C.; da Costa, L.; Inaba, K.; da Luz, L.T.; Nascimento, B.; Perez, A.; et al. Catecholamines as outcome markers in isolated traumatic brain injury: The COMA-TBI study. Crit. Care 2017, 21, 37. [Google Scholar] [CrossRef] [PubMed]
- Tapp, Z.M.; Godbout, J.P.; Kokiko-Cochran, O.N. A Tilted Axis: Maladaptive Inflammation and HPA Axis Dysfunction Contribute to Consequences of TBI. Front. Neurol. 2019, 10, 345. [Google Scholar] [CrossRef]
- Ekdahl, C.T.; Kokaia, Z.; Lindvall, O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience 2009, 158, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Duman, R.S. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. USA 2008, 105, 751–756. [Google Scholar] [CrossRef]
- Vallieres, L.; Campbell, I.L.; Gage, F.H.; Sawchenko, P.E. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J. Neurosci. 2002, 22, 486–492. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Araujo, D.M.; Hefti, F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience 1993, 53, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, C.T.; Claasen, J.H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637. [Google Scholar] [CrossRef]
- Zheng, W.; ZhuGe, Q.; Zhong, M.; Chen, G.; Shao, B.; Wang, H.; Mao, X.; Xie, L.; Jin, K. Neurogenesis in adult human brain after traumatic brain injury. J. Neurotrauma 2013, 30, 1872–1880. [Google Scholar] [CrossRef]
- Corne, R.; Besson, V.; Slimane, S.A.S.; Coutan, M.; Palhas, M.L.C.; Shen, F.X.; Marchand-Leroux, C.; Ogier, M.; Mongeau, R. Insulin-like Growth Factors may be Markers of both Traumatic Brain Injury and Fear-Related Stress. Neuroscience 2021, 466, 205–221. [Google Scholar] [CrossRef]
- Sinson, G.; Perri, B.R.; Trojanowski, J.Q.; Flamm, E.S.; McIntosh, T.K. Improvement of cognitive deficits and decreased cholinergic neuronal cell loss and apoptotic cell death following neurotrophin infusion after experimental traumatic brain injury. J. Neurosurg. 1997, 86, 511–518. [Google Scholar] [CrossRef]
- Sherer, M.; Nakase-Thompson, R.; Yablon, S.A.; Gontkovsky, S.T. Multidimensional assessment of acute confusion after traumatic brain injury. Arch. Phys. Med. Rehabil. 2005, 86, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Wickwire, E.M.; Williams, S.G.; Roth, T.; Capaldi, V.F.; Jaffe, M.; Moline, M.; Motamedi, G.K.; Morgan, G.W.; Mysliwiec, V.; Germain, A.; et al. Sleep, Sleep Disorders, and Mild Traumatic Brain Injury. What We Know and What We Need to Know: Findings from a National Working Group. Neurotherapeutics 2016, 13, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Makley, M.J.; Johnson-Greene, L.; Tarwater, P.M.; Kreuz, A.J.; Spiro, J.; Rao, V.; Celnik, P.A. Return of memory and sleep efficiency following moderate to severe closed head injury. Neurorehabil. Neural Repair 2009, 23, 320–326. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. InTBIR Participants and Investigators. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef]
- Cáceres, E.; Olivella, J.C.; Di Napoli, M.; Raihane, A.S.; Divani, A.A. Immune Response in Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2024, 24, 593–609. [Google Scholar] [CrossRef]
- Relja, B.; Land, W.G. Damage-associated molecular patterns in trauma. Eur. J. Trauma Emerg. Surg. 2020, 46, 751–775. [Google Scholar] [CrossRef]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef]
- Loane, D.J.; Kumar, A.; Stoica, B.A.; Cabatbat, R.; Faden, A.I. Progressive neurodegeneration after experimental brain trauma: Association with chronic microglial activation. J. Neuropathol. Exp. Neurol. 2014, 73, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136 Pt 1, 28–42. [Google Scholar] [CrossRef]
- Samatra, D.P.G.P.; Pratiwi, N.M.D.; Widyadharma, I.P.E. High Il-1β Serum as a Predictor of Decreased Cognitive Function in Mild Traumatic Brain Injury Patients. Open Access Maced. J. Med. Sci. 2018, 6, 1674–1677. [Google Scholar] [CrossRef]
- Di Battista, A.P.; Rhind, S.G.; Hutchison, M.G.; Hassan, S.; Shiu, M.Y.; Inaba, K.; Topolovec-Vranic, J.; Neto, A.C.; Rizoli, S.B.; Baker, A.J. Inflammatory cytokine and chemokine profiles are associated with patient outcome and the hyperadrenergic state following acute brain injury. J. Neuroinflamm. 2016, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Ponsford, J.L.; Spitz, G.; McKenzie, D. Using Post-Traumatic Amnesia to Predict Outcome after Traumatic Brain Injury. J. Neurotrauma 2016, 33, 997–1004. [Google Scholar] [CrossRef]
- Pascual, A.; Hidalgo-Figueroa, M.; Piruat, J.I.; Pintado, C.O.; Gómez-Díaz, R.; López-Barneo, J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat. Neurosci. 2008, 11, 755–761. [Google Scholar] [CrossRef]
- Ibáñez, C.F. Catecholaminergic neuron survival: Getting hooked on GDNF. Nat. Neurosci. 2008, 11, 735–736. [Google Scholar] [CrossRef]
- DeKosky, S.T.; Goss, J.R.; Miller, P.D.; Styren, S.D.; Kochanek, P.M.; Marion, D. Upregulation of nerve growth factor following cortical trauma. Exp. Neurol. 1994, 130, 173–177. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Sprunger, D.B.; Campeau, S.; Higgins, E.A.; Watkins, L.R.; Rudy, J.W.; Maier, S. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience 2003, 121, 847–853. [Google Scholar] [CrossRef]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef]
- Kalmbach, D.A.; Conroy, D.A.; Falk, H.; Rao, V.; Roy, D.; Peters, M.E. Poor sleep is linked to impeded recovery from traumatic brain injury. Sleep 2018, 41, zsy147. [Google Scholar] [CrossRef]
- Levin, H.S.; O’Donnell, V.M.; Grossman, R.G. The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. J. Nerv. Ment. Dis. 1979, 167, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Mitjans, M.; Van der Maren, S.; Gosselin, N.; Duclos, C.; Frenette, A.J.; Arbour, C.; Burry, L.; Williams, V.; Bernard, F.; Williamson, D.R. Use of actigraphy for monitoring agitation and rest-activity cycles in patients with acute traumatic brain injury in the ICU. Brain Inj. 2024, 38, 692–698. [Google Scholar] [CrossRef] [PubMed]
| Variable | Patients with PTCS | Patients Without PTCS | Healthy Controls | p-Value |
|---|---|---|---|---|
| Age, years | 42.6 ± 19.5 | 48.2 ± 15.6 | 52.5 ± 1.7 | - |
| Gender (M:F) | 16:1 | 9:3 | 16:18 | - |
| Days from injury | 35.4 ± 5.5 | 33.5 ± 3.5 | - | <0.05 |
| Disability rating scale at admission | 17 (15–19) | 13 (8–16) | - | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piancone, F.; La Rosa, F.; Hernis, A.; Marventano, I.; Arcuri, P.; Rabuffetti, M.; Navarro, J.; Saresella, M.; Clerici, M.; Comanducci, A. Neuroinflammatory Signature of Post-Traumatic Confusional State: The Role of Cytokines in Moderate-to-Severe Traumatic Brain Injury. Int. J. Mol. Sci. 2025, 26, 8593. https://doi.org/10.3390/ijms26178593
Piancone F, La Rosa F, Hernis A, Marventano I, Arcuri P, Rabuffetti M, Navarro J, Saresella M, Clerici M, Comanducci A. Neuroinflammatory Signature of Post-Traumatic Confusional State: The Role of Cytokines in Moderate-to-Severe Traumatic Brain Injury. International Journal of Molecular Sciences. 2025; 26(17):8593. https://doi.org/10.3390/ijms26178593
Chicago/Turabian StylePiancone, Federica, Francesca La Rosa, Ambra Hernis, Ivana Marventano, Pietro Arcuri, Marco Rabuffetti, Jorge Navarro, Marina Saresella, Mario Clerici, and Angela Comanducci. 2025. "Neuroinflammatory Signature of Post-Traumatic Confusional State: The Role of Cytokines in Moderate-to-Severe Traumatic Brain Injury" International Journal of Molecular Sciences 26, no. 17: 8593. https://doi.org/10.3390/ijms26178593
APA StylePiancone, F., La Rosa, F., Hernis, A., Marventano, I., Arcuri, P., Rabuffetti, M., Navarro, J., Saresella, M., Clerici, M., & Comanducci, A. (2025). Neuroinflammatory Signature of Post-Traumatic Confusional State: The Role of Cytokines in Moderate-to-Severe Traumatic Brain Injury. International Journal of Molecular Sciences, 26(17), 8593. https://doi.org/10.3390/ijms26178593








