Integrated Multi-Omics Analysis Reveals the Role of Resveratrol in Regulating the Intestinal Function of Megalobrama amblycephala via m6A Methylation
Abstract
1. Introduction
2. Results
2.1. Effects of RES on the Growth Performance and Lipid Metabolism of Juveniles Fed with an HF Diet
2.2. Intestinal Microbiota Analysis
2.3. Role of RES in Maintaining Intestinal Barrier Health in Juvenile M. amblycephala Fed with an HF Diet
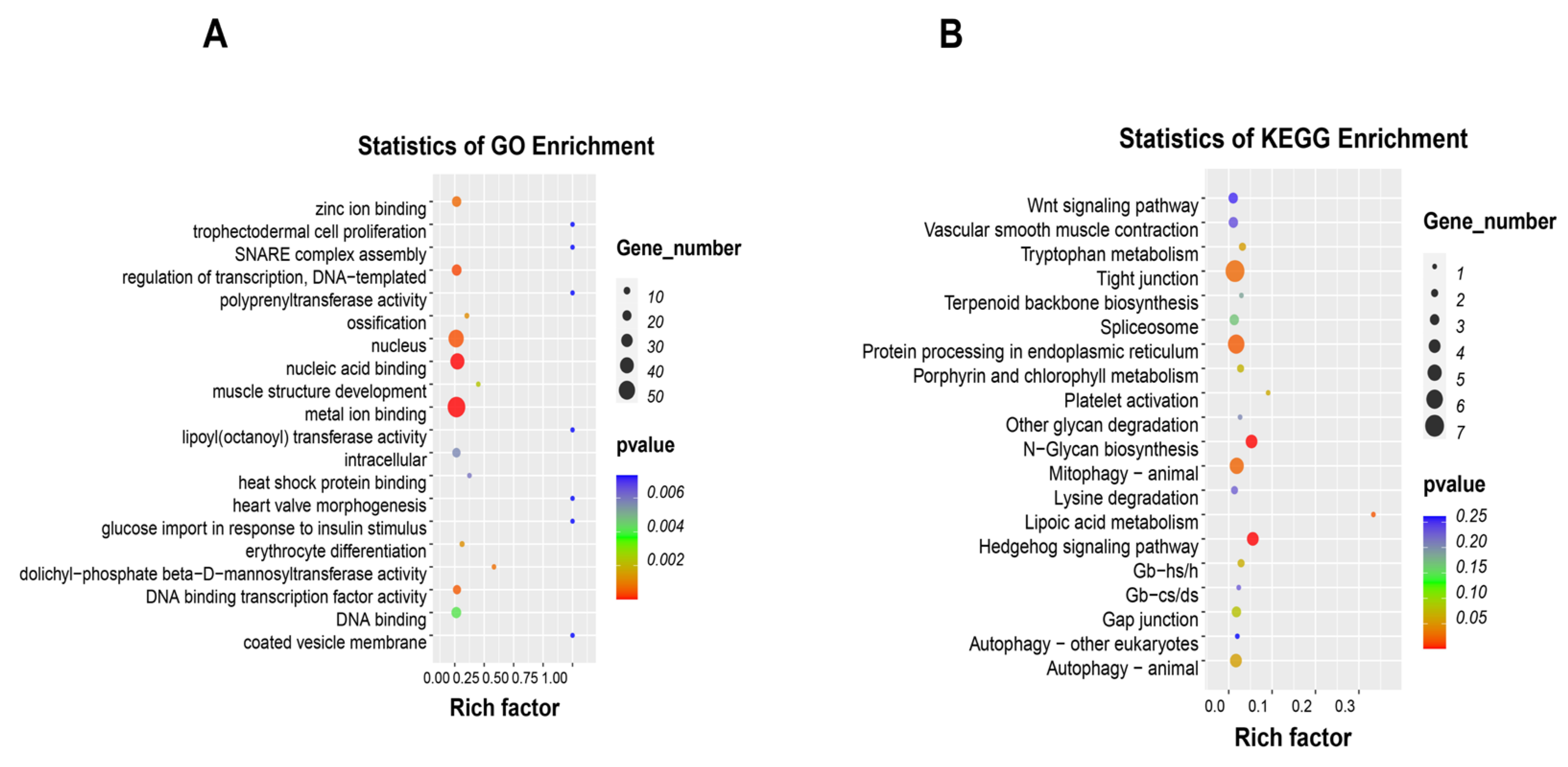
2.4. MeRIP-seq of the Intestinal Tissues
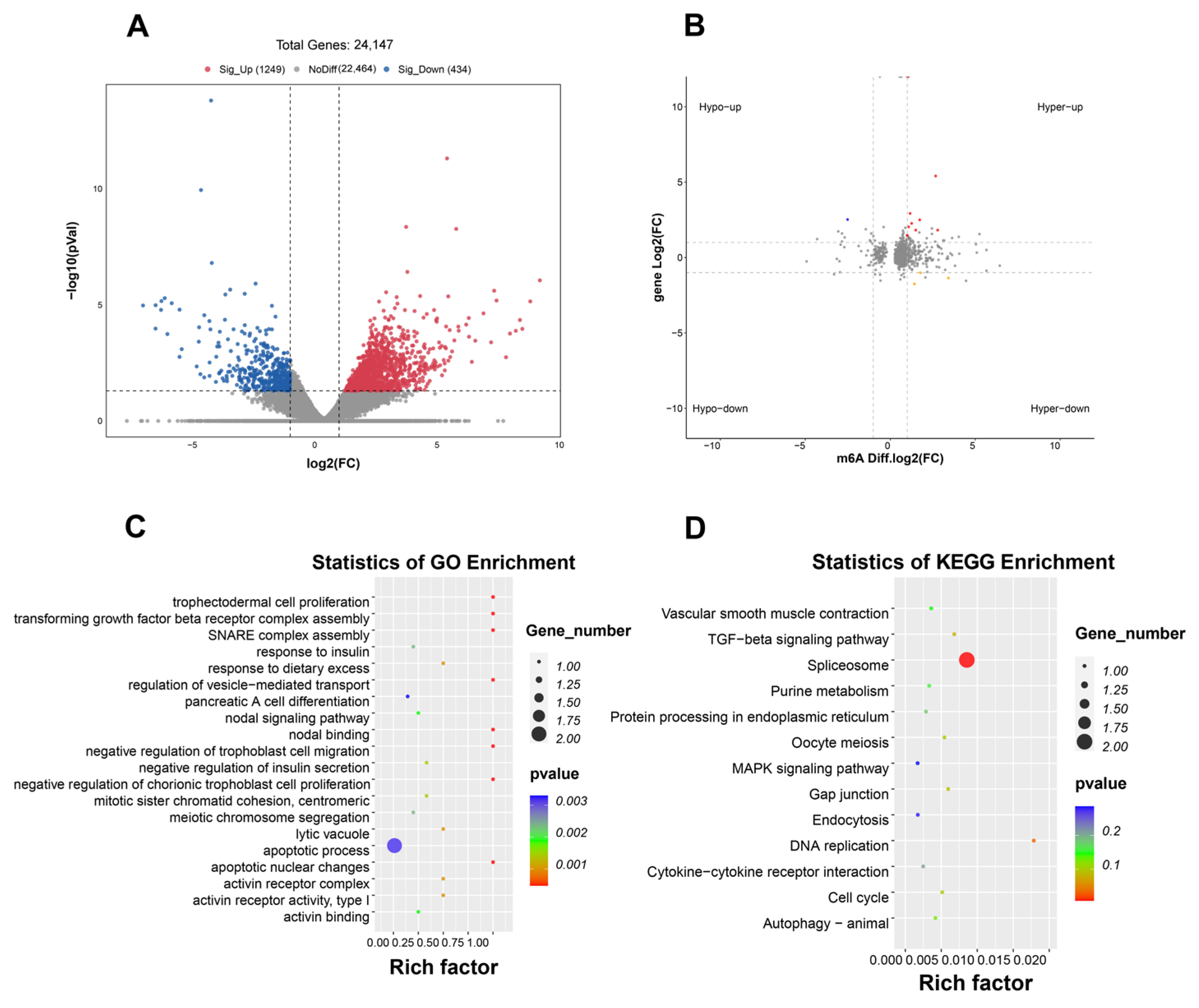
2.5. Differential m6A Peaks Identification and Functional Analysis in HF and HF-RES Groups
2.6. Association Analysis Between DEGs and Differential m6A Peaks
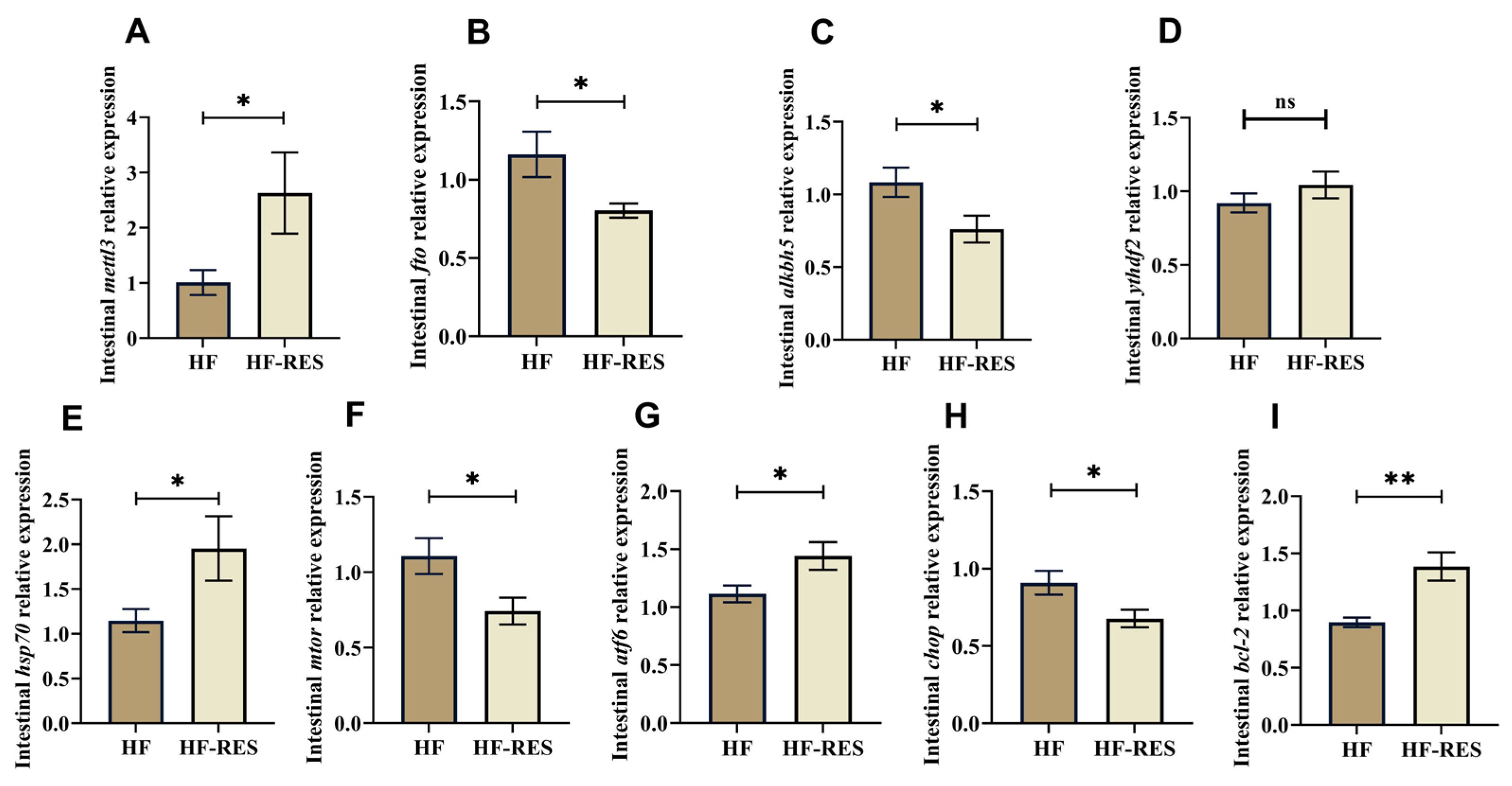
2.7. Expression Analysis of Intestinal m6A Methylase and Endoplasmic Reticulum Stress-Related Genes
2.8. Intestinal Microbiota, m6A Methylation, Intestinal Barrier, and Lipid Metabolism Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Diets
4.2. Fish and Feeding Trial
4.3. Sample Collection
4.4. Parameter Measurement
4.4.1. Growth Performance Analysis
4.4.2. Biochemical Index Analysis
4.5. Histological and Fluorescence Staining
4.6. Microbiota Analysis in Intestinal Contents
4.7. MeRIP-seq
4.7.1. RNA Extraction, Library Preparation, and Sequencing
4.7.2. Bioinformatics Analysis
4.8. Real-Time PCR Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, S.; Xie, X.; Zhang, W.; Cao, Z. The nuntrition of Silurus meridionalis: III. protein-sparing effect og dietary lipid. Acta Hydrobiol. Sin. 2001, 25, 70–75. [Google Scholar] [CrossRef]
- Lu, K.; Xu, W.; Li, J.; Li, X.; Huang, G.; Liu, W. Alterations of liver histology and blood biochemistry in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish. Sci. 2013, 79, 661–671. [Google Scholar] [CrossRef]
- Wei, M.; Song, L.; Yuan, X.; Li, H.; Ji, H.; Sun, J. Dietary supplementation with a PPARγ agonist promotes adipocyte hyperplasia and improves high-fat diet tolerance and utilization in grass carp (Ctenopharyngodon idellus). Aquaculture 2023, 578, 740081. [Google Scholar] [CrossRef]
- Wei, M.; Yuan, X.; Song, L.; Li, H.; Ji, H.; Sun, J. Comparative proteomic analysis of pathological characterization of adipose tissue remodeling induced by high-fat diet and high-carbohydrate diet in grass carp (Ctenopharyngodon idellus). Aquaculture 2024, 590, 741079. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, J.; Qin, Q.; Liu, J.; Xu, J.; Xu, W. Berberine improved intestinal barrier function by modulating the intestinal microbiota in blunt snout bream (Megalobrama amblycephala) under dietary high-fat and high-carbohydrate stress. Fish Shellfish Immunol. 2020, 102, 336–349. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Yang, S.; Li, Z.; Zhong, J.; Fang, R. Epidermal growth factor and intestinal barrier function. Mediat. Inflamm. 2016, 2016, 1927348. [Google Scholar] [CrossRef]
- González-Garzón, A.C.; Ramón-Ugalde, J.P.; Ambríz-García, D.A.; Vazquez-Avendaño, J.R.; Hernández-Pichardo, J.E.; Rodríguez-Suastegui, J.L.; Cortez-Romero, C.; Navarro-Maldonado, M.D.C. Resveratrol reduces ROS by increasing GSH in vitrified sheep embryos. Animals 2023, 13, 3602. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Tian, R.; Yan, X.; Zhou, Y. Resveratrol alleviates amyloid β-induced neuronal apoptosis, inflammation, and oxidative and endoplasmic reticulum stress by circ_0050263/miR-361-3p/PDE4A axis during Alzheimer’s disease. Chem. Biol. Drug Des. 2023, 102, 1121–1132. [Google Scholar] [CrossRef]
- Posey, K.L. Curcumin and resveratrol: Nutraceuticals with so much potential for pseudoachondroplasia and other ER-stress conditions. Biomolecules 2024, 14, 154. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, X.; Zheng, Y.; Qiu, L.; Hu, G.; Bing, X.; Chen, J. The effect of resveratrol on intestinal histology and inflammatory factor content in red tilapia. Chin. J. Anim. Nutr. 2022, 34, 589–599. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, X.; Liu, L.; Wang, X.; Li, H.; Zhu, J.; Cao, Y.; Zhu, H. Gut microbiota modulation by plant polyphenols in koi carp (Cyprinus carpio L.). Front. Microbiol. 2022, 13, 977292. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Yu, J.; Gan, Z.; Wei, W.; Wang, C.; Zhang, L.; Wang, T.; Zhong, X. Resveratrol attenuates high-fat diet induced hepatic lipid homeostasis disorder and decreases m6A RNA methylation. Front. Pharmacol. 2020, 11, 568006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, S.; Ma, J.; Liu, Z.; Liu, H. REW-ISA V2: A biclustering method fusing homologous information for analyzing and mining epi-transcriptome data. Front. Genet. 2021, 12, 654820. [Google Scholar] [CrossRef]
- Gan, Z.; Wei, W.; Wu, J.; Zhao, Y.; Zhang, L.; Wang, T.; Zhong, X. Resveratrol and curcumin improve intestinal mucosal integrity and decrease m6A RNA methylation in the intestine of weaning piglets. ACS Omega 2019, 4, 17438–17446. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ren, Z.; Zeng, H.; Yao, B. Apparent digestibility of various feedstuffs for bluntnose black bream Megalobrama amblycephala Yih. Aquac. Nutr. 2008, 14, 153–165. [Google Scholar] [CrossRef]
- Sun, D.; Huang, F. Analysis of the current situation and development strategies of the aquaculture of Megalobrama amblyocephala in Gehu. J. Aquac. 2019, 40, 47–49. [Google Scholar] [CrossRef]
- Jin, M.; Pan, T.; Cheng, X.; Zhu, T.T.; Sun, P.; Zhou, F.; Ding, X.; Zhou, Q. Effects of supplemental dietary l-carnitine and bile acids on growth performance, antioxidant and immune ability, histopathological changes and inflammatory response in juvenile black seabream (Acanthopagrus schlegelii) fed high-fat diet. Aquaculture 2019, 504, 199–209. [Google Scholar] [CrossRef]
- Xie, J.; Liao, S.; Wang, R.; He, X.; Fang, H.; Zhuang, Z.; Wei, D.; Tian, L.; Liu, Y.; Niu, J. Molecular cloning, functional characterization and expression analysis of p65 subunit of golden pompano (Trachinotus ovatus) and response to high fat diet and LPS administration. Aquaculture 2020, 514, 734508. [Google Scholar] [CrossRef]
- Deol, P.; Ruegger, P.; Logan, G.D.; Shawki, A.; Li, J.; Mitchell, J.D.; Yu, J.; Piamthai, V.; Radi, S.H.; Hasnain, S.; et al. Diet high in linoleic acid dysregulates the intestinal endocannabinoid system and increases susceptibility to colitis in Mice. Gut Microbes 2023, 15, 229945. [Google Scholar] [CrossRef]
- de Roos, B.; Mavrommatis, Y.; Brouwer, I.A. Long-chain n-3 polyunsaturated fatty acids: New insights into mechanisms relating to inflammation and coronary heart disease. Br. J. Pharmacol. 2009, 158, 413–428. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, N.; Jiang, H.; Xue, W.; Guo, X.; Xu, X.; Fang, W.; Wang, H.; Hua, E. Resveratrol treatment improves plasma and blood glucose concentration and lipid metabolism in high-fat-fed C57BL/6J mice. Eur. Food Res. Technol. 2016, 592, 741170. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Fan, Z.; Wang, L.; Zheng, X. Resveratrol ameliorates oxidative stress, inflammatory response and lipid metabolism in common carp (Cyprinus carpio) fed with high-fat diet. Front. Immunol. 2022, 13, 965954. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, Y.; Tian, H.; Jiang, G.; Li, X.; Liu, W. Resveratrol supplementation improves lipid and glucose metabolism in high-fat diet-fed blunt snout bream. Fish Physiol. Biochem. 2017, 44, 163–173. [Google Scholar] [CrossRef]
- Tong, L.; Chen, Z.; Li, Y.; Wang, X.; Yang, C.; Li, Y.; Zhu, Y.; Lu, Y.; Liu, Q.; Xu, N.; et al. Transketolase promotes MAFLD by limiting inosine-induced mitochondrial activity. Cell Metab. 2024, 36, 1013–1029. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shi, Y.; Yang, X.; Gao, J.; Nie, Z.; Xu, G. Effects of resveratrol on lipid metabolism in liver of red tilapia Oreochromis niloticus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 261, 109408. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhao, H.; Shu, L.; Xing, H.; Wang, C.; Lu, C.; Song, G. Effect of resveratrol on intestinal tight junction proteins and the gut microbiome in high-fat diet-fed insulin resistant mice. Int. J. Food Sci. Nutr. 2020, 71, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, Y.; Lin, X.; Huang, J.; Zhang, F.; Chen, C.; Ren, H.; Zheng, S.; Yang, J.; Hui, S. Resveratrol improves diabetic kidney disease by modulating the gut microbiota-short chain fatty acids axis in db/db mice. Int. J. Food Sci. Nutr. 2024, 75, 264–276. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut firmicutes: Relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 63, 12073–12088. [Google Scholar] [CrossRef]
- Li, X.; Sun, F.; Zhang, Y.; Guo, Y.; Men, Q. Analysis of gut microbiota in rats with non-alcoholic fatty liver disease based on 16S rRNA gene sequencing technology. Chin. J. Hepatol. 2023, 15, 39–46. [Google Scholar] [CrossRef]
- Qiao, B.; Liu, J.; Deng, N.; Cai, Y.; Bian, Y.; Wu, Y.; Tan, Z. Gut content microbiota dysbiosis and dysregulated lipid metabolism in diarrhea caused by high-fat diet in a fatigued state. Food Funct. 2023, 14, 3880. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, Y.; Wang, L.; Han, J.; Song, Z.; Hu, G.; Li, G.; Fan, Z. The effects of resveratrol on growth, lipid metabolism, and antioxidant capacity of rats fed with high-fat diet. Acta Vet. Zootech. Sin. 2017, 48, 2216–2224. [Google Scholar] [CrossRef]
- Zeisel, M.B.; Dhawan, P.; Baumert, T.F. Tight junction proteins in gastrointestinal and liver disease. Gut 2018, 68, 316906. [Google Scholar] [CrossRef] [PubMed]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-fat, western-style diet, systemic inflammation, and gut microbiota: A narrative review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yu, X.; Zhao, P.; Huang, J.; Huang, X. Effects of resveratrol on tight junction proteins and the Notch1 pathway in an HT-29 cell model of inflammation induced by lipopolysaccharide. Inflammation 2022, 45, 2449–2464. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, J.; Ke, W.; Wang, J.; Li, D.; Liu, R.; Jia, Y.; Wang, X.; Chen, X.; Chen, F.; et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic. Biol. Med. 2020, 156, 83–98. [Google Scholar] [CrossRef]
- Dominissini, D.; Moshitch-Moshkovitz, S.; Schwartz, S.; Salmon-Divon, M.; Ungar, L.; Osenberg, S.; Cesarkas, K.; Jacob-Hirsch, J.; Amariglio, N.; Kupiec, M.; et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 2012, 485, 201–206. [Google Scholar] [CrossRef]
- Huang, J.; Yin, H.; Zhang, Y.; Zhang, C.; Qiao, H.; Wang, J.; Liu, L. Regulation of resveratrol on intestinal mucosal barrier function based on TGF-β signaling pathway. Feed Res. 2019, 42, 112–114. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, B.; Li, Q.; Zhao, T.; Xu, P.; Song, Y.; Luo, Z. Dietary lecithin attenuates adverse effects of high fat diet on growth performance, lipid metabolism, endoplasmic reticulum stress and antioxidant capacity in the intestine of largemouth bass (Micropterus salmoides). Aquaculture 2024, 595, 741688. [Google Scholar] [CrossRef]
- Zhu, H.; Li, X.; Qiao, M.; Sun, X.; Li, G. Resveratrol alleviates inflammation and ER stress through SIRT1/NRF2 to delay ovarian aging in a short-lived fish. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 596–602. [Google Scholar] [CrossRef]
- Wang, B.; Ge, S.; Xiong, W.; Xue, Z. Effects of resveratrol pretreatment on endoplasmic reticulum stress and cognitive function after surgery in aged mice. BMC Anesthesiol. 2018, 18, 141. [Google Scholar] [CrossRef]
- Buck, T.M.; Wright, C.M.; Brodsky, J.L. The activities and function of molecular chaperones in the endoplasmic reticulum. Semin. Cell Dev. Biol. 2007, 18, 751–761. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Y.; Bai, J.; Yang, Y.; Wang, F.; Feng, Y.; Zhang, R.; Li, F.; Zhang, P.; Lv, N.; et al. Blockade of HSP70 by VER-155008 synergistically enhances bortezomib-induced cytotoxicity in multiple myeloma. Cell Stress Chaperones 2020, 25, 357–367. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, C.; Guo, T.; Liu, J.; Yang, B.; Lu, Z. Molecular Mechanism of m6A Methylation Modification Genes METTL3 and FTO in Regulating Heat Stress in Sheep. Int. J. Mol. Sci. 2023, 24, 11926. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, Y. Endoplasmic reticulum stress and liver injury in non-alcoholic fatty liver disease. Chin. Hepatol. 2023, 28, 633–636. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, Y.; Hu, G. Research progress on the relationship between endoplasmic reticulum stress and hepatic lipid metabolism. J. Toxicol. 2015, 29, 231–234. [Google Scholar] [CrossRef]
- Ke, X.; You, K.; Pichaud, M.; Haiser, H.J.; Graham, D.B.; Vlamakis, H.; Porter, J.A.; Xavier, R.J. Gut bacterial metabolites modulate endoplasmic reticulum stress. Genome Biol. 2021, 22, 292. [Google Scholar] [CrossRef]
- Duan, J.; Matute, J.D.; Unger, L.W.; Hanley, T.; Schnell, A.; Lin, X.; Krupka, N.; Griebel, P.; Lambden, C.; Sit, B.; et al. Endoplasmic reticulum stress in the intestinal epithelium initiates purine metabolite synthesis and promotes Th17 cell differentiation in the gut. Immunity 2023, 56, 1115–1131. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y. Effects of Resveratrol and Quercetin on Lipid Metabolism, Growth Performance, and Antioxidant Capacity of Megalobrama amblycephala Fed High-Fat Diet. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2017. [Google Scholar]
- Shen, S.; Liao, Q.; Zhang, T.; Pan, R.; Lin, L. Myricanol modulates skeletal muscle-adipose tissue crosstalk to alleviate high-fat diet-induced obesity and insulin resistance. Br. J. Pharmacol. 2019, 176, 3983–4001. [Google Scholar] [CrossRef]
- Kamyab-Hesary, K.; Ghanadan, A.; Balighi, K.; Mousavinia, S.F.; Nasimi, M. Immunohistochemical Staining in the Assessment of Melanoma Tumor Thickness. Pathol. Oncol. Res. 2019, 26, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Lu, S.; Jiang, W.; Mu, Q.; Lin, Y.; Gu, Z.; Wu, Y.; Ge, X.; Miao, L. Lactobacillus plantarum Alters Gut Microbiota and Metabolites Composition to Improve High Starch Metabolism in Megalobrama amblycephala. Animals 2025, 15, 583. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef]
- Jiang, W.; Lin, Y.; Qian, L.; Lu, S.; Gu, Z.; Ge, X.; Miao, L. m6A Methylation Mediated Autophagy and Nucleotide-Binding Oligomerization Domain-like Receptors Signaling Pathway Provides New Insight into the Mitigation of Oxidative Damage by Mulberry Leaf Polysaccharides. Int. J. Mol. Sci. 2025, 26, 4345. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]






| Gene | GeneID | DiffModLog2FC 1 | p-Value | Chromsome | Start | End | Peak-Length | Region |
|---|---|---|---|---|---|---|---|---|
| nynrin | LOC125243639 | 6.44 | 0.00 | NC_063056.1 | 7,889,886 | 7,890,097 | 212 | 3′UTR |
| histone H3. v1 | LOC125250419 | 5.66 | 0.00 | NC_063060.1 | 41,658,950 | 41,662,658 | 3709 | 3′UTR |
| neu4 | LOC125252118 | 5.66 | 0.00 | NC_063061.1 | 11,575,766 | 11,576,660 | 895 | exonic |
| ceacam1 | LOC125263488 | 5.25 | 0.03 | NC_063045.1 | 9,519,250 | 9,519,431 | 182 | exonic |
| otud7a | otud7a | 5.09 | 0.04 | NC_063058.1 | 9,780,589 | 9,780,739 | 151 | 3′UTR |
| irf4 | LOC125253556 | 5.04 | 0.00 | NC_063061.1 | 7,605,408 | 7,612,562 | 7155 | 3′UTR |
| trim39 | LOC125263520 | −4.92 | 0.00 | NC_063045.1 | 10,315,588 | 10,315,953 | 366 | exonic |
| ttc38 | LOC125253910 | −4.30 | 0.00 | NC_063062.1 | 22,714,721 | 22,715,566 | 846 | 5′UTR |
| znf184 | LOC125248635 | 4.06 | 0.01 | NC_063059.1 | 40,038,026 | 40,049,733 | 354 | 3′UTR |
| slit1a | slit1a | 4.03 | 0.00 | NC_063053.1 | 37,082,927 | 37,183,886 | 691 | 3′UTR |
| slc2a12 | slc2a12 | 3.81 | 0.02 | NC_063067.1 | 15,936,237 | 15,936,508 | 272 | exonic |
| zfy1 | LOC125266323 | 3.64 | 0.02 | NC_063047.1 | 3,706,888 | 3,707,277 | 390 | exonic |
| bmp1a | bmp1a | 3.64 | 0.03 | NC_063055.1 | 35,350,248 | 35,350,428 | 181 | 3′UTR |
| cyyr1 | cyyr1 | 3.54 | 0.01 | NC_063050.1 | 56,166,843 | 56,167,050 | 208 | 5′UTR |
| mtres1 | mtres1 | 3.42 | 0.03 | NC_063053.1 | 20,679,502 | 20,682,293 | 2792 | 5′UTR |
| LOC125253164 | LOC125253164 | 3.39 | 0.01 | NC_063061.1 | 32,536,181 | 32,536,362 | 182 | exonic |
| nfe2l1b | nfe2l1b | 3.38 | 0.02 | NC_063063.1 | 20,348,636 | 20,348,876 | 241 | exonic |
| znf692 | znf692 | 3.32 | 0.03 | NC_063055.1 | 13,164,878 | 13,165,057 | 180 | exonic |
| LOC125260582 | LOC125260582 | −3.30 | 0.00 | NC_063067.1 | 4,134,964 | 4,135,136 | 173 | exonic |
| ri | LOC125250829 | −3.14 | 0.02 | NC_063060.1 | 47,937,450 | 47,937,624 | 175 | 3′UTR |
| Ingredient | CON | HF | HF + 0.06%RES | HF + 0.3%RES | HF + 0.6%RES |
|---|---|---|---|---|---|
| Fishmeal 1 | 6.00 | 6.00 | 6.00 | 6.00 | 6.00 |
| Soybean meal (46%) 1 | 30.00 | 30.00 | 30.00 | 30.00 | 30.00 |
| Rapeseed meal 1 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Cottonseed meal 1 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Cottonseed protein concentrate1 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| Wheat flour 1 | 18.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Rice bran 1 | 6.00 | 7.00 | 7.00 | 7.00 | 7.00 |
| Soybean oil 2 | 4.50 | 11.50 | 11.50 | 11.50 | 11.50 |
| Calcium dihydrogen phosphate 1 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Mineral premix 1 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin premix 1 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin C 1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Choline chloride 1 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Microcrystalline cellulose 1 | 5.00 | 5.00 | 4.94 | 4.70 | 4.40 |
| RES | 0 | 0 | 0.06 | 0.30 | 0.60 |
| Total | 100.00 | 100.00 | 100 | 100 | 100 |
| Nutrition composition (air dry basis) | |||||
| Crude protein, % | 32.75 | 31.79 | 31.94 | 32.03 | 31.68 |
| Crude fat, % | 7.70 | 14.76 | 14.46 | 14.35 | 14.58 |
| Gross energy (KJ/g) | 16.23 | 15.01 | 14.73 | 15.16 | 14.91 |
| Gene | Primer Sequence (5′-3′) | Product Length (bps) | Accession No. |
|---|---|---|---|
| tlr4 | F: GAATGCTGGACAAGGACAGGA | 102 | XM_048204248.1 |
| R: GTGATAGGAAGACTGCTGGGA | |||
| nf-κb | F: AGTCCGATCCATCCGCACTA | 85 | XM_048176853.1 |
| R: ACTGGAGCCGGTCATTTCAG | |||
| il-1β | F: ACCAGCACGACCTTGCAGTG | 174 | XM_048181166.1 |
| R: CTGGGATGCATTCGGTTTGA | |||
| jam2 | F: CCTCCGTGGTGTTACACAGA | 105 | XM_048193479.1 |
| R: AGCACATTGAGGGTGACGAT | |||
| zo1 | F: CCTCTGGTGATGTGTGGTCC | 75 | XM_048184358.1 |
| R: AGACGCACAATGAGGTAGGC | |||
| claudin41 | F: TTGTGATTGGGATCCTGGGC | 84 | XM_048167602.1 |
| R: TGGTTTTGGAGCTCTCGTCC | |||
| ppar-α | F: GTGCCAATACTGTCGCTTTCAG | 104 | XM_048158021.1 |
| R: CCGCCTTTAACCTCAGCTTCT | |||
| ppar-β | F: CATCCTCACGGGCAAGAC | 150 | XM_048209548.1 |
| R: TGGCAGCGGTAGAAGACA | |||
| lpl | F: TCTGATGGGATCTGGCAC | 85 | XM_048164066.1 |
| R: GTTTCTGGATTTGGGTCG | |||
| cyp7a1 | F: TTTCCGTCAGACGCTTCAGG | 123 | XM_048186424.1 |
| R: CCCTTCTTCAAGCCAGTCGT | |||
| hsp70 | F: CCAGGTGTACGAGGGAGAGA | 113 | XM_048209656.1 |
| R: AGGTCACTTCAATCTGCGGG | |||
| atf6 | F: CGATCAGGATGGAGAGTGGGATA | 108.30 | XM_048207041.1 |
| R: AGGGCTACTCCACAATGGGT | |||
| chop | F: ATGTGGTGCAGAGTTGGAGG | 108.20 | XM_048198700.1 |
| R: CACATCCAGAAACTCGGGCT | |||
| bcl-2 | F: CGTCTACCTGGACAACCACA | 106.50 | XM_048179299.1 |
| R: GCGTTTCTGTGCAATGAGTG | |||
| mtor | F: ACGGTCTCTACTCTGCCAGT | 106.30 | XM_048206869.1 |
| R: ACCAGGGGGCATAAAACTCG | |||
| mettl3 | F: GTTTGCAGTGGTGATGGCTG | 103.20 | XM_048185876.1 |
| R: TTTCGTGTGCCTGGAGACAG | |||
| fto | F: GATTCTGCAGCTGGTGGACT | 103.50 | XM_048184627.1 |
| R: GTCTGTCTGTGCTGCTGTCT | |||
| alkbh5 | F: GATCGATGAGGTGGTTGCCA | 109.20 | XM_048197746.1 |
| R: TACGTGTAGCCCTCTCCGAA | |||
| ythdf2 | F: GGACAAGTGGAAGGGACGTT | 100.40 | XM_048191909.1 |
| R: TCCAGTGGAACCTCCTGAGT | |||
| β-actin | F: TCGTCCACCGCAAATGCTTCTA | 152 | AY170122.2 |
| R: CCGTCACCTTCACCGTTCCAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Mu, Q.; Qian, L.; Lin, Y.; Jiang, W.; Lu, S.; Miao, L.; Ge, X. Integrated Multi-Omics Analysis Reveals the Role of Resveratrol in Regulating the Intestinal Function of Megalobrama amblycephala via m6A Methylation. Int. J. Mol. Sci. 2025, 26, 8587. https://doi.org/10.3390/ijms26178587
Gu Z, Mu Q, Qian L, Lin Y, Jiang W, Lu S, Miao L, Ge X. Integrated Multi-Omics Analysis Reveals the Role of Resveratrol in Regulating the Intestinal Function of Megalobrama amblycephala via m6A Methylation. International Journal of Molecular Sciences. 2025; 26(17):8587. https://doi.org/10.3390/ijms26178587
Chicago/Turabian StyleGu, Zhengyan, Qiaoqiao Mu, Linjie Qian, Yan Lin, Wenqiang Jiang, Siyue Lu, Linghong Miao, and Xianping Ge. 2025. "Integrated Multi-Omics Analysis Reveals the Role of Resveratrol in Regulating the Intestinal Function of Megalobrama amblycephala via m6A Methylation" International Journal of Molecular Sciences 26, no. 17: 8587. https://doi.org/10.3390/ijms26178587
APA StyleGu, Z., Mu, Q., Qian, L., Lin, Y., Jiang, W., Lu, S., Miao, L., & Ge, X. (2025). Integrated Multi-Omics Analysis Reveals the Role of Resveratrol in Regulating the Intestinal Function of Megalobrama amblycephala via m6A Methylation. International Journal of Molecular Sciences, 26(17), 8587. https://doi.org/10.3390/ijms26178587








