Abstract
Despite intensive worldwide research efforts and multiple available therapeutic schemes for cancer treatment, cancer still remains a challenge, rendering the need for the discovery of new therapeutic approaches imperative. Photodynamic therapy (PDT) is a novel, non-invasive anti-cancer treatment that relies on the generation of reactive oxygen species (ROS) that are cytotoxic to cancer cells. ROS are generated by the interaction between a photosensitizer (PS) drug, a light source (primarily a laser), and oxygen. Although PDT offers the advantage of using non-ionizing radiation and bears great therapeutic potential, it has not yet been widely adopted in clinical practice. This review summarizes the new developments in the use of PDT in combination with chemotherapy, immunotherapy, and radiotherapy, giving emphasis to the combination of PDT with a novel type of therapy that also takes into account the tumor microenvironment (TME) to enhance treatment efficacy. TME-targeting therapies include strategies like hypoxia modulation, vascular normalization, and immune cell reprogramming. Interestingly, when combined with PDT, these therapies can improve therapeutic outcomes while reducing side effects, and nanoparticle-based delivery systems have demonstrated the potential to enhance PDT selectivity and efficiency. This review highlights PDT’s enormous potential in treating various cancer types and underscores the need for continued exploration of combination therapies to maximize its clinical impact.
1. Introduction
1.1. Cancer and Tumor Microenvironment (TME)
Cancer is characterized by abnormal cell proliferation, eventually leading to the formation of a tumor. When the tumor becomes malignant, cells acquire the capacity to dissociate from the original tumor mass, migrate, and invade through surrounding tissues and finally establish a new metastatic tumor in distant parts of the body. Although a tumor was originally thought to include only cancer cells, it is now known that cancer cells comprise only a part of what is known as the tumor microenvironment (TME) [1]. In fact, the TME is highly heterogeneous among patients, as although the main components are present in all tumors, the exact amount may differ significantly between tumors. Thus, various tumors may differ in the content of the TME, which may include varying amounts and compositions of extracellular matrix (ECM), the presence of immune cells [T and B lymphocytes, dendritic cells, natural killer cells, neutrophils, myeloid-derived suppressor cells (MDSC), and tumor-associated macrophages (TAMs)], cells of the vasculature (pericytes and endothelial cells), as well as stromal cells, such as fibroblasts and cancer-associated fibroblasts (CAFs) [2]. All TME components contribute to the cancer phenotype and are responsible for the observed inter-patient heterogeneity [2,3]. TME composition is therefore crucial for tumor progression and metastasis, while the amount of ECM and the resulting tumor stiffness have also been associated with increased tumor invasiveness and metastasis as well as therapy resistance [3].
Although extensive research has been conducted on cancer biology and the discovery of novel therapeutic approaches, effective cancer treatment is often a true challenge, posing more problems than offering a cure [4]. More specifically, conventional therapies such as chemotherapy and radiotherapy are usually accompanied by substantial toxic effects that affect the patients’ quality of life [5,6]. Moreover, despite the promising results of immunotherapy, this treatment modality can also be challenging, as its efficacy cannot be predicted, while developing resistance is also quite common. Thus, more research is imperative to develop more targeted and less toxic anti-cancer therapies or enhance the combination of existing therapies and reduce their side effects.
Among the novel anti-cancer strategies, photodynamic therapy (PDT) is gaining ground as an attractive therapeutic approach that could be easily combined with other therapies to enhance therapeutic outcome. PDT combines the use of a photosensitizer (PS) drug, a light source, primarily laser, and oxygen, and it can therefore be combined both with classical therapies, such as chemotherapy and radiotherapy, and with novel therapeutic approaches, such as TME-targeting therapies that aim to disrupt its pro-tumorigenic properties, and include antiangiogenic agents, ECM-modulating treatments, and immune-targeting strategies [3], as well as inhibitors of hypoxia-inducible factor-1 (HIF-1) [2]. In this review, we briefly present the key characteristics of PDT and its principles and highlight the effects of combining PDT with other therapeutic approaches, giving emphasis to the emerging TME-targeting therapies.
We, thus, explore the potential of PDT in combination with other cancer therapies, with a primary focus on TME-targeting strategies. This review aims to evaluate how these combination approaches enhance PDT efficacy, mitigate its limitations, and improve overall cancer treatment outcomes, highlighting promising avenues for future clinical application.
1.2. An Overview of Photodynamic Therapy (PDT)
PDT is a non-invasive treatment that relies on the use of non-toxic components to generate reactive oxygen species (ROS) that are cytotoxic to cancer cells. Specifically, in PDT, there is interaction between a PS, a light source, and oxygen, which individually are non-toxic, to generate ROS [7]. Since each component is non-toxic on its own, PDT provides a targeted therapeutic effect with minimal systemic toxicity. The PS, a light-activated drug, can be administered orally, intravenously, or locally. During the drug-to-light interval (DLI), it selectively accumulates in tumor tissues, allowing precise activation when exposed to light of a specific wavelength [8]. Figure 1 shows the general concept of the application of PDT in the clinical setting.
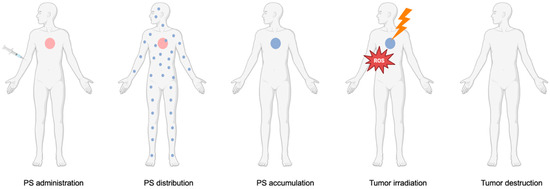
Figure 1.
Application of PDT in the clinical setting. The PS is administered to the patient and distributed systemically. Once it accumulates in the tumor, the targeted area is irradiated with a light source, leading to ROS production and subsequent tumor destruction.
While the exact mechanisms for the accumulation of PSs in the tumor tissues remain unclear, several factors have been proposed to contribute to the selective uptake by tumor sites [9]. One of these factors is the enhanced permeability and retention (EPR) effect that is characteristic of the TME, where blood vessels appear leaky with poor organization, resulting in easier PS accumulation than in normal tissues, where the vasculature is well organized and non-leaky. The EPR effect is accompanied by defective lymphatic drainage in tumors that increases PS retention at the tumor site [10]. PSs tend to bind to low-density lipoprotein (LDL) receptors, so another factor that affects the selective accumulation of PSs could be the increased expression of LDL receptors present in various cancer types [9].
Interestingly, compared to conventional cancer treatments such as chemotherapy and radiotherapy, PDT offers several advantages. More specifically, it is minimally invasive, does not require overnight hospitalization of the patient, and has fewer systemic side effects due to its localized nature [8]. Additionally, PDT can be applied repeatedly without significant toxicity, making it a viable option for long-term cancer management. Beyond its direct cytotoxic effects, PDT can stimulate immune responses and disrupt pathways involved in drug resistance, further enhancing its therapeutic potential [11]. Moreover, because PS activation occurs only in the presence of light, systemic toxicity remains low [12]. Finally, PDT has also been associated with impressive cosmetic outcomes, particularly in surface/skin cancers [8].
Despite these advantages, PDT also has certain limitations in its application. First, the depth of light penetration is restricted, making it less effective for treating deep-seated tumors. Another limitation is that PDT cannot be used for treating large tumors because light cannot penetrate deeply into biological tissues, while singlet oxygen, which is produced from the PDT reaction, has a short half-life, so PDT cannot be applied in large tissue areas. Moreover, since PDT is a localized treatment, it cannot be applied to cancer of advanced stage that has already metastasized, so it can only be used for treating regional malignancies and lesions of precancerous stage [13]. Finally, PDT has been associated with skin photosensitivity in patients after treatment [8], especially with certain PSs of the so-called first-generation PSs, as patients had to avoid light exposure, even from the sun, with clear consequences on patients’ life quality.
To address these limitations, current research efforts are focusing not only on developing better PSs and appropriate light sources but also on combining PDT with other cancer therapies, including chemotherapy, radiotherapy, and immunotherapy. In fact, since PDT targets different cellular mechanisms from chemotherapy and radiotherapy, the combined therapy can enhance treatment efficacy while reducing drug dose and associated side effects. Additionally, PDT has also been used in combination with antibody-based immunotherapies to improve tumor targeting [13]. Overall, these synergistic strategies have been shown to improve PDT’s therapeutic index, allowing for better tumor management and fewer side effects [14].
1.3. PDT’s Mechanism of Action
As mentioned above, one of the key components of PDT is the use of PSs, which, depending on their characteristics, can be categorized into three generations. The first generation emerged in the 19th century and consists of hematoporphyrin (Hp) and its derivatives (HpD). Hp has a heterotypical nature, and large doses were required to be effective as a cancer diagnostic tool, so it was purified further in the form of photofrin [7,15,16].
The second generation of PS overcame some serious disadvantages of the first generation, like short light wavelength and prolonged accumulation of the PS in normal tissues, and was based on chlorin and porphyrin structures that have a longer wavelength than those of the first generation [15]. These PSs have a high absorbance in the deep red, so they have an increased penetration of light, resulting in a more effective action towards the tumor [8]. One of the major disadvantages of second-generation PSs, however, is their low water solubility, which severely restricts their intravenous administration, presenting several challenges [16].
The third-generation PSs have a higher selectivity for regions of tumor tissues by conjugating or encapsulating PSs in carriers that can be transported to targeted tissues [15]. Antibodies, liposomes, carbohydrates, and proteins can be used as carriers to improve the chemical, physical, and therapeutic abilities of PSs [8]. Finally, third-generation PSs are designed to have better absorption of the ideal wavelength to improve tissue penetration [7]. A summary of the key characteristics of each PS generation is shown in Table 1 below.

Table 1.
Summary of the key characteristics of PDT.
Another key component of PDT is the selection of the appropriate light source. Various light sources have been employed so far based on the tumor’s location and the PS used, with lamps and lasers being the most commonly used. More recently, light-emitting diodes (LEDs) have emerged as a promising alternative for enhancing treatment flexibility and efficiency [17]. LEDs are suitable for PSs sharing the same wavelength and can be made portable. However, their large beam size and poor quality limit their use in endoscopic applications, as they cannot be paired with flexible optical fibers. For deeper tumors, lasers and LEDs are preferred due to their compatibility with flexible fibers. Natural sunlight can also be used for PDT, primarily in the treatment of skin lesions. When light penetrates the tumor tissue, it undergoes scattering, reflection, or absorption, which is affected by tissue type and light wavelength. Due to tissue heterogeneity, light absorption varies across different regions [7]. Longer wavelengths (600–850 nm), such as red light, penetrate tissues more effectively, while shorter wavelengths (<600 nm) have limited penetration and increase skin photosensitivity. Wavelengths beyond 850 nm cannot excite oxygen to its singlet state, leading to insufficient ROS production [8].
Oxygen, another component of PDT, is essential for ROS generation, while its availability within tumor tissues directly impacts treatment effectiveness. However, oxygen levels can vary significantly between different tumor types and even within regions of the same tumor [8]. In fact, hypoxia in tumor tissues can impair the tumor vasculature, ultimately affecting the delivery route of the PS. Thus, hypoxic tumor areas are considered obstacles to PDT efficacy and are sometimes resistant to PDT [7].
The photodynamic reaction is initiated when photons from the light source are absorbed by the PS (Figure 2). This illumination transforms the ground state PS, which is called the singlet state (1PS), to its excited singlet state (1PS*) [18]. The excited singlet state lasts for a few nanoseconds, thus being very short-lived and unstable. Subsequently, and through intersystem crossing (ISC), the PS can transform into its triplet state, 3PS* [17]. This triplet state is long-lived and more stable than the singlet state, and it can undergo two types of reactions, namely Type l and Type ll. A Type l reaction occurs when the triplet state reacts with different substrates, yielding free radicals or radical ions. The reaction between the generated radicals and O2 results in the production of ROS like hydroxyl radical (HO•), hydrogen peroxide (H2O2), and superoxide anion (O2−•), which cause oxidative damage to the cells. In Type II reactions, the longer lifetime of the triplet state provides enough time for energy to be directly transferred to molecular oxygen (O2), leading to the production of singlet oxygen (1O2) [8].
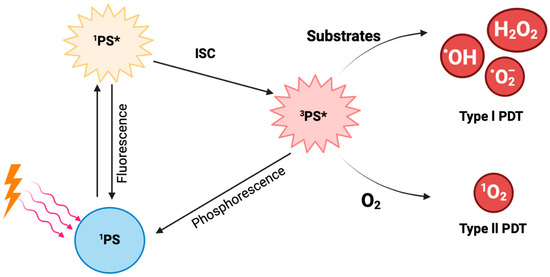
Figure 2.
Diagrammatic representation of the photodynamic reaction from the moment the PS absorbs photons from the light source to the generation of free radicals.
Interestingly, the two reaction types can occur at the same time, although it is widely accepted that most PSs mainly use the Type II reaction [17]. The rate by which these two reactions occur depends on oxygen concentration, the PS, the substrate present, and the PS’s binding affinity to the substrate [8].
Most PDT treatments that are currently applied are based on Type II reactions, so a large amount of oxygen is required during treatment. Consequently, Type II PDT reactions have the potential to make hypoxia worse and lead to a less effective therapeutic outcome [19]. The exact role of oxygen in Type I PDT remains debated, but studies indicate that Type I PDT is more effective than Type II in hypoxic environments. A way to increase the presence of oxygen in tumor tissues is the use of hyperbaric oxygen (HBO) treatment, where patients are provided with pure oxygen in a pressurized chamber. This method increases oxygen availability and alleviates hypoxia in tumor tissues [20]. Another approach involves directly transporting oxygen to the tumor using carriers like hemoglobin and perfluorocarbons [8].
2. Combination of PDT with Other Therapies
In order to maximize the benefits from PDT treatment while overcoming current limitations, PDT is given in combination with other therapeutic approaches. More specifically, PDT has been used in combination with the following:
A. Chemotherapy: In chemotherapy, cytotoxic agents are administered to the patient, aiming to destroy the tumor [21]. Several studies have combined chemotherapeutic agents with PDT. Morita et al. (2020) investigated the safety and efficacy of combining PDT with chemotherapy [22]. The study demonstrated that combining bexarotene, used for cutaneous T-cell lymphoma treatment, with PDT resulted in a better therapeutic outcome compared to bexarotene monotherapy. Along the same line, Zhang et al. (2023) investigated a pH-responsive drug delivery system based on conjugated polymers (PFE-DOX-2) for effective synergistic chemotherapy combined with PDT for treating breast cancer [23]. Doxorubicin (DOX), a widely used chemotherapeutic agent, was attached to the polymers to prevent premature drug leakage and enhance tumor cell uptake. The combined treatment with PDT significantly reduced cell viability and increased necrosis, demonstrating the enhanced therapeutic potential of this approach. The combination of DOX with PDT was also used by Chilakamarthi et al. (2023), where low-dose DOX was combined with a porphyrin-based PS (P-nap) to treat colorectal cancer in mice [24]. The combination therapy, along with irradiation, led to significant tumor reduction by increasing the Bax/Bcl-xL ratio and p53 expression and promoting apoptosis. Additionally, the downregulation of Phosphoinositide 3-kinase (PI3K) and Cyclooxygenase 2 (COX-2) pathways contributed to tumor suppression and relapse prevention. Other chemotherapeutic agents have also been explored, such as ruthenium-based complexes used to generate PSs for colorectal cancer treatment. The ruthenium-enhanced drug internalization led to a higher number of cancer cells arrested in the S phase, while upon irradiation, the treated cell lines showed reduced viability, increased ROS production, and enhanced apoptosis [25].
B. Immunotherapy: In immunotherapy, the tumor is destroyed by stimulating distinct components of the patient’s immune system [26]. As immunotherapy emerges as a promising strategy for treating various cancer types, its combination with other therapeutic modalities, including PDT, has gathered significant scientific interest due to its potential to enhance treatment efficacy. One promising example is the combination of nanoparticles loaded with the PS drug Temoporfin and a PD-L1 blockade antibody, which has demonstrated the ability to inhibit primary tumor growth and delay the progression of distant tumors in colorectal cancer [27]. Since PDT often upregulates PD-L1 expression, allowing cancer cells to evade immune detection, the addition of a PD-L1 inhibitor effectively counteracts this mechanism by enhancing immune recognition and promoting apoptosis. This combination not only improved tumor suppression but also promoted long-term survival by significantly enhancing the activation and response of CD8+ T cells, a crucial component of the antitumor immune response [27]. PDT was also combined with an autophagy inhibitor (CQ) for treating colorectal cancer and resulted in the enhancement of the maturation of infiltrating CD8+ T cells and DCs [28]. Two other studies utilized the combination of PDT with monoclonal antibodies and its effectiveness as a cancer therapeutic. One of them used an immunotoxin (saporin) conjugated to cetuximab, an Epidermal Growth Factor Receptor (EGFR) inhibitor, causing cell death, inhibiting recurrence, and increasing antitumor cytotoxicity for the treatment of lung cancer [29]. Similarly, Yamashita et al. (2023) showed that the use of monoclonal antibodies (panitumumab or trastuzumab) along with PDT induces cell death 24 h after radiation and binds specifically to cancer cells, sparing the neighboring tissues from any undesired effects of the treatment [30]. Additionally, the combination of PDT with sorafenib, a multi-kinase inhibitor known to induce necrosis, has been explored. When delivered via nanoparticles that enhance drug internalization, this combination demonstrated an improved antitumor effect in thyroid and breast cancers. In the study, nanoparticles loaded with the PS drug chlorin e6 and sorafenib significantly reduced cell viability and tumor volume. The treatment also promoted increased recruitment of cytotoxic T cells and enhanced inflammation, contributing to tumor suppression in both primary and distant lesions [31].
Another way of combining immunotherapy and PDT is with the use of vaccines. Cancer cells treated with PDT were used to prime dendritic cells (DC) to create vaccines. This method was shown to enhance the response of cytotoxic T cells (CTL) against tumors [32], increase survival, decrease tumor size [33], and increase the number of CD8+ T cells in the lymph nodes surrounding the tumor area [34]. PDT-treated cancer cells were used to create vaccines by another team that used a stimulant of immune activities (GC) along with the vaccine to treat tumors. The results suggest that this combination has an additive therapeutic effect as the number of dead cells almost doubled and the progression of the tumor was delayed [35]. Lastly, the combination of PDT with another vaccine type (FlaB-Vax) was shown to extend survival, suppress both primary and secondary tumors, and induce systemic CD8+ T-cell tumor-specific responses [36].
However, although combining PDT with chemotherapy, radiotherapy, or immunotherapy has shown significant promise, additional combined therapies have also been suggested to include other targeted treatments. For instance, the combination of PDT with vismodegib, a drug targeting the Sonic Hedgehog signaling pathway, has been explored in basal-cell carcinoma patients. This combination resulted in excellent cosmetic outcomes with minimal adverse effects on patients’ daily activities [37].
C. Radiotherapy: In radiotherapy, ionizing radiation is used to destroy cancer cells, inhibiting their growth [38]. Recent years have seen growing scientific interest in combining PDT with radiotherapy. To the best of our knowledge, only a couple of research articles present the combination of PDT with radiotherapy. For instance, Bulin et al. (2019) combined PDT with X-rays to treat colorectal cancer [39]. Cells treated with both modalities showed significantly higher necrosis compared to those treated with either PDT or radiotherapy alone. The combination also led to a greater reduction in tumor size, inhibiting cancer cell growth, while PDT induced necrosis, creating a synergistic therapeutic effect when applied together. Another study by Mayahi et al. (2019) showed that PDT followed by X-ray radiation increased cell death in MCF-7 cells and significantly decreased cell survival [40]. Liu et al. (2021) used nanoparticles (RGD-PEG-PAA-MN@LM) for PDT combined with radiotherapy [41]. Their findings indicate that using these nanoparticles for PDT followed by X-ray irradiation can increase ROS production and decrease the tumor size. Lastly, the team of Zhou et al. (2023) developed a nanoplatform containing red blood cell-coated nanoparticles loaded with the PS Ce6 for PDT and radiotherapy combination [42]. This system enabled deep-tissue treatment and triggered antitumor immune responses, effectively suppressing tumor growth and metastasis.
D. As part of triple-combination strategies: Several studies have compared different therapeutic combinations or explored triple-combination approaches. One study examined a triple combination of PDT with radiotherapy and immunotherapy. PDT-killed SCC7 cells were used to prime dendritic cells (DC) to create vaccines. Mice with squamous-cell carcinoma (SCC7) tumors treated with the DC-based vaccine showed improved survival rates and delayed tumor growth compared to PDT alone. In fact, when irradiation was performed around the time of vaccination, tumor growth inhibition was further enhanced compared to pre-vaccination irradiation [43]. Another study demonstrated that combining PDT with chemotherapy and immunotherapy was also effective in inhibiting tumor growth in both primary and metastatic lung lesions of triple-negative breast cancer (TNBC). The combination therapy utilized a prodrug capable of releasing DOX and caspase-3, which promoted apoptosis. When paired with immune checkpoint inhibition (anti-PD-L1), it further enhanced the maturation of dendritic cells and the activation of cytotoxic T lymphocytes [44]. Furthermore, a study by Kim et al. 2021 investigated the combination of PDT, a PD-1/PD-L1 immune checkpoint inhibitor, and the rho-kinase (ROCK) inhibitor ripasudil, resulting in significant tumor growth inhibition in both primary and metastatic lesions [45].
A summary of the studies that combined PDT with chemotherapy, radiotherapy, or immunotherapy is presented in Table 2 below.

Table 2.
Summary of studies combining PDT with chemotherapy, radiotherapy, or immunotherapy.
3. PDT and TME-Targeted Therapies
3.1. TME-Targeting Therapies
A novel anti-cancer therapeutic approach that is gaining ground is TME-targeting therapy.
TME-targeting therapy includes various strategies, all of which target different TME components.
- (a)
- One strategy is to try to reduce the ECM content of the tumor that usually affects drug delivery by compressing blood vessels. In this strategy, ECM components such as collagen are specifically targeted with angiotensin ll receptor agonists such as Losartan, which has been shown to reduce collagen l secretion [46].
- (b)
- Another strategy is using inhibitors of HIF-1to tackle hypoxia present in the TME, such as Topotecan, a topoisomerase 1 inhibitor, and Digoxin, which targets HIG1A [2,47].
- (c)
- A third strategy is to target pericytes and endothelial cells to prevent neovascularization using Anti-Vascular Endothelial Growth Factor (VEFG) antibodies, such as Bevacizumab, and mTOR inhibitors to decrease angiogenesis [48,49]. In fact, Bevacizumab, a humanized monoclonal antibody for VEGFA, was the first antiangiogenic therapy to receive FDA approval in 2004 for treating the TME of different cancers such as glioblastoma and cervical cancer [3].
- (d)
- The diverse immune composition within the TME can also be targeted through various routes. One approach is decreasing TAM and myeloid-derived suppressive cell (MDSC) infiltration by using small-molecule inhibitors or neutralizing antibodies against CDF-1 or CD204 [50,51,52]. IL-1 receptor antagonists, like Anakinra and Canakinumab, can be used for targeting chronic inflammation, which is closely related to carcinogenesis [53,54].
- (e)
- Finally, other targetable components of the TME are CAFs, with many strategies currently under investigation to modulate their activity, such as the targeting of fibroblast activation protein α (FAP) with an interleukin-2 variant (RO6874281), which targets FAP [2].
Thus, TME-targeting strategies encompass a wide range of approaches that interfere with distinct components of the tumor environment. These include reducing extracellular matrix density to improve vascular perfusion; alleviating hypoxia through HIF-1 inhibition or oxygen delivery; suppressing angiogenesis by targeting VEGF signaling; modulating immune infiltration, such as TAMs and MDSCs; and regulating CAF activity. Together, these strategies reshape the tumor milieu, enhance drug delivery, and create conditions that support the efficacy of complementary treatments such as PDT (Figure 3).
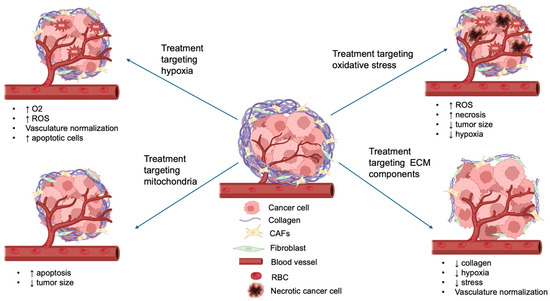
Figure 3.
Schematic overview of the TME components targeted by different therapeutic strategies in combination with PDT. The diagram highlights representative approaches against hypoxia, oxidative stress, extracellular matrix (ECM) components, and mitochondria, illustrating how these interventions enhance PDT efficacy (↑ indicates increase; ↓ indicates decrease).
Notably, TME-targeted therapies offer distinct advantages compared to other targeted strategies. Unlike conventional targeted therapies that act on single molecular drivers, TME-targeted approaches modulate the entire tumor ecosystem. By altering various TME components, these therapies can improve drug penetration and enhance the efficacy of co-administered treatments. Importantly, they also address tumor heterogeneity, which often limits the success of therapies directed against single oncogenic pathways. This broader mode of action makes TME-targeted therapy a highly complementary partner for PDT, providing synergistic effects that are less likely to be overcome by resistance mechanisms.
3.2. Combination of TME-Targeting Therapies
3.2.1. Targeting Hypoxia and TME Vasculature
With the growing interest in developing novel therapeutics that target the TME, combining TME-targeting therapies with PDT is a rather appealing approach. In fact, there have been several studies combining PDT with various TME-targeting therapies. Specifically, Lin et al. (2022) [55] used polymer-encapsulated carbonized hemin nanoparticles (P-CHNPs) as the PS drug to tackle the oxidative stress generated in the TME. Hemin is a Fe(III)-containing proto-porphyrin IX that can catalyze oxidation reactions and promote the production of ROS. Cells treated with P-CHNPs and irradiated exhibited an increased extent of necrosis, and the size of the tumors significantly decreased. The light from the irradiation during PDT further enhanced the conversion of H2O2 to HO• or O2, resulting in decreased hypoxia and enhancing the anti-cancer effect. Interestingly, the regulation of oxidative stress was more targeted to the TME than the normal surrounding tissues, and the tumor size was significantly reduced in contrast to when PDT is used alone, where the tumor growth is suppressed, but a decrease in tumor size is hard to achieve.
Moreover, several studies have highlighted the impact of using nanoparticles to reduce hypoxia, which is a fundamental characteristic of the TME. More specifically, conjugated nanoparticles linked to hemoglobin (Hb) and encapsulated into liposomes (Hb–NPs@liposome, which was the PS) were used by Jiang et al. (2019) [56] to increase the oxygen production in the TME. No external light source was used because luminol and H2O2 were added, and Hb can activate luminol to emit blue light. The results showed that the hemoglobin nanoparticles can be internalized by the cancer cells when the liposome interacts with them. As a result, ROS production was increased, and the cell viability of cervical cancer cells was reduced [56]. Lee et al. (2022) [57] used nanoparticles loaded with a chemotherapeutic drug that targets microtubules, named paclitaxel (PTX), and the PS drug chlorin e6 (Ce6) for treating TME hypoxia and showed a reduction in cell viability and inhibition in tumor growth via activation of the p53 signaling pathway. The nanoparticles were created by using paclitaxel loaded in human serum albumin (HSA) conjugated with Azo, which is a linker that reacts to hypoxia. These nanoparticles also contained Ce6 and RGD derivatives (RP/CA/PHNPs). The Azo group facilitated the distribution and release of Ce6, increasing the efficacy of PDT and ROS production. This increase in the production of ROS resulted in the activation of the p53 signaling pathway and the decrease in tumor development [57].
Similarly, Zhang et al. (2022) [58] aimed to reduce hypoxia by using nanoparticles (AFZDA) to deliver the PS to the tumor site. These nanoparticles contained gold, zinc, iron, DOX, and angiogenin-2, which is thought to promote the formation of blood vessels, which helps in the normalization of the tumor vasculature. The results indicated that by using these nanoparticles, there was increased oxygen and drug delivery to the tumor site by normalizing the tumor vasculature through changes in their structure and morphology, ultimately resulting in extending the life of the treated mice [58]. Zhu et al. (2020) [59] used nanovesicles combined with the glucocorticosteroid dexamethasone to alleviate hypoxia by vascular normalization. By combining the nanovesicles with dexamethasone and laser irradiation, tumor growth inhibition was achieved, and ROS production was increased due to successful vascular normalization with no side effects [59]. Another study also used dexamethasone (Dex) to treat colon cancer in mice. When PDT was applied, survival pathways such as HIF-1α and NF-κB were found to be activated, and Dex was reported to inhibit these pathways. In this study, a porphyrin-based PS containing methoxy naphthalene (P-nap) was used. The result of using Dex along with PDT was an increase in the ratio of the pro-apoptotic Bax over the anti-apoptotic Bcl-xL towards apoptosis, as well as an increase in the expression of tumor suppressor p53. Moreover, a downregulation in HIF-1α and c-Myc was observed, which is regulated by NF-κB, enhancing tumor suppression and increasing the survival rate [60].
Another example is the use of different kinds of nanoparticles (PFOB@IMHNPs) that can deliver oxygen, PS (mTHPC), and a photothermal agent (IR780) into the site of the tumor. The results of Kv et al. (2022) [61] showed that after laser irradiation, PFOB@IMHNPs suppressed tumors and relieved hypoxia in the TME, resulting in full inhibition of tumor growth both in vitro and in vivo. This was achieved by increasing ROS production and temperature in the TME through the PS and the photothermal agent [61]. Liang et al. (2020) [62] used a nanoplatform (PDA-Pt-CD@RuFc) that could produce oxygen and ROS. After the samples were incubated with the nanoplatform and irradiated with a laser, the number of apoptotic cells increased dramatically, and inflammatory TME markers as well as genes related to hypoxia were downregulated [62].
Notably, the combination of PDT with nanoparticles and chemotherapeutic agents was explored in two studies. Yang et al. (2022) [63] used a nanoplatform (Fe3O4/Au NCs@LCPAA-TPP) responsive to TME that contained gold nanoclusters as PS along with hydrogels, ferric oxide (Fe3O4), and mitochondria-localized triphenylphosphine derivatives (TPP). Irradiation of the nanoplatform with a laser resulted in tumor volume reduction as the absorption of its contents was broadened by Fe3O4, and consequently, apoptosis of the tumor cells was triggered [63].
3.2.2. Targeting Mitochondria and Other ECM Components
A nanosystem (MND-IR@RESV) that reacts in the presence of ROS and accumulates in mitochondria was created by Peng et al. (2023) [64]. When stimulated by an NIR laser, the nanosystem selectively accumulated in the tumor and improved the cancer cells’ mortality as the volume of the tumor was decreased and apoptosis was induced. The nanosystem had minor side effects, and its toxicity was low in normal tissues [64]. Finally, the team of Wang et al. (2020) [65] used an anti-fibrotic drug loaded into a polymer to target components of the ECM, like collagen l and hyaluronic acid. The results indicated that this nanosystem can selectively accumulate in the TME, and the amount of collagen l and hyaluronic acid decreased dramatically. Therefore, the solid stress of the tumor was decreased due to the destruction of the ECM, and hypoxia was reduced as the vasculature of the tumor became less leaky. Moreover, the nanosystem had negligible systemic toxicity in mice as there was no fluctuation in their weight, and no metastatic lesions were observed [65].
Table 3 below summarizes the studies discussed above that combined PDT with TME-targeting therapies.

Table 3.
Summary of studies combining PDT with therapies targeting the TME.
4. Discussion
PDT has been studied extensively for cancer treatment over the past years, as it offers some important advantages, including localized action, minimal side effects, repeatability, and excellent cosmetic outcomes. However, despite its potential, it remains underutilized in clinical settings. In this review, we studied the current literature on the combination of PDT with conventional cancer therapies (chemotherapy, radiotherapy, and immunotherapy) as well as with the novel TME-targeting strategies to enhance overall therapeutic efficacy.
The findings demonstrate that combining PDT with these treatments improves outcomes by enhancing therapeutic effects while reducing the adverse effects associated with conventional therapies. Such combinations have the potential to overcome limitations in standard treatments and optimize the benefits of PDT, making it a promising strategy for future cancer therapeutics.
The combination of PDT with chemotherapy has proven more effective than chemotherapy alone, as demonstrated by studies involving cancer patients, mice, and cell lines [22,23,25,44]. Similarly, combining PDT with radiotherapy showed enhanced tumor suppression in mice, with further improvements observed when DC vaccination was incorporated into the treatment scheme [39,43]. The combination of PDT with immunotherapy also showed promising results across various cancer types, with half of the reviewed studies reporting therapeutic effects not only in primary tumors but also in distant lesions [27,28,29,31,36,45].
Moreover, we gave emphasis to studies that combined PDT with TME-targeting therapies, as the unique characteristics of the TME pose significant obstacles to PDT’s effectiveness. Targeting key features such as hypoxia can help overcome these barriers and enhance PDT’s therapeutic potential. The results of studies combining PDT with TME-targeting therapies demonstrated that targeting specific components of the TME can effectively enhance tumor destruction and improve therapeutic outcomes. Using therapeutic approaches that increase the production of ROS, essential for the photodynamic reaction, or normalizing the vasculature present in the tumor site are some of the methods proven to have the ability to increase the cytotoxicity towards cancer cells and, as a result, inhibit the growth of the tumor and enhance the photodynamic effect. Using nanoparticle systems to deliver the PS inside cells results in improvements of targeted delivery to specific cells or tissues, so the accumulation and uptake of the drug by the target cells is enhanced, while the rest of the tissues remain relatively or even completely unaffected.
According to the findings discussed above, a summary of studies using a combination of PDT and TME-targeting therapies is presented in Figure 4 below.
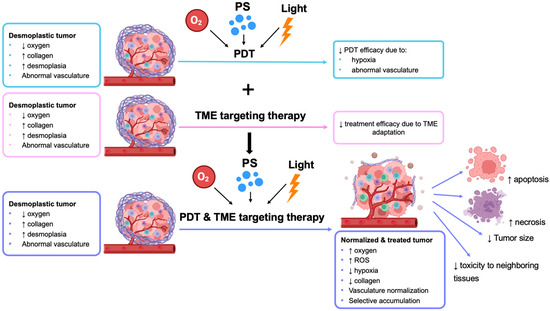
Figure 4.
PDT combined with TME-targeting therapy. The figure shows the limitations of PDT monotherapy, TME targeting therapy alone, and the advantages of combining PDT with TME-targeting therapy (↑ indicates increase; ↓ indicates decrease).
Notably, though, further research is needed for these methods to be used in the clinical setting, as, so far, all the evidence of their effectiveness is derived from experiments on cell lines and mouse models.
Despite the promising results of PDT combined with other therapies in preclinical settings, the path to clinical success remains uncertain and challenging. While animal models and cell-based experiments demonstrate significant tumor regression and reduced side effects, translating these findings into human trials has proven inconsistent [66]. Many preclinical studies fail to account for the complexity and variability of human tumors, including patient-specific factors such as genetic heterogeneity, immune responses, and tumor microenvironment dynamics. As a result, what works in controlled laboratory conditions often falls short in real-world applications, raising concerns about the robustness of current combination approaches. For example, modulating hypoxia may unintentionally lead to adaptive tumor responses, such as enhanced blood vessel formation or metabolic changes, which can reduce the effectiveness of the treatment. Moreover, nanoparticle-based delivery systems, while theoretically improving PS uptake and specificity, face obstacles related to systemic toxicity, immune clearance, and insufficient drug penetration in solid tumors. Most of the studies discussed relied on mice or cell models, highlighting the need for further research for a successful transition to clinical application [23,24,25,27,28,29,30,31,32,33,34,35,39,43,44,45]. Additional studies and clinical trials are essential to evaluate the effectiveness of these combination therapies in patients, enabling their potential integration into clinical practice and enhancing cancer treatment outcomes.
The analysis of the reviewed studies highlights the significant potential of combining PDT with other cancer treatments or TME-targeting therapies for treating various cancer types. Actually, the combination of PDT and TME-targeting therapies has demonstrated remarkable therapeutic effects in cell lines and animal models, suggesting promising prospects for future clinical application [55,56,57,58,59,60,61,62,63,64,65]. Further research should focus on deepening the understanding of TME dynamics and exploring innovative ways to enhance PDT’s efficacy, such as targeting CAFs, reprogramming immune cells within the TME, or leveraging nanotechnology for more precise and effective treatment delivery.
The strategies depicted in Figure 3 offer certain therapeutic advantages, but they also bear certain limitations.
For instance, treatments targeting hypoxia have shown promise as they increase oxygen, enhance ROS production, and also increase the number of apoptotic cells, but at the same time, the majority of these therapies have not reached late-stage clinical trials either due to complications pertaining to tumor heterogeneity or induced resistance [67]. Along the same line, oxidative stress treatments have the ability to elevate ROS production and decrease hypoxia and tumor size, but they also lead to necrosis, which can be toxic for healthy neighboring cells.
Treatments that target mitochondria seem to be more promising as they lead to increased antitumor activity both in vitro and in vivo with minimal toxicity and low side effects in normal tissues. Similarly, treatments targeting components of the extracellular matrix decompress blood vessels, enhancing drug delivery and promoting ROS generation while also enhancing the PDT effect. Moreover, this modality has been shown to decrease the metastatic ability of the cells with low systemic side effects [65]. To address the above-mentioned limitations, future research could focus on combining different treatments, such as HIF inhibitors and vasculature normalization, to increase treatment efficacy. Future research should also explore developing medical equipment that can easily deliver light to deep tissues so the effect of each of these therapies, as well as the efficacy of PDT, is maximized.
While the majority of studies report positive outcomes for PDT-based combination therapies, a deeper comparative analysis reveals several important points. Strategies targeting hypoxia and vascular normalization consistently show strong preclinical efficacy, but conflicting results highlight the risk of adaptive responses such as compensatory angiogenesis, which may reduce long-term benefits. Similarly, nanoparticle-based delivery platforms demonstrate enhanced PS accumulation and ROS production, yet they face challenges with systemic toxicity, immune clearance, and poor scalability, raising concerns about their translational feasibility. By contrast, immune-targeting approaches, particularly those that combine PDT with checkpoint inhibitors, have shown the most durable and systemic antitumor effects, although variability in response rates across tumor models underscores the need for better patient stratification. Overall, while TME-directed approaches hold great promise, those integrating PDT with immunotherapy appear most likely to advance toward clinical success, whereas nanoparticle-heavy strategies remain limited by manufacturing and regulatory hurdles. A clearer understanding of these limitations and conflicts will be essential for prioritizing future research directions and guiding rational clinical trial design.
The clinical translation of PDT, particularly in combination with TME-targeting strategies, encounters several barriers. Regulatory challenges arise from the diversity of approval pathways and the complexity of evaluating PDT as a drug–device combination [68]. Different regions’ jurisdictions, including the United States, Europe, and Japan, apply inconsistent standards for premarket evaluation, registration procedures, and optimization of therapeutic parameters [69]. For instance, PS sensitizers, such as Photofrin, BPD-MA, ALA, and MAL, have received approval for distinct cancer indications depending on regional criteria, yet significant discrepancies remain in drug–light interval assessment and light power density optimization between U.S. and Japanese studies [70]. In addition, inconsistent trial registration and incomplete evidence reporting reduce transparency and hinder post-market surveillance [71]. Beyond regulatory issues, manufacturing and scalability present further challenges, particularly for nanoparticle-based PDT systems. PS-loaded nanoplatforms frequently face hydrophobicity, solubility, and aggregation problems, while complex synthesis pathways limit reproducibility and large-scale production [72]. The absence of standardized long-term toxicity evaluations remains a significant obstacle, as noted in preclinical studies on nanostructure-based systems [73,74]. Addressing these challenges is critical for the safe and effective advancement of PDT–TME approaches.
Despite these barriers, PDT is making progress in clinical evaluation. Several trials are actively investigating its role in immunomodulation, chemotherapy enhancement, and TME modulation. For example, a clinical trial in basal-cell carcinoma is studying how PDT alters the immune microenvironment [75]. In lung cancer, photoimmunotherapy with ASP-1929 combined with cemiplimab is being tested for refractory, inoperable, and metastatic non-small-cell lung cancer [76]. In pancreatic cancer, verteporfin-mediated photoradiation is being combined with pembrolizumab and chemotherapy to evaluate local and systemic immune responses [77]. In cholangiocarcinomas, PDT is under evaluation as both a comparator to radiofrequency ablation and as a neoadjuvant approach to improve resectability [78,79]. Other active studies include glioblastoma [80], recurrent prostate cancer [81], intermediate-risk prostate cancer [82], recurrent superficial esophageal carcinoma [83], and extrahepatic cholangiocarcinoma [78,84]. Collectively, these studies illustrate a growing clinical interest in PDT, particularly when integrated with immunotherapy or TME-targeted strategies, and highlight the need for harmonized regulatory frameworks and robust manufacturing solutions to facilitate broader clinical adoption.
5. Conclusions
This literature review highlights the usefulness of combining PDT with chemotherapy, radiotherapy, and immunotherapy, producing a synergistic therapeutic effect across various cancer types. The main focus, however, was on the combination of PDT with TME-targeting therapies, which address critical barriers to treatment success, such as increased ECM deposition, hypoxia, abnormal vasculature, and immune suppression. By targeting these features, TME-focused combinations enhance the effectiveness of PDT, leading to improved tumor destruction, increased apoptosis, and reduced resistance to treatment. Additionally, innovative delivery systems, such as nanoparticles, improve PS uptake, boost ROS production, and minimize off-target effects. These promising preclinical outcomes underscore the need for further research and clinical trials to bridge the gap between research and clinical application. With continued development, PDT-TME combination therapies hold the potential to overcome current limitations and redefine cancer treatment strategies.
Future research should prioritize the integration of PDT with TME-targeted strategies that specifically enhance antitumor immunity. Approaches that remodel the ECM, normalize tumor vasculature, alleviate hypoxia, or reprogram immunosuppressive cell populations can create a more favorable environment for immunotherapy. Combining these interventions with PDT has the potential to increase immune infiltration, improve antigen presentation, and promote durable systemic responses. Parallel advances in imaging-guided PDT could enable real-time monitoring of treatment efficacy and PS distribution, while tumor profiling may support personalized selection of photosensitizers and delivery platforms. These directions are expected to accelerate the translation of PDT–TME therapies into clinical practice and maximize their therapeutic potential in cancer treatment.
Author Contributions
Conceptualization, A.S.; methodology, A.S.; software, S.T.; formal analysis, S.T.; investigation, S.T.; resources, S.T. and V.G.; writing—original draft preparation, S.T.; writing—review and editing, A.S., S.T. and V.G.; visualization, S.T.; supervision, A.S. and V.G.; project administration, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef] [PubMed]
- Yuzhalin, A.E. Redefining cancer research for therapeutic breakthroughs. Br. J. Cancer 2024, 130, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Verginadis, I.I.; Citrin, D.E.; Ky, B.; Feigenberg, S.J.; Georgakilas, A.G.; Hill-Kayser, C.E.; Koumenis, C.; Maity, A.; Bradley, J.D.; Lin, A. Radiotherapy toxicities: Mechanisms, management, and future directions. Lancet 2025, 405, 338–352. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401, Erratum in Genes Dis. 2024, 11, 101211. [Google Scholar] [CrossRef] [PubMed]
- Van Straten, D.; Mashayekhi, V.; De Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Sorrin, A.J.; Kemal Ruhi, M.; Ferlic, N.A.; Karimnia, V.; Polacheck, W.J.; Celli, J.P.; Huang, H.C.; Rizvi, I. Photodynamic Therapy and the Biophysics of the Tumor Microenvironment. Photochem. Photobiol. 2020, 96, 232–259. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Juarranz, Á.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer—A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Huis in ‘t Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. Immunother. Cancer 2021, 9, e001926, Erratum in J. Immunother. Cancer 2021, 9, e001926corr1. [Google Scholar] [CrossRef]
- Qin, S.; Xu, Y.; Li, H.; Chen, H.; Yuan, Z. Recent advances in in situ oxygen-generating and oxygen-replenishing strategies for hypoxic-enhanced photodynamic therapy. Biomater. Sci. 2022, 10, 51–84. [Google Scholar] [CrossRef]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef] [PubMed]
- Nygren, P. What is cancer chemotherapy? Acta Oncol. 2001, 40, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Tateishi, C.; Muramatsu, S.; Kubo, R.; Yonezawa, E.; Kato, H.; Nishida, E.; Tsuruta, D. Efficacy and safety of bexarotene combined with photo(chemo)therapy for cutaneous T-cell lymphoma. J. Dermatol. 2020, 47, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, Q.; Zhang, Z.; Tang, Y. A pH-Responsive Drug Delivery System Based on Conjugated Polymer for Effective Synergistic Chemo-/Photodynamic Therapy. Molecules 2023, 28, 399. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Mahadik, N.S.; Koteshwar, D.; Krishna, N.V.; Giribabu, L.; Banerjee, R. Potentiation of novel porphyrin based photodynamic therapy against colon cancer with low dose doxorubicin and elucidating the molecular signalling pathways responsible for relapse. J. Photochem. Photobiol. B Biol. 2023, 238, 112625. [Google Scholar] [CrossRef]
- Massoud, J.; Pinon, A.; Gallardo-Villagrán, M.; Paulus, L.; Ouk, C.; Carrion, C.; Antoun, S.; Diab-Assaf, M.; Therrien, B.; Liagre, B. A Combination of Ruthenium Complexes and Photosensitizers to Treat Colorectal Cancer. Inorganics 2023, 11, 451. [Google Scholar] [CrossRef]
- Dhar, R.; Seethy, A.; Singh, S.; Pethusamy, K.; Srivastava, T.; Talukdar, J.; Rath, G.K.; Karmakar, S. Cancer immunotherapy: Recent advances and challenges. J. Cancer Res. Ther. 2021, 17, 834–844. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, G.; Wu, H.; Liu, C.; Zhan, Y.; Qiu, Y.; Shou, C.; Gao, F.; Zhang, J.; Yin, P.; et al. Photodynamic therapy synergizes with PD-L1 checkpoint blockade for immunotherapy of CRC by multifunctional nanoparticles. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 2931–2948. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhao, M.; Wang, W.; Hong, L.; Wu, Z.; Luo, G.; Lu, S.; Tang, Y.; Li, J.; Wang, J.; et al. 5-ALA mediated photodynamic therapy with combined treatment improves anti-tumor efficacy of immunotherapy through boosting immunogenic cell death. Cancer Lett. 2023, 554, 216032. [Google Scholar] [CrossRef]
- Sonokawa, T.; Obi, N.; Usuda, J.; Sudo, Y.; Hamakubo, T. Development of a new minimally invasive phototherapy for lung cancer using antibody–toxin conjugate. Thorac. Cancer 2023, 14, 645–653. [Google Scholar] [CrossRef]
- Yamashita, S.; Kojima, M.; Onda, N.; Shibutani, M. In Vitro Comparative Study of Near-Infrared Photoimmunotherapy and Photodynamic Therapy. Cancers 2023, 15, 3400. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.; Mao, K.; Wu, C.; Chen, H.; Wang, J.; Wang, X.; Cong, X.; Li, Y.; Meng, X.; et al. Photodynamic therapy produces enhanced efficacy of antitumor immunotherapy by simultaneously inducing intratumoral release of sorafenib. Biomaterials 2020, 240, 119845. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Wang, X.; Shi, L.; Fan, Z.; Zhang, G.; Yang, D.; Bahavar, C.F.; Zhou, F.; Chen, W.R.; et al. Antitumor Effects of DC Vaccine With ALA-PDT-Induced Immunogenic Apoptotic Cells for Skin Squamous Cell Carcinoma in Mice. Technol. Cancer Res. Treat. 2018, 17, 1533033818785275. [Google Scholar] [CrossRef]
- Trempolec, N.; Doix, B.; Degavre, C.; Brusa, D.; Bouzin, C.; Riant, O.; Feron, O. Photodynamic Therapy-Based Dendritic Cell Vaccination Suited to Treat Peritoneal Mesothelioma. Cancers 2020, 12, 545. [Google Scholar] [CrossRef]
- Vedunova, M.; Turubanova, V.; Vershinina, O.; Savyuk, M.; Efimova, I.; Mishchenko, T.; Raedt, R.; Vral, A.; Vanhove, C.; Korsakova, D.; et al. DC vaccines loaded with glioma cells killed by photodynamic therapy induce Th17 anti-tumor immunity and provide a four-gene signature for glioma prognosis. Cell Death Dis. 2022, 13, 1062. [Google Scholar] [CrossRef]
- Korbelik, M.; Banáth, J.; Zhang, W.; Gallagher, P.; Hode, T.; Lam, S.S.K.; Chen, W.R. N-dihydrogalactochitosan as immune and direct antitumor agent amplifying the effects of photodynamic therapy and photodynamic therapy-generated vaccines. Int. Immunopharmacol. 2019, 75, 105764. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Cherukula, K.; Bang, Y.J.; Vijayan, V.; Moon, M.J.; Thiruppathi, J.; Puth, S.; Jeong, Y.Y.; Park, I.-K.; Lee, S.E.; et al. Combination of Photodynamic Therapy and a Flagellin-Adjuvanted Cancer Vaccine Potentiated the Anti-PD-1-Mediated Melanoma Suppression. Cells 2020, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, J.M.; Segal, R.J.; Zeitouni, N.C. Combination vismodegib and photodynamic therapy for multiple basal cell carcinomas. Photodiagnosis Photodyn. Ther. 2018, 21, 58–62. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Bulin, A.L.; Broekgaarden, M.; Simeone, D.; Hasan, T. Low dose photodynamic therapy harmonizes with radiation therapy to induce beneficial effects on pancreatic heterocellular spheroids. Oncotarget 2019, 10, 2625–2643. [Google Scholar] [CrossRef]
- Mayahi, S.; Neshasteh-Riz, A.; Pornour, M.; Eynali, S.; Montazerabadi, A. Investigation of combined photodynamic and radiotherapy effects of gallium phthalocyanine chloride on MCF-7 breast cancer cells. JBIC J. Biol. Inorg. Chem. 2020, 25, 39–48. [Google Scholar] [CrossRef]
- Liu, T.; Song, Y.; Huang, Z.; Pu, X.; Wang, Y.; Yin, G.; Gou, L.; Weng, J.; Meng, X. Photothermal photodynamic therapy and enhanced radiotherapy of targeting copolymer-coated liquid metal nanoparticles on liver cancer. Colloids Surf. B Biointerfaces 2021, 207, 112023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Gao, Y.; Yang, Z.; Wang, N.; Ge, J.; Cao, X.; Kou, D.; Gu, Y.; Li, C.; Afshari, M.J.; et al. Biomimetic Upconversion Nanoplatform Synergizes Photodynamic Therapy and Enhanced Radiotherapy against Tumor Metastasis. ACS Appl. Mater. Interfaces 2023, 15, 26431–26441. [Google Scholar] [CrossRef]
- Doix, B.; Trempolec, N.; Riant, O.; Feron, O. Low Photosensitizer Dose and Early Radiotherapy Enhance Antitumor Immune Response of Photodynamic Therapy-Based Dendritic Cell Vaccination. Front. Oncol. 2019, 9, 2019. [Google Scholar] [CrossRef]
- Yao, D.; Wang, Y.; Bian, K.; Zhang, B.; Wang, D. A self-cascaded unimolecular prodrug for pH-responsive chemotherapy and tumor-detained photodynamic-immunotherapy of triple-negative breast cancer. Biomaterials 2023, 292, 121920. [Google Scholar] [CrossRef]
- Kim, S.; Kim, S.A.; Nam, G.-H.; Hong, Y.; Kim, G.B.; Choi, Y.; Lee, S.; Cho, Y.; Kwon, M.; Jeong, C.; et al. In situ immunogenic clearance induced by a combination of photodynamic therapy and rho-kinase inhibition sensitizes immune checkpoint blockade response to elicit systemic antitumor immunity against intraocular melanoma and its metastasis. J. Immunother. Cancer 2021, 9, e001481, Erratum in J. Immunother. Cancer 2021, 9, e001481corr1. [Google Scholar] [CrossRef]
- Coulson, R.; Liew, S.H.; Connelly, A.A.; Yee, N.S.; Deb, S.; Kumar, B.; Vargas, A.C.; O’Toole, S.A.; Parslow, A.C.; Poh, A.; et al. The angiotensin receptor blocker, Losartan, inhibits mammary tumor development and progression to invasive carcinoma. Oncotarget 2017, 8, 18640–18656. [Google Scholar] [CrossRef]
- Paolicchi, E.; Gemignani, F.; Krstic-Demonacos, M.; Dedhar, S.; Mutti, L.; Landi, S. Targeting hypoxic response for cancer therapy. Oncotarget 2016, 7, 13464–13478. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor microenvironment abnormalities: Causes, consequences, and strategies to normalize. J. Cell. Biochem. 2007, 101, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, J.; You, Q.; Huang, H.; Chen, Y.; Liu, K. The mTOR/AP-1/VEGF signaling pathway regulates vascular endothelial cell growth. Oncotarget 2016, 7, 53269–53276. [Google Scholar] [CrossRef] [PubMed]
- Komohara, Y.; Fujiwara, Y.; Ohnishi, K.; Takeya, M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv. Drug Deliv. Rev. 2016, 99, 180–185. [Google Scholar] [CrossRef]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Vizler, C.; Nagy, L.I.; Kitajka, K.; Puskas, L.G. Pro-Tumoral Inflammatory Myeloid Cells as Emerging Therapeutic Targets. Int. J. Mol. Sci. 2016, 17, 1958. [Google Scholar] [CrossRef] [PubMed]
- Tulotta, C.; Ottewell, P. The role of IL-1B in breast cancer bone metastasis. Endocr. Relat. Cancer 2018, 25, R421–R434. [Google Scholar] [CrossRef]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J.; Ridker, P.; Lorenzatti, A.; Krum, H.; Varigos, J.; et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- Lin, L.; Pang, W.; Jiang, X.; Ding, S.; Wei, X.; Gu, B. Light amplified oxidative stress in tumor microenvironment by carbonized hemin nanoparticles for boosting photodynamic anticancer therapy. Light Sci. Appl. 2022, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Bai, H.; Liu, L.; Lv, F.; Ren, X.; Wang, S. Luminescent, Oxygen-Supplying, Hemoglobin-Linked Conjugated Polymer Nanoparticles for Photodynamic Therapy. Angew. Chem. Int. Ed. 2019, 58, 10660–10665. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dey, D.K.; Kim, K.; Kim, S.; Kim, E.; Kang, S.C.; Bajpai, V.K.; Huh, Y.S. Hypoxia-responsive nanomedicine to overcome tumor microenvironment-mediated resistance to chemo-photodynamic therapy. Mater. Today Adv. 2022, 14, 100218. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Zhang, Z.; He, D.; Zhu, J.; Chen, Y.; Zhang, Y. Remodeling of tumor microenvironment for enhanced tumor chemodynamic/photothermal/chemo-therapy. J. Nanobiotechnol. 2022, 20, 388. [Google Scholar] [CrossRef]
- Zhu, D.; Duo, Y.; Suo, M.; Zhao, Y.; Xia, L.; Zheng, Z.; Li, Y.; Tang, B.Z. Tumor-Exocytosed Exosome/Aggregation-Induced Emission Luminogen Hybrid Nanovesicles Facilitate Efficient Tumor Penetration and Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 13836–13843. [Google Scholar] [CrossRef]
- Chilakamarthi, U.; Mahadik, N.S.; Bhattacharyya, T.; Gangadhar, P.S.; Giribabu, L.; Banerjee, R. Glucocorticoid receptor mediated sensitization of colon cancer to photodynamic therapy induced cell death. J. Photochem. Photobiol. B Biol. 2024, 251, 112846. [Google Scholar] [CrossRef]
- Kv, R.; Liu, T.-I.; Lu, I.L.; Liu, C.-C.; Chen, H.-H.; Lu, T.-Y.; Chiang, W.-H.; Chiu, H.-C. Tumor microenvironment-responsive and oxygen self-sufficient oil droplet nanoparticles for enhanced photothermal/photodynamic combination therapy against hypoxic tumors. J. Control. Release 2020, 328, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-H.; Zheng, Y.; Wu, X.-W.; Tan, C.-P.; Ji, L.-N.; Mao, Z.-W. A Tailored Multifunctional Anticancer Nanodelivery System for Ruthenium-Based Photosensitizers: Tumor Microenvironment Adaption and Remodeling. Adv. Sci. 2020, 7, 1901992. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Shi, R.; Zhao, Z.; Xie, A.; Shen, Y.; Zhu, M. Design of the tumor microenvironment-multiresponsive nanoplatform for dual-targeting and photothermal imaging guided photothermal/photodynamic/chemodynamic cancer therapies with hypoxia improvement and GSH depletion. Chem. Eng. J. 2022, 441, 136042. [Google Scholar] [CrossRef]
- Peng, Y.; Cheng, L.; Luo, C.; Xiong, F.; Wu, Z.; Zhang, L.; Zhan, P.; Shao, L.; Luo, W. Tumor microenvironment-responsive nanosystem achieves reactive oxygen species self-cycling after photothermal induction to enhance efficacy of antitumor therapy. Chem. Eng. J. 2023, 463, 142370. [Google Scholar] [CrossRef]
- Wang, S.-B.; Chen, Z.-X.; Gao, F.; Zhang, C.; Zou, M.-Z.; Ye, J.-J.; Zeng, X.; Zhang, X.-Z. Remodeling extracellular matrix based on functional covalent organic framework to enhance tumor photodynamic therapy. Biomaterials 2020, 234, 119772. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Codony, V.L.; Tavassoli, M. Hypoxia-induced therapy resistance: Available hypoxia-targeting strategies and current advances in head and neck cancer. Transl. Oncol. 2021, 14, 101017. [Google Scholar] [CrossRef]
- Bown, S.G. Taking PDT into mainstream clinical practice. In Photodynamic Therapy: Back to the Future; World Congress of the International Photodynamic Association, Proceedings of SPIE: Bellingham, WA, USA, 2009; Volume 7380, pp. 23–28. [Google Scholar]
- Kawase, Y.; Iseki, H. Parameter-finding studies of photodynamic therapy for approval in Japan and the USA. Photodiagn. Photodyn. Ther. 2013, 10, 434–445. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Frochot, C.; Mordon, S.R. Update of the situation of clinical photodynamic therapy in Europe in the 2003–2018 period. J. Porphyr. Phthalocyan. 2019, 23, 347–357. [Google Scholar] [CrossRef]
- Abdel Gaber, S.A.; Fadel, M. Nanotechnology and photodynamic therapy from a clinical perspective. Transl. Biophotonics 2023, 5, e202200016. [Google Scholar] [CrossRef]
- Voon, S.H.; Kiew, L.V.; Lee, H.-B.; Lim, S.H.; Noordin, M.I.; Kamkaew, A.; Burgess, K.; Chung, L.Y. In vivo studies of nanostructure-based photosensitizers for photodynamic cancer therapy. Small 2014, 10, 4993–5013. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and Photothermal Therapies: Synergy Opportunities for Nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Alteration of the Immune Microenvironment in Basal Cell Carcinoma (BCC) Following Photodynamic Therapy (PDT). Available online: https://www.medifind.com/articles/clinical-trial/313313790 (accessed on 28 August 2025).
- Phase II Trial: Photoimmunotherapy and Anti-PD1 in Patients with Refractory Inoperable and Metastatic Non-Small Cell Lung Cancer. 2025. Available online: https://adisinsight.springer.com/trials/700382233 (accessed on 28 August 2025).
- Photodynamic Priming to Facilitate Immunologic Activity of Anti-PD1 in Patients with Pancreatic Cancer. 2024. Available online: https://adisinsight.springer.com/trials/700373061 (accessed on 28 August 2025).
- Cholangiocarcinoma Treatment with Radiofrequency Ablation or Photodynamic Therapy: A Randomized Controlled Trial. 2022. Available online: https://www.medifind.com/articles/clinical-trial/347963332 (accessed on 27 August 2025).
- A Prospective Randomized Controlled Study of Neoadjuvant PDT in the Treatment of Cholangiocarcinoma. 2021. Available online: https://www.clinicaltrials.gov/study/NCT04824742?term=A%20Prospective%20Randomized%20Controlled%20Study%20of%20Neoadjuvant%20PDT%20in%20the%20Treatment%20of%20Cholangiocarcinoma&rank=1: (accessed on 28 August 2025).
- Photodynamic Therapy for Glioblastoma Multiforme Based on Metaverse and Yellow Fluorescence. 2025. Available online: https://ichgcp.net/clinical-trials-registry/NCT06939400 (accessed on 27 August 2025).
- Open-Label Clinical Study to Assess the Safety and Efficacy of the SpectraCure P18 System (Interstitial Multiple Diode Lasers and IDOSE® Software) and Verteporfin for Injection (VFI) for the Treatment of Recurrent Prostate Cancer. 2017. Available online: https://www.urotoday.com/clinical-trials/prostate-cancer/115457-open-label-clinical-study-to-assess-the-safety-and-adequacy-of-effectiveness-of-the-spectracure-p18-system-interstitial-multiple-diode-lasers-and-idose-software-and-verteporfin-for-injection-vfi-for-the-treatment-of-recurrent-prostate-cancer.html (accessed on 28 August 2025).
- Study of the Efficacy, Safety and Quality of Life After TOOKAD® Soluble Vascular Targeted Photodynamic Therapy (VTP) for Minimally Invasive Treatment of Localized Intermediate Risk Prostate Cancer. Study of the Efficacy, Safety & Quality of Life After TOOKAD® Soluble. 2017. Available online: https://www.clinicaltrials.gov/study/NCT01875393?term=TOOKAD&rank=2 (accessed on 27 August 2025).
- Real-World Study of Hematoporphyrin Injection-Based Photodynamic Therapy in Patients with Recurrent or Residual Superficial Esophageal Cancer. 2024. Available online: https://www.clinicaltrials.gov/study/NCT06437288?cond=Esophageal%20Cancer&term=Hematoporphyrin&intr=Photodynamic%20Therapy%20&rank=1 (accessed on 28 August 2025).
- Clinical Effect and Safety of Photodynamic Therapy Versus Radiofrequency Ablation Versus Photodynamic Therapy Plus Radiofrequency Ablation for Unresectable Extrahepatic Cholangiocarcinoma. 2022. Available online: https://www.clinicaltrials.gov/study/NCT05519319?cond=Unresectable%20Extrahepatic%20Cholangiocarcinoma&term=Radiofrequency%20Ablation%20&intr=Photodynamic%20Therapy%20&rank=2 (accessed on 28 August 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).