Raman Monitoring of Staphylococcus aureus Osteomyelitis: Microbial Pathogenesis and Bone Immune Response
Abstract
1. Introduction
2. Results
2.1. Osteomyelitis Rat Model and Microscopy Observations
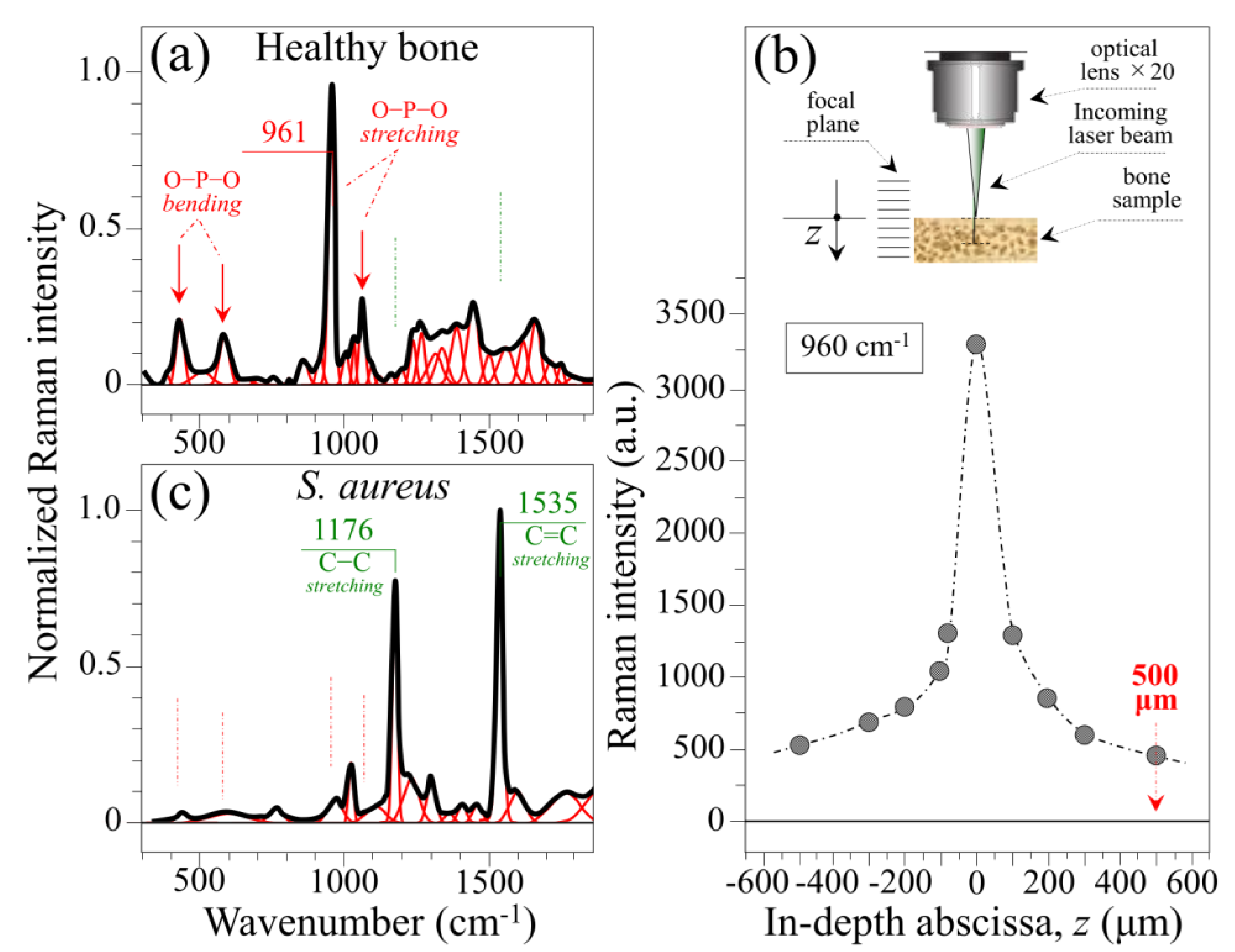
2.2. Reference Raman Spectra and Probe Depth Assessment
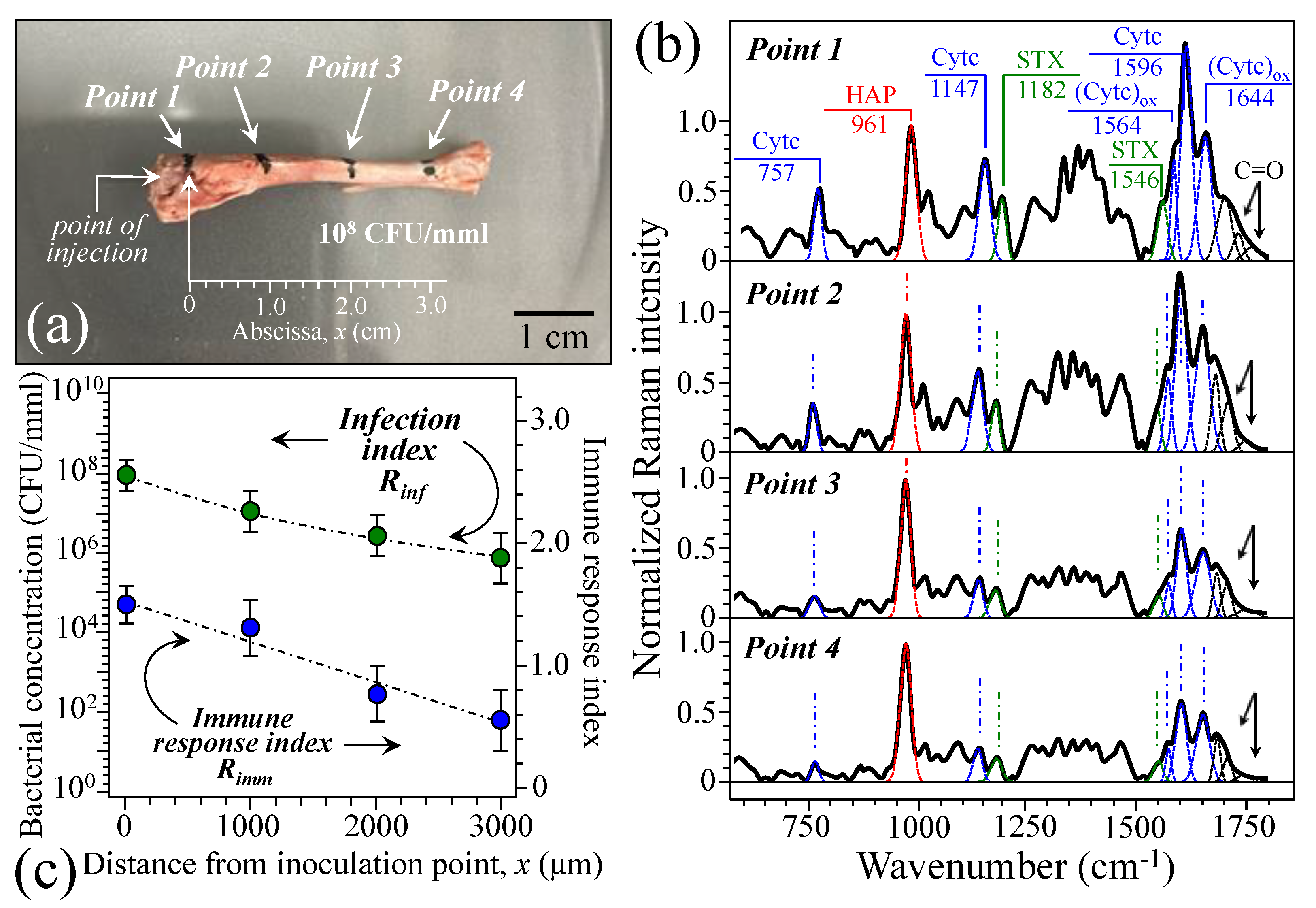
2.3. Raman Spectra of Bone at the Acute Stage of Osteomyelitis
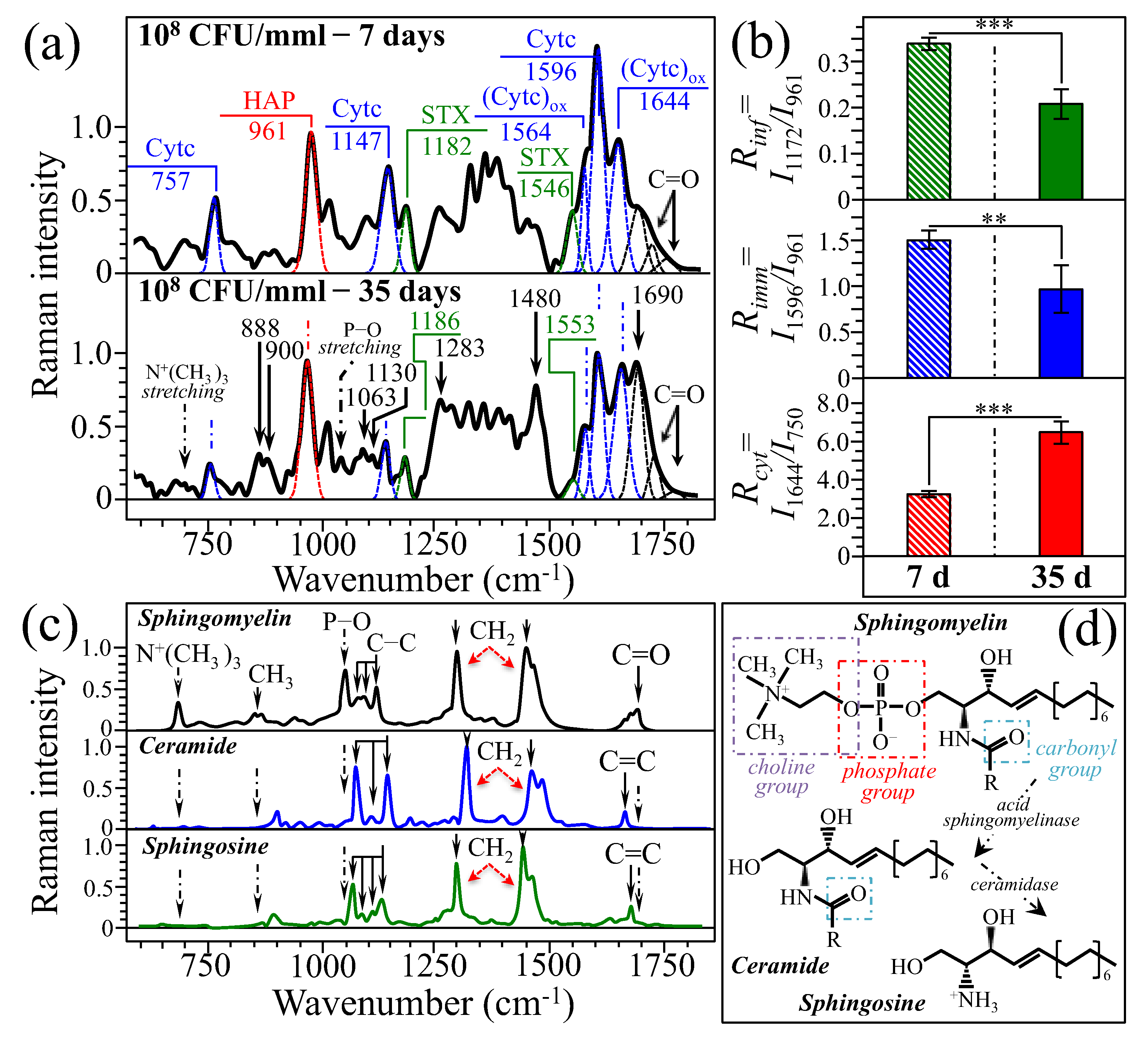
2.4. Raman Spectra of Bone at the Subacute Stage of Osteomyelitis
3. Discussion
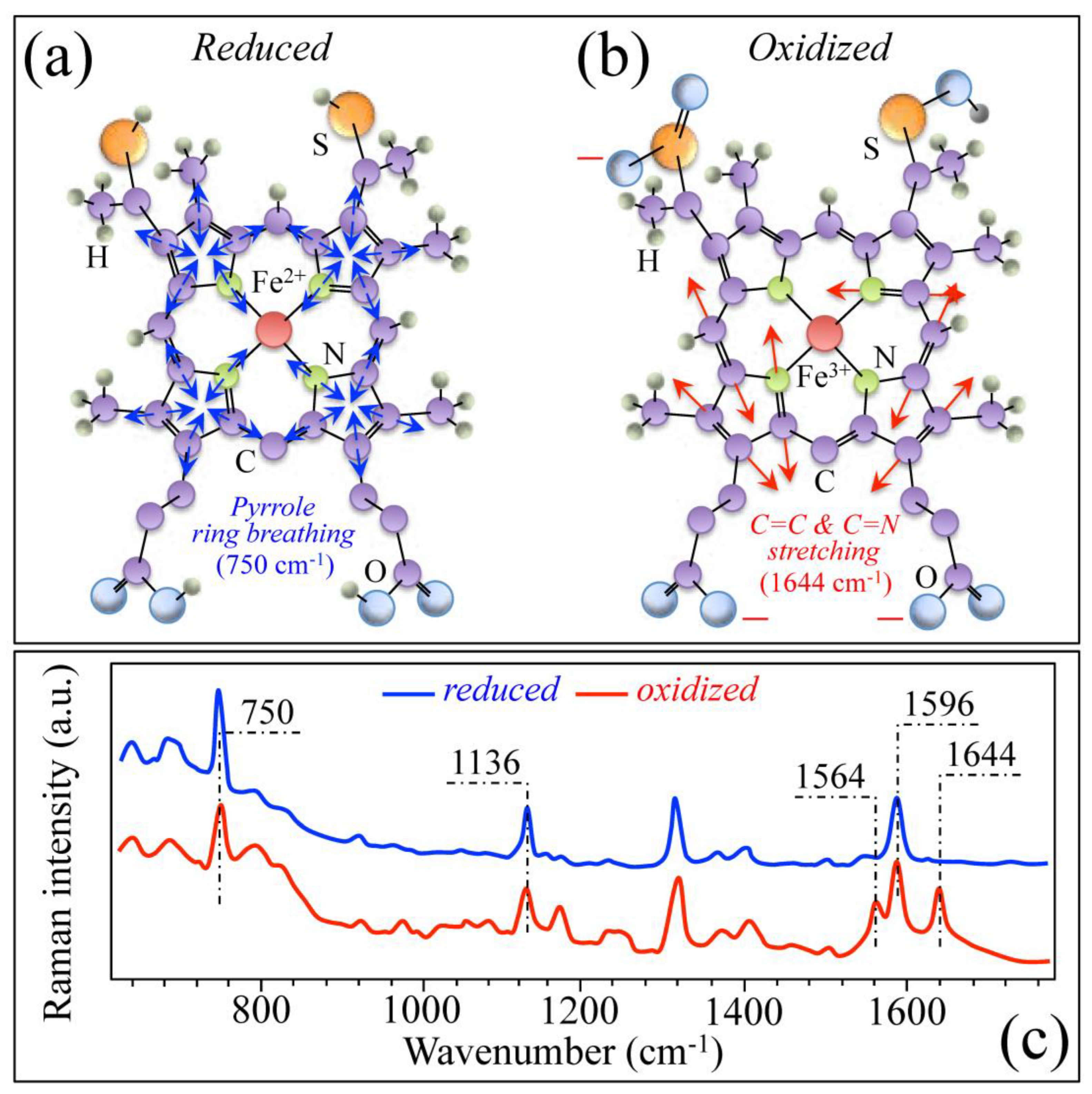
3.1. Osteomyelitis and Its Link to Bone Immune Response
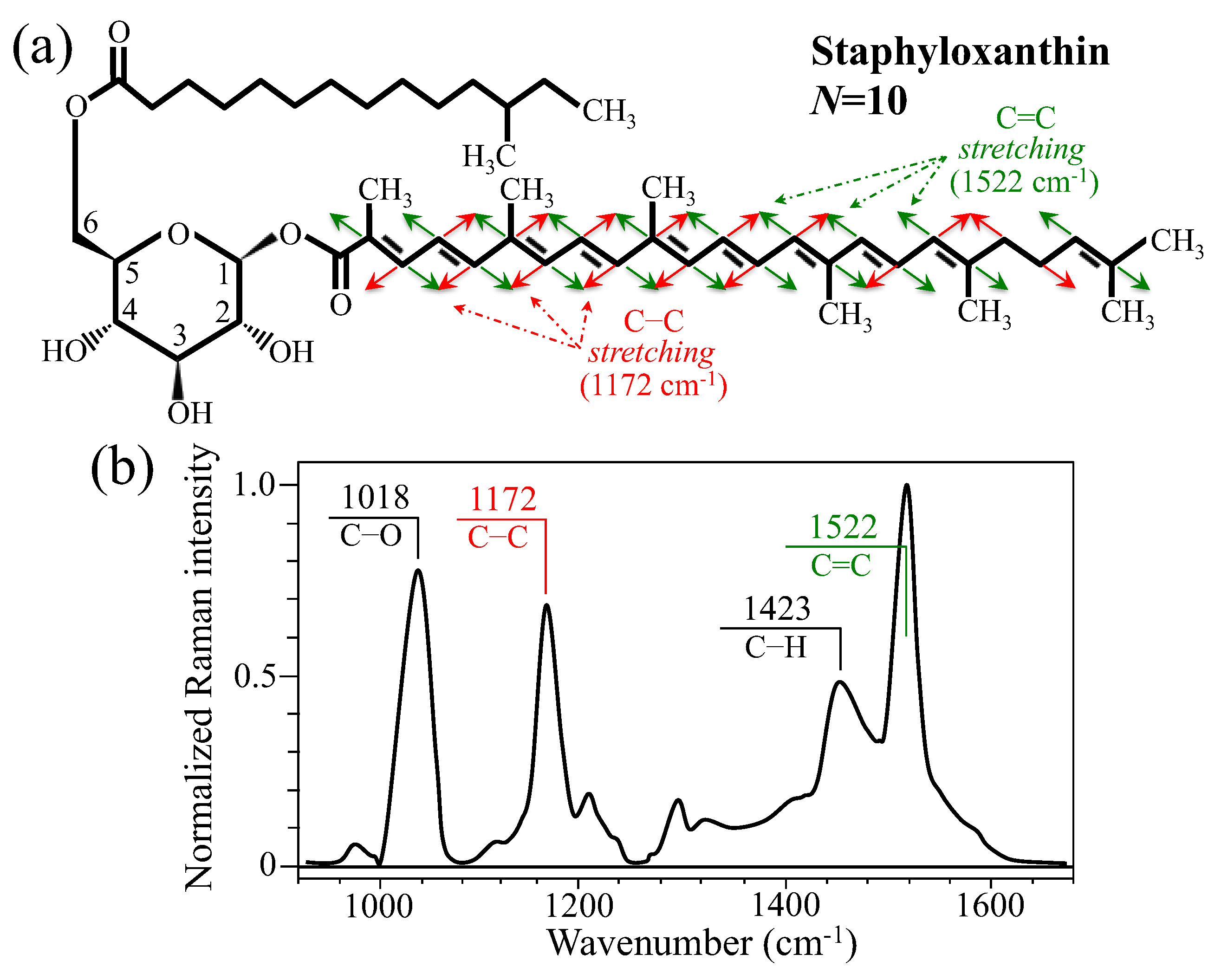
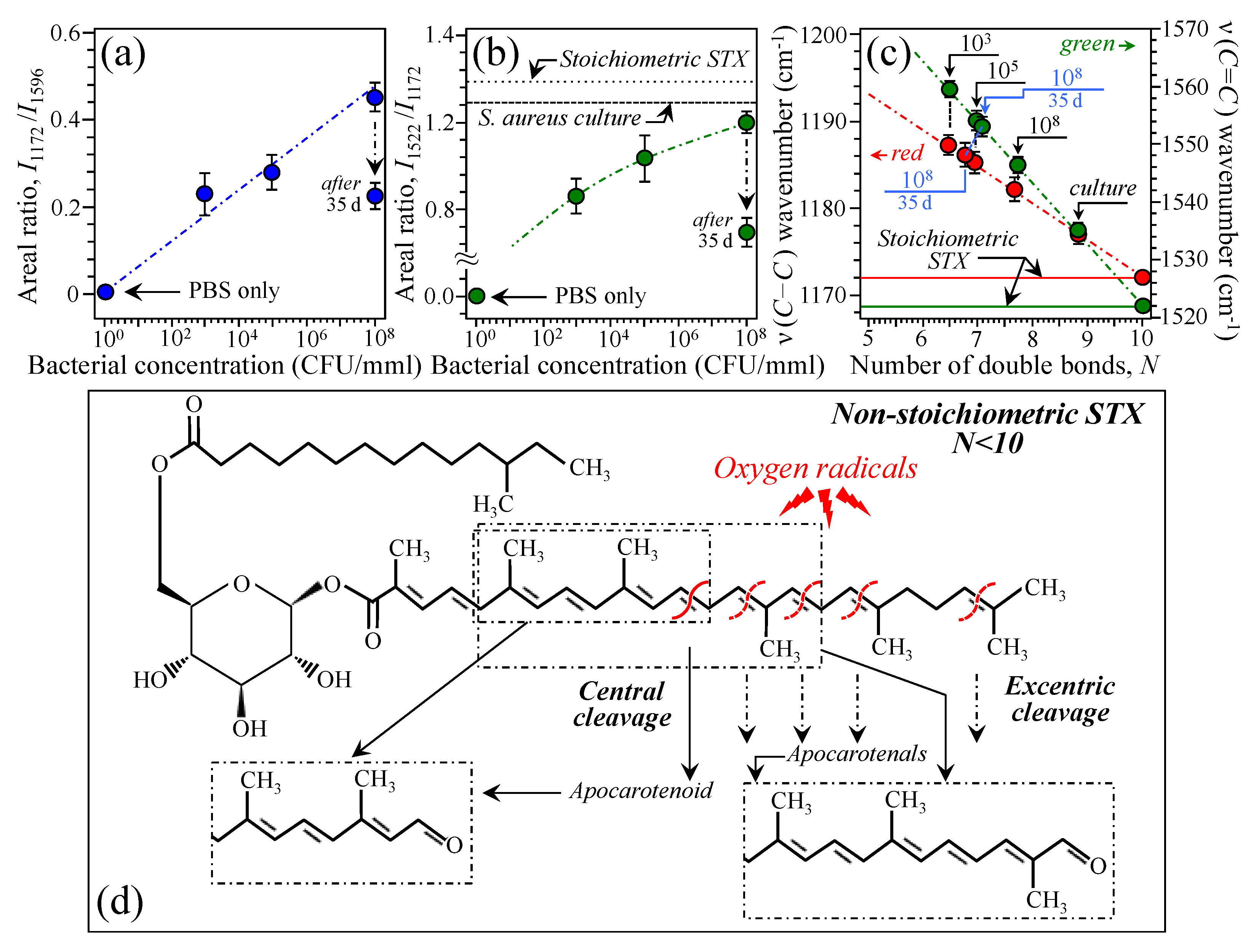
3.2. Bacteria Structural Modification in Response to Host Immune Response
3.3. Raman Insights into S. aureus Persistence and Immunity Manipulation
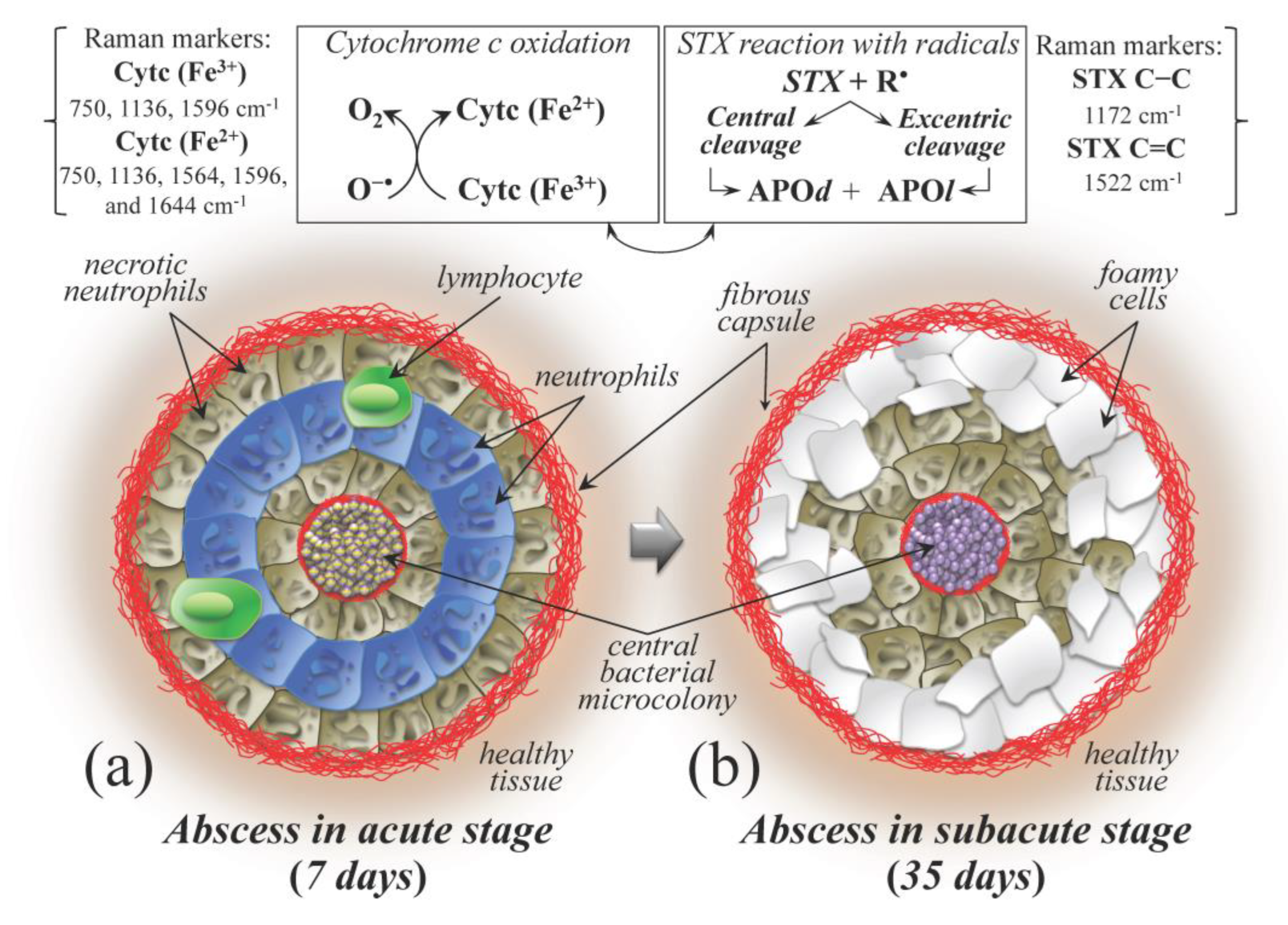
3.4. The Potential Benefits of a Raman Probe in the Surgery Room
3.5. Limitations of This Study
4. Methods and Materials
4.1. Animal Experiments and Sample Preparation
4.2. Raman Spectroscopic Assessments and Spectroscopic Data Treatments
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Higgins, E.; Suh, G.A.; Tande, A.J. Enhancing diagnostics in orthopedic infections. J. Clin. Microbiol. 2022, 60, e02196-21. [Google Scholar] [CrossRef] [PubMed]
- Demehri, S.; Baffour, F.I.; Klein, J.G.; Ghotbi, E.; Ibad, H.A.; Moradi, K.; Taguchi, K.; Fritz, J.; Carrino, J.A.; Guermazi, A.; et al. Musculoskeletal CT imaging: State-of-the-art advancements and future directions. Radiology 2023, 308, e230344. [Google Scholar] [CrossRef]
- Sambri, A.; Spinnato, P.; Tedeschi, S.; Zamparini, E.; Fiore, M.; Zucchini, R.; Giannini, C.; Caldari, E.; Crombé, A.; Viale, P.; et al. Bone and joint infections: The role of imaging in tailoring diagnosis to improve patients’ care. J. Pers. Med. 2021, 11, 1317. [Google Scholar] [CrossRef]
- Lee, Y.J.; Sadigh, S.; Mankad, K.; Kapse, N.; Rajeswaran, G. The imaging of osteomyelitis. Quant. Imaging Med. Surg. 2016, 6, 184–198. [Google Scholar] [CrossRef]
- Wu, J.S.; Gorbachova, T.; Morrison, W.B.; Haims, A.H. Imaging-guided bone biopsy for osteomyelitis: Are there factors associated with positive or negative cultures? Am. J. Roentgenol. 2007, 188, 1529–1534. [Google Scholar] [CrossRef]
- Sousa, R.; Carvalho, A.; Santos, A.C.; Araujo Abreu, M. Optimal microbiological sampling for the diagnosis of osteoarticular infection. EFFORT Open Rev. 2021, 6, 390–398. [Google Scholar] [CrossRef]
- Monsen, T.; Loevgren, E.; Widerstroem, M.; Wallinder, L. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J. Clin. Microbiol. 2009, 47, 2496–2501. [Google Scholar] [CrossRef]
- Xian, C.; Liu, Y.; Zhou, L.; Ding, T.; Chen, J.; Wang, T.; Gao, J.; Hao, X.; Bi, L. Optimal ultrasonic treatment frequency and duration parameters were used to detect the pathogenic bacteria of orthopedic implant-associated infection by ultrasonic oscillation. J. Infect. Chemother. 2024, 30, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, T.; Niciejewski, K.; Kozielski, M.; Szybowicz, M.; Siatkowski, M.; Krauss, H. Identifying compositional and structural changes in spongy and subchondral bone from the hip joints of patients with osteoarthritis using Raman spectroscopy. J. Biomed. Opt. 2012, 17, 017007. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Bora, T.; Al Ghaithi, A.; Thukral, S.; Dutta, J. Raman spectroscopy detects changes in bone mineral quality and collagen cross-linkage in Staphylococcus infected human bone. Sci. Rep. 2018, 8, 9417. [Google Scholar] [CrossRef]
- Pezzotti, G.; Rondinella, A.; Marin, E.; Zhu, W.; Aldini, N.N.; Ulian, G.; Valdré, G. Raman spectroscopic investigation on the molecular structure of apatite and collagen in osteoporotic cortical bone. J. Mech. Behav. Biomed. Mater. 2017, 65, 264–273. [Google Scholar] [CrossRef]
- Rebrosova, K.; Siler, M.; Samek, O.; Ruzicka, F.; Bernatova, S.; Hola, V.; Jezek, J.; Zemanek, P.; Sokolova, J.; Petras, P. Rapid identification of staphylococci by Raman spectroscopy. Sci. Rep. 2017, 7, 14846. [Google Scholar] [CrossRef]
- Pezzotti, G. Raman spectroscopy in cell biology and microbiology. J. Raman Spectrosc. 2021, 52, 2348–2443. [Google Scholar] [CrossRef]
- Lindtner, R.A.; Wurm, A.; Pirchner, E.; Putzer, D.; Arora, R.; Coraca-Huber, D.C.; Schirmer, M.; Badzoka, J.; Kappacher, C.; Huck, C.W.; et al. Enhancing bone infection diagnosis with Raman handheld spectroscopy: Pathogen discrimination and diagnostic potential. Int. J. Mol. Sci. 2023, 25, 541. [Google Scholar] [CrossRef]
- Wurm, A.; Kuhn, J.; Kugel, K.; Putzer, D.; Arora, R.; Coraca-Huber, D.C.; Zelger, P.; Badzoka, J.; Kappacher, C.; Huck, C.W.; et al. Raman microscopic spectroscopy as a diagnostic tool to detect Staphylococcus epidermidis in bone grafts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121570. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Tannert, A.; Ebert, C.; Guliev, R.R.; Ozegowski, Y.; Carvalho, L.; Wildemann, B.; Eiserloh, S.; Coldewey, S.M.; Loeffler, B.; et al. Insight into S. aureus-induced bone deformation in a mouse model of chronic osteomyelitis using fluorescence and Raman imaging. Int. J. Mol. Sci. 2023, 24, 9762. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.; Zheng, W.; Ma, Y.; Huang, Z. Real-time monitoring of pharmacokinetics of antibiotics in biofilm with Raman-tagged hyperspectral stimulated Raman scattering microscopy. Theranostics 2019, 9, 1348–1357. [Google Scholar] [CrossRef]
- Dulude, J.-P.; Le Moel, A.; Dallaire, F.; Doyon, J.; Urmey, K.; Maple, E.; Leblanc, G.; Basile, G.; Mottard, S.; Isler, M.; et al. Intraoperative use of high-speed Raman spectroscopy during soft tissue sarcoma resection. Sci. Rep. 2025, 15, 8789. [Google Scholar] [CrossRef]
- Al Ghaithi, A.K.; Al Maskari, S.M.; Al Mutani, M.M.; Al Bimani, A.M.; Al Jabri, Z.; Al Badi, K.S.; Husband, J. Specific discrimination of pathogenic bacteria causing septic arthritis using Raman spectroscopy: In-vitro study. Diagn. Microbiol. Infect. Dis. 2024, 109, 116339. [Google Scholar] [CrossRef]
- Rebrosova, K.; Samek, O.; Kizovsky, M.; Bernatova, S.; Hola, V.; Ruzicka, F. Raman spectroscopy—A novel method for identification and characterization of microbes on a single-cell level in clinical settings. Front. Cell. Infect. Microbiol. 2022, 12, 866463. [Google Scholar] [CrossRef]
- Al Ghaithi, A.; Al Bimani, A.; Al Maskari, S. Investigating the growth of Pseudomonas aeruginosa and its influence on osteolysis in human bone: An in vitro study. Strateg. Trauma. Limb Reconstr. 2021, 16, 127–131. [Google Scholar] [CrossRef]
- Mendoza Bertelli, A.; Delpino, M.V.; Lattar, S.; Giai, C.; Noto Llana, M.; Sanjuan, N.; Cassat, J.E.; Sordelli, D.; Gomez, M. Staphylococcus aureus protein A enhances osteoclastogenesis via TNFR1 and EGFR signaling. Biochim. Biophys. Acta 2019, 1862, 1975–1983. [Google Scholar] [CrossRef]
- Pezzotti, G.; Zhu, W.; Boffelli, M.; Adachi, T.; Ichioka, H.; Yamamoto, T.; Marunaka, Y.; Kanamura, N. Vibrational algorithms for quantitative crystallographic analyses of hydroxyapatite-based biomaterials: I. Theoretical foundations. Anal. Bioanal. Chem. 2015, 407, 3325–3342. [Google Scholar] [CrossRef]
- Adachi, T.; Pezzotti, G.; Yamamoto, T.; Ichioka, H.; Boffelli, M.; Zhu, W.; Kanamura, N. Vibrational algorithms for quantitative crystallographic analyses of hydroxyapatite-based biomaterials: II. Application to decayed human teeth. Anal. Bioanal. Chem. 2015, 407, 3343–3356. [Google Scholar] [CrossRef]
- de Mul, F.F.M.; Otto, C.; Greve, J.; Arends, J.; ten Bosch, J.J. Calculation of the Raman line broadening on carbonation in synthetic hydroxyapatite. J. Raman Spectros. 1988, 19, 13–21. [Google Scholar] [CrossRef]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- McElderry, J.-D.P.; Zhu, P.; Mroue, K.H.; Xu, J.; Pavan, B.; Fang, M.; Zhao, G.; McNerny, E.; Kohn, D.H.; Franceschi, R.T.; et al. Crystallinity and compositional changes in carbonated apatites: Evidence from 31P solid-state NMR, Raman, and AFM analysis. J. Solid. State Chem. 2013, 206, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.G.; Bullock, A.J.; MacNeil, S.; Rehman, I.U. Characterisation of structural changes in collagen with Raman spectroscopy. Appl. Spectrosc. Rev. 2019, 54, 509–542. [Google Scholar] [CrossRef]
- Wan, K.S.; Tochino, S.; Zhu, W.L.; Ohtsuka, S.; Pezzotti, G. Quantitative evaluation of probe response functions for Raman and fluorescence bands of single-crystalline and polycrystalline Al2O3. J. Phys. D Appl. Phys. 2010, 43, 205501. [Google Scholar] [CrossRef]
- Ayala, O.D.; Wakeman, C.A.; Pence, I.J.; Gaddy, J.A.; Slaughter, J.C.; Skaar, E.P.; Mahadevan-Jansen, A. Drug-resistant Staphylococcus aureus strain reveal distinct biochemical features with Raman microspectroscopy. ACS Infect. Dis. 2018, 4, 1197–1210. [Google Scholar] [CrossRef]
- Dong, P.-T.; Mohammad, H.; Hui, J.; Leanse, L.G.; Li, J.; Liang, L.; Dai, T.; Saleem, M.N.; Cheng, J.-X. Photolysis of Staphyloxanthin in methicillin-resistant Staphylococcus aureus potentiates killing by reactive oxygen species. Adv. Sci. 2019, 6, 1900030. [Google Scholar] [CrossRef]
- Maquelin, K.; Hoogenboezem, T.; Jachtenberg, J.W.; Dumke, R.; Jacobs, E.; Puppels, G.J.; Hartwig, N.G.; Vink, C. Raman spectroscopic typing reveals the presence of carotenoids in mycoplasma pneumoniae. Microbiology 2009, 155, 2068–2077. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Okada, M.; Smith, N.I.; Palonpon, A.F.; Endo, H.; Kawata, S.; Sodeoka, M.; Fujita, K. Label-free Raman observation of cytochrome c dynamics during apoptosis. Proc. Natl. Acad. Sci. USA 2012, 109, 28–32. [Google Scholar] [CrossRef]
- Mostafa, A.; Kanehira, Y.; Tapio, K.; Bald, I. From bulk to single molecules—Surface enhanced Raman scattering of cytochrome c using plasmonic DNA origami nanoantennas. Nano Lett. 2024, 24, 6916–6923. [Google Scholar] [CrossRef]
- Takahashi, T.; Ogura, T. Resonance Raman spectra of cytochrome c oxidase in whole mitochondria. Bull. Chem. Soc. Jpn. 2002, 75, 1001–1004. [Google Scholar] [CrossRef]
- Piccoli, C.; Perna, G.; Scrima, R.; Cela, O.; Rinaldi, R.; Boffoli, D.; Capozzi, V.; Capitanio, N. A novel redox state Heme a marker in cytochrome c oxidase revealed by Raman spectroscopy. Phys. Scr. 2005, 2005, 199–204. [Google Scholar] [CrossRef]
- Hu, S.; Morris, I.K.; Singh, J.P.; Smith, K.M.; Spiro, T.G. Complete assignment of cytochrome c resonance Raman spectra via enzymatic reconstitution with isotopically labeled hemes. J. Am. Chem. Soc. 1993, 115, 12446–12458. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Tian, L.; Zhang, Q.; Guan, Y.; Chen, L.; Liu, G.; Yu, H.-q.; Tian, Y.; Huang, Q. Raman micro-spectroscopy monitoring of cytochrome c redox state in Candida utilis during cell death under low-temperature plasma-induced oxidative stress. Analyst 2020, 145, 3922. [Google Scholar] [CrossRef] [PubMed]
- Pezzotti, G.; Adachi, T.; Imamura, H.; Ikegami, S.; Kitahara, R.; Yamamoto, T.; Kanamura, N.; Zhu, W.; Ishibashi, K.-I.; Okuma, K.; et al. Raman spectroscopic algorithms for assessing virulence in oral candidiasis: The fight-or-flight response. Int. J. Mol. Sci. 2024, 25, 11410. [Google Scholar] [CrossRef]
- George, D.; Pallu, S.; Bourzac, C.; Wazzani, R.; Allena, R.; Remond, Y.; Portier, H. Prediction of cortical bone thickness variations in the tibial diaphysis of running rats. Life 2022, 12, 233. [Google Scholar] [CrossRef]
- Perilli, E.; Le, V.; Ma, B.; Salmon, P.; Reynolds, K.; Fazzalari, N.L. Detecting early bone changes using in vivo micro-CT in ovariectomized, zoledronic acid-treated and sham-operated rats. Osteoporos. Int. 2010, 21, 1371–1382. [Google Scholar] [CrossRef]
- Peltola, H.; Vahvanen, V. A comparative study of osteomyelitis and purulent arthritis with special reference to aetiology and recovery. Infection 1984, 12, 75–79. [Google Scholar] [CrossRef]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Raudenkolb, S.; Wartewig, S.; Brezesinski, G.; Funari, S.S.; Neubert, R.H.H. Hydration properties of N-(α-hydroxyacyl)-sphingosine: X-ray powder diffraction and FT-Raman spectroscopic studies. Chem. Phys. Lipids 2005, 136, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ghonaim, H.M.; Periasamy, N.; Noro, M.G.; Anwar, J. Towards a simplified model membrane of skin lipids: Preparation and characterisation of a ternary lipid mixture. Int. J. Pharm. Pharm. Sci. 2014, 6, 148–152. [Google Scholar]
- Bowen, R.D.; Edwards, H.G.M.; Varnali, T. Influence of a methyl substituent on the Raman spectrum of but-3-enyl methyl ether. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2012, 93, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lennon, A.M.; Brune, L.; Techert, S.; Buchalla, W. Fluorescence spectroscopy shows porphyrins produced by cultural oral bacteria differ depending on composition of growth media. Caries Res. 2022, 57, 74–86. [Google Scholar] [CrossRef]
- Garzoni, C.; Kelley, W.L. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009, 17, 59–65. [Google Scholar] [CrossRef]
- Lazzarini, L.; Mader, J.T.; Calhoun, J.H. Osteomyelitis in long bones. J. Bone Jt. Surg. Am. 2004, 86A, 2305–2318. [Google Scholar] [CrossRef]
- Marriott, I.; Gray, D.L.; Tranguch, S.L.; Fowler, V.G.; Stryjewski, M., Jr.; Levin, L.S.; Hudson, M.C.; Bost, K.L. Osteoblasts express the inflammatory cytokine interleukin-6 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Am. J. Pathol. 2004, 164, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Meghji, S.; Williams, R.J.; Henderson, B.; Brock, J.H.; Nair, S.P. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect. Immun. 2001, 69, 2872–2877. [Google Scholar] [CrossRef]
- Cheng, A.G.; DeDent, A.C.; Schneewind, O.; Missiakas, D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol. 2011, 19, 225–232. [Google Scholar] [CrossRef]
- Muthukrishnan, G.; Masters, E.A.; Daiss, J.L.; Schwarz, E.M. Mechanisms of immune evasion and bone tissue colonization that make Staphylococcus aureus the primary pathogen in osteomyelitis. Curr. Osteoporos. Rep. 2019, 17, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Hofstee, M.I.; Riool, M.; Terjajevs, I.; Thompson, K.; Stoddart, M.J.; Richards, R.G.; Zaat, S.A.J.; Moriarty, T.F. 3-dimensional in vitro Staphylococcus aureus abscess communities display antibiotic tolerance and protection from neutrophil clearance. Infect. Immun. 2020, 88, e00293-20. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Liakopoulos, V.; Stefanidis, I. Cytochrome c as a potentially useful marker of mitochondrial and cellular damage. Front. Immunol. 2016, 7, 279. [Google Scholar] [CrossRef]
- Szafranska, A.K.; Oxley, A.P.A.; Chaves-Moreno, D.; Horst, S.A.; Rosslenbroich, S.; Peters, G.; Goldmann, O.; Rohde, M.; Sinha, B.; Pieper, D.H.; et al. Medina, High-resolution transcriptomic analysis of the adaptive response of Staphylococcus aureus during acute and chronic phases of osteomyelitis. MBio 2014, 5, e01775-14. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Liakopoulos, V.; Stefanidis, I.; Lawson, B.R. Toll-like receptors and their role in renal pathologies. Inflamm. Allergy Drug Targets 2012, 11, 464–477. [Google Scholar] [CrossRef]
- Jemmerson, R.; LaPlante, B.; Treeful, A. Release of intact, monomeric cytochrome c from apoptotic and necrotic cells. Cell Death Differ. 2002, 9, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Codina, R.; Vanasse, A.; Kelekar, A.; Vezys, V.; Jemmerson, R. Cytochrome c-induced lymphocyte death from the outside in: Inhibition by serum leucine-rich alpha-2-glycoprotein-1. Apoptosis 2010, 15, 139–152. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef]
- Green, D.R. Apoptotic pathways: Ten minutes to dead. Cell 2005, 121, 671–674. [Google Scholar] [CrossRef]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Does the redox status of cytochrome c act as a fail-safe mechanism in the regulation of programmed cell death? Free. Radic. Biol. Med. 2001, 31, 697–703. [Google Scholar] [CrossRef]
- Pan, Z.; Voehringer, D.W.; Meyn, R.E. Analysis of redox regulation of cytochrome c-induced apoptosis in a cell-free system. Cell Death Differ. 1999, 6, 683–688. [Google Scholar] [CrossRef]
- Suto, D.; Sato, K.; Ohba, Y.; Yoshimura, T.; Fuji, J. Suppression of the pro-apoptotic unction of cytochrome c by singlet oxygen via a heme redox state-independent mechanism. Biochem. J. 2005, 392, 399–406. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Regulation of apoptosis by the redox state of cytochrome c. Biochim. Biophys. Acta 2008, 1777, 877–881. [Google Scholar] [CrossRef]
- Borutaite, V.; Brown, G.C. Mitochondrial regulation of caspase activation by cytochrome oxidase and tetramethylphenylenediamine via cytosolic cytochrome c redox state. J. Biol. Chem. 2007, 282, 31124–31130. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Orii, Y. Resonance Raman studies of cytochrome oxidase. J. Biochem. 1978, 84, 1245–1252. [Google Scholar] [CrossRef]
- Abramczyk, H.; Surmacki, J.M.; Kopec, M.; Jarczewska, K.; Romanowska-Pietrasiak, B. Hemoglobin and cytochrome c: Reinterpreting the origins of oxygenation and oxidation in erythrocytes and in vivo cancer lung cells. Sci. Rep. 2023, 13, 14731. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive oxygen species (ROS) regulates different types of cell death by acting as a rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed]
- Mocsai, A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 2013, 210, 1283–1299. [Google Scholar] [CrossRef] [PubMed]
- Marriott, I. Apoptosis-associated uncoupling of bone formation and resorption in osteomyelitis. Front. Cell. Infect. Microbiol. 2013, 3, 101. [Google Scholar] [CrossRef] [PubMed]
- Bury, D.C.; Rogers, T.S.; Dickman, M.M. Osteomyelitis: Diagnosis and treatment. Am. Fam. Physician 2021, 104, 395–402. [Google Scholar]
- Masters, E.A.; Ricciardi, B.F.; de Mesy Bentley, K.L.; Moriarty, T.F.; Schwarz, E.M.; Muthukrishnan, G. Skeletal infections: Microbial pathogenesis, immunity and clinical management. Nat. Rev. Microbiol. 2022, 20, 385–400. [Google Scholar] [CrossRef]
- Miller, L.G.; Kaplan, S.L. Staphylococcus aureus: A community pathogen. Infect. Dis. Clin. N. Am. 2009, 23, 35–52. [Google Scholar]
- Pietrocola, G.; Nobile, G.; Rindi, S.; Speziale, P. Staphylococcus aureus manipulates innate immunity through own and host-expressed proteases. Front. Cell. Infect. Microbiol. 2017, 7, 166. [Google Scholar] [CrossRef]
- Missiakas, D.; Winstel, V. Selective host cell death by Staphylococcus aureus: A strategy for bacterial persistence. Front. Immunol. 2021, 11, 621733. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Pathogenesis of Staphylococcus aureus abscesses. Am. J. Pathol. 2015, 185, 1518–1527. [Google Scholar] [CrossRef]
- Dinges, M.M.; Orwin, P.M.; Schlievert, P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000, 13, 16–34. [Google Scholar] [CrossRef]
- Cheng, A.G.; Kim, H.K.; Burts, M.L.; Krausz, T.; Schneewind, O.; Missiakas, D.M. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissue. J. Fed. Am. Soc. Exp. Biol. 2009, 23, 3393–3404. [Google Scholar]
- Kim, H.K.; DeDent, A.; Cheng, A.G.; McAdow, M.; Bagnoli, F.; Missiakas, D.M.; Schneewind, O. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 2010, 28, 6382–6392. [Google Scholar] [CrossRef]
- Good, C.J.; Butrico, C.E.; Colley, M.E.; Gibson-Corley, K.N.; Cassat, J.E.; Spraggins, J.M.; Caprioli, R.M. In situ lipidomics of Staphylococcus aureus osteomyelitis using imaging mass spectrometry. bioRxiv 2023. [Google Scholar] [CrossRef]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta BBA–Mol. Basis Dis. 2012, 1822, 1442–1452. [Google Scholar] [CrossRef]
- Perry, W.J.; Grunenwald, C.M.; Van De Plas, R.; Witten, J.C.; Martin, D.R.; Apte, S.S.; Cassat, J.E.; Pettersson, G.B.; Caprioli, R.M.; Skaar, E.P.; et al. Visualizing Staphylococcus aureus pathogenic membrane modification within the host infection environment by multimodal imaging mass spectrometry. Cell Chem. Biol. 2022, 29, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-H.; Groseclose, R.; Mininger, C.; Bergstrom, M.; Zhang, L.; Lenhard, S.C.; Skedzielewski, T.; Kelley, Z.D.; Comroe, D.; Hong, H.; et al. Multimodal imaging distribution assessment of a liposomal antibiotic in an infectious disease model. J. Control. Release 2022, 352, 199–210. [Google Scholar] [CrossRef]
- Mueller, H.W.; O’Flaherty, J.T.; Greene, D.G.; Samuel, M.P.; Wykle, R.L. 1-O-alkyl-linked glycerophospholipids of human neutrophils: Distribution of arachidonate and other acyl residues in the ether-linked and diacyl species. J. Lipid Res. 1984, 25, 383–388. [Google Scholar] [CrossRef]
- Ivanova, P.T.; Milne, S.B.; Brown, H.A. Identification of atypical ether-linked glycerophospholipid species in macrophages by mass spectrometry. J. Lipid Res. 2010, 51, 1581–1590. [Google Scholar] [CrossRef]
- Mitroulis, I.; Kalafati, L.; Hajishengallis, G.; Chavakis, T. Myelopoiesis in the context of innate immunity. J. Innate Immun. 2018, 10, 365–372. [Google Scholar] [CrossRef]
- Qureshi, A.; Subathra, M.; Grey, A.; Schey, K.; Del Poeta, M.; Luberto, C. Role of sphingomyelin synthase in controlling the antimicrobial activity of neutrophils against Cryptococcus neoformans. PLoS ONE 2010, 5, e15587. [Google Scholar] [CrossRef]
- Hinkovska-Galcheva, V.T.; Boxer, L.A.; Mansfield, P.J.; Harsh, D.; Blackwood, A.; Shayman, J.A. The formation of ceramide-1-phosphate during neutrophil phagocytosis and its role in liposome fusion. Sci. Rep. 1998, 273, 33203–33209. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Bratton, D.L.; Frasch, S.C.; Warner, M.L.; Henson, P.M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef]
- Isogai, N.; Shiono, Y.; Kuramoto, T.; Yoshioka, K.; Ishiyama, H.; Funao, H.; Nakamura, M.; Matsumoto, M.; Ishii, K. Potential osteomyelitis biomarkers identified by plasma metabolome analysis in mice. Cell Death Differ. 2020, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Gatt, S. Enzymic hydrolysis and synthesis of ceramides. J. Biol. Chem. 1963, 238, 3131–3133. [Google Scholar] [CrossRef] [PubMed]
- Goni, F.M.; Alonso, A. Sphingomyelinases: Enzymology and membrane activity. FEBS Lett. 2002, 531, 38–46. [Google Scholar] [CrossRef]
- Marchesini, N.; Hannun, Y.A. Acid and neutral sphingomyelinases: Roles and mechanisms of regulation. Biochem. Cell Biol. 2004, 82, 27–44. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An overview of sphingolipid metabolism: From synthesis to breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar]
- Kennedy, S.; Kane, K.A.; Pyne, N.J.; Pyne, S. Targeting sphingosine-1-phosphate signalling for cardioprotection. Curr. Opin. Pharmacol. 2009, 9, 194–201. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Claus, R.A. Keep your friends close, but your enemies closer: Role of acid sphingomyelinase during infection and host response. Front. Med. 2021, 7, 616500. [Google Scholar] [CrossRef]
- Scheel-Toellner, D.; Wang, K.; Craddock, R.; Webb, P.R.; McGettrick, H.M.; Assi, K.; Parkes, N.; Clough, L.E.; Gulbins, E.; Salmon, M.; et al. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood 2004, 104, 2557–2564. [Google Scholar] [CrossRef]
- Fox, S.; Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Neutrophil apoptosis: Relevance to the innate immune response and inflammation disease. J. Innate Immun. 2010, 2, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Brostjan, C.; Oehler, R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 2020, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, N.; Ryan, E.J.; Widaa, A.; Sexton, G.; Fennell, J.; O’Rourke, S.; Cahill, K.C.; Kearney, C.J.; O’Brien, F.J.; Kerrigan, S.W. Staphylococcal osteomyelitis: Disease progression, treatment challenges, and future directions. Clin. Microbiol. Rev. 2018, 31, e00084-17. [Google Scholar] [CrossRef]
- Gimza, B.D.; Cassat, J.E. Mechanisms of antibiotic failure during Staphylococcus aureus osteomyelitis. Front. Immunol. 2021, 12, 303. [Google Scholar] [CrossRef]
- Pelz, A.; Wieland, K.-P.; Putzbach, K.; Hentschel, P.; Albert, K.; Goetz, F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J. Biol. Chem. 2005, 280, 32493–32498. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.E.; McCready-Vangi, A.R.; Uberoi, A.; Murga-Garrido, S.M.; Lovins, V.M.; White, E.K.; Pan, J.T.-C.; Knight, S.A.B.; Morgenstern, A.R.; Bianco, C.; et al. Variable staphyloxanthin production by Staphylococcus aureus drives strain-dependent effects on diabetic wound-healing outcomes. Cell Rep. 2023, 42, 113281. [Google Scholar] [CrossRef] [PubMed]
- Clauditz, A.; Resch, A.; Wieland, K.-P.; Peschel, A.; Goetz, F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect. Immun. 2006, 74, 4950–4953. [Google Scholar] [CrossRef]
- Liu, G.Y.; Essex, A.; Buchanan, J.T.; Datta, V.; Hoffman, H.M.; Bastian, J.F.; Fierer, J.; Nizet, V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005, 202, 209–215. [Google Scholar] [CrossRef]
- Schaffer, H.E.; Chance, R.R.; Silbey, R.J.; Knoll, K.; Schrock, R.R.J. Conjugation length dependence of Raman scattering in a series of linear polyenes: Implications for polyacetylene. Chem. Phys. 1991, 94, 4161–4170. [Google Scholar] [CrossRef]
- Thomas, D.B.; McGoverin, C.M. The analytical niche for Raman spectroscopy in biological pigment research. Spectrosc. Eur. 2016, 28, 6–9. [Google Scholar]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.M.; de Oliveira, L.F.C. Raman spectra of carotenoids in natural products. Spectrochim. Acta Part. A 2003, 59, 2207–2212. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Yeum, K.-J. Carotenoid—Radical interactions. Biochem. Biophys. Res. Comm. 2003, 305, 754–760. [Google Scholar] [CrossRef]
- Handelman, G.J.; van Kuijk, F.J.G.M.; Chatterjee, A.; Krinsky, N.I. Characterization of products formed during the autoxidation of β-carotene. Free Radic. Biol. Med. 1991, 10, 427–437. [Google Scholar] [CrossRef]
- Kennedy, T.A.; Liebler, D.C. Peroxyl radical oxidation of β-carotene: Formation of b-carotene epoxides. Chem. Res. Toxicol. 1991, 4, 290–295. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Wang, X.-D.; Tang, G.; Russell, R.M. Mechanism of carotenoid cleavage to retinoids. In Carotenoids in Human Health; Canfield, L.M., Krinsky, N.I., Olson, J.A., Eds.; New York Academy of Science: New York, NY, USA, 1993; pp. 156–166. [Google Scholar]
- Gao, G.; Wei, C.C.; Jeevarajan, A.S.; Kispert, L.D. Geometrical isomerization of carotenoids mediated by cation radical/dication formation. J. Phys. Chem. 1996, 100, 5362–5366. [Google Scholar] [CrossRef]
- Khaled, M.; Hadjipetrou, A.; Kispert, L.D. Electrochemical and electron paramagnetic resonance studies of carotenoid cation radicals and dications: Effect of deuteration. J. Phys. Chem. 1990, 94, 5164–5169. [Google Scholar] [CrossRef]
- Thammavongsa, V.; Missiakas, D.M.; Schneewind, O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 2013, 342, 863–866. [Google Scholar] [CrossRef]
- Winstel, V.; Missiakas, D.; Schneewind, O. Staphylococcus aureus targets the purine salvage pathway to kill phagocytes. Proc. Natl. Acad. Sci. USA 2018, 115, 6846–6851. [Google Scholar] [CrossRef] [PubMed]
- Winstel, V.; Schneewind, O.; Missiakas, D. Staphylococcus aureus exploits the host apoptotic pathway to persist during infection. MBio 2019, 10, e02270-19. [Google Scholar] [CrossRef] [PubMed]
- Thomer, L.; Schneewind, O.; Missiakas, D. Pathogenesis of Staphylococcus aureus bloodstream infections. Annu. Rev. Pathol. 2016, 11, 343–364. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, W.; Liu, L.; Huang, Y.; He, J.; Xie, W.; Wu, P.; Du, C. Baseline correction for Raman spectra using an improved asymmetric least squares method. Anal. Methods 2014, 6, 4402–4407. [Google Scholar] [CrossRef]
- Pezzotti, G.; Kobara, M.; Asai, T.; Nakaya, T.; Miyamoto, N.; Adachi, T.; Yamamoto, T.; Kanamura, N.; Ohgitani, E.; Marin, E.; et al. Raman imaging of pathogenic Candida auris: Visualization of structural characteristics and machine-learning identification. Front. Microbiol. 2021, 12, 769597. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, S.; Horie, N.; Ikegami, S.; Imamura, H.; Zhu, W.; Ikegaya, H.; Mazda, O.; Pezzotti, G.; Takahashi, K. Raman Monitoring of Staphylococcus aureus Osteomyelitis: Microbial Pathogenesis and Bone Immune Response. Int. J. Mol. Sci. 2025, 26, 8572. https://doi.org/10.3390/ijms26178572
Fujii S, Horie N, Ikegami S, Imamura H, Zhu W, Ikegaya H, Mazda O, Pezzotti G, Takahashi K. Raman Monitoring of Staphylococcus aureus Osteomyelitis: Microbial Pathogenesis and Bone Immune Response. International Journal of Molecular Sciences. 2025; 26(17):8572. https://doi.org/10.3390/ijms26178572
Chicago/Turabian StyleFujii, Shun, Naoyuki Horie, Saki Ikegami, Hayata Imamura, Wenliang Zhu, Hiroshi Ikegaya, Osam Mazda, Giuseppe Pezzotti, and Kenji Takahashi. 2025. "Raman Monitoring of Staphylococcus aureus Osteomyelitis: Microbial Pathogenesis and Bone Immune Response" International Journal of Molecular Sciences 26, no. 17: 8572. https://doi.org/10.3390/ijms26178572
APA StyleFujii, S., Horie, N., Ikegami, S., Imamura, H., Zhu, W., Ikegaya, H., Mazda, O., Pezzotti, G., & Takahashi, K. (2025). Raman Monitoring of Staphylococcus aureus Osteomyelitis: Microbial Pathogenesis and Bone Immune Response. International Journal of Molecular Sciences, 26(17), 8572. https://doi.org/10.3390/ijms26178572






