The Sex Hormone Precursors Dehydroepiandrosterone (DHEA) and Its Sulfate Ester Form (DHEAS): Molecular Mechanisms and Actions on Human Body
Abstract
1. Introduction
2. Method of Literature Review and Retrieval
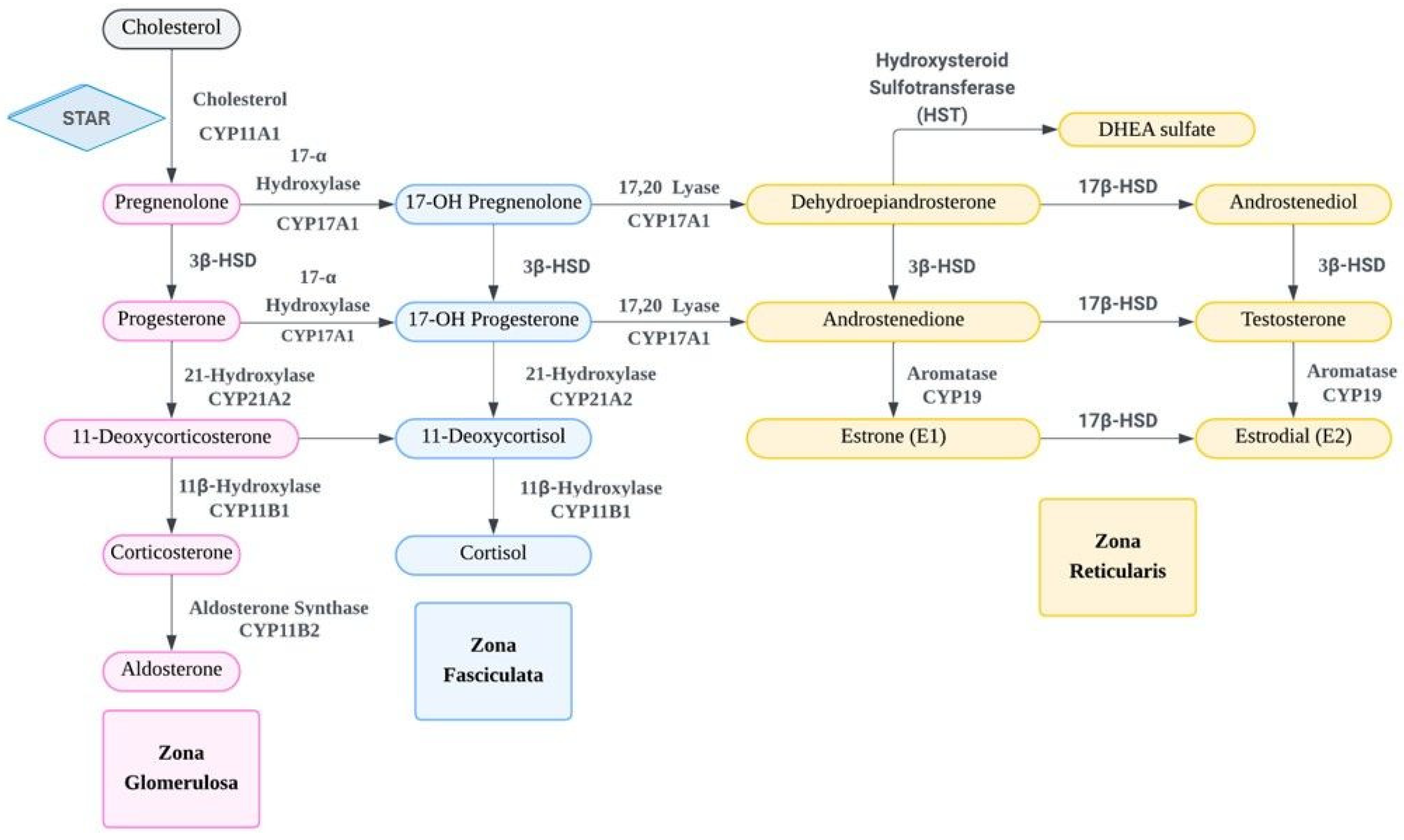
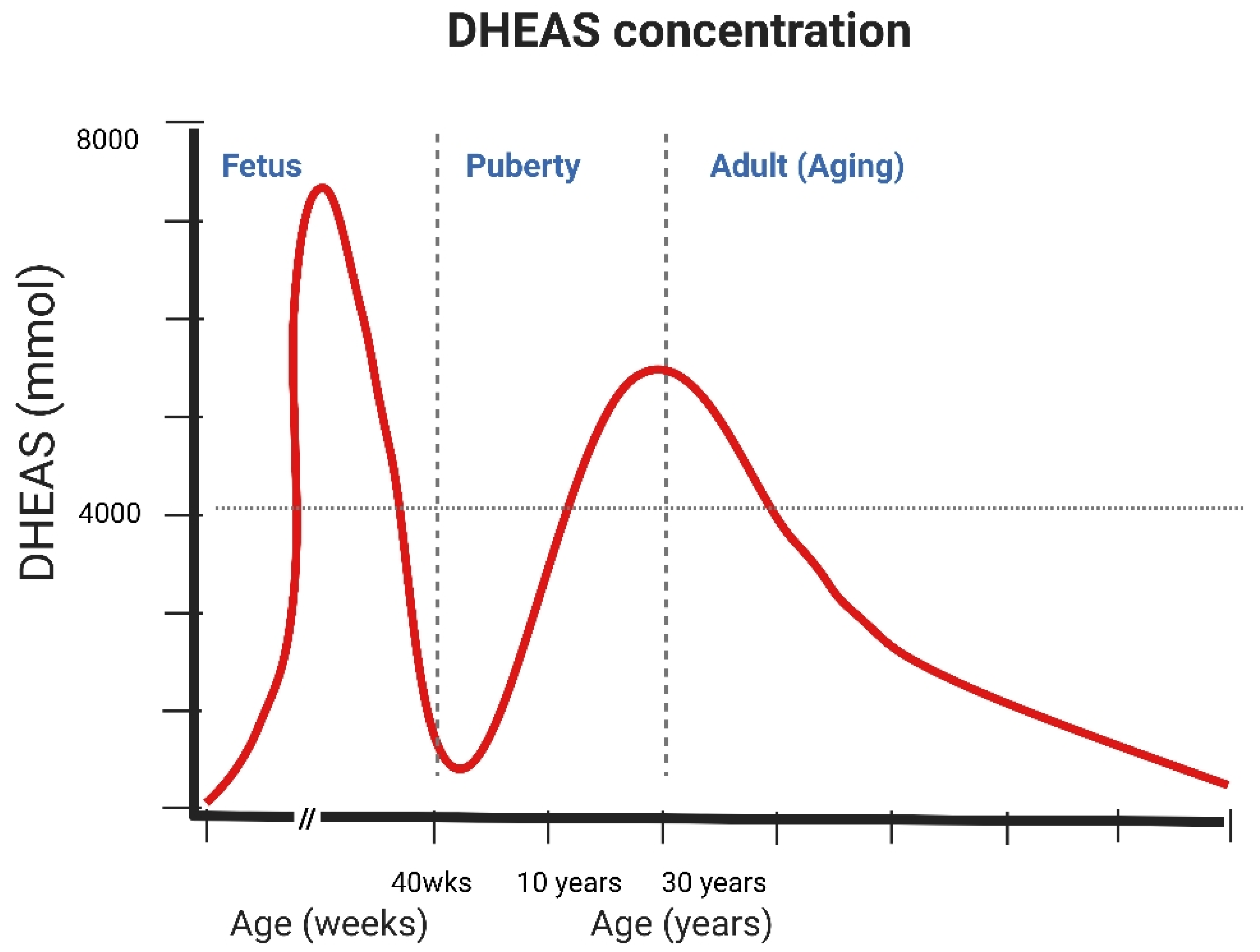
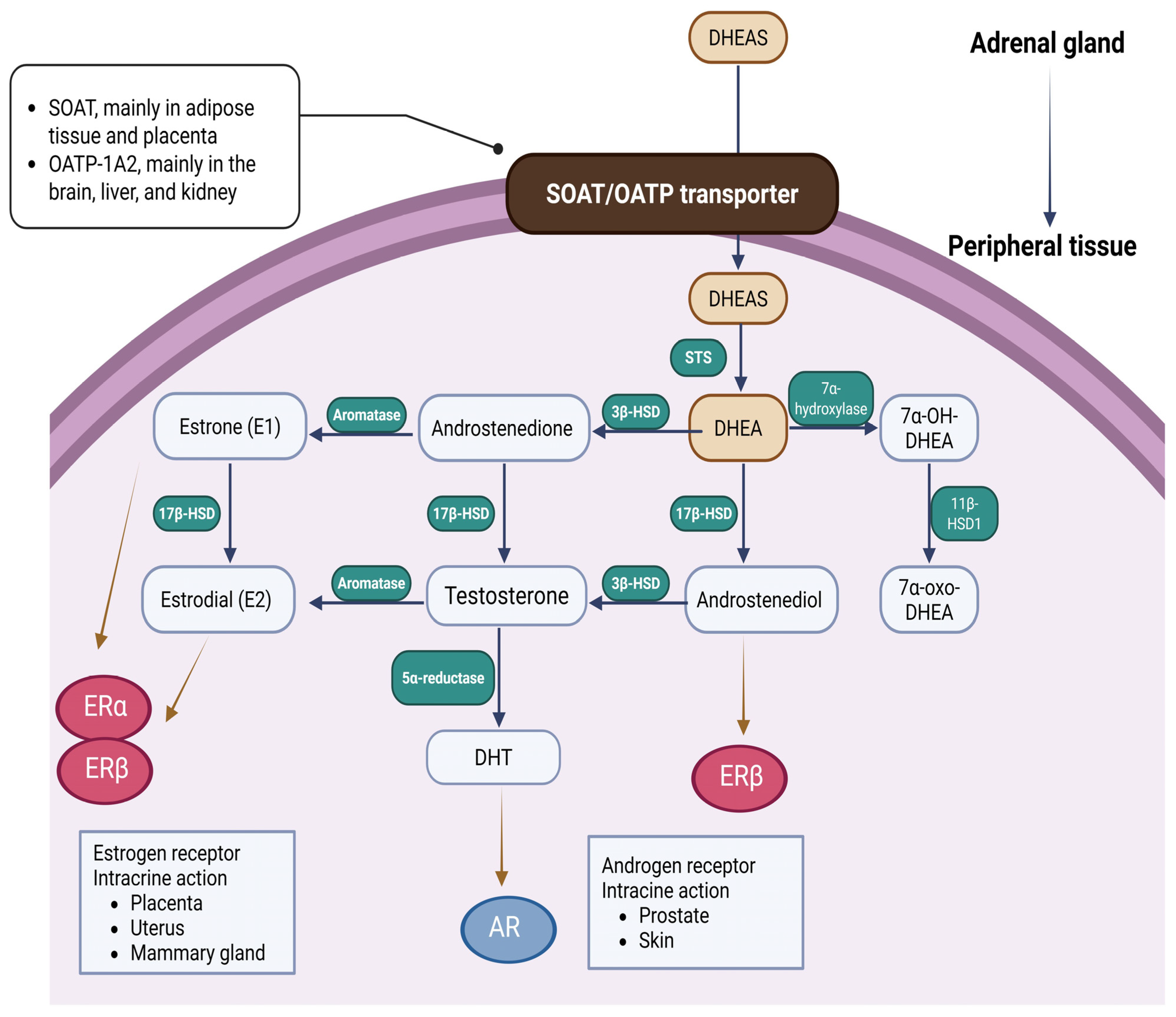
3. The Molecular and Cellular Mechanism of DHEA/DHEAS Production, Conversion, and Action
4. Clinical Associations with DHEA and DHEAS Actions
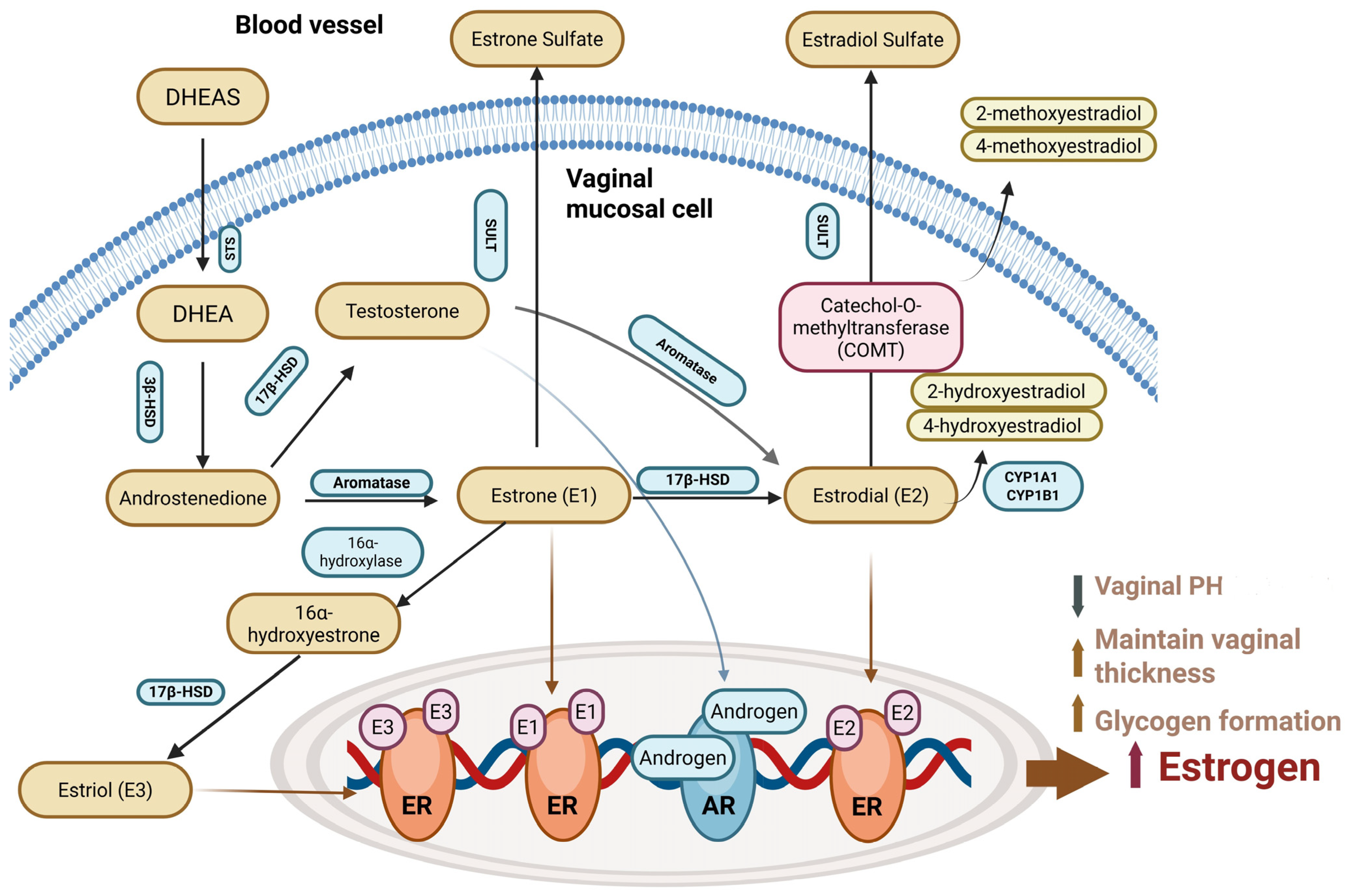
4.1. DHEA on Menopausal Symptoms
4.2. DHEA in Central Nervous System
4.3. DHEA in Metabolic System
4.4. DHEA in Musculoskeletal System
4.5. DHEA in Cardiovascular System
4.6. DHEA in Immune System
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Butenandt, A.; Dannenbaum, H. Über Androsteron. III. Isolierung eines neuen, physiologisch unwirksamen Sterinderivates aus Männerharn, seine Verknüpfung mit Dehydro-androsteron und Androsteron: Ein Beitrag zur Konstitution des Androsterons. Hoppe-Seyler’s Z. Physiol. Chem. 1934, 229, 192–208. [Google Scholar] [CrossRef]
- Munson, P.; Gallagher, T.; Koch, F. Isolation of dehydroisoandrosterone sulfate from normal male urine. J. Biol. Chem. 1944, 152, 67–77. [Google Scholar] [CrossRef]
- Rutkowski, K.; Sowa, P.; Rutkowska-Talipska, J.; Kuryliszyn-Moskal, A.; Rutkowski, R. Dehydroepiandrosterone (DHEA): Hypes and hopes. Drugs 2014, 74, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Deneve, L.; Vermeulen, A. The determination of 17-oxosteroid sulphates in human plasma. J. Endocrinol. 1965, 32, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, M.F.; Cambiasso, M.J.; Holschbach, M.A.; Cabrera, R. Oestrogens and progestagens: Synthesis and action in the brain. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef]
- Roth, G.S.; Lane, M.A.; Ingram, D.K.; Mattison, J.A.; Elahi, D.; Tobin, J.D.; Muller, D.; Metter, E.J. Biomarkers of caloric restriction may predict longevity in humans. Science 2002, 297, 811. [Google Scholar] [CrossRef]
- Tannenbaum, C.; Barrett-Connor, E.; Laughlin, G.A.; Platt, R.W. A longitudinal study of dehydroepiandrosterone sulphate (DHEAS) change in older men and women: The Rancho Bernardo Study. Eur. J. Endocrinol. 2004, 151, 717–725. [Google Scholar] [CrossRef][Green Version]
- Stocco, D.M.; Zhao, A.H.; Tu, L.N.; Morohaku, K.; Selvaraj, V. A brief history of the search for the protein(s) involved in the acute regulation of steroidogenesis. Mol. Cell. Endocrinol. 2017, 441, 7–16. [Google Scholar] [CrossRef]
- Auchus, R.J.; Rainey, W.E. Adrenarche—Physiology, biochemistry and human disease. Clin. Endocrinol. 2004, 60, 288–296. [Google Scholar] [CrossRef]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef]
- Wang, T.; Cook, I.; Falany, C.N.; Leyh, T.S. Paradigms of sulfotransferase catalysis: The mechanism of SULT2A1. J. Biol. Chem. 2014, 289, 26474–26480. [Google Scholar] [CrossRef]
- Jefcoate, C. High-flux mitochondrial cholesterol trafficking, a specialized function of the adrenal cortex. J. Clin. Investig. 2002, 110, 881–890. [Google Scholar] [CrossRef][Green Version]
- Dhatariya, K.K.; Nair, K.S. Dehydroepiandrosterone: Is there a role for replacement? Mayo Clin. Proc. 2003, 78, 1257–1273. [Google Scholar] [CrossRef]
- Longcope, C. The metabolism of dehydroepiandrosterone sulfate and dehydroepiandrosterone. Aging Male 1998, 1, 51–55. [Google Scholar] [CrossRef]
- Rosenfeld, R.S.; Rosenberg, B.J.; Hellman, L. Direct analysis of dehydroisoandrosterone in plasma. Steroids 1975, 25, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Witchel, S.F.; Topaloglu, A.K. Puberty: Gonadarche and adrenarche. In Yen and Jaffe’s Reproductive Endocrinology, 8th ed.; Strauss, J.F., Barbieri, R.L., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 394–446.e16. [Google Scholar] [CrossRef]
- Guazzo, E.; Kirkpatrick, P.; Goodyer, I.; Shiers, H.; Herbert, J. Cortisol, dehydroepiandrosterone (DHEA), and DHEA sulfate in the cerebrospinal fluid of man: Relation to blood levels and the effects of age. J. Clin. Endocrinol. Metab. 1996, 81, 3951–3960. [Google Scholar] [PubMed]
- Lin, H.; Li, L.; Wang, Q.; Wang, Y.; Wang, J.; Long, X. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA supplementation of bone mineral density in healthy adults. Gynecol. Endocrinol. 2019, 35, 924–931. [Google Scholar] [CrossRef]
- Samaras, N.; Samaras, D.; Frangos, E.; Forster, A.; Philippe, J. A review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: Is treatment beneficial? Rejuvenation Res. 2013, 16, 285–294. [Google Scholar] [CrossRef]
- Hornsby, P.J. Aging of the human adrenal cortex. Ageing Res. Rev. 2002, 1, 229–242. [Google Scholar] [CrossRef]
- Labrie, F.; Luu-The, V.; Labrie, C.; BélAnger, A.; Simard, J.; Lin, S.-X.; Pelletier, G. Endocrine and intracrine sources of androgens in women: Inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr. Rev. 2003, 24, 152–182. [Google Scholar] [CrossRef]
- Crawford, S.; Santoro, N.; Laughlin, G.A.; Sowers, M.F.; McConnell, D.; Sutton-Tyrrell, K.; Weiss, G.; Vuga, M.; Randolph, J.; Lasley, B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J. Clin. Endocrinol. Metab. 2009, 94, 2945–2951. [Google Scholar] [CrossRef]
- Klinge, C.M.; Clark, B.J.; Prough, R.A. Dehydroepiandrosterone research: Past, current, and future. In Vitamins and Hormones; Litwack, G., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 108, pp. 1–28. [Google Scholar] [CrossRef]
- Labrie, F.; Martel, C.; Belanger, A.; Pelletier, G. Androgens in women are essentially made from DHEA in each peripheral tissue according to intracrinology. J. Steroid Biochem. Mol. Biol. 2017, 168, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Couzinet, B.; Nahoul, K.; Brailly, S.; Chanson, P.; Baulieu, E.E.; Schaison, G. Panhypopituitarism as a model to study the metabolism of dehydroepiandrosterone (DHEA) in humans. J. Clin. Endocrinol. Metab. 1997, 82, 2578–2585. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Horton, R.; Hawks, D.; Lobo, R. 3α,17β-Androstanediol glucuronide in plasma. A marker of androgen action in idiopathic hirsutism. J. Clin. Investig. 1982, 69, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.F.; Kroboth, P.D.; Kroboth, F.J.; Stone, R.A.; Folan, M.; Salek, F.S.; Pollock, B.G.; Linares, A.M.; Hakala, C. Sex differences in the pharmacokinetics of dehydroepiandrosterone (DHEA) after single- and multiple-dose administration in healthy older adults. J. Clin. Pharmacol. 2000, 40, 596–605. [Google Scholar] [CrossRef]
- Portman, D.J.; Gass, M.L.S. Genitourinary syndrome of menopause: New terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. Climacteric 2014, 17, 557–563. [Google Scholar] [CrossRef]
- Gebhart, J.B.; Rickard, D.J.; Barrett, T.J.; Lesnick, T.G.; Webb, M.J.; Podratz, K.C.; Spelsberg, T.C. Expression of estrogen receptor isoforms α and β messenger RNA in vaginal tissue of premenopausal and postmenopausal women. Am. J. Obstet. Gynecol. 2001, 185, 1325–1331. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.R.; Chen, K.H. The Utilization of Dehydroepiandrosterone as a Sexual Hormone Precursor in Premenopausal and Postmenopausal Women: An Overview. Pharmaceuticals 2021, 15, 46. [Google Scholar] [CrossRef]
- Barton, D.L.; Shuster, L.T.; Dockter, T.; Atherton, P.J.; Thielen, J.; Birrell, S.N.; Sood, R.; Griffin, P.; Terstriep, S.A.; Mattar, B.; et al. Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance). Support. Care Cancer 2018, 26, 1335–1343. [Google Scholar] [CrossRef]
- Dobs, A.S.; Nguyen, T.; Pace, C.; Roberts, C.P. Differential effects of oral estrogen versus oral estrogen-androgen replacement therapy on body composition in postmenopausal women. J. Clin. Endocrinol. Metab. 2002, 87, 1509–1516. [Google Scholar] [CrossRef]
- Davis, S.R.; Panjari, M.; Stanczyk, F.Z. Clinical review: DHEA replacement for postmenopausal women. J. Clin. Endocrinol. Metab. 2011, 96, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Bélanger, A.; Bélanger, P.; Bérubé, R.; Martel, C.; Cusan, L.; Gomez, J.; Candas, B.; Chaussade, V.; Castiel, I.; et al. Metabolism of DHEA in postmenopausal women following percutaneous administration. J. Steroid Biochem. Mol. Biol. 2007, 103, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Portman, D.J.; Labrie, F.; Archer, D.F.; Bouchard, C.; Cusan, L.; Girard, G.; Ayotte, N.; Koltun, W.; Blouin, F.; Young, D.; et al. Lack of effect of intravaginal dehydroepiandrosterone (DHEA, prasterone) on the endometrium in postmenopausal women. Menopause 2015, 22, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L. The therapeutic effect of dehydroepiandrosterone (DHEA) on vulvovaginal atrophy. Pharmacol. Res. 2021, 166, 105509. [Google Scholar] [CrossRef]
- Barton, D.L.; Sloan, J.A.; Shuster, L.T.; Gill, P.; Griffin, P.; Flynn, K.; Terstriep, S.A.; Rana, F.N.; Dockter, T.; Atherton, P.J.; et al. Evaluating the efficacy of vaginal dehydroepiandosterone for vaginal symptoms in postmenopausal cancer survivors: NCCTG N10C1 (Alliance). Support. Care Cancer 2018, 26, 643–650. [Google Scholar] [CrossRef]
- Bouchard, C.; Labrie, F.; Archer, D.F.; Portman, D.J.; Koltun, W.; Elfassi, É.; Grainger, D.A.; Ayotte, N.; Cooper, T.A.; Martens, M.; et al. Decreased efficacy of twice-weekly intravaginal dehydroepiandrosterone on vulvovaginal atrophy. Climacteric 2015, 18, 590–607. [Google Scholar] [CrossRef]
- Peixoto, C.; Carrilho, C.G.; Barros, J.A.; Ribeiro, T.T.; Silva, L.M.; Nardi, A.E.; Cardoso, A.; Veras, A.B. The effects of dehydroepiandrosterone on sexual function: A systematic review. Climacteric 2017, 20, 129–137. [Google Scholar] [CrossRef]
- Elraiyah, T.; Sonbol, M.B.; Wang, Z.; Khairalseed, T.; Asi, N.; Undavalli, C.; Nabhan, M.; Altayar, O.; Prokop, L.; Montori, V.M.; et al. Clinical review: The benefits and harms of systemic dehydroepiandrosterone (DHEA) in postmenopausal women with normal adrenal function: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 3536–3542. [Google Scholar] [CrossRef]
- Collà Ruvolo, C.; Gabrielli, O.; Formisano, C.; Califano, G.; Manna, P.; Venturella, R.; Di Carlo, C. Prasterone in the treatment of mild to moderate urge incontinence: An observational study. Menopause 2022, 29, 957–962. [Google Scholar] [CrossRef]
- Misasi, G.; Russo, E.; Montt Guevara, M.M.; Tomatis, V.; Fidecicchi, T.; Luisi, S.; Giannini, A.; Mannella, P.; Caretto, M.; Pomara, G.; et al. Effects of vaginal DHEA on stress urinary incontinence in postmenopausal women with vulvovaginal atrophy. Maturitas 2025, 196, 108232. [Google Scholar] [CrossRef]
- Baulieu, E.E. Neurosteroids: Of the nervous system, by the nervous system, for the nervous system. Recent Prog. Horm. Res. 1997, 52, 1–32. [Google Scholar] [PubMed]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Grégoire, C.; Espallergues, J. Neuro(active)steroids actions at the neuromodulatory sigma1 (σ1) receptor: Biochemical and physiological evidences, consequences in neuroprotection. Pharmacol. Biochem. Behav. 2006, 84, 581–597. [Google Scholar] [CrossRef]
- Pérez-Neri, I.; Montes, S.; Ojeda-López, C.; Ramírez-Bermúdez, J.; Ríos, C. Modulation of neurotransmitter systems by dehydroepiandrosterone and dehydroepiandrosterone sulfate: Mechanism of action and relevance to psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Laurine, E.; Lafitte, D.; Grégoire, C.; Sérée, E.; Loret, E.; Douillard, S.; Michel, B.; Briand, C.; Verdier, J.M. Specific binding of dehydroepiandrosterone to the N terminus of the microtubule-associated protein MAP2. J. Biol. Chem. 2003, 278, 29979–29986. [Google Scholar] [CrossRef] [PubMed]
- Charalampopoulos, I.; Margioris, A.N.; Gravanis, A. Neurosteroid dehydroepiandrosterone exerts anti-apoptotic effects by membrane-mediated, integrated genomic and non-genomic pro-survival signaling pathways. J. Neurochem. 2008, 107, 1457–1469. [Google Scholar] [CrossRef]
- Lazaridis, I.; Charalampopoulos, I.; Alexaki, V.I.; Avlonitis, N.; Pediaditakis, I.; Efstathopoulos, P.; Calogeropoulou, T.; Castanas, E.; Gravanis, A. Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis. PLoS Biol. 2011, 9, e1001051. [Google Scholar] [CrossRef]
- Melchior, C.L.; Ritzmann, R.F. Dehydroepiandrosterone is an anxiolytic in mice on the plus maze. Pharmacol. Biochem. Behav. 1994, 47, 437–441. [Google Scholar] [CrossRef]
- Sousa, A.; Ticku, M.K. Interactions of the neurosteroid dehydroepiandrosterone sulfate with the GABA(A) receptor complex reveals that it may act via the picrotoxin site. J. Pharmacol. Exp. Ther. 1997, 282, 827–833. [Google Scholar] [CrossRef]
- Gonzalez-Alvear, G.M.; Werling, L.L. Regulation of [3H]dopamine release from rat striatal slices by sigma receptor ligands. J. Pharmacol. Exp. Ther. 1994, 271, 212–219. [Google Scholar] [CrossRef]
- Schverer, M.; Lanfumey, L.; Baulieu, E.E.; Froger, N.; Villey, I. Neurosteroids: Non-genomic pathways in neuroplasticity and involvement in neurological diseases. Pharmacol. Ther. 2018, 191, 190–206. [Google Scholar] [CrossRef]
- Shoae-Hassani, A.; Mortazavi-Tabatabaei, S.A.; Sharif, S.; Rezaei-Khaligh, H.; Verdi, J. DHEA provides a microenvironment for endometrial stem cells neurogenesis. Med. Hypotheses 2011, 76, 843–846. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.; Tagashira, H.; Fukunaga, K. Dehydroepiandrosterone-mediated stimulation of sigma-1 receptor activates Akt-eNOS signaling in the thoracic aorta of ovariectomized rats with abdominal aortic banding. Cardiovasc. Ther. 2011, 29, 219–230. [Google Scholar] [CrossRef]
- Savineau, J.P.; Marthan, R.; Dumas de la Roque, E. Role of DHEA in cardiovascular diseases. Biochem. Pharmacol. 2013, 85, 718–726. [Google Scholar] [CrossRef]
- Kurata, K.; Takebayashi, M.; Morinobu, S.; Yamawaki, S. β-Estradiol, dehydroepiandrosterone, and dehydroepiandrosterone sulfate protect against N-methyl-D-aspartate-induced neurotoxicity in rat hippocampal neurons by different mechanisms. J. Pharmacol. Exp. Ther. 2004, 311, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Strac, D.S.; Konjevod, M.; Perkovic, M.N.; Tudor, L.; Erjavec, G.N.; Pivac, N. Dehydroepiandrosterone (DHEA) and its sulphate (DHEAS) in Alzheimer’s disease. Curr. Alzheimer Res. 2020, 17, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Urani, A.; Privat, A.; Maurice, T. The modulation by neurosteroids of the scopolamine-induced learning impairment in mice involves an interaction with sigma1 (σ1) receptors. Brain Res. 1998, 799, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Weill-Engerer, S.; David, J.P.; Sazdovitch, V.; Liere, P.; Eychenne, B.; Pianos, A.; Schumacher, M.; Delacourte, A.; Baulieu, E.E.; Akwa, Y. Neurosteroid quantification in human brain regions: Comparison between Alzheimer’s and nondemented patients. J. Clin. Endocrinol. Metab. 2002, 87, 5138–5143. [Google Scholar] [CrossRef]
- Aldred, S.; Mecocci, P. Decreased dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) concentrations in plasma of Alzheimer’s disease (AD) patients. Arch. Gerontol. Geriatr. 2010, 51, e16–e18. [Google Scholar] [CrossRef]
- Li, L.; Xu, B.; Zhu, Y.; Chen, L.; Sokabe, M.; Chen, L. DHEA prevents Aβ25-35-impaired survival of newborn neurons in the dentate gyrus through a modulation of PI3K-Akt-mTOR signaling. Neuropharmacology 2010, 59, 323–333. [Google Scholar] [CrossRef]
- Sultana, F.; Davis, S.R.; Islam, R.M. Effect of dehydroepiandrosterone therapy on cognitive performance among postmenopausal women: A systematic review of randomized clinical trial data. Menopause 2023, 30, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Jenaway, A.; Paykel, E.S.; Herbert, J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol. Psychiatry 2000, 48, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhong, W.; An, H.; Fu, M.; Chen, Y.; Zhang, Z.; Xiao, Z. Attenuated DHEA and DHEA-S response to acute psychosocial stress in individuals with depressive disorders. J. Affect. Disord. 2017, 215, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.; Schmidt, P.J.; Danaceau, M.; Murphy, J.; Nieman, L.; Rubinow, D.R. Dehydroepiandrosterone treatment of midlife dysthymia. Biol. Psychiatry 1999, 45, 1533–1541. [Google Scholar] [CrossRef]
- Schmidt, P.J.; Daly, R.C.; Bloch, M.; Smith, M.J.; Danaceau, M.A.; St. Clair, L.S.; Murphy, J.H.; Haq, N.A.; Rubinow, D.R. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch. Gen. Psychiatry 2005, 62, 154–162. [Google Scholar] [CrossRef]
- Ferrero, H.; Fernández-Irigoyen, J.; Laínez, S.; Robles, A.; Matorras, R.; Fernández-Santos, J.M.; Prieto, B.; Escrivá, F.; Gutiérrez-Adán, A.; Casis, L.; et al. Effect of DHEA administration on the ovarian reserve: Molecular study of the ovarian environment. Reprod. Biol. Endocrinol. 2016, 14, 1–10. [Google Scholar]
- Zhang, M.; Niu, W.; Wang, Y.; Zhang, J.; Dang, R.; Wang, W.; Liu, S.; Yu, H.; Liu, M.; Xu, Y. DHEA prevents testicular apoptotic cell death induced by STZ in type 1 diabetic rats: Role of Akt and NF-κB. Oxid. Med. Cell. Longev. 2017, 2017, 1–11. [Google Scholar]
- Rosenfeld, R.G.; Rosenbloom, A.L.; Guevara-Aguirre, J. Growth hormone (GH) insensitivity due to primary GH receptor deficiency. Endocr. Rev. 1994, 15, 369–390. [Google Scholar] [CrossRef]
- Guevara-Aguirre, J.; Balasubramanian, P.; Guevara-Aguirre, M.; Wei, M.; Madia, F.; Cheng, C.W.; Hwang, D.; Martin-Montalvo, A.; Saavedra, J.; Ingles, S.; et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011, 3, 70ra13. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Hinson, J.P.; Vinson, G.P. The role of DHEA in adrenal androgens and aging. Aging Cell 2002, 1, 140–145. [Google Scholar] [CrossRef]
- Baulieu, E.E.; Robel, P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc. Natl. Acad. Sci. USA 1998, 95, 4089–4091. [Google Scholar] [CrossRef] [PubMed]
- Weksler, M.E. Changes in the B-cell repertoire with age. Vaccine 2000, 18, 1624–1628. [Google Scholar] [CrossRef]
- Phillips, A.C.; Carroll, D.; Gale, C.R.; Lord, J.M.; Arlt, W.; Batty, G.D. Cortisol, DHEAS, their ratio and the metabolic syndrome: Evidence from the Vietnam Experience Study. Eur. J. Endocrinol. 2010, 162, 919–923. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef]
- Aoki, K.; Terauchi, Y. Effect of Dehydroepiandrosterone (DHEA) on Diabetes Mellitus and Obesity. Vitam. Horm. 2018, 108, 355–365. [Google Scholar] [CrossRef]
- Labrie, F.; Bélanger, A.; Cusan, L.; Candas, B.; Labrie, C. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J. Clin. Endocrinol. Metab. 1997, 82, 2396–2402. [Google Scholar] [CrossRef]
- Morales, A.J.; Nolan, J.J.; Nelson, J.C.; Yen, S.S. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J. Clin. Endocrinol. Metab. 1994, 78, 1360–1367. [Google Scholar] [CrossRef]
- Ibáñez, L.; Valls, C.; Ferrer, A.; Marcos, M.V.; Rodriguez-Hierro, F.; de Zegher, F. Sensitization to Insulin Induces Ovulation in Nonobese Adolescents with Anovulatory Hyperandrogenism. J. Clin. Endocrinol. Metab. 2001, 86, 3400–3403. [Google Scholar] [CrossRef]
- Casson, P.R.; Andersen, R.N.; Herrod, H.G.; Stentz, F.B.; Straughn, A.B.; Abraham, G.E.; Buster, J.E. Oral dehydroepiandrosterone in physiologic doses modulates immune function in postmenopausal women. Am. J. Obstet. Gynecol. 1993, 169, 1536–1539. [Google Scholar] [CrossRef]
- Morales, A.J.; Haubrich, R.H.; Hwang, J.Y.; Asakura, H.; Yen, S.S. The effect of six months treatment with 50 mg daily of DHEA on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin. Endocrinol. 1998, 49, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.E. Dehydroepiandrosterone (DHEA): A fountain of youth? J. Clin. Endocrinol. Metab. 1996, 81, 3147–3151. [Google Scholar] [CrossRef] [PubMed]
- Reiter, E.; Slominski, A.; Tobin, D.J.; Paus, R. DHEA—Hormone, neurotransmitter, neurosteroid: Fact or fantasy? J. Investig. Dermatol. Symp. Proc. 2015, 17, 44–49. [Google Scholar] [CrossRef]
- Labrie, F. Intracrinology. Mol. Cell. Endocrinol. 1991, 78, C113–C118. [Google Scholar] [CrossRef]
- Hammer, F.; Subtil, S.; Lux, P.; Maser-Gluth, C.; Stewart, P.M.; Allolio, B.; Arlt, W. No evidence for hepatic conversion of DHEA to active androgens in women. J. Clin. Endocrinol. Metab. 2005, 90, 723–728. [Google Scholar] [CrossRef][Green Version]
- Labrie, F.; Luu-The, V.; Lin, S.X.; Simard, J.; Labrie, C.; Bélanger, A.; Pelletier, G. The key role of 17β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 1997, 62, 148–158. [Google Scholar] [CrossRef]
- Arlt, W.; Justl, H.G.; Callies, F.; Reincke, M.; Hörmann, R.; Allolio, B.; Schneider, P.; Hübler, D.; Ernst, M.; Schulte, H.M. Oral dehydroepiandrosterone in women with adrenal insufficiency. N. Engl. J. Med. 1999, 341, 1013–1020. [Google Scholar] [CrossRef]
- Stárka, L.; Dušková, M.; Hill, M. Dehydroepiandrosterone: A neuroactive steroid. J. Steroid Biochem. Mol. Biol. 2015, 145, 254–260. [Google Scholar] [CrossRef]
- Raber, J. DHEA and DHEAS in the aging brain. J. Steroid Biochem. Mol. Biol. 1999, 69, 319–327. [Google Scholar]
- Orentreich, N.; Brind, J.L.; Rizer, R.L.; Vogelman, J.H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J. Clin. Endocrinol. Metab. 1984, 59, 551–555. [Google Scholar] [CrossRef]
- Labrie, F.; Bélanger, A.; Labrie, C.; Simard, J.; Cusan, L.; Gomez, J.L.; Candas, B. Bioavailability of DHEA and DHEA sulfate following percutaneous administration. J. Clin. Endocrinol. Metab. 2007, 92, 1542–1548. [Google Scholar]
- Svec, F.; Porter, J.R. The actions of exogenous dehydroepiandrosterone in animals and humans. Proc. Soc. Exp. Biol. Med. 1998, 218, 174–191. [Google Scholar] [CrossRef]
- Longcope, C. Dehydroepiandrosterone metabolism. J. Endocrinol. 1996, 150, S125–S127. [Google Scholar] [CrossRef]
- Arlt, W. Androgen therapy in women. J. Endocrinol. 2006, 190, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F. DHEA, important source of sex steroids in men and even more in women. Prog. Brain Res. 2010, 182, 97–148. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.L.; Boudreau, R.M.; Cappola, A.R.; Arnold, A.M.; Robbins, J.; Cushman, M.; Newman, A.B. Cardiovascular disease is associated with greater incident dehydroepiandrosterone sulfate decline in the oldest old: The Cardiovascular Health Study All Stars Study. J. Am. Geriatr. Soc. 2010, 58, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Cappola, A.R.; O’Meara, E.S.; Guo, W.; Bartz, T.M.; Fried, L.P.; Newman, A.B. Trajectories of dehydroepiandrosterone sulfate predict mortality in older adults: The Cardiovascular Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1268–1274. [Google Scholar] [CrossRef]
- Ohlsson, C.; Nethander, M.; Norlén, A.K.; Poutanen, M.; Gudmundsson, E.F.; Aspelund, T.; Sigurdsson, S.; Ryberg, H.; Gudnason, V.; Tivesten, Å. Serum DHEA and testosterone levels associate inversely with coronary artery calcification in elderly men. J. Clin. Endocrinol. Metab. 2023, 108, 3272–3279. [Google Scholar] [CrossRef]
- Liu, D.; Dillon, J.S. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Gα(i2,3). J. Biol. Chem. 2002, 277, 21379–21388. [Google Scholar] [CrossRef]
- Chevalier, M.; Gilbert, G.; Lory, P.; Marthan, R.; Quignard, J.F.; Savineau, J.P. Dehydroepiandrosterone (DHEA) inhibits voltage-gated T-type calcium channels. Biochem. Pharmacol. 2012, 83, 1530–1539. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, S.; Paulin, R.; Sutendra, G.; Dromparis, P.; Roy, M.; Watson, K.O.; Nagendran, J.; Haromy, A.; Dyck, J.R.; Michelakis, E.D. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-β/NFAT axis. Circulation 2009, 120, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Chen, Y.; Zhou, Y.; Adi, D.; Zheng, Y.-Y.; Liu, F.; Ma, Y.-T.; Xie, X. Prognostic value of dehydroepiandrosterone sulfate for patients with cardiovascular disease: A systematic review and meta-analysis. J. Am. Heart Assoc. 2017, 6, e004896. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Sun, C.; Tang, O.; Gorlov, I.; Nambi, V.; Virani, S.S.; Villareal, D.T.; Taffet, G.E.; Yu, B.; Bressler, J.; et al. Plasma dehydroepiandrosterone sulfate and cardiovascular disease risk in older men and women. J. Clin. Endocrinol. Metab. 2020, 105, e4304–e4327. [Google Scholar] [CrossRef]
- Baird, G.L.; Archer-Chicko, C.; Barr, R.G.; Bluemke, D.A.; Foderaro, A.E.; Fritz, J.S.; Hill, N.S.; Kawut, S.M.; Klinger, J.R.; Lima, J.A.C.; et al. Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease– and congenital heart disease–associated pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1800467. [Google Scholar] [CrossRef]
- Rain, S.; Andersen, S.; Najafi, A.; Gammelgaard Schultz, J.; da Silva Gonçalves Bós, D.; Handoko, M.L.; Bogaard, H.J.; Vonk-Noordegraaf, A.; Andersen, A.; van der Velden, J.; et al. Right ventricular myocardial stiffness in experimental pulmonary arterial hypertension: Relative contribution of fibrosis and myofibril stiffness. Circ. Heart Fail. 2016, 9, e002636. [Google Scholar] [CrossRef]
- Straub, R.H.; Schölmerich, J.; Zietz, B. Replacement therapy with DHEA plus corticosteroids in patients with chronic inflammatory diseases—Substitutes of adrenal and sex hormones. Z. Rheumatol. 2000, 59, 108–118. [Google Scholar] [CrossRef]
- Cutolo, M.; Straub, R.H. Stress as a risk factor in the pathogenesis of rheumatoid arthritis. Neuroimmunomodulation 2006, 13, 277–282. [Google Scholar] [CrossRef]
- Skare, T.L.; Hauz, E.; de Carvalho, J.F. Dehydroepiandrosterone (DHEA) supplementation in rheumatic diseases: A systematic review. Mediterr. J. Rheumatol. 2023, 34, 292–301. [Google Scholar] [CrossRef]
- Imrich, R.; Vlcek, M.; Kerlik, J.; Vogeser, M.; Kirchhoff, F.; Penesova, A.; Radikova, Z.; Lukac, J.; Rovensky, J. Determinants of adrenal androgen hypofunction in premenopausal females with rheumatoid arthritis. Physiol. Res. 2014, 63, 321–329. [Google Scholar] [CrossRef]
- Auci, D.; Kaler, L.; Subramanian, S.; Huang, Y.; Frincke, J.; Reading, C.; Offner, H. A new orally bioavailable synthetic androstene inhibits collagen-induced arthritis in the mouse: Androstene hormones as regulators of regulatory T cells. Ann. N. Y. Acad. Sci. 2007, 1110, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Derksen, R.H. Dehydroepiandrosterone (DHEA) and systemic lupus erythematosus. Semin. Arthritis Rheum. 1998, 27, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, D.; Black, C.; McIntyre, L.; Royle, P.L.; Thomas, S. Dehydroepiandrosterone for systemic lupus erythematosus. Cochrane Database Syst. Rev. 2007, 4, CD005114. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.M.; Lan, J.L.; Lin, H.Y.; Luo, S.F. Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: A multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002, 46, 2924–2927. [Google Scholar] [CrossRef]
- Porola, P.; Straub, R.H.; Virkki, L.M.; Konttinen, Y.T.; Nordström, D.C. Failure of oral DHEA treatment to increase local salivary androgen outputs of female patients with Sjögren’s syndrome. Scand. J. Rheumatol. 2011, 40, 387–390. [Google Scholar] [CrossRef]
- Pillemer, S.R.; Brennan, M.T.; Sankar, V.; Leakan, R.A.; Smith, J.A.; Grisius, M.; Ligier, S.; Radfar, L.; Kok, M.R.; Kingman, A.; et al. Pilot clinical trial of dehydroepiandrosterone (DHEA) versus placebo for Sjögren’s syndrome. Arthritis Rheum. 2004, 51, 601–604. [Google Scholar] [CrossRef]
- Virkki, L.M.; Porola, P.; Forsblad-d’Elia, H.; Valtysdottir, S.; Solovieva, S.A.; Konttinen, Y.T. Dehydroepiandrosterone (DHEA) substitution treatment for severe fatigue in DHEA-deficient patients with primary Sjögren’s syndrome. Arthritis Care Res. 2010, 62, 118–124. [Google Scholar] [CrossRef]
- Cutolo, M. Androgens in rheumatoid arthritis: When are they effectors? Arthritis Res. Ther. 2009, 11, 126. [Google Scholar] [CrossRef]
- Forslind, K.; Hafström, I.; Ahlmén, M.; Svensson, B.; BARFOT Study Group. Sex: A major predictor of remission in early rheumatoid arthritis? Ann. Rheum. Dis. 2007, 66, 46–52. [Google Scholar] [CrossRef]
- Masi, A.T.; Da Silva, J.A.; Cutolo, M. Perturbations of hypothalamic-pituitary-gonadal (HPG) axis and adrenal androgen (AA) functions in rheumatoid arthritis. Baillière’s Clin. Rheumatol. 1996, 10, 295–332. [Google Scholar] [CrossRef]
- Capellino, S.; Montagna, P.; Villaggio, B.; Soldano, S.; Straub, R.H.; Cutolo, M. Hydroxylated estrogen metabolites influence the proliferation of cultured human monocytes: Possible role in synovial tissue hyperplasia. Clin. Exp. Rheumatol. 2008, 26, 903–909. [Google Scholar]
- Marx, C.; Wolkersdörfer, G.W.; Bornstein, S.R. A new view on immune-adrenal interactions: Role for Fas and Fas ligand? Neuroimmunomodulation 1998, 5, 5–8. [Google Scholar] [CrossRef]
- Masi, A.T.; Chrousos, G.P.; Bornstein, S.R. Enigmas of adrenal androgen and glucocorticoid dissociation in premenopausal-onset rheumatoid arthritis. J. Rheumatol. 1999, 26, 247–250. [Google Scholar] [PubMed]
- Sandoughi, M.; Kaykhaei, M.A.; Langarizadeh, E.; Dashipour, A. Effects of dehydroepiandrosterone on quality of life in premenopausal women with rheumatoid arthritis: A preliminary randomized clinical trial. Int. J. Rheum. Dis. 2020, 23, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F.; Labrie, C. DHEA and intracrinology at menopause, a positive choice for evolution of the human species. Climacteric 2013, 16, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Baber, R.J.; Panay, N.; Fenton, A.; IMS Writing Group. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016, 19, 109–150. [Google Scholar] [CrossRef]
- Faubion, S.S.; Kingsberg, S.A.; Clark, A.L.; Kaunitz, A.M.; Spadt, S.K.; Larkin, L.C.; Mitchell, C.M.; Shifren, J.L.; Simon, J.A.; Thurston, R.C.; et al. The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause 2020, 27, 976–992. [Google Scholar] [CrossRef]
- Labrie, F.; Archer, D.F.; Koltun, W.; Vachon, A.; Young, D.; Frenette, L.; Portman, D.; Montesino, M.; Côté, I.; Parent, J.; et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause 2018, 25, 1339–1353. [Google Scholar] [CrossRef]
- Archer, D.F. Dehydroepiandrosterone intravaginal administration for the management of postmenopausal vulvovaginal atrophy. J. Steroid Biochem. Mol. Biol. 2015, 145, 139–143. [Google Scholar] [CrossRef]
- Sauer, U.; Talaulikar, V.; Davies, M.C. Efficacy of intravaginal dehydroepiandrosterone (DHEA) for symptomatic women in the peri- or postmenopausal phase. Maturitas 2018, 116, 79–82. [Google Scholar] [CrossRef]
- Bouchard, C.; Labrie, F.; Derogatis, L.; Girard, G.; Ayotte, N.; Gallagher, J.; Cusan, L.; Archer, D.F.; Portman, D.; Lavoie, L.; et al. Effect of intravaginal dehydroepiandrosterone (DHEA) on female sexual function in postmenopausal women: ERC-230 open-label study. Horm. Mol. Biol. Clin. Investig. 2016, 25, 181–190. [Google Scholar] [CrossRef]
- Lupien, S.J.; Nair, N.P.; Brière, S.; Maheu, F.; Tu, M.T.; Lemay, M.; McEwen, B.S.; Meaney, M.J. Increased cortisol levels and impaired cognition in human aging: Implication for depression and dementia in later life. Rev. Neurosci. 1999, 10, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Kalimi, M.; Shafagoj, Y.; Loria, R.; Padgett, D.; Regelson, W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol. Cell. Biochem. 1994, 131, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Vanitallie, T.B. Stress: A risk factor for serious illness. Metabolism 2002, 51, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Sulli, A.; Villaggio, B.; Seriolo, B.; Accardo, S. Relations between steroid hormones and cytokines in rheumatoid arthritis and systemic lupus erythematosus. Ann. Rheum. Dis. 1998, 57, 573–577. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-Y.; Chen, J.-H.; Chen, K.-H. The Sex Hormone Precursors Dehydroepiandrosterone (DHEA) and Its Sulfate Ester Form (DHEAS): Molecular Mechanisms and Actions on Human Body. Int. J. Mol. Sci. 2025, 26, 8568. https://doi.org/10.3390/ijms26178568
Lin H-Y, Chen J-H, Chen K-H. The Sex Hormone Precursors Dehydroepiandrosterone (DHEA) and Its Sulfate Ester Form (DHEAS): Molecular Mechanisms and Actions on Human Body. International Journal of Molecular Sciences. 2025; 26(17):8568. https://doi.org/10.3390/ijms26178568
Chicago/Turabian StyleLin, Hsin-Yi, Jie-Hong Chen, and Kuo-Hu Chen. 2025. "The Sex Hormone Precursors Dehydroepiandrosterone (DHEA) and Its Sulfate Ester Form (DHEAS): Molecular Mechanisms and Actions on Human Body" International Journal of Molecular Sciences 26, no. 17: 8568. https://doi.org/10.3390/ijms26178568
APA StyleLin, H.-Y., Chen, J.-H., & Chen, K.-H. (2025). The Sex Hormone Precursors Dehydroepiandrosterone (DHEA) and Its Sulfate Ester Form (DHEAS): Molecular Mechanisms and Actions on Human Body. International Journal of Molecular Sciences, 26(17), 8568. https://doi.org/10.3390/ijms26178568






