The Interplay Between Oxidative Stress and Lipid Composition in Obesity-Induced Inflammation: Antioxidants as Therapeutic Agents in Metabolic Diseases
Abstract
1. Introduction
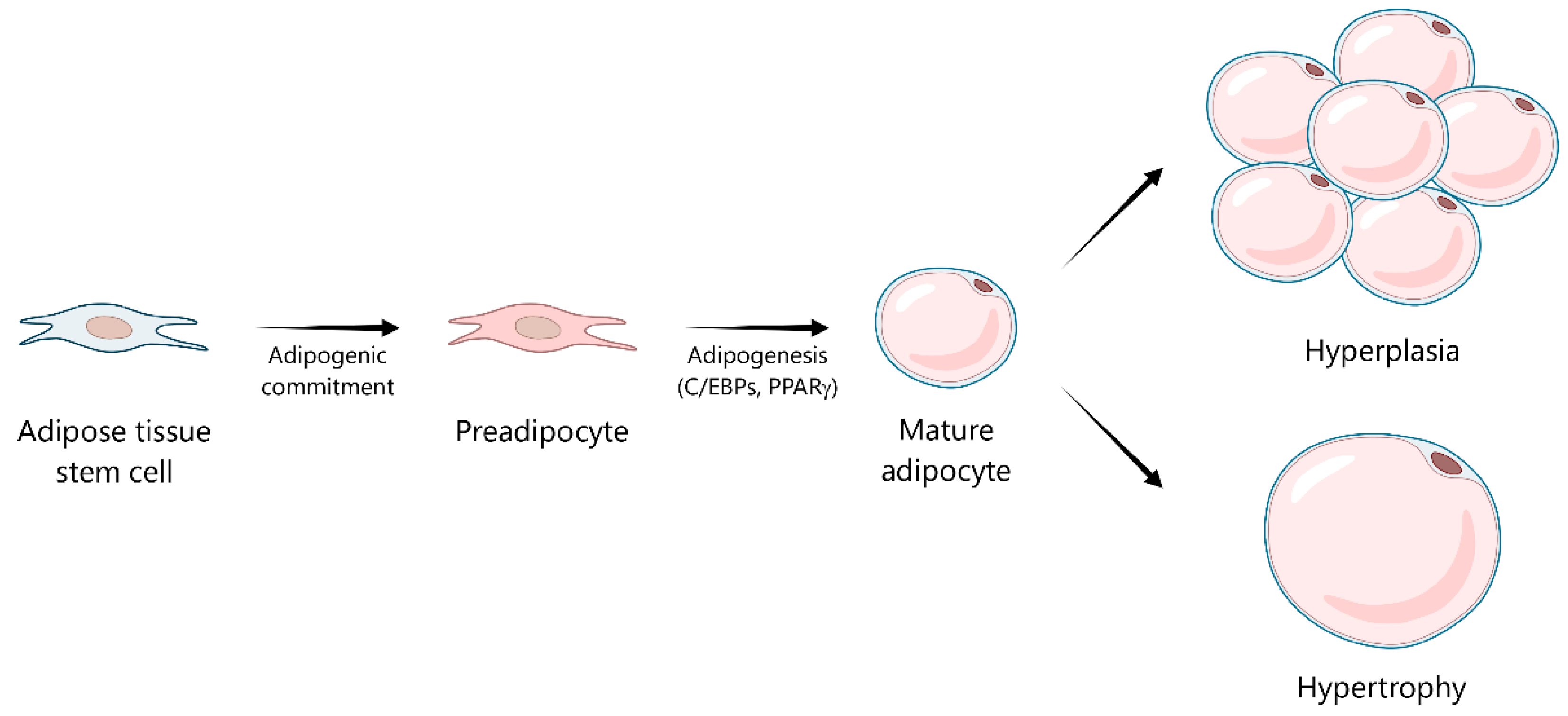
2. Metabolic and Endocrine Functions of Adipose Tissue
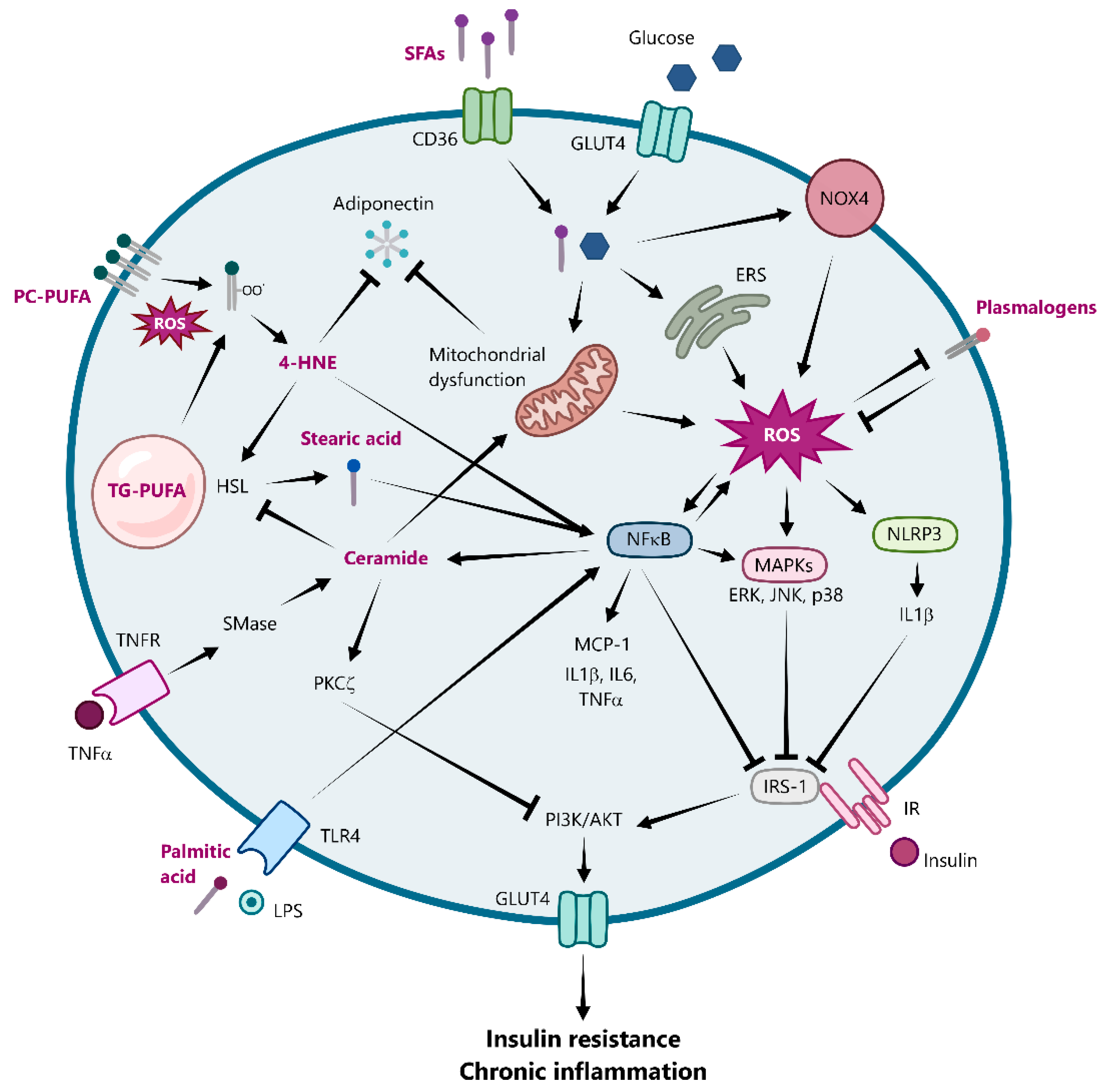
3. Adipose Tissue Dysfunction: The Role of Reactive Oxygen Species and Oxidative Stress in Obesity
4. The Link Between Oxidative Stress with Inflammation and Insulin Resistance in Obese Adipose Tissue
5. The Crosstalk Between Lipid Mediators and Oxidative Stress in Adipose Tissue Inflammation
| Lipid Class | Characteristics | Molecular Targets | Mechanism of Action | References |
|---|---|---|---|---|
| Polyunsaturated fatty acids (PUFAs) | Contain carbon–carbon double bonds; abundant in cell membranes; susceptible to lipid peroxidation | Nrf2, NFκB, MAPKs, SREBP1, PPARγ, ubiquitin–proteasome system, PKA, AMPK, Akt, PKC | 4-HNE (secondary aldehyde): Induces ROS production and inflammation; impairs adipogenesis through SREBP1; induces insulin resistance through IRS-1 dephosphorylation; upregulates adiponectin gene expression through PPARγ, and its degradation via the ubiquitin–proteasome system; induces lipolysis via cAMP/PKA/HSL pathway and inhibiting AMPK, contributing to FFA efflux. | [6,88,89,90] |
| Saturated fatty acids (SFAs) | No carbon–carbon double bonds; key signaling molecules | ER stress (via PERK, BiP, IRE1α, CHOP), E-FABP, TLR4, NF-κB, JNK, MAPKs, NLRP3 inflammasome, NOX enzymes | Stearic acid (18:0): Activates JNK and NF-κB via ER stress; increases pro-inflammatory cytokines (TNF-α, IL-6, IL-β, MCP-1); promotes macrophage polarization and differentiation via retinoic acid receptor-signaling and E-FABP. Palmitic acid (C16:0): Activates TLR4-mediated signaling (NF-κB, MAPKs and NLRP3 inflammasome); induces ROS production via NOX enzymes; increases pro-inflammatory cytokines (IL-β, IL-6, IL-8, TNF-α); reduces anti-inflammatory mediators (IL-10, adiponectin); impairs insulin sensitivity through IRS-1 serine phosphorylation. | [39,91,92,93,94,95,96,97,98,99,100,101,102,103,104] |
| Phospholipids | Glycerol backbone with two fatty acid chains and a phosphate group; primary components of cellular membranes. Plasmalogens: Glycerophospholipids with a vinyl ether bond at the sn-1 position and an ester-linked fatty acid at the sn-2 position | ROS, NLRP3 inflammasome, mitochondria, AMPK, insulin receptor | Plasmalogens: Scavenge and neutralize ROS. Phosphatidylcholine: Reduced PC metabolism attenuates inflammation via NLRP3 attenuation; preserves mitochondrial integrity via AMPK-dependent mitophagy and reduced mitochondrial ROS; enhances insulin sensitivity through insulin receptor activation and GLUT4 translocation. Oxidized phospholipids: Truncated OxPLs induce the expression of antioxidant genes (Ho1, Txnrd1, Gclm); full-length OxPLs upregulate pro-inflammatory genes (Il1β, Il6, Cxcl1). | [5,87,105,106,107,108,109,110] |
| Sphingolipids | Sphingosine backbone linked to a fatty acid; structural membrane components and signaling molecules | NLRP3 inflammasome, Akt (via atypical PKCζ), mitochondrial respiration, HSL | Ceramides: Activate NLRP3 inflammasome and promote IL-1β secretion; inhibit Akt activation and impair glucose uptake; suppress mitochondrial respiration and HSL-mediated lipolysis. | [7,111,112,113,114,115,116,117,118,119,120] |
6. Lipid Signatures, Oxidative Markers, and Inflammatory Mediators as Biomarkers in Human Obesity
6.1. Lipid Signatures in Obesity
6.2. Oxidative Markers in Obesity
6.3. Inflammatory Markers in Obesity
7. Therapeutic Modulation of Lipid Signaling and Inflammation in Obesity Using Antioxidants
| Antioxidant Compound (Family) | Structure Type | Molecular Targets | Mechanism of Action | References |
|---|---|---|---|---|
| Resveratrol (Stilbenes/Polyphenols) | 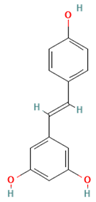 | SIRT1, NF-κB, NLRP3 inflammasome | Activates SIRT1 (inhibits NF-κB, enhances adiponectin secretion); suppresses NLRP3 inflammasome activation (attenuates IL-1β, reduces macrophage infiltration) | [141,142,143,144] |
| Epigallocatechin gallate (EGCG) (Catechins/Polyphenols) |  | Lipid rafts, TLR-4, NLRP3 inflammasome | Disrupts lipid raft formation in macrophages (prevents TLR-4 dimerization, blocks inflammatory cascade) | [148,149,150,151] |
| Curcumin (Curcuminoids) |  | Cytochrome P450, soluble epoxide hydrolase, SPMs | Enhances cytochrome P450 epoxygenase activity (increases EETs); inhibits soluble epoxide hydrolase (inhibits EET degradation); modulates specialized SPMs | [157,158,159,160,161,162] |
| Astaxanthin (Xanthophyll carotenoids) |  | Cell membranes, CD36 receptors, PPARγ | Membrane-stabilizing (prevents lipid peroxidation, OxPLs formation); inhibits interaction between oxidized phospholipids and CD36 receptors; enhances PPARγ signaling | [169,170] |
| Quercetin (Flavonoids/Polyphenols) | 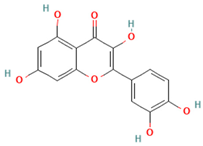 | Ceramide synthesis pathway, ceramidase, S1P | Inhibits ceramide synthesis, enhances ceramidase activity (reduces hepatic ceramide); reduces S1P production (counteracts pro-inflammatory signaling) | [173,174] |
| Anthocyanins (Flavonoids/Polyphenols) | 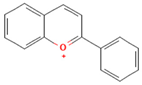 | Lipid rafts, inflammatory signaling platforms | Modulate lipid raft composition in human endothelial cells (disrupt pro-inflammatory platforms, enhance anti-inflammatory effect) | [177] |
| Sulforaphane (Isothiocyanates) | 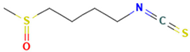 | Nrf2, PPARγ, pattern recognition receptors | Activates Nrf2 (induces antioxidant enzymes, regulates lipid metabolism via PPARγ); increases adiponectin expression; suppresses lipogenesis; reduces lipotoxicity; disrupts oxidized lipid-receptor crosstalk | [180,181,182] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-Hydroxynonenal |
| AGE | Advanced glycation end product |
| Akt/PKB | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| ATGL | Adipose triglyceride lipase |
| ATMs | Adipose tissue macrophages |
| BMI | Body mass index |
| C/EBP | CCAAT/enhancer-binding protein |
| CAT | Catalase |
| CHOP | C/EBP homologous protein |
| CRP | C-reactive protein |
| DNL | De novo lipogenesis |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinase |
| GPX | Glutathione peroxidase |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| GLUT4 | Glucose transporter type 4 |
| H2O2 | Hydrogen peroxide |
| HDL | High-density lipoprotein cholesterol |
| HSL | Hormone-sensitive lipase |
| IκBα | Inhibitor of kappa B alpha |
| IKK | IκB kinase |
| IL-6 | Interleukin-6 |
| IRS | Insulin receptor substrate |
| JNK | c-Jun N-terminal kinases |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | Nucleotide-binding domain leucine-rich-containing family pyrin domain-containing-3 |
| NOX | NADPH oxidase |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| OxPL | Oxidized phospholipid |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PI3K | Phosphoinositide 3-kinase |
| PPAR | Peroxisome proliferator-activated receptor |
| PTP | Protein tyrosine phosphatase |
| PUFA | Polyunsaturated fatty acid |
| ROS | Reactive oxygen species |
| SFAs | Saturated fatty acids |
| SOD | Superoxide dismutase |
| SPM | Specialized pro-resolving mediator |
| SREBP1 | Sterol regulatory element-binding protein 1 |
| SVF | Stromal-vascular fraction |
| TAGs | Triacylglycerol |
| T2DM | Type 2 diabetes mellitus |
| TNF-α | Tumor necrosis factor-alpha |
| OH | Hydroxyl radical |
| O2·− | Superoxide anion |
References
- NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [CrossRef]
- Neeland, I.J.; Lim, S.; Tchernof, A.; Gastaldelli, A.; Rangaswami, J.; Ndumele, C.E.; Powell-Wiley, T.M.; Després, J.P. Metabolic syndrome. Nat. Rev. Dis. Primers 2024, 10, 77. [Google Scholar] [CrossRef]
- Blüher, M. Understanding Adipose Tissue Dysfunction. J. Obes. Metab. Syndr. 2024, 33, 275–288. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Cho, Y.K.; Lee, S.; Lee, J.; Doh, J.; Park, J.H.; Jung, Y.S.; Lee, Y.H. Lipid remodeling of adipose tissue in metabolic health and disease. Exp. Mol. Med. 2023, 55, 1955–1973. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Chaurasia, B.; Talbot, C.L.; Summers, S.A. Adipocyte Ceramides-The Nexus of Inflammation and Metabolic Disease. Front. Immunol. 2020, 11, 576347. [Google Scholar] [CrossRef] [PubMed]
- Fock, K.M.; Khoo, J. Diet and exercise in management of obesity and overweight. J. Gastroenterol. Hepatol. 2013, 28 (Suppl. S4), 59–63. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Mead, J.R.; Irvine, S.A.; Ramji, D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. 2002, 80, 753–769. [Google Scholar] [CrossRef]
- Feingold, K.R. Lipid and Lipoprotein Metabolism. Endocrinol. Metab. Clin. N. Am. 2022, 51, 437–458. [Google Scholar] [CrossRef]
- Harris, C.A.; Haas, J.T.; Streeper, R.S.; Stone, S.J.; Kumari, M.; Yang, K.; Han, X.; Brownell, N.; Gross, R.W.; Zechner, R.; et al. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J. Lipid Res. 2011, 52, 657–667. [Google Scholar] [CrossRef]
- Ameer, F.; Scandiuzzi, L.; Hasnain, S.; Kalbacher, H.; Zaidi, N. De novo lipogenesis in health and disease. Metabolism 2014, 63, 895–902. [Google Scholar] [CrossRef]
- Cho, C.H.; Patel, S.; Rajbhandari, P. Adipose tissue lipid metabolism: Lipolysis. Curr. Opin. Genet. Dev. 2023, 83, 102114. [Google Scholar] [CrossRef]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Mei, R.; Qin, W.; Zheng, Y.; Wan, Z.; Liu, L. Role of Adipose Tissue Derived Exosomes in Metabolic Disease. Front. Endocrinol. 2022, 13, 873865. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, J.; Meng, H.; Zhang, X. Adipose Tissue-Resident Immune Cells in Obesity and Type 2 Diabetes. Front. Immunol. 2019, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 2019, 1, 189–200. [Google Scholar] [CrossRef]
- Hoffstedt, J.; Arner, E.; Wahrenberg, H.; Andersson, D.P.; Qvisth, V.; Löfgren, P.; Rydén, M.; Thörne, A.; Wirén, M.; Palmér, M.; et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 2010, 53, 2496–2503. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schön, M.R.; Kern, M.; Stumvoll, M.; Blüher, M. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E506–E515. [Google Scholar] [CrossRef] [PubMed]
- Esser, N.; L’Homme, L.; De Roover, A.; Kohnen, L.; Scheen, A.J.; Moutschen, M.; Piette, J.; Legrand-Poels, S.; Paquot, N. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia 2013, 56, 2487–2497. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, S.; Suzuki, K.; Ruhee, R.T. A Brief Overview of Oxidative Stress in Adipose Tissue with a Therapeutic Approach to Taking Antioxidant Supplements. Antioxidants 2021, 10, 594. [Google Scholar] [CrossRef]
- Masschelin, P.M.; Cox, A.R.; Chernis, N.; Hartig, S.M. The Impact of Oxidative Stress on Adipose Tissue Energy Balance. Front. Physiol. 2019, 10, 1638. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress, 1st ed.; Academic Press: London, UK, 1985. [Google Scholar]
- Loh, K.; Deng, H.; Fukushima, A.; Cai, X.; Boivin, B.; Galic, S.; Bruce, C.; Shields, B.J.; Skiba, B.; Ooms, L.M.; et al. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009, 10, 260–272. [Google Scholar] [CrossRef]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef]
- Han, C.Y. Roles of Reactive Oxygen Species on Insulin Resistance in Adipose Tissue. Diabetes Metab. J. 2016, 40, 272–279. [Google Scholar] [CrossRef]
- Shin, S.K.; Cho, H.W.; Song, S.E.; Im, S.S.; Bae, J.H.; Song, D.K. Oxidative stress resulting from the removal of endogenous catalase induces obesity by promoting hyperplasia and hypertrophy of white adipocytes. Redox Biol. 2020, 37, 101749. [Google Scholar] [CrossRef]
- Baldini, F.; Fabbri, R.; Eberhagen, C.; Voci, A.; Portincasa, P.; Zischka, H.; Vergani, L. Adipocyte hypertrophy parallels alterations of mitochondrial status in a cell model for adipose tissue dysfunction in obesity. Life Sci. 2021, 265, 118812. [Google Scholar] [CrossRef]
- Trayhurn, P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 2013, 93, 1–21. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.W.; Osborne, O.; Oh, D.Y.; Sasik, R.; Schenk, S.; Chen, A.; Chung, H.; Murphy, A.; Watkins, S.M.; et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 2014, 157, 1339–1352. [Google Scholar] [CrossRef]
- DeVallance, E.; Li, Y.; Jurczak, M.J.; Cifuentes-Pagano, E.; Pagano, P.J. The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology. Antioxid. Redox Signal. 2019, 31, 687–709. [Google Scholar] [CrossRef] [PubMed]
- Mahadev, K.; Motoshima, H.; Wu, X.; Ruddy, J.M.; Arnold, R.S.; Cheng, G.; Lambeth, J.D.; Goldstein, B.J. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 2004, 24, 1844–1854. [Google Scholar] [CrossRef]
- Schröder, K.; Wandzioch, K.; Helmcke, I.; Brandes, R.P. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Y.; Umemoto, T.; Omer, M.; Den Hartigh, L.J.; Chiba, T.; LeBoeuf, R.; Buller, C.L.; Sweet, I.R.; Pennathur, S.; Abel, E.D.; et al. NADPH oxidase-derived reactive oxygen species increases expression of monocyte chemotactic factor genes in cultured adipocytes. J. Biol. Chem. 2012, 287, 10379–10393. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Mouche, S.; Sajic, T.; Veyrat-Durebex, C.; Supale, R.; Pierroz, D.; Ferrari, S.; Negro, F.; Hasler, U.; Feraille, E.; et al. Deficiency in the NADPH oxidase 4 predisposes towards diet-induced obesity. Int. J. Obes. 2012, 36, 1503–1513. [Google Scholar] [CrossRef]
- Den Hartigh, L.J.; Omer, M.; Goodspeed, L.; Wang, S.; Wietecha, T.; O’Brien, K.D.; Han, C.Y. Adipocyte-Specific Deficiency of NADPH Oxidase 4 Delays the Onset of Insulin Resistance and Attenuates Adipose Tissue Inflammation in Obesity. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 466–475. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Choo, H.J.; Kim, J.H.; Kwon, O.B.; Lee, C.S.; Mun, J.Y.; Han, S.S.; Yoon, Y.S.; Yoon, G.; Choi, K.M.; Ko, Y.G. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 2006, 49, 784–791. [Google Scholar] [CrossRef]
- Gao, C.L.; Zhu, C.; Zhao, Y.P.; Chen, X.H.; Ji, C.B.; Zhang, C.M.; Zhu, J.G.; Xia, Z.K.; Tong, M.L.; Guo, X.R. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 2010, 320, 25–33. [Google Scholar] [CrossRef]
- Lindinger, P.W.; Christe, M.; Eberle, A.N.; Kern, B.; Peterli, R.; Peters, T.; Jayawardene, K.J.; Fearnley, I.M.; Walker, J.E. Important mitochondrial proteins in human omental adipose tissue show reduced expression in obesity. J. Proteom. 2015, 124, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Schöttl, T.; Schempp, C.; Fromme, T.; Hauner, H.; Klingenspor, M.; Skurk, T. Inverse relationship between body mass index and mitochondrial oxidative phosphorylation capacity in human subcutaneous adipocytes. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E380–E387. [Google Scholar] [CrossRef]
- Heinonen, S.; Jokinen, R.; Rissanen, A.; Pietiläinen, K.H. White adipose tissue mitochondrial metabolism in health and in obesity. Obes. Rev. 2020, 21, e12958. [Google Scholar] [CrossRef]
- Vankoningsloo, S.; De Pauw, A.; Houbion, A.; Tejerina, S.; Demazy, C.; de Longueville, F.; Bertholet, V.; Renard, P.; Remacle, J.; Holvoet, P.; et al. CREB activation induced by mitochondrial dysfunction triggers triglyceride accumulation in 3T3-L1 preadipocytes. J. Cell Sci. 2006, 119, 1266–1282. [Google Scholar] [CrossRef]
- Kaaman, M.; Sparks, L.M.; van Harmelen, V.; Smith, S.R.; Sjölin, E.; Dahlman, I.; Arner, P. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia 2007, 50, 2526–2533. [Google Scholar] [CrossRef]
- Koh, E.H.; Park, J.Y.; Park, H.S.; Jeon, M.J.; Ryu, J.W.; Kim, M.; Kim, S.Y.; Kim, M.S.; Kim, S.W.; Park, I.S.; et al. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes 2007, 56, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wang, C.C.; Huang, H.C.; Wei, Y.H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 2013, 280, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, A.; Korac, A.; Srdic-Galic, B.; Buzadzic, B.; Otasevic, V.; Stancic, A.; Vucetic, M.; Markelic, M.; Velickovic, K.; Golic, I.; et al. Differences in the redox status of human visceral and subcutaneous adipose tissues--relationships to obesity and metabolic risk. Metabolism 2014, 63, 661–671. [Google Scholar] [CrossRef]
- Monzo-Beltran, L.; Vazquez-Tarragón, A.; Cerdà, C.; Garcia-Perez, P.; Iradi, A.; Sánchez, C.; Climent, B.; Tormos, C.; Vázquez-Prado, A.; Girbés, J.; et al. One-year follow-up of clinical, metabolic and oxidative stress profile of morbid obese patients after laparoscopic sleeve gastrectomy. 8-oxo-dG as a clinical marker. Redox Biol. 2017, 12, 389–402. [Google Scholar] [CrossRef]
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6973. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhai, X.; Qiu, Y.; Lu, X.; Jiao, Y. The Nrf2 in Obesity: A Friend or Foe? Antioxidants 2022, 11, 2067. [Google Scholar] [CrossRef] [PubMed]
- Yeop Han, C.; Kargi, A.Y.; Omer, M.; Chan, C.K.; Wabitsch, M.; O’Brien, K.D.; Wight, T.N.; Chait, A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: Dissociation of adipocyte hypertrophy from inflammation. Diabetes 2010, 59, 386–396. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef]
- Li, C.; Menoret, A.; Farragher, C.; Ouyang, Z.; Bonin, C.; Holvoet, P.; Vella, A.T.; Zhou, B. Single cell transcriptomics based-MacSpectrum reveals novel macrophage activation signatures in diseases. JCI Insight 2019, 5, e126453. [Google Scholar] [CrossRef]
- Wentworth, J.M.; Naselli, G.; Brown, W.A.; Doyle, L.; Phipson, B.; Smyth, G.K.; Wabitsch, M.; O’Brien, P.E.; Harrison, L.C. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes 2010, 59, 1648–1656. [Google Scholar] [CrossRef]
- Jaitin, D.A.; Adlung, L.; Thaiss, C.A.; Weiner, A.; Li, B.; Descamps, H.; Lundgren, P.; Bleriot, C.; Liu, Z.; Deczkowska, A.; et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019, 178, 686–698.E14. [Google Scholar] [CrossRef]
- Pepping, J.K.; Freeman, L.R.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E392–E404. [Google Scholar] [CrossRef] [PubMed]
- Coats, B.R.; Schoenfelt, K.Q.; Barbosa-Lorenzi, V.C.; Peris, E.; Cui, C.; Hoffman, A.; Zhou, G.; Fernandez, S.; Zhai, L.; Hall, B.A.; et al. Metabolically Activated Adipose Tissue Macrophages Perform Detrimental and Beneficial Functions during Diet-Induced Obesity. Cell Rep. 2017, 20, 3149–3161. [Google Scholar] [CrossRef]
- Robblee, M.M.; Kim, C.C.; Porter Abate, J.; Valdearcos, M.; Sandlund, K.L.; Shenoy, M.K.; Volmer, R.; Iwawaki, T.; Koliwad, S.K. Saturated Fatty Acids Engage an IRE1α-Dependent Pathway to Activate the NLRP3 Inflammasome in Myeloid Cells. Cell Rep. 2016, 14, 2611–2623. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J. Am. Heart Assoc. 2018, 7, e007442. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.G.; Møller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yan, X.; Zhao, Y.; Wang, J.; Liu, B.; Yu, S.; Fu, J.; Liu, Y.; Su, J. Macrophage Polarization Mediated by Mitochondrial Dysfunction Induces Adipose Tissue Inflammation in Obesity. Int. J. Mol. Sci. 2022, 23, 9252. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Sanlioglu, S.; Williams, C.M.; Samavati, L.; Butler, N.S.; Wang, G.; McCray, P.B., Jr.; Ritchie, T.C.; Hunninghake, G.W.; Zandi, E.; Engelhardt, J.F. Lipopolysaccharide induces Rac1-dependent reactive oxygen species formation and coordinates tumor necrosis factor-alpha secretion through IKK regulation of NF-kappa B. J. Biol. Chem. 2001, 276, 30188–30198. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Wiegmann, K.; Farid, A.; Wolf, A.; Utermöhlen, O.; Krut, O.; Krönke, M.; Schramm, M. Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of NEMO. Sci. Signal. 2019, 12, eaar5926. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, A.; Ichijo, H. Redox control of cell fate by MAP kinase: Physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim. Biophys. Acta 2008, 1780, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Shrivastava, A.; Tan, T.H. Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene 2001, 20, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K.; Cheung, S.W.; Cheng, K.K. NLRP3 Inflammasome Activation in Adipose Tissues and Its Implications on Metabolic Diseases. Int. J. Mol. Sci. 2020, 21, 4184. [Google Scholar] [CrossRef]
- Rheinheimer, J.; de Souza, B.M.; Cardoso, N.S.; Bauer, A.C.; Crispim, D. Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism 2017, 74, 1–9. [Google Scholar] [CrossRef]
- Dominic, A.; Le, N.T.; Takahashi, M. Loop Between NLRP3 Inflammasome and Reactive Oxygen Species. Antioxid. Redox Signal. 2022, 36, 784–796. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Park, J.E.; Kang, E.; Han, J.S. HM-chromanone attenuates TNF-α-mediated inflammation and insulin resistance by controlling JNK activation and NF-κB pathway in 3T3-L1 adipocytes. Eur. J. Pharmacol. 2022, 921, 174884. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, J.K.; Han, J.S. Betulinic acid improves TNF-α-induced insulin resistance by inhibiting negative regulator of insulin signalling and inflammation-activated protein kinase in 3T3-L1 adipocytes. Arch. Physiol. Biochem. 2024, 130, 452–459. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Zhao, J.; Guo, C. JNK downregulation improves olanzapine-induced insulin resistance by suppressing IRS1(Ser307) phosphorylation and reducing inflammation. Biomed. Pharmacother. 2021, 142, 112071. [Google Scholar] [CrossRef]
- Rotter, V.; Nagaev, I.; Smith, U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003, 278, 45777–45784. [Google Scholar] [CrossRef] [PubMed]
- Jager, J.; Grémeaux, T.; Cormont, M.; Le Marchand-Brustel, Y.; Tanti, J.F. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology 2007, 148, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Angelidou, G.; Ni, Z.; Criscuolo, A.; Schiller, J.; Blüher, M.; Fedorova, M. AdipoAtlas: A reference lipidome for human white adipose tissue. Cell Rep. Med. 2021, 2, 100407. [Google Scholar] [CrossRef] [PubMed]
- Elrayess, M.A.; Almuraikhy, S.; Kafienah, W.; Al-Menhali, A.; Al-Khelaifi, F.; Bashah, M.; Zarkovic, K.; Zarkovic, N.; Waeg, G.; Alsayrafi, M.; et al. 4-hydroxynonenal causes impairment of human subcutaneous adipogenesis and induction of adipocyte insulin resistance. Free Radic. Biol. Med. 2017, 104, 129–137. [Google Scholar] [CrossRef]
- Wang, Z.; Dou, X.; Gu, D.; Shen, C.; Yao, T.; Nguyen, V.; Braunschweig, C.; Song, Z. 4-Hydroxynonenal differentially regulates adiponectin gene expression and secretion via activating PPARγ and accelerating ubiquitin-proteasome degradation. Mol. Cell. Endocrinol. 2012, 349, 222–231. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Li, J.; Gu, D.; Li, S.; Shen, C.; Song, Z. Increased 4-hydroxynonenal formation contributes to obesity-related lipolytic activation in adipocytes. PLoS ONE 2013, 8, e70663. [Google Scholar] [CrossRef]
- Gowda, S.G.B.; Gao, Z.J.; Chen, Z.; Abe, T.; Hori, S.; Fukiya, S.; Ishizuka, S.; Yokota, A.; Chiba, H.; Hui, S.P. Untargeted Lipidomic Analysis of Plasma from High-fat Diet-induced Obese Rats Using UHPLC-Linear Trap Quadrupole-Orbitrap MS. Anal. Sci. 2020, 36, 821–828. [Google Scholar] [CrossRef]
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated Fatty Acids in Obesity-Associated Inflammation. J. Inflamm. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Anderson, E.K.; Hill, A.A.; Hasty, A.H. Stearic acid accumulation in macrophages induces toll-like receptor 4/2-independent inflammation leading to endoplasmic reticulum stress-mediated apoptosis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1687–1695. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Y.; Hao, J.; Sun, Y.; Liu, S.; Bernlohr, D.A.; Sauter, E.R.; Cleary, M.P.; Suttles, J.; Li, B. Stearic Acid Induces CD11c Expression in Proinflammatory Macrophages via Epidermal Fatty Acid Binding Protein. J. Immunol. 2018, 200, 3407–3419. [Google Scholar] [CrossRef]
- Shi, H.; Kokoeva, M.V.; Inouye, K.; Tzameli, I.; Yin, H.; Flier, J.S. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Investig. 2006, 116, 3015–3025. [Google Scholar] [CrossRef]
- Davis, J.E.; Gabler, N.K.; Walker-Daniels, J.; Spurlock, M.E. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity 2008, 16, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef]
- Nicholas, D.A.; Zhang, K.; Hung, C.; Glasgow, S.; Aruni, A.W.; Unternaehrer, J.; Payne, K.J.; Langridge, W.H.R.; De Leon, M. Palmitic acid is a toll-like receptor 4 ligand that induces human dendritic cell secretion of IL-1β. PLoS ONE 2017, 12, e0176793. [Google Scholar] [CrossRef]
- Snodgrass, R.G.; Huang, S.; Choi, I.W.; Rutledge, J.C.; Hwang, D.H. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J. Immunol. 2013, 191, 4337–4347. [Google Scholar] [CrossRef] [PubMed]
- Håversen, L.; Danielsson, K.N.; Fogelstrand, L.; Wiklund, O. Induction of proinflammatory cytokines by long-chain saturated fatty acids in human macrophages. Atherosclerosis 2009, 202, 382–393. [Google Scholar] [CrossRef]
- Ajuwon, K.M.; Spurlock, M.E. Palmitate activates the NF-kappaB transcription factor and induces IL-6 and TNFalpha expression in 3T3-L1 adipocytes. J. Nutr. 2005, 135, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.; Brickey, W.J.; Ting, J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef]
- Rodriguez-Pacheco, F.; Gutierrez-Repiso, C.; Garcia-Serrano, S.; Alaminos-Castillo, M.A.; Ho-Plagaro, A.; Valdes, S.; Garcia-Arnes, J.; Gonzalo, M.; Andrade, R.J.; Moreno-Ruiz, F.J.; et al. The pro-/anti-inflammatory effects of different fatty acids on visceral adipocytes are partially mediated by GPR120. Eur. J. Nutr. 2017, 56, 1743–1752. [Google Scholar] [CrossRef]
- Yang, L.; Qian, Z.; Ji, H.; Yang, R.; Wang, Y.; Xi, L.; Sheng, L.; Zhao, B.; Zhang, X. Inhibitory effect on protein kinase Ctheta by Crocetin attenuates palmitate-induced insulin insensitivity in 3T3-L1 adipocytes. Eur. J. Pharmacol. 2010, 642, 47–55. [Google Scholar] [CrossRef]
- Palau-Rodriguez, M.; Marco-Ramell, A.; Casas-Agustench, P.; Tulipani, S.; Miñarro, A.; Sanchez-Pla, A.; Murri, M.; Tinahones, F.J.; Andres-Lacueva, C. Visceral Adipose Tissue Phospholipid Signature of Insulin Sensitivity and Obesity. J. Proteome Res. 2021, 20, 2410–2419. [Google Scholar] [CrossRef]
- Engelmann, B. Plasmalogens: Targets for oxidants and major lipophilic antioxidants. Biochem. Soc. Trans. 2004, 32, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Zhong, Z.; Stubelius, A.; Sweeney, S.R.; Booshehri, L.M.; Antonucci, L.; Liu-Bryan, R.; Lodi, A.; Terkeltaub, R.; Lacal, J.C.; et al. Choline Uptake and Metabolism Modulate Macrophage IL-1β and IL-18 Production. Cell Metab. 2019, 29, 1350–1362.E7. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Li, Z.; Tung, V.S.K.; Pan, M.; Han, X.; Evgrafov, O.; Jiang, X.C. Inhibiting Phosphatidylcholine Remodeling in Adipose Tissue Increases Insulin Sensitivity. Diabetes 2023, 72, 1547–1559. [Google Scholar] [CrossRef]
- Serbulea, V.; DeWeese, D.; Leitinger, N. The effect of oxidized phospholipids on phenotypic polarization and function of macrophages. Free Radic. Biol. Med. 2017, 111, 156–168. [Google Scholar] [CrossRef]
- Serbulea, V.; Upchurch, C.M.; Schappe, M.S.; Voigt, P.; DeWeese, D.E.; Desai, B.N.; Meher, A.K.; Leitinger, N. Macrophage phenotype and bioenergetics are controlled by oxidized phospholipids identified in lean and obese adipose tissue. Proc. Natl. Acad. Sci. USA 2018, 115, E6254–E6263. [Google Scholar] [CrossRef]
- Li, Y.; Talbot, C.L.; Chaurasia, B. Ceramides in Adipose Tissue. Front. Endocrinol. 2020, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Turpin, S.M.; Nicholls, H.T.; Willmes, D.M.; Mourier, A.; Brodesser, S.; Wunderlich, C.M.; Mauer, J.; Xu, E.; Hammerschmidt, P.; Brönneke, H.S.; et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014, 20, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, P.; Brüning, J.C. Contribution of specific ceramides to obesity-associated metabolic diseases. Cell Mol. Life Sci. 2022, 79, 395. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Ahmad, Z.; Thomas, R.; Melhem, M.; Snider, A.J.; Obeid, L.M.; Al-Mulla, F.; Hannun, Y.A.; Ahmad, R. Neutral sphingomyelinase 2 regulates inflammatory responses in monocytes/macrophages induced by TNF-α. Sci. Rep. 2020, 10, 16802. [Google Scholar] [CrossRef]
- Holland, W.L.; Bikman, B.T.; Wang, L.P.; Yuguang, G.; Sargent, K.M.; Bulchand, S.; Knotts, T.A.; Shui, G.; Clegg, D.J.; Wenk, M.R.; et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Investig. 2011, 121, 1858–1870. [Google Scholar] [CrossRef]
- Schilling, J.D.; Machkovech, H.M.; He, L.; Sidhu, R.; Fujiwara, H.; Weber, K.; Ory, D.S.; Schaffer, J.E. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J. Biol. Chem. 2013, 288, 2923–2932. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011, 17, 179–188. [Google Scholar] [CrossRef]
- Powell, D.J.; Hajduch, E.; Kular, G.; Hundal, H.S. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol. 2003, 23, 7794–7808. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, P.; Ostkotte, D.; Nolte, H.; Gerl, M.J.; Jais, A.; Brunner, H.L.; Sprenger, H.G.; Awazawa, M.; Nicholls, H.T.; Turpin-Nolan, S.M.; et al. CerS6-Derived Sphingolipids Interact with Mff and Promote Mitochondrial Fragmentation in Obesity. Cell 2019, 177, 1536–1552.E23. [Google Scholar] [CrossRef]
- Chaurasia, B.; Tippetts, T.S.; Mayoral Monibas, R.; Liu, J.; Li, Y.; Wang, L.; Wilkerson, J.L.; Sweeney, C.R.; Pereira, R.F.; Sumida, D.H.; et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 2019, 365, 386–392. [Google Scholar] [CrossRef] [PubMed]
- García-Fontana, B.; Morales-Santana, S.; Díaz Navarro, C.; Rozas-Moreno, P.; Genilloud, O.; Vicente Pérez, F.; Pérez del Palacio, J.; Muñoz-Torres, M. Metabolomic profile related to cardiovascular disease in patients with type 2 diabetes mellitus: A pilot study. Talanta 2016, 148, 135–143. [Google Scholar] [CrossRef]
- Huynh, K.; Barlow, C.K.; Jayawardana, K.S.; Weir, J.M.; Mellett, N.A.; Cinel, M.; Magliano, D.J.; Shaw, J.E.; Drew, B.G.; Meikle, P.J. High-Throughput Plasma Lipidomics: Detailed Mapping of the Associations with Cardiometabolic Risk Factors. Cell Chem. Biol. 2019, 26, 71–84.E4. [Google Scholar] [CrossRef]
- Pomares-Bri, I.; Roca, M.; Borrás, F.; Wabitsch, M.; Lahoz, A.; Micol, V.; Herranz-López, M. Polyphenols reverse hyperglycemia-induced adipocyte dysfunction: A Metabolomic and Lipidomic study of efficacy. Food Res. Int. 2025, 211, 116453. [Google Scholar] [CrossRef]
- Russo, S.B.; Ross, J.S.; Cowart, L.A. Sphingolipids in obesity, type 2 diabetes, and metabolic disease. Handb. Exp. Pharmacol. 2013, 216, 373–401. [Google Scholar] [CrossRef]
- Neeland, I.J.; Singh, S.; McGuire, D.K.; Vega, G.L.; Roddy, T.; Reilly, D.F.; Castro-Perez, J.; Kozlitina, J.; Scherer, P.E. Relation of plasma ceramides to visceral adiposity, insulin resistance and the development of type 2 diabetes mellitus: The Dallas Heart Study. Diabetologia 2018, 61, 2570–2579. [Google Scholar] [CrossRef]
- Hou, B.; Zhao, Y.; He, P.; Xu, C.; Ma, P.; Lam, S.M.; Li, B.; Gil, V.; Shui, G.; Qiang, G.; et al. Targeted lipidomics and transcriptomics profiling reveal the heterogeneity of visceral and subcutaneous white adipose tissue. Life Sci. 2020, 245, 117352. [Google Scholar] [CrossRef] [PubMed]
- Kartsoli, S.; Kostara, C.E.; Tsimihodimos, V.; Bairaktari, E.T.; Christodoulou, D.K. Lipidomics in non-alcoholic fatty liver disease. World J. Hepatol. 2020, 12, 436–450. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Dai, Q.; Milne, G.; Long, J.; Cai, Q.; Chen, Q.; Zhang, X.; Lan, Q.; Rothman, N.; et al. Lipid peroxidation biomarkers associated with height and obesity measures in the opposite direction in women. Obesity 2022, 30, 1257–1267. [Google Scholar] [CrossRef]
- Le Lay, S.; Simard, G.; Martinez, M.C.; Andriantsitohaina, R. Oxidative stress and metabolic pathologies: From an adipocentric point of view. Oxid. Med. Cell. Longev. 2014, 2014, 908539. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Y.; Liu, B.; Jia, X.; Wang, R.; Lu, Q. Associations of plasma 8-iso-prostaglandin F(2α)levels with fasting blood glucose (FBG) and intra-abdominal fat (IAF) area in various Glycometabolism populations. BMC Endocr. Disord. 2021, 21, 215. [Google Scholar] [CrossRef] [PubMed]
- Shabalala, S.C.; Johnson, R.; Basson, A.K.; Ziqubu, K.; Hlengwa, N.; Mthembu, S.X.H.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Cirilli, I.; et al. Detrimental Effects of Lipid Peroxidation in Type 2 Diabetes: Exploring the Neutralizing Influence of Antioxidants. Antioxidants 2022, 11, 2071. [Google Scholar] [CrossRef]
- Pillon, N.J.; Croze, M.L.; Vella, R.E.; Soulère, L.; Lagarde, M.; Soulage, C.O. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology 2012, 153, 2099–2111. [Google Scholar] [CrossRef]
- Jankovic, A.; Korac, A.; Buzadzic, B.; Stancic, A.; Otasevic, V.; Ferdinandy, P.; Daiber, A.; Korac, B. Targeting the NO/superoxide ratio in adipose tissue: Relevance to obesity and diabetes management. Br. J. Pharmacol. 2017, 174, 1570–1590. [Google Scholar] [CrossRef]
- Choi, L.S.; Ahmed, K.; Kim, Y.S.; Yim, J.E. Skin accumulation of advanced glycation end products and cardiovascular risk in Korean patients with type 2 diabetes mellitus. Heliyon 2022, 8, e09571. [Google Scholar] [CrossRef]
- Korac, B.; Kalezic, A.; Pekovic-Vaughan, V.; Korac, A.; Jankovic, A. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol. 2021, 42, 101887. [Google Scholar] [CrossRef]
- Yoshimura, A.; Ohnishi, S.; Orito, C.; Kawahara, Y.; Takasaki, H.; Takeda, H.; Sakamoto, N.; Hashino, S. Association of peripheral total and differential leukocyte counts with obesity-related complications in young adults. Obes. Facts 2015, 8, 1–16. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef]
- Unamuno, X.; Izaguirre, M.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Becerril, S.; Valentí, V.; Moncada, R.; Silva, C.; Salvador, J.; et al. Increase of the Adiponectin/Leptin Ratio in Patients with Obesity and Type 2 Diabetes after Roux-en-Y Gastric Bypass. Nutrients 2019, 11, 2069. [Google Scholar] [CrossRef] [PubMed]
- López-Vicario, C.; Rius, B.; Alcaraz-Quiles, J.; García-Alonso, V.; Lopategi, A.; Titos, E.; Clària, J. Pro-resolving mediators produced from EPA and DHA: Overview of the pathways involved and their mechanisms in metabolic syndrome and related liver diseases. Eur. J. Pharmacol. 2016, 785, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.U.; Schulze, M.B. Metabolically healthy obesity: The low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2018, 6, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.P.; Ka, S.M.; Hsu, W.H.; Chen, A.; Chao, L.K.; Lin, C.C.; Hsieh, C.C.; Chen, M.C.; Chiu, H.W.; Ho, C.L.; et al. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J. Cell. Physiol. 2015, 230, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gomez, Y.; Mattison, J.A.; Pearson, K.J.; Martin-Montalvo, A.; Palacios, H.H.; Sossong, A.M.; Ward, T.M.; Younts, C.M.; Lewis, K.; Allard, J.S.; et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013, 18, 533–545. [Google Scholar] [CrossRef]
- García-Martínez, B.I.; Ruiz-Ramos, M.; Pedraza-Chaverri, J.; Santiago-Osorio, E.; Mendoza-Núñez, V.M. Effect of resveratrol on markers of oxidative stress and sirtuin 1 in elderly adults with type 2 diabetes. Int. J. Mol. Sci. 2023, 24, 7422. [Google Scholar] [CrossRef]
- Molani-Gol, R.; Rafraf, M. Effects of resveratrol on the anthropometric indices and inflammatory markers: An umbrella meta-analysis. Eur. J. Nutr. 2024, 63, 1023–1040. [Google Scholar] [CrossRef]
- Berman, A.Y.; Motechin, R.A.; Wiesenfeld, M.Y.; Holz, M.K. The therapeutic potential of resveratrol: A review of clinical trials. NPJ Precis. Oncol. 2017, 1, 35. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential adverse effects of resveratrol: A literature review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, M.; Yao, W.; Du, K.; He, M.; Jin, X.; Jiao, L.; Ma, G.; Wei, B.; Wei, M. Epigallocatechin-3-Gallate Attenuates Microglial Inflammation and Neurotoxicity by Suppressing the Activation of Canonical and Noncanonical Inflammasome via TLR4/NF-κB Pathway. Mol. Nutr. Food Res. 2019, 63, e1801230. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Gao, Q.; Wang, T.; Kan, Z.; Li, X.; Hu, L.; Peng, C.Y.; Qian, F.; Wang, Y.; Granato, D. Green tea polyphenols and epigallocatechin-3-gallate protect against perfluorodecanoic acid induced liver damage and inflammation in mice by inhibiting NLRP3 inflammasome activation. Food Res. Int. 2020, 127, 108628. [Google Scholar] [CrossRef]
- Hou, H.; Yang, W.; Bao, S.; Cao, Y. Epigallocatechin Gallate Suppresses Inflammatory Responses by Inhibiting Toll-like Receptor 4 Signaling and Alleviates Insulin Resistance in the Livers of High-fat-diet Rats. J. Oleo Sci. 2020, 69, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, Y.; Ling, F.; Guan, Y.; Zhang, D.; Zhu, Q.; Liu, J.; Wu, Y.; Niu, Y. The phytochemical epigallocatechin gallate prolongs the lifespan by improving lipid metabolism, reducing inflammation and oxidative stress in high-fat diet-fed obese rats. Aging Cell 2020, 19, e13199. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, H.; Yu, J.; Sui, J.; Pan, D.; Wang, S.; Liao, W.; Yang, L.; Sun, G. Effects of green tea catechin on the blood pressure and lipids in overweight and obese population-a meta-analysis. Heliyon 2023, 9, e21228. [Google Scholar] [CrossRef] [PubMed]
- Schuldesz, A.C.; Tudor, R.; Nandarge, P.S.; Elagez, A.; Cornea, A.; Ion, R.; Bratosin, F.; Prodan, M.; Simu, M. The effects of epigallocatechin-3-gallate nutritional supplementation in the management of multiple sclerosis: A systematic review of clinical trials. Nutrients 2024, 16, 2723. [Google Scholar] [CrossRef] [PubMed]
- Nakadate, K.; Kawakami, K.; Yamazaki, N. Synergistic effect of beta-cryptoxanthin and epigallocatechin gallate on obesity reduction. Nutrients 2024, 16, 2344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Z.; Zhou, F.; Ouyang, J.; Wang, Q.Y.; Li, Y.L.; Wu, J.L.; Huang, J.A.; Liu, Z.H. Combined use of epigallocatechin-3-gallate (EGCG) and caffeine in low doses exhibits marked anti-obesity synergy through regulation of gut microbiota and bile acid metabolism. Food Funct. 2021, 12, 4105–4116. [Google Scholar] [CrossRef]
- Most, J.; Timmers, S.; Warnke, I.; Jocken, J.W.; van Boekschoten, M.; de Groot, P.; Bendik, I.; Schrauwen, P.; Goossens, G.H.; Blaak, E.E. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 215–227. [Google Scholar] [CrossRef]
- Rodríguez Castaño, P.; Parween, S.; Pandey, A.V. Bioactivity of Curcumin on the Cytochrome P450 Enzymes of the Steroidogenic Pathway. Int. J. Mol. Sci. 2019, 20, 4606. [Google Scholar] [CrossRef]
- Yarru, L.P.; Settivari, R.S.; Gowda, N.K.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G.E. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poult. Sci. 2009, 88, 2620–2627. [Google Scholar] [CrossRef]
- Unhapipatpong, C.; Polruang, N.; Shantavasinkul, P.C.; Julanon, N.; Numthavaj, P.; Thakkinstian, A. The effect of curcumin supplementation on weight loss and anthropometric indices: An umbrella review and updated meta-analyses of randomized controlled trials. Am. J. Clin. Nutr. 2023, 117, 1005–1016. [Google Scholar] [CrossRef]
- Thota, R.N.; Acharya, S.H.; Garg, M.L. Curcumin and/or omega-3 polyunsaturated fatty acids supplementation reduces insulin resistance and blood lipids in individuals with high risk of type 2 diabetes: A randomised controlled trial. Lipids Health Dis. 2019, 18, 31. [Google Scholar] [CrossRef]
- Fiala, M.; Terrando, N.; Dalli, J. Specialized Pro-Resolving Mediators from Omega-3 Fatty Acids Improve Amyloid-β Phagocytosis and Regulate Inflammation in Patients with Minor Cognitive Impairment. J. Alzheimers Dis. 2015, 48, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Bayram, S.; Kızıltan, G. The Role of Omega- 3 Polyunsaturated Fatty Acids in Diabetes Mellitus Management: A Narrative Review. Curr. Nutr. Rep. 2024, 13, 527–551. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Hosseini, M.S.; Khalili, N.; Naimi, E.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of curcumin on serum cytokine concentrations in subjects with metabolic syndrome: A post-hoc analysis of a randomized controlled trial. Biomed. Pharmacother. 2016, 82, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin supplementation on markers of inflammation and oxidative stress among healthy overweight and obese girl adolescents: A randomized placebo-controlled clinical trial. Phytother. Res. 2019, 33, 2015–2022. [Google Scholar] [CrossRef]
- Ganjali, S.; Sahebkar, A.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.; Parizadeh, S.M.R.; Ghayour-Mobarhan, M. Investigation of the effects of curcumin on serum cytokines in obese individuals: A randomized controlled trial. Sci. World J. 2014, 2014, 898361. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of turmeric and its principal bioactive curcumin on human health: Pharmaceutical, medicinal, and food applications: A comprehensive review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef]
- Vors, C.; Couillard, C.; Paradis, M.E.; Gigleux, I.; Marin, J.; Vohl, M.C.; Couture, P.; Lamarche, B. Supplementation with resveratrol and curcumin does not affect the inflammatory response to a high-fat meal in older adults with abdominal obesity: A Randomized, Placebo-Controlled Crossover Trial. J. Nutr. 2018, 148, 379–388. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Si, H. Synergistic anti-inflammatory effects and mechanisms of the combination of resveratrol and curcumin in human vascular endothelial cells and rodent aorta. J. Nutr. Biochem. 2022, 108, 109083. [Google Scholar] [CrossRef]
- Pereira, C.P.M.; Souza, A.C.R.; Vasconcelos, A.R.; Prado, P.S.; Name, J.J. Antioxidant and anti-inflammatory mechanisms of action of astaxanthin in cardiovascular diseases. Int. J. Mol. Med. 2021, 47, 37–48. [Google Scholar] [CrossRef]
- Nawaz, A.; Nishida, Y.; Takikawa, A.; Fujisaka, S.; Kado, T.; Aminuddin, A.; Bilal, M.; Jeelani, I.; Aslam, M.R.; Nishimura, A.; et al. Astaxanthin, a Marine Carotenoid, Maintains the Tolerance and Integrity of Adipose Tissue and Contributes to Its Healthy Functions. Nutrients 2021, 13, 4374. [Google Scholar] [CrossRef]
- Xia, W.; Tang, N.; Kord-Varkaneh, H.; Low, T.Y.; Tan, S.C.; Wu, X.; Zhu, Y. The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: A meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 161, 105113. [Google Scholar] [CrossRef]
- Medoro, A.; Davinelli, S.; Milella, L.; Willcox, B.J.; Allsopp, R.C.; Scapagnini, G.; Willcox, D.C. Dietary astaxanthin: A promising antioxidant and anti-inflammatory agent for brain aging and adult neurogenesis. Mar. Drugs 2023, 21, 643. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Jeon, S. The Impact of Phytochemicals in Obesity-Related Metabolic Diseases: Focus on Ceramide Metabolism. Nutrients 2023, 15, 703. [Google Scholar] [CrossRef]
- Momchilova, A.; Nikolaev, G.; Pankov, S.; Vassileva, E.; Krastev, N.; Robev, B.; Krastev, D.; Pinkas, A.; Pankov, R. Effect of Quercetin and Fingolimod, Alone or in Combination, on the Sphingolipid Metabolism in HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 13916. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Noshadi, N.; Bonyadian, A.; Hojati, A.; Abbasalizad-Farhangi, M.; Heidari, M.; Darzi, M.; Seyedhosseini-Ghaheh, H.; Khajeh, M.; Pourteymour Fard Tabrizi, F.; Vajdi, M.; et al. The effect of quercetin supplementation on the components of metabolic syndrome in adults: A systematic review and dose–response meta-analysis of randomized controlled trials. J. Funct. Foods. 2024, 116, 106175. [Google Scholar] [CrossRef]
- Xia, M.; Ling, W.; Zhu, H.; Wang, Q.; Ma, J.; Hou, M.; Tang, Z.; Li, L.; Ye, Q. Anthocyanin prevents CD40-activated proinflammatory signaling in endothelial cells by regulating cholesterol distribution. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 519–524. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Mendoza-Córdova, M.Y.; Mahady, G.B.; Luna-Vital, D.A.; Gutiérrez-Uribe, J.A.; Chuck-Hernández, C. Consumption of dietary anthocyanins and their association with a reduction in obesity biomarkers and the prevention of obesity. Trends Food Sci. Technol. 2023, 140, 104140. [Google Scholar] [CrossRef]
- Noman, A.M.; Sultan, M.T.; Maaz, M.; Mazhar, A.; Tariq, N.; Imran, M.; Hussain, M.; Mujtaba, A.; Abdelgawad, M.A.; Mostafa, E.M.; et al. Nutraceutical potential of anthocyanins: A comprehensive treatise. Food Sci. Nutr. 2025, 13, e70164. [Google Scholar] [CrossRef]
- Tiwari, V.; Sharma, S.; Tiwari, A.; Sheoran, B.; Kaur, S.; Sharma, A.; Yadav, M.; Bhatnagar, A.; Garg, M. Effect of dietary anthocyanins on biomarkers of type 2 diabetes and related obesity: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2024, 64, 7517–7534. [Google Scholar] [CrossRef]
- Baralić, K.; Živanović, J.; Marić, Đ.; Bozic, D.; Grahovac, L.; Antonijević Miljaković, E.; Ćurčić, M.; Buha Djordjevic, A.; Bulat, Z.; Antonijević, B.; et al. Sulforaphane-A Compound with Potential Health Benefits for Disease Prevention and Treatment: Insights from Pharmacological and Toxicological Experimental Studies. Antioxidants 2024, 13, 147. [Google Scholar] [CrossRef]
- Lee, J.; Ahn, H.; Hong, E.J.; An, B.S.; Jeung, E.B.; Lee, G.S. Sulforaphane attenuates activation of NLRP3 and NLRC4 inflammasomes but not AIM2 inflammasome. Cell. Immunol. 2016, 306–307, 53–60. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Axelsson, A.S.; Tubbs, E.; Mecham, B.; Chacko, S.; Nenonen, H.A.; Tang, Y.; Fahey, J.W.; Derry, J.M.J.; Wollheim, C.B.; Wierup, N.; et al. Sulforaphane reduces hepatic glucose production and improves glucose control in patients with type 2 diabetes. Sci. Transl. Med. 2017, 9, eaah4477. [Google Scholar] [CrossRef] [PubMed]
- Dwibedi, C.; Axelsson, A.S.; Abrahamsson, B.; Fahey, J.W.; Asplund, O.; Hansson, O.; Ahlqvist, E.; Tremaroli, V.; Bäckhed, F.; Rosengren, A.H. Effect of broccoli sprout extract and baseline gut microbiota on fasting blood glucose in prediabetes: A randomized, placebo-controlled trial. Nat. Microbiol. 2025, 10, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Kensler, T.W. The challenges of designing and implementing clinical trials with broccoli sprouts… and turning evidence into public health action. Front. Nutr. 2021, 8, 648788. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Villela-Castrejón, J.; Serna-Saldívar, S.O.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Synergistic combinations of curcumin, sulforaphane, and dihydrocaffeic acid against human colon cancer cells. Int. J. Mol. Sci. 2020, 21, 3108. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares-Vicente, M.; Herranz-López, M. The Interplay Between Oxidative Stress and Lipid Composition in Obesity-Induced Inflammation: Antioxidants as Therapeutic Agents in Metabolic Diseases. Int. J. Mol. Sci. 2025, 26, 8544. https://doi.org/10.3390/ijms26178544
Olivares-Vicente M, Herranz-López M. The Interplay Between Oxidative Stress and Lipid Composition in Obesity-Induced Inflammation: Antioxidants as Therapeutic Agents in Metabolic Diseases. International Journal of Molecular Sciences. 2025; 26(17):8544. https://doi.org/10.3390/ijms26178544
Chicago/Turabian StyleOlivares-Vicente, Mariló, and María Herranz-López. 2025. "The Interplay Between Oxidative Stress and Lipid Composition in Obesity-Induced Inflammation: Antioxidants as Therapeutic Agents in Metabolic Diseases" International Journal of Molecular Sciences 26, no. 17: 8544. https://doi.org/10.3390/ijms26178544
APA StyleOlivares-Vicente, M., & Herranz-López, M. (2025). The Interplay Between Oxidative Stress and Lipid Composition in Obesity-Induced Inflammation: Antioxidants as Therapeutic Agents in Metabolic Diseases. International Journal of Molecular Sciences, 26(17), 8544. https://doi.org/10.3390/ijms26178544






