Contribution of Second-Shell Residues to PLP-Dependent Transaminase Catalysis: A Case Study of D-Amino Acid Transaminase from Desulfomonile tiedjei
Abstract
1. Introduction
2. Results
2.1. Enzyme Identification, Expression, and Purification
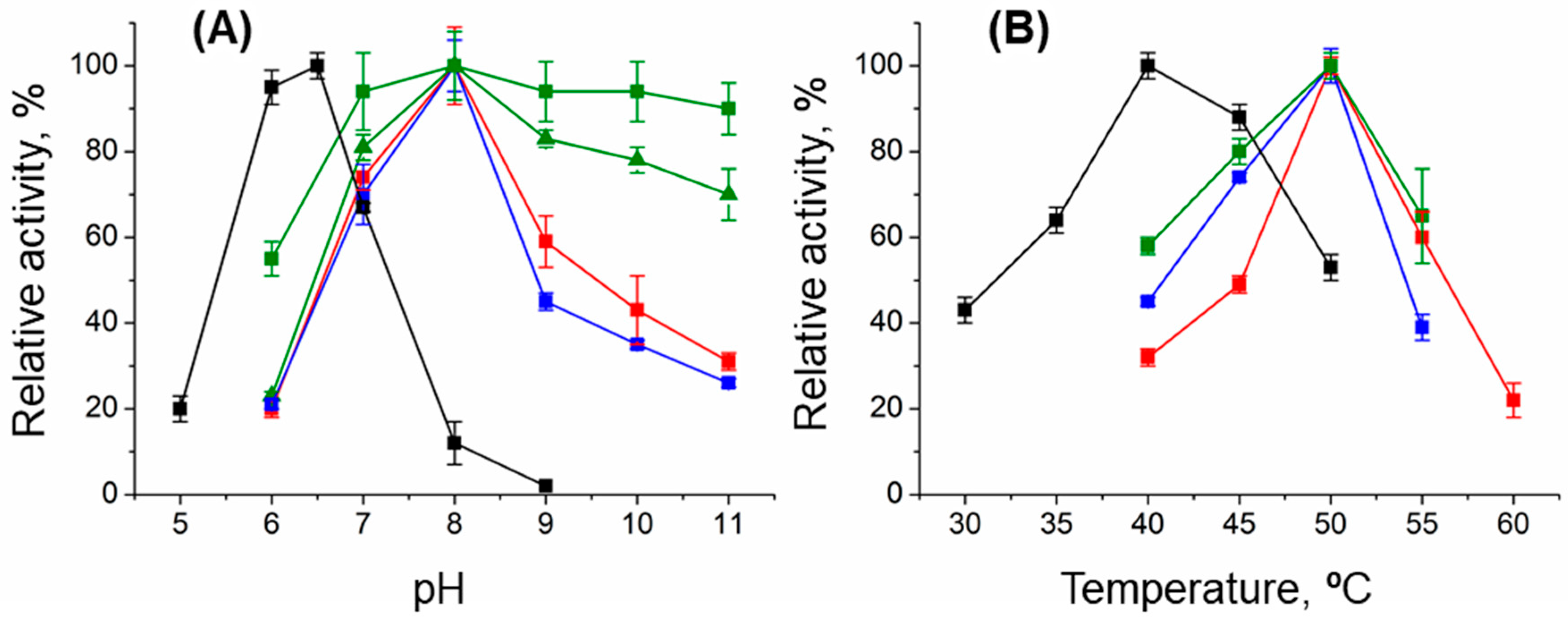
2.2. Substrate Specificity, Enantioselectivity, and Stability
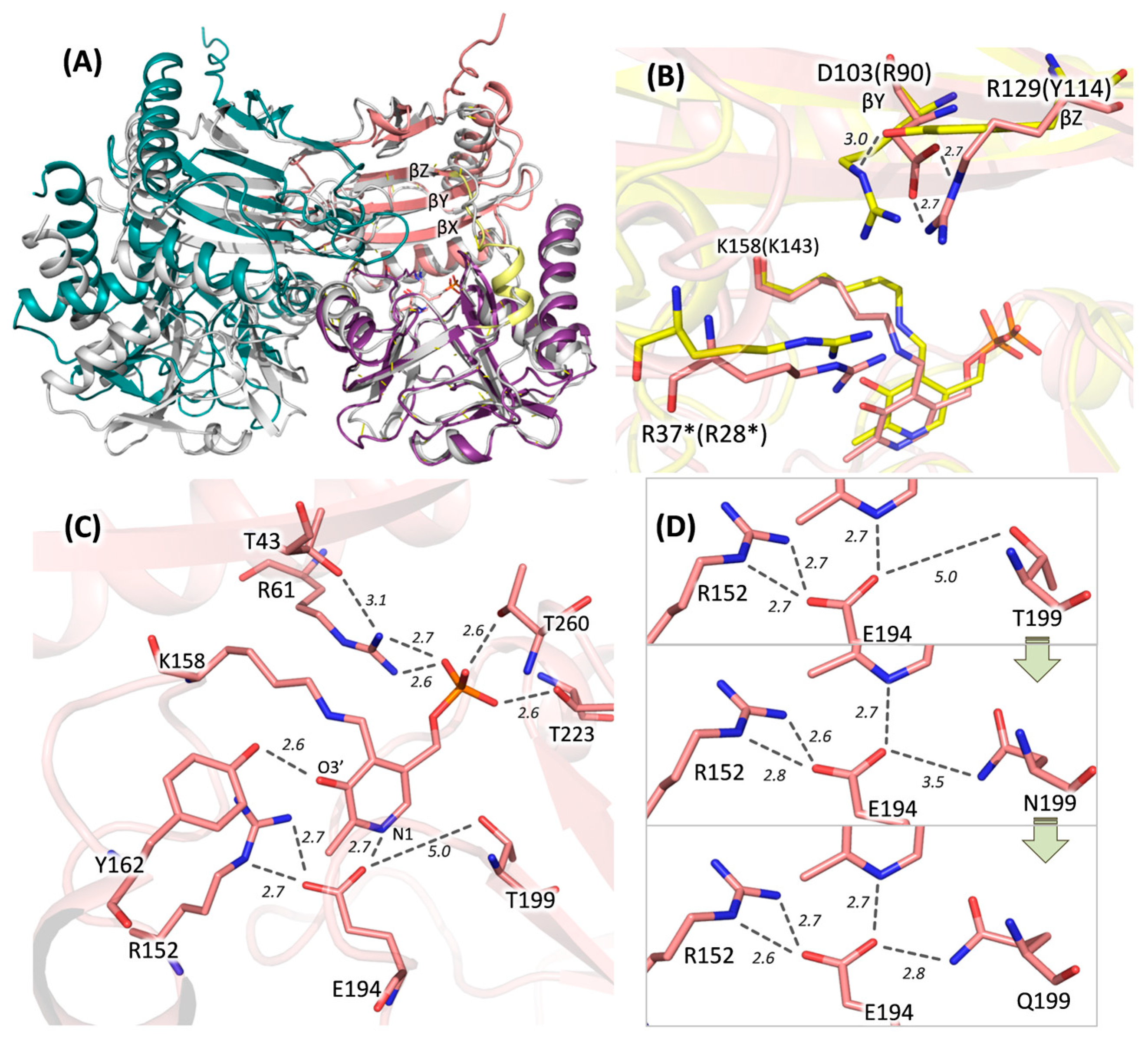
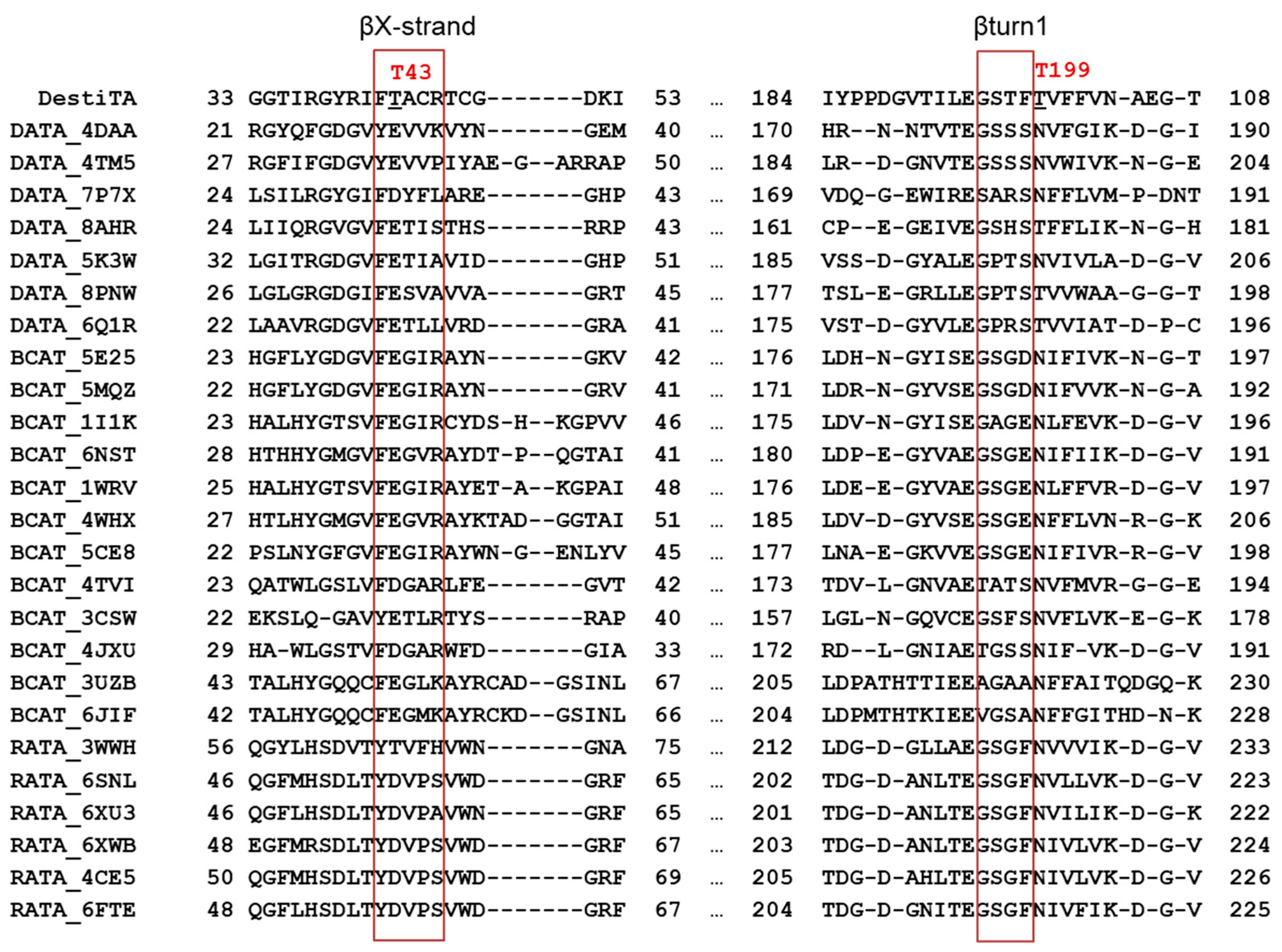
2.3. Overall Structure of DestiTA. PLP Coordination in the Active Site
2.4. Characterization of the DestiTA Variants: Thermal Stability and Catalytic Function
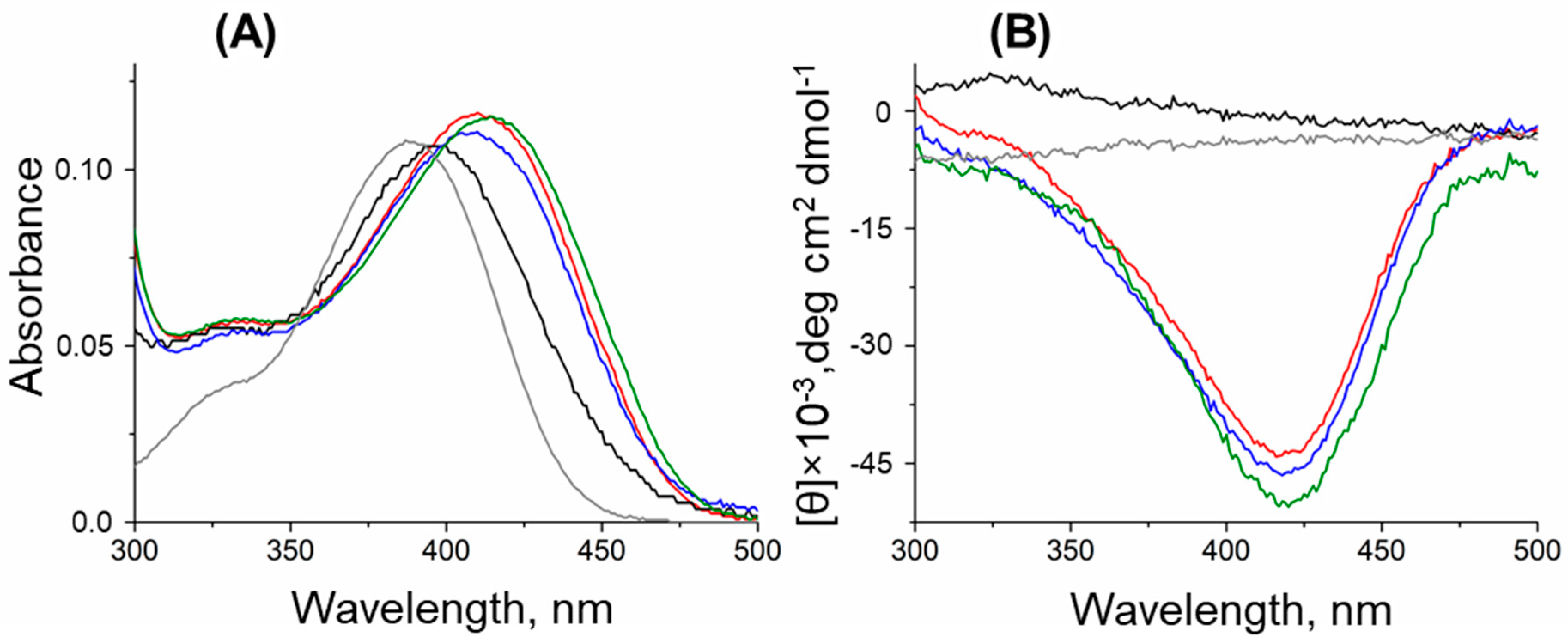
2.5. Characterization of the DestiTA Variants: Cofactor Binding and Internal Aldimine Formation
3. Discussion
4. Materials and Methods
4.1. Preparation and Purification of the Recombinant DestiTA and Its Variants
4.2. Enzyme Activity Assay
4.3. Effect of pH and Temperature on the Transamination Reaction
4.4. Spectral Analysis
4.5. Analysis of the Enantiomeric Excess in the Transamination Reaction
4.6. ThermoFluor Assay
4.7. Determination of the Equilibrium Dissociation Constant of the Holoenzyme
4.8. Molecular Modeling and Structure Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toney, M.D. Reaction specificity in pyridoxal phosphate enzymes. Arch Biochem Biophys. 2005, 433, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, A.E. Amino Group Transfer. In The Enzymes; Boyer, P.D., Ed.; Academic Press: London, UK, 1973; pp. 379–481. [Google Scholar]
- Eliot, A.C.; Kirsch, J.F. Pyridoxal Phosphate Enzymes: Mechanistic, Structural, and Evolutionary Considerations. Annu. Rev. Biochem. 2004, 73, 383–415. [Google Scholar] [CrossRef] [PubMed]
- Savile, C.K.; Janey, J.M.; Mundorff, E.C.; Moore, J.C.; Tam, S.; Jarvis, W.R.; Colbeck, J.C.; Krebber, A.; Fleitz, F.J.; Brands, J.; et al. Biocatalytic Asymmetric Synthesis of Chiral Amines from Ketones Applied to Sitagliptin Manufacture. Science 2010, 329, 305–309. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic synthesis for industrial applications. Angew. Chemie Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Winkler, C.K.; Schrittwieser, J.H.; Kroutil, W. Power of Biocatalysis for Organic Synthesis. ACS Cent. Sci. 2021, 7, 55–71. [Google Scholar] [CrossRef]
- Bell, E.L.; Finnigan, W.; France, S.P.; Green, A.P.; Hayes, M.A.; Hepworth, L.J.; Lovelock, S.L.; Niikura, H.; Osuna, S.; Romero, E.; et al. Biocatalysis. Nat. Rev. Methods Prim. 2021, 1, 46. [Google Scholar] [CrossRef]
- Grishin, N.V.; Phillips, M.A.; Goldsmith, E.J. Modeling of the spatial structure of eukaryotic ornithine decarboxylases. Protein Sci. 1995, 4, 1291–1304. [Google Scholar] [CrossRef]
- Steffen-Munsberg, F.; Vickers, C.; Kohls, H.; Land, H.; Mallin, H.; Nobili, A.; Skalden, L.; van den Bergh, T.; Joosten, H.-J.; Berglund, P.; et al. Bioinformatic analysis of a PLP-dependent enzyme superfamily suitable for biocatalytic applications. Biotechnol. Adv. 2015, 33, 566–604. [Google Scholar] [CrossRef]
- Bezsudnova, E.Y.; Popov, V.O.; Boyko, K.M. Structural insight into the substrate specificity of PLP fold type IV transaminases. Appl. Microbiol. Biotechnol. 2020, 104, 2343–2357. [Google Scholar] [CrossRef]
- Telzerow, A.; Paris, J.; Håkansson, M.; González-Sabín, J.; Ríos-Lombardía, N.; Gröger, H.; Morís, F.; Schürmann, M.; Schwab, H.; Steiner, K. Expanding the Toolbox of R-Selective Amine Transaminases by Identification and Characterization of New Members. ChemBioChem 2021, 22, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Han, Q.; Tan, Y.; Ding, H.; Li, J. Current Advances on Structure-Function Relationships of Pyridoxal 5′-Phosphate-Dependent Enzymes. Front. Mol. Biosci. 2019, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Höhne, M.; Schätzle, S.; Jochens, H.; Robins, K.; Bornscheuer, U.T. Rational assignment of key motifs for function guides in silico enzyme identification. Nat. Chem. Biol. 2010, 6, 807–813. [Google Scholar] [CrossRef]
- Gu, J.; Xu, Y.; Nie, Y. Role of distal sites in enzyme engineering. Biotechnol. Adv. 2023, 63, 108094. [Google Scholar] [CrossRef]
- Chaturvedi, S.S.; Bím, D.; Christov, C.Z.; Alexandrova, A.N. From random to rational: Improving enzyme design through electric fields, second coordination sphere interactions, and conformational dynamics. Chem. Sci. 2023, 14, 10997–11011. [Google Scholar] [CrossRef] [PubMed]
- Toney, M.D.; Kirsch, J.F. Kinetics and equilibria for the reactions of coenzymes with wild type and the Y70F mutant of Escherichia coli aspartate aminotransferase. Biochemistry 1991, 30, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Turbeville, T.D.; Zhang, J.; Hunter, G.A.; Ferreira, G.C. Histidine 282 in 5-aminolevulinate synthase affects substrate binding and catalysis. Biochemistry 2007, 46, 5972–5981. [Google Scholar] [CrossRef][Green Version]
- Yano, T.; Mizuno, T.; Kagamiyama, H. A hydrogen-bonding network modulating enzyme function: Asparagine-194 and tyrosine-225 of Escherichia coli aspartate aminotransferase. Biochemistry 1993, 32, 1810–1815. [Google Scholar] [CrossRef]
- Van Ophem, P.W.; Pospischil, M.A.; Ringe, D.; Peisach, D.; Petsko, G.; Soda, K.; Manning, J.M. Catalytic ability and stability of two recombinant mutants of D-amino acid transaminase involved in coenzyme binding. Protein Sci. 1995, 4, 2578–2586. [Google Scholar] [CrossRef]
- Chan-Huot, M.; Dos, A.; Zander, R.; Sharif, S.; Tolstoy, P.M.; Compton, S.; Fogle, E.; Toney, M.D.; Shenderovich, I.; Denisov, G.S.; et al. NMR studies of protonation and hydrogen bond states of internal aldimines of pyridoxal 5′-phosphate acid-base in alanine racemase, aspartate aminotransferase, and poly-L-lysine. J. Am. Chem. Soc. 2013, 135, 18160–18175. [Google Scholar] [CrossRef]
- Liu, W.; Peterson, P.E.; Carter, R.J.; Zhou, X.; Langston, J.A.; Fisher, A.J.; Toney, M.D. Crystal structures of unbound and aminooxyacetate-bound Escherichia coli gamma-aminobutyrate aminotransferase. Biochemistry 2004, 43, 10896–10905. [Google Scholar] [CrossRef]
- Goto, M.; Miyahara, I.; Hayashi, H.; Kagamiyama, H.; Hirotsu, K. Crystal structures of branched-chain amino acid aminotransferase complexed with glutamate and glutarate: True reaction intermediate and double substrate recognition of the enzyme. Biochemistry 2003, 42, 3725–3733. [Google Scholar] [CrossRef]
- Yano, Y.; Kuramitsu, S.; Tanase, S.; Morino, Y.; Kagamiyama, H. Role of Asp222 in the Catalytic Mechanism of Escherichia coli Aspartate Aminotransferase: The Amino Acid Residue Which Enhances the Function of the Enzyme-Boun Coenzyme Pyridoxal 5′-Phpsphate. Biochemistry 1992, 31, 5878–5887. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Fogle, E.; Toney, M.D.; Denisov, G.S.; Shenderovich, I.G.; Buntkowsky, G.; Tolstoy, P.M.; Huot, M.C.; Limbach, H.H. NMR localization of protons in critical enzyme hydrogen bonds. J. Am. Chem. Soc. 2007, 129, 9558–9559. [Google Scholar] [CrossRef] [PubMed]
- Roura Padrosa, D.; Alaux, R.; Smith, P.; Dreveny, I.; López-Gallego, F.; Paradisi, F. Enhancing PLP-Binding Capacity of Class-III ω-Transaminase by Single Residue Substitution. Front. Bioeng. Biotechnol. 2019, 7, 282. [Google Scholar] [CrossRef]
- Dajnowicz, S.; Parks, J.M.; Hu, X.; Gesler, K.; Kovalevsky, A.Y.; Mueser, T.C. Direct evidence that an extended hydrogen-bonding network influences activation of pyridoxal 5′-phosphate in aspartate aminotransferase. J. Biol. Chem. 2017, 292, 5970–5980. [Google Scholar] [CrossRef] [PubMed]
- Bakunova, A.K.; Matyuta, I.O.; Minyaev, M.E.; Boyko, K.M.; Popov, V.O.; Bezsudnova, E.Y. Incorporation of pyridoxal-5′-phosphate into the apoenzyme: A structural study of D-amino acid transaminase from Haliscomenobacter hydrossis. Biochim. Biophys. Acta Proteins Proteom. 2025, 1873, 141056. [Google Scholar] [CrossRef]
- Bakunova, A.K.; Matyuta, I.O.; Minyaev, M.E.; Isaikina, T.Y.; Boyko, K.M.; Popov, V.O.; Bezsudnova, E.Y. Multifunctionality of arginine residues in the active sites of non-canonical D-amino acid transaminases. Arch. Biochem. Biophys. 2024, 756, 110011. [Google Scholar] [CrossRef]
- Xiang, C.; Ao, Y.-F.; Höhne, M.; Bornscheuer, U.T. Shifting the pH Optima of (R)-Selective Transaminases by Protein Engineering. Int. J. Mol. Sci. 2022, 23, 15347. [Google Scholar] [CrossRef]
- Gloss, L.M.; Kirsch, J.F. Use of Site-Directed Mutagenesis and Alternative Substrates To Assign the Prototropic Groups Important to Catalysis by Escherichia coli Aspartate Aminotransferase. Biochemistry 1995, 34, 3999–4007. [Google Scholar] [CrossRef]
- Toney, M.D. Controlling reaction specificity in pyridoxal phosphate enzymes. Biochim. Biophys. Acta Proteins Proteom. 2011, 1814, 1407–1418. [Google Scholar] [CrossRef]
- Shilova, S.A.; Matyuta, I.O.; Petrova, E.S.; Nikolaeva, A.Y.; Rakitina, T.V.; Minyaev, M.E.; Boyko, K.M.; Popov, V.O.; Bezsudnova, E.Y. Expanded substrate specificity in D-amino acid transaminases: A case Study of transaminase from Blastococcus saxobsidens. Int. J. Mol. Sci. 2023, 24, 16194. [Google Scholar] [CrossRef] [PubMed]
- Pavkov-Keller, T.; Strohmeier, G.A.; Diepold, M.; Peeters, W.; Smeets, N.; Schürmann, M.; Gruber, K.; Schwab, H.; Steiner, K. Discovery and structural characterisation of new fold type IV-transaminases exemplify the diversity of this enzyme fold. Sci. Rep. 2016, 6, 38183. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, M.; Sekine, M.; Katane, M.; Furuchi, T.; Yohda, M.; Yoshikawa, T.; Homma, H. Cloning and functional characterization of Arabidopsis thaliana D-amino acid aminotransferase–D-aspartate behavior during germination. FEBS J. 2008, 275, 1188–1200. [Google Scholar] [CrossRef]
- Shilova, S.A.; Khrenova, M.G.; Matyuta, I.O.; Nikolaeva, A.Y.; Rakitina, T.V.; Klyachko, N.L.; Minyaev, M.E.; Boyko, K.M.; Popov, V.O.; Bezsudnova, E.Y. To the Understanding of Catalysis by D-Amino Acid Transaminases: A Case Study of the Enzyme from Aminobacterium colombiense. Molecules 2023, 28, 2109. [Google Scholar] [CrossRef]
- Bakunova, A.K.; Nikolaeva, A.Y.; Rakitina, T.V.; Isaikina, T.Y.; Khrenova, M.G.; Boyko, K.M.; Popov, V.O.; Bezsudnova, E.Y. The Uncommon Active Site of D-Amino Acid Transaminase from Haliscomenobacter hydrossis: Biochemical and Structural Insights into the New Enzyme. Molecules 2021, 26, 5053. [Google Scholar] [CrossRef]
- Tanizawa, K.; Masu, Y.; Asano, S.; Tanaka, H.; Soda, K. Thermostable D-amino acid aminotransferase from a thermophilic Bacillus species. J. Biol. Chem. 1989, 264, 2445–2449. [Google Scholar] [CrossRef]
- Kobayashi, J.; Shimizu, Y.; Mutaguchi, Y.; Doi, K.; Ohshima, T. Characterization of d-amino acid aminotransferase from Lactobacillus salivarius. J. Mol. Catal. B Enzym. 2013, 94, 15–22. [Google Scholar] [CrossRef]
- Peisach, D.; Chipman, D.M.; Van Ophem, P.W.; Manning, J.M.; Ringe, D. Crystallographic study of steps along the reaction pathway of D-amino acid aminotransferase. Biochemistry 1998, 37, 4958–4967. [Google Scholar] [CrossRef]
- Sugio, S.; Petsko, G.A.; Manning, J.M.; Soda, K.; Ringe, D. Crystal Structure of a D-Amino Acid Aminotransferase: How the Protein Controls Stereoselectivity. Biochemistry 1995, 34, 9661–9669. [Google Scholar] [CrossRef]
- Shilova, S.A.; Matyuta, I.O.; Khrenova, M.G.; Nikolaeva, A.Y.; Klyachko, N.L.; Minyaev, M.E.; Khomutov, A.R.; Boyko, K.M.; Popov, V.O.; Bezsudnova, E.Y. In search for structural targets for engineering D-amino acid transaminase: Modulation of pH optimum and substrate specificity. Biochem. J. 2023, 480, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- DeWeerd, K.; Mandelco, L.; Tanner, R.; Woese, C.; Suflita, J. Desulfomonile tiedjei gen. nov. and sp. nov., a novel anaerobic, dehalogenating, sulfate-reducing bacterium. Arch. Microbiol. 1990, 154, 23–30. [Google Scholar] [CrossRef]
- Metzler, C.M.; Cahill, A.; Metzler, D.E. Equilibriums and absorption spectra of Schiff bases. J. Am. Chem. Soc. 1980, 102, 6075–6082. [Google Scholar] [CrossRef]
- Ro, H.S.; Hong, S.P.; Seo, H.J.; Yoshimura, T.; Esaki, N.; Soda, K.; Kim, H.S.; Sung, M.H. Site-directed mutagenesis of the amino acid residues in β-strand III [Val30-Val36] of D-amino acid aminotransferase of Bacillus sp. YM-1. FEBS Lett. 1996, 398, 141–145. [Google Scholar] [CrossRef]
- Cai, K.; Schirch, D.; Schirch, V. The affinity of pyridoxal 5′-phosphate for folding intermediates of Escherichia coli serine hydroxymethyltransferase. J. Biol. Chem. 1995, 270, 19294–19299. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Mizuguchi, H.; Kagamiyama, H. The imine-pyridine torsion of the pyridoxal 5′-phosphate Schiff base of aspartate aminotransferase lowers its pKa in the unliganded enzyme and is crucial for the successive increase in the pKa during catalysis. Biochemistry 1998, 37, 15076–15085. [Google Scholar] [CrossRef] [PubMed]
- Bezsudnova, E.Y.; Boyko, K.M.; Nikolaeva, A.Y.; Zeifman, Y.S.; Rakitina, T.V.; Suplatov, D.A.; Popov, V.O. Biochemical and structural insights into PLP fold type IV transaminase from Thermobaculum terrenum. Biochimie 2019, 158, 130–138. [Google Scholar] [CrossRef]
- Yu, E.; Dibrova, D.V.V.; Yu, A.; Rakitina, T.V.V.; Popov, V.O.O.; Bezsudnova, E.Y.Y.; Dibrova, D.V.V.; Nikolaeva, A.Y.Y.; Rakitina, T.V.V.; Popov, V.O.O. Identification of branched-chain amino acid aminotransferases active towards (R)-(+)-1-phenylethylamine among PLP fold type IV transaminases. J. Biotechnol. 2018, 271, 26–28. [Google Scholar] [CrossRef]
- Hult, K.; Berglund, P. Enzyme promiscuity: Mechanism and applications. Trends Biotechnol. 2007, 25, 231–238. [Google Scholar] [CrossRef]
- Toney, M.D. Aspartate aminotransferase: An old dog teaches new tricks. Arch. Biochem. Biophys. 2014, 544, 119–127. [Google Scholar] [CrossRef]
- Cassimjee, K.E.; Humble, M.S.; Land, H.; Abedi, V.; Berglund, P. Chromobacterium violaceum ω-transaminase variant Trp60Cys shows increased specificity for (S)-1-phenylethylamine and 4′-substituted acetophenones, and follows Swain–Lupton parameterisation. Org. Biomol. Chem. 2012, 10, 5466. [Google Scholar] [CrossRef]
- Kelly, S.A.; Mix, S.; Moody, T.S.; Gilmore, B.F. Transaminases for industrial biocatalysis: Novel enzyme discovery. Appl. Microbiol. Biotechnol. 2020, 104, 4781–4794. [Google Scholar] [CrossRef] [PubMed]
- Slabu, I.; Galman, J.L.; Lloyd, R.C.; Turner, N.J. Discovery, Engineering, and Synthetic Application of Transaminase Biocatalysts. ACS Catal. 2017, 7, 8263–8284. [Google Scholar] [CrossRef]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef]
- Mikhailova, A.G.; Rakitina, T.V.; Timofeev, V.I.; Karlinsky, D.M.; Korzhenevskiy, D.A.; Agapova, Y.K.; Vlaskina, A.V.; Ovchinnikova, M.V.; Gorlenko, V.A.; Rumsh, L.D. Activity modulation of the oligopeptidase B from Serratia proteamaculans by site-directed mutagenesis of amino acid residues surrounding catalytic triad histidine. Biochimie 2017, 139, 125–136. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef] [PubMed]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.M.; Mittal, J.; Feig, M.; MacKerell, A.D. Optimization of the Additive CHARMM All-Atom Protein Force Field Targeting Improved Sampling of the Backbone ϕ, ψ and Side-Chain χ1 and χ2 Dihedral Angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Chen, X.; Briozzo, P.; Machover, D.; Simonson, T. A Computational Model for the PLP-Dependent Enzyme Methionine γ-Lyase. Front. Mol. Biosci. 2022, 9, 886358. [Google Scholar] [CrossRef]
- Konagurthu, A.S.; Whisstock, J.C.; Stuckey, P.J.; Lesk, A.M. MUSTANG: A multiple structural alignment algorithm. Proteins 2006, 64, 559–574. [Google Scholar] [CrossRef]

| TA | Amino Acid Motifs in the Active Site [13] | ||
|---|---|---|---|
| BCAT from Escherichia coli | 31YxxxxFxGxR40 | 95YxR97 | 107MxV109 |
| (R)-ATA from Aspergillus fumigatus | 53HxxxxYxVxS62 | 113FxE115 | 125xRx127 |
| DATA from Bacillus sp. YM-1 | 26FxxxxYxVxK35 | 88HxY90 | 98RxH100 |
| DATA from Aminobacterium colombiense | 27RxxxxFxTxS36 | 86MxR88 | - |
| DATA from Curtobacterium pusillum | 46RxxxxFxTxA60 | 115FxK117 | - |
| DATA from Blastococcus saxobsidence | 34RxxxxFxSxA43 | 94VxR96 | - |
| DATA from Haliscomenobacter hydrossis | 28RxxxxFxYxL37 | 88GxR90 | - |
| DATA from Desulfomonile tiedjei | 37RxxxxFxAxR37 | 101LxD103 | - |
| Substrate | Co-Substrate | Parameters | T0.5 1, °C | ||
|---|---|---|---|---|---|
| kcat, s−1 | Km, mM | kcat/Km, s−1 M−1 | |||
| WT DestiTA | |||||
| D-alanine | α-ketoglutarate | 5.8 ± 0.2 | 0.5 ± 0.1 | 11,600 ± 3000 | 55.0 ± 0.4 (64.4 ± 0.2) |
| α-ketoglutarate | D-alanine | 0.11 ± 0.01 | 53,000 ± 8000 | ||
| (R)-PEA | α-ketoglutarate | 0.7 ± 0.1 | 17 ± 2 | 70 ± 20 | |
| α-ketoglutarate | (R)-PEA | 0.36 ± 0.05 | 3300 ± 400 | ||
| The T43E variant | |||||
| D-alanine | α-ketoglutarate | 0.15 ± 0.01 | 2.9 ± 0.8 | 52 ± 20 | 60.2 ± 0.3 (61.1 ± 0.4) |
| α-ketoglutarate | D-alanine | 2.8 ± 0.9 | 54 ± 30 | ||
| The T199N variant | |||||
| D-alanine | α-ketoglutarate | 5.0 ± 0.2 | 0.52 ± 0.07 | 10,000 ± 2000 | 54.7 ± 0.3 (63.2 ± 0.3) |
| α-ketoglutarate | D-alanine | 0.21 ± 0.03 | 24,000 ± 4000 | ||
| The T199Q variant | |||||
| D-alanine | α-ketoglutarate | 8.4 ± 0.4 | 50 ± 6 | 170 ± 30 | 50.9 ± 0.1 (65.7 ± 0.1) |
| α-ketoglutarate | D-alanine | 0.45 ± 0.07 | 19,000 ± 4000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakunova, A.K.; Rudina, I.V.; Popov, V.O.; Bezsudnova, E.Y. Contribution of Second-Shell Residues to PLP-Dependent Transaminase Catalysis: A Case Study of D-Amino Acid Transaminase from Desulfomonile tiedjei. Int. J. Mol. Sci. 2025, 26, 8536. https://doi.org/10.3390/ijms26178536
Bakunova AK, Rudina IV, Popov VO, Bezsudnova EY. Contribution of Second-Shell Residues to PLP-Dependent Transaminase Catalysis: A Case Study of D-Amino Acid Transaminase from Desulfomonile tiedjei. International Journal of Molecular Sciences. 2025; 26(17):8536. https://doi.org/10.3390/ijms26178536
Chicago/Turabian StyleBakunova, Alina K., Iuliia V. Rudina, Vladimir O. Popov, and Ekaterina Yu. Bezsudnova. 2025. "Contribution of Second-Shell Residues to PLP-Dependent Transaminase Catalysis: A Case Study of D-Amino Acid Transaminase from Desulfomonile tiedjei" International Journal of Molecular Sciences 26, no. 17: 8536. https://doi.org/10.3390/ijms26178536
APA StyleBakunova, A. K., Rudina, I. V., Popov, V. O., & Bezsudnova, E. Y. (2025). Contribution of Second-Shell Residues to PLP-Dependent Transaminase Catalysis: A Case Study of D-Amino Acid Transaminase from Desulfomonile tiedjei. International Journal of Molecular Sciences, 26(17), 8536. https://doi.org/10.3390/ijms26178536









