Aqueous Dispersion of Unmodified Fullerene C60: Stimulation of Hair Growth and Study of a New Molecular Target for Interaction
Abstract
1. Introduction
2. Results and Discussion
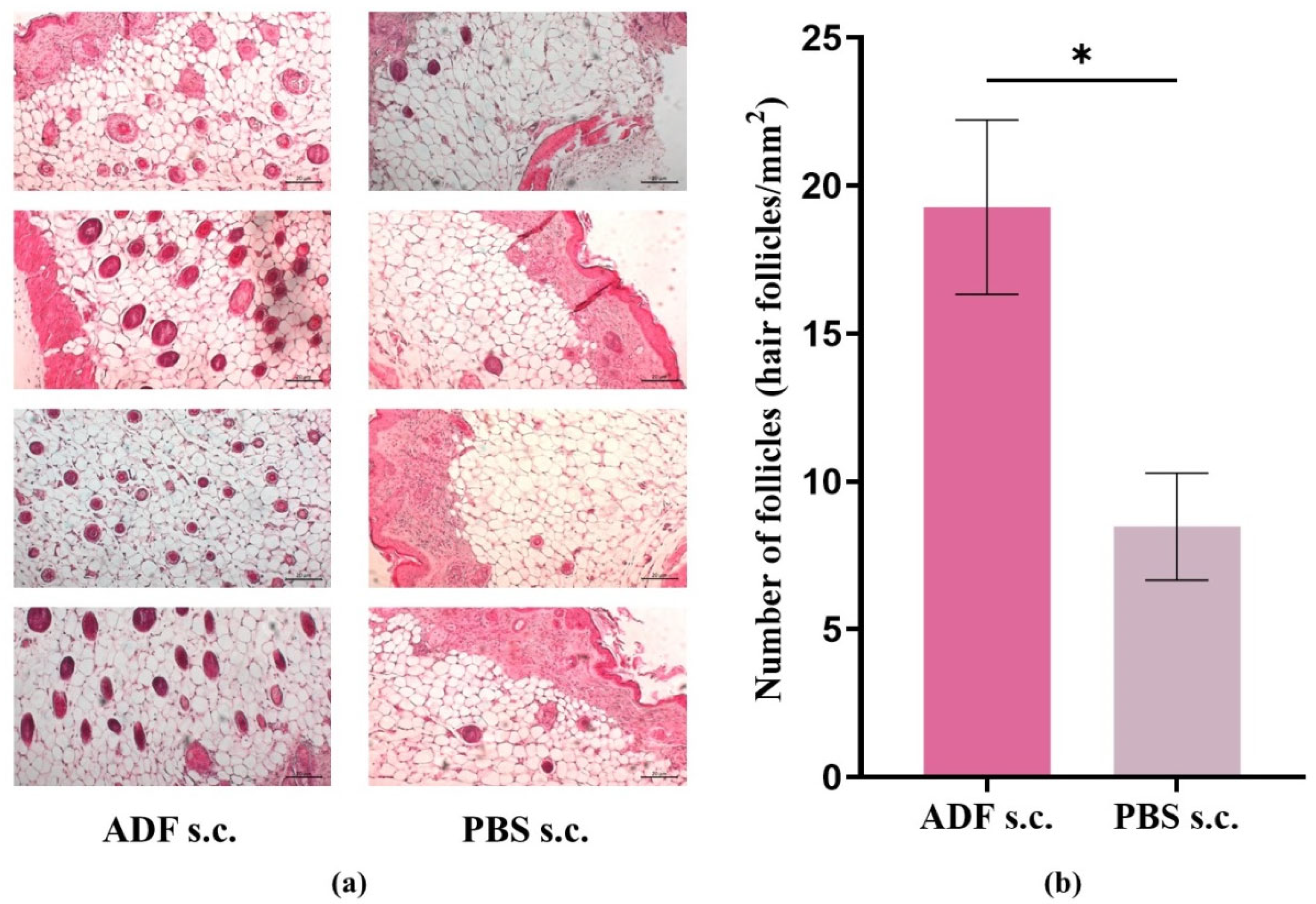
2.1. ADF Stimulates Hair Growth
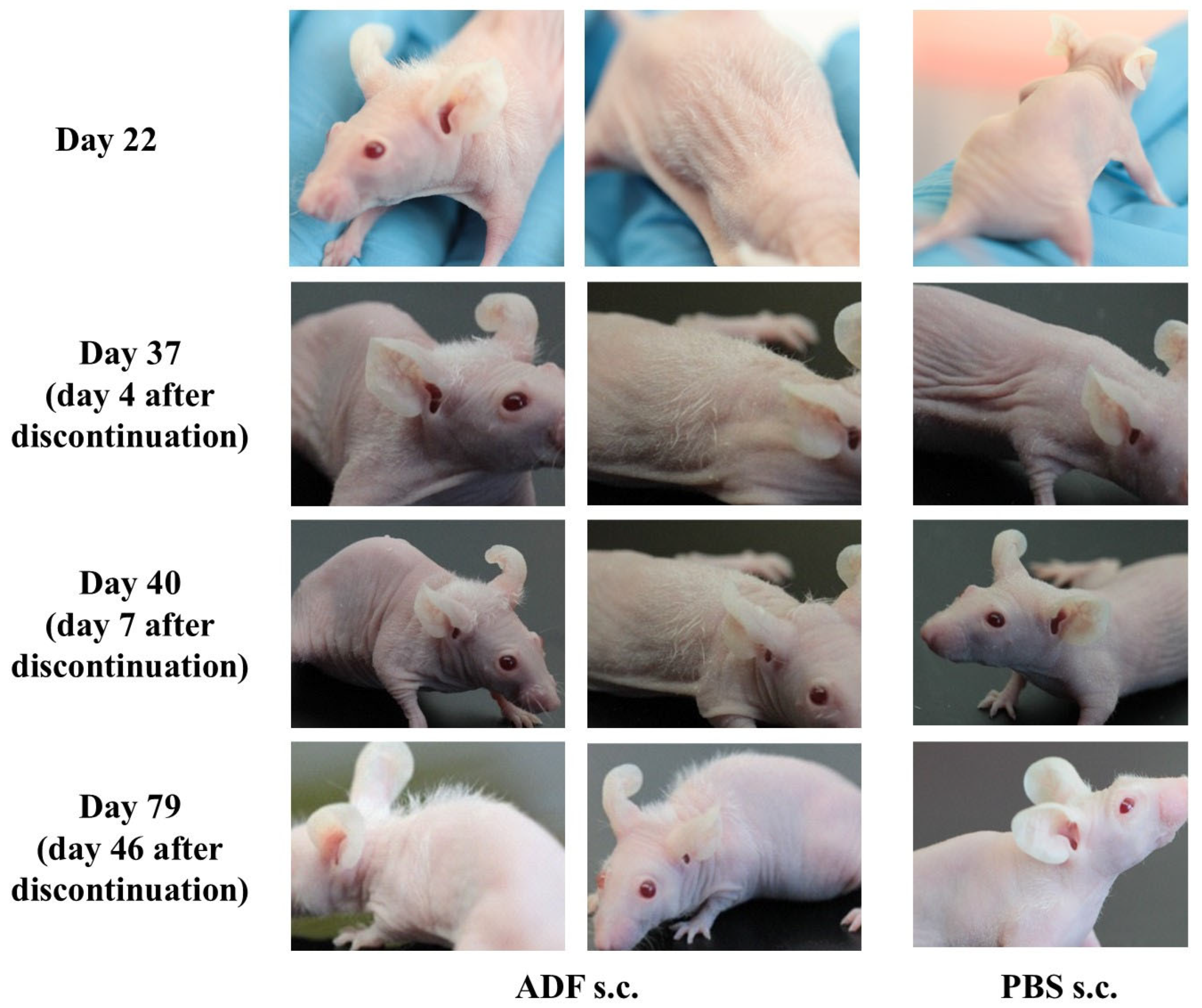
2.2. The Effect of Stimulating Hair Growth Persists After Discontinuation of ADF Administration
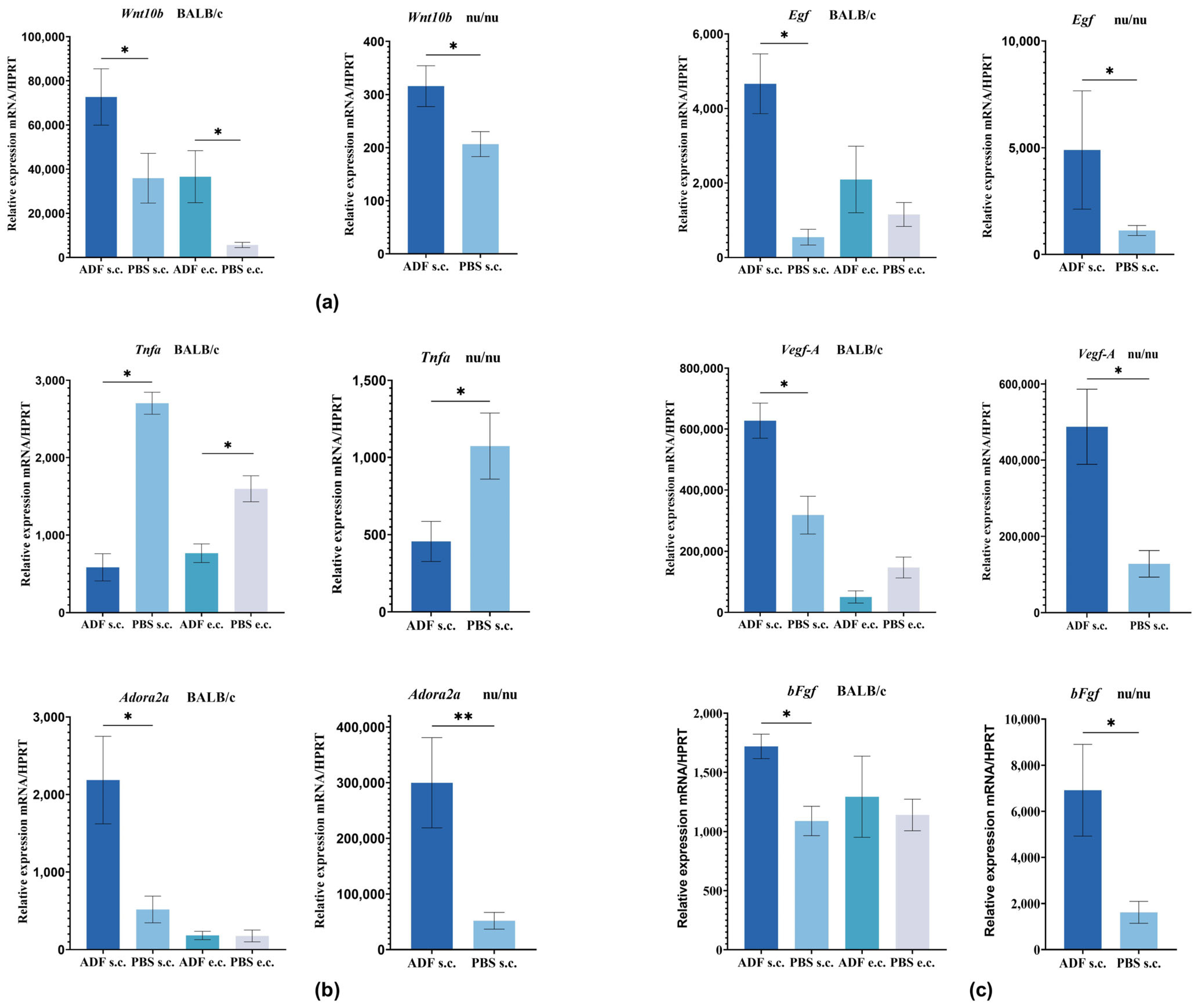
2.3. Evaluation of Gene Expression Involved in the Development and Functioning of Hair Follicles
2.4. Hypothesis About Possibility of Interaction of Fullerene C60 with Adenosine A2A Receptor
2.5. The Proposed Mechanism for Stimulating Hair Growth by Fullerene C60
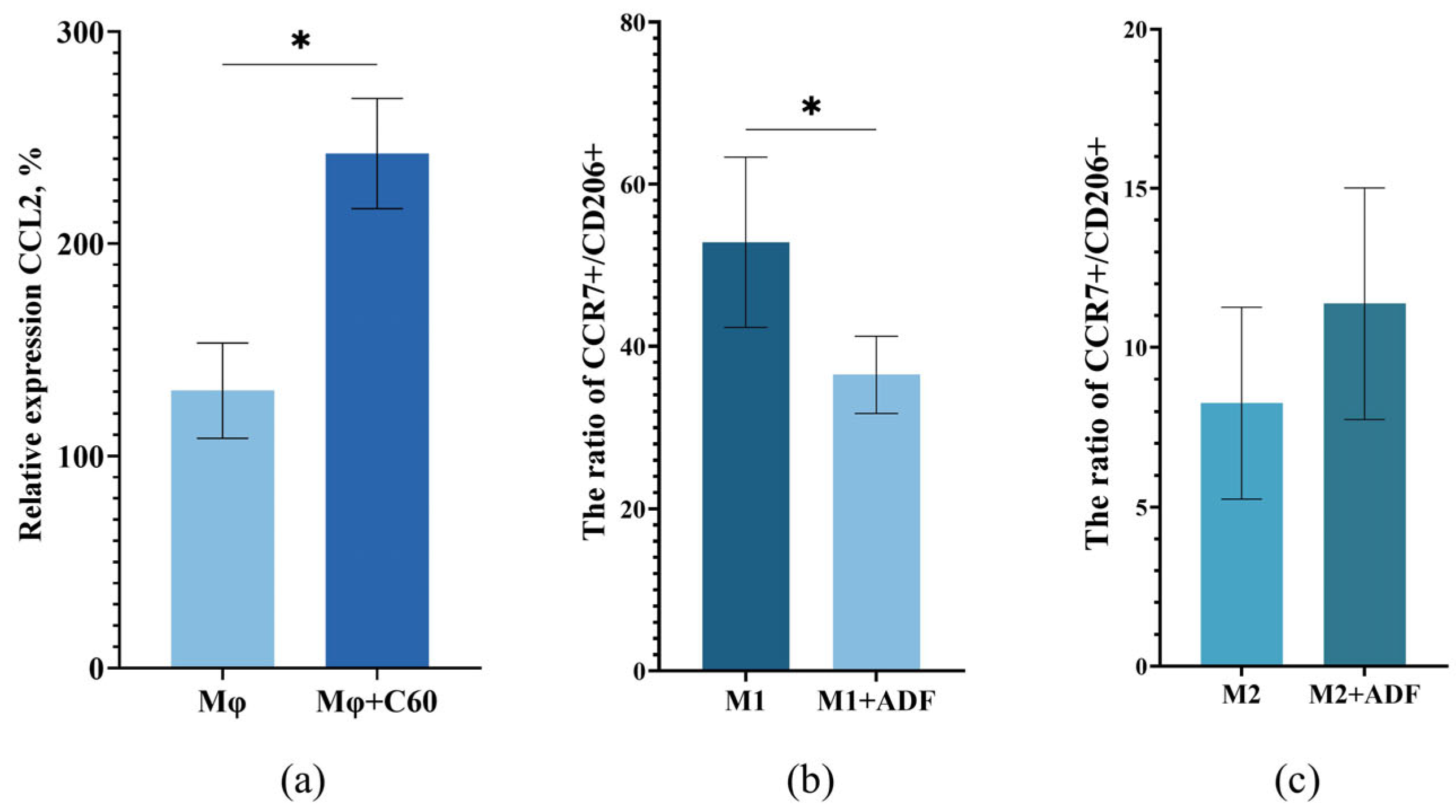
2.6. Hypothesis About the Interaction of ADF with Macrophages
3. Materials and Methods
3.1. Mice
3.2. The Aqueous Dispersion of Fullerene C60 (ADF)
3.3. Experimental Protocol
3.4. Skin Biopsy for Histological Analysis and qRT-PCR
3.5. Quantitative Real-Time PCR
3.6. Histological Analysis and Counting of the Number of Hair Follicles
3.7. Computational Modeling of Protein Structures
3.8. Macrophage Chemotaxis Assay
3.9. Isolation of Peripheral Blood Mononuclear Cells
3.10. Differentiation of PBMCs
3.11. Flow Cytometry
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | Aqueous dispersion of unmodified fullerene C60 |
| PBS | Phosphate-buffered saline |
| HF | Hair follicle |
| VEGF-A | Vascular endothelial growth factor alpha |
| EGF | Epidermal growth factor |
| bFGF | Basic fibroblast growth factor |
| ADORA2A | Adenosine A2A receptor |
| ROS | Reactive oxygen species |
| DPC | Dermal papilla cells |
| LPO | Lipid peroxidation |
| Mφ | Macrophages |
| M1 | Macrophages of the M1 phenotype |
| M2 | Macrophages of the M2 phenotype |
| TNFα | Tumor necrosis factor alpha |
| ATP | Adenosine triphosphate |
| TGFβ | Transforming growth factor beta |
| IL-10 | Interleukin-10 |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| NF-κB | Nuclear interleukin-1β factor kappa-light-chain-enhancer of activated B cells |
| IL-1β | Interleukin-1β |
| e.c. | Epicutaneously |
| s.c. | Subcutaneously |
References
- Alessandrini, A.; Bruni, F.; Piraccini, B.M.; Starace, M. Common causes of hair loss—clinical manifestations, trichoscopy and therapy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 629–640. [Google Scholar] [CrossRef]
- Lin, R.L.; Garibyan, L.; Kimball, A.B.; Drake, L.A. Systemic causes of hair loss. Ann. Med. 2016, 48, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Phillips, T.G.; Slomiany, W.P.; Allison, R. Hair loss: Common causes and treatment. Am. Fam. Physician 2017, 96, 371–378. [Google Scholar] [PubMed]
- Ocampo-Garza, J.; Griggs, J.; Tosti, A. New investigational drugs for the treatment of alopecia. Expert Opin. Drug Res. 2019, 28, 275–284. [Google Scholar] [CrossRef]
- Vary, J.C. Selected disorders of skin appendages—acne, alopecia, hyperhidrosis. Med. Clin. N. Am. 2015, 99, 1195–1211. [Google Scholar] [CrossRef]
- Gokce, N.; Basgoz, N.; Kenanoglu, S.; Akalin, H.; Ozkul, Y.; Ergoren, M.C.; Beccari, T.; Bertelli, M.; Dundar, M. An overview of the genetic aspects of hair loss and its connection with nutrition. J. Prev. Med. Hyg. 2022, 63, E228. [Google Scholar] [PubMed]
- Nanda, S.; De Bedout, V.; Miteva, M. Alopecia as a systemic disease. Clin. Dermatol. 2019, 37, 618–628. [Google Scholar] [CrossRef]
- Nalluri, R.; Harries, M. Alopecia in general medicine. Clin. Med. 2016, 16, 74–78. [Google Scholar] [CrossRef]
- Rossi, A.; Fortuna, M.C.; Caro, G.; Pranteda, G.; Garelli, V.; Pompili, U.; Carlesimo, M. Chemotherapy-induced alopecia management: Clinical experience and practical advice. J. Cosmet. Dermatol. 2017, 16, 537–541. [Google Scholar] [CrossRef]
- Bae, J.M.; Jung, H.M.; Goo, B.; Park, Y.M. Hair regrowth through wound healing process after ablative fractional laser treatment in a murine model. Lasers Surg. Med. 2015, 47, 433–440. [Google Scholar] [CrossRef]
- Bai, L.; Sun, H.; Jiang, W.; Yang, L.; Liu, G.; Zhao, X. DNA methylation and histone acetylation are involved in Wnt10b expression during the secondary hair follicle cycle in Angora rabbits. J. Anim. Physiol. Anim. Nutr. 2021, 105, 599–609. [Google Scholar] [CrossRef]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible Dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- du Cros, D.L. Fibroblast growth factor and Epidermal growth factor in hair development. J. Investig. Dermatol. 1993, 101, 106S–113S. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Ansel, J.; Perry, P.; Brown, J.; Damm, D.; Phan, T.; Hart, C.; Luger, T.; Hefeneider, S. Cytokine modulation of keratinocyte cytokines. J. Investig. Dermatol. 1990, 94, 101S–107S. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A.; Sitkovsky, M. Role of G-protein-coupled Adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001, 414, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Sivak, K.V.; Vasin, A.V.; Egorov, V.V.; Tsevtkov, V.B.; Kuzmich, N.N.; Savina, V.A.; Kiselev, O.I. Adenosine receptor A 2A as a drug target for the treatment of sepsis. Mol. Biol. 2016, 50, 231–245. [Google Scholar] [CrossRef]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Liu, M.; Liu, X.; Wang, Y.; Sui, Y.; Liu, F.; Liu, Z.; Zou, F.; Zuo, K.; Wang, Z.; Sun, W.; et al. Intrinsic ros drive hair follicle cycle progression by modulating DNA damage and repair and subsequently hair follicle apoptosis and macrophage polarization. Oxidative Med. Cell. Longev. 2022, 8279269, 35. [Google Scholar] [CrossRef]
- Wier, E.M.; Garza, L.A. Through the lens of hair follicle neogenesis, a new focus on mechanisms of skin regeneration after wounding. Semin. Cell Dev. Biol. 2020, 100, 122–129. [Google Scholar] [CrossRef]
- Ding, J.; Lei, L.; Liu, S.; Zhang, Y.; Yu, Z.; Su, Y.; Ma, X. Macrophages are necessary for skin regeneration during tissue expansion. J. Transl. Med. 2019, 17, 36. [Google Scholar] [CrossRef]
- Kasuya, A.; Ito, T.; Tokura, Y. M2 macrophages promote wound-induced hair neogenesis. J. Dermatol. Sci. 2018, 91, 250–255. [Google Scholar] [CrossRef]
- Cai, B.; Zheng, Y.; Liu, X.; Yan, J.; Wang, J.; Yin, G. A crucial role of fibroblast growth factor 2 in the differentiation of hair follicle stem cells toward endothelial cells in a STAT5-dependent manner. Differentiation 2020, 111, 70–78. [Google Scholar] [CrossRef]
- Ardizzone, A.; Bova, V.; Casili, G.; Repici, A.; Lanza, M.; Giuffrida, R.; Colarossi, C.; Mare, M.; Cuzzocrea, S.; Esposito, E.; et al. Role of Basic Fibroblast Growth Factor in Cancer: Biological Activity, Targeted Therapies, and Prognostic Value. Cells 2023, 12, 1002. [Google Scholar] [CrossRef]
- Rittmaster, R.S. Finasteride. N. Engl. J. Med. 1994, 330, 120–125. [Google Scholar] [PubMed]
- Gupta, A.K.; Talukder, M.; Venkataraman, M.; Bamimore, M.A. Minoxidil: A comprehensive review. J. Dermatolog. Treat. 2022, 33, 1896–1906. [Google Scholar] [CrossRef]
- Roque, L.V.; Dias, I.S.; Cruz, N.; Rebelo, A.; Roberto, A.; Rijo, P.; Reis, C.P. Design of finasteride-loaded nanoparticles for potential treatment of alopecia. Skin. Pharmacol. Physiol. 2017, 30, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.S.; Oliveira, P.M.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Latanoprost loaded in polymeric nanocapsules for effective topical treatment of alopecia. AAPS PharmSciTech 2020, 21, 305. [Google Scholar] [CrossRef] [PubMed]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Zhou, Z.; Lenk, R.; Dellinger, A.; MacFarland, D.; Kumar, K.; Wilson, S.R.; Kepley, C.L. Fullerene nanomaterials potentiate hair growth. Nanomedicine 2009, 5, 202–207. [Google Scholar] [CrossRef]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative stress-associated senescence in dermal papilla cells of men with androgenetic alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef]
- Shershakova, N.N.; Andreev, S.M.; Tomchuk, A.A.; Makarova, E.A.; Nikonova, A.A.; Turetskiy, E.A.; Petukhova, O.A.; Kamyshnikov, O.Y.; Ivankov, O.I.; Kyzyma OAet, a.l. Wound healing activity of aqueous dispersion of fullerene C60 produced by “green technology”. Nanomedicine 2023, 47, 102619. [Google Scholar] [CrossRef]
- Galkina, A.A.; Bolyakina, D.K.; Shatilova, A.V.; Shatilov, A.A.; Babikhina, M.O.; Golomidova, A.K.; Andreev, S.M.; Shershakova, N.N.; Khaitov, M.R. Developing and evaluating the effectiveness of wound-healing compounds based on cationic peptides and fullrene. Extrem. Med. 2023, 3, 56–64. [Google Scholar]
- Bosi, S.; Da Ros, T.; Spalluto, G.; Prato, M. Fullerene derivatives: An attractive tool for biological applications. Eur. J. Med. Chem. 2003, 38, 913–923. [Google Scholar] [CrossRef]
- Masalova, O.V.; Lesnova, E.I.; Andreev, S.M.; Shershakova, N.N.; Kozlov, V.V.; Permyakova, K.Y.; Demidova, N.A.; Valuev-Elliston, V.T.; Turetskiy, E.A.; Ivanov, A.V.; et al. Adjuvant effect of dispersed fullerene C60 on the immune response to constructs harboring amino acid and nucleotide sequences of Hepatitis C virus nonstructural NS5B protein. Vopr. Virusol. 2023, 67, 516–526. (In Russian) [Google Scholar] [CrossRef]
- Shershakova, N.; Baraboshkina, E.; Andreev, S.; Purgina, D.; Struchkova, I.; Kamyshnikov, O.; Nikonova, A.; Khaitov, M. Anti-inflammatory effect of fullerene C60 in a mice model of atopic dermatitis. J. Nanobiotechnol. 2016, 14, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Fan, C.H.; Zhu, H.S. Photo-induced cytotoxicity of malonic acid C60 fullerene derivatives and its mechanism. Toxicol. In Vitro 2002, 16, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Klimova, R.; Andreev, S.; Momotyuk, E.; Demidova, N.; Fedorova, N.; Chernoryzh, Y.; Yurlov, K.; Turetskiy, E.; Baraboshkina, E.; Shershakova, N.; et al. Aqueous fullerene C60 solution suppresses Herpes simplex virus and Cytomegalovirus infections. Fuller. Nanotub. Carbon Nanostruct. 2019, 28, 487–499. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lei, H.Y.; Luh, T.Y.; Chou, C.-K.; Liu, H.S. Light independent inactivation of Dengue-2 virus by carboxyfullerene C Isomer. Virology 2000, 275, 258–262. [Google Scholar] [CrossRef]
- Tsao, N.; Luh, T.Y.; Chou, C.K.; Chang, T.Y.; Wu, J.J.; Liu, C.C.; Lei, H.Y. In Vitro action of carboxyfullerene. J. Antimicrobal. Chemother. 2002, 49, 641–649. [Google Scholar] [CrossRef]
- Wang, I.C.; Tai, L.A.; Lee, D.D.; Kanakamma, P.P.; Shen, C.K.; Luh, T.Y.; Cheng, C.H.; Hwang, K.C. C60 and water-soluble fullerene derivatives as antioxidants against radicalinitiated lipid peroxidation. J. Med. Chem. 1999, 4, 4614–4620. [Google Scholar] [CrossRef]
- Li, W.; Li, N.; Sui, B.; Yang, D. Anti-aging effect of fullerenol on skin aging through derived stem cells in a mouse model. Exp. Ther. Med. 2017, 14, 5045–5050. [Google Scholar] [CrossRef]
- Dugan, L.L.; Lovett, E.G.; Quick, K.L.; Lotharius, J.; Lin, T.T.; O’Malley, K.L. Fullerene-based antioxidants and neurodegenerative disorders. Park. Relat. Disord. 2001, 7, 243–246. [Google Scholar] [CrossRef]
- Vileno, B.; Sienkiewicz, A.; Lekka, M.; Kulik, A.J.; Forró, L. In Vitro assay of singlet oxygen generation in the presence of water-soluble derivatives of C60. Carbon 2004, 42, 1195–1198. [Google Scholar] [CrossRef]
- Andreev, I.M.; Romanova, V.S.; Petrukhina, A.O.; Andreev, S.M. Amino acid derivatives of fullerene C60 behave as lipophilic ions that penetrate biological membranes. Solid State Phys. 2002, 44, 658–660. [Google Scholar] [CrossRef]
- Piotrovsky, L.B.; Kiselev, O.I. Fullerenes in Biology; Rostok Publishing House: St. Petersburg, Russia, 2006; 336p. (In Russian) [Google Scholar]
- Aoshima, H.S.; Saytoh, Y.; Ito, S.; Yamana, S.; Miwa, N. Safety evaluation of highly purifiedfullerenes (HPFs): Based on screening of eye and skin damage. J. Toxicol. Sci. 2009, 4, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, O.D.; Morozova, O.V.; Zherdev, A.V.; Yaropolov, A.I.; Klochkov, S.G.; Bachurin, S.O.; Dzantiev, B.B. Study of distribution and biological effects of fullerene C60 after single and multiple intragastrical administrations to rats. Fullerenes. Nanotub. Carbon Nanostruct. 2015, 23, 658–668. [Google Scholar] [CrossRef]
- Litasova, E.V.; Iljin, V.V.; Sokolov, A.V.; Vasilyev, V.B.; Dumpis, M.A.; Piotrovskiy, L.B. The biodegradation of fullerene C60 by myeloperoxidase. Dokl. Biochem. Biophys. 2016, 471, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Fortner, J.D.; Kim, D.I.; Boyd, A.M.; Falkner, J.C.; Moran, S.; Colvin, V.L.; Hughes, J.B.; Kim, J.H. Reaction of water-stable C60 aggregates with ozone. Environ. Sci. Technol. 2007, 41, 7497–7502. [Google Scholar] [CrossRef]
- Schreiner, K.M.; Filley, T.R.; Blanchette, R.A.; Bowen, B.B.; Bolskar, R.D.; Hockaday, W.C.; Masiello, C.A.; Raebiger, J.W. White-rot basidiomycete-mediated decomposition of C60 fullerol. Environ. Sci. Technol. 2009, 43, 3162–3168. [Google Scholar] [CrossRef]
- Shershakova, N.N.; Baraboshkina, E.N.; Andreev, S.M.; Shabanova, D.D.; Smirnov, V.V.; Kamishnikov, Y.O.; Khaitov, M.R. Absence of acute toxicity in an aqueous solution of C60 fullerene. Immunology 2016, 37, 325–329. (In Russian) [Google Scholar]
- Bosticardo, M.; Yamazaki, Y.; Cowan, J.; Giardino, G.; Corsino, C.; Scalia, G.; Prencipe, R.; Ruffner, M.; Hill, D.A.; Sakovich, I.; et al. Heterozygous FOXN1 variants cause low TRECs and severe T Cell lymphopenia, revealing a crucial role of FOXN1 in supporting early thymopoiesis. Am. J. Hum. Genet. 2019, 105, 549–561. [Google Scholar] [CrossRef]
- Begum, S.; Gu, L.J.; Lee, M.R.; Li, Z.; Li, J.J.; Hossain, M.J.; Wang, Y.B.; Sung, C.K. In vivo hair growth-stimulating effect of medicinal plant extract on BALB/c nude mice. Pharm. Biol. 2015, 53, 1098–1103. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, N.X.; Huang, K.; Cai, B.Z.; Zeng, Y.; Xu, Y.M.; Liu, Y.; Yuan, Y.P.; Lin, C.M. iTRAQ-Based Quantitative Proteomic Comparison of Early- and Late-Passage Human Dermal Papilla Cell Secretome in Relation to Inducing Hair Follicle Regeneration. PLoS ONE 2016, 11, e0167474. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Guo, H.; Qiu, W.; Lai, X.; Yang, T.; Widelitz, R.B.; Chuong, C.M.; Lian, X.; Yang, L. Modulating hair follicle size with Wnt10b/DKK1 during hair regeneration. Exp. Dermatol. 2014, 23, 407–413. [Google Scholar] [CrossRef]
- Ouji, Y.; Yoshikawa, M.; Shiroi, A.; Ishizaka, S. Promotion of hair follicle development and trichogenesis by Wnt-10b in cultured embryonic skin and inreconstituted skin. Biochem. Biophys. Res. Commun. 2006, 345, 581–587. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, K.; Ye, J.X.; Lian, X.H.; Yang, T. Wnt10b promotes growth of hair follicles via a canonical Wnt signaling pathway. Clin. Exp. Dermatol. 2011, 36, 534–540. [Google Scholar] [CrossRef]
- Leiros, G.J.; Attoresi, A.I.; Balana, M.E. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/Beta-catenin and androgen signaling in dermal papilla cells from patients with androgenetic alopecia. Br. J. Dermatol. 2012, 166, 1035–1042. [Google Scholar] [CrossRef]
- Mak, K.K.; Chan, S.Y. Epidermal growth factor as a biological switch in hair growth cycle. J. Biol. Chem. 2003, 278, 26120–26126. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Kuwahara, M.; Yoshinaga, Y.; Shirakusa, T. Basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) levels, as prognosis indicators in NSCLC. Eur. J. Cardiothorac. Susg. 2004, 25, 442–448. [Google Scholar]
- Ruckert, R.; Lindner, G.; Bulfone-Paus, S.; Paus, R. High-dose proinflammatory cytokines induce apoptosis of hair bulb keratinocytes In Vivo. Br. J. Dermatol. 2000, 14, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, M.L. TNF superfamily in skin appendage development. Cytokine Growth Factor Rev. 2008, 19, 219–230. [Google Scholar] [CrossRef]
- Philpott, M.P.; Sanders, D.A.; Bowen, J.; Kealy, T. Effects of interleukins, colony-stimulating factor and tumor necrosis factor on human hair follicle growth In Vitro: A possible role for interleukin-1 and tumor necrosis factor-alpha in alopecia areata. Br. J. Dermatol. 1996, 6, 942–948. [Google Scholar] [CrossRef]
- Tosti, A.; Peluso, A.M.; Misciali, C.; Venturo, N.; Patrizi, A.; Fanti, P.A. Loose anagen hair. Arch Dermatol. 1997, 133, 1089–1093. [Google Scholar] [CrossRef]
- Soma, T.; Ogo, M.; Suzuki, J.; Takahashi, T.; Hibino, T. Analysis of apoptotic cell death in human hair follicles In Vivo and In Vitro. J. Investig. Dermatol. 1998, 6, 948–954. [Google Scholar] [CrossRef]
- Shah, R.; Abraham, B.; Hou, J.; Sellin, J. Frequency and associated factors of hair loss among patients with inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 229–232. [Google Scholar] [CrossRef]
- Gorcey, L.; Gordon Spratt, E.A.; Leger, M.C. Alopecia universalis successfully treated with Adalimumab. JAMA Dermatol. 2014, 150, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Marubayashi, A.; Nakaya, Y.; Fukui, K.; Arase, S. Minoxidil-induced hair growth is mediated by adenosine in cultured dermal papilla cells: Possible involvement of sulfonylurea receptor 2B as a target of Minoxidil. J. Investig. Dermatol. 2001, 117, 1594–1600. [Google Scholar] [CrossRef]
- Haskó, G.; Pacher, P. Regulation of macrophage function by Adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G. Adenosine: An endogenous regulator of innate immunity. Trends Immunol. 2004, 25, 33–39. [Google Scholar] [CrossRef]
- Pinhal-Enfield, G.; Ramanathan, M.; Hasko, G.; Vogel, S.N.; Salzman, A.L.; Boons, G.J.; Leibovich, S.J. An angiogenic switch in macrophages involving synergy between Toll-like receptors 2, 4, 7, and 9 and Adenosine A(2A) receptors. Am. J. Pathol. 2003, 163, 711–721. [Google Scholar] [CrossRef]
- Csoka, B.; Selmeczy, Z.; Koscso, B.; Nemeth, Z.H.; Pacher, P.; Murray, P.J.; Kepka-Lenhart, D.; Morris, S.M., Jr.; Gause, W.C.; Leibovich, S.J.; et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012, 26, 376–386. [Google Scholar] [CrossRef]
- Shershakova, N. Fullerene C60, Mechanism of Biological Activity and Development of Approaches to the Treatment of Diseases Associated with Oxidative Stress. Ph.D. Dissertation, Higher Attestation Commission (HAC), Moscow, Russia, 2024. (In Russian). [Google Scholar]
- Conductier, G.; Blondeau, N.; Guyon, A.; Nahon, J.-L.; Rovère, C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J. Neuroimmunol. 2010, 224, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Jena, M.K.; Nayak, N.; Chen, K.; Nayak, N.R. Role of Macrophages in Pregnancy and Related Complications. Arch. Immunol. Ther. Exp. 2019, 67, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Ding, V.M.; Ling, L.; Natarajan, S.; Yap, M.; Cool, S.; Choo, A. FGF-2 modulates Wnt signal-ing in undifferentiated hESC and iPS cells through activated PI3-K/GSK3beta signaling. J. Cell. Physiol. 2010, 225, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Chou, C.H.; Huang, H.D.; Yen, M.H.; Hong, H.C.; Chao, P.H.; Wang, Y.H.; Chen, P.Y.; Nian, S.X.; Chen, Y.R.; et al. Mechanical stretch induces hair regeneration through the alternative activation of macrophages. Nat. Commun. 2019, 10, 1524. [Google Scholar] [CrossRef]
- Carrasco, E.; Calvo, M.I.; Blázquez-Castro, A.; Vecchio, D.; Zamarrón, A.; de Almeida, I.J.D.; Stockert, J.C.; Hamblin, M.R.; Juarranz, Á.; Espada, J. Photoactivation of ROS production in situ transiently activates cell proliferation in mouse skin and in the hair follicle stem cell niche promoting hair growth and wound healing. J. Investig. Dermatol. 2015, 135, 2611–2622. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Zhou, R.; Ma, G.; Dekker, J.D.; Tucker, H.O.; Yao, Z.; Guo, X. Foxp1 regulates the proliferation of hair follicle stem cells in response to oxidative stress during hair cycling. PLoS ONE 2015, 10, e0131674. [Google Scholar] [CrossRef]
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Kang, J.I.; Park, K.M.; Park, K.D. In Situ forming and reactive oxygen species-scavenging gelatin hydrogels for enhancing wound healing efficacy. Acta Biomater. 2020, 103, 142–152. [Google Scholar] [CrossRef]
- Bolshakova, O.; Zherebyatieva, O.; Sarantseva, S.V. Fullerenes In Vivo. Toxicity and protective effects. Nanotoxicology 2025, 19, 233–258. [Google Scholar] [CrossRef]
- Andreev, S.M.; Purgina, D.D.; Bashkatova, E.N.; Garshev, A.V.; Maerle, A.V.; Khaitov, M.R. Facile preparation of aqueous fullerene C60 nanodispersions. Nanotechnol. Russ. 2014, 9, 369–379. [Google Scholar] [CrossRef]
- Coudert, E.; Gehant, S.; de Castro, E.; Pozzato, M.; Baratin, D.; Neto, T.; Sigrist, C.J.A.; Redaschi, N.; Bridge, A. UniProt consortium. Annotation of biologically relevant ligands in UniProtKB using ChEBI. Bioinformatics 2023, 39, 793. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold protein structure database in 2024, providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 2024, 52, D368–D375. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Schöning-Stierand, K.; Diedrich, K.; Ehrt, C.; Flachsenberg, F.; Graef, J.; Sieg, J.; Penner, P.; Poppinga, M.; Ungethüm, A.; Rarey, M. ProteinsPlus: A comprehensive collection of web-based molecular modeling tools. Nucleic Acids Res. 2022, 50, W611–W615. [Google Scholar] [CrossRef] [PubMed]
- Schöning-Stierand, K.; Diedrich, K.; Fährrolfes, R.; Flachsenberg, F.; Meyder, A.; Nittinger, E.; Steinegger, R.; Rarey, M. ProteinsPlus: Interactive analysis of protein–ligand binding interfaces. Nucleic Acids Res. 2020, 48, W48–W53. [Google Scholar] [CrossRef]
- Fährrolfes, R.; Bietz, S.; Flachsenberg, F.; Meyder, A.; Nittinger, E.; Otto, T.; Volkamer, A.; Rarey, M. ProteinsPlus: A web portal for structure analysis of macromolecules. Nucleic Acids Res. 2017, 45, W337–W343. [Google Scholar] [CrossRef]
- Graef, J.; Ehrt, C.; Rarey, M. Binding site detection remastered: Enabling fast, robust, and reliable binding site detection and descriptor calculation with DoGSite3. J. Chem. Inf. Model. 2023, 63, 3128–3137. [Google Scholar] [CrossRef]
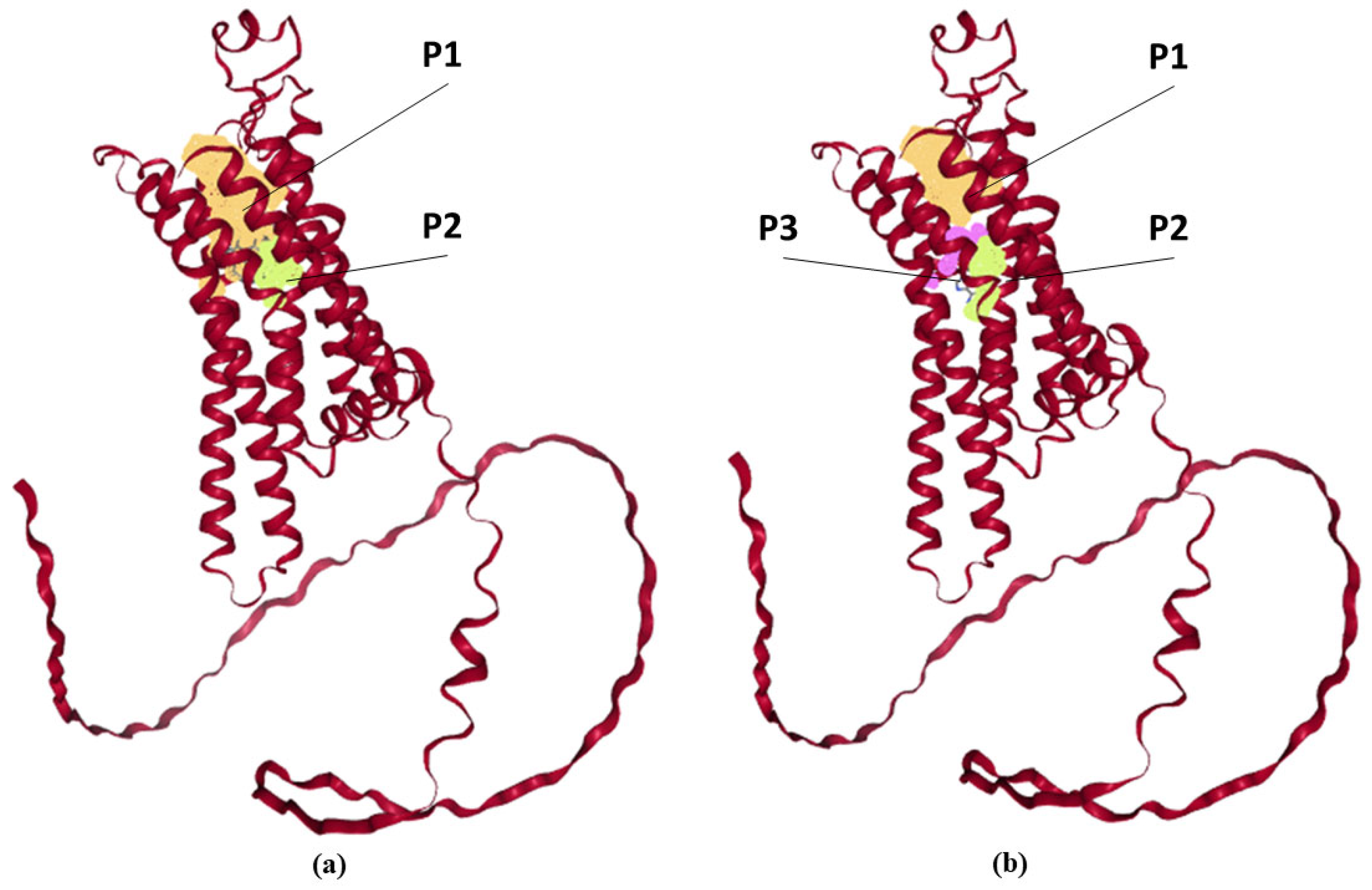

| Ligand | Predicted Pockets | ||||
|---|---|---|---|---|---|
| Name | Size and Shape Descriptors | Element and Functional Group Descriptors | Amino Acid (AA) Composition | AA Residues | |
Fullerene C60 sphere 7 Å | P1 (orange) | Volume 457.216 [Å3] Surface 494.781 [Å2] Depth 22.127 [Å] | Pocket atoms 195 Hydrogen bond donors 9 Hydrogen bond acceptors 11 Aromatic atoms 39 Hydrophobicity ratio 0.74878 | Apolar AA 27 Polar AA 12 Acidic AA 1 Basic AA 3 | Tyr 6, Ala 56, Phe 59, Ala 60, Ile 63, Ser 64, Phe 77, Ala 78, Val 81, Leu 82, Val 83, Thr 85, Gln 86, Ile 89, Ser 129, Ile 132, Gly 133, Leu 162, Phe 163, Glu 164, Tyr 171, Met 172, Asn 176, Phe 177, Phe 180, Val 181, Leu 185, Phe 237, Ala 238, Cys 240, Trp 241, Leu 242, Leu 244, His 245, Asn 248, His 259, Pro 262, Met 265, Tyr 266, Ala 268, Ile 269, Ser 272, His 273 |
| P2 (light green) | Volume 75.776 [Å3] Surface 51.825 [Å2] Depth 9.499 [Å] | Pocket atoms 64 Hydrogen bond donors 5 Hydrogen bond acceptors 6 Aromatic atoms 12 Hydrophobicity ratio 0.7188 | Apolar AA 8 Polar AA 5 Acidic AA 1 Basic AA 1 | Leu 45, Ala 48, Asp 49, Val 52, Ala 56, Val 81, Leu 84, Thr 85, Ser 88, Phe 237, Trp 241, Ser 272, His 273, Asn 275, Ser 276 | |
| Adenosine ligand size 10 × 6 Å  | P1 (orange) | Volume 335.36 [Å3] Surface 408.501 [Å2] Depth 14.333 [Å] | Pocket atoms 92 Hydrogen bond donors 8 Hydrogen bond acceptors 9 Aromatic atoms 21 Hydrophobicity ratio 0.7826 | Apolar AA 16 Polar AA 5 Acidic AA 1 Basic AA 2 | Tyr 6, Ala 56, Phe 59, Ala 60, Ile 63, Ser 64, Phe 77, Ala 78, Val 81, Leu 82, Leu 162, Phe 163, Glu 164, Met 172, Trp 241, Leu 244, Asn 248, His 259, Pro 262, Met 265, Tyr 266, Ile 269, Ser 272, His 273 |
| P2 (light green) | Volume 79.36 [Å3] Surface 52.698 [Å2] Depth 10.911 [Å] | Pocket atoms 69 Hydrogen bond donors 4 Hydrogen bond acceptors 6 Aromatic atoms 11 Hydrophobicity ratio 0.7101 | Apolar AA 9 Polar AA 6 Acidic AA 1 Basic AA 1 | Leu 45, Ala 48, Asp 49, Val 52, Val 81, Leu 84, Thr 85, Ser 88, Leu 92, Ile 233, Phe 237, Trp 241, Ser 272, His 273, Asn 275, Ser 276, Asn 279 | |
| P3 (pink) | Volume 61.44 [Å3] Surface 32.276 [Å2] Depth 12.825 [Å] | Pocket atoms 81 Hydrogen bond donors 1 Hydrogen bond acceptors 2 Aromatic atoms 17 Hydrophobicity ratio 0.7407 | Apolar AA 12 Polar AA 6 Acidic AA 0 Basic AA 1 | Leu 82, Val 83, Thr 85, Gln 86, Ile 89, Ser 129, Ile 132, Gly 133, Tyr 171, Met 172, Asn 176, Phe 180, Val 181, Leu 185, Phe 237, Ala 238, Trp 240, Leu 242, His 245 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shershakova, N.; Baraboshkina, E.; Khochenkov, D.; Turetskiy, E.; Nikonova, A.; Kamyshnikov, O.; Bolyakina, D.; Parshina, V.; Shabanova, D.; Makarova, E.; et al. Aqueous Dispersion of Unmodified Fullerene C60: Stimulation of Hair Growth and Study of a New Molecular Target for Interaction. Int. J. Mol. Sci. 2025, 26, 8517. https://doi.org/10.3390/ijms26178517
Shershakova N, Baraboshkina E, Khochenkov D, Turetskiy E, Nikonova A, Kamyshnikov O, Bolyakina D, Parshina V, Shabanova D, Makarova E, et al. Aqueous Dispersion of Unmodified Fullerene C60: Stimulation of Hair Growth and Study of a New Molecular Target for Interaction. International Journal of Molecular Sciences. 2025; 26(17):8517. https://doi.org/10.3390/ijms26178517
Chicago/Turabian StyleShershakova, Nadezda, Elena Baraboshkina, Dmitry Khochenkov, Evgeny Turetskiy, Alexandra Nikonova, Oleg Kamyshnikov, Daria Bolyakina, Veronika Parshina, Daria Shabanova, Evelina Makarova, and et al. 2025. "Aqueous Dispersion of Unmodified Fullerene C60: Stimulation of Hair Growth and Study of a New Molecular Target for Interaction" International Journal of Molecular Sciences 26, no. 17: 8517. https://doi.org/10.3390/ijms26178517
APA StyleShershakova, N., Baraboshkina, E., Khochenkov, D., Turetskiy, E., Nikonova, A., Kamyshnikov, O., Bolyakina, D., Parshina, V., Shabanova, D., Makarova, E., Andreev, S., Kudlay, D., & Khaitov, M. (2025). Aqueous Dispersion of Unmodified Fullerene C60: Stimulation of Hair Growth and Study of a New Molecular Target for Interaction. International Journal of Molecular Sciences, 26(17), 8517. https://doi.org/10.3390/ijms26178517






